Abstract
Recent studies focused on the pharmacology and feasibility of herbal compounds as a potential strategy to target a variety of human diseases ranging from metabolic to brain disorders. Accordingly, bioactive ingredients which are found within a variety of herbal compounds are reported to produce both neuroprotective and psychotropic activities which may help to combat mental disorders such as depression, anxiety, sleep disturbances and cognitive alterations. In the present manuscript, we focus on three herbs which appear effective in mitigating anxiety or depression with favourable risk-benefit profiles, namely Scutellaria baicalensis (S. baicalensis), Hericium erinaceus (H. erinaceus) and Rhodiola rosea (R. rosea). These three traditional folk medicinal herbs target the main biochemical events that are implicated in mental disorders, mimicking, to some extent, the mechanisms of action of conventional antidepressants and mood stabilizers with a wide margin of tolerability. In detail, they rescue alterations in neurotransmitter and neuro-endocrine systems, stimulate neurogenesis and the synthesis of neurotrophic factors, and they counteract oxidative stress, mitochondrial dysfunction and inflammation. Albeit the encouraging results that emerge from both experimental and clinical evidence, further studies are needed to confirm and better understand the mental-health promoting, and specifically, the antidepressant effects of these herbs.
Keywords: phytochemicals, depression, anxiety, monoamines, neuroprotection, neurogenesis, neurotrophic factors, antioxidant, anti-inflammatory
1. Introduction
A growing number of people worldwide suffer from age- or stress-related mental disorders such as depression, anxiety and insomnia. Since many conventional medications possess either side effects or limited efficacy, many patients increasingly prefer herbal compounds for such mood symptoms relief [1]. Accordingly, recent investigations have focused on the psychopharmacology of naturally-occurring compounds as a potential strategy to target mental disorders. Wide evidence indicates that bioactive ingredients found within a variety of phytochemical compounds are endowed with multiple, yet intricate, psychotropic activities which may help to combat depression, anxiety, sleep disorders and cognitive alterations [2]. Several phytochemicals are reported to produce mental benefits that are comparable to standard anxiolytics and antidepressants in the absence of overt adverse effects [1,2]. As such, they appear as an optimal, first-choice therapy in mild-to-moderate depressive disorders. Furthermore, some herbal extracts may be also combined or enriched with conventional antidepressants and mood stabilizers to alleviate some of their common side effects while potentiating their efficacy [3,4].
In the present manuscript we focus on three neuroprotective herbs which appear effective in mitigating anxiety or depression with favourable risk-benefit profiles, namely Scutellaria baicalensis (S. baicalensis) [5,6,7,8,9], Hericium erinaceus (H. erinaceus) [10,11,12,13,14] and Rhodiola rosea (R. rosea) [15,16,17,18,19,20]. We chose these specific herbs in order to analyse both their overlapping and complementary properties, posing the provocative issue of whether their combined administration may yield synergistic antidepressant-like effects. The general health-promoting effects of S. baicalensis, H. erinaceus and R. rosea in humans and animals are mainly attributable to anti-inflammatory and antioxidant properties [6,7,8,16,17,18,19,21,22,23,24,25,26,27,28,29,30,31].
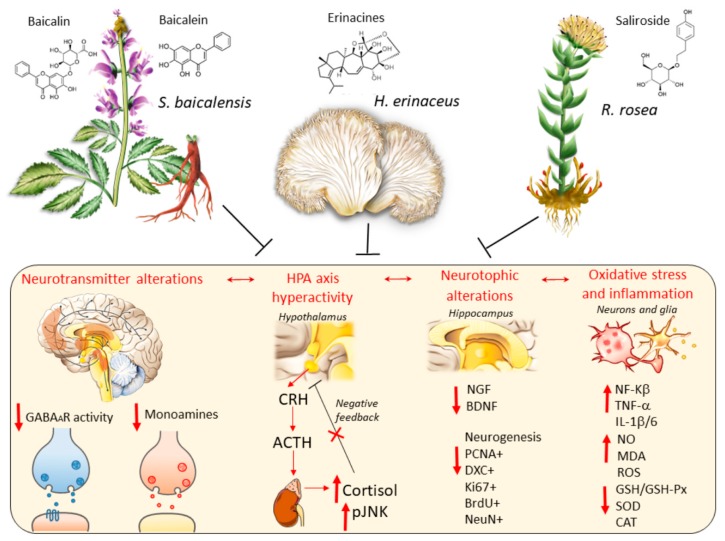
At a first glance, the mechanisms of action of these herbal compounds in mental diseases appear puzzling and often overlapping. The bioactive ingredients found within S. baicalensis, H. erinaceus and R. rosea target the main biochemical events which are implicated in psychiatric conditions [32,33,34] mimicking at some extent the mechanisms of action of conventional antidepressants and mood stabilizers in the absence of serious adverse effects [5,10,13,15,20,35,36] (Figure 1). Far from being the aim of the present manuscript is an attempt to deal with the neurobiology of depression, which is a complex, multifactorial disorder [33], here we limit to reviewing and discussing potential biochemical and molecular mechanisms through which the abovementioned compounds produce anxiolytic/antidepressant-like effects. In detail, bioactive compounds within S. baicalensis, H. erinaceus and R. rosea rescue alterations in monoamine and GABA neurotransmission, and they stimulate neurogenesis and the synthesis of neurotrophic factors while counteracting oxidative stress, mitochondrial dysfunction and inflammation (Figure 1) [5,6,7,8,9,10,11,12,13,14,15,20]. These herbs also modulate neuro-immune and neuro-endocrine functions by targeting hypothalamic-pituitary-adrenal (HPA) axis hyper-activation, which is implicated in mental disorders (Figure 1) [1,19,33]. Nonetheless, at a closer examination, each plant possesses specific effects by acting on different neurotransmitter systems and molecular pathways. After briefly summarizing the main herbal-related neuroprotective mechanisms which may be relevant for depressive disorders, we move to discuss evidence centred on the anxiolytic/antidepressant potential of each herb, as assessed specifically in experimental and clinical studies.
Figure 1.
A general view on the mechanisms of action of S. baicalensis, H. herinaceum and R. rosea against the main biological pathways that are altered in depression and depressive-like behavior (yellowish box). Hypotheses on the neurobiology of depression and anxiety are largely based on dysregulations of (i) neurotransmitter systems, including GABA and GABAA receptors [8], as well as monoamines (dopamine, norepinephrine and serotonin) [9]; (ii) hypothalamic-pituitary-adrenal (HPA) axis consisting of an abnormal release of corticotropin-releasing factor (CRH), adrenocorticotropin (ACTH) secretion from the anterior pituitary, abnormal secretion of glucocorticoids (cortisol in humans and corticosterone in rodents), activation of phosphorylated c-Jun kinase (pJNK), and aberrant negative feedback HPA axis [9]. (iii) impaired neurotrophic mechanisms consisting of low expression of nerve growth factor (NGF) and brain-derived neurotrophic factor (BDNF), impaired neurogenesis and plasticity [9]; (iv) chronic oxidative stress and neuroinflammation [10]. Black T-shaped lines indicate inhibition. Red bold arrows indicate downregulation or hyperactivation. Red thin arrows indicate sequential molecular events.
The still limited, although encouraging, results which emerge from both experimental and clinical studies underline the need for further investigations aimed at dissecting the fine molecular mechanisms of action as well as the safety and pharmacokinetic profiles of these herbal compounds.
2. S. baicalensis, H. erinaceus and R. rosea: Toxicology and Neuroprotective Effects which may be Relevant for their Antidepressant Potential
S. baicalensis, H. erinaceus and R. rosea are folk traditional medicinal herbs that gained increasing popularity for their health-promoting properties including antitumor, hepatoprotective, antimicrobial, anti-inflammatory, anti-hyperlipidemic, antidiabetic, cardio-protective, neurotrophic and neuroprotective effects [5,10,15,16,35,37,38,39,40,41,42,43,44,45,46,47]. In the brain, the beneficial effects of each herb are due to different bioactive compounds, some of which are able to cross the blood-brain-barrier (BBB). For S. baicalensis these correspond to two major flavonoids, namely baicalin (glucuronide) and baicalein (aglycon), being purified from the plant’s dry roots (Scutellariae radix) [48]. H. erinaceus possesses three main classes of bioactive compounds, namely polysaccharides, hericenones and erinacines, with the first two being extracted from the fruit bodies and the latter from the mycelia. Despite the widely reported beneficial effects of these H. erinaceus bioactive ingredients in the brain, to date only erinacines have been documented to cross the BBB [49]. Eventually, the main bioactive compound of R. rosea is salidroside glycone, which has been detected in the brain tissue as well [29,50,51].
Based on toxicological and clinical studies, S. baicalensis, H. erinaceus and R. rosea are generally considered to be safe and well tolerated [40,52,53,54,55,56,57,58,59,60,61,62,63]. In experimental studies, the estimated median lethal dose (LD50) of some compounds varies according to the extraction method and administration route [54]. In fact, as reported by pioneer toxicological studies, following subcutaneous injection in mice, the median lethal dose (LD50) of both S. baicalensis ethanolic extract and isolated baicalin is 6 g/kg [54]. Instead, the LD50 of isolated baicalin following intraperitoneal injection is 3.081 g/kg [54]. More recently, 2.5 g/kg of S. baicalensis ethanol extracts were shown to be safe in rats, though some reversible inflammatory changes were detected in the liver [55]. Baicalin was shown to inhibit the proliferation of embryonic stem cells at half maximal inhibitory concentration (IC50) values up to 135.9 mg/l, suggesting that it may induce low embryonic toxicity at high concentrations [56]. In humans, oral intake of S. baicalensis extracts and baicalin at the daily doses of 300 mg and 200–800 mg respectively, is generally safe and well tolerated [57,58].
As far as it concerns H. erinaceus, the acute oral LD50 of its erinacine-A-enriched mycelia is higher than 5 g/kg in rats [59]. As shown by sub-chronic toxicology studies, erinacine-A-enriched H. erinaceus administered daily for 28 days or 13 weeks is safe and not teratogenic at doses up to 3 g/kg and 2.625 g/kg, respectively, which is nearly 171 times the recommended daily intake for humans (1.05 g/60 kg of body weight/day) [60,61]. Moreover, H. erinaceus mycelium is not mutagenic in the bacterial reverse mutation test (Ames test), in vitro chromosome aberration test, and in vivo erythrocyte micronucleus test [61]. In line with these studies, in rats, no toxicity signs are observed following oral administration of an H. erinaceus mycelia extract at 2.395 g/kg [49]. In humans, no adverse effects are reported following oral intake of H. erinaceus extracts at the cumulative dose of 1.650 g/day (of which 80% bulk mycelia, and 20% fruiting body extracts) [62].
Pioneer acute toxicity studies showed that R. rosea possesses low toxicity in rats, with LD50 being estimated as 3.360 g/kg [63]. However, in more recent toxicological studies on R. rosea-treated mice and A. salina brine shrimp, neither the LD50 >5.000 g/kg nor the LC50 >1.000 mg/ml exhibited toxic effects [64]. Salidroside, the main active component of R. rosea is not genotoxic at doses up to 1.5 g/kg in mice and it does not lead to maternal or embryonic toxicity at 0.125 g/kg in rats [65,66]. In summary, based on known toxicological data on animal studies, R. rosea is generally evidenced to be safe, with no acute and chronic toxicity under the experimental conditions at its therapeutic window [53,64]. In line with this, oral intake of R. rosea at does up to 680 mg/day does not produce any serious adverse effects in humans [67].
2.1. Common Neuroprotective Effects of S. baicalensis, H. erinaceus and R. rosea
S. baicalensis, H. erinaceus and R. rosea produce neuroprotective effects in models of Parkinson’s disease (PD) [6,7,12,17,68,69], Alzheimer’s disease (AD) [11,70,71,72,73,74,75,76,77,78,79], Huntington disease (HD) [18], hypoxia/hypo-perfusion/stroke [51,80,81], nerve and brain injury [82,83], glutamate-induced neurotoxicity [16,75,84], and epilepsy [85,86,87]. The in vitro and in vivo neuroprotective effects of these herbal compounds, administered as either full/enriched herbal extracts and/or as single bioactive ingredients, are largely attributable to antioxidant, mitochondrial-protecting and anti-inflammatory activities (Figure 2).
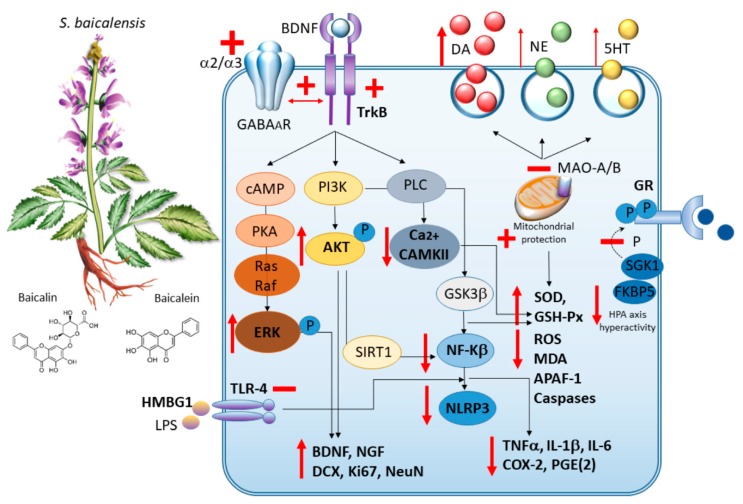
Figure 2.
S. baicalensis: molecular mechanisms underlying neuroprotection and anxiolytic/antidepressant-like effects. By acting as MAO A/B inhibitors, S. baicalensis and its bioactive ingredients (mostly baicalin and baicalein) induce monoamine, and mostly DA release. At the same time, they act as partial, subtype-selective GABAA receptor ligands and also foster the interaction of GABAA receptors with TrkB to potentiate GABA-induced signaling. By increasing cAMP/pERK and PI3K/pAKT signaling, they promote the synthesis of neurotrophic factors (BDNF and NGF) as well as neurogenesis. S. baicalensis promotes anti-inflammatory effects through inhibition of HMBG1/TLR4/NF-k, AKT/SIRT1/NF-k, and GSK3/NF-k axes. This leads to a reduction in NLRP3 and proinflammatory cytokines levels. At the same time, S. baicalensis promotes anti-oxidant effects which are bound to both Ca2+/CAMKII pathway inhibition and mitochondrial protection. By downregulating SGK1 and FKBP5, S. baicalensis also counteracts alterations in glucocorticoid receptors (GR) associated with phosphorylation of its crucial serine residues and HPA axis hyperactivity.
In detail, common antioxidant properties of these compounds consist of reducing the levels of reactive oxygen and nitrogen species (ROS and RNS, respectively), enhancing superoxide dismutase (SOD), glutathione (GSH), glutathione peroxidase (GSH-Px) and catalase (CAT) activities along with heat shock proteins 70 (HSP70), heme oxygenase-1 (HO-1) and thioredoxin levels, and eventually, decreasing lipid peroxidation assessed as reduction of malondialdehyde (MDA) content and lipoxygenase (LPX) inhibition [6,7,12,17,26,27,28,69,70,73,75,78,79,81,85,86,87]. They also counteract mitochondrial alterations, endoplasmic reticulum (ER) stress, and apoptosis by improving mitochondrial membrane potential (MMP) depolarization and ATP production while promoting mitophagy and mitochondrial biogenesis, and by decreasing the levels of C/EBP Homologous Protein (CHOP), pJNK, p-p38, Bax/Bcl-2 ratio, caspases 3, 6 and 9, and cytochrome-c release [12,16,51,68,69,71,73,75,77,78,81,83]. Again, S. baicalensis, H. erinaceus and R. rosea counteract inflammation by decreasing glial cells activation, inducible nitric oxide synthase (iNOS) and NF- κB levels, as well as the production of pro-inflammatory cytokines TNF-α and IL-β1 and IL-6 [8,16,29,74,79,83] (Figure 3). Despite being assessed in neurotoxicity models, these effects may be also key for depressive-related disorders, where oxidative stress, mitochondrial alterations and inflammation are widely implicated [34]. As a further common effect which is relevant to both neurodegenerative and mental disorders, these herbs also promote neurogenesis, neuronal differentiation and the synthesis of neurotrophic factors [8,12,72,74], and they enhance the release of dopamine (DA), norepinephrine (NE) and serotonin (5-HT), in part by acting as monoamine oxidase (MAO) inhibitors [6,7,12,88,89,90,91]. Nonetheless, at a closer look, each herb also possesses specific effects which may complementarily contribute to their mood-stabilizing potential beyond neuroprotection. These effects are discussed in the following section before moving to experimental and clinical studies on depression.
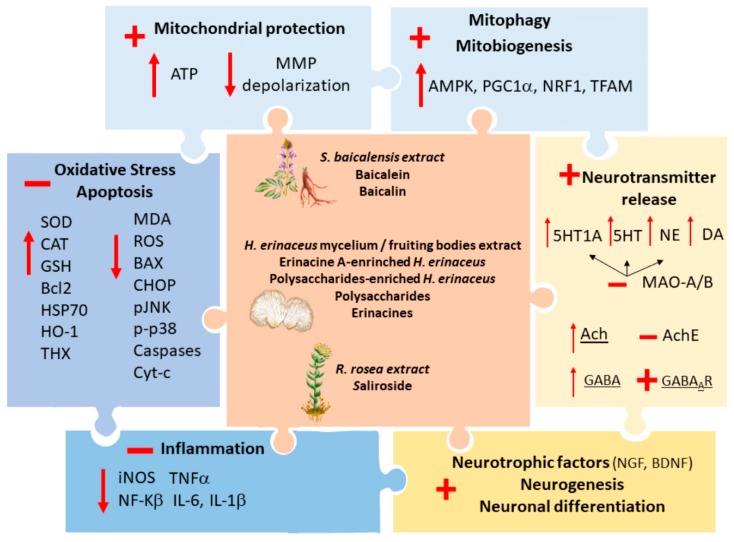
Figure 3.
Mechanisms underlying the neuroprotective effects of S. baicalensis, H. erinaceus and R. rosea. The neuroprotective effects of S. baicalensis, H. erinaceus and R. rosea, administered as either full/enriched herbal extracts and/or as single bioactive ingredients (central box), are bound to antioxidant, mitochondrial-protecting, anti-inflammatory, neurotransmitter release-stimulating and neurotrophic/neurogenic activities. These effects are here represented as pieces of a puzzle where S. baicalensis, H. erinaceus and R. rosea perfectly fit in the middle. Antioxidant properties of these compounds consist of reducing the levels of reactive oxygen species (ROS), enhancing superoxide dismutase (SOD), glutathione (GSH) and catalase (CAT) activities along with heat shock proteins 70 (HSP70), heme oxygenase-1 (HO-1) and thioredoxin (THX) levels, while decreasing lipid peroxidation (malondialdehyde, MDA, content) and counteracting apoptosis through reduction of Bax, C/EBP Homologous Protein (CHOP), pJNK, pp38, caspases 3, 6 and 9, and cytochrome-c release. These effects are closely associated to mitochondrial protection, namely improvement of mitochondrial membrane potential (MMP) depolarization and ATP production, and concomitant enhancement of mitophagy and mitochondrial biogenesis via increasing 5’ AMP-activated protein kinase (AMPK), Peroxisome proliferator-activated receptor gamma coactivator 1-alpha (PGC1α), Nuclear respiratory factor 1 (NRF1) and Mitochondrial transcription factor A (TFAM). At the same time the three herbs promote anti-inflammatory effects by decreasing NF-kβ and iNOS enzymes levels while inhibiting the release of pro-inflammatory cytokines TNF-α, IL-6 and IL-1β. These herbs also target alterations of neurotransmitter systems, by enhancing the release of monoamine through MAO inhibition, acetylcholine (Ach) via inhibition of acetylcholine esterase (AchE), and GABA-induced inhibitory currents by promoting stimulation of GABAA receptors.
2.2. Complementary Effects of S. baicalensis, H. erinaceus and R. rosea
S. baicalensis primarily targets alterations of the DA system, which are implicated in various neurological and mental disorders, and also in their comorbidity [92]. Immediately after intravenous administration, total flavonoids from Scutellariae radix are able to cross the BBB, with the striatum and hippocampus being the most prominent targets [88]. Remarkably, baicalin distributes most specifically to the DA system and it induces an increase in DA levels in the rat striatum, hippocampus and cortex [88,89,93]. Accordingly, recent studies suggest that baicalin and baicalein may induce beneficial effects in DA-related brain disorders by increasing DA levels in the brain besides protecting dopaminergic neurons from mitochondrial- and oxidative-related toxicity [6,7,94]. In fact, baicalein fully prevents 6-OHDA- and MPTP-induced behavioral alterations by preventing reductions of striatal DA levels, the increase in DOPAC/DA and HVA/DA ratios and the loss of striatal tyrosine hydroxylase (TH) [6,7]. Similarly, baicalin prevents methamphetamine (METH)-induced alterations, namely the loss of DA and DA transporter (DAT) in the striatum, which play an important role in the pathogenesis of mental disorders [94]. Baicalin also ameliorates synaptogenesis and memory-related dysfunctions which are associated with GABAA receptor downregulation following abnormal stimulation of DA D1 receptors (D1Rs) [95]. In detail, baicalin prevents the reduction of GABAA-induced currents which occurs following the in vivo administration of exogenous DA and abnormal stimulation of D1Rs [95]. Mechanistically, baicalin fosters the interaction of GABAAR with tyrosine kinase receptor B (TrkB) and AKT thus reversing the DA-induced decrease in the expression of GABAAR/TrkB/AKT pathway. Thus, baicalin plays a key role in modulating the GABAergic system. This is supported by the emerging role of baicalin as a potential anxiolytic by acting as a partial, subtype-selective GABAA receptor ligand [9]. These findings are in line with increasing evidence centered on the neuroprotective and nootropic action of Scutellariae radix extracts in substance-induced addiction, attention deficit hyperactivity disorders, depression and anxiety [93,95,96].
H. erinaceus has a prominent effect on neurotrophic factors’ induction and modulation of NE system. In detail, H. erinaceus bioactive compounds, especially erinacines and hericones, possesses strong nerve growth factor (NGF)-stimulating properties, showing remarkable neurite outgrowth activities in various cell lines and in dissociated cells of brain, spinal cord, and retina [40,97,98,99,100,101,102,103,104,105,106,107,108]. In detail, H. erinaceus enhances NGF-mediated neurite outgrowth via activation of the Trk/MEK/ERK and PI3K-Akt signalling pathways [105,106]. Recently, two novel H. erinaceus cyathane diterpenoid derivatives were shown to promote BDNF expression in vitro, suggesting a common, yet unknown, upstream target for both NGF and BDNF induction [109]. Intriguingly, in vivo administration of erinacine A-enriched H. erinaceus extract produces an increase in NGF levels which is detected specifically within the major NE-producing brainstem nucleus Locus Coeruleus (LC) and within the hippocampus of rats [90]. Remarkably, such an effect matches the increase in H. erinaceus-induced NE levels within the LC and hippocampus, suggesting that H. erinaceus, and mostly erinacines, may modulate neurotrophin-neurotransmitter interactions, especially in the LC-hippocampal axis. This is key since NE-LC neurons are critically involved in stress-response, depression and sleep disturbances; in fact, different stress-related molecular changes related to the LC have been detected in patients with mood disorders, and LC-CA1 hippocampal projections are decreased in mice models of chronic social defeat stress (CSDS) and chronic footshock stress (CFS) [110,111]. Remarkably, an early degeneration of NE-LC neurons occurs in AD [112], and such a phenomenon was recently linked to depressive symptoms characterizing early AD stages [113]. In detail, a minimal loss of NE-LC neuronal population following a bilateral infusion of the neurotoxin 6-OHDA produces depressive-like behaviors in mice, which can be reversed by administration of NE precursors [113]. Thus, by stimulating NE and NGF/BDNF synthesis, H. erinaceus may produce plastic effects which are expected to counteract behavioural alterations besides neurotoxicity. In fact, H. erinaceus reverses early learning and memory deficits which are induced by amyloid beta peptides independently of neuropathology in AD mice models [114].
Eventually, R. rosea possesses remarkable multi-target activities on both cellular and systemic levels of stress-response regulation [115]. As assessed through RNA microarray studies in neuroglial cell lines, R. rosea modulates the transcription of various biological and molecular mediators that are associated with emotional behavior, particularly aggressive behaviour [116]. In detail, R. rosea regulates various components of the antioxidant, anti-inflammatory, neuroendocrine, neurotrophic and neurotransmitter receptor pathways, which are likely associated with both its neuroprotective potential and beneficial effects on mood [15]. R. rosea increases the levels of both monoamines and acetylcholine (Ach) in nerve terminals and is likely bound to its mental health-promoting effects concerning both mood and cognition [91,115,116,117,118,119,120,121]. However, compared with S. baicalenis and H. erinaceus, R. rosea produces more significant effects at the level of the 5-HT system, by increasing 5-HT and 5-HT1A receptor levels [121,122]. R. rosea also produces remarkable anti-stress effects by modulating HPA axis and opioid peptides release and by acting as a corticotrophin-releasing factor (CRF) antagonist, thus blunting cortisol release [37,115,118]. Again, R. rosea inhibits stress-induced molecular events including abnormal cortisone release, nitric oxide production and pJNK expression [123]. At the molecular levels, R. rosea inhibits stress-induced over-activation of the pSAPK/pJNK pathway, which is bound to oxidative stress as well as altered synaptic plasticity and glucocorticoid receptors (GR) responsivity, and this is specifically implicated in its antidepressant-like effects [36,123]. At the same time, R. rosea stimulates the release of stress-related molecules, namely neuropeptide Y (NPY) and Hsp72, which likely represents a defense cellular response aimed at increasing tolerance and adaptation to stress [124]. These findings are in line with a growing body of both preclinical and clinical evidence indicating its potential use in the prevention and treatment of stress- and age-related cognitive and mood alterations, including fatigue, weakness, depression and anxiety [15,43,110,115,117,125,126,127].
3. S. baicalensis, H. erinaceus and R. rosea: Anxiolytic/Antidepressant Effects in Experimental and Clinical Studies
3.1. S. baicalensis in Experimental Models
3.1.1. Anxiety
The anxiolytic-like effects of S. baicalensis have been demonstrated since the early 1990s [128]. Administration of S. baicalensis extract and baicalin was shown to normalize all of the hormonal metabolic disturbances which develop in rats exposed to stress by fixation. These include alterations in insulin, urea, glucose, corticotrophin and hydroxycorticosteroids levels, which are bound to alterations of hypothalamic and adrenocortical functional activity [128].
Recently, baicalin emerged as an alternative, GABAA receptor benzodiazepine (BZ)-site ligand possessing minimal side effects along with a selective activity profile compared with BZs [9,129,130,131]. Despite common prescription of conventional BZs as potent anxiolytics, the numerous side effects they produce such as sedation, myorelaxation, amnesia, and addiction, have continuously prompted the search for alternative BZ site ligands with less side effects. Baicalin was identified as a BZ-site ligand since the early 2000s [129], and this was associated with anxiolytic-like activity occurring in the absence of sedative and myorelaxant side effects, as assessed in mice through Vogel conflict test and elevated plus maze test [130,131].
Subsequent studies also assessed the effects of baicalin on GABAA receptor binding selectivity as well as on the cognitive impairment, anticonvulsant and motor incoordination side effects that are associated with conventional anxiolytics [9]. Contrarily to diazepam (3 mg/kg), baicalin (3.3–30 mg/kg) acts as a subtype-selective partial agonist of GABAA receptors, exhibiting no amnesic, anticonvulsant, and motor incoordination activities in mice, as assessed through the step-through passive avoidance test, picrotoxin-induced seizure test and rotarod test, respectively. Contrarily to the full agonist diazepam, baicalin shows a selectivity profile by acting through α2- and α3-containing GABAA receptor subtypes [9]. Electrophysiological studies showed that baicalin mildly potentiates GABA-induced currents, and this can be abolished by co-application of the BZ site antagonist flumazenil. Thus, baicalin acts a partial BZ-site agonist, which may explain its anxiolytic activity with limited side effects.
The anxiolytic-like effects of centrally administered baicalein were also evaluated in mice [132]. Baicalein exerts an anxiolytic-like effect already at low doses (0.02, 0.2 pmol), increasing the time spent in open arms and the head-dipping while reducing the stretched-attend postures in the elevated plus-maze. These effects are abolished by pretreatment with dehydroepiandrosterone sulfate (DHEAS) and pentylenetetrazol (PTZ) but not dl-p-chlorophenilalanine ethyl ester (PCPA) and flumazenil (FMZ), suggesting that the pharmacological activities of baicalein may dependent on GABAergic ligand sites other than BZ ones [132].
3.1.2. Chronic Corticosterone-Induced Depression
In mice models of chronic corticosterone (CORT)-induced depression, baicalin (40, 80, and 160 mg/kg) administered either orally or intragastrically produces anxyolitic- and antidepressant-like effects which are reproduced by the conventional antidepressant fluoxetine [133,134]. In fact, baicalin rescues the behavioral alterations induced by chronic CORT, namely the decreased time spent in the center and non-periphery zone in the open-field test, the increased immobility time in tail suspension test and forced swimming test, as well as the decreased time spent in open arms in the elevated plus maze test [133,134]. In chronic CORT-treated mice, baicalin restores the aberrant negative feedback of HPA axis, as assessed by dexamethasone suppression test [133]. By applying proteomics and systems biology, biological processes related to glucocorticoid receptor (GR) signaling were identified as potential molecular targets. In fact, baicalin selectively targets CORT-induced alterations in the nuclear/cytoplasmic GR distribution in the hippocampus. In detail, baicalin selectively reverses the CORT-induced decrease of GR levels in cytoplasm and the concomitant increase of GR levels in nucleus [133]. On the other hand, the CORT-induced enhancement of GR in nucleus, as well as the increased GR phosphorylation status at its crucial serine residues are similarly decreased by both baicalin and fluoxetine [133]. Baicalin, similar to fluoxetine, restores normal GR function by normalizing the levels of two GR-phosphorylating proteins, namely FK506-binding protein 51 (FKBP5) and serum- and glucocorticoid-inducible kinase 1 (SGK1) [134]. The antidepressant-like effects of baicalin are also associated with neurogenic activity. In fact, baicalin reverses the CORT-induced decrease in Ki67- and doublecortin (DCX)-positive cells in the dentate gyrus of the hippocampus [134].
3.1.3. Olfactory Bulbectomy-Induced Depression
In olfactory bulbectomy (OBX) mice models of depression, baicalin treatment (20 and 40 mg/kg) significantly reverses the abnormal levels of serum corticosterone, while normalizing behavioural alterations assessed through sucrose consumption, open field test, and forced swimming test [135]. This is associated with downregulation of the inflammatory factors IL-1β, IL-6, and TNF-α in the hippocampus and hypothalamus, which occurs through inhibition of the SIRT1-NF-kB pathway [135]. The antidepressant-like effects of baicalin (20 and 40 mg/kg) in OBX rat models are also associated with anti-oxidant and antiapoptotic activity. In fact, baicalin reverses OBX-induced alterations in the levels or activity of MDA and GSH-Px while preventing apoptotic protease-activating factor-1 (APAF-1) expression and subsequent caspase-mediated signalling cascades [136].
In the context of olfactory dysfunction-related depression, the effects of baicalin were evaluated in a transgenic mice model featuring hyperactivation of APPL2 (adaptor protein phosphotyrosine interacting with PH domain and leucine zipper 2). This mutation leads to depressive-like behaviour due to abnormal GR activity, impaired neurogenesis at olfactory system and loss of olfactory sensitivity [137]. Baicalin treatment blunts APPL2/GR signaling pathway and it improves neurogenesis at the subventrivular zone, olfactory bulb, and hippocampus in APPL2-Tg mice and also in a chronic CORT model. Behavioral tests revealed that baicalin similarly attenuates depressive- and anxiety-like behavior while improving olfactory functions both in APPL2-Tg mice and the chronic CORT depression model [137].
3.1.4. Streptozotocin-Induced Depression
S. baicalensis bioactive compounds produce antidepressant-like and cognitive-enhancing effects by targeting systemic metabolic alterations which are commonly associated with neurological and mental disorders [8]. In detail, in rat models of diabetes mellitus established via intraperitoneal injection of streptozotocin, baicalin (50, 100 and 200 mg/kg) administered daily for 7 weeks reverses depressive-like and cognitive alterations such as decreased percentage of time spent in the target quadrant, the number of times of crossing the platform in the water maze test, as well as the increase in escape latency and mean path length in the water maze test [8]. At the molecular level, baicalin counteracts streptozotocin-induced neuronal cell loss as well as the decline and increase of hippocampal acetylcholine transporter (ChAT) and acetylcholinesterase (AChE) levels, respectively. This is accompanied by increased levels of pERK, Bcl 2, and BDNF. At the same time, baicalin decreases plasma glucose levels and counteracts apoptosis by reversing the increase in phosphorylated c-Jun N-terminal kinase (pJNK), p38, caspase 3 and Bax levels [8].
3.1.5. Chronic (unrestraint) Mild Stress-Induced Depression
In mice models of chronic (unrestraint) mild stress (CUMS), baicalin (25, 50 and 60 mg/kg) alleviates depression-like behavior by increasing sucrose consumption and reducing immobility times in the tail suspension and forced swim tests [138,139,140]. These effects are associated with a reduction of inflammatory cytokines IL-1β, IL-6, and TNF-α levels in serum and in the hippocampus [138,139]. In mice subjected to CUMS baicalin also abrogates the increase in NMDAR/NR2B and Ca2+/calmodulin-dependent protein kinase II (CaMPK-II) as well as the decrease in pERK, while counteracting reactive oxygen species (ROS) production [138]. Baicalin administration also counteracts CUMS- and neuroinflammation (LPS)-induced depressive-like behavior in mice, and this occurs through downregulation of TLR4 via the HMBG1/NF-kb [139] and PI3K/AKT/FoxO1 pathways [140].
Baicalin administration exerts antidepressant-like and neuroprotective effects by counteracting oxidative stress and apoptosis in CUMS models [141]. In fact, it reduces the level of MDA, caspase-1 and IL-1β while increasing SOD in the hippocampus. These effects are mediated by inhibition of glycogen synthase kinase-3 (GSK3β)/ NF-κB / NLRP3 (Nucleotide-binding domain, leucine-rich repeat, pyrin domain containing protein 3) signaling pathway [141]. This is in line with a reduction of NLRP3 inflammasome levels which is detected in the prefrontal cortex of CUMS mice following administration of baicalin (20, 40mg/kg) [142].
Chronic CMS models of depression have been also employed to compare the effects of baicalin with fluoxetine [35,143]. Mice from both the baicalin and fluoxetine groups show a decrease in depression-like behavior compared with controls. Both baicalin (25, 50, 100 mg/kg) and fluoxetine (10 mg/kg) induce plastic changes by counteracting the CMS-induced decrease in the expression levels of the synaptic proteins synaptophysin (SYP), postsynaptic density protein-95 (PSD95), as well as TrkB, Rac1, cofilin and BDNF [143]. Remarkably, baicalin treatment alleviates the ultrastructural alterations occurring in the hippocampal CA3 area of the CMS group [143].
In another study on the CMS rat model, the effect of orally administered baicalin at the dose of 25 mg was as potent as that of fluoxetine 20 mg/kg [35]. This is associated with inhibition of monoamine oxidase A and B (MAO A/B) activity by baicalin [35]. In line with the beneficial effects of baicalin in counteracting monoamine-related alterations, these results suggest that baicalin may produce an antidepressant-like effect in vivo, at least in part, through MAO inhibition [35]. In CMS models, the antidepressant-like effects of chronic baicalin treatment (10, 20, 40 mg/kg) are also associated with anti-inflammatory activity, consisting of a reduction in the mRNA expression and activity of cyclooxygenase-2 (COX-2), as well as prostaglandin E(2) (PGE(2)) levels in the frontal cortex and hippocampus [144].
In CUMS mice, baicalin administration, through activation of the Akt/FOXG1 pathway, also promotes neurogenesis by increasing the number of DCX-positive cells, while fostering neuronal maturation, differentiation and survival [145]. These findings are reproduced in CUMS mice which are administered with full Radix Scuellariae extract (500, 1000 mg/kg) [146], which markedly reverses the shortened escape latency in morris maze test, the reduced immobility time in tail suspension test and in forced swimming test, as well as the increased sucrose consumption in sucrose preference test. These effects are associated with an enhancement of cAMP/PKA-dependent neurogenesis, as shown by the reversal of CUMS-induced reduction in BrdU, DCX and NeuN in the mice hippocampi [146].
Overall, these findings suggest that the antidepressant-like effects of baicalin are due to inhibition neuroinflammation and oxidative stress, potentiation of neurogenesis and neuronal differentiation, as well as amelioration of alterations associated with HPA axis hyperactivity, GABA and DA systems (Figure 2). Encouraging results are also achieved using S. baicalensis radix extract [146], though further experimental studies are needed to confirm its antidepressant-like action compared with baicalin alone.
3.2. S. baicalensis in clinical studies
To date, clinical studies assessing specifically the antidepressant effects of S. baicalensis are still lacking. In the literature, there are only two available studies reporting the tolerability and the cognitive enhancing properties of S. baicalensis in humans. In detail, Pang et al. investigated the pharmacokinetics, safety and tolerability of baicalein after a multiple-ascending-dose protocol in thirty-three healthy Chinese volunteers. Participants were randomized to receive baicalein chewable tablets (n = 8 per dose regimen) or placebo (n = 2 per dose regimen). Dosing regimens were 200, 400, and 800 mg once daily on days 1 and 10, and twice daily on days 3–9. In the dose range of 200–800 mg, multiple-dose oral baicalein administration was safe and well tolerated [57].
In a human clinical trial, subjects who were orally given 300 mg of S. baicalensis extract (formulation UP326) for 30 days showed a marked improvement in speed and accuracy of processing complex information in computer tasks [58]. They also showed a reduced standard deviation of performance compared to baseline and the placebo group. All study compounds were well tolerated with no reports of serious or unexpected adverse effects. Thus, S. baicalensis may help to maintain memory, sustain speed of processing, and reduce the number or ageing-associated memory decline. Nonetheless, it cannot be argued that S. baicalensis has antidepressant effects in humans. This is also based on a much higher concentration of baicalin used in experimental compared with clinical studies.
3.3. H. erinaceus in Experimental Studies
Recent preclinical and clinical studies have shown that besides enhancing cognitive function and conferring neuroprotection, H. erinaceus also improves depression, anxiety, and sleep disturbances [39,115,147,148]. In line with this, recent studies have also been exploring the feasibility of mushrooms as potential fortified foods enriched with lithium, a well-known and gold standard mood stabilizer [4]. Co-cultivation of H. erinaceus with 0.25–1.0 mM lithium chloride results in a concentration-dependent uptake of lithium and its accumulation in H. erinaceus fruiting bodies. Such a supplementation does neither alter mushroom biomass, appearance, shape or size nor does it produce significant effects on mineral composition. As calculated, consumption of 100 g dry weight of H. erinaceus fruiting bodies supplemented with 1.0 mM lithium would constitute 69% of the provisional recommended dietary daily intake of lithium (1.0 mg). This suggests that H. erinaceus deserves to be further studied in experimental models and eventually, human studies in terms of both safety and potential synergistic activity with other herbs or conventional mood stabilizers [4].
Compared with saline-treated mice, dietary administration of H. erinaceus ethanolic fruit body extracts at 60 mg/kg once a day for 4 weeks reduces anxiety and depressive-like behaviour as assessed through elevated plus-maze, tail-suspension and forced swimming tests [149]. This is associated with increased proliferation of hippocampal progenitors and enhanced neurogenesis, which was evaluated by immunohistochemistry of proliferating cell nuclear antigen (PCNA) and Ki67 in the subgranular zone of the hippocampus, and BrdU/NeuN-positive cells in the dentate gyrus [149]. However, the present study carries an inherent limitation since healthy mice instead of depression mice models were employed as positive controls [149].
3.3.1. Inflammation-Related Depression
The effects of H. erinaceus were evaluated in a mouse model of inflammation-induced depression consisting of intraperitoneal LPS administration [13]. Oral administration of the H. erinaceus fruit body extract amycenone (200 mg/kg) prior to a single administration of LPS (0.5 mg/kg) improves depressive-like behaviour compared with LPS-treated mice, as shown by a reduction in the immobility time measured at tail-suspension test and forced swimming tests. At the molecular level, H. erinaceus reduces serum levels of the pro-inflammatory marker TNF-α while increasing the anti-inflammatory cytokine interleukin-10 (IL-10). These effects are reproduced by the administration of paroxetine (30 mg/kg) suggesting that H. erinaceus extracts may be beneficial in inflammation-related depression [13].
3.3.2. Restraint Stress-Induced Depression
The findings on the anti-inflammatory and neurotrophic properties of H. erinaceus in relation with its antidepressant-like efficacy were recently reproduced in a mice model of repeated restraint stress (RS) [14]. Mice were orally administered H. erinaceus mycelium ethanolic extract at the daily dose of 100, 200 or 400 mg/kg b.w. for 4 weeks, and RS protocol was started at 2 weeks of H. erinaceus administration. Compared with saline-treated animals subject to RS, H. erinaceus, especially at the highest doses, could reverse the depressive-like behaviour caused by RS, namely the increased immobility time in the tail suspension and forced swimming tests, as well as the increased number of entries and the time spent in the open arm. This was accompanied by an increase in monoamine neurotransmitters levels along with a reduction of the pro-inflammatory factors IL-6, TNF-α and NF-κB, and the upregulation of BDNF [14]. The molecular mechanisms of action of H. erinaceus are summarized in Figure 4.
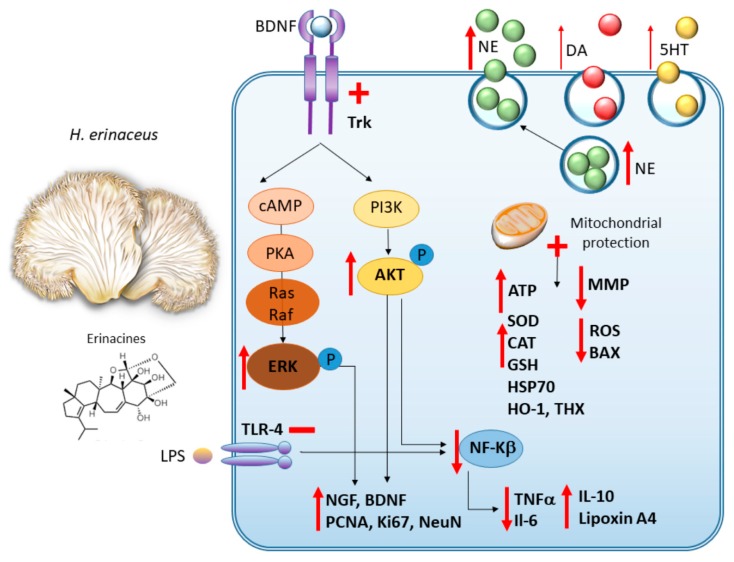
Figure 4.
H. erinaceus: molecular mechanisms underlying neuroprotective and antidepressant-like effects. H. erinaceus and its bioactive ingredients (mostly erinacines) potentiate monoamine, and mostly norepinephrine (NE) synthesis and release. At the same time, they promote TrkB-related increase in pERK and pAKT and the subsequent synthesis of neurotrophic factors (BDNF and NGF) along with neurogenesis. H. erinaceus promotes anti-inflammatory effects through inhibition of TLR4/NF-kb, PI3K/AKT/Nf-κB axes. H. erinaceus also protects from apoptosis, mitochondrial damage and promotes anti-oxidant effects.
3.4. H. erinaceus in Clinical Studies
In thirty females suffering from depression, menopause, sleep disturbances and indefinite complaints, the effects of H. erinaceus intake were evaluated by using the Kupperman Menopausal Index (KMI), the Center for Epidemiologic Studies Depression Scale (CES-D), the Pittsburgh Sleep Quality Index (PSQI), and the Indefinite Complaints Index (ICI) [150]. H. erinaceus cookies containing 0.5 g of fruit bodies powder or placebo cookies containing no powder were taken for 4 weeks. Following H. erinaceus treatment, each of the CES-D and the ICI score was significantly lower than that before treatment. Moreover, compared with placebo, H. erinaceus-receiving group showed significantly lower scores associated with insensitivity, agitation, irritation, palpitation and anxiety, suggesting that H. erinaceus may hold potential in reducing depression and anxiety [93]. However, no differences were observed between the two groups in the menopause and sleep-quality indexes.
A recent study carried out on 77 volunteers affected by overweight or obesity reported that a daily, 8-week oral supplementation with H. erinaceus (80% mycelium extract and 20% fruiting body extract), coupled with a low calorie diet regimen improves depression, anxiety, sleep, and binge eating compared with subjects undergoing low calorie diet only [91]. This is correlated with increased circulating pro-BDNF levels and pro-BDNF/BDNF ratio, despite the lack of any significant changes in BDNF circulating levels.
In an 86-year-old male patient with recurrent depressive disorder and mild cognitive impairment which developed during antidepressant therapy, Mirtazapine treatment was combined with H. erinaceus extract (formulation Amyloban®3399) [151]. At 6 months of H. erinaceus daily intake, the patient was free of depression and he showed improved cognitive function and body weight in the absence of adverse reactions, suggesting that H. erinaceus could be a useful antidepressant, especially in geriatric depression [151].
H. erinaceus extract (Amyloban®3399) intake for 4 weeks was also shown to counteract sleep disturbances in a pilot study on female undergraduate students [152]. This was assessed through subjective sleep-quality and well-being questionnaires GHQ-28 and PSQI (Pittsburg Sleep Quality Index) and also through the levels of salivary free- 3-methoxy-4-hydroxyphenylglycol, an index of chronic stress and depressive symptoms reflecting sympathetic nervous system activity. The latter was found to be increased after awakening, strengthening the evidence for an improvement in anxiety and sleep quality following H. erinaceus intake [152].
3.5. R. rosea in Experimental Studies
3.5.1. Behavioral Despair-Related Depression
The anti-depressant-like activities of R. rosea root’s extract have been assessed in a behavioral despair rat model of depression consisting of repeatedly forced swimming in a restricted space [36]. This leads to immobility reflecting a state of despair that is effectively reversed by antidepressant compounds in humans. Thus, the effects of R. rosea extracts were compared with the standard anti-depressant imipramine and with the naturally-occurring H. perforatum extract [36]. The herbal compounds were administered three times, once immediately after the initial exposure to the forced swimming assay, as well as 24 and 1 h prior to re-exposure. Single doses of standard anti-depressants were administered 30 min prior to stress re-exposure. R. rosea root extract (10, 20 and 50 mg/kg) administered either orally or intravenously increases the swimming time of rats in a dose dependent manner, and remarkably, its anti-depressant-like effects are greater compared with those produced by imipramine and H. perforatum. The behavior despair assay was also applied to study the effects of five single bioactive compounds isolated from R. rosea roots, each one administered at the dose of 0.26 mg/kg (namely rhodioloside/saliroside, tyrosol, rosavin, rosarin and rosin). Rhodioloside/saliroside exhibits higher anti-depressant effect compared with other compounds; however, the strongest effect is produced by the fixed combination of the five compounds, indicating a synergistic mechanism of action of R. rosea bioactive ingredients [36,99].
3.5.2. Olfactory Bulbectomy-Induced Depression
Saliroside oral administration (20, 40 mg/kg) for 2 weeks also alleviates OBX-induced depressive-like behavior in rats, as assessed through sucrose consumption, open field test, forced swimming test and tail suspension test [19,20]. This is associated with reduced TNF-α, IL-1β, IL-6 and NF-κB levels in the hippocampus and prefrontal cortex along with a concomitant increase in BDNF expression in the hippocampus [19,20]. Besides, similarly to the antidepressant amitriptyline (10 mg/kg), saliroside markedly restores the depletion of 5-HT and NE levels in the prefrontal cortex of OBX rats [20]. Saliroside also attenuates CRF expression in hypothalamus while reducing serum corticosterone levels [19].
3.5.3. Inflammation-Related Depression
In a mice model of LPS-induced depression, a five-day pre-treatment with saliroside (12 and 24 mg/kg), similar to fluoxetine (20 mg/kg) produces antidepressant-like behavior through anti-inflammatory, monoamine-enhancing and neurotrophic effects [153]. In detail, saliroside reduces IL-6 and TNF-α levels in the serum and NF-κB levels in the mice hippocampi. At the same time, saliroside upregulates TrkB/BDNF levels and restores 5-HT and NE levels in the prefrontal cortex of LPS-depressed mice compared with the vehicle group [153]. A recent study also evaluated the effects of both single and combined oral administration of R. rosea (250, 500 mg/kg) and Curcuma longa (250, 500 mg/kg) extracts in a CMS mice model of depression followed by LPS-induced inflammation [154]. The combination of R. rosea 500 mg/kg and Curcuma 500 mg/kg remarkably reverses depression-like behavioral changes while providing synergistic anti-inflammatory effects, namely reduced levels of TNF-α and IL-6 in CMS exposed and LPS-challenged mice [154].
3.5.4. Chronic Mild Stress-Induced Depression
The antidepressant-like effects of R. rosea (1.5 g/kg/day for three weeks) in CMS rat models are also bound to stimulation of neuronal stem cells proliferation [155]. In detail, R. rosea improves depressive-like behavior while increasing the amount of healthy, PCNA-positive cells and preventing the occurrence of damaged, Caspase 3-positive neuronal cells [155].
In female CMS rat models, daily R. rosea administration (10, 15 and 20 mg/kg) for three weeks produces antidepressant-like behaviors which are comparable with those induced by fluoxetine (10 mg/kg). In detail, R. rosea reverses the CMS-induced decrease in sucrose intake, moving behaviour, minimized weight gain and dysregulation of their oestrous cycle [118].
In rat models of CMS-induced depression, R. rosea extract (1.5, 3 and 6 g/kg) administration for 3 weeks fully recovers 5-HT hippocampal levels already at the lowest dose. This is accompanied by potentiation of neural stem cell proliferation as assessed through BrdU immunohistochemistry [121]. Thus, serotonin may be crucially involved in the beneficial effects of R. rosea on mood disorders. These findings are reproduced in mice models showing depressive-like behavior following nicotine withdrawal [122]. In detail, in rats previously treated for 14 days with nicotine (2 mg/kg) and the selective 5-HT1A receptor antagonist WAY 100635 (1 mg/kg), R. rosea (5, 10, 20, and 40 mg/kg) oral administration prevents depressive-like behaviors related with nicotine withdrawal, as assessed through the abstinence scale, the immobility time on forced swimming test and marble burying test. These effects are associated with an increase in the diencephalic content of 5-HT and the expression of 5-HT1A receptor [122].
3.5.5. Prepulse Inhibition-Related Depression and Psychosis
R. rosea effects were recently evaluated in murine models of prepulse inhibition, an established operational measure of sensorimotor gating which is impaired in a variety of mental disorders featuring psychosis [156]. In detail, R. rosea extract robustly reverses prepulse inhibition deficits which are induced by either the dopamine D2 receptor agonist apomorphine or the NMDA receptor antagonist dizocilpine. Thus, R. rosea may possess antipsychotic effects, though the underlying molecular mechanisms remain to be elucidated [156].
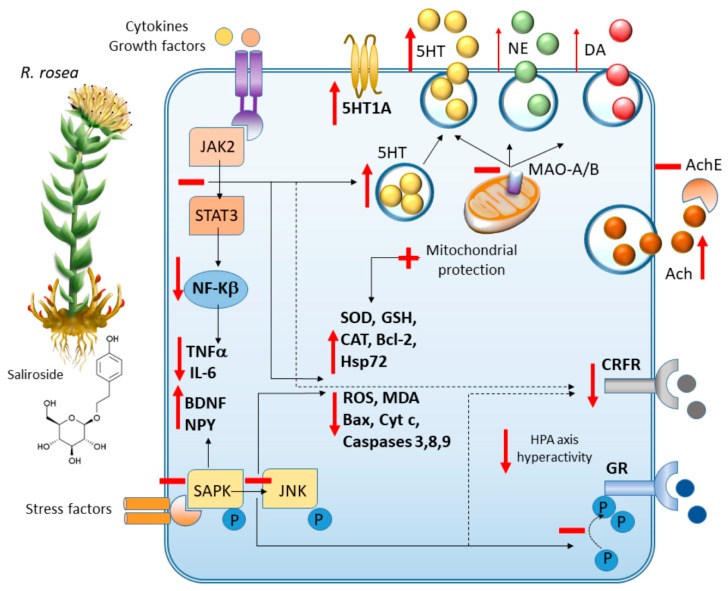
In summary, the antidepressant mechanisms of R. rosea and saliroside might be associated with antioxidant and anti-inflammatory effects and the regulation of monoamine and cholinergic systems and HPA axis activity (Figure 5).
Figure 5.
R. rosea: molecular mechanisms underlying neuroprotective, cognitive enhancing and anxiolytic/antidepressant-like effects. By acting as MAO A/B inhibitor, R. rosea and its main bioactive ingredient (saliroside) induce monoamine, and mostly 5-HT release while potentiating 5-HT synthesis and the expression of 5-HT1A receptors. R. rosea also potentiates Ach release while acting as an Ach esterase (AchE) inhibitor. At the same time, R. rosea acts as a JAK/STAT and SAPK/JNK pathway inhibitor to promote the synthesis of neurotrophic factors along with anti-inflammatory and antioxidant effects. JAK/STAT and SAPK/JNK pathway inhibition is also associated with R. rosea-induced downregulation of CRF receptors and inhibition of GR phosphorylation at crucial serine residues related to HPA axis activity.
3.6. R. rosea in Clinical Studies
In humans, R. rosea modulates cortical plasticity by preventing the activity-dependent reduction in neuronal synaptic efficacy [157]. A transcranial Direct Current Stimulation (DST) study evaluated the effect of a single R. rosea extract dose on the plastic effects induced by anodal and cathodal transcranial stimulation in twenty-eight healthy volunteers receiving 500 mg of either R. rosea or placebo. Acute R. rosea intake prevents cathodal DST-induced LTD-like plasticity, despite not affecting cortical excitability [157]. Thus, the adaptogenic, mental health-promoting effects of R. rosea are likely bound to plastic mechanisms of action in the brain.
3.6.1. Anti-Fatigue and Anti-Stress Effects
The antiasthenic effects of R. rosea were evaluated by a randomized double-blind cross-over trial on a group of 56 young, healthy physicians during night duty [158]. The effects were measured as total mental performance calculated as Fatigue Index and through different perceptive and cognitive tests which were performed before and after night duty. In the R. rosea treatment group, significant improvements were observed in all the tests during the first two weeks’ period, and no side-effects were reported [158].
The effects of a standardized R. rosea extract (SHR-5, 50 mg) were also assessed in foreign students during a stressful examination period of 20 days [159]. The study was performed as a double-blind, randomized and placebo-controlled trial with low repeated dose regime. The physical and mental performance, which was assessed before and after the period, was based on objective as well as on subjective evaluation. Compared with placebo, significant improvements were observed in the R. rosea group as it concerns physical fitness and mental fatigue. The self-assessment of the general well-being was also significantly improved in the R. rosea group [159]. Despite these encouraging results of R. rosea intake, its dosage appeared suboptimal in improving mental work performance evaluated through the correction of text tests or neuro-muscular tapping test [159].
This issue was addressed by a subsequent clinical study which was carried out to assess the differences between the effects of two R. rosea doses on mental work capacity during periods of fatigue and stress [160]. This consisted of a randomized, double-blind, placebo-controlled, parallel-group clinical study on 161 cadets who were randomized into four groups, namely R. rosea (2x185.0 mg)-, R. rosea (3x185.0 mg)-, placebo-receiving and non-treatment group. The results showed a pronounced antifatigue effect along with improved work performance for both R. rosea dosages compared with the placebo group. No significant differences were observed between the two dosage groups. However, there was a slight trend in favour of the lower dose in the psychometric tests, which was not observed in the physiological tests [160]. Thus, R. rosea can effectively reduce general fatigue and anxiety under stressful conditions.
3.6.2. Anxiolytic and Antidepressant Effects
A pilot study on ten adults with a diagnosis of generalized anxiety disorder (GAD) was conducted to specifically evaluate the anxiolytic efficacy of R. rosea [161]. Subjects receiving a daily dose of 340 mg of R. rosea extract for 10 weeks show significant decreases in mean Hamilton Anxiety Rating Scale (HARS) scores at endpoint, witnessing for significant improvement in GAD symptoms. Adverse events were generally mild or moderate in severity, the most common being dizziness and dry mouth [161].
Clinical trials of the late 1980s demonstrated that, when administered together with tricyclic anti-depressants, R. rosea leads to a marked reduction in the side effects of the drugs, while producing additional beneficial effects on depressive symptoms [162,163].
More recently, the antidepressant effects of R. rosea extract were confirmed by a phase III clinical trial which was carried out as a randomized double-blind placebo-controlled study over a period of six weeks [69]. Participants fulfilling diagnostic criteria of mild-to-moderate depression were randomized into three groups: the first (31 patients) receiving two tablets daily of R. rosea (SHR-5, 340 mg/day); the second (29 patients) receiving two R. rosea tablets twice per day (SHR-5 680 mg/day), and the third (29 patients) receiving two placebo tablets daily. The efficacy of SHR-5 extract with respect to depressive complaints was assessed on days 0 and 42 of the study period through Beck Depression Inventory (BDI) and Hamilton Rating Scale for Depression (HAMD) scores. In the groups receiving either 340 or 680 mg/day R. rosea, there was a significant overall improvement in depression, together with insomnia, emotional instability and somatization. No serious side-effects were reported in any of the groups, concluding that R. rosea exhibits anti-depressive efficacy in patients with mild to moderate depression [69].
A subsequent, randomized double-blind, 12-week, proof-of-concept trial reproduced safety and efficacy data on the antidepressant action of R. rosea compared with the conventional antidepressant sertraline in adult outpatients with mild to moderate depression [164,165]. Patients were randomized in three groups receiving capsules of either R. rosea powdered extract (SHR-5, 340 mg), sertraline (50 mg) or placebo (i.e., lactose monohydrate). The outcomes were evaluated through HRSD, BDI and Clinical Global Impression (CGI) scores. For the first two weeks all patients received one capsule daily. Subjects showing ≤ 50% reduction in HRSD score compared to baseline had the dose increased to 2 capsules daily during the following two weeks of therapy. This procedure was continued every 2 weeks for subjects with ≤ 50% reduction in HRSD score compared to baseline, up to a dose of 4 capsules daily for the last six weeks of therapy. Outcome measurements were obtained at baseline and after 2, 4, 6, 8 and 12 weeks of treatment. No statistically significant differences were observed over time for HRSD, BDI or CGI scores among treatment groups. A slightly greater decline in HRSD scores by week 12 was observed for sertraline compared with R. rosea and placebo. Nonetheless, clinically meaningful odds ratios of global improvement emerged by week 12 for R. rosea and sertraline compared with placebo; in detail, compared with placebo the odds of improvement were 1.4 and 1.9 times for R. rosea and sertraline, respectively. Remarkably, more subjects reported study-related adverse for sertraline (63.2%) compared with R. rosea (30.0%) and placebo (16.7%). While two subjects prematurely discontinued sertraline treatment, no subject prematurely discontinued R. rosea or placebo. In summary, R. rosea possesses milder antidepressant efficacy, yet, higher tolerability compared with conventional antidepressants [164,165].
The impact of an R. rosea extract (200 mg Vitano®) on self-reported anxiety, stress, cognition, and other mood symptoms was evaluated by a non-placebo controlled trial on eighty mildly anxious participants who were randomized into two groups, one receiving R. rosea twice a day and one receiving no treatments [166]. Self-report measures and cognitive tests were completed at four testing sessions over a period of 14 days. The R. rosea-receiving group self-reported a significant reduction in anxiety, stress, anger, confusion and depression at 14 days and a significant improvement in total mood, though no relevant differences in cognitive performance between the groups were observed. Moreover, R. rosea presented a favourable safety/tolerability profile [166].
In a recent preliminary observational study, forty-five adults suffering from mild or moderate depression were supplemented with a combination of R. rosea and saffron extracts (one tablet, 154 mg of R. rosea and 15 mg of saffron) twice a day for 6 weeks [167]. After 6 weeks (D42) of supplementation, the HARS scores decreased by 58% in nearly 85% of patients. A significant drop in both Hospital Anxiety and Depression Scale anxiety and depression scores was also observed at D42, the decrease being significant from 2 weeks of supplementation. At the end of the study, both general practitioners and patients noticed significant improvements in depression symptoms. Safety was excellent, and no serious adverse effects were recorded, suggesting that combination of R. rosea with other herbal compounds deserves further investigation as a potential strategy for the management of mild-moderate depression [167].
3.6.3. Obstructive Sleep Apnea- and Menopause-Related Mood Alterations
In patients with obstructive sleep apnea (OSA), R. rosea was shown to relieve anxiety and depressive symptoms by inhibiting oxygen free radicals and lipid peroxidation [168]. This was assessed in ninety patients with moderate and severe OSA presenting with negative emotions diagnosed by self-rating depression scale (SDS) and self-rating anxiety scale (SAS). Compared with untreated controls, R. rosea intake for 3 months significantly improved SDS and SAS scores, which is associated with an increase in serum levels of SOD and a concomitant reduction of serum MDA levels [168].
As recently reviewed, R. rosea may also act as a potential selective estrogen receptor modulator (SERM) in the prevention and treatment of menopause-related mood disturbances, including fatigue, depression, as well as cognitive decline and memory impairment, cardiovascular disease, and osteoporosis [169]. The mechanisms of action through which R. rosea ameliorates menopause-related alterations include activation of intra-cellular signal transduction pathways downstream of estrogen receptors, potentiation of antioxidant defence and anti-inflammatory effects through counteracting NF-Kβ and TNF-α production [169].
A summary of both experimental and clinical studies on the antidepressant effects of S. baicalensis, H. erinaceus and R. rosea is provided in Table 1.
Table 1.
Experimental and clinical studies centered on the antidepressant-like mechanisms and potential of S. baicalensis, H. erinaceus and R. rosea.
| Experimental Models of Depression | S. baicalensis | H. erinaceus | R. rosea |
|---|---|---|---|
| Chronic corticosterone-induced depression (CORT) |
Baicalin (40, 80, and 160 mg/kg) [133,134] ↑cytoplasmic GR levels ↓nuclear GR levels ↓GR phosphorylation ↑negative feedback of HPA axis [133] ↑neurogenesis (Ki67- DCX-positive cells) in the dentate gyrus of the hippocampus [134]. |
||
| Olfactory bulbectomy-induced depression (OBX) |
Baicalin (20 and 40 mg/kg) [135,136,137] ↓serum corticosterone [135] ↓IL-1β, IL-6, and TNF-α in the brain via inhibition of the SIRT1-NF-kB [135] ↓oxidative stress and apoptosis [136] ↑GSH-Px and ↓MDA, APAF-1 and caspases ↑neurogenesis and olfactory function via APPL2/GR signaling pathway [137]. |
Saliroside (20 and 40 mg/kg) [19,20] ↓TNF-α, IL-1β, IL-6 and NF-κB in the hippocampus and prefrontal cortex ↑BDNF expression in the hippocampus [19,20] ↑5-HT and NE levels in the prefrontal cortex [20] ↓CRF expression in hypothalamus and ↓serum corticosterone levels [19]. |
|
| Chronic (unrestraint) mild stress-induced depression (CUMS) and Chronic mild stress + inflammation (CUMS+LPS) |
Baicalin (10, 20, 25, 40, 50, 60, and 100 mg/kg) [35,138,139,140,141,142,143,144,145] ↓monoamine oxidase A and B (MAO A/B) activity [35] ↓IL-1β, IL-6, and TNF-α in serum and in the hippocampus [138,139] ↓COX-2 and PGE(2) in the brain [144] ↓TLR4 via the HMBG1/NF-kb and PI3K/AKT/FoxO1 pathways [139,140]. ↑SOD and ↓ROS, MDA and caspase 1 [138,141] via ↓NMDAR/NR2B, ↓Ca2+/CaMPK-II and ↑pERK [139], and ↓GSK3β/ NF-κB / NLRP3 [141,142] ↓ultrastructural hippocampal alterations ↑synaptic proteins SYP PSD95, TrkB, Rac1, cofilin ↑BDNF [143] ↑neurogenesis (DCX-positive cells) ↑neuronal maturation, differentiation and survival [145]. Radix Scuellariae extract (500 and 1000 mg/kg) [146] ↑cAMP/PKA-dependent neurogenesis, ↑BrdU, DCX and NeuN in the mice hippocampi [146]. |
R. rosea extract (1.5 g/kg/day) [155] ↑neuronal stem cell proliferation ↑PCNA-positive cells [155] ↓Caspase 3-positive neuronal cells [155]. R. rosea extract (1.5, 3 and 6 g/kg) [121] ↑5-HT hippocampal levels already at the lowest dose. ↑neural stem cell proliferation ↑BrdU-positive cells [121]. R. rosea extract (250 and 500 mg/kg) [154] ↓TNF-α and IL-6 in CMS exposed and LPS-challenged mice [154] Potentiated effects when administered with curcuma longa (500 mg/kg) [154]. |
|
| Streptozotocin (diabetes)-induced depression | Baicalin (50, 100 and 200 mg/kg) [8] ↑hippocampal acetylcholine transporter (ChAT) ↓acetylcholinesterase (AChE) ↑pERK) ↑Bcl 2 ↑BDNF ↓pJNK) ↓p-p38, ↓caspase 3 and ↓Bax [8]. |
||
| Inflammation (LPS)-induced depression | Baicalin (25, 50 and 60 mg/kg) [139,140] ↓TLR4 via the HMBG1/NF-kb and PI3K/AKT/FoxO1 pathways [139,140]. |
H. erinaceus fruit body extract amycenone (200mg/kg) [13] ↓serum TNF-α ↑IL-10 [13]. |
Saliroside (12 and 24 mg/kg) [153] ↓serum IL-6 and TNF-α ↓NF-κB in the brain ↑TrkB/BDNF levels ↑5-HT and NE levels in the prefrontal cortex [153]. |
| Restraint stress-induced depression |
H. erinaceus mycelium ethanolic extract (100, 200 and 400 mg7kg) [14] ↑monoamines levels ↓IL-6, TNF-α and NF-κB ↑BDNF [14]. |
||
| Clinical studies | S. baicalensis | H. erinaceus | R.rosea |
| Baicalein chewable tablets (200, 400, and 800 mg once daily on days 1 and 10, and twice daily on days 3–9). Safe and well tolerated. Related mood effects were not analysed/reported [57]. S. baicalensis extract (300 mg daily for 30 days). Safe and well tolerated. Marked improvement in speed and accuracy of processing complex information in computer tasks [58]. |
H. erinaceus cookies (0.5 g of fruit bodies powder for 4 weeks). Lower scores associated with insensitivity, agitation, irritation, palpitation and anxiety in H. erinaceus-receiving women compared with placebo group [155]. H. erinaceus (1.650 g/day, 80% mycelium extract and 20% fruiting body extract for 8 weeks). Safe and well tolerated. Coupled with a low calorie diet improves depression, anxiety, sleep, and binge eating compared with subjects undergoing low calorie diet only [91]. Increases circulating pro-BDNF levels and pro-BDNF/BDNF ratio [91]. H. erinaceus extract (AmylobanⓇ) daily for 6 months combined with Mirtazapine. Combats depression, and improves cognitive function and body weight in the absence of adverse reactions [151]. H. erinaceus extract (AmylobanⓇ3399) intake for 4 weeks counteracts sleep disturbances in a pilot study on female undergraduate students. It increases the levels of salivary free- 3-methoxy-4-hydroxyphenylglycol, an index of chronic stress and depressive symptoms reflecting sympathetic nervous system activity [152]. |
R. rosea extract (340 mg/day for 10 weeks). Improvement of general anxiety disorder symptoms evaluated by HARS scores. Generally mild adverse effects, the most common being dizziness and dry mouth [161]. R. rosea extract (SHR-5, 340 or 680 mg/day for six weeks). Safe and well tolerated. Improvement of depressive symptoms, insomnia, emotional instability and somatization compared with placebo group [69]. R. rosea powdered extract (SHR-5 capsule, 340 mg, one capsule/day for the first week, two capsules/day for the following two weeks, up to up to 4 capsules/day for the last six weeks). Improves depressive symptoms compared with placebo and produces antidepressant effects which are comparable with sertraline (50 mg). Fewer adverse effects were reported for R. rosea compared with sertraline [164,165]. R. rosea extract (Vitano®, 200 mg twice a day for 14 days). Safe and well tolerated. Reduces self-reported anxiety, stress, anger, confusion and depression, and overall improvement in mood [166] R. rosea extract (one tablet, 154mg, combined with saffron tablet 15 mg, twice a day for 6 weeks). Excellent safety, no serious adverse effects. Improvements in HARS scores and depression symptoms reported by both general practitioners and patients [167]. |
4. Conclusions and Future Perspectives
The evidence here reviewed suggests that S. baicalensis, H. erinaceus and R. rosea deserve to be further investigated for their antidepressant/anxiolytic potential since they promote mental-health with a wide margin of safety. As common neuroprotective and antidepressant-like effects, these compounds promote synaptic plasticity and neurogenesis while counteracting oxidative stress, mitochondrial alterations, and neuro-inflammation. These three herbs also improve cognitive functions by rescuing Ach neurotransmission. These considerations are key in the light of the close association that exists among depression, cognitive alterations and neurodegeneration, being the latter often accompanied or even preceded by mood alterations [170,171]. In view of their antioxidant and mitochondrial protecting effects, these herbs could be considered as an adjunct to standard antidepressant drugs which possess side effects related to mitochondrial toxicity [172].
Despite common properties, each herb also produces specific, and potentially complementary effects, mostly concerning modulation of neurotransmitter systems, HPA axis, and the synthesis of neurotrophic factors. This leads to consider the chance that the combination of these different herbs may produce potentially synergistic, mood-stabilizing efficacy, which remains to be investigated. Despite a growing number of commercially available herbal formulations owning promising therapeutic potential, most of them remain untested and none have been turned into registered drugs. To date, there are many difficulties and obstacles in developing plant-derived medicines and this is due to an inadequate knowledge of their mode of action, potential adverse reactions, contraindications, and interactions with other drugs [173]. Thus, further studies are needed to deepen our understanding and knowledge on both the safety profile and fine molecular mechanisms underlying the beneficial effects of S. baicalensis, H. erinaceus and R. rosea in the brain. In fact, these herbal compounds may also act through yet unexplored biological pathways that are implicated in both mood and neurological disorders. For instance, some recent studies indicate that a variety of herbal compounds, including S. baicalensis and R. rosea, may produce neuroprotective effects by modulating autophagy, which is key to maintain intracellular homeostasis under oxidative stress conditions by degrading abnormal proteins or organelles, especially mitochondria [174,175,176,177]. For instance, baicalin-induced autophagy is associated with inhibition of the mitochondrial apoptotic pathway and protection against experimental traumatic brain injury [178], while saliroside-induced autophagy counteracts alpha-synuclein aggregation and toxicity [179]. The link among S. baicalensis, H. erinaceus, R. rosea and autophagy has not been demonstrated in depressive disorders specifically. However, such an issue deserves further investigation since autophagy alterations occur in both psychiatric and neurodegenerative disorders, and autophagy modulation is bound to the mechanisms of action of some conventional antidepressants and mood stabilizers [170,178,179,180].
Abbreviations
| 6-OHDA | 6-hydroxydopamine |
| AChE | acetylcholinesterase |
| AD | Alzheimer’s disease |
| APPL2 | adaptor protein phosphotyrosine interacting with PH domain and leucine zipper 2 |
| BBB | blood-brain barrier |
| BDI | Beck Depression Inventory |
| BDNF | brain-derived neurotrophic factor |
| BZ | benzodiazepine |
| CAT | catalase |
| CES-D | Center for Epidemiologic Studies Depression Scale |
| CGI | Clinical Global Impression |
| ChAT | acetylcholine transporter |
| CORT | corticosterone |
| COX-2 | cyclooxygenase-2 |
| CRF | corticotrophin-releasing factor |
| CRH | corticotropin-releasing hormone |
| CUMS | chronic (unpredictable) mild stress |
| D1Rs | DA D1 receptors |
| DA | dopamine |
| DAT | DA transporter |
| DCX | doublecortin |
| DPPH | 1-diphenyl-2-picrylhydrazyl |
| DST | Direct Current Transcranial Stimulation |
| GABA | Gamma-Aminobutyric acid |
| GABAAR | GABA A subtype receptor |
| GAD | generalized anxiety disorder |
| GR | glucocorticoid receptor |
| GSH | glutathione |
| GSH-Px | glutathione peroxidase |
| GSK3β | glycogen synthase kinase-3 |
| H2O2 | hydrogen peroxide |
| HAMD | Hamilton Rating Scale for Depression |
| HARS | Hamilton Anxiety Rating Scale |
| HD | Huntington’s disease |
| HMBG1 | High Mobility Group Box 1 |
| HPA | hypothalamic-pituitary-adrenal axis |
| HSP70 | heat shock proteins 70 |
| ICI | Indefinite Complaints Index |
| IL-1β | interleukin 1 beta |
| KMI | Kupperman Menopausal Index |
| LC | Locus Coeruleus |
| LPS | Lipopolysaccharide |
| MAO A/B | monoamine oxidase A/B |
| MDA | malondialdehyde |
| METH | methamphetamine |
| MMP | mitochondrial membrane potential |
| MPTP | 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine |
| NE | norepinephrine |
| NF-κB | nuclear factor kappa-light-chain-enhancer of activated B cells |
| NGF | nerve growth factor |
| NLRP3 | nucleotide-binding domain, leucine-rich repeat, pyrin domain containing protein 3 |
| NMDAR/NR2B | N-methyl D-aspartate receptor subtype 2B |
| NPY | neuropeptide Y |
| OBX | olfactory bulbectomy |
| OSA | obstructive sleep apnea |
| PCNA | proliferating cell nuclear antigen |
| PD | Parkinson’s disease |
| pERK | phosphorylated Extracellular signal-regulated kinase |
| PGE(2) | prostaglandin E(2) |
| pJNK | phosphorylated c-Jun N-terminal kinases |
| PSD95 | postsynaptic density protein-95 |
| PSQI | Pittsburgh Sleep Quality Index |
| ROS | reactive oxygen species |
| RS | repeated restraint stress |
| SAS | self-rating anxiety scale |
| SDS | self-rating depression scale |
| SERM | selective oestrogen receptor modulator |
| SOD | superoxide dismutase |
| SYP | synaptophysin |
| TLR4 | toll-like receptor 4 |
| TNF-α | tumor necrosis factor alpha |
| TrkB | tyrosine kinase receptor B |
Author Contributions
Writing—original draft preparation, F.L. and F.F.; writing—review and editing, and art-work, F.L., F.B., C.L.B., and M.P.; conceptualization and intellectual content, C.F., M.P., and F.F.; supervision, F.F; All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Ministero della Salute, Ricerca Corrente 2020.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Liu L., Liu C., Wang Y., Wang P., Li Y., Li B. Herbal Medicine for Anxiety, Depression and Insomnia. Curr. Neuropharmacol. 2015;13:481–493. doi: 10.2174/1570159X1304150831122734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yeung K.S., Hernandez M., Mao J.J., Haviland I., Gubili J. Herbal medicine for depression and anxiety: A systematic review with assessment of potential psycho-oncologic relevance. Phytother. Res. 2018;32:865–891. doi: 10.1002/ptr.6033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Iovieno N., Dalton E.D., Fava M., Mischoulon D. Second-tier natural antidepressants: Review and critique. J. Affect. Disord. 2011;130:343–357. doi: 10.1016/j.jad.2010.06.010. [DOI] [PubMed] [Google Scholar]
- 4.Rzymski P., Niedzielski P., Siwulski M., Mleczek M., Budzyńska S., Gąsecka M., Poniedziałek B. Lithium biofortification of medicinal mushrooms Agrocybe cylindracea and Hericium erinaceus. J. Food Sci. Technol. 2017;54:2387–2393. doi: 10.1007/s13197-017-2679-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sowndhararajan K., Deepa P., Kim M., Park S.J., Kim S. Neuroprotective and Cognitive Enhancement Potentials of Baicalin: A Review. Brain Sci. 2018;8:104. doi: 10.3390/brainsci8060104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Im H.I., Joo W.S., Nam E., Lee E.S., Hwang Y.J., Kim Y.S. Baicalein prevents 6-hydroxydopamine-induced dopaminergic dysfunction and lipid peroxidation in mice. J. Pharmacol. Sci. 2005;98:185–189. doi: 10.1254/jphs.SC0050014. [DOI] [PubMed] [Google Scholar]
- 7.Mu X., He G.R., Yuan X., Li X.X., Du G.H. Baicalein protects the brain against neuron impairments induced by MPTP in C57BL/6 mice. Pharmacol. Biochem. Behav. 2011;98:286–291. doi: 10.1016/j.pbb.2011.01.011. [DOI] [PubMed] [Google Scholar]
- 8.Ma P., Mao X.Y., Li X.L., Ma Y., Qiao Y.D., Liu Z.Q., Zhou H.H., Cao Y.G. Baicalin alleviates diabetes-associated cognitive deficits via modulation of mitogen-activated protein kinase signaling, brain-derived neurotrophic factor and apoptosis. Mol. Med. Rep. 2015;12:6377–6383. doi: 10.3892/mmr.2015.4219. [DOI] [PubMed] [Google Scholar]
- 9.Wang F., Xu Z., Ren L., Tsang S.Y., Xue H. GABA A receptor subtype selectivity underlying selective anxiolytic effect of baicalin. Neuropharmacology. 2008;55:1231–1237. doi: 10.1016/j.neuropharm.2008.07.040. [DOI] [PubMed] [Google Scholar]
- 10.Friedman M. Chemistry, Nutrition, and Health-Promoting Properties of Hericium erinaceus (Lion’s Mane) Mushroom Fruiting Bodies and Mycelia and Their Bioactive Compounds. J. Agric. Food Chem. 2015;63:7108–7123. doi: 10.1021/acs.jafc.5b02914. [DOI] [PubMed] [Google Scholar]
- 11.Liu Z., Wang Q., Cui J., Wang L., Xiong L., Wang W., Li D., Liu N., Wu Y., Mao C. Systemic Screening of Strains of the Lion’s Mane Medicinal Mushroom Hericium erinaceus (Higher Basidiomycetes) and Its Protective Effects on Aβ-Triggered Neurotoxicity in PC12 Cells. Int. J. Med. Mushrooms. 2015;17:219–229. doi: 10.1615/IntJMedMushrooms.v17.i3.20. [DOI] [PubMed] [Google Scholar]
- 12.Kuo H.C., Lu C.C., Shen C.H., Tung S.Y., Hsieh M.C., Lee K.C., Lee L.Y., Chen C.C., Teng C.C., Huang W.S., et al. Hericium erinaceus mycelium and its isolated erinacine A protection from MPTP-induced neurotoxicity through the ER stress, triggering an apoptosis cascade. J. Transl. Med. 2016;14:78. doi: 10.1186/s12967-016-0831-y. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 13.Yao W., Zhang J.C., Dong C., Zhuang C., Hirota S., Inanaga K., Hashimoto K. Effects of amycenone on serum levels of tumor necrosis factor-α, interleukin-10, and depression-like behavior in mice after lipopolysaccharide administration. Pharmacol. Biochem. Behav. 2015;136:7–12. doi: 10.1016/j.pbb.2015.06.012. [DOI] [PubMed] [Google Scholar]
- 14.Chiu C.H., Chyau C.C., Chen C.C., Lee L.Y., Chen W.P., Liu J.L., Lin W.H., Mong M.C. Erinacine A-Enriched Hericium erinaceus Mycelium Produces Antidepressant-Like Effects through Modulating BDNF/PI3K/Akt/GSK-3β Signaling in Mice. Int. J. Mol. Sci. 2018;19:341. doi: 10.3390/ijms19020341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Amsterdam J.D., Panossian A.G. Rhodiola rosea, L as a putative botanical antidepressant. Phytomedicine. 2016;23:770–783. doi: 10.1016/j.phymed.2016.02.009. [DOI] [PubMed] [Google Scholar]
- 16.Lee Y., Jung J.C., Jang S., Kim J., Ali Z., Khan I.A., Oh S. Anti-Inflammatory and Neuroprotective Effects of Constituents Isolated from Rhodiola rosea. Evid. Based Complement. Altern. Med. 2013;2013:514049. doi: 10.1155/2013/514049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jacob R., Nalini G., Chidambaranathan N. Neuroprotective effect of Rhodiola rosea Linn against MPTP induced cognitive impairment and oxidative stress. Ann. Neurosci. 2013;20:47–51. doi: 10.5214/ans.0972.7531.200204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xiao L., Li H., Zhang J., Yang F., Huang A., Deng J., Liang M., Ma F., Hu M., Huang Z. Salidroside protects Caenorhabditis elegans neurons from polyglutamine-mediated toxicity by reducing oxidative stress. Molecules. 2014;19:7757–7769. doi: 10.3390/molecules19067757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yang S.J., Yu H.Y., Kang D.Y., Ma Z.Q., Qu R., Fu Q., Ma S.P. Antidepressant-like effects of salidroside on olfactory bulbectomy-induced pro-inflammatory cytokine production and hyperactivity of HPA axis in rats. Pharmacol. Biochem. Behav. 2014;124:451–457. doi: 10.1016/j.pbb.2014.07.015. [DOI] [PubMed] [Google Scholar]
- 20.Zhang X., Du Q., Liu C., Yang Y., Wang J., Duan S., Duan J. Rhodioloside ameliorates depressive behavior via up-regulation of monoaminergic system activity and anti-inflammatory effect in olfactory bulbectomized rats. Int. Immunopharmacol. 2016;36:300–304. doi: 10.1016/j.intimp.2016.05.008. [DOI] [PubMed] [Google Scholar]
- 21.Wu Y., Wang F., Fan L., Zhang W., Wang T., Du Y., Bai X. Baicalin alleviates atherosclerosis by relieving oxidative stress and inflammatory responses via inactivating the NF-κB and p38 MAPK signaling pathways. Biomed. Pharmacother. 2018;97:1673–1679. doi: 10.1016/j.biopha.2017.12.024. [DOI] [PubMed] [Google Scholar]
- 22.Abdullah N., Ismail S.M., Aminudin N., Shuib A.S., Lau B.F. Evaluation of Selected Culinary-Medicinal Mushrooms for Antioxidant and ACE Inhibitory Activities. Evid. Based Complement. Altern. Med. 2012;2012:464238. doi: 10.1155/2012/464238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Han Z.H., Ye J.M., Wang G.F. Evaluation of in vivo antioxidant activity of Hericium erinaceus polysaccharides. Int. J. Biol. Macromol. 2013;52:66–71. doi: 10.1016/j.ijbiomac.2012.09.009. [DOI] [PubMed] [Google Scholar]
- 24.Wong J.Y., Abdulla M.A., Raman J., Phan C.W., Kuppusamy U.R., Golbabapour S., Sabaratnam V. Gastroprotective Effects of Lion’s Mane Mushroom Hericium erinaceus (Bull.:Fr.) Pers. (Aphyllophoromycetideae) Extract against Ethanol-Induced Ulcer in Rats. Evid. Based Complement. Altern. Med. 2013;2013:492976. doi: 10.1155/2013/492976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang Z., Lv G., Pan H., Pandey A., He W., Fan L. Antioxidant and hepatoprotective potential of endo-polysaccharides from Hericium erinaceus grown on tofu whey. Int. J. Biol. Macromol. 2012;51:1140–1146. doi: 10.1016/j.ijbiomac.2012.09.002. [DOI] [PubMed] [Google Scholar]
- 26.Trovato A., Siracusa R., Di Paola R., Scuto M., Ontario M.L., Bua O., Di Mauro P., Toscano M.A., Petralia C.C.T., Maiolino L., et al. Redox modulation of cellular stress response and lipoxin A4 expression by Hericium Erinaceus in rat brain: Relevance to Alzheimer’s disease pathogenesis. Immun. Ageing. 2016;13:23. doi: 10.1186/s12979-016-0078-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jiang S., Wang Y., Zhang X. Comparative studies on extracts from Hericium erinaceus by different polarity reagents to gain higher antioxidant activities. Exp. Ther. Med. 2016;12:513–517. doi: 10.3892/etm.2016.3279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kushairi N., Phan C.W., Sabaratnam V., David P., Naidu M. Lion’s Mane Mushroom, Hericium erinaceus (Bull.: Fr.) Pers. Suppresses H(2)O(2)-Induced Oxidative Damage and LPS-Induced Inflammation in HT22 Hippocampal Neurons and BV2 Microglia. Antioxidants. 2019;8:261. doi: 10.3390/antiox8080261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhu L., Chen T., Chang X., Zhou R., Luo F., Liu J., Zhang K., Wang Y., Yang Y., Long H., et al. Salidroside ameliorates arthritis-induced brain cognition deficits by regulating Rho/ROCK/NF-κB pathway. Neuropharmacology. 2016;103:134–142. doi: 10.1016/j.neuropharm.2015.12.007. [DOI] [PubMed] [Google Scholar]
- 30.Cai L., Wang H., Li Q., Qian Y., Yao W. Salidroside inhibits H2O2-induced apoptosis in PC12 cells by preventing cytochrome c release and inactivating of caspase cascade. Acta Biochim. Biophys. Sin. 2008;40:796–802. doi: 10.1111/j.1745-7270.2008.00463.x. [DOI] [PubMed] [Google Scholar]
- 31.Qu Z.Q., Zhou Y., Zeng Y.S., Li Y., Chung P. Pretreatment with Rhodiola rosea extract reduces cognitive impairment induced by intracerebroventricular streptozotocin in rats: Implication of anti-oxidative and neuroprotective effects. Biomed. Environ. Sci. 2009;22:318–326. doi: 10.1016/S0895-3988(09)60062-3. [DOI] [PubMed] [Google Scholar]
- 32.Möhler H. The GABA system in anxiety and depression and its therapeutic potential. Neuropharmacology. 2012;62:42–53. doi: 10.1016/j.neuropharm.2011.08.040. [DOI] [PubMed] [Google Scholar]
- 33.Nestler E.J., Barrot M., DiLeone R.J., Eisch A.J., Gold S.J., Monteggia L.M. Neurobiology of depression. Neuron. 2002;34:13–25. doi: 10.1016/S0896-6273(02)00653-0. [DOI] [PubMed] [Google Scholar]
- 34.Czarny P., Wigner P., Galecki P., Sliwinski T. The interplay between inflammation, oxidative stress, DNA damage, DNA repair and mitochondrial dysfunction in depression. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2018;80:309–321. doi: 10.1016/j.pnpbp.2017.06.036. [DOI] [PubMed] [Google Scholar]
- 35.Zhu W., Ma S., Qu R., Kang D., Liu Y. Antidepressant effect of baicalin extracted from the root of Scutellaria baicalensis in mice and rats. Pharm. Biol. 2006;44:503–510. doi: 10.1080/13880200600878684. [DOI] [Google Scholar]
- 36.Panossian A., Nikoyan N., Ohanyan N., Hovhannisyan A., Abrahamyan H., Gabrielyan E., Wikman G. Comparative study of Rhodiola preparations on behavioral despair of rats. Phytomedicine. 2008;15:84–91. doi: 10.1016/j.phymed.2007.10.003. [DOI] [PubMed] [Google Scholar]
- 37.Liang B., Guo Z., Xie F., Zhao A. Antihyperglycemic and antihyperlipidemic activities of aqueous extract of Hericium erinaceus in experimental diabetic rats. BMC Complement. Altern. Med. 2013;13:253. doi: 10.1186/1472-6882-13-253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lai P.L., Naidu M., Sabaratnam V., Wong K.H., David R.P., Kuppusamy U.R., Abdullah N., Malek S.N. Neurotrophic properties of the Lion’s mane medicinal mushroom, Hericium erinaceus (Higher Basidiomycetes) from Malaysia. Int. J. Med. Mushrooms. 2013;15:539–554. doi: 10.1615/IntJMedMushr.v15.i6.30. [DOI] [PubMed] [Google Scholar]
- 39.He X., Wang X., Fang J., Chang Y., Ning N., Guo H., Huang L., Huang X., Zhao Z. Structures, biological activities, and industrial applications of the polysaccharides from Hericium erinaceus (Lion’s Mane) mushroom: A review. Int. J. Biol. Macromol. 2017;97:228–237. doi: 10.1016/j.ijbiomac.2017.01.040. [DOI] [PubMed] [Google Scholar]
- 40.Li I.C., Lee L.Y., Tzeng T.T., Chen W.P., Chen Y.P., Shiao Y.J., Chen C.C. Neurohealth Properties of Hericium erinaceus Mycelia Enriched with Erinacines. Behav. Neurol. 2018;2018:5802634. doi: 10.1155/2018/5802634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dimpfel W., Schombert L., Panossian A.G. Assessing the Quality and Potential Efficacy of Commercial Extracts of Rhodiola rosea L. by Analyzing the Salidroside and Rosavin Content and the Electrophysiological Activity in Hippocampal Long-Term Potentiation, a Synaptic Model of Memory. Front. Pharmacol. 2018;9:425. doi: 10.3389/fphar.2018.00425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhong Z., Han J., Zhang J., Xiao Q., Hu J., Chen L. Pharmacological activities, mechanisms of action, and safety of salidroside in the central nervous system. Drug Des. Dev. Ther. 2018;12:1479–1489. doi: 10.2147/DDDT.S160776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cifani C., Micioni Di B.M.V., Vitale G., Ruggieri V., Ciccocioppo R., Massi M. Effect of salidroside, active principle of Rhodiola rosea extract, on binge eating. Physiol. Behav. 2010;101:555–562. doi: 10.1016/j.physbeh.2010.09.006. [DOI] [PubMed] [Google Scholar]
- 44.Wang H., Ding Y., Zhou J., Sun X., Wang S. The in vitro and in vivo antiviral effects of salidroside from Rhodiola rosea L. against coxsackievirus B3. Phytomedicine. 2009;16:146–155. doi: 10.1016/j.phymed.2008.07.013. [DOI] [PubMed] [Google Scholar]
- 45.Wu D., Yang X., Zheng T., Xing S., Wang J., Chi J., Bian F., Li W., Xu G., Bai X., et al. A novel mechanism of action for salidroside to alleviate diabetic albuminuria: Effects on albumin transcytosis across glomerular endothelial cells. Am. J. Physiol. Endocrinol. Metab. 2016;310:E225–E237. doi: 10.1152/ajpendo.00391.2015. [DOI] [PubMed] [Google Scholar]
- 46.Xing S., Yang X., Li W., Bian F., Wu D., Chi J., Xu G., Zhang Y., Jin S. Salidroside stimulates mitochondrial biogenesis and protects against H2O2-induced endothelial dysfunction. Oxidative Med. Cell. Longev. 2014;2014:904834. doi: 10.1155/2014/904834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Li Y., Pham V., Bui M., Song L., Wu C., Walia A., Uchio E., Smith-Liu F., Zi X. Rhodiola rosea L.: An herb with anti-stress, anti-aging, and immunostimulating properties for cancer chemoprevention. Curr. Pharmacol. Rep. 2017;3:384–395. doi: 10.1007/s40495-017-0106-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fong S.Y.K., Li C., Ho Y.C., Li R., Wang Q., Wong Y.C., Xue H., Zuo Z. Brain Uptake of Bioactive Flavones in Scutellariae Radix and Its Relationship to Anxiolytic Effect in Mice. Mol. Pharm. 2017;14:2908–2916. doi: 10.1021/acs.molpharmaceut.7b00029. [DOI] [PubMed] [Google Scholar]
- 49.Hu J.H., Li I.C., Lin T.W., Chen W.P., Lee L.Y., Chen C.C., Kuo C.F. Absolute Bioavailability, Tissue Distribution, and Excretion of Erinacine S in Hericium erinaceus Mycelia. Molecules. 2019;24:1624. doi: 10.3390/molecules24081624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Guo N., Hu Z., Fan X., Zheng J., Zhang D., Xu T., Yu T., Wang Y., Li H. Simultaneous determination of salidroside and its aglycone metabolite p-tyrosol in rat plasma by liquid chromatography-tandem mass spectrometry. Molecules. 2012;17:4733–4754. doi: 10.3390/molecules17044733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Barhwal K., Das S.K., Kumar A., Hota S.K., Srivastava R.B. Insulin receptor A and Sirtuin 1 synergistically improve learning and spatial memory following chronic salidroside treatment during hypoxia. J. Neurochem. 2015;135:332–346. doi: 10.1111/jnc.13225. [DOI] [PubMed] [Google Scholar]
- 52.Zhao T., Tang H., Xie L., Zheng Y., Ma Z., Sun Q., Li X. Scutellaria baicalensis Georgi. (Lamiaceae): a review of its traditional uses, botany, phytochemistry, pharmacology and toxicology. J. Pharm. Pharmacol. 2019;71:1353–1369. doi: 10.1111/jphp.13129. [DOI] [PubMed] [Google Scholar]
- 53.Tao H., Wu X., Cao J., Peng Y., Wang A., Pei J., Xiao J., Wang S., Wang Y. Rhodiola species: A comprehensive review of traditional use, phytochemistry, pharmacology, toxicity, and clinical study. Med. Res. Rev. 2019;39:1779–1850. doi: 10.1002/med.21564. [DOI] [PubMed] [Google Scholar]
- 54.Chang H.M., But P.P.H., editors; Yeung S.C.S., Yao S.C., Wang L.L., translators. Pharmacology and Applications of Chinese Materia Medica. Volume 2. World Scientific; Singapore: 1987. pp. 1022–1028. [Google Scholar]
- 55.Yi Y., Zhao Y., Li C., Zhang Y., Bin Y., Yuan Y., Pan C., Wang L., Liang C. Potential chronic liver toxicity in rats orally administered an ethanol extract of Huangqin (Radix Scutellariae baicalensis) J. Tradit. Chin. Med. 2018;38:242–256. [PubMed] [Google Scholar]
- 56.Zhang W., Song D.R., Wang Y.N., Zhu Z. Evaluation of embryotoxicity of baicalin based on embryonic stem cell test system. Chin. J. Pharmacol. Toxicol. 2012;26:864–869. [Google Scholar]
- 57.Pang H., Xue W., Shi A., Li M., Li Y., Cao G., Yan B., Dong F., Xiao W., He G., et al. Multiple-Ascending-Dose Pharmacokinetics and Safety Evaluation of Baicalein Chewable Tablets in Healthy Chinese Volunteers. Clin. Drug Investig. 2016;36:713–724. doi: 10.1007/s40261-016-0418-7. [DOI] [PubMed] [Google Scholar]
- 58.Yimam M., Burnett B.P., Brownell L., Jia Q. Clinical and Preclinical Cognitive Function Improvement after Oral Treatment of a Botanical Composition Composed of Extracts from Scutellaria baicalensis and Acacia catechu. Behav. Neurol. 2016;2016:7240802. doi: 10.1155/2016/7240802. Erratum in: Behav. Neurol.2017, 2017, 3493086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Li I.C., Chen W.P., Chen Y.P., Lee L.Y., Tsai Y.T., Chen C.C. Acute and developmental toxicity assessment of erincine A-enriched Hericium erinaceus mycelia in Sprague-Dawley rats. Drug Chem. Toxicol. 2018:1–6. doi: 10.1080/01480545.2017.1381110. [DOI] [PubMed] [Google Scholar]
- 60.Li I.C., Chen Y.L., Lee L.Y., Chen W.P., Tsai Y.T., Chen C.C., Chen C.S. Evaluation of the toxicological safety of erinacine A-enriched Hericium erinaceus in a 28-day oral feeding study in Sprague-Dawley rats. Food Chem. Toxicol. 2014;70:61–67. doi: 10.1016/j.fct.2014.04.040. [DOI] [PubMed] [Google Scholar]
- 61.Lee L.Y., Li I.C., Chen W.P., Tsai Y.T., Chen C.C., Tung K.C. Thirteen-Week Oral Toxicity Evaluation of Erinacine AEnriched Lion’s Mane Medicinal Mushroom, Hericium erinaceus (Agaricomycetes), Mycelia in Sprague-Dawley Rats. Int. J. Med. Mushrooms. 2019;21:401–411. doi: 10.1615/IntJMedMushrooms.2019030320. [DOI] [PubMed] [Google Scholar]
- 62.Vigna L., Morelli F., Agnelli G.M., Napolitano F., Ratto D., Occhinegro A., Di Iorio C., Savino E., Girometta C., Brandalise F., et al. Hericium erinaceus Improves Mood and Sleep Disorders in Patients Affected by Overweight or Obesity: Could Circulating Pro-BDNF and BDNF Be Potential Biomarkers? Evid. Based Complement. Altern. Med. 2019;2019:7861297. doi: 10.1155/2019/7861297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kurkin V.A., Zapesochnaya G.G., Shchavlinskii A.N., Nukhi-movskii E.L., Vandyshev V.V. Method of analysis of identity and quality of Rhodiola rosea rhizome. Khim. Farm. Zh. 1985;19:185–190. [Google Scholar]
- 64.Montiel-Ruiz R.M., Roa-Coria J.E., Patiño-Camacho S.I., Flores-Murrieta F.J., Déciga-Campos M. Neuropharmacological and toxicity evaluations of ethanol extract from Rhodiola rosea. Drug Dev. Res. 2012;73:106–113. doi: 10.1002/ddr.21001. [DOI] [Google Scholar]
- 65.Zhu Y., Zhu J., Ma X., Tian Y., Wan X., Zhang T. Evaluation for developmental toxicity of rhodioside injection in rats. Chin. J. New Drugs. 2009;18:2068–2071. [Google Scholar]
- 66.Zhu J., Wan X., Zhu Y., Ma X., Zheng Y., Zhang T. Evaluation of salidroside in vitro and in vivo genotoxicity. Drug Chem. Toxicol. 2010;33:220–226. doi: 10.3109/01480540903373654. [DOI] [PubMed] [Google Scholar]
- 67.Darbinyan V., Aslanyan G., Amroyan E., Gabrielyan E., Malmström C., Panossian A. Clinical trial of Rhodiola rosea L. extract SHR-5 in the treatment of mild to moderate depression. Nord. J. Psychiatry. 2007;61:343–348. doi: 10.1080/08039480701643290. Erratum in: Nord. J. Psychiatry2007, 61, 503. [DOI] [PubMed] [Google Scholar]
- 68.Zhang X., Du L., Zhang W., Yang Y., Zhou Q., Du G. Therapeutic effects of baicalein on rotenone-induced Parkinson’s disease through protecting mitochondrial function and biogenesis. Sci. Rep. 2017;7:9968. doi: 10.1038/s41598-017-07442-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wang S., He H., Chen L., Zhang W., Zhang X., Chen J. Protective effects of salidroside in the MPTP/MPP(+)-induced model of Parkinson’s disease through ROS-NO-related mitochondrion pathway. Mol. Neurobiol. 2015;51:718–728. doi: 10.1007/s12035-014-8755-0. [DOI] [PubMed] [Google Scholar]
- 70.Lebeau A., Esclaire F., Rostène W., Pélaprat D. Baicalein protects cortical neurons from beta-amyloid (25–35) induced toxicity. Neuroreport. 2001;12:2199–2202. doi: 10.1097/00001756-200107200-00031. [DOI] [PubMed] [Google Scholar]
- 71.Wang R., Shen X., Xing E., Guan L., Xin L. Scutellaria baicalensis stem-leaf total flavonoid reduces neuronal apoptosis induced by amyloid beta-peptide (25–35) Neural Regen. Res. 2013;8:1081–1090. doi: 10.3969/j.issn.1673-5374.2013.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zhao J., Lu S., Yu H., Duan S., Zhao J. Baicalin and ginsenoside Rb1 promote the proliferation and differentiation of neural stem cells in Alzheimer’s disease model rats. Brain Res. 2018;1678:187–194. doi: 10.1016/j.brainres.2017.10.003. [DOI] [PubMed] [Google Scholar]
- 73.Cheng J.H., Tsai C.L., Lien Y.Y., Lee M.S., Sheu S.C. High molecular weight of polysaccharides from Hericium erinaceus against amyloid beta-induced neurotoxicity. BMC Complement. Altern. Med. 2016;16:170. doi: 10.1186/s12906-016-1154-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Tsai-Teng T., Chin-Chu C., Li-Ya L., Wan-Ping C., Chung-Kuang L., Chien-Chang S., Chi-Ying H.F., Chien-Chih C., Shiao Y.J. Erinacine A-enriched Hericium erinaceus mycelium ameliorates Alzheimer’s disease-related pathologies in APPswe/PS1dE9 transgenic mice. J. Biomed. Sci. 2016;23:49. doi: 10.1186/s12929-016-0266-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zhang J., An S., Hu W., Teng M., Wang X., Qu Y., Liu Y., Yuan Y., Wang D. The Neuroprotective Properties of Hericium erinaceus in Glutamate-Damaged Differentiated PC12 Cells and an Alzheimer’s Disease Mouse Model. Int. J. Mol. Sci. 2016;17:1810. doi: 10.3390/ijms17111810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Nabavi S.F., Braidy N., Orhan I.E., Badiee A., Daglia M., Nabavi S.M. Rhodiola rosea L. and Alzheimer’s disease: From farm to pharmacy. Phytother. Res. 2016;30:532–539. doi: 10.1002/ptr.5569. [DOI] [PubMed] [Google Scholar]
- 77.Liao Z.L., Su H., Tan Y.F., Qiu Y.J., Zhu J.P., Chen Y., Lin S.S., Wu M.H., Mao Y.P., Hu J.J., et al. Salidroside protects PC-12 cells against amyloid β-induced apoptosis by activation of the ERK1/2 and AKT signaling pathways. Int. J. Mol. Med. 2019;43:1769–1777. doi: 10.3892/ijmm.2019.4088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zhang L., Yu H., Zhao X., Lin X., Tan C., Cao G., Wang Z. Neuroprotective effects of salidroside against beta-amyloid-induced oxidative stress in SH-SY5Y human neuroblastoma cells. Neurochem. Int. 2010;57:547–555. doi: 10.1016/j.neuint.2010.06.021. [DOI] [PubMed] [Google Scholar]
- 79.Li Q., Wang J., Li Y., Xu X. Neuroprotective effects of salidroside administration in a mouse model of Alzheimer’s disease. Mol. Med. Rep. 2018;17:7287–7292. doi: 10.3892/mmr.2018.8757. [DOI] [PubMed] [Google Scholar]
- 80.Dai J., Qiu Y.M., Ma Z.W., Yan G.F., Zhou J., Li S.Q., Wu H., Jin Y.C., Zhang X.H. Neuroprotective effect of baicalin on focal cerebral ischemia in rats. Neural Regen. Res. 2018;13:2129–2133. doi: 10.4103/1673-5374.241464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lee K.F., Chen J.H., Teng C.C., Shen C.H., Hsieh M.C., Lu C.C., Lee K.C., Lee L.Y., Chen W.P., Chen C.C., et al. Protective effects of Hericium erinaceus mycelium and its isolated erinacine A against ischemia-injury-induced neuronal cell death via the inhibition of iNOS/p38 MAPK and nitrotyrosine. Int. J. Mol. Sci. 2014;15:15073–15089. doi: 10.3390/ijms150915073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wong K.H., Naidu M., David R.P., Abdulla M.A., Abdullah N., Kuppusamy U.R., Sabaratnam V. Functional recovery enhancement following injury to rodent peroneal nerve by Lion’s Mane mushroom, Hericium erinaceus (Bull.: Fr.) Pers. (Aphyllophoromycetideae) Int. J. Med. Mushrooms. 2009;11:225–236. doi: 10.1615/IntJMedMushr.v11.i3.20. [DOI] [Google Scholar]
- 83.Chen S.F., Tsai H.J., Hung T.H., Chen C.C., Lee C.Y., Wu C.H., Wang P.Y., Liao N.C. Salidroside improves behavioral and histological outcomes and reduces apoptosis via PI3K/Akt signaling after experimental traumatic brain injury. PLoS ONE. 2012;7:e45763. doi: 10.1371/annotation/1f110857-27d7-4e83-9eb3-4e5f51950a26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Chang C.H., Chen Y., Yew X.X., Chen H.X., Kim J.X., Chang C.C., Peng C.C., Peng R.Y. Improvement of erinacine A productivity in Hericium erinaceus mycelia and its neuroprotective bioactivity against the glutamate-insulted apoptosis. LWT Food Sci. Technol. 2016;65:1100–1108. doi: 10.1016/j.lwt.2015.08.014. [DOI] [Google Scholar]
- 85.Li Q., Li Q.Q., Jia J.N., Sun Q.Y., Zhou H.H., Jin W.L., Mao X.Y. Baicalein Exerts Neuroprotective Effects in FeCl(3)-Induced Posttraumatic Epileptic Seizures via Suppressing Ferroptosis. Front. Pharmacol. 2019;10:638. doi: 10.3389/fphar.2019.00638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Liu Y.F., Gao F., Li X.W., Jia R.H., Meng X.D., Zhao R., Jing Y.Y., Wang Y., Jiang W. The anticonvulsant and neuroprotective effects of baicalin on pilocarpine-induced epileptic model in rats. Neurochem. Res. 2012;37:1670–1680. doi: 10.1007/s11064-012-0771-8. [DOI] [PubMed] [Google Scholar]
- 87.Si P.P., Zhen J.L., Cai Y.L., Wang W.J., Wang W.P. Salidroside protects against kainic acid-induced status epilepticus via suppressing oxidative stress. Neurosci. Lett. 2016;618:19–24. doi: 10.1016/j.neulet.2016.02.056. [DOI] [PubMed] [Google Scholar]
- 88.Zhang L., Xing D., Wang W., Wang R., Du L. Kinetic difference of baicalin in rat blood and cerebral nuclei after intravenous administration of Scutellariae Radix extract. J. Ethnopharmacol. 2006;103:120–125. doi: 10.1016/j.jep.2005.07.013. [DOI] [PubMed] [Google Scholar]
- 89.Chen L., Zhang L., Wang X., Lin H., Du L. Determination of dopamine and its relativity of baicalin in rat nuclei after intravenous administration of flavonoids from Scutellariae radix. Biomed. Chromatogr. 2007;21:84–88. doi: 10.1002/bmc.722. [DOI] [PubMed] [Google Scholar]
- 90.Shimbo M., Kawagishi H., Yokogoshi H. Erinacine A increases catecholamine and nerve growth factor content in the central nervous system of rats. Nutr. Res. 2005;25:617–623. doi: 10.1016/j.nutres.2005.06.001. [DOI] [Google Scholar]
- 91.van Diermen D., Marston A., Bravo J., Reist M., Carrupt P.A., Hostettmann K. Monoamine oxidase inhibition by Rhodiola rosea L. roots. J. Ethnopharmacol. 2009;122:397–401. doi: 10.1016/j.jep.2009.01.007. [DOI] [PubMed] [Google Scholar]
- 92.Zhou R., Han X., Wang J., Sun J. Baicalin may have a therapeutic effect in attention deficit hyperactivity disorder. Med. Hypotheses. 2015;85:761–764. doi: 10.1016/j.mehy.2015.10.012. [DOI] [PubMed] [Google Scholar]
- 93.Zhou R., Wang J., Han X., Ma B., Yuan H., Song Y. Baicalin regulates the dopamine system to control the core symptoms of ADHD. Mol. Brain. 2019;12:11. doi: 10.1186/s13041-019-0428-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Wu P.H., Shen Y.C., Wang Y.H., Chi C.W., Yen J.C. Baicalein attenuates methamphetamine-induced loss of dopamine transporter in mouse striatum. Toxicology. 2006;226:238–245. doi: 10.1016/j.tox.2006.06.015. [DOI] [PubMed] [Google Scholar]
- 95.Ding S., Zhuge W., Hu J., Yang J., Wang X., Wen F., Wang C., Zhuge Q. Baicalin reverses the impairment of synaptogenesis induced by dopamine burden via the stimulation of GABA(A)R-TrkB interaction in minimal hepatic encephalopathy. Psychopharmacology. 2018;235:1163–1178. doi: 10.1007/s00213-018-4833-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Su G.Y., Yang J.Y., Wang F., Ma J., Zhang K., Dong Y.X., Song S.J., Lu X.M., Wu C.F. Antidepressant-like effects of Xiaochaihutang in a rat model of chronic unpredictable mild stress. J. Ethnopharmacol. 2014;152:217–226. doi: 10.1016/j.jep.2014.01.006. [DOI] [PubMed] [Google Scholar]
- 97.Kawagishi H., Ando M., Sakamoto H., Yoshida S., Ojima F., Ishiguro Y., Ukai N., Furukawa S. Hericenones C, D and E, stimulators of nerve growth factor (NGF)-synthesis, from the mushroom Hericium erinaceum. Tetrahedron Lett. 1991;32:4561–4564. doi: 10.1016/0040-4039(91)80039-9. [DOI] [Google Scholar]
- 98.Kawagishi H., Ishiyama D., Mori H., Sakamoto H., Ishiguro Y., Furukawa S., Jingxuan L. Dictyophorines A and B, two stimulators of NGF-synthesis from the mushroom Dictyophora indusiata. Phytochemistry. 1997;45:1203–1205. doi: 10.1016/S0031-9422(97)00144-1. [DOI] [PubMed] [Google Scholar]
- 99.Kawagishi H., Shimada A., Hosokawa S., Mori H., Sakamoto H., Ishiguro Y., Sakemi S., Bordner J., Kojima N., Furukawa S. Erinacines E, F, and G, stimulators of nerve growth factor (NGF)-synthesis, from the mycelia of Hericium erinaceum. Tetrahedron Lett. 1996;37:7399–7402. doi: 10.1016/0040-4039(96)01687-5. [DOI] [Google Scholar]
- 100.Kawagishi H., Shimada A., Shirai R., Okamoto K., Ojima F., Sakamoto H., Ishiguro Y., Furukawa S. Erinacines A, B and C, strong stimulators of nerve growth factor (NGF)-synthesis, from the mycelia of Hericium erinaceum. Tetrahedron Lett. 1994;35:1569–1572. doi: 10.1016/S0040-4039(00)76760-8. [DOI] [Google Scholar]
- 101.Kawagishi H., Simada A., Shizuki K., Mori H., Okamoto K., Sakamoto H., Furukawa S. Erinacine D, a stimulator of NGF-synthesis, from the mycelia of Hericium erinaceum. Heterocycl. Commun. 1996;2:51–54. doi: 10.1515/HC.1996.2.1.51. [DOI] [Google Scholar]
- 102.Lee E.W., Shizuki K., Hosokawa S., Suzuki M., Suganuma H., Inakuma T., Li J., Ohnishi-Kameyama M., Nagata T., Furukawa S., et al. Two novel diterpenoids, erinacines H and I from the Mycelia of Hericium erinaceum. Biosci. Biotechnol. Biochem. 2000;64:2402–2405. doi: 10.1271/bbb.64.2402. [DOI] [PubMed] [Google Scholar]
- 103.Ma B.J., Shen J.W., Yu H.Y., Ruan Y., Wu T.T., Zhao X. Hericenones and erinacines: Stimulators of nerve growth factor (NGF) biosynthesis in Hericium erinaceus. Mycology. 2010;1:92–98. doi: 10.1080/21501201003735556. [DOI] [Google Scholar]
- 104.Hao-Yu T., Xia Y., Cheng-Chen Z., Qian J., Jin-Ming G. Structure Diversity, Synthesis, and Biological Activity of Cyathane Diterpenoids in Higher Fungi. Curr. Med. Chem. 2015;22:2375–2391. doi: 10.2174/0929867322666150521091333. [DOI] [PubMed] [Google Scholar]
- 105.Zhang C.C., Cao C.Y., Kubo M., Harada K., Yan X.T., Fukuyama Y., Gao J.M. Chemical Constituents from Hericium erinaceus Promote Neuronal Survival and Potentiate Neurite Outgrowth via the TrkA/Erk1/2 Pathway. Int. J. Mol. Sci. 2017;18:1659. doi: 10.3390/ijms18081659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Phan C.W., Lee G.S., Hong S.L., Wong Y.T., Brkljača R., Urban S., Abd Malek S.N., Sabaratnam V. Hericium erinaceus (Bull.: Fr) Pers. Cultivated under tropical conditions: Isolation of hericenones and demonstration of NGF-mediated neurite outgrowth in PC12 cells via MEK/ERK and PI3K-Akt signaling pathways. Food Funct. 2014;5:3160–3169. doi: 10.1039/C4FO00452C. [DOI] [PubMed] [Google Scholar]
- 107.Phan C.W., David P., Naidu M., Wong K.H., Sabaratnam V. Neurite outgrowth stimulatory effects of culinary-medicinal mushrooms and their toxicity assessment using differentiating Neuro-2a and embryonic fibroblast BALB/3T3. BMC Complement. Altern. Med. 2013;13:261. doi: 10.1186/1472-6882-13-261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Samberkar S., Gandhi S., Naidu M., Wong K.H., Raman J., Sabaratnam V. Lion’s mane, hericium erinaceus and tiger milk, lignosus rhinocerotis (Higher basidiomycetes) medicinal mushrooms stimulate neurite outgrowth in dissociated cells of brain, spinal cord, and retina: An in vitro study. Int. J. Med. Mushrooms. 2015;17:1047–1054. doi: 10.1615/IntJMedMushrooms.v17.i11.40. [DOI] [PubMed] [Google Scholar]
- 109.Rupcic Z., Rascher M., Kanaki S., Köster R.W., Stadler M., Wittstein K. Two new cyathane diterpenoids from mycelial cultures of the medicinal mushroom Hericium erinaceus and the rare species, Hericium flagellum. Int. J. Mol. Sci. 2018;19:740. doi: 10.3390/ijms19030740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Guo L., Stormmesand J., Fang Z., Zhu Q., Balesar R., van Heerikhuize J., Sluiter A., Swaab D., Bao A.M. Quantification of Tyrosine Hydroxylase and ErbB4 in the Locus Coeruleus of Mood Disorder Patients Using a Multispectral Method to Prevent Interference with Immunocytochemical Signals by Neuromelanin. Neurosci. Bull. 2019;35:205–215. doi: 10.1007/s12264-019-00339-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Zhang Q., Hu D.X., He F., Li C.Y., Qi G.J., Cai H.W., Li T.X., Ming J., Zhang P., Chen X.Q., et al. Locus coeruleus-CA1 projections are involved in chronic depressive stress-induced hippocampal vulnerability to transient global ischaemia. Nat. Commun. 2019;10:2942. doi: 10.1038/s41467-019-10795-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Giorgi F.S., Ryskalin L., Ruffoli R., Biagioni F., Limanaqi F., Ferrucci M., Busceti C.L., Bonuccelli U., Fornai F. The Neuroanatomy of the Reticular Nucleus Locus Coeruleus in Alzheimer’s Disease. Front. Neuroanat. 2017;11:80. doi: 10.3389/fnana.2017.00080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Szot P., Franklin A., Miguelez C., Wang Y., Vidaurrazaga I., Ugedo L., Sikkema C., Wilkinson C.W., Raskind M.A. Depressive-like behavior observed with a minimal loss of locus coeruleus (LC) neurons following administration of 6-hydroxydopamine is associated with electrophysiological changes and reversed with precursors of norepinephrine. Neuropharmacology. 2016;101:76–86. doi: 10.1016/j.neuropharm.2015.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Mori K., Obara Y., Moriya T., Inatomi S., Nakahata N. Effects of Hericium erinaceus on amyloid β(25–35) peptide-induced learning and memory deficits in mice. Biomed. Res. 2011;32:67–72. doi: 10.2220/biomedres.32.67. [DOI] [PubMed] [Google Scholar]
- 115.Panossian A., Wikman G., Sarris J. Rosenroot (Rhodiola rosea): Traditional use, chemical composition, pharmacology and clinical efficacy. Phytomedicine. 2010;17:481–493. doi: 10.1016/j.phymed.2010.02.002. [DOI] [PubMed] [Google Scholar]
- 116.Panossian A., Hamm R., Wikman G., Efferth T. Mechanism of action of Rhodiola, salidroside, tyrosol and triandrin in isolated neuroglial cells: An interactive pathway analysis of the downstream effects using RNA microarray data. Phytomedicine. 2014;21:1325–1348. doi: 10.1016/j.phymed.2014.07.008. [DOI] [PubMed] [Google Scholar]
- 117.Mattioli L., Titomanlio F., Perfumi M. Effects of a Rhodiola rosea L. extract on the acquisition, expression, extinction, and reinstatement of morphine-induced conditioned place preference in mice. Psychopharmacology. 2012;221:183–193. doi: 10.1007/s00213-012-2686-0. [DOI] [PubMed] [Google Scholar]
- 118.Mattioli L., Funari C., Perfumi M. Effects of Rhodiola rosea L. extract on behavioural and physiological alterations induced by chronic mild stress in female rats. J. Psychopharmacol. 2009;23:130–142. doi: 10.1177/0269881108089872. [DOI] [PubMed] [Google Scholar]
- 119.Vasileva L.V., Getova D.P., Doncheva N.D., Marchev A.S., Georgiev M.I. Beneficial effect of commercial Rhodiola extract in rats with scopolamine-induced memory impairment on active avoidance. J. Ethnopharmacol. 2016;193:586–591. doi: 10.1016/j.jep.2016.10.011. [DOI] [PubMed] [Google Scholar]
- 120.Hillhouse B., Ming D.S., French C., Towers G.H. Acetylcholine esterase inhibitors in Rhodiola rosea. Pharm. Biol. 2004;42:68–72. doi: 10.1080/13880200490505636. [DOI] [Google Scholar]
- 121.Chen Q.G., Zeng Y.S., Qu Z.Q., Tang J.Y., Qin Y.J., Chung P., Wong R., Hägg U. The effects of Rhodiola rosea extract on 5-HT level, cell proliferation and quantity of neurons at cerebral hippocampus of depressive rats. Phytomedicine. 2009;16:830–838. doi: 10.1016/j.phymed.2009.03.011. [DOI] [PubMed] [Google Scholar]
- 122.Mannucci C., Navarra M., Calzavara E., Caputi A.P., Calapai G. Serotonin involvement in Rhodiola rosea attenuation of nicotine withdrawal signs in rats. Phytomedicine. 2012;19:1117–1124. doi: 10.1016/j.phymed.2012.07.001. [DOI] [PubMed] [Google Scholar]
- 123.Panossian A., Hambardzumyan M., Hovhanissyan A., Wikman G. The adaptogens rhodiola and schizandra modify the response to immobilization stress in rabbits by suppressing the increase of phosphorylated stress-activated protein kinase, nitric oxide and cortisol. Drug Target Insights. 2007;2:39–54. doi: 10.1177/117739280700200011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Panossian A., Wikman G., Kaur P., Asea A. Adaptogens stimulate neuropeptide y and hsp72 expression and release in neuroglia cells. Front. Neurosci. 2012;6:6. doi: 10.3389/fnins.2012.00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Titomanlio F., Perfumi M., Mattioli L. Rhodiola rosea L. extract and its active compound salidroside antagonized both induction and reinstatement of nicotine place preference in mice. Psychopharmacology. 2014;231:2077–2086. doi: 10.1007/s00213-013-3351-y. [DOI] [PubMed] [Google Scholar]
- 126.Palmeri A., Mammana L., Tropea M.R., Gulisano W., Puzzo D. Salidroside, a bioactive compound of rhodiola rosea, ameliorates memory and emotional behavior in adult mice. J. Alzheimers Dis. 2016;52:65–75. doi: 10.3233/JAD-151159. [DOI] [PubMed] [Google Scholar]
- 127.Jin H., Pei L., Shu X., Yang X., Yan T., Wu Y., Wei N., Yan H., Wang S., Yao C., et al. Therapeutic Intervention of Learning and Memory Decays by Salidroside Stimulation of Neurogenesis in Aging. Mol. Neurobiol. 2016;53:851–866. doi: 10.1007/s12035-014-9045-6. [DOI] [PubMed] [Google Scholar]
- 128.Udintsev S.N., Krylova S.G., Konovalova O.N. Correction by natural adaptogens of hormonal-metabolic status disorders in rats during the development of adaptation syndrome using functional tests with dexamethasone and ACTH. Biull. Eksp. Biol. Med. 1991;112:599–601. doi: 10.1007/BF00840509. [DOI] [PubMed] [Google Scholar]
- 129.Hui K.M., Wang X.H., Xue H. Interaction of flavones from the roots of Scutellaria baicalensis with the benzodiazepine site. Planta Med. 2000;66:91–93. doi: 10.1055/s-0029-1243121. [DOI] [PubMed] [Google Scholar]
- 130.Liao J.F., Hung W.Y., Chen C.F. Anxiolytic-like effects of baicalein and baicalin in the Vogel conflict test in mice. Eur. J. Pharmacol. 2003;464:141–146. doi: 10.1016/S0014-2999(03)01422-5. [DOI] [PubMed] [Google Scholar]
- 131.Xu Z., Wang F., Tsang S.Y., Ho K.H., Zheng H., Yuen C.T., Chow C.Y., Xue H. Anxiolytic-like effect of baicalin and its additivity with other anxiolytics. Planta Med. 2006;72:189–192. doi: 10.1055/s-2005-873193. [DOI] [PubMed] [Google Scholar]
- 132.de Carvalho R.S., Duarte F.S., de Lima T.C. Involvement of GABAergic non-benzodiazepine sites in the anxiolytic-like and sedative effects of the flavonoid baicalein in mice. Behav. Brain Res. 2011;221:75–82. doi: 10.1016/j.bbr.2011.02.038. [DOI] [PubMed] [Google Scholar]
- 133.Zhang K., He M., Wang F., Zhang H., Li Y., Yang J., Wu C. Revealing Antidepressant Mechanisms of Baicalin in Hypothalamus Through Systems Approaches in Corticosterone- Induced Depressed Mice. Front. Neurosci. 2019;13:834. doi: 10.3389/fnins.2019.00834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Zhang K., Pan X., Wang F., Ma J., Su G., Dong Y., Yang J., Wu C. Baicalin promotes hippocampal neurogenesis via SGK1- and FKBP5-mediated glucocorticoid receptor phosphorylation in a neuroendocrine mouse model of anxiety/depression. Sci. Rep. 2016;6:30951. doi: 10.1038/srep30951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Yu H., Zhang F., Guan X. Baicalin reverse depressive-like behaviors through regulation SIRT1-NF-kB signalling pathway in olfactory bulbectomized rats. Phytother. Res. 2019;33:1480–1489. doi: 10.1002/ptr.6340. [DOI] [PubMed] [Google Scholar]
- 136.Yu H.Y., Yin Z.J., Yang S.J., Ma S.P., Qu R. Baicalin Reverses Depressive-Like Behaviours and Regulates Apoptotic Signalling Induced by Olfactory Bulbectomy. Phytother. Res. 2016;30:469–475. doi: 10.1002/ptr.5550. [DOI] [PubMed] [Google Scholar]
- 137.Gao C., Du Q., Li W., Deng R., Wang Q., Xu A., Shen J. Baicalin Modulates APPL2/Glucocorticoid Receptor Signaling Cascade, Promotes Neurogenesis, and Attenuates Emotional and Olfactory Dysfunctions in Chronic Corticosterone-Induced Depression. Mol. Neurobiol. 2018;55:9334–9348. doi: 10.1007/s12035-018-1042-8. [DOI] [PubMed] [Google Scholar]
- 138.Zhong J., Li G., Xu H., Wang Y., Shi M. Baicalin ameliorates chronic mild stress-induced depression-like behaviors in mice and attenuates inflammatory cytokines and oxidative stress. Braz. J. Med. Biol. Res. 2019;52:e8434. doi: 10.1590/1414-431x20198434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Liu L., Dong Y., Shan X., Li L., Xia B., Wang H. Anti-Depressive Effectiveness of Baicalin In Vitro and In Vivo. Molecules. 2019;24:326. doi: 10.3390/molecules24020326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Guo L.T., Wang S.Q., Su J., Xu L.X., Ji Z.Y., Zhang R.Y., Zhao Q.W., Ma Z.Q., Deng X.Y., Ma S.P. Baicalin ameliorates neuroinflammation-induced depressive-like behavior through inhibition of toll-like receptor 4 expression via the PI3K/AKT/FoxO1 pathway. J. Neuroinflammation. 2019;16:95. doi: 10.1186/s12974-019-1474-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Zhang C.Y., Zeng M.J., Zhou L.P., Li Y.Q., Zhao F., Shang Z.Y., Deng X.Y., Ma Z.Q., Fu Q., Ma S.P., et al. Baicalin exerts neuroprotective effects via inhibiting activation of GSK3β/NF-κB/NLRP3 signal pathway in a rat model of depression. Int. Immunopharmacol. 2018;64:175–182. doi: 10.1016/j.intimp.2018.09.001. [DOI] [PubMed] [Google Scholar]
- 142.Liu X., Liu C. Baicalin ameliorates chronic unpredictable mild stress-induced depressive behavior: Involving the inhibition of NLRP3 inflammasome activation in rat prefrontal cortex. Int. Immunopharmacol. 2017;48:30–34. doi: 10.1016/j.intimp.2017.04.019. [DOI] [PubMed] [Google Scholar]
- 143.Lu Y., Sun G., Yang F., Guan Z., Zhang Z., Zhao J., Liu Y., Chu L., Pei L. Baicalin regulates depression behavior in mice exposed to chronic mild stress via the Rac/LIMK/cofilin pathway. Biomed. Pharmacother. 2019;116:109054. doi: 10.1016/j.biopha.2019.109054. [DOI] [PubMed] [Google Scholar]
- 144.Li Y.C., Shen J.D., Li J., Wang R., Jiao S., Yi L.T. Chronic treatment with baicalin prevents the chronic mild stress-induced depressive-like behavior: Involving the inhibition of cyclooxygenase-2 in rat brain. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2013;40:138–143. doi: 10.1016/j.pnpbp.2012.09.007. [DOI] [PubMed] [Google Scholar]
- 145.Zhang R., Ma Z., Liu K., Li Y., Liu D., Xu L., Deng X., Qu R., Ma Z., Ma S. Baicalin exerts antidepressant effects through Akt/FOXG1 pathway promoting neuronal differentiation and survival. Life Sci. 2019;221:241–248. doi: 10.1016/j.lfs.2019.02.033. [DOI] [PubMed] [Google Scholar]
- 146.Zhang R., Guo L., Ji Z., Li X., Zhang C., Ma Z., Fu Q., Qu R., Ma S. Radix Scutellariae Attenuates CUMS-Induced Depressive-Like Behavior by Promoting Neurogenesis via cAMP/PKA Pathway. Neurochem. Res. 2018;43:2111–2120. doi: 10.1007/s11064-018-2635-3. [DOI] [PubMed] [Google Scholar]
- 147.Mori K., Inatomi S., Ouchi K., Azumi Y., Tuchida T. Improving effects of the mushroom Yamabushitake (Hericium erinaceus) on mild cognitive impairment: A double-blind placebo-controlled clinical trial. Phytother. Res. 2009;23:367–372. doi: 10.1002/ptr.2634. [DOI] [PubMed] [Google Scholar]
- 148.Chong P.S., Fung M.L., Wong K.H., Lim L.W. Therapeutic Potential of Hericium erinaceus for Depressive Disorder. Int. J. Mol. Sci. 2019;21:163. doi: 10.3390/ijms21010163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Ryu S., Kim H.G., Kim J.Y., Kim S.Y., Cho K.O. Hericium erinaceus Extract Reduces Anxiety and Depressive Behaviors by Promoting Hippocampal Neurogenesis in the Adult Mouse Brain. J. Med. Food. 2018;21:174–180. doi: 10.1089/jmf.2017.4006. [DOI] [PubMed] [Google Scholar]
- 150.Nagano M., Shimizu K., Kondo R., Hayashi C., Sato D., Kitagawa K., Ohnuki K. Reduction of depression and anxiety by 4 weeks Hericium erinaceus intake. BioMed Res. 2010;31:231–237. doi: 10.2220/biomedres.31.231. [DOI] [PubMed] [Google Scholar]
- 151.Inanaga K. Marked improvement of neurocognitive impairment after treatment with compounds from Hericium erinaceum: A case study of recurrent depressive disorder. Pers. Med. Universe. 2014;3:46–48. doi: 10.1016/j.pmu.2014.02.004. [DOI] [Google Scholar]
- 152.Okamura H., Anno N., Tsuda A., Inokuchi T., Uchimura N., Inanaga K. The effects of Hericium erinaceus (Amyloban® 3399) on sleep quality and subjective well-being among female undergraduate students: A pilot study. Pers. Med. Universe. 2015;4:76–78. doi: 10.1016/j.pmu.2015.03.006. [DOI] [Google Scholar]
- 153.Zhu L., Wei T., Gao J., Chang X., He H., Miao M., Yan T. Salidroside attenuates lipopolysaccharide (LPS) induced serum cytokines and depressive-like behavior in mice. Neurosci. Lett. 2015;606:1–6. doi: 10.1016/j.neulet.2015.08.025. [DOI] [PubMed] [Google Scholar]
- 154.Vasileva L.V., Saracheva K.E., Ivanovska M.V., Petrova A.P., Sucouglu E., Murdjeva M.A., Getova-Spasova D.P. Beneficial Effect of Chronic Treatment with Extracts from Rhodiola Rosea L. and Curcuma Longa L. on the Immunoreactivity of Animals Subjected to a Chronic Mild Stress Model. Folia Med. 2017;59:443–453. doi: 10.1515/folmed-2017-0046. [DOI] [PubMed] [Google Scholar]
- 155.Kamel Ismail Z.M., Morcos M.A., Eldin Mohammad M.D., Gamal Aboulkhair A. Enhancement of Neural Stem Cells after Induction of Depression in Male Albino Rats (A histological & Immunohistochemical Study) Int. J. Stem Cells. 2014;7:70–78. doi: 10.15283/ijsc.2014.7.2.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Coors A., Brosch M., Kahl E., Khalil R., Michels B., Laub A., Franke K., Gerber B., Fendt M. Rhodiola rosea root extract has antipsychotic-like effects in rodent models of sensorimotor gating. J. Ethnopharmacol. 2019;235:320–328. doi: 10.1016/j.jep.2019.02.031. [DOI] [PubMed] [Google Scholar]
- 157.Concerto C., Infortuna C., Muscatello M.R.A., Bruno A., Zoccali R., Chusid E., Aguglia E., Battaglia F. Exploring the effect of adaptogenic Rhodiola Rosea extract on neuroplasticity in humans. Complement. Ther. Med. 2018;41:141–146. doi: 10.1016/j.ctim.2018.09.013. [DOI] [PubMed] [Google Scholar]
- 158.Darbinyan V., Kteyan A., Panossian A., Gabrielian E., Wikman G., Wagner H. Rhodiola rosea in stress induced fatigue – a double blind cross-over study of a standardized extract SHR-5 with a repeated low-dose regimen on the mental performance of healthy physicians during night duty. Phytomedicine. 2000;7:365–371. doi: 10.1016/S0944-7113(00)80055-0. [DOI] [PubMed] [Google Scholar]
- 159.Spasov A.A., Wikman G.K., Mandrikov V.B., Mironova I.A., Neumoin V.V. A double-blind, placebo-controlled pilot study of the stimulating and adaptogenic effect of Rhodiola rosea SHR-5 extract on the fatigue of students caused by stress during an examination period with a repeated low-dose regimen. Phytomedicine. 2000;7:85–89. doi: 10.1016/S0944-7113(00)80078-1. [DOI] [PubMed] [Google Scholar]
- 160.Shevtsov V.A., Zholus B.I., Shervarly V.I., Vol’skij V.B., Korovin Y.P., Khristich M.P., Roslyakova N.A., Wikman G. A randomized trial of two different doses of a SHR-5 Rhodiola rosea extract versus placebo and control of capacity for mental work. Phytomedicine. 2003;10:95–105. doi: 10.1078/094471103321659780. [DOI] [PubMed] [Google Scholar]
- 161.Bystritsky A., Kerwin L., Feusner J.D. A pilot study of Rhodiola rosea (Rhodax) for generalized anxiety disorder (GAD) J. Altern. Complement. Med. 2008;14:175–180. doi: 10.1089/acm.2007.7117. [DOI] [PubMed] [Google Scholar]
- 162.Brichenko V.S., Kupriyanova I.E., Skorokhodova T.F. The use of herbal adaptogens together with tricyclic antidepressants in patients with psychogenic depressions. In Modern Problems of Pharmacology and Search for New Medicines; Goldsberg ED, Tomsk, Russia, 1986; Volume 2, pp. 58–60
- 163.Brichenko V.S., Skorokhodova T.F. Clinical and Organisational Aspects of early Manifestations of Nervous and Mental diseases. Altajskoe Kniže; Barnaul, Russia: 1987. Herbal Adaptogens in Rehabilitation of Patients with depression; p. 15. [Google Scholar]
- 164.Mao J.J., Li Q.S., Soeller I., Xie S.X., Amsterdam J.D. Rhodiola rosea therapy for major depressive disorder: A study protocol for a randomized, double-blind, placebo-controlled trial. J. Clin. Trials. 2014;4:170. doi: 10.4172/2167-0870.1000170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Mao J.J., Xie S.X., Zee J., Soeller I., Li Q.S., Rockwell K., Amsterdam J.D. Rhodiola rosea versus sertraline for major depressive disorder: A randomized placebo-controlled trial. Phytomedicine. 2015;22:394–399. doi: 10.1016/j.phymed.2015.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Cropley M., Banks A.P., Boyle J. The Effects of Rhodiola rosea L. Extract on Anxiety, Stress, Cognition and Other Mood Symptoms. Phytother. Res. 2015;29:1934–1939. doi: 10.1002/ptr.5486. [DOI] [PubMed] [Google Scholar]
- 167.Bangratz M., Ait Abdellah S., Berlin A., Blondeau C., Guilbot A., Dubourdeaux M., Lemoine P. A preliminary assessment of a combination of rhodiola and saffron in the management of mild-moderate depression. Neuropsychiatr. Dis. Treat. 2018;14:1821–1829. doi: 10.2147/NDT.S169575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Yu H.L., Zhang P.P., Zhang C., Zhang X., Li Z.Z., Li W.Q., Fu A.S. Effects of rhodiola rosea on oxidative stress and negative emotional states in patients with obstructive sleep apnea. Lin Chung Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2019;33:954–957. doi: 10.13201/j.issn.1001-1781.2019.10.013. [DOI] [PubMed] [Google Scholar]
- 169.Gerbarg P.L., Brown R.P. Pause menopause with Rhodiola rosea, a natural selective estrogen receptor modulator. Phytomedicine. 2016;23:763–769. doi: 10.1016/j.phymed.2015.11.013. [DOI] [PubMed] [Google Scholar]
- 170.Limanaqi F., Biagioni F., Ryskalin L., Busceti C.L., Fornai F. Molecular Mechanisms Linking ALS/FTD and Psychiatric Disorders, the Potential Effects of Lithium. Front. Cell. Neurosci. 2019;13:450. doi: 10.3389/fncel.2019.00450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Galts C.P.C., Bettio L.E.B., Jewett D.C., Yang C.C., Brocardo P.S., Rodrigues A.L.S., Thacker J.S., Gil-Mohapel J. Depression in neurodegenerative diseases: Common mechanisms and current treatment options. Neurosci. Biobehav. Rev. 2019;102:56–84. doi: 10.1016/j.neubiorev.2019.04.002. [DOI] [PubMed] [Google Scholar]
- 172.Hargreaves I.P., Al Shahrani M., Wainwright L., Heales S.J. Drug-Induced Mitochondrial Toxicity. Drug Saf. 2016;39:661–674. doi: 10.1007/s40264-016-0417-x. [DOI] [PubMed] [Google Scholar]
- 173.Ekor M. The growing use of herbal medicines: Issues relating to adverse reactions and challenges in monitoring safety. Front. Pharmacol. 2014;4:177. doi: 10.3389/fphar.2013.00177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Limanaqi F., Biagioni F., Busceti C.L., Ryskalin L., Polzella M., Frati A., Fornai F. Phytochemicals Bridging Autophagy Induction and Alpha-Synuclein Degradation in Parkinsonism. Int. J. Mol. Sci. 2019;20:3274. doi: 10.3390/ijms20133274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Liu F., Zhang J., Qian J., Wu G., Ma Z. Baicalin attenuates liver hypoxia/reoxygenation injury by inducing autophagy. Exp. Ther. Med. 2018;16:657–664. doi: 10.3892/etm.2018.6284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Fang J., Zhu Y., Wang H., Cao B., Fei M., Niu W., Zhou Y., Wang X., Li X., Zhou M. Baicalin Protects Mice Brain From Apoptosis in Traumatic Brain Injury Model Through Activation of Autophagy. Front. Neurosci. 2019;12:1006. doi: 10.3389/fnins.2018.01006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Chen S., Cai F., Wang J., Yang Z., Gu C., Wang G., Mao G., Yan J. Salidroside protects SH-SY5Y from pathogenic α-synuclein by promoting cell autophagy via mediation of mTOR/p70S6K signaling. Mol. Med. Rep. 2019;20:529–538. doi: 10.3892/mmr.2019.10285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Gassen N.C., Rein T. Is There a Role of Autophagy in Depression and Antidepressant Action? Front. Psychiatry. 2019;10:337. doi: 10.3389/fpsyt.2019.00337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Ryskalin L., Limanaqi F., Frati A., Busceti C.L., Fornai F. mTOR-Related Brain Dysfunctions in Neuropsychiatric Disorders. Int. J. Mol. Sci. 2018;19:2226. doi: 10.3390/ijms19082226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Limanaqi F., Biagioni F., Gambardella S., Ryskalin L., Fornai F. Interdependency Between Autophagy and Synaptic Vesicle Trafficking: Implications for Dopamine Release. Front. Mol. Neurosci. 2018;11:299. doi: 10.3389/fnmol.2018.00299. [DOI] [PMC free article] [PubMed] [Google Scholar]