Abstract
The apical Na+-K+-2Cl− cotransporter (NKCC2) mediates NaCl reabsorption by the thick ascending limb (TAL). The free radical superoxide () stimulates TAL NaCl absorption by enhancing NKCC2 activity. In contrast, nitric oxide (NO) scavenges and inhibits NKCC2. NKCC2 activity depends on the number of NKCC2 transporters in the TAL apical membrane and its phosphorylation. We hypothesized that stimulates NKCC2 activity by enhancing apical surface NKCC2 expression. We measured surface NKCC2 expression in rat TALs by surface biotinylation and Western blot analysis. Treatment of TALs with produced by exogenous xanthine oxidase (1 mU/ml) and hypoxanthine (500 µM) stimulated surface NKCC2 expression by ~18 ± 5% (P < 0.05). -stimulated surface NKCC2 expression was blocked by the scavenger tempol (50 µM). Scavenging H2O2 with 100 U/ml catalase did not block the stimulatory effect of xanthine oxidase-hypoxanthine (22 ± 8% increase from control, P < 0.05). Inhibition of endogenous NO production with Nω-nitro-l-arginine methyl ester enhanced surface NKCC2 expression by 21 ± 6% and, when added together with xanthine oxidase-hypoxanthine, increased surface NKCC2 by 41 ± 10% (P < 0.05). Scavenging with superoxide dismutase (300 U/ml) decreased this stimulatory effect by 60% (39 ± 4% to 15 ± 10%, P < 0.05). Protein kinase C inhibition with Gö-6976 (100 nM) blocked -stimulated surface NKCC2 expression (P < 0.05). did not affect NKCC2 phosphorylation at Thr96/101 or its upstream kinases STE20/SPS1-related proline/alanine-rich kinase-oxidative stress-responsive kinase 1. We conclude that increases surface NKCC2 expression by stimulating protein kinase C and that this effect is blunted by endogenous NO. -stimulated apical trafficking of NKCC2 may be involved in the enhanced surface NKCC2 expression observed in Dahl salt-sensitive rats.
Keywords: free radicals, hypertension, ion transport, Na+-K+-2Cl− cotransporter, nephron, nitric oxide, protein kinase C, thick ascending limb
INTRODUCTION
The thick ascending limb (TAL) plays an important role in the maintenance of NaCl homeostasis by absorbing 30% of the filtered NaCl load through the apical Na+-K+-2Cl− transporter (NKCC2) while being water impermeable (3, 8, 9, 23, 28). NaCl absorption in the TAL occurs via a secondary active transport mechanism that includes entry of NaCl through apical NKCC2 and active extrusion of Na+ through basolateral Na+-K+-ATPase (38).
Superoxide () is a free radical produced by the one-electron reduction of molecular oxygen (15) and plays an important role in Na+ reabsorption in the kidney (17, 26, 30, 41, 47, 50). Enhanced production of in the renal medulla contributes to the pathogenesis of salt-sensitive hypertension (17, 26, 27, 48, 50). However, the mechanism by which renal medullary contributes to hypertension is not clear. We and others have shown that increases TAL Na+ reabsorption (30, 32, 41). -stimulated TAL NaCl transport is in part due to direct stimulation of NKCC2 activity (32). To reabsorb NaCl from the tubule lumen, NKCC2 must be present at the apical surface of the TAL. However, it is not known whether increases the amount of NKCC2 at the apical surface. In addition, phosphorylation of STE20/SPS1-related proline/alanine-rich kinase (SPAK) and oxidative stress-responsive kinase 1 (OSR1) phosphorylates NKCC2 at Thr96/101, which simulates ion transport (45, 46). Therefore, we hypothesized that stimulates NKCC2 activity by enhancing the surface expression of NKCC2 in the TAL and also studied the effect of on the phosphorylation of NKCC2.
In the TAL, endogenously produced nitric oxide (NO) inhibits TAL NaCl absorption (42, 44) in part by directly inhibiting NKCC2 activity (48). NO also scavenges in the TAL (40, 41, 42), where it exerts an opposite effect in TAL NaCl transport (40, 41, 42). Thus, we also tested the hypothesis that endogenous NO could decrease the effect of on surface NKCC2 expression.
MATERIALS AND METHODS
Isolation of medullary TALs.
Male Sprague-Dawley rats weighing 220−250 g were anesthetized with ketamine (100 mg/kg body wt ip) and xylazine (20 mg/kg body wt ip). Medullary TAL suspensions were prepared according to previously described methods (2–6, 8–12, 24, 25). In brief, kidneys were perfused via the aorta with a perfusion solution [containing (in mM) 130 NaCl, 2.5 NaH2PO4, 4.0 KCl, 1.2 MgSO4, 6 l-alanine, 1.0 disodium citrate, 5.5 glucose, 2.0 calcium lactate, and 10 HEPES; pH 7.4] with 0.1% collagenase (Sigma, St. Louis, MO) and 100 units heparin. To obtain TAL suspensions, the outer medulla was dissected, minced, and digested in collagenase.
Treatment of TAL suspensions with drugs and cell surface biotinylation.
TAL cell surface proteins were biotinylated as previously described in detail (2–6, 10–12, 24, 25, 39). TAL suspensions were equilibrated at 37°C for 10 min followed by treatment with agonists for 20 min. They were oxygenated every 2 min with 100% O2 with gentle mixing. Inhibitors were added at the time of equilibration so that all of the receptors or enzymes were blocked before agonists were added. To increase production, xanthine oxidase (Xo; EMD Millipore Chemicals) and hypoxanthine (Hy; Sigma) were added to the suspension. Next, suspensions were cooled rapidly to 4°C, washed two times with chilled perfusion solution, and centrifuged at 100 g for 2 min. TAL suspensions were incubated 30 min at 4°C with biotinylation solution [containing (in mM) 10 HEPES, 130 NaCl, 2 MgSO4, 1 CaCl2, and 5.5 glucose; pH: 7.8–8.0] with 0.9 mg/ml NHS-SS-biotin (Pierce Biotechnology) in a gentle rocker. After biotinylation, tubules were washed three times at 4°C: one time with perfusion solution and two times with perfusion solution containing 100 mM glycine to remove excess NHS-SS-biotin. TAL suspensions were centrifuged (100 g) and lysed in buffer containing 150 mM NaCl, 50 mM HEPES, 5 mM EDTA, 2% Triton X-100, and 0.2% SDS with protease inhibitors (10 µg/ml aprotinin, 5 µg/ml leupeptin, 4 mmol/l benzamidine, 5 µg/ml chymostatin, and 5 µg/ml pepstatin A, Sigma).
Total protein content in each sample was measured in duplicate by a colorimetric assay using Bradford's method (Pierce Biotechnology). To measure surface NKCC2, 80 µg biotinylated TAL suspension proteins from each experimental sample were incubated in two rounds with 100 µl streptavidin-coated agarose beads (Pierce Biotechnology). Surface proteins were extracted from the beads by boiling in 40 µl SDS-loading buffer containing 50 µM dl-dithiothreitol and 5% β-mercaptoethanol as described in detail in our previous studies (2–6, 10–12, 24, 25, 39). For intracellular NKCC2 measurement, 1/10 fraction of the supernatant obtained from the pulldown reaction (equivalent to 8 µg intracellular nonbiotinylated proteins) was loaded on the same gels from each experiment. Proteins eluted from streptavidin beads or obtained from TAL lysates were centrifuged for 1 min at 10,000 g, loaded in each lane of a 6.5% SDS-polyacrylamide gel, separated by electrophoresis, and transferred to Immobilon-P PVDF membranes (Millipore, Bedford, MA).
Western blot analysis.
TAL lysates for total protein and TAL lysate proteins for surface protein eluted from streptavidin beads were centrifuged for 1 min at 10,000 g, resolved by SDS-PAGE in 6.5% gel, and transferred to PVDF membrane. The PVDF membrane was incubated for 60 min in blocking buffer containing 50 mM Tris, 150 mM NaCl, 3% BSA (Equitech-bio), and 0.1% Tween 20. Next, 1:1,000 dilution of anti-NKCC2 raised in the chicken was incubated overnight at 4°C in blocking buffer. The membrane was incubated with anti-chicken secondary antibody (1:10,000 conjugated with horseradish peroxidase) (24). Bands were developed with ECL solution (GE Healthcare). Optical densities from surface and intracellular protein fractions were used to calculate NKCC2 levels and to calculate the ratio of surface to total protein. Although total proteins were measured in all samples, as a loading control for total NKCC2, we used GAPDH. To control equal protein loading for surface NKCC2 measurements, we measured total NKCC2 in each sample in the same gel in continuous lanes. To control that the surface fraction contains primarily surface proteins, we measured intracellular GAPDH, which is not biotinylated by NHS-SS-biotin that reacts with lysines in surface proteins at pH 7.6 or higher (21). As expected, GAPDH was easily detectable in the intracellular fractions but not in the surface fractions. For GAPDH immunoblot analysis, all of the experiments were blocked with a mixture of 2.5% BSA for 60 min. We used primary monoclonal anti-GAPDH (Millipore) at 1:160,000 for 120 min at room temperature in 3% BSA. Finally, anti-mouse secondary antibody conjugated to horseradish peroxidase (Amersham) was used at 1:12,000 for 60 min at room temperature in 3% BSA (Equitech-bio).
Measurement of the phospho-NKCC2-to-total NKCC2 ratio and phospho-SPAK-to-total SPAK ratio.
For measurements of the phosphorylated-to-total ratio for NKCC2 and SPAK, we isolated TAL suspensions as we did for previous experiments (2–6, 10–12, 24, 25, 39). TAL suspensions were separated into aliquots in two tubes and stabilized for 10 min at 37°C followed by 20 min of treatment. To inhibit NO production, we used 5 mM Nω-nitro-l-arginine methyl ester (l-NAME) during stabilization and then added perfusion solution for control and Xo-Hy for production. Next, TALs were washed and lysed in lysis buffer (same composition as above) with the addition of phosphatase inhibitor cocktail (Roche). For phospho-NKCC2 and total NKCC2, we used 6.5% polyacrylamide gels; for phospho-SPAK and total SPAK, we used 10% gels. TAL (15 µg) proteins were loaded in each lane, resolved, and then transferred to PVDF membranes. For immunoblot analysis, we used 3% BSA for total NKCC2 during blocking and for both primary and secondary antibodies. We used 1:100,000 dilution primary antibody at 4°C overnight and anti-rabbit secondary antibody for 1 h at 1:2,500 dilutions. For phospho-SPAK, total SPAK, and phospho-NKCC2, we used 5% BSA for blocking, primary antibody, and secondary antibody. For phospho-NKCC2 blot analysis, we used 1:5,000 dilution for 2 h, and secondary anti-rabbit antibody was used at 1:2,500 dilution. The affinity-purified rabbit anti-NKCC2 antibody was produced by GenScript (Piscataway, NJ) and recognizes amino acids 859–873 in the COOH-terminus of rat NKCC2. We and others have characterized this antibody in the past (1, 4, 11). For phospho-NKCC2 at Thr96/101, we used rabbit polyclonal antibody affinity purified against double (Thr96/101) phosphopeptide, which only reacts with NKCC2 when phosphorylated at both Thr96/101. This antibody was generated in the rabbit using the R5 antibody sequence of Giménez and Forbush (19, 20) optimized for the rat (4, 11). For blot analysis of total SPAK (which was a generous gift from Dr. Shinichi Uchida), 1:5,000 dilution was used at 4°C overnight, and anti-rabbit secondary antibody was used for 1 h at 1:2,500 dilution. Primary antibody for phospho-SPAK-OSR1 (phospho-SPAK Ser373/phospho-OSR1 Ser325, Millipore) was used at 1:4,000 dilution for 2 h and secondary anti-rabbit antibody for 1 h.
Statistics.
Results are expressed as means ± SE. Differences between means were evaluated with one-way ANOVA with Bonferroni post hoc testing. P values of <0.05 were considered significant.
RESULTS
Effect of production in TAL surface NKCC2 expression.
We have previously shown that exogenously produced stimulates NKCC2 activity and TAL Cl− reabsorption. To produce exogenous , we used 1 mU/ml Xo (7, 22, 41, 43) in the presence of 500 µM Hy as a substrate. To investigate whether increases apical surface NKCC2 expression, rat TAL suspensions were divided into four aliquots and incubated with vehicle (perfusion solution), the scavenger tempol, the -generating system Xo-Hy, and Xo-Hy plus tempol for 20 min. After treatment, we chilled TAL suspensions to stop NKCC2 trafficking and then measured surface NKCC2 as described above in materials and methods. Data are presented as percentages of control (vehicle). We found that Xo-Hy enhanced surface NKCC2 expression by 18%, and this effect was completely blocked by the scavenger tempol at 50 µM (control: 100%, tempol: 89 ± 10%, Xo-Hy: 118 ± 5%, and tempol + Xo-Hy: 99 ± 6%, n = 5, P < 0.05 vs. Xo-Hy; Fig. 1). There was no significant change in total NKCC2 expression (control: 100%, tempol: 102 ± 4%, Xo-Hy: 105 ± 5%, and tempol + Xo-Hy: 106 ± 6%), suggesting that has no effect on total NKCC2 expression but enhanced NKCC2 trafficking to the apical membrane. Loading control experiments showed absence of the intracellular protein GAPDH in surface fractions (Fig. 1).
Fig. 1.

Effect of extracellular superoxide () production by xanthine oxidase and hypoxanthine (Xo-Hy) on surface Na+-K+-2Cl− cotransporter (NKCC2) in rat thick ascending limbs (TALs). Top, representative Western blot for NKCC2 and GAPDH measured from surface (lanes 3–6) and intracellular (lanes 1, 2, 7, and 8) fractions. GAPDH in the intracellular fractions was not different between control and treated samples. We loaded 80 µg protein for surface NKCC2 measurement from control, tempol, Xo-Hy, and tempol + Xo-Hy after 20 min of treatment (50 µM tempol, 1 mU/ml Xo, and 500 µM Hy were used). For intracellular NKCC2 measurements, 8 µg protein was loaded. Bottom, cumulative data showing production by Xo-Hy increased surface NKCC2, which was blocked by the scavenger tempol. n = 5. *P < 0.05 vs. control; #P < 0.05 vs. Xo-Hy.
Xo also produces H2O2 in addition to in the presence of Hy and oxygen (43). To assure the effect was because of , we tested the effect of catalase on -stimulated surface NKCC2 expression. Scavenging H2O2 with catalase had no significant effect on -stimulated surface NKCC2 expression (control: 100%, catalase: 113 ± 8%, Xo-Hy: 117 ± 3%, catalase + Xo-Hy: 122 ± 7%, n = 5, P < 0.05 vs. control; Fig. 2). Total NKCC2 expression was not changed in rat TAL suspensions by any of the treatments (control: 100%, catalase: 101 ± 6%, Xo-Hy: 103 ± 5%, and catalase + Xo-Hy: 104 ± 5%). GAPDH was used as loading control for total NKCC2 in all experiments and was not different between control and treatments.
Fig. 2.

Effect of catalase on superoxide ()-stimulated thick ascending limb (TAL) apical surface Na+-K+-2Cl− cotransporter (NKCC2) expression. Top, representative Western blot for NKCC2 and GAPDH measured from surface (lanes 3–6) and intracellular (lanes 1, 2, 7, and 8) fractions. Surface NKCC2 was measured from TAL suspensions treated with control (perfusion solution), catalase (100 U/ml), xanthine oxidase and hypoxanthine (Xo-Hy), and catalase + Xo-Hy for 20 min (1 mU/ml Xo and 500 µM Hy were used). We loaded 80 µg TAL protein for surface NKCC2 measurements. Bottom, cumulative data showing that removal of H2O2 by catalase failed to block -stimulated surface NKCC2 expression. n = 5. *P < 0.05 vs. control.
Effect of exogenous in TAL surface NKCC2 expression during NO synthase inhibition.
NO produced by NO synthase (NOS) decreases NKCC2 activity (42, 47) and NaCl reabsorption by the TAL. NO also decreases the bioavailability of (35, 40) and inhibits the effect of on NKCC2-mediated NaCl reabsorption. Therefore, we investigated whether inhibition of NOS by l-NAME (5 mM) would enhance -stimulated surface NKCC2 expression. Adult rat TAL suspensions were separated into aliquots in four different tubes. They were treated with vehicle as control, l-NAME, Xo-Hy, and Xo-Hy plus l-NAME. Surface NKCC2 measured from those samples were 100% (control), 121 ± 5% (l-NAME), 121 ± 6% (Xo-Hy), and 141 ± 10% (l-NAME + Xo-Hy), respectively (n = 5, P < 0.05 vs. control; Fig. 3). Thus, production by Xo-Hy enhanced surface NKCC2 expression by 21%. Inhibition of endogenous NO production by l-NAME by itself increased surface NKCC2 by 20% and, when incubated together with Xo-Hy, enhanced surface NKCC2 by 41% (P < 0.05 from baseline). However, this did not reach statistical significance from l-NAME alone (P = 0.14, l-NAME vs. Xo-Hy + l-NAME). These data suggest that endogenous NO blunts surface NKCC2 levels and exhibits a tendency to inhibit -stimulated surface NKCC2 levels. No significant change in total NKCC2 expression was observed in response to any of these treatments (control: 100%, l-NAME: 99 ± 2%, Xo-Hy: 95 ± 7%, and l-NAME + Xo-Hy: 99 ± 8%).
Fig. 3.

Effect of nitric oxide synthase (NOS) inhibition by Nω-nitro-l-arginine methyl ester (l-NAME) on superoxide ()-stimulated thick ascending limb (TAL) surface Na+-K+-2Cl− cotransporter (NKCC2) expression. Top, representative Western blot for NKCC2 and GAPDH measured from surface (lanes 3–6) and intracellular (lanes 1, 2, 7, and 8) fractions. Surface NKCC2 was measured from TAL suspensions after 20 min of treatment with control, l-NAME, xanthine oxidase and hypoxanthine (Xo-Hy), and l-NAME + Xo-Hy (5 mM l-NAME, 1 mU/ml Xo, and 500 µM Hy were used). Bottom, cumulative data showing that endogenous NO decreased surface NKCC2 and tended to blunt the stimulatory effect of on surface NKCC2 expression. n = 5. *P < 0.05 vs. control.
Effect of endogenous on surface NKCC2 expression during NOS inhibition: role of tempol.
Because NOS inhibition by 5 mM l-NAME alone increased surface NKCC2 expression by 21% and this could be because of stimulated levels (Fig. 3), we investigated the role of endogenous on surface NKCC2. To scavenge , we used 25, 50, or 100 µM tempol in a different group. TAL suspensions were separated into aliquots in four tubes and pretreated with 5 mM l-NAME for 10 min. Next, they were treated with vehicle or 25, 50, or 100 µM tempol. Surface NKCC2 measured from this group of rats was not statistically different from l-NAME alone (l-NAME: 100%, l-NAME + 25 µM tempol: 108 ± 6%, l-NAME + 50 µM tempol: 104 ± 4%, and l-NAME + 100 µM tempol: 103 ± 3%, n = 5; data not shown). These data suggest that endogenous does not alter surface NKCC2 expression during NOS inhibition.
Effect of superoxide dismutase on -stimulated surface NKCC2 expression.
To make sure the effect of Xo-Hy was the result of extracellular , we used superoxide dismutase (SOD) in a different group of tubules. In this group of experiments, isolated TAL suspensions were separated into four tubes and treated with l-NAME to inhibit endogenous NOS. During NOS inhibition, Xo-Hy enhanced surface NKCC2 expression by 39 ± 4% (n = 5, P < 0.05 vs. control; Fig. 4), and that effect was significantly blunted by SOD pretreatment (15 ± 10%, P < 0.05 vs. Xo-Hy). SOD alone had no significant effect on surface NKCC2 expression (6 ± 5%, P = not significant vs. control). Total NKCC2 expression was not changed (control: 100%, l-NAME + SOD: 102 ± 4%, l-NAME + Xo-Hy: 103 ± 5%, and SOD + l-NAME + Xo-Hy: 104 ± 6%).
Fig. 4.

Effect of superoxide dismutase (SOD) on superoxide ()-stimulated surface Na+-K+-2Cl− cotransporter (NKCC2) in rat medullary thick ascending limbs (TALs) during nitric oxide synthase (NOS) inhibition. Top, representative Western blot for NKCC2 and GAPDH measured from surface (lanes 3–6) and intracellular (lanes 1, 2, 7, and 8) fractions. Surface NKCC2 was measured from TAL suspensions treated with control, SOD (300 U/ml), xanthine oxidase and hypoxanthine (Xo-Hy), and SOD + Xo-Hy for 20 min [5 mM Nω-nitro-l-arginine methyl ester (l-NAME), 1 mU/ml Xo, and 500 µM Hy were used]. To inhibit endogenous nitric oxide production, we treated all samples with 5 mM l-NAME 10 min before the experiment. Bottom, cumulative data showing that SOD blocked -stimulated surface NKCC2 expression. n = 5. *P < 0.05 vs. control; #P < 0.05 vs. Xo-Hy.
Effect of protein kinase C-α/β1 inhibition on -stimulated surface NKCC2 expression.
We and others have shown that increases TAL NaCl absorption by activation of protein kinase C (PKC) (29, 47), specially the PKC-α or PKC-β1 pathway. Therefore, we used PKC-α or PKC-β1 inhibitor (Gö-6976) to test whether -stimulated surface NKCC2 trafficking is mediated via the PKC pathway. TAL suspensions were obtained from rats and separated into four tubes. Next, l-NAME was added to inhibit endogenous NO production. -stimulated surface NKCC2 measured in this group was 37%, and this was significantly blocked by 100 nM Gö-6976 (l-NAME: 100%, Gö-6976 + l-NAME: 105 ± 1%, l-NAME + Xo-Hy: 137 ± 10%, and l-NAME + Gö-6976 + Xo-Hy: 105 ± 8%, n = 5, P < 0.05; Fig. 5). Total NKCC2 expression was not statistically different from control (l-NAME: 100%, l-NAME + Xo-Hy: 96 ± 6%, l-NAME + Gö6976 + Xo-Hy: 104 ± 8%, and l-NAME + Gö6976: 98 ± 2%).
Fig. 5.

Effect of the PKC-α/β1 inhibitor Gö-6976 on superoxide ()-stimulated thick ascending limb (TAL) surface Na+-K+-2Cl− cotransporter (NKCC2) expression. Top, representative Western blot for NKCC2 and GAPDH measured from surface (lanes 3–6) and intracellular (lanes 1, 2, 7, and 8) fractions. Surface NKCC2 expression was measured from TAL suspensions treated with control, 100 nM Gö-6976, xanthine oxidase and hypoxanthine (Xo-Hy), and Gö-6976 + Xo-Hy for 20 min [5 mM Nω-nitro-l-arginine methyl ester (l-NAME), 1 mU/ml Xo, and 500 µM Hy were used]. To inhibit endogenous nitric oxide production, we treated all of the samples with 5 mM l-NAME 10 min before the experiment. Bottom, cumulative data showing that PKC inhibition by Gö-6976 completely blocked -stimulated surface NKCC2 expression, whereas Gö-6976 alone had no effect. n = 5. *P < 0.05 vs. control; #P < 0.05 vs. Xo-Hy.
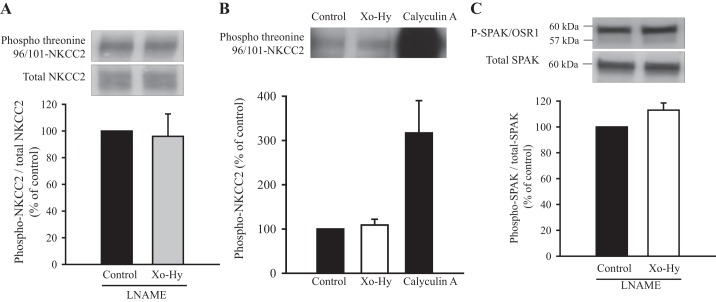
Effect of on the phosphorylation of NKCC2 and SPAK.
NKCC2 phosphorylation at Thr96/101 is an important mechanism that could mediate enhanced NKCC2 activity (45, 46), other than an increase in surface NKCC2 expression (4, 19, 20, 24). We tested whether produced by Xo-Hy stimulates NKCC2 phosphorylation. Phospho-NKCC2 and total NKCC2 were measured in total TALs (not in surface) treated with Xo-Hy for 20 min. We found that this treatment did not change the phospho-NKCC2-to-total NKCC2 ratio (control: 100% vs. Xo-Hy: 96 ± 16.8%; Fig. 6A) or total NKCC2 expression. As a positive control, we treated TALs with a protein phosphatase inhibitor (calyculin-A, 25 μM, 20 min) and found that calyculin-A dramatically enhanced phospho-NKCC2 by 4.6 ± 0.3-fold (P < 0.01; Fig. 6B). Thus, our data suggest that does not increase phosphorylation of NKCC2 at Thr96/101. In the same samples, we measured phospho-SPAK and total SPAK and found that did not affect phospho-SPAK or total SPAK (control: 100% vs. Xo-Hy: 113 ± 5.5%; Fig. 6C). In Fig. 6C, in the uppermost phospho-SPAK band, there is a lighter band close to the 57-kDa mark; this indicates OSR1. OSR1 (~58 kDa) was not changed in response to .
Fig. 6.
Effect of superoxide () production by xanthine oxidase and hypoxanthine (Xo-Hy) on the phosphorylation of STE20/SPS1-related proline/alanine-rich kinase (SPAK) and Na+-K+-2Cl− cotransporter (NKCC2) in rat thick ascending limbs (TALs) [5 mM Nω-nitro-l-arginine methyl ester (l-NAME), 1 mU/ml Xo, and 500 µM Hy were used]. A, top: representative Western blot for phospho-NKCC2 and total NKCC2. Bottom, the cumulative ratio of phospho-NKCC2 to total NKCC2 was not increased by treatment with Xo-Hy. n = 6. B, top, representative Western blot for phospho-NKCC2. Bottom, cumulative data in response to and 25 μM calyculin-A. n = 3. C, top: representative Western blot for phospho-SPAK [phospho-SPAK/oxidative stress-responsive kinase 1 (P-SPAK/OSR1)] and total SPAK. Bottom, cumulative data showing that the phospho-SPAK/OSR1-to-total SPAK ratio was not increased by 20 min of treatment with Xo-Hy. n = 8.
DISCUSSION
We and others have shown that enhances NaCl reabsorption and stimulates NKCC2 activity and NaCl reabsorption by the TAL (41, 47). However, the mechanism by which stimulates NKCC2 activity is not known. Here, we show that produced by Xo increased TAL apical surface NKCC2 expression (Fig. 1). -stimulated surface NKCC2 was completely blocked by SOD or the SOD mimetic tempol and enhanced by decreasing endogenous NO production in TALs. Hence, these data suggest that stimulates NKCC2 trafficking to the apical surface. We have previously reported that produced by Xo-Hy stimulates TAL NaCl reabsorption by 30% (41, 47) in isolated perfused TALs. Our present observation that increases surface NKCC2 expression by 20–30% is in agreement with stimulation of NKCC2 and NaCl reabsorption as previously described (41, 47). We also found that did not increase NKCC2 phosphorylation at Thr96/101. Thus, our data indicate that increased surface NKCC2 likely accounts for most of the stimulatory effect of on NKCC2 activity.
Xo catalyzes the formation of both and H2O2 in the presence of oxygen and Hy (43). Therefore, we tested whether H2O2 contributed to the stimulation of surface NKCC2 expression. We found that adding catalase to the bath, which converts H2O2 to water and oxygen (13, 15), did not affect baseline surface NKCC2 or decreased Xo-Hy-stimulated surface NKCC2 expression (Fig. 2). These data suggest that Xo-Hy-stimulated surface NKCC2 is primarily the result of . We also studied whether decreasing endogenous NO production would increase -stimulated surface NKCC2. We found that pretreatment of TALs with the NOS inhibitor l-NAME enhanced baseline surface NKCC2 by 21% and also tended to increase -stimulated surface NKCC2 to 40% of baseline, although this did not reach statistical significance (P = 0.14; Fig. 3). These data suggest that endogenous NO tonically inhibits surface NKCC2. These findings are in agreement with our previous data showing that NO inhibits NKCC2 activity (42) and that cGMP (2), the second messenger of NO, potently decreases surface NKCC2. l-NAME alone increased surface NKCC2 by ~20%, and adding Xo-Hy after l-NAME tended to increased surface NKCC2 to 40%. It could be argued that the effect of is additive to that of decreasing NO or that NO scavenges produced exogenously. To eliminate the influence of endogenous NO and study the signaling induced by alone, all subsequent experiments included l-NAME in the bath. Under these conditions, we often found that Xo-Hy increased surface NKCC2 by 30–40%, suggesting that endogenous NO blunts part of the response induced by Xo-Hy. These findings are of relevance to the control of blood pressure since Kopkan and Majid (33) showed that, in a NO-deficient model of hypertension, the effect of contributes a large part to the development of salt sensitivity.
There is a reasonable explanation for why l-NAME caused a small increase in surface NKCC2. We have previously shown that NO inhibits NKCC2 (42) and have also shown that the second messenger of NO, cGMP, potently decreases surface NKCC2 (2). Thus, in TAL suspensions, we expect some endogenous NO production, which, when inhibited by l-NAME, would decrease cGMP, thereby increasing surface NKCC2. Different from NO, endogenous (unstimulated) was not sufficient to increase surface NKCC2 because our data show that tempol, by itself, did not decrease surface NKCC2 even at a high concentration (100 µM). Our data suggest that baseline production of endogenous (in the absence of stimuli/agonist or hormones) may not be sufficient to enhance surface NKCC2.
In the rat renal medulla, infusion of SOD inhibitor increases levels, which decrease urinary Na+ and water excretion (50). In anesthetized dogs, renal infusion of SOD inhibitor decreased urine flow and fractional Na+ excretion, suggesting that stimulates NaCl absorption (35). We found that SOD did not increase surface NKCC2 expression but completely blocked -stimulated surface NKCC2 expression. Thus, most if not all of the effect of Xo-Hy is the result of and not H2O2. In agreement with these data, we have previously reported that produced by Xo-Hy but not H2O2 (up to 200 nM) stimulates TAL Cl− transport (41). Taken together, our data support the idea that but not H2O2 produced exogenously stimulate apical trafficking of NKCC2, which is associated with higher Cl− transport.
In vivo, scavenging in the renal medulla with the SOD mimetic tempol increases Na+ excretion and urine flow, suggesting that produced in some cell type in the medulla stimulates NaCl and water reabsorption (50). We found that increases Cl− transport in isolated TALs, and this effect is inhibited when is scavenged by tempol (41). Here, we found that tempol blocked the stimulatory effect Xo-Hy but did not change baseline surface NKCC2 levels by itself at 25, 50, or 100 µM. These data support the role of as a stimulatory factor. However, in isolated perfused TALs, tempol (50 µM) decreased baseline Cl− reabsorption (41). There are many potential explanations for this apparent discrepancy. It is possible that tempol inhibits Cl− absorption in perfused TALs by a mechanism that does not involve a decrease in surface NKCC2. Second, it is possible that, in perfused TALs, production is much higher than in our TAL suspensions because luminal flow stimulates production in part by activating NAD(P)H oxidase NOX4 (29). TAL suspensions do not have luminal flow; thus, tempol had no effect in the absence of exogenous . This is a limitation of our study, which relied on TALs without luminal flow. Another possibility is that, in the presence of l-NAME, decreasing with tempol does not enhance NO bioavailability, since there is no NO production. In isolated perfused TAL experiments, l-NAME was not present; thus, tempol could decrease surface NKCC2 by increasing NO and cGMP, which, as we have previously shown, inhibit surface NKCC2 levels (2).
-stimulated TAL surface NKCC2 expression was completely blocked using a nonselective PKC-α/β inhibitor (Gö-6976), whereas the inhibitor had no effect alone (Fig. 5). These data suggest that a PKC-mediated pathway is involved in enhanced surface NKCC2 levels. Our present finding that -stimulated surface NKCC2 expression is mediated by PKC is supported by observations of Silva et al. (47), who showed that Gö6976 blocked the stimulatory effect of Xo-Hy on net Cl− reabsorption in isolated perfused TALs. Thus, our present data that PKC inhibition blocks -stimulated TAL surface NKCC2 support an important role for PKC in stimulation of surface NKCC2 levels.
The mechanisms by which or PKC-α/β stimulate surface NKCC2 levels in TALs are unknown. We have previously shown that surface NKCC2 levels are maintained by a balance between exocytic delivery, endocytosis, and recycling (6, 10). could potentially enhance the rate of exocytic delivery or inhibit endocytosis. We speculate that PKC-α or PKC-β stimulates exocytic delivery, since there is some evidence that this occurs in other cells outside the kidney (18, 49). PKC induces signaling by phosphorylating a very large number of substrates. To our knowledge, there are no reports indicating that any of the PKC isoforms phosphorylate NKCC2 directly, so it is unlikely that the effect of occurs via NKCC2 phosphorylation. However, PKC could potentially activate SPAK or OSR1 and enhance NKCC2 phosphorylation at Thr96/101. This is one of the reasons we studied NKCC2 phosphorylation, which was unchanged by . The direct mechanism by which PKC increases surface NKCC2 is an important question to study. It is plausible to hypothesize that PKC-α or PKC-β increases the rate of NKCC2 exocytic delivery to the membrane, perhaps by phosphorylating a protein within the SNARE complex, which, as we have previously shown, mediates NKCC2 trafficking (11, 12, 14, 36, 37).
Phosphorylation of NKCC2 at Thr96/101 is also a potential mechanism for the stimulation of NKCC2 activity (4, 34, 45, 46). To date, there have been no studies on the effect of or other reactive oxygen species on NKCC2 phosphorylation. We found that Xo-Hy did not change phospho-NKCC2 (Fig. 6, A and B), whereas inhibition of endogenous protein phosphatases with calyculin-A dramatically enhanced Thr96/101 phosphorylation, as we have previously reported (24). The upstream kinases that phosphorylate Thr96/101 are SPAK (~60 kDa) and OSR1 (~58 kDa), and they are activated by phosphorylation (45, 46). Using an antibody that recognizes both phospho-SPAK and phospho-OSR1 (Fig. 6C) (4), we found that Xo-Hy did not increase their phosphorylation. These data suggest that -stimulated surface NKCC2 is not associated with an increase in phosphorylation of NKCC2 at Thr96/101. It is usually assumed that NKCC2 phosphorylated at Thr96/101 is the active form of the transporter. However, this has not been demonstrated directly in TALs. To our knowledge, evidence that phosphorylation directly affects NKCC2 activity is provided by mutation of the phosphorylated sites to alanines, which dramatically reduce NKCC2 activity in oocytes (20) or human embryonic kidney cells (46). Most evidence showing that increased phosphorylation activates the transporter is correlative with higher activity. We think that surface expression levels are more important to the control of net NKCC2-mediated NaCl absorption by the TAL and that phosphorylation plays a role in controlling trafficking rates. We have previously observed in many cases (cAMP, cGMP, and Dahl salt-sensitive rats) that net Cl− reabsorption by the TAL correlates better with changes in surface NKCC2 levels (2, 4, 10, 24, 25) than with the magnitude of change in phosphorylation, and, in some cases, they change in the same direction and magnitude. We think that this occurs because trafficking and phosphorylation are controlled by different signaling mechanisms, scaffolding proteins, and subcellular localization of each protein in the cascade. Thus, we do not find it odd that the activity of NKCC2 increases without a change in its Thr96/101 phosphorylation. We did observe that NKCC2 shows a baseline level of phosphorylation at Thr96/101 that is easily detectable in TALs, and this level is maintained after treatment. Thus, a fraction of the transporter is phosphorylated in the absence of stimuli. We have recently reported a rat model where surface NKCC2 levels, but not its phosphorylation, drive higher NKCC2 activity (31). Similarly, we observed this when we stimulated NKCC2 with extracellular fructose (5). Our data do not exclude the concept that enhanced phosphorylation of NKCC2 increases its net activity. However, our data show that NKCC2 activity can be increased without enhancing its phosphorylation levels. We have argued this extensively in the past (3).
We conclude that increases surface NKCC2 levels in TALs but not its phosphorylation at Thr96/101, suggesting that trafficking is the main mechanism for the -stimulated NaCl absorption that we and others have previously reported (16, 29, 40, 47).
GRANTS
This work was supported in part by National Institute of Diabetes and Digestive and Kidney Diseases Grant RO1-DK-105818A1 (to P. Ortiz).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
M.Z.H. and P.A.O. conceived and designed research; M.Z.H. performed experiments; M.Z.H. and P.A.O. analyzed data; M.Z.H. and P.A.O. interpreted results of experiments; M.Z.H. prepared figures; M.Z.H. drafted manuscript; M.Z.H. and P.A.O. edited and revised manuscript; M.Z.H. and P.A.O. approved final version of manuscript.
REFERENCES
- 1.Alshahrani S, Di Fulvio M. Expression of the Slc12a1 gene in pancreatic β-cells: molecular characterization and in silico analysis. Cell Physiol Biochem 30: 95–112, 2012. doi: 10.1159/000339050. [DOI] [PubMed] [Google Scholar]
- 2.Ares GR, Caceres P, Alvarez-Leefmans FJ, Ortiz PA. cGMP decreases surface NKCC2 levels in the thick ascending limb: role of phosphodiesterase 2 (PDE2). Am J Physiol Renal Physiol 295: F877–F887, 2008. doi: 10.1152/ajprenal.00449.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ares GR, Caceres PS, Ortiz PA. Molecular regulation of NKCC2 in the thick ascending limb. Am J Physiol Renal Physiol 301: F1143–F1159, 2011. doi: 10.1152/ajprenal.00396.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ares GR, Haque MZ, Delpire E, Ortiz PA. Hyperphosphorylation of Na-K-2Cl cotransporter in thick ascending limbs of Dahl salt-sensitive rats. Hypertension 60: 1464–1470, 2012. doi: 10.1161/HYPERTENSIONAHA.112.202101. [DOI] [PubMed] [Google Scholar]
- 5.Ares GR, Kassem KM, Ortiz PA. Fructose acutely stimulates NKCC2 activity in rat thick ascending limbs by increasing surface NKCC2 expression. Am J Physiol Renal Physiol 316: F550–F557, 2019. doi: 10.1152/ajprenal.00136.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ares GR, Ortiz PA. Dynamin2, clathrin, and lipid rafts mediate endocytosis of the apical Na/K/2Cl cotransporter NKCC2 in thick ascending limbs. J Biol Chem 287: 37824–37834, 2012. doi: 10.1074/jbc.M112.386425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Blake DR, Stevens CR, Sahinoglu T, Ellis G, Gaffney K, Edmonds S, Benboubetra M, Harrison R, Jawed S, Kanczler J, Millar TM, Winyard PG, Zhang Z. Xanthine oxidase: four roles for the enzyme in rheumatoid pathology. Biochem Soc Trans 25: 812–816, 1997. doi: 10.1042/bst0250812. [DOI] [PubMed] [Google Scholar]
- 8.Burg MB. Thick ascending limb of Henle’s loop. Kidney Int 22: 454–464, 1982. doi: 10.1038/ki.1982.198. [DOI] [PubMed] [Google Scholar]
- 9.Burg MB, Orloff J. Oxygen consumption and active transport in separated renal tubules. Am J Physiol 203: 327–330, 1962. doi: 10.1152/ajplegacy.1962.203.2.327. [DOI] [PubMed] [Google Scholar]
- 10.Caceres PS, Ares GR, Ortiz PA. cAMP stimulates apical exocytosis of the renal Na+-K+-2Cl− cotransporter NKCC2 in the thick ascending limb: role of protein kinase A. J Biol Chem 284: 24965–24971, 2009. doi: 10.1074/jbc.M109.037135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Caceres PS, Mendez M, Haque MZ, Ortiz PA. Vesicle-associated membrane protein 3 (VAMP3) mediates constitutive trafficking of the renal co-transporter NKCC2 in thick ascending limbs: role in renal function and blood pressure. J Biol Chem 291: 22063–22073, 2016. doi: 10.1074/jbc.M116.735167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Caceres PS, Mendez M, Ortiz PA. Vesicle-associated membrane protein 2 (VAMP2) but Not VAMP3 mediates cAMP-stimulated trafficking of the renal Na+-K+-2Cl− co-transporter NKCC2 in thick ascending limbs. J Biol Chem 289: 23951–23962, 2014. doi: 10.1074/jbc.M114.589333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chelikani P, Fita I, Loewen PC. Diversity of structures and properties among catalases. Cell Mol Life Sci 61: 192–208, 2004. doi: 10.1007/s00018-003-3206-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chung SH, Polgar J, Reed GL. Protein kinase C phosphorylation of syntaxin 4 in thrombin-activated human platelets. J Biol Chem 275: 25286–25291, 2000. doi: 10.1074/jbc.M004204200. [DOI] [PubMed] [Google Scholar]
- 15.DiGuiseppi J, Fridovich I, McCord JM. The toxicology of molecular oxygen. Crit Rev Toxicol 12: 315–342, 1984. doi: 10.3109/10408448409044213. [DOI] [PubMed] [Google Scholar]
- 16.Edwards A, Layton AT. Modulation of outer medullary NaCl transport and oxygenation by nitric oxide and superoxide. Am J Physiol Renal Physiol 301: F979–F996, 2011. doi: 10.1152/ajprenal.00096.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Evans RG, Fitzgerald SM. Nitric oxide and superoxide in the renal medulla: a delicate balancing act. Curr Opin Nephrol Hypertens 14: 9–15, 2005. doi: 10.1097/00041552-200501000-00003. [DOI] [PubMed] [Google Scholar]
- 18.Ge X, Low B, Liang M, Fu J. Angiotensin II directly triggers endothelial exocytosis via protein kinase C-dependent protein kinase D2 activation. J Pharmacol Sci 105: 168–176, 2007. doi: 10.1254/jphs.FP0070858. [DOI] [PubMed] [Google Scholar]
- 19.Giménez I, Forbush B. Regulatory phosphorylation sites in the NH2 terminus of the renal Na-K-Cl cotransporter (NKCC2). Am J Physiol Renal Physiol 289: F1341–F1345, 2005. doi: 10.1152/ajprenal.00214.2005. [DOI] [PubMed] [Google Scholar]
- 20.Giménez I, Forbush B. The residues determining differences in ion affinities among the alternative splice variants F, A, and B of the mammalian renal Na-K-Cl cotransporter (NKCC2). J Biol Chem 282: 6540–6547, 2007. doi: 10.1074/jbc.M610780200. [DOI] [PubMed] [Google Scholar]
- 21.Gottardi CJ, Caplan MJ. Cell surface biotinylation in the determination of epithelial membrane polarity. J Tissue Cult Methods 14: 173–180, 1992. doi: 10.1007/BF01409008. [DOI] [Google Scholar]
- 22.Granger DN. Role of xanthine oxidase and granulocytes in ischemia-reperfusion injury. Am J Physiol 255: H1269–H1275, 1988. doi: 10.1152/ajpheart.1988.255.6.H1269. [DOI] [PubMed] [Google Scholar]
- 23.Greger R. Ion transport mechanisms in thick ascending limb of Henle’s loop of mammalian nephron. Physiol Rev 65: 760–797, 1985. doi: 10.1152/physrev.1985.65.3.760. [DOI] [PubMed] [Google Scholar]
- 24.Haque MZ, Ares GR, Caceres PS, Ortiz PA. High salt differentially regulates surface NKCC2 expression in thick ascending limbs of Dahl salt-sensitive and salt-resistant rats. Am J Physiol Renal Physiol 300: F1096–F1104, 2011. doi: 10.1152/ajprenal.00600.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Haque MZ, Caceres PS, Ortiz PA. β-Adrenergic receptor stimulation increases surface NKCC2 expression in rat thick ascending limbs in a process inhibited by phosphodiesterase 4. Am J Physiol Renal Physiol 303: F1307–F1314, 2012. doi: 10.1152/ajprenal.00019.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Haque MZ, Majid DS. Assessment of renal functional phenotype in mice lacking gp91PHOX subunit of NAD(P)H oxidase. Hypertension 43: 335–340, 2004. doi: 10.1161/01.HYP.0000111137.15873.4a. [DOI] [PubMed] [Google Scholar]
- 27.Haque MZ, Majid DS. High salt intake delayed angiotensin II-induced hypertension in mice with a genetic variant of NADPH oxidase. Am J Hypertens 24: 114–118, 2011. doi: 10.1038/ajh.2010.173. [DOI] [PubMed] [Google Scholar]
- 28.Hebert SC, Reeves WB, Molony DA, Andreoli TE. The medullary thick limb: function and modulation of the single-effect multiplier. Kidney Int 31: 580–589, 1987. doi: 10.1038/ki.1987.38. [DOI] [PubMed] [Google Scholar]
- 29.Herrera M, Silva GB, Garvin JL. Angiotensin II stimulates thick ascending limb superoxide production via protein kinase C(α)-dependent NADPH oxidase activation. J Biol Chem 285: 21323–21328, 2010. doi: 10.1074/jbc.M110.109157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hong NJ, Garvin JL. Flow increases superoxide production by NADPH oxidase via activation of Na-K-2Cl cotransport and mechanical stress in thick ascending limbs. Am J Physiol Renal Physiol 292: F993–F998, 2007. doi: 10.1152/ajprenal.00383.2006. [DOI] [PubMed] [Google Scholar]
- 31.Jaykumar AB, Caceres PS, King-Medina KN, Liao TD, Datta I, Maskey D, Naggert JK, Mendez M, Beierwaltes WH, Ortiz PA. Role of Alström syndrome 1 in the regulation of blood pressure and renal function. JCI Insight pii: 30385718, 2018. doi: 10.1172/jci.insight.95076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Juncos R, Garvin JL. Superoxide enhances Na-K-2Cl cotransporter activity in the thick ascending limb. Am J Physiol Renal Physiol 288: F982–F987, 2005. doi: 10.1152/ajprenal.00348.2004. [DOI] [PubMed] [Google Scholar]
- 33.Kopkan L, Majid DS. Superoxide contributes to development of salt sensitivity and hypertension induced by nitric oxide deficiency. Hypertension 46: 1026–1031, 2005. doi: 10.1161/01.HYP.0000174989.39003.58. [DOI] [PubMed] [Google Scholar]
- 34.Lin SH, Yu IS, Jiang ST, Lin SW, Chu P, Chen A, Sytwu HK, Sohara E, Uchida S, Sasaki S, Yang SS. Impaired phosphorylation of Na+-K+-2Cl− cotransporter by oxidative stress-responsive kinase-1 deficiency manifests hypotension and Bartter-like syndrome. Proc Natl Acad Sci USA 108: 17538–17543, 2011. doi: 10.1073/pnas.1107452108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Majid DS, Nishiyama A. Nitric oxide blockade enhances renal responses to superoxide dismutase inhibition in dogs. Hypertension 39: 293–297, 2002. doi: 10.1161/hy0202.104137. [DOI] [PubMed] [Google Scholar]
- 36.Martín-Ávila A, Medina-Tamayo J, Ibarra-Sánchez A, Vázquez-Victorio G, Castillo-Arellano JI, Hernández-Mondragón AC, Rivera J, Madera-Salcedo IK, Blank U, Macías-Silva M, González-Espinosa C. Protein tyrosine kinase Fyn regulates TLR4-elicited responses on mast cells controlling the function of a PP2A-PKCα/β signaling node leading to TNF secretion. J Immunol 196: 5075–5088, 2016. doi: 10.4049/jimmunol.1501823. [DOI] [PubMed] [Google Scholar]
- 37.Martin TD, Mitin N, Cox AD, Yeh JJ, Der CJ. Phosphorylation by protein kinase Cα regulates RalB small GTPase protein activation, subcellular localization, and effector utilization. J Biol Chem 287: 14827–14836, 2012. doi: 10.1074/jbc.M112.344986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Molony DA, Reeves WB, Andreoli TE. Na+:K+:2Cl− cotransport and the thick ascending limb. Kidney Int 36: 418–426, 1989. doi: 10.1038/ki.1989.211. [DOI] [PubMed] [Google Scholar]
- 39.Ortiz PA. cAMP increases surface expression of NKCC2 in rat thick ascending limbs: role of VAMP. Am J Physiol Renal Physiol 290: F608–F616, 2006. doi: 10.1152/ajprenal.00248.2005. [DOI] [PubMed] [Google Scholar]
- 40.Ortiz PA, Garvin JL. Interaction of and NO in the thick ascending limb. Hypertension 39: 591–596, 2002. doi: 10.1161/hy0202.103287. [DOI] [PubMed] [Google Scholar]
- 41.Ortiz PA, Garvin JL. Superoxide stimulates NaCl absorption by the thick ascending limb. Am J Physiol Renal Physiol 283: F957–F962, 2002. doi: 10.1152/ajprenal.00102.2002. [DOI] [PubMed] [Google Scholar]
- 42.Ortiz PA, Hong NJ, Garvin JL. NO decreases thick ascending limb chloride absorption by reducing Na+-K+-2Cl− cotransporter activity. Am J Physiol Renal Physiol 281: F819–F825, 2001. doi: 10.1152/ajprenal.0075.2001. [DOI] [PubMed] [Google Scholar]
- 43.Parks DA, Granger DN. Xanthine oxidase: biochemistry, distribution and physiology. Acta Physiol Scand Suppl 548: 87–99, 1986. [PubMed] [Google Scholar]
- 44.Plato CF, Stoos BA, Wang D, Garvin JL. Endogenous nitric oxide inhibits chloride transport in the thick ascending limb. Am J Physiol Renal Physiol 276: F159–F163, 1999. doi: 10.1152/ajprenal.1999.276.1.F159. [DOI] [PubMed] [Google Scholar]
- 45.Ponce-Coria J, San-Cristobal P, Kahle KT, Vazquez N, Pacheco-Alvarez D, de Los Heros P, Juárez P, Muñoz E, Michel G, Bobadilla NA, Gimenez I, Lifton RP, Hebert SC, Gamba G. Regulation of NKCC2 by a chloride-sensing mechanism involving the WNK3 and SPAK kinases. Proc Natl Acad Sci USA 105: 8458–8463, 2008. doi: 10.1073/pnas.0802966105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Richardson C, Sakamoto K, de los Heros P, Deak M, Campbell DG, Prescott AR, Alessi DR. Regulation of the NKCC2 ion cotransporter by SPAK-OSR1-dependent and -independent pathways. J Cell Sci 124: 789–800, 2011. doi: 10.1242/jcs.077230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Silva GB, Ortiz PA, Hong NJ, Garvin JL. Superoxide stimulates NaCl absorption in the thick ascending limb via activation of protein kinase C. Hypertension 48: 467–472, 2006. doi: 10.1161/01.HYP.0000236646.83354.51. [DOI] [PubMed] [Google Scholar]
- 48.Wangensteen R, Rodríguez-Gomez I, Moreno JM, Vargas F, Alvarez-Guerra M. Chronic nitric oxide blockade modulates renal Na-K-2Cl cotransporters. J Hypertens 24: 2451–2458, 2006. doi: 10.1097/01.hjh.0000251907.93298.44. [DOI] [PubMed] [Google Scholar]
- 49.Xue R, Zhao Y, Chen P.. Involvement of PKC alpha in PMA-induced facilitation of exocytosis and vesicle fusion in PC12 cells. Biochem Biophys Res Commun 380: 371–376, 2009. doi: 10.1016/j.bbrc.2009.01.105. [DOI] [PubMed] [Google Scholar]
- 50.Zou AP, Li N, Cowley AW Jr. Production and actions of superoxide in the renal medulla. Hypertension 37: 547–553, 2001. doi: 10.1161/01.HYP.37.2.547. [DOI] [PubMed] [Google Scholar]