Abstract
This study aimed to investigate the impact of sarcopenia and body composition on survival outcomes in Korean patients with advanced-stage high-grade serous ovarian carcinoma (HGSOC). We retrospectively identified patients diagnosed with and treated for International Federation of Gynecology and Obstetrics stage III-IV HGSOC. Skeletal muscle index (SMI) was measured using pre-treatment computed tomography scans at the third lumbar vertebra. Sarcopenia was defined as SMI <39.0 cm2/m2. Patients’ clinicopathologic characteristics and survival outcomes were compared according to sarcopenia presence. For subgroup analysis, we also measured the total fat area from the same image. In total, 76 and 103 patients were assigned to the sarcopenia and control groups, respectively. Comorbidities, stage, serum CA-125 levels, and size of residual tumor after surgery were similar between both groups. After a median follow up of 42.7 months, both groups showed similar progression-free survival (PFS) and overall survival (OS). In subgroup analysis confined to the sarcopenia group, patients with high fat-to-muscle ratio (FMR; ≥2.1, n = 38) showed significantly worse OS than those with low FMR (<2.1, n = 38) (5-year survival rate, 44.7% vs. 80.0%; p = 0.046), whereas PFS was not different (p = 0.365). Multivariate analyses identified high FMR as an independent poor prognostic factor for OS in this group (adjusted hazard ratio, 3.377; 95% confidence interval, 1.170–9.752; p = 0.024). In conclusion, sarcopenia did not influence recurrence rates and survival in Korean patients with advanced-stage HGSOC. However, among the patients with sarcopenia, high FMR was associated with decreased OS.
Keywords: ovarian neoplasms, high-grade serous carcinoma, sarcopenia, body composition, prognosis, survival
1. Introduction
Ovarian cancer, one of the deadliest gynecologic malignancies, causes more than 150,000 deaths worldwide each year [1]. The incidence of ovarian cancer is higher among high Human Development Index countries, and it is gradually increasing in Korea [2]. Owing to the absence of cancer-specific symptoms and effective screening tools, ovarian cancer tends to be diagnosed at an advanced-stage and thus has a high recurrence rate and poor five-year survival rate despite intensive treatment [3].
Sarcopenia, characterized by loss of skeletal muscle mass and function, does not occur exclusively in the elderly but is also commonly observed in cancer patients [4]. Previous studies have suggested sarcopenia as a prognostic factor associated with poor survival and increased resistance and toxicity to chemotherapy in patients with various malignancies, including breast, small cell lung, urothelial, and gastric cancers [5,6,7,8]. In ovarian cancer, conflicting results have been reported: while some studies concluded that sarcopenia adversely affected patients’ progression-free survival (PFS) or overall survival (OS) [9,10], others could not determine a significant association of sarcopenia with survival outcomes [11,12]. There were differences in study design, population, disease setting, and definition of sarcopenia among the studies; therefore, careful attention is required to interpret the study results. Moreover, considering the fact that body composition is different among the Organisation for Economic Co-operation and Development (OECD) member countries [13], sarcopenia and its impact on cancer prognosis may vary by geographical regions and ethnicities.
To determine sarcopenia, recent studies have suggested utilization of computed tomography (CT) scans. A cross-sectional image of CT scans at the level of the third lumbar vertebra (L3) is known to represent an individual’s body composition, such as total body skeletal muscle and adipose tissues and fat distribution [14,15]. Moreover, CT scans are acquired routinely as part of cancer patients’ care, so quantification of body composition using CT scans is quite possible.
To our knowledge, clinical significance of sarcopenia in Korean ovarian cancer patients has not been explored. Thus, we aimed to investigate impact of sarcopenia on survival outcomes in Korean patients with advanced-stage high-grade serous ovarian carcinoma (HGSOC), which is the predominant histologic type of ovarian cancer. In this study, sarcopenia was determined based on the pre-treatment CT scan; the fat composition was also measured considering the fact that the Asian population generally has a higher body fat percentage than the Western population at the same body mass index (BMI) [16].
2. Results
2.1. Analysis in All Patients
Patients’ clinicopathologic characteristics are presented in Table 1. The sarcopenia group (n = 76) had significantly lower pre-treatment body mass index (BMI) (mean, 22.1 vs. 24.7 kg/m2; p < 0.001) and received neoadjuvant chemotherapy (NAC) less frequently (17.1% vs. 30.1%; p = 0.046), compared to the control group (n = 103). Other characteristics showed no significant difference between two groups. The patients’ initial body composition and laboratory results are presented in Table 2. The sarcopenia group showed less skeletal muscle area (median, 88.1 vs. 106.1 cm2; p < 0.001) and total fat area (median, 188.5 vs. 230.7 cm2; p < 0.001). Among the various calculated body composition indices, all others except skeletal muscle index (SMI) were similar between the sarcopenia and control groups. There were no differences in the laboratory results, inflammatory indices, and nutritional index between the groups.
Table 1.
Clinicopathologic characteristics of all patients.
| Characteristics | All (n = 179, %) | No Sarcopenia Group (n = 103, %) | Sarcopenia Group (n = 76, %) | p |
|---|---|---|---|---|
| Age, years | ||||
| Mean ± SD | 57.5 ± 10.6 | 57.8 ± 11.1 | 57.0 ± 9.9 | 0.615 |
| BMI 1, kg/m2 | ||||
| Mean ± SD | 23.6 ± 3.2 | 24.7 ± 3·3 | 22.1 ± 2.3 | <0.001 |
| Normal (18.5−22.9) | 81 (45.3) | 35 (34.0) | 46 (60.5) | <0.001 |
| Overweight (23.0–24.9) | 52 (29.1) | 30 (29.1) | 22 (28.9) | |
| Obesity (≥25.0) | 46 (25.7) | 38 (36.9) | 8 (10.5) | |
| Comorbidities | ||||
| Hypertension | 48 (26.8) | 28 (27.2) | 20 (26.3) | 0.897 |
| Diabetes | 15 (8.4) | 10 (9.7) | 5 (6.6) | 0.455 |
| Dyslipidemia | 21 (11.7) | 15 (14.6) | 6 (7.9) | 0.171 |
| ASA score | 0.080 | |||
| 1 | 63 (35.2) | 31 (30.1) | 32 (42.1) | |
| 2 | 104 (58.1) | 67 (65.0) | 37 (48.7) | |
| 3 | 12 (6.7) | 5 (4.9) | 7 (9.2) | |
| FIGO stage | 0.653 | |||
| IIIA1 | 8 (4.5) | 5 (4.9) | 3 (3.9) | |
| IIIA2 | 6 (3.4) | 4 (3.9) | 2 (2.6) | |
| IIIB | 17 (9.5) | 9 (8.7) | 8 (10.5) | |
| IIIC | 91 (50.8) | 50 (48.5) | 41 (53.9) | |
| IVA | 10 (5.6) | 4 (3.9) | 6 (7.9) | |
| IVB | 47 (26.3) | 31 (30.1) | 16 (21.1) | |
| CA-125, IU/ml | ||||
| Median (range) | 801.0 (5.1–24720.0) | 833.0 (7.0–10000.0) | 793.0 (5.1–24720.0) | 0.829 |
| Primary treatment strategy | 0.046 | |||
| PDS | 135 (75.4) | 72 (69.9) | 63 (82.9) | |
| NAC | 44 (24.6) | 31 (30.1) | 13 (17.1) | |
| Residual tumour after PDS/IDS | 0.336 | |||
| No gross | 114 (63.7) | 67 (65.0) | 47 (61.8) | |
| <1 cm | 44 (24.6) | 26 (25.2) | 18 (23.7) | |
| 1–2 cm | 10 (5.6) | 3 (2.9) | 7 (9.2) | |
| ≥2 cm | 11 (6.1) | 7 (6.8) | 4 (5.3) | |
| Regimen of first-line chemotherapy | 0.368 | |||
| Paclitaxel-Carboplatin | 161 (89.9) | 93 (90.3) | 68 (89.5) | 0.393 |
| Docetaxel-Carboplatin | 14 (7.8) | 9 (8.7) | 5 (6.6) | |
| Paclitaxel-Carboplatin-Bevacizumab | 4 (2.2) | 1 (1.0) | 3 (3.9) | |
| Main cycles of first-line chemotherapy | ||||
| Median (range) | 6 (4–12) | 6 (4–12) | 6 (4–12) | 0.438 |
| 4–6 | 123 (68.7) | 70 (68.0) | 53 (69.7) | |
| 7–9 | 50 (27.9) | 31 (30.1) | 19 (25.0) | |
| 10–12 | 6 (3.4) | 2 (1.9) | 4 (5.3) | |
| Recurrence | 140 (78.2) | 78 (75.7) | 62 (81.6) | 0.349 |
| PSR 2 | 95 (53.1) | 47 (45.6) | 48 (63.2) | 0.031 |
| PRR | 45 (25.1) | 31 (30.1) | 14 (18.4) | |
| Platinum sensitivity | 0.075 | |||
| Platinum-sensitive 3 | 134 (74.9) | 72 (69.9) | 62 (81.6) | |
| Platinum-resistant | 45 (25.1) | 31 (30.1) | 14 (18.4) |
1 In this study, underweight patients (BMI <18.5 kg/m2) were excluded in analysis. 2 PSR was defined as relapse ≥6 months after completion of taxane- and platinum-based chemotherapy, whereas PRR as relapse <6 months.3 In addition to PSR, the patients who completed taxane- and platinum-based chemotherapy and did not experience disease recurrence during at least six months of follow-up period were considered platinum-sensitive. Abbreviations: ASA, American Society of Anesthesiologists; BMI, body mass index; CA-125, cancer antigen 125; FIGO, International Federation of Gynecology and Obstetrics; IDS, interval debulking surgery; NAC, neoadjuvant chemotherapy; PDS, primary debulking surgery; PRR, platinum-resistant recurrence; PSR, platinum-sensitive recurrence; SD, standard deviation.
Table 2.
Body composition and laboratory results of all patients.
| Characteristics | All (n = 179, %) | No Sarcopenia Group (n = 103, %) |
Sarcopenia Group (n = 76, %) |
p |
|---|---|---|---|---|
| Body composition at diagnosis 1 | ||||
| Skeletal muscle area, cm2 | 98.0 (64.1–209.8) | 106.1 (84.8–209.8) | 88.1 (64.1–109.0) | <0.001 |
| Total fat area, cm2 | 211.8 (42.2–612.5) | 230.7 (78.8–612.5) | 188.5 (42.2–458.2) | <0.001 |
| Subcutaneous fat | 131.7 (34.4–310.8) | 154.0 (55.8–310.8) | 119.8 (34.4–252.0) | <0.001 |
| Visceral fat | 70.4 (6.6–289.4) | 81.5 (11.2–289.4) | 59.6 (6.6–213.0) | 0.001 |
| Muscle fat | 6.2 (0.7–36.2) | 6.5 (0.7–36.2) | 5.3 (1.2–31.6) | 0.103 |
| Calculated body composition index 1 | ||||
| Skeletal muscle index (SMI), cm2/m2 | 40.3 (27.1–79.2) | 42.6 (39.0–79.2) | 36.3 (27.1–39.0) | <0.001 |
| Fat-to-muscle ratio (FMR) | 2.1 (0.5–6.5) | 2.1 (0.8–6.5) | 2.1 (0.5–4.9) | 0.508 |
| Visceral-to-subcutaneous fat ratio (VSR) | 0.5 (0.1–2.9) | 0.5 (0.1–1.4) | 0.4 (0.1–2.9) | 0.212 |
| Skeletal muscle mass-to-visceral fat ratio (SVR) | 1.4 (0.3–14.2) | 1.3 (0.3–8.7) | 1.5 (0.5–14.2) | 0.178 |
| Laboratory test at diagnosis 1 | ||||
| Hemoglobin, g/dL | 12.2 (8.3–14.9) | 12.2 (9.1–14.9) | 12.4 (8.3–14.6) | 0.491 |
| WBC count, 103/uL | 7.0 (1.5–17.0) | 6.9 (1.5–14.7) | 7.1 (3.5–17.0) | 0.417 |
| Neutrophil (%) | 68.9 (28.0–92.0) | 68.9 (28.0–92.0) | 68.9 (47.0–83.0) | 0.734 |
| Lymphocyte (%) | 21.7 (5.0–57.0) | 22.2 (5.0–57.0) | 21.2 (9.4–42.9) | 0.772 |
| Monocyte (%) | 6.8 (0.7–20.9) | 6.8 (0.7–20.9) | 6.9 (3.7–16.0) | 0.335 |
| Platelet count, 103/uL | 316.5 (95.0–698.0) | 312.0 (95.0–698.0) | 323.0 (159.0–634.0) | 0.355 |
| Albumin, g/dL | 3.9 (2.3–5.1) | 3.8 (2.3–4.6) | 4.0 (2.4–5.1) | 0.128 |
| Calculated inflammatory index 1 | ||||
| Neutrophil-to-lymphocyte ratio (NLR) | 3.2 (0.5–18.4) | 3.1 (0.5–18.4) | 3.2 (1.2–8.8) | 0.945 |
| Monocyte-to-lymphocyte ratio (MLR) | 0.3 (0.1–0.9) | 0.3 (0.1–0.9) | 0.3 (0.1–0.9) | 0.378 |
| Platelet-to-lymphocyte ratio (PLR) | 204.9 (71.6–768.5) | 208.3 (71.6–768.5) | 204.7 (77.2–628.1) | 0.923 |
| Calculated nutritional index | ||||
| Prognostic nutritional index (PNI) 2 | ||||
| Mean ± SD | 46.0 ± 7.0 | 45.3 ± 7.0 | 47.0 ± 6.9 | 0.100 |
1 Median (range). 2 PNI, 10 × serum albumin (g/dL) + 0.005 × peripheral blood lymphocyte count (/uL). Abbreviations: SD, standard deviation; WBC, white blood cell.
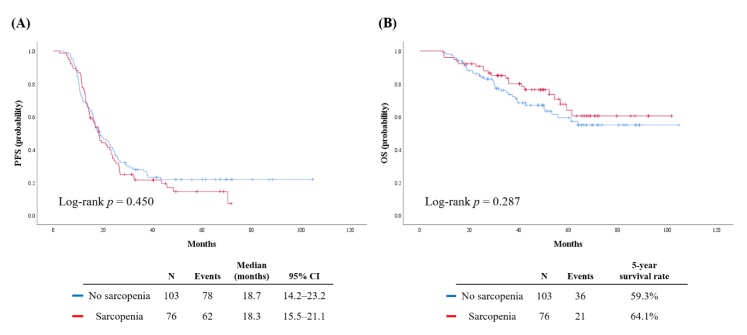
The median length of observation was 42.7 months, and it was not different between both groups (45.9 vs. 41.5 months; p = 0.497). During this period, 140 patients (78.2%) experienced disease recurrence, and 57 patients (31.8%) died of disease. Patients in the sarcopenia and control groups showed similar PFS (median, 18.3 vs. 18.7 months; p = 0.450; Figure 1A) and OS (five-year survival rate, 64.1% vs. 59.3%; p = 0.287; Figure 1B).
Figure 1.
Survival outcomes of patients. (A) Progression-free survival; (B) overall survival.
Multivariate analyses adjusting patients’ age, International Federation of Gynecology and Obstetrics (FIGO) stage, serum CA-125 levels, primary treatment strategy, residual tumor size after surgery, and BMI revealed that pre-treatment sarcopenia status did not influence patients’ PFS and OS (Table 3). Instead, age ≥58 years (adjusted hazard ratio (aHR), 1.458; 95% confidence interval (CI), 1.024–2.077; p = 0.037) and gross residual tumor (aHR, 1.504; 95% CI, 1.068–2.119; p = 0.020) were identified as independent poor prognostic factors for PFS. For OS, NAC rather than primary debulking surgery (PDS; aHR, 2.000; 95% CI, 1.096–3.649; p = 0.024) and gross residual tumor (aHR, 2.142; 95% CI, 1.258–3.647; p = 0.005) were the poor prognostic factors.
Table 3.
Factors associated with patients’ survival outcomes.
| Characteristics | n | (A) Progression-Free Survival | (B) Overall Survival | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Univariate Analysis | Multivariate Analysis | Univariate Analysis | Multivariate Analysis | ||||||||||
| HR | 95% CI | p | aHR | 95% CI | p | HR | 95% CI | p | aHR | 95% CI | p | ||
| Age, years | |||||||||||||
| <58 | 94 | 1 | − | − | 1 | − | − | 1 | − | − | 1 | − | − |
| ≥58 | 85 | 1.558 | 1.116–2.175 | 0.009 | 1.458 | 1.024–2.077 | 0.037 | 1.551 | 0.919–2.618 | 0.101 | 1.213 | 0.692–2.127 | 0.500 |
| FIGO stage | |||||||||||||
| III | 122 | 1 | − | − | 1 | − | − | 1 | − | − | 1 | − | − |
| IV | 57 | 1.342 | 0.944–1.908 | 0.101 | 1.216 | 0.820–1.805 | 0.330 | 1.490 | 0.861–2.579 | 0.154 | 1.256 | 0.690–2.288 | 0.456 |
| CA-125, IU/ml | |||||||||||||
| <800 | 89 | 1 | − | − | 1 | − | − | 1 | − | − | 1 | − | − |
| ≥800 | 90 | 1.164 | 0.835–1.622 | 0.370 | 1.140 | 0.811–1.602 | 0.451 | 1.110 | 0.660–1.867 | 0.695 | 0.964 | 0.560–1.660 | 0.894 |
| Primary treatment strategy | |||||||||||||
| PDS | 135 | 1 | − | − | 1 | − | − | 1 | − | − | 1 | − | − |
| NAC | 44 | 1.669 | 1.151–2.419 | 0.007 | 1.380 | 0.902–2.113 | 0.138 | 2.376 | 1.392–4.057 | 0.002 | 2.000 | 1.096–3.649 | 0.024 |
| Residual tumor after PDS/IDS | |||||||||||||
| No gross | 114 | 1 | − | − | 1 | − | − | 1 | − | − | 1 | − | − |
| Gross | 65 | 1.568 | 1.119–2.198 | 0.009 | 1.504 | 1.068–2.119 | 0.020 | 2.169 | 1.286–3.658 | 0.004 | 2.142 | 1.258–3.647 | 0.005 |
| BMI, kg/m2 | |||||||||||||
| Normal (18.5−22.9) | 81 | 1 | − | − | 1 | − | − | 1 | − | − | 1 | − | − |
| Overweight (23.0−24.9) | 52 | 0679 | 0.449–1.029 | 0.068 | 0.656 | 0.429–1.004 | 0.052 | 0.728 | 0.366–1.450 | 0.367 | 0.707 | 0.347–1.437 | 0.338 |
| Obesity (≥25.0) | 46 | 1.184 | 0.799–1.755 | 0.399 | 1.132 | 0.742–1.726 | 0.564 | 1.638 | 0.909–2.951 | 0.100 | 1.261 | 0.661–2.405 | 0.481 |
| Sarcopenia | |||||||||||||
| No | 103 | 1 | − | − | 1 | − | − | 1 | − | − | 1 | − | − |
| Yes | 76 | 0.879 | 0629–1.228 | 0.451 | 1.292 | 0.906–1.843 | 0.157 | 0.747 | 0.436–1.280 | 0.289 | 0.870 | 0.488–1.550 | 0.636 |
Abbreviations: aHR, adjusted hazard ratio; BMI, body mass index; CA-125, cancer antigen 125; CI, confidence interval; FIGO, International Federation of Gynecology and Obstetrics; HR, hazard ratio; IDS, interval debulking surgery; NAC, neoadjuvant chemotherapy; PDS, primary debulking surgery.
2.2. Subgroup Analysis in Sarcopenia Patients
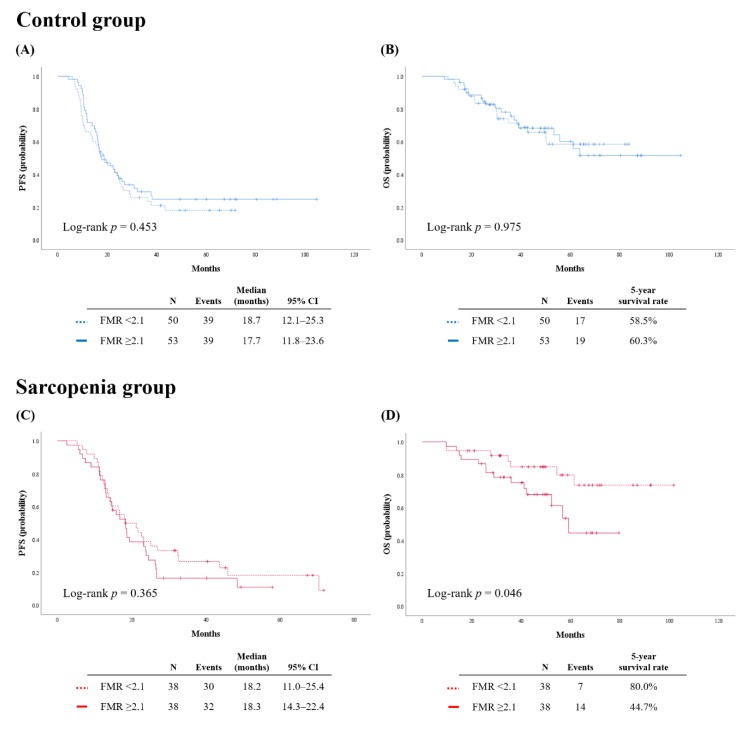
Because we focused on patients’ relative fat mass, we adopted fat-to-muscle ratio (FMR) among the calculated body composition indices. As the median FMR of all patients was 2.1, we subdivided the patients into FMR low (<2.1) and high (≥2.1) groups. Among the patients without sarcopenia (n = 103), no differences in PFS and OS were observed between the FMR low and high groups (p = 0.453 and p = 0.975, respectively) (Figure 2A,B).
Figure 2.
Survival outcomes of patients by fat-to-muscle ratio. (Upper) Control group; (Lower) sarcopenia group. (A,C) Progression-free survival; (B,D) overall survival.
Next, we performed subgroup analysis confined to the sarcopenia group (n = 76). Patients’ clinicopathologic characteristics are presented in Table 4. Compared to patients with low FMR, patients with high FMR were significantly older (mean, 60.1 vs. 54.0 years; p = 0.006), and had higher pre-treatment BMI (mean, 23.6 vs. 20.7 kg/m2; p < 0.001) and prevalence of dyslipidemia (15.8% vs. 0%; p = 0.025). Other characteristics were similar between the FMR high and low groups. Sarcopenia patients’ initial body composition and laboratory results are presented in Table 5. Compared to the FMR low group, the FMR high group showed higher total fat area (median, 228.1 vs. 141.5 cm2; p < 0.001) and visceral-to-subcutaneous fat ratio (VSR; median, 0.6 vs. 0.3; p = 0.001), and lower skeletal muscle mass-to-visceral fat ratio (SVR; median, 1.1 vs. 2.5; p < 0.001). However, skeletal muscle area as well as SMI were similar between both groups. There were no differences in the laboratory results, inflammatory indices, and nutritional index between the two groups.
Table 4.
Clinicopathologic characteristics of sarcopenia patients. 1 In this study, underweight patients (BMI < 18.5 kg/m2) were excluded in analysis. 2 PSR was defined as relapse ≥6 months after completion of taxane- and platinum-based chemotherapy, whereas PRR as relapse <6 months. 3 In addition to PSR, the patients who completed taxane- and platinum-based chemotherapy and did not experience disease recurrence during at least six months of follow-up period were considered platinum-sensitive.
| Characteristics | FMR Low Group (n = 38, %) |
FMR High Group (n = 38, %) | p |
|---|---|---|---|
| Age, years | |||
| Mean ± SD | 54.0 ± 8.2 | 60.1 ± 10.6 | 0.006 |
| BMI 1, kg/m2 | |||
| Mean ± SD | 20.7 ± 1.5 | 23.6 ± 1.9 | <0.001 |
| Normal (18.5–22.9) | 33 (86.8) | 13 (34.2) | <0.001 |
| Overweight (23.0–24.9) | 5 (13.2) | 17 (44.7) | |
| Obesity (≥25.0) | 0 | 8 (21.1) | |
| Comorbidities | |||
| Hypertension | 7 (18.4) | 13 (34.2) | 0.118 |
| Diabetes | 3 (7.9) | 2 (5.3) | >0.999 |
| Dyslipidemia | 0 | 6 (15.8) | 0.025 |
| ASA score | 0.466 | ||
| 1 | 16 (42.1) | 16 (42.1) | |
| 2 | 20 (52.6) | 17 (44.7) | |
| 3 | 2 (5.3) | 5 (13.2) | |
| FIGO stage | 0.613 | ||
| III | 28 (73.7) | 26 (68.4) | |
| IV | 10 (26.3) | 12 (31.6) | |
| CA-125, IU/ml | |||
| Median (range) | 793.0 (13.0–24720.0) | 712.5 (5.1–7821.0) | 0.949 |
| Primary treatment strategy | 0.361 | ||
| PDS | 33 (86.8) | 30 (78.9) | |
| NAC | 5 (13.2) | 8 (21.1) | |
| Residual tumour after PDS/IDS | 0.533 | ||
| No gross | 23 (60.5) | 24 (63.2) | |
| <1 cm | 11 (28.9) | 7 (18.4) | |
| 1–2 cm | 2 (5.3) | 5 (13.2) | |
| ≥2 cm | 2 (5.3) | 2 (5.3) | |
| Regimen of first-line chemotherapy | 0.306 | ||
| Paclitaxel-Carboplatin | 36 (94.7) | 32 (84.2) | |
| Docetaxel-Carboplatin | 1 (2.6) | 4 (10.5) | |
| Paclitaxel-Carboplatin-Bevacizumab | 1 (2.6) | 2 (5.3) | |
| Main cycles of first-line chemotherapy | |||
| Median (range) | 6 (4–12) | 6 (4–12) | 0.374 |
| 4–6 | 28 (73.7) | 25 (65.8) | 0.725 |
| 7–9 | 8 (21.1) | 11 (28.9) | |
| 10–12 | 2 (5.3) | 2 (5.3) | |
| Recurrence | 30 (78.9) | 32 (842) | 0.554 |
| PSR 2 | 24 (63.2) | 24 (63.2) | 0.638 |
| PRR | 6 (15.8) | 8 (21.1) | |
| Platinum sensitivity | 0.554 | ||
| Platinum-sensitive 3 | 32 (84.2) | 30 (78.9) | |
| Platinum-resistant | 6 (15.8) | 8 (21.1) |
Abbreviations: ASA, American Society of Anesthesiologists; BMI, body mass index; CA-125, cancer antigen 125; FIGO, International Federation of Gynecology and Obstetrics; IDS, interval debulking surgery; NAC, neoadjuvant chemotherapy; PDS, primary debulking surgery; PRR, platinum-resistant recurrence; PSR, platinum-sensitive recurrence; SD, standard deviation.
Table 5.
Body composition and laboratory results of sarcopenia patients.1 Median (range). 2 PNI, 10 × serum albumin (g/dL) + 0.005 × peripheral blood lymphocyte count (/uL).
| Characteristics | FMR low group (n = 38, %) |
FMR high group (n = 38, %) |
p |
|---|---|---|---|
| Body composition at diagnosis 1 | |||
| Skeletal muscle area, cm2 | 89.8 (74.5–109.0) | 86.4 (64.1–104.8) | 0.094 |
| Total fat area, cm2 | 141.5 (42.2–199.3) | 228.1 (166.0–458.2) | <0.001 |
| Subcutaneous fat | 97.1 (34.4–165.4) | 138.3 (54.6–252.0) | <0.001 |
| Visceral fat | 35.2 (6.6–79.4) | 82.1 (30.1–213.0) | <0.001 |
| Muscle fat | 3.8 (1.2–15.2) | 7.8 (2.3–31.6) | <0.001 |
| Calculated body composition index 1 | |||
| Skeletal muscle index (SMI), cm2/m2 | 36.0 (27.1–39.0) | 37.4 (28.7–39.0) | 0.228 |
| Fat-to-muscle ratio (FMR) | 1.6 (0.5–2.1) | 2.6 (2.1–4.8) | <0.001 |
| Visceral-to-subcutaneous fat ratio (VSR) | 0.3 (0.1–1.3) | 0.6 (0.2–2.9) | 0.001 |
| Skeletal muscle mass-to-visceral fat ratio (SVR) | 2.5 (1.0–14.2) | 1.1 (0.5–2.6) | <0.001 |
| Laboratory test at diagnosis 1 | |||
| Hemoglobin, g/dL | 12.1 (8.3–14.6) | 12.5 (9.2–14.3) | 0.569 |
| WBC count, 103/uL | 7.4 (3.5–15.3) | 6.9 (4.1–17.0) | 0.971 |
| Neutrophil (%) | 69.7 (47.0–83.0) | 68.4 (49.7–81.2) | 0.646 |
| Lymphocyte (%) | 21.5 (9.4–37.0) | 21.2 (9.7–42.9) | 0.893 |
| Monocyte (%) | 7.2 (3.7–16.0) | 6.5 (4.5–13.5) | 0.557 |
| Platelet count, 103/uL | 323.5 (159.0–634.0) | 3225 (202.0–564.0) | 0.383 |
| Albumin, g/dL | 3.9 (2.8–5.0) | 4.0 (2.4–5.1) | 0.521 |
| Calculated inflammatory index 1 | |||
| Neutrophil-to-lymphocyte ratio (NLR) | 3.2 (1.4–8.8) | 3.3 (1.2–8.4) | 0.884 |
| Monocyte-to-lymphocyte ratio (MLR) | 0.3 (0.1–0.8) | 0.3 (0.1–0.9) | 0.771 |
| Platelet-to-lymphocyte ratio (PLR) | 201.0 (77.2–547.0) | 211.2 (97.3–682.1) | 0.633 |
| Calculated nutritional index | |||
| Prognostic nutritional index (PNI) 2 | |||
| Mean ± SD | 46.7 (34.5–59.1) | 48.2 (27.7–64.0) | 0.357 |
Abbreviations: SD, standard deviation; WBC, white blood cell.
In the sarcopenia group, patients with FMR showed significantly worse OS than those with low FMR (five-year survival rate, 44.7% vs. 80.0%; p = 0.046), whereas PFS was not different (p = 0.365) (Figure 2C,D). Multivariate analyses identified high FMR as an independent poor prognostic factor for OS in this group (aHR, 3.377; 95% CI, 1.170–9.752; p = 0.024), whereas high FMR did not influence patients’ PFS (p = 0.825) (Table 6). Other poor prognostic factors for OS were NAC rather than PDS (aHR, 3.310; 95% CI, 1.096–10.000; p = 0.034) and gross residual tumor after surgery (aHR, 4.377; 95% CI, 1.655–11.578; p = 0.003).
Table 6.
Factors associated with sarcopenia patients’ survival outcomes.
| Characteristics | n | (A) Progression-Free Survival | (B) Overall Survival | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Univariate Analysis | Multivariate Analysis | Univariate Analysis | Multivariate Analysis | ||||||||||
| HR | 95% CI | p | aHR | 95% CI | p | HR | 95% CI | p | aHR | 95% CI | p | ||
| Age, years | |||||||||||||
| <58 | 44 | 1 | − | − | 1 | − | − | 1 | − | − | 1 | − | − |
| ≥58 | 32 | 1.934 | 1.158–3.229 | 0.012 | 1.905 | 1.065–3.407 | 0.030 | 1.593 | 0.669–3.795 | 0.293 | 1.041 | 0.417–2.598 | 0.932 |
| FIGO stage | |||||||||||||
| III | 54 | 1 | − | − | 1 | − | − | 1 | − | − | 1 | − | − |
| IV | 22 | 1.063 | 0.612–1.845 | 0.829 | 0.917 | 0.484–1.739 | 0.791 | 1.394 | 0.557–3.488 | 0.487 | 0.947 | 0.345–2.594 | 0.915 |
| CA-125, IU/ml | |||||||||||||
| <800 | 39 | 1 | − | − | 1 | − | − | 1 | − | − | 1 | − | − |
| ≥800 | 37 | 0.933 | 0.563–1.546 | 0.787 | 0.863 | 0.492–1.514 | 0.608 | 0.999 | 0.424–2.354 | 0.998 | 1.171 | 0.414–3.314 | 0.766 |
| Primary treatment strategy | |||||||||||||
| PDS | 63 | 1 | − | − | 1 | − | − | 1 | − | − | 1 | − | − |
| NAC | 13 | 1.456 | 0.773–2.742 | 0.245 | 1.254 | 0.594–2.644 | 0.553 | 2.933 | 1.177–7.309 | 0.021 | 3.310 | 1.096–10.000 | 0.034 |
| Residual tumor after PDS/IDS | |||||||||||||
| No gross | 47 | 1 | − | − | 1 | − | − | 1 | − | − | 1 | − | − |
| Gross | 29 | 2.274 | 1.363–3.795 | 0.002 | 2.270 | 1.334–3.861 | 0.003 | 3.587 | 1.442–8.922 | 0.006 | 4.377 | 1.655–11.578 | 0.003 |
| BMI, kg/m2 | |||||||||||||
| Normal (18.5−22.9) | 46 | 1 | − | − | 1 | − | − | 1 | − | − | 1 | − | − |
| Overweight (23.0−24.9) | 22 | 0.921 | 0.517–1.641 | 0.780 | 0.846 | 0.440–1.624 | 0.615 | 1.024 | 0.383–2.740 | 0.962 | 0.783 | 0.244–2.517 | 0.682 |
| Obesity (≥25.0) | 8 | 1.407 | 0.648–3.051 | 0.388 | 0.937 | 0.370–2.376 | 0.892 | 1.726 | 0.482–6.178 | 0.401 | 0.356 | 0.065–1.935 | 0.232 |
| Fat-to-muscle ratio (FMR) | |||||||||||||
| <2.1 | 38 | 1 | − | − | 1 | − | − | 1 | − | − | 1 | − | − |
| ≥2.1 | 38 | 1.262 | 0.762–2.092 | 0.366 | 1.073 | 0.576–1.999 | 0.825 | 2.476 | 0.989–6.199 | 0.053 | 3.377 | 1.170–9.752 | 0.024 |
Abbreviations: aHR, adjusted hazard ratio; BMI, body mass index; CA-125, cancer antigen 125; CI, confidence interval; FIGO, International Federation of Gynecology and Obstetrics; HR, hazard ratio; IDS, interval debulking surgery; NAC, neoadjuvant chemotherapy; PDS, primary debulking surgery.
2.3. Correlations between Body Compositio and Systemic Inflammatory Indices
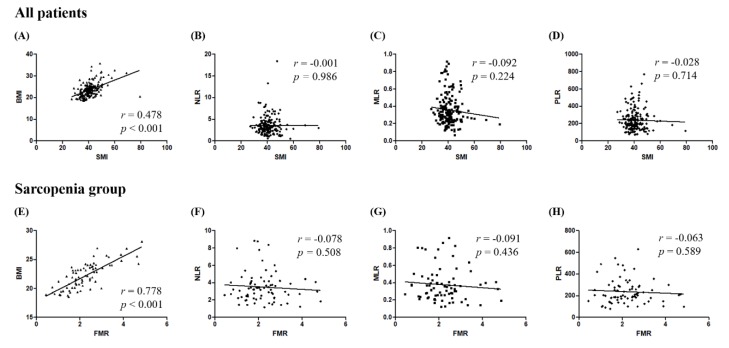
We investigated the correlations between SMI and the three systemic inflammatory indices, neutrophil-to-lymphocyte ratio (NLR), monocyte-to-lymphocyte ratio (MLR), and platelet-to-lymphocyte ratio (PLR). While SMI was significantly associated with BMI (Pearson’s correlation coefficient r = 0.478; p < 0.001), there were no correlations between SMI and either NLR, MLR, or PLR (Figure 3A–D).
Figure 3.
Correlations between body composition and systemic inflammatory indices. (Upper) Analyses according to skeletal muscle index in all patients; (Lower) analyses according to fat to muscle ratio in sarcopenia patients. (A,E) Body mass index; (B,F) neutrophil-to-lymphocyte ratio; (C,G) monocyte-to-lymphocyte ratio; (D,H) platelet-to-lymphocyte ratio.
To elucidate the underlying mechanisms of high FMR and poor survival outcome in sarcopenia patients, correlations between FMR and the three systemic inflammatory indices—NLR, MLR, and PLR—were also investigated. While FMR was significantly associated with BMI (Pearson’s correlation coefficient r = 0.778; p < 0.001), significant correlations were not observed between FMR and NLR, between FMR and MLR, and between FMR and PLR (Figure 3E–H).
3. Discussion
In this study, we investigated the impact of pre-treatment sarcopenia on survival outcomes in patients with advanced-stage HGSOC and revealed that there was no significant association between sarcopenia and recurrence rate or survival. However, further subgroup analysis identified high FMR as a poor prognostic factor for OS in sarcopenia patients.
Unlike other malignancies in which sarcopenia is associated with decreased OS and increased post-operative morbidity [17,18], inconsistent results on the relationship between sarcopenia and survival outcome are observed among the studies regarding ovarian cancer. There are two representative retrospective studies: while Bronger et al. reported the baseline sarcopenia is an independent poor prognostic factor for PFS and OS in advanced-stage serous ovarian cancer [10], Rutten et al. demonstrated that sarcopenia was not a prognostic factor for OS or major complications in ovarian cancer patients undergoing PDS [11]. Most studies were conducted in Western populations whose body composition is different from that of Asians. Recently, a Japanese retrospective study showed results similar to those of our study; pre-treatment SMI was not associated with ovarian cancer patients’ PFS and OS [19]. However, that study included early-stage disease and histologic types other than HGSOC, which is definitely different compared to our study.
To date, researches on sarcopenia in cancer patients have been conducted in the context of cancer cachexia. Patients with HGSOC are at high risk of sarcopenia and cachexia. First, as the disease is often detected in a much-progressed state, the patients might already have cachexia at the time of diagnosis. Second, an enlarging tumor mass induces metabolic dysfunction towards catabolism, while bowel obstructions during disease progression cause anorexia or reduced food intake [20]. Third, newly diagnosed patients undergo aggressive cytoreductive surgery followed by taxane- and platinum-based chemotherapy as an established standard of care, which further aggravate anorexia and loss of body weight [21]. Consequently, poor nutritional status and loss of muscle mass and strength is highly expected in patients with ovarian cancer. Previously, our research team reported that underweight status, one of the representative features of cachexia, was a poor prognostic factor in patients with advanced-stage ovarian cancer [22]. In the current study, rather than cancer cachexia, we focused on sarcopenia itself which may be incidentally discovered at the time of diagnosis of ovarian cancer. For this purpose, we excluded pre-treatment underweight patients in whom cancer cachexia could already be dominant.
CT scans are known to distinguish fat and muscle tissue accurately with high reproducibility by using specific attenuation of each tissue [23]. The most commonly used and validated Hounsfield unit (HU) range for adipose tissue is −190 to −30. However, there is an inconsistency between the literature with the HU range for muscle tissue, which starts from either 0 or −29 and ends at 100 or 150. Exclusion of the area ranging from −29 to 0 HU may result in significant loss of the total muscle area. Instead, we used −29 to 150 HU for muscle tissue so as not to miss the low attenuation muscle, same as that used in previous studies [24,25].
Although there was no statistical difference in PFS and OS between the sarcopenia and control groups, we found that high FMR is an independent prognostic factor for OS in the sarcopenia group. The coexistence of sarcopenia and obesity (sarcopenic obesity) seems to affect patients’ survival outcomes equal to or greater than the sum of the respective risks of obesity and sarcopenia alone [26]. A previous study has reported that the presence of sarcopenic obesity increased patients’ mortality in colorectal cancer [27]. In the current study, we focused on amount of the fat relative to the muscle, rather than BMI, considering the fact that Asians have a higher body fat percentage than Westerners at the same BMI [16], and similar results were found with the previous studies.
One remarkable observation in the current study is that we tried to elucidate the mechanisms underlying the relationship between high FMR and decreased survival in sarcopenic patients with advanced-stage HGSOC. Previously, our research team reported that adipose stem cells from visceral and subcutaneous fat facilitated the growth and migration of ovarian cancer cells via IL-6/JAK2/STAT3 pathway [28]. Adding to this, other researchers have reported that visceral obesity is associated with a chronic inflammatory state, which leads to adverse metabolic consequences [29]. The relationship between sarcopenia and systemic inflammation has been also reported [30]. In this context, we hypothesized that systemic inflammatory indices (NLR, MLR, and PLR) would be different between the high and low FMR groups. However, there were no differences between both groups, and correlations were not observed between FMR and the three systemic inflammatory indices. Similar correlations were also observed between SMI and the inflammatory indices. These findings might be related to the small sample size or exclusion of underweight patients. Moreover, investigation of other systemic inflammatory markers and adipose tissue-derived cytokines, such as leptin, IL-6 and TNF-α, may answer our hypothesis exactly.
In keeping with the era of precision medicine, early identification of adverse body composition which might influence patients’ survival outcome would be one of the important issues. For patients who have high FMR, aerobic exercises may be recommended to reduce adipose tissue. To date, intervention studies to prevent sarcopenia or maintain skeletal muscle mass in patients with ovarian cancer is still insufficient. Nevertheless, as recommended by various societies, prescription of resistance-type exercise training and a protein-rich diet or protein supplement should be also considered for HGSOC patients with sarcopenia. Hormone replacement therapy or vitamin D may be given, but more evidence is needed [31,32,33]. For those who have chemotherapy-induced nausea and vomiting, adequate anti-emetics as well as parenteral nutrition should be provided. If patients suffer from dyspepsia or abdominal distention owing to large amount of ascites, drainage of ascitic fluid may improve patients’ symptoms as well as nutritional status. If there is long persistent seeding ileus, procedures such as stoma formation may be considered as well. Prior to administering these interventions, all HGSOC patients should be screened for sarcopenia and adiposity at the time of diagnosis. As pre-treatment or baseline CT scans are commonly performed to determine the severity of disease and to establish a treatment plan in most patients, routine screening for body composition would be available and practical.
The current study has several limitations. First, a small sample size with possible selection bias that originates from the retrospective study design might be problematic. Second, the sequential change of body composition in each individual was not considered. Third, associations between sarcopenia and surgery or chemotherapy-related complications were not investigated. Finally, although muscle mass was successfully measured by using CT scans, muscle quality was hard to know by this imaging modality. Decreased muscle quality is known to be associated with the fatty degeneration or fatty infiltration of the muscle (i.e., myosteatosis). Currently, magnetic resonance imaging (MRI) is the best modality to evaluate the muscle quality and myosteatosis. In addition, MRI may also provide information on inflammation, edema, fibrosis, and atrophy in the muscle [34,35,36]. However, because of its high cost, limited availability, and long image acquisition time, MRI-based body composition assessment is not a routine clinical practice. Most of our study population did not undergo pre-treatment MRI, so accurate assessment of muscle quality was unavailable. Despite this study’s limitations, the current study is the first study to adopt CT-based body composition measurement techniques to identify prognostic factors in Korean ovarian cancer patients.
4. Materials and Methods
This retrospective cohort study was approved by the Institutional Review Board of Seoul National University Hospital (SNUH; No. H-1911-171-1082) which waived the requirement to obtain informed consent.
4.1. Study Population
From the Ovarian Cancer Cohort Database, we searched patients who met the following inclusion criteria: (1) patients older than 18 years of age, (2) those with HGSOC diagnosed and primarily treated at SNUH between January 2010 and December 2017, and (3) those with FIGO stage III-IV disease. However, patients with the following conditions were excluded: (1) patients with any malignancy other than HGSOC, (2) those with insufficient clinical data, (3) those who did not undergo pre-treatment CT scans, and (4) those who were underweight based on pre-treatment BMI (<18.5 kg/m2). In total, 179 patients who met these criteria were included in this analysis.
4.2. CT Image Analysis and Definition of Sarcopenia
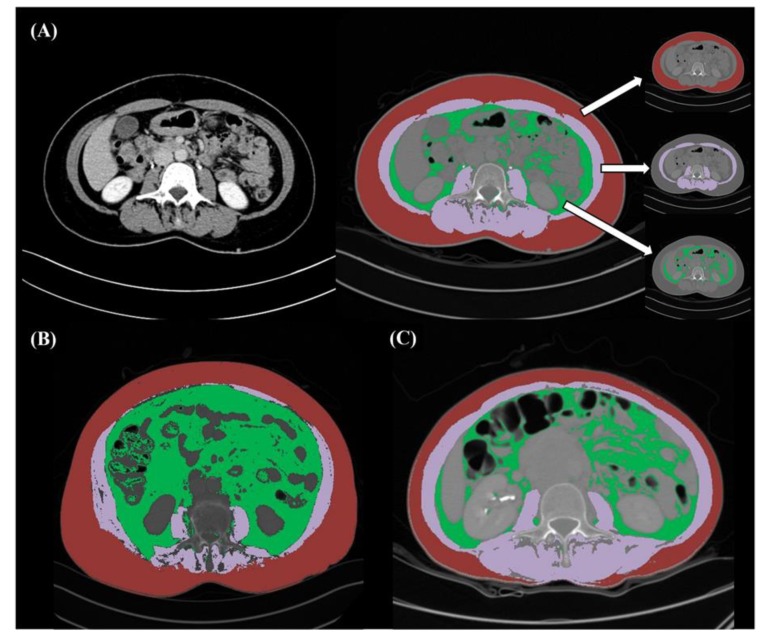
For the evaluation of sarcopenia, a cross-sectional area of the muscle at the level of L3 vertebral body was measured using baseline CT scans. Applying previously validated boundaries of −190 to −30 HU for fat tissue and −29 to 150 HU for skeletal muscle [37], an experienced radiologist (T.M.K., 5 years of genitourinary imaging experience) who was blinded to the clinical outcome measured total abdominal muscle area (cm2), intramuscular fat area (cm2), visceral fat area (cm2), and subcutaneous fat area (cm2). This CT image analysis was conducted by semi-automatic technique using AsanJ-Morphometry software (Asan Image Metrics, Seoul, Korea) (Figure 4A–C).
Figure 4.
Evaluation of body composition using CT image. Preoperative axial CT image at the level of L3 vertebral body level. (A) A 52-year old woman with newly diagnosed high-grade serous ovarian carcinoma. Total abdominal muscle area (purple), visceral fat area (green), and subcutaneous fat area (red) are segmented by the semi-automatic technique; (B) A 73-year old woman with sarcopenia and high fat-to-muscle ratio (4.6); (C) A 53-year old woman with sarcopenia and low fat-to-muscle ratio (1.5).
Total abdominal muscle area (cm2) was normalized for height (m2) and reported as lumbar SMI. To date, the sex-specific cut-off values of SMI for sarcopenia have not been validated in Korean healthy individuals. Adoption of the cut-off values suggested by Japanese study groups was deterred because they were developed in different study populations (e.g., patients with liver disease, [38]) or had age limitations (e.g., <50 years, [39]). In addition, proportions of populations with overweight-obesity are even different between Korea and Japan according to the OECD Health Statistics 2019 [13]. Therefore, we defined sarcopenia as SMI of <39.0 cm2/m2 according to the proposed cut-off value by an international consensus, and divided patients into sarcopenia group (<39.0 cm2/m2) and no sarcopenia group (control group; ≥39.0 cm2/m2) [40]. We also calculated other body composition indices, such as FMR, VSR, and SVR.
4.3. Data Collection
We collected patients’ clinicopathologic characteristics including age, co-morbidities such as hypertension or diabetes, American Society of Anesthesiologists score, FIGO stage, NAC, residual tumor size after PDS or interval debulking surgery, and regimens and cycles of adjuvant chemotherapy. Patients treated with neoadjuvant chemotherapy received 3–4 cycles of taxane- and platinum-based chemotherapy before surgery, and optimal debulking surgery was considered when no gross residual tumor was achieved.
Patients’ pre-treatment BMI was calculated as body weight (kg) divided by height squared (m2), which were measured at the time of diagnosis. All patients were classified into three groups based on the following BMI criteria suggested by the World Health Organization for the Asian population: normal (≥18.5 kg/m2 and <23.0 kg/m2), overweight (≥23.0 kg/m2 and <25.0 kg/m2), and obese (≥25.0 kg/m2) [16].
Data acquisition also included serum CA-125 levels, hemoglobin, albumin, and differential blood cell counts including neutrophils, lymphocytes, monocytes, and platelets at initial diagnosis, less than a month prior to either PDS or the start date of NAC. As systemic inflammatory indices, we calculated the NLR, MLR, and PLR. As a pre-treatment nutritional index, we calculated the prognostic nutritional index (PNI) as follows: 10 × serum albumin (g/dL) + 0.005 × peripheral blood lymphocyte count (/uL) [41].
In terms of survival data, OS was defined as the time interval between the date of diagnosis and the date of cancer-related death or the end of the study. During the surveillance, patients received CT scanning routinely every three to four months for the first two years, every six months for the next two years, and thereafter, every year or when symptoms or examination findings were suspicious for recurrence. Therefore, we defined PFS as the time interval between the start date of primary treatment and the date of image-confirmed disease progression, which was assessed based on the Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1 [42].
4.4. Statistical Analysis
We compared the patients’ clinicopathologic characteristics and survival outcomes between the sarcopenia and control groups. We used Student’s t-test and the Mann-Whitney U test for comparisons of continuous variables and Pearson’s chi-squared and Fisher’s exact test for categorical variables. For survival analysis, we conducted the Kaplan-Meier methods with log-rank test. Multivariate analysis was performed using a Cox proportional-hazards model, and aHRs and 95% CIs were calculated. We used IBM SPSS Statistics software (version 25·0; SPSS Inc., Chicago, IL, USA) for these analyses. Correlation values were calculated by the Pearson’s correlation coefficient test using the GraphPad Prism 5 software (GraphPad Inc., La Jolla, CA, USA). A p value <0.05 was considered statistically significant.
5. Conclusions
In conclusion, we investigated the clinical significance of sarcopenia in Korean patients with advanced-stage HGSOC and found that sarcopenia did not influence patients’ recurrence rates and survival. However, among the sarcopenia patients, those who had relatively high levels of fat compared to muscle mass showed worse OS. Further translational researches and prospective studies are warranted.
Author Contributions
Conceptualization, S.I.K. and Y.S.S.; Formal analysis, S.I.K. and T.M.K.; Investigation, S.I.K., T.M.K., M.L., and H.S.K.; Methodology, S.I.K., T.M.K., J.Y.C., and Y.S.S.; Project administration, Y.S.S.; Supervision, Y.S.S.; Validation, H.H.C. and J.Y.C.; Writing—original draft, S.I.K. and T.M.K.; Writing—review and editing, all authors. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by a grant from the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health and Welfare, Republic of Korea (No. HI16C2037).
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Bray F., Ferlay J., Soerjomataram I., Siegel R.L., Torre L.A., Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.Lim M.C., Won Y.J., Ko M.J., Kim M., Shim S.H., Suh D.H., Kim J.W. Incidence of cervical, endometrial, and ovarian cancer in Korea during 1999–2015. J. Gynecol. Oncol. 2019;30:e38. doi: 10.3802/jgo.2019.30.e38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Smith L.H., Morris C.R., Yasmeen S., Parikh-Patel A., Cress R.D., Romano P.S. Ovarian cancer: can we make the clinical diagnosis earlier? Cancer. 2005;104:1398–1407. doi: 10.1002/cncr.21310. [DOI] [PubMed] [Google Scholar]
- 4.Cruz-Jentoft A.J., Bahat G., Bauer J., Boirie Y., Bruyère O., Cederholm T., Schneider S.M. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing. 2019;48:16–31. doi: 10.1093/ageing/afy169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Caan B.J., Feliciano E.M.C., Prado C.M., Alexeeff S., Kroenke C.H., Bradshaw P., Chen W.Y. Association of Muscle and Adiposity Measured by Computed Tomography With Survival in Patients With Nonmetastatic Breast Cancer. JAMA Oncol. 2018;4:798–804. doi: 10.1001/jamaoncol.2018.0137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kim E.Y., Kim Y.S., Park I., Ahn H.K., Cho E.K., Jeong Y.M. Prognostic Significance of CT-Determined Sarcopenia in Patients with Small-Cell Lung Cancer. J. Thorac. Oncol. 2015;10:1795–1799. doi: 10.1097/JTO.0000000000000690. [DOI] [PubMed] [Google Scholar]
- 7.Fukushima H., Yokoyama M., Nakanishi Y., Tobisu K., Koga F. Sarcopenia as a prognostic biomarker of advanced urothelial carcinoma. PLoS ONE. 2015;10:e0115895. doi: 10.1371/journal.pone.0115895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee J.S., Kim Y.S., Kim E.Y., Jin W. Prognostic significance of CT-determined sarcopenia in patients with advanced gastric cancer. PLoS ONE. 2018;13:e0202700. doi: 10.1371/journal.pone.0202700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kumar A., Moynagh M.R., Multinu F., Cliby W.A., McGree M.E., Weaver A.L., Jatoi A. Muscle composition measured by CT scan is a measurable predictor of overall survival in advanced ovarian cancer. Gynecol. Oncol. 2016;142:311–316. doi: 10.1016/j.ygyno.2016.05.027. [DOI] [PubMed] [Google Scholar]
- 10.Bronger H., Hederich P., Hapfelmeier A., Metz S., Noël P.B., Kiechle M., Schmalfeldt B. Sarcopenia in Advanced Serous Ovarian Cancer. Int. J. Gynecol. Cancer. 2017;27:223–232. doi: 10.1097/IGC.0000000000000867. [DOI] [PubMed] [Google Scholar]
- 11.Rutten I.J.G., Ubachs J., Kruitwagen R.F.P.M., van Dijk D.P.J., Beets-Tan R.G.H., Massuger L.F.A.G., Van Gorp T. The influence of sarcopenia on survival and surgical complications in ovarian cancer patients undergoing primary debulking surgery. Eur. J. Surg Oncol. 2017;43:717–724. doi: 10.1016/j.ejso.2016.12.016. [DOI] [PubMed] [Google Scholar]
- 12.Staley SA M., Tucker K., Newton M., Ertel M., Zhang Y., Doherty I., Gehrig P.A. Sarcopenia as a predictor of survival in patients with epithelial ovarian cancer (EOC) receiving platinum and taxane-based chemotherapy. J. Clin. Oncol. 2019;37:e17030. doi: 10.1200/JCO.2019.37.15_suppl.e17030. [DOI] [PubMed] [Google Scholar]
- 13.OECD Health Statistic. [(accessed on 22 February 2020)];2019 Available online: http://www.oecd.org/els/health-systems/health-data.htm.
- 14.Mourtzakis M., Prado C.M., Lieffers J.R., Reiman T., McCargar L.J., Baracos V.E. A practical and precise approach to quantification of body composition in cancer patients using computed tomography images acquired during routine care. Appl. Physiol. Nutr. Metab. 2008;33:997–1006. doi: 10.1139/H08-075. [DOI] [PubMed] [Google Scholar]
- 15.Shen W., Punyanitya M., Wang Z., Gallagher D., St-Onge M.P., Albu J., Heshka S. Total body skeletal muscle and adipose tissue volumes: estimation from a single abdominal cross-sectional image. J. Appl. Physiol. 2004;97:2333–2338. doi: 10.1152/japplphysiol.00744.2004. [DOI] [PubMed] [Google Scholar]
- 16.WHO Expert Consultation Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet. 2004;363:157–163. doi: 10.1016/S0140-6736(03)15268-3. [DOI] [PubMed] [Google Scholar]
- 17.Levolger S., Van Vugt J.L.A., De Bruin R.W.F., IJzermans J.N.M. Systematic review of sarcopenia in patients operated on for gastrointestinal and hepatopancreatobiliary malignancies. Br. J. Surg. 2015;102:1448–1458. doi: 10.1002/bjs.9893. [DOI] [PubMed] [Google Scholar]
- 18.Shachar S.S., Williams G.R., Muss H.B., Nishijima T.F. Prognostic value of sarcopenia in adults with solid tumours: A meta-analysis and systematic review. Eur. J. Cancer. 2016;57:58–67. doi: 10.1016/j.ejca.2015.12.030. [DOI] [PubMed] [Google Scholar]
- 19.Nakayama N., Nakayama K., Nakamura K., Razia S., Kyo S. Sarcopenic Factors May Have No Impact on Outcomes in Ovarian Cancer Patients. Diagnostics. 2019;9:206. doi: 10.3390/diagnostics9040206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gadducci A., Cosio S., Fanucchi A., Genazzani A.R. Malnutrition and cachexia in ovarian cancer patients: pathophysiology and management. Anticancer Res. 2001;21:2941–2947. [PubMed] [Google Scholar]
- 21.Bristow R.E., Tomacruz R.S., Armstrong D.K., Trimble E.L., Montz F.J. Survival effect of maximal cytoreductive surgery for advanced ovarian carcinoma during the platinum era: a meta-analysis. J. Clin. Oncol. 2002;20:1248–1259. doi: 10.1200/JCO.20.5.1248. [DOI] [PubMed] [Google Scholar]
- 22.Kim S.I., Kim H.S., Kim T.H., Suh D.H., Kim K., No J.H., Song Y.S. Impact of underweight after treatment on prognosis of advanced-stage ovarian cancer. J. Immunol. Res. 2014;2014:349546. doi: 10.1155/2014/349546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee K., Shin Y., Huh J., Sung Y.S., Lee I.S., Yoon K.H., Kim K.W. Recent Issues on Body Composition Imaging for Sarcopenia Evaluation. Korean J. Radiol. 2019;20:205–217. doi: 10.3348/kjr.2018.0479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Antoun S., Lanoy E., Iacovelli R., Albiges-Sauvin L., Loriot Y., Merad-Taoufik M., Escudier B. Skeletal muscle density predicts prognosis in patients with metastatic renal cell carcinoma treated with targeted therapies. Cancer. 2013;119:3377–3384. doi: 10.1002/cncr.28218. [DOI] [PubMed] [Google Scholar]
- 25.Martin L., Birdsell L., MacDonald N., Reiman T., Clandinin M.T., McCargar L.J., Baracos V.E. Cancer cachexia in the age of obesity: skeletal muscle depletion is a powerful prognostic factor, independent of body mass index. J. Clin. Oncol. 2013;31:1539–1547. doi: 10.1200/JCO.2012.45.2722. [DOI] [PubMed] [Google Scholar]
- 26.Baracos V.E., Arribas L. Sarcopenic obesity: hidden muscle wasting and its impact for survival and complications of cancer therapy. Ann. Oncol. 2018;29:ii1–ii9. doi: 10.1093/annonc/mdx810. [DOI] [PubMed] [Google Scholar]
- 27.Malietzis G., Currie A.C., Athanasiou T., Johns N., Anyamene N., Glynne-Jones R., Jenkins J.T. Influence of body composition profile on outcomes following colorectal cancer surgery. Br. J. Surg. 2016;103:572–580. doi: 10.1002/bjs.10075. [DOI] [PubMed] [Google Scholar]
- 28.Kim B., Kim H.S., Kim S., Haegeman G., Tsang B.K., Dhanasekaran D.N., Song Y.S. Adipose Stromal Cells from Visceral and Subcutaneous Fat Facilitate Migration of Ovarian Cancer Cells via IL-6/JAK2/STAT3 Pathway. Cancer Res. Treat. 2017;49:338–349. doi: 10.4143/crt.2016.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wisse B.E. The inflammatory syndrome: the role of adipose tissue cytokines in metabolic disorders linked to obesity. J. Am. Soc. Nephrol. 2004;15:2792–2800. doi: 10.1097/01.ASN.0000141966.69934.21. [DOI] [PubMed] [Google Scholar]
- 30.Dalle S., Rossmeislova L., Koppo K. The Role of Inflammation in Age-Related Sarcopenia. Front. Physiol. 2017;8:1045. doi: 10.3389/fphys.2017.01045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dent E., Morley J.E., Cruz-Jentoft A.J., Arai H., Kritchevsky S.B., Guralnik J., Ruiz J. International Clinical Practice Guidelines for Sarcopenia (ICFSR): Screening, Diagnosis and Management. J. Nutr. Health Aging. 2018;22:1148–1161. doi: 10.1007/s12603-018-1139-9. [DOI] [PubMed] [Google Scholar]
- 32.Burton L.A., Sumukadas D. Optimal management of sarcopenia. Clin. Interv. Aging. 2010;5:217–228. doi: 10.2147/cia.s11473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rolland Y., Czerwinski S., Van Kan G.A., Morley J.E., Cesari M., Onder G., Chumlea W.M.C. Sarcopenia: its assessment, etiology, pathogenesis, consequences and future perspectives. J. Nutr. Health Aging. 2008;12:433–450. doi: 10.1007/BF02982704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dalakas M.C. Inflammatory muscle diseases. N. Engl. J. Med. 2015;372:1734–1747. doi: 10.1056/NEJMra1402225. [DOI] [PubMed] [Google Scholar]
- 35.Seabolt L.A., Welch E.B., Silver H.J. Imaging methods for analyzing body composition in human obesity and cardiometabolic disease. Ann. N. Y. Acad. Sci. 2015;1353:41–59. doi: 10.1111/nyas.12842. [DOI] [PubMed] [Google Scholar]
- 36.Erlandson M.C., Lorbergs A.L., Mathur S., Cheung A.M. Muscle analysis using pQCT, DXA and MRI. Eur. J. Radiol. 2016;85:1505–1511. doi: 10.1016/j.ejrad.2016.03.001. [DOI] [PubMed] [Google Scholar]
- 37.Aubrey J., Esfandiari N., Baracos V.E., Buteau F.A., Frenette J., Putman C.T., Mazurak V.C. Measurement of skeletal muscle radiation attenuation and basis of its biological variation. Acta Physiol. 2014;210:489–497. doi: 10.1111/apha.12224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nishikawa H., Shiraki M., Hiramatsu A., Moriya K., Hino K., Nishiguchi S. Japan Society of Hepatology guidelines for sarcopenia in liver disease (1st edition): Recommendation from the working group for creation of sarcopenia assessment criteria. Hepatol. Res. 2016;46:951–963. doi: 10.1111/hepr.12774. [DOI] [PubMed] [Google Scholar]
- 39.Hamaguchi Y., Kaido T., Okumura S., Kobayashi A., Shirai H., Yao S., Uemoto S. Proposal for new selection criteria considering pre-transplant muscularity and visceral adiposity in living donor liver transplantation. J. Cachexia Sarcopenia Muscle. 2018;9:246–254. doi: 10.1002/jcsm.12276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fearon K., Strasser F., Anker S.D., Bosaeus I., Bruera E., Fainsinger R.L., Davis M. Definition and classification of cancer cachexia: an international consensus. Lancet Oncol. 2011;12:489–495. doi: 10.1016/S1470-2045(10)70218-7. [DOI] [PubMed] [Google Scholar]
- 41.Feng Z., Wen H., Ju X., Bi R., Chen X., Yang W., Wu X. The preoperative prognostic nutritional index is a predictive and prognostic factor of high-grade serous ovarian cancer. BMC Cancer. 2018;18:883. doi: 10.1186/s12885-018-4732-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Eisenhauer E.A., Therasse P., Bogaerts J., Schwartz L.H., Sargent D., Ford R., Rubinstein L. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) Eur. J. Cancer. 2009;45:228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]