Abstract
Traditional concepts of life sciences consider oxidative stress as a fundamental process of aging and various diseases including cancer, whereas traditional medicine recommends dietary intake of iron to support physiological functions of the organism. However, due to its strong pro-oxidative capacity, if not controlled well, iron can trigger harmful oxidative stress manifested eventually by toxic chain reactions of lipid peroxidation. Such effects of iron are considered to be major disadvantages of uncontrolled iron usage, although ferroptosis seems to be an important defense mechanism attenuating cancer development. Therefore, a variety of iron-containing nanoparticles were developed for experimental radio-, chemo-, and photodynamic as well as magnetic dynamic nanosystems that alter redox homeostasis in cancer cells. Moreover, studies carried over recent decades have revealed that even the end products of lipid peroxidation, represented by 4-hydroxynonenal (4-HNE), could have desirable effects even acting as kinds of selective anticancer substances produced by non-malignant cells for defense again invading cancer. Therefore, advanced nanotechnologies should be developed for using iron to trigger targeted lipid peroxidation as an anticancer option of integrative biomedicine.
Keywords: iron, nanomedicine, reactive oxygen species, ferroptosis, 4-hydroxynonenal
1. Introduction
One of the most challenging health conditions is cancer, and nanotechnology has provided new opportunities for the development of more efficient treatment procedures. The potential biomedical benefits of nanoparticles were first suggested in the 1970s, and the era of nanomedicine started at the turn of this century. Iron containing nanoparticles including iron oxide nanoparticles (IONPs), are promising tools for anticancer therapies. On the basis of the different oxidation states and crystalline structures, IONPs comprise magnetite (Fe3O4), maghemite (γ-Fe2O3), and hematite (α-Fe2O3). IONPs made up of magnetite and maghemite have great biocompatibility with superparamagnetic properties. Recently, a variety of iron containing nanosystems, as potent agents in anticancer treatment, have been described in the literature. Iron oxide nanoparticle properties have been reported to be useful for various biomedical purposes, such are targeted drug delivery, bioimaging, biosensors, and hyperthermia [1], while some are approved by Food and Drug Administration (FDA) [2,3]. Hence, NanoTherm for management of glioblastoma and Ferumoxytol for iron deficiency treatment are among the FDA approved iron oxide containing nanomedicines.
Iron has crucial roles in biological systems, such as the immune system, neural system, and muscular system. Iron also plays an important role in carcinogenesis and altered iron metabolism in cancer has been well documented [4,5]. Some of the regulatory roles of iron are described below.
2. Iron Regulated Cellular Processes
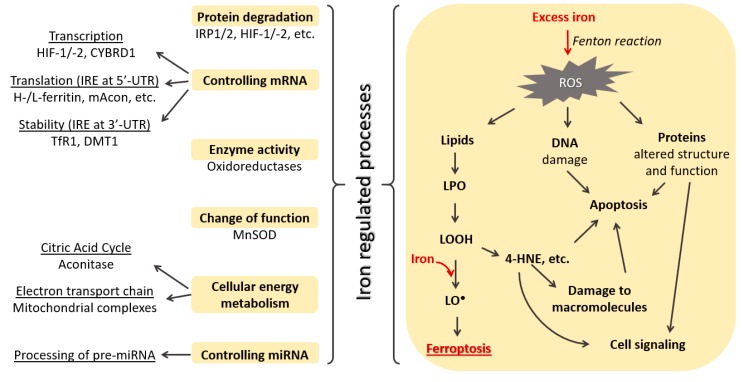
Iron is an essential nutrient and in the form of ferric ions (Fe3+) is bound to transferrin and carried through the body. When diferric transferrin is bound to the transferrin receptor the complex is endocytosed. In the endosome, Fe3+ is reduced to ferrous iron (Fe2+) by a metalloreductase six-transmembrane epithelial antigen of prostate 3 (STEAP3). This is followed by Fe2+ translocation to cytoplasm via divalent metal transporter 1 (DMT1), where it enters a transient pool of metabolically active iron [6]. Intracellularly iron can regulate a variety of processes (Figure 1).
Figure 1.
Some cellular processes regulated by iron.
Iron can modulate cellular energy metabolism by influencing the citric acid cycle and oxidative phosphorylation [7]. Iron promotes the activity of citric acid cycle enzymes, such are mitochondrial aconitase (mAcon), citrate synthase, isocitric dehydrogenase, and succinate dehydrogenase [7]. When iron is transported to the mitochondria it can be utilized for heme synthesis or iron–sulfur (Fe-S) cluster formation which are crucial components of mitochondrial complexes and other proteins needed for mitochondrial respiration and ATP generation [8,9,10].
Another important role of iron is its ability to control mRNA transcription, translation, and stability [6]. Low iron concentration promotes hypoxia-inducible factor (HIF) transcription, while under conditions of iron repletion they are hydroxylated by the action of HIF prolyl hydroxylase domain proteins (PHDs) followed by protein degradation [11]. Furthermore, changes in iron concentration regulate mRNA containing an iron responsive element (IRE) in the 3′ and 5′ untranslated regions (UTRs). Binding of iron-regulatory protein (IRP) to IRE located in the 3′-UTR of the mRNA for transferrin receptor 1 [12] and DMT1 [13] enhances its stability against nuclease attack. IRPs also have high affinity towards IRE located at the 5′-UTR of the mRNA for mitochondrial aconitase [14] and heavy and light ferritin [15], suppressing its translation. These processes are described in detail in an excellent review by Lawen and Lane [6]. Additionally, iron is also involved in the control of processing of miRNA precursors through poly(C)-binding protein 2, affecting miRNA pathway activity [16].
In addition to being an essential nutrient, iron can also have potential toxic effects on cells. The toxicity is due to their pro-oxidant capacities leading to the generation of reactive oxygen species (ROS) that can affect macromolecules and alter cell signaling pathways [17]. It has been well recognized that ROS act as a double edge sword and that their activity depends on the concentration and type of ROS, as well as on their diffusion distance from the target molecules [18,19]. Excessive ROS, based on the amount, have dual effects on tumor growth [20]. Exposure of cervical cancer cells to Fe3+ induces ROS formation at lower concentrations and affects oncogene E6/E7 expression [21]. Iron and the consequent ROS were shown to modulate nuclear factor-kappa B (NF-κB) signaling pathway [22]. By inducing NF-κB signaling and affecting serum interleukin-6 and serotonin levels, iron exhibits an important immunomodulatory role [23].
Recently, it was reported that, under both physiological and pathological conditions, iron can be incorporated into mitochondrial superoxide dismutase (SOD)2. Iron incorporation in SOD2 leads to a switch in the enzyme activity from antioxidant function of superoxide dismutase to prooxidant peroxidase. This switch can further contribute to iron toxicity by affecting cellular signaling and metabolism and eventually the development of cancer [24].
Under normal physiological conditions, superoxide (O2●−) and hydrogen peroxide (H2O2), derived from a variety of cellular processes, are detoxified by cellular antioxidant systems. The O2●− is first converted to H2O2, either spontaneously or by the action of SOD, while glutathione peroxidase (GPX), thioredoxin peroxidase, and catalase further decompose H2O2 to molecular oxygen and water. However, in an excess of iron, O2●− and H2O2 take part in the Fenton reaction yielding highly reactive hydroxyl radical (OH●) [25]. Additionally, iron promotes the generation of reactive nitrogen species (RNS). Both, RNS and OH● promote damage of proteins and lipid peroxidation [26,27,28,29]. Depending on the amount of lipid peroxidation products formed, they have either an important signaling role or exhibit cytotoxic effects for the cells and tissues [30,31]. The consequences of iron induced lipid peroxidation are described in detail below. Thus, it is evident that maintaining iron and redox homeostasis is essential.
3. Ferroptosis as a Target for Oncotherapy
Some of the mechanisms of the action of iron containing anticancer therapies account for their ability to induce ferroptosis (Figure 1). In 2012, ferroptosis was defined as a regulated nonapoptotic and iron dependent form of cell death [32], which depends on the intracellular concentration of iron as well as on the amount of ROS and level of lipid peroxidation. The process of ferroptosis is induced by the inhibition of cysteine import, inactivation of the phospholipid peroxidase glutathione peroxidase 4 (GPX4) activity and glutathione (GSH) depletion, lipid peroxidation, and excessive accumulation of lipid-based ROS (LO●) [33,34]. Today, different classes of ferroptosis induction compounds (FINs) are recognized [35]. Those responsible for inducing ferroptosis by depletion of intracellular glutathione are classified as Class I FINs. Ferroptosis can be triggered by GPX4 inactivation either directly (Class II FINs) or indirectly (Class III FINs). Additionally, an increase in the LIP via increased activity of heme oxygenase or directly via iron overload, can also trigger ferroptosis (Class IV FINs) [35].
An abundance of cellular NADPH can determine the sensitivity to ferroptosis as it has an important role in the elimination of lipid hydroperoxides (LOOH). Nevertheless, the abundance of NADP(H) has been shown to be inversely correlated with sensitivity of numerous cancer cell lines to ferroptosis [36].
The importance of ferroptosis in the management of neoplastic diseases is well recognized. Cancer cells harbor an increased iron pool and yet manage to escape ferroptosis, due to the fine balance between iron and thiols [37]. However, the fact that neoplastic cells have higher requirements of iron favors ferroptosis as an anticancer process opposing tumor development. Indeed, ferroptosis has been reported as one of the underlying mechanisms in the inhibition of numerous malignancies [38,39].
IONPs were found to reduce cellular antioxidant defense systems (e.g., activities of SOD, GSH, and catalase) and to induce ROS generation, lipid peroxidation, and caspase-3 activity promoting breast cancer cell death [40]. Exposure of human hepatocarcinoma cells to IONPs leads to the formation of 8-oxo-2’-deoxyguanosine (8-oxo-dG), and oxidative DNA damage markers. The administration of alternating magnetic field (AMF) induced magnetic hyperthermia and further contributed to an elevated 8-oxo-dG level and malignant destruction [41].
One of the adaptive mechanisms of tumor cells is adaptation to reduced levels of oxygen upregulating the expression of HIF-1. Sanazole, a hypoxic cell radiosensitizer, in a complex with IONP and the cytotoxic drug Berberine reduced the tumor volume of Dalton lymphoma ascites in a murine model [42]. The same study suggested that the observed effect was due to the downregulation of HIF-1 and the upregulation of extrinsic apoptotic pathway.
Recently, Sang and coworkers developed ferroptosis amplifier nanodevices for cancer treatment. Mitochondria targeted the nanophotosensitizer complex containing superparamagnetic IONPs (SPIONs), and when loaded with Sorafenib, exhibited antitumor activity by inducing ROS formation, lipid peroxidation, and ferroptosis of therapy resistant epithelial-to-mesenchymal transition cells [43]. The same group later described another mitochondrial membrane that targeted a photosensitive nanodevice. Black hole quencher-based fluorescence loaded with magnetic IONPs and Sorafenib was intracellularly disassembled by GSH and anchored to the mitochondrial membrane. Near-infrared (NIR) laser irradiation triggered ferroptosis and inhibited tumor growth both in vitro and in vivo [44]. A variety of iron containing nanopraticles that improve photodynamic therapy (PDT) effects have been successfully synthesized [45,46]. Antitumor activity of IONPs can be enhanced by coating, as in the case of maghemite coated with poly(N,N-dimethylacrylamide) [47].
4. The Lipid Peroxidation Product 4-Hydroxynonenal
In addition to the above-mentioned importance of iron-induced and ROS-mediated lipid peroxidation and LO● in ferroptosis of neoplastic cells, lipid peroxidation also has an important role in cell regulation. The peroxidation of polyunsaturated fatty acids (PUFAs) and subsequent decomposition of lipid hydroperoxides (LOOH) leads to formation of reactive aldehydes, among which 4-hydroxynonenal (4-HNE) is of particular interest. Namely, due to its bioactive properties, 4-HNE can either directly or indirectly affect macromolecules and modulate cellular processes and function, thus, resembling bioactivities of ROS [48,49]. Known also as a second messenger of free radicals, which interferes with bioactivities of various cytokines, 4-HNE acts in concentration-dependent and cell-type dependent manners [50,51]. Generally speaking, if present at low, physiological concentrations, 4-HNE mostly acts as a growth regulating factor affecting proliferation, differentiation, and antioxidant capacities of the cells, whereas its presence at higher supraphysiological levels usually induces apoptosis or even necrosis. One of major reasons for such multifunctional bioactivities of 4-HNE is its high affinity to bind to proteins, modulating their structure and function and forming a kind of reservoir of 4-HNE-proten adducts, which are hardly metabolized and could offer an explanation for the adverse effects of very different medical remedies, either synthetic or of natural origin [49,52,53,54]. It is commonly believed that 4-HNE possesses mutagenic and carcinogenic affects, and thus resembles negative carcinogenic activities of free radicals. However, due to the altered lipid metabolism and hypoxia, cancer cells develop different antioxidant capacities as compared with their counterpart non-malignant cells, but eventually that makes them more susceptible to the cytotoxic effects of 4-HNE [55,56,57]. Complex interactions between invading cancer and surrounding tissue can lead to the enhanced production of 4-HNE by normal, stromal, and especially inflammatory cells. Then, elevated 4-HNE acts as a kind of anticancer substance [58,59]. The fact that all rapidly proliferating cells, including those that are malignant, require higher levels of iron than is needed for the resting cells, while the iron-triggered and self-catalyzed chain reaction of lipid peroxidation generates 4-HNE, could present novel opportunities to use iron and other transition metals as potential anticancer substances, especially if packed in the form of relatively stable micro- or nanoparticles [60].
5. Iron-Containing Nanoparticles in Oncotherapy
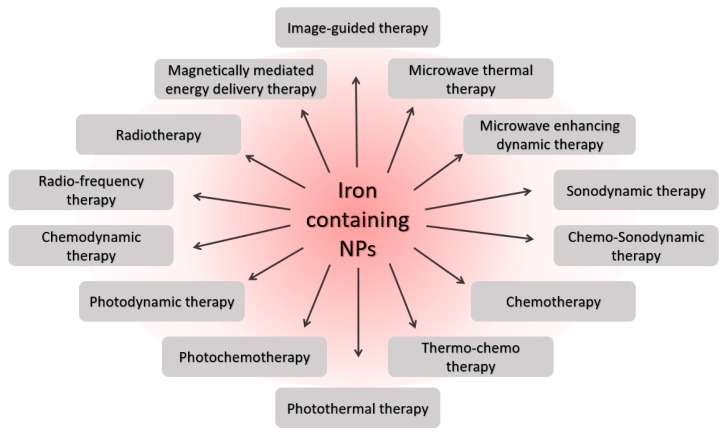
Ferroptosis, as a promising mechanism for the demise of cancer cells, has become a promising strategy in cancer nanomedicine. Due their advantages, iron-containing nanoparticles are used in numerous nanomedicine strategies in oncology (Figure 2).
Figure 2.
Biomedical therapies involving iron containing nanoparticles.
Chemodynamic therapy (CDT) with a high specificity towards a tumor microenvironment (TME) is a promising strategy for the management of cancer. The anticancer properties of CDT are due to their ability to promote conversion of endogenous H2O2 via Fenton reaction into ●OH triggering lipid peroxidation and ferroptosis of cancer cells [61,62]. In recent years, an effort has been made to develop nanoparticles with encapsulated iron for ferroptosis assisted CDT. Conjugated polymer nanoparticles (CPNPs) containing iron(III) and targeted to endothelin-B receptors selectively trigger ferroptosis of melanoma cells with no significant effect on normal cells [63]. Wang and colleagues developed redox- and light-responsive (RLR) nanoparticles that contained iron and were programmed to degrade and self-activate in an acidic environment with excessive glutathione, as is the case for a tumor microenvironment (TME). The constructed RLR nanoparticles were loaded with doxorubicin (DOX) and modified with iRGD peptide that binds to αvβ3 integrins. Such nanoparticles showed specificity in targeting integrin overexpressing cancer cells as compared with normal cells [64]. Further specificity towards cancer cells has been confirmed in the in vivo tumor model.
Magnetic hyperthermia is another important application of iron containing nanoparticles for cancer treatment. Magnetic hyperthermia is based on the effects of AMF on the magnetization properties of iron causing a heat release in the surrounding space. The simultaneous action of chemodynamic therapy with magnetic-photo hyperthermia has an inhibitory effect on tumor progression, as reported for Fe3O4-Pd Janus nanoparticles [65]. The mechanism of anticancer activity included both amplified magnetic-photo heating, as well as elevated ROS production and ferroptosis of cancer cells. Furthermore, nanotheranostic potential of iron oxide nanoparticles was shown for α-Fe2O3 nanoparticles coated with ultrasmall gold (Au) nanoseeds. The α-Fe2O3 with Au nanoparticles resembled good photothermal efficacy and upon irradiation, with low laser power, yielded ROS generation and DNA damage, suppressing tumor growth [66]. Compared to ferromagnetics, superparamagnetic iron oxide nanoparticles generate more heat and are extensively used in cancer therapy. Superparamagnetic properties of iron oxide nanoparticles have shown promising results in the hyperthermia treatment of solid tumors such as prostate cancer [67,68] and glioblastoma [69,70]. Impressively, almost complete glioblastoma regression was observed after multiple applications of magnetic hyperthermia [70]. For further reading on the medical applications of SPIONs, a comprehensive review has been published last year [71]. Xiong and colleagues have recently developed a nanosystem containing DOX, FeCl3, and tannic acid. Photothermal excitation triggered drug release from the nanosystem, while laser irradiation promoted ROS generation, lipid peroxidation, ferroptosis, reduced intracellular glutathione (GSH) level, and inhibited tumor progression, as shown both in vitro and in vivo on an ER+ breast carcinoma model [72].
The ability of polydopamine nanoparticles to chelate metal ions has been used in developing ultrasmall poly(ethylene glycol) (PEG)-modified polydopamine nanoparticles containing either Fe2+ or Fe3+ [73]. Such nanoparticles showed potent anticancer activity by ferroptosis via induction of ROS formation and lipid peroxidation and inhibition of GPX4 activity. Furthermore, the specific design of the study identified that Fe2+ and Fe3+ ion preferred anticancer mechanisms. Nanoparticles containing Fe2+ had a preference for ROS induced ferroptosis, whereas those containing Fe3+ had a preference for lipid peroxidation-dependent ferroptosis [73].
Recently, nanozymes with iron containing nanoparticles have been demonstrated to be good tools for cancer therapy. Nanozymes are nanomaterials exhibiting intrinsic enzyme-like properties. The peroxidase-like activity of iron containing nanoparticles was first reported in 2007 [74]. To date, iron containing nanozymes with various enzyme mimetic properties have been reported, such as catalase, peroxidase, or SOD-like activities. The classification of nanozymes, together with their catalytic properties and application, have been recently described in an excellent review by Huang and colleagues [75]. One example of nanozymes used for the anticancer strategies, is the recently developed nanozymes biodegradable in the acidic environment with peroxidase-like catalytic activity having microwave enhancing dynamic therapy (MEDT) and microwave thermal therapy (MTT) effects [76]. Microwave irradiation accelerated ●OH generation in the tumor microenvironment. Novel nanozymes, under microwave irradiation, were able to locally increase temperature, as well as promote ROS formation, in particular ●OH, leading to malignant cell destruction and inhibition of a tumor.
The scientific community is continuously making efforts to improve current, or developing novel more efficient, nanosystems for oncotherapy. Table 1 highlights the recent animal studies with iron nanodevices for cancer therapy by altering cellular redox homeostasis.
Table 1.
Recent animal studies with variety of iron containing nanoparticles for cancer therapy by altering cellular redox homeostasis.
| Tumor Type | Animal Tumor Model | Type of Nanoparticles | Reported Therapy | Effect | Ref. |
|---|---|---|---|---|---|
| Breast cancer | Breast tumor xenograft mouse model | RLR nanoparticle composed of Fe3O4 nanoparticles and a nanoflower-like MnO2 shell | Chemodynamic therapy | Nanoparticles are degraded under acidic environment and excessive GSH yielding accelerated ROS production and tumoricidal effect. | [64] |
| Breast cancer | 4T1 tumor-bearing mice | Fe3O4-Pd Janus nanoparticles (JNPs) | Simultaneous magnetic-photo hyperthermia and chemodynamic therapy | Dual exposure of nanoparticles to AMF and laser irradiation induces enhanced temperature and ROS generation that are via Fenton reaction converted to OH●. | [77] |
| Breast cancer | 4T1 tumor-bearing mice | α-Fe2O3 nanoparticles coated with ultrasmall gold nanoseeds | Magnetic resonance imaging, photothermal therapy and radiosensitization | Upon NIR irradiation nanoparticles showed enhanced photothermal therapy and sensitized radiotherapy by inducing ROS formation and tumor inhibition. | [66] |
| Breast cancer | 4T1 tumor-bearing mice | Ultrasmall PEG-modified polydopamine nanoparticles containing Fe2+/3+ | Chemotherapy, Ferroptosis therapy | Fe2+ containing nanoparticles induce ROS dependent ferroptosis while Fe2+ containing nanoparticles induce lipid peroxidation-dependent ferroptosis. | [73] |
| Breast cancer | 4T1 tumor-bearing mice | Nanoparticles porphyrin-based metal–organic framework and MnFe2O4 nanoparticles as the nanoenzyme | Enhanced Photodynamic Therapy | Nanodevice exhibits catalase-like and GPX-like activity, In the tumor, upon irradiation, continuously promotes ROS formation via Fenton reaction, and reduces GSH modulating tumor microenvironment. | [78] |
| Breast cancer | 4T1 tumor-bearing mice | Silica nanoparticles with MnO2 nanoparticles and FeCO | Synergistic Gas therapy (GT) and chemodynamic therapy (CDT) | Under acidic environment MnO2 promotes ROS that further triggers decomposition of FeCO into CO. | [79] |
| Breast cancer | MCF-7 tumor-bearing mice | Nanogel loaded with magnetic IONPs and 10-hydroxy camptothecin | Enhanced photothermal-chemotherapy | Nanogel represents a good anticancer drug delivery system and can also serve as nanocarrier for photothermal therapy due to its absorption at NIR region. Furthermore, magnetic IONPs in the presence of macrophages promote ROS formation. | [80] |
| Breast cancer | 4T1 tumor-bearing mice | Nanosystem containing Fe(OH)3 modified upconversion nanoparticles | Synergetic chemo- and photodynamic therapy | INR irradiation promotes ROS formation in cancer cells. | [81] |
| Breast cancer | 4T1 tumor-bearing mice | IONPs modified with glucose oxidase and polydopamine | Photothermal therapy | NIR irradiation induces heat generation and formation of H2O2. H2O2 is then via Fenton reaction converted to OH● inducing apoptosis of cancer cells. | [82] |
| Breast cancer | 4T1 tumor-bearing mice | ROS nanoreactor based on core-shell-structured iron carbide nanoparticles | Magnetic Resonance Imaging Guided Cancer Therapy | In the acidic tumor microenvironment Fe2+ are released in acidic environments where, via Fenton reaction, generate OH● and inhibit tumor. | [83] |
| Breast cancer | 4T1 tumor-bearing mice | FeOOH nanoparticles coated with poly(norepinephrine) and loaded with artemisinin (Art) | Photothermal-chemical combination therapy | Exposure to NIR promotes heat generation and synchronous release of iron ions and Art in the acidic tumor microenvironment promotes generation of ROS and subsequent generation of OH● via Fenton reaction having high toxicity for tumor and low for normal tissue. | [84] |
| Breast cancer | 4T1 tumor-bearing mice | Mitochondrial membrane targeted nanophotosensitizer complex containing SPION and sorafenib | Ferroptosis as cancer therapy | Activated nanoparticles consume GSH, induce excessive ROS production and release SPION and sorafenib, promoting ferroptosis. Efficacy was also shown for the drug resistant in vitro model. | [43] |
| Breast cancer | 4T1 tumor-bearing mice | Mitochondrial membrane targeted nanophotosensitizer complex containing magnetic IONPs and sorafenib | Ferroptosis as cancer therapy | Activated nanoparticles downregulate GPX-4 and xCT inducing ferroptosis. | [44] |
| ER+ breast cancer | MCF7 tumor xenograft model Balbc | Drug-organics-inorganics self-assembled (DFTA) nanosystem with DOX, FeCl3 and tannic acid | Chemotherapy, Photothermal therapy and Ferroptosis therapy | Photothermal excitation triggers DOX release, activates SOD-like reaction and reduces GSH through excessive ROS production. | [72] |
| Glioma | Ectopic glioma tumor-bearing mice | Fe3O4-IR806 superparticles | Photothermal-photodynamic therapy | Photothermal conversion efficacy upon NIR irradiation was enhanced and promoted excessive ROS formation exhibiting tumor toxicity. | [85] |
| Hepato-cellular carcinoma | H22-tumor bearing mice and HepG2 tumor-bearing nude mice | Nanozymes containing Fe-metal organic framework nanoparticles | Microwave enhancing dynamic therapy, Microwave thermal therapy | Microwave irradiation promotes excessive ROS formation, in particular OH●, inducing cell death. In the presence of gold nanoclusters, the same can have application in imaging and microwave thermal therapy. | [76] |
| Hepato-cellular carcinoma | H22-tumor bearing mice and HepG2 tumor-bearing nude mice | PEG-modified nanoparticles loaded with photosensitizer and MnFe2O4 and silica upconversion nanoparticles | Photodynamic therapy | Loading efficiency of photosensitizer is increased NIR irradiation activates luminescence form upconversion nanoparticles yielding activation of photosensitizer and consequent ROS formation that take part in Fenton reaction eliciting tumoricidal effect. | [46] |
| Lung adenocarcima | A549 tumor-bearing nude mice | Modified IONPs with β-lapachone encapsulated in the nanostructure formed by H2O2-responsive polyprodrug and pH-responsive polymer (LaCIONPs) | Chemo/chemodynamic combination therapy | Acidic environment of tumor cells triggers LaCIONPs decomposition triggering excessive H2O2. H2O2 further via Fenton reaction produces OH● and also activates the release of drug eliciting tumoricidal effect. | [86] |
| Prostatic cancer | PC3 tumor-bearing nude mice | γ-Fe2O3 with copper sulfide shell | Photothermal Therapy, Magnetic Hyperthermia and Photodynamic Therapy | NIR exposure and magnetic stimulation promotes heat generation and ROS formation with tumoricidal effects. | [68] |
6. Approaches to Attenuate the Effects of Iron-Containing Nanoparticles on Healthy Cells and Tissue
In addition to the beneficial anticancer activities of iron-containing nanoparticles, one should also consider the potential adverse effects for healthy tissue. Toxicological potential of a variety nanoparticles [87], including the ones containing iron, have been reported [88]. Most of the metal nanoparticles induce generation of ROS and affect cellular redox mechanisms that could lead to their genotoxicity [89]. Iron-containing nanoparticles were shown to induce oxidative stress and dermal toxicity in vitro [88]. Janus Fe3O4-TiO4 nanoparticles, a potential agent for magnetic resonance imaging and PDT, when used at a higher dose (25 μg/cm2), alter cellular redox homeostasis of normal hepatic cells inducing lipid peroxidation while decreasing cell viability and ATP levels. However, if used at a lower dose (6.25 μg/cm2), such effects are not observed [90]. Additionally, particle size and different coatings on their surface can be detrimental for their activity [91]. Therefore, it is of crucial importance to develop strategies that will attenuate the adverse effects of iron containing nanoparticles for healthy tissue. Moreover, one should bear in mind that cytotoxicity of nanomaterials is dose dependent and concentrations that showed beneficial effects in vitro could have no effect or adverse effects in vivo and could also differ depending on the target tissue. Supplementation with antioxidants, such as thymoquinone, showed promising effects both in vitro and in vivo [92]. Indocyanine green used for PDT was found to be more stable and safer for healthy tissue when loaded to 3-aminopropyltrimethoxysilane coated cationic SPIONs and used as PTT and PDT dual therapy [77]. Functionalization of the IONPs surface with small molecules or polymers could improve IONPs stability and biocompatibility [1,93]. Functionalization can also improve or add new properties to IONPs such as antioxidant and antimicrobial activity [94], enabling thermal therapy [93], or promoting ROS-induced anticancer activity [95].
7. Conclusions
Iron is necessary for numerous physiological processes but could also be an important trigger of oxidative stress, especially of lipid peroxidation, thus, contributing either to carcinogenesis or to toxicity of anticancer treatments. Hence, ferroptosis acts as a potential natural anticancer defense mechanism that uses iron to generate ROS and lipid peroxidation and eventually destroy cancer cells. Furthermore, recent findings indicate that the end products of lipid peroxidation, notably 4-HNE, could be generated by non-malignant cells as a defense mechanism against cancer complementary to ferroptosis. Although numerous promising iron-containing nanoparticles were developed for experimental radio-, chemo-, and photodynamic, as well as magnetic dynamic nanosystems that induce oxidative stress and ferroptosis, they do not exploit selective cytotoxicity of 4-HNE as a potentially novel anticancer activity principle. Namely, binding of 4-HNE to (extra)cellular proteins could result in persistent lipid peroxidation selectively destroying cancer cells due to the differences in lipid metabolism and antioxidant systems between cancer and non-malignant cells. Therefore, nanosystems should be developed that use iron to trigger ferroptosis and target lipid peroxidation as anticancer options of integrative biomedicine utilizing the benefits of advanced nanotechnologies and of natural anticancer defense mechanisms of the host.
Author Contributions
Conceptualization, M.J. and N.Z.; writing—original draft preparation, M.J., S.B.S., and N.Z.; writing—review and editing, M.J., S.B.S. and N.Z.; visualization, M.J. and N.Z.; supervision, M.J. and N.Z.; funding acquisition, N.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Ansari S.A.M.K., Ficiarà E., Ruffinatti F.A., Stura I., Argenziano M., Abollino O., Cavalli R., Guiot C., D’Agata F. Magnetic Iron Oxide Nanoparticles: Synthesis, Characterization and Functionalization for Biomedical Applications in the Central Nervous System. Materials. 2019;12:465. doi: 10.3390/ma12030465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bobo D., Robinson K.J., Islam J., Thurecht K.J., Corrie S.R. Nanoparticle-Based Medicines: A Review of FDA-Approved Materials and Clinical Trials to Date. Pharm. Res. 2016;33:2373–2387. doi: 10.1007/s11095-016-1958-5. [DOI] [PubMed] [Google Scholar]
- 3.Sainz V., Conniot J., Matos A.I., Peres C., Zupancic E., Moura L., Silva L.C., Florindo H.F., Gaspar R.S. Regulatory aspects on nanomedicines. Biochem. Biophys. Res. Commun. 2015;468:504–510. doi: 10.1016/j.bbrc.2015.08.023. [DOI] [PubMed] [Google Scholar]
- 4.Kukulj S., Jaganjac M., Boranic M., Krizanac S., Santic Z., Poljak-Blazi M. Altered iron metabolism, inflammation, transferrin receptors, and ferritin expression in non-small-cell lung cancer. Med. Oncol. 2010;27:268–277. doi: 10.1007/s12032-009-9203-2. [DOI] [PubMed] [Google Scholar]
- 5.Wang Y., Yu L., Ding J., Chen Y. Iron Metabolism in Cancer. Int. J. Mol. Sci. 2018;20:95. doi: 10.3390/ijms20010095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lawen A., Lane D.J. Mammalian iron homeostasis in health and disease: Uptake, storage, transport, and molecular mechanisms of action. Antioxid. Redox Signal. 2013;18:2473–2507. doi: 10.1089/ars.2011.4271. [DOI] [PubMed] [Google Scholar]
- 7.Oexle H., Gnaiger E., Weiss G. Iron-dependent changes in cellular energy metabolism: Influence on citric acid cycle and oxidative phosphorylation. Biochim. Biophys. Acta. 1999;1413:99–107. doi: 10.1016/S0005-2728(99)00088-2. [DOI] [PubMed] [Google Scholar]
- 8.Stehling O., Lill R. The role of mitochondria in cellular iron-sulfur protein biogenesis: Mechanisms, connected processes, and diseases. Cold Spring Harb. Perspect. Biol. 2013;5:a011312. doi: 10.1101/cshperspect.a011312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Horowitz M.P., Greenamyre J.T. Mitochondrial iron metabolism and its role in neurodegeneration. J. Alzheimers Dis. 2010;20:S551–S568. doi: 10.3233/JAD-2010-100354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xu W., Barrientos T., Andrews N.C. Iron and copper in mitochondrial diseases. Cell Metab. 2013;17:319–328. doi: 10.1016/j.cmet.2013.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nandal A., Ruiz J.C., Subramanian P., Ghimire-Rijal S., Sinnamon R.A., Stemmler T.L., Bruick R.K., Philpott C.C. Activation of the HIF prolyl hydroxylase by the iron chaperones PCBP1 and PCBP2. Cell Metab. 2011;14:647–657. doi: 10.1016/j.cmet.2011.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Casey J.L., Koeller D.M., Ramin V.C., Klausner R.D., Harford J.B. Iron regulation of transferrin receptor mRNA levels requires iron-responsive elements and a rapid turnover determinant in the 3’ untranslated region of the mRNA. EMBO J. 1989;8:3693–3699. doi: 10.1002/j.1460-2075.1989.tb08544.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gunshin H., Allerson C.R., Polycarpou-Schwarz M., Rofts A., Rogers J.T., Kishi F., Hentze M.W., Rouault T.A., Andrews N.C., Hediger M.A. Iron-dependent regulation of the divalent metal ion transporter. FEBS Lett. 2001;509:309–316. doi: 10.1016/S0014-5793(01)03189-1. [DOI] [PubMed] [Google Scholar]
- 14.Kim H.Y., LaVaute T., Iwai K., Klausner R.D., Rouault T.A. Identification of a conserved and functional iron-responsive element in the 5’-untranslated region of mammalian mitochondrial aconitase. J. Biol. Chem. 1996;271:24226–24230. doi: 10.1074/jbc.271.39.24226. [DOI] [PubMed] [Google Scholar]
- 15.Sammarco M.C., Ditch S., Banerjee A., Grabczyk E. Ferritin L and H subunits are differentially regulated on a post-transcriptional level. J. Biol. Chem. 2008;283:4578–4587. doi: 10.1074/jbc.M703456200. [DOI] [PubMed] [Google Scholar]
- 16.Li Y., Lin L., Li Z., Ye X., Xiong K., Aryal B., Xu Z., Paroo Z., Liu Q., He C., et al. Iron homeostasis regulates the activity of the microRNA pathway through poly(C)-binding protein 2. Cell Metab. 2012;15:895–904. doi: 10.1016/j.cmet.2012.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tisma V.S., Basta-Juzbasic A., Jaganjac M., Brcic L., Dobric I., Lipozencic J., Tatzber F., Zarkovic N., Poljak-Blazi M. Oxidative stress and ferritin expression in the skin of patients with rosacea. J. Am. Acad. Dermatol. 2009;60:270–276. doi: 10.1016/j.jaad.2008.10.014. [DOI] [PubMed] [Google Scholar]
- 18.Jaganjac M., Tirosh O., Cohen G., Sasson S., Zarkovic N. Reactive aldehydes--second messengers of free radicals in diabetes mellitus. Free Radic. Res. 2013;47:39–48. doi: 10.3109/10715762.2013.789136. [DOI] [PubMed] [Google Scholar]
- 19.Jaganjac M., Cipak A., Schaur R.J., Zarkovic N. Pathophysiology of neutrophil-mediated extracellular redox reactions. Front. Biosci. (Landmark Ed.) 2016;21:839–855. doi: 10.2741/4423. [DOI] [PubMed] [Google Scholar]
- 20.Jaganjac M., Poljak-Blazi M., Zarkovic K., Schaur R.J., Zarkovic N. The involvement of granulocytes in spontaneous regression of Walker 256 carcinoma. Cancer Lett. 2008;260:180–186. doi: 10.1016/j.canlet.2007.10.039. [DOI] [PubMed] [Google Scholar]
- 21.Poljak-Blazi M., Jaganjac M., Sabol I., Mihaljevic B., Matovina M., Grce M. Effect of ferric ions on reactive oxygen species formation, cervical cancer cell lines growth and E6/E7 oncogene expression. Toxicol. In Vitro. 2011;25:160–166. doi: 10.1016/j.tiv.2010.10.013. [DOI] [PubMed] [Google Scholar]
- 22.Xiong S., She H., Takeuchi H., Han B., Engelhardt J.F., Barton C.H., Zandi E., Giulivi C., Tsukamoto H. Signaling role of intracellular iron in NF-kappaB activation. J. Biol. Chem. 2003;278:17646–17654. doi: 10.1074/jbc.M210905200. [DOI] [PubMed] [Google Scholar]
- 23.Poljak-Blazi M., Jaganjac M., Mustapic M., Pivac N., Muck-Seler D. Acute immunomodulatory effects of iron polyisomaltosate in rats. Immunobiology. 2009;214:121–128. doi: 10.1016/j.imbio.2008.07.005. [DOI] [PubMed] [Google Scholar]
- 24.Ganini D., Santos J.H., Bonini M.G., Mason R.P. Switch of Mitochondrial Superoxide Dismutase into a Prooxidant Peroxidase in Manganese-Deficient Cells and Mice. Cell Chem. Biol. 2018;25:413–425.e6. doi: 10.1016/j.chembiol.2018.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Winterbourn C.C. Toxicity of iron and hydrogen peroxide: The Fenton reaction. Toxicol. Lett. 1995;82–83:969–974. doi: 10.1016/0378-4274(95)03532-X. [DOI] [PubMed] [Google Scholar]
- 26.Stojanović S., Stanić D., Nikolić M., Spasić M., Niketić V. Iron catalyzed conversion of NO into nitrosonium (NO+) and nitroxyl (HNO/NO−) species. Nitric Oxide. 2004;11:256–262. doi: 10.1016/j.niox.2004.09.007. [DOI] [PubMed] [Google Scholar]
- 27.O’Donnell V.B., Eiserich J.P., Chumley P.H., Jablonsky M.J., Krishna N.R., Kirk M., Barnes S., Darley-Usmar V.M., Freeman B.A. Nitration of unsaturated fatty acids by nitric oxide-derived reactive nitrogen species peroxynitrite, nitrous acid, nitrogen dioxide, and nitronium ion. Chem. Res. Toxicol. 1999;12:83–92. doi: 10.1021/tx980207u. [DOI] [PubMed] [Google Scholar]
- 28.Beckman J.S., Ischiropoulos H., Zhu L., van der Woerd M., Smith C., Chen J., Harrison J., Martin J.C., Tsai M. Kinetics of superoxide dismutase- and iron-catalyzed nitration of phenolics by peroxynitrite. Arch Biochem. Biophys. 1992;298:438–445. doi: 10.1016/0003-9861(92)90432-V. [DOI] [PubMed] [Google Scholar]
- 29.Rubbo H., Radi R. Protein and lipid nitration: Role in redox signaling and injury. Biochim. Biophys. Acta. 2008;1780:1318–1324. doi: 10.1016/j.bbagen.2008.03.007. [DOI] [PubMed] [Google Scholar]
- 30.Elrayess M.A., Almuraikhy S., Kafienah W., Al-Menhali A., Al-Khelaifi F., Bashah M., Zarkovic K., Zarkovic N., Waeg G., Alsayrafi M., et al. 4-hydroxynonenal causes impairment of human subcutaneous adipogenesis and induction of adipocyte insulin resistance. Free Radic. Biol. Med. 2017;104:129–137. doi: 10.1016/j.freeradbiomed.2017.01.015. [DOI] [PubMed] [Google Scholar]
- 31.Al-Menhali A.S., Banu S., Angelova P.R., Barcaru A., Horvatovich P., Abramov A.Y., Jaganjac M. Lipid peroxidation is involved in calcium dependent upregulation of mitochondrial metabolism in skeletal muscle. Biochim. Biophys. Acta Gen. Subj. 2020;1864:129487. doi: 10.1016/j.bbagen.2019.129487. [DOI] [PubMed] [Google Scholar]
- 32.Dixon S.J., Lemberg K.M., Lamprecht M.R., Skouta R., Zaitsev E.M., Gleason C.E., Patel D.N., Bauer A.J., Cantley A.M., Yang W.S., et al. Ferroptosis: An iron-dependent form of nonapoptotic cell death. Cell. 2012;149:1060–1072. doi: 10.1016/j.cell.2012.03.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yang W.S., SriRamaratnam R., Welsch M.E., Shimada K., Skouta R., Viswanathan V.S., Cheah J.H., Clemons P.A., Shamji A.F., Clish C.B., et al. Regulation of ferroptotic cancer cell death by GPX4. Cell. 2014;156:317–331. doi: 10.1016/j.cell.2013.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stockwell B.R., Friedmann Angeli J.P., Bayir H., Bush A.I., Conrad M., Dixon S.J., Fulda S., Gascón S., Hatzios S.K., Kagan V.E., et al. Ferroptosis: A Regulated Cell Death Nexus Linking Metabolism, Redox Biology, and Disease. Cell. 2017;171:273–285. doi: 10.1016/j.cell.2017.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hassannia B., Vandenabeele P., Vanden Berghe T. Targeting Ferroptosis to Iron Out Cancer. Cancer Cell. 2019;35:830–849. doi: 10.1016/j.ccell.2019.04.002. [DOI] [PubMed] [Google Scholar]
- 36.Shimada K., Hayano M., Pagano N.C., Stockwell B.R. Cell-Line Selectivity Improves the Predictive Power of Pharmacogenomic Analyses and Helps Identify NADPH as Biomarker for Ferroptosis Sensitivity. Cell Chem. Biol. 2016;23:225–235. doi: 10.1016/j.chembiol.2015.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Toyokuni S., Ito F., Yamashita K., Okazaki Y., Akatsuka S. Iron and thiol redox signaling in cancer: An exquisite balance to escape ferroptosis. Free Radic. Biol. Med. 2017;108:610–626. doi: 10.1016/j.freeradbiomed.2017.04.024. [DOI] [PubMed] [Google Scholar]
- 38.Mou Y., Wang J., Wu J., He D., Zhang C., Duan C., Li B. Ferroptosis, a new form of cell death: Opportunities and challenges in cancer. J. Hematol. Oncol. 2019;12:34. doi: 10.1186/s13045-019-0720-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lu B., Chen X.B., Ying M.D., He Q.J., Cao J., Yang B. The Role of Ferroptosis in Cancer Development and Treatment Response. Front. Pharmacol. 2018;8:992. doi: 10.3389/fphar.2017.00992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Alarifi S., Ali D., Alkahtani S., Alhader M.S. Iron oxide nanoparticles induce oxidative stress, DNA damage, and caspase activation in the human breast cancer cell line. Biol. Trace Elem. Res. 2014;159:416–424. doi: 10.1007/s12011-014-9972-0. [DOI] [PubMed] [Google Scholar]
- 41.Cellai F., Munnia A., Viti J., Doumett S., Ravagli C., Ceni E., Mello T., Polvani S., Giese R.W., Baldi G., et al. Magnetic Hyperthermia and Oxidative Damage to DNA of Human Hepatocarcinoma Cells. Int. J. Mol. Sci. 2017;18:939. doi: 10.3390/ijms18050939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sreeja S., Krishnan Nair C.K. Tumor control by hypoxia-specific chemotargeting of iron-oxide nanoparticle - Berberine complexes in a mouse model. Life Sci. 2018;195:71–80. doi: 10.1016/j.lfs.2017.12.036. [DOI] [PubMed] [Google Scholar]
- 43.Sang M., Luo R., Bai Y., Dou J., Zhang Z., Liu F., Feng F., Xu J., Liu W. Mitochondrial membrane anchored photosensitive nano-device for lipid hydroperoxides burst and inducing ferroptosis to surmount therapy-resistant cancer. Theranostics. 2019;9:6209–6223. doi: 10.7150/thno.36283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sang M., Luo R., Bai Y., Dou J., Zhang Z., Liu F., Feng F., Liu W. BHQ-Cyanine-Based “Off-On” Long-Circulating Assembly as a Ferroptosis Amplifier for Cancer Treatment: A Lipid-Peroxidation Burst Device. ACS Appl. Mater. Interfaces. 2019;11:42873–42884. doi: 10.1021/acsami.9b12469. [DOI] [PubMed] [Google Scholar]
- 45.Hou H., Huang X., Wei G., Xu F., Wang Y., Zhou S. Fenton Reaction-Assisted Photodynamic Therapy for Cancer with Multifunctional Magnetic Nanoparticles. ACS Appl. Mater. Inter. 2019;11:29579–29592. doi: 10.1021/acsami.9b09671. [DOI] [PubMed] [Google Scholar]
- 46.Ding B., Shao S., Xiao H., Sun C., Cai X., Jiang F., Zhao X., Ma P., Lin J. MnFe2O4-decorated large-pore mesoporous silica-coated upconversion nanoparticles for near-infrared light-induced and O2 self-sufficient photodynamic therapy. Nanoscale. 2019;11:14654–14667. doi: 10.1039/C9NR04858H. [DOI] [PubMed] [Google Scholar]
- 47.Horák D., Pustovyy V.I., Babinskyi A.V., Palyvoda O.M., Chekhun V.F., Todor I.N., Kuzmenko O.I. Enhanced antitumor activity of surface-modified iron oxide nanoparticles and an α-tocopherol derivative in a rat model of mammary gland carcinosarcoma. Int. J. Nanomed. 2017;12:4257–4268. doi: 10.2147/IJN.S137574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zarkovic N., Cipak A., Jaganjac M., Borovic S., Zarkovic K. Pathophysiological relevance of aldehydic protein modifications. J. Proteom. 2013;92:239–247. doi: 10.1016/j.jprot.2013.02.004. [DOI] [PubMed] [Google Scholar]
- 49.Jaganjac M., Milkovic L., Gegotek A., Cindric M., Zarkovic K., Skrzydlewska E., Zarkovic N. The relevance of pathophysiological alterations in redox signaling of 4-hydroxynonenal for pharmacological therapies of major stress-associated diseases. Free Radic. Biol. Med. 2019 doi: 10.1016/j.freeradbiomed.2019.11.023. [DOI] [PubMed] [Google Scholar]
- 50.Borović Šunjić S., Čipak A., Rabuzin F., Wildburger R., Žarković N. The Influence of 4-Hydroxy-2-nonenal on Proliferation, Differentiation and Apoptosis of Human Osteosarcoma Cells. Biofactors. 2005;24:141–148. doi: 10.1002/biof.5520240117. [DOI] [PubMed] [Google Scholar]
- 51.Mouthuy P.A., Snelling S.J.B., Dakin S.G., Milković L., Čipak Gašparović A., Carr A.J., Žarković N. Biocompatibility of implantable materials: An oxidative stress viewpoint. Biomaterials. 2016;109:55–68. doi: 10.1016/j.biomaterials.2016.09.010. [DOI] [PubMed] [Google Scholar]
- 52.Sovic A., Borović S., Lončarić I., Kreuzer T., Žarković K., Vuković T., Wäg G., Hraščan R., Wintersteiger R., Klinger R., et al. The carcinostatic and proapoptotic potential of 4-Hydroxynonenal in HeLa cells is associated with its conjugation to cellular proteins. Anticancer Res. 2001;21:1997–2004. [PubMed] [Google Scholar]
- 53.Milkovic L., Cipak Gasparovic A., Zarkovic N. Overview on major lipid peroxidation bioactive factor 4-hydroxynonenal as pluripotent growth regulating factor. Free Radic. Res. 2015;49:850–860. doi: 10.3109/10715762.2014.999056. [DOI] [PubMed] [Google Scholar]
- 54.Cesar V., Jozić I., Begović L., Vuković T., Mlinarić S., Lepeduš H., Borović Šunjić S., Žarković N. Cell-Type-Specific Modulation of Hydrogen Peroxide Cytotoxicity and 4-Hydroxynonenal Binding to Human Cellular Proteins In Vitro by Antioxidant Aloe vera Extract. Antioxidants. 2018;7:125. doi: 10.3390/antiox7100125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Borović S., Čipak A., Meinitzer A., Kejla Z., Perovic D., Waeg G., Žarković N. Differential effect of 4-hydroxynonenal on normal and malignant mesenchimal cells. Redox Rep. 2007;207:50–54. doi: 10.1179/135100007X162194. [DOI] [PubMed] [Google Scholar]
- 56.Zarkovic K., Jakovcevic A., Zarkovic N. Contribution of the HNE-Immunohistochemistry to Modern Pathological Concepts of Major Human Diseases. Free Radic. Biol. Med. 2017;111:110–125. doi: 10.1016/j.freeradbiomed.2016.12.009. [DOI] [PubMed] [Google Scholar]
- 57.Bauer G., Zarkovic N. Revealing mechanisms of selective, concentration-dependent potentials of 4-hydroxy-2-nonenal to induce apoptosis in cancer cells through inactivation of membrane-associated catalase. Free Radic. Biol. Med. 2015;81:128–144. doi: 10.1016/j.freeradbiomed.2015.01.010. [DOI] [PubMed] [Google Scholar]
- 58.Zhong H., Xiao M., Zarkovic K., Zhu M., Sa R., Lu L., Tao Y., Chen Q., Xia L., Cheng S., et al. Mitochondrial Control of Apoptosis through Modulation of Cardiolipin Oxidation in Hepatocellular Carcinoma: A Novel Link between Oxidative Stress and Cancer. Free Radic. Biol. Med. 2017;176:67–76. doi: 10.1016/j.freeradbiomed.2016.10.494. [DOI] [PubMed] [Google Scholar]
- 59.Piskač Živković N., Petrovečki M., Tomasović Lončarić Č., Nikolić I., Waeg G., Jaganjac M., Žarković K., Žarković N. Positron Emission Tomography-Computed Tomography and 4-Hydroxynonenal-histidine Immunohistochemistry Reveal Differential Onset of Lipid Peroxidation in Primary Lung Cancer and in Pulmonary Metastasis of Remote Malignancies. Redox Biol. 2017;11:600–605. doi: 10.1016/j.redox.2017.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Žarković N., Žarković K., Kralj M., Borović S., Sabolović S., Poljak Blaži M., Čipak A., Pavelić K. Anticancer and antioxidative effects of micronized zeolite clinoptilolite. Anticancer Res. 2003;23:1589–1596. [PubMed] [Google Scholar]
- 61.Wang L., Huo M., Chen Y., Shi J. Tumor Microenvironment-Enabled Nanotherapy. Adv. Healthc. Mater. 2018;7:e1701156. doi: 10.1002/adhm.201701156. [DOI] [PubMed] [Google Scholar]
- 62.Tang Z., Liu Y., He M., Bu W. Chemodynamic Therapy: Tumour Microenvironment-Mediated Fenton and Fenton-like Reactions. Angew. Chem. Int. Ed. Engl. 2019;58:946–956. doi: 10.1002/anie.201805664. [DOI] [PubMed] [Google Scholar]
- 63.Jasim K.A., Gesquiere A.J. Ultrastable and Biofunctionalizable Conjugated Polymer Nanoparticles with Encapsulated Iron for Ferroptosis Assisted Chemodynamic Therapy. Mol. Pharm. 2019 doi: 10.1021/acs.molpharmaceut.9b00737. in press. [DOI] [PubMed] [Google Scholar]
- 64.Wang S., Yang L., Cho H.Y., Dean Chueng S.T., Zhang H., Zhang Q., Lee K.B. Programmed degradation of a hierarchical nanoparticle with redox and light responsivity for self-activated photo-chemical enhanced chemodynamic therapy. Biomaterials. 2019;224:119498. doi: 10.1016/j.biomaterials.2019.119498. [DOI] [PubMed] [Google Scholar]
- 65.Ma X., Wang Y., Liu X.-L., Ma H., Li G., Li Y., Gao F., Peng M., Fan H.M., Liang X.-J. Fe3O4-Pd Janus nanoparticles with amplified dual-mode hyperthermia and enhanced ROS generation for breast cancer treatment. Nanoscale Horiz. 2019;4:1450–1459. doi: 10.1039/C9NH00233B. [DOI] [Google Scholar]
- 66.Zhong D., Zhao J., Li Y., Qiao Y., Wei Q., He J., Xie T., Li W., Zhou M. Laser-triggered aggregated cubic α-Fe2O3@Au nanocomposites for magnetic resonance imaging and photothermal/enhanced radiation synergistic therapy. Biomaterials. 2019;219:119369. doi: 10.1016/j.biomaterials.2019.119369. [DOI] [PubMed] [Google Scholar]
- 67.Johannsen M., Thiesen B., Wust P., Jordan A. Magnetic nanoparticle hyperthermia for prostate cancer. Int. J. Hyperth. 2010;26:790–795. doi: 10.3109/02656731003745740. [DOI] [PubMed] [Google Scholar]
- 68.Curcio A., Silva A.K.A., Cabana S., Espinosa A., Baptiste B., Menguy N., Wilhelm C., Abou-Hassan A. Iron Oxide Nanoflowers @ CuS Hybrids for Cancer Tri-Therapy: Interplay of Photothermal Therapy, Magnetic Hyperthermia and Photodynamic Therapy. Theranostics. 2019;9:1288–1302. doi: 10.7150/thno.30238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Rego G.N.A., Mamani J.B., Souza T.K.F., Nucci M.P., Silva H.R.D., Gamarra L.F. Therapeutic evaluation of magnetic hyperthermia using Fe3O4-aminosilane-coated iron oxide nanoparticles in glioblastoma animal model. Einstein (Sao Paulo) 2019;17:eAO4786. doi: 10.31744/einstein_journal/2019AO4786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Rego G.N.A., Nucci M.P., Mamani J.B., Oliveira F.A., Marti L.C., Filgueiras I.S., Ferreira J.M., Real C.C., Faria D.P., Espinha P.L., et al. Therapeutic Efficiency of Multiple Applications of Magnetic Hyperthermia Technique in Glioblastoma Using Aminosilane Coated Iron Oxide Nanoparticles: In Vitro and In Vivo Study. Int. J. Mol. Sci. 2020;21:958. doi: 10.3390/ijms21030958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Dulińska-Litewka J., Łazarczyk A., Hałubiec P., Szafrański O., Karnas K., Karewicz A. Superparamagnetic Iron Oxide Nanoparticles-Current and Prospective Medical Applications. Materials. 2019;12:617. doi: 10.3390/ma12040617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Xiong H., Wang C., Wang Z., Jiang Z., Zhou J., Yao J. Intracellular cascade activated nanosystem for improving ER+ breast cancer therapy through attacking GSH-mediated metabolic vulnerability. J. Control Release. 2019;309:145–157. doi: 10.1016/j.jconrel.2019.07.029. [DOI] [PubMed] [Google Scholar]
- 73.Chen L., Lin Z., Liu L., Zhang X., Shi W., Ge D., Sun Y. Fe2+/Fe3+ Ions Chelated with Ultrasmall Polydopamine Nanoparticles Induce Ferroptosis for Cancer Therapy. ACS Biomater. Sci. Eng. 2019;5:4861–4869. doi: 10.1021/acsbiomaterials.9b00461. [DOI] [PubMed] [Google Scholar]
- 74.Gao L., Zhuang J., Nie L., Zhang J., Zhang Y., Gu N., Wang T., Feng J., Yang D., Perrett S., et al. Intrinsic peroxidase-like activity of ferromagnetic nanoparticles. Nat. Nanotechnol. 2007;2:577–583. doi: 10.1038/nnano.2007.260. [DOI] [PubMed] [Google Scholar]
- 75.Huang Y., Ren J., Qu X. Nanozymes: Classification, Catalytic Mechanisms, Activity Regulation, and Applications. Chem. Rev. 2019;119:4357–4412. doi: 10.1021/acs.chemrev.8b00672. [DOI] [PubMed] [Google Scholar]
- 76.Ma X., Ren X., Guo X., Fu C., Wu Q., Tan L., Li H., Zhang W., Chen X., Zhong H., et al. Multifunctional iron-based Metal-Organic framework as biodegradable nanozyme for microwave enhancing dynamic therapy. Biomaterials. 2019;214:119223. doi: 10.1016/j.biomaterials.2019.119223. [DOI] [PubMed] [Google Scholar]
- 77.Bilici K., Muti A., Sennaroğlu A., Yagci Acar H. Indocyanine green loaded APTMS coated SPIONs for dual phototherapy of cancer. J. Photochem. Photobiol. B. 2019;201:111648. doi: 10.1016/j.jphotobiol.2019.111648. [DOI] [PubMed] [Google Scholar]
- 78.Yin S.-Y., Song G., Yang Y., Zhao Y., Wang P., Zhu L.-M., Yin X., Zhang X.-B. Persistent Regulation of Tumor Microenvironment via Circulating Catalysis of MnFe2O4@Metal–Organic Frameworks for Enhanced Photodynamic Therapy. Adv. Funct. Mater. 2019;29:1901417. doi: 10.1002/adfm.201901417. [DOI] [Google Scholar]
- 79.Zhao B., Zhao P., Jin Z., Fan M., Meng J., He Q. Programmed ROS/CO-releasing nanomedicine for synergetic chemodynamic-gas therapy of cancer. J. Nanobiotechnol. 2019;17:75. doi: 10.1186/s12951-019-0507-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Li W., Xue B., Shi K., Qu Y., Chu B., Qian Z. Magnetic iron oxide nanoparticles/10-hydroxy camptothecin co-loaded nanogel for enhanced photothermal-chemo therapy. Appl. Mater. Today. 2019;14:84–95. doi: 10.1016/j.apmt.2018.11.008. [DOI] [Google Scholar]
- 81.Wu X., Yan P., Ren Z., Wang Y., Cai X., Li X., Deng R., Han G. Ferric Hydroxide-Modified Upconversion Nanoparticles for 808 nm NIR-Triggered Synergetic Tumor Therapy with Hypoxia Modulation. ACS Appl. Mater. Interfaces. 2019;11:385–393. doi: 10.1021/acsami.8b18427. [DOI] [PubMed] [Google Scholar]
- 82.Zhang T., Li Y., Hong W., Chen Z., Peng P., Yuan S., Qu J., Xiao M., Xu L. Glucose oxidase and polydopamine functionalized iron oxide nanoparticles: Combination of the photothermal effect and reactive oxygen species generation for dual-modality selective cancer therapy. J. Mater. Chem. B. 2019;7:2190–2200. doi: 10.1039/C8TB03320J. [DOI] [PubMed] [Google Scholar]
- 83.Yu J., Zhao F., Gao W., Yang X., Ju Y., Zhao L., Guo W., Xie J., Liang X.J., Tao X., et al. Magnetic Reactive Oxygen Species Nanoreactor for Switchable Magnetic Resonance Imaging Guided Cancer Therapy Based on pH-Sensitive Fe5C2@Fe3O4 Nanoparticles. ACS Nano. 2019;13:10002–10014. doi: 10.1021/acsnano.9b01740. [DOI] [PubMed] [Google Scholar]
- 84.He Z., Su H., Shen Y., Shi W., Liu X., Liu Y., Zhang F., Zhang Y., Sun Y., Ge D. Poly(norepinephrine)-coated FeOOH nanoparticles as carriers of artemisinin for cancer photothermal-chemical combination therapy. RSC Adv. 2019;9:9968–9982. doi: 10.1039/C9RA01289C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wang X., Li Z., Ding Y., Wang K., Xing Z., Sun X., Guo W., Hong X., Zhu X., Liu Y. Enhanced photothermal-photodynamic therapy for glioma based on near-infrared dye functionalized Fe3O4 superparticles. Chem. Eng. J. 2020;381:122693. doi: 10.1016/j.cej.2019.122693. [DOI] [Google Scholar]
- 86.Wang S., Wang Z., Yu G., Zhou Z., Jacobson O., Liu Y., Ma Y., Zhang F., Chen Z.Y., Chen X. Tumor-Specific Drug Release and Reactive Oxygen Species Generation for Cancer Chemo/Chemodynamic Combination Therapy. Adv. Sci. (Weinh.) 2019;6:1801986. doi: 10.1002/advs.201801986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Mauro M., Crosera M., Bianco C., Adami G., Montini T., Fornasiero P., Jaganjac M., Bovenzi M., Larese Filon F. Permeation of platinum and rhodium nanoparticles through intact and damaged human skin. J. Nanopart. Res. 2015;17:253. doi: 10.1007/s11051-015-3052-z. [DOI] [Google Scholar]
- 88.Murray A.R., Kisin E., Inman A., Young S.H., Muhammed M., Burks T., Uheida A., Tkach A., Waltz M., Castranova V., et al. Oxidative stress and dermal toxicity of iron oxide nanoparticles in vitro. Cell Biochem. Biophys. 2013;67:461–476. doi: 10.1007/s12013-012-9367-9. [DOI] [PubMed] [Google Scholar]
- 89.Mortezaee K., Najafi M., Samadian H., Barabadi H., Azarnezhad A., Ahmadi A. Redox interactions and genotoxicity of metal-based nanoparticles: A comprehensive review. Chem. Biol. Interact. 2019;312:108814. doi: 10.1016/j.cbi.2019.108814. [DOI] [PubMed] [Google Scholar]
- 90.Su H., Li Z., Lazar L., Alhamoud Y., Song X., Li J., Wang Y., Fiati Kenston S.S., Lqbal M.Z., Wu A., et al. In vitro evaluation of the toxicity and underlying molecular mechanisms of Janus Fe3O4-TiO2 nanoparticles in human liver cells. Environ. Toxicol. 2018;33:1078–1088. doi: 10.1002/tox.22631. [DOI] [PubMed] [Google Scholar]
- 91.Valdiglesias V., Kiliç G., Costa C., Fernández-Bertólez N., Pásaro E., Teixeira J.P., Laffon B. Effects of iron oxide nanoparticles: Cytotoxicity, genotoxicity, developmental toxicity, and neurotoxicity. Environ. Mol. Mutagen. 2015;56:125–148. doi: 10.1002/em.21909. [DOI] [PubMed] [Google Scholar]
- 92.Ansari M.O., Parveen N., Ahmad M.F., Wani A.L., Afrin S., Rahman Y., Jameel S., Khan Y.A., Siddique H.R., Tabish M., et al. Evaluation of DNA interaction, genotoxicity and oxidative stress induced by iron oxide nanoparticles both in vitro and in vivo: Attenuation by thymoquinone. Sci. Rep. 2019;9:6912. doi: 10.1038/s41598-019-43188-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Frimpong R.A., Hilt J.Z. Magnetic nanoparticles in biomedicine: Synthesis, functionalization and applications. Nanomedicine (Lond.) 2010;5:1401–1414. doi: 10.2217/nnm.10.114. [DOI] [PubMed] [Google Scholar]
- 94.Shah S.T., Yehya W.A., Saad O., Simarani K., Chowdhury Z., Alhadi A.A., Al-Ani L.A. Surface Functionalization of Iron Oxide Nanoparticles with Gallic Acid as Potential Antioxidant and Antimicrobial Agents. Nanomaterials. 2017;7:306. doi: 10.3390/nano7100306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Mai T., Hilt J.Z. Functionalization of iron oxide nanoparticles with small molecules and the impact on reactive oxygen species generation for potential cancer therapy. Colloid Surface A. 2019;576:9–14. doi: 10.1016/j.colsurfa.2019.05.003. [DOI] [Google Scholar]