Abstract
Reactive oxygen species (ROS) constitute a homeostatic rheostat that modulates signal transduction pathways controlling cell turnover. Most oncogenic pathways activated in cancer cells drive a sustained increase in ROS production, and cancer cells are strongly addicted to the increased activity of scavenging pathways to maintain ROS below levels that produce macromolecular damage and engage cell death pathways. Consistent with this notion, tumor cells are more vulnerable than their normal counterparts to pharmacological treatments that increase ROS production and inhibit ROS scavenging. In the present review, we discuss the recent advances in the development of integrated anticancer therapies based on nanoparticles engineered to kill cancer cells by raising their ROS setpoint. We also examine nanoparticles engineered to exploit the metabolic and redox alterations of cancer cells to promote site-specific drug delivery to cancer cells, thus maximizing anticancer efficacy while minimizing undesired side effects on normal tissues.
Keywords: nanoparticles, ROS, cancer therapy, redox homeostasis
1. Importance of Reactive Oxygen Species in Cancer Therapy
Selectively killing cancer cells while avoiding systemic toxicity is the primary challenge in cancer therapy. Standard chemotherapeutic drugs and radiotherapy often fail to eradicate tumors and do not discriminate between cancer and highly proliferating healthy cells. Furthermore, most drugs employed in standard chemotherapy are mutagenic and lead to the potential onset of secondary, therapy-induced malignancies in cancer survivors [1,2]. Even therapies designed to target specific signaling pathways altered in cancer cells are not devoid of undesired side effects. The development of novel strategies aimed at increasing the cancer-specific targeting and the therapeutic window of antineoplastic compounds is thus in high demand.
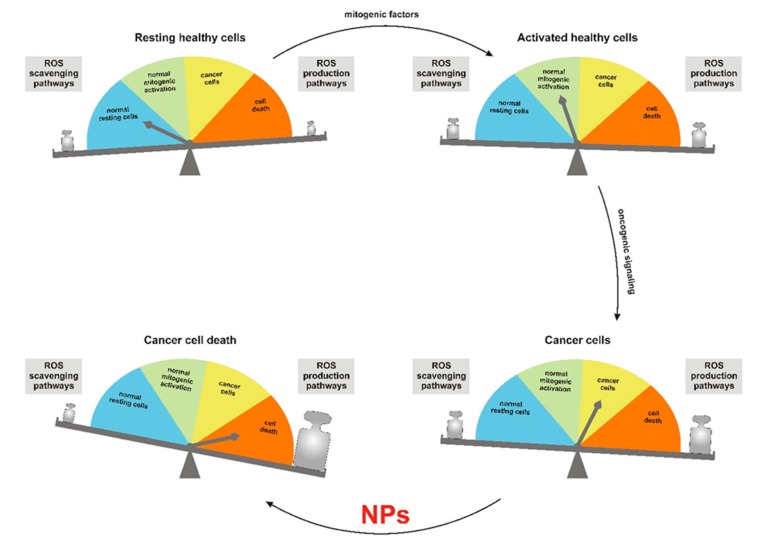
In this context, the elevated levels of reactive oxygen species (ROS) observed in cancer cells compared to their normal counterparts represent a promising therapeutic strategy to target malignant cells selectively [3]. ROS are reactive molecules derived from excitation and univalent reduction of molecular oxygen (O2), which lead to the generation of superoxide (O2•−), hydroxyl radical (•OH) and hydrogen peroxide (H2O2). ROS are produced in cells by several oxidases and may act as secondary messengers controlling different signal transduction pathways. According to the theory of the ROS rheostat [4], ROS regulate cell fate in a dose-dependent manner (Figure 1). While low/medium levels of ROS promote mitogenic signaling through reversible oxidation of cysteines to sulfenic acid [5] and disulfide bonds [6], high levels of ROS exert cytotoxic effects by inducing base oxidation in nucleic acids and lipid peroxidation, resulting in cell death, which may trigger inflammation and fibrosis. In cancer cells, activation of oncogenic pathways boosts ROS production by the mitochondrial electron transport chain (ETC) [7] and nonmitochondrial oxidases. The increased activity of ROS-scavenging pathways partly curbs such an increase in ROS production. The combined effects of these pathways reset the homeostatic ROS setpoint to a higher level, which provides cancer cells with a proliferative advantage but also makes them more vulnerable to a further increase of ROS that will trigger macromolecular damage and cell death.
Figure 1.
The reactive oxygen species (ROS) rheostat affects cell fate. NPs: nanoparticles.
Low ROS levels are associated with resting healthy cells (Figure 1, upper left). Physiologic stimulation with mitogenic factors induces an increase in ROS levels, which drive cell proliferation (Figure 1, upper right). Aberrant activation of oncogenic signals results in increased ROS generation, with concomitant upregulation of scavenging systems, which results in a higher ROS setpoint in cancer cells (Figure 1, lower right). NPs are a powerful tool to further increase ROS levels beyond the threshold triggering cell death. Cancer cells are selectively vulnerable to this treatment due to their higher ROS setpoint (Figure 1, lower left).
Consistent with this notion, we recently provided evidence for a ROS-based strategy to selectively kill T-cell acute lymphoblastic leukemia (T-ALL) cells and sensitize them to glucocorticoid-based therapies, while sparing healthy thymocytes [8]. Other evidence points to the anticancer efficacy of therapeutic strategies aimed at inducing oxidative stress [9,10]. Moreover, several anticancer drugs, such as cisplatin [11], doxorubicin [12] and taxanes [13], kill cancer cells partly by increasing ROS levels. Although ROS-inducing compounds are thus likely to be intrinsically selective for cancer cells, their performance could be further enhanced by strategies aimed at confining their damaging activity to the tumor microenvironment.
To this effect, nanotechnologies may provide novel and powerful tools to both alter redox homeostasis in cancer cells and improve the targeting of anticancer drugs to tumor cells by exploiting the unique features of their microenvironment, which include high ROS levels and the acidic pH that results from the glycolytic rewiring of tumor metabolism (Warburg effect). Nanomedicine is based on the use of synthetic particles of 1–1000 nm diameter (nanoparticles, NPs), which can be classified into six main groups: carbon NPs, metal NPs, ceramic NPs, semiconductor NPs, polymeric NPs and lipid-based NPs [14]. In particular, carbon NPs employ the different allotropic forms of carbon, including caged structures (e.g., fullerenes), tubular structures (e.g., carbon nanotubes (CNTs), sheet structures (e.g., graphene and nanodots) [15,16].
2. Modulation of Redox Homeostasis by Nanoparticles
Some NPs promote an imbalance in redox homeostasis by both depleting scavenging pathways and by generating ROS [17]. NPs may produce ROS due to their physico-chemical characteristics and the presence of transition metals (e.g., iron or copper) in their composition. Metal-containing NPs promote the generation of ROS via the Haber-Weiss and Fenton-type reactions [18].
In addition to transition metals, the lanthanide cerium also has interesting redox properties, as it can switch between the 4+ and 3+ oxidation states [19]. Cerium has been employed in nanomedicine mainly in the form of cerium oxide NPs (CeO-NPs), which exhibit redox-modulatory activities that mimic both superoxide dismutase and catalase activities. Interestingly, catalase, but not superoxide dismutase, activity of CeO-NPs is significantly decreased at low pH, suggesting that it could induce oxidative stress and cytotoxicity in the acidic microenvironment that characterizes highly glycolytic tumors, while, at the neutral pH of normal tissues, CeO-NPs could exert antioxidant (i.e., protective) effects [19]. Consistent with this notion, Alili et al. tested the anticancer effects of CeO-NPs in human melanoma cells both in vitro and in vivo. Results showed that CeO-NPs induced apoptosis in cancer cells but not in normal tissues and reduced tumor growth and invasiveness in a xenograft animal model in vivo [20]. CeO-NPs were also effective in killing high-grade astrocytoma cells but not normal endothelial cells, which were inhibited in their migratory capacity, suggesting that tumor angiogenesis might also be effectively targeted by this treatment [21]. In addition, CeO-NPs were also used in combination with the anticancer compound doxorubicin, which exerts its cytotoxic effect in part by inducing ROS [12]. Consistent with its dual effect on ROS in normal vs. cancer tissues, CeO-NPs enhanced the anticancer effects of doxorubicin while significantly reducing the undesired side effects of this compound on normal cells, including myocardiocytes. This is a significant finding, given the relevance of myocardiotoxicity of doxorubicin in cancer patients [22]. In a recent study, Hijaz et al. combined CeO-NPs with folic acid to target ovarian cancer cells that overexpress a membrane-bound isoform of folate receptor with cisplatin, which exerts its anticancer activity partly by enhancing ROS levels [11]; results showed a significant anticancer effect both in vitro and in a xenograft mouse model [23].
Small NPs may also enter subcellular organelles and enhance ROS production by activating the ETC and NADPH-dependent oxidases [24]. In this context, super-paramagnetic iron oxide NPs (SPIONs) are endocytosed by cells, internalized in lysosomes and dissociated to release iron ions, which, in turn, translocate into the mitochondria where they target ETC components Fe-S clusters and heme, resulting in O2•− production [25]. Similarly, manganese oxide NPs (MnO2-NPs) increase ROS levels in isolated mitochondria by affecting ETC complexes I and III, as well as reducing the activity of complexes II and IV, resulting in an impairment of mitochondrial activity [26]. Iron oxide magnetic NPs (IOMNPs) can activate plasma membrane proteins, such as NADPH oxidases [27], inducing the generation of O2•− [28]. Arsenic NPs (As-NPs), which are tested for breast cancer treatment [29], increase ROS generation by inhibiting complexes I and II of the ETC [30].
Carbon NPs (e.g., fullerenes, CNTs, carbon nanodots) produce ROS through redox-active functional groups attached to the NPs. In the alternative, transition metal contaminants resulting from the synthesis process of CNTs may catalyze the production of ROS [31]. In addition, due to their large surface area, carbon NPs can absorb other chemicals, which, upon biotransformation, can be oxidized to redox-active quinones and reduced to semiquinones and O2•−. The latter can be dismutated into H2O2, which, upon reacting with transition metals, produces hydroxyl radicals [24]. Interestingly, carbon NPs may exert indirect, non-cell-autonomous antitumor effects [32,33], which may result from (i) a decrease of matrix metalloproteinases (MMPs) expression levels, which inhibits tumor metastasis [34] and (ii) enhancement of antitumor immunity [32], partly due to their ability to trigger ROS production by NADPH oxidase and activation of toll-like receptors (TLRs) in professional phagocytes [35]. The use of carbon NPs as photosensors (PSs) in photodynamic therapy (PDT) is described below.
3. Nanoparticles in Photodynamic Therapy (PDT)
PDT is a minimally invasive treatment that exploits the physical properties of organic PSs. The majority of the PSs approved for clinical use by the FDA are excited in the far-red spectrum (630–690 nm wavelengths) [36]. PSs are classified into four groups: porphyrins, chlorins, phthalocyanines and a mixed group including hypericin, hypocrellin, indocyanine and methylene blue. The synthetic derivatives of protoporphyrin IX (e.g., taloporfin, temoporfin, verteporfin and photofrin) are the most commonly used PSs in PDT. Unlike radiotherapy, the photons used to trigger the PSs are nonionizing and thus less harmful to the normal tissues [36]. The photoactivation of PSs may initiate two types of reactions termed type I and type II [36,37], both of which generate ROS. Most of the PSs are strongly hydrophobic molecules, which limits their use in the clinic. PSs currently employed in therapy are administrated with a lipid (cremophor) or organic (dextrose, polyethylene glycol) vehicles that are suitable for topical use only. The intravenous inoculation of these pharmacological compositions may lead to severe side effects, including allergic reactions, hypersensitivity and prolonged photosensitization [38]. The suboptimal biodistribution of PSs is another potential problem for routine clinical use. The temoporfin Foscan, a second-generation PS that showed promising results in in vitro experiments, was proposed for the treatment of head and neck cancer. However, Foscan was not approved for clinical use due to its poor tumor selectivity and significant side effects [38].
NPs may significantly improve the solubility of PSs, allowing their systemic administration [39]. Cancer-specific targeting of NPs may be obtained, taking advantage of the enhanced glycolytic rate and extracellular acidification that are characteristic of most tumors. Selective targeting of the neoplastic cells can also be achieved by conjugating NPs to metabolites (e.g., folic acid, thiamine), small peptides (e.g., integrin-binding motifs), antibodies, polysaccharides, fatty acids and nucleic acids that either recognize cancer-specific surface molecules or are preferentially taken up by cancer cells [40]. Van Driel and colleagues developed EGF-conjugated NPs containing IRDye700DX as a PS [41]. These NPs were effective in killing head and neck cancer cells in an in vivo mouse model. A similar strategy was used to target leukemia-initiating cells (LIC) with NPs conjugated to anti-CD117 antibodies in a mouse model of chronic myelogenous leukemia [42].
Due to their limited penetration in tissues in humans, the therapeutic use of infrared photons in PDT is limited to skin cancers. However, light delivery in deep tissues was recently obtained using an LED-equipped microdevice implanted in the cancer tissue and switched on using a wireless signal. The emitted light was sufficient to illuminate a volume of 5 mm in diameter and photoactivated a PS in the liver of an adult pig [43].
The beneficial effects of PDT may not be limited to cancer cells only, as ROS produced by photoactivation could activate the immune system and stimulate it to recognize and attack cancer cells. In addition, ROS could damage the blood vessels that feed the tumor, inducing a hypoxic condition [44].
Recently, graphene quantum nanodots (GQNs) were employed as a multifunctional system for both tumor imaging and as electron donors for tumor killing. Tabish et al. showed that, following irradiation with 670 nm photons, GQNs generate singlet oxygen and heat [16]. The resulting combination of photodynamic and photothermal action of GQNs successfully killed MDA-MB-231 breast cancer cells in vitro and in a xenograft mouse model [45]. Efficacy of ROS generation, in part, depends on the presence of functional groups on the surface of nanodots (e.g., ketonic carbonyl, hydroxyl and carboxyl) [46]. In addition, fullerene modified with chitosan and PDT enhanced ROS production by the ETC in human malignant melanoma cells [47].
4. Use of Nanoparticles for Redox-Controlled Drug Delivery
Many anticancer drugs have pharmaceutical limitations, including poor solubility, low bioavailability and high cytotoxicity for noncancer cells [48]. A critical application of NPs is to enhance tumor-specific drug delivery while minimizing undesired side effects on healthy cells. To this effect, the high surface area and functional loading capacity of CNTs were successfully employed in vitro and in vivo to deliver anticancer compounds [49] and protect loaded drugs from adverse microenvironment conditions that could inhibit their activity [48].
NPs may be engineered to regulate drug release in response to both exogenous (as in PTD) and endogenous stimuli exploiting the characteristic alterations of tumor metabolism [50]. Doxorubicin linked by disulfide bonds to hyaluronic acid conjugated with single-walled CNTs (SWCNTs) represents an example of a complex redox-sensitive tumor-specific delivery system [51]. Increased levels of hyaluronidase and glutathione in tumor cells [52] trigger hyaluronic acid cleavage and reduction of disulfide bonds, respectively, resulting in the tumor-selective release of doxorubicin [53]. A study from Farjadian and colleagues showed that poly(N-vinylcaprolactam) cross-linked with poly(ethylene glycol) diacrylate forms NPs that release doxorubicin in breast cancer in response to acidic pH and elevated temperature [54].
To overcome the problem of acquired resistance to doxorubicin [55] due to increased extrusion by the ATP-binding cassette (ABC) proteins, doxorubicin was conjugated with bovine serum albumin (BSA) NPs in association with cyclopamine, an inhibitor of the hedgehog pathway, which decreases the expression of ABC proteins. This approach led to the intracellular accumulation of doxorubicin and restored the sensitivity of breast cancer cells to doxorubicin [56]. In a recent work, Yang and colleagues [57] used mesoporous silica NPs (MSNPs), which are internalized by endocytosis, to target a human acute lymphoblastic leukemia (ALL) cell line. MSNPs were loaded with doxorubicin, and their surface was decorated with aptamer Sgc8, which specifically recognizes the protein tyrosine kinase-7 (PTK-7) on the membrane of ALL cells [57]. This system allows specific doxorubicin uptake by leukemia cells, and, as MSNPs release the drug slowly and preferentially under the acidic conditions, it results in sustained drug accumulation at tumor sites, thus enhancing the specific anticancer effect of doxorubicin. Many tumors, including brain, bladder and ovarian cancers, are treated with the platinum-based drug cisplatin, which presents several side effects, such as ototoxicity, nephrotoxicity, hemolysis, neurotoxicity and impaired gametogenesis [58]. Interestingly, platinum NPs (Pt-NPs) accumulate in the tumor site and are internalized into the endosomes of cancer cells, where low endosomal pH and the high ROS levels within cancer cells induce the release of toxic Pt2+ ions, leading to DNA damage, cell cycle arrest and apoptosis [59].
Although tumor neo-angiogenesis is a hallmark of cancer [60], tumor vasculature is chaotic and disorganized, leading to inconstant blood flow [61]. Solid tumors are characterized by a high interstitial fluid pressure (IFP), which arises as a consequence of the lack of a functional draining lymphatic system and the elevated density of tumor stroma [62]. The defect of lymphatic drainage in tumor tissues accounts for the enhanced permeability and retention (EPR) effect, which leads to tumor-specific accumulation of macromolecules and NPs [63]. The EPR effect can be enhanced using NPs that release vasodilating agents such as nitric oxide (NO). To this effect, Song and colleagues incorporated polymer functionalized with nitrate into self-assembling d-α-tocopheryl/polyethylene glycol succinate micelles (TNO3) that release NO selectively in tumor cells, due to their high levels of glutathione compared to normal cells [52]. Using TNO3 loaded with doxorubicin, the authors obtained enhanced delivery of this compound to hepatocellular carcinoma cells in vivo [64], resulting in a synergistic anticancer effect of doxorubicin and TNO3 [64]. Furthermore, doxorubicin-derived O2•− reacts with NO, generating the reactive nitrogen species (RNS) peroxynitrite (ONOO−), a potent oxidant that kills cancer cells [65]. In addition, NO induces the degradation of the anti-apoptotic protein MCL-1, thus activating BAX/BAK-dependent apoptosis [66]. Similarly, Deepagan and colleagues observed boosted EPR effect upon administration of NO-generating NPs (NO-NPs), composed by hydrophilic polyethylene glycol and hydrophobic nitrated dextran, which release NO upon reduction by glutathione [67]. This ensures tumor site-specific vasodilatation that enhances the further uptake of NO-NPs. Doxorubicin-loaded NO-NPs efficiently blocked the growth of HT29 colon cancer cells in vivo by delivering high amounts of doxorubicin specifically to cancer cells. This approach has two interesting implications: (i) glutathione is oxidized during release of NO by nitrated dextran, depowering antioxidant defenses of cancer cells; (ii) although NO is rapidly inactivated to nitrate and nitrite ions, the latter are converted back to NO only in the acidic and hypoxic microenvironment that is characteristic of tumor tissues [68]. Thus, glutathione-sensitive NO-releasing NPs accumulate in tumor tissues due to the EPR effect, promote tumor -specific vasodilatation and drug delivery to cancer cells. The depletion of glutathione, along with the sensitization to ROS-inducing drugs by high levels of NO, maximize anticancer efficacy while sparing normal tissues.
Several other NO-releasing NPs have been developed, including zwitterionic diazeniumdiolate (NONOate)-loaded liposomes, which selectively release NO in the tumor microenvironment [69] due to its low pH. Glucose oxidase (GOx) and l-arginine co-loaded mesoporous organosilica NPs exploit the glucose avidity of cancer cells to generate H2O2 from GOx-mediated conversion of glucose to gluconic acid and consequent H2O2-mediated oxidation of l-arginine into NO [70]. These NPs, besides generating a high amount of NO, exert their antitumor effects by inducing glucose starvation of cancer cells [70].
5. Conclusions
Rewiring of tumor metabolism and redox homeostasis are critical hallmarks of cancer. In recent years, many studies have built on this knowledge to develop redox-based pharmacological approaches aimed at exploiting the vulnerability of cancer cells to compounds that increase ROS production and inhibit ROS scavenging. The integration of this approach with redox-active NPs (Table 1) may thus prove to be a significant breakthrough in cancer therapy in the near future. To this effect, cerium oxide-based NPs appear especially promising due to their peculiar dual redox properties to induce oxidative stress in cancer cells and exert protective antioxidant effects on normal cells. While photodynamically-triggered NPs appear to be most effective for the treatment of superficial cancers (e.g., skin, head and neck), future developments aimed at delivering photons into deep tissues might significantly extend the use of PTD to many neoplasms. NPs engineered to deliver their cargo in response to acidic pH and glutathione concentration may also prove to be powerful tools to increase the therapeutic window on many anticancer compounds. Rigorous in vivo testing using both genetically-driven and xenograft-based preclinical models as well as clinical trials will be instrumental in defining the real effectiveness and fostering the further development of these combinatorial anticancer strategies.
Table 1.
Summary of the different types of NPs described in the text.
| Nanoparticle Systems | Nanoparticle Composition | Mechanism of Action | Model Used for Validation | References |
|---|---|---|---|---|
| ROS Modulators | ||||
| CeO-NPs | Cerium oxide | ROS scavenging or generation depending on pH | In vitro cell lines and xenograft animal model | [19,20,21,22,23] |
| SPIONs | Super-paramagnetic iron oxide | Generation of O2•− by the ETC through the release of iron ions | SG-7701, Raw264.7, NIH3T3, HUVEC and HK2 normal cells; N2a, GY7703, HepG2, CNE1 and CNE2 cancer cells | [25] |
| MnO2-NPs | Manganese oxide | Impairment of the ETC and increased ROS production | Evaluation of pharmacokinetics and toxicity of NPs in C57 mice organs | [26] |
| IOMNPs | Magnetic iron oxide | Activation of NADPH oxidases and generation of O2•− | Several human cancer cell lines and in vivo animal models | [28] |
| As-NPs | Arsenic | Inhibition of complex I and II of the ETC | MDA-MB-231, MCF-7 (human breast cancer) cell lines in vitro; isolated rat liver mitochondria | [29,30] |
| Carbon-NPs | Fullerenes, carbon nanotubes, carbon nanodots | Transition metal-catalyzed generation of ROS and activation of NADPH oxidases and TLRs in professional phagocytes | Human hepatoma (H22) murine model; human pancreatic tumor xenografts mice; U937 (human myeloid lineage cells), nonsensitized human peripheral blood phagocytes | [32,34,35] |
| PDT Mediators | ||||
| Ce6 | Cerium 6 activated by LED-equipped microdevice | Wireless-activated infrared irradiation | Bladder cancer mouse xenograft and adult pig | [43] |
| GQNs | Graphene quantum nanodots | Generation of singlet oxygen and heat upon irradiation with 670 nm photons | MDA-MB-231 breast cancer cells, breast cancer xenograft mouse model | [45,46] |
| Redox-Based Delivery Systems | ||||
| SWCNTs | Carbon nanotubes + hyaluronic acid + doxorubicin | Release of doxorubicin in the presence of high levels of hyaluronidase and glutathione | MCF-7 (human breast cancer cells), breast cancer xenograft mouse model | [51] |
| BSA-NPs | Bovine serum albumin + doxorubicin + cyclopamine | Release of doxorubicin and decreased expression of ABC proteins | MDA-MB-231 and MCF-7 (breast cancer cells) in vitro, breast cancer xenograft mouse model | [56] |
| MSNPs | Mesoporous silica + doxorubicin + Sgc8 aptamer | Specific release of doxorubicin in T-ALL cells after PTK-7 binding | CEM (T-ALL), Ramos (Burkitt lymphoma), Lo2 (normal liver), 293T (human embryonic kidney) cell lines in vitro | [57] |
| Pt-NPs | Water-soluble platinum NPs capped with polyvinyl alcohol | Release of Pt2+ ions at low endosomal pH | Human glioblastoma U251 cell line | [59] |
| TNO3 | d-α-tocopheryl/polyethylene glycol succinate micelles + doxorubicin | Release of NO in the presence of high levels of glutathione and synergistic anticancer effect with doxorubicin | Hepatocellular carcinoma cells in vivo | [64] |
| NO-NPs | Hydrophilic polyethylene glycol + hydrophobic nitrated dextran | Boosted EPR effect by releasing NO upon reduction by glutathione | In vitro release of NO in the presence of glutathione, HT29 human colon carcinoma cell line in vitro, HT29 tumor-bearing mice | [67] |
| NONOate-loaded liposomes | Liposomes + zwitterionic diazeniumdiolate | Release of NO in tumor microenvironment due to low pH | Acellular system with controlled pH | [69] |
| Mesoporous organosilica NPs | Mesoporous organosilica + glucose oxidase + l-arginine | Release of high amount of NO in the presence of glucose and consequent glucose starvation of cancer cells | U87MG mouse xenograft model | [70] |
NPs: nanoparticles; CeO-NPs: cerium oxide NPs; SPIONs: super-paramagnetic iron oxide NPs; MnO2-NPs: manganese oxide NPs; IOMNPs: iron oxide magnetic NPs; As-NPs: arsenic NPs; Carbon-NPs: carbon-based NPs; Ce6: cerium oxide NPs under the size of 6 nm; GQNs: graphene quantum nanodots; SWCNTs: single-walled carbon nanotubes; BSA-NPs: bovine serum albumin NPs; MSNPs: mesoporous silica NPs; Pt-NPs: platinum NPs; TNO3: d-α-tocopheryl/polyethylene glycol succinate micelles; NO-NPs: nitric oxide-releasing NPs; ETC: electron transport chain; NADPH: nicotinamide adenine dinucleotide phosphate; TLRs: Toll-like receptors; ABC: ATP-binding cassette; T-ALL: T-cell acute lymphoblastic leukemia; PTK-7: protein tyrosine kinase-7; EPR: enhanced permeability and retention effect.
Author Contributions
All the authors contributed equally to this work. All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded by Veneto Institute of Oncology IOV-IRCCS (5 × 1000 “Sinergia tra oncologia molecolare e clinica”) and by a grant (IG 17794) from the Italian Association for Cancer Research.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Vega-Stromberg T. Chemotherapy-induced secondary malignancies. J. Infus. Nurs. 2003;26:353–361. doi: 10.1097/00129804-200311000-00004. [DOI] [PubMed] [Google Scholar]
- 2.Dracham C.B., Shankar A., Madan R. Radiation induced secondary malignancies: A review article. Radiat. Oncol. J. 2018;36:85–94. doi: 10.3857/roj.2018.00290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Trachootham D., Alexandre J., Huang P. Targeting cancer cells by ROS-mediated mechanisms: A radical therapeutic approach? Nat. Rev. Drug Discov. 2009;8:579–591. doi: 10.1038/nrd2803. [DOI] [PubMed] [Google Scholar]
- 4.Maryanovich M., Gross A. A ROS rheostat for cell fate regulation. Trends Cell Biol. 2013;23:129–134. doi: 10.1016/j.tcb.2012.09.007. [DOI] [PubMed] [Google Scholar]
- 5.Sena L.A., Chandel N.S. Physiological roles of mitochondrial reactive oxygen species. Mol. Cell. 2012;48:158–167. doi: 10.1016/j.molcel.2012.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cremers C.M., Jakob U. Oxidant Sensing by Reversible Disulfide Bond Formation. J. Biol. Chem. 2013;288:26489–26496. doi: 10.1074/jbc.R113.462929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Raimondi V., Ciccarese F., Ciminale V. Oncogenic pathways and the electron transport chain: A dangeROS liaison. Br. J. Cancer. 2020;122:168–181. doi: 10.1038/s41416-019-0651-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Silic-Benussi M., Scattolin G., Cavallari I., Minuzzo S., Del Bianco P., Francescato S., Basso G., Indraccolo S., D’Agostino D.M., Ciminale V. Selective killing of human T-ALL cells: An integrated approach targeting redox homeostasis and the OMA1/OPA1 axis. Cell Death Dis. 2018;9:822. doi: 10.1038/s41419-018-0870-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shaw A.T., Winslow M.M., Magendantz M., Ouyang C., Dowdle J., Subramanian A., Lewis T.A., Maglathin R.L., Tolliday N., Jacks T. Selective killing of K-ras mutant cancer cells by small molecule inducers of oxidative stress. Proc. Natl. Acad. Sci. USA. 2011;108:8773–8778. doi: 10.1073/pnas.1105941108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Iskandar K., Rezlan M., Yadav S.K., Foo C.H., Sethi G., Qiang Y., Bellot G.L., Pervaiz S. Synthetic Lethality of a Novel Small Molecule Against Mutant KRAS-Expressing Cancer Cells Involves AKT-Dependent ROS Production. Antioxid. Redox Signal. 2016;24:781–794. doi: 10.1089/ars.2015.6362. [DOI] [PubMed] [Google Scholar]
- 11.Casares C., Ramirez-Camacho R., Trinidad A., Roldan A., Jorge E., Garcia-Berrocal J.R. Reactive oxygen species in apoptosis induced by cisplatin: Review of physiopathological mechanisms in animal models. Eur. Arch. Otorhinolaryngol. 2012;269:2455–2459. doi: 10.1007/s00405-012-2029-0. [DOI] [PubMed] [Google Scholar]
- 12.Tsang W.P., Chau S.P., Kong S.K., Fung K.P., Kwok T.T. Reactive oxygen species mediate doxorubicin induced p53-independent apoptosis. Life Sci. 2003;73:2047–2058. doi: 10.1016/S0024-3205(03)00566-6. [DOI] [PubMed] [Google Scholar]
- 13.Alexandre J., Hu Y., Lu W., Pelicano H., Huang P. Novel action of paclitaxel against cancer cells: Bystander effect mediated by reactive oxygen species. Cancer Res. 2007;67:3512–3517. doi: 10.1158/0008-5472.CAN-06-3914. [DOI] [PubMed] [Google Scholar]
- 14.Khan I., Saeed K., Khan I. Nanoparticles: Properties, applications and toxicities. Arab. J. Chem. 2019 doi: 10.1016/j.arabjc.2017.05.011. [DOI] [Google Scholar]
- 15.Tabish T.A., Zhang S., Winyard P.G. Developing the next generation of graphene-based platforms for cancer therapeutics: The potential role of reactive oxygen species. Redox Biol. 2018;15:34–40. doi: 10.1016/j.redox.2017.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tabish T.A. Graphene-based materials: The missing piece in nanomedicine? Biochem. Biophys. Res. Commun. 2018;504:686–689. doi: 10.1016/j.bbrc.2018.09.029. [DOI] [PubMed] [Google Scholar]
- 17.Kwon S., Ko H., You D.G., Kataoka K., Park J.H. Nanomedicines for Reactive Oxygen Species Mediated Approach: An Emerging Paradigm for Cancer Treatment. Acc. Chem. Res. 2019;52:1771–1782. doi: 10.1021/acs.accounts.9b00136. [DOI] [PubMed] [Google Scholar]
- 18.Manke A., Wang L., Rojanasakul Y. Mechanisms of nanoparticle-induced oxidative stress and toxicity. Biomed. Res. Int. 2013;2013 doi: 10.1155/2013/942916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xu C., Qu X. Cerium oxide nanoparticle: A remarkably versatile rare earth nanomaterial for biological applications. NPG Asia Mater. 2014;6 doi: 10.1038/am.2013.88. [DOI] [Google Scholar]
- 20.Alili L., Sack M., von Montfort C., Giri S., Das S., Carroll K.S., Zanger K., Seal S., Brenneisen P. Downregulation of tumor growth and invasion by redox-active nanoparticles. Antioxid. Redox Signal. 2013;19:765–778. doi: 10.1089/ars.2012.4831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sack-Zschauer M., Bader S., Brenneisen P. Cerium Oxide Nanoparticles as Novel Tool in Glioma Treatment: An In vitro Study. J. Nanomed. Nanotechnol. 2017;8:1–9. doi: 10.4172/2157-7439.1000474. [DOI] [Google Scholar]
- 22.Sulthana S., Banerjee T., Kallu J., Vuppala S.R., Heckert B., Naz S., Shelby T., Yambem O., Santra S. Combination Therapy of NSCLC Using Hsp90 Inhibitor and Doxorubicin Carrying Functional Nanoceria. Mol. Pharm. 2017;14:875–884. doi: 10.1021/acs.molpharmaceut.6b01076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hijaz M., Das S., Mert I., Gupta A., Al-Wahab Z., Tebbe C., Dar S., Chhina J., Giri S., Munkarah A., et al. Folic acid tagged nanoceria as a novel therapeutic agent in ovarian cancer. BMC Cancer. 2016;16:220. doi: 10.1186/s12885-016-2206-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fu P.P., Xia Q., Hwang H.M., Ray P.C., Yu H. Mechanisms of nanotoxicity: Generation of reactive oxygen species. J. Food Drug Anal. 2014;22:64–75. doi: 10.1016/j.jfda.2014.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.He C., Jiang S., Jin H., Chen S., Lin G., Yao H., Wang X., Mi P., Ji Z., Lin Y., et al. Mitochondrial electron transport chain identified as a novel molecular target of SPIO nanoparticles mediated cancer-specific cytotoxicity. Biomaterials. 2016;83:102–114. doi: 10.1016/j.biomaterials.2016.01.010. [DOI] [PubMed] [Google Scholar]
- 26.Ashrafi Hafez A., Naserzadeh P., Mortazavian A.M., Mehravi B., Ashtari K., Seydi E., Salimi A. Comparison of the effects of MnO2-NPs and MnO2-MPs on mitochondrial complexes in different organs. Toxicol. Mech. Methods. 2019;29:86–94. doi: 10.1080/15376516.2018.1512693. [DOI] [PubMed] [Google Scholar]
- 27.Amara N., Bachoual R., Desmard M., Golda S., Guichard C., Lanone S., Aubier M., Ogier-Denis E., Boczkowski J. Diesel exhaust particles induce matrix metalloprotease-1 in human lung epithelial cells via a NADP(H) oxidase/NOX4 redox-dependent mechanism. Am. J. Physiol. Lung Cell Mol. Physiol. 2007;293:L170–L181. doi: 10.1152/ajplung.00445.2006. [DOI] [PubMed] [Google Scholar]
- 28.Pisanic T.R., II, Sungho J., Shubayev V.I. Nanotoxicity: From In Vivo and In Vitro Models to Health Risks. Wiley; Hoboken, NJ, USA: 2020. Iron Oxide Magnetic Nanoparticle Nanotoxicity: Incidence and Mechanisms—Nanotoxicity—Wiley Online Library. [DOI] [Google Scholar]
- 29.Subastri A., Arun V., Sharma P., Preedia Babu E., Suyavaran A., Nithyananthan S., Alshammari G.M., Aristatile B., Dharuman V., Thirunavukkarasu C. Synthesis and characterisation of arsenic nanoparticles and its interaction with DNA and cytotoxic potential on breast cancer cells. Chem. Biol. Interact. 2018;295:73–83. doi: 10.1016/j.cbi.2017.12.025. [DOI] [PubMed] [Google Scholar]
- 30.Hosseini M.J., Shaki F., Ghazi-Khansari M., Pourahmad J. Toxicity of Arsenic (III) on Isolated Liver Mitochondria: A New Mechanistic Approach. Iran. J. Pharm. Res. 2013;12:121–138. [PMC free article] [PubMed] [Google Scholar]
- 31.Baldrighi M., Trusel M., Tonini R., Giordani S. Carbon Nanomaterials Interfacing with Neurons: An In vivo Perspective. Front. Neurosci. 2016;10:250. doi: 10.3389/fnins.2016.00250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen C., Xing G., Wang J., Zhao Y., Li B., Tang J., Jia G., Wang T., Sun J., Xing L., et al. Multihydroxylated [Gd@C82(OH)22]n nanoparticles: Antineoplastic activity of high efficiency and low toxicity. Nano Lett. 2005;5:2050–2057. doi: 10.1021/nl051624b. [DOI] [PubMed] [Google Scholar]
- 33.Wang J., Hu Z., Xu J., Zhao Y. Therapeutic applications of low-toxicity spherical nanocarbon materials. NPG Asia Mater. 2014;6 doi: 10.1038/am.2013.79. [DOI] [Google Scholar]
- 34.Kang S.G., Zhou G., Yang P., Liu Y., Sun B., Huynh T., Meng H., Zhao L., Xing G., Chen C., et al. Molecular mechanism of pancreatic tumor metastasis inhibition by Gd@C82(OH)22 and its implication for de novo design of nanomedicine. Proc. Natl. Acad. Sci. USA. 2012;109:15431–15436. doi: 10.1073/pnas.1204600109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Skivka L.M., Prylutska S.V., Rudyk M.P., Khranovska N.M., Opeida I.V., Hurmach V.V., Prylutskyy Y.I., Sukhodub L.F., Ritter U. C60 fullerene and its nanocomplexes with anticancer drugs modulate circulating phagocyte functions and dramatically increase ROS generation in transformed monocytes. Cancer Nanotechnol. 2018;9:8. doi: 10.1186/s12645-017-0034-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vankayala R., Hwang K.C. Near-Infrared-Light-Activatable Nanomaterial-Mediated Phototheranostic Nanomedicines: An Emerging Paradigm for Cancer Treatment. Adv. Mater. 2018;30:e1706320. doi: 10.1002/adma.201706320. [DOI] [PubMed] [Google Scholar]
- 37.Pass H.I. Photodynamic therapy in oncology: Mechanisms and clinical use. J. Natl. Cancer Inst. 1993;85:443–456. doi: 10.1093/jnci/85.6.443. [DOI] [PubMed] [Google Scholar]
- 38.Master A., Livingston M., Sen Gupta A. Photodynamic nanomedicine in the treatment of solid tumors: Perspectives and challenges. J. Control. Release. 2013;168:88–102. doi: 10.1016/j.jconrel.2013.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hinger D., Grafe S., Navarro F., Spingler B., Pandiarajan D., Walt H., Couffin A.C., Maake C. Lipid nanoemulsions and liposomes improve photodynamic treatment efficacy and tolerance in CAL-33 tumor bearing nude mice. J. Nanobiotechnol. 2016;14:71. doi: 10.1186/s12951-016-0223-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yan S., Huang Q., Chen J., Song X., Chen Z., Huang M., Xu P., Zhang J. Tumor-targeting photodynamic therapy based on folate-modified polydopamine nanoparticles. Int. J. Nanomed. 2019;14:6799–6812. doi: 10.2147/IJN.S216194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.van Driel P., Boonstra M.C., Slooter M.D., Heukers R., Stammes M.A., Snoeks T.J.A., de Bruijn H.S., van Diest P.J., Vahrmeijer A.L., van Bergen En Henegouwen P.M.P., et al. EGFR targeted nanobody-photosensitizer conjugates for photodynamic therapy in a pre-clinical model of head and neck cancer. J. Control. Release. 2016;229:93–105. doi: 10.1016/j.jconrel.2016.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Barth B.M., Altinoğlu E.I., Shanmugavelandy S.S., Kaiser J.M., Crespo-Gonzalez D., DiVittore N.A., McGovern C., Goff T.M., Keasey N.R., Adair J.H., et al. Targeted indocyanine-green-loaded calcium phosphosilicate nanoparticles for in vivo photodynamic therapy of leukemia. ACS Nano. 2011;5:5325–5337. doi: 10.1021/nn2005766. [DOI] [PubMed] [Google Scholar]
- 43.Bansal A., Yang F., Xi T., Zhang Y., Ho J.S. In vivo wireless photonic photodynamic therapy. Proc. Natl. Acad. Sci. USA. 2018;115:1469–1474. doi: 10.1073/pnas.1717552115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kobayashi H., Choyke P.L. Near-Infrared Photoimmunotherapy of Cancer. Acc. Chem. Res. 2019 doi: 10.1021/acs.accounts.9b00273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nurunnabi M., Khatun Z., Reeck G.R., Lee D.Y., Lee Y.K. Photoluminescent graphene nanoparticles for cancer phototherapy and imaging. ACS Appl. Mater. Interfaces. 2014;6:12413–12421. doi: 10.1021/am504071z. [DOI] [PubMed] [Google Scholar]
- 46.Zhou Y., Sun H., Wang F., Ren J., Qu X. How functional groups influence the ROS generation and cytotoxicity of graphene quantum dots. Chem. Commun. 2017;53:10588–10591. doi: 10.1039/C7CC04831A. [DOI] [PubMed] [Google Scholar]
- 47.Li Q., Liu C., Li H. Induction of Endogenous Reactive Oxygen Species in Mitochondria by Fullerene-Based Photodynamic Therapy. J. Nanosci. Nanotechnol. 2016;16:5592–5597. doi: 10.1166/jnn.2016.11717. [DOI] [PubMed] [Google Scholar]
- 48.Sun T., Zhang Y.S., Pang B., Hyun D.C., Yang M., Xia Y. Engineered nanoparticles for drug delivery in cancer therapy. Angew. Chem. Int. Ed. Eng. 2014;53:12320–12364. doi: 10.1002/anie.201403036. [DOI] [PubMed] [Google Scholar]
- 49.Augustine S., Singh J., Srivastava M., Sharma M., Das A., Malhotra B.D. Recent advances in carbon based nanosystems for cancer theranostics. Biomater. Sci. 2017;5:901–952. doi: 10.1039/C7BM00008A. [DOI] [PubMed] [Google Scholar]
- 50.Tang Q., Yu B., Gao L., Cong H., Song N., Lu C. Stimuli Responsive Nanoparticles for Controlled Anti-cancer Drug Release. Curr. Med. Chem. 2018;25:1837–1866. doi: 10.2174/0929867325666180111095913. [DOI] [PubMed] [Google Scholar]
- 51.Hou L., Yang X., Ren J., Wang Y., Zhang H., Feng Q., Shi Y., Shan X., Yuan Y., Zhang Z. A novel redox-sensitive system based on single-walled carbon nanotubes for chemo-photothermal therapy and magnetic resonance imaging. Int. J. Nanomed. 2016;11:607–624. doi: 10.2147/ijn.s98476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Estrela J.M., Ortega A., Obrador E. Glutathione in cancer biology and therapy. Crit. Rev. Clin. Lab. Sci. 2006;43:143–181. doi: 10.1080/10408360500523878. [DOI] [PubMed] [Google Scholar]
- 53.Li R., Xie Y. Nanodrug delivery systems for targeting the endogenous tumor microenvironment and simultaneously overcoming multidrug resistance properties. J. Control. Release. 2017;251:49–67. doi: 10.1016/j.jconrel.2017.02.020. [DOI] [PubMed] [Google Scholar]
- 54.Farjadian F., Rezaeifard S., Naeimi M., Ghasemi S., Mohammadi-Samani S., Welland M.E., Tayebi L. Temperature and pH-responsive nano-hydrogel drug delivery system based on lysine-modified poly (vinylcaprolactam) Int. J. Nanomed. 2019;14:6901–6915. doi: 10.2147/IJN.S214467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Dvorak P., Hlavac V., Mohelnikova-Duchonova B., Liska V., Pesta M., Soucek P. Downregulation of ABC Transporters in Non-neoplastic Tissues Confers Better Prognosis for Pancreatic and Colorectal Cancer Patients. J. Cancer. 2017;8:1959–1971. doi: 10.7150/jca.19364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lu Y.L., Ma Y.B., Feng C., Zhu D.L., Liu J., Chen L., Liang S.J., Dong C.Y. Co-delivery of Cyclopamine and Doxorubicin Mediated by Bovine Serum Albumin Nanoparticles Reverses Doxorubicin Resistance in Breast Cancer by Down-regulating P-glycoprotein Expression. J. Cancer. 2019;10:2357–2368. doi: 10.7150/jca.30323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yang Y., Zhao W., Tan W., Lai Z., Fang D., Jiang L., Zuo C., Yang N., Lai Y. An Efficient Cell-Targeting Drug Delivery System Based on Aptamer-Modified Mesoporous Silica Nanoparticles. Nanoscale Res. Lett. 2019;14:390. doi: 10.1186/s11671-019-3208-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Dasari S., Tchounwou P.B. Cisplatin in cancer therapy: Molecular mechanisms of action. Eur. J. Pharmacol. 2014;740:364–378. doi: 10.1016/j.ejphar.2014.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Asharani P.V., Xinyi N., Hande M.P., Valiyaveettil S. DNA damage and p53-mediated growth arrest in human cells treated with platinum nanoparticles. Nanomedicine. 2010;5:51–64. doi: 10.2217/nnm.09.85. [DOI] [PubMed] [Google Scholar]
- 60.Hanahan D., Weinberg R.A. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/S0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 61.Chaplin D.J., Olive P.L., Durand R.E. Intermittent blood flow in a murine tumor: Radiobiological effects. Cancer Res. 1987;47:597–601. [PubMed] [Google Scholar]
- 62.Jain R.K. Barriers to drug delivery in solid tumors. Sci. Am. 1994;271:58–65. doi: 10.1038/scientificamerican0794-58. [DOI] [PubMed] [Google Scholar]
- 63.Greish K. Enhanced permeability and retention (EPR) effect for anticancer nanomedicine drug targeting. Methods Mol. Biol. 2010;624:25–37. doi: 10.1007/978-1-60761-609-2_3. [DOI] [PubMed] [Google Scholar]
- 64.Song Q., Tan S., Zhuang X., Guo Y., Zhao Y., Wu T., Ye Q., Si L., Zhang Z. Nitric oxide releasing d-alpha-tocopheryl polyethylene glycol succinate for enhancing antitumor activity of doxorubicin. Mol. Pharm. 2014;11:4118–4129. doi: 10.1021/mp5003009. [DOI] [PubMed] [Google Scholar]
- 65.Fraszczak J., Trad M., Janikashvili N., Cathelin D., Lakomy D., Granci V., Morizot A., Audia S., Micheau O., Lagrost L., et al. Peroxynitrite-dependent killing of cancer cells and presentation of released tumor antigens by activated dendritic cells. J. Immunol. 2010;184:1876–1884. doi: 10.4049/jimmunol.0900831. [DOI] [PubMed] [Google Scholar]
- 66.Snyder C.M., Shroff E.H., Liu J., Chandel N.S. Nitric oxide induces cell death by regulating anti-apoptotic BCL-2 family members. PLoS ONE. 2009;4:e7059. doi: 10.1371/journal.pone.0007059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Deepagan V.G., Ko H., Kwon S., Rao N.V., Kim S.K., Um W., Lee S., Min J., Lee J., Choi K.Y., et al. Intracellularly Activatable Nanovasodilators To Enhance Passive Cancer Targeting Regime. Nano Lett. 2018;18:2637–2644. doi: 10.1021/acs.nanolett.8b00495. [DOI] [PubMed] [Google Scholar]
- 68.Fukumura D., Kashiwagi S., Jain R.K. The role of nitric oxide in tumour progression. Nat. Rev. Cancer. 2006;6:521–534. doi: 10.1038/nrc1910. [DOI] [PubMed] [Google Scholar]
- 69.Tai L.A., Wang Y.C., Yang C.S. Heat-activated sustaining nitric oxide release from zwitterionic diazeniumdiolate loaded in thermo-sensitive liposomes. Nitric Oxide. 2010;23:60–64. doi: 10.1016/j.niox.2010.04.003. [DOI] [PubMed] [Google Scholar]
- 70.Fan W., Lu N., Huang P., Liu Y., Yang Z., Wang S., Yu G., Hu J., He Q., Qu J., et al. Glucose-Responsive Sequential Generation of Hydrogen Peroxide and Nitric Oxide for Synergistic Cancer Starving-Like/Gas Therapy. Angew. Chem. Int. Ed. Eng. 2017;56:1229–1233. doi: 10.1002/anie.201610682. [DOI] [PubMed] [Google Scholar]