Abstract
Post-translational modifications (PTM) of proteins are crucial for fine-tuning a cell’s response to both intracellular and extracellular cues. ADP-ribosylation is a PTM, which occurs in two flavours: modification of a target with multiple ADP-ribose moieties (poly(ADP-ribosyl)ation or PARylation) or with only one unit (MARylation), which are added by the different enzymes of the PARP family (also known as the ARTD family). PARylation has been relatively well-studied, particularly in the DNA damage response. This has resulted in the development of PARP inhibitors such as olaparib, which are increasingly employed in cancer chemotherapeutic approaches. Despite the fact that the majority of PARP enzymes catalyse MARylation, MARylation is not as well understood as PARylation. MARylation is a dynamic process: the enzymes reversing intracellular MARylation of acidic amino acids (MACROD1, MACROD2, and TARG1) were discovered in 2013. Since then, however, little information has been published about their physiological function. MACROD1, MACROD2, and TARG1 have a ‘macrodomain’ harbouring the catalytic site, but no other domains have been identified. Despite the lack of information regarding their cellular roles, there are a number of studies linking them to cancer. However, some of these publications oppose each other, some rely on poorly-characterised antibodies, or on aberrant localisation of overexpressed rather than native protein. In this review, we critically assess the available literature on a role for the hydrolases in cancer and find that, currently, there is limited evidence for a role for MACROD1, MACROD2, or TARG1 in tumorigenesis.
Keywords: ADP-ribosylation, cancer, macrodomain, ADP-ribosyl hydrolase, PARP, ARTD, MACROD1, MACROD2, TARG1
1. ADP-Ribosylation Reactions
Post-translational modifications of proteins can have a myriad of consequences: changing interactomes, stability, localisation, or activity are just a few examples. To date, more than 200 types of modification are known [1]. The addition of ADP-ribose moieties to a protein was first reported in the 1960s when chains of (poly)ADP-ribose (PAR) were identified on target proteins, termed poly(ADP-ribosyl)ation (PARylation) [2]. Since then, PARylation has been intensively studied, leading to the identification of crucial roles in the repair of DNA damage and to the development of specific inhibitors that are utilised in the clinic to treat diverse cancers [3]. In contrast, much less is known about its smaller sibling, mono(ADP-ribosyl)ation (MARylation), where only single ADP-ribose units (MAR) are conjugated into proteins. In 2008, it was first noted that PARP10/ARTD10 has MARylation rather than PARylation activity [4] and, lately, it has become clear that only the minority of PARP enzymes are capable of PARylation [5]. Moreover, not only protein, but also DNA has been identified as a target for ADP-ribosylation catalysed by PARP3 [6,7]. Following PARP1 and PARP2, PARP3 has also been described as a potential therapeutic target in certain cancers [8,9,10,11]. The most recent addition to the spectrum of ADP-ribosylated molecules is RNA, which can be modified in vitro by multiple PARPs [12].
One of the best-studied but still barely understood MARylating enzymes is PARP10. PARP10 was initially identified as an interactor of MYC [13] and, later, of ubiquitinated proliferating cell nuclear antigen (PCNA) [14,15]. Its overexpression leads to cell death in HeLa cells through an unknown mechanism [16]. In contrast, in non-transformed RPE-1 cells, overexpression leads to stimulation of cell growth [17]. It has been given a role in replication and DNA damage [15,17,18] as well as mitochondrial metabolism [19], but it is not clear what the relevant substrates of this enzyme are. Potential substrates were identified using protein microarrays [20], which was performed for the PARylating PARP2 [21]. However, partially due to a lack of antibodies at that time, these modifications were not verified to occur in cells. PARP10 was also reported to be a regulator of NF-kB signaling [22]. PARP14 functions as a co-activator of STAT6 and has been described as an anti-cancer and anti-inflammatory target, reviewed in more detail elsewhere [23,24]. PARP16 is located at the endoplasmic reticulum (ER) and plays a role in the unfolded protein response [25,26]. The most recent function identified for MARylation is its role in the anti-viral defence. PARP7, PARP10, and PARP12 gene expression is induced by interferon-α and ADP-ribosylation mediated by these PARPs may function to counteract viral proliferation [27,28]. In addition to enzymes of the PARP family, several sirtuins (SIRTs) appear to be able to ADP-ribosylate substrates in addition to their better-studied NAD+-dependent deacylase activity [29,30]. The first research showing that SIRTs possess MARylation activity described it as a very weak activity [31]. Other papers, focusing on single enzymes, showed that the mitochondrial SIRT4 MARylates glutamate dehydrogenase to downregulate insulin secretion [32]. The nuclear SIRT6 modifies PARP1 [33] and BAF10 [34] to regulate their activities and, lastly, the nucleolar SIRT7 was reported to MARylate histones p53 and ELK4 [35]. To date, very little information is available about the MARylating activity of the SIRTs. The functional consequences of MARylation in normal cell physiology, thus, appear to be very diverse, reviewed extensively elsewhere [36]. Herein, we focus on the enzymes reversing the intracellular MARylation reaction: the macrodomain-containing hydrolases.
2. Macrodomains
Intimately linked to ADP-ribosylation is a protein structural module known as the macrodomain, named for the unusually large histone macroH2A from which it was identified [37]. The macrodomain is a protein fold formed around a central β-sheet, flanked by α-helices that forms a pocket where ADP-ribose can bind [38,39]. These pockets are slightly different between the macrodomain-containing proteins with the consequence that the macrodomain-containing protein family can be subdivided. Some macrodomains bind to PAR and others bind to MAR [40,41,42,43].
Macrodomains not only serve as binding modules to mediate protein interactions depending on ADP-ribosylation, but a number of them have catalytic activity. Poly(ADP-ribosyl)glycohydrolase (PARG) is able to degrade PAR chains by cleaving the glycosidic bond between adjacent ADP-ribose moieties. However, it cannot remove the final ADP-ribose attached to the protein [44]. Three other proteins, MACROD1, MACROD2, and TARG1, are not active toward PAR but are capable of removing the ADP-ribose moiety connected to the protein partner, presumably by cleaving an ester bond between ADP-ribose and an acidic receptor amino acid [45,46,47]. They can, therefore, both reverse any MARylation as generated by PARP enzymes, and also remove the last ADP-ribose left behind by PARG. Of the 12 human macrodomain-containing proteins known today, 11 proteins were identified by sequence homology [39,43]. The macrodomain of PARG was only recognised after the structure had been solved [44], which indicates the possibility that more macrodomain-containing proteins remain to be identified. A recent bioinformatics approach has suggested that the largely uncharacterised protein C12orf4 harbours a macrodomain, or at least a macro-like domain, but this remains to be confirmed experimentally [48].
The macrodomain is an evolutionary well-conserved module [39]. For instance, recent work has shown that certain alpha-viruses encode a protein with a macrodomain-like fold [49], which displays catalytic activity towards MARylated proteins [50,51,52]. The existence of such proteins enhances the evidence connecting existing links between MARylation and immunity [53]. In this review, we will focus specifically on potential oncogenic functions of the mono(ADP-ribosyl)hydrolases MACROD1, MACROD2, and TARG1. It should also be noted, however, that other macrodomain-containing proteins such as PARG and CHD1L (ALC1) have been previously linked closely to cancer [54,55].
3. Macrodomain-Containing Hydrolases: MACROD1, MACROD2, and TARG1
MACROD1, MACROD2, and TARG1 are relatively small proteins (Table 1) without any notable other domains beside the macrodomain. The first catalytic activity of MACROD1, MACROD2, and TARG1 that was recognised was their deacetylation of O-acetyl-ADP-ribose (OAADPR) to generate acetyl and ADP-ribose [56,57]. OAADPR arises as a by-product of SIRT-mediated deacetylation in which the acetyl group is transferred onto ADP-ribose while releasing nicotinamide, and may have important biological functions [58]. Two years later, three labs reported the activity of MACROD1, MACROD2, and TARG1 in removing ADP-ribose from protein substrates, which was proposed to be limited to removal of ADP-ribose from acidic amino acids [43,45,46,47]. Serine and arginine MARylation were suggested to be removed by ARH3 and ARH1, respectively [59,60,61,62], which are structurally different proteins that do not contain a macrodomain. In 2017, MACROD1, MACROD2, and TARG1 were reported as being capable of removing ADP-ribose from both modified single-stranded DNA [63] and, in 2018, also being capable of removing ADP-ribose from MARylated single-stranded RNA [12]. Lastly, in 2019, the hydrolysis of α-NAD was added to the list of their in vitro activities [64].
Table 1.
Summary of nomenclature, expression, and localisation data of MACROD1, MACROD2, and TARG1.
| Protein Name | Alternative Names | Gene Name | MW Predicted | MW Observed | Intracellular Localisation | Expression | Regulation |
|---|---|---|---|---|---|---|---|
| MACROD1 | LRP16 | MACROD1 | 35.5 kDa | 27 kDa* [70,71] | Mitochondrial [40,70,71] | Ubiquitous expression, enriched in skeletal muscle [70,71] | mRNA expression is induced by oestrogen [72,73,74] |
| MACROD2 | C20orf133 | MACROD2 | 47 kDa | 50 kDa [70] | Diffuse nuclear, cytoplasmic [70,75] | So far detected only in the brain [70,76] | Phosphorylation by ATM upon DNA damage induces translocation to cytoplasm [75] |
| TARG1 | C6orf130 | OARD1 | 17 kDa | 17 kDa [14,70] | Nuclear, nucleolar, stress granular [14,45,70] | Ubiquitous expression [70] | Leaves nucleoli upon DNA damage [14] |
* The predicted molecular weight of MACROD1 is 35 kDa. However, due to cleavage of the N-terminus upon translocation into mitochondria, the protein detected in western blotting (WB) is smaller [70].
To date, it is not known which of these activities is relevant for their functionality in cells, either in normal physiology nor in pathological conditions. This is predominantly due to the fact that it is not well known how prevalent their in vitro substrates are in cells or what their cellular functions may be. This is partially due to the fact that antibodies or modules recognising MARylation have been developed only recently [65,66,67]. Before these technical developments, it was not possible to confirm with antibodies that MARylation of proteins takes place in cells, and, hence, reversal mediated by these macrodomain-containing proteins could also not be assessed. Currently, it is still not straightforward to estimate endogenous MARylation levels in cells, as no inhibitors were available for MACROD1, MACROD2, or TARG1. During experimental procedures such as cell fixation or lysis, MARylation could possibly be removed by these enzymes and, therefore, go undetected. One study was performed to identify MACROD1 inhibitors using an AlphaScreen assay, where inhibitors in the micromolar range were identified. These can serve as lead compounds for further optimisation, but are likely not specific enough yet to be used for studies in cells due to their other activities at this concentration [68]. Another study identified an allosteric inhibitor of macrodomain 2 of PARP14, which blocks its binding to ADP-ribose. However, it was not tested whether the identified compound might inhibit one of the macrodomains with catalytic activity [69].
The roles of MACROD1, MACROD2, and TARG1 in normal cell biological processes are not well understood. They appear to be expressed and localised in different tissues and intracellular locations, which clearly gives them unique roles to play despite their biochemical similarity. This also explains why loss of TARG1 alone can lead to a neurodegenerative phenotype [45]. MACROD1 resides in mitochondria [40,70,71], but, depending on the tag used for overexpression, will reside exclusively in the nucleus instead [70]. MACROD2 displays a more diffuse cytoplasmic/nuclear distribution [70,75] and overexpressed TARG1 appears to be present in nucleoplasm, nucleoli, and cytoplasmic stress granules [14,70]. TARG1 and MACROD1 are expressed throughout the different tissues with an enrichment of MACROD1 in skeletal muscle at both the protein and RNA level [70]. MACROD2 has, thus far, only been detected in human neuroblastoma cells and in mouse cortical neurons [70,76]. Several reports have correlated polymorphisms or deletions within the MACROD2 gene with autism-spectrum disorders [77,78,79,80]. It is not clear, however, whether the MACROD2 gene product itself or surrounding genes are responsible for this association even though elevated protein expression in neurons supports a potential brain-specific function of MACROD2 [76]. However, a long non-coding RNA has been identified within an intron of the MACROD2 gene, which is more highly expressed in most tissues investigated than the MACROD2 mRNA, and, thereby, potentially confuses correlations of mutations in the MACROD2 gene and phenotypes [81]. In another genome-wide association study, MACROD2 was identified as a factor influencing vascular-adhesion protein-1 (VAP-1) levels, which the authors confirmed by knockdown of MACROD2. This leads to lower VAP-1 expression in adipocytes, presumably through the transcriptional regulation of VAP-1 [82]. MACROD2 was also reported to leave the nucleus upon DNA damage, dependent on phosphorylation by ATM [75], whereas TARG1 localises from nucleoli to nuclear sites of damage [14], which both have an unclear functional relevance. More data may be found on MACROD1, which is also known as leukaemia-related protein 16 or LRP16. MACROD1 has been attributed to a number of functions in the nucleus, such as co-activation of the androgen receptor [83], counteracting PARP7-mediated MARylation in the nucleus [84,85], and activation of NF-kB signalling [86,87,88]. MACROD1 was also reported as an enhancer of oestrogen receptor signalling [89], and is upregulated after stimulation of cells with oestrogen [72,74,90]. No regulatory factors have been identified yet for MACROD2 and TARG1. A BioID interaction screen, which identifies proteins in close proximity to the protein of interest [91], identified many proteins involved in nuclear/cytoplasmic and mitochondrial nucleic acid metabolism as interactors of TARG1 and MACROD1, respectively [70]. Whether these proteins are MARylated and serve as a substrate of TARG1/MACROD1 remains to be determined. It is possible that, despite spatio-temporal restrictions, MACROD1 and TARG1 in their respective compartments are involved in similar signalling networks, converging on the regulation of cellular nucleic acids. The physiological functions of the three enzymes remains elusive. Most puzzling perhaps is that, at the moment, despite the mitochondrial localisation of MACROD1, the majority of reports describe nuclear functions. Future work will need to address this apparent discrepancy.
4. Mono(ADP-ribosyl)ation in Cancer
The post-translational modification poly(ADP-ribosyl)ation has been intimately linked to cancer before [92,93], as have other post-translational modifications such as phosphorylation [94]. In BRCA1/BRCA2-deficient patients, PARP1 inhibitors have been shown effective specifically against the tumour cells applied in the clinic. However, this is one of the rare examples of a synthetic lethal interaction [95]. Better understanding of the processes regulated by MARylation will provide opportunities for further drug development, as is exemplified by current research into the potential of PARP14 as a drug target [24]. Little is known about the potential role of the mono(ADP-ribosyl)hydrolases in cancer. MACROD1, MACROD2, and OARD1 (TARG1) exhibit mutations only in 0.9%, 2.6%, and 1% of cancer patient samples, respectively, from over 1000 samples in the cBioPortal curated dataset [96]. The fact that MACROD2 mutation rates are twice the rates seen for MACROD1 and OARD1 likely reflects that the MACROD2 gene is larger (Figure 1), and is located at a known fragile region [97]. A specific recurring deletion of exon 6 has been observed in esophageal squamous cell carcinoma and gastric cancer [98]. For MACROD1, a RUNX-MACROD1 fusion was identified in leukaemia [99] and, for MACROD2, a PDGFRA-MACROD2 fusion in a pleomorphic sarcoma [100]. Despite the low number of identified mutations in patient tumour samples, several reports have correlated MACROD1 or MACROD2 expression levels with the clinical outcome, as described in the next paragraphs.
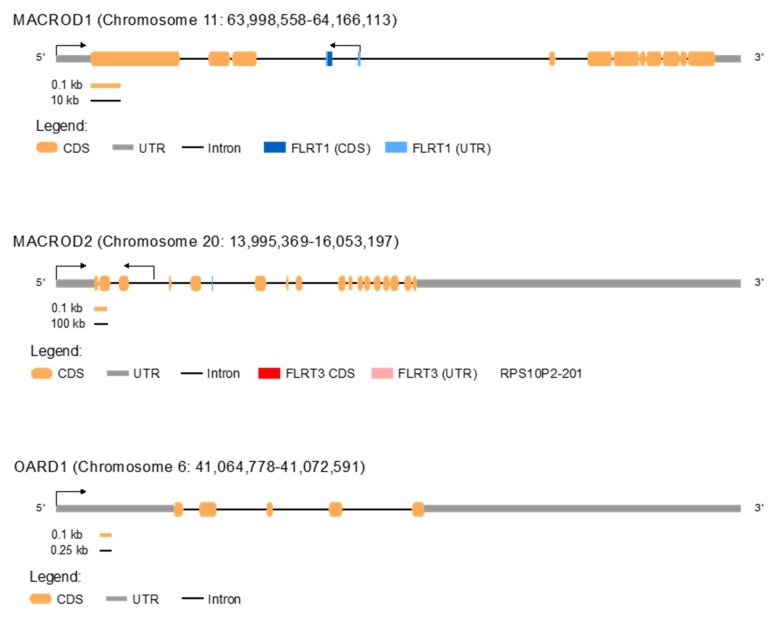
Figure 1.
Overview of the MACROD1, MACROD2, and OARD1 gene structure.
The gene structure of MACROD1, MACROD2, and OARD1 is shown schematically. More lncRNAs are present. However, only RPS10P2-201 is displayed since it has been shown to be relevant RPS10P2-201 [81]. CDS = coding sequence. UTR = untranslated region.
5. MACROD1 in Cancer
A number of studies have addressed a potential oncogenic function of MACROD1 and started deciphering the molecular mechanism underlying observed effects. MACROD1 expression was shown to be upregulated by oestrogen [73,74], which leads to several studies of the role of MACROD1 in tumours with a differential oestrogen status. MACROD1 overexpression in the oestrogen and progesterone receptor positive Ishikawa cells, derived from an endometrial cancer, had no effect on cell proliferation. It did, however, enhance the invasiveness of these cells as measured by transwell assays [101]. Mechanistically, the authors propose a mechanism wherein MACROD1, dependent on oestrogen, blocks recruitment of ERα to the E-cadherin promoter, which lowers E-cadherin expression and, through this, enhances invasiveness. shRNA-mediated knockdown of MACROD1 achieved the opposite effect by enhancing E-cadherin expression [101]. This implies that MACROD1 can be an important factor in metastasis. These findings appear to contradict an earlier report where overexpression of MACROD1 in MCF7 cells, which are oestrogen-responsive breast cancer cells, showed an effect on cell growth. It led to enhanced proliferation [74]. A later report studying MACROD1 in 293T cells showed that knockdown of MACROD1 sensitized cells to TNFα-induced apoptosis [86]. From these studies, it is, thus, not clear what effect loss or gain of MACROD1 has on cell physiology. Recent work has demonstrated that fusing an N-terminal tag, such as GFP, to MACROD1 leads to a nuclear localisation, whereas C-terminally labelled and an endogenous protein appear to be exclusively localised in mitochondria [70]. Mass spectrometry datasets have also detected MACROD1 in the mitochondria [102]. This does not exclude the possibility that, under specific circumstances, MACROD1 may re-localise to the nucleus. In pathogenic conditions, such as the presence of a RUNX-MACROD1 fusion protein that was identified in leukaemia [99], the protein likely also localises to the nucleus instead of the mitochondria, as the RUNX fusion will mask the mitochondrial targeting sequence, comparable to the localisation after labelling with an N-terminal GFP tag. Unfortunately, some of the studies investigating MACROD1 have either used N-terminally tagged fusion constructs [84,85,88], or have not stated clearly how the fusion proteins were generated [103]. Furthermore, the majority of applied antibodies show multiple bands in the western blot and, hence, are not suitable for immunohistochemistry (IHC) or immunofluorescence (IF) (Table 2). It will be worthwhile to repeat some of the studies of MACROD1′s molecular function in carcinogenesis to clarify whether unlabeled MACROD1 overexpression leads to enhanced cell growth, or whether this effect depends on the tumour background and also to study knockdown/knockout systems more thoroughly.
Table 2.
Summary of studies studying the effect of MACROD1 and MACROD2 protein levels on cell and/or tumour growth.
| Cancer Tissue | Protein | Expression | Effect/Prognosis | Antibody Used | Reference |
|---|---|---|---|---|---|
| Neuroendocrine lung tumours | MACROD1 | Elevated | Poorer survival | Monoclonal rabbit antibody against LRP16 Not further specified or validated |
[104] |
| Hepatocellular carcinoma | MACROD1 | Overexpressed: N- or C- tag not specified | Lower cell and tumour growth | Santa Cruz goat polyclonal This antibody is not available anymore. Whole blots are not shown. It was not validated with siRNA |
[103] |
| Pancreatic carcinoma | MACROD1 | Overexpressed: N- or C- tag not specified | Higher cell and tumour growth | Abcam rabbit polyclonal This antibody recognises multiple bands in WB and is thus not suitable for IHC/IF |
[105] |
| Colorectal carcinoma | MACROD1 | Elevated | Poorer survival | Polyclonal rabbit antibody generated by the authors’ institute Not further specified or validated |
[106] |
| Gastric carcinoma | MACROD1 | Elevated | Poorer survival | Polyclonal rabbit antibody generated by the authors’ institute Not further specified or validated |
[107] |
| Breast cancer | MACROD1 | MACROD1 expression quantified as either positive or negative | MACROD1 expression was higher in patients with advanced stages | LRP16 rabbit anti-human antibody, source not given Not further specified or validated |
[108] |
| Endometrial cancer | MACROD1 | Overexpressed | No effect on proliferation but enhanced invasion | Antiserum generated in rabbits against amino acids 83-324 | [101] |
| Breast cancer | MACROD1 | Overexpression | Increased proliferation | Not specified | [74] |
| Colorectal carcinoma | MACROD1 | Non-tagged and N-terminal flag-tagged overexpression | Confers resistance to chemotherapeutics | Antibody used for IHC and WB not specified, recognises bands at ±35 and ±45 kDa | [88] |
| Tumours induced in mice by sublethal irradiation | MACROD2 | Knockout mice | No difference between wildtype and MacroD2-/- | Thermofisher PA5-45950 This antibody recognises bands at ±38 kDa and ±22 kDa |
[109] |
Despite these technical challenges, multiple studies have correlated tumour growth with MACROD1 expression in either xenograft models or in patient cohorts. Overexpression of MACROD1 in the hepatocellular carcinoma lines HepG2 and MHCC-97L leads to a decrease in cell growth and metastatic potential, as measured by transwell assays [103]. Furthermore, when delivered into nude mice, cells overexpressing MACROD1 lead to a decreased tumour volume and have a lower metastatic potential compared to cells without overexpressed MACROD1 [103]. With the information available, it cannot be distinguished whether this is a genuine effect or an artefact due to forced nuclear localisation of the overexpression construct [103]. In a similar set of experiments in the pancreatic carcinoma cell lines, Panc1, CFPAC1, Bxpc3, SW1990, AsPC1, and HPDE6-C7, opposite results were achieved [105]. Knockdown of MACROD1 leads to enhanced apoptosis and decreased cell growth. However, only one shRNA construct was used, so any effects seen could be potentially off-target. Cells overexpressing the same MACROD1 construct as in the study described before [103] grew faster and were more resistant to apoptosis. Xenograft experiments show the same trend. Cells lacking MACROD1 had a lower tumour-forming potential and higher survival rate. Cells overexpressing MACROD1 had a higher tumour volume and a lower survival rate [105]. The authors do not comment on the opposing effects in these two tumour types, but agree that larger-scale studies are required to verify these findings. A third study shows IHC of lung tumour samples with an antibody of an unknown source and specificity [104]. High MACROD1 expression, as measured by IHC, was reported to correlate with a negative outcome in colorectal carcinoma and in gastric carcinoma. However, the antibody used was generated by the authors and not validated by a western blot [106,107]. CRISPR-mediated MACROD1 knockout rhabdomyosarcoma cells [70] and MACROD1 knockout mice appear viable [71], which makes it unlikely that loss of MACROD1 has a drastic growth inhibitory or developmental effect.
In conclusion, most of the data available on an oncogenic function of MACROD1 rely on poorly characterised antibodies, unclear overexpression constructs or a single shRNA construct. The majority of these studies would need to be reproduced with a more thorough characterisation and description of the materials used to be able to draw deeper conclusions. Several studies agree that MACROD1 expression can be induced by oestrogen [73,74,89,90,101], but it remains unclear what the effect of this overexpression is on cells. The RUNX-MACROD1 fusion identified in leukaemia may provide an important hint at a potential pathologic function. It is possible that the physiological mitochondrial localisation of MACROD1 can turn into a pathogenic nuclear one, where it aberrantly acts as a transcriptional activator. It will be interesting to see whether more instances can be identified where such fusions are present. Alternatively, other masking events may occur in cells, such as binding by interaction partners or PTMs, which, thereby, redirects the protein to the nucleus for a physiological function.
6. MACROD2 in Cancer
A number of analyses suggest MACROD2 may play a potential role in cancer. MACROD2 copy number is increased in three different tamoxifen-resistant MCF7 breast cancer cell lines, prompting the authors to analyse MACROD2 expression in patient samples. In oestrogen receptor-positive tissues of breast cancer patients with a recorded tamoxifen-resistance, however, three patients displayed a decreased copy number, whereas the other two patients showed an increase. Using IHC with a custom antibody, varying levels of MACROD2 were detected in primary and secondary tumour tissues of these patients, collectively showing that MACROD2 may be overexpressed in cancer tissues. MCF7 and T47D cells with exogenously overexpressed MACROD2 grow faster than control cells in media containing tamoxifen, which implies that MACROD2 confers tamoxifen-resistance to the cells and appear to be stimulated by tamoxifen, as demonstrated by faster growth in medium with tamoxifen than without [110]. Conversely, tamoxifen-resistant MCF7 clones with shRNA-mediated knockdown of MACROD2 become more sensitive even though it does not completely reverse resistance [110]. This may be mediated by the activation of oestrogen-regulated genes in response to tamoxifen in cells overexpressing MACROD2. Lastly, cells stably expressing shRNA to knockdown MACROD2 grow markedly slower in nude mice [110]. The authors argue that, as a cancer specific fragile site, the MACROD2 gene can be lost, but this fragility also allows for amplification in the specific case of ER-positive breast cancers treated with tamoxifen to incur resistance. A more recent study showed that, in approximately one-third of colorectal carcinomas investigated, heterozygous or homozygous losses were mapped within the MACROD2 gene in which the majority are intragenic microdeletions mapping to a region in exons 4 to 5 [111]. To test whether MACROD2 deficiency can promote tumourigenesis, MACROD2 knockout was introduced into an adenomatous polyposis coli protein (Apc) mouse model. Mutations in the APC tumour suppressor are a major driver of sporadic colorectal cancers, where it could be shown that even haploinsufficiency of MACROD2 leads to more and larger adenoma formation [111]. Furthermore, human cells transplanted into nude mice displayed increased tumour growth when lacking MACROD2, but a reduced tumour growth when MACROD2 was overexpressed [111]. The underlying mechanism was suggested to be impaired PARP1 activity in the MACROD2-/- cells, which leads to increased sensitivity of DNA damage and, ultimately, causes enhanced chromosomal instability [111,112].
These findings appear paradoxical with the previous report, where loss of MACROD2 impairs cell growth from a loss of resistance to tamoxifen. MacroD2 knockout mice do not show altered survival rates after sub-lethal irradiation compared to wildtype mice, which indicates that loss of MACROD2 alone is not sufficient to drive tumourigenesis triggered by DNA damage [109]. It is not clear whether loss or overexpression of MACROD2 contributes to tumourigenesis, or whether both can drive tumour growth dependent on conditions, such as the functionality of the DNA damage repair systems or the oestrogen receptor status. In an investigation of stage-III colon cancer, MACROD2 expression determined by immunohistochemistry was found to correlate with poor survival [113]. A human protein atlas antibody (HPA049076) was used for this work, which has been retracted by the company in the meantime and did not appear to be validated in any way, such as western blotting or siRNA-mediated knockdown. It is, thus, not clear whether this antibody recognises MACROD2 at all, or whether it recognises additional proteins, which may be upregulated in the samples analysed. Altogether, it appears that loss of MACROD2 as such is not sufficient to drive tumourigenesis, but may have an additive effect in models prone to tumour formation such as loss of APC in colorectal cells. More studies are urgently needed to clarify the exact role of MACROD2 in both the onset of cancer as well as in the response of existing tumours to therapies.
7. TARG1 in Cancer
The only phenotype associated with TARG1 expression is neurodegeneration, occurring due to a mutation that leads to a truncated protein with a disrupted macrodomain, and, thereby, loss of catalytic activity [45]. It is not clear whether this neurodegeneration is a result of a potentially toxic truncated or unfolded protein or from loss of hydrolase activity. Knockdown of TARG1 leads to a decrease in 293T cell proliferation and a slight increase in senescence in U2OS cells, which are derived from an osteosarcoma [45]. CRISPR-mediated knockdown of TARG1 does not influence HeLa or U2OS proliferation [14,70], which leaves it unclear in which setting TARG1 is required for cell growth. Overexpression does not lead to changes in cell proliferation [14,70]. We could not identify any data linking TARG1 to cancer, nor see elevated expression or mutation in databases such as COSMIC. Based on expression levels in databases and these experimental results, at this stage, it does appear unlikely that TARG1 is involved in cancer even though further experimentation is required.
8. Conclusions
Despite the presence of several publications reporting a correlation between MACROD1 or MACROD2 levels and cancer development or progression, it is not clear at the moment that they have a causative role. MACROD2 is potentially relevant in ER-positive, tamoxifen resistant breast cancers where it may confer resistance to treatment. However, larger cohorts of patient samples need to be analysed to further substantiate these initial findings. Loss of MACROD2 in an APC null background potentially stimulates tumour formation. However, loss of MacroD2 alone does not have any effect in a knockout mouse model. Detailed studies are required to determine how loss of MACROD2 might cooperate with loss of APC to drive cancer. MACROD1 levels are upregulated by oestrogen with unclear consequences for cells. Yet, it may down-regulate E-cadherin and, thereby, promote metastasis. If confirmed, this may be an attractive therapeutic target intending to keep the cancer dormant and prevent metastasis.
Conflicting data show that overexpression and knockdown of MACROD1 have no effect, no stimulus, and no inhibition of cell growth, which implies that, perhaps, inhibiting MACROD1 may not have detrimental effects for the whole organism but rather may be dependent on the tumour background and, thus, may represent a valid drug target. The effects observed may, however, be dependent on the constructs used as well as on the specific cell-types and have to be studied in more detail. Future work will have to dissect in which context loss or gain of MACROD1 may be driving aspects of cancer growth. The recent development and characterisation of more specific antibodies, the ongoing improvements of mass spectrometric measurements of MARylation, and also the attempts at making specific inhibitors for both transferases and hydrolases should allow a more detailed analysis of their (patho-)physiological function in the near future. The partially paradoxical findings described will undoubtedly be clarified with better validated tools to determine the extent to which MACROD1, MACROD2, and TARG1 are relevant for tumourigenesis in order to establish their potential as drug candidates.
Author Contributions
Writing, review, and editing: K.L.H.F., C.D.O.C., and R.Ž. All authors have read and agreed to the published version of the manuscript.
Funding
The START Program of the Medical Faculty, RWTH Aachen University to K.L.H.F. (10/18) and to R.Z (13/20) funded this research.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Minguez P., Letunic I., Parca L., Garcia-Alonso L., Dopazo J., Huerta-Cepas J., Bork P. PTMcode v2: A resource for functional associations of post-translational modifications within and between proteins. Nucleic Acids Res. 2015;43:D494–D502. doi: 10.1093/nar/gku1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Virag L. 50 years of poly(ADP-ribosyl)ation. Mol. Asp. Med. 2013;34:1043–1045. doi: 10.1016/j.mam.2013.05.002. [DOI] [PubMed] [Google Scholar]
- 3.Slade D. PARP and PARG inhibitors in cancer treatment. Genes Dev. 2020 doi: 10.1101/gad.334516.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kleine H., Poreba E., Lesniewicz K., Hassa P.O., Hottiger M.O., Litchfield D.W., Shilton B.H., Luscher B. Substrate-Assisted catalysis by PARP10 limits its activity to mono-ADP-ribosylation. Mol. Cell. 2008;32:57–69. doi: 10.1016/j.molcel.2008.08.009. [DOI] [PubMed] [Google Scholar]
- 5.Vyas S., Matic I., Uchima L., Rood J., Zaja R., Hay R.T., Ahel I., Chang P. Family-Wide analysis of poly(ADP-ribose) polymerase activity. Nat. Commun. 2014;5:4426. doi: 10.1038/ncomms5426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Belousova E.A., Ishchenko A.A., Lavrik O.I. DNA is a New Target of Parp3. Sci. Rep. 2018;8:4176. doi: 10.1038/s41598-018-22673-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zarkovic G., Belousova E.A., Talhaoui I., Saint-Pierre C., Kutuzov M.M., Matkarimov B.T., Biard D., Gasparutto D., Lavrik O.I., Ishchenko A.A. Characterization of DNA ADP-ribosyltransferase activities of PARP2 and PARP3: New insights into DNA ADP-ribosylation. Nucleic Acids Res. 2018;46:2417–2431. doi: 10.1093/nar/gkx1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rodriguez-Vargas J.M., Nguekeu-Zebaze L., Dantzer F. PARP3 comes to light as a prime target in cancer therapy. Cell Cycle. 2019;18:1295–1301. doi: 10.1080/15384101.2019.1617454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Beck C., Rodriguez-Vargas J.M., Boehler C., Robert I., Heyer V., Hanini N., Gauthier L.R., Tissier A., Schreiber V., Elofsson M., et al. PARP3, a new therapeutic target to alter Rictor/mTORC2 signaling and tumor progression in BRCA1-associated cancers. Cell Death Differ. 2019;26:1615–1630. doi: 10.1038/s41418-018-0233-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sharif-Askari B., Amrein L., Aloyz R., Panasci L. PARP3 inhibitors ME0328 and olaparib potentiate vinorelbine sensitization in breast cancer cell lines. Breast Cancer Res. Treat. 2018;172:23–32. doi: 10.1007/s10549-018-4888-6. [DOI] [PubMed] [Google Scholar]
- 11.Oplustil O’Connor L., Rulten S.L., Cranston A.N., Odedra R., Brown H., Jaspers J.E., Jones L., Knights C., Evers B., Ting A., et al. The PARP inhibitor AZD2461 provides insights into the role of PARP3 inhibition for both synthetic lethality and tolerability with chemotherapy in preclinical models. Cancer Res. 2016;76:6084–6094. doi: 10.1158/0008-5472.CAN-15-3240. [DOI] [PubMed] [Google Scholar]
- 12.Munnur D., Bartlett E., Mikolcevic P., Kirby I.T., Matthias Rack J.G., Mikoc A., Cohen M.S., Ahel I. Reversible ADP-ribosylation of RNA. Nucleic Acids Res. 2019;47:5658–5669. doi: 10.1093/nar/gkz305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yu M., Schreek S., Cerni C., Schamberger C., Lesniewicz K., Poreba E., Vervoorts J., Walsemann G., Grotzinger J., Kremmer E., et al. PARP-10, a novel Myc-interacting protein with poly(ADP-ribose) polymerase activity, inhibits transformation. Oncogene. 2005;24:1982–1993. doi: 10.1038/sj.onc.1208410. [DOI] [PubMed] [Google Scholar]
- 14.Butepage M., Preisinger C., von Kriegsheim A., Scheufen A., Lausberg E., Li J., Kappes F., Feederle R., Ernst S., Eckei L., et al. Nucleolar-Nucleoplasmic shuttling of TARG1 and its control by DNA damage-induced poly-ADP-ribosylation and by nucleolar transcription. Sci. Rep. 2018;8:6748. doi: 10.1038/s41598-018-25137-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shahrour M.A., Nicolae C.M., Edvardson S., Ashhab M., Galvan A.M., Constantin D., Abu-Libdeh B., Moldovan G.L., Elpeleg O. PARP10 deficiency manifests by severe developmental delay and DNA repair defect. Neurogenetics. 2016;17:227–232. doi: 10.1007/s10048-016-0493-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Herzog N., Hartkamp J.D., Verheugd P., Treude F., Forst A.H., Feijs K.L., Lippok B.E., Kremmer E., Kleine H., Luscher B. Caspase-Dependent cleavage of the mono-ADP-ribosyltransferase ARTD10 interferes with its pro-apoptotic function. FEBS J. 2013;280:1330–1343. doi: 10.1111/febs.12124. [DOI] [PubMed] [Google Scholar]
- 17.Schleicher E.M., Galvan A.M., Imamura-Kawasawa Y., Moldovan G.L., Nicolae C.M. PARP10 promotes cellular proliferation and tumorigenesis by alleviating replication stress. Nucleic Acids Res. 2018;46:8908–8916. doi: 10.1093/nar/gky658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nicolae C.M., Aho E.R., Vlahos A.H., Choe K.N., De S., Karras G.I., Moldovan G.L. The ADP-ribosyltransferase PARP10/ARTD10 interacts with proliferating cell nuclear antigen (PCNA) and is required for DNA damage tolerance. J. Biol. Chem. 2014;289:13627–13637. doi: 10.1074/jbc.M114.556340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Marton J., Fodor T., Nagy L., Vida A., Kis G., Brunyanszki A., Antal M., Luscher B., Bai P. PARP10 (ARTD10) modulates mitochondrial function. PLoS ONE. 2018;13:e0187789. doi: 10.1371/journal.pone.0187789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Feijs K.L., Kleine H., Braczynski A., Forst A.H., Herzog N., Verheugd P., Linzen U., Kremmer E., Luscher B. ARTD10 substrate identification on protein microarrays: Regulation of GSK3beta by mono-ADP-ribosylation. Cell Commun. Signal. 2013;11:5. doi: 10.1186/1478-811X-11-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Troiani S., Lupi R., Perego R., Depaolini S.R., Thieffine S., Bosotti R., Rusconi L. Identification of candidate substrates for poly(ADP-ribose) polymerase-2 (PARP2) in the absence of DNA damage using high-density protein microarrays. FEBS J. 2011;278:3676–3687. doi: 10.1111/j.1742-4658.2011.08286.x. [DOI] [PubMed] [Google Scholar]
- 22.Verheugd P., Forst A.H., Milke L., Herzog N., Feijs K.L., Kremmer E., Kleine H., Luscher B. Regulation of NF-kappaB signalling by the mono-ADP-ribosyltransferase ARTD10. Nat. Commun. 2013;4:1683. doi: 10.1038/ncomms2672. [DOI] [PubMed] [Google Scholar]
- 23.Schweiker S.S., Tauber A.L., Sherry M.E., Levonis S.M. Structure, function and inhibition of poly(ADP-ribose)polymerase, member 14 (PARP14) Mini Rev. Med. Chem. 2018;18:1659–1669. doi: 10.2174/1389557518666180816111749. [DOI] [PubMed] [Google Scholar]
- 24.Qin W., Wu H.J., Cao L.Q., Li H.J., He C.X., Zhao D., Xing L., Li P.Q., Jin X., Cao H.L. Research progress on PARP14 as a drug target. Front. Pharmacol. 2019;10:172. doi: 10.3389/fphar.2019.00172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jwa M., Chang P. PARP16 is a tail-anchored endoplasmic reticulum protein required for the PERK-and IRE1α-mediated unfolded protein response. Nat. Cell Biol. 2012;14:1223–1230. doi: 10.1038/ncb2593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Di Paola S., Micaroni M., Di Tullio G., Buccione R., Di Girolamo M. PARP16/ARTD15 is a novel endoplasmic-reticulum-associated mono-ADP-ribosyltransferase that interacts with, and modifies karyopherin-ss1. PLoS ONE. 2012;7:e37352. doi: 10.1371/journal.pone.0037352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Atasheva S., Frolova E.I., Frolov I. Interferon-Stimulated poly(ADP-Ribose) polymerases are potent inhibitors of cellular translation and virus replication. J. Virol. 2014;88:2116–2130. doi: 10.1128/JVI.03443-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Atasheva S., Akhrymuk M., Frolova E.I., Frolov I. New PARP gene with an anti-alphavirus function. J. Virol. 2012;86:8147–8160. doi: 10.1128/JVI.00733-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Imai S.I., Guarente L. It takes two to tango: NAD+ and sirtuins in aging/longevity control. NPJ Aging Mech. Dis. 2016;2:16017. doi: 10.1038/npjamd.2016.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Feldman J.L., Dittenhafer-Reed K.E., Denu J.M. Sirtuin catalysis and regulation. J. Biol. Chem. 2012;287:42419–42427. doi: 10.1074/jbc.R112.378877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Du J., Jiang H., Lin H. Investigating the ADP-ribosyltransferase activity of sirtuins with NAD analogues and 32P-NAD. Biochemistry. 2009;48:2878–2890. doi: 10.1021/bi802093g. [DOI] [PubMed] [Google Scholar]
- 32.Haigis M.C., Mostoslavsky R., Haigis K.M., Fahie K., Christodoulou D.C., Murphy A.J., Valenzuela D.M., Yancopoulos G.D., Karow M., Blander G., et al. SIRT4 inhibits glutamate dehydrogenase and opposes the effects of calorie restriction in pancreatic beta cells. Cell. 2006;126:941–954. doi: 10.1016/j.cell.2006.06.057. [DOI] [PubMed] [Google Scholar]
- 33.Mao Z., Hine C., Tian X., Van Meter M., Au M., Vaidya A., Seluanov A., Gorbunova V. SIRT6 promotes DNA repair under stress by activating PARP1. Science. 2011;332:1443–1446. doi: 10.1126/science.1202723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rezazadeh S., Yang D., Tombline G., Simon M., Regan S.P., Seluanov A., Gorbunova V. SIRT6 promotes transcription of a subset of NRF2 targets by mono-ADP-ribosylating BAF170. Nucleic Acids Res. 2019;47:7914–7928. doi: 10.1093/nar/gkz528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mitra N., Dey S. Biochemical characterization of mono ADP ribosyl transferase activity of human sirtuin SIRT7 and its regulation. Arch. Biochem. Biophys. 2019;680:108226. doi: 10.1016/j.abb.2019.108226. [DOI] [PubMed] [Google Scholar]
- 36.Luscher B., Butepage M., Eckei L., Krieg S., Verheugd P., Shilton B.H. ADP-Ribosylation, a multifaceted posttranslational modification involved in the control of cell physiology in health and disease. Chem. Rev. 2018;118:1092–1136. doi: 10.1021/acs.chemrev.7b00122. [DOI] [PubMed] [Google Scholar]
- 37.Pehrson J.R., Fried V.A. MacroH2A, a core histone containing a large nonhistone region. Science. 1992;257:1398–1400. doi: 10.1126/science.1529340. [DOI] [PubMed] [Google Scholar]
- 38.Timinszky G., Till S., Hassa P.O., Hothorn M., Kustatscher G., Nijmeijer B., Colombelli J., Altmeyer M., Stelzer E.H., Scheffzek K., et al. A macrodomain-containing histone rearranges chromatin upon sensing PARP1 activation. Nat. Struct. Mol. Biol. 2009;16:923–929. doi: 10.1038/nsmb.1664. [DOI] [PubMed] [Google Scholar]
- 39.Rack J.G., Perina D., Ahel I. Macrodomains: Structure, function, evolution, and catalytic activities. Annu. Rev. Biochem. 2016;85:431–454. doi: 10.1146/annurev-biochem-060815-014935. [DOI] [PubMed] [Google Scholar]
- 40.Neuvonen M., Ahola T. Differential activities of cellular and viral macro domain proteins in binding of ADP-ribose metabolites. J. Mol. Biol. 2009;385:212–225. doi: 10.1016/j.jmb.2008.10.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Karras G.I., Kustatscher G., Buhecha H.R., Allen M.D., Pugieux C., Sait F., Bycroft M., Ladurner A.G. The macro domain is an ADP-ribose binding module. EMBO J. 2005;24:1911–1920. doi: 10.1038/sj.emboj.7600664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kustatscher G., Hothorn M., Pugieux C., Scheffzek K., Ladurner A.G. Splicing regulates NAD metabolite binding to histone macroH2A. Nat. Struct. Mol. Biol. 2005;12:624–625. doi: 10.1038/nsmb956. [DOI] [PubMed] [Google Scholar]
- 43.Feijs K.L., Forst A.H., Verheugd P., Luscher B. Macrodomain-Containing proteins: Regulating new intracellular functions of mono(ADP-ribosyl)ation. Nat. Rev. Mol. Cell Biol. 2013;14:443–451. doi: 10.1038/nrm3601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Slade D., Dunstan M.S., Barkauskaite E., Weston R., Lafite P., Dixon N., Ahel M., Leys D., Ahel I. The structure and catalytic mechanism of a poly(ADP-ribose) glycohydrolase. Nature. 2011;477:616–620. doi: 10.1038/nature10404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sharifi R., Morra R., Appel C.D., Tallis M., Chioza B., Jankevicius G., Simpson M.A., Matic I., Ozkan E., Golia B., et al. Deficiency of terminal ADP-ribose protein glycohydrolase TARG1/C6orf130 in neurodegenerative disease. EMBO J. 2013;32:1225–1237. doi: 10.1038/emboj.2013.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jankevicius G., Hassler M., Golia B., Rybin V., Zacharias M., Timinszky G., Ladurner A.G. A family of macrodomain proteins reverses cellular mono-ADP-ribosylation. Nat. Struct. Mol. Biol. 2013;20:508–514. doi: 10.1038/nsmb.2523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rosenthal F., Feijs K.L., Frugier E., Bonalli M., Forst A.H., Imhof R., Winkler H.C., Fischer D., Caflisch A., Hassa P.O., et al. Macrodomain-Containing proteins are new mono-ADP-ribosylhydrolases. Nat. Struct. Mol. Biol. 2013;20:502–507. doi: 10.1038/nsmb.2521. [DOI] [PubMed] [Google Scholar]
- 48.Dudkiewicz M., Pawlowski K. A novel conserved family of macro-like domains-putative new players in ADP-ribosylation signaling. PeerJ. 2019;7:e6863. doi: 10.7717/peerj.6863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Egloff M.P., Malet H., Putics A., Heinonen M., Dutartre H., Frangeul A., Gruez A., Campanacci V., Cambillau C., Ziebuhr J., et al. Structural and functional basis for ADP-ribose and poly(ADP-ribose) binding by viral macro domains. J. Virol. 2006;80:8493–8502. doi: 10.1128/JVI.00713-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Eckei L., Krieg S., Butepage M., Lehmann A., Gross A., Lippok B., Grimm A.R., Kummerer B.M., Rossetti G., Luscher B., et al. The conserved macrodomains of the non-structural proteins of Chikungunya virus and other pathogenic positive strand RNA viruses function as mono-ADP-ribosylhydrolases. Sci. Rep. 2017;7:41746. doi: 10.1038/srep41746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fehr A.R., Channappanavar R., Jankevicius G., Fett C., Zhao J., Athmer J., Meyerholz D.K., Ahel I., Perlman S. The conserved Coronavirus macrodomain promotes virulence and suppresses the innate immune response during severe acute respiratory syndrome Coronavirus infection. mBio. 2016:7. doi: 10.1128/mBio.01721-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Li C., Debing Y., Jankevicius G., Neyts J., Ahel I., Coutard B., Canard B. Viral macro domains reverse protein ADP-ribosylation. J. Virol. 2016;90:8478–8486. doi: 10.1128/JVI.00705-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Daugherty M.D., Young J.M., Kerns J.A., Malik H.S. Rapid evolution of PARP genes suggests a broad role for ADP-ribosylation in host-virus conflicts. PLoS Genet. 2014;10:e1004403. doi: 10.1371/journal.pgen.1004403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tanuma S.I., Shibui Y., Oyama T., Uchiumi F., Abe H. Targeting poly(ADP-ribose) glycohydrolase to draw apoptosis codes in cancer. Biochem. Pharm. 2019;167:163–172. doi: 10.1016/j.bcp.2019.06.004. [DOI] [PubMed] [Google Scholar]
- 55.Ma N.F., Hu L., Fung J.M., Xie D., Zheng B.J., Chen L., Tang D.J., Fu L., Wu Z., Chen M., et al. Isolation and characterization of a novel oncogene, amplified in liver cancer 1, within a commonly amplified region at 1q21 in hepatocellular carcinoma. Hepatology. 2008;47:503–510. doi: 10.1002/hep.22072. [DOI] [PubMed] [Google Scholar]
- 56.Peterson F.C., Chen D., Lytle B.L., Rossi M.N., Ahel I., Denu J.M., Volkman B.F. Orphan macrodomain protein (human C6orf130) is an O-acyl-ADP-ribose deacylase: Solution structure and catalytic properties. J. Biol. Chem. 2011;286:35955–35965. doi: 10.1074/jbc.M111.276238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chen D., Vollmar M., Rossi M.N., Phillips C., Kraehenbuehl R., Slade D., Mehrotra P.V., von Delft F., Crosthwaite S.K., Gileadi O., et al. Identification of macrodomain proteins as novel O-acetyl-ADP-ribose deacetylases. J. Biol. Chem. 2011;286:13261–13271. doi: 10.1074/jbc.M110.206771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tong L., Denu J.M. Function and metabolism of sirtuin metabolite O-acetyl-ADP-ribose. Biochim. Biophys. Acta. 2010;1804:1617–1625. doi: 10.1016/j.bbapap.2010.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wang M., Yuan Z., Xie R., Ma Y., Liu X., Yu X. Structure-Function analyses reveal the mechanism of the ARH3-dependent hydrolysis of ADP-ribosylation. J. Biol. Chem. 2018;293:14470–14480. doi: 10.1074/jbc.RA118.004284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Abplanalp J., Leutert M., Frugier E., Nowak K., Feurer R., Kato J., Kistemaker H.V.A., Filippov D.V., Moss J., Caflisch A., et al. Proteomic analyses identify ARH3 as a serine mono-ADP-ribosylhydrolase. Nat. Commun. 2017;8:2055. doi: 10.1038/s41467-017-02253-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Fontana P., Bonfiglio J.J., Palazzo L., Bartlett E., Matic I., Ahel I. Serine ADP-ribosylation reversal by the hydrolase ARH3. eLife. 2017;6 doi: 10.7554/eLife.28533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Moss J., Stanley S.J., Nightingale M.S., Murtagh J.J., Jr., Monaco L., Mishima K., Chen H.C., Williamson K.C., Tsai S.C. Molecular and immunological characterization of ADP-ribosylarginine hydrolases. J. Biol. Chem. 1992;267:10481–10488. [PubMed] [Google Scholar]
- 63.Munnur D., Ahel I. Reversible mono-ADP-ribosylation of DNA breaks. FEBS J. 2017;284:4002–4016. doi: 10.1111/febs.14297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Stevens L.A., Kato J., Kasamatsu A., Oda H., Lee D.Y., Moss J. The ARH and macrodomain families of α-ADP-ribose-acceptor hydrolases catalyze α-NAD+ hydrolysis. ACS Chem. Biol. 2019;14:2576–2584. doi: 10.1021/acschembio.9b00429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gibson B.A., Conrad L.B., Huang D., Kraus W.L. Generation and characterization of recombinant antibody-like ADP-ribose binding proteins. Biochemistry. 2017;56:6305–6316. doi: 10.1021/acs.biochem.7b00670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lu A.Z., Abo R., Ren Y., Gui B., Mo J.R., Blackwell D., Wigle T., Keilhack H., Niepel M. Enabling drug discovery for the PARP protein family through the detection of mono-ADP-ribosylation. Biochem. Pharmacol. 2019;167:97–106. doi: 10.1016/j.bcp.2019.05.007. [DOI] [PubMed] [Google Scholar]
- 67.Butepage M., Krieg S., Eckei L., Li J., Rossetti G., Verheugd P., Luscher B. Assessment of intracellular auto-modification levels of ARTD10 using mono-ADP-ribose-specific macrodomains 2 and 3 of murine Artd8. Methods Mol. Biol. 2018;1813:41–63. doi: 10.1007/978-1-4939-8588-3_4. [DOI] [PubMed] [Google Scholar]
- 68.Haikarainen T., Maksimainen M.M., Obaji E., Lehtio L. Development of an inhibitor screening assay for mono-ADP-ribosyl hydrolyzing macrodomains using AlphaScreen technology. SLAS Discov. 2018;23:255–263. doi: 10.1177/2472555217737006. [DOI] [PubMed] [Google Scholar]
- 69.Moustakim M., Riedel K., Schuller M., Gehring A.P., Monteiro O.P., Martin S.P., Fedorov O., Heer J., Dixon D.J., Elkins J.M., et al. Discovery of a novel allosteric inhibitor scaffold for polyadenosine-diphosphate-ribose polymerase 14 (PARP14) macrodomain 2. Bioorg. Med. Chem. 2018;26:2965–2972. doi: 10.1016/j.bmc.2018.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Žaja R., Aydin G., Lippok B.E., Lüscher B., Feijs K.L. A comparative analysis of MacroD1, MACROD2 and TARG1 expression, localisation and interactome. Sci. Rep. doi: 10.1038/s41598-020-64623-y. under review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Agnew T., Munnur D., Crawford K., Palazzo L., Mikoc A., Ahel I. MacroD1 is a promiscuous ADP-ribosyl hydrolase localized to mitochondria. Front. Microbiol. 2018;9:20. doi: 10.3389/fmicb.2018.00020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Han W.D., Si Y.L., Zhao Y.L., Li Q., Wu Z.Q., Hao H.J., Song H.J. GC-Rich promoter elements maximally confers estrogen-induced transactivation of LRP16 gene through ERalpha/Sp1 interaction in MCF-7 cells. J. Steroid Biochem. Mol. Biol. 2008;109:47–56. doi: 10.1016/j.jsbmb.2007.12.009. [DOI] [PubMed] [Google Scholar]
- 73.Zhao Y.L., Han W.D., Li Q., Mu Y.M., Lu X.C., Yu L., Song H.J., Li X., Lu J.M., Pan C.Y. Mechanism of transcriptional regulation of LRP16 gene expression by 17-beta estradiol in MCF-7 human breast cancer cells. J. Mol. Endocrinol. 2005;34:77–89. doi: 10.1677/jme.1.01628. [DOI] [PubMed] [Google Scholar]
- 74.Han W.D., Mu Y.M., Lu X.C., Xu Z.M., Li X.J., Yu L., Song H.J., Li M., Lu J.M., Zhao Y.L., et al. Up-Regulation of LRP16 mRNA by 17beta-estradiol through activation of estrogen receptor alpha (ERalpha), but not ERbeta, and promotion of human breast cancer MCF-7 cell proliferation: A preliminary report. Endocr. Relat. Cancer. 2003;10:217–224. doi: 10.1677/erc.0.0100217. [DOI] [PubMed] [Google Scholar]
- 75.Golia B., Moeller G.K., Jankevicius G., Schmidt A., Hegele A., Preisser J., Tran M.L., Imhof A., Timinszky G. ATM induces MACROD2 nuclear export upon DNA damage. Nucleic Acids Res. 2017;45:244–254. doi: 10.1093/nar/gkw904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ito H., Morishita R., Mizuno M., Kawamura N., Tabata H., Nagata K.I. Biochemical and morphological characterization of a neurodevelopmental disorder-related mono-ADP-ribosylhydrolase, MACRO domain containing 2. Dev. Neurosci. 2018;40:278–287. doi: 10.1159/000492271. [DOI] [PubMed] [Google Scholar]
- 77.Lombardo B., Esposito D., Iossa S., Vitale A., Verdesca F., Perrotta C., Di Leo L., Costa V., Pastore L., Franze A. Intragenic deletion in MACROD2: A family with complex phenotypes including microcephaly, intellectual disability, polydactyly, renal and pancreatic malformations. Cytogenet. Genome Res. 2019;158:25–31. doi: 10.1159/000499886. [DOI] [PubMed] [Google Scholar]
- 78.Jones R.M., Cadby G., Blangero J., Abraham L.J., Whitehouse A.J.O., Moses E.K. MACROD2 gene associated with autistic-like traits in a general population sample. Psychiatr. Genet. 2014;24:241–248. doi: 10.1097/YPG.0000000000000052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Curran S., Bolton P., Rozsnyai K., Chiocchetti A., Klauck S.M., Duketis E., Poustka F., Schlitt S., Freitag C.M., Lee I., et al. No association between a common single nucleotide polymorphism, rs4141463, in the MACROD2 gene and autism spectrum disorder. Am. J. Med. Genet. B Neuropsychiatr. Genet. 2011;156:633–639. doi: 10.1002/ajmg.b.31201. [DOI] [PubMed] [Google Scholar]
- 80.Anney R., Klei L., Pinto D., Regan R., Conroy J., Magalhaes T.R., Correia C., Abrahams B.S., Sykes N., Pagnamenta A.T., et al. A genome-wide scan for common alleles affecting risk for autism. Hum. Mol. Genet. 2010;19:4072–4082. doi: 10.1093/hmg/ddq307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Bilinovich S.M., Lewis K., Grepo N., Campbell D.B. The long noncoding RNA RPS10P2-AS1 is implicated in autism spectrum disorder risk and modulates gene expression in human neuronal progenitor cells. Front. Genet. 2019;10:970. doi: 10.3389/fgene.2019.00970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Chang Y.C., Hee S.W., Lee W.J., Li H.Y., Chang T.J., Lin M.W., Hung Y.J., Lee I.T., Hung K.Y., Assimes T., et al. Genome-Wide scan for circulating vascular adhesion protein-1 levels: MACROD2 as a potential transcriptional regulator of adipogenesis. J. Diabetes Investig. 2018;9:1067–1074. doi: 10.1111/jdi.12805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Yang J., Zhao Y.L., Wu Z.Q., Si Y.L., Meng Y.G., Fu X.B., Mu Y.M., Han W.D. The single-macro domain protein LRP16 is an essential cofactor of androgen receptor. Endocr. Relat. Cancer. 2009;16:139–153. doi: 10.1677/ERC-08-0150. [DOI] [PubMed] [Google Scholar]
- 84.Bindesboll C., Tan S., Bott D., Cho T., Tamblyn L., MacPherson L., Gronning-Wang L., Nebb H.I., Matthews J. TCDD-Inducible poly-ADP-ribose polymerase (TIPARP/PARP7) mono-ADP-ribosylates and co-activates liver X receptors. Biochem. J. 2016;473:899–910. doi: 10.1042/BJ20151077. [DOI] [PubMed] [Google Scholar]
- 85.Ahmed S., Bott D., Gomez A., Tamblyn L., Rasheed A., Cho T., MacPherson L., Sugamori K.S., Yang Y., Grant D.M., et al. Loss of the mono-ADP-ribosyltransferase, Tiparp, increases sensitivity to dioxin-induced steatohepatitis and lethality. J. Biol. Chem. 2015;290:16824–16840. doi: 10.1074/jbc.M115.660100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wu Z., Li Y., Li X., Ti D., Zhao Y., Si Y., Mei Q., Zhao P., Fu X., Han W. LRP16 integrates into NF-kappaB transcriptional complex and is required for its functional activation. PLoS ONE. 2011;6:e18157. doi: 10.1371/journal.pone.0018157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wu Z., Wang C., Bai M., Li X., Mei Q., Li X., Wang Y., Fu X., Luo G., Han W. An LRP16-containing preassembly complex contributes to NF-kappaB activation induced by DNA double-strand breaks. Nucleic Acids Res. 2015;43:3167–3179. doi: 10.1093/nar/gkv161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Li X., Wu Z., An X., Mei Q., Bai M., Hanski L., Li X., Ahola T., Han W. Blockade of the LRP16-PKR-NF-kappaB signaling axis sensitizes colorectal carcinoma cells to DNA-damaging cytotoxic therapy. eLife. 2017;6 doi: 10.7554/eLife.27301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Han W.D., Zhao Y.L., Meng Y.G., Zang L., Wu Z.Q., Li Q., Si Y.L., Huang K., Ba J.M., Morinaga H., et al. Estrogenically regulated LRP16 interacts with estrogen receptor alpha and enhances the receptor’s transcriptional activity. Endocr. Relat. Cancer. 2007;14:741–753. doi: 10.1677/ERC-06-0082. [DOI] [PubMed] [Google Scholar]
- 90.Tian L., Wu Z., Zhao Y., Meng Y., Si Y., Fu X., Mu Y., Han W. Differential induction of LRP16 by liganded and unliganded estrogen receptor alpha in SKOV3 ovarian carcinoma cells. J. Endocrinol. 2009;202:167–177. doi: 10.1677/JOE-09-0054. [DOI] [PubMed] [Google Scholar]
- 91.Roux K.J., Kim D.I., Burke B. BioID: A screen for protein-protein interactions. Curr. Protoc. Protein Sci. 2013;74 doi: 10.1002/0471140864.ps1923s74. [DOI] [PubMed] [Google Scholar]
- 92.Curtin N.J. PARP inhibitors for cancer therapy. Expert Rev. Mol. Med. 2005;7:1–20. doi: 10.1017/S146239940500904X. [DOI] [PubMed] [Google Scholar]
- 93.Davar D., Beumer J.H., Hamieh L., Tawbi H. Role of PARP inhibitors in cancer biology and therapy. Curr. Med. Chem. 2012;19:3907–3921. doi: 10.2174/092986712802002464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Rush J., Moritz A., Lee K.A., Guo A., Goss V.L., Spek E.J., Zhang H., Zha X.M., Polakiewicz R.D., Comb M.J. Immunoaffinity profiling of tyrosine phosphorylation in cancer cells. Nat. Biotechnol. 2005;23:94–101. doi: 10.1038/nbt1046. [DOI] [PubMed] [Google Scholar]
- 95.Ashworth A., Lord C.J. Synthetic lethal therapies for cancer: What’s next after PARP inhibitors? Nat. Rev. Clin. Oncol. 2018;15:564–576. doi: 10.1038/s41571-018-0055-6. [DOI] [PubMed] [Google Scholar]
- 96.Gao J., Aksoy B.A., Dogrusoz U., Dresdner G., Gross B., Sumer S.O., Sun Y., Jacobsen A., Sinha R., Larsson E., et al. Integrative analysis of complex cancer genomics and clinical profiles using the cBioPortal. Sci. Signal. 2013;6:pl1. doi: 10.1126/scisignal.2004088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Bradley W.E., Raelson J.V., Dubois D.Y., Godin E., Fournier H., Prive C., Allard R., Pinchuk V., Lapalme M., Paulussen R.J., et al. Hotspots of large rare deletions in the human genome. PLoS ONE. 2010;5:e9401. doi: 10.1371/journal.pone.0009401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Hu N., Kadota M., Liu H., Abnet C.C., Su H., Wu H., Freedman N.D., Yang H.H., Wang C., Yan C., et al. Genomic landscape of somatic alterations in esophageal squamous cell carcinoma and gastric cancer. Cancer Res. 2016;76:1714–1723. doi: 10.1158/0008-5472.CAN-15-0338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Imagama S., Abe A., Suzuki M., Hayakawa F., Katsumi A., Emi N., Kiyoi H., Naoe T. LRP16 is fused to RUNX1 in monocytic leukemia cell line with t(11;21)(q13;q22) Eur. J. Haematol. 2007;79:25–31. doi: 10.1111/j.1600-0609.2007.00858.x. [DOI] [PubMed] [Google Scholar]
- 100.Zheng B., Zhang S., Cai W., Wang J., Wang T., Tang N., Shi Y., Luo X., Yan W. Identification of novel fusion transcripts in undifferentiated pleomorphic sarcomas by transcriptome sequencing. Cancer Genom. Proteom. 2019;16:399–408. doi: 10.21873/cgp.20144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Meng Y.G., Han W.D., Zhao Y.L., Huang K., Si Y.L., Wu Z.Q., Mu Y.M. Induction of the LRP16 gene by estrogen promotes the invasive growth of Ishikawa human endometrial cancer cells through the downregulation of E-cadherin. Cell Res. 2007;17:869–880. doi: 10.1038/cr.2007.79. [DOI] [PubMed] [Google Scholar]
- 102.Smith A.C., Robinson A.J. MitoMiner v4.0: An updated database of mitochondrial localization evidence, phenotypes and diseases. Nucleic Acids Res. 2019;47:D1225–D1228. doi: 10.1093/nar/gky1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Shao L., Jing W., Wang L., Pan F., Wu L., Zhang L., Yang P., Hu M., Fan K. LRP16 prevents hepatocellular carcinoma progression through regulation of Wnt/β-catenin signaling. J. Mol. Med. 2018;96:547–558. doi: 10.1007/s00109-018-1639-4. [DOI] [PubMed] [Google Scholar]
- 104.Shao Y., Li X., Lu Y., Liu L., Zhao P. Aberrant LRP16 protein expression in primary neuroendocrine lung tumors. Int. J. Clin. Exp. Pathol. 2015;8:6560–6565. [PMC free article] [PubMed] [Google Scholar]
- 105.Mo Z., Hu M., Yu F., Shao L., Fan K., Jiao S. Leukemia-Related protein 16 (LRP16) promotes tumor growth and metastasis in pancreatic cancer. OncoTargets Ther. 2018;11:1215–1222. doi: 10.2147/OTT.S157914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Xi H.Q., Zhao P., Han W.D. Clinicopathological significance and prognostic value of LRP16 expression in colorectal carcinoma. World J. Gastroenterol. 2010;16:1644–1648. doi: 10.3748/wjg.v16.i13.1644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Li Y.Z., Zhao P., Han W.D. Clinicopathological significance of LRP16 protein in 336 gastric carcinoma patients. World J. Gastroenterol. 2009;15:4833–4837. doi: 10.3748/wjg.15.4833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Yao D.J., Qiao S., Zhang Y., Zhao Y.T., Yuan C.H. Correlation between expression of LRP16, Ki67 and EGFR and breast cancer clinical pathologic factors and prognosis. Eur. Rev. Med. Pharmacol. Sci. 2017;21:47–51. [PubMed] [Google Scholar]
- 109.Lo Re O., Mazza T., Vinciguerra M. Mono-ADP-ribosylhydrolase MACROD2 is dispensable for murine responses to metabolic and genotoxic insults. Front. Genet. 2018;9:654. doi: 10.3389/fgene.2018.00654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Mohseni M., Cidado J., Croessmann S., Cravero K., Cimino-Mathews A., Wong H.Y., Scharpf R., Zabransky D.J., Abukhdeir A.M., Garay J.P., et al. MACROD2 overexpression mediates estrogen independent growth and tamoxifen resistance in breast cancers. Proc. Natl. Acad. Sci. USA. 2014;111:17606–17611. doi: 10.1073/pnas.1408650111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Sakthianandeswaren A., Parsons M.J., Mouradov D., MacKinnon R.N., Catimel B., Liu S., Palmieri M., Love C., Jorissen R.N., Li S., et al. MACROD2 haploinsufficiency impairs catalytic activity of PARP1 and promotes chromosome instability and growth of intestinal tumors. Cancer Discov. 2018;8:988–1005. doi: 10.1158/2159-8290.CD-17-0909. [DOI] [PubMed] [Google Scholar]
- 112.Sakthianandeswaren A., Parsons M.J., Mouradov D., Sieber O.M. MACROD2 deletions cause impaired PARP1 activity and chromosome instability in colorectal cancer. Oncotarget. 2018;9:33056–33058. doi: 10.18632/oncotarget.25887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Van den Broek E., den Uil S.H., Coupe V.M.H., Delis-van Diemen P.M., Bolijn A.S., Bril H., Stockmann H., van Grieken N.C.T., Meijer G.A., Fijneman R.J.A. MACROD2 expression predicts response to 5-FU-based chemotherapy in stage III colon cancer. Oncotarget. 2018;9:29445–29452. doi: 10.18632/oncotarget.25655. [DOI] [PMC free article] [PubMed] [Google Scholar]