Abstract
Background and Aims: Statins are the first-line medication to treating hypercholesterolemia. Several studies have investigated the impact of statins on the risk of hepatocellular carcinoma (HCC). However, the extent to which statins may prevent HCC remains uncertain. Therefore, we performed a meta-analysis of relevant studies to quantify the magnitude of the association between statins use and the risk of HCC. Methods: A systematic literature search of PubMed, EMBASE, Google Scholar, Web of Science, and Scopus was performed for studies published between January 1, 1990, and September 1, 2019, with no restriction of language. Two reviewers independently evaluated the literature and included observational and experimental studies that reported the association between statin use and HCC risk. The random-effect model was used to calculate the overall risk ratio (RR) with a 95% confidence interval (CI), and the heterogeneity among the studies was assessed using the Q statistic and I2 statistic. The Newcastle Ottawa Scale (NOS) was also used to evaluate the quality of the included studies. Results: A total of 24 studies with 59,073 HCC patients was identified. Statin use was associated with a reduced risk of HCC development (RR: 0.54, 95% CI: 0.47–0.61, I2 = 84.39%) compared with nonusers. Moreover, the rate of HCC reduction was also significant among patients with diabetes (RR: 0.44, 95% CI: 0.28–0.70), liver cirrhosis (RR: 0.36, 95% CI: 0.30–0.42), and antiviral therapy (RR: 0.21, 95% CI: 0.08–0.59) compared with nonusers. Conclusion: This study serves as additional evidence supporting the beneficial inhibitory effect of statins on HCC incidence. The subgroup analyses of this study also highlight that statins are significantly associated with a reduced risk of HCC and may help to direct future prevention efforts. Additional large clinical studies are needed to determine whether statins are associated with a lower risk of HCC.
Keywords: Hepatocellular carcinoma, liver cancer, liver cirrhosis, fatty liver, liver fibrosis, statins
1. Introduction
Hepatocellular carcinoma (HCC) is a growing public health issue worldwide and the most common primary malignancy of the liver [1]. HCC has received close attention for being the sixth most frequent type of cancer and the second leading cause of cancer-related mortality worldwide [2]. The incidence rate of HCC patients has increased significantly, predicted to rise to 22 million by 2032 [3]. HCC often develops in patients with chronic liver disease [4]. Chronic liver diseases, such as hepatitis C virus (HCV), hepatitis B virus, nonalcoholic fatty liver disease, autoimmune liver disease, and alcoholic liver disease lead to liver cirrhosis and eventually to HCC [5,6,7]. Liver cirrhosis is present in approximately 98% of HCC patients [8]. Previous studies also reported that patients with diabetes had a greater (two-fold) risk of HCC incidence than those without diabetes [4,9]. Several studies have reported an inverse association between statin use and chronic liver diseases [10,11], including HCC development [12].
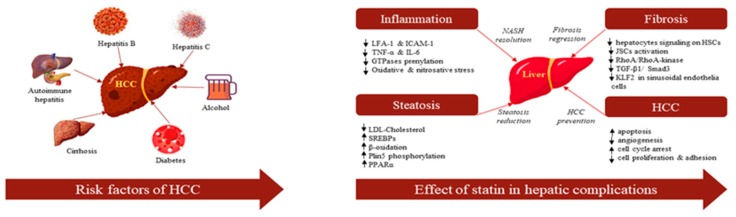
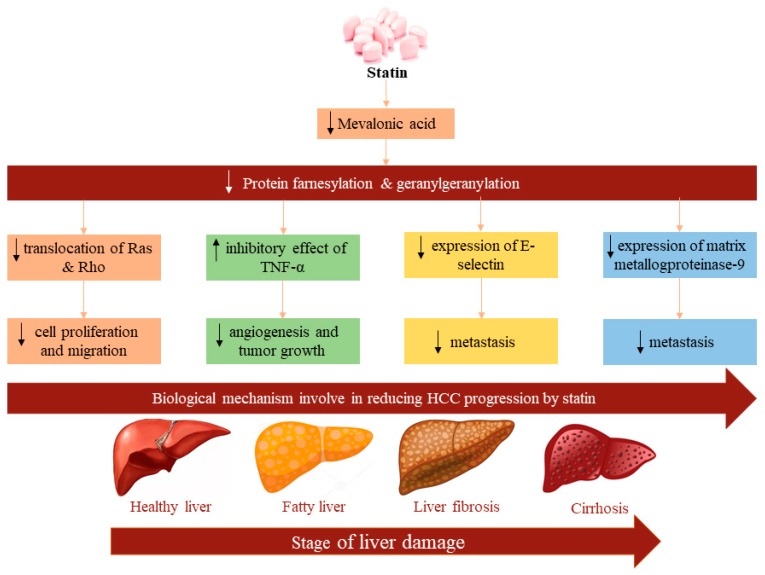
Statins, 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase inhibitors, are commonly used as standard therapy for the management and prevention of cardiovascular disease and stroke by reducing blood cholesterol level [13]. A meta-analysis of ten epidemiological studies involving 4298 patients with HCC demonstrated that statin use reduced HCC incidence (Odd Ratio (OR): 0.63, 95% confidence interval (CI): 0.52–0.76) compared to nonusers [14]. Besides their efficiency in cholesterol reduction in vitro preclinical studies showed that statins also have antiangiogenic, immunomodulatory, antiproliferative, and antifibrotic properties that probably reduce tumor growth or HCC development [15,16]. Moreover, in vivo studies showed promising results in antitumor effects such as the inhibition of cell proliferation and the promotion of tumor cell differentiation in various animal models [17,18]. Statins perhaps help to prevent HCC by suppressing oncogenic pathways including Rho-dependent kinase [19], tumor necrosis factor (TNF)-mediated interleukin (IL6) production [20], Akt [21], Myc-medicated cell proliferation, and so on [22] (Figure 1).
Figure 1.
Risk factors of hepatocellular carcinoma (HCC) and the effect of statins in hepatic diseases.
The beneficial effects of statin use have ubiquitously been reported for liver cancer patients. Therefore, it is important to evaluate their effects in various doses, types, regions, and other disease conditions. To better evaluate the extent to which statins may reduce the risk of HCC, we surveyed recently published relevant studies that investigated the association between statin use and the risk of HCC development. Our primary objective was to resolve discrepancies and to measure the nature and magnitude of the association between statins and the risk of HCC development.
2. Results
2.1. Literature Screening
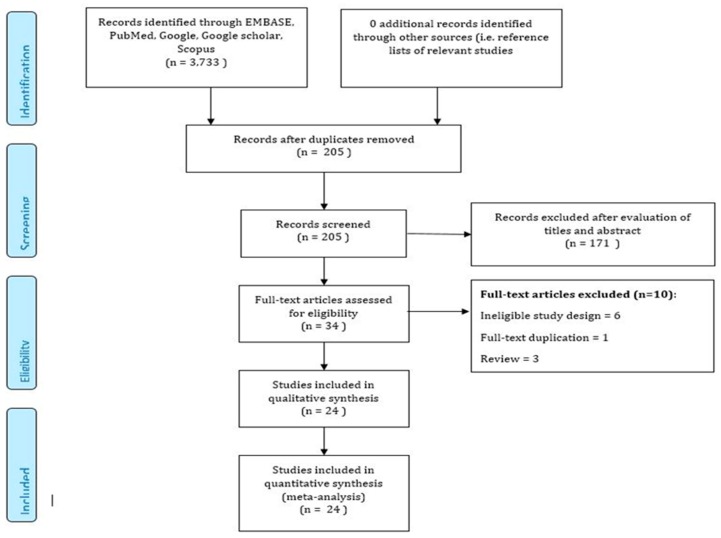
The initial publications search of the electronic databases yielded 3245 publications. After eliminating duplication, a total of 3113 studies were excluded based on the predefined exclusion criteria which left 32 articles for full-text review. Furthermore, two articles were added after screening the reference lists of the 32 relevant articles. Based on the review criteria, another 10 articles were excluded, which left a total of 24 publications for our present meta-analysis [23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46] (Figure 2).
Figure 2.
PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) flow diagram for study selection.
2.2. Study Characteristics
Table 1 shows a summary of the included 24 publications. The publications comprised 2,674,298 participants, with 59,073 HCC participants. Twelve publications were case-control studies [23,24,26,27,30,32,33,34,35,39,40,43], ten publications were cohort studies [23,25,28,29,31,36,37,38,41,42], and three publications were randomized control trial studies [44,45,46]. Of these, twelve publications were from Western [23,24,28,30,32,33,38,40,41,42,43,44,46] countries and twelve publications were from Asian [25,26,27,29,31,34,35,36,37,39,44,45] countries. All the studies used the International Classification of Diseases (ICD) code to identify HCC patients and the ATC (Anatomical Therapeutic Chemical Classification System) code to identify statin users.
Table 1.
Characteristics of included studies.
| Author | Year | Country | Study Design | Study Duration | % Male | Total Pts | Total HCC | Inclusion Criteria | Exclusion Criteria | Outcome | Adjusted with DM |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Tran | 2019 | UK | Nested CC | 2000–2011 | 67.3 | 2537 | 434 | Having the first diagnosis of primary liver cancer, including HCC and IBDC) |
Participants with a cancer diagnosis prior to baseline or in the year after baseline | OR = 0.61 (95% CI: 0.42–0.86) | Yes |
| UK | Prospective Cohort | 2006–2010 | 62.6 | 471,851 | 182 | ICD-10 code C22 | Participants with a cancer diagnosis prior to baseline or in the year after baseline | HR: 0.48 (95% CI: 0.24–0.94) | Yes | ||
| German | 2019 | USA | CC | 2002–2016 | 102 | 34 | ICD-9 | Index cases, liver masses other than HCC, and etiologies of liver diseases other than NAFLD | OR = 0.20 (95% CI: 0.07–0.60) | Yes | |
| Goh | 2019 | South Korea | Retrospective cohort | 2008–2012 | 67.6 | 7713 | 702 | ICD-9 | Follow-up less than 6 months, missing data on cholesterol, age < 18, and history of HCC before the index date | HR: 0.36 (95%: 0.19–0.68) | Yes |
| Kim | 2018 | S Korea | Nested CC | 2002–2013 | 83.6 | 514,866 | 8210 | ICD-10 | Pts without supporting clinical codes, indicating the presence of HCC including any liver diagnostic tests (biopsy or arteriography of hepatic artery) and any treatments of the liver (hepatectomy, liver transplantation, radiofrequency ablation, arterial embolization, radiotherapy, or chemotherapy). | OR = 0.44 (95% CI: 0.33–0.58) | Yes |
| Kim | 2017 | South Korea | Nested CC | 2002–2013 | 81.4 | 1374 | 229 | ICD-10 | Pts whose first antidiabetic drug was insulin and patients aged <40 years during first antidiabetic prescription | OR: 0.36 (95% CI: 0.22–0.60) | Yes |
| Simon | 2016 | USA | Cohort | 2001–2014 | 95.2 | 9135 | 239 | ICD-9 | Pts with human immunodeficiency virus (HIV) and those who had a positive hepatitis B surface antigen (HBsAg), baseline cirrhosis, or HCC |
HR = 0.60 (95% CI: 0.53–0.68) | Yes |
| Hsiang | 2015 | China | Retrospective cohort | 2000–2012 | 67.9 | 53,513 | 6,883 | ICD-9 | Pts with HCV or HIV coinfections, missing statin prescriptions, interferon exposure, HBsAg seroclearance within 6 months of the baseline, and age < 18 | HR: 0.68 (95%0.48–0.97) | Yes |
| Björkhem-Bergman | 2014 | Sweden | CC | 2006–2010 | 52 | 23,964 | 3994 | ICD-9 | NR | OR = 0.88 (95%0.81–0.96) | Yes |
| Chen | 2015 | Taiwan | Cohort | 2000–2008 | 54.9 | 71,824 | 1735 | ICD-9 | Gender not clear, age < 20, patients diagnosed with cancer prior to the diagnosis of HBV | HR = 0.34 (95% 0.27–0.42) | Yes |
| McGlynn | 2014 | USA | CC | 1999–2010 | 74.4 | 562 | 94 | ICD-9 | Pts with HCC or any cancer before the index date | OR = 0.32 (95% CI: 0.15–0.67) | Yes |
| McGlynn | 2015 | USA | CC | 1998–2011 | 71.6 | 5835 | 1195 | ICD-9 | Pts with HCC or any cancer before the index date | OR: 0.55 (95% CI: 0.45–0.69) | Yes |
| Lai | 2013 | Taiwan | CC | 2000–2009 | 72.6 | 17,400 | 3480 | ICD-9 | Pts with HCC or any cancer before the index date | OR = 0.72 (95% 0.59–0.88) | Yes |
| Leung | 2013 | Taiwan | CC | 2000–2008 | 46.3 | 34,205 | 27,364 | ICD-9 | Pts diagnosed with cancer before index date, follow-up less than 6 months, and having any prior record of mastectomy | HR: 0.44 (95% CI: 0.296–0.72) | Yes |
| Tsan | 2012 | Taiwan | Cohort | 1997–2008 | 58.2 | 33,413 | 1,021 | ICD-9 | Previously diagnosed HCC | HR = 0.47 (95% CI: 0.36–0.61) | No |
| Tsan | 2013 | Taiwan | Cohort | 1999–2010 | 49.2 | 295,887 | 27,883 | ICD-9 | Previously diagnosed HCC | HR = 0.53 (95% CI: 0.49–0.57) | Yes |
| Marelli | 2011 | USA | Cohort | 1990–2009 | ~52.2 | 91,714 | 105 | ICD-9 | Pts had insufficient history in the database | HR: 0.87 (95% CI: 0.60–1.26) | Yes |
| Chiu | 2010 | Taiwan | CC | 2005–2008 | 68.8 | 2332 | 1166 | ICD-9 | Pts with wrist and hip fractures and previous history of HCC | OR = 0.62 (95% CI: 0.41–0.91) | Yes |
| B. EL–SERAG | 2009 | USA | CC | 1997–2002 | 99.0 | 6515 | 1303 | ICD-9 | Previous history of liver disease | OR = 0.74 (95% CI: 0.64–0.78) | Yes |
| Friedman | 2008 | USA | Cohort | 1994–2003 | NR | 361,859 | 42 | ICD-9 | NR | HRmale = 0.49 (95% CI: 0.33–0.70) HRfemale = 0.40 (95% CI: 0.21–0.75) |
No |
| Friis | 2005 | Denmark | Cohort | 1989–2002 | 56.6 | 334,754 | 171 | ICD-9 | Pts with a history of cancer before study entry | HR = 1.16 (95% CI: 0.46–2.92) | No |
| Khurana | 2005 | USA | CC | 1997–2002 | NR | 480,306 | 409 | ICD-9 | NR | OR = 0.52 (95% CI: 0.41–0.66) | No |
| Matsushita | 2010 | Japan | RCT | 2010 | 31.5 | 13,724 | 12 | ICD-9 | Pts with previous history of cancer | HR: 0.58 (95% CI: 0.18–1.86) | Yes |
| CTT | 2012 | Europe, Australia, North America | RCT | 2012 | NR | 134,537 | 68 | ICD-9 | Pts with nonfatal nonmelanoma skin cancers and benign neoplasm | HR: 1.06 (95% CI: 0.65–1.70) | No |
| Sato | 2006 | Japan | RCT | 1991–1995 | NR | 263 | 1 | Osaka Cancer Registry | Pts who resided outside the Osaka prefecture at entry were excluded | HR: 0.63 (95% CI: 0.11–3.54) | No |
Note: NR = Not reported, CC = Case-Control, RCT = Randomized Control Trial, ICD = International Classification of Diseases, Pts = Participants, OR = Odd Ratio, HR = Hazard Ratio, CI = confidence interval, HCC = Hepatocellular Carcinoma, DM = Diabetic Mellitus, IBDC: Inclusion Body Disease of Cranes, NAFLD: Non-alcoholic fatty liver disease, HCV: Hepatitis C virus, HBV: Hepatitis B virus.
2.3. Study Quality
We utilized the Newcastle Ottawa scale to assess the quality of each study, which is usually applied for non-randomized studies and has been recommended by the Cochrane collaboration [47]. We also assessed randomized study quality with the Cochrane tools (Cochrane Community, London, UK) [48]. The range of the NOS (The Newcastle-Ottawa Scale) score was 7–9, and therefore, the level of evidence was high.
2.4. Meta-Analysis
2.4.1. Primary Analysis
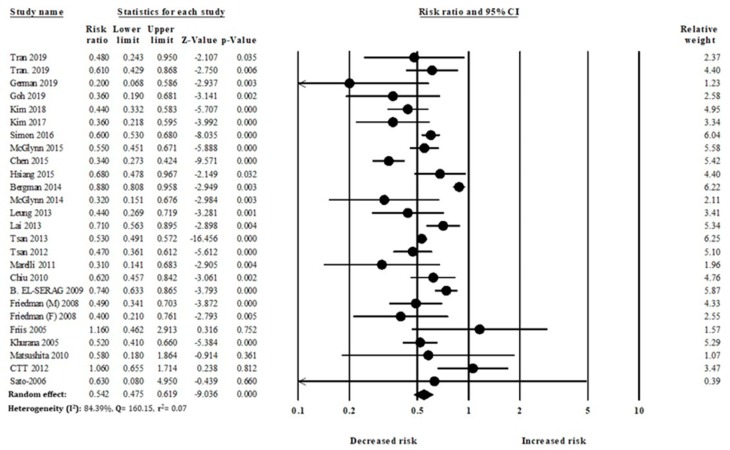
Our meta-analysis comprised 24 studies with 59,073 HCC individuals. In the pooled analysis, overall statin use was significantly associated with a reduced risk of HCC development (RR: 0.54, 95% CI: 0.47–0.61), compared to nonusers (Figure 3).
Figure 3.
Statin use and hepatocellular carcinoma (HCC) risk.
2.4.2. Secondary Analysis
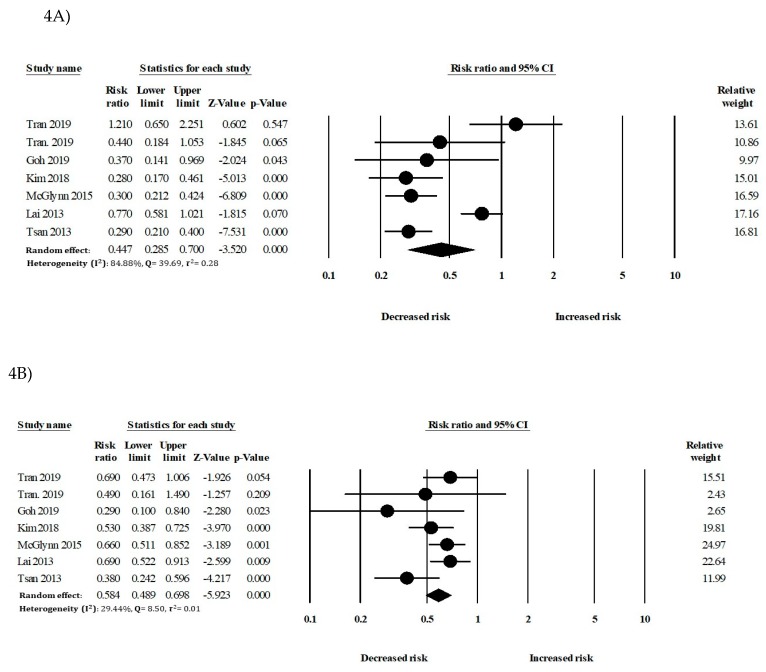
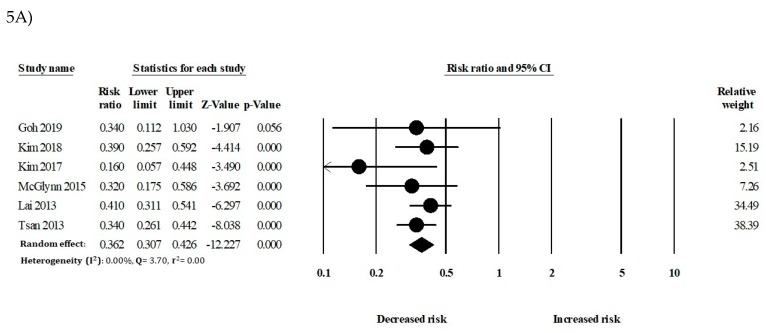
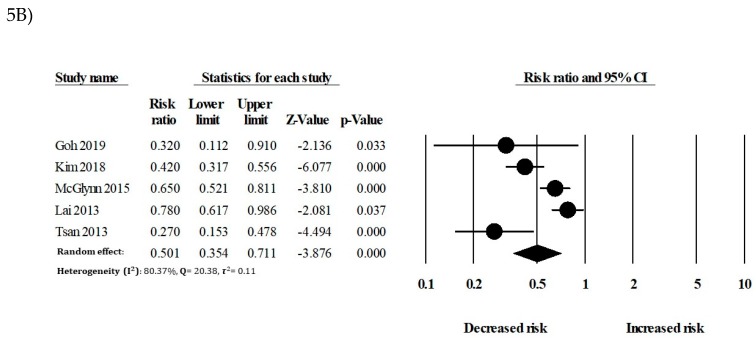
In the secondary analysis, the relationship between statin use and HCC development in patients with DM (Diabetes Mellitus), liver diseases, or antiviral therapy was considered. In patients with DM, statin use was significantly associated with a reduced risk of HCC (RR: 0.44, 95% CI: 0.28–0.70) compared to that of nonusers. In patients without DM, statin use also showed a significant risk reduction of HCC (RR: 0.58, 95% CI: 0.48–0.69) (Figure 4). In patients with liver cirrhosis, statins had an inverse association with HCC (RR: 0.36, 95% CI: 0.30–0.42). In patients without liver cirrhosis, statin use also showed a significant reduction of HCC incidence (RR: 0.50, 95% CI: 0.35–0.71) (Figure 5).
Figure 4.
Statin use and the risk of hepatocellular carcinoma (HCC) in patients with (A) DM and (B) without diabetes mellitus (DM).
Figure 5.
Statin use and the risk of hepatocellular carcinoma (HCC) in patients with (A) liver cirrhosis and (B) without liver cirrhosis.
2.5. Subgroup Analysis
The study design, region, dose, and different types of statins were considered in the subgroup analysis (Table 2). The overall pooled Risk Ratio (RR) for the observational study design was 0.52 (95% CI: 0.46–0.60), and the overall pooled RR for the RCT study design was 0.95 (95% CI: 0.61–1.47). Furthermore, we investigated the association between statin use and HCC development among patients from different regions. The pooled RR for Asian and Western populations were 0.49 (95% CI: 0.42–0.57), and 0.59 (95% CI: 0.49–0.70), respectively.
Table 2.
Subgroup analysis.
| Subgroup Analysis | No. of Studies | Adjusted RR | 95% CI | p-Value | Test of Heterogeneity | |||
|---|---|---|---|---|---|---|---|---|
| Q | I2 (%) | Tau2 | p-Value | |||||
| Study design | ||||||||
| Observational | 21 | 0.52 | 0.46–0.60 | <0.001 | 155.14 | 85.82 | 0.07 | <0.05 |
| Case-control | 12 * | 0.56 | 0.46–0.67 | <0.001 | 70.44 | 84.38 | 0.07 | <0.001 |
| Cohort | 10 * | 0.49 | 0.42–0.57 | <0.001 | 28.87 | 65.36 | 0.02 | 0.001 |
| RCT | 3 | 0.95 | 0.61–1.47 | 0.82 | 1.03 | 0 | 0 | 0.59 |
| Study location | ||||||||
| Asian | 12 | 0.49 | 0.42–0.57 | <0.001 | 30.61 | 64.06 | 0.03 | 0.001 |
| Western | 12 | 0.59 | 0.49–0.70 | <0.001 | 71.47 | 81.81 | 0.06 | <0.001 |
| Dose | ||||||||
| ≤365 cDDD | 8 | 0.55 | 0.46–0.65 | <0.001 | 5.84 | 0 | 0 | 0.55 |
| >365 cDDD | 6 | 0.47 | 0.36–0.61 | <0.001 | 4.51 | 0 | 0 | 0.47 |
| Type of statin | ||||||||
| Atorvastatin | 6 | 0.55 | 0.43–0.69 | <0.001 | 6.74 | 25.84 | 0.02 | 0.24 |
| Lovastatin | 3 | 0.43 | 0.21–0.86 | 0.01 | 3.03 | 34.18 | 0.14 | 0.21 |
| Cerivastatin | 2 | 0.61 | 0.26–1.42 | 0.25 | 0.89 | 0 | 0 | 0.34 |
| Fluvastatin | 4 | 0.41 | 0.25–0.66 | <0.001 | 1.61 | 0 | 0 | 0.65 |
| Pravastatin | 5 | 0.76 | 0.56–1.03 | 0.08 | 2.48 | 0 | 0 | 0.64 |
| Rosuvastatin | 5 | 0.47 | 0.26–0.84 | 0.01 | 5.09 | 21.44 | 0.09 | 0.27 |
| Simvastatin | 7 | 0.54 | 0.46–0.63 | <0.001 | 0.85 | 0 | 0 | 0.99 |
Note: * one study contained both case-control and cohort study design. RCT = Randomized Control Trial, cDDD = cumulative defined daily doses, RR = Risk Ratio, CI = confidence interval.
In the dose-dependent analysis, risk reduction was accentuated with an increase of the cumulative defined daily doses (cDDD) compared with nonusers (RR 0.55 (95% CI: 0.46–0.65) and RR 0.47 (95% CI: 0.36–0.61) for ≤365 cDDD and >365 cDDD, respectively; p for trend <0.0001). An analysis of different types of statins showed protective effects, particularly for fluvastatin (RR 0.41, 95% CI: 0.25–0.66, p < 0.001), lovastatin (RR 0.43, 95% CI: 0.21–0.86, p = 0.01), rosuvastatin (RR 0.47, 95% CI: 0.26–0.84, p = 0.01), simvastatin (RR 0.54, 95% CI:0.46–0.63, p < 0.001), and atorvastatin (RR 0.55, 95% CI: 0.43–0.69, p < 0.001). However, cerivastatin and pravastatin showed protective effects (RR 0.61, 95% CI: 0.26–1.42, p = 0.25 and RR 0.76, 95% CI: 0.56–1.03, p = 0.08) but were statistically insignificant (Supplementary Figure S1–12).
2.6. Publication Bias
To detect publication bias, the pooled analysis or subgroup analyses were not sufficient. Therefore, we used the Egger test and the Begg test to identify publication bias. However, visual inspection of the funnel plot revealed no publication bias, later confirmed by the Begg adjusted rank correlation test (Supplementary Figure S13).
3. Discussion
3.1. Major Findings
In this meta-analysis of 24 studies with a total of 59,073 HCC individuals, statins were significantly associated with reductions in the risk of HCC (RR: 0.54, 95% CI: 0.47–0.61, I2 = 84.39%). We also analyzed the beneficial effect of statins on HCC risk reduction in patients with high-risk factors (Supplementary Figure S14), including diabetes and liver cirrhosis. Previous studies reported that patients with diabetes and liver cirrhosis were significantly associated with an increased risk of HCC. However, findings of our study showed that statins significantly reduced the risk of HCC in patients with diabetes and liver cirrhosis. Moreover, the rate of HCC reduction with statins use was greater in patients with diabetes or liver cirrhosis than in patients without diabetes or liver cirrhosis. Higher cumulative doses of statins use were associated with greater risk reductions than lower cumulative doses of statins. The use of fluvastatin, lovastatin, and rosuvastatin showed greater effects for reducing the risk of HCC than the use of other statins.
3.2. Comparison with Other Studies
The findings of this study on HCC risk are similar to three previous systematic reviews and meta-analyses. A study in 2013 found that statins decreased HCC in analyses of 10 studies with a total of 1,459,417 participants with 4298 HCC patients (OR: 0.63, 95% CI: 0.52–0.76) [14]. In the next year, a meta-analysis that included 12 studies with a total of 5,640,313 participants also suggested a reduction of HCC (RR: 0.58, 95% CI: 0.51–0.67) [49]. Another meta-analysis of five observational studies with 87,127 participants also evaluated different types of statin use and reduction of HCC risk [50] and found that fluvastatin is the most effective for reducing HCC risk (RR: 0.55, 95% CI: 0.26–1.11) compared with the reductions associated with other types of statins. Our study has updated and extended the evidence of these prior systematic reviews and meta-analyses in three ways. First, we included more observational studies from different continents. Second, we included more subgroup analyses than previous studies. Finally, we evaluated several additional factors associated with statins use and HCC risk, e.g., diabetes and liver cirrhosis, to examine any possible bias or influence of these additional factors.
3.3. Biological Plausibility
There are several convincing biological explanations that statin use can reduce the risk of HCC. Statins indeed have several pleiotropic effects to reduce the risk of HCC, including antioxidative, anti-inflammatory, endothelial function, and anti-fibrotic properties (Figure 6). Statins are currently the ultimate choice to treat hypercholesterolemia because they inhibit the mevalonate pathway which is mainly responsible for stimulating cholesterol synthesis [51]. However, the use of statins actually reduces the expression of downstream metabolites of the cholesterol synthesis pathway, including farnesyl pyrophosphate, and geranyl pyrophosphate, thereby slowing the prenylation of GTPase down, which decreases the translocation of Ras and Rho and their functions, decreasing cell proliferation and migration [52]. Steatosis-induced HSC activation is suppressed by statins, through the paracrine effect of hepatocytes. Statins can also downregulate profibrogenic gene expressions (TGF-β1, α-SMA, and tissue inhibitor of metalloproteinases-1) and protein expression of αSMA in HSCs; thus, it reduces liver fibrosis [53]. Furthermore, chronic hepatic injury is also an important factor in HCC development [54]. Statin use further induces hepatocyte apoptosis by secreting inflammation-mediated damage occurring molecular patterns including IL-6, IL-1β, TNF, and reactive oxygen species (ROS) [55]. However, statins have the ability to mitigate hepatic inflammation by deactivating IL-6 and TNF-α expression and by significantly attenuating metalloproteinases activity and production of ROS [53,56]. Intratumor heterogeneity supplies energy for tumor evolution and drug resistance [57,58]. It is both intrinsically timely and important to properly address the current use of statins and to provide an outlook for future use, where statins may be applied to a wider range of HCC reduction [59,60]. Finally, statins activate the protooncogenic transcription factor Myc [61] and accelerate the expression of the suppressor miRNA-145 [62], which ultimately controls tumor cell migration and invasion.
Figure 6.
Mechanism of statins to prevent hepatocellular carcinoma (HCC).
3.4. Quality of the Evidence
This study is a large updated meta-analysis on this topic, including both randomized controlled trials and observational studies (case-control and cohort studies). All the included studies were of higher quality with their respective NOS scores ranging between 7 and 9. All the effect sizes ware adjusted with potential confounding factors (Supplementary Figure S15), and the heterogeneity was relatively low in both primary and subgroup analyses.
3.5. Study Limitations
This meta-analysis has several limitations that need to be addressed. First, all the included studies were adjusted with potential confounding factors, but these confounding factors were not the same. For example, some failed to adjust their study for factors such as the severity of liver disease (classified with the Child–Pugh score), alcohol consumption, diabetes, or different viral diseases. Second, most included studies were observational (cohort and case-control study) and the HCC patients were identified by the ICD code. Therefore, it is not clear that the HCC patients who used statins were followed properly and what was the medication compliance of these populations. Third, some studies used the same databases to assess the association between statin use and HCC risk. Even though the study duration was not the same for any of the included studies, some patients might overlap in the included studies.
3.6. Research and Clinical Implications
Our updated meta-analysis included a large number of studies with 59,073 HCC patients from diverse populations (four continents), and the pooled risk ratio was less than 0.5 with a narrow confidence interval. Our findings therefore strongly suggest that statin use has a significant and clinically relevant effect on HCC reduction, including with various conditions such as diabetes and other types of liver diseases. Recently, concern has been raised about the potential hepatotoxicity of statins, and several guidelines regarding statin use also persistently warn of these risks. However, many studies have also reported that statin-induced hepatotoxicity is extremely low (less than 3% of all patients taking statins). A significant amount of retrospective studies mentioned that statins are safe for use in patients with cirrhosis, even if statins are more effective in reducing liver decompensation and hepatocellular carcinoma. However, prescribing statins ubiquitously is not recommended in patients with all types of cirrhosis. A study by Kaplan et al. [62] suggested that patients with serious cirrhosis/advanced decompensated cirrhosis (CTP C) may experience more unwanted consequences than benefits associated with initiating or continuing statin therapy. If the patients are diagnosed with decompensated cirrhosis, then statins should be prescribed with caution at low doses and should be accompanied with timely monitoring of creatinine phosphokinase levels [63]. Furthermore, Abraldes et al. [64] reported that the use of statins may be risky in Child–Pugh B or Child–Pugh C patients whose total bilirubin levels are quite high. Patients with Child–Pugh A cirrhosis can get more benefits from statin use but there is no specific evidence for dose and duration. However, longer duration of statin use is recommended to get better beneficial effects.
Our results support the hypothesis that statins could be used as an anticancer therapy targeting the mevalonate pathway. The current study found that lipophilic statins, such as lovastatin or simvastatin, have a higher beneficial effect on HCC than hydrophilic statins such as pravastatin. Another finding from our subgroup analyses suggested that statins showed a higher beneficial effect in the Western population compared with the Asian population. Variation in genetic structure or polymorphism between Asian and Western people can influence statins’ pharmacokinetics and pharmacodynamics properties [64]. More importantly, it showed a chemopreventive role in different kinds of populations. According to these findings, a prospective evaluation should be carried out on whether statins can be used as anticancer drugs. The chemopreventive effects of statins have only been shown in observational studies, but the pooled estimate of three RCTs did not show a significant chemopreventive effect. However, these studies included only a small number of individuals (81 participants) and shorter duration of follow-up. It is also important to address that the primary outcome of these RCTs focused on the effect of statins on cardiovascular mortality. Moreover, the patients included in these RCTs had at low risk for development of HCC. Therefore, these studies were not well designed to distinguish significant differences in the two groups (placebo vs statin) with regard to HCC development. They did not screen the HCC patients because the incidence of HCC was the secondary outcome. Thus, post hoc analysis of these trials was not to judge the protective effect of statins against HCC. To make this issue clear, additional prospective randomized controlled trials are warranted to justify the findings from our primary and subgroup analyses.
However, two large clinical trials are ongoing in the United States and Taiwan which have immense potential to justify or nullify our findings in our updated meta-analysis. In the prospective randomized control trial Phase-II (NCT02968810) of the United States study, researchers are evaluating the preventive effect of statin on liver cancer in patients with liver cirrhosis. Similarly, in Taiwan, another prospective randomized clinical trial phase IV (NCT03024684) is evaluating preventive HCC recurrence after curative treatment. Since it takes a long time and is expensive to develop a new drug, drug repurposing is getting researchers’ attention in order to find new indications. Therefore, the results of these clinical trials and our updated meta-analysis bring new hope to patients with HCC.
4. Methods
4.1. Meta-Analytic Guidelines
In this study, we followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flow diagram for study inclusion and exclusion. Moreover, we also considered the Meta-analysis of Observational Studies in Epidemiology (MOOSE) guidelines for observational studies [65].
4.2. Databases and Search Strategy
We systematically searched for relevant studies in PubMed, EMBASE, Web of Science, Google Scholar, and Scopus for studies published between January 1, 1990, and September 1, 2019, with no restriction of language. The following search terms were used to finds potential studies: “hepatocellular carcinoma”, OR “liver cancer”, and “statins” OR “simvastatin”, OR “atorvastatin”, OR “lovastatin”, OR “HMG-CoA reductase inhibitor(s)”, OR “pravastatin”, OR “rosuvastatin” OR “cerivastatin” OR “pitavastatin” (Supplementary Table S1). Our initial evaluation was separately conducted by two authors (M.M.I. and T.N.P.). They cross-checked all the reference lists from retrieved articles to find additional relevant articles.
4.3. Eligibility Criteria
Eligibility was restricted to large observational (≥200 participants) and clinical trials only which investigated the association between statin use and the risk of HCC as the primary outcome. Studies were included if they met the following inclusion criteria: a) a large observational study with at least a 6-month follow-up time; b) the study reported the development of HCC in adult individuals (≥18 years old) with statin use versus non-statin use; c) the study clearly defined statin exposure and the identification of HCC in patients; and d) the study clearly estimated the risk of HCC as a hazard ratio (HR), odds ratio (OR), and risk ratio (RR) with 95% CIs. However, studies were excluded if they are only review articles, letters to the editors, or case reports. Studies including fewer participants (<200 participants) were also excluded.
4.4. Selection Process
The same two authors (M.M.I. and T.N.P.) independently screened the titles and abstracts of the previously selected literature. They followed prespecified inclusion and exclusion criteria developed through the discussion with all authors to identify relevant studies. Any disagreements during that reviewing process were resolved by the consideration of the prior guidelines; any remaining conflict was then resolved by discussion with the main investigator (Y.C.L.). The same two authors (M.M.I. and T.N.P.) independently conducted the data collection process and checked for study duplication, population sizes, and date of publications.
4.5. Data Extraction
The primary outcome measures were ORs and HRs with 95% CIs for the association between statin use and the risk of HCC. Two authors (M.M.I. and T.N.P.) identified and recorded the effect sizes reflecting the higher degree of adjustment variables for possible confounding factors. Unadjusted findings and the effect of statins on HCC with other potential diseases were also extracted. Other information extracted from the included studies were author names, publication years, study designs, number of participants, number of HCC patients, process of HCC patient’s identifications, statins user’s definitions, doses information, effects of different types of statins on HCC, follow-up times, settings, and regions.
4.6. Assessment of Bias Risk
The Newcastle Ottawa Scale (NOS) was applied for evaluating the individual quality of each study (Supplementary Table S2). The heterogeneity among the studies was calculated using the Q statistic and the I2 statistic. Moreover, publication bias was assessed by the funnel plot-based (Egger test and the Begg test).
4.7. Statistical Analysis
We performed statistical analyses with the Comprehensive Meta-analysis (CMA) software (version: 3, Biostat, Englewood, NJ, USA). The risk ratio with 95% confidence intervals (CIs) was calculated to assess outcomes, and a p-value of less than 0.05 was considered significant. Furthermore, the random effect model was used to pool the effect size and funnel plots were drawn to present the effect sizes visually. We only considered adjusted effect sizes reported in studies for analysis to account for confounding variables.
5. Conclusions
To our knowledge, this is the most extensive meta-analysis so far that evaluated the beneficial effects of statins on HCC. The findings of this meta-analysis provided additional evidence because they showed that statin use can provide a 46% risk reduction in HCC development. Furthermore, a larger mechanistic study is warranted to confirm or refute our findings.
Acknowledgments
We want to thank our colleagues who are native English speakers for editing our manuscripts.
Supplementary Materials
The following are available online at https://www.mdpi.com/2072-6694/12/3/671/s1, Figure S1: Statin use and the risk of HCC in patients (case-control study), Figure S2: Statin use and the risk of HCC in patients (cohort study), Figure S3: Statin use and the risk of HCC in patients (randomized control trials), Figure S4: Statin use and the risk of HCC in patients (Asian continent), Figure S5: Statin use and the risk of HCC in patients (Western continent), Figure S6: Statin use and the risk of HCC in patients (>365cDDD), Figure S7: Atorvastatin use and the risk of HCC in patients, Figure S8: Lovastatin use and the risk of HCC in patients, Figure S9: Cerevastatin use and the risk of HCC in patients, Figure S10: Pravastatin use and the risk of HCC in patients, Figure S11: Rosuvastatin use and the risk of HCC in patients, Figure S12: Simvastatin use and the risk of HCC in patients, Figure S13: Funnel plot, Figure S14 Adjusted risk factors, Table S1: Searching words, Table S2: Methodological quality of included studies based on the Newcastle Ottawa scale.
Author Contributions
M.M.I., T.N.P., H.-C.Y. and Y.-C.(J.)L. conceived the idea for and designed the study, supervised the study intervention, and revised drafts of article; M.M.I. designed the study, analyzed the data, interpreted the data, wrote the first draft, and revised the article; T.N.P. designed the study, analyzed the data, performed the statistical analysis, interpreted the data, and revised the article. B.A.W. proofread the study. All authors have read and agreed to the published version of the manuscript.
Funding
This research is granted in part by the Ministry of Education (MOE) under grant MOE 108-6604-001-400 and Ministry of Science and Technology (MOST) under grant MOST 109-2222-E-038-002-MY2.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Rawla P., Sunkara T., Muralidharan P., Raj J.P. Update in global trends and aetiology of hepatocellular carcinoma. Contemp. Oncol. 2018;22:141. doi: 10.5114/wo.2018.78941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chan A.W., Zhong J., Berhane S., Toyoda H., Cucchetti A., Shi K., Tada T., Chong C.C., Xiang B.D., Li L.Q. Development of pre and post-operative models to predict early recurrence of hepatocellular carcinoma after surgical resection. J. Hepatol. 2018;69:1284–1293. doi: 10.1016/j.jhep.2018.08.027. [DOI] [PubMed] [Google Scholar]
- 3.Ghouri Y.A., Mian I., Rowe J.H. Review of hepatocellular carcinoma: Epidemiology, etiology, and carcinogenesis. J. Carcinog. 2017;16:1. doi: 10.4103/jcar.JCar_9_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.El-serag H.B., Tran T., Everhart J.E. Diabetes increases the risk of chronic liver disease and hepatocellular carcinoma. Gastroenterology. 2004;126:460–468. doi: 10.1053/j.gastro.2003.10.065. [DOI] [PubMed] [Google Scholar]
- 5.Perz J.F., Armstrong G.L., Farrington L.A., Hutin Y.J., Bell B.P. The contributions of hepatitis B virus and hepatitis C virus infections to cirrhosis and primary liver cancer worldwide. J. Hepatol. 2006;45:529–538. doi: 10.1016/j.jhep.2006.05.013. [DOI] [PubMed] [Google Scholar]
- 6.Preda C., Popescu C., Baicus C., Constantinescu I., Oproiu A., Voiosu T., Diculescu M., Negreanu L., Gheorghe L., Sporea I. Risk of hepatitis B virus reactivation in hepatitis B virus+ hepatitis C virus-co-infected patients with compensated liver cirrhosis treated with ombitasvir, paritaprevir/r+ dasabuvir+ ribavirin. J. Viral. Hepat. 2018;25:834–841. doi: 10.1111/jvh.12872. [DOI] [PubMed] [Google Scholar]
- 7.Lee M.H., Yang H.I., Liu J., Batrla-Utermann R., Jen C.L., Iloeje U.H., Lu S.N., You S.L., Wang L.Y., Chen C.J. Prediction models of long-term cirrhosis and hepatocellular carcinoma risk in chronic hepatitis B patients: Risk scores integrating host and virus profiles. Hepatology. 2013;58:546–554. doi: 10.1002/hep.26385. [DOI] [PubMed] [Google Scholar]
- 8.Fattovich G., Stroffolini T., Zagni I., Donato F. Hepatocellular carcinoma in cirrhosis: Incidence and risk factors. Gastroenterology. 2004;127:35–50. doi: 10.1053/j.gastro.2004.09.014. [DOI] [PubMed] [Google Scholar]
- 9.Davila J., Morgan R., Shaib Y., McGlynn K., El-Serag H. Diabetes increases the risk of hepatocellular carcinoma in the United States: A population based case control study. Gut. 2005;54:533–539. doi: 10.1136/gut.2004.052167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Simon T.G., King L.Y., Zheng H., Chung R.T. Statin use is associated with a reduced risk of fibrosis progression in chronic hepatitis C. J. Hepatol. 2015;62:18–23. doi: 10.1016/j.jhep.2014.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yang Y.H., Chen W.C., Tsan Y.T., Chen M.J., Shih W.T., Tsai Y.H., Chen P.C. Statin use and the risk of cirrhosis development in patients with hepatitis C virus infection. J. Hepatol. 2015;63:1111–1117. doi: 10.1016/j.jhep.2015.07.006. [DOI] [PubMed] [Google Scholar]
- 12.Undela K., Shah C.S., Mothe R.K. Statin use and risk of cancer: An overview of meta-analyses. World J. Meta-Anal. 2017;26:41–53. doi: 10.13105/wjma.v5.i2.41. [DOI] [Google Scholar]
- 13.Baigent C., Blackwell L., Emberson J., Holland L., Reith C., Bhala N., Peto R., Barnes E., Keech A., Simes J. Efficacy and Safety of More Intensive Lowering of LDL Cholesterol: A Meta-Analysis of Data from 170,000 Participants in 26 Randomised Trials. Lancet. 2010;376:1670–1681. doi: 10.1016/S0140-6736(10)61350-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Singh S., Singh P.P., Singh A.G., Murad M.H., Sanchez W. Statins are associated with a reduced risk of hepatocellular cancer: A systematic review and meta-analysis. Gastroenterology. 2013;144:323–332. doi: 10.1053/j.gastro.2012.10.005. [DOI] [PubMed] [Google Scholar]
- 15.Souk K., Al-Badri M., Azar S. The safety and benefit of statins in liver cirrhosis: A review. Exp. Clin. Endocrinol. Diabetes. 2015;123:577–580. doi: 10.1055/s-0035-1564093. [DOI] [PubMed] [Google Scholar]
- 16.Kubatka P., Kruzliak P., Rotrekl V., Jelinkova S., Mladosievicova B. Statins in oncological research: From experimental studies to clinical practice. Crit. Rev. Oncol. Hematol. 2014;92:296–311. doi: 10.1016/j.critrevonc.2014.08.002. [DOI] [PubMed] [Google Scholar]
- 17.Inano H., Suzuki K., Onoda M., Wakabayashi K. Anti-carcinogenic activity of simvastatin during the promotion phase of radiation-induced mammary tumorigenesis of rats. Carcinogenesis. 1997;18:1723–1727. doi: 10.1093/carcin/18.9.1723. [DOI] [PubMed] [Google Scholar]
- 18.Björkhem-Bergman L., Acimovic J., Torndal U.B., Parini P., Eriksson L.C. Lovastatin prevents carcinogenesis in a rat model for liver cancer. Effects of ubiquinone supplementation. Anticancer Res. 2010;30:1105–1112. [PubMed] [Google Scholar]
- 19.Relja B., Meder F., Wang M., Blaheta R., Henrich D., Marzi I., Lehnert M. Simvastatin modulates the adhesion and growth of hepatocellular carcinoma cells via decrease of integrin expression and ROCK. Int. J. Oncol. 2011;38:879–885. doi: 10.3892/ijo.2010.892. [DOI] [PubMed] [Google Scholar]
- 20.Wang J., Tokoro T., Higa S., Kitajima I. Anti-inflammatory effect of pitavastatin on NF-κB activated by TNF-α in hepatocellular carcinoma cells. Biol. Pharm. Bull. 2006;29:634–639. doi: 10.1248/bpb.29.634. [DOI] [PubMed] [Google Scholar]
- 21.Ghalali A., Martin-Renedo J., Högberg J., Stenius U. Atorvastatin decreases HBx-induced phospho-Akt in hepatocytes via P2X receptors. Mol. Cancer Res. 2017;15:714–722. doi: 10.1158/1541-7786.MCR-16-0373. [DOI] [PubMed] [Google Scholar]
- 22.Cao Z., Fan-Minogue H., Bellovin D.I., Yevtodiyenko A., Arzeno J., Yang Q., Gambhir S.S., Felsher D.W. MYC phosphorylation, activation, and tumorigenic potential in hepatocellular carcinoma are regulated by HMG-CoA reductase. Cancer Res. 2011;71:2286–2297. doi: 10.1158/0008-5472.CAN-10-3367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tran K.T., McMenamin Ú.C., Coleman H.G., Cardwell C.R., Murchie P., Iversen L., Lee A.J., Thrift A.P. Statin use and risk of liver cancer: Evidence from two population-based studies. Int. J. Cancer. 2019;146:1250–1260. doi: 10.1002/ijc.32426. [DOI] [PubMed] [Google Scholar]
- 24.German M.N., Lutz M.K., Pickhardt P.J., Bruce R.J., Said A. Statin use is protective against hepatocellular carcinoma in patients with nonalcoholic fatty liver disease: A case-control study. J. Clin. Gastroenterol. 2019 doi: 10.1097/MCG.0000000000001260. [DOI] [PubMed] [Google Scholar]
- 25.Goh M.J., Sinn D.H., Kim S., Woo S.Y., Cho H., Kang W., Gwak G.Y., Paik Y.H., Choi M.S., Lee J., et al. Statin use and the risk of hepatocellular carcinoma in patients with chronic hepatitis B. Hepatology. 2019 doi: 10.1002/hep.30973. [DOI] [PubMed] [Google Scholar]
- 26.Kim G., Jang S.Y., Nam C.M., Kang E.S. Statin use and the risk of hepatocellular carcinoma in patients at high risk: A nationwide nested case-control study. J. Hepatol. 2018;68:476–484. doi: 10.1016/j.jhep.2017.10.018. [DOI] [PubMed] [Google Scholar]
- 27.Kim G., Jang S.Y., Han E., Lee Y.h., Park S.y., Nam C.M., Kang E.S. Effect of statin on hepatocellular carcinoma in patients with type 2 diabetes: A nationwide nested case-control study. Int. J. Cancer. 2017;140:798–806. doi: 10.1002/ijc.30506. [DOI] [PubMed] [Google Scholar]
- 28.Simon T.G., Bonilla H., Yan P., Chung R.T., Butt A.A. Atorvastatin and fluvastatin are associated with dose-dependent reductions in cirrhosis and hepatocellular carcinoma, among patients with hepatitis C virus: Results from ERCHIVES. Hepatology. 2016;64:47–57. doi: 10.1002/hep.28506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hsiang J.C., Wong G.L.H., Tse Y.K., Wong V.W.S., Yip T.C.F., Chan H.L.Y. Statin and the risk of hepatocellular carcinoma and death in a hospital-based hepatitis B-infected population: A propensity score landmark analysis. J. Hepatol. 2015;63:1190–1197. doi: 10.1016/j.jhep.2015.07.009. [DOI] [PubMed] [Google Scholar]
- 30.Björkhem-Bergman L., Backheden M., Söderberg Löfdal K. Safety d: Statin treatment reduces the risk of hepatocellular carcinoma but not colon cancer—results from a nationwide case-control study in Sweden. Pharmacoepidemiol. Drug Saf. 2014;23:1101–1106. doi: 10.1002/pds.3685. [DOI] [PubMed] [Google Scholar]
- 31.Chen C.I., Kuan C.F., Fang Y.A., Liu S.H., Liu J.C., Wu L.L., Chang C.J., Yang H.C., Hwang J., Miser J.S., et al. Cancer risk in HBV patients with statin and metformin use: A population-based cohort study. Medicine. 2015;94:462. doi: 10.1097/MD.0000000000000462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McGlynn K.A., Hagberg K., Chen J., Graubard B.I., London W.T., Jick S., Sahasrabuddhe V.V. Statin use and risk for primary liver cancer in the clinical practice research datalink. J. Natl. Cancer Inst. 2015;107 doi: 10.1093/jnci/djv009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McGlynn K.A., Divine G.W., Sahasrabuddhe V.V., Engel L.S., VanSlooten A., Wells K., Yood M.U., Alford S.H. Statin use and risk of hepatocellular carcinoma in a US population. Cancer Epidemiol. 2014;38:523–527. doi: 10.1016/j.canep.2014.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lai S.W., Liao K.F., Lai H.C., Muo C.H., Sung F.C., Chen P.-C. Statin use and risk of hepatocellular carcinoma. Cancer Epidemiol. 2013;28:485–492. doi: 10.1007/s10654-013-9806-y. [DOI] [PubMed] [Google Scholar]
- 35.Leung H.W., Chan A.L., Lo D., Leung J.H., Chen H.L. Common cancer risk and statins: A population-based case–control study in a Chinese population. Expert Opin. Drug Saf. 2013;12:19–27. doi: 10.1517/14740338.2013.744392. [DOI] [PubMed] [Google Scholar]
- 36.Tsan Y.T., Lee C.H., Ho W.C., Lin M.H., Wang J.D., Chen P.C. Statins and the risk of hepatocellular carcinoma in patients with hepatitis C virus infection. J. Clin. Oncol. 2013;31:1514–1521. doi: 10.1200/JCO.2012.44.6831. [DOI] [PubMed] [Google Scholar]
- 37.Tsan Y.T., Lee C.H., Wang J.D., Chen P.C. Statins and the risk of hepatocellular carcinoma in patients with hepatitis B virus infection. J. Clin. Oncol. 2012;30:623–630. doi: 10.1200/JCO.2011.36.0917. [DOI] [PubMed] [Google Scholar]
- 38.Marelli C., Gunnarsson C., Ross S., Haas S., Stroup D.F., Cload P., Clopton P., DeMaria A.N. Statins and risk of cancer: A retrospective cohort analysis of 45,857 matched pairs from an electronic medical records database of 11 million adult Americans. J. Am. Coll. Cardiol. 2011;58:530–537. doi: 10.1016/j.jacc.2011.04.015. [DOI] [PubMed] [Google Scholar]
- 39.Chiu H.F., Ho S.C., Chen C.C., Yang C.Y. Statin use and the risk of liver cancer: A population-based case–control study. Am. J. Gastroenterol. 2011;106:894. doi: 10.1038/ajg.2010.475. [DOI] [PubMed] [Google Scholar]
- 40.El–Serag H.B., Johnson M.L., Hachem C., Morgana R.O. Statins are associated with a reduced risk of hepatocellular carcinoma in a large cohort of patients with diabetes. Am. J. Gastroenterol. 2009;136:1601–1608. doi: 10.1053/j.gastro.2009.01.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Friedman G.D., Flick E.D., Udaltsova N., Chan Pharm D.J., Quesenberry C.P., Jr., Habel L.A. Screening statins for possible carcinogenic risk: Up to 9 years of follow-up of 361 859 recipients. Pharmacoepidemiol. Drug Saf. 2008;17:27–36. doi: 10.1002/pds.1507. [DOI] [PubMed] [Google Scholar]
- 42.Friis S., Poulsen A.H., Johnsen S.P., McLaughlin J.K., Fryzek J.P., Dalton S.O., Sørensen H.T., Olsen J.H. Cancer risk among statin users: A population-based cohort study. J. Cancer. 2005;114:643–647. doi: 10.1002/ijc.20758. [DOI] [PubMed] [Google Scholar]
- 43.Khurana V., Saluja A., Caldito G., Fort C., Schiff E. Statins are protective against hepatocellular cancer in patients with hepatitis C virus infection: Half a million US veterans’ study. Gastroenterology. 2005;128:714. [Google Scholar]
- 44.Matsushita Y., Sugihara M., Kaburagi J., Ozawa M., Iwashita M., Yoshida S., Saito H., Hattori Y.J.P. Pravastatin use and cancer risk: A meta-analysis of individual patient data from long-term prospective controlled trials in Japan. Pharmacoepidemiol. Drug Saf. 2010;19:196–202. doi: 10.1002/pds.1870. [DOI] [PubMed] [Google Scholar]
- 45.Sato S., Ajiki W., Kobayashi T., Awata N. Pravastatin use and the five-year incidence of cancer in coronary heart disease patients: From the prevention of coronary sclerosis study. J. Epidemiol. 2006;16:201–206. doi: 10.2188/jea.16.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Trialists C.T. Lack of effect of lowering LDL cholesterol on cancer: Meta-analysis of individual data from 175,000 people in 27 randomised trials of statin therapy. PLoS ONE. 2012;7:e29849. doi: 10.1371/journal.pone.0029849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur. J. Epidemiol. 2010;25:603–605. doi: 10.1007/s10654-010-9491-z. [DOI] [PubMed] [Google Scholar]
- 48.Higgins J.P., Altman D.G., Gøtzsche P.C., Jüni P., Moher D., Oxman A.D., Savović J., Schulz K.F., Weeks L., Sterne J.A. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:5928. doi: 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shi M., Zheng H., Nie B., Gong W., Cui X. Statin use and risk of liver cancer: An update meta-analysis. BMJ Open. 2014;4:e005399. doi: 10.1136/bmjopen-2014-005399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhou Y.Y., Zhu G.Q., Wang Y., Zheng J.N., Ruan L.Y., Cheng Z., Hu B., Fu S.W., Zheng M.H. Systematic review with network meta-analysis: Statins and risk of hepatocellular carcinoma. Oncotarget. 2016;7:21753. doi: 10.18632/oncotarget.7832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Iannelli F., Lombardi R., Milone M.R., Pucci B., De Rienzo S., Budillon A., Bruzzese F. Targeting mevalonate pathway in cancer treatment: Repurposing of statins. Recent Pat. AntiCancer Drug Discov. 2018;13:184–200. doi: 10.2174/1574892812666171129141211. [DOI] [PubMed] [Google Scholar]
- 52.Gazzerro P., Proto M.C., Gangemi G., Malfitano A.M., Ciaglia E., Pisanti S., Santoro A., Laezza C., Bifulco M. Pharmacological actions of statins: A critical appraisal in the management of cancer. Pharmacol. Rev. 2012;64:102–146. doi: 10.1124/pr.111.004994. [DOI] [PubMed] [Google Scholar]
- 53.Chong L.W., Hsu Y.C., Lee T.F., Lin Y., Chiu Y.T., Yang K.C., Wu J.C., Huang Y.T. Fluvastatin attenuates hepatic steatosis-induced fibrogenesis in rats through inhibiting paracrine effect of hepatocyte on hepatic stellate cells. BMC Gastroenterol. 2015;15:22. doi: 10.1186/s12876-015-0248-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hoshida Y., Fuchs B., Tanabe K. Prevention of hepatocellular carcinoma: Potential targets, experimental models, and clinical challenges. Curr. Cancer Drug Targets. 2012;12:1129–1159. [PMC free article] [PubMed] [Google Scholar]
- 55.Higashi T., Friedman S.L., Hoshida Y. Hepatic stellate cells as key target in liver fibrosis. Adv. Drug Deliv. Rev. 2017;121:27–42. doi: 10.1016/j.addr.2017.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kwak B., Mulhaupt F., Myit S., Mach F. Statins as a newly recognized type of immunomodulator. Nat. Med. 2000;6:1399. doi: 10.1038/82219. [DOI] [PubMed] [Google Scholar]
- 57.Herrero J.I., Sangro B., Quiroga J., Pardo F., Herraiz M., Cienfuegos J.A., Prieto J. Influence of tumor characteristics on the outcome of liver transplantation among patients with liver cirrhosis and hepatocellular carcinoma. Liver Transplant. 2001;7:631–636. doi: 10.1053/jlts.2001.25458. [DOI] [PubMed] [Google Scholar]
- 58.Jamal-Hanjani M., Wilson G.A., McGranahan N., Birkbak N.J., Watkins T.B., Veeriah S., Shafi S., Johnson D.H., Mitter R., Rosenthal R. Tracking the evolution of non–small-cell lung cancer. N. Engl. J. Med. 2017;376:2109–2121. doi: 10.1056/NEJMoa1616288. [DOI] [PubMed] [Google Scholar]
- 59.Grootveld M., Percival B., Gibson M., Osman Y., Edgar M., Molinari M., Mather M.L., Casanova F., Wilson P.B. Progress in low-field benchtop NMR spectroscopy in chemical and biochemical analysis. Anal. Chim. Acta. 2019;1067:11–30. doi: 10.1016/j.aca.2019.02.026. [DOI] [PubMed] [Google Scholar]
- 60.Dongiovanni P., Petta S., Mannisto V., Mancina R.M., Pipitone R., Karja V., Maggioni M., Kakela P., Wiklund O., Mozzi E. Statin use and non-alcoholic steatohepatitis in at risk individuals. J. Hepatol. 2015;63:705–712. doi: 10.1016/j.jhep.2015.05.006. [DOI] [PubMed] [Google Scholar]
- 61.Montero J., Morales A., Llacuna L., Lluis J.M., Terrones O., Basanez G., Antonsson B., Prieto J., García-Ruiz C., Colell A. Mitochondrial cholesterol contributes to chemotherapy resistance in hepatocellular carcinoma. Cancer Res. 2008;68:5246–5256. doi: 10.1158/0008-5472.CAN-07-6161. [DOI] [PubMed] [Google Scholar]
- 62.Docrat T.F., Nagiah S., Krishnan A., Naidoo D.B., Chuturgoon A.A. Atorvastatin induces MicroRNA-145 expression in HEPG2 cells via regulation of the PI3K/AKT signalling pathway. Chem. Biol. Interact. 2018;287:32–40. doi: 10.1016/j.cbi.2018.04.005. [DOI] [PubMed] [Google Scholar]
- 63.Moctezuma-Velázquez C., Abraldes J.G., Montano-Loza A.J. The use of statins in patients with chronic liver disease and cirrhosis. Curr. Treat. Options Gastroenterol. 2018;16:226–240. doi: 10.1007/s11938-018-0180-4. [DOI] [PubMed] [Google Scholar]
- 64.Abraldes J.G., Albillos A., Bañares R., Turnes J., González R., García–Pagán J.C., Bosch J. Simvastatin lowers portal pressure in patients with cirrhosis and portal hypertension: A randomized controlled trial. Gastroenterology. 2009;136:1651–1658. doi: 10.1053/j.gastro.2009.01.043. [DOI] [PubMed] [Google Scholar]
- 65.Moher D., Liberati A., Tetzlaff J., Altman D.G. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. Ann. Intern. Med. 2009;151:264–269. doi: 10.7326/0003-4819-151-4-200908180-00135. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.