Abstract
An alternative control regimen for drug-resistant parasites is combination deworming, where two drugs with different modes of action are administered simultaneously to target the same parasite. Few studies have investigated this in equine cyathostomins. We previously reported that an oxibendazole (OBZ) and pyrantel pamoate (PYR) combination was not sustainable against a cyathostomin population with high levels of OBZ and PYR resistance. This study consisted of a field study and two computer simulations to evaluate the efficacy of a moxidectin-oxibendazole (MOX-OBZ) combination against the same cyathostomin population. In the field study, anthelmintic treatments occurred when ten horses exceeded 100 eggs per gram. Fecal egg counts and efficacy evaluations were performed every two weeks. The two simulations utilized weather data as well as equine and parasite population parameters from the field study. The first simulation repeated the treatment schedule used in the field study over a 40 year period. The second evaluated efficacies of combination treatments using selective therapy over 40 years. In the field study, efficacies of MOX and both combination treatments were 100%. The egg reappearance period for MOX was 16 weeks, and the two combination treatments were 12 and 18 weeks. The first (46.7%) and last (40.1%) OBZ efficacies were not significantly different from each other. In the simulation study, the combination treatment delayed MOX resistance development compared to when MOX was used as a single active. This occurred despite the low efficacy of OBZ. The second set of simulations identified combination treatments used with selective therapy to be the most effective at delaying MOX resistance. Overall, this study supports the use of combination treatment against drug-resistant cyathostomins, when one of the actives exhibits high efficacy, and demonstrates benefits of this approach despite substantially lowered efficacy of the other active ingredient.
Keywords: Moxidectin, Combination, Deworming, Cyathostomin, Oxibendazole, Equine
Graphical abstract
Highlights
-
•
Oxibendazole-moxidectin combination treatments were 100% effective.
-
•
Oxibendazole efficacies (<50%) did not differ pre and post combination treatment.
-
•
The model observed oxibendazole-moxidectincombinationto delaymoxidectin resistance.
-
•
Combination use in selective therapy delayed resistance most effectively.
1. Introductionintroduction
Cyathostomins are the most prevalent (Herd, 1990) and abundant (Nielsen et al., 2010) helminth parasites infecting horses, and can cause the syndrome of larval cyathostominosis. Most horses do not show clinical signs of infection, but larval cyathostominosis has been reported to be fatal in 50% of cases (Reid et al., 1995). Presently, three anthelmintic drug classes are available for treating equine cyathostomins, namely the benzimidazoles (BZ), tetrahydropyrimidines (TP), and the macrocyclic lactones (ML). In the Northern Hemisphere, MLs are comprised of ivermectin (IVM) and moxidectin (MOX), where the latter exhibits larvicidal efficacy (Nielsen et al., 2019a). Cyathostomins have wide-spread resistance to the BZ and TP drug classes, and some farms report multi-drug resistance (Peregrine et al., 2014). Reports of shortened egg-reappearance periods (ERP) following ML treatment exist, indicating that resistance is developing to this last remaining drug class as well (Peregrine et al., 2014).
Combination deworming, or the simultaneous administration of two drugs with different modes of action targeting the same parasite, is proposed as a method for parasite control while managing anthelmintic resistance (Barnes et al., 1995; Leathwick, 2012), and has proven useful against trichostrongylid parasites in sheep (Bartley et al., 2004, 2005; Entrocasso et al., 2008; Le Jambre et al., 2010). Combination treatments have the greatest advantage for sustainable parasite control and preserving anthelmintic efficacy when little or no resistance exists to the drug classes combined (Barnes et al., 1995; Leathwick, 2012). Also, realizing the benefit of combinations is heavily dependent upon maintaining a large refugia population, or a portion of the parasites that are not exposed to treatment (Leathwick, 2012). The maintenance of refugia provides a source of susceptible genetic alleles to dilute the resistant alleles surviving treatment (Leathwick et al., 2012; Muchiut et al., 2018). Collectively, these studies suggest that combining a new and presumably effective drug in combination with a drug, to which resistance exists, may decrease the rate of resistance development to the new drug.
Despite minimal scientific evidence, combination products are marketed for cyathostomin treatment in some countries and used off label in others (Bartram et al., 2012; Scott et al., 2015; Lyons et al., 2016; Wilkes et al., 2017). Kaplan et al. (2014) found an additive effect for drug efficacy against equine cyathostomins when oxibendazole (OBZ) and pyrantel pamoate (PYR) were used in combination for a single treatment, where the starting efficacies were >80% for both drugs. In contrast, we (Scare et al., 2018a) observed the effects of repeated combination treatments of OBZ and PYR against a cyathostomin population with known high-level resistance to both the BZ and TP drug classes (Lyons, 2003). Starting efficacies of each drug were much lower in the latter study than those reported by Kaplan et al. (2014). We found the first combination treatment to demonstrate an additive effect with an efficacy of 76%, however, three subsequent combination treatments resulted in significantly lower efficacies of around 40% (Scare et al., 2018a). Overall, even the initial combination treatment achieved an efficacy well below that expected from treatment of a susceptible population. Therefore, the benefits of combination deworming against cyathostomin parasites already displaying significant levels of resistance to both actives were marginal, and the complex lifecycle presents a challenge for control parasites while managing anthelmintic resistance.
Since resistance exists to all actives available for equine use, it is currently unknown if combining an active to which resistance exists with a new active having a novel mode of action would provide any benefit for control against drug-resistant equine cyathostomins. The current study implemented both field data and computer simulations to observe the effects of OBZ combined with MOX to target a cyathostomin population (Population S) harboring significant resistance to the BZ drug class, but which has never previously been exposed to an ML drug. Specifically, the aims were: 1) to generate field data using a MOX/OBZ combination to target a cyathostomin population with established resistance to both BZ and TP products, but remains ML naïve, and 2) to use the results from the field data to perform computer simulations to observe the likely long-term effects of this treatment regimen under different management conditions.
2. Materials and methods
2.1. Ponies
A band of Shetland ponies, established as a closed herd in 1974, at the University of Kentucky was used in this study. The herd currently consists of 20 mares, ranging from ages 5–23 years. The herd harbors a population of cyathostomin parasites, otherwise known as Population S, with documented resistance to the BZ and TP drug classes, (Lyons, 2003). The ponies are maintained outside year-round. During the warmer months (March to October), the ponies were kept in a dry lot with restricted access to stripped grazing and were provided grass hay, consisting of either timothy or orchard grass. Restricted access to grazing is routinely implemented to avoid the onset of laminitis. Hay was continuously provided during the winter months in addition to pasture access. Salt and mineral blocks were available ad libitum. The research was conducted under the approval from the University of Kentucky's Institutional Animal Care and Use Committee (IACUC) under protocol number 2012-1046.
2.2. Study design
This study took place between August 2016 and December 2018. Fecal samples were collected at the time of each treatment and every two weeks thereafter. Ponies were weighed on an electronic scale prior to each treatment and anthelmintics were orally administered at 110% of their body weight. Treatments were administered when ten ponies exceeded 100 eggs per gram (EPG) or at 40 weeks post treatment. A total of five treatments were administered and all ponies received the same treatments. In order to establish the single active baseline efficacies, all ponies were first treated with OBZ on June 21, 2016 and then with MOX on August 24, 2016. All ponies were then administered a combination of MOX and OBZ for treatments three and four, which occurred on March 28, 2017 and January 4, 2018, respectively. All ponies received OBZ for the fifth treatment on October 8, 2018 to observe any potential changes in its efficacy after the combination treatments. Drug efficacies were determined every two weeks by the fecal egg count reduction (FECR) test using the following formula:
The FECR test was performed using the total herd pre- and post-treatment FECs. Egg reappearance periods were determined when the total herd efficacy was <85% (Reinemeyer and Nielsen, 2018).
2.3. Anthelmintics
A paste formulation of OBZ (Anthelcide EQ, Zoetis, Kalamazoo, MI, USA) and a gel formulation of MOX (Quest, Zoetis, Kalamazoo, MI, USA) were used in this study to represent the BZ and ML drug classes, respectively. Oxibendazole was selected for consistency with the previous study observing the effects of combination treatment with OBZ and PYR against the same cyathostomin population (Scare et al., 2018a). Moxidectin was chosen as the ML to observe the impact of larvicidal efficacy. Anthelmintics were administered according to the labeled dosages, at 10 mg/kg bodyweight for OBZ and at 0.4 mg/kg bodyweight for MOX. Anthelmintics were prepared by weighing the calculated dose on an electronic scale and placing in a second syringe for administration to account for variability due to the existence of air bubbles within syringes.
2.4. Fecal egg counts
The Mini-FLOTAC technique, with a detection limit of 5 EPG, was used to perform all FECs in this study (Cringoli et al., 2017). Counts were performed in triplicate to obtain a mean egg count. Samples were prepared as described by Noel et al. (2016). Briefly, 5 g of feces were homogenized within the Fill-FLOTAC containing 45 mL of glucose-NaCl flotation medium with a specific gravity of at least 1.24. Both chambers of the Mini-FLOTAC slide w were filled with the fecal slurry and allowed to rest for 10 min to allow for adequate flotation before microscopic examination and enumeration.
2.5. Coprocultures, Baermann procedure, and larval identification
To characterize the strongyle population, ten ponies were randomly selected and fecal samples were collected for coproculture and subsequent larval identification. Only fecal samples collected at the time of treatment and at two weeks post-treatment were used. The cultures were set up individually as described by Henriksen and Korsholm (1983) using 10 g of feces. The cultures were placed in an incubator at 24 °C for 14 days and moistened with tap water as needed. Subsequently, samples were placed in a Baermann apparatus for 48 h at room temperature for sedimentation. After this, the sediment were collected and were stored at 4 °C and processed within two weeks. The harvested larvae were placed in a nematode counting chamber (Chalex Corp. Ketchum, ID, USA). The slide was heated to 55 °C for approximately 3 min in order to inactivate the larvae. All larvae present in the sample were then examined at 100X and identified to stage, genus, and species where applicable, as described by Russel (1948).
2.6. Statistical analysis
Using the triplicate counts, mean FECs and 95% confidence intervals (α = 0.05) were determined for each sample at all time points using Microsoft Excel 2016 (Redmond, WA, USA). Drug efficacies were determined at each timepoint using the two FECRT methods as previously described in section 2.2.
Further statistical analyses were performed using SAS software (version 9.4, SAS Institute, Cary, North Carolina, USA). The individual horse FECs and FECR tests were used for these analyses. Any negative efficacies were replaced with 0%, and horses that had 0 EPG pre-treatment were excluded from the FECR analyses. All FECs and FECR tests were first log-transformed to achieve a normal distribution. All statistical analyses were interpreted at α = 0.05. Because the shortest treatment interval was 30 weeks when ten horses reached the >100 EPG threshold, the analyses run did not include data beyond 30 weeks for the other treatments.
2.6.1. Analyses for fecal egg counts
Two mixed linear models with repeated measures over time were used to evaluate individual horse FECs pre- and post-treatment. In both models, the terms ‘replicate’ and ‘date’ were kept as random effects. The first model evaluated FECs following the single active MOX treatment and both combination treatments, while the second evaluated FECs following the two OBZ treatments. The categorical variables for both models were ‘treatment,’ ‘weeks post-treatment,’ and an interaction term of ‘weeks post treatment’ and ‘treatment.’ Whenever the interaction term of ‘treatment’ and ‘weeks post-treatment’ was found significant, a ‘least squares means’ analysis was used for a Tukey's pairwise comparison (α = 0.05).
2.6.2. Analyses for efficacies
Two mixed linear models with repeated measures over time were used to evaluate the drug efficacies per horse. ‘Date’ was kept as the random effect for both models. The first model compared the efficacies of MOX and the two combination treatments, and the second model compared the efficacies of the two OBZ treatments. For both models, the terms ‘treatment,’ ‘weeks post-treatment,’ and the interaction term ‘weeks post treatment’ and ‘treatment’ were the categorical variables. Whenever the interaction term ‘weeks post treatment’ and ‘treatment’ was found significant, a ‘least squares means’ analysis was used for a Tukey's pairwise comparison (α = 0.05).
2.7. Computer simulations
All simulations made use of a previously published cyathostomin model (Leathwick et al., 2015, 2019; Sauermann et al., 2019). Weather data for Lexington, Kentucky ranging from 2002 to 2011 were sourced from the National Centers for Environmental Information (www.ncdc.noaa.gov) and repeated four times to allow for a total simulation period of 40 years. A herd of 20 ponies was modeled to mimic the study population with the same age distribution and starting strongyle fecal egg count level. It was assumed that resistance was the result of a single mutation in one gene, which was represented by three genotypes; a homozygous susceptible (SS), a homozygous resistant (RR) and a heterozygote (RS). The initial resistance (R) allele frequency was calculated to match the desired initial efficacies following the Hardy Weinberg equilibrium (Table 4).
Table 4.
Survival rates following anthelmintic treatment against various cyathostomin stages.
| Active | Genotype | Survival Rate |
|||
|---|---|---|---|---|---|
| Adults | L4 lumen | L4 mucosa | EL3 | ||
| BZ | SS | 0.04 | 0.2 | 1 | 1 |
| BZ | RS | 0.5 | 0.6 | 1 | 1 |
| BZ | RR | 0.95 | 1 | 1 | 1 |
| MOX | SS | 0.01 | 0.01 | 0.25 | 0.4 |
| MOX | RS | 0.5 | 0.5 | 0.6 | 0.67 |
| MOX | RR | 0.95 | 0.95 | 0.95 | 0.95 |
Abbreviations: BZ, benzimidazole; MOX, moxidectin; SS, homozygous susceptible; RS, heterozygous; RR, homozygous resistant; L4, fourth larval stage; EL3, encysted third larval stage.
Two sets of simulation were performed to observe the time it took for resistance to develop to MOX under different treatment regimens. The first set of simulations replicated the treatment regimen performed in the field study where all horses were treated with a MOX and BZ combination at the same time. The simulations included a spread of BZ efficacies ranging between 99% and 25%. The combination with BZ 50% efficacy represented the field study. The simulated treatment dates corresponded with the dates used in the field study (January 1st and March 31st) and a treatment regimen in which the time of treatment alternated annually between January 1st and March 31st. All scenarios were simulated for 40 years.
The second set of simulations evaluated a treatment regimen employing selective treatments at various EPG thresholds (200, 300, 400, and 500 EPG). The two treatments simulated were either a combination of MOX and BZ where BZ efficacy was initially 50% (as in the field study) or a MOX single-active treatment. All scenarios were simulated for 40 years.
3. Results
3.1. Fecal egg counts
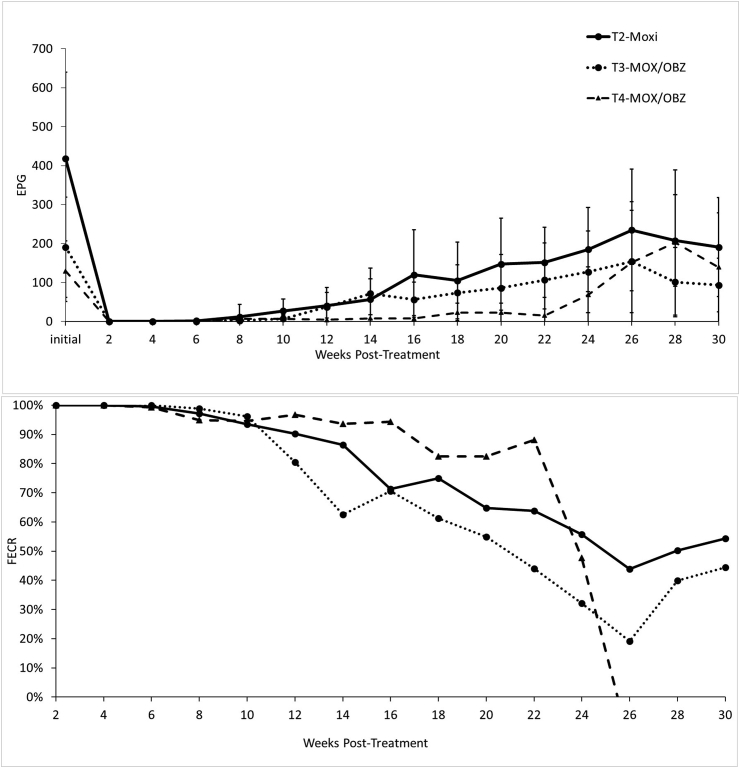
The mean strongyle egg counts prior to each treatment were higher at the beginning of this study and declined over the subsequent treatments, however these differences were not significant (Fig. 1 and Table 1).
Fig. 1.
Graphical representations of the single active moxidectin (MOX) treatment and the combination treatments of oxibendazole (OBZ) and MOX. Figure A shows fecal egg counts as eggs per gram (EPG). Error bars represent 95% confidence intervals (α = 0.05). Figure B shows the percent efficacy of the treatments using the fecal egg count reduction (FECR) test calculated using the total herd fecal egg counts pre- and post-treatment.
Table 1.
Results of OBZ treatments administered before (Treatment 1) and after (Treatment 5) a single MOX and two MOX/OBZ combination treatments. The top portion of the table shows results as the mean of individual pony FECs, and the bottom portion shows results of the FECRT. 95% confidence intervals are included in parenthesis (α = 0.05).
| Mean of individual EPG | ||
| Initial | 2 weeks PT | |
| Treatment 1-OBZ | 447.8 (200.4–695.2)a | 260.6 (125.2–396.1)a |
| Treatment 5-OBZ |
169.6 (31.7–307.5)b |
110.3 (19.1–201.5)b |
| Mean herd efficacy (FECRT) | ||
| 2 weeks PT (%) | ||
| Treatment 1-OBZ | 46.7 | |
| Treatment 5-OBZ | 40.1 | |
Abbreviations: OBZ, oxibendazole; MOX, moxidectin; EPG, eggs per gram feces; PT, post treatment; FECRT, fecal egg count reduction test.
Superscript letters indicate significant differences between time points (α = 0.05).
Fecal egg counts at two weeks post-treatment following MOX and both combination treatments were 0 EPG. The ERPs and associated efficacies can be found in Table 2. Both OBZ treatments resulted in significantly lower FECs at two weeks post-treatment (p < 0.0001, Table 1).
Table 2.
Egg reappearance periods following moxidectin single active and two combination treatments. The ERPs are defined when the mean heard efficacy was <85%. The actual percent efficacies are included in parentheses.
| Treatment | Time of ERP in weeks |
|---|---|
| MOX, single active | 16 (67.2%) |
| 1st Combination Treatment (MOX/OBZ) | 12 (80.4%) |
| 2nd Combination Treatment (MOX/OBZ) | 18 (82.5%) |
Abbreviations: MOX, moxidectin; OBZ, oxibendazole; ERP, egg reappearance period.
3.2. Anthelmintic efficacy
The efficacies of MOX alone and both combination treatments were 100% (Fig. 1). The efficacies of the first and last single active OBZ treatments were 46.7% and 40.1%, respectively, and these were not significantly different (p = 0.989) (Table 1). There were no significant differences between the MOX and combination treatments.
3.3. Larval counts
Larval counts from the coprocultures are presented in Table 3. Anytime MOX was used, whether alone or in combination, the larval counts were reduced to zero. On the other hand, larval counts increased after the first OBZ treatment, and only decreased 76.7% after the final OBZ treatment.
Table 3.
Total larval counts (percent of total number of larvae recovered) following coprocultures of ten individual samples collected at pre- and two weeks post-treatment. Treatments were with oxibendazole (10 mg/kg), moxidectin (0.4 mg/kg), or a combination of the two. No strongylin species were encountered.
| OBZ-1 (Pre) | OBZ-1 (Post) | MOX (Pre) | MOX (Post) | Combo-1 (Pre) | Combo-1 (Post) | Combo-2 (Pre) | Combo-2 (Post) | OBZ-2 (Pre) | OBZ-2 (Post) | |
|---|---|---|---|---|---|---|---|---|---|---|
| Total Larvae | 1512 | 3062 | 1350 | 0 | 246 | 0 | 216 | 0 | 43 | 10 |
| Cyathostominae | 1509 | 3054 | 1340 | 0 | 242 | 0 | 196 | 0 | 43 | 10 |
| L1 Strongyles | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| L2 Strongyles | 3 | 8 | 10 | 0 | 4 | 0 | 20 | 0 | 0 | 0 |
3.4. Computer simulations
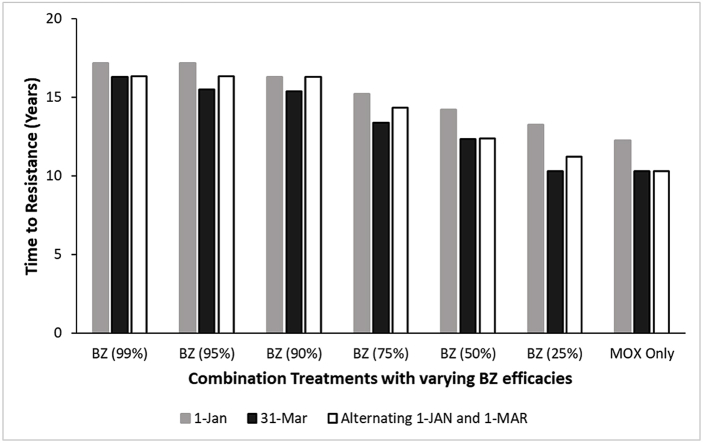
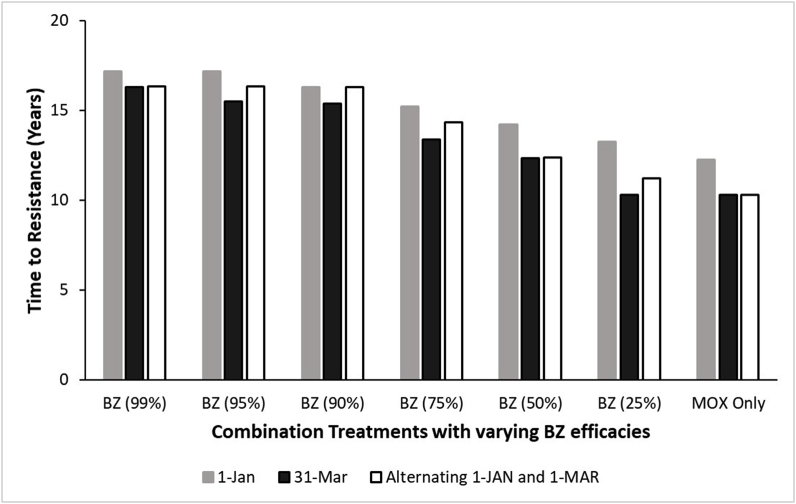
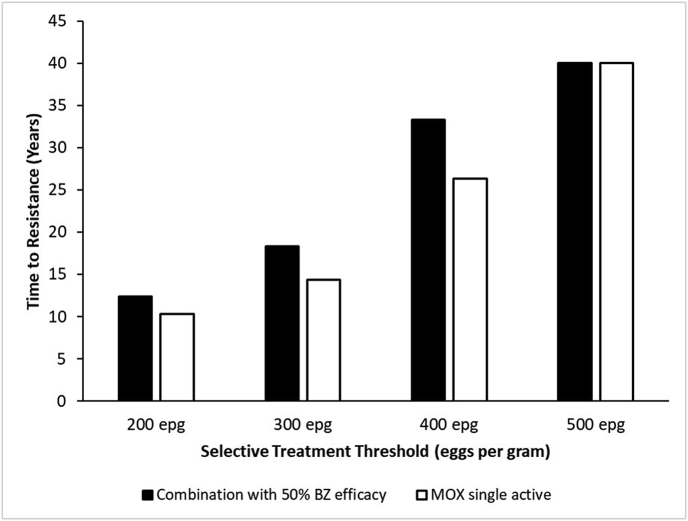
In comparison to MOX single active, combination treatment with 50% BZ efficacy slowed the rate of resistance development to MOX regardless of time of treatment (Fig. 2). Regardless of BZ efficacy used in the combination, one treatment per year on January 1st resulted in a slower rate of resistance development than the other treatment times (Fig. 2). Regarding selective treatment regimens, the combination treatments (50% BZ) slowed the rate of MOX resistance development at the 200, 300, and 400 EPG thresholds in comparison to MOX single active using the same thresholds (Fig. 3).
Fig. 2.
Results from the first simulation study implementing treatment parameters used in the field study and the effect on the rate of MOX resistance development. Treatments were administered one time per year either on January 1st (gray bars) or March 31st (black bars), or the annual treatments rotated between the dates (white bars). Treatments were a combination of MOX with varying BZ efficacies, or MOX single active.
Fig. 3.
Results from the second simulation study implementing selective therapy at various treatment thresholds and the effect on the rate of MOX resistance development. Treatments administered were either a combination of MOX and BZ (50% efficacy) as in the field study, or MOX single active.
4. Discussion
This study found that MOX used as a single active or in combination with OBZ (50%) achieved 100% efficacy against a BZ resistant yet ML naïve cyathostomin population (Fig. 1). When the treatment schedule used in this study was implemented in a 40-year computer simulation, combination treatment with BZ efficacy as low as 50% slowed the development of resistance to MOX. Furthermore, the simulations illustrated that combining selective therapy with use of combinations (BZ 50%) delayed the onset of resistance to MOX more effectively than when selective treatments used MOX alone. This finding is in agreement with studies for sheep parasites in which one of the actives in combination demonstrated reduced efficacy (Learmount et al., 2012) even as low as 50% (Leathwick et al., 2012).
Given the long-term resistance status of Population S cyathosomins to the BZ and TP drug classes, it appears that these resistance mechanisms did not affect the efficacy of MOX and no evidence of cross resistance was observed. In the field study, the ERP estimates for the three treatments using MOX were variable, ranging from 12 to 18 weeks (Fig. 1). Historic ERPs reported for MOX were between 16 and 22 weeks (Jacobs et al., 1995; Demeulenaere et al., 1997; DiPietro et al., 1997), but a couple aspects must be considered. First of all, previous studies lacked consensus in methodology used for determining ERP, and secondly the Population S ponies utilized in the present study demonstrated moderate to low fecal egg counts and a declining trend over the course of the study. The latter may well be a consequence of efficacious MOX treatments and lowered reinfection pressure which was also influenced by the grazing restrictions the ponies were under. Low starting egg counts are likely to add variability to the ERP determination, which could explain the findings made in this study. Seasonality is also known to significantly affect strongyle egg shedding (Chapman et al., 2003; Wood et al., 2012), and this likely added a source of variability to the ERPs as well.
The first simulation study employing the treatment dates utilized in the field study found that resistance developed more quickly with a 31-March annual treatment date compared to the 1-January or alternating annual treatments between the two dates. This suggests that treatments occurring when environmental conditions favor larval development (spring in the Northern Hemisphere) introduce a greater selection pressure for resistance. This is in agreement with recently reported findings (Nielsen et al., 2019b).
The benefits of combination treatments are contingent upon the size of the refugia population (Leathwick et al., 2012). Regarding cyathostomins, there are three possible sources of refugia, which are the free-living stages on pasture, luminal stages in untreated horses, and the encysted stages when a larvicidal drug is not used. In this study, the refugia population was minimal as all ponies received treatment simultaneously, and MOX provides some larvicidal efficacy. This study design represents similar situations on managed horse farms in the United States. Despite the AAEP equine parasite control recommendations for implementing selective therapy and refugia (Nielsen et al., 2019a), most horse farms still follow traditional deworming practices in which all horses are treated simultaneously for general prevention (Robert et al., 2015; Nielsen et al., 2018; Scare et al., 2018b).
In summary, this study identified combination deworming with MOX and BZ (~50%) as a possible control method against BZ resistant and macrocyclic lactone naïve cyathostomins. Refugia maintenance and seasonal timing of treatments remain important components in delaying the onset of resistance to all actives. Overall, the use of MOX-OBZ delayed the onset of resistance to MOX compared to when MOX single-active was used. While more long-term field studies are needed, this study provides initial support for combination treatment including a high-efficacy active against drug resistant cyathostomins, and demonstrates benefits of this approach despite substantially lowered efficacy of the other active ingredient. Further support for this approach would be obtained by studies investigating the combined efficacy of actives against parasites with only low levels of anthelmintic resistance.
Declaration of competing interest
The authors declare no conflicts of interest.
Acknowledgements
The field study was financially supported by Zoetis, and individual donors who supported Dr. Nielsen's latest crowdfunding campaign in support of the ‘Historic Parasitology Herds.’ We are grateful to the farm staff who provide continuous care for this special research herd. This study would not have been possible without the help from numerous very dedicated undergraduate students, namely Libby Wehling, Taylour Butler, Haley Zynda, Haley Anderson, Megan Bauer, Avery Martin, John Hines, Deanna Muscarello, Alyssa Carpenter, Morgan McVey, Samantha Naughton, Liz Bennett, and Aly McGuire.
References
- Barnes E.H., Dobson R.J., Barger I.A. Worm control and anthelmintic resistance: adventures with a model. Parasitol. Today. 1995;11:56–63. doi: 10.1016/0169-4758(95)80117-0. [DOI] [PubMed] [Google Scholar]
- Bartley D.J., Jackson F., Jackson E., Sargison N. Characterisation of two triple resistant field isolates of Teladorsagia from Scottish lowland sheep farms. Vet. Parasitol. 2004;123:189–199. doi: 10.1016/j.vetpar.2004.06.018. [DOI] [PubMed] [Google Scholar]
- Bartley D.J., Jackson E., Sargison N., Jackson F. Further characterization of a triple resistant field isolate of Teladorsagia from a Scottish lowland sheep farm. Vet. Parasitol. 2005;134:261–266. doi: 10.1016/j.vetpar.2005.07.017. [DOI] [PubMed] [Google Scholar]
- Bartram D.J., Leathwick D.M., Taylor M.A., Geurden T., Maeder S.J. The role of combination anthelmintic formulations in the sustainable control of sheep nematodes. Vet. Parasitol. 2012;186:151–158. doi: 10.1016/j.vetpar.2011.11.030. [DOI] [PubMed] [Google Scholar]
- Chapman M.R., French D.D., Klei T.R. Prevalence of strongyle nematodes in naturally infected ponies of different ages and during different seasons of the year in Louisiana. J. Parasitol. 2003;89:309–314. doi: 10.1645/0022-3395(2003)089[0309:POSNIN]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Cringoli G., Maurelli M.P., Levecke B., Bosco A., Vercruysse J., Utzinger J., Rinaldi L. The Mini-FLOTAC technique for the diagnosis of helminth and protozoan infections in humans and animals. Nat. Protoc. 2017;12(9):1723–1732. doi: 10.1038/nprot.2017.067. [DOI] [PubMed] [Google Scholar]
- Demeulenaere D., Vercruysse J., Dorny P., Claerebout E. Comparative studies of ivermectin and moxidectin in the control of naturally acquired cyathostome infections in horses. Vet. Rec. 1997;15:383–386. doi: 10.1136/vr.141.15.383. [DOI] [PubMed] [Google Scholar]
- DiPietro J.A., Hutchens D.E., Lock T.F., Walker K., Paul A.J., Shipley C., Rulli D. Clinical trial of moxidectin oral gel in horses. Vet. Parasitol. 1997;72:167–177. doi: 10.1016/s0304-4017(97)01108-4. [DOI] [PubMed] [Google Scholar]
- Entrocasso C., Alvarez L., Manazza J., Lifschitz A., Borda B., Virkel G., Mottier L., Lanusse C. Clinical efficacy assessment of the albendazole-ivermectin combination in lambs parasitized with resistant nematodes. Vet. Parasitol. 2008;155:249–256. doi: 10.1016/j.vetpar.2008.04.015. [DOI] [PubMed] [Google Scholar]
- Henriksen S.A., Korsholm H. A method for culture and recovery of gastrointestinal strongyle larvae. Nord. Veterinaermed. 1983;35:429–430. [PubMed] [Google Scholar]
- Herd R.P. The changing world of worms: the rise of the cyathostomes and the decline of Strongylus vulgaris. Comp. Cont. Educ. Pract. 1990;12:732–734. [Google Scholar]
- Jacobs D.E., Hutchinson M.J., Parker L., Gibbons L.M. Equine cyathostome infection – suppression of faecal egg output with moxidectin. Vet. Rec. 1995;137:545. doi: 10.1136/vr.137.21.545. [DOI] [PubMed] [Google Scholar]
- Kaplan R.M., West E.M., Norat-Collazo L.M., Vargas J. A combination treatment strategy using pyrantel pamoate and oxibendazole demonstrates additive effects for controlling equine cyathostomins. Equine Vet. Educ. 2014;26:485–491. [Google Scholar]
- Le Jambre L.F., Martin P.J., Johnston A. Efficacy of combination anthelmintics against multiple resistant strains of sheep nematodes. Anim. Prod. Sci. 2010;50:946–952. [Google Scholar]
- Learmount J., Taylor M.A., Bartram D.J. A computer simulation study to evaluate resistance development with a derquantel–abamectin combination on UK sheep farms. Vet. Parasitol. 2012;187:244–253. doi: 10.1016/j.vetpar.2011.12.033. [DOI] [PubMed] [Google Scholar]
- Leathwick D.M. Modelling the benefits of a new class of anthelmintic in combination. Vet. Parasitol. 2012;186:93–100. doi: 10.1016/j.vetpar.2011.11.050. [DOI] [PubMed] [Google Scholar]
- Leathwick D.M., Waghorn T.S., Miller C.M., Candy P.M., Oliver A.M.B. Managing anthelmintic resistance - use of a combination anthelmintic and leaving some lambs untreated to slow the development of resistance to ivermectin. Vet. Parasitol. 2012;187:285–294. doi: 10.1016/j.vetpar.2011.12.021. [DOI] [PubMed] [Google Scholar]
- Leathwick D.M., Donecker J.M., Nielsen M.K. A model for the dynamics of the free-living stages of equine cyathostomins. Vet. Parasitol. 2015;209:210–220. doi: 10.1016/j.vetpar.2015.02.031. [DOI] [PubMed] [Google Scholar]
- Leathwick D.M., Sauermann C.W., Reinemeyer C.R., Nielsen M.K. A model for the dynamics of the parasitic stages of equine cyathostomins. Vet. Parasitol. 2019;268:53–60. doi: 10.1016/j.vetpar.2019.03.004. [DOI] [PubMed] [Google Scholar]
- Lyons E. Population-S benzimidazole- and tetrahydropyrimidine-resistant small strongyles in a pony herd in Kentucky (1977-1999): effects of anthelmintic treatment on the parasites as determined in critical tests. Parasitol. Res. 2003;91:407–411. doi: 10.1007/s00436-003-0983-6. [DOI] [PubMed] [Google Scholar]
- Lyons E.T., Dorton A.R., Tolliver S.C. Evaluation of activity of fenbendazole, oxibendazole, piperazine, and pyrantel pamoate alone and combinations against ascarids, strongyles, and strongyloides in horse foals in field tests on two farms in Central Kentucky in 2014 and 2015. Vet. Parasitol. Reg. Stud. Rep. 2016;3–4:23–26. doi: 10.1016/j.vprsr.2016.05.007. [DOI] [PubMed] [Google Scholar]
- Muchiut S.M., Fernández A.S., Steffan P.E., Eliana R., Fiel C.A. Anthelmintic resistance: management of parasite refugia for Haemonchus contortus through replacement of resistant with susceptible populations. Vet. Parasitol. 2018;254:43–48. doi: 10.1016/j.vetpar.2018.03.004. [DOI] [PubMed] [Google Scholar]
- Nielsen M.K., Baptiste K.E., Tolliver S.C., Collins S.S., Lyons E.T. Analysis of multiyear studies in horses in Kentucky to ascertain whether counts of eggs and larvae per gram of feces are reliable indicators of numbers of strongyles and ascarids present. Vet. Parasitol. 2010;174:77–84. doi: 10.1016/j.vetpar.2010.08.007. [DOI] [PubMed] [Google Scholar]
- Nielsen M.K., Branan M.A., Wiedenheft A.M., Digianantonio R., Garber L.P., Kopral C.A., Phillippi-Taylor A.M., Traub-Dargatz J.L. Parasite control strategies used by equine owners in the United States: a national survey. Vet. Parasitol. 2018;250:45–51. doi: 10.1016/j.vetpar.2017.12.012. [DOI] [PubMed] [Google Scholar]
- Nielsen M.K., Mittel L., Grice A., Erskine M., Graves E., Vaala W., Tully R.C., French D.D., Bowman R., Kaplan R.M. American Association of Equine Practitioners; 2019. AAEP Parasite Control Guidelines.www.aaep.org [Google Scholar]
- Nielsen M.K., Sauermann C.W., Leathwick D.M. The effect of climate, season, and treatment intensity on anthelmintic resistance in cyathostomins: a modelling exercise. Vet. Parasitol. 2019;269:7–12. doi: 10.1016/j.vetpar.2019.04.003. [DOI] [PubMed] [Google Scholar]
- Noel M.L., Scare J.A., Bellaw J.L., Nielsen M.K. Accuracy and precision of mini-FLOTAC and McMaster techniques for determining equine strongyle egg counts. J. Equine Vet. Sci. 2016;48:182–187. [Google Scholar]
- Peregrine A.S., Molento M.B., Kaplan R.M., Nielsen M.K. Anthelmintic resistance in important parasites of horses: does it really matter? Vet. Parasitol. 2014;201:1–8. doi: 10.1016/j.vetpar.2014.01.004. [DOI] [PubMed] [Google Scholar]
- Reid S.W.J., Mair T.S., Hillyer M.H., Love S. Epidemiological risk factors associated with a diagnosis of clinical cyathostomiasis in the horse. Equine Vet. J. 1995;27:127–130. doi: 10.1111/j.2042-3306.1995.tb03048.x. [DOI] [PubMed] [Google Scholar]
- Reinemeyer C.R., Nielsen M.K. second ed. John Wiley & Sons, Inc.; Hoboken, NJ: 2018. Handbook of Equine Parasite Control. [Google Scholar]
- Robert M., Hu W., Nielsen M.K., Stowe C.J. Attitudes towards implementation of surveillance-based parasite control on Kentucky Thoroughbred farms – Current strategies, awareness and willingness-to-pay. Equine Vet. J. 2015;47:694–700. doi: 10.1111/evj.12344. [DOI] [PubMed] [Google Scholar]
- Russel A.F. The development of helminthiasis in Thoroughbred foals. J. Comp. Pathol. Ther. 1948;58:107–127. doi: 10.1016/s0368-1742(48)80009-3. [DOI] [PubMed] [Google Scholar]
- Sauermann C.W., Nielsen M.K., Leathwick D.M. Modelling the development of anthelmintic resistance in cyathostomin parasites: the importance of genetic and fitness parameters. Vet. Parasitol. 2019;269:28–33. doi: 10.1016/j.vetpar.2019.04.007. [DOI] [PubMed] [Google Scholar]
- Scare J.A., Lyons E.T., Wielgus K.M., Nielsen M.K. Combination deworming for the control of double-resistant cyathostomin parasites – short and long term consequences. Vet. Parasitol. 2018;251:112–118. doi: 10.1016/j.vetpar.2018.01.010. [DOI] [PubMed] [Google Scholar]
- Scare J.A., Steuer A.E., Gravatte H.S., Kálmán C., Ramires L., Dias de Castro L.L., Norris J.K., Miller F., Camargo F., Lawyer A., De Pedro P., Jolly B., Nielsen M.K. Management practices associated with strongylid parasite prevalence on horse farms in rural counties of Kentucky. Vet. Parasitol. Reg. Stud. Rep. 2018;14:25–31. doi: 10.1016/j.vprsr.2018.08.002. [DOI] [PubMed] [Google Scholar]
- Scott I., Bishop R.M., Pomroy W.E. Anthelmintic resistance in equine helminth parasites – a growing issue for horse owners and veterinarians in New Zealand? N. Z. Vet. J. 2015;63:188–198. doi: 10.1080/00480169.2014.987840. [DOI] [PubMed] [Google Scholar]
- Wilkes E.J.A., McConaghy F.F., Thompson R.L., Dawson K., Sangster N.C., Hughes K.J. Efficacy of a morantel–abamectin combination for the treatment of resistant ascarids in foals. Aust. Vet. J. 2017;95:85–88. doi: 10.1111/avj.12559. [DOI] [PubMed] [Google Scholar]
- Wood E.D.L., Matthews J.B., Stevenson S., Slote M., Nussey D.H. Variation in fecal egg counts in horses managed for conservation purposes: individual egg shedding consistency, age effects, and seasonal variation. Parasitology. 2012;140:115–128. doi: 10.1017/S003118201200128X. [DOI] [PubMed] [Google Scholar]