Abstract
The chemotherapeutics sorafenib and regorafenib inhibit shedding of MHC class I-related chain A (MICA) from hepatocellular carcinoma (HCC) cells by suppressing a disintegrin and metalloprotease 9 (ADAM9). MICA is a ligand for natural killer (NK) group 2 member D (NKG2D) and is expressed on tumor cells to elicit attack by NK cells. This study measured ADAM9 mRNA levels in blood samples of advanced HCC patients (n = 10). In newly diagnosed patients (n = 5), the plasma ADAM9 mRNA level was significantly higher than that in healthy controls (3.001 versus 1.00, p < 0.05). Among four patients treated with nivolumab therapy, two patients with clinical response to nivolumab showed significant decreases in fold changes of serum ADAM9 mRNA level from 573.98 to 262.58 and from 323.88 to 85.52 (p < 0.05); however, two patients with no response to nivolumab did not. Using the Cancer Genome Atlas database, we found that higher expression of ADAM9 in tumor tissues was associated with poorer survival of HCC patients (log-rank p = 0.00039), while ADAM10 and ADAM17 exhibited no such association. In addition, ADAM9 expression showed a positive correlation with the expression of inhibitory checkpoint molecules. This study, though small in sample size, clearly suggested that ADAM9 mRNA might serve as biomarker predicting clinical response and that the ADAM9-MICA-NKG2D system can be a good therapeutic target for HCC immunotherapy. Future studies are warranted to validate these findings.
Keywords: hepatocellular carcinoma, a disintegrin and metalloprotease 9, nivolumab, natural killer, immunotherapy
1. Introduction
Hepatocellular carcinoma (HCC) is the fifth most prevalent cancer and the third leading cause of cancer associated mortality worldwide [1,2]. Advanced stage HCC accompanied with portal vein invasion, distant metastasis, or lymph node metastasis is hard to treat due to the underlying liver cirrhosis, frequent recurrence, or multiple occurrence [3,4]. Currently, the tyrosine kinase inhibitors (TKI) sorafenib and lenvatinib are the first-line treatments in advanced HCC [5,6,7,8]. Regorafenib, nivolumab, cabozantinib, and ramucirumab were approved as second line therapies following their recent successes in several clinical trials [9,10,11,12,13,14,15]. Despite these breakthroughs, many patients still do not survive advanced HCC.
Among the recent breakthroughs in HCC treatments, immunotherapy is particularly notable. As a leading therapeutic in immunotherapy targeting programmed cell death 1 protein (PD-1), nivolumab demonstrated a 20% objective response rate and a 64% disease control rate in HCC patients who progressed on sorafenib [16]. Adoptive cellular immunotherapy using natural killer (NK) cells, cytokine induced killer (CIK) cells, or dendritic cells have also been studied [3]. Some adoptive cellular immunotherapy treatment modalities exhibited survival benefit in HCC patients [17]. These encouraging results were attributed to the immunological characteristics of the liver, which shelters a large pool of immune cells belonging to both the innate and acquired immune systems. The interactions between HCC and immune cells play a major role in tumor escape from immune surveillance resulting in HCC progression [18]. Inadequate co-stimulation, failure of tumor associated antigens (TAA) processing and presentation by dendritic cells, along with the suppression of effector T and NK cells are proposed mechanisms by which the HCC tumor cells evade the host immune system [19]. At the same time, HCC is indicated as a hot tumor that is characterized by increased expression of checkpoint molecules such as PD-1 and PD-1 ligand 1 (PD-L1) proteins, a large pool of tumor infiltrating immune cells, and high tumor mutational burdens, giving rise to ample amount of TAAs [20]. These immunological features enable HCC to benefit from immunotherapy. Thus, harnessing these immune characters through combination immunotherapy is proposed as the next important step in treatment of advanced HCC [4,13,14,15,21,22].
In this regard, a disintegrin and metalloproteases 9 (ADAM9) pathway in relation to MHC class I-related chain A (MICA) may provide a strategic ground for combination immunotherapy. Cell membrane-bound MICA (mMICA) is a ligand for NK group 2 member D (NKG2D), a stimulatory receptor on NK cells. The mMICA expressed in human HCC cells signals NK cells and other immune cells to kill the transformed hepatoma cells [23,24]. However, ADAM9, a matrix metalloproteinase (MMP) expressed by cancer cells, cleaves mMICA, releasing soluble MICA (sMICA). This sMICA acts as a decoy, weakening the cytotoxic immunity provided by NK cells and CD8+ T cells. This mMICA shedding by ADAM9 protease is identified as a mechanism of HCC escape from the host immune surveillance. Fortunately, sorafenib and regorafenib inhibit the expression of ADAM9 mRNA [23,24], restoring the host immunity against HCC and generating a room for synergistic action by adoptive cell therapy with NK cells or CD8+ T cells. Thus, the ADAM9-MICA-NKG2D system may provide a strategic target for a novel chemoimmunotherapy combining adoptive NK cell therapy and sorafenib or regorafenib [24].
In this pilot observational study, we aimed to characterize ADAM9 mRNA expression in blood samples of advanced HCC patients according to their clinical courses. To support our findings, we probed the role of ADAM9 as a prognostic biomarker for HCC using the Cancer Genome Atlas (TCGA) database. Furthermore, we present the case of a patient who achieved complete remission with regorafenib and autologous NK cell combination immunotherapy.
2. Results
2.1. Patient Characteristics
This study was conducted in CHA Bundang Hospital between January 2017 and November 2019. Advanced HCC patients eligible for this study, who were to be treated with sorafenib, regorafenib, or nivolumab as standard-of-care therapy and met our inclusion and exclusion criteria, were invited to the present study. A total of 10 patients participated in this study. The demographic and clinical details of each participant are listed in Table 1.
Table 1.
Demographic details and clinical characteristics of study participants (n = 10).
| Subject No. | Age | Sex | Etiology | Antiviral Therapy | Serum AFP (ng/mL) | Child-Pugh Class | Tumor Size (cm) | PV Invasion (Vp) * | mUICC Stage | Extra-Hepatic Metastasis | Type of Therapy |
|---|---|---|---|---|---|---|---|---|---|---|---|
| #1 | 58 | M | HBV | TDF | 97,387 | A | 8 | 4 | IVb | Yes | Sorafenib |
| #2 | 61 | M | HBV | ETV | >200,000 | B | 22 | 2 | Ivb | Yes | Sorafenib |
| #3 | 61 | M | HBV | ETV | 190 | B | 5.2 | 3 | Ivb | Yes | Sorafenib |
| #4 | 55 | M | HBV | TDF | 71.7 | A | 3 | 0 | III | No | TACE, Sorafenib |
| #5 | 58 | M | HBV | ETV | 14.7 | A | 10 | 2 | Ivb | Yes | Sorafenib, Nivolumab |
| #6 | 59 | M | HBV | ETV | 82.4 | A | 4 | 2 | Iva | No | Sorafenib, Nivolumab + NK cell therapy, Regorafenib + NK cell therapy |
| #7 | 45 | M | HBV | TDF | 154.7 | B | 11 | 4 | Iva | No | Sorafenib, Nivolumab |
| #8 | 44 | F | HCV | DAC/SUN | 6519.4 | A | 4.5 | 0 | Ivb | Yes | Sorafenib, Regorafenib, Nivolumab |
| #9 | 58 | F | HBV | TDF | 66 | A | 9 | 2 | Ivb | Yes | Sorafenib, Nivolumab Regorafenib |
| #10 | 76 | M | NASH | none | 4594.1 | A | 5.2 | 1 | Ivb | Yes | Sorafenib, Nivolumab |
Abbreviations: No, number; M, male; F, female; AFP, alpha-fetoprotein; PV, portal vein; mUICC, modified Union for International Cancer Control; HBV, hepatitis B virus; HCV, hepatitis C virus; NASH, nonalcoholic steatohepatitis; TACE, transarterial chemoembolization; NK, natural killer; TDF, tenofovir disoproxil fumarate; ETV, entecavir; DAC, daclatasvir; SUN, asunaprevir. * The extent of portal vein invasion (Vp) by tumor thrombosis was documented according to the Liver Cancer Study Group of Japan classification: Vp0 = no portal vein invasion, Vp1 = segmental portal vein invasion, Vp2 = right anterior/posterior portal vein, Vp3 = right/left portal vein and Vp4 = main trunk [25].
The 10 participants comprised eight chronic hepatitis B (CHB), one chronic hepatitis C (CHC) and one non-viral HCC patients. At the time of enrollment, five participants (Subject No. (#) 1 to #5) were newly diagnosed and treatment-naïve; they were subjected to sorafenib as first-line therapy. In addition, four patients (Subject #7 to #10) had failed first-line sorafenib therapy and were subjected to nivolumab therapy, whereas one patient (Subject #6) was enrolled after the patient had already reached near complete remission.
The baseline characteristics of the participants and their HCC are summarized in Table S1. The mean age was 57.4 years, and 80% of the participants were male. The modified Union for International Cancer Control (mUICC) TNM stage was III in 1 patient (10%), IVa in 2 patients (20%), and IVb in 7 patients (70%). The Barcelona Clinic Liver Cancer (BCLC) stage was B in 1 patient (10%) and C in 9 patients (90%). Most patients (70%) had well-preserved liver function (Child–Pugh class A).
2.2. Overexpression of Plasma ADAM9 mRNA in Untreated HCC Patients
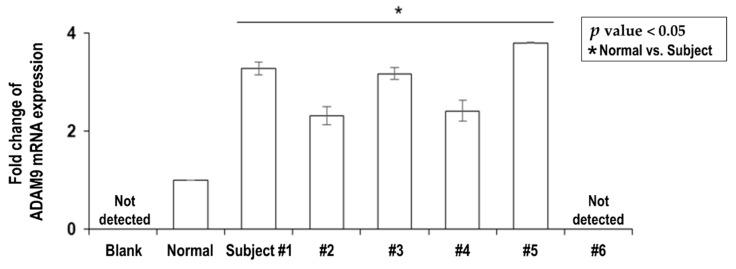
The plasma levels of ADAM9 mRNA were tested in the five newly diagnosed and treatment-naïve HCC patients (Table 1, Subject #1 to #5) and expressed as fold changes compared to the healthy control group (n = 5, 100% female, mean age 34.2 years). The mean value of pre-treatment plasma ADAM9 mRNA levels in the HCC patients was significantly higher than that in the healthy controls (3.001 ± 0.279 vs. 1.00 ± 0.005, p < 0.05) (Figure 1).
Figure 1.
Expression level of ADAM9 mRNA in plasma samples of treatment naïve advanced HCC patients and one HCC patient with near complete remission. Each patient (Subject #1 to #5) with newly diagnosed and treatment naïve HCC had significantly elevated expression of ADAM9 mRNA in plasma compared with normal controls (3.001 ± 0.279 vs. 1.00 ± 0.005, p < 0.05). The HCC patient who had reached near complete remission (Subject #6) did not express ADAM9 mRNA in plasma at all.
2.3. Decreased ADAM9 mRNA Expression Correlated with Response to Nivolumab
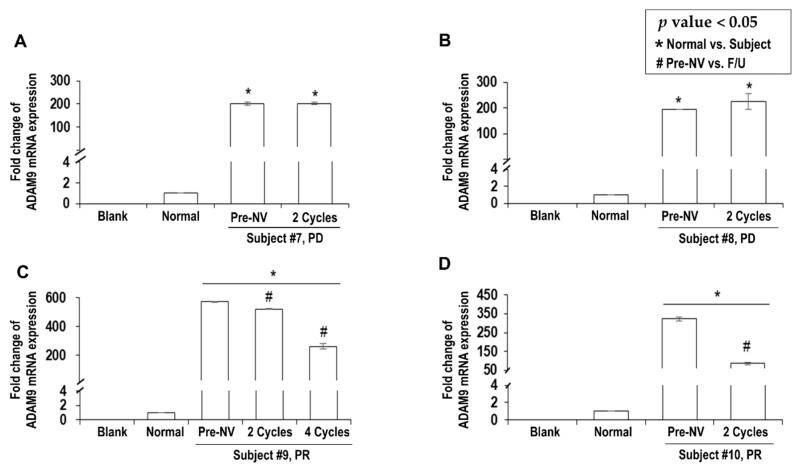
In four patients who were to receive nivolumab as second- or third-line treatment (Table 1, Subject #7 to #10), ADAM9 mRNA expression in serum was significantly elevated compared to that in the healthy controls (Figure 2; pre-nivolumab mean, 323.39 ± 88.67 vs. 1.00 ± 0.000003, p < 0.05). To investigate the functional relevance between the change in ADAM9 mRNA expression and clinical response, the serum levels of ADAM9 mRNA were followed during the nivolumab therapy.
Figure 2.
Expression level changes of serum ADAM9 mRNA in advanced HCC patients treated with nivolumab. The number of nivolumab cycles completed prior to follow-up blood sampling is denoted on the X-axis. After 2 cycles of nivolumab therapy, non-responders did not show significant change in ADAM9 mRNA (A, Subject #7; B, Subject #8). In contrast, responders exhibited a significant decrease in ADAM9 mRNA (C, Subject #9, from 573.98 ± 5.16 to 523.85 ± 7.0 (p < 0.05) after 2 cycles, and further down to 262.58 ± 20.13 (p < 0.05) after 4 cycles; D, Subject #10, from 323.88 ± 10.67 to 85.52 ± 5.59 (p < 0.05) after 2 cycles). Abbreviations: NV, nivolumab; F/U, follow-up; PD, progressive disease; PR, partial response.
After three and four cycles of nivolumab therapy, respectively, Subjects #7 and #8 showed progressive disease (PD) on follow-up computed tomography (CT) scans. In these patients, serum levels of ADAM9 mRNA in the follow-up were not significantly different from the pre-treatment levels (p > 0.05) (Figure 2A,B). Both patients succumbed to HCC progression and liver failure within 6 months since the start of nivolumab therapy.
In contrast, Subjects #9 and #10 started to reveal tumor regression after four cycles of nivolumab therapy. Their clinical courses are presented in Figures S1 and S2. Later, both patients exhibited partial response (PR) and survived longer than 6 months after the nivolumab therapy. In these patients, serum levels of ADAM9 mRNA decreased significantly. In Subject #9, the serum ADAM9 mRNA dropped from the pre-treatment level of 573.98 ± 5.16 to 523.85 ± 7.07 (p < 0.05) after two cycles, and further dropped to 262.58 ± 20.13 (p < 0.05) after 4 cycles of nivolumab therapy (Figure 2C). In Subject #10, it dropped from 323.88 ± 10.67 to 85.52 ± 5.59 (p < 0.05, Figure 2D) after two cycles of nivolumab therapy.
2.4. Immunophenotype Changes following Nivolumab Therapy
Lymphocyte immunophenotypes were tested before and after three cycles of nivolumab therapy for Subject #7 and four cycles for the rest (Table 2 and Figures S3–S6). Before nivolumab therapy, NK cells in Subjects #8 and #9 and cytotoxic T cells in Subject #9 were depleted, and these were therefore not detected for inhibitory checkpoint markers. In subject #8 and #10, PD-1 or T cell immunoglobulin- and mucin-domain-containing molecule-3 (TIM-3) positive cytotoxic T cells decreased significantly, but this change was not correlated with response to nivolumab. In contrast, TIM-3 positive helper T cells decreased in responders, but increased in non-responders.
Table 2.
Lymphocyte immunophenotypes of the 4 patients who received nivolumab therapy (%).
| Cell Type | Phenotype Marker | Non-Responders | Responders | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Subject #7 | Subject #8 | Subject #9 | Subject #10 | ||||||
| Pre-NV | 3 Cycles | Pre-NV | 4 Cycles | Pre-NV | 4 Cycles | Pre-NV | 4 Cycles | ||
| T cells | CD3+ | 63.62 | 52.98 | 74.53 | 84.57 | 6.18 | 19.02 | 45.01 | 53.65 |
| Helper T cells | CD3+CD4+ | 27.46 | 26.36 | 59.91 | 52.43 | 5.51 | 10.58 | 30.57 | 35.58 |
| Cytotoxic T cells | CD3+CD8+ | 34.15 | 20.03 | 13.95 | 30.7 | 0.73 | 7.41 | 14.57 | 18.75 |
| B cells | CD19+ | 10.15 | 7.27 | 1.88 | 1.06 | 3.21 | 60.7 | 19.1 | 5.84 |
| NK cells | CD3−CD56+ | 10.77 | 5 | 0.21 | 5.52 | 0.61 | 7.49 | 19.87 | 18.88 |
| NKT cells | CD3+CD56+ | 4 | 2.37 | 2.03 | 2.59 | 0.23 | 0.78 | 6.68 | 4.63 |
| Cytotoxic T cells | CD3+CD8+PD-1+ | 3.95 | 6.48 | 22.34 | 2.87 | ND | 0.12 | 24.65 | 7.99 |
| CD3+CD8+TIM3+ | 21.05 | 52.11 | 15.06 | 5.3 | ND | 45.32 | 11.57 | 6.73 | |
| CD3+CD8+LAG3+ | 6.58 | 16.47 | 38.57 | 37.64 | ND | 3.33 | 29.95 | 30.63 | |
| CD3+CD8+BTLA+ | 14.47 | 11.32 | 29.87 | 54.32 | ND | 6.7 | 2.7 | 1.79 | |
| Helper T cells | CD3+CD4+PD-1+ | 0 | 1.22 | 15.44 | 2.15 | 7.69 | 7.34 | 13.09 | 3.3 |
| CD3+CD4+TIM3+ | 19.81 | 37.3 | 3.42 | 11.89 | 69.74 | 45.52 | 10.2 | 4.01 | |
| CD3+CD4+LAG3+ | 0.94 | 16.51 | 35.16 | 25.04 | 8.21 | 2.42 | 6.93 | 4.53 | |
| CD3+CD4+BTLA+ | 13.16 | 9.4 | 24.81 | 29.19 | 0.51 | 2 | 1.94 | 11.14 | |
| NK cells | CD3−CD56+PD-1+ | 8.7 | 1.24 | ND | 1.58 | ND | 1.19 | 4.39 | 3.94 |
| CD3−CD56+TIM-3+ | 4.35 | 22.39 | ND | 21.45 | ND | 33.6 | 25.45 | 14.6 | |
| CD3−CD56+LAG3+ | 0 | 2.74 | ND | 14.51 | ND | 5.06 | 46.1 | 25.15 | |
| CD3−CD56+BTLA+ | 47.83 | 26.18 | ND | 9.15 | ND | 3.45 | 1.63 | 1.47 | |
Blood samples for the follow-up were acquired after three cycles of nivolumab therapy for Subject #7 and four cycles for the rest. Abbreviations: NV, nivolumab; HCC, hepatocellular carcinoma; NK, natural killer; NKT, natural killer-T; CD, cluster differentiation; ND, not detected; PD-1, programmed cell death 1; TIM-3, T cell immunoglobulin- and mucin-domain-containing molecule 3; LAG-3, lymphocyte activation gene 3; BTLA, B and T lymphocyte attenuator.
2.5. Serum ADAM9 mRNA Expression was Completely Suppressed in Complete Response of HCC
Plasma ADAM9 mRNA level was completely suppressed in one patient (Table 1 and Figure 1, Subject #6), who achieved complete response (CR). The clinical treatment course and the outcomes are detailed in Figures S7 and S8. This patient was a 59-year-old man with CHB HCC and had tumor recurrence after surgical resection. Since the HCC progressed on sorafenib and radiotherapy, nivolumab was administered in combination with activated autologous NK cell therapy (2–6 × 109 cells/100 mL, intravenous infusion every 4 weeks). With serum AFP level increasing continuously, disease progression at the molecular level was suspected that nivolumab was switched to the third-line chemotherapy, regorafenib. To allow synergistic action of NK cells upon suppression of ADAM9 protease and sMICA by regorafenib [24], the patient continued to receive the NK cell therapy every 4 weeks up to six times and then every 8 weeks up to a total of 15 times. At 6 months after beginning regorafenib with concomitant NK cell therapy, the patient achieved nearly complete regression of HCC. At this point, we acquired the patient’s blood sample for analysis and found that ADAM9 mRNA was not detectable at all in his plasma (Figure 1). From then on, immunophenotype changes were also checked and a decrease in inhibitory checkpoint molecules was observed (Table S2).
2.6. ADAM9 was Associated with HCC Prognosis in TCGA Database
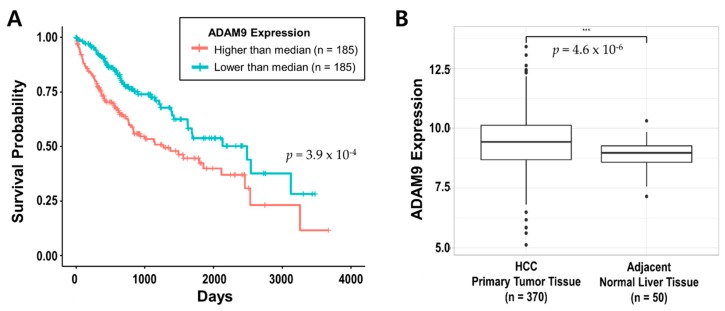
To evaluate the effect of ADAM9 expression on HCC prognosis, we performed in-silico analyses of 370 HCC patients from the TCGA database. Kaplan-Meier plots revealed that the group with ADAM9 expression the higher than the median had a significantly poorer overall survival rate (Log-rank test p = 3.9 × 10−4) (Figure 3A). In addition, ADAM9 was significantly upregulated in primary tumor tissues of HCC (n = 370) compared with adjacent normal liver tissues (n = 50) (t-test p = 4.6 × 10−6) (Figure 3B). Unlike ADAM9, other ADAM family genes ADAM10 and ADAM17 neither differed in their expression levels between HCC tumor tissues and adjacent normal liver tissues, nor showed significant correlation with survival analysis (Figures S9 and S10).
Figure 3.
Effect of ADAM9 expression on HCC prognosis in the TCGA database. (A) Kaplan–Meier plot of HCC patients (n = 370) according to ADAM9 expressions level higher or lower than median (n = 185 for each group). (B) Box-plot comparing ADAM9 expression between HCC primary tumor (n = 370) and adjacent normal liver tissue (n = 50). Abbreviations: HCC, hepatocellular carcinoma; TCGA, The Cancer Genome Atlas.
2.7. ADAM9 Expression is Positively Correlated with PD-1, TIM-3 and BTLA
The correlation between ADAM9 expression and expression of immune checkpoint molecules (PD-1, TIM-3, lymphocyte activation gene-3 (LAG-3) and B and T lymphocyte attenuator (BTLA)) in HCC patients (n = 370) from TCGA database was analyzed. ADAM9 expression was positively correlated with the expression of PD-1, TIM-3, and BTLA but not with that of LAG-3 (Figure 4). TIM-3 had the strongest positive correlation with ADAM9 (Correlation coefficient r = 0.37 and p = 1.3 × 10−13) (Figure 4B).
Figure 4.
Correlation between ADAM9 expression and four immune checkpoint genes PD-1 (A), TIM-3 (B), LAG-3 (C), and BTLA (D). Abbreviations: r, Pearson’s correlation coefficient; PD-1, programmed cell death 1; TIM-3, T cell immunoglobulin- and mucin-domain-containing molecule 3; LAG-3, lymphocyte activation gene 3; BTLA, B and T lymphocyte attenuator.
3. Discussion
In the present study, we found that the ADAM9 blood mRNA level was significantly elevated in HCC patients compared to healthy controls. Furthermore, the magnitude of this elevation was much greater in the patients with previous treatment failure than in the newly diagnosed treatment-naïve patients. These findings suggested that ADAM9 expression increase as HCC progresses, and that the immune evasion mechanisms involving ADAM9 protease probably aggravate as HCC progresses. In addition, serum ADAM9 mRNA levels decreased significantly in the two patients (one CHB and one non-viral HCC) who responded to nivolumab. Furthermore, ADAM9 mRNA was not detected at all in the plasma of one HCC patient who achieved CR with regorafenib and NK cell combination immunotherapy. As such, the present study demonstrated, for the first time, the functional relevance of blood ADAM9 mRNA levels with clinical response to HCC treatments. Since the sample size was too small in our study, we probed the TCGA data to find supporting evidence and found that higher ADAM9 expression level was significantly associated with poor prognosis of HCC. In contrast, ADAM10 and ADAM17, which were also reportedly related to MICA shedding [26], showed no such association.
In our study, the change of serum ADAM9 mRNA was easily correlated with the clinical response to nivolumab, while the observed lymphocyte immunophenotype changes were intriguing but not enough to draw a solid conclusion. Namely, Subject #8 did show some favorable changes in her lymphocyte immunophenotypes (mainly PD-1+ or TIM-3+ cytotoxic T cells) but tumor progression was still seen. Another interesting finding was the change of TIM-3 positive helper T cell; responders (Subjects #9 and #10) showed a decrease while non-responders (Subjects #7 and #8) showed an increase. In addition, ADAM9 was strongly correlated with TIM-3 in the TCGA database. These findings altogether suggest that key features of lymphocyte immunophenotype changes that can predict treatment response early on may exist. Our study indicated that the proportion of TIM-3 positive helper T cells may be a good candidate marker, and that unleashing helper T cell from exhaustion may be more important than unleashing cytotoxic T cells. Future studies are needed to elucidate the interplay between the ADAM9-MICA-NKG2D system and lymphocyte immunophenotypes, and to find the relevance between such factors and clinical outcome.
ADAMs belong to the zinc protease superfamily, and they are usually transmembrane proteins [27]. Containing disintegrin and metalloprotease domains, ADAMs take part in multiple cellular functions including cell adhesion and migration, proteolysis of the extracellular matrix and shedding of membrane proteins [27,28]. Several studies have indicated that ADAMs are involved in tumor development and progression of HCC [23,24,27,28,29,30,31,32,33,34,35,36,37,38,39,40]. Though the pathogenesis of HCC is multifactorial, it is largely due to hepatitis B (HBV) or C virus (HCV) infection and alcoholic or non-alcoholic fatty liver disease. These underlying liver conditions result in chronic inflammation and liver fibrosis, causing continuous remodeling of the extracellular matrix [27]. ADAMs are involved in this inflammatory process that leads to development of HCC [27]. Among several ADAMs associated with HCC, those related to MICA shedding are noteworthy as MICA is a critical part of cytotoxic cellular immunity. Our study revealed that only ADAM9 was significantly associated with prognosis of HCC while others, ADAM10 and ADAM17, were not. Regarding ADAM9, previous studies demonstrated that transcriptional suppression of ADAM9 led to inhibition of proliferation and invasion activities of HCC cell lines [23,24,36,37,38,39,40]. Inhibition of ADAM9 protease also showed similar results [41]. Some of these results were backed by the increased mMICA expression and subsequently increased susceptibility of HCC cells to NK cells [23,24,40]. On the other hand, treatment with interleukin (IL)-1β on HCC cell lines increased the expression of ADAM9 and sMICA, and the IL-1β-treated HCC cells became more resistant to the cytolytic activity of NK cells [42].
MICA is a ligand of NKG2D, and it triggers NK cell or CD8+ T cell-mediated cytokine release and cytotoxicity towards the target cells [24]. MICA expression is induced in response to various types of stress such as heat, DNA damage, and viral infection [43]. The ADAM9 protease that interferes with the MICA-NKG2D system was overexpressed in human HCC tissues [24]. In vitro, ADAM9 knockdown increased mMICA expression, decreased sMICA production, and increased the cytolytic activity of NK cells against HCC cells [23]. Sorafenib suppresses the expression of ADAM9. Therefore, sorafenib treatment in vitro showed the same results as the ADAM9 knockdown [23]. This important phenomenon was reproduced by another group. This time regorafenib was tested. Regorafenib suppressed the expression of ADAM9 and ADAM10 to a greater extent than sorafenib [24]. This suppression led to mMICA accumulation and inhibition of sMICA production [24]. The authors suggested that regorafenib is superior to sorafenib as regorafenib suppresses not only ADAM9 but also ADAM10 [24].
Beyond these experimental findings, the involvement of the MICA-NKG2D system was described in HCC patients. Both mMICA and sMICA showed elevated expression in human HCC [44,45,46]. Serum levels of sMICA were significantly elevated in chronic liver disease and HCC patients compared to healthy controls [44]. Furthermore, there was a stepwise increase in the level of sMICA as liver disease progressed from chronic hepatitis to cirrhosis, low-grade HCC and high-grade HCC [44]. In CHC patients, serum IL-1β levels were positively correlated with serum sMICA level, and serum IL-1β levels were significantly higher in CHC patients with HCC than those without HCC [42]. On the other hand, sMICA decrease could suggest favorable response to HCC treatment. For example, serum sMICA levels decreased, and NKG2D expression on NK cells and CD8+ T cells increased significantly after transcatheter arterial embolization therapy [44]. This could be related to our finding that ADAM9 mRNA decreased in HCC patients who responded to treatments. Interestingly, there were divergent findings on the level of sMICA and its association with incidence or prognosis of HCC. In HCC with CHB, higher sMICA was correlated with vascular invasion and poor prognosis and was associated with a G allele of single nucleotide polymorphism (SNP) rs2596542, a risk allele for HBV-induced HCC (p = 0.029, odds ratio = 1.19) [47]. In contrast, A allele of SNP rs2596542 was significantly associated with the higher risk of HCV-induced HCC (p = 4.21 × 10−13, odds ratio = 1.39) as well as low levels of sMICA [48]. These findings seemingly contradict each other, but could be explained by the fact that there may be an interplay between HCC etiology, i.e., the type of hepatitis virus or genetic factors and ADAM9-MICA-NKG2D system.
HCC occurrence in HBV infection can be partly ascribed to the perturbation of signaling pathways by HBV-encoded X protein (HBx) incorporated into the human genome [49]. HBx was associated with enhanced expression of MMPs such as ADAM9 and ADAM10 [50,51]. More MMPs expressed could mean more MICA shedding. Therefore, this viral factor explains the association between the higher sMICA levels and the poorer prognosis of HCC with CHB [43,47]. In the absence of such interactions between virus and MMPs, the level of sMICA may, more or less, linearly follow that of mMICA expression, as mMICA is the source of sMICA [48]. In this case, low sMICA levels reflect low mMICA expression resulted from host genetic factor, which may indicate weakened cytotoxic immune surveillance, as was observed in HCV-induced HCC [48]. In addition, hepatitis B surface antigen is known to inhibit MICA expression via induction of cellular micro RNAs [52].
Anti-viral treatment for HBV and HCV was much improved by the development of new antiviral agents. This notwithstanding, the development of HCC still remains a concern even after HBV suppression [53,54] or HCV eradication [55]. In these patients, NK cells were usually observed to have depressed function with decreased capacity of interferon-γ production, impaired IL-15 production, or decreased expression of NK cell activation receptors including NKG2D [51,56,57]. Representing 30–50% of all hepatic lymphocytes, NK cells increase up to 90% in patients with hepatic malignancy. Therefore, ADAM9-MICA-NKG2D system may also serve as a good target for HCC prevention strategy in patients chronically infected with HBV or HCV. In this regard, it is encouraging that one group found an approved drug for anti-alcoholism, disulfiram, and showed that it effectively restored mMICA expression by inhibiting ADAM10 and did not have unfavorable off-target effects [58].
One of the unmet needs in this era of immunologic treatments for HCC or other cancers is a biomarker that can predict treatment response well in advance. It would be very useful if the marker could dictate the best cancer immunotherapy course. Despite the proven efficacy of nivolumab in HCC, expression of a known immune marker, PD-L1, in tumor tissues, fell short of predicting treatment response [16]. Our and previous studies have suggested that ADAM9 mRNA may fill this unmet need [23,24,43]. ADAM9 transcript elevation might suggest the existence of an ADAM9 associated immune evasion mechanism in tumors. Decrease or increase of ADAM9 mRNA after 2–4 cycles of treatment may help predict treatment response or failure early on. Finally, a decrease of ADAM9 mRNA may indicate a candidate who can expect synergistic effects by adding adoptive cell therapy. Adoptive cell therapy composed of NK or CD8+ T cells have shown efficacy in treatment of HCC [3,17,22,59,60,61]. However, the patient population who had survival benefits was mostly restricted to those with minimal tumor burden after treatments with curative modality [17,22,62]. Therefore, there remains significant room for improvement of the adoptive cell therapy in patients with high burden of HCC [17,22,62]. This combination strategy may greatly enhance the survival of advanced HCC patients, as was shown in Subject #6. Thus, ADAM9 mRNA may help select the patients who may benefit from such combination immunotherapy.
There were limitations in our study. The population size was too small. We did not investigate whether the findings of our study could be applied for other agents approved for advanced HCC or not. To complement such weaknesses, we probed the TCGA data and retrieved encouraging results. This pilot study demonstrates that ADAM9 mRNA is associated with clinical response to HCC treatment and that there is an important link between the MICA-NKG2D system and prognosis of HCC, by showing how ADAM9 mRNA expression changes over time as HCC patients received nivolumab therapy. Thus, ADAM9 mRNA has potential as a biomarker to predict the clinical response of HCC patients, and the ADAM9-MICA-NKG2D system may be a good therapeutic target in HCC immunotherapy. Restoration of MICA-NKG2D signaling by suppressing ADAM9 mRNA and its influence on the tumor microenvironment and its potential as a prognostic marker of human HCC patients should be investigated in future studies. The knowledge garnered will help us to formulate better strategies to manage the advanced HCC patients.
4. Materials and Methods
4.1. Patients
This study was conducted in CHA Bundang Hospital between January 2017 and October 2019. HCC patients who were to be treated with sorafenib, regorafenib or nivolumab as the standard-of-care therapy were eligible for this study. Patients who were 19 years of age or older and were willing to participate in this study were enrolled. For enrollment, the patient must have met the inclusion criteria which included presentation of HCC, diagnosed histologically or radiologically, in accordance with the guidelines of the American Association for the Study of Liver Diseases or the European Association for the Study of the Liver [5,10]. Other inclusion criteria were Child-Pugh score ≤ 7, BCLC stage B or C, Eastern Cooperative Oncology Group (ECOG) performance status score of 0 or 1, adequate bone marrow (hemoglobin ≥ 9 g/dL, granulocyte count >1000/mm3 and platelet count ≥ 40,000/mm3), adequate liver function (serum aspartate aminotransferase and alanine aminotransferase < 5 times of upper normal limit, bilirubin ≤ 3 mg/dL, prothrombin time international normalized ratio (PT INR) < 1.5) and adequate renal function (serum creatinine < 1.5 times the upper normal limit). In addition, HCC lesions must be measurable by CT or magnetic resonance imaging (MRI) and there should be at least one lesion ≥ 1.0 cm in maximum diameter. Exclusion criteria were evidence of malignant tumor of other than HCC, uncontrolled ascites, hepatic encephalopathy, major bleeding event within 30 days, severe bacterial infection, infection with HIV, major cardiac disease, anticoagulation therapy, pregnant or breast-feeding woman, dysphagia precluding drug administration, and other contraindications for systemic HCC therapy. Both written and oral consent was obtained prior to sample collection. The study was approved by the institutional review board (IRB) of the CHA Bundang Medical Center (IRB protocol: 2016-03-039-019).
4.2. Study Design and Protocol
This study was a prospective observational clinical research study. After enrollment, the first blood samples were drawn within 3 weeks before and 4 days after the commencement of chemotherapy with sorafenib, regorafenib or nivolumab. Afterwards, follow-up blood samples were acquired at the 5th, 10th and 20th week or when chemotherapy was terminated. Blood samples were drawn into two plain bottles (BD Vacutainer®, 5 mL), two EDTA bottles (BD Vacutainer®, 3 mL) and one heparin bottle (BD Vacutainer®, 10 mL). Immune cells acquired from the EDTA and heparin bottles were immediately subjected to examination of immunophenotypes and functional assays. The plasma samples in the heparin bottles and serum samples in the plain bottles were stored at −80 °C for further ADAM9 mRNA quantification.
Patients’ age, sex, etiology of HCC and Child–Pugh score were recorded. Clinical and TNM stages were classified according to the BCLC clinical stage and the mUICC stages, respectively [5,63]. Tumor response (CR, PR, stable disease, and PD) was evaluated in accordance with the modified Response Evaluation Criteria in Solid Tumors (mRECIST) [64] every 10–12 weeks for more than 1 year via dynamic liver CT or MRI. Complete blood cell count, blood chemistry (liver function, renal function, metabolic function, electrolyte), PT INR, and tumor markers (AFP and PIVKA-II) were regularly checked as part of standard care.
4.3. mRNA Isolation and Real-Time PCR
To evaluate the serum or plasma ADAM9 mRNA expression levels, quantitative real-time reverse transcription-polymerase chain reaction (RT-PCR) was performed using a CFX Connect real-time system (Bio-Rad, Hercules, CA, USA). Total RNA was isolated from serum or plasma samples using the miRNeasy Serum/Plasma Kit (Qiagen, Hilden, Germany). For normal controls, we used banking serum and plasma from women (n = 5, mean age 34.2 years, range 29–41 years) who delivered at term (≥35 gestational weeks) because we had difficulty with collection of normal serum. The collection and use of these samples for research purposes was approved by the IRB of CHA Hospital (Seoul, Korea) (IRB protocol: 2006-12). The mRNA levels of ADAM9 and glyceraldehyde phosphate dehydrogenase (GAPDH) were determined by quantitative real-time RT-PCR using SYBR green mastermix (Roche, Basel, Switzerland) according to the manufacturer’s instructions. PCR reactions were performed by denaturation at 95 °C for 10 min followed by 45 cycles of amplification at 95 °C for 10 s, 55 °C for 15 s, and then melting curves were performed after PCR amplification under the following conditions: 60 °C to 95 °C with a temperature transition rate of 1 °C/s. The sequence of the PCR primers used for detection of ADAM9 and GAPDH were as follows: ADAM9 forward primer, 5′-GGAAACTGCCTT CTTAATATTCCAAA-3′, ADAM9 reverse primer, 5′-CCCAGCGTC CACCAACTTAT-3′, GAPDH forward primer, 5′- CTCCTCTTCGGCAGCACA-3′, GAPDH reverse primer, 5′- AACGCTTCACCTA ATTTG CG T -3′. GAPDH mRNA from each sample was quantified as an endogenous control of internal RNA. All experiments were performed in duplicate.
4.4. Flow Cytometric Analysis
HCC patient’s blood in the heparin bottle was transferred to a 15 mL conical tube containing 5 mL filcol-paque plus (GE Healthcare, Chicago, IL, USA) and centrifuged at 2000 rpm for 10 min. Plasma was transferred to a 5 mL cryovial (Corning Inc, Corning, NY, USA) and stored at −80 ℃. The buffy coat layer was transferred to a new 15 mL conical tube washed 2 times with PBS. Peripheral blood mononuclear cells (PBMCs) were stained with anti-CD3-eFluor 506 (ebioscience, San Diego, CA, USA), anti-CD3-eFluor 450 (ebioscience, USA), anti-CD4-eFluor 450 (ebioscience, USA), anti-CD4-PE (ebioscience), anti-CD8-Alexa Fluor 700 (ebioscience), anti-CD19-PE (ebioscience), anti-CD56-Alexa Fluor 700 (ebioscience), anti-CD16-PerCP/Cy5.5 (BioLegend, San Diego, CA, USA), anti-NKG2D-FITC (ebioscience), anti-CD158b-PerCP/Cy5.5 (BioLegend), anti-TIM3-FITC (ebioscience), anti-LAG-3-PE (BioLegend), anti-BTLA-APC/Cy7 (BioLegend), anti-PD-1-APC (BioLegend), anti-CTLA4-APC (Miltenyi Biotec, Bergisch Gladbach, Germany), anti-mouse IgG1 kappa isotype control-FITC (ebioscience, USA), anti-mouse IgG1 kappa isotype control-PE (ebioscience), anti-mouse IgG1 kappa isotype control-PerCP/Cy5.5 (BioLegend), REA control-APC (Miltenyi Biotec), anti-mouse IgG1 kappa isotype control-Alexa Fluor 700 (ebioscience), anti-mouse IgG1 kappa isotype control-APC/Cy7 (BioLegend), anti-mouse IgG1 kappa isotype control-eFluor 506 (ebioscience), anti-mouse IgG1 kappa isotype control-eFluor 450 (ebioscience). Stained cells were analyzed with CytoFLEX flow cytometry (Beckman Coulter, Brea, CA, USA) and resulting data were analyzed using Kaluza version 1.5a analysis software (Beckman Coulter).
4.5. Statistical Analysis
Data are expressed as the mean (± standard deviation) or frequencies (percentages), as appropriate. The statistical significance of differences between the groups was determined by Student t test or two-sample t test. A p-value of <0.05 was considered statistically significant. Statistical analyses were performed with SPSS 22.0 (SPSS Inc., Chicago, IL, USA).
4.6. In-Silico Analysis with TCGA Database
We downloaded transcriptomic, survival and clinical data of HCC patients (indexed as LIHC) from the Xena TCGA database hub (https://xenabrowser.net). The transcriptomic data included 370 patients and was generated by the University of North Carolina TCGA genome characterization center. The survival data includes information on overall survival. Statistical analyses were performed with t-test, Pearson’s correlation analysis, Cox-regression and log-rank analysis. All statistical analyses with TCGA dataset were performed with Python (Version 2.7.10) and R-studio (Version 1.1.456).
5. Conclusions
ADAM9 mRNA was overexpressed in blood samples of patients with advanced HCC. Decreased levels of ADAM9 mRNA in the blood was significantly associated with clinical response to HCC treatment with nivolumab. Also, using the TCGA database, we found that higher expression of ADAM9 in HCC tumor tissues is associated with poor survival of HCC patients. Therefore, ADAM9 mRNA has a potential as a biomarker predicting clinical response, and the ADAM9-MICA-NKG2D system may serve as a therapeutic target of HCC immunotherapy.
Acknowledgments
The authors thank ‘Immunotherapy Development Team of CHA Biolab (Seongnam, Korea)’ for their technical assistance.
Supplementary Materials
The following are available online at https://www.mdpi.com/2072-6694/12/3/745/s1, Table S1: Baseline patient and tumor characteristics of the study population (n = 10), Table S2: Changes in the proportions of immune checkpoint molecules and immune cells in the HCC patient (Subject #6), who showed complete tumor response following regorafenib and NK cell combination therapy, Figures S1 and S2: Clinical course and serum AFP levels of Subjects #9 and #10, Figures S3–S6: Lymphocyte distribution and inhibitory checkpoint molecule expression before and after nivolumab therapy in Subjects #7-#10. Figure S7: Clinical course and serum AFP levels of Subject #6, Figure S8: Completely resolved HCC after regorafenib and autologous NK cell combined therapy in one patient (Subject #6), Figure S9–S10: Effect of ADAM10 and ADAM17 expression on HCC prognosis.
Author Contributions
S.O. and J.L. contributed to the study design, data collection, study analysis, manuscript writing, critical review of the manuscript, and final approval of the manuscript submission. S.O., Y.P., J.L., H.-J.L., S.-H.L., Y.-S.B., K.K. and G.J.K. performed the experiments, analyzed the data, and wrote the manuscript. S.-M.L. and S.-K.C. assisted in performing the experiments. Y.C., Y.-E.C., Y.H., M.K., S.-G.H. and K.K. assisted in analyzing and interpreting the data. S.O., J.L., K.K. and G.J.K. contributed to data interpretation, critical review of the manuscript, and final approval of the manuscript submission. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by a grant from the “Gyeonggi-Incheon study group of the Korean Association for the Study of the Liver.”
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Chon Y.E., Park H., Hyun H.K., Ha Y., Kim M.N., Kim B.K., Lee J.H., Kim S.U., Kim D.Y., Ahn S.H., et al. Development of a New Nomogram Including Neutrophil-to-Lymphocyte Ratio to Predict Survival in Patients with Hepatocellular Carcinoma Undergoing Transarterial Chemoembolization. Cancers. 2019;11:509. doi: 10.3390/cancers11040509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ferlay J., Soerjomataram I., Dikshit R., Eser S., Mathers C., Rebelo M., Parkin D.M., Forman D., Bray F. Cancer incidence and mortality worldwide: Sources, methods and major patterns in GLOBOCAN 2012. Int. J. Cancer. 2015;136:E359–E386. doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- 3.Lee J.-H., Oh S.-Y., Kim J.Y., Nishida N. Cancer immunotherapy for hepatocellular carcinoma. [(accessed on 20 March 2020)];Hepatoma. Res. 2018 4:51. doi: 10.20517/2394-5079.2018.78. Available online: https://hrjournal.net/article/view/2776. [DOI] [Google Scholar]
- 4.Nishida N., Kudo M. Immune checkpoint blockade for the treatment of human hepatocellular carcinoma. Hepatol. Res. 2018;48:622–634. doi: 10.1111/hepr.13191. [DOI] [PubMed] [Google Scholar]
- 5.European Association for the Study of the Liver EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. J. Hepatol. 2018;69:182–236. doi: 10.1016/j.jhep.2018.03.019. [DOI] [PubMed] [Google Scholar]
- 6.Kudo M., Finn R.S., Qin S., Han K.H., Ikeda K., Piscaglia F., Baron A., Park J.W., Han G., Jassem J., et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: A randomised phase 3 non-inferiority trial. Lancet. 2018;391:1163–1173. doi: 10.1016/S0140-6736(18)30207-1. [DOI] [PubMed] [Google Scholar]
- 7.Saffo S., Taddei T.H. Systemic Management for Advanced Hepatocellular Carcinoma: A Review of the Molecular Pathways of Carcinogenesis, Current and Emerging Therapies, and Novel Treatment Strategies. Dig. Dis. Sci. 2019;64:1016–1029. doi: 10.1007/s10620-019-05582-x. [DOI] [PubMed] [Google Scholar]
- 8.Ueshima K., Nishida N., Hagiwara S., Aoki T., Minami T., Chishina H., Takita M., Minami Y., Ida H., Takenaka M., et al. Impact of Baseline ALBI Grade on the Outcomes of Hepatocellular Carcinoma Patients Treated with Lenvatinib: A Multicenter Study. Cancers. 2019;11:952. doi: 10.3390/cancers11070952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Daher S., Massarwa M., Benson A.A., Khoury T. Current and Future Treatment of Hepatocellular Carcinoma: An Updated Comprehensive Review. J. Clin. Transl. Hepatol. 2018;6:69–78. doi: 10.14218/JCTH.2017.00031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Heimbach J.K., Kulik L.M., Finn R.S., Sirlin C.B., Abecassis M.M., Roberts L.R., Zhu A.X., Murad M.H., Marrero J.A. AASLD guidelines for the treatment of hepatocellular carcinoma. Hepatology. 2018;67:358–380. doi: 10.1002/hep.29086. [DOI] [PubMed] [Google Scholar]
- 11.Kulik L., El-Serag H.B. Epidemiology and Management of Hepatocellular Carcinoma. Gastroenterology. 2019;156:477–491.e471. doi: 10.1053/j.gastro.2018.08.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bteich F., Di Bisceglie A.M. Current and Future Systemic Therapies for Hepatocellular Carcinoma. Gastroenterol. Hepatol. 2019;15:266–272. [PMC free article] [PubMed] [Google Scholar]
- 13.Liu X., Qin S. Immune Checkpoint Inhibitors in Hepatocellular Carcinoma: Opportunities and Challenges. Oncologist. 2019;24:S3–S10. doi: 10.1634/theoncologist.2019-IO-S1-s01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kudo M. Combination Cancer Immunotherapy with Molecular Targeted Agents/Anti-CTLA-4 Antibody for Hepatocellular Carcinoma. Liver Cancer. 2019;8:1–11. doi: 10.1159/000496277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Marrero J.A., Kulik L.M., Sirlin C.B., Zhu A.X., Finn R.S., Abecassis M.M., Roberts L.R., Heimbach J.K. Diagnosis, Staging, and Management of Hepatocellular Carcinoma: 2018 Practice Guidance by the American Association for the Study of Liver Diseases. Hepatology. 2018;68:723–750. doi: 10.1002/hep.29913. [DOI] [PubMed] [Google Scholar]
- 16.El-Khoueiry A.B., Sangro B., Yau T., Crocenzi T.S., Kudo M., Hsu C., Kim T.Y., Choo S.P., Trojan J., Welling T.H.R., et al. Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): An open-label, non-comparative, phase 1/2 dose escalation and expansion trial. Lancet. 2017;389:2492–2502. doi: 10.1016/S0140-6736(17)31046-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee J.H., Lee J.H., Lim Y.S., Yeon J.E., Song T.J., Yu S.J., Gwak G.Y., Kim K.M., Kim Y.J., Lee J.W., et al. Adjuvant immunotherapy with autologous cytokine-induced killer cells for hepatocellular carcinoma. Gastroenterology. 2015;148:1383–1391.e1386. doi: 10.1053/j.gastro.2015.02.055. [DOI] [PubMed] [Google Scholar]
- 18.Schmidt N., Neumann-Haefelin C., Thimme R. Cellular immune responses to hepatocellular carcinoma: Lessons for immunotherapy. Dig. Dis. 2012;30:483–491. doi: 10.1159/000341697. [DOI] [PubMed] [Google Scholar]
- 19.Kudo M. Immune Checkpoint Inhibition in Hepatocellular Carcinoma: Basics and Ongoing Clinical Trials. Oncology. 2017;92(Suppl. 1):50–62. doi: 10.1159/000451016. [DOI] [PubMed] [Google Scholar]
- 20.Chen D.S., Mellman I. Elements of cancer immunity and the cancer-immune set point. Nature. 2017;541:321–330. doi: 10.1038/nature21349. [DOI] [PubMed] [Google Scholar]
- 21.Li L., Li W., Wang C., Yan X., Wang Y., Niu C., Zhang X., Li M., Tian H., Yao C., et al. Adoptive transfer of natural killer cells in combination with chemotherapy improves outcomes of patients with locally advanced colon carcinoma. Cytotherapy. 2018;20:134–148. doi: 10.1016/j.jcyt.2017.09.009. [DOI] [PubMed] [Google Scholar]
- 22.Oh S., Lee J.H., Kwack K., Choi S.W. Natural Killer Cell Therapy: A New Treatment Paradigm for Solid Tumors. Cancers. 2019;11:1534. doi: 10.3390/cancers11101534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kohga K., Takehara T., Tatsumi T., Ishida H., Miyagi T., Hosui A., Hayashi N. Sorafenib inhibits the shedding of major histocompatibility complex class I-related chain A on hepatocellular carcinoma cells by down-regulating a disintegrin and metalloproteinase 9. Hepatology. 2010;51:1264–1273. doi: 10.1002/hep.23456. [DOI] [PubMed] [Google Scholar]
- 24.Arai J., Goto K., Stephanou A., Tanoue Y., Ito S., Muroyama R., Matsubara Y., Nakagawa R., Morimoto S., Kaise Y., et al. Predominance of regorafenib over sorafenib: Restoration of membrane-bound MICA in hepatocellular carcinoma cells. J. Gastroenterol. Hepatol. 2018;33:1075–1081. doi: 10.1111/jgh.14029. [DOI] [PubMed] [Google Scholar]
- 25.Kudo M., Izumi N., Ichida T., Ku Y., Kokudo N., Sakamoto M., Takayama T., Nakashima O., Matsui O., Matsuyama Y. Report of the 19th follow-up survey of primary liver cancer in Japan. Hepatol. Res. 2016;46:372–390. doi: 10.1111/hepr.12697. [DOI] [PubMed] [Google Scholar]
- 26.Waldhauer I., Goehlsdorf D., Gieseke F., Weinschenk T., Wittenbrink M., Ludwig A., Stevanovic S., Rammensee H.G., Steinle A. Tumor-associated MICA is shed by ADAM proteases. Cancer Res. 2008;68:6368–6376. doi: 10.1158/0008-5472.CAN-07-6768. [DOI] [PubMed] [Google Scholar]
- 27.Mazzocca A., Giannelli G., Antonaci S. Involvement of ADAMs in tumorigenesis and progression of hepatocellular carcinoma: Is it merely fortuitous or a real pathogenic link? Biochim. Biophys. Acta. 2010;1806:74–81. doi: 10.1016/j.bbcan.2010.02.002. [DOI] [PubMed] [Google Scholar]
- 28.Seals D.F., Courtneidge S.A. The ADAMs family of metalloproteases: Multidomain proteins with multiple functions. Genes Dev. 2003;17:7–30. doi: 10.1101/gad.1039703. [DOI] [PubMed] [Google Scholar]
- 29.Xia C., Zhang D., Li Y., Chen J., Zhou H., Nie L., Sun Y., Guo S., Cao J., Zhou F., et al. Inhibition of hepatocellular carcinoma cell proliferation, migration, and invasion by a disintegrin and metalloproteinase-17 inhibitor TNF484. J. Res. Med. Sci. 2019;24:26. doi: 10.4103/jrms.JRMS_129_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shiu J.S., Hsieh M.J., Chiou H.L., Wang H.L., Yeh C.B., Yang S.F., Chou Y.E. Impact of ADAM10 gene polymorphisms on hepatocellular carcinoma development and clinical characteristics. Int. J. Med. Sci. 2018;15:1334–1340. doi: 10.7150/ijms.27059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li Y., Ren Z., Wang Y., Dang Y.Z., Meng B.X., Wang G.D., Zhang J., Wu J., Wen N. ADAM17 promotes cell migration and invasion through the integrin beta1 pathway in hepatocellular carcinoma. Exp. Cell Res. 2018;370:373–382. doi: 10.1016/j.yexcr.2018.06.039. [DOI] [PubMed] [Google Scholar]
- 32.Honda H., Takamura M., Yamagiwa S., Genda T., Horigome R., Kimura N., Setsu T., Tominaga K., Kamimura H., Matsuda Y., et al. Overexpression of a disintegrin and metalloproteinase 21 is associated with motility, metastasis, and poor prognosis in hepatocellular carcinoma. Sci. Rep. 2017;7:15485. doi: 10.1038/s41598-017-15800-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu Y., Zhang W., Liu S., Liu K., Ji B., Wang Y. miR-365 targets ADAM10 and suppresses the cell growth and metastasis of hepatocellular carcinoma. Oncol. Rep. 2017;37:1857–1864. doi: 10.3892/or.2017.5423. [DOI] [PubMed] [Google Scholar]
- 34.Li S.Q., Wang D.M., Zhu S., Ma Z., Li R.F., Xu Z.S., Han H.M. The important role of ADAM8 in the progression of hepatocellular carcinoma induced by diethylnitrosamine in mice. Hum. Exp. Toxicol. 2015;34:1053–1072. doi: 10.1177/0960327114567767. [DOI] [PubMed] [Google Scholar]
- 35.Liu S., Zhang W., Liu K., Ji B., Wang G. Silencing ADAM10 inhibits the in vitro and in vivo growth of hepatocellular carcinoma cancer cells. Mol. Med. Rep. 2015;11:597–602. doi: 10.3892/mmr.2014.2652. [DOI] [PubMed] [Google Scholar]
- 36.Dong Y., Wu Z., He M., Chen Y., Chen Y., Shen X., Zhao X., Zhang L., Yuan B., Zeng Z. ADAM9 mediates the interleukin-6-induced Epithelial-Mesenchymal transition and metastasis through ROS production in hepatoma cells. Cancer Lett. 2018;421:1–14. doi: 10.1016/j.canlet.2018.02.010. [DOI] [PubMed] [Google Scholar]
- 37.Hu D., Shen D., Zhang M., Jiang N., Sun F., Yuan S., Wan K. MiR-488 suppresses cell proliferation and invasion by targeting ADAM9 and lncRNA HULC in hepatocellular carcinoma. Am. J. Cancer Res. 2017;7:2070–2080. [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 38.Wan D., Shen S., Fu S., Preston B., Brandon C., He S., Shen C., Wu J., Wang S., Xie W., et al. miR-203 suppresses the proliferation and metastasis of hepatocellular carcinoma by targeting oncogene ADAM9 and oncogenic long non-coding RNA HULC. Anti-Cancer Agents Med. Chem. 2016;16:414–423. doi: 10.2174/1871520615666150716105955. [DOI] [PubMed] [Google Scholar]
- 39.Zhou C., Liu J., Li Y., Liu L., Zhang X., Ma C.Y., Hua S.C., Yang M., Yuan Q. microRNA-1274a, a modulator of sorafenib induced a disintegrin and metalloproteinase 9 (ADAM9) down-regulation in hepatocellular carcinoma. FEBS Lett. 2011;585:1828–1834. doi: 10.1016/j.febslet.2011.04.040. [DOI] [PubMed] [Google Scholar]
- 40.Kohga K., Tatsumi T., Takehara T., Tsunematsu H., Shimizu S., Yamamoto M., Sasakawa A., Miyagi T., Hayashi N. Expression of CD133 confers malignant potential by regulating metalloproteinases in human hepatocellular carcinoma. J. Hepatol. 2010;52:872–879. doi: 10.1016/j.jhep.2009.12.030. [DOI] [PubMed] [Google Scholar]
- 41.Itabashi H., Maesawa C., Oikawa H., Kotani K., Sakurai E., Kato K., Komatsu H., Nitta H., Kawamura H., Wakabayashi G., et al. Angiotensin II and epidermal growth factor receptor cross-talk mediated by a disintegrin and metalloprotease accelerates tumor cell proliferation of hepatocellular carcinoma cell lines. Hepatol. Res. 2008;38:601–613. doi: 10.1111/j.1872-034X.2007.00304.x. [DOI] [PubMed] [Google Scholar]
- 42.Kohga K., Tatsumi T., Tsunematsu H., Aono S., Shimizu S., Kodama T., Hikita H., Yamamoto M., Oze T., Aketa H., et al. Interleukin-1beta enhances the production of soluble MICA in human hepatocellular carcinoma. Cancer Immunol. Immunother. CII. 2012;61:1425–1432. doi: 10.1007/s00262-012-1208-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Goto K., Kato N. MICA SNPs and the NKG2D system in virus-induced HCC. J. Gastroenterol. 2015;50:261–272. doi: 10.1007/s00535-014-1000-9. [DOI] [PubMed] [Google Scholar]
- 44.Kohga K., Takehara T., Tatsumi T., Ohkawa K., Miyagi T., Hiramatsu N., Kanto T., Kasugai T., Katayama K., Kato M., et al. Serum levels of soluble major histocompatibility complex (MHC) class I-related chain A in patients with chronic liver diseases and changes during transcatheter arterial embolization for hepatocellular carcinoma. Cancer Sci. 2008;99:1643–1649. doi: 10.1111/j.1349-7006.2008.00859.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jinushi M., Takehara T., Tatsumi T., Kanto T., Groh V., Spies T., Kimura R., Miyagi T., Mochizuki K., Sasaki Y., et al. Expression and role of MICA and MICB in human hepatocellular carcinomas and their regulation by retinoic acid. Int. J. Cancer. 2003;104:354–361. doi: 10.1002/ijc.10966. [DOI] [PubMed] [Google Scholar]
- 46.Groh V., Rhinehart R., Secrist H., Bauer S., Grabstein K.H., Spies T. Broad tumor-associated expression and recognition by tumor-derived gamma delta T cells of MICA and MICB. Proc. Natl. Acad. Sci. USA. 1999;96:6879–6884. doi: 10.1073/pnas.96.12.6879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kumar V., Yi Lo P.H., Sawai H., Kato N., Takahashi A., Deng Z., Urabe Y., Mbarek H., Tokunaga K., Tanaka Y., et al. Soluble MICA and a MICA variation as possible prognostic biomarkers for HBV-induced hepatocellular carcinoma. PLoS ONE. 2012;7:e44743. doi: 10.1371/journal.pone.0044743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kumar V., Kato N., Urabe Y., Takahashi A., Muroyama R., Hosono N., Otsuka M., Tateishi R., Omata M., Nakagawa H., et al. Genome-wide association study identifies a susceptibility locus for HCV-induced hepatocellular carcinoma. Nat. Genet. 2011;43:455–458. doi: 10.1038/ng.809. [DOI] [PubMed] [Google Scholar]
- 49.Feitelson M.A., Bonamassa B., Arzumanyan A. The roles of hepatitis B virus-encoded X protein in virus replication and the pathogenesis of chronic liver disease. Expert Opin. Ther. Targets. 2014;18:293–306. doi: 10.1517/14728222.2014.867947. [DOI] [PubMed] [Google Scholar]
- 50.Ou D.P., Tao Y.M., Tang F.Q., Yang L.Y. The hepatitis B virus X protein promotes hepatocellular carcinoma metastasis by upregulation of matrix metalloproteinases. Int. J. Cancer. 2007;120:1208–1214. doi: 10.1002/ijc.22452. [DOI] [PubMed] [Google Scholar]
- 51.Lunemann S., Malone D.F., Hengst J., Port K., Grabowski J., Deterding K., Markova A., Bremer B., Schlaphoff V., Cornberg M., et al. Compromised function of natural killer cells in acute and chronic viral hepatitis. J. Infect. Dis. 2014;209:1362–1373. doi: 10.1093/infdis/jit561. [DOI] [PubMed] [Google Scholar]
- 52.Wu J., Zhang X.J., Shi K.Q., Chen Y.P., Ren Y.F., Song Y.J., Li G., Xue Y.F., Fang Y.X., Deng Z.J., et al. Hepatitis B surface antigen inhibits MICA and MICB expression via induction of cellular miRNAs in hepatocellular carcinoma cells. Carcinogenesis. 2014;35:155–163. doi: 10.1093/carcin/bgt268. [DOI] [PubMed] [Google Scholar]
- 53.Chotiyaputta W., Lok A.S. Hepatitis B virus variants. Nat. Rev. Gastroenterol. Hepatol. 2009;6:453–462. doi: 10.1038/nrgastro.2009.107. [DOI] [PubMed] [Google Scholar]
- 54.Abu-Amara M., Feld J.J. Does antiviral therapy for chronic hepatitis B reduce the risk of hepatocellular carcinoma? Semin. Liver Dis. 2013;33:157–166. doi: 10.1055/s-0033-1345719. [DOI] [PubMed] [Google Scholar]
- 55.Morgan R.L., Baack B., Smith B.D., Yartel A., Pitasi M., Falck-Ytter Y. Eradication of hepatitis C virus infection and the development of hepatocellular carcinoma: A meta-analysis of observational studies. Ann. Intern. Med. 2013;158:329–337. doi: 10.7326/0003-4819-158-5-201303050-00005. [DOI] [PubMed] [Google Scholar]
- 56.Li Y., Wang J.J., Gao S., Liu Q., Bai J., Zhao X.Q., Hao Y.H., Ding H.H., Zhu F., Yang D.L., et al. Decreased peripheral natural killer cells activity in the immune activated stage of chronic hepatitis B. PLoS ONE. 2014;9:e86927. doi: 10.1371/journal.pone.0086927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jinushi M., Takehara T., Tatsumi T., Kanto T., Groh V., Spies T., Suzuki T., Miyagi T., Hayashi N. Autocrine/paracrine IL-15 that is required for type I IFN-mediated dendritic cell expression of MHC class I-related chain A and B is impaired in hepatitis C virus infection. J. Immunol. 2003;171:5423–5429. doi: 10.4049/jimmunol.171.10.5423. [DOI] [PubMed] [Google Scholar]
- 58.Goto K., Arai J., Stephanou A., Kato N. Novel therapeutic features of disulfiram against hepatocellular carcinoma cells with inhibitory effects on a disintegrin and metalloproteinase 10. Oncotarget. 2018;9:18821–18831. doi: 10.18632/oncotarget.24568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Takayama T., Makuuchi M., Sekine T., Terui S., Shiraiwa H., Kosuge T., Yamazaki S., Hasegawa H., Suzuki K., Yamagata M., et al. Distribution and therapeutic effect of intraarterially transferred tumor-infiltrating lymphocytes in hepatic malignancies. A preliminary report. Cancer. 1991;68:2391–2396. doi: 10.1002/1097-0142(19911201)68:11<2391::AID-CNCR2820681110>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 60.Kawata A., Une Y., Hosokawa M., Wakizaka Y., Namieno T., Uchino J., Kobayashi H. Adjuvant chemoimmunotherapy for hepatocellular carcinoma patients. Adriamycin, interleukin-2, and lymphokine-activated killer cells versus adriamycin alone. Am. J. Clin. Oncol. 1995;18:257–262. doi: 10.1097/00000421-199506000-00014. [DOI] [PubMed] [Google Scholar]
- 61.Takayama T., Sekine T., Makuuchi M., Yamasaki S., Kosuge T., Yamamoto J., Shimada K., Sakamoto M., Hirohashi S., Ohashi Y., et al. Adoptive immunotherapy to lower postsurgical recurrence rates of hepatocellular carcinoma: A randomised trial. Lancet. 2000;356:802–807. doi: 10.1016/S0140-6736(00)02654-4. [DOI] [PubMed] [Google Scholar]
- 62.Liu D., Staveley-O’Carroll K.F., Li G. Immune-based therapy clinical trials in hepatocellular carcinoma. J. Clin. Cell. Immunol. 2015;6:376. doi: 10.4172/2155-9899.1000376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ueno S., Tanabe G., Nuruki K., Hamanoue M., Komorizono Y., Oketani M., Hokotate H., Inoue H., Baba Y., Imamura Y., et al. Prognostic performance of the new classification of primary liver cancer of Japan (4th edition) for patients with hepatocellular carcinoma: A validation analysis. Hepatol. Res. 2002;24:395–403. doi: 10.1016/S1386-6346(02)00144-4. [DOI] [PubMed] [Google Scholar]
- 64.Lencioni R., Llovet J.M. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin. Liver Dis. 2010;30:52–60. doi: 10.1055/s-0030-1247132. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.