Abstract
The aim of this study was to investigate mesenchymal stem cell (MSC) function on novel type hybrid organic/inorganic microparticles (MPs) for application to bone regeneration. The MPs were based on chitosan (CS) and consisted of inorganic components, such as dibasic calcium phosphate (CaHPO4) or calcium carbonate (CaCO3). The MPs were crosslinked using tripolyphosphate. Four types of hybrid MPs were fabricated: CS; CS–10% CaHPO4; CS–20% CaHPO4; and CS–10% CaCO3. The MSCs were attached to all the types of MPs at day 1 and started to spread and proliferate further by days 2 and 7, as analysed by fluorescence microcopy. Cell proliferation was measured at days 7, 14, 21 and 28 by counting the cells attached on the MPs. The number of proliferated cells increased significantly for all types of MPs as time increased. MSC differentiation was analysed using osteoblast (OB) phenotype markers, including alkaline phosphatase activity (ALP), collagen I (COLLI) and osteocalcin (OCN) at days 7, 14, 21 and 28, using quantitative real-time PCR. The normalized mRNA expression of ALP for all MPs was observed only at day 7. The normalized mRNA expression of COLLI and OCN was significantly increased for all types of hybrid MPs at each time point compared to the control samples. Collectively, our results proved that hybrid organic/inorganic MPs were non-cytotoxic and supported MSC attachment, spreading, proliferation and differentiation into the OB phenotype. These hybrid MPs have great potential for application as bone-void fillers or bone tissue engineering scaffolds in bone regeneration.
Keywords: hybrid microparticles, injectable, mesenchymal stem cells, proliferation, gene expression, osteoblasts
1. Introduction
Bone has an intrinsic capacity to repair itself, which is best observed in healing in bone fractures. However, when bone defects occur as a result of severe injury, healing may not commence spontaneously. Over 500 000 bone-grafting procedures are performed annually in the USA (Bucholz, 2002; Ludwig et al., 2000). These numbers will grow as the life expectancy of the population increases. The estimated direct and indirect costs of treating fractures and bone defects in the USA are approximately $20 billion/year (Goldstein, 2006; Lohmann et al., 2007). The need for surgical reconstruction or replacement is often the result of trauma, pathological degeneration or congenital deformity of the tissue. The ability to generate new bone for skeletal use is a significant clinical need.
The two main types of bone grafts currently used are autografts and allografts (Friedlander, 1987; Rees and Haddad, 2003). An autograft is a section of bone taken from the patient’s own body, whereas an allograft is taken from a cadaver. These types of grafts are limited, due to some uncontrollable factors. For autografts, the key limitation is donor site morbidity (Younger and Chapman, 1989; Silber et al., 2003). A limitation of allografts is the immunological response to the foreign tissue of the graft (Boyce, 1999; Khan et al., 2008).
Because of these uncontrollable limitations, it is necessary to develop alternative bone substitute materials to use in bone defects. A large number of three-dimensional (3D) scaffolds prepared using natural and synthetic biomaterials have been studied, using tissue engineering techniques, to apply to bone regeneration (Griffith, 2002; Ohgushi, 2003; Burg et al., 2000). However, injectable biomaterials have advantages over 3D scaffolds, since they can be applied using minimally invasive surgery (Lee et al., 2007; Latta, 2003).
We have selected natural polysaccharide chitosan (CS) as a base material to fabricate the microparticles (MPs). CS is used in several medical applications, such as both systemic and local drug delivery (Wang et al., 2008; Orienti et al., 2002; Portero et al., 2002) and wound dressing (Kato et al., 2003). A recent study has reported that the HemCon® bandage for the wound dressing, prepared using chitosan acetate, is highly active in killing bacteria in mouse wounds (Burkatovskaya, 2006). Specifically we have selected CS for this study because of its unique property, intrinsic antibacterial activity, which directly benefits infected trauma bone defects. CS scaffolds appear to favour the differentiation of osteoprogenitor cells and bone formation (Conti et al., 2000; Kong et al., 2007; Seol et al., 2004). In general, these materials evoke a minimal foreign body reaction (Arpornmaeklong, 2007; Di Martino et al., 2005; Hoemann et al., 2005; Li, 2005) with little or no fibrous encapsulation. Biodegradability and biocompatibility are very important properties that make CS a useful material for bone regeneration.
However, in order to use CS in bone tissue engineering, structural integrity should be improved. Therefore, we designed a hybrid organic/inorganic MPs approach. In our previous study, we optimized the crosslinking density to obtain structurally strong solid-form MPs (Jayasuriya and Bhat, 2009a). The crosslink interaction between CS and tripolyphosphate (TPP) improves the structural integrity of these MPs. We selected TPP as a crosslinking agent for CS because it is not a toxic chemical crosslinker; it is a non-toxic multivalent ionic crosslinking agent and is even used for food preservation. Our previous studies and other studies have shown enhanced mechanical properties of the polymeric scaffolds incorporated with calcium phosphate, due to a reinforcement effect in the presence of calcium phosphate (Jayasuriya et al., 2003; Xu et al., 2004; Li et al., 2005; Chesnutt et al., 2009). Since calcium is one of the main components in bone mineral, inorganic compounds containing calcium may favour the function of osteoblasts (OB) during implantation. Calcium phosphate and calcium carbonate provides bone with its stiffness and strength. Therefore, we incorporated dicalcium phosphate (CaHPO4) and calcium carbonate (CaCO3) into the hybrid MPs. As a preliminary study, we incorporated 10% CaHPO4, 20% CaHPO4 or 10% CaCO3 (w/w) into CS in the MPs.
In our previous studies, we focused on scale-up fabrication of microparticles (Jayasuriya and Bhat, 2009a) and characterization of hybrid microparticles (Jayasuriya and Bhat, 2009b). Therefore, the aim of this study was to investigate mesenchymal stem cell (MSC) functions, such as attachment, proliferation and differentiation into OBs, on four different types of novel crosslinked hybrid organic/inorganic MPs.
2. Materials and methods
2.1. Materials and characterization
2.1.1. Materials
The CS (85% deacetylated), tripolyphosphate, cotton seed oil, hexane, acetic acid and Span 85 which were used to fabricate MPs were purchased from Sigma Chemical Co. (Milwaukee, USA).
2.1.2. Hybrid microparticle scaffold fabrication
Hybrid MPs were fabricated using a scale-up procedure as described previously (Jayasuriya and Bhat, 2009a). Briefly, CS solution (1.5%, w/v) was prepared by dissolving CS in dilute acetic acid (1%, v/v) at room temperature and filtering through nylon cloth to remove any insoluble components. CaHPO4 or CaCO3 was incorporated directly into the CS solution by dissolving to a final concentration of 10% or 20% (w/w of CS) and stirring for 1 h to obtain homogeneity. The CS solution (25 ml) was mixed with an equal volume of acetone. The mixture (36 ml) was then added dropwise into 600 ml cottonseed oil, which was mixed with 4 ml Span 85. The oil suspension was stirred for 14 h at 37 °C and an agitation speed of 870 rpm. 64% (w/w) of TPP was mixed with 4 ml distilled water and added to the reaction mixture; 4 h after the addition of TPP, the CS MPs were purified, using hexane followed by vacuum filtration and air drying. Four types of hybrid MPs were fabricated: CS; CS–10% CaHPO4; CS–20% CaHPO4; CS–10% CaCO3.
2.1.3. Digital camera pictures
A Canon digital SLR camera, Model Rebel xTi, with a resolution of 10 Mpi, was used to take the digital pictures of hybrid MPs.
2.1.4. Morphology of hybrid microparticles – scanning electron microscope (SEM)
Morphological images of the hybrid MPs were obtained using a Hitachi S3200 SEM operating with 20 kV accelerating voltage under high vacuum. A conventional secondary electron scintillator detector with a tungsten filament was used. The MPs were coated with a 20 nm gold layer, using a Denton vacuum model Desk II sputter coater.
2.2. Isolation and culture of bone marrow stromal cells (BMSCs)
2.2.1. Isolation
C57/BL-6 strain male mice, 6 weeks old, were purchased from the Charles Rivers Laboratory (Wilmington, MA, USA). The mice were housed in the animal care facilities of the University of Toledo Health Science Campus. The mice were euthanized by CO2 inhalation, performed according to the American Veterinary Medical Association (AVMA) panel on euthanasia and the University of Toledo guidelines. The BMSCs were isolated from mouse femurs using previously published procedures (Jayasuriya and Shah, 2008).
2.2.2. Culture and expansion of BMSCs in T-75 flasks
Isolated BMSCs were plated on T-75 flasks and incubated at 37 °C in a humidified 5% CO2/95% air atmosphere in a medium including α-minimum essential medium (αMEM), 10% fetal bovine serum (FBS), 100 U/ml penicillin, 100 μg/ml streptomycin sulphate. The cells were monitored and the medium was changed on day 5. At this stage the haematopoietic and fat cells were eliminated and the MSCs adhered to the flask. When the flask had confluent MSCs, the first passage was performed at day 10. The medium was changed every 2 days. After 5 days from the first passage, the adherent MSCs were harvested as follows: the cells were washed twice with Hank’s balanced salt solution, treated with two consecutive applications of trypsin/EDTA for 3–5 min each at room temperature, and washed with the growth medium.
2.3. Cell attachment, spreading and proliferation – fluorescence microscopy
Cellular experiments were performed using four different groups of hybrid MPs: CS MPs, CS–10% CaHPO4 MPs, CS–20% CaHPO4 MPs, and CS-CaCO3 MPs. Polystyrene wells were used as negative controls. Cell attachment, proliferation and differentiation was studied as described below. For each cellular experiment, three replicates was used per group. Hybrid MPs were sterilized under UV light for 15 min before starting all cellular experiments.
Samples of 6 mg of the four different types of sterilized MPs were placed in 96-well plates; since the MPs produced autofluorescence, 6 mg of MPs were used instead of 12 mg in the 96-well plates. Prior to cell seeding, 50 μl cell culture medium was added to each well to ensure that the MPs settled down at the bottom of the plate. The cell density used was 20 000 cells/well. Attachment of cells onto the MPs was studied using the live/dead cell assay (Molecular Probes). At days 1, 2 and 7 the cells were washed with 100 μl D-PBS. 100 μl 2μM calcein was added to each well, followed by incubation at room temperature for 20 min. Cells seeded in wells without MPs served as controls. Fluorescence images of the cells were taken using a Leica fluorescence microscope at ×100 original magnification.
2.4. Cell proliferation
2.4.1. Cell count – hemacytometer
A sample of 12 mg of sterilized MPs was added to each well in a 96-well plate. The MPs were sterilized under UV prior to cell seeding. A 50 μl cell suspension containing 20 000 cells was added to each well. 50 μl α-MEM was added to each well to bring the total volume up to 100 μl. The cell medium was changed once every 3 days. Proliferation of MSCs was estimated from measurements of cell count and DNA at 7, 14, 21 and 28 days. At each time point, the cells were trypsinized and counted using a hemacytometer. The number of attached cells at each time point was then normalized to the number of cells seeded initially. DNA was isolated from the cells at various time points, using the genomic DNA Mini Kit (Invitrogen) and the total amount of DNA was measured fluorometrically, using the DNA Quantification Kit (Sigma).
2.4.2. DNA quantitation assay
In order to confirm cell growth, the DNA content of the constructs was determined using a DNA Quantitation Kit, Fluorescence Assay (Sigma). Cell proliferation samples stored in a − 80 °C freezer were thawed to room temperature for 45–60 min and then lysed, using celLytic-M (Sigma) for 15 min. These cell lysate samples were then purified using a PureLink™ Genomic DNA Purification Kit (Invitrogen) according to the manufacturer’s instructions. These purified DNA samples were used to measure DNA concentration in the samples. DNA concentration amounts were measured at 346 nm excitation and 460 nm emission at ambient temperature in triplicate aliquots of the same sample, using DNA Quantitation Kit, Fluorescence Assay (Sigma) according to the manufacturer’s instructions, using a fluorometer instrument. The cell growth in the constructs was measured as the amount of DNA per construct (ng/ml cell lysate).
2.5. MSC differentiation studies
2.5.1. MSC differentiation using gene expression
Osteogenic differentiation was induced after the first passage by culturing MSCs in osteogenic medium (α-MEM supplemented with 10% FBS, 1% penicillin streptomycin, 50 μg/ml L-ascorbic acid, 10 nm dexamethasone, 10 mM β-glycerol phosphate). For each type of MP, 60 mg of weight, which is proportional to the 12 mg of MPs in 96-well plates, was added into each well of a 24-well plate. 500 μl medium was added to each well to allow the particles to settle down to the bottom of the plate. Primary MSCs were seeded on MPs in 24-well plates containing osteogenic medium at a cell density of 200 000 cells/ml. The level of gene expression was determined at days 7, 14, 21 and 28.
2.5.2. mRNA isolation
The total mRNA was extracted from the culture at 7, 14, 21 and 28 days, using RNEasy MiniPrep Kit (Qiagen). The cells attached to the MPs were lysed by adding RLT buffer directly to the 24-well plates. The supernatants were removed and mRNA was isolated according to the kit protocol. mRNA concentration at each time point was calculated from the OD260 value. The mRNAs isolated at days 14, 21, 28 were diluted to match the concentration of day 7. RNA (4 μl template) was mixed with 3 μl 0.5 μg/μl oligodT (Invitrogen, Cat. No. 18418–012) in a 0.6 ml microtube. The tubes were incubated at 70 °C for 5 min, then quickly chilled on ice. The mastermix (15 μl), containing nuclease-free water, MMLV 5 X buffer (Promega, Cat. No. M531A), 10 mM dNTP and reverse transcriptase (Promega, Cat. No. M314A) (9 : 4:1 : 1 ratio) was added to each tube, and the tubes were incubated at room temperature for 5 min and then placed at 42 °C for 1 h, followed by 70 °C for 15 min. OB-specific gene marker expression, such as alkaline phosphatase (ALP), collagen I (COLLI) and osteocalcin (OCN), was assessed by real-time RT–PCR, using primers (Fu et al., 2007; Moerman et al., 2004) as described in Table 1.
Table 1.
Primer sequences used for quantitative real-time PCR
| Gene name | Primer sequence | Number of base pairs | Primer concentration (nM) |
|---|---|---|---|
| ALP | |||
| Forward | 5′-GTGCCAGAGAAAGAGAGAGAC-3′ | 21 | 32.6 |
| Reverse | 5′-GACGCCCATACCATCTCC-3′ | 18 | 37.1 |
| OCN | |||
| Forward | 5′-GAGTCTGACAAAGCCTTCA-3′ | 19 | 35.4 |
| Reverse | 5′-AGCCATACTGGTCTGATAG-3′ | 19 | 36.4 |
| COLLI | |||
| Forward | 5′-ACTGTCCCAACCCCCAAAG-3′ | 19 | 33 |
| Reverse | 5′-CGTATTCTTCCGGGCAGAAA-3′ | 20 | 33.3 |
| GAPDH | |||
| Forward | 5′-GTCGGTGTGAACGGATTTG-3′ | 19 | 33.1 |
| Reverse | 5′-GAACATGTAGACCATGTAGTTG-3′ | 22 | 34.4 |
2.5.3. Quantitative real-time PCR
The DNA samples were amplified using lightcycler Quantitative real-time PCR (Roche Diagnostics). A mastermix was prepared by mixing 4.75 μl nuclease-free water, 0.9 μl enzyme diluent (Idaho Technology, Cat. No. 1773), 1 μl 10× dNTP (Idaho Technology, Cat. No. 1774), 1 μl 10× buffer (Idaho Technology, Cat. No. 1770), 0.2 μl primers (see primer sequences, listed in Table 1), 0.5 μl SYBR green and 0.1 μl 5 U/μl Taq Polymerase (Invitrogen, Cat. No. 10966–026) for each sample. 8 μl mastermix was mixed with 2 μl template in a 20 μl light-cycler capillary. The amplification programme consisted of 40 cycles of melting at 94 °C, annealing at 55 °C for 4 s, extension at 72 °C for 10 s. GAPDH was used as the normalized gene. The normalized expression levels were calculated as the ratio of GAPDH Ct value (threshold cycle) to the Ct value of the target gene (Leong et al., 2007). Minitab version 15 software was used for statistical analysis.
2.6. Statistical analysis
The data for proliferated cells, DNA, COLLI, ALP and OCN were reported as mean ± standard deviation (SD). Two-way analysis of variance (ANOVA) for the effects of different types of MPs and time, followed by Tukey post hoc multiple comparisons using statistical analysis, was performed using Minitab software, version 15. p < 0.05 was considered statistically significant.
3. Results
3.1. Physical nature of hybrid MPs
A digital camera image of the physical nature of the CS MPs is shown in Figure 1A. The CS MPs appeared light yellowish in colour, similar to the demineralized human bone powder. All other types of MPs also appeared in light yellowish colour as CS MPs (data not shown). The morphology of all types of hybrid MPs, including size, shape and surface properties, was examined using a SEM. SEM analyses of each type of MP are shown in Figure 1B–E, with higher magnification. The SEM images revealed that all types of MPs were approximately spherical in shape, with a diameter range of 30–60 μm. All types of MPs showed a smooth outer surface without any porous structure. Although either CaHPO4 or CaCO3 was added to the MPs, there was no difference in the surface appearance of MPs containing CaHPO4 or CaCO3 compared to CS MPs.
Figure 1.
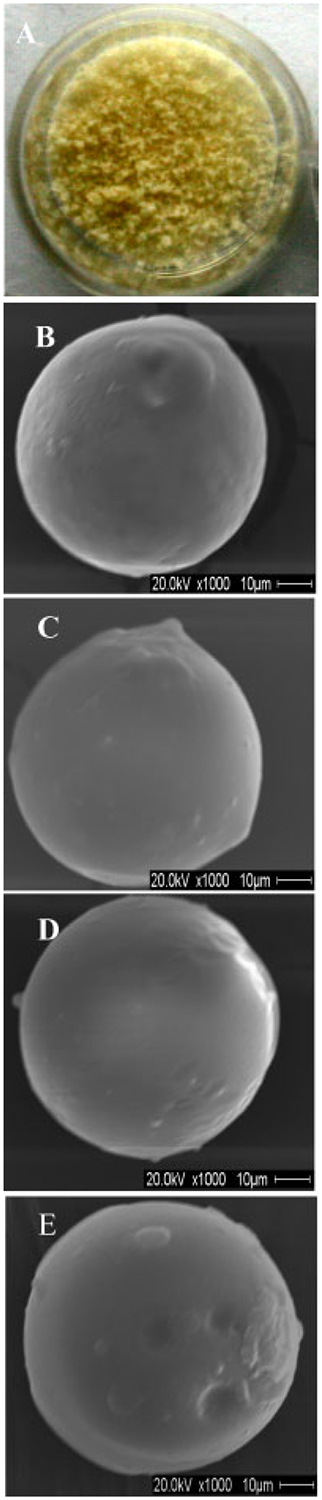
(A) Digital camera image of the CS MPs. SEM images of surfaces of hybrid MPs: (B) CS MPs; (C) CS–10% CaHPO4 MPs; (D) CS–20% CaHPO4 MPs; (E) CS–10% CaCO3 MPs
3.2. MSC attachment, spreading and proliferation – fluorescence microscopy
MSC attachment, spreading and proliferation on controls and the four types of MPs was visualized using fluorescence microcopy (Figure 2). The MSCs were attached onto the control wells (Figure 2A) and all types of MPs (Figure 2B–E). The morphology of MSCs attached to the controls and MPs was circular in shape at day 1. The number of attached MSCs increased with time for both controls and MPs. The MSCs started to spread and proliferate on both controls and MPs at day 2 and further spread and proliferated MSCs could be observed at day 7. The MSCs on controls exhibited slightly more spreading compared to the MPs at day 7. MSC spreading and proliferation appeared to be similar on all types of MPs at day 7.
Figure 2.
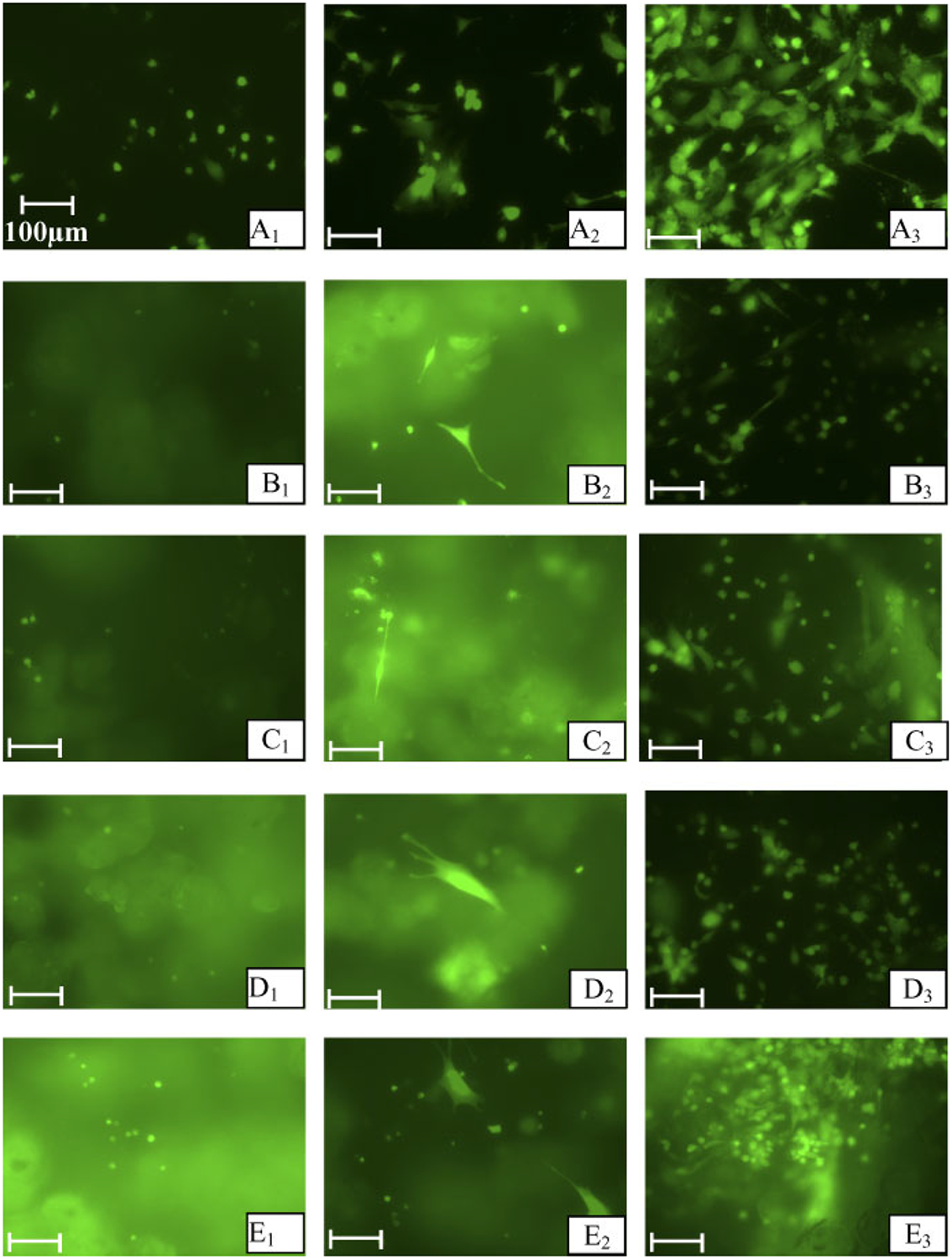
Florescent microcopy images of MSCs attached on controls and four types of MPs when treated with live/dead assay (original magnification, ×200). (A) Control wells; (B) CS-MPs; (C) CS–10% CaHPO4 MPs; (D) CS–20% CaHPO4 MPs, and (E) CS-CaCO3 MPs. The subscripts indicate day 1, 2 and 7 time points. Bar = 100 μm
3.3. MSC proliferation – cell count
Cell proliferation was measured at days 7, 14, 21 and 28 in the 96-well plates by counting the attached cells on the MPs and measuring the amount of DNA/ml cell lysate of MPs (12 mg). The number of cells proliferated on the hybrid MPs and control wells was plotted by normalizing the number of proliferated cells at each time point to the initial cell seeding number on MPs (Figure 3). The number of proliferated cells increased significantly for all types of hybrid MPs as time progressed. However, no significant difference was observed between the different types of MPs and controls at day 7, 14 and 21. At day 28, the number of proliferated cells in the control wells was significantly increased compared to all types of hybrid MPs. No significant difference was observed for the proliferated cells on hybrid MP groups. At day 7, approximately 50% of the initial number of cells seeded was attached to the hybrid MPs and control wells. At day 28, this number increase three-fold.
Figure 3.
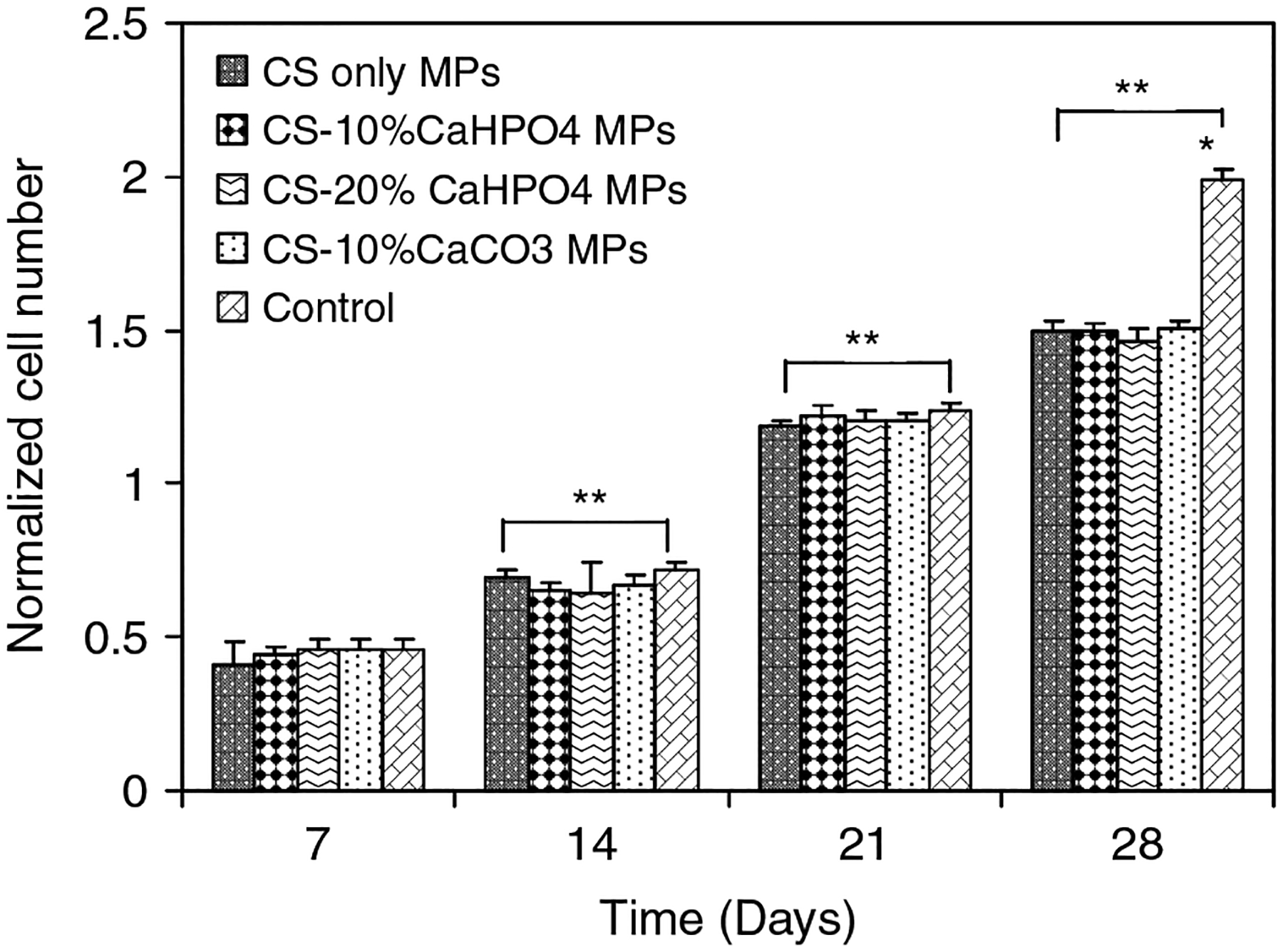
Normalized number of MSCs proliferated on four different types ofMPs and controls as counted by hemacytometer on days 7, 14, 21 and 28. *Significant difference between the cell number on MPs and control well. **Significant increase in cell number with respect to day 7 cell numbers
The amounts of DNA for all types of MPs and controls are plotted in Figure 4. Similar to the number of cells proliferated (Figure 3), the amount of DNA in the hybrid MPs and control wells at days 7,14, 21 and 28 was significantly increased. No significant DNA amount was observed in the different types of MPs at each time until day 21. At day 28, a significant amount of DNA was expressed for the control samples compared to the types of hybrid MP. DNA content was approximately doubled at day 28 for all types of hybrid MP compared to that at day 7.
Figure 4.
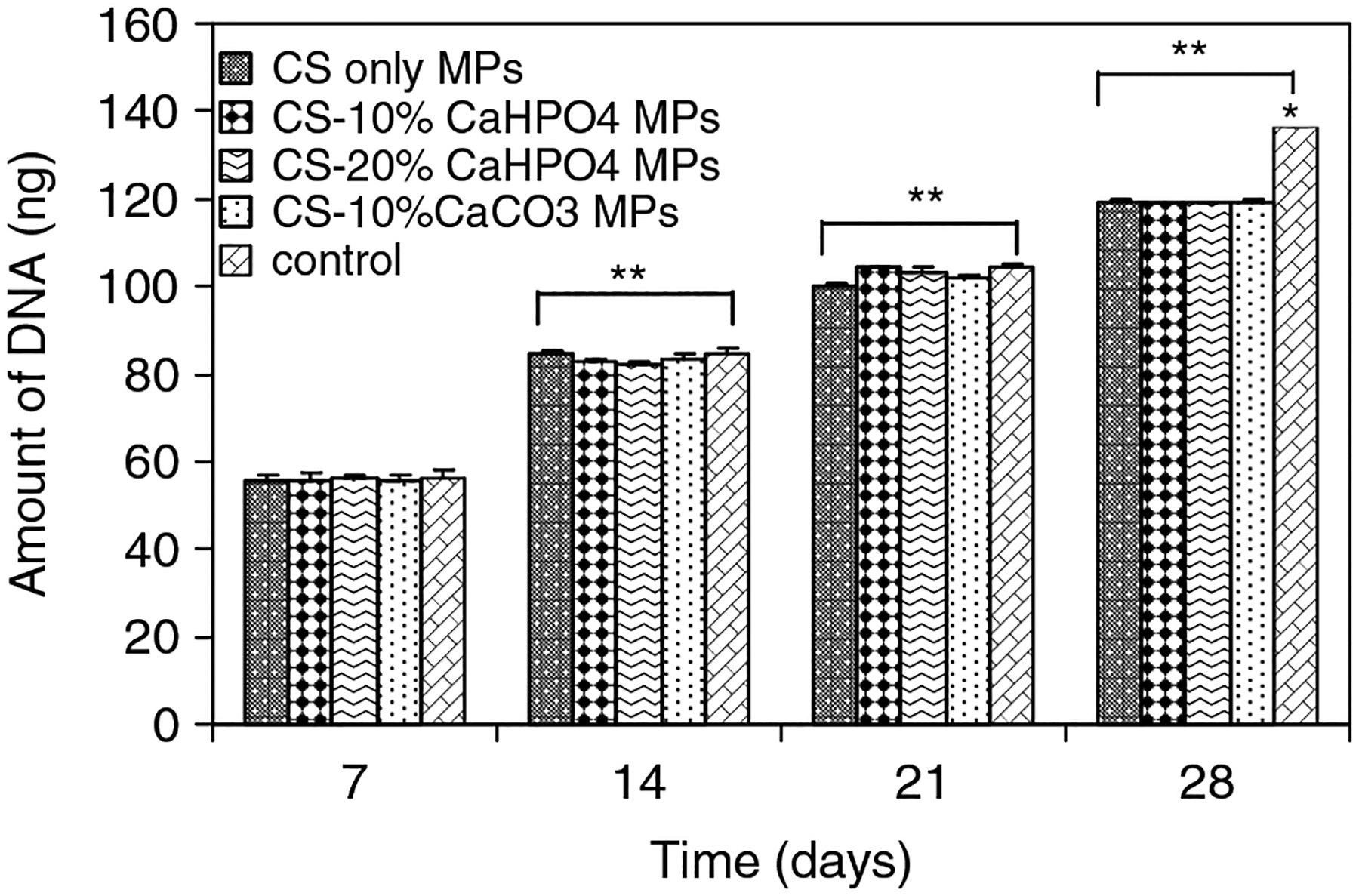
Amount of DNA of MSCs seeded on four different types of MPs and controls as determined by DNA quantitation assay on days 7, 14, 21 and 28. *Significant difference between the cell number on MPs and control well. **Significant increase in cell number with respect to day 7 cell numbers
3.4. OB phenotype – COLLI, ALP and OCN
MSC differentiation into OB phenotype was analysed using gene expression, including ALP, COLLI and OCN content. The expression of ALP is a time-honoured marker of OB cell function and represents one of the earliest indicators of cells that can form a mineralized matrix (Ishaug-Riley et al., 1997; zur Nieden et al., 2003). The normalized mRNA expression of ALP was plotted for all types of hybrid MPs and control wells in 24-well plates (Figure 5). The normalized mRNA expression of ALP was observed only at day 7 for all types of MP and controls. We were unable to observe any normalized mRNA expression of ALP in any of the groups, including controls, at days 14, 21 and 28. The normalized mRNA expression of ALP for all types of hybrid MPs was significantly higher than control samples at day 7 (p < 0.05).
Figure 5.
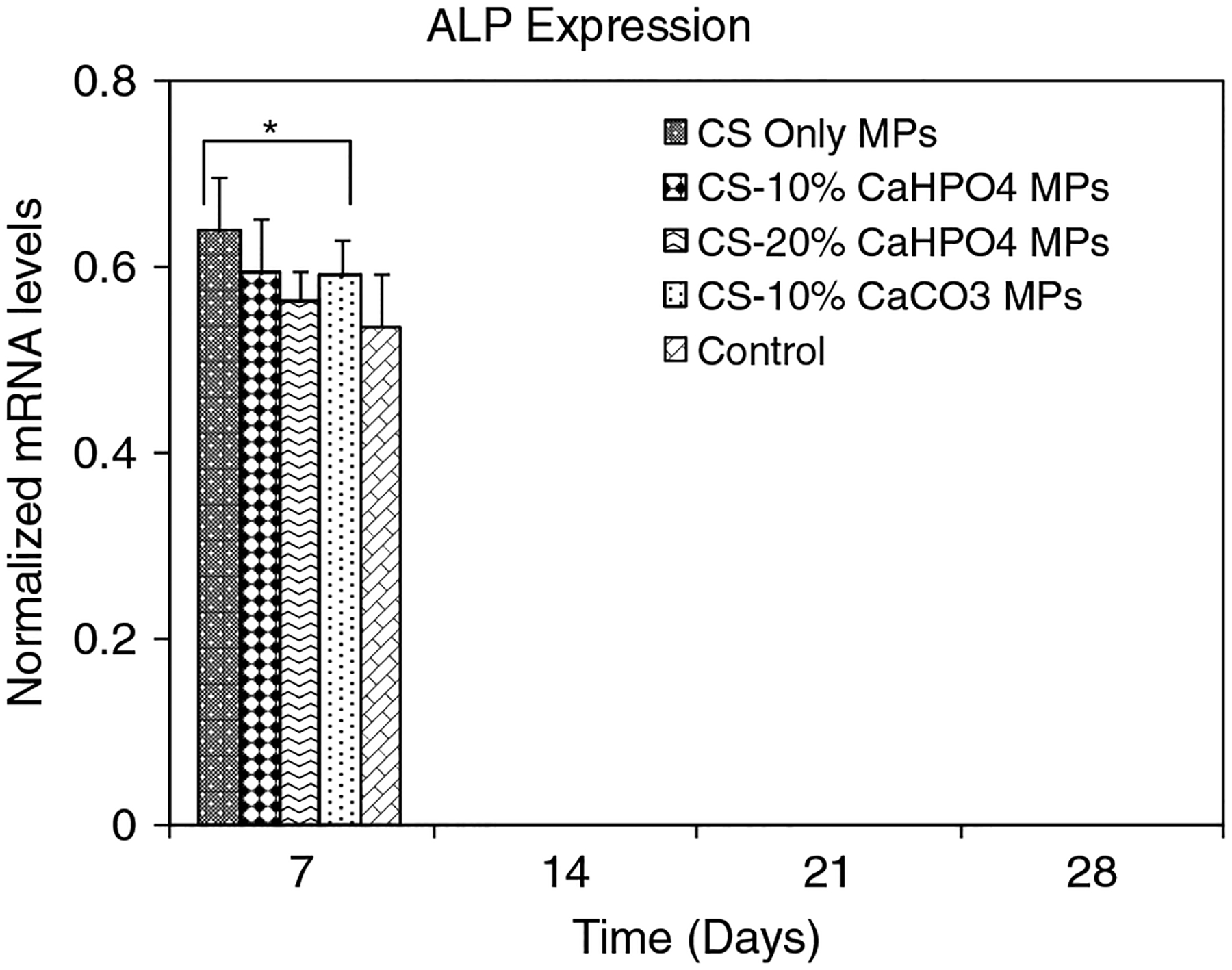
Normalized mRNA expression of ALP for MSCs seeded on four different types of MPs and controls on days 7, 14, 21, and 28. The mRNA levels were normalized to housekeep gene GAPDH. *Significant difference in normalized mRNA expression between the MPs and control group
Normalized mRNA expression of COLLI was plotted for all types of MPs and controls at days 7, 14, 21 and 28 (Figure 6). Normalized mRNA expression of COLLI was significantly increased between each time point for all groups. There was no significant difference of normalized mRNA expression of COLLI observed among the different types of MPs at days 7, 14, 21 and 28. However, there was a significant increase of normalized mRNA expression of COLLI for all types of MPs compared to the control samples (p < 0.05) at each time point.
Figure 6.
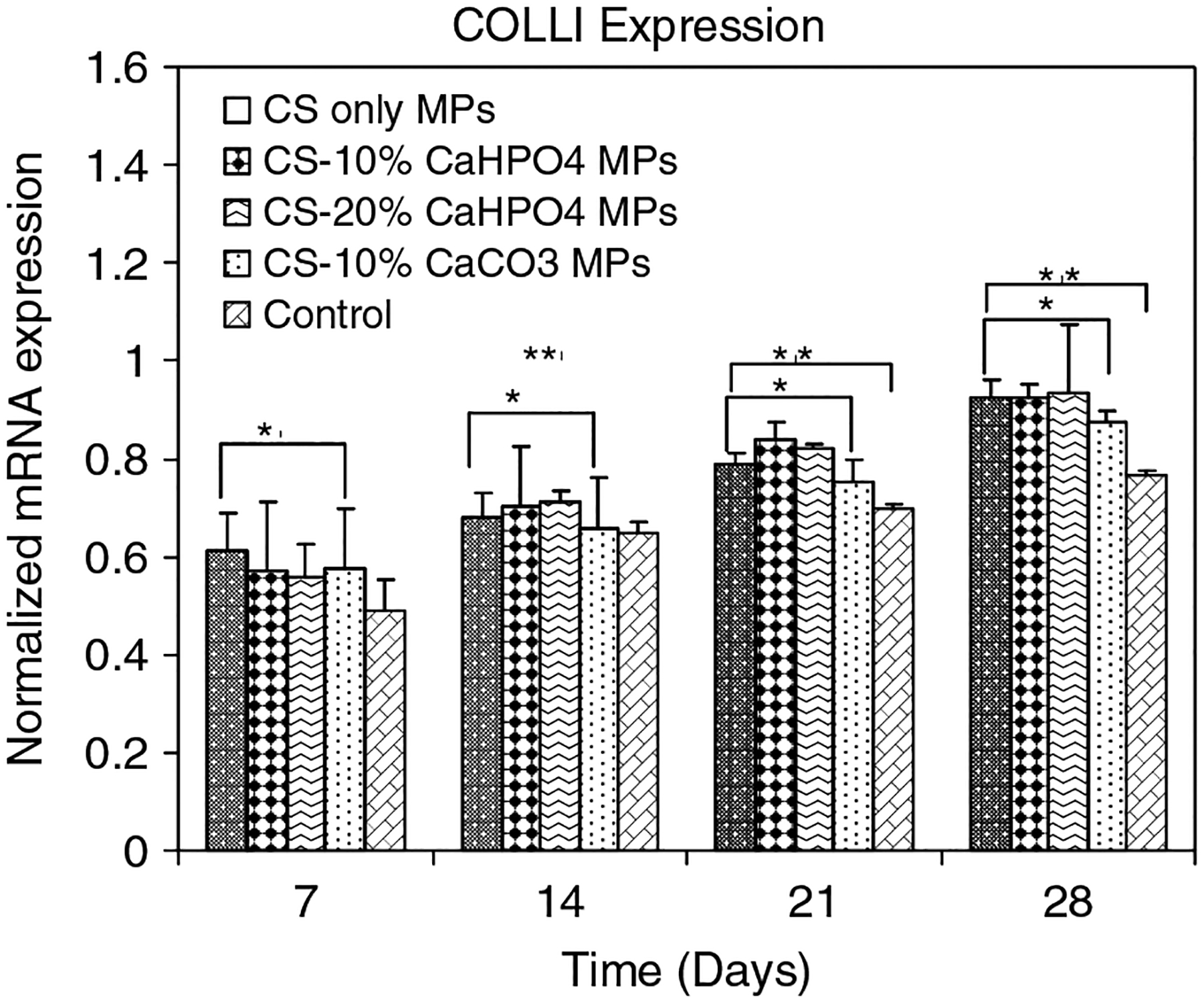
Normalized mRNA expression of COLLI for MSCs seeded on four different types of MPs and controls on days 7, 14, 21 and 28. The mRNA levels were normalized to housekeep gene GAPDH. *Significant difference in normalized mRNA expression between theMPs and control group. **Significant difference with respect to day 7 expression
Expression of OCN in the mature organism is restricted to bone, dentine and cementum. OCN, also known as Gla protein, is the most abundant non-collagenous protein in mature bone and is synthesized by mature OBs and incorporated into the mineralized matrix (Cowles et al., 1998). Normalized mRNA expression of OCN for all types of hybrid MPs and control wells are shown in Figure 7. There was a significant increase of mRNA expression of OCN for all types of hybrid MPs with increasing time (p < 0.05). Normalized mRNA expression of OCN in the hybrid MP groups was significantly increased compared to control wells at each time point. Normalized mRNA expression of OCN in 10% CaCO3 MPs was significantly different from the 10% and 20% CaHPO4 MPs (p < 0.05).
Figure 7.
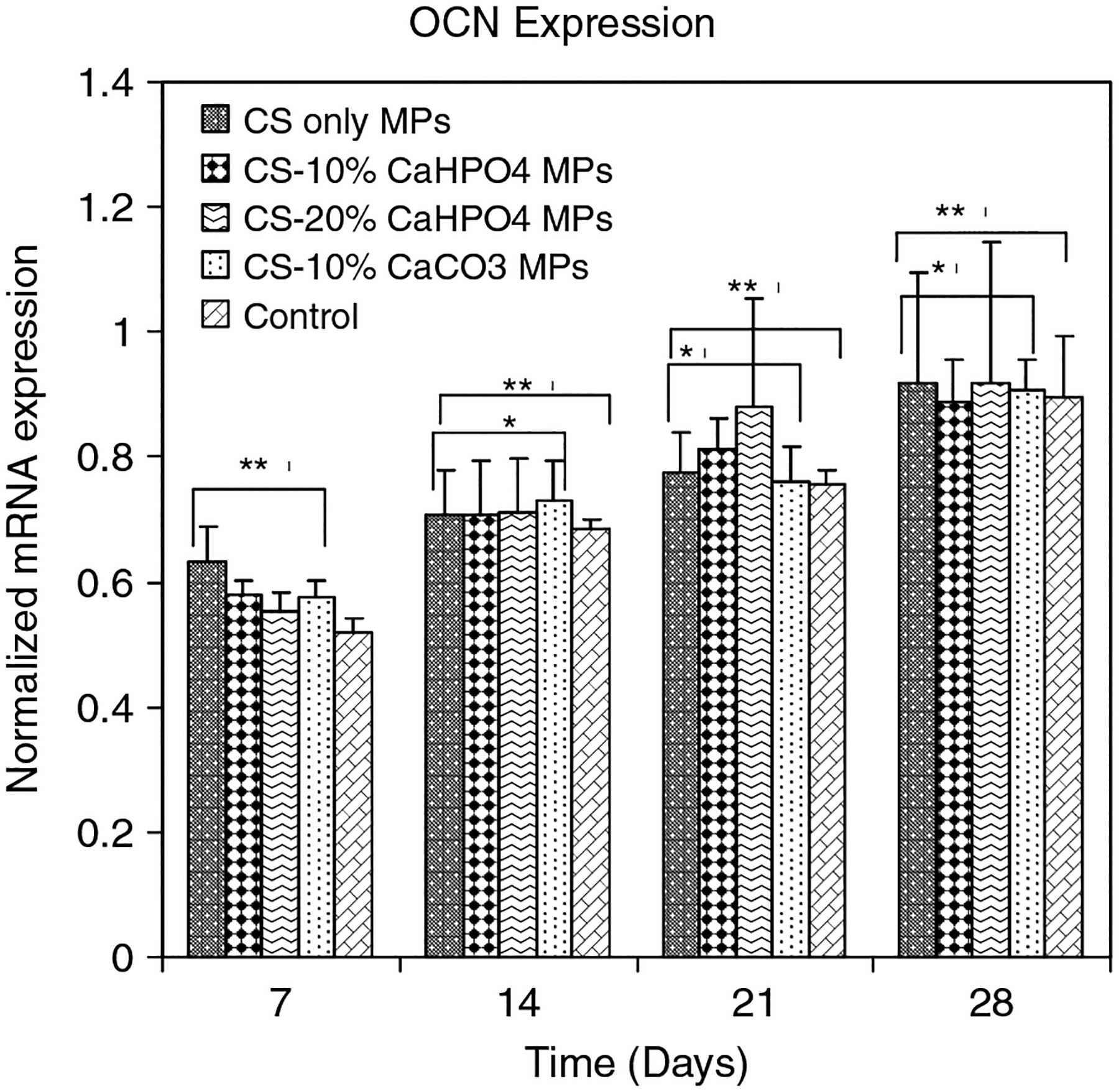
Normalized mRNA expression of OCN for MSCs seeded on four different types of MPs and controls on days 7, 14, 21 and 28. The mRNA levels were normalized to housekeep gene GAPDH. *Significant difference in normalized mRNA expression between theMPs and control group. **Significant difference with respect to day 7 expression
4. Discussion
In this study we investigated MSC functions, such as attachment, spreading, proliferation and differentiation into OBs, on novel hybrid organic/inorganic MPs. The hybrid MPs were based on CS and ionically crosslinked with TPP in order to achieve structural integrity. CS is a deacetylated derivative of chitin, a linear polysaccharide with high molecular weight and the second most abundant natural biopolymer, commonly found in the shells of marine crustaceans and the cell walls of fungi (Kato et al., 2003; Arpornmaeklong et al., 2007).
The fluorescence microscopy results (Figure 2) indicated evidence of MSC attachment, spreading and proliferation on all types of hybrid MPs. The MSCs started to spread and proliferate at day 2, similar to controls. However, more spread cells could be observed at day 7 in controls compared to that in hybrid MPs. It should be taken into consideration that the MPs produced autofluorescence in the calcein reagent. This autofluorescence may have interfered with correct imaging of cell spreading on the MPs at day 7 compared to that of controls.
There was no significant difference in the number of MSCs proliferating on controls and all types of hybrid MPs at day 7 (Figure 3). Similar results were observed for the amount of DNA at day 7 for all groups (Figure 4). However, a significant difference in the numbers of proliferated MSCs was shown between the controls and all types of MPs at day 28. A similar trend was observed for the amount of DNA at day 28 between controls and all types of MPs. It should be taken into account that these MPs are not porous. However, the void spaces formed between the MPs would facilitate MSC migration and conduction. We used 96-well plates to seed the cells with 12 mg of MPs. The smaller number of proliferated cells on the hybrid MPs at day 28 may be attributed to less void space being avaiable in the wells containing MPs compared to the control wells.
Several investigators have shown cell attachment and proliferation on CS-coated surfaces (Wang et al., 2008; Lahiji et al., 2000), CS MPs (Hoemann et al., 2005) and CS scaffolds (Seol et al., 2004; Arpornmaeklong et al., 2007; Li et al., 2005; Zhang et al., 2003), supporting our results in this study. A recent study has reported that OB proliferation was significantly increased on microsphere-based chitosan/nanocrystalline calcium phosphate composite scaffolds compared to plain chitosan scaffolds (Chesnutt et al., 2009).
When treated with β-glycerophosphate, ascorbic acid and dexamethasone, MSCs can differentiate into the OB phenotype and produce a calcified matrix, recapitulating in vivo conditions (Maniatopoulos et al., 1988). Osteoprogenitor differentiation was characterized by the expression of early and late bone-specific markers. OB-specific gene expression, including COLLI, ALP and OCN content in the cultured cells, was determined at days 7, 14, 21 and 28, using quantitative real-time PCR. These early and late bone-specific markers have been well documented in previous reports (Ishaug-Riley et al., 1997; zur Nieden et al., 2003; Cowles et al., 2003; Owen et al., 1990).
ALP is an early OB differentiation marker and normalized mRNA expression of ALP for all groups was observed only at day 7 (Figure 5). The level of ALP expression declines as cultures progress toward mineralization (Owen et al., 1990). This result indicated that the MSCs started to differentiate into OBs when they were treated with osteogenic medium, as early as day 7 (Figure 5). The normalized mRNA expression of COLLI (Figure 6) and OCN (Figure 7) was significantly increased at each time point compared to that of the control samples (p < 0.05). A recent study has shown that human OBs propagated on CS films continue to express COLLI (Lahiji et al., 2000). OCN is a highly expressed gene during the mineralization stage of bone development (Cowles et al., 1998; Lahiji et al., 2000). Our gene expression results have shown that all types of hybrid MPs favoured the OB-specific differentiation of MSCs compared to the control wells. A recent study has shown that both ALP and COLLI expression was increased, and both the bone sialoprotein and OCN genes were upregulated when MC3T3-E1 cells were cultured on electrodeposited calcium phosphate/chitosan coatings (Wang et al., 2008). Chitosan/tricalcium phosphate sponges supported the proliferation of osteoblastic cells as well as their differentiation, as indicated by high ALP activity and deposition of mineralized matrices by the cells (Lee et al., 2000).
The main advantage of hybrid MPs compared with the traditional block scaffolds is that micro-scale particles can be combined with a vehicle and be administered by injection, thus giving the possibility of filling defects of different geometries and sizes (Xu et al., 2004; Kruyt et al., 2006). The developed MP–OB constructs can be injected by minimally invasive surgery, unlike conventional 3D scaffolds. Collectively, our results have proved that the novel type of hybrid organic/inorganic MPs were non-cytotoxic and supported MSC attachment, spreading, proliferation and differentiation into the OB phenotype. This system can be potentially applied for a variety of applications to treat damaged bone due to trauma, including orthopaedic segmental defects, non-unions, complex fractures and spine fusion.
Acknowledgements
We would like to thank the National Science Foundation (NSF; Grant No. 0652024) for providing financial support to accomplish this work.
References
- Arpornmaeklong P, Suwatwirote N, Pripatnanont P, et al. 2007; Growth and differentiation of mouse osteoblasts on chitosan-collagen sponges. Int J Oral Maxillofac Surg 36: 328–337. [DOI] [PubMed] [Google Scholar]
- Bucholz RW. 2002; Nonallograft osteoconductive bone graft substitutes. Clin Orthop Relat Res 395: 44–52. [DOI] [PubMed] [Google Scholar]
- Boyce T, Edwards J, Scarborough N. 1999; Allograft bone: the influence of processing on safety and performance. Orthop Clin North Am 30: 571–581. [DOI] [PubMed] [Google Scholar]
- Burg KJ, Poter S, Kellam JF. 2000; Biomaterial developments for bone tissue engineering. Biomaterials 21: 3247–2359. [DOI] [PubMed] [Google Scholar]
- Burkatovskaya M, Tegos GP, Swietlik E, et al. 2006; Use of chitosan bandage to prevent fatal infections developing from highly contaminated wounds in mice. Biomaterials 27: 4157–4164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chesnutt BM, Viano AM, Yuan Y, et al. 2009; Design and characterization of a novel chitosan/nanocrystalline calcium phosphate composite scaffold for bone regeneration. J Biomed Mater Res A 88: 491–502. [DOI] [PubMed] [Google Scholar]
- Conti B, Giunchedi P, Genta I, et al. 2000; The preparation and in vivo evaluation of the wound-healing properties of chitosan microspheres. STP Pharma Sci 10: 101–104. [Google Scholar]
- Cowles EA, DeRome ME, Pastizzo G, et al. 1998; Mineralization and the expression of matrix proteins during in vivo bone development. Calcif Tissue Int 62: 74–82. [DOI] [PubMed] [Google Scholar]
- Di Martino A, Sittinger M, Risbud MV. 2005; Chitosan: a versatile biopolymer for orthopaedic tissue-engineering. Biomaterials 26: 5983–5990. [DOI] [PubMed] [Google Scholar]
- Friedlander GE. 1987; Currents concepts review: bone grafts: the basic science rationale for clinical applications. J Bone Joint Surg 69A: 786–790. [PubMed] [Google Scholar]
- Goldstein SA. 2006; Tissue engineering solutions for traumatic bone loss. J Am Acad Orthop Surg 14(10 suppl): S152–156. [DOI] [PubMed] [Google Scholar]
- Griffith LG. 2002; Emerging design principles in biomaterials and scaffolds for tissue engineering. Ann NY Acad Sci 961: 83–95. [DOI] [PubMed] [Google Scholar]
- Hoemann CD, Sun J, Legare A, et al. 2005; Tissue engineering of cartilage using an injectable and adhesive chitosan-based cell-delivery vehicle. Osteoarthr Cartilage 13: 318–329. [DOI] [PubMed] [Google Scholar]
- Fu Huihua, Doll Bruce, McNelis Tim. 2007; Osteobalst differentiation in vitro and in vivo promoted by osterix. J Biomed Mat Res Part A 83: 770–778. [DOI] [PubMed] [Google Scholar]
- Ishaug-Riley SL, Crane GM, Miller MJ, et al. 1997; Bone formation by three-dimensional stromal osteoblast culture in biodegradable polymer scaffolds. J Biomed Mater Res 36: 17–28. [DOI] [PubMed] [Google Scholar]
- Jayasuriya AC, Bhat A. 2009a; Optimization of scaled-up chitosan microparticles for bone regeneration. Biomed Mater 4: 55006. [DOI] [PubMed] [Google Scholar]
- Jayasuriya AC, Bhat A. 2009b; Fabrication and characterization of novel hybrid organic/inorganic microparticles to apply in bone regeneration. J Biomed Mater Res A 2009 Oct 13 (Epub ahead of print). [DOI] [PubMed] [Google Scholar]
- Jayasuriya AC, Gallagher SP, Shin K, et al. 2003; Development of environmentally responsive micro porous poly(lactide-coglycolide) scaffolds for bone tissue engineering. Trans. Soc Biomater 29: 637. [Google Scholar]
- Jayasuriya AC, Shah C. 2008; Controlled release of insulin-like growth factor-1 and bone marrow stromal cell function of bone-like mineral layer-coated poly(lactic-co-glycolic acid) scaffolds. J Tissue Eng Regen Med 2: 43–49. [DOI] [PubMed] [Google Scholar]
- Kato Y, Onishi H, Machida Y. 2003; Application of chitin and chitosan derivatives in the pharmaceutical field. Curr Pharm Biotechnol 4: 303–309. [DOI] [PubMed] [Google Scholar]
- Khan Y, Yaszemski MJ, Mikos AG, et al. 2008; Tissue engineering of bone: material and matrix considerations. J Bone Joint Surg Am 90(suppl 1): 36–42. [DOI] [PubMed] [Google Scholar]
- Kong L, Ao Q, Wang A, et al. 2007; Preparation and characterization of a multilayer biomimetic scaffold for bone tissue engineering. J Biomater Appl 22: 223–239. [DOI] [PubMed] [Google Scholar]
- Kruyt MC, Persson C, Johansson G, et al. 2006; Towards injectable cell-based tissue-engineered bone: the effect of different calcium phosphate microparticles and preculturing. Tissue Eng 12: 309–317. [DOI] [PubMed] [Google Scholar]
- Lahiji A, Sohrabi A, Hungerford DS, et al. 2000; Chitosan supports the expression of extracellular matrix proteins in human osteoblasts and chondrocytes. J Biomed Mater Res 51: 586–595. [DOI] [PubMed] [Google Scholar]
- Latta LL, Sarmiento A, Zych GA. 2003; Principles of nonoperative fracture treatment In Skeletal Trauma: Basic Science, Management, and Reconstruction, 3rd edn, Browner BD, Jupiter JB, Levine AM, et al. (eds). Saunders: Philadelphia, PA; 159–194. [Google Scholar]
- Leong DT, Gupta A, Bai HF, et al. 2007; Absolute quantification of gene expression in biomaterials research using real-time PCR. Biomaterials 28: 203–210. [DOI] [PubMed] [Google Scholar]
- Li Z, Ramay HR, Hauch KD, et al. 2005; Chitosan–alginate hybrid scaffolds for bone tissue engineering. Biomat 26: 3919–3928. [DOI] [PubMed] [Google Scholar]
- Li Z, Yubao L, Aiping Y, et al. 2005; Preparation and in vitro investigation of chitosan/nano-hydroxyapatite composite used as bone substitute materials. J Mater Sci Mater Med 16: 213–219. [DOI] [PubMed] [Google Scholar]
- Lee JY, Choo JE, Park HJ, et al. 2007; Injectable gel with synthetic collagen-binding peptide for enhanced osteogenesis in vitro and in vivo. Biochem Biophys Res Commun 357: 68–74. [DOI] [PubMed] [Google Scholar]
- Lee YM, Park YJ, Lee SJ, et al. 2000; Tissue engineered bone formation using chitosan/tricalcium phosphate sponges. J Periodontol 71: 410–417. [DOI] [PubMed] [Google Scholar]
- Lohmann H, Grass G, Rangger C, et al. 2007; Economic impact of cancellous bone grafting in trauma surgery. Arch Orthop Trauma Surg 127: 345–348. [DOI] [PubMed] [Google Scholar]
- Ludwig SC, Kowalski JM, Boden SD. 2000; Osteoinductive bone graft substitutes. Eur Spine J 9(suppl 1): S119–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maniatopoulos C, Sodek J, Meleher AH. 1988; Bone formation in vitro by stromal cells obtained from bone marrow of young adult rats. Cell Tissue Res 254: 317–330. [DOI] [PubMed] [Google Scholar]
- Moerman EJ, Teng K, Lipschitz DA, et al. 2004; Aging activates adipogenic and suppresses osteogenic programs in mesenchymal marrow stroma/stem cells: the role of PPAR-γ 2 transcription factor and TGF-β/BMP signaling pathways. Aging Cell 3: 379–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohgushi H, Miyake J, Tateishi T. 2003; Mesenchymal stem cells and bioceramics: strategies to regenerate the skeleton. Novartis Found Symp 249: 118–127. [PubMed] [Google Scholar]
- Orienti I, Cerchiara T, Luppi B, et al. 2002; Influence of different chitosan salts on the release of sodium diclofenac in colon-specific delivery. Int J Pharm 238: 51–59. [DOI] [PubMed] [Google Scholar]
- Owen TA, Aronow M, Shalhoub V, et al. 1990; Progressive development of the rat osteoblast phenotype in vitro: reciprocal relationships in expression of genes associated with osteoblast proliferation and differentiation during formation of the bone extracellular matrix. J Cell Physiol 143: 420–430. [DOI] [PubMed] [Google Scholar]
- Portero A, Remunan-Lopez C, Criado MT, et al. 2002; Reacetylated chitosan microspheres for controlled delivery of antimicrobial agents to the gastric mucosa. J Microencap 19: 797–809. [DOI] [PubMed] [Google Scholar]
- Rees DC, Haddad FS. 2003; Bone transplantation. Hosp Med 64: 205–209. [DOI] [PubMed] [Google Scholar]
- Seol YJ, Lee JY, Park YJ, et al. 2004; Chitosan sponges as tissue engineering scaffolds for bone formation. Biotechnol Lett 26: 1037–1041. [DOI] [PubMed] [Google Scholar]
- Silber JS, Anderson DG, Daffner SD, et al. 2003; Donor site morbidity after anterior iliac crest bone harvest for single-level anterior cervical discectomy and fusion. Spine 28: 134–139. [DOI] [PubMed] [Google Scholar]
- Wang J, de Boer J, de Groot K. 2008; Proliferation and differentiation of MC3T3-E1 cells on calcium phosphate/chitosan coatings. J Dent Res 87: 650–654. [DOI] [PubMed] [Google Scholar]
- Xu JW, Zaporojan V, Peretti GM, et al. 2004; Injectable tissue-engineered cartilage with different chondrocyte sources. Plast Reconstr Surg 113: 1361–1371. [DOI] [PubMed] [Google Scholar]
- Xu HH, Quinn JB, Takagi S, et al. 2004; Synergistic reinforcement of in situ hardening calcium phosphate composite scaffold for bone tissue engineering. Biomaterials 25: 1029–1037. [DOI] [PubMed] [Google Scholar]
- Younger EM, Chapman MW. 1989; Morbidity at bone graft donor sites. J Orthop Trauma 3: 192–195. [DOI] [PubMed] [Google Scholar]
- zur Nieden NI, Kempka G, Ahr HJ. 2003; In vitro differentiation of embryonic stem cells into mineralized osteoblasts. Differentiation 71: 18–27. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Ni M, Zhang M, et al. 2003; Calcium phosphate–chitosan composite scaffolds for bone tissue engineering. Tissue Eng 9: 337–345. [DOI] [PubMed] [Google Scholar]


