Abstract
Venous thromboembolism (VTE) remains highly prevalent in medically ill patients, and often leads to increased mortality and cost burden during hospitalization and post-discharge. Nearly half of all VTEs occur during or after hospitalization, with pulmonary embolism accounting for 10% of inpatient mortality. Appropriate prophylaxis in high-risk medically ill patients has been shown to reduce risk of VTE and related mortality. Despite current evidence-based guidelines, VTE prophylaxis has been under-used. This owes greatly to ambiguity and concerns related to appropriate patient and prophylactic agent selection, and duration of prophylaxis. Because many acutely ill medical patients have multiple comorbidities, the risk of major bleeding must be considered when choosing to implement pharmacological VTE prophylaxis. Multiple risk assessment models have been developed and validated to help estimate VTE and bleeding risks in this population. While studies have shown that the risk for VTE often extends far beyond hospital discharge, there is no evidence to support extending prophylaxis after hospital discharge. The appropriate selection of VTE prophylaxis requires consideration for cost, availability, patient preference, compliance, and underlying comorbidities. Our paper reviews the current evidence and reasoning for appropriate selection of VTE prophylaxis in acutely medical ill patients, and highlights our own approach and recommendations.
Keywords: venous thromboembolism, thromboprophylaxis, inpatient
Introduction
Venous thromboembolism (VTE) including deep vein thrombosis (DVT) and pulmonary embolism (PE) remains a major health problem with a reported high mortality rate and economic toll to the United States (U.S.) Health System. It is highly prevalent and is considered among the major causes for death in the U.S. Nearly one third of patients will have a recurrent event in their lifetime.1) It is estimated that 100,000 people die each year of VTE.1) Almost half of VTEs occur during or after hospitalization, with PE accounting for 10% of inpatient mortality.2) Hospitalized medically ill patients are at increased risk for VTE during and after their hospital stay.3,4) Although VTE prophylaxis for medically ill inpatients is crucially important, and despite the existence of published guidelines, we continue to see low adoption of such recommendations and lack of a standardized approach in many health systems. This document provides up-to-date and evidence-based recommendations for VTE prophylaxis in medically ill hospitalized patients, and presents our own approach to address this critical issue.
Epidemiology
While VTE remains the most common preventable cause of death in hospitalized medical patients, pharmacologic prophylaxis has been proven to reduce PE risk by 57%.5) Never the less VTE prophylaxis remains underused and inappropriately prescribed. Up to 900,000 patients experience their first VTE while hospitalized.6) Factors that increase risk include age, immobility, hypercoagulability, and renal insufficiency.7) Among hospitalized medically ill patients, 75% have multiple risk factors leading to an 8-fold increase in VTE risk when compared to the general population.7,8) Around 21% of PE cases are fatal, translating into 40,000 deaths yearly, with 75% of fatal VTE occurring in medically ill hospitalized patients.7,9)
Medically ill patients have increased VTE-related readmission rates that reach up to 28% six months post hospital admission.10) Based on a large retrospective analysis, more than 50% of cumulative six months VTE events were diagnosed within the first month post-hospitalization despite receiving prophylaxis, 57% of these events occurred post-discharge.11)
Pathophysiology
The basic principles of Virchow’s triad are fundamental when determining VTE risk in medically ill patients. Circulatory stasis or immobility, endothelial damage or inflammation, and overall hypercoagulable state, including prior history of thrombophilia or VTE, all cumulatively increase risk for VTE.12,13) Hospitalization alone is still considered the single most important risk factor for developing VTE, as these patients are more likely to be obese, elderly, immobile, and with active inflammation as a consequence of their underlying comorbidities and acute illness (e.g., sepsis, shock).14,15)
Medical conditions associated with a moderate to high risk of VTE include history of VTE or thrombophilia, malignancy, respiratory, infectious or cardiac disease (e.g. congestive heart failure (CHF)), cerebrovascular accident, autoimmune disorders and or renal insufficiency. Patients with active cancer or metastatic disease, who have received chemoradiotherapy within six months prior to admission are also considered high risk.16) In a post hoc analysis of the data from the MEDENOX study, immobile patients (defined as an autonomous walking distance of less than ten meters at the end of the treatment period) in the placebo group had a 20.3% incidence of VTE.17) Further analysis of the MEDENOX data confirmed reduction of VTE risk with using thromboprophylaxis agents.18)
Extended Prophylaxis after Discharge
Patients hospitalized for medical illness are at increased risk of VTE post-discharge based on their age, comorbidities, and continued immobility. Despite the increased risk in the post-discharge period, multiple trials have shown inconsistent results when addressing the efficacy and safety of continued pharmacological prophylaxis. Prophylaxis regimens were found to be effective when provided for a duration of hospitalization up to 6–14 days, however the average length hospital stay is currently much shorter.19,20) Prescribing post-discharge thromboprophylaxis for acutely ill medical patients has many challenges including route of administration, refusal of injectable agents, compliance, and cost.21–25) Based on one study, risk of VTE in hospitalized medically ill patients was highest within the first 19 days after hospital admission (a period that may encompass both duration of hospitalization and period after discharge).20) In a large real-world analysis of more than 11,000 acutely ill medical patients, the risk for VTE was cumulative, with 57% of VTEs occurred thirty days post-admission.10,11,25) In another study, 66.9% of patients who experienced DVT and/or PE events were diagnosed within the first month after hospital discharge, with 19.9% between months 1 and 2, and 13.2% between months 2 and 3.26) In the International Medical Prevention Registry on Venous Thromboembolism (IMPROVE) registry of 15,156 patients, the median time for all VTE events was 17 days (interquartile range 6–43 days), and the median time for post-discharge VTE events was 44 days (interquartile range 25–68 days).27)
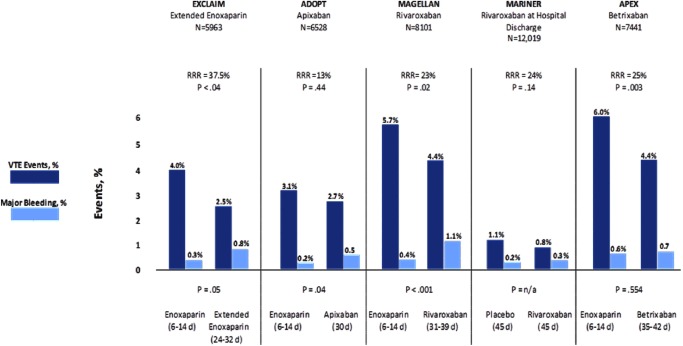
In an effort to increase compliance and safety in preventing VTEs post-hospitalization, multiple randomized control trials have been performed examining the utility and safety of several novel oral anticoagulants in the use of extended duration VTE prophylaxis. In 2017, betrixaban was food drug administration (FDA) approved for VTE prophylaxis in acutely ill medical patients based on results from the APEX trial. The APEX trial was a large randomized, double-blinded, multicenter Phase 3 study that compared standard enoxaparin dosage versus oral betrixaban (a novel oral anticoagulant, factor Xa inhibitor) for VTE prophylaxis for duration of 35 to 42 days.28) In this study, daily betrixaban 80 mg demonstrated a 25% relative risk reduction in VTE and VTE related death vs. enoxaparin.28) There was no significant difference (p=.003) in major bleeding events, but clinical non-major bleeding events were increased (Fig. 1), thus proving its utility in extended VTE prophylaxis.25)
Fig. 1 Trial results addressing extended prophylaxis treatment.
In October 2019, rivaroxaban also received FDA approval for VTE prophylaxis in acutely ill medical patients with low bleeding risk based on a sub-analysis of the Phase 3 MAGELLAN trial. MAGELLAN, is a multicenter, randomized, double-blind trial that evaluated the efficacy and safety of oral rivaroxaban 10 mg for 10±4 days or 35±4 days as compared to standard subcutaneous enoxaparin dosage in medically ill hospitalized patients.25) Rivaroxaban 10 mg was shown to be non-inferior at ten days and superior at 35 days compared to enoxaparin for composite asymptomatic proximal or symptomatic VTE, but with increased major bleeding events at both 10 and 35 days (Fig. 1).25) MARINER is another trial that was conducted in medically ill patients which showed that rivaroxaban 10 mg daily, given to medical patients for 45 days after hospital discharge, was not associated with a significantly lower risk of symptomatic VTE and related death compared to placebo.29)
Though there are now several options for extended duration VTE prophylaxis in acutely ill medical patients, the utilization of this practice within our current medical system remains limited. Additionally, this practice has not yet been adopted into current standard VTE prophylaxis recommendations.30)
Risk Stratification
To optimize outcomes, risk for bleeding should be estimated when considering pharmacological prophylaxis in medically ill patients. The strongest risk factors to estimate bleeding risk in medical hospitalized patients are active gastrointestinal ulcer, bleeding within three months prior to admission, and a platelet count of less than 50×109/L. Other risk factors include age >85 years, hepatic failure, severe renal failure, and/or critical care unit admission.7) Additionally, those with central venous catheter placement, rheumatic disease, malignancy, and male gender are considered to be at increased risk for bleeding during hospital admission.31)
Despite the known prevalence and associated mortality related to VTE in medically ill hospitalized patients, prevention has remained suboptimal. Multiple risk assessment models have been studied to help promote appropriate utilization of thromboprophylaxis modalities in medically ill patients. Perhaps the most studied of these are the Padua and IMPROVE risk assessment models, both of which have now been externally validated.32) In making recommendations regarding DVT prophylaxis in hospitalized medical patients, the American College of Chest Physicians (ACCP) recommended individualized approach based on balancing the benefit of reducing VTE with the risk of bleeding using risk assessment models. ACCP 2012 guidelines used Padua Prediction Scoring System (Table 1), however, the American Society of Hematology (ASH) 2018 guidelines referred to Padua and IMPROVE as RAMs that may also be useful in predicting VTE and bleeding risk.16)
Table 1 Padua predictive score for VTE among hospitalized medical patients.
| VTE risk factor | Points |
|---|---|
| Decreased mobility | 3 |
| Thrombophilia | 3 |
| Previous trauma or surgery within the last month | 2 |
| Age ≥70 | 1 |
| Heart or respiratory failure | 1 |
| Ischemic stroke or acute myocardial Infarction | 1 |
| Acute rheumatologic disorder and/or acute infection | 1 |
| Obesity | 1 |
| Hormonal therapy | 1 |
VTE: venous thromboembolism Low risk: score <4 High risk: score ≥4
The Padua VTE RAM used an 11-factor model appointing one to three points per factor in a binary fashion: high risk of VTE was designated with a score of four or more warranting pharmacologic prophylaxis, and low VTE risk was designated with a score less than four.16) Risk factors taken into account included active cancer, previous VTE, reduced mobility, known thrombotic condition, recent trauma/surgery, age >70, heart or respiratory failure, acute myocardial infraction or stroke, acute infection or rheumatologic disorder, body mass index >30, and/or use of hormone replacement therapy.
The IMPROVE VTE RAM was derived from a large international registry of 15,156 hospitalized, acutely ill medical patients. The RAM consisted of seven independent VTE risk factors that were designated one to three points each, depending on their strength of association with VTE risk.33) Risk factors include age>60 years, prior VTE, intensive care unit or coronary care unit stay, lower limb paralysis, immobility, known thrombophilia, and/or cancer. Two groups were identified within the cohort and were divided by VTE incidence rate. Low VTE risk (VTE event rate <1.0%) was designated a score of zero to one, at-risk, or moderate VTE risk (VTE event rate of∼1.0–1.5%) a score of two to three, and high VTE risk (VTE event rate of 4% or more) a score of four or more (Table 2).3) Recent large-scale external validation studies of the associative IMPROVE RAM have shown good calibration and discrimination suggesting that the IMPROVE associative VTE RAM may reliably stratify risk for VTE.23,34,35)
Table 2 The International Medical Prevention Registry on Venous Thromboembolism (IMPROVE-VTE) score.
| VTE risk factor | Points |
|---|---|
| Previous VTE | 3 |
| Known thrombophilia | 2 |
| Cancer | 2 |
| Current lower limb paralysis | 2 |
| Immobilization | 1 |
| ICU/CCU stay | 1 |
| Age >60 | 1 |
VTE: venous thromboembolism, ICU: intensive care unit, CCU: coronary care unit Low risk: score 0–1 (VTE risk <1.0%) Moderate risk: score 2–3 (VTE risk 1.0–1.5%) High risk: score of ≥4 (VTE risk >4%)
The evidence-derived IMPROVE Bleed RAM used 13 clinical and laboratory factors, and designated a score of seven or more to identify a patient cohort (∼10% of the population) at high risk of bleeding (major bleed risk 4.1% vs 0.4%) (Table 3).3) Patients with a score of less than seven were considered at lower risk for bleeding.
Table 3 IMPROVE-BLEED risk score.
| Bleeding risk factor | Points |
|---|---|
| Active gastric or duodenal ulcer | 4.5 |
| Prior bleeding within the last 3 months | 4 |
| Thrombocytopenia (<50×109/L) | 4 |
| Age ≥85 years | 3.5 |
| Liver failure (INR >1.5) | 2.5 |
| Severe kidney failure (GFR<30 mL/min/m2) | 2.5 |
| Admission to the ICU/CCU | 2.5 |
| Central venous catheter | 2 |
| Rheumatic disease | 2 |
| Active malignancy | 2 |
| Age: 40–84 years old | 1.5 |
| Male | 1 |
| Moderate kidney failure (GFR: 30–59 mL/min/m2) | 1 |
INR: international normalization ration, GFR: glomerular filtration rate, ICU: intensive care unit, CCU: coronary care unit Low risk: score <7 - Major bleed risk=0.4% High risk: score ≥7 - ∼10% of the population - Major bleed risk=4.1%
The above mentioned validated VTE and bleeding risk scores can be used at bed side during hospital admission to help providers tailor safe and patient-centric thromboprophylaxis plan.3)
Current Evidence
Pharmacologic prophylaxis
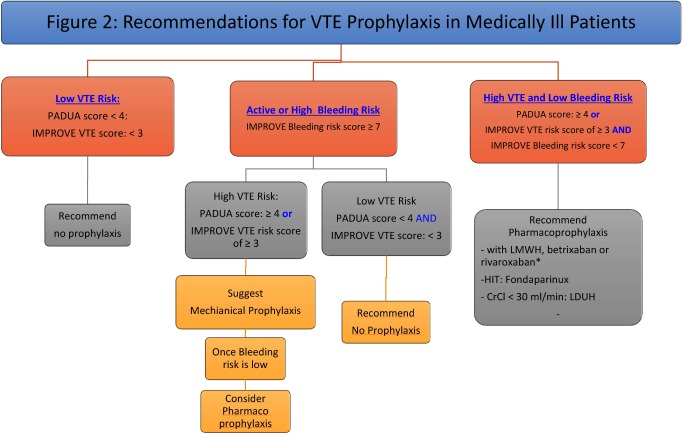
Both the 2018 ASH and 2012 ACCP VTE guidelines recommended the use of low molecular weight heparin (LMWH), low dose (twice daily [BID] or three times daily [TID]) unfractionated heparin (LDUH), or fondaparinux in acutely ill hospitalized patients at increased risk for thrombosis (Grade 1B).30,35) Additionally, the ASH guidelines recommended pharmacoprophylaxis or mechanical VTE prophylaxis over combined therapies.30) These guidelines were based on trials which included acutely ill hospitalized patients (mean age was >65 years) admitted for CHF, severe respiratory disease, or acute infectious, rheumatic, or inflammatory conditions who were immobilized and had at least one additional risk factor (e.g. age >40, active cancer, previous VTE, or serious infection). Duration of prophylaxis use ranged from 6–21 days or discharge from hospital, whichever came first.7) Meta-analysis of multiple trials demonstrated that anticoagulant thromboprophylaxis was associated with significant reduction in fatal PE and symptomatic DVT rates, but did not show a substantial difference in non-fatal PE, major bleeding, and all-cause mortality.7) Based on pooled analysis data, there was no significant difference seen between LDUH and LMWH for DVT, PE, overall mortality and heparin induced thrombocytopenia. However, there was a decrease in bleeding events seen with LMWH.7) There is no compelling data to suggest that LDUH TID, compared with BID dosing, reduces VTE or results in increased bleeding.7) In summary, there is no clear evidence in the current literature to support choosing one form of pharmacoprophylaxis over another in the medical population based on outcomes or from a cost-effectiveness standpoint (Figs. 1 and 2).
Fig. 2 Recommendations for VTE prophylaxis in medically ill patients.
ESRD: end stage renal disease, HIT: heparin induced thrombocytopenia, LDUH: low dose unfractionated heparin, LMWH: low molecular weight heparin, VTE: venous thromboembolism. * We suggest using betrixaban or rivaroxaban as an alternative to LMWH based on medication coverage and convenience (oral vs. injectable).
Mechanical prophylaxis
Based on pooled analyses, graduated compression stockings (GCS) did not show significant reduction in symptomatic VTE, but did increase risk for skin breakdown.7) There are no high quality studies of intermittent pneumatic compression (IPC) or venous flow pumps (VFP) devices in hospitalized medical patients.7) However, despite the uncertain benefit, mechanical thromboprophylaxis with GCS or IPC devices have been suggested by guidelines over no prophylaxis in patients at risk for VTE who are at high risk for bleeding.7) IPC devices have several limitations in medical populations: mechanical devices must be worn continuously which restricts patient mobility and are often considered to be uncomfortable resulting in patient deferral. This paradoxically may increase the risk of VTE.36) Overall risk reduction with the utilization of pneumatic compression devices is minimal. A recent study that included critically ill patients who received pharmacologic thromboprophylaxis in addition to IPC did not result in a significantly lower incidence of proximal lower-limb DVT than pharmacologic thromboprophylaxis alone (p=0.74).37)
Medically ill patients with a Padua VTE score of ≥4 or an IMPROVE VTE score of ≥3, provided that their IMPROVE-BLEED risk score is <7, should be offered pharmacologic prophylaxis during their hospital stay. Those with an IMPROVE-BLEED risk score of seven or more may benefit from mechanical means of prophylaxis until there is a reduction in their bleeding risk.3)
Our Approach
Because of the prevalence and risk associated with VTE, further efforts should be made across the country to help reduce the risk of in-hospital VTE related death. Allina Health, a large, not-for-profit health system with over 12 hospitals and 90 clinics has committed to providing up-to-date and evidence-based recommendations to address this serious health issue.
The following recommendations were created based on collaboration between vascular medicine, hospitalist, and intensive care unit specialists.
Recommendations (Fig. 2)
- For acutely ill hospitalized medical patients at increased risk of thrombosis (Padua score of ≥4 or IMPROVE VTE risk score of ≥3), and low risk for bleeding (IMPROVE-BLEED risk score of <7), we recommend anticoagulant thromboprophylaxis with LMWH, LDUH (BID or TID), fondaparinux or betrixaban.
- We suggest using LMWH over LDUH.
- In patients with history of heparin induced thrombocytopenia (HIT), we suggest using fondaparinux.
- In patients with end stage renal disease (ESRD), we suggest using LDUH.
- We suggest using betrixaban or rivaroxaban as an alternative to LMWH based on medication coverage and convenience (oral vs. injectable).
- We suggest not using other direct oral anticoagulants.
For acutely ill hospitalized medical patients at low risk of thrombosis (Padua score of <4 or IMPROVE VTE risk score of <3), we recommend against pharmacologic or mechanical thromboprophylaxis.
- For acutely ill hospitalized medical patients with increased risk of VTE (Padua score of ≥4 or IMPROVE VTE risk score of ≥3), who are bleeding or at risk for bleeding (IMPROVE-BLEED risk score of ≥7)
- We recommend against anticoagulant prophylaxis.
- We suggest optimal use of mechanical thrombophylaxis with GCS, or IPC.
- When bleeding risk decreases, and VTE risk persists, we suggest that pharmacologic thrombophylaxis substituted for mechanical prophylaxis.
- In acutely ill hospitalized patients who receive an initial course of thrombophylaxis:
- We suggest against extending the duration of thrombophylaxis beyond the period of patient immobilization or acute hospitalization when heparin is used.
- We recommend extended thrombophylaxis to 35–42 days with betrixaban or to 31–39 days when rivaroxaban is used.
We suggest against using thrombophylaxis in chronically immobilized patients including nursing home residents who do not have other indications for anticoagulation.
As part of Allina Health’s continued efforts toward risk reduction of inpatient VTE, a tailored order-set which implements both the IMPROVE and IMPROVE-BLEED scoring systems has been introduced to the hospitalist teams. Additionally, a Quality Improvement project has been initiated to further evaluate clinician’s selection of appropriate VTE thromboprophylaxis. With multidisciplinary support and a continually evolving administrative plan, it is our hope that VTE rates will continue to decline amongst our hospital admissions, and that our model for VTE prophylaxis may serve as a model for other medical institutions in the future.
Conclusion
Although VTE complications related to medical admissions are prevalent, fatal, and potentially preventable, appropriate measures to screen, assess, and initiate prophylactic therapy in patients at risk are lacking.38) Because of the significance of VTE related morbidity and mortality in medically hospitalized patients, we recommend appropriate and evidence-based thromboprophylaxis protocols focusing on a balance between VTE and bleeding risk.
Although hospitalized medically ill patients are at increased risk for VTE during and after their hospital stay, we recommend against extended pharmacoprophylaxis after discharge unless betrixaban or rivaroxaban are the agents of choice. Based on the current evidence, when indicated, it is prudent to recommend LMWH, LDUH, or fondaparinux for up to 6–14 days, oral betrixaban for 35–42 days or oral rivaroxaban for 31–39 days.
In addressing the need for implementing VTE prophylaxis in medically ill hospitalized patients, the use of the Padua, IMPROVE, and IMPROVE-BLEED RAMs should be encouraged in guiding clinical decision-making. When implemented, RAMs have been shown to decrease overall rates of VTE in hospitalized medical patients while balancing the risk for bleeding complications. We agree with the current guideline recommendations to individualize VTE prophylaxis selection based on patient preference, compliance, cost, and ease of administration (e.g., oral vs. injection, daily vs. BID vs. TID dosing).7)
More research is warranted to standardize risk assessment tools, streamline the selection of appropriate prophylactic agent, and to determine the appropriate duration of prophylaxis.
Disclosure Statement
All authors have no conflict of interest.
Author Contributions
Study conception: NS, EW
Data collection: NS, EW
Analysis: NS, EW
Investigation: NS, EW
Writing: NS, EW
Funding acquisition: not applicable
Critical review and revision: all authors
Final approval of the article: all authors
Accountability for all aspects of the work: all authors
References
- 1.Beckman MG, Hooper WC, Critchley SE, et al. Venous thromboembolism: a public health concern. Am J Prev Med 2010; 38 Suppl: S495-501. [DOI] [PubMed] [Google Scholar]
- 2.Mukhi N, Sidhu G, Nabors C, et al. Thromboprophylaxis use in medical inpatients and the impact of electronic risk assessment tool. Blood 2014; 124: 3511.25477483 [Google Scholar]
- 3.Spyropoulos AC, Raskob GE. New paradigms in venous thromboprophylaxis of medically ill patients. Thromb Haemost 2017; 117: 1662-70. [DOI] [PubMed] [Google Scholar]
- 4.Ageno W, Hunt BJ. Reducing the burden of venous thromboembolism in the acute medically ill population with extended-duration thromboprophylaxis. Eur Heart J Suppl 2018; 20 Suppl E: E6-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bozzato S, Galli L, Ageno W. Thromboprophylaxis in surgical and medical patients. Semin Respir Crit Care Med 2012; 33: 163-75. [DOI] [PubMed] [Google Scholar]
- 6.Dobesh PP, Ahuja T, Davis GA, et al. Venous thromboembolism in acute medically ill patients: identifying unmet needs and weighing the value of prophylaxis. Am J Manag Care 2018; 24 Suppl: S468-74. [PubMed] [Google Scholar]
- 7.Kahn SR, Lim W, Dunn AS, et al. Prevention of VTE in nonsurgical patients: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2012; 141 Suppl: e195S-226S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Khoury H, Welner S, Kubin M, et al. Disease burden and unmet needs for prevention of venous thromboembolism in medically ill patients in Europe show underutilization of preventive therapies. Thromb Haemost 2011; 106: 600-8. [DOI] [PubMed] [Google Scholar]
- 9.Piazza G, Fanikos J, Zayaruzny M, et al. Venous thromboembolic events in hospitalised medical patients. Thromb Haemost 2009; 102: 505-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Baser O, Liu X, Phatak H, et al. Venous thromboembolism prophylaxis and clinical consequences in medically ill patients. Am J Ther 2013; 20: 132-42. [DOI] [PubMed] [Google Scholar]
- 11.Amin AN, Varker H, Princic N, et al. Duration of venous thromboembolism risk across a continuum in medically ill hospitalized patients. J Hosp Med 2012; 7: 231-8. [DOI] [PubMed] [Google Scholar]
- 12.Xu J, Lupu F, Esmon CT. Inflammation, innate immunity and blood coagulation. Hamostaseologie 2010; 30: 5-6, 8-9. [PubMed] [Google Scholar]
- 13.Samama MM. An epidemiologic study of risk factors for deep vein thrombosis in medical outpatients: the Sirius study. Arch Intern Med 2000; 160: 3415-20. [DOI] [PubMed] [Google Scholar]
- 14.Office of the Surgeon General (US); National Heart, Lung, and Blood Institute (US). The Surgeon General’s Call to Action to Prevent Deep Vein Thrombosis and Pulmonary Embolism. Rockville (MD): Office of the Surgeon General (US); 2008. Available from: https://www.ncbi.nlm.nih.gov/books/NBK44178/ [PubMed]
- 15.Kaplan D, Casper TC, Elliott CG, et al. VTE incidence and risk factors in patients with severe sepsis and septic shock. Chest 2015; 148: 1224-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Barbar S, Noventa F, Rossetto V, et al. A risk assessment model for the identification of hospitalized medical patients at risk for venous thromboembolism: the Padua Prediction Score. J Thromb Haemost 2010; 8: 2450-7. [DOI] [PubMed] [Google Scholar]
- 17.Alikhan R, Cohen AT, Combe S, et al. Prevention of venous thromboembolism in medical patients with enoxaparin: a subgroup analysis of the MEDENOX study. Blood Coagul Fibrinolysis 2003; 14: 341-6. [DOI] [PubMed] [Google Scholar]
- 18.Cohen AT, Edmondson RA, Phillips MJ, et al. The changing pattern of venous thromboembolic disease. Pathophysiol Haemost Thromb 1996; 26: 65-71. [DOI] [PubMed] [Google Scholar]
- 19.HCUP NIS Related Reports. Healthcare Cost and Utilization Project (HCUP); 2011. Available from: https://www.hcup-us.ahrq.gov/reports/statbriefs/sb166.jsp.
- 20.Amin AN, Varker H, Princic N, et al. Duration of venous thromboembolism risk across a continuum in medically ill hospitalized patients. J Hosp Med 2012; 7: 231-8. [DOI] [PubMed] [Google Scholar]
- 21.Spyropoulos AC, Ageno W, Albers GW, et al. Rivaroxaban for thromboprophylaxis after hospitalization for medical illness. N Engl J Med 2018; 379: 1118-27. [DOI] [PubMed] [Google Scholar]
- 22.Peidro-Garcés L, Otero-Fernandez R, Lozano-Lizarraga L, et al. Adherence to and satisfaction with oral outpatient thromboembolism prophylaxis compared to parenteral: SALTO study. Rev Esp Cir Ortop Traumatol 2013; 57: 53-60. (English Edition) [DOI] [PubMed] [Google Scholar]
- 23.Mahan CE, Liu Y, Turpie AG, et al. External validation of a risk assessment model for venous thromboembolism in the hospitalised acutely-ill medical patient (VTE-VALOURR). Thromb Haemost 2014; 112: 692-9. [DOI] [PubMed] [Google Scholar]
- 24.Hull RD, Schellong SM, Tapson VF, et al. Extended-duration venous thromboembolism prophylaxis in acutely ill medical patients with recently reduced mobility: a randomized trial. Ann Intern Med 2010; 153: 8-18. [DOI] [PubMed] [Google Scholar]
- 25.Cohen AT, Spiro TE, Büller HR, et al. Rivaroxaban for thromboprophylaxis in acutely ill medical patients. N Engl J Med 2013; 368: 513-23. [DOI] [PubMed] [Google Scholar]
- 26.Popoola VO, Tavakoli F, Lau BD, et al. Exploring the impact of route of administration on medication acceptance in hospitalized patients: implications for venous thromboembolism prevention. Thromb Res 2017; 160: 109-13. [DOI] [PubMed] [Google Scholar]
- 27.Spencer FA, Lessard D, Emery C, et al. Venous thromboembolism in the outpatient setting. Arch Intern Med 2007; 167: 1471-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bevyxxa (package insert). South San Francisco, CA: Portola Pharmaceuticals, Inc.
- 29.Aryal MR, Gosain R, Donato A, et al. Systematic review and meta-analysis of the efficacy and safety of apixaban compared to rivaroxaban in acute VTE in the real world. Blood Adv 2019; 3: 2381-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schünemann HJ, Cushman M, Burnett AE, et al. American Society of Hematology 2018 guidelines for management of venous thromboembolism: prophylaxis for hospitalized and nonhospitalized medical patients. Blood Adv 2018; 2: 3198-225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Decousus H, Tapson VF, Bergmann JF, et al. Factors at admission associated with bleeding risk in medical patients: findings from the IMPROVE investigators. Chest 2011; 139: 69-79. [DOI] [PubMed] [Google Scholar]
- 32.Huang W, Anderson FA, Spencer FA, et al. Risk-assessment models for predicting venous thromboembolism among hospitalized non-surgical patients: a systematic review. J Thromb Thrombolysis 2013; 35: 67-80. [DOI] [PubMed] [Google Scholar]
- 33.Rosenberg D, Eichorn A, Alarcon M, et al. External validation of the risk assessment model of the International Medical Prevention Registry on Venous Thromboembolism (IMPROVE) for medical patients in a tertiary health system. J Am Heart Assoc 2014; 3: e001152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cohen AT, Spiro TE, Spyropoulos AC, et al. D-dimer as a predictor of venous thromboembolism in acutely ill, hospitalized patients: a subanalysis of the randomized controlled MAGELLAN trial. J Thromb Haemost 2014; 12: 479-87. [DOI] [PubMed] [Google Scholar]
- 35.Guyatt GH, Akl EA, Crowther M, et al. Executive summary: antithrombotic therapy and prevention of thrombosis: 9th ed, American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2012; 141 Suppl: 7S-47S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Turpie AGG, Leizorovicz A. Prevention of venous thromboembolism in medically ill patients: a clinical update. Postgrad Med J 2006; 82: 806-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Arabi YM, Al-Hameed F, Burns KEA, et al. Adjunctive intermittent pneumatic compression for venous thromboprophylaxis. N Engl J Med 2019; 380: 1305-15. [DOI] [PubMed] [Google Scholar]
- 38.Futterman LG, Lemberg L. A silent killer—often preventable. Am J Crit Care 2004; 13: 431-6. [PubMed] [Google Scholar]