Short abstract
Objective
Sepsis is a serious complication of acute cholangitis. We aimed to establish a nomogram for predicting the probability of sepsis in patients with acute cholangitis.
Methods
Subjects were patients with acute cholangitis in the Medical Information Mart for Intensive Care database. Extraneous variables were excluded based on stepwise regression. The nomogram was established using logistic regression.
Results
The predictive model comprised five variables: age (odds ratio [OR]: 1.03, 95% confidence interval [CI]: 1.01–1.04), ventilator-support time (OR: 1.004, 95% CI: 1.001–1.008), diabetes (OR: 10.74, 95% CI: 2.80–70.57), coagulopathy (OR: 2.92, 95% CI: 1.83–4.73) and systolic blood pressure (OR: 0.62, 95% CI: 0.41–0.93). The areas under the receiver operating characteristic curve of the nomogram for the training and validation sets were 0.700 and 0.647, respectively. The Hosmer–Lemeshow goodness-of-fit test revealed high concordance between the predicted and observed probabilities for both the training and validation sets. The calibration plot also demonstrated good agreement between the predicted and observed outcomes for both the training and validation sets.
Conclusions
We developed and validated a risk-prediction model for sepsis in patients with acute cholangitis. Our results will be helpful for preventing sepsis in patients with acute cholangitis.
Keywords: Sepsis, acute cholangitis, nomogram, prediction model, Medical Information Mart for Intensive Care database, logistic regression
Introduction
Acute cholangitis is a systemic infectious disease characterized by biliary obstruction and bile bacterial growth that cause acute inflammation and infection of the bile duct.1 Acute cholangitis was first described by Charcot as ‘hepatic fever’ in 1877; therefore, the typical signs and symptoms of acute cholangitis (intermittent fever with chills, right-upper-quadrant pain and jaundice) are known as Charcot’s triad.2
The prevalence of cholelithiasis varies from race to race. Of the many patients hospitalized for gallstones, 6% to 9% are diagnosed with acute cholangitis.3 Men and women are affected equally and the average age of patients with acute cholangitis is between 50 and 60 years old.4 If acute cholangitis is not properly treated, it rapidly develops into systemic inflammatory response syndrome, sepsis and mortality. In the 1990s, the mortality rate for severe acute cholangitis was between 11% and 27%.5
Sepsis is a clinical syndrome that results from dysregulation of the inflammatory response to infection that leads to organ dysfunction.6 Greater understanding of sepsis has led some researchers to suggest that sepsis is essentially a loss of control of the immune response produced by the body to fight pathogens, resulting in multiple organ dysfunction.7,8 Sepsis is one of the most common diseases in intensive care units worldwide9 and is associated with high mortality and costs. There are an estimated 18 million cases of sepsis worldwide each year; despite advances in intensive care and support technology, the mortality rate from sepsis remains between 15% and 80%.10 The annual cost of sepsis in the United States has been estimated to exceed $20 billion.11 The timing of treatment for sepsis is very important; delaying the start of treatment, even by 1 hour, increases the chance of adverse outcomes.12 Sepsis is a serious complication of acute cholangitis. The early identification and risk assessment of sepsis in patients with acute cholangitis are crucial to improving the treatment and prognosis of these patients.
A nomogram is a graphical tool for determining the probability of a clinical event in an individual patient based on a statistical predictive model.13 However, nomograms for predicting the risk of sepsis in cholangitis patients have been rarely reported. In this study, we aimed to develop a nomogram for predicting the risk of sepsis for patient with cholangitis.
Methods
Patients
The MIMIC (Medical Information Mart for Intensive Care) database is funded by the National Institutes of Health and was jointly launched by the Computational Physiology Laboratory of Massachusetts Institute of Technology, Beth Israel Deaconess Medical Center (BIDMC), and Philips Medical. The database collects and organizes clinical diagnosis and treatment information for more than 40,000 real patients who stayed in the intensive care unit of BIDMC from 2001 to 2012.14 The development of this database provides a large amount of systematic clinical diagnosis and treatment data for clinical medical workers to conduct scientific research. The MIMIC database is constantly updated, and the latest version is MIMIC-III (version 1.4).15,16 This study focused on building the library and extracting data on relevant patients from the MIMIC database using PostgreSQL software, version 9.6 (The PostgreSQL Global Development Group).
Research method
We extracted the hadm_id identifiers of 662 patients with a diagnosis of acute cholangitis from the MIMIC database using the icd9 code. These 662-person-time patients comprised the study subjects. Taking the hadm_id identifiers of 662 person-times as an index, we extracted the following information from the MIMIC-III database: age, sex, heart rate, systolic blood pressure (SBP), diastolic blood pressure, albumin, bilirubin, glucose, haemoglobin, lactate, neutrophil percentage, pH, sequential organ failure score, hypertension, diabetes, renal failure, alcohol use, coagulopathy, enteral nutrition, parenteral nutrition, ventilator-support time, frequency of ventilator usage, temperature, length of stay and sepsis.
The classification of variables is presented in Table 1. This study was approved by the institutional review boards of Massachusetts Institute of Technology and BIDMC, and was granted a waiver of the need to obtain informed consent.
Table 1.
Classification of variables.
| Variables | Range24 | Classification |
|---|---|---|
| Albumin (g/dL) | ||
| 3.5–5.5 | normal | |
| other | abnormal | |
| Bilirubin (mg/dL) | ||
| 0.1–1.0 | normal | |
| other | abnormal | |
| Glucose (mg/dL) | ||
| 70–140 | normal | |
| other | abnormal | |
| Haemoglobin (g/dL) | ||
| male | 13.5–17.5 | normal |
| other | abnormal | |
| female | 12.0–16 | normal |
| other | abnormal | |
| Lactate (mmol/L) | ||
| 0.5–1.6 | normal | |
| other | abnormal | |
| NEUT % | ||
| 45–62 | normal | |
| other | abnormal | |
| Heart rate (per minute) | ||
| 60–100 | normal | |
| other | abnormal | |
| SBP (mmHg) | ||
| 90–140 | normal | |
| other | abnormal | |
| DBP (mmHg) | ||
| 60–90 | normal | |
| other | abnormal | |
| pH | ||
| 7.35–7.45 | normal | |
| other | abnormal | |
| Sex | ||
| male | 1 | |
| female | 2 |
SBP: systolic blood pressure; DBP: diastolic blood pressure; NEUT %: neutrophil percentage.
Statistical analysis
Continuous variables conforming to a normal distribution are expressed as mean and standard deviation values; other continuous variables are expressed as median (25th–75th percentile) values. Categorical variables are expressed as percentages. Stepwise regression and lasso regression were used when selecting variables to enter into the model. The results from the more sensitive of the two methods were chosen. Logistic regression was used to construct predictive models. The nomogram was used to predict the probability of sepsis in patients with acute cholangitis. For nomogram construction and validation, we randomly assigned 70% of the patients to the training set and 30% to the validation set.
The area under the receiver operating characteristic curve (AUC) was used to evaluate the discriminative ability of the nomogram. The AUC ranges from 0 to 1, with 1 indicating perfect concordance and 0.5 indicating that the concordance is no better than chance. Calibration of the nomogram model, defined as concordance between predicted and observed probabilities, was established using the Hosmer–Lemeshow goodness-of-fit test (P > 0.05) and a calibration plot.
All statistical analyses were performed using SPSS (version 24.0, IBM Corp., Armonk, NY, USA) and R software (R Foundation for Statistical Computing, Vienna, Austria). All tests were two-sided and P ≤ 0.05 was considered indicative of statistical significance.
Results
Basic characteristics of the study subjects
The randomization process resulted in 463 and 199 patients with acute cholangitis being enrolled in the training and validation sets, respectively. The basic characteristics of the patients are shown in Table 2.
Table 2.
Basic characteristics of study subjects.
| Variable | All (n = 662) | Training cohorts (n = 463) | Validation cohorts (n = 199) |
|---|---|---|---|
| Age (mean ± SD) | 70.7 ± 14.7 | 70.5 ± 14.8 | 71 ± 14.7 |
| Temperature (mean ± SD) | 37.1 ± 3.9 | 37.2 ± 3.1 | 36.8 ± 5.3 |
| SOFA, median (25th–75th percentile) | 6 (4–8) | 6 (4–8) | 6 (4–8) |
| Ventilator-support time, median (25th–75th percentile) | 0 (0–19.4) | 2 (2–70) | 2 (2–53) |
| LOS, median (25th–75th percentile) | 8 (5–16) | 8 (5–17) | 8 (5–14) |
| Frequency of ventilator usage, median (25th–75th percentile) | 0 (0–1) | 0 (0–1) | 0 (0–1) |
| Sex (%) | |||
| male | 374 (56.5) | 261 (56.4) | 113 (56.8) |
| female | 288 (43.5) | 202 (43.6) | 86 (43.2) |
| Albumin (%) | |||
| normal | 99 (15) | 71 (15.3) | 28 (14.1) |
| abnormal | 563 (85) | 392 (84.7) | 171 (85.9) |
| Bilirubin (%) | |||
| normal | 165 (24.9) | 114 (24.6) | 51 (25.6) |
| abnormal | 497 (75.1) | 349 (75.4) | 148 (74.4) |
| Hypertension (%) | |||
| yes | 90 (13.6) | 58 (12.5) | 32 (16.1) |
| no | 572 (86.4) | 405 (87.5) | 167 (83.9) |
| Diabetes (%) | |||
| yes | 23 (3.1) | 15 (3.2) | 8 (4) |
| no | 639 (95.6) | 448 (96.8) | 191 (96) |
| Renal failure (%) | |||
| yes | 110 (16.6) | 75 (16.2) | 35 (17.6) |
| no | 552 (83.4) | 388 (83.8) | 164 (82.4) |
| Alcohol use (%) | |||
| yes | 26 (3.9) | 18 (3.9) | 8 (4) |
| no | 636 (96.1) | 445 (96.1) | 191 (96) |
| Coagulopathy (%) | |||
| yes | 146 (22.1) | 106 (22.9) | 40 (20.1) |
| no | 516 (77.9) | 357 (77.1) | 159 (79.9) |
| EN (%) | |||
| yes | 100 (15.1) | 77 (16.6) | 23 (11.6) |
| no | 562 (84.9) | 386 (83.4) | 176 (88.4) |
| Glucose (%) | |||
| normal | 470 (71) | 312 (67.4) | 158 (79.4) |
| abnormal | 192 (29) | 151 (32.6) | 41 (20.6) |
| Haemoglobin (%) | |||
| normal | 41 (6.2) | 30 (6.5) | 11 (5.5) |
| abnormal | 621 (93.8) | 433 (93.5) | 188 (94.5) |
| Lactate (%) | |||
| normal | 282 (42.6) | 183 (39.5) | 99 (49.7) |
| abnormal | 380 (57.4) | 280 (60.5) | 100 (50.3) |
| NEUT (%) | |||
| normal | 28 (4.2) | 18 (3.9) | 10 (5) |
| abnormal | 634 (95.8) | 445 (96.1) | 189 (95) |
| pH (%) | |||
| normal | 268 (40.5) | 193 (41.7) | 75 (37.7) |
| abnormal | 394 (59.5) | 270 (58.3) | 124 (62.3) |
| PN (%) | |||
| normal | 560 (84.6) | 383 (82.7) | 177 (88.9) |
| abnormal | 102 (15.4) | 80 (17.3) | 22 (11.1) |
| Heart rate (%) | |||
| normal | 518 (78.2) | 373 (80.6) | 145 (72.9) |
| abnormal | 144 (21.8) | 90 (19.4) | 54 (27.1) |
| SBP (%) | |||
| normal | 288 (43.5) | 205 (44.3) | 83 (41.7) |
| abnormal | 374 (56.5) | 258 (55.7) | 116 (58.3) |
| DBP (%) | |||
| normal | 455 (68.7) | 312 (67.4) | 143 (71.9) |
| abnormal | 207 (31.3) | 151 (32.6) | 56 (28.1) |
| Sepsis (%) | |||
| yes | 379 (51.3) | 201 (43.4) | 82 (41.2) |
| no | 283 (42.7) | 262 (56.6) | 117 (58.8) |
SD: standard deviation; SOFA: sequential organ failure score; LOS: length of stay; EN: enteral nutrition; PN: parenteral nutrition; SBP: systolic blood pressure; DBP: diastolic blood pressure; NEUT (%): neutrophil percentage.
Predictive nomogram for the probability of sepsis in patients with acute cholangitis
The AUC values of the lasso regression and stepwise regression were 0.688 and 0.700, respectively. Therefore, the stepwise regression method was used to screen for variables. The logistic regression results are shown in Table 3. Multivariate logistic regression was conducted for variables with P values that were less than 0.2 in the univariate logistic regression, and for variables with P values that were less than 0.05 in the multivariate logistic regression. These variables were entered into the prediction model. The following five variables were entered into the predictive model based on the results of stepwise regression: age (odds ratio [OR] = 1.03, 95% confidence interval [CI] = 1.01–1.04, P < 0.01), ventilator-support time (OR = 1.004, 95% CI = 1.001–1.008, P = 0.01), diabetes (OR = 10.74, 95% CI = 2.80–70.57, P < 0.01), coagulopathy (OR = 2.92, 95% CI = 1.83–4.73, P < 0.01) and SBP (OR = 0.62, 95% CI = 0.41–0.93, P = 0.01). A nomogram was established based on these five selected variables. The nomogram is used by scoring each variable on its corresponding score scale. The scores for all variables are then summed to obtain the total score, and a vertical line is drawn from the total-points row to indicate the estimated probability of sepsis being present (Figure 1).
Table 3.
The results of logistic regression.
| Variables | P value for univariate logistic regression | P value for multivariate logistic regression |
|---|---|---|
| Age | <0.01 | <0.01 |
| Diabetes | 0.01 | <0.01 |
| Coagulopathy | <0.01 | <0.01 |
| Haemoglobin | 0.13 | 0.13 |
| Length of stay | 0.09 | 0.09 |
| Hypertension | 0.19 | 0.06 |
| Enteral nutrition | 0.09 | 0.06 |
| Systolic blood pressure | 0.01 | 0.01 |
| Ventilator-support time | 0.14 | 0.01 |
| Sex | 0.91 | |
| Heart rate | 0.61 | |
| Diastolic blood pressure | 0.71 | |
| Albumin | 0.37 | |
| Bilirubin | 0.64 | |
| Glucose | 0.37 | |
| Lactate | 0.41 | |
| Neutrophil percentage | 0.76 | |
| pH | 0.72 | |
| Sequential organ failure score | 0.71 | |
| Renal failure | 0.66 | |
| Alcohol use | 0.68 | |
| Parenteral nutrition | 0.87 | |
| Frequency of ventilator usage | 0.27 | |
| Temperature | 0.50 |
Boldface values in table 3 means that P value is less than 0.05.
Figure 1.
Predictive nomogram for the probability of sepsis in patients with acute cholangitis.
SBP: systolic blood pressure.
Discriminative ability of the nomogram
The AUC was used to validate the nomogram. The AUCs of the training set (Figure 2) and the validation set (Figure 3) were 0.700 and 0.647, respectively.
Figure 2.
ROC curve for the training set.
AUC: area under the ROC curve; ROC: receiver operating characteristic.
Figure 3.
ROC curve for the validation set.
AUC: area under the ROC curve; ROC: receiver operating characteristic.
Nomogram calibration
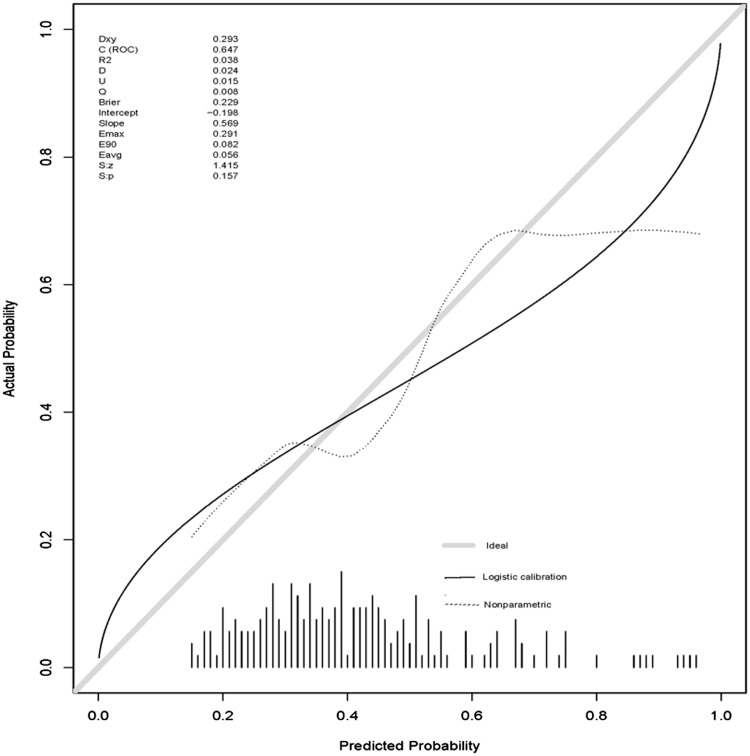
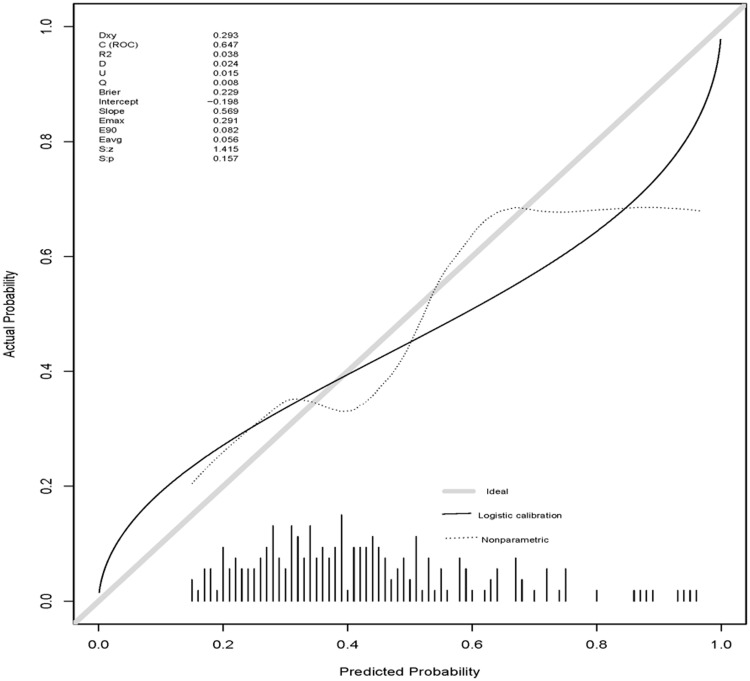
The nomogram model was calibrated using the Hosmer–Lemeshow goodness-of-fit test and a calibration plot. The Hosmer–Lemeshow goodness-of-fit test revealed the presence of a high concordance between the predicted and observed probabilities both for the training set (x2 = 13.52, df = 8, P = 0.09) and the validation set (x2 = 3.03, df = 8, P = 0.93). The calibration plot also showed good agreement between the predicted and observed outcomes both for the training set (Figure 4) and the validation set (Figure 5).
Figure 4.
Calibration plot for the training set.
Figure 5.
Calibration plot for the validation set.
Discussion
Bacterial infection that occurs in the presence of biliary obstruction can lead to acute cholangitis, a condition characterized by fever, abdominal pain and jaundice.17 The biliary tree is normally sterile, but a combination of obstruction and infection of the biliary tract can lead to acute cholangitis.18 A normal biliary blood flow results in normal concentrations of bacteria and a normal pressure inside the catheter. However, obstruction of the biliary tract increases the intraductal pressure; the resulting reduced bile flow will allow any bacteria present more time to multiply, possibly leading to infection. Patients with acute cholangitis present with a wide disease spectrum: some are mildly ill and others have severe sepsis and shock. Sepsis is the tenth leading cause of death in the United States.19 Previous studies have shown that the early detection of sepsis and early goal-directed therapy can improve prognosis and reduce mortality in patients with severe sepsis and septic shock.20 It is therefore necessary to establish an early predictive model of sepsis in patients with acute cholangitis.
In this study, a predictive model was implemented using a nomogram. Nomograms are used to transform statistical equations into simplified graphs and they have become a reliable and convenient tool for quantifying risk. To our knowledge, the present study is the first to use the MIMIC-III database to establish a predictive model for sepsis in patients with acute cholangitis. The AUCs for the training and validation sets both exceeded 0.5, indicating that the nomogram developed in this study has good discrimination performance. However, only 662 acute cholangitis patients from the MIMIC-III database were included in this study. Future studies could improve the logistic regression model and nomogram by using larger samples. The Hosmer–Lemeshow goodness-of-fit test and the calibration plot further indicated that the model provided a good fit to the real data.
We found that in patients with acute cholangitis, sepsis is positively associated with age, ventilator-support time, diabetes, coagulopathy and an SBP less than 90 mmHg or greater than 140 mmHg; these findings are consistent with previous research.21–23 Clinical treatments should focus on patients with these characteristics, and early treatment with antibiotics should be used to prevent the occurrence of sepsis.
The present study had some limitations. First, it used a retrospective design; thus, additional prospective validation studies are needed. Second, external validation from other institutions could facilitate the wider use of the developed prognostic nomogram. Third, our nomogram was based on only five available predictors, so the accuracy of our model might be improved by adding other relevant variables. These limitations could restrict the usefulness of our nomogram in real clinical practice.
Conclusion
We used logistic regression to develop and validate a risk-prediction model for sepsis in patients with acute cholangitis. The developed nomogram will be valuable in helping to prevent sepsis in patients with acute cholangitis.
Declaration of conflicting interest
The authors declare that there is no conflict of interest.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
References
- 1.Mosler P. Management of acute cholangitis. Gastroenterol Hepatol (NY) 2011; 7: 121–123. [PMC free article] [PubMed] [Google Scholar]
- 2.Mosler P. Diagnosis and management of acute cholangitis. Curr Gastroenterol Rep 2011; 13: 166–172. [DOI] [PubMed] [Google Scholar]
- 3. What if it's acute cholangitis? Drug Ther Bull 2005; 43: 62–64. [DOI] [PubMed] [Google Scholar]
- 4.Ahmed M. Acute cholangitis - an update. World J Gastrointest Pathophysiol 2018; 9: 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lan Cheong Wah D, Christophi C, Muralidharan V. Acute cholangitis: current concepts. ANZ J Surg 2017; 87: 554–559. [DOI] [PubMed] [Google Scholar]
- 6.Taeb AM, Hooper MH, Marik PE. Sepsis: current definition, pathophysiology, diagnosis, and management. Nutr Clin Pract 2017; 32: 296–308. [DOI] [PubMed] [Google Scholar]
- 7.Rittirsch D, Flierl MA, Ward PA. Harmful molecular mechanisms in sepsis. Nat Rev Immunol 2008; 8: 776–787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Parrish WR, Gallowitsch-Puerta M, Czura CJ, et al. Experimental therapeutic strategies for severe sepsis: mediators and mechanisms. Ann N Y Acad Sci 2008; 1144: 210–236. [DOI] [PubMed] [Google Scholar]
- 9.Marik PE. Early management of severe sepsis: concepts and controversies. Chest 2014; 145: 1407–1418. [DOI] [PubMed] [Google Scholar]
- 10.Cheng B, Hoeft AH, Book M, et al. Sepsis: pathogenesis, biomarkers, and treatment. Biomed Res Int 2015; 2015: 846935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Torio CM, Andrews RM. National inpatient hospital costs: the most expensive conditions by payer, 2011: Statistical brief #160. Healthcare Cost and Utilization Project (HCUP) statistical briefs. Rockville (MD): Agency for Healthcare Research and Quality (US), 2006. [PubMed] [Google Scholar]
- 12.Cohen J. Diagnosing sepsis: does the microbiology matter? Crit Care 2008; 12: 145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guo CG, Zhao DB, Liu Q, et al. A nomogram to predict lymph node metastasis in patients with early gastric cancer. Oncotarget 2017; 8: 12203–12210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Saeed M, Villarroel M, Reisner AT, et al. Multiparameter Intelligent Monitoring in Intensive Care II: a public-access intensive care unit database. Crit Care Med 2011; 39: 952–960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Johnson AE, Pollard TJ, Shen L, et al. MIMIC-III, a freely accessible critical care database. Sci Data 2016; 3: 160035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang Z. Accessing critical care big data: a step by step approach. J Thorac Dis 2015; 7: 238–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee JG. Diagnosis and management of acute cholangitis. Nat Rev Gastroenterol Hepatol 2009; 6: 533–541. [DOI] [PubMed] [Google Scholar]
- 18.Lipsett PA, Pitt HA. Acute cholangitis. Front Biosci 2003; 8: s1229–s1239. [DOI] [PubMed] [Google Scholar]
- 19.Danai P, Martin GS. Epidemiology of sepsis: recent advances. Curr Infect Dis Rep 2005; 7: 329–334. [DOI] [PubMed] [Google Scholar]
- 20.Rivers E, Nguyen B, Havstad S, et al. Early goal-directed therapy in the treatment of severe sepsis and septic shock. N Engl J Med 2001; 345: 1368–1377. [DOI] [PubMed] [Google Scholar]
- 21.Ruiz-Mesa JD, Marquez-Gomez I, Sena G, et al. Factors associated with severe sepsis or septic shock in complicated pyelonephritis. Medicine (Baltimore) 2017; 96: e8371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xuan W, Zhou Q, Yao S, et al. Mechanical ventilation induces an inflammatory response in preinjured lungs in late phase of sepsis. Oxid Med Cell Longev 2015; 2015: 364020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.West TE, Wikraiphat C, Tandhavanant S, et al. Patient characteristics, management, and predictors of outcome from severe community-onset staphylococcal sepsis in Northeast Thailand: a prospective multicenter study. Am J Trop Med Hyg 2017; 96: 1042–1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wikipedia. Reference ranges for blood tests, https://en.wikipedia.org/wiki/Reference_ranges_for_blood_tests (2019, accessed 10 April 2019).