Abstract
Background:
Maternal obesity is a well-known risk factor for significant obstetric and neonatal complications. The influence of the gastrointestinal microbiome in the setting of maternal obesity during pregnancy is less understood. The purpose of this systematic review is to synthesize the literature on the relationships between maternal obesity and excessive gestational weight gain (EGWG) and the composition of maternal and child gastrointestinal microbiomes.
Method:
We searched CINHAL, OVID Medline, Web of Science, and PubMed for relevant literature using medical subject heading terms related to obesity, pregnancy, and the gastrointestinal microbiome. We assessed 249 articles for potential inclusion using the preferred reporting items for systematic review and meta-analyses framework and deemed 11 articles as relevant for this review.
Results:
Maternal obesity was associated with significant microbial changes in both maternal and infant fecal microbiome biospecimens including increases in Bacteroidetes, Firmicutes, and the Actinobacteria phyla and decreases in Bifidobacteria. However, inconsistencies in uniform taxonomic results across all studies mean that evidence of specific microbial associations with obesity and EGWG is inconclusive.
Conclusion:
Our findings suggest that both maternal and child gastrointestinal microbiome composition is altered in the setting of maternal obesity and EGWG during pregnancy. Future microbiome studies should concentrate on the investigation of metagenomic sequencing to elucidate microbial function rather than solely taxonomic composition. More diverse populations of mothers should be sampled to address health disparities and adverse outcomes of underrepresented populations. Finally, analytic pipelines should be standardized across studies to aid in reproducibility.
Keywords: microbiome, obesity, pregnancy, maternal–child health, gestational weight gain
In 2007, the National Institutes of Health (NIH) allocated over US$150 million to pursue research on the role of the microbiota in healthy and pathophysiological states (Proctor, 2016; Stulberg et al., 2016). Microbiota refers to all the microorganisms living on or within the human body. The human gastrointestinal tract is largely inhabited by microbes, including viruses, fungi, and bacteria, that are integral to daily functioning (Human Microbiome Project Consortium, 2012). Microbes present in the gastrointestinal tract and other body tissues such as the skin, vagina, and oral mucosa facilitate important biological processes embedded within our metabolic, endocrine, immune, and nervous systems (D’Argenio & Salvatore, 2015; Heintz-Buschart & Wilmes, 2018). Research on the microbiome has proliferated to include a wide range of health disorders including cancer (Goodman & Gardner, 2018), cardiovascular disease (Tang & Hazen, 2017), inflammatory bowel disease (Lane, Zisman, & Suskind, 2017), and mental health conditions such as anxiety and depression (Foster, Rinaman, & Cryan, 2017).
Considering the rising prevalence of obesity in the United States (Hales, Carroll, Fryar, & Ogden, 2017), research in humans has focused on how the stress of obesity influences the gastrointestinal microbiota. Obesity in adult, nonpregnant populations decreases microbial α diversity, the species diversity in an individual’s gastrointestinal tract, leading to low-grade inflammation, which can further exacerbate microbial dysregulation (Mosca, Leclerc, & Hugot, 2016). In addition, obesity is associated with increases in β diversity, the diversity of species between individuals. A recently published systematic review on nonpregnant women reported a significant increase in bacterial species from the Firmicutes phylum in obese individuals compared to a larger proportion of bacteria in the Bacteroidetes phylum in lean individuals, illustrating specific differences in gastrointestinal microbiota between obese and lean phenotypes (Castaner et al., 2018). In contrast, Koliada et al. (2017) found inverse relationships between both Firmicutes and Bacteroidetes and increasing body mass index (BMI), illustrating the divergent results of taxonomy-based studies.
Maternal obesity is a well-documented risk factor for obstetric complications and increased risk of subsequent chronic disease in offspring (Monasta et al., 2010; Patel et al., 2012; Ruager-Martin, Hyde, & Modi, 2010; Van Lieshout, Taylor, & Boyle, 2011). The American College of Obstetricians and Gynecologists (2013) defines obesity during pregnancy as a BMI at the first prenatal appointment equal to or above 30 kg/m2. Recommendations from the Institute of Medicine in 2009 state that women with a BMI ≥ 30 kg/m2 should gain between 11 and 20 pounds between the time of conception and the onset of labor (Gilmore & Redman, 2015). Excessive gestational weight gain (EGWG) is defined as gaining more than recommended by health standards during pregnancy (Rasmussen et al., 2010). Given the rising prevalence of maternal obesity and growing evidence implicating the role of the microbiome in obesity in nonpregnant adults, a review of the currently published literature investigating maternal obesity and the gastrointestinal microbiome throughout pregnancy and the postpartum period is necessary.
The central objective of the present review is to synthesize the literature on the relationship between maternal obesity and EGWG and the composition of maternal and child gastrointestinal microbiota. More specifically, we extracted, assessed, and discuss below the maternal and child fecal microbiota composition associated with (1) maternal obesity and (2) EGWG during pregnancy.
Method
Eligibility Criteria
We conducted this review using the preferred reporting items for systematic reviews and meta-analyses recommendations and organized it using a web-based consensus tool called Covidence (www.covidence.org). To be eligible for inclusion, studies had to (1) be conducted with human biospecimens, (2) include analysis of maternal or child gastrointestinal microbiome biospecimens (i.e., fecal swab, stool), and (3) include maternal overweight or obesity as a primary variable of interest. Studies were limited to those written in English and published in peer-reviewed journals. Because the microbiome is an emerging topic, we did not limit the search criteria to a specific date range.
Literature Search Strategy
We used several strategies for the literature search including electronic searches, ancestral searches, and hand searches of journals known to focus on content related to maternal–child health. For the electronic search, we used the online databases CINHAL, OVID Medline, Web of Science, and PubMed using keywords and medical subject heading (MeSH) terms in three main topic areas: microbiome, obesity, and pregnancy. MeSH terms used in the advanced search included (“microbiota” OR “microbiome”) AND (“obesity” OR “obese”) AND (“pregnancy”). The first author (C.D.) implemented the database search and pulled the relevant citations, abstracts, and full texts into Covidence. After the removal of duplicates between databases, two authors (C.D. and S.P.) assessed abstracts for inclusion criteria. The same two authors conducted a full-text article review for final consensus regarding inclusion.
Data Extraction and Quality Assessment
The two authors (C.D. and S.P.) extracted data from each article and reviewed them through the Covidence system. Because the included articles were not intervention studies, protocols were not available for extraction. Data extracted included PICO statements (population, intervention, comparison/control, outcome), number of participants, biospecimen source and time point of collection, operational definitions for prepregnancy obesity and GWG, and significant microbiota associated with maternal obesity and EGWG. We assessed all of the full-text articles included in this review for customized quality metrics to aid in the interpretation of quasi-experimental and observational studies. These quality assessment criteria focused on study design (i.e., Level I = randomized controlled trial or experimental study, Level II = quasi-experimental, Level III = nonexperimental, Level IV = qualitative) and three categories of quality assessment (A = high, B = good, or C = low quality; Keim-Malpass, Letzkus, & Kennedy, 2015). The risk assessment considers generalizability, sufficiency of the sample size, adequacy of the control group, and consistency with current scientific recommendations for sequencing and analysis. The same two authors (C.D. and S.P.) who evaluated the articles for inclusion also completed the quality assessment.
Results
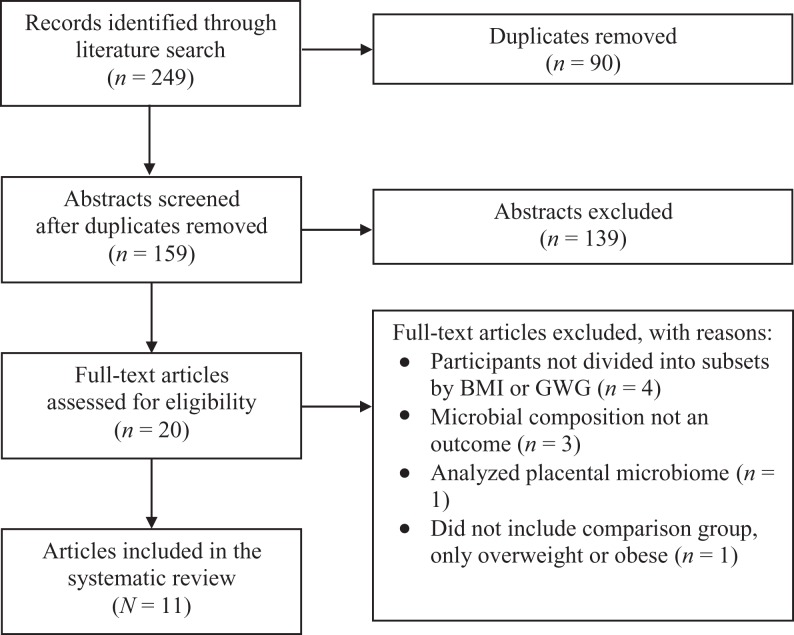
We initially identified 249 records for this systematic review on the interaction between maternal obesity and EGWG and the gastrointestinal microbiome. After removing duplicates and reviewing abstracts and full-text articles for inclusion criteria, we had a final selection of 11 articles to review (see Figure 1).
Figure 1.
Preferred reporting items for systematic reviews and meta-analyses diagram of literature search and inclusion process. BMI = body mass index; GWG = gestational weight gain.
Study Characteristics and Sampling
Table 1 lists data extracted from each study, including author, PICO statement, number of participants and biospecimens, time point of microbiome collection, and the data source (i.e., primary data collection in a cohort study or collection from a specimen biobank or repository). We have categorized studies in the table by population from which biospecimens were collected: five studies collected biospecimens from mothers only (Collado, Isolauri, Laitinen, & Salminen, 2008; Gomez-Arango et al., 2016a; Houttu, Mokkala, & Laitinen, 2017; Santacruz et al., 2010; Smid et al., 2018), four sequenced biospecimens from children only (Collado, Isolauri, Laitinen, & Salminen, 2010; Galley, Bailey, Kamp Dush, Schoppe-Sullivan, & Christian, 2014; Mueller et al., 2016; Robinson et al., 2017), and two included biospecimens from both mothers and their infants (Chu, Antony, et al., 2016; Stanislawski et al., 2017). The average time point for collection of maternal gastrointestinal microbiome biospecimens was 17.2 weeks’ gestation (range = 10−24 weeks’ gestation), while two studies conducted follow-up microbiome collection at 30−39 weeks’ gestation (range = 30−39 weeks; Collado et al., 2008; Smid et al., 2018). Only two of the studies used biospecimens from a common biobank or biorepository (Robinson et al., 2017; Stanislawski et al., 2017). Table 1 further provides operationalization of key variables across each of the included studies. Of the 11 studies, 5 reported how they defined prepregnancy BMI (Collado et al., 2008, 2010; Houttu et al., 2017; Smid et al., 2018; Stanislawski et al., 2017), 4 of which used maternal self-report of weight and height prior to conception as the definition (Collado et al., 2008, 2010; Houttu et al., 2017; Stanislawski et al., 2017).
Table 1.
Study Characteristics.
| Author(s) | PICO Statement | Participants and Comparison Groups | Definition of Prepregnancy BMI and Obesity | Time Point for Microbiome Collection | Sample Sourcea | Quality Assessmentb |
|---|---|---|---|---|---|---|
| Biospecimens collected from mothers only | ||||||
| Collado et al. (2008) | Do BMI and GWG alter the maternal gastrointestinal microbiota before delivery? |
N = 54 women:
|
BMI: Maternal self-report of weight at first clinic
visit Overweight/obesity: BMI > 30 kg/m2 |
First (10–15 weeks’ gestation) and third (30–35 weeks’ gestation) trimesters of pregnancy | Study | IIB |
| Houttu et al. (2017) | Does the degree of overweight alter the composition of the maternal gastrointestinal microbiome during pregnancy? |
N = 99 women
|
BMI: Maternal self-report of weight at first clinic visit Obesity: BMI > 30 kg/m2 |
< 17 weeks’ gestation | Study | IIB |
| Gomez-Arango et al. (2016a, 2016b) | Is there a difference in gut microbiome composition and circulating metabolic hormones between OW and OB pregnant women? |
N = 70 women
|
BMI: not reported Obesity: BMI > 30 kg/m2 |
16 weeks’ gestation | Study | IIB |
| Santacruz et al. (2010) | Do body weight and GWGc alter the composition of the maternal gastrointestinal microbiome during pregnancy? |
N = 50 women
|
BMI: not reported Obesity: BMI > 25 kg/m2 |
24 weeks’ gestation | Study | IIB |
| Smid et al. (2018) | Does EGWG change the maternal gastrointestinal microbiome during pregnancy? |
N = 31 women Groups: Above and below median GWG for this cohort, baseline and follow-up |
BMI: Weight at first clinic visit Obesity: BMI > 30 kg/m2 |
Baseline: < 20 weeks’ gestation Follow-up: 36−39 weeks’ gestation |
Study | IIIB |
| Biospecimens collected from mothers and infants | ||||||
| Chu et al. (2016) | Does maternal obesity alter the neonatal and infant gut microbiome in early life? |
N = 157 women, 157 infants Groups: High fat intake and control |
Not reported | First maternal and infant stool samples at 24−48 hr after delivery and second sample at 4−6 weeks of life | Study | IIB |
| Stanislawski et al. (2017) | Is maternal prepregnancy overweight/obesity associated with differences in the maternal gastrointestinal microbiota at the time of delivery or in the gastrointestinal microbiota of their infants during the first 2 years of life? |
N = 169 women, 181 infants Groups: NW and OW/OB mothers |
BMI: Maternal self-report of weight at first clinic visit Overweight and obesity: BMI ≥ 25 kg/m2 |
Maternal sample 4 days after delivery, newborn samples at 6 time points over the first 2 years of life | Biobank | IIA |
| Biospecimens collected from infants only | ||||||
| Collado et al. (2010) | Do maternal BMI status and EGWG alter the microbiota of their infants? |
N = 42 infants, 26 NW mothers, 16 OW mothers Groups: OW and NW |
BMI: Maternal self-report of weight at first clinic visit Overweight: BMI ≥ 25 kg/m2 |
1 and 6 months of life | Study | IIA |
| Galley et al. (2014) | Is maternal obesity associated with differences in the composition of the gut microbiome in children in early life? |
N = 77 infants, 25 OB mothers, 51 non-OB mothers Groups: OW and OB |
BMI: Not reported Obesity: BMI > 30 kg/m2 |
18–27 months of age | Study | IIB |
| Mueller et al. (2016) | Is maternal prepregnancy BMI associated with differences in the gastrointestinal microbiota between infants delivered vaginally and those delivered via ECS? |
N = 74 neonates
|
BMI: Not reported Overweight and Obesity: > 25 kg/m2 |
2 days after delivery | Study | IIB |
| Robinson et al. (2017) | Is GWGc associated with changes in infant fecal microbiota composition, bacterial-community richness, and Shannon diversity index? |
N = 84 infants Groups: GWG categories |
Not reported | <1 year of age | Biobank | IIB |
Note. Studies are arranged to highlight the population studied. BMI = body mass index; ECS = elective cesarean section; EGWG = excessive gestational weight gain; GWG = gestational weight gain; NW = normal weight; OB = obese; OW = overweight; PICO = population, intervention, comparison/control, outcome; VD = vaginal delivery.
aSamples were either sourced directly from a biobank or from a prospective cohort study. bThe quality assessment score includes level (Level I = randomized controlled trial or experimental study, Level II = quasi-experimental, Level III = nonexperimental, Level IV = qualitative) and quality assessment (A = high, B = good, or C = low quality) which was adapted from Keim-Malpass, Letzkus, and Kennedy (2015). cGWG was defined by the Institute of Medicine guidelines.
Objective 1: Microbiota Alterations Associated With Maternal Prepregnancy Obesity
Table 2 presents the phyla, families, genera, and species (when reported) of the microbiota the reviewed studies found to be significantly impacted by maternal prepregnancy weight status. The nine studies that categorized maternal participants by overweight or obesity status are arranged by populations from which they drew the biospecimens (i.e., maternal, child, or both) to illustrate potential trends across the sampling source. There was little agreement among studies regarding which specific microbes, at any level of taxonomy including phylum, are significantly altered in the setting of maternal obesity.
Table 2.
Significant Microbiota and Their Association With Maternal Obesity.
| Microbe | Biospecimen Sampling and Study | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Infant | Maternal | Both | ||||||||||
| Mueller et al. (2016) a | Collado et al. (2010) | Galley et al. (2014) | Gomez-Arango et al. (2016) | Houttu et al. (2017) | Collado et al. (2008) | Santacruz et al. (2010) | Stanislawski et al. (2017) | Chu et al. (2016) | ||||
| Phylum | Family | Genus | Species | |||||||||
| Actinobacteria | — | — | — | − | + | + | ||||||
| Bifidobacteriaceae | Bifidobacteria | — | − | − | − | |||||||
| Coriobacteriaceae | Collinsella | — | + | |||||||||
| Bacteroidetes | — | — | — | +, −a | −b, +c | + | −d | + | ||||
| Bacteroidaceae | Bacteroides | bacteroides | + | − | − | |||||||
| Prevotellaceae | Provotella | — | − | +c | + | + | ||||||
| Porphyromonadaceae | Parabacteroides | paracteroides sp. | +c | − | ||||||||
| Rikenellaceae | — | — | +d | + | ||||||||
| Firmicutes | Lachnospiraceae | Blautia | blautia sp. | −b | + | |||||||
| — | — | +d | + | |||||||||
| Sporobacteria | WAL 1855D | + | ||||||||||
| Clostridiaceae | Faecalibacteria | — | + | |||||||||
| — | — | − | ||||||||||
| Clostridium | C. histolytica | + | + | |||||||||
| C. leptum | + | |||||||||||
| C. perfringens | − | |||||||||||
| C. coccoides | + | − | − | − | ||||||||
| Christensenellaceae | — | — | − | |||||||||
| Enterobacteriaceae | Enterococcus | — | − | + | ||||||||
| Eubacteriaceae | Eubacteria | — | − | |||||||||
| Peptoniphilaceae | Finegoldia | finegoldia sp. | − | |||||||||
| — | — | — | + | |||||||||
| Oscillospiraceae | Oscillibacter | — | +c | |||||||||
| Ruminococcaceae | — | — | − | |||||||||
| Ruminoccocus | ruminoccocus sp. | − | ||||||||||
| Staphylococcaceae | Staphylococcus | S. aureus | + | + | + | |||||||
| Proteobacteria | Enterobacteriaceae | Escherichia | E. coli | + | ||||||||
| Enterobactor | — | + | ||||||||||
| Hydrogenophilaceae | Hydrogenophilus | — | − | |||||||||
| — | — | — | +a | |||||||||
| Pseudomonadaceae | Psuedomonas | — | − | |||||||||
| Xanthomonadace | — | — | − | |||||||||
| Tenericutes | — | — | — | +e | ||||||||
| Verrucomicrobia | Verrucomicrobiaceae | Akkermansia | akkermansia sp. | + | ||||||||
| A. muciniphila | + | + | ||||||||||
| Microbial diversity | ||||||||||||
| α | +c | − | − | |||||||||
| β | +c | + | ||||||||||
Note. The table visualizes associations between microbe populations and maternal prepregnancy obesity as reported in the reviewed studies that used prepregnancy overweight or obesity as an independent variable. Dashes in the family, genus, or species columns indicate the studies did not report the lower taxonomic categories for that microbe. + indicates a positive association between obesity/overweight and changes to the microbe population. – indicates a negative association between obesity/overweight and changes to the microbe population. A blank cell indicates that the study did not report any significant changes to that microbe population.
a Mueller et al. (2016) also grouped infants by type of delivery. Findings associated with cesarean delivery are indicated with superscript alphabet “a.” All others associated with vaginal delivery. bAssociated with low income. cAssociated with high income. dAssociation with maternal overweight and obesity. eAssociation with overweight group only.
Of the nine studies, three found decreases in Clostridium coccoides in the setting of maternal obesity (Collado et al., 2008; Santacruz et al., 2010; Stanislawski et al., 2017), two found increases in Staphylococcus aureus in obese women prior to pregnancy (Collado et al., 2008; Santacruz et al., 2010), and one found increased S. aureus in the feces of infants at 6 months of life (Collado et al., 2010) in the setting of maternal prepregnancy obesity. Collado, Isolauri, Laitinen, and Salminen (2008) and Stanislawski et al. (2017) found that maternal prepregnancy overweight and obesity reduced α diversity of the maternal gastrointestinal microbiota, while three other studies found no difference in α diversity by maternal prepregnancy weight category (Gomez-Arango et al., 2016b; Houttu et al., 2017; Smid et al., 2018).
Assessing differences in the microbiota that are independently associated with maternal prepregnancy obesity is difficult due to confounding variables such as mode of delivery, antibiotic use, diet, and socioeconomic status, which are known to influence gastrointestinal microbiota composition (Mueller, Bakacs, Combellick, Grigoryan, & Dominguez-Bello, 2015). For example, Mueller et al. (2016) reported that bacteria in the Bacteroidetes phylum were enriched after vaginal delivery and decreased after cesarean delivery in infants whose mothers were overweight or obese. Galley, Bailey, Kamp Dush, Schoppe-Sullivan, and Christian (2014) found that levels of organisms in Bacteroidetes, including those in the genera Prevotella and Parabacteroides, were increased in the infants of high-income obese women and decreased, at the phylum level, in low-income women. Prepregnancy obesity may also confound analysis of the composition of microbiota associated with obesity acquired during gestation, as two studies found that mothers with prepregnancy obesity had increased abundance of organisms from the Prevotella genus and no difference in α diversity compared to women with prepregnancy overweight (Collado et al., 2008; Houttu et al., 2017).
Objective 2: Microbiota Alterations Associated With EGWG
Of the five studies that assessed the impact of EGWG on the gastrointestinal microbiome (Table 3), three characterized maternal biospecimens (Collado et al., 2008; Santacruz et al., 2010; Smid et al., 2018), one characterized child biospecimens (Robinson et al., 2017), and one included both maternal and child biospecimens (Collado et al., 2010). Studies reported positive associations between EGWG and abundance of Clostridium histolyticum, Bacteroidetes (phylum), Enterobacter (genus), E. coli, and Escherichia sp. and negative associations between EGWG and abundance of Akkermansia muciniphila and Bacteroides bacteroides. However, there are inconsistencies in the directionality of association across studies for organisms in the Prevotella genus, with Collado et al. (2008) finding a positive association with EGWG and Collado, Isolauri, Laitinen, and Salminen (2010) reporting a negative association. Likewise, Collado et al. (2008) and Santacruz et al. (2010) found a negative association between EGWG and abundance of organisms in the Bifidobacteria genus in maternal biospecimens during pregnancy, while Robinson et al. (2017) reported a positive association in infants younger than 1 year of life, demonstrating a potential divergent relationship between the maternal and infant microbiomes.
Table 3.
Significant Microbiota and Their Association With Excessive Gestational Weight Gain.
| Microbe | Biospecimen Sampling and Study | |||||||
|---|---|---|---|---|---|---|---|---|
| Maternal | Both | Infant | ||||||
| Collado et al. (2008) | Santacruz et al. (2010) | Smid et al. (2018) | Collado et al. (2010) | Robinson et al. (2017) | ||||
| Phylum | Family | Genus | Species | |||||
| Bacteroidetes | Prevotellaceae | Provotella | — | + | − | |||
| Bacteroidaceae | Bacteroides | bacteroides | − | |||||
| — | — | — | + | + | ||||
| Actinobacteria | Bifidobacteriaceae | Bifidobacteria | — | − | − | + | ||
| Firmicutes | Clostridiaceae | Clostridium | C. histolyticum | + | + | |||
| Proteobacteria | Enterobacteriaceae | Enterobactor | — | + | + | |||
| Escherichia | E. coli | + | ||||||
| escherichia sp. | + | |||||||
| Verrucomicrobia | Verrucomicrobiaceae | Akkermansia | A. muciniphila | − | ||||
| Microbial diversity | ||||||||
| α | + | − | ||||||
| β | ||||||||
Note. The table visualizes associations between microbe populations and excessive gestational weight gain (EGWG) as reported in the reviewed studies that used EGWG as an independent variable. Dashes in the family, genus, and species columns indicate the studies did not report the lower taxonomic categories for that microbe. + indicates a positive association between obesity/overweight and changes to the microbe population. – indicates a negative association between obesity/overweight and changes to the microbe population. A blank cell indicates that the study did not report any significant changes to that microbe population.
Discussion
Research on the association between the composition and diversity of gastrointestinal microbiota and obesity in humans has been conducted largely outside of pregnancy. In the present systematic review, we examine the state of the science exploring the effects of maternal obesity and EGWG on maternal and infant microbiota during this unique time period. Overall, the studies found that the compositional diversity of the microbiota and relative abundances of specific microbes are significantly altered in maternal prepregnancy obesity and EGWG compared to normal weight and adequate GWG. However, results may be confounded by variables such as antibiotic use and delivery method, which are known to influence microbiota composition and could result in inconsistencies in the directionality of the association between maternal obesity and/or EGWG and compositional changes in the microbiota.
Our synthesis of these articles indicates that, despite the lack of differences in macronutrient consumption among weight groups (i.e., obese, overweight, normal weight; Gomez-Arango et al., 2016a, 2016b; Houttu et al., 2017; Santacruz et al., 2010), there are differences in reported maternal and child microbiomes. Although the differences in microbiome composition among these weight groups remain unclear, patterns in both maternal and child microbiome composition suggest that the relationships among GWG, composition of the microbiome, and host metabolism during pregnancy may have significant functional impacts. For example, two of the reviewed studies demonstrated that the microbiota may be influencing insulin resistance and glucose homeostasis in mothers (Gomez-Arango et al., 2016b; Houttu et al., 2017), suggesting that bacterial functional differences may contribute to obesogenic changes in maternal metabolism. Thus, bacterial taxonomy may be less important than functional genetic components common to several bacterial species that may be driving changes in maternal metabolism during gestation.
Modulation of Maternal Obesity by the Microbiome
While the articles included in this review examined the influence of maternal obesity and GWG on microbiota composition, conversely, some researchers have argued that the microbiome could be a key mechanism in the development of maternal and child obesity risk (Ley et al., 2005; Mohammadkhah, Simpson, Patterson, & Ferguson, 2018; Suez, Shapiro, & Elinav, 2016). Experimental evidence for the influence of the gastrointestinal microbiome on the development of obesity comes from animal studies comparing germ-free mice to conventional mice reared in a pathogen-free environment (Bäckhed, Manchester, Semenkovich, & Gordon, 2007). In a landmark study by Bäckhed et al. (2004), the distal gut microbiota from pathogen-free mice were transplanted into germ-free mice, resulting in a 60% increase in body fat within 2 weeks. More importantly, there was no increase in calorie consumption or changes in energy expenditure, suggesting that the gastrointestinal microbiota affects phenotypic characteristics related to obesity of the host (Bäckhed et al., 2004). In addition, several studies in both human and animal models have implicated microbial composition as an important modulator for obesity, noting, for instance, reduced microbial diversity in obese, nonpregnant women (Ley et al., 2005; Suez et al., 2016).
Inflammation and Metabolic Syndrome
Gastrointestinal microbiota contribute to the regulation of energy homeostasis; thus, disruption in these microbiota can cause metabolic diseases (Smith et al., 2011). First, increased intestinal permeability (called leaky gut syndrome) and dysbiosis of the microbiota drive chronic and widespread inflammation in the tissue (Turner, Nedjai, Hurst, & Pennington, 2014). This inflammatory process stimulates the activation of macrophages, which are essential components of protective immunity (Ang & Ding, 2016; Turner et al., 2014). The largest population of macrophages is located in the intestines (Ang & Ding, 2016). In noninflamed intestinal tissue, macrophages kill predatory microbes without any inflammatory response (den Besten et al., 2013). However, in the presence of dysbiosis of the microbiota, the macrophages attract inflammatory immune cells (typically monocytes derived from the innate immune system) that secrete interleukin-8 and transforming growth factor-β (den Besten et al., 2013; Turner et al., 2014). Secreted chemokines and cytokines perpetuate the inflammatory process, resulting in immune cell infiltration of adipose tissue and immune exhaustion leading to lower resistance to infection (Sze & Schloss, 2016). Future research should address the role of the gastrointestinal microbiota and the immune system in the progression of obesity as well as insulin resistance, impaired glucose tolerance, and the resulting cardiovascular disease risk.
Research has not yet fully deconvoluted the complex interplay among obesity, weight gain, and metabolic syndrome during childbearing years and pregnancy. Rather than obesity alone, it is a combination of the effects of maternal behaviors (e.g., diet, exercise), biological factors (e.g., microbiota composition and function, epigenetics), and the environment (e.g., food security, structural inequality) that likely influence metabolic outcomes.
Fetal Programming and Transgenerational Obesity
The investigation into the gastrointestinal microbiota’s influence on maternal obesity is important due to the known effects of obesity on fetal programming (Neri & Edlow, 2015). Research examining fetal programming provides compelling evidence about how early in utero development may predispose a child to chronic metabolic concerns later in life (Kwon & Kim, 2017; Rinaudo & Wang, 2012). The critical period in fetal development coincides with rapid cell differentiation and proliferation (Widdowson & McCance, 1975). The maternal microbial milieu has the potential to influence maternal obesity, thereby increasing birth weight, which could lead to chronic cardiometabolic health concerns such as childhood obesity, hypertension, renal disease, and diabetes (Rinaudo & Wang, 2012). Research suggests that one of the impacts of maternal obesity and EGWG is an increase in facultative anaerobes, such as Proteobacteria, Actinobacteria, in the child’s gastrointestinal microbiome that delay maturation of the microbiota (Chong, Bloomfield, & O’Sullivan, 2018). Facultative anaerobes, normal early colonizers in the neonatal gut, consume oxygen and facilitate the establishment of ubiquitous microbiota such as Bifidobacteria and Bacteroides (Chong et al., 2018). Initiation of the colonization of Bifidobacteria and Bacteroides shows the adaptive colonization that occurs as the immune system and microbiota mature (Chong et al., 2018). In the setting of maternal obesity, maturation of microbiota, which normally occurs over the first 2 years of childhood, may be prevented or delayed as facultative anaerobes fail to give way to the strict anaerobes normally inhabiting the gastrointestinal compartment of adults and older children (Rodríguez et al., 2015).
Various studies have shown a difference in gastrointestinal microbiota composition between obese and normal-weight children (Bervoets et al., 2013; Ignacio et al., 2016; White et al., 2013). Ignacio et al. (2016) found significantly higher abundances of Bifidobacteria in lean compared to overweight children. Research has shown that Bifidobacteria have immune system benefits including host protection against pathogens (O’Callaghan & van Sinderen, 2016). Using a novel type of time-series analysis, White et al. (2013) detected significant associations between specific gastrointestinal microbiota and child growth trajectory measured by child weight-for-age Z-scores. However, the microbiome is only one potential determinant of childhood obesity. Other factors, both modifiable and unmodifiable, include genotype and environmental factors (Siega-Riz, Siega-Riz, & Laraia, 2006).
16S Ribosomal RNA (16S rRNA) Gene Sequencing and Bioinformatics Analysis
Studying the microbiome composition requires the extraction of DNA from the biospecimen, often from a fecal sample or swabbing of the area of interest. The most common method for classifying microbial taxonomy and phylogeny is 16S rRNA gene sequencing (Janda & Abbott, 2007), which uses short strands of DNA called primers that are designed to target specific variable regions of the 16s rRNA gene. The 16s rRNA gene is highly conserved, or passed through generations, and acts as a microbe-specific genetic signature. These signatures allow researchers to characterize the abundance of specific microbes and the overall phylogenetic diversity of the microbiota present in a biospecimen. In eight of the reviewed studies (Chu, Meyer, Prince, & Aagaard, 2016; Galley et al., 2014; Gomez-Arango et al., 2016b; Houttu et al., 2017; Mueller et al., 2016; Robinson et al., 2017; Smid et al., 2018; Stanislawski et al., 2017), investigators used 16S rRNA gene sequencing across two different platforms (i.e., Illumnia and Ion Torrent) to characterize the microbiota. Limitations of 16S rRNA sequencing include low resolution and sensitivity of taxonomic identity compared to shotgun metagenomic sequencing, which comprehensively samples genes from the entire microbial genome, resulting in information about which microorganisms are present and their functional role in the community (Poretsky, Rodriguez-R, Luo, Tsementzi, & Konstantinidis, 2014). For studies focused on complex phenotypes, such as obesity, information about both microbial composition and function is needed to enhance scientific understanding.
Variations across the reviewed studies regarding the microbiota reported to be associated with maternal obesity or EGWG could be due, at least in part, to variations in the bioinformatics pipelines (i.e., Quantitative Insights Into Microbial Ecology [QIIME], mothur, MetaGenome Rapid Annotation using Subsystem Technology), reference genomes (i.e., SILVA, GreenGenes), and software (i.e., statistical software, SPSS, R) investigators used to guide analyses of the biospecimens. Inconsistencies across studies, therefore, could be due to bioinformatic limitations with paired-end sequencing reads with reference genomes resulting in unclassified microbiota or only broad, phylum-level classification. In all of the studies except for four (Collado et al., 2008, 2010; Robinson et al., 2017; Santacruz et al., 2010), authors reported using QIIME, big data, open-source software built for microbiome analysis from raw FASTQ sequencing data on Illumina platforms (Caporaso et al., 2010). Just under half of the studies (5/11) reported the reference database they used and in all five it was GreenGenes (Galley et al., 2014; Gomez-Arango et al., 2016b; Mueller et al., 2016; Smid et al., 2018; Stanislawski et al., 2017). Authors should always report these methodological pipelines and associated code to increase the transparency of results and contribute to the larger body of knowledge in obesity and microbiome research (Kelsey, Dreisbach, Alhusen, & Grossmann, 2019). Most of the studies used either SPSS or R for their analysis, but two studies (Mueller et al., 2016; Smid et al., 2018) did not report this information. In conducting future studies, investigators should consider measures to increase reproducibility including making data available for public use, publishing in open science journals, and using processing and analysis code that is freely available.
Limitations
Importantly, there are several limitations across the studies included in this review that restrict our ability to draw conclusions about associations between maternal obesity and EGWG and maternal and infant microbiota. First, the studies were not consistent in the composition of their comparison groups. While most of the studies used the categories of both maternal overweight (25−29.9 kg/m2) and obesity (above 30 kg/m2), two grouped obesity and overweight together into one comparison group with a BMI above 25 kg/m2 (Gomez-Arango et al., 2016b; Houttu et al., 2017), making comparisons across studies difficult. The literature has shown that the risk of adverse pregnancy and neonatal outcomes is higher among obese mothers compared to overweight mothers; thus, this difference in classification is an important distinction (Gilmore & Redman, 2015; Siega-Riz et al., 2009). In addition, several studies did not include a normal-weight control group (Gomez-Arango et al., 2016a, 2016b; Houttu et al., 2017). On the other hand, all of the studies that examined GWG used the recently revised IOM guidelines, making comparisons among studies easier to conduct (Collado et al., 2008, 2010; Robinson et al., 2017; Santacruz et al., 2010; Smid et al., 2018). Second, most of the reviewed studies did not fully describe the baseline demographic characteristics of participants. For the studies in which investigators did describe these characteristics, most biospecimens were from participants of Scandinavian descent. Given the reported disparities in risks associated with prepregnancy and parity-related obesity for African American and Hispanic populations and the continued ethnic and racial disparities in maternal morbidity and mortality (Harper, Dugan, Espeland, Martinez-Borges, & Mcquellon, 2007), researchers should attempt to recruit participant samples that are racially and ethnically representative of the study setting’s population and should also acknowledge the racial and ethnic bias inherent in their samples.
Limitations of our systematic review process include the following: (1) we only included published data, (2) our original search criteria did not include gestational weight gain as a search term, (3) Covidence does not allow for further delineation of database imports, that is, the database from which we drew each study is not identified, and (4) our ability to make comparisons across studies was limited because not all collected both maternal and child biospecimens.
Nursing Implications
The impact of the gastrointestinal microbiome on health, particularly during pregnancy, is not fully understood. Nurses need to be competent in the biological underpinnings of microbiology in order to understand its relation to direct patient care. The emergence of new frontiers in personalized nutrition, obesity management, fecal transplants, and direct-to-consumer microbiome sequencing means that nurses will be at the front line of educating patients and their families. More research is required on prebiotics and probiotics before recommendations for specific therapies for influencing the maternal microbiota can be made.
Conclusion
In this systematic review, we synthesized data from 11 articles on the interaction between maternal obesity and/or EGWG and the maternal and infant microbiome. We found evidence that the composition of both maternal and child gastrointestinal microbiomes is associated with maternal obesity, but the directionality, mechanisms, and significant microbes have yet to be fully determined. Further research using advanced methods, such as shotgun metagenomic sequencing, should be conducted to discern the functional relevance of the microbiota in association with metabolism during gestation. Current research is largely focused on the characterization and taxonomic composition of microbial communities. An important step in this area of research would be an enhanced understanding of the cardiometabolic and immune outcomes associated with dysbiosis. Future research in this area should implement standardized approaches to biospecimen collection, sequencing platforms, and analytic methods, which will more easily allow for the interpretation of findings in terms of clinical relevance.
Footnotes
Author Contributions: Caitlin Dreisbach contributed to conception, design, acquisition, analysis, and interpretation; drafted the article; critically revised the article; gave final approval; and agreed to be accountable for all aspects of work ensuring integrity and accuracy. Stephanie Prescott contributed to acquisition, analysis, and interpretation; critically revised the article; gave final approval; and agreed to be accountable for all aspects of work ensuring integrity and accuracy. Jeanne Alhusen contributed to conception and design; critically revised the article; gave final approval; and agreed to be accountable for all aspects of work ensuring integrity and accuracy.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work is funded by CD’s National Research Service Award through the National Institute for Nursing Research (F31NR017821) and an Association for Women’s Health, Obstetric, and Neonatal Nurses 2018 March of Dimes Margaret Comerford Freda “Saving Babies, Together®” Award.
ORCID iD: Caitlin Dreisbach  https://orcid.org/0000-0003-3964-3161
https://orcid.org/0000-0003-3964-3161
References
- American College of Obstetricians and Gynecologists. (2013). ACOG committee opinion no. 549: Obesity in pregnancy. Obstetrics and Gynecology, 121, 213–217. doi:10.1097/01.AOG.0000425667.10377.60 [DOI] [PubMed] [Google Scholar]
- Ang Z., Ding J. L. (2016). GPR41 and GPR43 in obesity and inflammation—Protective or causative? Frontiers in Immunology, 7, 28 doi:10.3389/fimmu.2016.00028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bäckhed F., Ding H., Wang T., Hooper L. V., Koh G. Y., Nagy A.…Gordon J. I. (2004). The gut microbiota as an environmental factor that regulates fat storage. Proceedings of the National Academy of Sciences of the United States of America, 101, 15718–15723. doi:10.1073/pnas.0407076101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bäckhed F., Manchester J. K., Semenkovich C. F., Gordon J. I. (2007). Mechanisms underlying the resistance to diet-induced obesity in germ-free mice. Proceedings of the National Academy of Sciences of the United States of America, 104, 979–984. doi:10.1073/pnas.0605374104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bervoets L., Van Hoorenbeeck K., Kortleven I., Van Noten C., Hens N., Vael C.…Vankerckhoven V. (2013). Differences in gut microbiota composition between obese and lean children: A cross-sectional study. Gut Pathogens, 5, 10 doi:10.1186/1757-4749-5-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caporaso J. G., Kuczynski J., Stombaugh J., Bittinger K., Bushman F. D., Costello E. K.…Knight R. (2010). QIIME allows analysis of high-throughput community sequencing data. Nature Methods, 7, 335–336. doi:10.1038/nmeth.f.303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castaner O., Goday A., Park Y.-M., Lee S.-H., Magkos F., Shiow S.-A. T. E., Schröder H. (2018). The gut microbiome profile in obesity: A systematic review. International Journal of Endocrinology, 2018, 4095789 doi:10.1155/2018/4095789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chong C. Y. L., Bloomfield F. H., O’Sullivan J. M. (2018). Factors affecting gastrointestinal microbiome development in neonates. Nutrients, 10, E274 doi:10.3390/nu10030274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu D. M., Antony K. M., Ma J., Prince A. L., Showalter L., Moller M., Aagaard K. M. (2016). The early infant gut microbiome varies in association with a maternal high-fat diet. Genome Medicine, 8, 77 doi:10.1186/s13073-016-0330-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu D. M., Meyer K. M., Prince A. L., Aagaard K. M. (2016). Impact of maternal nutrition in pregnancy and lactation on offspring gut microbial composition and function. Gut Microbes, 7, 459–470. doi:10.1080/19490976.2016.1241357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collado M. C., Isolauri E., Laitinen K., Salminen S. (2008). Distinct composition of gut microbiota during pregnancy in overweight and normal-weight women. The American Journal of Clinical Nutrition, 88, 894–899. doi:10.1093/ajcn/88.4.894 [DOI] [PubMed] [Google Scholar]
- Collado M. C., Isolauri E., Laitinen K., Salminen S. (2010). Effect of mother’s weight on infant’s microbiota acquisition, composition, and activity during early infancy: A prospective follow-up study initiated in early pregnancy. The American Journal of Clinical Nutrition, 92, 1023–1030. doi:10.3945/ajcn.2010.29877 [DOI] [PubMed] [Google Scholar]
- D’Argenio V., Salvatore F. (2015). The role of the gut microbiome in the healthy adult status. Clinica Chimica Acta, 451, 97–102. doi:10.1016/j.cca.2015.01.003 [DOI] [PubMed] [Google Scholar]
- den Besten G., van Eunen K., Groen A. K., Venema K., Reijngoud D.-J., Bakker B. M. (2013). The role of short-chain fatty acids in the interplay between diet, gut microbiota, and host energy metabolism. Journal of Lipid Research, 54, 2325–2340. doi:10.1194/jlr.R036012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster J. A., Rinaman L., Cryan J. F. (2017). Stress & the gut-brain axis: Regulation by the microbiome. Neurobiology of Stress, 7, 124–136. doi:10.1016/j.ynstr.2017.03.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galley J. D., Bailey M., Kamp Dush C., Schoppe-Sullivan S., Christian L. M. (2014). Maternal obesity is associated with alterations in the gut microbiome in toddlers. PLoS One, 9, e113026 doi:10.1371/journal.pone.0113026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilmore L. A., Redman L. M. (2015). Weight gain in pregnancy and application of the 2009 IOM guidelines: Toward a uniform approach. Obesity, 23, 507–511. doi:10.1002/oby.20951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez-Arango L. F., Barrett H. L., McIntyre H. D., Callaway L. K., Morrison M., Dekker Nitert M., & SPRING Trial Group. (2016. a). Connections between the gut microbiome and metabolic hormones in early pregnancy in overweight and obese women. Diabetes, 65, 2214–2223. doi:10.2337/db16-0278 [DOI] [PubMed] [Google Scholar]
- Gomez-Arango L. F., Barrett H. L., McIntyre H. D., Callaway L. K., Morrison M., Dekker Nitert M, & SPRING Trial Group. (2016. b). Increased systolic and diastolic blood pressure is associated with altered gut microbiota composition and butyrate production in early pregnancy. Hypertension, 68, 974–981. doi:10.1161/HYPERTENSIONAHA.116.07910 [DOI] [PubMed] [Google Scholar]
- Goodman B., Gardner H. (2018). The microbiome and cancer. The Journal of Pathology, 244, 667–676. doi:10.1002/path.5047 [DOI] [PubMed] [Google Scholar]
- Hales C. M., Carroll M. D., Fryar C. D., Ogden C. L. (2017). Prevalence of obesity among adults and youth: United States, 2015–2016. NCHS Data Brief, 1–8. [PubMed] [Google Scholar]
- Harper M., Dugan E., Espeland M., Martinez-Borges A., Mcquellon C. (2007). Why African-American women are at greater risk for pregnancy-related death. Annals of Epidemiology, 17, 180–185. doi:10.1016/j.annepidem.2006.10.004 [DOI] [PubMed] [Google Scholar]
- Heintz-Buschart A., Wilmes P. (2018). Human gut microbiome: Function matters. Trends in Microbiology, 26, 563–574. doi:10.1016/j.tim.2017.11.002 [DOI] [PubMed] [Google Scholar]
- Houttu N., Mokkala K., Laitinen K. (2017). Overweight and obesity status in pregnant women are related to intestinal microbiota and serum metabolic and inflammatory profiles. Clinical Nutrition, 37, 1955–1966. doi:10.1016/j.clnu.2017.12.013 [DOI] [PubMed] [Google Scholar]
- Human Microbiome Project Consortium. (2012). Structure, function and diversity of the healthy human microbiome. Nature, 486, 207–214. doi:10.1038/nature11234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ignacio A., Fernandes M. R., Rodrigues V. A. A., Groppo F. C., Cardoso A. L., Avila-Campos M. J., Nakano V. (2016). Correlation between body mass index and faecal microbiota from children. Clinical Microbiology and Infection, 22, 258.e1–8 doi:10.1016/j.cmi.2015.10.031 [DOI] [PubMed] [Google Scholar]
- Janda J. M., Abbott S. L. (2007). 16S rRNA gene sequencing for bacterial identification in the diagnostic laboratory: Pluses, perils, and pitfalls. Journal of Clinical Microbiology, 45, 2761–2764. doi:10.1128/JCM.01228-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keim-Malpass J., Letzkus L. C., Kennedy C. (2015). Parent/caregiver health literacy among children with special health care needs: A systematic review of the literature. BMC Pediatrics, 15, 92 doi:10.1186/s12887-015-0412-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelsey C., Dreisbach C., Alhusen J., Grossmann T. (2019). A primer on investigating the role of the microbiome in brain and cognitive development. Developmental Psychobiology, 61, 341–349. doi:10.1002/dev.21778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koliada A., Syzenko G., Moseiko V., Budovska L., Puchkov K., Perederiy V.…Vaiserman A. (2017). Association between body mass index and Firmicutes/Bacteroidetes ratio in an adult Ukrainian population. BMC Microbiology, 17, 120 doi:10.1186/s12866-017-1027 -1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon E. J., Kim Y. J. (2017). What is fetal programming? A lifetime health is under the control of in utero health. Obstetrics & Gynecology Science, 60, 506–519. doi:10.5468/ogs.2017.60.6.506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane E. R., Zisman T. L., Suskind D. L. (2017). The microbiota in inflammatory bowel disease: Current and therapeutic insights. Journal of Inflammation Research, 10, 63–73. doi:10.2147/JIR.S116088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ley R. E., Bäckhed F., Turnbaugh P., Lozupone C. A., Knight R. D., Gordon J. I. (2005). Obesity alters gut microbial ecology. Proceedings of the National Academy of Sciences of the United States of America, 102, 11070–11075. doi:10.1073/pnas.0504978102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohammadkhah A. I., Simpson E. B., Patterson S. G., Ferguson J. F. (2018). Development of the gut microbiome in children, and lifetime implications for obesity and cardiometabolic disease. Children (Basel, Switzerland), 5, 160 doi:10.3390/children5120160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monasta L., Batty G. D., Cattaneo A., Lutje V., Ronfani L., Van Lenthe F. J., Brug J. (2010). Early-life determinants of overweight and obesity: A review of systematic reviews. Obesity Reviews, 11, 695–708. doi:10.1111/j.1467-789X.2010.00735.x [DOI] [PubMed] [Google Scholar]
- Mosca A., Leclerc M., Hugot J. P. (2016). Gut microbiota diversity and human diseases: Should we reintroduce key predators in our ecosystem? Frontiers in Microbiology, 7, 455 doi:10.3389/fmicb.2016.00455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller N. T., Bakacs E., Combellick J., Grigoryan Z., Dominguez-Bello M. G. (2015). The infant microbiome development: Mom matters. Trends in Molecular Medicine, 21, 109–117. doi:10.1016/j.molmed.2014.12.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller N. T., Shin H., Pizoni A., Werlang I. C., Matte U., Goldani M. Z.…Dominguez-Bello M. G. (2016). Birth mode-dependent association between pre-pregnancy maternal weight status and the neonatal intestinal microbiome. Scientific Reports, 6, 23133 doi:10.1038/srep23133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neri C., Edlow A. G. (2015). Effects of maternal obesity on fetal programming: Molecular approaches. Cold Spring Harbor Perspectives in Medicine, 6, a026591 doi:10.1101/cshperspect.a026591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Callaghan A., van Sinderen D. (2016). Bifidobacteria and their role as members of the human gut microbiota. Frontiers in Microbiology, 7, 925 doi:10.3389/fmicb.2016.00925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel S. P., Rodriguez A., Little M. P., Elliott P., Pekkanen J., Hartikainen A.-L.…Järvelin M.-R. (2012). Associations between pre-pregnancy obesity and asthma symptoms in adolescents. Journal of Epidemiology and Community Health, 66, 809–814. doi:10.1136/jech.2011.133777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poretsky R., Rodriguez-R L. M, Luo C., Tsementzi D., Konstantinidis K. T. (2014). Strengths and limitations of 16S rRNA gene amplicon sequencing in revealing temporal microbial community dynamics. PLoS One, 9, e93827 doi:10.1371/journal.pone.0093827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proctor L. M. (2016). The national institutes of health human microbiome project. Seminars in Fetal & Neonatal Medicine, 21, 368–372. doi:10.1016/j.siny.2016.05.002 [DOI] [PubMed] [Google Scholar]
- Rasmussen K. M., Abrams B., Bodnar L. M., Butte N. F., Catalano P. M., Maria Siega-Riz A. (2010). Recommendations for weight gain during pregnancy in the context of the obesity epidemic. Obstetrics and Gynecology, 116, 1191–1195. doi:10.1097/AOG.0b013e3181f60da7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinaudo P., Wang E. (2012). Fetal programming and metabolic syndrome. Annual Review of Physiology, 74, 107–130. doi:10.1146/annurev-physiol-020911-153245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson A., Fiechtner L., Roche B., Ajami N. J., Petrosino J. F., Camargo C. A.…Hasegawa K. (2017). Association of maternal gestational weight gain with the infant fecal microbiota. Journal of Pediatric Gastroenterology and Nutrition, 65, 509–515. doi:10.1097/MPG.0000000000001566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodríguez J. M., Murphy K., Stanton C., Ross R. P., Kober O. I., Juge N.…Collado M. C. (2015). The composition of the gut microbiota throughout life, with an emphasis on early life. Microbial Ecology in Health and Disease, 26, 26050 doi:10.3402/mehd.v26.26050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruager-Martin R., Hyde M. J., Modi N. (2010). Maternal obesity and infant outcomes. Early Human Development, 86, 715–722. doi:10.1016/j.earlhumdev.2010.08.007 [DOI] [PubMed] [Google Scholar]
- Santacruz A., Collado M. C., García-Valdés L., Segura M. T., Martín-Lagos J. A., Anjos T.…Sanz Y. (2010). Gut microbiota composition is associated with body weight, weight gain and biochemical parameters in pregnant women. The British Journal of Nutrition, 104, 83–92. doi:10.1017/S0007114510000176 [DOI] [PubMed] [Google Scholar]
- Siega-Riz A. M., Viswanathan M., Moos M.-K., Deierlein A., Mumford S., Knaack J.…Lohr K. N. (2009). A systematic review of outcomes of maternal weight gain according to the Institute of Medicine recommendations: Birthweight, fetal growth, and postpartum weight retention. American Journal of Obstetrics and Gynecology, 201, 339.e1–14 doi:10.1016/j.ajog.2009.07.002 [DOI] [PubMed] [Google Scholar]
- Siega-Riz A.-M., Siega-Riz A.-M., Laraia B. (2006). The implications of maternal overweight and obesity on the course of pregnancy and birth outcomes. Maternal and Child Health Journal, 10, S153–6. doi:10.1007/s10995-006-0115-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smid M. C., Ricks N. M., Panzer A., Mccoy A. N., Azcarate-Peril M. A., Keku T. O., Boggess K. A. (2018). Maternal gut microbiome biodiversity in pregnancy. American Journal of Perinatology, 35, 24–30. doi:10.1055/s-0037-1604412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith P. D., Smythies L. E., Shen R., Greenwell-Wild T., Gliozzi M., Wahl S. M. (2011). Intestinal macrophages and response to microbial encroachment. Mucosal Immunology, 4, 31–42. doi:10.1038/mi.2010.66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanislawski M. A., Dabelea D., Wagner B. D., Sontag M. K., Lozupone C. A., Eggesbø M. (2017). Pre-pregnancy weight, gestational weight gain, and the gut microbiota of mothers and their infants. Microbiome, 5, 113 doi:10.1186/s40168-017-0332-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stulberg E., Fravel D., Proctor L. M., Murray D. M., LoTempio J., Chrisey L.…Records A. (2016). An assessment of U.S. microbiome research. Nature Microbiology, 1, 15015 doi:10.1038/nmicrobiol.2015.15 [DOI] [PubMed] [Google Scholar]
- Suez J., Shapiro H., Elinav E. (2016). Role of the microbiome in the normal and aberrant glycemic response. Clinical Nutrition Experimental, 6, 59–73. doi:10.1016/j.yclnex.2016.01.001 [Google Scholar]
- Sze M. A., Schloss P. D. (2016). Looking for a signal in the noise: Revisiting obesity and the microbiome. MBio, 7 doi:10.1128/mBio.01018-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang W. H. W., Hazen S. L. (2017). The gut microbiome and its role in cardiovascular diseases. Circulation, 135, 1008–1010. doi:10.1161/CIRCULATIONAHA.116.024251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner M. D., Nedjai B., Hurst T., Pennington D. J. (2014). Cytokines and chemokines: At the crossroads of cell signalling and inflammatory disease. Biochimica et Biophysica Acta, 1843, 2563–2582. doi:10.1016/j.bbamcr.2014.05.014 [DOI] [PubMed] [Google Scholar]
- Van Lieshout R. J., Taylor V. H., Boyle M. H. (2011). Pre-pregnancy and pregnancy obesity and neurodevelopmental outcomes in offspring: A systematic review. Obesity Reviews, 12, e548–59. doi:10.1111/j.1467-789X.2010.00850.x [DOI] [PubMed] [Google Scholar]
- White R. A., Bjørnholt J. V., Baird D. D., Midtvedt T., Harris J. R., Pagano M.…Eggesbø M. (2013). Novel developmental analyses identify longitudinal patterns of early gut microbiota that affect infant growth. PLoS Computational Biology, 9, e1003042 doi:10.1371/journal.pcbi.1003042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widdowson E. M., McCance R. A. (1975). A review: New thoughts on growth. Pediatric Research, 9, 154–156. doi:10.1203/00006450-197503000-00010 [DOI] [PubMed] [Google Scholar]