Short abstract
Objective
We examined the ability of anthropometric measures to predict the risk of metabolic syndrome (MetS) and its components in Peruvian adults.
Methods
Participants were 1,815 Peruvian adults. Body mass index (BMI), waist circumference (WC), A Body Shape Index (ABSI), Body Roundness Index (BRI) and Visceral Adiposity Index were examined. MetS components were defined using the National Cholesterol Education Program’s Adult Treatment Panel III. Logistic regression was used to calculate odds ratios of MetS and MetS components in relation to increases in anthropometric measures. Receiver operating characteristic curves (and area under the curve) were calculated to compare each measure’s power to predict MetS and MetS components.
Results
BRI performed similar to or better than BMI and WC at predicting MetS and MetS components. ABSI underperformed other measures. In men, the odds of MetS and its components increased with unit increases in the anthropometric measures (e.g. a unit increase in BRI was associated with 2.43-fold increased odds of MetS; 95% confidence interval [CI]: 1.95–3.02). A similar association was found for women (odds ratio: 1.89; 95% CI: 1.68–2.12).
Conclusion
Our study is the first to identify BRI as a potentially useful clinical predictor of MetS in Peruvian adults.
Keywords: Metabolic syndrome, anthropometric measures, Peru, body mass index, waist circumference, A Body Shape Index, Body Roundness Index, Visceral Adiposity Index
Introduction
Metabolic syndrome (MetS), a clustering of several cardiovascular risk factors, is an important predictor of all-cause mortality and premature mortality from cardiovascular events.1 The prevalence of MetS in low- and middle-income countries ranges from 10% to 47%.2 Owing to the individual and societal burden of cardiovascular disease (CVD) in these countries, it is crucial to identify individuals with MetS. Early identification of at-risk individuals facilitates the design of programs to modify risk factors and prevent the onset and progression of MetS later in life. A growing body of epidemiologic evidence shows that simple and inexpensive anthropometric measures can be used to predict MetS. These include measures such as body mass index (BMI) and waist circumference (WC), which have been used in clinical practice for decades, as well as novel measures such as the Body Roundness Index (BRI),3 A Body Shape Index (ABSI)4 and the Visceral Adiposity Index (VAI).5
Previous study findings on the association between anthropometric measures and MetS are inconsistent. In a large cross-sectional study in the USA, Mooney et al.6 found that BMI was the best predictor of blood pressure (BP), and central adiposity measures (including WC) were the best predictors of blood glucose. In Iranian adults, BMI was a better predictor than ABSI of MetS.7 A recent study in China found that BRI performed similarly to WC and BMI as a predictor of diabetes mellitus and risk factors, and these measures all outperformed ABSI.8 Another study by Maessen et al.9 of a Dutch population yielded similar results. However, there are few studies on anthropometric measures as predictors of MetS in South American populations. A recent study on women in Cartagena, Colombia, found little predictive power of anthropometric measures on MetS: only waist-to-height ratio was weakly predictive of MetS.10
MetS rates in South America are steadily rising, motivating the identification of cost-effective methods of identifying at-risk individuals.11–14 Given the rise in MetS risk in South America, we sought to compare the predictive power of different anthropometric measures to detect MetS in a Peruvian cohort. We examined the predictive power of BMI, WC, BRI, ABSI and VAI to identify individuals with MetS and its components.
Methods
Study population
The study population was residents of Lima and Callao, Peru, who participated in the Prevalencia de Factores de Riesgo de Enfermedades No-Transmissibles (FRENT). FRENT is a population-based study investigating the prevalence of risk factors for non-communicable diseases. The FRENT study has been described in detail previously.13 For the present study purposes, we excluded participants taking antidiabetic drugs (n = 30), lipid lowering drugs (n = 33) or antihypertensive drugs (n = 81). Data were finally analysed from 1,518 participants, 952 women (62.7%) and 566 men (37.3%). All participants provided written informed consent and all research protocols were approved by the institutional review boards of the National Institute of Health in Peru, Dos De Mayo Hospital in Peru and the University of Washington in Seattle, WA, USA.
Data collection and variable specification
Trained interviewers used a structured questionnaire validated by the Pan American Health Organization to assess the prevalence of non-communicable disease risk factors.15 Interviewers collected information on participants’ age, sex, educational attainment and medical history. Height and weight were measured with light clothing and no shoes. BP was measured using a mercury desk sphygmomanometer. BP was taken after participants had been seated for 5 minutes and again 30 minutes into the interview; these two values were averaged to obtain a BP reading. A blood sample was also obtained following an 8-hour fast. The blood samples were used to obtain measurements of serum triglycerides (TG), total cholesterol, high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol, fasting blood glucose (FBG) and insulin at the Peruvian National Institute of Health Laboratory in Lima, Peru. All laboratory assays were completed without prior knowledge of participants’ medical history. Lipid, lipoprotein and glucose concentrations are all reported in mg/dL.
Anthropometric indices
The anthropometric measures used in this analysis were BMI, WC, BRI, ABSI and VAI. BMI was measured in kg/m2. WC was measured at the sides midway between the iliac crest and the lower ribs. BRI, ABSI and VAI were calculated using the following equations.3–5
It should be noted that VAI differs from the other anthropometric measures, as it is invasive (requires blood draws to assess HDL-C and TG) and is calculated directly from two MetS components.
Metabolic syndrome
MetS components were defined in accordance with the Adult Treatment Panel III of the National Cholesterol Education Program:16,17 (1) elevated BP (mean systolic BP [SBP] ≥130 mm Hg and/or mean diastolic BP [DBP] ≥85 mm Hg); (2) abdominal obesity (WC > 102 cm in men or WC > 88 cm in women); (3) reduced HDL-C (<40 mg/dL in men or <50 mg/dL in women); (4) elevated TG (≥150 mg/dL) and elevated FBG (≥100 mg/dL or current antidiabetic medication use).17 Consistent with the criteria, MetS was defined when at least three of the above were present.
Statistical analyses
Sociodemographic characteristics were examined by sex and reported in percentages. Chi-squared tests were used to compare the distributions of categorical variables by sex. Continuous variables were expressed as mean ± standard deviation. Student’s t-tests were used to evaluate differences in mean distributions by sex. Spearman’s rank correlation coefficients were calculated to test the association between anthropometric measures (BMI, WC, BRI, ABSI and VAI) and CVD risk factors (FBG, TG, HDL-C, SBP, and DBP). Spearman’s rank correlation coefficients were used, as the data were not normally distributed. Binary logistic regression was used to evaluate the unadjusted and adjusted associations between anthropometric measures and MetS components. Adjustments were made for age, alcohol use and smoking status. Odds ratios (ORs) and 95% confidence intervals (CI) were calculated. The ORs examined the change in odds per unit increase in the anthropometric measures, except for ABSI, which was scaled by a factor of 100 owing to its small range. Receiver operating characteristic (ROC) curves with area under curve (AUC) and 95% CI were created for anthropometric measures as predictors of MetS components. All data analyses were performed using SAS 9.2 (SAS Institute, Inc., Cary, NC, USA). All reported P-values were two-tailed and statistical significance was set at α = 0.05.
Results
The characteristics of the study population by sex are shown in Table 1. Overall, women had fewer years of postsecondary education (women: 35.78% vs. men: 41.74%, P < 0.001). Men were more likely than women to be current or former smokers (men: 44.35% vs. women: 15.96%, P < 0.001), currently employed (men: 65.60% vs. women: 44.15%, P < 0.001) and moderate or high alcohol consumers (men: 65.01% vs. women: 39.92%, P < 0.001). Men and women also differed with respect to mean WC (men: 92.0 cm vs. women: 88.8 cm, P < 0.001), mean BRI (men: 4.5 vs. women: 5.1, P < 0.001), mean VAI (men: 5.3 vs. women: 5.8, P = 0.045) and MetS (men: 23.50% vs. women: 28.56%, P = 0.038 (Table 1). Because of these significant differences between men and women, we conducted the main analyses separately by sex.
Table 1.
Characteristics of the study population.
| Characteristic | Men (N = 566) (%) | Women (N = 952) (%) | P-valuea |
|---|---|---|---|
| Age (years) | |||
| Mean ± SD | 38.3 ± 15.96 | 39.9 ± 14.49 | 0.057 |
| ≤24 | 27.52 | 18.78 | 0.002 |
| 25–34 | 30.67 | 29.10 | |
| 35–44 | 22.90 | 28.73 | |
| 45–54 | 17.65 | 21.52 | |
| ≥55 | 1.26 | 1.87 | |
| Education | |||
| 6 years or less | 10.71 | 17.84 | <0.001 |
| 7–12 years | 47.55 | 46.38 | |
| More than 12 years | 41.74 | 35.78 | |
| Currently employed | |||
| No | 34.40 | 55.85 | <0.001 |
| Yes | 65.60 | 44.15 | |
| Smoking status | |||
| Never smoker | 55.65 | 84.03 | <0.001 |
| Former smoker | 12.19 | 6.30 | |
| Current smoker | 32.16 | 9.66 | |
| Alcohol consumption | |||
| Low | 34.98 | 60.08 | <0.001 |
| Moderate | 58.30 | 38.97 | |
| High | 6.71 | 0.95 | |
| BMI (kg/m2) | |||
| Underweight (≤18.5) | 1.77 | 0.84 | 0.002 |
| Normal (18.5–24.9) | 38.69 | 42.23 | |
| Overweight (25.0–29.9) | 43.64 | 35.61 | |
| Obese (≥30.0) | 15.90 | 21.32 | |
| Leisure time physical activity | |||
| No | 29.68 | 22.79 | 0.002 |
| Yes, <150 minutes/week | 57.95 | 66.91 | |
| Yes, ≥150 minutes/week | 12.37 | 10.29 | |
| Waist circumference (cm) | |||
| Mean ± SD | 92.0 ± 10.61 | 88.8 ± 11.59 | <0.001 |
| A Body Shape Index (ABSI) | |||
| Mean ± SD | 8.1 ± 0.54 | 8.1 ± 0.59 | 0.111 |
| Body Roundness Index (BRI) | |||
| Mean ± SD | 4.5 ± 1.36 | 5.1 ± 1.84 | <0.001 |
| Visceral Adiposity Index (VAI) | |||
| Mean ± SD | 5.3 ± 4.32 | 5.8 ± 5.62 | 0.045 |
| Metabolic syndrome | |||
| No | 76.50 | 71.64 | 0.038 |
| Yes | 23.50 | 28.36 |
aP-values for continuous variables were calculated using Student’s t-tests and for categorical variables using chi-squared tests of independence.
We evaluated the association between anthropometric measures and MetS components (Table 2). Among men, BMI was strongly correlated with FBG (r = 0.33; P < 0.05) and DBP (r = 0.33; P < 0.05). In addition, WC was strongly correlated with SBP (r = 0.30; P < 0.05). As expected, VAI was strongly correlated with HDL-C (r = −0.66; P < 0.05) and TG (r = 0.95; P < 0.05). We observed similar results in women. BMI had a strong positive correlation with FBG (r = 0.31; P < 0.05) and DBP (r = 0.27; P < 0.05), and WC was positively correlated with SBP (r = 0.32; P < 0.05). Of the adiposity measures, VAI was most strongly correlated with HDL-C (r = −0.62; P < 0.05) and TG (r = 0.93; P < 0.05). Overall, ABSI showed the weakest correlation with MetS components (Table 2).
Table 2.
Spearman’s rank correlation coefficients for anthropometric measures and cardiovascular disease risk factors
| BMI (kg/m2) | WC (cm) | ABSI | BRI | VAI | |
|---|---|---|---|---|---|
| Men | |||||
| Fasting blood glucose (mg/dL) | 0.330 | 0.292 | 0.031a | 0.304 | 0.222 |
| Triglycerides (mg/dL) | 0.462 | 0.461 | 0.097 | 0.439 | 0.948 |
| HDL-C (mg/dL) | −0.291 | −0.268 | −0.032a | −0.246 | −0.664 |
| Systolic blood pressure (mm Hg) | 0.273 | 0.301 | 0.088 | 0.291 | 0.188 |
| Diastolic blood pressure (mm Hg) | 0.331 | 0.330 | 0.063 | 0.316 | 0.247 |
| Women | |||||
| Fasting blood glucose (mg/dL) | 0.306 | 0.301 | 0.060a | 0.301 | 0.250 |
| Triglycerides (mg/dL) | 0.437 | 0.455 | 0.152 | 0.451 | 0.933 |
| HDL-C (mg/dL) | −0.220 | −0.209 | −0.068 | −0.213 | −0.618 |
| Systolic blood pressure (mm Hg) | 0.296 | 0.323 | 0.120 | 0.304 | 0.271 |
| Diastolic blood pressure (mm Hg) | 0.265 | 0.265 | 0.063a | 0.262 | 0.227 |
BMI: body mass index; WC: waist circumference; ABSI: A Body Shape Index; BRI: Body Roundness Index; VAI: Visceral Adiposity Index; HDL-C: high-density lipoprotein cholesterol. aNot significant (all other coefficients were significant at the 0.05 level).
Table 3 shows that for both sexes, the odds of prevalent MetS; elevated FBG, BP and TG; and reduced HDL increased progressively with a unit increase in adiposity measures. For instance, in men, a unit increase in BMI was associated with 1.38-fold increased odds of MetS (aOR: 1.38; 95% CI: 1.28–1.48) and a unit increase in BRI was associated with a 2.43-fold increase in odds of MetS (aOR: 2.43; 95% CI: 1.95–3.02) (Table 3). In women, a unit increase in BMI was associated with a 1.21-fold increase in odds of MetS (aOR: 1.21; 95% CI: 1.17–1.26) and a unit increase in BRI was associated with 1.89-fold increased odds of MetS (aOR: 1.89; 95% CI: 1.68–2.12).
Table 3.
Odds ratios (95% confidence intervals) for anthropometric measures and metabolic syndrome components
| BMI (kg/m2) | WC (cm) | ABSI (× 100) | BRI | VAI | |
|---|---|---|---|---|---|
| Men | |||||
| High blood glucose | |||||
| Unadjusted OR (95% CI) | 1.18 (1.11–1.27) | 1.06 (1.03–1.09) | 1.00 (0.95-1.06) | 1.59 (1.30–1.93) | 1.14 (1.08–1.20) |
| Adjusted OR (95% CI) | 1.17 (1.09–1.25) | 1.05 (1.02–1.08) | 0.94 (0.89–1.01) | 1.44 (1.16–1.79) | 1.13 (1.07–1.20) |
| High triglycerides | |||||
| Unadjusted OR (95% CI) | 1.21 (1.15–1.27) | 1.09 (1.06–1.11) | 1.03 (0.99–1.06) | 1.72 (1.48–1.98) | 5.70 (4.06–8.01) |
| Adjusted OR (95% CI) | 1.19 (1.14–1.26) | 1.08 (1.05–1.10) | 0.99 (0.95–1.03) | 1.61 (1.37–1.88) | 5.80 (4.10–8.21) |
| Low HDL-C | |||||
| Unadjusted OR (95% CI) | 1.15 (1.10–1.20) | 1.05 (1.03–1.07) | 1.00 (0.97–1.03) | 1.44 (1.26–1.64) | 1.50 (1.39–1.62) |
| Adjusted OR (95% CI) | 1.15 (1.10–1.21) | 1.05 (1.03–1.07) | 0.99 (0.95–1.02) | 1.49 (1.28–1.73) | 1.54 (1.42–1.68) |
| High blood pressure | |||||
| Unadjusted OR (95% CI) | 1.15 (1.10–1.21) | 1.06 (1.04–1.08) | 1.01 (0.98–1.05) | 1.48 (1.28–1.71) | 1.06 (1.02–1.10) |
| Adjusted OR (95% CI) | 1.14 (1.08–1.20) | 1.05 (1.03–1.07) | 0.98 (0.94–1.02) | 1.41 (1.21–1.66) | 1.04 (1.00–1.09) |
| Metabolic syndrome | |||||
| Unadjusted OR (95% CI) | 1.37 (1.28–1.47) | 1.16 (1.12–1.20) | 1.15 (1.11–1.19) | 3.65 (2.97–4.48) | 1.36 (1.27–1.45) |
| Adjusted OR (95% CI) | 1.38 (1.28–1.48) | 1.15 (1.12–1.19) | 0.99 (0.95–1.04) | 2.43 (1.95–3.02) | 1.34 (1.26–1.43) |
| Women | |||||
| High blood glucose | |||||
| Unadjusted OR (95% CI) | 1.14 (1.10–1.19) | 1.07 (1.05–1.09) | 1.06 (1.02–1.10) | 1.46 (1.31–1.63) | 1.12 (1.09–1.16) |
| Adjusted OR (95% CI) | 1.12 (1.07–1.17) | 1.06 (1.04–1.08) | 1.03 (0.99–1.07) | 1.35 (1.20–1.52) | 1.10 (1.06–1.14) |
| High triglycerides | |||||
| Unadjusted OR (95% CI) | 1.16 (1.12–1.19) | 1.08 (1.06–1.09) | 1.06 (1.03–1.09) | 1.57 (1.44–1.72) | 2.88 (2.47–3.37) |
| Adjusted OR (95% CI) | 1.13 (1.09–1.17) | 1.06 (1.04–1.08) | 1.03 (1.01–1.06) | 1.43 (1.30–1.57) | 2.84 (2.42–3.32) |
| Low HDL-C | |||||
| Unadjusted OR (95% CI) | 1.08 (1.05–1.12) | 1.03 (1.02–1.05) | 1.01 (0.99–1.03) | 1.23 (1.14–1.33) | 1.63 (1.50–1.76) |
| Adjusted OR (95% CI) | 1.08 (1.05–1.12) | 1.03 (1.02–1.05) | 1.01 (0.99–1.03) | 1.25 (1.15–1.36) | 1.81 (1.65–1.99) |
| High blood pressure | |||||
| Unadjusted OR (95% CI) | 1.13 (1.09–1.17) | 1.07 (1.05–1.09) | 1.07 (1.04–1.10) | 1.48 (1.35–1.62) | 1.08 (1.05–1.11) |
| Adjusted OR (95% CI) | 1.09 (1.05–1.13) | 1.05 (1.03–1.07) | 1.04 (1.01–1.07) | 1.29 (1.16–1.42) | 1.03 (1.00–1.06) |
| Metabolic syndrome | |||||
| Unadjusted OR (95% CI) | 1.24 (1.19–1.29) | 1.13 (1.11–1.15) | 1.09 (1.06–1.12) | 2.06 (1.84–2.30) | 1.70 (1.58–1.83) |
| Adjusted OR (95% CI) | 1.21 (1.17–1.26) | 1.11 (1.09–1.13) | 1.07 (1.04–1.10) | 1.89 (1.68–2.12) | 1.64 (1.52–1.77) |
Odds ratios were adjusted for age, smoking status and alcohol use. Components are defined as follows: High blood glucose: fasting blood glucose ≥100 mg/dL; high triglycerides: triglycerides ≥150 mg/dL; low HD: HDL < 50 mg/dL for women and < 40 mg/dL for men; high blood pressure: systolic blood pressure ≥130 or diastolic blood pressure ≥85. BMI: body mass index; WC: waist circumference; ABSI: A Body Shape Index; BRI: Body Roundness Index; VAI: Visceral Adiposity Index; OR: odds ratio; CI: confidence interval; HDL-C: high-density lipoprotein cholesterol.
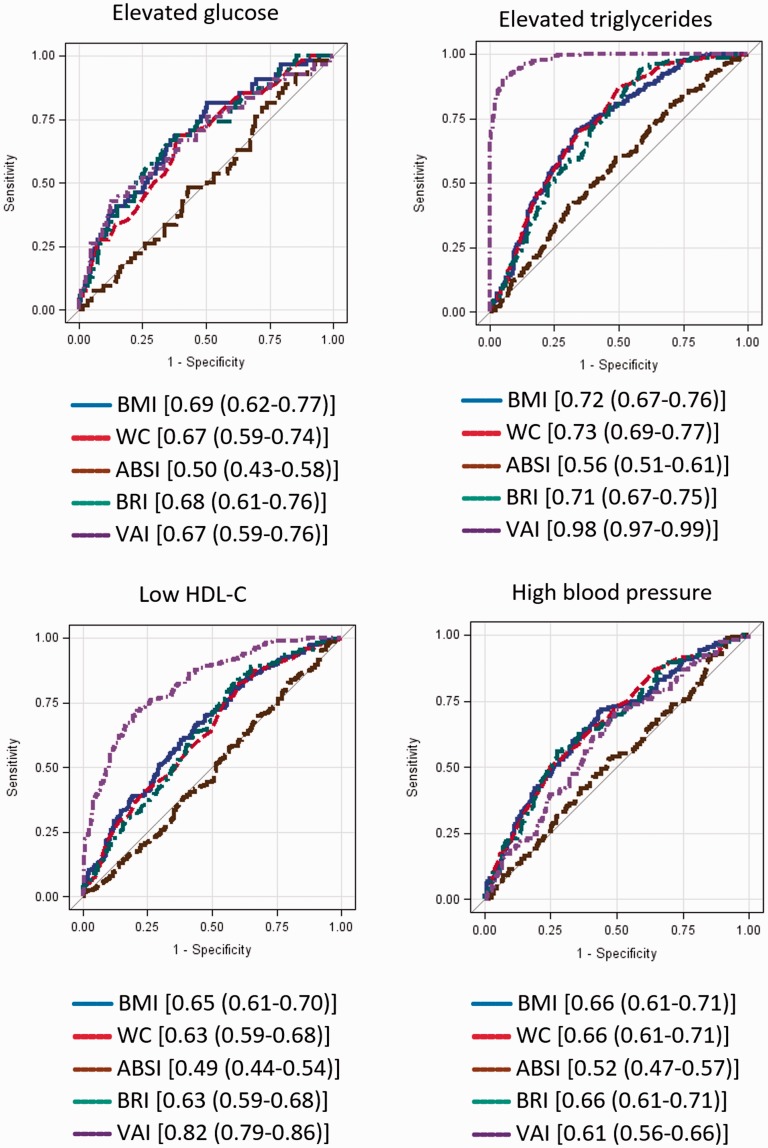
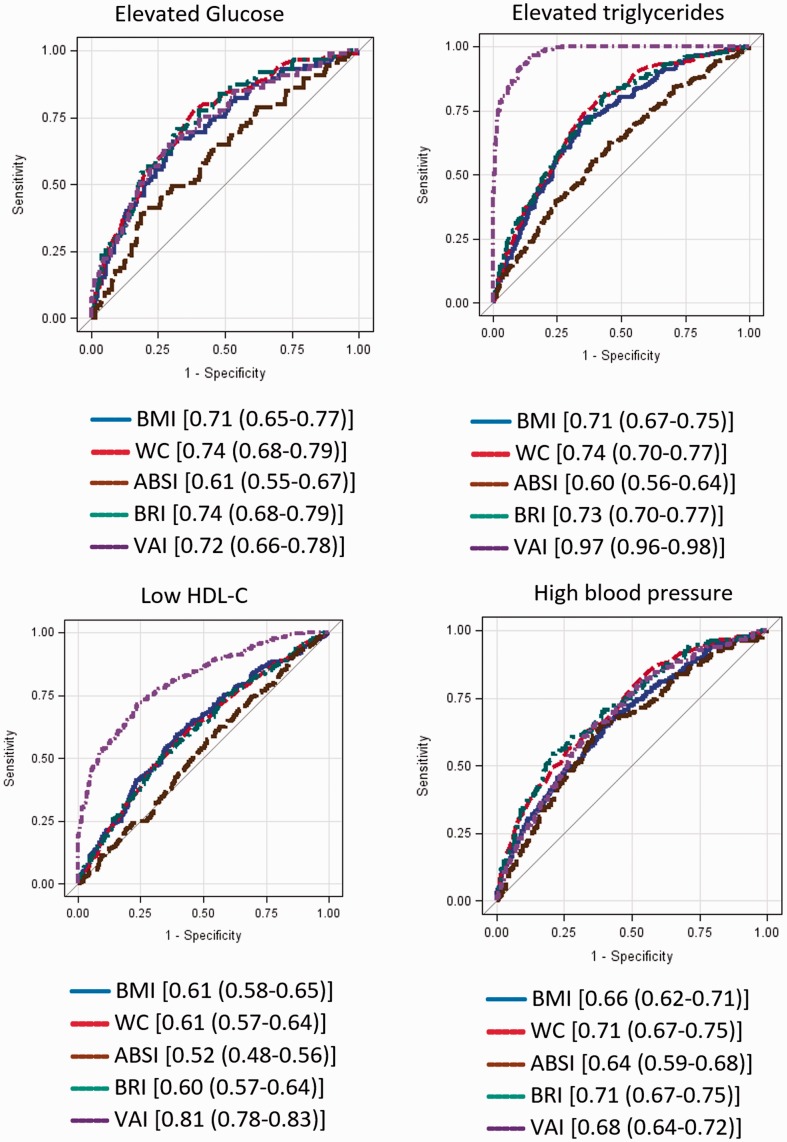
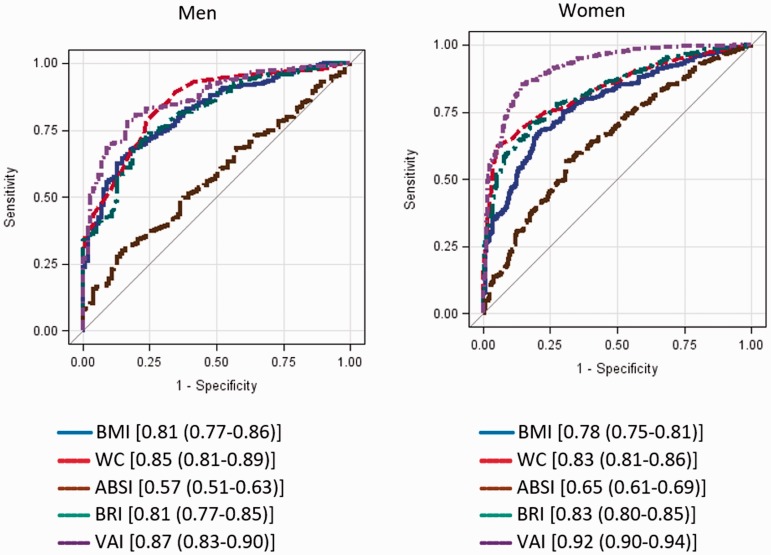
ROC curves for adiposity measures predicting MetS components in men and women are presented in Figures 1 and 2, respectively. In men, BMI, WC and BRI performed equally in predicting high BP (AUC = 0.61 for all), whereas BMI (AUC = 0.69; 95% CI: 0.62–0.77) and BRI (AUC = 0.68; 95% CI: 0.61–0.76) predicted elevated glucose. WC (AUC = 0.73; 95% CI: 0.69–0.77), BMI (AUC = 0.72; 95% CI: 0.67–0.76) and BRI (AUC = 0.71; 95% CI: 0.67–0.75) predicted elevated TG. BMI, WC and BRI were equally predictive of low HDL. VAI accurately identified low HDL-C, high TG and MetS. In women, BMI (AUC = 0.71; 95% CI: 0.65–0.77) and BRI (AUC = 0.74; 95% CI: 0.68–0.79) identified risk of elevated glucose. WC (AUC = 0.74; 95% CI: 0.70–0.77) and BRI (AUC = 0.73; 95% CI: 0.70–0.77) identified elevated TG. BMI (AUC = 0.61), WC (AUC = 0.61) and BRI (AUC = 0.60) performed similarly well in predicting low HDL-C. WC and BRI (AUC = 0.71 in both cases) accurately predicted high BP. VAI was the most accurate predictor of elevated TG, low HDL-C and MetS. For men, BRI was a strong predictor of MetS, with an AUC of 0.81, compared with 0.81 for BMI and 0.85 for WC. For women, BRI as a predictor of MetS had an AUC of 0.83, compared with 0.78 for BMI and 0.83 for WC (Appendix Figure 1).
Figure 1.
ROC curves (AUC) for metabolic syndrome and metabolic syndrome components for men.
Components are defined as follows: High blood glucose: fasting blood glucose ≥100 mg/dL; high triglycerides: triglycerides ≥150 mg/dL; low HDL: HDL < 50 mg/dL for women and <40 mg/dL for men; high blood pressure: systolic blood pressure ≥130 or diastolic blood pressure ≥85. Legends show [AUC (95% Wald confidence limits)]. BMI: body mass index; WC: waist circumference; ABSI: A Body Shape Index; BRI: Body Roundness Index; VAI: Visceral Adiposity Index; HDL-C: high-density lipoprotein cholesterol; ROC: receiver operating characteristic; AUC: area under the curve.
Figure 2.
ROC curves (AUC) for metabolic syndrome and metabolic syndrome components for women.
Components are defined as follows: High blood glucose: fasting blood glucose ≥ 100 mg/dL; high triglycerides: triglycerides ≥ 150 mg/dL; low HDL: HDL < 50 mg/dL for women and < 40 mg/dL for men; high blood pressure: systolic blood pressure ≥ 130 or diastolic blood pressure ≥ 85. Legends show [AUC (95% Wald confidence limits)]. BMI: body mass index; WC: waist circumference; ABSI: A Body Shape Index; BRI: Body Roundness Index; VAI: Visceral Adiposity Index; HDL-C: high-density lipoprotein cholesterol; ROC: receiver operating characteristic; AUC: area under the curve.
Discussion
We found statically significant associations between anthropometric measures and MetS and associated risk factors in a Peruvian population. As expected, VAI was the best anthropometric predictor of MetS. This is partly because of the measure’s high degree of correlation with TG and HDL-C. We found that BRI, a novel and non-invasive anthropometric measure, consistently performed as well as or better than the standard measures (BMI and WC) as a predictor of MetS and MetS risk factors. These findings demonstrate that BRI is an effective predictor of MetS risk among Peruvian adults.
Our findings are in general agreement with those of some previous studies8,9 but not all.6 Among rural residents in northeastern China, Chang et al.8 found that that BRI performed similarly to BMI and WC in predicting diabetes mellitus, and ABSI showed the weakest predictive ability. Similar findings were reported by Maessen et al.9 in their population-based study in Nijmegen, the Netherlands. The authors noted that BRI was as good a predictor of CVD risk presence (although not superior) as established anthropometric indices such as BMI and WC. However, ABSI was not a good predictor of CVD risk. We found that BMI, WC and BRI outperformed ABSI in predicting MetS and MetS components, as previously reported for an Iranian population.7 In contrast to results previously reported for an American population by Mooney,6 we found that the best predictors of high BP and high blood glucose differed by sex. BMI, BRI and WC performed similarly well as predictors of high BP in men, but BRI and WC performed better than BMI in women. BMI outperformed other measures in predicting high blood glucose in men, whereas BRI and WC were better predictors in women. Despite the heterogeneity in population characteristics and geographical differences, the results of our study and those of others show that simple anthropometric measures have global utility in identifying individuals with high risk of developing MetS.
Some limitations should be considered when interpreting our results. First, the cross-sectional study design did not allow us to establish temporality between the adiposity measures and MetS. Future studies need to evaluate the longitudinal relation between anthropometric measures and MetS risk and to establish clinical tools such as cut points for predicting MetS risk. Second, all our participants were urban, and therefore our results may not be generalizable to the whole Peruvian population. Individuals in rural areas may have different diets and physical activity patterns. Last, despite controlling for confounders in the multivariate regression models, residual confounding by unmeasured or imprecisely measured covariates was possible.
Our study contributes to a growing body of research comparing the effectiveness of different anthropometric measures as predictors of MetS risk in populations worldwide. Combined with family history of cardiometabolic risk, findings such as these are important, as they can inform clinical practice and public health counselling and help to identify at-risk individuals in need of preventive interventions.
Acknowledgements
This study was supported by the Direccion General de Epidemiologia Ministerio de Salud, Peru, by an award from the National Institutes of Health, National Center on Minority Health and Health Disparities (T37-MD001449), and by the Instituto Nacional de Salud, Peru.
Appendix
Figure A1.
ROC curves (AUC) for metabolic syndrome.
Legends show [AUC (95% Wald confidence limits)]. BMI: body mass index; WC: waist circumference; ABSI: A Body Shape Index; BRI: Body Roundness Index; VAI: Visceral Adiposity Index; ROC: receiver operating characteristic; AUC: area under the curve.
Declaration of conflicting interest
The authors declare that there is no conflict of interest.
Funding
This study was supported by National Center on Minority Health and Health Disparities( grant number T37-MD001449).
References
- 1.Mottillo S, Filion KB, Genest J, et al. The metabolic syndrome and cardiovascular risk a systematic review and meta-analysis. J Am Coll Cardiol 2010; 56: 1113–1132. [DOI] [PubMed] [Google Scholar]
- 2.Misra A, Khurana L. Obesity and the metabolic syndrome in developing countries. J Clin Endocrinol Metab 2008; 93: S9–S30. [DOI] [PubMed] [Google Scholar]
- 3.Thomas DM, Bredlau C, Bosy-Westphal A, et al. Relationships between body roundness with body fat and visceral adipose tissue emerging from a new geometrical model. Obesity (Silver Spring) 2013; 21: 2264–2271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Krakauer NY, Krakauer JC. A new body shape index predicts mortality hazard independently of body mass index. PLoS One 2012; 7: e39504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Amato MC, Giordano C, Galia M, et al. Visceral adiposity index: a reliable indicator of visceral fat function associated with cardiometabolic risk. Diabetes Care 2010; 33: 920–922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mooney SJ, Baecker A, Rundle AG. Comparison of anthropometric and body composition measures as predictors of components of the metabolic syndrome in a clinical setting. Obes Res Clin Pract 2013; 7: e55–e66. [DOI] [PubMed] [Google Scholar]
- 7.Haghighatdoost F, Sarrafzadegan N, Mohammadifard N, et al. Assessing body shape index as a risk predictor for cardiovascular diseases and metabolic syndrome among Iranian adults. Nutrition 2014; 30: 636–644. [DOI] [PubMed] [Google Scholar]
- 8.Chang Y, Guo X, Chen Y, et al. A body shape index and body roundness index: two new body indices to identify diabetes mellitus among rural populations in northeast China. BMC Public Health 2015; 15: 794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Maessen MF, Eijsvogels TM, Verheggen RJ, et al. Entering a new era of body indices: the feasibility of a body shape index and body roundness index to identify cardiovascular health status. PLoS One 2014; 9: e107212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mora-García GJ, Gómez-Camargo D, Mazenett E, et al. Anthropometric parameters cut-off points and predictive value for metabolic syndrome in women from Cartagena, Colombia. Salud Publica Mex 2014; 56: 146–153. [DOI] [PubMed] [Google Scholar]
- 11.Aballay LR, Eynard AR, Diaz Mdel P, et al. Overweight and obesity: a review of their relationship to metabolic syndrome, cardiovascular disease, and cancer in South America. Nutr Rev 2013; 71: 168–179. [DOI] [PubMed] [Google Scholar]
- 12.Mujica V, Leiva E, Icaza G, et al. Evaluation of metabolic syndrome in adults of Talca city, Chile. Nutr J 2008; 7: 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gelaye B, Revilla L, Lopez T, et al. Prevalence of metabolic syndrome and its relationship with leisure time physical activity among Peruvian adults. Eur J Clin Invest 2009; 39: 891–898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Baracco R, Mohanna S, Seclén S. A comparison of the prevalence of metabolic syndrome and its components in high and low altitude populations in Peru. Metab Syndr Relat Disord 2007; 5: 55–62. [DOI] [PubMed] [Google Scholar]
- 15.Pan American Health Organization. Countrywide Integrated Noncommunicable Diseases Intervention (CINDI) programme, www.paho.org/english/ad/dpc/nc/hcncindi.pdf (1995, accessed Day Month Year).
- 16.Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. Executive Summary of The Third Report of The National Cholesterol Education Program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (Adult Treatment Panel III). JAMA 2001; 285: 2486–2497. [DOI] [PubMed] [Google Scholar]
- 17.Grundy SM, Cleeman JI, Daniels SR, et al. Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, lung, and blood institute scientific statement. Circulation 2005; 112: 2735–2752. [DOI] [PubMed] [Google Scholar]