Abstract
Different aspects related to globalization together with the great capacity of the arthropod vectors to adapt to a changing world favour the emergence and reemergence of numerous infectious diseases transmitted by them. Diptera (mosquitoes and sandflies), ticks, fleas and lice, among others, cause a wide spectrum of diseases with relevance in public health. Herein, arthropod-borne disease are reviewed, with special emphasis on the existing risk to contract them in Spain according to different parameters, such as the presence of arthropod and the circulation or the possible circulation of the causative agents.
Keywords: Arthropod vector, Ticks, Mosquitoes, Fleas, Spain, Arboviruses
Abstract
Diferentes aspectos relacionados con la globalización junto a la gran capacidad de los artrópodos vectores para adaptarse a un mundo cambiante propician la emergencia y reemergencia de numerosos procesos infecciosos transmitidos por los mismos. Dípteros (culícidos y flebótomos), garrapatas, pulgas y piojos, entre otros, provocan un variado espectro de enfermedades con gran importancia en Salud Pública. En esta revisión se repasan las diferentes afecciones transmitidas por artrópodos vectores, haciendo un especial hincapié en el riesgo existente para contraerlas en España en función de diferentes parámetros, como la presencia del artrópodo y la circulación o posible circulación de los agentes causales.
Palabras clave: Artrópodo vector, Garrapatas, Mosquitos, Pulgas, España, Arbovirus
Arthropods are the most abundant invertebrates of the animal kingdom. Their classification is very complex and among them are very diverse orders (spiders, scorpions, ants, crabs, butterflies, lice, centipedes, mosquitoes, ticks, etc.). Without entering into their different actions and functions in nature, some of them have great importance in Public Health and Animal Health due to the diseases they are capable of transmitting and/or for their capacity to act as reservoirs of infectious processes.1
We can define an arthropod vector (AV) as an invertebrate that has a segmented body covered by a cuticle (exoskeleton), with articulated appendages, with the ability to transmit infectious agents. Most AVs belong to Insecta and Arachnida classes (Table 1 ).
Table 1.
Main arthropod vectors with health significance.
| Class | Order | Genus | Common name |
|---|---|---|---|
| Insecta | Siphonaptera | Ctenocephalides, Pulex | Fleas |
| Phthiraptera |
Pediculus Pthirus |
Lice Crab louse |
|
| Hemiptera |
Rhodnius, Triatoma Cimexa |
Triatoma infestans Bed bugs |
|
| Diptera |
Anopheles, Culex, Aedes, etc. Simulium Glossina Tabanus Phlebotomus, Lutzomya Culicoides, Leptoconos, etc. |
Mosquitoes Black flies Tse-tse flies Horseflies Phlebotominae Sand fly |
|
| Arachnida | Ixodida | Ixodes, Dermacentor, Rhipicephalus, Hyalomma, Amblyomma, Argas, Ornithodoros, etc. | Ticks |
| Trombidiforms Sarcoptiforms Mesostigmata |
Neotrombicula, Demodex Sarcoptes Dermanyssus |
Trombiculids, scabies mites, red bugs and other mites |
Vector capacity not demonstrated.
To review the broad subject of arthropod vector-borne diseases (AVBD) in Spain is a complex task. Just listing the arthropods that transmit diseases in our environment, such as dipterans (culicids and phlebotominae sand flies), fleas, lice, bed bugs and ticks, among others, and the diseases they transmit, or that they can transmit at any given time, would be a reason for one or several treatises. In any case, before discussing the subject, it should be remembered that the infections that they transmit are usually included in the so-called zoonoses. In this regard, the World Organization for Animal Health estimates that at least 60% of infections that affect humans have a zoonotic origin2 and, according to the Pandemic Emerging Threats Program of the American Agency for International Development, almost 75% of current threats also have this origin.3 One of the many known routes for the acquisition of zoonoses, apart from direct contact with animals or their products, faecal-oral or respiratory routes, bites or scratches, the consumption of undercooked products or the intake of milk, is AV transmission.4 It is difficult to limit this issue to our country, since the setting is global and very dynamic, and diseases do not understand political borders. Only a few months ago new threats appeared, such as the re-emergence of yellow fever in Brazil or the epidemic of plague in Madagascar.5 However, in this review we will put the focus on AVBD with greater risk to humans in our environment, without losing the “one health” perspective.
AVBD are subject to complex interactions (demographic, social and cultural changes, climate change, wars and famine or evolution of microorganisms) among which, undoubtedly, global transport systems and the consequent invasion of exotic species6 stand out. Generally speaking, travel, migration and globalization contribute to the emergence of infectious diseases. Its importance has been debated for many years and, possibly, dates back to ancient times.7 Humans carry their usual microbiota, pathogens, ectoparasites and other possible vectors, the immunological history of past infections and vaccines, the genetic load (greater or lesser susceptibility), cultural preferences, behaviours, habits and customs, as well as luggage (pets, goods and others).7 In the case of AVBD, the equation for the appearance of a certain disease would be the following: the presence of competent vector arthropods plus susceptible population, together with the presence of reservoirs and/or intermediate hosts (sick people), could give result an epidemic. The introduction of the tiger mosquito (Aedes albopictus) and the threat of its expansion could be a good example.
Diptera (mosquitoes and phlebotominae sand flies) overview in Spain and its impact on Public Health
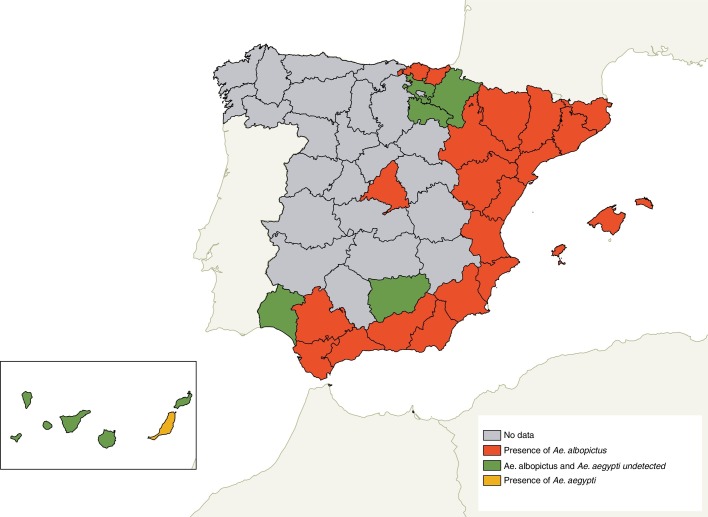
It is thought that A. albopictus was introduced in Europe in 1979 through Albania, by the trade of used tyres, although the first publication on its introduction in the European continent dates back to 1990 in Italy.8 Since then, A. albopictus has expanded throughout the Mediterranean area. In Spain, this aggressive mosquito is well established in Catalonia, Levante, in the coastal area of Murcia and Andalusia. It has also been detected in Guipúzcoa,9 and little by little it is introduced in other non-coastal areas such as Aragon, extending through travel routes (for example, motorways).10 The last detection was carried out in the Community of Madrid11 (Fig. 1 ). A few months ago, the first specimens of this species were also identified in the North of Portugal. This mosquito has been incriminated as a vector of numerous arboviruses in different parts of the world, including Europe. A. albopictus, is the chikungunya virus vector. In this regard, most of the cases in Europe are imported, although in the last two decades there have been different epidemic outbreaks in Italy12 and, recently, in France.13 In Spain, only imported cases have been reported.14 This mosquito (next to Aedes aegypti which, as will be detailed later, has been detected in a timely manner in Europe) can act as a vector of the dengue virus and with less effectiveness of the Zika virus.15 In France, A. albopictus was responsible for the first autochthonous cases of dengue16 and of those reported in the same country during the following years, one of them with a history of travel to Madeira, where a large outbreak had been reported (more than a thousand cases) between 2012 and 2013.17 In 2011, the occurrence of autochthonous cases of dengue in Croatia were also attributed to A. albopictus, establishing the transmission by this mosquito in Europe.18 In Spain we are only suffering from their annoying bites, for now.19
Fig. 1.
Updated distribution map of Aedes albopictus in Spain. Ae: Aedes.
Table 2 shows arbovirus infection and Table 3 shows other infections transmitted by dipterans worldwide, with the risk of transmission in Spain. We have evaluated the risk of emergence and/or re-emergence of these diseases in Spain according to the existing studies for each pathogen, the experience according to other diseases, previous immunity and other criteria, some of them subjective, that do not appear in the text.
Table 2.
Arboviruses transmitted by culicoides with impact on Public Health.
| Family/virus | Geographical distribution | Presence in Spain | Risk of emergence or re-emergence in Spain |
||||
|---|---|---|---|---|---|---|---|
| Disease cases | Presence of the vector | Virus pathogenicity | Risk | Risk according to authors | |||
| Bunyaviridae | |||||||
| Batai | Africa Asia Europe |
– | 1 | 1 | 1 | Moderate | Low |
| La Crosse encephalitis | North America | – | 0 | 0 | 0 | Low | Low |
| Rift Valley fever | Africa Middle East |
– | 0 | 1 | 0 | Moderate | Moderate |
| Inkoo | North Europe | – | 1 | 1 | 0 | High | Low |
| Tahyna | Africa Asia Europe |
Only serological evidence | 1 | 1 | 0 | High | Low |
| Flaviviridae | |||||||
| Dengue | Africa America Asia Oceania |
Present in the past | 1 | 1 | 0 | High | High |
| Murray Valley encephalitis | Oceania | – | 0 | 0 | 0 | Low | Low |
| Saint Louis encephalitis | America | – | 0 | 1 | 0 | Moderate | Low |
| Japanese encephalitis | Asia | – | 0 | 1 | 0 | Moderate | Low |
| Yellow fever | Africa South America |
– | 1 | 1 | 0 | High | Low |
| West Nile Virus | Africa Asia Europe North America Middle East Oceania |
Sporadic communication | 1 | 1 | 0 | High | High |
| Usutu | Africa Europe |
Sporadic communication | 1 | 1 | 1 | Moderate | Low |
| Zika | Africa America Asia Oceania |
– | 1 | 1 | 0 | High | Moderate |
| Togaviridae | |||||||
| Chikungunya | Africa America Asia Oceania |
– | 1 | 1 | 0 | High | High |
| Eastern equine encephalitis | America | – | 0 | 1 | 0 | Moderate | Low |
| Western equine encephalitis | America | – | 0 | 0 | 0 | Low | Low |
| Equine encephalitis of Venezuela | America | – | 0 | 1 | 0 | Moderate | Low |
| Mayaro | South America | 0 | 1 | 0 | Moderate | Low | |
| O’nyong-nyong | Africa | – | 0 | 0 | 0 | Low | Low |
| Ross River | Oceania | – | 0 | 0 | 0 | Low | Low |
| Sindbis | Africa Asia Europe Oceania |
Only serological evidence | 1 | 1 | 0 | High | Low |
The risk of emergence/re-emergence is calculated based on three factors: (a) presence of cases of the disease in humans in the last 5 years in Europe, Mediterranean, Central and South American countries with a significant relationship with Spain; (b) presence of the vector in Spain; (c) pathogenicity of the virus for humans. Each factor is scored with one point (presence in the first two factors and low pathogenicity in the third factor) or with zero points (absence in the first two factors and high pathogenicity in the third factor). The first two factors are added and the third is subtracted. The total score can range between 0 and 2, with 0 being: low risk; 1: moderate risk; 2: high risk.
Table 3.
Main human diseases transmitted by diptera.
| Disease | Causal agent | Diptera |
Geographical distribution | Endemic presence in Spain | Risk of emergence or re-emergence in Spain |
||||
|---|---|---|---|---|---|---|---|---|---|
| Family | Genus/species | Disease cases | Presence of the vector | Risk | Risk according to authors | ||||
| Oropuche fever | Oropuche virus | Ceratopogonidae | Culicoides paraensis | Caribbean, South America |
No | 0 | 0 | Low | Low |
| Mansonelosis | Mansonella ozzardi | Ceratopogonidae | Culicoides Leptoconos equaerti | Caribbean, South America | No | 0 | 0 | Low | Low |
| M. ozzardi | Simuliidae | Simulium | South America, Panama | No | 0 | 0 | Low | Low | |
| Mansonella perstans | Ceratopogonidae | Culicoides | Africa, Caribbean, South America | No | 0 | 0 | Low | Low | |
| Mansonella streptocera | Ceratopogonidae | Culicoides | Africa | No | 0 | 0 | Low | Low | |
| Malaria | Plasmodium spp. | Culicidae | Anopheles | Africa, Asia, Central and South America, Pacific | No | 1 | 1 | High | Moderate |
| Filariasis | Wuchereria bancrofti, Brugia malayi, Brugia timori | Culicidae | Aedes/Ochlerotatus, Anopheles, Culex, Mansonia | Africa, Asia, Caribbean, Western Pacific, South America | No | 0 | 0 | Low | Low |
| Trypanosomiasis | Trypanosoma gambiense, T. rhodesiense | Glossinidae | Tse fly (Glossina) | Africa | No | 0 | 0 | Low | Low |
| Leishmaniasis | Leishmania spp. | Physicodidae | Phlebotomus/Lutzomya | Africa, Asia, Europe, Central and South America | Yes | 1 | 1 | High | High |
| Bartonellosis | Bartonella bacilliformis | Physicodidae | Lutzomya | Central and South America | No | 0 | 0 | Low | Low |
| Fever by phlebotominae sand flies | Virus Toscana, Naples, Sicily, Granada, etc. | Physicodidae | Phlebotomus/Lutzomya | Central and South America, China, Mediterranean, North Africa | Yesa | 1 | 1 | High | High |
| Vesicular stomatitis | Vesicular stomatitis virus | Physicodidae | Lutzomya | America | No | 0 | 0 | Low | Low |
| Onchocerciasis | Onchocerca volvulus | Simuliidae | Simulium | Africa, Central and South America | No | 0 | 0 | Low | Low |
| Loiasis | Loa | Tabanidae | Chrysops | Tropical Africa | No | 0 | 0 | Low | Low |
| Tularemia | Francisella tularensis | Tabanidae | Chrysops | North America, Russia, Japan | No | 0 | 0 | Low | Low |
There are 7 serotypes included in the phlebotomy fever group that have been isolated in Europe; Toscana and Granada viruses have been detected in Spain.
A. aegypti is the main Zika vector and also a transmitter of yellow fever, dengue and chikungunya. In principle, this species is not a problem because it is not settled in Europe, although it has been detected in some areas, such as in an airport in the Netherlands and, more recently, in Fuerteventura20 (Canary Islands) (Fig. 1).
In view of the Zika epidemic in the Americas, the Ministry of Health, Social Services and Equality, in collaboration with the Carlos III Health Institute and the Autonomous Regions, has established a Zika virus disease surveillance in Spain. To date (last update in July 2017), 325 confirmed cases have been reported, all imported, except 4 congenital cases whose mothers were infected in the risk zone and 2 autochthonous cases of sexual transmission.21
It should be noted that it is not necessary to resort to invasive or exotic species to refer to AVBD. Thus, Anopheles atroparvus is present in Spain, a good malaria vector. The official map of the distribution of this mosquito is not updated; however, although the risk of a malarial outbreak is low in our country,22 recent events have involved this species in the two cases of autochthonous malaria registered in the north of the peninsula.23 Spain was an endemic country of malaria until 1964, when WHO declared it a free zone. In Europe, after the eradication of malaria, most cases are imported, although sporadic cases have recently been reported in many Mediterranean countries such as France, Italy, Greece.24 The emergence of autochthonous cases in Greece between 2009 and 2017 has raised doubts about the real situation in Europe as a malaria-free zone.25
Other diptera present in Spain which we cannot ignore are phlebotominae sand flies. The species Phlebotomus perniciosus and Phlebotomus ariasi, are the competent vectors of the leishmaniasis agent (Leishmania infantum). There are also other potential vectors of L. infantum in Spain, as they are Phlebotomus papatasi and Phlebotomus sergenti. Leishmaniasis is endemic throughout the Mediterranean basin in Europe and its geographic scope is spreading. Usually, the epidemiology of leishmaniasis was linked to the rural habitat with the presence of dogs. The great epidemic outbreak of Fuenlabrada with a high number of cases in an urban area, in which leishmaniasis was not common, showed the dangers of changing the urban model in Spain. Many houses were built in rural agricultural areas with gardens and peri-urban green spaces where wildlife was present. In these places the concentration of phlebotominae sand flies was high, and, in their environment, there were not only dogs but also other L. infantum reservoirs, such as hares and rabbits. This fact together with mild temperatures in recent years, decreasing the mortality of the vector, seems to be what caused the great outbreak.26, 27, 28 Undoubtedly, the presence of phlebotominae sand flies throughout the Iberian Peninsula is a great threat, since not only do they transmit leishmania, but they are also Toscana virus vectors, which is causing numerous cases of meningoencephalitis in some areas of Spain29 and other phleboviruses such as the Granada virus (without proven pathogenic power), the Naples virus or the Sicily virus.30
Another species of mosquito to which special attention is to be paid is Culex pipiens. This species, which is distributed and well represented throughout the Iberian Peninsula, is capable of transmitting the West Nile virus (WNV). West Nile fever is becoming a serious problem in some areas of Europe, as in Greece, where there has been an outbreak with numerous neuroinvasive forms,31 and in 2017 cases have been reported in France, Italy, Romania, Hungary, Croatia, Serbia and Austria.32 In Spain, according to the data from the situation report and WNV risk assessment, published in 2013, there are several species of mosquitoes capable of virus transmission. For example, Culex modestus, Culex perexiguus and Culex theileri show a high vector competence, while C. pipiens and A. albopictus have a moderate vector competence.33 The first human case of neuroinvasive disease due to WNV in our country was identified, retrospectively, in a patient diagnosed with meningitis in September 2004 who, in the days before the onset of symptoms, visited a village in Badajoz, Extremadura (Spain).34 In 2010, the Ministry of the Environment reported the detection of WNV in 36 equine farms in the provinces of Cádiz, Sevilla and Málaga.35 Through this active surveillance, 15 suspected cases were investigated and two human cases of WNV meningoencephalitis were confirmed.35 Between 2011 and 2016, virus activity was detected in equines, suggesting that the virus is endemic in our country.36 In addition, there was previous evidence about its circulation in birds that were considered for the development of a predictive virus circulation model in our country.37
Influence of climate change
Another factor that clearly influences infections transmitted by arthropod vectors is climate change. In 2017 the International Conference on Climate Change and Health was held in Atlanta, which revolved around the idea that: “Health is the human face of climate change”. Following this meeting, a special article was published in the New England Journal of Medicine which stated that the distribution of infectious diseases such as Lyme borreliosis, rickettsiosis or West Nile fever are expanding at the same rate as their AVs.38 We know that climate variations and extreme weather events have a profound impact on AVBD.39
Mosquitoes and ticks are devoid of temperature regulation mechanisms and, for this reason, fluctuations in temperature greatly affect their reproduction and survival.40 In our country, it is more than possible that the great increase in the number of ticks in recent years is due to the fact that winters, in general, are much milder than years ago. Just to mention an example, Ixodes ricinus, the tick that most frequently bites people in the north of Spain, is very sensitive to climate warming. This, among other factors, is increasing its survival.41 This species of tick transmits very prevalent diseases in Europe, such as Lyme disease or tick-borne encephalitis, or others such as Rickettsia monacensis infection, human anaplasmosis, and babesiosis.42, 43 In Spain, human cases of all of them have been described, except for tick-borne encephalitis. Although there is a high suspicion of the circulation of the virus in Spain, the molecular screening of hundreds of ticks has been carried out in our laboratory, with negative results.43 In addition, other pathogens have been detected in I. ricinus specimens collected in Spain, such as Rickettsia helvetica, Candidatus Neoehrlichia mikurensis or Borrelia miyamotoi, which leads us to be alert to the possible occurrence of human cases.44, 45, 46 As a consequence of climate change, the hypothesis about the probable changes in the distribution of another species of tick, Hyalomma marginatum, which is the Crimean-Congo hemorrhagic fever (CCHF) vector in Europe, has also been established. Under warmer climate conditions, according to prediction models, it is expected that the distribution of this tick species will extend to new areas which were previously free of the vector. In relation to this issue, the epidemiology of Mediterranean spotted fever seems clearly associated with climate change, especially with low rainfall values47 and it has been shown that warming causes greater aggressiveness in its AVs. Table 4 shows the tick-borne diseases throughout the world, with the prediction of risk for Spain (subjective assessments).
Table 4.
Main tick-borne diseases with impact on Public Health.
|
Pathogen Family Infection or disease |
Geographical distribution | Presence in Spain | Risk of emergence or re-emergence in Spain |
|||
|---|---|---|---|---|---|---|
| Disease cases | Presence of the vector | Risk | Risk according to authors | |||
| Bacteria | ||||||
| Rickettsiaceae | ||||||
| DEBONEL/TIBOLA | Europe | Endemic | 1 | 1 | High | High |
| African tick-bite fever | Sub-Saharan Africa, Caribbean, Oceania, Turkey | – | 1 | 0 | Moderate | Low |
| Mediterranean spotted fever | Mediterranean | Endemic | 1 | 1 | High | High |
| Australian tick-bite fever | Australia | – | 0 | 0 | Low | Low |
| Japanese spotted fever | Japan, Thailand | – | 0 | 0 | Low | Low |
| Flinders Island spotted fever | Australia, Thailand, Nepal | – | 0 | 0 | Low | Low |
| Rocky Mountain spotted fever | America | – | 1 | 1 | High | Low |
| Pacific Coast tick fever | USA | – | 0 | 0 | Low | Low |
| Rickettsia sibirica mongolitimonae infection (LAR) | Africa, Southern Europe | Endemic | 1 | 1 | High | High |
| Far-Eastern tick-borne rickettsiosis | China, Japan, Russia | – | 0 | 0 | Low | Low |
| Queensland Tick Typhus | Australia | – | 0 | 0 | Low | Low |
| Siberian tick typhus | Siberia, Mongolia | – | 0 | 1 | Moderate | Moderate |
| Rickettsia helvetica infection | Central Asia, Europe | Endemic | 1 | 1 | High | Moderate |
| Rickettsia aeschlimannii infection | Africa, Mediterranean | Endemic | 1 | 1 | High | Moderate |
| Rickettsia parkeri infection | America | – | 1 | 0 | Moderate | Low |
| Rickettsia massiliae infection | America, Mediterranean | Endemic | 1 | 1 | High | Moderate |
| ‘Candidatus Rickettsia kellyi’ infection | India | – | 0 | 0 | Low | Low |
| ‘Candidatus Rickettsia tarasevichiae’ infection | China | – | 0 | 0 | Low | Low |
| Anaplasmataceae | ||||||
| Human Anaplasmosis | Europe | Endemic | 1 | 1 | High | Low |
| Anaplasma capra infection | China | – | 0 | 0 | Low | Low |
| Ehrlichia ewingii infection | America | – | 0 | 0 | Low | Low |
| Ehrlichia chaffeensis infection | America | – | 0 | 0 | Low | Low |
| Ehrlichia canis infection | America | – | 0 | 1 | Moderate | Low |
| Ehrlichia muris infection | Asia, USA | – | 0 | 1 | Moderate | Low |
| ‘Candidatus Neoehrlichia mikurensis’ infection | Asia, Europe | Endemic | 1 | 1 | High | High |
| Borreliaceae | ||||||
| Lyme borreliosis | America, Asia, Europe | Endemic | 1 | 1 | High | High |
| Borrelia miyamotoi infection | America, Asia, Europe | Endemic | 1 | 1 | High | High |
| Borrelia mayonii infection | North America | – | 0 | 0 | Low | Low |
| Tick-borne relapsing fever | Africa, Asia, America, Mediterranean | Endemica | 1 | 1 | High | High |
| Francisellaceae | ||||||
| Tularemia | America, Asia, Europe | Endemic | 1 | 1 | High | High |
| Virus | ||||||
| Bunyaviridae | ||||||
| Avalon virus infection | Canada, Russia | – | 0 | 0 | Low | Low |
| Bhanja virus infection | Africa, Asia, Europe | Endemic | 1 | 1 | High | Low |
| Crimean-Congo haemorrhagic fever | Europe, Africa, Asia | Sporadic communication | 1 | 1 | High | High |
| Heartland virus infection | USA | – | 0 | 0 | Low | Low |
| Issyk-kul virus infection | Kyrgyzstan, Tajikistan, Kazakhstan | – | 0 | 1 | Moderate | Low |
| Severe fever with thrombocytopenia syndrome or Huaiyangshan | China | – | 0 | 0 | Low | Low |
| Flaviviridae | ||||||
| Alkahumra virus infection | Saudi Arabia | – | 0 | 0 | Low | Low |
| European subtype tick-borne encephalitis | Europe, South Korea | – | 1 | 1 | High | Moderate |
| Far Eastern and Siberian subtype tick-borne encephalitis | Asia | – | 0 | 0 | Low | Low |
| Kyasanur Forest disease | India | – | 0 | 0 | Low | Low |
| Omsk hemorrhagic fever | Siberia | – | 0 | 1 | Moderate | Low |
| Louping ill virus infection | Europe | Endemic | 1 | 1 | High | Low |
| Powassan virus infection | North America | – | 0 | 1 | Moderate | Low |
| Tyuleni virus infection | Europe, USA, Russia | – | 1 | 0 | Moderate | Low |
| Orthomyxoviridae | ||||||
| Bourbon virus infection | USA | – | 0 | 0 | Low | Low |
| Dhori virus infection | Mediterranean | – | 1 | 1 | High | Low |
| Thogoto virus infection | Africa, Asia, Europe | – | 1 | 1 | High | Low |
| Reoviridae | ||||||
| Eyach virus infection | Europe | – | 1 | 1 | High | Low |
| Colorado tick fever | North America | – | 0 | 0 | Low | Low |
| Kemerovo virus infection | Egypt, Slovakia, Russia | – | 1 | 1 | High | Low |
| Tribec virus infection | Europe | – | 1 | 1 | High | Low |
| Protozoa | ||||||
| Babesiidae | ||||||
| Human babesiosis | America, Europe | Endemic | 1 | 1 | High | High |
DEBONEL/TIBOLA: Dermacentor-borne necrosis, erythema and lymphadenopathy/tick-borne lymphadenopathy; LAR: rickettsiosis associated with lymphangitis.
In Spain, Borrelia hispanica relapsing fever.
The risk of emergence/re-emergence is calculated based on two factors: (a) presence of cases of the disease in humans in the last 5 years in Europe, Mediterranean countries and Central and South American countries with a significant relationship with Spain; (b) presence of the vector in Spain.
Each factor is scored with one point (presence in the two factors) or zero points (absence in the two factors) and both are added. The total score can range between 0 and 2, with 0 being: low risk, 1: moderate risk and 2: high risk.
Surveillance and diagnosis of infections transmitted by arthropod vectors
To be able to show a perspective on the AVBD, it is essential to monitor and identify microorganisms in vertebrates and arthropods, designing strategies before their transmission to humans. Early detection and implementation of control strategies allow minimizing the impact on the population. Between 1990 and 2010, 91% of emerging infections spread from a wild-type focal point.2 Occasionally, the infection is spread directly from reservoirs such as bats, rats or chimpanzees to domestic animals, which amplify the infection, or to people; other times, infection dissemination (spill-over) occurs through AV such as ticks, fleas or mosquitoes. In any case, it is essential to carry out a surveillance and to know the microorganisms carried by AV. A recent example in Spain is the detection of CCHFV in ticks of the species Hyalomma lusitanicum collected in deer a few years ago48 in the province of Caceres and the explanation of one of the possible ways of the arrival of the virus to our country.49 Both findings contributed to the early detection of the disease that appeared in 2016 as, to some extent, it was predictable. On September 1, 2016, the Ministry of Health issued a press release reporting the death of a man by CCHF and the contagion of the nurse who had taken care of him in the ICU of the Vallecas Hospital where he had been treated. The first two autochthonous cases of CCHF in Spain were confirmed50 (Fig. 2 ). In the last CCHFV situation report and transmission risk assessment in Spain,51 the virus has been found in a low percentage of ticks of the species H. lusitanicum. This tick does not bite people very often and does not seem to be a good virus vector, although it shares an ecological niche with another very active species (H. marginatum) which bites humans more often. Given this situation, two scenarios could be developed: that of Turkey, where in 2002 the first case was reported and there are currently around 1,000 cases/year, or the scenario in Greece, where the first case was reported in 1975; the second, in 2008 and to date, no new cases have been reported.52
Fig. 2.
Transmission cycle of Crimean-Congo haemorrhagic fever in Spain, 2016 (spill-over). CCHF: Crimean-Congo haemorrhagic fever.
The diagnosis of tick-borne diseases is not always easy. We must bear in mind that a tick bite history is usually absent in at least half of the cases and that incubation periods can be very long. Depending on the size of the tick, they can be very difficult to see (they can simulate a small mole), and their bite is painless. Unless there is awareness or a high index of suspicion when faced with certain signs and/or clinical symptoms, who is going to think of a tick-borne disease? It is clear, “what is not sought, is not found”.52 In 2018, WHO revised the list of emerging pathogens that could cause serious epidemics in the future and those that need to be investigated, including CCHFV, Ebola/Marburg virus, Zika virus, Middle East respiratory syndrome coronavirus (MERS-CoV), severe acute respiratory syndrome coronavirus (SARS-CoV), Lassa virus, Nipah virus and Rift Valley fever virus. In addition, this year the list is completed with disease X, referring to an international epidemic that could be caused by a pathogen whose pathogenic potential and route of transmission is unknown at the moment. This list takes into account the transmissibility between humans, the severity of cases and the percentage of mortality, the difficulty of control and diagnosis and the context of public health and global expansion. In addition, there are other diseases that need more attention as soon as possible: hemorrhagic fevers by other arenaviruses, chikungunya virus, diseases by other highly pathogenic coronaviruses or by emerging enteroviruses and febrile syndrome with severe thrombocytopenia.53 Many of these diseases are AV-borne.
Other arthropod vectors (lice, fleas and ticks)
Another AV that is said to have killed more people than all wars together is the body louse (Human pediculus), transmitting exanthematic or epidemic typhus (Rickettsia prowazekii), endemic recurrent fever (Borrelia recurrentis) and the trench fever (Bartonella quintana). Body lice have been a serious public health problem until recently. They live in the seams of clothes and multiply in cold weather, lack of hygiene and war conditions. A person can be infested with thousands of lice, and each specimen is capable of biting an average of five times a day. It is said that body lice were one of the main problems during the Russian Revolution, where three million people affected by exanthematic typhus died. Thus, Vladimir Ilyich Lenin (1870–1924) stated: Either Socialism defeats the louse, or the louse will defeat Socialism”. Here in Spain, it was also a problem during the post-war period and was used as propaganda by Franco's regime. We can ask ourselves: Is there a risk of an epidemic or an epidemic outbreak of exanthematic typhus? It could happen, as it occurred in Burundi in 1996, when a large epidemic affected more than one hundred thousand patients.54 All the alarms went off when a Red Cross nurse was diagnosed after returning from work in the affected country. Body lice are not seen on the body surface but live in the seams of clothing, in 20 ± 2 °C temperatures. Parasitism by body lice should be suspected in persons with signs of scratching and lack of hygiene, more frequently in cold times of the year. At present, body lice have reappeared in refugee camps in Europe, as in the Second World War. In November 2015, the European Center for Disease Prevention and Control reported the emergence of 27 cases of recurrent fever due to body lice at different points along the route followed by refugees arriving in Italy from the Syrian war.55 In Western Europe, although we have not experienced any epidemic since the post-war period, and the fact that the condition had been eradicated, occasionally, there are R. prowazekii and B. quintana infection reports in homeless people parasitized by lice56 and cases of Brill–Zinsser disease have been described in people who have suffered from exanthematic typhus which could lead to an epidemic outbreak in certain conditions.57
Fleas are other bloodsucking insects of worldwide distribution with an impact in Public Health. Rickettsia can be transmitted to humans by at least two species: the rat flea (Xenopsilla cheopis), which is the endemic or murine typhus vector (caused by Rickettsia typhi), and the cat flea (Ctenocephalides felis), which is the key vector of Rickettsia felis and, occasionally, of R. typhi. As far as we know, rickettsias are not transmitted by the human flea (Pulex irritans). In Europe, murine typhus is a common AVBD in Mediterranean countries such as Greece, Cyprus, Croatia and Spain, including the Canary Islands.58 It develops as a non-specific febrile disease, with or without rash, which is often underdiagnosed. In clinical practice, murine typhus should be included in the differential diagnosis of any patient with fever of intermediate duration, that is, in a patient with fever (more than 38 °C) of more than 7 and less than 28 days of progression, without focality to guide the diagnosis, which remains without a diagnosis after an initial evaluation that includes a complete clinical history, physical examination, complete blood count, chest X-ray and biochemical blood and urine tests.59 R. felis infection is another rickettsiosis with characteristics similar to murine typhus, of which cases have also been published in Spain60 and that should be considered in patients with fever and/or rash, with a history of contact with cats or flea bites. Despite the fact that there have been no reports of autochthonous Yersinia pestis infection transmitted by the rat flea (X. cheopis) in Spain, there is an alert for travellers at the time of writing this manuscript due to an epidemic outbreak in Madagascar that has affected several thousand people.5
Finally, we should remember the phrase written by Hans Zinsser in 1934 in his book entitled: Rats, lice and history: “Nothing in the world of living creatures remains constant. Infectious diseases are constantly changing, new ones are in the process of development and the oldest ones are changing or disappearing”.
Currently, ticks are considered the most dangerous AVs in the world, because of their ease in moving from animals to people, because of their ubiquitous nature (they are present on all continents, including Antarctica) and because of their capacity to agglutinate inside them a host of pathogenic microorganisms potentially transmissible through their hematophagous habits. The human tampering of ecosystems (deforestation, erosion of geographical limits to facilitate travel, etc.) or climate change are some of the factors that are favouring a greater contact between wild animals (with their ticks and the diseases they transmit) and people, facilitating the expansion of ticks to new previously unoccupied areas. Diptera, ticks or other AV may play the leading role in the next pandemic. Therefore, anticipating the next Public Health crisis is in our hands.
Conflict of interests
The authors declare no conflict of interest.
Acknowledgements
We wish to thank Jorge García Labeaga, of URBE Ingeniería, for his collaboration in the preparation of Fig. 1.
Footnotes
Please cite this article as: Portillo A, Ruiz-Arrondo I, Oteo JA. Artrópodos vectores en España y sus enfermedades transmisibles. Med Clin (Barc). 2018;151:450–459.
References
- 1.Marquardt W.C. 2nd ed. Elsevier Academic Press; 2005. Biology of disease vectors. [Google Scholar]
- 2.Karesh W.B., Dobson A., Lloyd-Smith J.O., Lubroth J., Dixon M.A., Bennett M., et al. Ecology of zoonoses: natural and unnatural histories. Lancet. 2012;380:1936–1945. doi: 10.1016/S0140-6736(12)61678-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.USAID (U.S. Agency for International Development) 2016. Emerging pandemic threats. Available from: https://www.usaid.gov/news-information/fact-sheets/emerging-pandemic-threats-program [accessed 21.05.18] [Google Scholar]
- 4.Miller A. In: Infectious diseases. Cohen J., Powderly W.G., editors. Mosby Elsevier; London: 2003. Recreational infections; pp. 955–960. [Google Scholar]
- 5.Plague – Madagascar . 2017. Disease outbreak news. http://www.who.int/csr/don/02-november-2017-plague-madagascar/es [accessed 01.08.18] [Google Scholar]
- 6.Tabachnick W.J. Challenges in predicting climate and environmental effects on vector-borne disease episystems in a changing world. J Exp Biol. 2010;213:946–954. doi: 10.1242/jeb.037564. [DOI] [PubMed] [Google Scholar]
- 7.Wilson M.E. Travel and the emergence of infectious diseases. Emerg Infect Dis. 1995;1:39–46. doi: 10.3201/eid0102.950201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sabatini A., Raineri V., Trovato G., Coluzzi M. Aedes albopictus in Italy and possible diffusion of the species into the Mediterranean area. Parassitologia. 1990;32:301–304. [PubMed] [Google Scholar]
- 9.Collantes F., Delacour S., Delgado J.A., Bengoa M., Torrell-Sorio A., Guinea H., et al. Updating the known distribution of Aedes albopictus (Skuse, 1984) in Spain 2015. Acta Trop. 2016;164:64–68. doi: 10.1016/j.actatropica.2016.08.023. [DOI] [PubMed] [Google Scholar]
- 10.Eritja R., Palmer J.R.B., Roiz D., Sanpera-Calbet I., Bartumeus F. Direct evidence of adult Aedes albopictus dispersal by car. Sci Rep. 2017;7:14399. doi: 10.1038/s41598-017-12652-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Melero-Alcibar R., Tello Fierro A., Marino E., Vázquez M.A. Aedes (Stegomyia) albopictus (Skuse, 1894) (Diptera, Culicidae) primera cita para la Comunidad de Madrid, España. Boln Asoc Esp Ent. 2017;41:515–519. [Google Scholar]
- 12.Rezza G. Chikungunya is back in Italy: 2007–2017. J Travel Med. 2018;25 doi: 10.1093/jtm/tay004. [DOI] [PubMed] [Google Scholar]
- 13.Calba C., Guerbois-Galla M., Franke F., Jeannin C., Auzet-Caillaud M., Grard G., et al. Preliminary report of an autochthonous chikungunya outbreak in France, July to September 2017. Euro Surveill. 2017;22 doi: 10.2807/1560-7917.ES.2017.22.39.17-00647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Velasco E., Cimas M., Díaz O. Enfermedad por virus Chikungunya en España. Bol Epidemiol. 2014;22:219–235. [Google Scholar]
- 15.Epelboin Y., Talaga S., Epelboin L., Dusfour I. Zika virus: an updated review of comp or naturally infected mosquitoes. PLoS Negl Trop Dis. 2017;11:e0005933. doi: 10.1371/journal.pntd.0005933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.La Ruche G., Souarès Y., Armengaud A., Peloux-Petiot F., Delaunay P., Desprès P., et al. First two autochthonous dengue virus infections in metropolitan France, September 2010. Euro Surveill. 2010;15:19676. [PubMed] [Google Scholar]
- 17.European Centre for Disease Prevention and Control . ECDC; Stockholm: 2014. Dengue outbreak in Madeira, Portugal, March 2013. [Google Scholar]
- 18.Gjenero-Margan I., Aleraj B., Krajcar D., Lesnikar V., Klobučar A., Pem-Novosel I., et al. Autochthonous dengue fever in Croatia, August–September 2010. Euro Surveill. 2011;16:19805. [PubMed] [Google Scholar]
- 19.Roiz D., Eritja R., Molina R., Melero-Alcibar R., Lucientes J. Initial distribution assessment of Aedes albopictus (Diptera: Culicidae) in the Barcelona, Spain, area. J Med Entomol. 2008;45:347–352. doi: 10.1603/0022-2585(2008)45[347:idaoaa]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 20.2017. Identificación del mosquito Aedes aegypti en Fuerteventura. Evaluación rápida de riesgo. Available from: http://www.msssi.gob.es/profesionales/saludPublica/ccayes/alertasActual/Eventos_de_salud_publica_en_seguimiento.htm [accessed 01.08.18] [Google Scholar]
- 21.Zika . 2017. Casos diagnosticados en España. Available from: http://www.msssi.gob.es/profesionales/saludPublica/zika/casosDiagnosticados/home.htm [accessed 01.08.18] [Google Scholar]
- 22.Vázquez M.C., Santa Olalla P., Cortés M., Sierra M.J., Amela Heras C., Lucientes J., et al. Centro de Coordinación de Alertas y Emergencias sanitarias (CCAES), Ministerio de Sanidad, Servicios Sociales e Igualdad; 2015. Informe de situación y evaluación del riesgo para España de Paludismo. [Google Scholar]
- 23.Ruiz-Arrondo I., Hernández Traiana L., Oteo Revuelta J.A. Fauna de mosquitos (Diptera, Culicidae) presentes en el humedal de La Grajera (Logroño) y sus implicaciones en Salud Pública. Zubía. 2017;35:123–140. [Google Scholar]
- 24.2016. Malaria – Annual Epidemiological Report 2016 [2014 data] Available from: https://ecdc.europa.eu/en/publications-data/malaria-annual-epidemiological-report-2016-2014-data [accessed 01.08.18] [Google Scholar]
- 25.Olaso A., López-Ballero M.F. Really malaria-free Europe? Enferm Infecc Microbiol Clin. 2018;36:251–255. doi: 10.1016/j.eimc.2017.07.008. [DOI] [PubMed] [Google Scholar]
- 26.Arce A., Estirado A., Ordobas M., Sevilla S., García N., Moratilla L., et al. Re-emergence of leishmaniasis in Spain: community outbreak in Madrid, Spain, 2009 to 2012. Euro Surveill. 2013;18:20546. doi: 10.2807/1560-7917.es2013.18.30.20546. [DOI] [PubMed] [Google Scholar]
- 27.Antoniou M., Gramiccia M., Molina R., Dvorak V., Volf P. The role of indigenous phlebotomine sandflies and mammals in the spreading of leishmaniasis agents in the Mediterranean region. Euro Surveill. 2013;18:20540. doi: 10.2807/1560-7917.es2013.18.30.20540. [DOI] [PubMed] [Google Scholar]
- 28.Leishmaniasis en la Comunidad de Madrid . 2014. Documentos Técnicos de Salud Pública. Subdirección de Promoción de la Salud y Prevención. Dirección General de Atención Primaria; p. 4. [Google Scholar]
- 29.Sanbonmatsu-Gámez S., Pérez-Ruiz M., Collao X., Sánchez-Seco M.P., Morillas-Márquez F., de la Rosa-Fraile M., et al. Toscana virus in Spain. Emerg Infect Dis. 2005;11:1701–1707. doi: 10.3201/eid1111.050851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Moriconi M., Rugna G., Calzolari M., Bellini R., Albieri A., Angelini P., et al. Phlebotomine sand-fly-borne pathogens in the Mediterranean Basin: human leishmaniasis and phlebovirus infections. PLoS Negl Trop Dis. 2017;11:e0005660. doi: 10.1371/journal.pntd.0005660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Papa A., Papadopoulou E. Acute viral infections of the central nervous system, 2014–2016, Greece. J Med Virol. 2017 doi: 10.1002/jmv.24997. [DOI] [PubMed] [Google Scholar]
- 32.2018. Surveillance and disease data for West Nile fever. Available from: https://ecdc.europa.eu/en/west-nile-fever/surveillance-and-disease-data [accessed 01.08.18] [Google Scholar]
- 33.Sánchez A., Amela C., Santos S., Suárez B., Simón F., Sierra M.J., et al. Centro de Coordinación de Alertas y Emergencias Sanitarias (CCAES), Ministerio de Sanidad, Servicios Sociales e Igualdad; 2013. Informe de situación y evaluación del riesgo para España de Virus del Nilo Occidental. [Google Scholar]
- 34.Kaptoul D., Viladrich P.F., Domingo C., Niubó J., Martínez-Yélamos S., De Ory F., et al. West Nile virus in Spain: report of the first diagnosed case (in Spain) in a human with aseptic meningitis. Scand J Infect Dis. 2007;39:70–71. doi: 10.1080/00365540600740553. [DOI] [PubMed] [Google Scholar]
- 35.García-Bocanegra I., Jaén-Téllez J.A., Napp S., Arenas-Montes A., Fernández-Morente M., Fernández-Molera V., et al. West Nile fever outbreak in horses and humans, Spain, 2010. Emerg Infect Dis. 2011;17:2397–2399. doi: 10.3201/eid1712.110651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.García-Bocanegra I., Belkhiria J., Napp S., Cano-Terriza D., Jiménez-Ruiz S., Martínez-López B. Epidemiology and spatio-temporal analysis of West Nile virus in horses in Spain between 2010 and 2016. Transbound Emerg Dis. 2017 doi: 10.1111/tbed.12742. [DOI] [PubMed] [Google Scholar]
- 37.Sánchez-Gómez A., Amela C., Fernández-Carrión E., Martínez-Avilés M., Sánchez-Vizcaíno J.M., Sierra-Moros M.J. Risk mapping of West Nile virus circulation in Spain, 2015. Acta Trop. 2017;169:163–169. doi: 10.1016/j.actatropica.2017.02.022. [DOI] [PubMed] [Google Scholar]
- 38.Hunter D.J., Frumkin H., Jha A. Preventive medicine for the planet and its peoples. N Engl J Med. 2017;376:1605–1607. doi: 10.1056/NEJMp1702378. [DOI] [PubMed] [Google Scholar]
- 39.Semenza J.C., Suk L.E. Vector-borne diseases and climate change: a European perspective. FEMS Microbiol Lett. 2018;365 doi: 10.1093/femsle/fnx244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Patz J.A., Campbell-Lendrum D., Holloway T., Foley J.A. Impact of regional climate change on human health. Nature. 2005;438:310–317. doi: 10.1038/nature04188. [DOI] [PubMed] [Google Scholar]
- 41.Medlock J.M., Hansford K.M., Bormane A., Derdakova M., Estrada-Peña A., George J.C., et al. Driving forces for changes in geographical distribution of Ixodes ricinus ticks in Europe. Parasites Vectors. 2013;6:1. doi: 10.1186/1756-3305-6-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Oteo J.A., Portillo A. Tick-borne rickettsioses in Europe. Ticks Tick Borne Dis. 2012;3:271–278. doi: 10.1016/j.ttbdis.2012.10.035. [DOI] [PubMed] [Google Scholar]
- 43.Palomar A.M., Portillo A., Eiros J.M., Oteo J.A. Virology II – advanced issues. iConcept Press; Hong Kong: 2014. The risk of introducing tick-borne encephalitis and Crimean-Congo hemorrhagic fever into Southwestern Europe (Iberian Peninsula) [Google Scholar]
- 44.Palomar A.M., Santibáñez P., Mazuelas D., Roncero L., Santibáñez S., Portillo A., et al. Role of birds in dispersal of etiologic agents of tick-borne zoonoses, Spain, 2009. Emerg Infect Dis. 2012;18:1188–1191. doi: 10.3201/eid1807.111777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Palomar A.M., García-Álvarez L., Santibáñez S., Portillo A., Oteo J.A. Detection of tick-borne ‘Candidatus Neoehrlichia mikurensis’ and Anaplasma phagocytophilum in Spain in 2013. Parasites Vectors. 2014;7:57. doi: 10.1186/1756-3305-7-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Palomar A.M., Portillo A., Santibáñez P., Santibáñez S., Oteo J.A. Borrelia miyamotoi: should this pathogen be considered for the diagnosis of tick-borne infectious diseases in Spain? Enferm Infecc Microbiol Clin. 2017 doi: 10.1016/j.eimc.2017.10.020. [DOI] [PubMed] [Google Scholar]
- 47.De Sousa R., Luz T., Parreira P., Santos-Silva M., Bacellar F. Boutonneuse fever and climate variability. Ann N Y Acad Sci. 2006;1078:162–169. doi: 10.1196/annals.1374.029. [DOI] [PubMed] [Google Scholar]
- 48.Estrada-Peña A., Palomar A.M., Santibáñez P., Sánchez N., Habela M.A., Portillo A., et al. Crimean-Congo hemorrhagic fever virus in ticks, Southwestern Europe, 2010. Emerg Infect Dis. 2012;18:179–180. doi: 10.3201/eid1801.111040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Palomar A.M., Portillo A., Santibáñez P., Mazuelas D., Arizaga J., Crespo A., et al. Crimean-Congo hemorrhagic fever virus in ticks from migratory birds, Morocco. Emerg Infect Dis. 2013;19:260–263. doi: 10.3201/eid1902.121193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Negredo A., de la Calle-Prieto F., Palencia-Herrejón E., Mora-Rillo M., Astray-Mochales J., Sánchez-Seco M.P., et al. Autochthonous Crimean-Congo hemorrhagic fever in Spain. N Engl J Med. 2017;377:154–161. doi: 10.1056/NEJMoa1615162. [DOI] [PubMed] [Google Scholar]
- 51.Sierra M.J., Suárez B., García San Miguel L., Palmera R., Reques L., Simón F., et al. Ministerio de Sanidad, Servicios Sociales e Igualdad; 2017. Informe de situación y evaluación del riesgo de transmisión de Fiebre Hemorrágica de Crimea-Congo (FHCC) en España. [Google Scholar]
- 52.Oteo J.A., Palomar A.M. Fiebre hemorrágica de Crimea-Congo: «lo que no se busca no se encuentra». Med Clin (Barc) 2018;150:266–267. doi: 10.1016/j.medcli.2017.07.003. [DOI] [PubMed] [Google Scholar]
- 53.2018. List of Blueprint priority diseases. Available from: http://www.who.int/blueprint/priority-diseases/en/ [accessed 01.08.18] [Google Scholar]
- 54.Raoult D., Ndihokubwayo J.B., Tissot-Dupont H., Roux V., Faugere B., Abegbinni R., et al. Outbreak of epidemic typhus associated with trench fever in Burundi. Lancet. 1998;352:353–358. doi: 10.1016/s0140-6736(97)12433-3. [DOI] [PubMed] [Google Scholar]
- 55.2015. Rapid risk assessment: communicable disease risks associated with the movement of refugees in Europe during the winter season. Available from: https://ecdc.europa.eu/en/publications-data/rapid-risk-assessment-communicable-disease-risks-associated-movement-refugees [accessed 01.08.18] [Google Scholar]
- 56.Brouqui P., Stein A., Dupont H.T., Gallian P., Badiaga S., Rolain J.M., et al. Ectoparasitism and vector-borne diseases in 930 homeless people from Marseilles. Medicine (Baltimore) 2005;84:61–68. doi: 10.1097/01.md.0000152373.07500.6e. [DOI] [PubMed] [Google Scholar]
- 57.Faucher J.F., Socolovschi C., Aubry C., Chirouze C., Hustache-Mathieu L., Raoult D., et al. Brill–Zinsser disease in Moroccan man, France, 2011. Emerg Infect Dis. 2012;18:171–172. doi: 10.3201/eid1801.111057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Portillo A., Santibáñez S., García-Álvarez L., Palomar A.M., Oteo J.A. Rickettsioses in Europe. Microbes Infect. 2015;17:834–838. doi: 10.1016/j.micinf.2015.09.009. [DOI] [PubMed] [Google Scholar]
- 59.Oteo J.A. Fever of intermediate duration: new times, new tools and change of spectrum. Enferm Infecc Microbiol Clin. 2010;28:407–408. doi: 10.1016/j.eimc.2010.04.003. [DOI] [PubMed] [Google Scholar]
- 60.Oteo J.A., Portillo A., Santibáñez S., Blanco J.R., Pérez-Martínez L., Ibarra V. Cluster of cases of human Rickettsia felis infection from Southern Europe (Spain) diagnosed by PCR. J Clin Microbiol. 2006;44:2669–2671. doi: 10.1128/JCM.00366-06. [DOI] [PMC free article] [PubMed] [Google Scholar]