Abstract
Hemorrhagic transformation (HT) is one of the most common adverse events related to acute ischemic stroke (AIS) that affects the treatment plan and clinical outcome. Identification of a sensitive radiological marker may influence the controversial thrombolytic decision in the setting of AIS and may at a minimum indicate more intensive monitoring or further prophylactic interventions. In this article we summarize possible radiological biomarkers and the role of different radiological modalities including computed tomography (CT), magnetic resonance imaging, angiography, and ultrasound in predicting HT. Different radiological indices of early ischemic changes, large ischemic lesion volume, severe blood flow restriction, blood-brain barrier disruption, poor collaterals and high blood flow velocities have been reported to be associated with higher risk of HT. The current levels of evidence of the available studies highlight the role of the different CT perfusion parameters in predicting HT. Further large standardized studies are recommended to compare the sensitivity and specificity of the different radiological markers combined and delineate the most reliable predictor.
Keywords: Computed tomography, Digital Subtraction Angiography, hemorrhagic transformation, magnetic resonance, predictors
Introduction
Hemorrhagic transformation (HT) is one of the most common and serious challenges in management of acute ischemic stroke (AIS) that affects both the treatment plan and the clinical prognosis. It is still one of the most feared complications of thrombolysis that restricts its indications. The identification of markers for HT might be helpful in improving the risk:benefit ratio of thrombolytic therapy.1
Definition, pathology and clinical presentation of HT
HT is defined as an infarcted, hypodense area in or around which various degrees of hyperdensity appear on unenhanced computed tomography (CT) scans. According to the European Cooperative Acute Stroke Study (ECASS II), HTs are radiologically classified according to their appearance on CT as hemorrhagic infarction (HI) or parenchymal hemorrhage (PH). HI1 is defined as small petechiae along the margins of the ischemia; HI2, as confluent petechiae within the infarcted zone but no mass effect; PH1, as blood clots in 30% or less of the ischemic zone with mild mass effect; and PH2, as blood clots in more than 30% of the ischemic area with a marked mass effect.2
The symptoms of HT vary widely from being asymptomatic to significant deterioration depending on its site and size. The ECASS II defines the intracranial hemorrhage to be symptomatic if causing an increase in the National Institutes of Health Stroke Scale (NIHSS) score of four or more points and if the hemorrhage is found to probably be the cause of clinical deterioration.2 László and Hortobágyi reported that generally HI can be considered as a sign of recanalization because it is mostly does not affect the prognosis or long-term clinical outcome; on the contrary, PH is considered a comorbidity because of its negative influence on the prognosis.3
HT pathophysiology is still not fully understood. Cerebral ischemia initiates a cascade of cellular, metabolic and inflammatory events that result in disruption of the blood-brain barrier (BBB) and the impairment of the autoregulatory capacity of the cerebral blood vessels, predisposing them to blood extravasation on reperfusion of the ischemic tissue.4 Various clinical factors have been reported to be associated with increased risk of HT, such as advancing age, pretreatment of hypertension, atrial fibrillation, cardioembolic stroke, high NIHSS, hyperglycemia, low lipid profile (total cholesterol and low-density lipoprotein), elevated globulin level, albuminuria, lower platelet count, delayed reperfusion, intravenous tissue plasminogen activator recombinant (IV rt-PA), concurrent use of antithrombotic agents or anticoagulants, fibrinolytic agents, and endovascular treatment.5,6 Massive cerebral infarction and the volume of gray-matter involvement are also among the most dangerous factors of HT development.6,7
Epidemiology and rate of HT
The appearance of HT ranges from between 10% and 40% on CT, and 40% to 70% on autopsy.5 This wide range is attributed to different individual risk factors. HIs represent 89% of the total HTs, whereas PHs account for the remainder.5 The incidence of symptomatic HT ranges from 0.6% to 20%.2,5
Management of patients with ischemic stroke and HT
HT represents the most feared complication and main limitation facing the use of thrombolytic therapy.1 History of intracranial hemorrhage (ICH), severe uncontrolled hypertension, serious head trauma or stroke in the previous three months, recent use of anticoagulation, thrombocytopenia and coagulopathy are considered as absolute contraindications for IV rt-PA use for treatment of AIS because of the associated high risk of HT.1 Multiple clinically based risk-predictive scores have been proposed to predict the risk of symptomatic HT.8–13 Despite the high sensitivity of these scores to symptomatic HT, high risk scores are not enough to withhold thrombolytic therapy because these patients are still likely to benefit from alteplase.14 These cases require close monitoring and follow-up. The general guidelines of treatment of HT are quite similar to the treatment of spontaneous ICH but increase the risk of worsening ischemia.14 The risk:benefit ratio of treatment has to be weighed; the use of reversal agents such as cryoprecipitate, platelets, fresh-frozen plasma, prothrombin complex concentrate, vitamin K, recombinant factor VIIa and antifibrinolytic agents are indicated in treatment of symptomatic ICH or asymptomatic cases with evidence of coagulopathy. Neurosurgical evacuation of the hematoma may be warranted in some cases.14 Moreover, HT also represents a challenge against initiating a secondary prophylactic antiplatelet or anticoagulants.15
Endovascular treatment was reported to be associated with higher risk of HT than thrombolytic therapy. The incidence of PH after endovascular treatment was recently reported to be 6.0%.16
Economic burden of HT
HT-induced increase in morbidity and mortality subsequently carries a higher economic and social burden than that already reported with ischemic stroke.17 The increased health-care cost including treatment, duration and quality of care and repeated imaging required for follow-up, and the non–health-care cost associated with decreased productivity, warrant further efforts to predict and prevent hemorrhagic adverse events.17,18
Aim of the work
The identification of markers for HT might be helpful in improving the risk:benefit ratio of thrombolytic therapy. Besides being a diagnostic and classifying tool, neuroimaging may also predict the occurrence of HT. In this article we review the possible radiological biomarkers and the role of different radiological modalities including CT, magnetic resonance imaging (MRI), digital subtraction angiography (DSA) and ultrasound (US) in predicting HT in light of administrated treatment, including intravenous (IV) thrombolysis and/or thrombolytic or mechanical intra-arterial (IA) therapy.
Methodology and search strategy
We conducted a systematic review of the literature using a database search of MEDLINE (Bethesda, MD), PubMed, Scopus, and Web of Science (Clarivate Analytics, PA) to identify studies addressing 1) AIS, 2) HT and 3) relevant neuroimaging methods. The search covered January 1990 to November 2019 using the following key terms: 1) “Hemorrhagic transformation” AND/ OR 2) “Predictors”, AND one of the following from 3): “CT or MR, or DSA, or US.” We expanded our retrieval to also include clinical trials, cohort studies, multicenter studies, comparative studies, case-control studies, and case reports having more than five participants for key questions regarding HT. Letters, hospital bulletins and single case reports were excluded.
The level of evidence (LOE) was adopted from Ackley et al. as follows: level 1: systematic review or meta-analysis, level II: randomized controlled trial, level III: nonrandomized controlled trials, level IV: case control studies, cohort studies, level V: meta-synthesis, and level VI: single descriptive or qualitative study.19
Discussion
Impact of CT imaging on the prediction of HT after AIS
Noncontrast CT
The availability, speed and accessibility of noncontrast CT of the brain are the main reasons that this examination remains the diagnostic procedure of choice in patients presenting with acute ischemic stroke (AIS) to exclude pretreatment intracranial hemorrhage.20
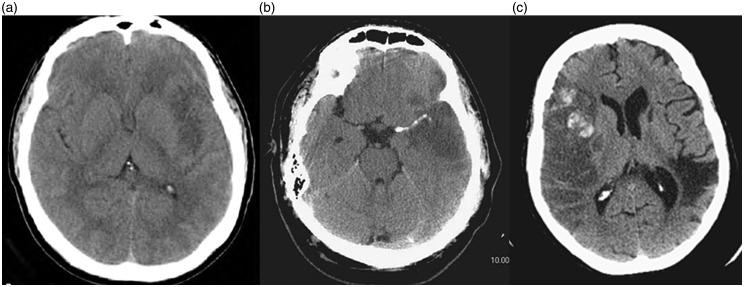
Despite being nonsensitive to early infarctions, some early subtle CT findings strongly predict HT, including the loss of differentiation of the lentiform nucleus and insular ribbon, and effacement of the sulci, either alone or with a hyperdense middle cerebral artery sign (HMCAS) (Figure 1).20 HMCAS is a common sign in the anterior circulation infarction and is independently predictive of HT after thrombolytic therapy; it is also associated with increased severity and more frequent early ischemic changes on the baseline CT scan than those without HMCAS (LOE: IV).21
Figure 1.
Noncontrast computed tomography (CT) of the brain showing (a) early sign of cerebral ischemia, (b) hyperdense middle cerebral artery sign and (c) Alberta Stroke Program Early CT Score 5 with evidence of hemorrhagic transformation.
Alberta Stroke Program Early CT Score (ASPECTS) is used as a quantitative CT score of acute anterior-circulation ischemic stroke (Figure 1). Low ASPECTS (<7) indicating large infarctions are associated with increased stroke severity and increased risk of HT (LOE: IV, II and IV, respectively).22–24
CT angiography
CT angiography (CTA) is available in most emergency departments and stroke centers, providing reliable, rapid and safe diagnosis of occlusion of major intracranial arteries.25
Several CTA imaging parameters related to ischemic lesions have been associated with an increased risk of HT such as proximal large-vessel occlusion and the extent of collaterals.
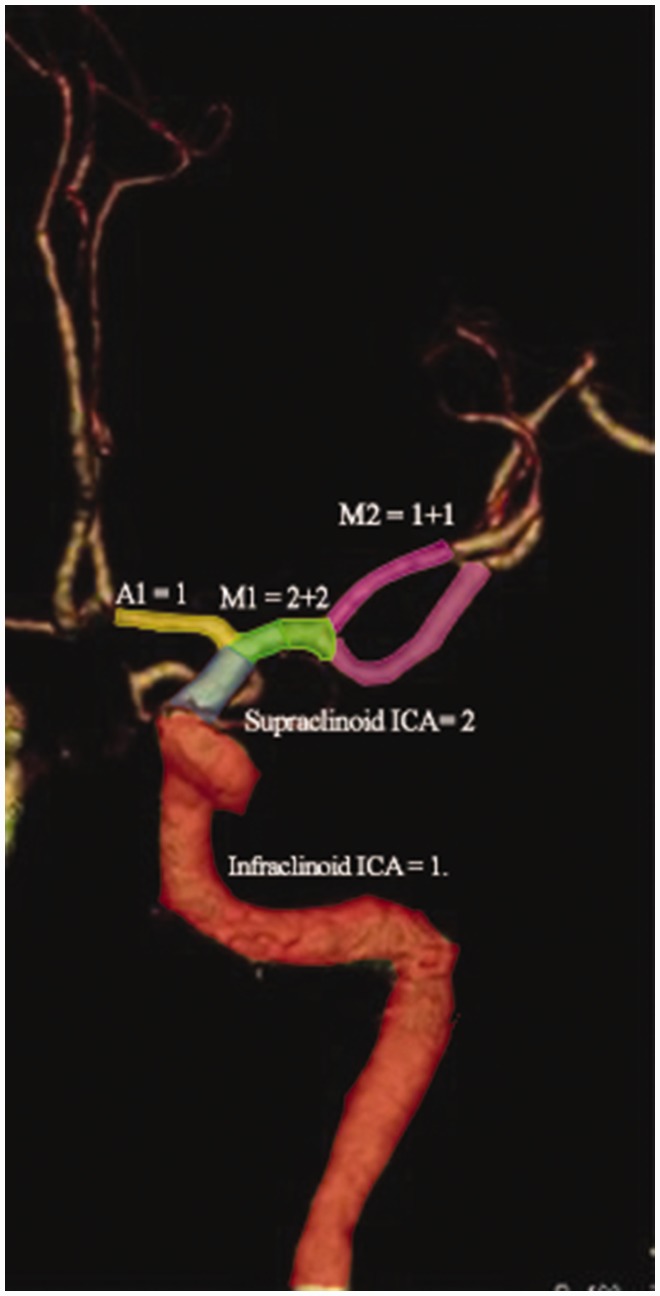
In 2008 Peutz and colleagues introduced the clot burden score (CBS), which scores major anterior circulation arteries at 10 points for presence of contrast opacification on CTA. Two points are subtracted for each thrombus preventing contrast opacification in the proximal M1, distal M1 or supraclinoid internal carotid artery (ICA) and one point each for M2 branches, A1 and infraclinoid ICA (Figure 2). Lower CBS were associated with lower follow-up ASPECTS and higher parenchymal hematoma rates (LOE: IV). The quantification of the extent of intracranial thrombus with the CBS acutely predicts the prognosis, final infarct volume and HT risk (LOE: IV).25 Similar results were reported by further studies (LOE: IV).26,27
Figure 2.
Assessment of clot burden score: Two points are subtracted for each thrombus preventing contrast opacification in the proximal M1, distal M1 or supraclinoid internal carotid artery (ICA) and one point each for M2 branches, A1 and infraclinoid ICA.
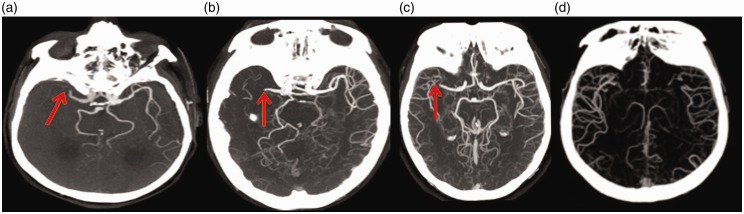
Large-vessel obliteration and poor collateral state on CTA have been shown to be associated with higher risk of HT (LOE: IV).24,26,28 In 2012 Lin et al. reviewed three variables in CTA: (1) distal ICA and/or M1 segment of the MCA, (2) ASPECTS and (3) collateral score.24 The collaterals were scored on reviewing the source images using a four-point scale from 0 to 3 as previously introduced by Kim and colleagues:29 “0”—absent collateral supply; “1”—collaterals filling between 0% and 50%; “2”—collateral supply filling from 50% to 100%; and “3”—collateral supply filling 100% of the occluded MCA territory in comparison to the other side (Figure 3). HT univariate predictors were proximal vessel occlusion (p = 0.049), low collateral score (p = 0.017) and low ASPECTS (p = 0.001). Multivariable analysis found ASPECTS to be the only independent predictor.24 More recent studies reported the low collateral score as an significant predictor of hemorrhagic transformation.26,30
Figure 3.
Computed tomography brain angiography showing M1 occlusion with (a) absent collaterals, collateral score 0; (b) collateral filling less than 50% (collateral score: 1); (c) collateral filling greater than 50% (collateral score: 2) and (d) collateral filling 100% (collateral score: 3).
CT perfusion
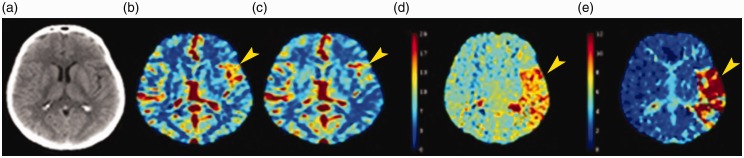
Computed tomography perfusion (CTP) can distinguish between the potentially salvageable “penumbra” and irreversibly damaged brain tissue “infarct core” via quantitative assessments of cerebral blood flow (CBF), cerebral blood volume (CBV) and mean transit time (MTT) (Figure 4). CBV is defined as the total blood volume in a given region of the brain. MTT is defined as the average transit time of blood through a given brain region. CBF is defined as the CBV per a specific amount of time (CBF = CBV/MTT).31 Absolute CTP parameters are values of a certain brain region, whereas the relative parameters are the values measured in the pathological hemisphere expressed as a percentage of the values measured in the contralateral normal hemisphere.31
Figure 4.
(a) Noncontrast computed tomography; computed tomography perfusion maps including (b) cerebral blood volume, (c) cerebral blood flow, (d) mean transit time and (e) Tmax showing a large area of matched deficit indicative of core infarct in the right middle cerebral artery territory.
There is no agreement on which CTP parameter is the most predictive of HT. Some studies have shown that CTP parameters of the whole infarct area involving the penumbra and infarct core could be used to predict HT, finding a relative CBV (rCBV) less than or equal to 1.09 and Tmax greater than 14 seconds, respectively, to be the most predictive of HT, with a relative CBF (rCBF) less than 30% also being of considerable utility in predicting HT (LOE: IV).5,32 Others found rCBF to have the highest HT predictive value (LOE: IV).33 One study found neither absolute CBF nor CBV to be significantly correlated to HT; relative CTP parameters were not examined.19 Another study focused on the absolute CTP parameters of the infarction core, finding a CBV of less than or equal to 0.5 ml/100 g to be predictive of HT, but not investigating relative CTP parameters (LOE: IV).34 The infarcted region volume derived from MTT maps and rCBV values at admission have been reported to be strongly associated with HT (LOE: IV).35,36
CTP also can assess the permeability surface product (PS). PS measures the rate by which the contrast material extravasates from the intravascular to the extravascular space through a disrupted BBB, reflecting the permeability of the BBB involved in the pathophysiology of HT of ischemic stroke. High PS has increased risk of occurrence of HT after AIS (LOE: IV).27,34,37,38
A recently published high LOE (I) systematic review found the CTP imaging appearance suggestive of high BBB permeability, and acute severe hypoperfusion (i.e. CBV < 0.5 ml/100 g; rCBV = 1.09; rCBF < 0.48; Tmax > 14 s; rMTT.1.3; TTP 0.27 s) are highly sensitive and predictive for the risk of HT.39
Single-photon emission computed tomography
Single-photon emission computed tomography (SPECT) is a nuclear medicine tomographic imaging technique using gamma rays that is able to create three-dimensional images. SPECT is used in cases of AIS to assess the ischemic defect along with residual blood flow.40,41 The CBF reduction severity in SPECT has also been reported to be associated with a higher risk of HT (LOE: IV).40–43
Brief conclusion
The reviewed LOE of the role of the different CT imaging modalities in the prediction of HT after AIS (summarized in Table 1) highlights the superiority of the different CT perfusion parameters in the prediction of HT after IV thrombolysis and/or thrombolytic or mechanical IA therapy (LOE: I).
Table 1.
Review of LOE of the role of CT imaging in the prediction of hemorrhagic transformation.
| Study | LOEa | No of Study Participants | Treatment Modality (%) | OR 95% CI, p | |
|---|---|---|---|---|---|
| Noncontrast CT | |||||
| Early ischemia sign | Jaillard et al.20 | IV | 237 | IV streptokinase | 2.6 (1.5–4.4), p = 0.002 |
| Hyperdense middle cerebral artery sign | Zou et al.21 | IV | 182 | IV thrombolysis (100) | 2.691 (1.231–5.882), p = 0.013 |
| Low Alberta Stroke Program Early CT Score | Barber et al.22 | IV | 203 | IV thrombolysis (100) | 14 (18–117), p = 0·012 |
| Dzialowski et al.23 | II | 800 | IV thrombolysis (100) | 18.9 (2.6–138) | |
| Lin et al.24 | IV | 83 | IV thrombolysis and/or thrombolytic or mechanical IA therapy (52.4) | 0.731 (0.561–0.953), p = 0.021 | |
| CTA | |||||
| Clot burden score | Puetz et al.25 | IV | 263 | IV thrombolysis and/or thrombolytic or mechanical IA therapy (49.8) | p = 0.003 |
| Fanou et al.26 | IV | 395 | IV thrombolysis (69.1) | 0.46 (0.23–0.90), p = 0.0253 | |
| Horsch et al.27 | IV | 545 | IV thrombolysis and/or thrombolytic or mechanical IA therapy (100) | 1.28, (1.16–1.41) | |
| Large-vessel occlusions | Sims et al.28 | IV | 47 | IV thrombolysis (100) | p = 0.04 |
| Lin et al.24 | IV | 83 | IV thrombolysis and/or thrombolytic or mechanical IA therapy (46.9) | p = 0.049 | |
| Fanou et al.26 | IV | 395 | IV thrombolysis (69.1) | 2.22 (1.21–4.12), p = 0.0102 | |
| Poor collateral vessels | Lin et al.24 | IV | 83 | IV thrombolysis and/or thrombolytic or mechanical IA therapy (46.9) | p = 0.017 |
| Fanou et al.26 | IV | 395 | IV thrombolysis (69.1) | 0.36 (0.14–0.88), p = 0.0284 | |
| van Kranendonk et al.30 | IV | 478 | IV thrombolysis and/or thrombolytic or mechanical IA therapy | 1.90 (1.05–3.42) | |
| CTP | |||||
| Decreased rCBF | Yassi et al.5 | IV | 132 | IV thrombolysis (53) | p = 0.021 |
| Langel and Popovic33 | IV | 75 | IV thrombolysis (100) | p < 0.001 | |
| Decreased CBV | Jain et al.32 | IV | 83 | IV thrombolysis (27.7) | 1.14 (1.05–1.25), p = 0.009 |
| Increased MTT | Yassi et al.5 | IV | 132 | IV thrombolysis (53) | p = 0.002 |
| Souza et al.35 | IV | 96 | IV thrombolysis and/or thrombolytic or mechanical IA therapy | 3.7, p = 0.007 | |
| Increased permeability surface product | Horsch et al.27 | IV | 545 | IV thrombolysis and/or thrombolytic or mechanical IA therapy (100) | 2.22 (1.46–3.37), p = 0.001 |
| Aviv et al.34 | IV | 41 | IV thrombolysis and/or thrombolytic or mechanical IA therapy (54) | 3.5 (1.69, 7.06) p = 0.0007 | |
| Hom et al.37 | IV | 32 | IV thrombolysis and/or thrombolytic or mechanical IA therapy | 1.71, p = 0.01 | |
| Ozkul-Wermester et al.38 | IV | 86 | IV thrombolysis (36) | 28 (1.75–452.98), p = 0.02 | |
| High BBB permeability and acute severe hypoperfusion (i.e. CBV < 0.5 ml/100 g; rCBV = 1.09; rCBF < 0.48; Tmax > 14 s; rMTT .1.3; TTP 0.27 s) | Adebayo and Culpan39 | I | 808 | IV thrombolysis and/or thrombolytic or mechanical IA therapy | (95% CI; 65%–97%) |
| SPECT | |||||
| CBF reduction | Hanson et al.40 | IV | 15 | NA | p < 0.005 |
| Ueda et al.41 | IV | 34 | IA thrombolysis | p < 0.05 | |
| Alexandrov et al.42 | IV | 185 | None | 17.40 (2.69–170.89) p < 0.05 | |
BBB: blood-brain barrier; CBF: cerebral blood flow; CBV: cerebral blood volume; CI: confidence interval; CT: computed tomography; CTA: computed tomography angiography; CTP: computed tomography perfusion; IA: intra-arterial; IV: intravenous; LOE: level of evidence; MTT: mean transit time; NA: not available; OR: odds ratio; rCBF: relative cerebral blood flow; rCBV: relative cerebral blood volume; rMTT: relative mean transit time; SPECT: single-photon emission computed tomography; TTP: time to peak. aLOE adopted from Ackley et al. as follows: level 1: systematic review or meta-analysis, level II: randomized controlled trial, level III: nonrandomized controlled trials, level IV: case-control studies, cohort studies, level V: meta-synthesis, and level VI: single descriptive or qualitative study.20
Impact of MRI on prediction of HT after AIS
Noncontrast MRI
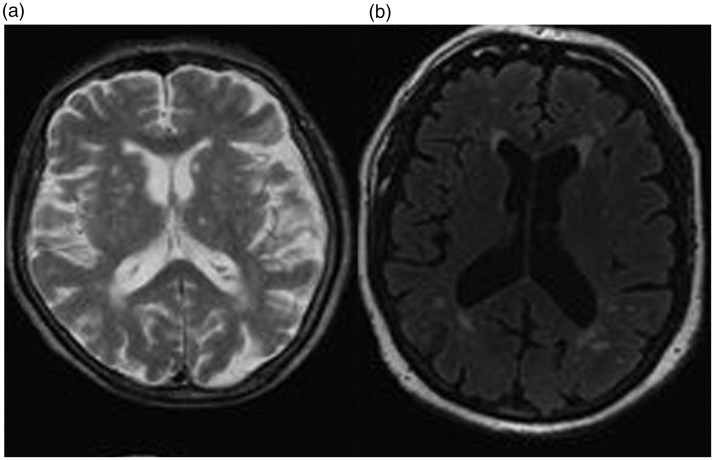
Leukoaraiosis (LA) is defined as a diffuse, confluent white-matter abnormality (with hyperintensity on T2-weighted or fluid-attenuated inversion recovery (FLAIR) MRI sequences) (Figure 5). LA of the deep white matter is an independent risk factor for HT after thrombolytic treatment for acute stroke (LOE: IV).44,45 Yang et al. could not find an association between LA and risk of symptomatic ICH.46
Figure 5.
Noncontrast magnetic resonance imaging showing evidence of leukoaraiosis in the form of hyperintensity both in T2 and fluid-attenuated inversion recovery sequences.
Contrast-enhanced MRI
Contrast enhancement at T1 after contrast media administration, which is indicative of a BBB leak, may be another important tool in assessing the risk of HT. Early parenchymal enhancement was observed in mice subjected to temporary MCA occlusion that subsequently developed petechial hemorrhage (LOE: IV).44,47,48 In ischemic stroke patients treated with thrombolysis, parenchymal enhancement shown on immediate post-treatment CT scans is well correlated with subsequent HT (LOE: IV).49–52
Another reported sign of BBB defect is extravasation of contrast agent into cerebrospinal fluid (CSF) resulting in poor fluid suppression on FLAIR MRI, and hyperintensity of the CSF space termed hyperintense acute reperfusion marker (HARM).53 Latour et al. retrospectively identified HARM in 53% of tPA-treated patients, 46% of whom developed HT, and found a correlation between HARM and reperfusion within one week (LOE: IV).54 Another study could not find enough evidence of HARM as a predictor of HT.52
Sulcal hyperintensity (cortical gadolinium enhancement) on FLAIR is another pattern attributed to BBB disruption. Cho et al. reported that such FLAIR hyperintensity was predictive of symptomatic hemorrhage after thrombolysis (LOE: IV).55 Sulcal hyperintensity on FLAIR was also documented to be significantly associated with HT after IA thrombolysis (LOE: IV).56
Diffusion-weighted MRI
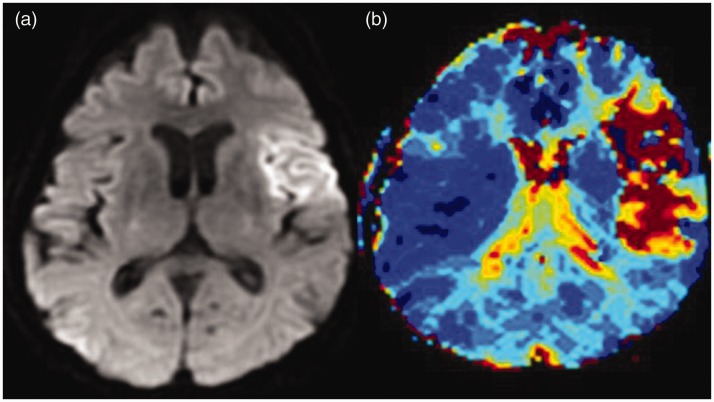
Recently, the use of diffusion-weighted MRI (DWI) has increased to guide therapeutic decisions regarding thrombolysis, especially for patients outside the three-hour window (Figure 6).57 Several studies suggest that DWI findings may help to identify patients who are at increased risk for HT after IV rt-PA. The size of infarction on DWI was reported as a predictive parameter of symptomatic HT by Singer et al., who applied the ASPECTS score to DWI images in a study that predicted HT risk after IV rt-PA (LOE: IV).58
Figure 6.
Magnetic resonance (a) diffusion and (b) perfusion showing evidence of restricted blood flow in the left middle cerebral artery territory.
Assessment of the water apparent diffusion coefficient (ADC) in the DWI lesions shows that significantly lower ADC values are found more in DWI lesion regions that develop ICH than in viable or necrotic regions after rt-PA. ADC values less than or equal to 550 × 10–6 mm2 per second were found to be a predictor of HT (LOE: IV).59,60 Another study reported that the volume of initial DWI lesion and ADC values both predict HT, but only ADC values less than or equal to 550 × 10–6 mm2 per second persisted as an independent predictor after multivariable analysis (LOE: IV).61
Very low CBV (VLCBV), defined as CBV less than the 2.5th percentile of normal contralateral brain, was reported to predict HT after thrombolysis better than did DWI or ADC volume (LOE: IV).62 Both baseline DWI lesion volume and VLCBV were more predictive of HT when compared to sulcal hyperintensity on FLAIR (LOE: IV).63
Perfusion-weighted MRI
Similar to diffusion-weighted (DWI), perfusion-weighted MRI (PWI) can detect tissue ischemia earlier than other conventional neuroimaging modalities in experimental and clinical settings (Figure 6). Prolonged perfusion deficit at three to six hours after the initial MRI scan was identified in significantly more of the area of subsequent HT (LOE: IV).60
PWI was also used to assess BBB integrity. Bang and colleagues initially reported that BBB derangements as shown by decreased signal intensity at later time points in T2* PWI can identify patients at risk for HT after recanalization therapy (LOE: IV).64 However, they later reported that the permeability of the BBB is a dynamic process and that HT types may differ to the extent that, in some patients, permeability abnormalities may not be associated with HT.65 In a prospective study, percentage recovery and the relative recirculation of the contrast agent as another BBB permeability marker, derived from T2* PWI, were shown to be significantly higher in patients with subsequent HT when compared to those without (LOE: IV).66 Kim et al. compared the degree of contribution of pretreatment perfusion status vs tissue status (as measured by size of DWI lesions) to the development of HT (LOE: IV).67 A large area of severe perfusion delay was found to be an independent predictor of HT irrespective of DWI lesion volume or CT changes. Thus, PWI can identify patients at risk even if the CT or DWI seems promising.67
Two prospective multicenter studies termed Diffusion and Perfusion Imaging for Understanding Stroke Evolution (DEFUSE) and Echoplanar Imaging Thrombolytic Evaluation Trial (EPITHET) used MRI to predict hemorrhage after TPA administration. The DEFUSE trial looked at severity of perfusion delay and DWI lesion volume in an attempt to identify risk factors for HT after recanalization therapy. “Malignant profile” was characterized by a large DWI lesion volume (100 ml or more) and/or a large PWI lesion volume (100 ml or more) with long delays on the Tmax (eight seconds or longer) map. Early reperfusion was associated with fatal intracranial hemorrhage in patients with a malignant profile.68 Although EPITHET used the same mismatch definitions as the DEFUSE study, it showed that patients with a malignant profile were not found to have an increased risk for HT when it is limited to parenchymal type of ICH.69
T2*-weighted gradient-echo sequences and susceptibility-weighted imaging MRI
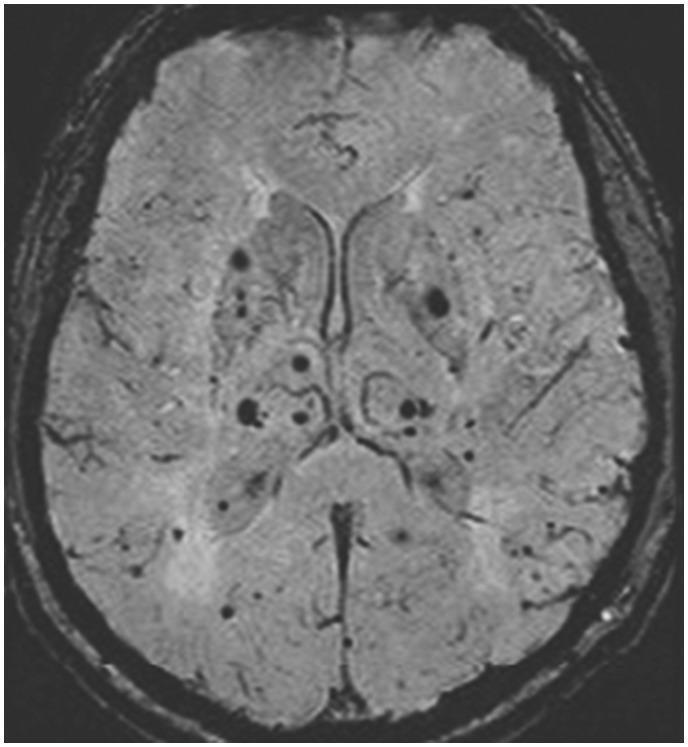
Cerebromicrobleeds (CMBs) are small, perivascular hemosiderin deposits (usually with macrophages) from leakage through cerebral small vessels, reflective of impaired small-vessel integrity. Radiologically, CMBs are visualized as small, rounded, homogeneous, and hypointense lesions on T2*-weighed gradient-recalled echo (GRE) or susceptibility-weighted imaging MRI (SWI) (Figure 7). Some studies that explored the association between CMBs and the risk of HT following IV rt-PA in patients of ischemic stroke have indicated possible links (LOE: IV).70,71
Figure 7.
Susceptibility-weighted imaging magnetic resonance imaging showing evidence of small, rounded, homogeneous, and hypointense lesions suggestive of cerebral microbleeds.
A drop of signal within transcerebral veins detected in T2*-GRE imaging during the early stage of cerebral ischemia was observed within the affected hemisphere in some patients with acute ICA territory ischemia. The abnormal visibility of transcerebral veins reflects severe hemodynamic impairment and was linked to higher risk of HT (LOE: IV).72
Brief conclusion
The reviewed LOE of the role of the different MRI modalities in the prediction of HT after AIS (summarized in Table 2) does not favor a certain MRI biomarker over the other.
Table 2.
Review of LOE of the role of MRI in the prediction of hemorrhagic transformation.
| Study | LOEa | No of Study Participants | Treatment Modality (%) | OR 95% CI, p | |
|---|---|---|---|---|---|
| Noncontrast MRI | |||||
| Leukoaraiosis (hyperintensity on T2 or FLAIR | Shi et al.45 | IV | 105 | IV thrombolysis and/or thrombolytic or mechanical IA therapy (100) | 3.4 (1.23–9.57), p = 0.019 |
| Contrast-enhanced MRI | |||||
| Contrast enhancement at T1-weighted | Yokogami et al.49 | IV | 35 | IA urokinase | p < 0.01 |
| Vo et al.50 | IV | 22 | IV thrombolysis (27.2) | p = 0.013 | |
| Kim et al.51 | IV | 55 | IV thrombolysis (27.2) | p = 0.003 | |
| Hjort et al.52 | IV | 33 | IV thrombolysis (100) | p = 0.043 | |
| Hyperintensity of CSF space termed hyperintense acute reperfusion marker on FLAIR | Latour et al.54 | IV | 144 | IV thrombolysis and/or thrombolytic or mechanical IA therapy (28.4) | 8.11 (2.85–23.1) p < 0.001 |
| Sulcal hyperintensity on FLAIR | Cho et al.55 | IV | 88 | IV thrombolysis and/or thrombolytic or mechanical IA therapy (100) | 13.64 (1.51–123.28) |
| Kim et al.56 | IV | 14 | IV thrombolysis and/or thrombolytic or mechanical IA therapy (100) | p = 0.031 | |
| DWI | |||||
| Large-sized lesion on DWI | Singer et al.58 | IV | 217 | IV thrombolysis and/or thrombolytic or mechanical IA therapy (100) | p = 0.004 |
| Selim et al.61 | IV | 29 | IV thrombolysis (100) | p = 0.032 | |
| Campbell et al.63 | IV | 49 | IV thrombolysis and/or thrombolytic or mechanical IA therapy | p = 0.003 | |
| ADC values ≤550 × 10−6 mm2/s | Tong et al.59 | IV | 17 | IV thrombolysis and/or thrombolytic or mechanical IA therapy (100) | p < 0.001 |
| Tong et al.60 | IV | 27 | IV thrombolysis and/or thrombolytic or mechanical IA therapy (59.2) | p = 0.02 | |
| Selim et al. 61 | IV | 29 | IV thrombolysis (100) | 1.176; p = 0.042 | |
| Very low CBV | Campbell et al. 62 | IV | 91 | IV thrombolysis (39.5) | 0.727 p = 0.0002 |
| Campbell et al.63 | IV | 49 | IV thrombolysis and/or thrombolytic or mechanical IA therapy | p = 0.002 | |
| PWI | |||||
| Prolonged perfusion deficit | Tong et al.60 | IV | 27 | IV thrombolysis and/or thrombolytic or mechanical IA therapy (59.2) | p = 0.03 |
| Decreased signal intensity at later time points in perfusion MRI acquisition | Bang et al.64 | IV | 32 | IV thrombolysis and/or thrombolytic or mechanical IA therapy | p < 0.001 |
| Increased relative recirculation of the contrast agent in T2* PWI | Thornhill et al.66 | IV | 18 | IV thrombolysis (44.4) | p = 0.006 |
| Large area of severe perfusion delay | Kim et al.67 | IV | 183 | IV thrombolysis and/or thrombolytic or mechanical IA therapy | 12.91 (3.69–45.17), p < 0.001 |
| T2*-weighted GRE sequences and SWI | |||||
| Cerebromicrobleeds: small, rounded, homogeneous, hypointense lesions | Nighoghossian et al.70 | IV | 100 | IV thrombolysis (27) | p < 0.001 |
| Kidwell et al.71 | IV | 41 | p < 0.05 | ||
| Abnormal visibility of transcerebral veins | Hermier et al.72 | IV | 49 | IV thrombolysis (100) | p = 0.001 |
ADC: apparent diffusion coefficient; CBV: cerebral blood volume; CSF: cerebrospinal fluid; DWI: diffusion-weighted MRI; FLAIR: fluid-attenuated inversion recovery; GRE: gradient-echo sequences; IA: intra-arterial; IV: intravenous; LOE: level of evidence; MRI: magnetic resonance imaging; PWI: perfusion-weighted MRI; SWI: susceptibility-weighted imaging.
LOE adopted from Ackley et al. as follows: level 1: systematic review or meta-analysis, level II: randomized controlled trial (RCT), level III: nonrandomized controlled trials, level IV: case control studies, cohort studies, level V: meta-synthesis, and level VI: single descriptive or qualitative study.20
Impact of DSA on the prediction of HT after AIS
Diagnostic DSA and intervention at admission
There are limited data on angiographic markers of HT. Based on the baseline angiography, the collateral grade is evaluated with the American Society of Interventional and Therapeutic Neuroradiology/Society of Interventional Radiology Collateral Flow Grading System. This angiographic scale assigns patients to: “Grade 0 (no collaterals visible to the ischemic site), 1 (slow collaterals to the periphery of the ischemic site with persistence of some of the defect), 2 (rapid collaterals to the periphery of ischemic site with persistence of some of the defect and to only a portion of the ischemic territory), 3 (collaterals with slow but complete angiographic blood flow of the ischemic bed by the late venous phase) and 4 (complete and rapid collateral blood flow to the vascular bed in the entire ischemic territory by retrograde perfusion).” Lower angiographic collateral flow grades strongly influence the rate of HT after therapeutic recanalization for AIS (LOE: IV).73
Follow-up DSA after recanalization
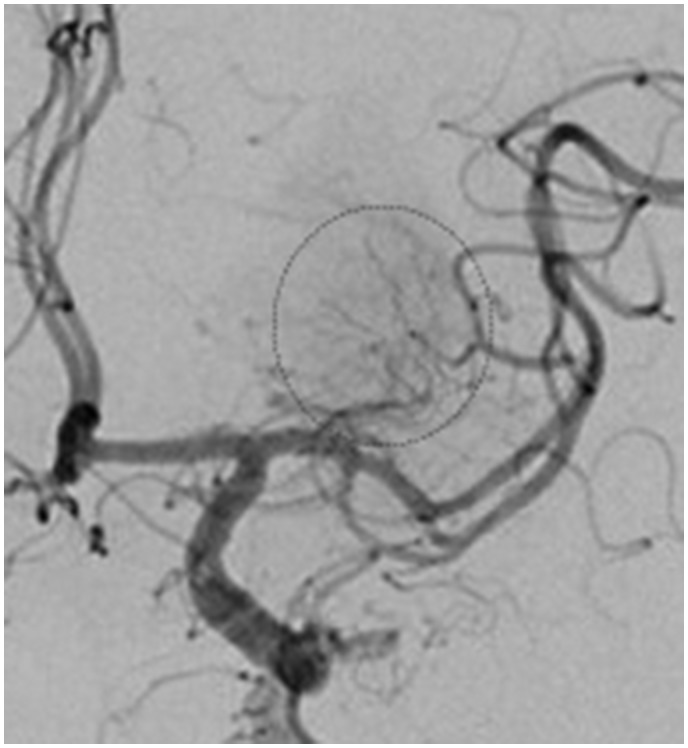
Prominent brain vascularity in the form of capillary blush with or without arteriovenous shunting and early venous drainage (so called angiographic blush) can be seen on angiography after acute recanalization of cerebral artery occlusion with mechanical thrombectomy (Figure 8). Presence of angiographic blush after mechanical thrombectomy was independently associated with the volume of HT (LOE: IV).74
Figure 8.
Angiography of the right internal carotid artery showing evidence of capillary blush.
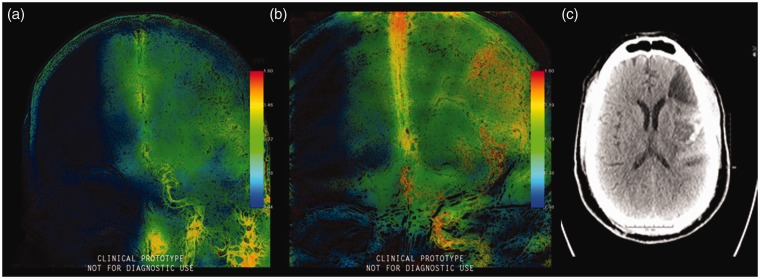
We previously reported on two-dimensional parametric parenchymal blood flow analysis software that could potentially act as an indicator for post-thrombectomy ICH. The software allows the separation of these two signals using band-pass and band-reject filtering to allow for greater visibility of the parenchyma. Elevated parametric parenchymal blood flow wash-in rates after thrombectomy may be associated with increased risk of HT (Figure 9) (LOE: IV).16
Figure 9.
(a) Pretherapeutic parametric blood flow wash-in rate color-coded images showing restricted diffusion in the middle cerebral artery territory, (b) post-therapeutic parametric blood flow wash-in rate color-coded images showing middle cerebral artery recanalization after treatment, and (c) noncontrast computed tomography showing evidence of hemorrhagic transformation.
Brief conclusion
The reviewed LOE of the role of the diagnostic, interventional or follow-up DSA in the prediction of HT after AIS (summarized in Table 3) does not favor one certain angiographic biomarker over another.
Table 3.
Review of LOE of the role of DSA in the prediction of hemorrhagic transformation.
| Study | LOEa | No of Study Participants | Treatment Modality (%) | OR 95% CI, p | |
|---|---|---|---|---|---|
| Poor collaterals | Bang et al.73 | IV | 222 | IV thrombolysis and/or thrombolytic or mechanical IA therapy (100) | 2.666 (1.163–6.113), p = 0.048 |
| Angiographic blush | Omran et al.74 | IV | 48 | IV thrombolysis and/or thrombolytic or mechanical IA therapy (100) | p = 0.01 |
| Elevated parametric parenchymal blood flow wash-in rates | Elsaid et al.16 | IV | 30 | IV thrombolysis and/or thrombolytic or mechanical IA therapy (100) | p = 0.024 |
CI: confidence interval; DSA: digital subtraction angiography; IA: intra-arterial; IV: intravenous; LOE: level of evidence; OR: odds ratio. aLOE adopted from Ackley et al. as follows: level 1: systematic review or meta-analysis, level II: randomized controlled trial (RCT), level III: nonrandomized controlled trials, level IV: case control studies, cohort studies, level V: meta-synthesis, and level VI: single descriptive or qualitative study.20
Impact of US imaging on prediction of HT after AIC
Among the diagnostic tools that can investigate the cerebral hemodynamic, transcranial Doppler (TCD) and transcranial color-coded sonography (TCCS) is a noninvasive technique that can be repeated bedside during the disease course.
Conventional TCD
Gaseous or solid microemboli within the MCA can be detected by TCD as high-intensity transient signals, also called microembolic signals (MES). They are characterized by: (1) having a duration, 300 ms; (2) an amplitude that is three dB higher than the background blood flow signal; (3) are typically unidirectional and occur randomly within the cardiac cycle; and (4) produce a characteristic sound like a “moan” or “chirp” on audio signal. Higher prevalence rates of MES have been reported in strokes caused by large-vessel disease and in cardioembolic strokes, which are in turn the major risk factors of HT (LOE: V).75
Color-coded Doppler TCD
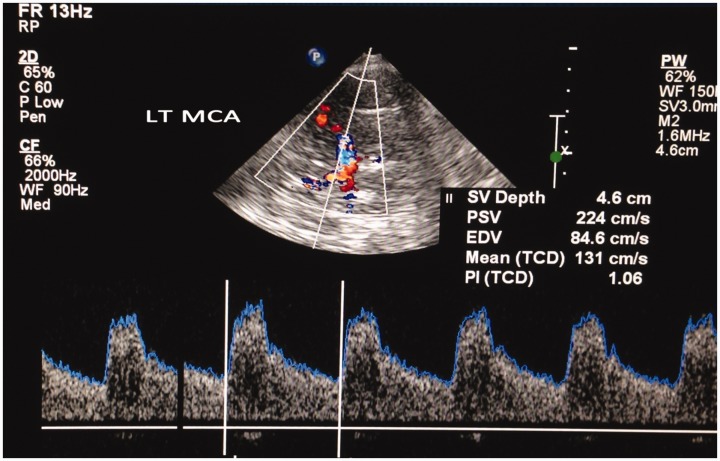
TCCS provides complementary information to periprocedural radiologic data and might help to gain more insight into the complex changes occurring not only after artery occlusion but also after successful recanalization. Changes of the flow velocities of TCCD in symptomatic MCA occlusive disease may be related to the occurrence of further vascular events after stroke (Figure 10).76 Early TCCS detection of a high peak systolic velocity (PSV) MCA ratio (recanalized MCA PSV/contralateral MCA PSV) in successfully recanalized stroke patients indicates an increased risk of ICH (LOE: IV).77 These results are consistent with a previous TCD study that reported high MCA mean blood flow velocity index after successful recanalization therapy for anterior circulation stroke as a risk for postinterventional ICH (LOE: IV).78
Figure 10.
Transtemporal transcranial color-coded duplex (TCCD) insonation of the left middle cerebral artery showing elevated peak systolic velocity. LT MCA: left middle cerebral artery, SV: sample volume, PSV: peak systolic velocity, EDV: end diastolic velocity, TCD: transcranial doppler, PI: Pulsatility Index.
Brief conclusion
The reviewed LOE of the role of the transcranial ultrasonography in the prediction of HT after AIS (summarized in Table 4) does not favor one certain sonographic biomarker over another.
Table 4.
Review of LOE of the role of ultrasound imaging in the prediction of hemorrhagic transformation.
| Study | LOEa | No of Study Participants | Treatment Modality (%) | OR 95% CI, p | |
|---|---|---|---|---|---|
| Microembolic signals | Sarkar et al. 75 | V | NS | NS | NS |
| High PSV:MCA ratio (recanalized MCA PSV/ contralateral MCA PSV) | Baracchini et al. 77 | IV | 226 | IV thrombolysis and/or thrombolytic or mechanical IA therapy (100) | p < 0.0001 |
| High MCA mean blood flow velocity index | Kneihsl et al. 78 | IV | 123 | IV thrombolysis and/or thrombolytic or mechanical IA therapy (100) | p < 0.001 |
CI: confidence interval; IA: intra-arterial; IV: intravenous; LOE: level of evidence; MCA: middle cerebral artery; NS: not stated; OR: odds ratio; PSV: peak systolic velocity. aLOE adopted from Ackley et al. as follows: level 1: systematic review or meta-analysis, level II: randomized controlled trial, level III: nonrandomized controlled trials, level IV: case control studies, cohort studies, level V: meta-synthesis, and level VI: single descriptive or qualitative study.20
Current imaging protocols and future research
Revision of the LOE of the previously reported possible biomarkers for HT shows that there are no current guidelines for imaging protocols for prediction of the risk of HT. Most of the studies were either retrospective or prospective cohort or case-control studies that did not provide the required LOE, but they do deserve a considerable degree of attention. The highest LOEs provided by a meta-analysis review article highlight the sensitivity, specificity and accuracy of the different CT perfusion parameters in predicting HT.39 Identification of a sensitive radiological marker may serve influence the controversial thrombolytic decision in the setting of AIS and may at a minimum indicate more intensive monitoring or further prophylactic interventions. Further large standardized studies are recommended to compare the sensitivity and specificity of these radiological markers and delineate the most reliable predictor.
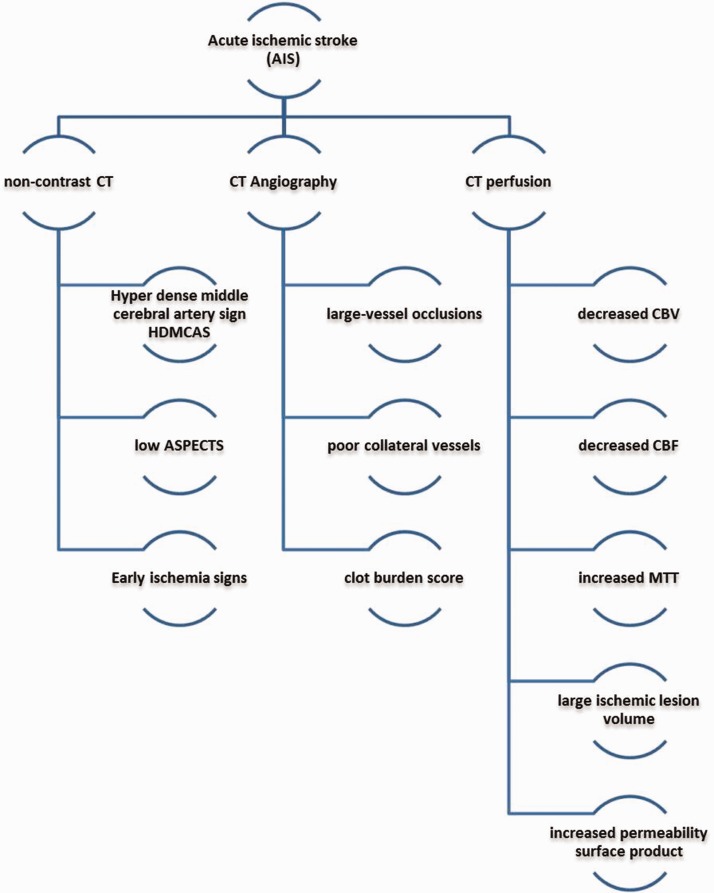
Being the standard initial tool of assessment of any stroke patient, the availability, the relative low cost and short scan duration, from our point of view, gives the CT with its different modalities an advantage over the other tools, especially in the absence of a superior LOE in favor of the others. In addition to the fact that the most used imaging protocol in the setting of AIS is noncontrast CT of the brain, CTA, and CTP, we propose that a combination of the HT radiological biomarkers in these modalities (Figure 11) may provide a more sensitive and predictive value of the risk of HT than a single biomarker. A combination of different biomarkers has already proved to be promising, as shown by Adebayo and Culpan.39 Analysis of the risk of HT in patients with multiple radiological biomarkers is warranted.
Figure 11.
Summary of the hemorrhagic transformation radiological biomarkers in noncontrast computed tomography (CT) brain, computed tomography angiography, and computed tomography perfusion. ASPECTS: Alberta Stroke Program Early CT Score; CBF: cerebral blood flow; CBV: cerebral blood volume; MTT: mean transit time.
Declaration of conflicting interests
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The authors received no financial support for the research, authorship, and/or publication of this article.
ORCID iD
Nada Elsaid https://orcid.org/0000-0002-2476-5652
References
- 1.Fugate JE, Rabinstein AA. Absolute and relative contraindications to IV rt-PA for acute ischemic stroke. Neurohospitalist 2015; 5: 110121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Larrue V, Von Kummer R, Muller A, et al. Risk factors for severe hemorrhagic transformation in ischemic stroke patients treated with recombinant tissue plasminogen activator: A secondary analysis of the European-Australasian Acute Stroke Study (ECASS II). Stroke 2001; 32: 438–441. [DOI] [PubMed] [Google Scholar]
- 3.László JM, Hortobágyi T. Hemorrhagic transformation of ischemic stroke. Vascul Dis Ther 2017; 2: 1–25. [Google Scholar]
- 4.Khatri R, McKinney AM, Swenson B, et al. Blood-brain barrier, reperfusion injury, and hemorrhagic transformation in acute ischemic stroke. Neurology 2012; 79: 52–57. [DOI] [PubMed] [Google Scholar]
- 5.Yassi N, Parsons M, Christensen S, et al. Prediction of poststroke hemorrhagic transformation using computed tomography perfusion. Stroke 2013; 44: 3039–3043. [DOI] [PubMed] [Google Scholar]
- 6.Zhang J, Yang Y, Sun H, et al. Hemorrhagic transformation after cerebral infarction: Current concepts and challenges. Ann Transl Med 2014; 2: 81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tan S, Wang D, Liu M, et al. Frequency and predictors of spontaneous hemorrhagic transformation in ischemic stroke and its association with prognosis. J Neurol 2014; 261: 905–912. [DOI] [PubMed] [Google Scholar]
- 8.Cucchiara B, Tanne D, Levine SR, et al. A risk score to predict intracranial hemorrhage after recombinant tissue plasminogen activator for acute ischemic stroke. J Stroke Cerebrovasc Dis 2008; 17: 331–333. [DOI] [PubMed] [Google Scholar]
- 9.Lou M, Safdar A, Mehdiratta M, et al. The HAT score: A simple grading scale for predicting hemorrhage after thrombolysis. Neurology 2008; 71: 1417–1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Strbian D, Engelter S, Michel P, et al. Symptomatic intracranial hemorrhage after stroke thrombolysis: The SEDAN score. Ann Neurol 2012; 71: 634–641. [DOI] [PubMed] [Google Scholar]
- 11.Mazya M, Egido JA, Ford GA, et al. Predicting the risk of symptomatic intracerebral hemorrhage in ischemic stroke treated with intravenous alteplase: Safe Implementation of Treatments in Stroke (SITS) symptomatic intracerebral hemorrhage risk score. Stroke 2012; 43: 1524–1531. [DOI] [PubMed] [Google Scholar]
- 12.Flint AC, Faigeles BS, Cullen SP, et al. THRIVE score predicts ischemic stroke outcomes and thrombolytic hemorrhage risk in VISTA. Stroke 2013; 44: 3365–3369. [DOI] [PubMed] [Google Scholar]
- 13.Saposnik G, Guzik AK, Reeves M, et al. Stroke prognostication using age and NIH stroke scale: SPAN-100. Neurology 2013; 80: 21–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yaghi S, Willey J, Cucchiara B, et al. Treatment and outcome of hemorrhagic transformation after intravenous alteplase in acute ischemic stroke: A scientific statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2017; 48: e343–e361. [DOI] [PubMed] [Google Scholar]
- 15.Paciaroni M, Bandini F, Agnelli G, et al. Hemorrhagic transformation in patients with acute ischemic stroke and atrial fibrillation: Time to initiation of oral anticoagulant therapy and outcomes. J Am Heart Assoc 2018; 7: e010133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Elsaid N, Saied A, Joshi K, et al. 2D parametric parenchymal blood flow as a predictor of the hemorrhagic events after endovascular treatment of acute ischemic stroke: A single-center retrospective study. Interv Neuroradiol 2018; 6: 522–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Donkor E. Stroke in the 21st century: A snapshot of the burden, epidemiology, and quality of life. Stroke Res Treat 2018; 2018: 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fattore G, Torbica A, Susi A, et al. The social and economic burden of stroke survivors in Italy: A prospective, incidence-based, multi-centre cost of illness study. BMC Neurology 2012; 12: 137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ackley BJ, Swan BA, Ladwig G, et al. Evidence-based nursing care guidelines: Medical-surgical interventions, 1st ed St Louis, MO: Mosby Elsevier, 2008. ; p.7. [Google Scholar]
- 20.Jaillard A, Cornu C, Durieux A, et al. Hemorrhagic transformation in acute ischemic stroke. The MAST-E study. MAST-E Group. Stroke 1999; 30: 1326–1332. [DOI] [PubMed] [Google Scholar]
- 21.Zou M, Churilov L, He A, et al. Hyperdense middle cerebral artery sign is associated with increased risk of hemorrhagic transformation after intravenous thrombolysis for patients with acute ischaemic stroke. J Clin Neurosci 2013; 20: 984–987. [DOI] [PubMed] [Google Scholar]
- 22.Barber PA, Demchuk AM, Zhang J, et al. Validity and reliability of a quantitative computed tomography score in predicting outcome of hyperacute stroke before thrombolytic therapy. ASPECTS Study Group Alberta Stroke Programme Early CT Score. Lancet 2000; 355: 1670–1674. [DOI] [PubMed] [Google Scholar]
- 23.Dzialowski I, Hill M, Coutts S, et al. Extent of early ischemic changes on computed tomography (CT) before thrombolysis: Prognostic value of the Alberta Stroke Program Early CT Score in ECASS II. Stroke 2006; 37: 973–978. [DOI] [PubMed] [Google Scholar]
- 24.Lin K, Zink WE, Tsiouris AJ, et al. Risk assessment of hemorrhagic transformation of acute middle cerebral artery stroke using multimodal CT. J Neuroimaging 2012; 22: 160–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Puetz V, Dzialowski I, Hill MD, et al. Intracranial thrombus extent predicts clinical outcome, final infarct size and hemorrhagic transformation in ischemic stroke: The clot burden score. Int J Stroke 2008; 3: 230–236. [DOI] [PubMed] [Google Scholar]
- 26.Fanou EM, Knight J, Aviv RI, et al. Effect of collaterals on clinical presentation, baseline imaging, complications, and outcome in acute stroke. AJNR Am J Neuroradiol 2015; 36: 2285–2291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Horsch A, Bennink E, van Seeters T, et al. Computed tomography perfusion derived blood-brain barrier permeability does not yet improve prediction of hemorrhagic transformation. Cerebrovasc Dis 2018; 45: 26–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sims JR, Rordorf G, Smith EE, et al. Arterial occlusion revealed by CT angiography predicts NIH stroke score and acute outcomes after IV Tpa treatment. AJNR Am J Neuroradiol 2005; 26: 246–251. [PMC free article] [PubMed] [Google Scholar]
- 29.Kim J, Fischbein N, Lu Y, et al. Regional angiographic grading system for collateral flow: Correlation with cerebral infarction in patients with middle cerebral artery occlusion. Stroke 2004; 35: 1340–1344. [DOI] [PubMed] [Google Scholar]
- 30.van Kranendonk K, Treurniet K, Boers A, et al. Clinical and imaging markers associated with hemorrhagic transformation in patients with acute ischemic stroke. Stroke 2019; 50: 2037–2043. [DOI] [PubMed] [Google Scholar]
- 31.Konstas AA, Goldmakher GV, Lee TY, et al. Theoretic basis and technical implementations of CT perfusion in acute ischemic stroke. Part 1. Theoretic basis. AJNR Am J Neuroradiol 2009; 30: 662–668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jain AR, Jain M, Kanthala AR, et al. Association of CT perfusion parameters with hemorrhagic transformation in acute ischemic stroke. AJNR Am J Neuroradiol 2013; 34: 1895–1900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Langel C, Popovic K. Infarct-core CT perfusion parameters in predicting post-thrombolysis hemorrhagic transformation of acute ischemic stroke. Radiol Oncol 2018; 53: 25–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Aviv RI, D’Esterre CD, Murphy BD, et al. Hemorrhagic transformation of ischemic stroke: Prediction with CT perfusion. Radiology 2009; 250: 867–877. [DOI] [PubMed] [Google Scholar]
- 35.Souza LC, Payabvash S, Wang Y, et al. Admission CT perfusion is an independent predictor of hemorrhagic transformation in acute stroke with similar accuracy to DWI. Cerebrovasc Dis 2012; 33: 8–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wintermark M, Sincic R, Sridhar D, et al. Cerebral perfusion CT: Technique and clinical applications. J Neuroradiol 2008; 35: 253–260. [DOI] [PubMed] [Google Scholar]
- 37.Hom J, Dankbaar JW, Soares BP, et al. Blood-brain barrier permeability assessed by perfusion CT predicts symptomatic hemorrhagic transformation and malignant edema in acute ischemic stroke. AJNR Am J Neuroradiol 2010; 32: 41–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ozkul-Wermester O, Guegan-Massardier E, Triquenot A, et al. Increased blood-brain barrier permeability on perfusion computed tomography predicts hemorrhagic transformation in acute ischemic stroke. Eur Neurol 2014; 72: 45–53. [DOI] [PubMed] [Google Scholar]
- 39.Adebayo O, Culpan G. Diagnostic accuracy of computed tomography perfusion in the prediction of haemorrhagic transformation and patient outcome in acute ischaemic stroke: A systematic review and meta-analysis. Eur Stroke J. Epub ahead of print 25 October 2019. DOI:10.1177/239698731988346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hanson SK, Grotta JC, Rhoades H, et al. Value of single-photon emission-computed tomography in acute stroke therapeutic trials. Stroke 1993; 24: 1322–1329. [DOI] [PubMed] [Google Scholar]
- 41.Ueda T, Hatakeyama T, Kumon Y, et al. Evaluation of risk of hemorrhagic transformation in local intraarterial thrombolysis in acute ischemic stroke by initial SPECT. Stroke 1994; 25: 298–303. [DOI] [PubMed] [Google Scholar]
- 42.Alexandrov AV, Black SE, Ehrlich LE, et al. Predictors of hemorrhagic transformation occurring spontaneously and on anticoagulants in patients with acute ischemic stroke. Stroke 1997; 28: 1198–1202. [DOI] [PubMed] [Google Scholar]
- 43.Umemura A, Suzuka T, Yamada K. Quantitative measurement of cerebral blood flow by 99mTc-HMPAO SPECT in acute ischemic stroke: Usefulness in determining therapeutic options. J Neurol Neurosurg Psychiatry 2000; 69: 472–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Neumann-Haefelin C, Brinker G, Uhlenkuken U, et al. Prediction of hemorrhagic transformation after thrombolytic therapy of clot embolism: An MRI investigation in rat brain. Stroke 2002; 33: 1392–1398. [DOI] [PubMed] [Google Scholar]
- 45.Shi ZS, Loh Y, Liebeskind DS, et al. Leukoaraiosis predicts parenchymal hematoma after mechanical thrombectomy in acute ischemic stroke. Stroke 2012; 43: 1806–1811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yang CM, Hung CL, Su HC, et al. Leukoaraiosis and risk of intracranial hemorrhage and outcome after stroke thrombolysis. PLoS One 2018; 13: e0196505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Knight RA, Barker PB, Fagan SC, et al. Prediction of impending hemorrhagic transformation in ischemic stroke using magnetic resonance imaging in rats. Stroke 1998; 29: 144–151. [DOI] [PubMed] [Google Scholar]
- 48.Dijkhuizen RM, Asahi M, Wu O, et al. Delayed rt-PA treatment in a rat embolic stroke model: Diagnosis and prognosis of ischemic injury and hemorrhagic transformation with magnetic resonance imaging. J Cereb Blood Flow Metab 2001; 21: 964–971. [DOI] [PubMed] [Google Scholar]
- 49.Yokogami K, Nakano S, Ohta H, et al. Prediction of hemorrhagic complications after thrombolytic therapy for middle cerebral artery occlusion: Value of pre- and post-therapeutic computed tomographic findings and angiographic occlusive site. Neurosurgery 1996; 39: 1102–1107. [DOI] [PubMed] [Google Scholar]
- 50.Vo K, Santiago F, Lin W, et al. MR Imaging enhancement patterns as predictors of hemorrhagic transformation in acute ischemic stroke. AJNR Am J Neuroradiol 2003; 24: 674–679. [PMC free article] [PubMed] [Google Scholar]
- 51.Kim EY, Na DG, Kim SS, et al. Prediction of hemorrhagic transformation in acute ischemic stroke: Role of diffusion weighted imaging and early parenchymal enhancement. AJNR Am J Neuroradiol 2005; 26: 1050–1055. [PMC free article] [PubMed] [Google Scholar]
- 52.Hjort N, Wu O, Ashkanian M, et al. MRI detection of early blood-brain barrier disruption parenchymal enhancement predicts focal hemorrhagic transformation after thrombolysis. Stroke 2008; 39: 1025–1028. [DOI] [PubMed] [Google Scholar]
- 53.Mathews VP, Caldemeyer KS, Lowe MJ, et al. Brain: Gadolinium-enhanced fast fluid-attenuated inversion-recovery MR imaging. Radiology 1999; 211: 257–263. [DOI] [PubMed] [Google Scholar]
- 54.Latour LL, Kang DW, Ezzeddine MA, et al. Early blood-brain barrier disruption in human focal brain ischemia. Ann Neurol 2004; 56: 468–477. [DOI] [PubMed] [Google Scholar]
- 55.Cho A, Kim J, Kim S, et al. Focal fluid-attenuated inversion recovery hyperintensity within acute diffusion-weighted imaging lesions is associated with symptomatic intracerebral hemorrhage after thrombolysis. Stroke 2008; 39: 3424–3226. [DOI] [PubMed] [Google Scholar]
- 56.Kim EY, Kim SS, Na DG, et al. Sulcal hyperintensity on fluid-attenuated inversion recovery imaging in acute ischemic stroke patients treated with intra-arterial thrombolysis: Iodinated contrast media as its possible cause and the association with hemorrhagic transformation. J Comput Assist Tomogr 2005; 29: 264–269. [DOI] [PubMed] [Google Scholar]
- 57.Parsons MW, Barber PA, Chalk J, et al. Diffusion and perfusion-weighted MRI response to thrombolysis in stroke. Ann Neurol 2002; 51: 28–37. [DOI] [PubMed] [Google Scholar]
- 58.Singer O, Kurre W, Humpich M, et al. Risk assessment of symptomatic intracerebral hemorrhage after thrombolysis using DWI-ASPECTS. Stroke 2009; 40: 2743–2748. [DOI] [PubMed] [Google Scholar]
- 59.Tong DC, Adami A, Moseley ME, et al. Relationship between apparent diffusion coefficient and subsequent hemorrhagic transformation following acute ischemic stroke. Stroke 2000; 31: 2378–2384. [DOI] [PubMed] [Google Scholar]
- 60.Tong DC, Adami A, Moseley ME, et al. Prediction of hemorrhagic transformation following acute stroke. Arch Neurol 2001; 58: 587–593. [DOI] [PubMed] [Google Scholar]
- 61.Selim M, Fink JN, Kumar S, et al. Predictors of hemorrhagic transformation after intravenous recombinant tissue plasminogen activator: Prognostic value of the initial apparent diffusion coefficient and diffusion weighted lesion volume. Stroke 2002; 33: 2047–2052. [DOI] [PubMed] [Google Scholar]
- 62.Campbell B, Christensen S, Butcher K, et al. Regional very low cerebral blood volume predicts hemorrhagic transformation better than diffusion-weighted imaging volume and thresholded apparent diffusion coefficient in acute ischemic stroke. Stroke 2010; 41: 82–88. [DOI] [PubMed] [Google Scholar]
- 63.Campbell BC, Costello C, Christensen S, et al. Fluid attenuated inversion recovery hyperintensity in acute ischemic stroke may not predict hemorrhagic transformation. Cerebrovasc Dis 2011; 32: 401–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bang O, Buck B, Saver J, et al. Prediction of hemorrhagic transformation after recanalization therapy using T2*-permeability magnetic resonance imaging. Ann Neurol 2007; 62: 170–176. [DOI] [PubMed] [Google Scholar]
- 65.Bang O, Saver J, Alger J, et al. Patterns and predictors of blood-brain barrier permeability derangements in acute ischemic stroke. Stroke 2009; 40: 454–461. [DOI] [PubMed] [Google Scholar]
- 66.Thornhill RE, Chen S, Rammo W, et al. Contrast-enhanced MR imaging in acute ischemic stroke: T2* measures of blood–brain barrier permeability and their relationship to T1 estimates and hemorrhagic transformation. AJNR Am J Neuroradiol 2010; 31: 1015–1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kim JH, Bang OY, Liebeskind DS, et al. Impact of baseline tissue status (diffusion-weighted imaging lesion) versus perfusion status (severity of hypoperfusion) on hemorrhagic transformation. Stroke 2010; 41: e135–e142. [DOI] [PubMed] [Google Scholar]
- 68.Albers G, Thijs V, Wechsler L, et al. Magnetic resonance imaging profiles predict clinical response to early reperfusion: The Diffusion and Perfusion Imaging Evaluation for Understanding Stroke Evolution (DEFUSE) study. Ann Neurol 2006; 60: 508–517. [DOI] [PubMed] [Google Scholar]
- 69.Davis S, Donnan G, Parsons M, et al. Effects of alteplase beyond 3 h after stroke in the Echoplanar Imaging Thrombolytic Evaluation Trial (EPITHET): A placebo-controlled randomised trial. Lancet Neurol 2008; 7: 299–309. [DOI] [PubMed] [Google Scholar]
- 70.Nighoghossian N, Hermier M, Adeleine P, et al. Old microbleeds are a potential risk factor for cerebral bleeding after ischemic stroke: A gradient-echo T2*-weighted brain MRI study. Stroke 2002; 33: 735–742. [DOI] [PubMed] [Google Scholar]
- 71.Kidwell CS, Saver JL, Villablanca JP, et al. Magnetic resonance imaging detection of microbleeds before thrombolysis: An emerging application. Stroke 2002; 33: 95–98. [DOI] [PubMed] [Google Scholar]
- 72.Hermier M, Nighoghossian N, Derex L, et al. Hypointense transcerebral veins at T2*-weighted MRI: A marker of hemorrhagic transformation risk in patients treated with intravenous tissue plasminogen activator. J Cereb Blood Flow Metab 2003; 23: 1362–1370. [DOI] [PubMed] [Google Scholar]
- 73.Bang O, Saver J, Kim S, et al. Collateral flow averts hemorrhagic transformation after endovascular therapy for acute ischemic stroke. Stroke 2011; 42: 2235–2239. [DOI] [PubMed] [Google Scholar]
- 74.Omran S, Boddu SR, Gusdon AM. Angiographic blush after mechanical thrombectomy is associated with hemorrhagic transformation of ischemic stroke. J Stroke Cerebrovasc Dis 2018; 27: 3124–3130. [DOI] [PubMed] [Google Scholar]
- 75.Sarkar S, Ghosh S, Ghosh SK, et al. Role of transcranial Doppler ultrasonography in stroke. Postgrad Med J 2007; 83: 683–689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Elsaid N, El-Mitwalli A, Farrag M, et al. Outcome and prognosis of middle cerebral artery occlusive disease in a sample of Egyptian patients. Neurology 2017; 88 : P3.255. [Google Scholar]
- 77.Baracchini C, Farina F, Pieroni A, et al. Ultrasound identification of patients at increased risk of intracranial hemorrhage after successful endovascular recanalization for acute ischemic stroke. World Neurosurg 2019; 125: e849–e855. [DOI] [PubMed] [Google Scholar]
- 78.Kneihsl M, Niederkorn K, Deutschmann H, et al. Increased middle cerebral artery mean blood flow velocity index after stroke thrombectomy indicates increased risk for intracranial hemorrhage. J Neurointerv Surg 2018; 10: 882–887. [DOI] [PubMed] [Google Scholar]