Abstract
Aim
To investigate multivariable analyses for noninvasive association of the isocitrate dehydrogenase (IDH) mutational status in grade II and III gliomas including evaluation of T2 mapping-sequences.
Methods
Magnetic resonance imaging (MRI) examinations with histopathologically proven World Health Organization grade II and III gliomas were retrospectively enrolled. Multivariate receiver operating characteristics (ROC) analyses to associate IDH mutational status were performed containing quantitative T2 mapping analyses and qualitative characteristics (sex, age, localization, heterogeneity, oedema, necrosis and diameter). Relaxation times were calculated pixelwise by means of standardized ROI analyses. Interobserver variability also was tested.
Results
Out of 32 patients (mean age: 50.7 years; range: 32–83), nine had grade II gliomas and 24 grade III, while 59.5% showed a positive IDH mutated state (IDHm) and 40.5% were wildtype (IDHw). Multivariable ROC analyses were calculated for relaxation time and range, localization and age with a cumulative 0.955 area under the curve (AUC) (p < 0.001), while central T2-relaxation time had by far the highest single variable sensitivity (AUC: 0.873; range: 0.762; age: 0.809; localization: 0.713). Age (cut off: 49 years; p = 0.031) and localization (p = 0.014) were the only qualitative parameters found to be significant as IDHw gliomas were older and IDHm gliomas were preferentially located fronto-temporal.
Conclusions
This is the first study evaluating quantitative T2 mapping sequences for association of the IDH mutational status in grade II and III gliomas demonstrating an association between relaxation time and mutational status. Analyses of T2 mapping relaxation times may even be suitable for predicting the correct IDH mutational state. Prognostic accuracy increases significantly in predicting the correct mutational state when combing T2 relaxation time characteristics and the qualitative MRI features age and localization.
Keywords: Glioma, T2 mapping, Multiparametric imaging, IDH1/2-state (isocitrate-dehydrogenase), MRI (magnetic resonance imaging)
Introduction
Gliomas are the most common brain tumour in adults and are classified following the World Health Organization (WHO) classification.1,2 These criteria, solely including the histologic morphology, remain the gold standard for treatment decision making and major prognostic parameters.1–3 Numerous prospective randomized trials and studies have shown different therapy outcome for tumours within the same WHO graduation, indicating a need for an extended ‘subhistological’ classification, including different molecular marker profiles, as gliomas within the same WHO grade can show highly different molecular marker profiles.4,5 As a consecutive reaction the revised WHO classification from 2016 firstly included genotypic parameters.5–7 These molecular parameters provided more accurate diagnostic, prognostic and maybe even predictive information regarding therapy response and outcome.6,7
Low-grade gliomas represent an entity with a highly heterogeneous tumour biology, behaviour and prognosis.8,9 Especially in grade II and III gliomas the isocitrate dehydrogenase (IDH) status seems to have a major predictive impact. The IDH wildtype (IDHw) (present in 10–20%) is associated with a significantly poorer prognosis, so that even histopathologically graded II gliomas can have a similar prognosis as high-grade glioblastomas (WHO °IV).4,5,10,11 Magnetic resonance imaging (MRI) is the modality of choice to evaluate gliomas and besides accurate assessment of tumour extent, MRI features are known to correlate with the histopathological grade.2,6,7,12 Though the future challenge lies in MRI-based tumour geno-/phenotyping and to implement these new, non-invasive possibilities of tumour characterization, into clinical routine.
Current literature states that the IDH state is associated with several imaging characteristics.5,10,13–18 The molecular differences and biological behaviour of the tumour might be represented in imaging features as, for example, genotypes seem to correlate with the localization, contrast enhancement (CE)-behaviour and homo- or heterogeneity of the tumour.5,10,13–17 There are several promising advanced MRI features such as spectroscopy, FLAIR volume measurement, dynamic susceptibility contrast perfusion imaging and analysis of apparent diffusion coefficient (ADC) maps; however, maps without distinct evidence in predicting the correct IDH state.19–21 Nevertheless, so far the results of quantitative MRI assessed through ADC analyses seem to be most promising.5,16–18,22
Newer MRI mapping techniques allow a quantification of the relaxation at different echo times pixelwise, giving a more accurate information about the tissue and its composition.23 The purpose of this study was to investigate quantitative evaluation of ‘T2 mapping’ relaxation times to predict and hereby assess a possible association with the IDH mutational state in low-grade gliomas non-invasively, and to compare these results with established qualitative MRI features.
Material and methods
Patients
All participants were enrolled retrospectively, and informed consent was waived. The institutional ethical board approved the present study (application number EA1/306/16). From April 2015 to March 2018, 33 patients with the diagnosis of a WHO grade II or III glioma were collected. Exclusion criteria were underage patients, imaging alterations caused by earlier surgery of the brain or insufficient image quality. Histopathological reports were available for all patients. IDH status was determined in 22 patients by immunohistochemistry, the remainder by Polymerase chain reaction. All patients had IDH1 mutations, 19 on codon R132H and one on codon R132L, so far, there were no IDH2 mutations. ATRX loss was present in 19 patients with IDH mutation (IDHm), in one it was not examined and no ATRX loss was demonstrated in all patients with IDHw. 1 p/19q-codeletion was not detectable in any patient.
Imaging
MRI with contrast media was performed preoperatively on 1.5 Tesla (Avanto Magnetom; Siemens, Erlangen, Germany) (n = 22) and 3 Tesla (Skyra; Siemens, Erlangen, Germany) (n = 11) MRI scanners. Since the T2 variation of brain tissue at 1.5 T and 3 T are not significantly different, imaging parameters were nearly identical. Gadolinium-DOTA (Dotarem, Guerbet, Villepinte, France) was used as a contrast agent. The evaluated sequences were: T1 magnetization-prepared rapid gradient-echo (MPRAGE) transversal (sagittal and coronar were reconstructed) and post-contrast (repetition time (TR) 2200, echo time (TE) 2.67, slice thickness 1 mm, inversion time 900, in-plane resolution 0.9766 × 0.9766 mm, acquisition matrix 256 × 246), T2/FLAIR-sequences (TE 88 and TR 9.000 ms and 3 mm slice thickness) and T2 mapping (TR 3100 TE 13.8–165.6 with 12 TEs: 13.8, 27.6, 41.4, 55.2, 69, 82.2, 96.6, 110.4, 124.2, 138, 151.8 and 165.6 ms). By use of MapIt (Siemens, Erlangen, Germany), T2 Maps were reconstructed online. The total acquisition time for the mapping sequence was 5 min 19 s.
Parameters
Radiological and clinical analyses parameters were as follows:
sex;
age;
tumour localization, divided into two compartments ‘fronto-temporal’ and ‘other’;
Contrast Enhancement (CE) = Increased signal intensity (SI) after a MRI contrast agent administration (more than 25%);
macroscopic pattern, homogenous or heterogeneous (yes/no);
necrosis;
T2w/FLAIR oedema;
maximal diameter in T1CEw and T2w/FLAIR images;
region of interest (ROI)-based tissue T2-map analysis with evaluation of relaxation times and ranges in the centre of the tumour.
A heterogenous pattern was defined visually as a tissue heterogeneity in T1w sequences of more than 25% within the tumour volume/in one representable slice. Oedema was defined as peritumoral hyperintensity in T2w/FLAIR sequences. According to the Response Assessment Group in Neuro-oncology (RANO), positive CE was defined as an increased signal intensity of more than 25% after contrast-agent administration.24 Necrosis was defined as hypointense tissue in T1-CE sequences within the tumour volume without any signs of CE behaviour. Images were post-processed with visage software tool (Visage Imaging/Pro Medicus Limited, Version 7.1.10). T2 map ROI were drawn manually by an experiences neuroradiologist (> 10 years of experience) plus a second reader (two years of experience) with a standardized diameter of 5 mm. The ROIs covered representable tissue of the solid tumour, while areas of necrosis and vessels were excluded (M.K., E.W.).
Inter-reader variability was tested. The slice with the largest tumour dimension was chosen, the anatomic centre of the tumour was delineated. A second ROI was automatically copied and pasted in the healthy-appearing white matter of the contralateral lobe with help of the image processing program to assure reliability of the measurements.
Statistics
The statistical analysis was performed with XLSTAT (Version 2011,0,01; Addinsoft SARL, New York, USA) and SPSS Software (IBM; New York, USA). Kolmogorov–Smirnov test was performed to compare single parameter distributions between glioma grade and mutational state cohorts and to identify suitable parameters for multivariable analyses of receiver operating characteristics (ROC). Subsequently significant or trending parameters were fed into the multivariate ROC approach and area under the curve (AUC) was evaluated. Cross validation was applied for the ROC analyses. To define the statistical influence of every single score category, standardized coefficients (95% confidence intervals (CI)) were evaluated and single AUC were created. A p-value of less than 0.05 was defined as statistically significant. Inter-observer variability was calculated using Cronbach’s alpha.
Results
Demographics and tumour characteristics
Out of 32 patients: 19 (59.5%) were classified as IDHm and 13 (40.5%) as IDHw; 28% (9/32) had grade II gliomas and 72% (23/32) grade III. Median age of the cohort was 50.7 years (range: 32–83); 36% (12/32) were female and 64% (20/32) male patients. In five patients (15.5%) pre-surgery and or pre-biopsy was performed without causing imaging alterations. Glioma localization was as followed: 65.5% (21/32) of all gliomas were located frontal and/or temporal, while 34.5% (11/32) were in other brain regions; 28% (9/32) showed a positive CE-behaviour while 72% (23/32) did not; 37.5% (12/32) of gliomas appearance was rated as heterogenous while 62.5% (20/33) were rated as homogenous. Necrosis was found in 9%. Oedema was described in 65.5% in the T2w/FLAIR sequences. Mean T1 perpendicular diameter was 40.1 ± 15.6 mm and T2w/FLAIR diameter was 50.1 ± 18.9 mm. Mean T2-Map relaxation time was 298.6 ± 121.9 ms in the central placed ROI-analyses and 82.3 ± 4.1 ms in the peripheral placed ROI analyses. The ROI placed in the contralateral brain tissue showed no significant variances. Inter-reader variability did not differ significantly. All results are listed in Table 1, and Figures 1 and 2 show two exemplary MRIs with IDHw and IDHm WHO grade III gliomas.
Table 1.
Patients characteristics.
| Overall (n = 32) | IDHm (n = 19) | IDHw (n = 13) | p-value | |
|---|---|---|---|---|
| Age (years) | 49.9 ± 2.6 | 43.9 ± 2.7 | 58.8 ± 4.3 | 0.031 |
| Sex (m/f) | 20/12 | 9/10 | 11/2 | 0.302 |
| Localization | ||||
| Fronto-temporal | 21 | 16 | 5 | 0.014 |
| Other | 11 | 3 | 8 | |
| Heterogeneity | ||||
| heterogeneity | 12 | 7 | 5 | 0.926 |
| homogeneity | 20 | 12 | 8 | |
| CE behavior | ||||
| Positive | 9 | 4 | 5 | 0.282 |
| Negative | 23 | 15 | 8 | |
| Oedema | ||||
| Positive | 21 | 14 | 7 | 0.246 |
| Negative | 11 | 5 | 6 | |
| Necrosis | ||||
| Positive | 3 | 2 | 1 | 0.751 |
| Negative | 29 | 17 | 12 | |
| Diameter T1 (mm) | 41.3 ± 2.8 | 42.4 ± 4.2 | 39.7 ± 3.2 | 0.565 |
| Diameter T2 (mm) | 50.6 ± 3.5 | 50.9 ± 4.5 | 50.3 ± 5.1 | 0.879 |
| T2 map (ms) | ||||
| Mean relaxation | 298.58 ± 21.3 | 363.2 ± 24.7 | 199.2 ± 14.2 | 0.004 |
| Range | 178.3 ± 21.6 | 226.1 ± 25.8 | 104.6 ± 28.2 | 0.008 |
CE: contrast enhancement; IDHm: isocitrate dehydrogenase mutation; IDHw: isocitrate dehydrogenase wildtype
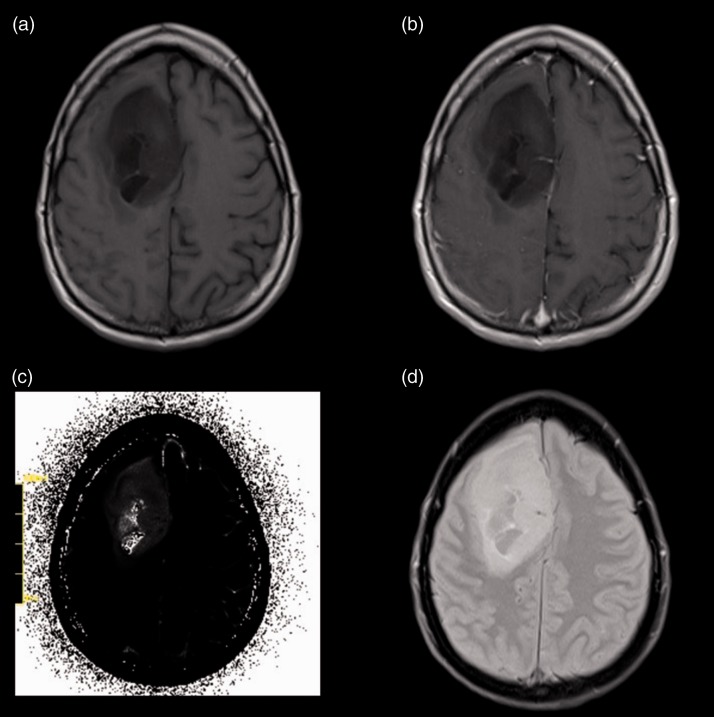
Figure 1.
Patient, 49 years old, with a heterogenous IDHm grade III glioma located in the right frontal region (a) and without a significant positive CE in the T1CE sequences (b). (c) Shows the original raw T2 Map while (d) shows the corresponding FLAIR-sequence without a large oedema.
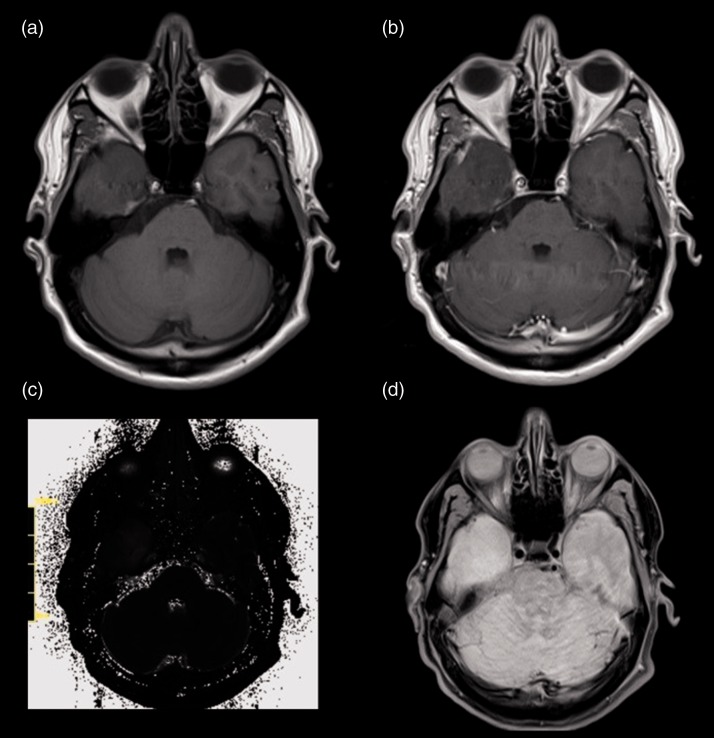
Figure 2.
Patient, 38 years old, with a homogenous IDHw grade III glioma located in the lower temporal lobe (a) and without a significant pos. CE in the T1CE-sequence (b). (c) Shows the original raw T2 Map while (d) shows the corresponding FLAIR-sequence without a large oedema.
Multivariable and single-variable analyses between IDHm and IDHw mutational status cohorts
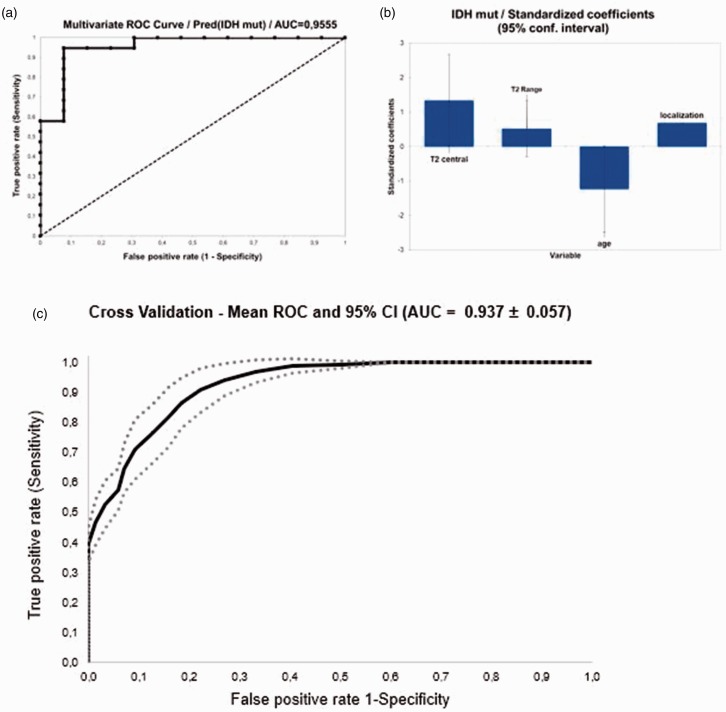
Kolmogorov–Smirnov test identified central T2-relaxation time (p = 0.002) and range (p = 0.008) as significant. For categorical variables only age (p = 0.031) and localization (p = 0.014) were found to be significantly associated with the IDH status. None of the other qualitative parameter were found to be significant (p > 0.05) (Table 1). Multivariate ROC analyses were performed for central relaxation time and range, localization and age with an 0.955 AUC and p < 0.001 (cut off: 0.601; sensitivity: 0.947; specificity: 0.923; positive predictive value (PPV): 0.947; negative predictive value (NPV): 0.923; accuracy: 0.937 and 95% CI 0.887–1.000) (Figure 3). Cross validation for the logistic regression model revealed a total accuracy of validation data of 0.710 ± 0.087 and mean AUC of the datasets created from cross-validation of 0.937 ± 0.057.
Figure 3.
Multivariate receiver operating characteristics (ROC) analyses. (a) ROC curve analyses. The black panel represents the margin for the are under the curve (AUC). The black dashed panel represents the 0.5 AUC. (b) The power of the standardized coefficients upon the multivariate model for (from left to right): T2 relaxation time (central), T2 Range, age and tumour localization. (c) The cross validation for the logistic regression model (total accuracy). (IDHm: isocitrate dehydrogenase mutation).
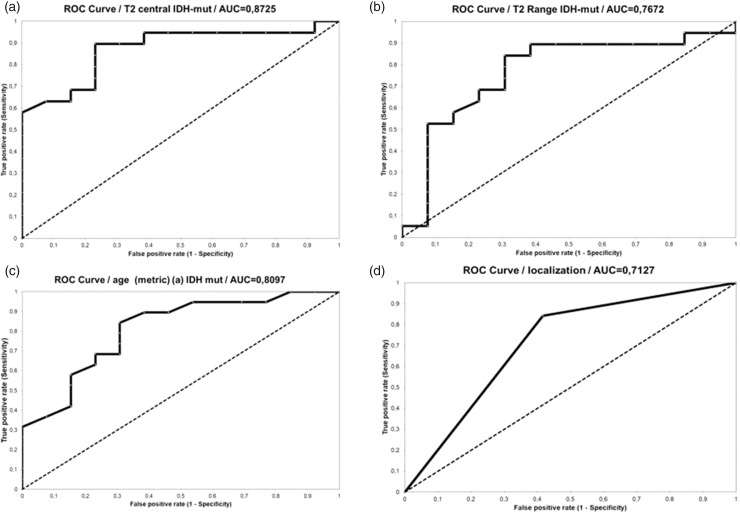
Single score category standardized coefficients (95% CI) showed the highest association for the central T2-relaxation time (Figure 4). AUC for central T2-relaxation times was 0.872 (cut off: 231.0; sensitivity: 0.895; specificity: 0.769; PPV: 0.850; NPV: 0.833; accuracy: 0.843 and 95% CI 0.750–0.995). AUC for T2 range was 0.767 (cut off: 99.0; sensitivity: 0.842; specificity: 0.692; PPV: 0.800; NPV: 0.750; accuracy: 0.781 and 95% CI 0.587–0.948). AUC for age as metric variable was 0.809 (cut off: 49.0; sensitivity: 0.842; specificity: 0.692; PPV: 0.800; NPV: 0.750; accuracy: 0.781 and 95% CI 0.657–0.963). AUC for localization was 0.717 (sensitivity: 0.947; specificity: 0.538; PPV: 0.750; NPV: 0.875; accuracy: 0.781 and 95% CI 0.545–0.881).
Figure 4.
Single variate receiver operating characteristics (ROC) analyses. The black panel represents the margin for the area under the curve (AUC). The black dashed panel represents the 0.5 AUC. (a) T2 relaxation time (central); (b) T2 range; (c) age; (d) localization. (IDHm: isocitrate dehydrogenase mutation).
Multivariable and single-variable analyses between WHO grade II and III glioma cohorts
Kolmogorov–Smirnov test and/or chi-square test showed no significance for any single parameter association with glioma grading (p > 0.05). Subsequently multivariable ROC analyses were not performed.
Discussion
As tumours within the same WHO grade but a different IDH mutational state may have totally divergent prognoses, the new WHO classification emphasizes the role of advanced MRI, predicting the correct mutational state.5 This study implicates an association between T2 relaxation times and IDH mutational state in grade II and III gliomas. Furthermore, T2 mapping may even be a promising technique to predict the correct IDH mutational state noninvasively. In this study multivariable ROC analyses were calculated, showing a significant and strong association of shortened T2 relaxation times and smaller ranges in the central tumour region of IDHw gliomas. Alongside the quantitative measurements only age and localization were identified to be associated positively with IDH status, as IDHw tumours were found more often in older patients and mutated tumours were preferentially located in the fronto-temporal region. Besides age and tumour localization no qualitative characteristic was significantly associated with IDH status.14,15
In the current literature, only few a data are published regarding quantitative T2 mapping and glioma. A recently published study by Hattingen et al. is using T2 mapping for monitoring therapy outcome under bevacizumab treatment in patients with glioblastoma.23 However, especially data regarding mapping sequences and gliomas’ mutational states are missing.
Therefore, our quantitative measurements are difficult to compare. Nevertheless, with p < 0.001 and AUC = 0.955, a strong statistical proof is indicated for our results (Figure 3). After identifying central T2 relaxation time (p < 0.004) and the range (p = 0.008) as significant, we decided to add the two statistically most powerful qualitative parameters, who also had been previously described in literature as useful for determining the mutational status (Table 1).14,15,17 Subdivided in each single parameter standardized coefficients showed that the quantitative ‘mapping’ measurements and patient’s age had the major impact (Figures 3 and 4).
As mentioned, literature is limited on the significance of T2 relaxation time in gliomas and why times should differ between IDH statuses. Lee et. al. investigated the tissue with the help of diffusion weighted imaging (DWI) histogram analysis and dynamic susceptibility perfusion weighed imaging in high-grade gliomas, concluding that mutated subtypes are more heterogenic on a microenvironmental level.22 This tissue heterogeneity may be represented by oedema, vascularization, angiogenesis or higher water content in the extracellular spaces, reflecting longer relaxation times and higher ranges in IDHm gliomas.
This seem to be plausible as the wildtypes, associated with worse prognosis and known out of DWI/ADC analysis have a higher tumour tissue cellularity which decreases the extracellular spaces and the microenvironmental heterogeneity.22,25–27 The overall longer T2 relaxation times in IDHm gliomas might be caused by the accumulation of D2HG, which is produced in large quantities by the altered function of IDH.5,22,28,29
In a recent study Bahrami et al. also introduced a quantitative approach by evaluating pixelwise tissue heterogeneity analysis and border distinctiveness (edge contrast) with the help of FLAIR sequences and radiomic features. The study highlights the potential of T2w/FLAIR tissue analysis in predicting the mutational status.30 Nevertheless, the study by Bahrami et al. was limited as the majority (> 65%) of the collective underwent subtotal resections leading to distortion of the quantitative analyses.30 In this study only the minority (15.5%) underwent pre-surgery and or pre-biopsy. There are several more texture-based approaches and neuroimaging bases algorithms to predict the mutational state in gliomas, all of them with promising results but without implementing mapping sequences.31,32
Tumour localization is correlated with the IDH mutational state, as described elsewhere, for example Wang et al. report a preferable frontal and parietal localization in IDHm gliomas;15 Qi et al. report an association between IDHm gliomas and a unilateral growth pattern, a homogenous signal intensity and no significant CE-behaviour.14
The underlying study assessed tumour localization for two compartments, either fronto-temporal or ‘other’, with a positively correlation for mutated gliomas located frontal and/or temporal, with a remarkable high sensitivity but a low specificity (Table 1 and Figure 4).
Villanueva-Meyer et al. noted that IDHw gliomas might be associated with an older age and defined 45 years as a cut off value throughout a regression analysis.17 Our results are in line with Villanueva-Meyer et al. as our regression analysis also showed a positive correlation between higher age and glioma with IDHw, cut off age was 49 years. Within the multivariate ROC analyses age as a metric variable was significant (p = 0.017) and showed an AUC of 0.8097 (Table 1 and Figure 4). In accordance with the results of Villanueva-Meyer et al., we conclude that age is the most powerful qualitative patient characteristic for IDHw mutation, with a cut-off age between 45 and 50 years. Other studies implemented ADC analyses or MR spectroscopy for assessing mutation status.5,16,17,22 Zang et al. achieved levels of accuracy up to 86% in predicting the IDH genotype of grade III and IV gliomas by using a machine-based algorithm, also containing MRI features such as ADC analyses and qualitative features such as size, shape and texture.16 Further studies comparing or combining ‘Mapping’ sequences with ADC analyses maybe a promising step towards predicting the mutational status even more precise and valid. It is also interesting that no statistical co-evidence could be described for prediction of WHO grades by using any of our variable analyses.
The future of non-invasive genotype/phenotype imaging in gliomas and brain tumours in common will be fully automatic analyses of multiparametric algorithms like in other tumour entities.12 It will be interesting to see what kind of role the analysis of mapping sequences will play.
Limitations
The retrospective and non-blind study design is a limiting factor. A further limitation of this study might be the relatively small patient cohort and a missing volume-based analysis, while this study used a regional based approach. However, the results of this study are promising and novel, indicating a correlation between relaxation time and IDH mutational state. Due to the small sample size though, the quality of the multivariate prediction model is limited. Therefore, larger patient numbers, examined with mapping techniques are required. Furthermore, even if only a minority of the examined cohort underwent pre-surgery and or pre-biopsy, the collective was not treatment naïve, which may cause distortion. Another limitation is the use of two different scanners. Nevertheless, although two different types of scanner were used, T2 values of brain parenchyma may not be affected significantly by the field strength.33
Conclusion
To the best of our knowledge this is the first study evaluating quantitative T2 mapping sequences for prediction/ association of the IDH mutational state in grade II and III gliomas, demonstrating an association between relaxation time and mutational state. T2 mapping may even be suitable for predicting the correct IDH mutational state, as wildtypes had significantly shorter relaxation times in this cohort than mutated gliomas. Furthermore, age and tumour localization were reproducible qualitative MRI features. Wildtype mutation was associated with older patients and mutated types were preferentially found in the fronto-temporal region. Prognostic accuracy for the correct mutational state is increasing significantly when combing T2 relaxation time characteristics and qualitative MRI features. Further investigations on MR mapping techniques for genotype and phenotype correlation are required, as this technique seems to be promising.
Declaration of conflicting interests
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The authors received no financial support for the research, authorship, and/or publication of this article.
ORCID iD
Timo A Auer https://orcid.org/0000-0002-5763-689X
References
- 1.Wen PY, Kesari S. Malignant gliomas in adults. New Eng J Med 2008; 359: 492–507. [DOI] [PubMed] [Google Scholar]
- 2.Louis DN, Ohgaki H, Wiestler OD, et al. The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol 2007; 114: 97–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Weller M, van den Bent M, Hopkins K, et al. EANO guideline for the diagnosis and treatment of anaplastic gliomas and glioblastoma. Lancet Oncol 2014; 15: e395–403. [DOI] [PubMed] [Google Scholar]
- 4.Riemenschneider MJ, Jeuken JW, Wesseling P, et al. Molecular diagnostics of gliomas: state of the art. Acta Neuropathol 2010; 120: 567–584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Smits M, van den Bent MJ. Imaging correlates of adult glioma genotypes. Radiology 2017; 284: 316–331. [DOI] [PubMed] [Google Scholar]
- 6.Banan R, Hartmann C. The new WHO 2016 classification of brain tumors-what neurosurgeons need to know. Acta Neurochir 2017; 159: 403–418. [DOI] [PubMed] [Google Scholar]
- 7.Louis DN, Perry A, Reifenberger G, et al. The 2016 World Health Organization classification of tumors of the central nervous system: a summary. Acta Neuropathol 2016; 131: 803–820. [DOI] [PubMed] [Google Scholar]
- 8.Yeh SA, Ho JT, Lui CC, et al. Treatment outcomes and prognostic factors in patients with supratentorial low-grade gliomas. Brit J Radiol 2005; 78: 230–235. [DOI] [PubMed] [Google Scholar]
- 9.Sanai N, Chang S, Berger MS. Low-grade gliomas in adults. J Neurosurg 2011; 115: 948–965. [DOI] [PubMed] [Google Scholar]
- 10.Metellus P, Coulibaly B, Colin C, et al. Absence of IDH mutation identifies a novel radiologic and molecular subtype of WHO grade II gliomas with dismal prognosis. Acta Neuropathol 2010; 120: 719–729. [DOI] [PubMed] [Google Scholar]
- 11.Claus EB, Walsh KM, Wiencke JK, et al. Survival and low-grade glioma: the emergence of genetic information. Neurosurg Focus 2015; 38: E6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Suh CH, Kim HS, Jung SC, et al. Imaging prediction of isocitrate dehydrogenase (IDH) mutation in patients with glioma: a systemic review and meta-analysis. Eur Radiol 2019; 29: 745–758. [DOI] [PubMed] [Google Scholar]
- 13.Darlix A, Deverdun J, Menjot de Champfleur N, et al. IDH mutation and 1p19q codeletion distinguish two radiological patterns of diffuse low-grade gliomas. J Neuro-Oncol 2017; 133: 37–45. [DOI] [PubMed] [Google Scholar]
- 14.Qi S, Yu L, Li H, et al. Isocitrate dehydrogenase mutation is associated with tumor location and magnetic resonance imaging characteristics in astrocytic neoplasms. Oncol Lett 2014; 7: 1895–1902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang Y, Zhang T, Li S, et al. Anatomical localization of isocitrate dehydrogenase 1 mutation: a voxel-based radiographic study of 146 low-grade gliomas. Eur Journal Neurol 2015; 22: 348–354. [DOI] [PubMed] [Google Scholar]
- 16.Zhang B, Chang K, Ramkissoon S, et al. Multimodal MRI features predict isocitrate dehydrogenase genotype in high-grade gliomas. Neuro-oncol 2017; 19: 109–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Villanueva-Meyer JE, Wood MD, Choi BS, et al. MRI Features and IDH mutational status of grade ii diffuse gliomas: impact on diagnosis and prognosis. AJR 2018; 210: 621–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sonoda Y, Shibahara I, Kawaguchi T, et al. Association between molecular alterations and tumor location and MRI characteristics in anaplastic gliomas. Brain Tumor Pathol 2015; 32: 99–104. [DOI] [PubMed] [Google Scholar]
- 19.Sahin N, Melhem ER, Wang S, et al. Advanced MR imaging techniques in the evaluation of nonenhancing gliomas: perfusion-weighted imaging compared with proton magnetic resonance spectroscopy and tumor grade. Neuroradiol J 2013; 26: 531–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Demerath T, Simon-Gabriel CP, Kellner E, et al. Mesoscopic imaging of glioblastomas: are diffusion, perfusion and spectroscopic measures influenced by the radiogenetic phenotype? Neuroradiol J 2017; 30: 36–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ranjith G, Parvathy R, Vikas V, et al. Machine learning methods for the classification of gliomas: initial results using features extracted from MR spectroscopy. Neuroradiol J 2015; 28: 106–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee S, Choi SH, Ryoo I, et al. Evaluation of the microenvironmental heterogeneity in high-grade gliomas with IDH1/2 gene mutation using histogram analysis of diffusion-weighted imaging and dynamic-susceptibility contrast perfusion imaging. J Neuro-Oncol 2015; 121: 141–150. [DOI] [PubMed] [Google Scholar]
- 23.Hattingen E, Jurcoane A, Daneshvar K, et al. Quantitative T2 mapping of recurrent glioblastoma under bevacizumab improves monitoring for non-enhancing tumor progression and predicts overall survival. Neuro-Oncol 2013; 15: 1395–1404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wen PY, Macdonald DR, Reardon DA, et al. Updated response assessment criteria for high-grade gliomas: response assessment in neuro-oncology working group. J Clin Oncol 2010; 28: 1963–1972. [DOI] [PubMed] [Google Scholar]
- 25.Kono K, Inoue Y, Nakayama K, et al. The role of diffusion-weighted imaging in patients with brain tumors. AJNR 2001; 22: 1081–1088. [PMC free article] [PubMed] [Google Scholar]
- 26.Di Costanzo A, Scarabino T, Trojsi F, et al. Proton MR spectroscopy of cerebral gliomas at 3 T: spatial heterogeneity, and tumour grade and extent. Eur Radiol 2008; 18: 1727–1735. [DOI] [PubMed] [Google Scholar]
- 27.Kang Y, Choi SH, Kim YJ, et al. Gliomas: Histogram analysis of apparent diffusion coefficient maps with standard- or high-b-value diffusion-weighted MR imaging-correlation with tumor grade. Radiology 2011; 261: 882–890. [DOI] [PubMed] [Google Scholar]
- 28.Verger A, Metellus P, Sala Q, et al. IDH mutation is paradoxically associated with higher (18)F-FDOPA PET uptake in diffuse grade II and grade III gliomas. Eur J Nucl Med Mol Imaging 2017; 44: 1306–1311. [DOI] [PubMed] [Google Scholar]
- 29.Isal S, Gauchotte G, Rech F, et al. A high (18)F-FDOPA uptake is associated with a slow growth rate in diffuse Grade II–III gliomas. Brit J Radiol 2018; 91: 20170803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bahrami N, Hartman SJ, Chang YH, et al. Molecular classification of patients with grade II/III glioma using quantitative MRI characteristics. J Neuro-Oncol 2018; 139: 633–642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Park YW, Han K, Ahn SS, et al. Prediction of IDH1-Mutation and 1p/19q-Codeletion Status Using Preoperative MR Imaging Phenotypes in Lower Grade Gliomas. AJNR 2018; 39: 37–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Batchala PP, Muttikkal TJE, Donahue JH, et al. Neuroimaging-based classification algorithm for predicting 1p/19q-codeletion status in IDH-mutant lower grade gliomas. AJNR 2019; 40: 426–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.West J, Blystad I, Engstrom M, et al. Application of quantitative MRI for brain tissue segmentation at 1.5 T and 3.0 T field strengths. PLoS One 2013; 8: e74795. [DOI] [PMC free article] [PubMed] [Google Scholar]