Abstract
Aim
In the present study, we aimed to characterise changes in functional brain networks in individuals who had sustained uncomplicated mild traumatic brain injury (mTBI). We assessed the progression of these changes into the chronic phase. We also attempted to explore how these changes influenced the severity of post-concussion symptoms as well as the cognitive profile of the patients.
Methods
A total of 65 patients were prospectively recruited for an advanced magnetic resonance imaging (MRI) scan within 7 days of sustaining mTBI. Of these, 25 were reassessed at 6 months post injury. Differences in functional brain networks were analysed between cases and age- and sex-matched healthy controls using independent component analysis of resting-state functional MRI.
Results
Our study revealed reduced functional connectivity in multiple networks, including the anterior default mode network, central executive network, somato-motor and auditory network in patients who had sustained mTBI. A negative correlation between network connectivity and severity of post-concussive symptoms was observed. Follow-up studies performed 6 months after injury revealed an increase in network connectivity, along with an improvement in the severity of post-concussion symptoms. Neurocognitive tests performed at this time point revealed a positive correlation between the functional connectivity and the test scores, along with a persistence of negative correlation between network connectivity and post-concussive symptom severity.
Conclusion
Our results suggest that uncomplicated mTBI is associated with specific abnormalities in functional brain networks that evolve over time and may contribute to the severity of post-concussive symptoms and cognitive deficits.
Keywords: Mild traumatic brain injury, resting-state functional MRI, post-concussion symptoms, neurocognitive deficits
Introduction
Mild traumatic brain injury (mTBI) has become a problem of global concern today and has been aptly labelled a silent epidemic.1 Since it generally requires no surgical intervention and often culminates in spontaneous recovery, it may not always receive adequate attention from the treating medical professionals. Nevertheless, a fraction of patients develop persistent symptoms (physical, emotional and cognitive) which constitute ‘post-concussion syndrome’.2 These symptoms, which could last from months to years, can seriously hamper the social and economic productivity of affected individuals.3,4 Chronic cognitive impairment is a known sequela of mTBI, with a recent meta-analysis reporting that up to half of individuals with a single mTBI demonstrate long-term cognitive impairment.5
Of late, there has been growing interest in developing a better understanding of the changes in the intrinsic architecture of the brain, which have a bearing on the spectrum of TBI-associated neurocognitive sequelae.6,7 Conventional neuroimaging techniques have limited ability to detect the changes which underlie the symptoms and neurocognitive deficits associated with TBI. For this reason, we specifically undertook to assess changes in those individuals who sustained mTBI but had a normal scan on conventional imaging.
Recent studies have demonstrated alteration in the functional connectivity of resting-state networks (RSNs) across the entire TBI spectrum, ranging from mild to moderate to severe.8,9 Some studies have also drawn a correlation between the level of functional connectivity and neurocognitive task performance.10 Attempts have also been made to study the network alterations in patients with post-concussion syndrome (PCS). There is a need to assess the evolution of changes in functional network connectivity over time and to investigate the role of these large-scale brain networks in the development of persistent cognitive deficits and PCS symptoms in mTBI. Although mTBI lies at the mildest end of the TBI spectrum, it constitutes the majority of individuals who have sustained TBI and is therefore a substantial source of morbidity not just for affected individuals and their families but also for society. The aims of this study were to assess the status of the RSNs after sustaining mTBI, to study its progression with time and to assess its relationship with the development of PCS and neurocognitive deficits. We hypothesised that there are abnormalities in functional brain networks in uncomplicated mTBI which contribute to the severity of post-concussive symptoms and cognitive deficits.
Methods
Participants
Sixty-five adult patients with mTBI were originally recruited prospectively from a tertiary health-care centre in the city of Delhi as part of a longitudinal observational study. The definition of mTBI was taken as per the criteria laid down by the American Congress of Rehabilitation Medicine.11 Accordingly, a person is said to have sustained mTBI provided he/she had a traumatically induced physiological disruption of brain function, as manifested by at least one of the following: (a) any period of loss of consciousness, (b) any loss of memory for events immediately before or after the accident, (c) any alteration in mental state at the time of the accident (e.g. feeling dazed, disoriented or confused) and (d) focal neurological deficit(s) that may or may not be transient but where the severity of the injury did not exceed the following: loss of consciousness of approximately ≤30 minutes; after 30 minutes, an initial Glasgow Coma Scale (GCS) of 13–15; and post-traumatic amnesia (PTA) not greater than 24 hours. All participants had sustained closed-head TBI through road traffic accidents and had a minimal recordable GCS score of 13–15. The study was conducted within the first week of sustaining injury. Only patients with uncomplicated mTBI (characterised by the absence of intracranial abnormalities on structural imaging) were included.12 Exclusion criteria were the presence of pre-existing neurological/psychiatric disorders, drug abuse, polytrauma, prior history of TBI and any contra-indication to magnetic resonance imaging (MRI). Patients with detectable lesions on conventional MRI were also excluded. Of the 65 cases, five patients had a suboptimal MRI scan. A total of 60 patients were thus finally included in the initial study. Their MRI data were compared with those of 60 age- and sex-matched healthy controls. The demographic profile of the study participants is appended in Table 1. Of the 60 patients who were initially assessed, 25 reported for a repeat study at 6 months. Of these, four patients had to be excluded due to suboptimal scans or due to inability to complete the neuropsychological tests. Written informed consent was obtained from all subjects. The institutional ethics committee of the institute where the study was performed approved the study.
Table 1.
Demographic characteristics of participants.
| Characteristics | Controls (N = 60) | Acute TBI (N = 60) | p-Value |
|---|---|---|---|
| Age | 30.82 ± 7.39 | 30.40 ± 10.34 | 0.787 |
| Sex | 41 male, 25 female | 40 male, 26 female | Not significant |
TBI: traumatic brain injury.
Assessment of post-concussion symptoms
The presence and severity of post-concussion symptoms were assessed by the Rivermead Post-Concussion Symptoms Questionnaire (RPSQ).13 The questionnaire is comprised of 16 symptoms that commonly occur after brain injury. Subjects were asked to rate the severity of each of these symptoms over the last 24 hours from 0 (no or no more symptoms than before the trauma) to 4 (symptoms of highest severity). The sum of the scores for the 16 symptoms was then obtained. The questionnaire was administered to the patients at both visits, namely within a week of injury as well as 6 months after.
Neuropsychological data
All patients who reported 6 months after injury were assessed using the Postgraduate Institute Battery of Brain Dysfunction (PGIBBD) for evaluation of their cognitive profile.14 This is a comprehensive battery administered in the local language consisting of five major tests: memory scale (including attention), performance scale, verbal adult intelligence scale, Bender Gestalt test and Nahor Benson test. The memory scale (consisting of 10 subtests) was selected to assess the long-term episodic memory, recent episodic memory, mental balance, working memory span (forward and backward), delayed and immediate recall, immediate recall of semantically related word pairs, immediate recall of arbitrarily related word pairs, visual retention and recognition of the objects. The same neuropsychological tests were performed for an equal number of controls. Neuropsychological test data for TBI patients and healthy subjects are summarised in Table 2.
Table 2.
Neuropsychological characteristics of chronic traumatic brain injury (TBI; follow-up) and healthy controls.
| Characteristics | Controls (N = 21) | Chronic TBI (N = 21) | p-Value |
|---|---|---|---|
| Memory scale | |||
| Long-term episodic memory | 5.95 ± 0.22 | 5.48 ± 0.98 | 0.065 |
| Recent episodic memory | 5.0 ± .00 | 4.86 ± 0.36 | 0.068 |
| Mental balance | 6.95 ± 1.53 | 6.50 ± 2.40 | 0.473 |
| Working memory span (forward and backward) | 11.33 ± 3.31 | 9.33 ± 2.11 | 0.029* |
| Delayed recall | 9.24 ± 1.14 | 8.05 ± 1.83 | 0.018* |
| Immediate recall | 9.86 ± 2.82 | 9.67 ± 2.01 | 0.914 |
| Immediate recall of semantically related word pairs | 4.95 ± 0.22 | 4.57 ± 0.68 | 0.030* |
| Immediate recall of arbitrarily related word pairs | 13.38 ± 2.18 | 9.95 ± 3.47 | 0.001* |
| Visual retention | 11.38 ± 1.40 | 9.52 ± 2.66 | 0.013* |
| Recognition of objects | 8.88 ± 1.63 | 8.10 ± 2.04 | 0.165 |
Mean and standard deviation (SD) for neuropsychological test score; *p < 0.05.
Image acquisition
MRI data were collected on a Siemens Skyra 3.0 T scanner with a circularly polarised 20 channel matrix head and neck coil and a 45 mT/m actively shielded gradient system. Each subject was initially scanned within 7 days of sustaining injury. The three-plane localiser imaging was followed by axial T1-weighted, T2-weighted and fluid attenuation inversion recovery (FLAIR) images, followed by sagittal T2-weighted, coronal FLAIR, susceptibility weighted images (SWI) and 3D magnetisation-prepared rapid acquisition gradient echo (MPRAGE) images. For resting-state functional MRI (fMRI), functional brain volumes were acquired using an echo-planar T2*-weighted imaging sequence. Each volume consisted of 30 interleaved 5-mm thick slices without inter-slice gap (time echo = 30 ms, time repetition = 2000 ms, field of view = 240 mm × 240 mm, flip angle = 90°, voxel size =3.75 mm × 3.75 mm × 5 mm). A total of 205 brain volumes were measured, with scanning time of 410 seconds. All subjects were imaged in the awake state with their eyes closed. The MRI protocol is summarised in Table 3. The total time taken for the entire scan was approximately 20 minutes. The same imaging protocol was repeated for patients 6 months after injury. Controls had only one MRI investigation (with an identical protocol as that of patients). They also underwent a single neuropsychological test session.
Table 3.
MR acquisition protocol.
| S. No. | Protocol | TR (ms) | TE (ms) | TI (ms) | Slice thickness (mm) | FOV (mm) | Matrix | Time (minutes) |
|---|---|---|---|---|---|---|---|---|
| 1 | T1 axial | 2000 | 12 | 859 | 4.0 | 179 × 220 | 195 × 320 | 1.58 |
| 2 | T2 axial | 5870 | 99 | 4.0 | 179 × 220 | 250 × 512 | 1.41 | |
| 3 | FLAIR axial | 9000 | 81 | 2500 | 4.0 | 172 × 220 | 175 × 320 | 2.44 |
| 4 | FLAIR coronal | 9000 | 81 | 2500 | 4.5 | 172 × 220 | 175 × 320 | 2.26 |
| 5 | T2 sagittal | 4550 | 87 | 4.0 | 220 × 220 | 288 × 384 | 1.28 | |
| 6 | SWI | 28 | 20 | 1.5 | 193 × 220 | 179 × 256 | 2.50 | |
| 7 | 3D MPRAGE | 1900 | 2.49 | 900 | 0.90 | 210 × 240 | 224 × 256 | 3.55 |
| 8 | rfMRI | 2000 | 30 | 5.0 | 240 × 240 | 64 × 64 | 6.83 |
MR: magnetic resonance; TR: repetition time; TE: echo time; TI: inversion time; FOV: field of view; FLAIR: fluid attenuation inversion recovery sequence; SWI: susceptibility weighted imaging; MPRAGE: magnetisation prepared rapid acquisition gradient echo; rfMRI: resting-state functional magnetic resonance imaging.
Data analysis
The structural brain MR images (T1- and T2-weighted, FLAIR and SWI images) were examined by an experienced neuroradiologist. All subjects with any abnormality noted on MRI were excluded from the study. The fMRI data were preprocessed using the FMRI Expert Analysis Tool (FEAT), which is a part of FSL (FMRIB Software Library; http://fsl.fmrib.ox.ac.uk). Functional brain volumes from individual subjects were corrected for slice timing and head movement. A Gaussian kernel of full-width at half-maximum of 5 mm was used for smoothing of data. The data were then preprocessed with high- and low-pass temporal filtering to enhance the signal-to-noise ratio. It was then co-registered onto the individual’s structural (MPRAGE) scan, as well as standard space images (Montreal Neurological Institute (MNI)-152 template) using FMRIB’s Linear Image Registration Tool and Nonlinear Registration Tool. After intensity normalisation of each functional volume, independent components (ICs) were generated using FSL FEAT to gain insight into unexpected artefacts in the data.15,16 Those ICs which showed a time course with high frequencies (>0.1 Hz), periodic pattern or prominent spikes were regarded as noise components and were removed using fsl_regfilt. The de-noised data were decomposed into a set of 35 time courses using the Multivariate Exploratory Linear Optimized Decomposition into Independent Components (MELODIC) algorithm within FSL. Associated spatial maps which jointly describe the temporal and spatial characteristics of components using probabilistic independent component analysis were generated.17 The noise components within the 35 ICs which were initially generated were removed using fsl_regfilt. The de-noised data were decomposed into a set of 20 independent components by running MELODIC again. A general linear model was used to generate a multi-subject design matrix for two groups (Healthy Control (HC) and mTBI) and contrast files (two contrasts: HC vs. mTBI and mTBI vs. HC). Age was mean centred (subtracting the overall mean age from each individual age) and added as a nuisance variable.
The dual regression technique was used for between-group analysis of the resting data for a voxel-wise comparison of resting-state functional connectivity.18 The set of spatial maps from the group-average analysis was used to generate individual subject’s spatial maps and associated time series using dual regression. First, for each subject, the group average set of spatial maps was regressed into the subject’s four-dimensional space–time data set. This resulted in a set of subject-specific time series, one per group-level spatial map. Next, those time series were regressed into the same four-dimensional data set, resulting in a set of subject-specific spatial maps, one per group-level spatial map. The spatial maps representing RSNs were selected using a two-step process. In the first step, the visual selection of the IC of interest was carried out by comparing them to those found in the literature.19,20 As a second step, the power spectra graph output of FSL elaborations that shows the variability of low frequency fluctuations in every IC was analysed. The power spectra graph of a network shows a typical mono-peak pattern in the 0.01–0.03 Hz region, whereas an artefact power spectrum shows a multi-peak pattern in the 0–0.1 Hz range.21 Voxel-wise analyses of the group differences between the mTBI and control group were carried out using FSL randomised non-parametric permutation testing with 10,000 permutations function per contrast for each IC of interest.22
Threshold-free cluster enhancement was used to control for multiple comparisons, and the significance threshold was set to p < 0.05 (familywise error). The results characterised the probabilistic statistical maps, representing the group differences in functional connectivity for all RSNs of interest: the mTBI group map was subtracted from the control group and vice versa for all RSNs in order to evaluate the region with an increase or decrease in intrinsic functional connectivity in the RSNs between the two groups. The statistical maps were then sampled to a standard MNI 1 mm brain Montreal Atlas to localise the areas of RSN alterations better. The Harvard–Oxford cortical and subcortical atlases (Harvard Center for Morphometric Analysis), which are provided with the FSL software, were used to identify the anatomical representation of the clusters of the resulting probabilistic independent component analysis maps that showed significant differences between the two groups using the ‘autoaq’ script.
The differences in participant demographics between patients and controls were tested for statistical significance using Student’s t-test for age and chi-square test for sex. An independent-samples t-test was performed to see the difference in neuropsychological test performance between the controls and patient groups. Two-tailed bootstrapping Pearson bivariate correlation analysis was performed between network functional connectivity and cognitive function scores, as well as between network functional connectivity and RPSQ scores, the threshold for both being set at p < 0.05. All statistical analyses were performed using IBM SPSS Statistics for Windows v20 (IBM Corp., Armonk, NY).
Results
Of the 60 cases who were initially recruited within the first 7 days of injury, most patients reported the presence of post-concussion symptoms on being administered the RPSQ questionnaire, with the RPSQ score ranging from 0 to 29 (mean 9.87 ± 5.85). Of the reported symptoms, headache was the commonest, followed by fatigability and sleep disturbances. A detailed follow-up study could be performed 6 months after injury on 21 patients. It was observed that there was a significant reduction in symptom severity, with the RPSQ score ranging from 0 to 20 (mean 7.05 ± 5.55; as against a mean of 10.81 ± 5.98 in the acute phase in purely this cohort).
Neuropsychological assessment
Cognitive performance was tested using the PGIBBD memory scale 6 months following injury. The TBI subjects showed a significant difference (p < 0.05) in test scores compared with controls in several domains. The tests included working memory span, delayed recall, immediate recall of semantically related word pairs, immediate recall of arbitrarily related word pairs and visual retention. No significant difference was noted in the other cognitive function tests of the memory scale. The results are summarised in Table 2.
Resting-state fMRI
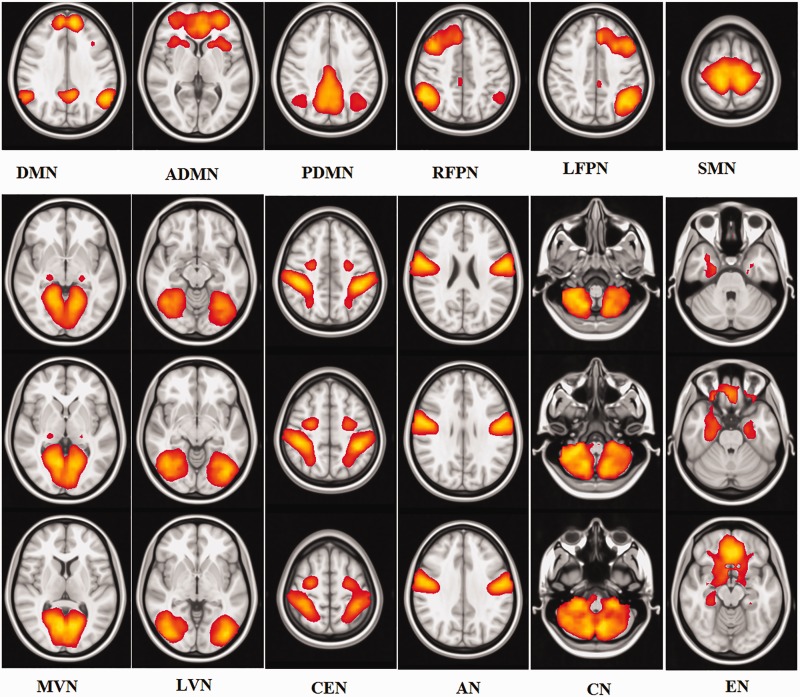
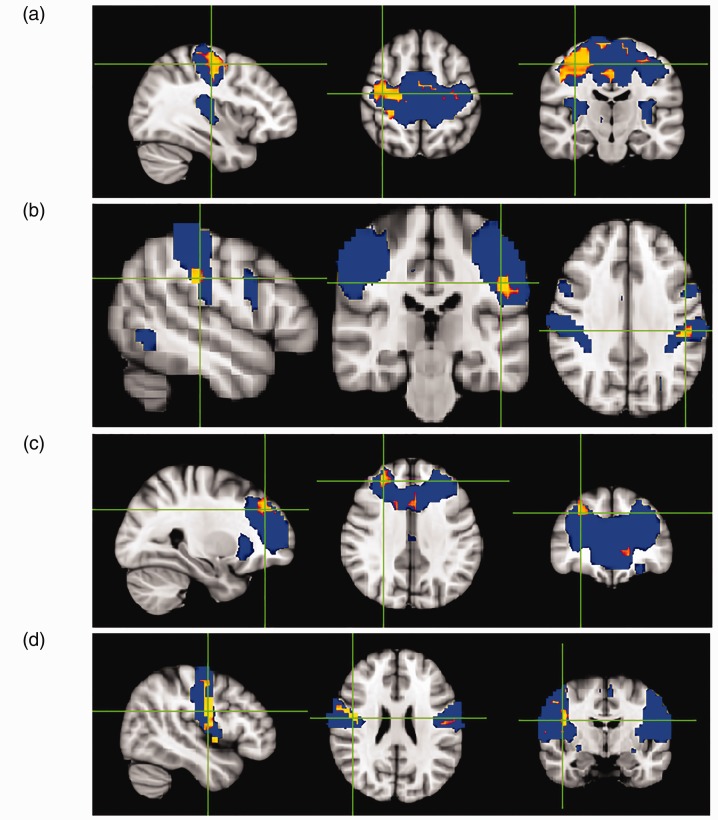
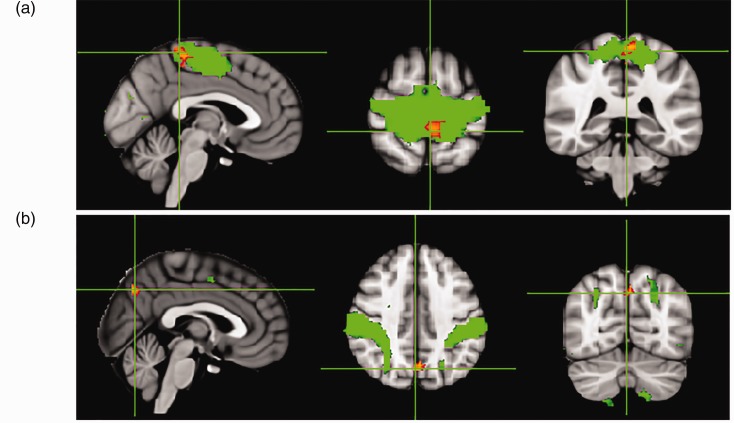
A total of 12 components were identified as RSNs from the group MELODIC output that included the default mode network (DMN), anterior DMN (ADMN), posterior DMN, right fronto-parietal network, left fronto-parietal network, sensorimotor network (SMN), medial visual network, lateral visual network, central executive network (CEN), auditory network (AN), cerebellar network and emotion network (Figure 1). The mTBI group showed significantly decreased intrinsic functional connectivity (p < 0.05) compared with the healthy control group in the regions of SMN (pre-central gyrus and cingulate gyrus), CEN (post-central gyrus), ADMN (frontal pole) and AN (insular cortex and post-central gyrus; Figure 2 and Table 4). No significant differences between mTBI and control subjects were found in the intrinsic connectivity of the other visualised networks. In the entire analysis, none of the RSNs showed any clusters wherein mTBI subjects had increased connectivity compared with healthy control subjects. On follow-up studies performed 6 months after injury, there was an increase in network connectivity in the follow-up group compared with the acute TBI group in subregions of the SMN (pre-central gyrus) and CEN (precuneus; Figure 3 and Table 5).
Figure 1.
Twelve intrinsic resting-state networks derived using independent component analysis. DMN: default mode network; ADMN: anterior DMN; PDMN: posterior DMN; RFPN: right fronto-parietal attention network; LFPN: left fronto-parietal attention network; SMN: somato-motor network; MVN: medial visual network; LVN: lateral visual network; CEN: central executive network; AN: auditory network; CN: cerebellar network; EN: emotional network.
Figure 2.
Group-level independent component analysis spatial maps of the resting state networks (RSNs) (blue), with an overlaid red-yellow colour representing significantly decreased functional connectivity within RSNs in cases of acute mild traumatic brain injury compared with healthy controls: (a) SMN, (b) CEN, (c) ADMN and (d) AN. Data are shown at p < 0.05 corrected for multiple comparisons threshold free cluster enhancement.
Table 4.
Difference between intra-RSN functional connectivity in acute mild TBI patients compared with healthy controls.
| RSN | Hemisphere | Cluster voxel | MNI coordinates (x, y, z) |
|---|---|---|---|
| SMN | |||
| Pre-central gyrus | R | 16,372 | 20, −17, 54 |
| Post-central gyrus | |||
| Superior frontal gyrus | |||
| Cingulate gyrus | L | 429 | −30, −19, 56 |
| ECN | |||
| Post-central gyrus | L | 433 | −50, −26, 32 |
| Supramarginal gyrus | |||
| Central opercular cortex | |||
| Parietal operculum cortex | |||
| DMN | |||
| Frontal pole | R | 671 | 28, 40, 34 |
| Middle frontal gyrus | |||
| Superior frontal gyrus | |||
| Frontal pole | L | 6 | −2, −18, 32 |
| AN | |||
| Insular cortex | R | 4492 | 46, −4, 24 |
| Central opercular cortex | |||
| Pre-central gyrus | |||
| Post-central gyrus | L | 658 | −58, −18, 36 |
| Supramarginal gyrus, anterior division | |||
p < 0.05 familywise error corrected.
RSN: resting-state network; MNI: Montreal Neurological Institute space; x, y, z: coordinates of primary peak locations in the MNI space; SMN: sensorimotor network; ECN: executive control network; DMN: default mode network; AN: auditory network.
Figure 3.
Group-level independent component analysis spatial maps of the resting state networks (RSNs) (green), with an overlaid red-yellow colour representing significantly improved functional connectivity within RSNs in cases of mild traumatic brain injury in the chronic phase compared with the acute phase: (a) SMN and (b) CEN. Data are shown at p < 0.05 corrected for multiple comparisons threshold free cluster enhancement.
Table 5.
Difference between intra RSN FC in mild TBI patients in chronic compared with acute phase.
| RSN | Hemisphere | Cluster voxel | MNI coordinates (x, y, z) |
|---|---|---|---|
| SMN | |||
| Pre-central gyrus | L | 1299 | −2, −38, 34 |
| Post-central gyrus | |||
| ECN | |||
| Precuneous cortex | R | 433 | 2, −68, 40 |
| Cuneal cortex | |||
p < 0.05 familywise error corrected.
ECN: executive control network; RSN: resting-state network; SMN: sensorimotor network.
Correlation analysis between functional connectivity and clinical parameters
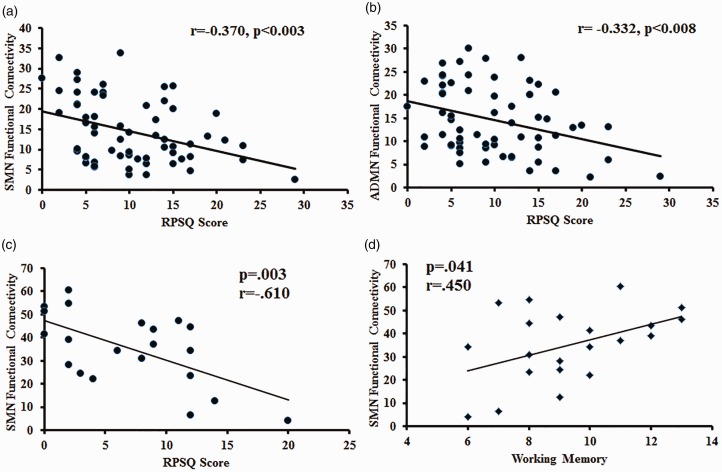
A negative correlation was observed between functional connectivity and symptom severity in mTBI patients assessed within a week of sustaining injury. The intrinsic SMN and ADMN connectivity negatively correlated with RPSQ scores (r = –0.370, p < 0.003, and r = –0.332, p < 0.008, respectively; Figure 4). Six months following injury, there was a significant improvement in functional connectivity which was accompanied by a concomitant improvement in symptom severity. There was persistence of a negative correlation between functional connectivity in SMN and symptom severity at this time point which was statistically significant (r = –0.610, p = 0.003; Figure 4). Neuropsychological tests performed at this time revealed a significant positive correlation between the intrinsic functional connectivity in the somato-motor network and the test scores in the domain of working memory (r = 0.450, p < 0.05; Figure 4).
Figure 4.
Scatter plot showing the significant correlation between intrinsic resting state network functional connectivity (FC) and clinical score within the traumatic brain injury group. (a) Somato-motor network (SMN) FC z-score and Rivermead Post-Concussion Symptoms Questionnaire (RPSQ) score at acute time point (r = −0.370, p < 0.003). (b) Right anterior default mode network FC z-score and RPSQ score at acute time point (r = −0.332, p < 0.008). (c) SMN FC z-score and RPSQ score at chronic time point (r = −0.610, p = 0.003). (d) SMN FC z-score and working memory score (r = 0.450, p = 0.003).
Discussion
The present study endeavoured to assess the changes in functional connectivity in uncomplicated mTBI, its evolution with time and its relationship to the severity of post-concussion symptoms and neurocognitive deficits. We used resting-state fMRI to determine the time-varying functional connectivity changes in patients who had sustained mTBI through road traffic accidents and had no detectable abnormality on conventional scans. The patients who were initially scanned within the first week of injury were reassessed at 6 months. The generalised decrease in brain connectivity noted in multiple networks at the initial scan showed significant recovery in the chronic phase, along with an improvement in the severity of post-concussive symptoms. A negative correlation between network connectivity and severity of post-concussion symptoms was observed at both time points. Subtle deficits in cognitive function were observed on detailed neuro-psychological evaluation which was performed 6 months after injury. These correlated with the brain connectivity changes observed on resting-state fMRI.
TBI is often accompanied by a constellation of symptoms and neurocognitive sequelae, which vary in nature and intensity. PCS, which may affect individuals who sustain TBI including mTBI, is characterised by the presence of physical symptoms such as headaches and sleep disturbance, affective problems such as irritability and cognitive complaints such as poor memory and concentration.23,24 There is uncertainty about the degree to which PCS is associated with brain lesions. Several studies have ascribed diffuse axonal injury to the sequelae associated with PCS.25–27 Disturbances of attention, memory and executive functioning are the most common neurocognitive consequences of TBI at all levels.28 Brain networks are believed to form an essential substrate for performing most cognitive functions.29 Disruption in these networks secondary to TBI could be associated with the spectrum of symptoms and cognitive deficits that underlie TBI. Our cohort of patients, comprised of a relatively homogeneous set of uncomplicated mTBI, also complained of a multitude of post-concussive symptoms, with headache, fatigability and sleep disturbances being the most common. There was a significant improvement in symptom severity on the follow-up study performed 6 months after injury. A detailed neuropsychological analysis in the memory domain performed at this time point revealed that the cohort of chronic mTBI patients had a decreased score in long-term episodic memory, working memory span, delayed recall, immediate recall of semantically related word pairs, immediate recall of arbitrarily related word pairs, visual retention and recognition of objects compared with controls.
Resting-state fMRI is an excellent technique for exploring brain networks.30,31 As the name suggests, it exploits the hemodynamic consequences of neuronal activity while the subject is at rest.32 The core brain networks, which are anatomically distinct, large-scale brain systems with distinct cognitive functions were first formally characterised by Mesulam.33 Since then, several major brain networks have been identified and characterised in both the resting and active brain.34,35 Recent works have highlighted the consequences of mTBI on functional brain networks. Several studies have observed alterations in network connectivity secondary to TBI involving DMN, motor, fronto-parietal and executive networks.8,9,36,37 These findings are in accordance with our study wherein significantly decreased functional connectivity was noted in the mTBI cases in the regions of the SMN, CEN, ADMN and AN.
Among all brain networks at the resting state, the DMN is the most widely studied network in mTBI. The DMN, a key RSN, shows alterations in connectivity across a wide pathophysiological spectrum of TBI. The midline components of the DMN are constituted mainly by the posterior cingulate cortex (PCC), precuneus and anterior cingulate cortex/medial prefrontal cortex.38 The DMN supports internally directed mental activity.39 It also has an important influence on cognitive control.40,41 Interaction of the DMN with other brain networks in the regulation of attention has been illustrated in previous studies.40,42 Previous reports studying brain networks subsequent to mTBI have revealed a reduction in connectivity in the DMN.9,36,43 Our study too reveals a reduced connectivity in the ADMN (frontal pole). The ADMN includes the anterior regions of the DMN (the dorsal and ventral middle prefrontal cortex) and is involved in self-referential mental thoughts. This reduced connectivity observed in our study within the first week of injury showed a significant correlation with the severity of post-concussive symptoms. Another recent study showed that altered frontal connectivity in the DMN has been observed to correlate negatively with post-traumatic symptoms (i.e. depression, anxiety, fatigue and PCS). Further, the reduced posterior connectivity in the DMN was seen to correlate positively with neurocognitive dysfunction.44 A correlation between the functional connectivity within the DMN and the degree of cognitive impairment secondary to TBI has been observed in other studies.45,46 In addition to the decreased intrinsic connectivity within the DMN, other studies have also reported increased extrinsic connectivity between the DMN and other regions such as the prefrontal cortex.9
In addition to the DMN, other prominent networks seen to be affected at the initial stage in our study include the CEN and SMN. The CEN, anchored in the dorsolateral prefrontal cortex and posterior parietal cortex, has also been implicated in the past in TBI.8 The CEN is critical for actively maintaining and manipulating information in working memory, and for judgement and decision making in the context of goal-directed behavior.47,48 The SMN is involved in movement planning and execution, sensory processing and motor learning, and it plays a role in disease-related functional alterations in task performance. The decreased connectivity observed in the SMN in our study at the initial time point showed a definite correlation with the severity of post-concussion symptoms. A significant improvement in connectivity in the SMN and CEN was observed in our subjects with time. There was concomitantly an improvement in the severity of the post-concussion symptoms, with a persistence of negative correlation between network connectivity in SMN and symptom severity. A subtle decrease in neuropsychological test performance scores was observed in the memory domain in our cohort of mTBI cases at the chronic time point. The test performance score for working memory correlated with the intrinsic connectivity of the SMN, thus reiterating the complex interplay between network disruption and cognitive deficit. Although the SMN is primarily concerned with sensory processing and motor learning, its role in higher-order executive functions, including working memory, has been elucidated in recent studies.49,50 Higher executive function scores have been seen to be associated with more positive connectivity between the somato-motor and default networks, as well as more negative connectivity between the somato-motor and dorsal attention networks.49 The authors suggest that executive functions may rely on broad patterns of connectivity across many brain systems, including the somato-motor network, as was seen in our case.
Deficits in intrinsic connectivity of other networks such as interhemispheric connectivity of hippocampal and frontal lobe circuits have also been observed in TBI, and an association of connectivity measures in these networks with functional and neurocognitive outcomes has also been demonstrated.45 Cross-network region of interest (ROI) analysis has reported disruption between networks in the context of mTBI-related memory deficits.51 Increased connectivity in temporal regions and decreased connectivity in frontal regions in mTBI patients with PCS was observed in another study which employed the graph theory approach.52 Differential connectivity of several brain regions in the acute phase of mTBI has been reported in other studies as well. One study using independent component analysis of resting-state fMRI demonstrated reduced functional connectivity in both PCC and precuneus regions of the DMN, with increased connectivity between thalamus, hippocampus and amygdala and other regions of the brain.53 Likewise, decreased motor striatal and increased fronto-parietal network connectivity and differential connectivity within the DMN have been reported by other groups.44,54 Our data demonstrate alterations of multiple brain networks at the resting state, which improves with time, but does not reveal any area of increased functional connectivity.
Time-varying brain connectivity changes seen predominantly in the frontal and parietal lobes post mTBI during a 6-month observation period have been reported in the past.55 ROI-based connectivity assessed in this study revealed 33 distinct ROI pairs, the majority of which revealed decreased connectivity in the initial studies but became comparable with controls in 6 months. Our findings are along similar lines, with partial restoration of connectivity comparable with controls. The patients also reported a substantial improvement in their symptom severity, which was statistically significant. However, persistent activation abnormalities have been observed in some studies, wherein no significant clinical recovery could be documented.56 Another longitudinal study in mTBI involving 27 patients did not find any significant differences between the two time points.9 However, in this case, the second study was performed at 3 months following injury, which may be an early time point for significant improvement to occur in connectivity.
There are several reasons for the heterogeneity in the results obtained from different studies. First, the pathology underlying TBI is vastly heterogeneous, with outcomes that range from death to severe cognitive disability to complete recovery. Second, the timing of imaging after injury is an important factor to explain the variability of results. Also, the mechanism of injury and its effect on the multiple networks is unique to each individual.57 Hence, functional connectivity findings across injury severities cannot be generalised. Other factors which induce variability include differences in analysis methods, as well as the compounding effects of medications, ageing, stress and so on.
Overall, our observations are in accordance with the literature. The novelty of our study lies in the fact that we have performed longitudinal studies in a group of uncomplicated mTBI cases to assess the functional connectivity changes, their progression with time and their relation to the severity of post-concussive symptoms as well as cognitive performance. A larger cohort of subjects with a longer period of longitudinal follow-up would enable a more statistically meaningful conclusion regarding the natural history of network changes over the course of spontaneous recovery.
Conclusions
The results of this study highlight the fact that uncomplicated mTBI results in alterations of functional connectivity patterns which evolve over time and have a bearing on the severity of post-concussion symptoms as well as the neurocognitive performance. This is in line with our hypothesis that uncomplicated mTBI may be associated with abnormalities in functional brain networks which contribute to symptom severity and cognitive deficits. Resting-state functional connectivity is thus a potential tool for improved detection of mTBI in the early stage, as well as an indicator of recovery in the chronic setting.
Acknowledgements
We thank Dr Subash Khushu for his guidance in execution of this study. This work was performed as a part of the Defence Research and Development Organization (DRDO), India, sponsored R&D project INM-322.
Declaration of conflicting interests
The authors declared no potential conflicts of interest with respect to the research, authorship and/or publication of this article.
Funding
The authors received no financial support for the research, authorship and/or publication of this article.
ORCID iD
Maria M D’Souza https://orcid.org/0000-0002-4107-2487
References
- 1.Cassidy JD, Carroll L, Peloso P, et al. Incidence, risk factors and prevention of mild traumatic brain injury: results of the WHO Collaborating Centre Task Force on Mild Traumatic Brain Injury. J Rehabil Med 2004; 43: 28–60. [DOI] [PubMed] [Google Scholar]
- 2.Iverson GL. Outcome from mild traumatic brain injury. Curr Opin Psychiatry 2005; 18: 301–317. [DOI] [PubMed] [Google Scholar]
- 3.Willer B, Leddy JJ. Management of concussion and post-concussion syndrome. Curr Treat Options Neurol 2006; 8: 415–426. [DOI] [PubMed] [Google Scholar]
- 4.Von Wild KR. Posttraumatic rehabilitation and one year outcome following acute traumatic brain injury (TBI): data from the well defined population based German Prospective Study 2000–2002. Acta Neurochir Suppl 2008; 101: 55–60. [DOI] [PubMed] [Google Scholar]
- 5.McInnes K, Friesen CL, MacKenzie DE, et al. Mild traumatic brain injury (mTBI) and chronic cognitive impairment: a scoping review. PLoS One 2017; 12: e0174847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dosenbach NU, Fair DA, Cohen AL. A dual-networks architecture of top-down control. Trends Cogn Sci 2008; 12: 99–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fox MD, Raichle ME. Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nat Rev Neurosci 2008; 8: 700–711. [DOI] [PubMed] [Google Scholar]
- 8.Hillary FG, Slocomb J, Hills EC. Changes in resting connectivity during recovery from severe traumatic brain injury. Int J Psychophysiol 2011; 82: 115–123. [DOI] [PubMed] [Google Scholar]
- 9.Mayer AR, Mannell MV, Ling J, et al. Functional connectivity in mild traumatic brain injury. Hum Brain Mapp 2011; 32: 1825–1835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bonnelle V, Leech R, Kinnunen KM, et al. Default mode network connectivity predicts sustained attention deficits after traumatic brain injury. J Neurosci 2011; 31: 13442–13451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kay T, Harrington DE, Adams R, et al. Definition of mild traumatic brain injury. J Head Trauma Rehabil 1993; 8: 86–87. [Google Scholar]
- 12.Iverson GL, Lange RT, Wäljas M, et al. Outcome from complicated versus uncomplicated mild traumatic brain injury. Rehabil Res Pract 2012; 415740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.King NS, Crawford S, Wenden FJ, et al. The Rivermead Post Concussion Symptoms Questionnaire: a measure of symptoms commonly experienced after head injury and its reliability. J Neurol 1995; 242: 587–592. [DOI] [PubMed] [Google Scholar]
- 14.Dwarka P, Santosh KV. Handbook of PGI battery of brain dysfunction (PGI-BBD), Agra, India: National Psychological Corporation Modern Printers, 1990, pp. 172. [Google Scholar]
- 15.Jenkinson M, Bannister P, Brady M, et al. Improved optimization for the robust and accurate linear registration and motion correction of brain images. NeuroImage 2002; 17: 825–841. [DOI] [PubMed] [Google Scholar]
- 16.Smith SM. Fast robust automated brain extraction. Hum Brain Mapp 2002; 17: 143–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Beckmann CF, DeLuca M, Devlin JT, et al. Investigations into resting-state connectivity using independent component analysis. Philos Trans R SocLond B Biol Sci 2005; 360: 1001–1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Beckmann CF, Mackay CE, Filippini N, et al. Group comparison of resting-state FMRI data using multi-subject ICA and dual regression. NeuroImage 2009; 47: S148. [Google Scholar]
- 19.Storti SF, Formaggio E, Nordio R, et al. Automatic selection of resting-state networks with functional magnetic resonance imaging. Front Neurosci 2013; 7: 72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sang L, Qin W, Liu Y, et al. Resting-state functional connectivity of the vermal and hemispheric subregions of the cerebellum with both the cerebral cortical networks and subcortical structures. NeuroImage 2012; 61: 1213–1225. [DOI] [PubMed] [Google Scholar]
- 21.Kelly RE, Alexopoulos GS, Wang Z, et al. Visual inspection of independent components: defining a procedure for artifact removal from fMRI data. J Neurosci Methods 2010; 189: 233–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nichols TE, Holmes AP. Nonparametric permutation tests for functional neuroimaging: a primer with examples. Hum Brain Mapp 2002; 15: 1–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dikmen S, McLean A, Temkin N. Neuropsychological and psychosocial consequences of minor head injury. J Neurol Neurosurg Psychiatry 1986; 11: 1227–1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Levin HS, Mattis S, Ruff RM, et al. Neurobehavioral outcome following minor head injury: a three-center study. J Neurosurg 1987; 66: 234–243. [DOI] [PubMed] [Google Scholar]
- 25.Messé A, Caplain S, Paradot G, et al. Diffusion tensor imaging and white matter lesions at the subacute stage in mild traumatic brain injury with persistent neurobehavioral impairment. Hum Brain Mapp 2011; 32: 999–1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Smits M, Houston GC, Dippel DW, et al. Microstructural brain injury in post-concussion syndrome after minor head injury. Neuroradiol 2011; 53: 553–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bazarian J, Zhong J, Blyth B, et al. Diffusion tensor imaging detects clinically important axonal damage after mild traumatic brain injury: a pilot study. J Neurotrauma 2007; 24: 1447–1459. [DOI] [PubMed] [Google Scholar]
- 28.Arciniegas DB, Held K, Wagner P. Cognitive impairment following traumatic brain injury. Curr Treat Options Neurol 2002; 4: 43–57. [DOI] [PubMed] [Google Scholar]
- 29.Bressler SL, Tognoli E. Operational principles of neurocognitive networks. Int J Psychophysiol 2006; 60: 139–148. [DOI] [PubMed] [Google Scholar]
- 30.Biswal B, Mennes M, Zuo XN, et al. Toward discovery science of human brain function. Proc Natl Acad Sci USA 2010; 107: 4734–4739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sporns O. Networks of the brain, Cambridge, MA: MIT Press, 2011. [Google Scholar]
- 32.Logothetis NK, Wandell BA. Interpreting the BOLD signal. Ann Rev Neurophysiol 2004; 66: 735–769. [DOI] [PubMed] [Google Scholar]
- 33.Mesulam MM. Large-scale neurocognitive networks and distributed processing for attention, language, and memory. Ann Neurol 1990; 28: 597–613. [DOI] [PubMed] [Google Scholar]
- 34.Seeley WW, Menon V, Schatzberg AF, et al. Dissociable intrinsic connectivity networks for salience processing and executive control. J Neurosci 2007; 27: 2349–2356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Toro R, Fox PT, Paus T. Functional coactivation map of the human brain. Cereb Cortex 2008; 18: 2553–2559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Johnson B, Zhang K, Gay M, et al. Alteration of brain default network in subacute phase of injury in concussed individuals: resting-state fMRI study. Neuroimage 2012; 59: 511–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kasahara M, Menon DK, Salmond CH, et al. Altered functional connectivity in the motor network after traumatic brain injury. Neurology 2010; 75: 168–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Washington SD, VanMeter JW. Anterior-posterior connectivity within the default mode network increases during maturation. Int J Med Biol Front 2015; 21: 207–218. [PMC free article] [PubMed] [Google Scholar]
- 39.Mason MF, Norton MI, Van Horn JD, et al. Wandering minds: the default network and stimulus-independent thought. Science 2007; 315: 393–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hampson M, Driesen NR, Skudlarski P, et al. Brain connectivity related to working memory performance. J Neurosci 2006; 26: 13338–13343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cabeza R, Prince SE, Daselaar SM, et al. Brain activity during episodic retrieval of autobiographical and laboratory events: an fMRI study using a novel photo paradigm. J Cogn Neurosci 2004; 16: 1583–1594. [DOI] [PubMed] [Google Scholar]
- 42.Leech R, Kamourieh S, Beckmann CF, et al. Fractionating the default mode network: distinct contributions of the ventral and dorsal posterior cingulate cortex to cognitive control. J Neurosci 2011; 31: 3217–3224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Slobounov S, Gay M, Zhang K, et al. Alteration of brain functional network at rest and in response to YMCA physical stress test in concussed athletes: RsFMRI study. Neuroimage 2011; 55: 1716–1727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhou Y, Milham MP, Lui Y, et al. Default-mode network disruption in mild traumatic brain injury. Radiology 2012; 265: 882–892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Marquez de la Plata CD, Garces J, Shokri Kojori E, et al. Deficits in functional connectivity of hippocampal and frontal lobe circuits after traumatic axonal injury. Arch Neurol 2011; 68: 74–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sharp DJ, Beckmann CF, Greenwood R, et al. Default mode network functional and structural connectivity after traumatic brain injury. Brain 2011; 134: 2233–2247. [DOI] [PubMed] [Google Scholar]
- 47.Muller NG, Knight RT. The functional neuroanatomy of working memory: contributions of human brain lesion studies. Neuroscience 2006; 139: 51–58. [DOI] [PubMed] [Google Scholar]
- 48.Koechlin E, Summerfield C. An information theoretical approach to prefrontal executive function. Trends Cogn Sci 2007; 11: 229–235. [DOI] [PubMed] [Google Scholar]
- 49.Reineberg AE, Gustavson DE, Benca C, et al. The relationship between resting state connectivity and individual differences in executive functions. Front Psychol 2018; 9: 1600–1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Braunlich K, Gomez-Lavin J, Seger CA. Frontoparietal networks involved in categorization and item working memory. Neuroimage 2015; 107: 146–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sours C, Zhuo J, Janowich J, et al. Default mode network interference in mild traumatic brain injury – a pilot resting state study. Brain Res 2013; 1537: 201–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Messé A, Caplain S, Pélégrini-Issac M, et al. Specific and evolving resting-state network alterations in post-concussion syndrome following mild traumatic brain injury. PLoS One 2013; 8: e65470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Iraji A, Benson RR, Welch RD, et al. Resting state functional connectivity in mild traumatic brain injury at the acute stage: independent component and seed-based analyses. J Neurotrauma 2015; 32: 1031–1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Shumskaya E, Andriessen TM, Norris DG, et al. Abnormal whole-brain functional networks in homogeneous acute mild traumatic brain injury. Neurology 2012; 79: 175–182. [DOI] [PubMed] [Google Scholar]
- 55.Bharath RD, Munivenkatappa A, Gohel S, et al. Recovery of resting brain connectivity ensuing mild traumatic brain injury. Front Hum Neurosci 2015; 9: 513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chen J-K, Johnston KM, Petrides M, et al. Recovery from mild head injury in sports: evidence from serial functional magnetic resonance imaging studies in male athletes. Clin J Sport Med 2008; 18: 241–247. [DOI] [PubMed] [Google Scholar]
- 57.Wang JY, Bakhadirov K, Abdi H, et al. Longitudinal changes of structural connectivity in traumatic axonal injury. Neurology 2011; 77: 818–826. [DOI] [PMC free article] [PubMed] [Google Scholar]