Abstract
Objectives
Patients with complex cerebral aneurysms can now be treated intravascularly with the help of flow-diverting stents. The primary purpose of this article is to document the clinical and angiographic outcomes in 80 patients who were treated with the Pipeline flow-diverting stent (PFS; Medtronic, Dublin, Ireland) and the obliteration mechanism was discussed.
Patients and methods
Between October 2015 and October 2019, 80 patients with 90 complex (undefined neck, large/giant, blood blister–like, and recurrent side-wall) cerebral aneurysms treated with the PFS were retrospectively reviewed. Forty-five patients were women and 35 were men, with a mean age of 52 years. Large or giant aneurysms were defined as 10 mm or larger and small aneurysms were defined as less than 10 mm at the largest diameter measured on angiogram.
Results
Forty-one aneurysms (45.6%) were large or giant, 41 (45.6%) were small, four (4.4%) were recurrent side-wall aneurysms and four (4.4%) were blood blister–like aneurysms. In total, 87 PFSs were placed in 80 patients with 90 aneurysms. In six patients, coexisting proximal stenosis of parent artery was also covered with PFS without balloon angioplasty. Adjunct coils were placed in 31 aneurysms (34%). One patient died of intracerebral hematoma after thrombolysis. There was one intrastent occlusion at six-month follow-up without any symptoms. The morbidity and the mortality rate is 0% and 1.3% (95% confidence interval (CI), 0%–3.7%). Control angiography was available in 74 (92.5%) patients with 83 aneurysms, and the aneurysm occlusion rate was 98.8% (95% CI, 96.5%–100%) in 6 to 12 months.
Conclusion
For wide-necked saccular, large/giant, blood blister–like aneurysms and recurrent side-wall aneurysms, PFS is a valid and safe treatment option.
Keywords: Aneurysm, cerebral, complex, Pipeline flow-diverting stent
Introduction
Patients with complex cerebral aneurysms can now be treated intravascularly with flow-diverting stents as an alternative treatment to coiling with or without stenting.1–6 The Medtronic Pipeline flow-diverting stent (PFS; Medtronic, Dublin, Ireland) is the leading braided flow-diverting stent on the market, and this device has had a positive impact on aneurysm repair in the past few years.4 In a systematic review of early studies (2009–2014),4 29 studies including 1524 patients with 3 to 62 months of follow-up were analyzed. The overall technical failure and complication rate was 9.3%. The rate of procedure-related complication was 14% and 6.6% for morbidity and mortality. Posterior circulation location, peripheral location and fusiform, dissecting and circumferential aneurysm were statistically significant risk factors for procedure-related complications. With the accumulation of experiences, in the following studies,7 thromboembolic complications were noted in 1.4% and hemorrhagic complications were found in 0.7% of procedures. The primary purpose of this article is to document the clinical and angiographic outcomes in 80 patients who were treated with the PFS and the obliteration mechanism was discussed.
Materials and Methods
This retrospective study included 90 aneurysms treated with PFSs in 80 consecutive patients (age mean ± SD, 52 ± 13 years; range, 15–73 years) between October 2015 and October 2019. Forty-five (56.3%) patients were women and 35 (43.7%) were men. Five patients presented with subarachnoid hemorrhage (SAH), four patients had recurrent aneurysms, 41 patients presented with headaches or neurological deficits caused by aneurysm mass effect and 41 patients were incidental.
Large or giant aneurysms were defined 10 mm or larger and small aneurysms were defined as less than 10 mm at the largest diameter measured on angiogram or magnetic resonance imaging. These cases were approved by the ethics board of our hospital. Cerebral aneurysms with undefined neck, large/giant in size, blood blister–like and recurrent side-wall aneurysms were treated with the PFS. Mostly, 65 of 90 (72.3%) of the aneurysms were located in the internal carotid artery (ICA), with nine of 90 (10%) in the vertebral artery, nine of 90 (10%) in the middle cerebral artery (MCA), four of 90 (4.4%) in the basilar artery and three of 90 (3.3%) in the posterior cerebral artery. In six patients, coexisting proximal stenosis of parent artery was also covered with PFS without balloon angioplasty.
Follow-up
Computed tomography scanning was obtained if a patient had ongoing headache. Every patient was scheduled for a control digital subtraction angiography (DSA) at six months. If the six-month control angiography revealed incomplete occlusion of the aneurysm, an additional control DSA was performed at 12 months.
Endovascular treatment
The decisions to use PFSs were undertaken based on literature reports by neurosurgeons. Endovascular treatments were performed with the patient under general anesthesia. Working projections were selected by three-dimensional (3D) rotational angiography in every patient. The length and diameter of targeted landing zone of the parent arteries were measured by using 3D reconstructions and two-dimensional working projection angiograms. In every patient, an 8F guiding catheter (Cordis Neurovascular, Miami Lakes, FL, USA) was placed proximally in the common carotid or subclavian artery. A 6F 115 cm or 5F 125 cm Navien intermediate catheter (Medtronic, USA) was navigated into the internal carotid or vertebral artery as distally as possible. A microcatheter 0.027 inches in diameter (Marksman; Medtronic, USA) was advanced over a 0.014-inch microguidewire (Traxcess14, Microvention, USA). The microcatheter tip was advanced distally enough so the PFS would not protrude into the aneurysm while it was advanced. In cases of a very wide neck of the large/giant aneurysm, for which the Marksman catheter could not be passed directly, the “exchanging technique” was used. Using the “exchanging technique,” the Marksman catheter was advanced on a 300 cm 0.014 inches microguidewire following a distally advanced 0.0175 inches microcatheter with a 200 cm 0.014 inches microguidewire.
The PFS releasing process should be as slow as possible for accurate PFS deployment. A single PFS was placed in most of cases. An additional PFS was necessary when the stent shortened and the aneurysm neck was not covered completely. We tried to avoid covering an artery bifurcation, such as the internal carotid artery bifurcation, basilar artery bifurcation, and vertebral artery bifurcation (Figure 1). Coils were used as an adjunct to the PFS placement in ruptured aneurysms and large or giant aneurysms (Figures 2 to 4).
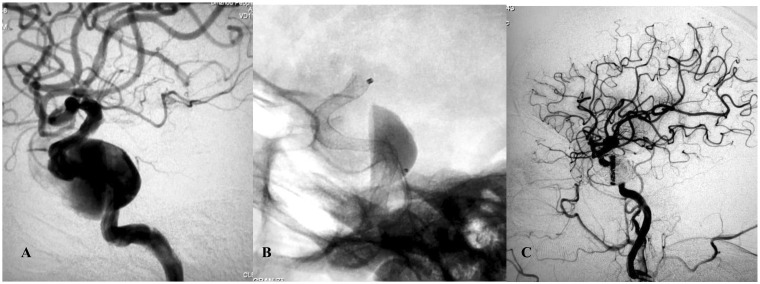
Figure 3.
(a) Frontal review of left internal carotid artery (ICA) angiogram showed a very wide-necked giant ICA aneurysm. (b) Intraoperative view showing the Pipeline flow-diverting stent (4.0 × 30 mm) opening to the normal size of the parent artery. Note the contrast stagnation within the sac. (c) Angiogram after additional coil placement. (d) Six-month control angiography showing total occlusion of the aneurysm and reconstruction of the parent artery.
Figure 1.
(a) Left vertebral angiogram showing the left posterior cerebral artery two small fusiform aneurysms (arrows). (b) Immediate postoperative view showing the single Pipeline flow-diverting stent (2.75 × 20 mm) placed in the left posterior cerebral artery, resulting in contrast stasis within the sac.
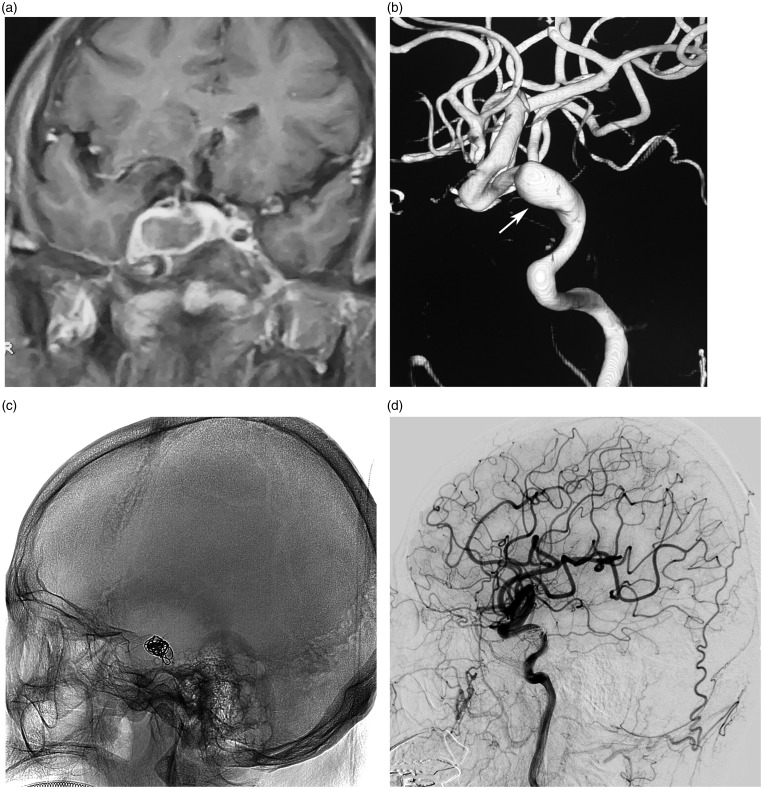
Figure 2.
(a) Enhanced magnetic resonance imaging, coronal view, showing a giant mass in the right cavernous sinus. (b) Three-dimensional reconstruction of right internal carotid artery angiography showing an aneurysm of the cavernous segment of internal carotid artery (arrow). (c) Fluoroscopic image showing a Pipeline flow-diverting stent (3.75 × 30 mm) and coils. (d) Seven-month follow-up angiogram showing the disappearance of the aneurysms and reconstruction of the internal carotid artery.
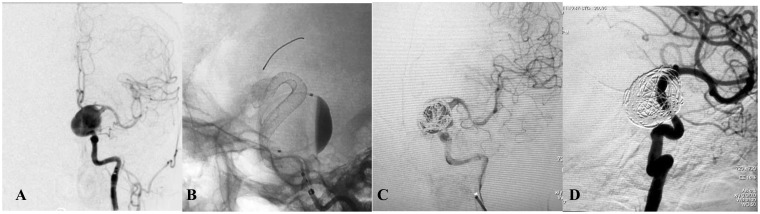
Figure 4.
(a) Lateral angiogram showing a giant cavernous internal carotid artery aneurysm. (b) Intraoperative view showing the Pipeline flow-diverting stent (3.75 × 30 mm) opening to the normal size of the parent artery. Note the contrast stagnation within the sac. (c) Angiography after placement of coils showing complete occlusion of the aneurysm and reconstruction of the parent artery.
Staged flow-diverting stent strategy
An acutely ruptured giant MCA aneurysm was treated by PFS scheduled one month after conventional coiling.
Medication
In the acute stage of SAH, four patients were premedicated with a loading dose of 300 mg clopidogrel and 300 mg aspirin, followed by 75 mg clopidogrel and 100 mg aspirin every day. Another 76 patients were premedicated with 75 mg clopidogrel and 100 mg aspirin daily for three to five days before treatment. Intravenous heparin was administered routinely during the treatment. A dose of 75 mg clopidogrel and 100 mg aspirin daily was maintained after the treatment for three months and clopidogrel was discontinued while aspirin was continued to six months.
Statistics
Proportions of aneurysm obliteration, morbidity and mortality pertinent to PFS treatment during follow-up and their 95% confidence interval (CI) were calculated. A Kaplan-Meier curve was constructed for aneurysm obliteration during follow-up (SPSS, 16.0).
Results
Seventy-five patients had 85 unruptured aneurysms, including four recurrent side-wall aneurysms. Only five patients presented with SAH caused by a blood blister–like aneurysm in four patients and a giant MCA aneurysm in one patient (Table 1). Forty-one aneurysms (45.6%) were large or giant, 41 (45.6%) were small, four (4.4%) were recurrent side-wall aneurysms (Figure 5) and four (4.4%) were blood blister–like aneurysms. Fifty-nine of 90 (65.6%) aneurysms were wide-necked saccular, and 31 of 90 (34.4%) were fusiform.
Table 1.
Ninety aneurysms in 80 patients treated using Pipeline flow-diverting stents.
| Characteristics | N (%) |
|---|---|
| Patient age, mean (range), y | 52 (15–73) |
| Female | 45 (56.3%) |
| Male | 35 (43.7%) |
| Ruptured | 5 (6.3%) |
| Unruptured | 75 (93.7%) |
| Aneurysm locations | |
| ICA | 65 (72.3%) |
| VA | 9 (10%) |
| BA | 4 (4.4%) |
| MCA | 9 (10%) |
| PCA | 3 (3.3%) |
| Aneurysm type | |
| Small < 10 mm | 41 (45.6%) |
| Large or giant | 41 (45.6%) |
| Recurrent side wall | 4 (4.4%) |
| Blood blister–like | 4 (4.4%) |
| Aneurysm morphology | |
| Saccular | 59 (65.6%) |
| Fusiform | 31 (34.4%) |
BA: basilar artery; ICA: internal carotid artery; MCA: middle cerebral artery; PCA: posterior cerebral artery; VA: vertebral artery.
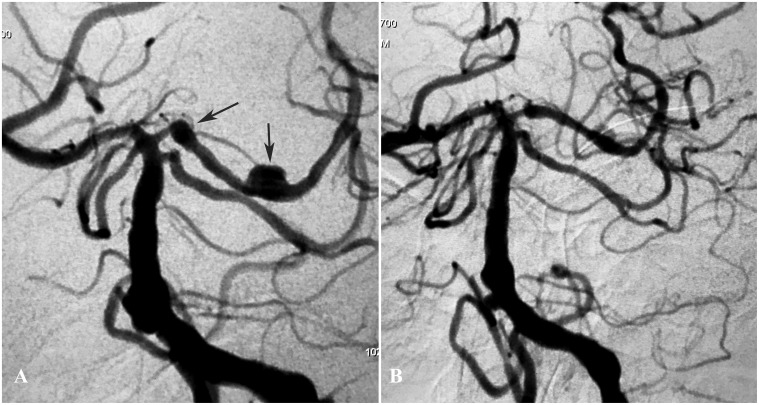
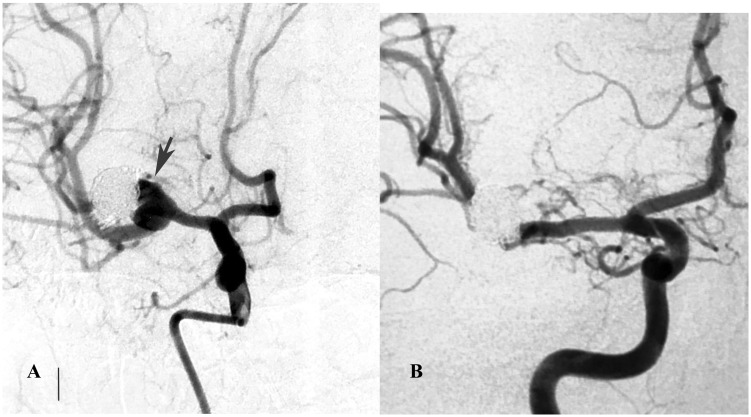
Figure 5.
(a) A right carotid angiogram showed a recurrent aneurysm in a patient who had a middle cerebral artery aneurysm previously treated with coiling (arrow). (b) Six-month control angiography showing occlusion of the aneurysm.
In 80 patients, 90 aneurysms were successfully treated with a total of 87 PFSs. There was no technical failure in this series. Only one PFS was used in 73 patients, one PFS was used to treat one aneurysm in 67 patients, and one PFS treated two aneurysms in six patients. In seven patients, a second PFS was necessary to cover the aneurysm neck. Among them, coils were placed in addition to the PFS in 31 aneurysms, 10 of which were giant, 15 were large, and only six were small. In the small aneurysms within this group, coils were used adjunctively because four blister-like aneurysms were treated in the acute stage of SAH. Four recurrent aneurysms had coils from previous treatments. Moreover, there was a self-expandable stent at the aneurysm neck in one patient.
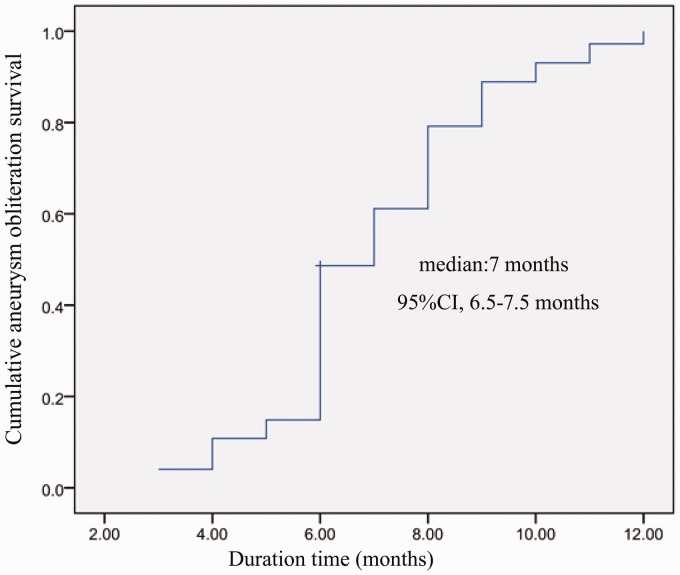
The overall adverse event rate was one of 80 (1.25%), with a mortality rate of one of 80 (1.25%; 95% CI, 0%–3.7%). There was no morbidity in this series. One patient died of thrombolysis from thrombus formation in the left ICA, resulting in an ipsilateral remote intraparenchymal hematoma. Control angiography was performed in 74 of the 80 patients (92.5%) with 83of 90 (92.2%) aneurysms at six and 12 months. Six patients with six aneurysms had their six-month DSA control pending. According to the control angiograms, 82 of 83 aneurysms were occluded for a total occlusion rate of 98.8% (95% CI, 96.5%–100%), with one MCA bifurcation aneurysm that did not show complete obliteration at the six-month control angiography. A Kaplan-Meier curve was created, showing median follow-up duration of 7.0 (95% CI, 6.5–7.5) months (Figure 6).
Figure 6.
A Kaplan-Meier curve shows the median follow-up duration of 7.0 (95% confidence interval, 6.5–7.5) months.
Discussion
In our series, the angiographic occlusion rates were 98.8% at 12 months, which was comparable with those in the previous PFS series,4 and our results confirmed the efficacy and safety of the PFS treatment in complex intracranial aneurysms. For small saccular aneurysms, conventional stent-assisted coiling and PFS are two equally efficacious modalities, such as the communicating segment aneurysms of the ICA.8,9 The aneurysm-healing mechanism of coiled aneurysms and PFS treatment is different.10 The main function of the flow diverter is to reduce blood flow and stasis in the aneurysm so that gradual thrombosis occurs within the aneurysm and subsequent healing.11,12 The aneurysm sac is prone to thrombosis because of the irregular patterns of blood flow and the potential for a low oxygen tension, especially during immobilization or long-haul travel.13
Early and delayed aneurysm ruptures following treatment with PFS have been analyzed by Lv et al.14 Although the definite causes of the aneurysm rupture are not yet proved, they suggested aggressive aneurysm thrombosis resulting in acute aneurysm growth cause of the delayed aneurysm rupture following flow-diverter treatment. Moreover, coil packing is not sufficient to spare the aneurysm from rupture. In large or giant aneurysms, a thrombus may have resulted from the slow blood flow and stagnation observed after PFS treatment. The thrombus continued to propagate in the aneurysm sac, causing intra-aneurysm hypertension and aneurysm rupture. The conditions for thrombus growth are that the blood can diffuse to the thrombus nucleation and supply the blood cells; the surface of the thrombus can accept the blood cells continuously and tightly. Because the thrombus has a higher osmolarity than blood, this will draw fluids and blood cells into the aneurysm sac, increasing the size of the lesion. Larger sizes of individual aneurysms vary and have a higher frequency of aneurysmal growth and rupture. It is proposed that a thrombus forms within the aneurysm sac, growing slowly over days or weeks and extending along the inside of the aneurysm wall, may eventually occlude the aneurysm, and even cause aneurysm growth and rupture.
In this series, adjuvant aneurysm filling with coils is considered in ruptured, large or giant aneurysms. A scheduled one-month, staged flow-diverting stent strategy was addressed in this study. We suggest that one potential treatment paradigm is to stage flow diversion after coiling in the acute phase to reduce early rebleeding risk and shrink the aneurysm volume; the aneurysm will be able to tolerate dual-antiplatelet therapy and flow-diversion treatment. This one-month scheduled flow-diversion concept is based on our greater understanding of delayed bleeding mechanisms of giant aneurysms.14 In our experience, it is important that the goal of this combined approach is to achieve just enough packing density to prevent early rebleeding and shrink the aneurysm volume by more than one-half. We have paid special attention to identifying the most likely point of rupture and targeted coiling with special emphasis on packing and thus protecting the rupture site. The “double-microcatheter technique” can distribute coils evenly in the aneurysm without confining the coils to one part of the aneurysms or risking coil prolapse. This strategy can also be used in patients with a giant intradural aneurysm, which are at risk of delayed rupture after flow-diverting treatment.
Parenchymal hemorrhage was another complication reported following flow-diverter treatment, with possible causes including wire perforation, aneurysm rupture, antiplatelet medication, hemorrhage within ischemic tissue, and so forth.4 Thromboembolic events may occur during treatment, during early follow-up, and also in the late phase.4 Possible causes include the following: (a) low response or resistance of antiplatelet drugs, (b) poor apposition to vessel wall, and (c) endothelium injuries. In a cohort of 37 vertebrobasilar aneurysms, the overall procedural complication rate was 14%, including three neurologically symptomatic complications. During the follow-up period of 14 months, 85% were completely or near-completely occluded.15
Flow diversion of bifurcation aneurysms is feasible, with low rates of permanent morbidity and mortality and high occlusion rates; however, nonocclusion may occur.16 A multivariable analysis identified older age (>70 years), higher maximal diameter (≥15 mm), and fusiform morphology were independently associated with higher rates of incomplete occlusion at follow-up.7 Some authors found smaller PFS diameter promotes occlusion of small anterior circulation intracranial saccular aneurysms, most likely due to the inherent greater metal density of smaller devices.1 For distal cerebral artery aneurysms, such as in the MCA, posterior cerebral artery, anterior cerebral artery (A1/A2, pericallosal artery), and the posterior inferior cerebellar artery, PFS use was also associated with good clinical outcome.17 Twenty procedures were performed on 18 patients, 11 with prior PFS, seven with a vascular reconstruction device, and two previously treated with both. Salvage PFS for previously stented aneurysms is feasible and offers good prospects of aneurysm obliteration with acceptable complication rates.18 In this series, stenosis proximal to the aneurysm was covered by the PFS without any adverse events.
Conclusion
For wide-necked saccular, large/giant, blood blister–like aneurysms and recurrent side-wall aneurysms, PFS is a valid and safe treatment option.
Declaration of conflicting interests
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This research was supported by the Beijing Municiple Administration of Hospitals Incubating Program PX2020039.
ORCID iD
Xianli Lv https://orcid.org/0000-0001-8270-8464
References
- 1.Kole MJ, Miller TR, Cannarsa G, et al. Pipeline embolization device diameter is an important factor determining the efficacy of flow diversion treatment of small intracranial saccular aneurysms. J Neurointerv Surg 2019; 11: 1004–1008. [DOI] [PubMed] [Google Scholar]
- 2.Kühn AL, Kan P, Srinivasan V, et al. Flow diverter for endovascular treatment of intracranial mirror segment internal carotid artery aneurysms. Interv Neuroradiol 2019; 25: 4–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lv X, Jiang C, Li Y, et al. Treatment of giant intracranial aneurysms. Interv Neuroradiol 2009; 15: 135–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lv X, Yang H, Liu P, et al. Flow-diverter devices in treatment of intracranial aneurysms: A meta-analysis and systematic review. Neuroradiol J 2016; 29: 66–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wallace AN, Delgado Almandoz JE, Kayan Y, et al. Pipeline treatment of intracranial aneurysms is safe and effective in patients with cutaneous metal allergy. World Neurosurg 2019; 123: e180–e185. [DOI] [PubMed] [Google Scholar]
- 6.Wu Z, Lv X, Li Y, et al. Endovascular treatment for complex intracranial aneurysms: Lessons learnt in five patients. Neuroradiol J 2010; 23: 459–466. [DOI] [PubMed] [Google Scholar]
- 7.Maragkos GA, Ascanio LC, Salem MM, et al. Predictive factors of incomplete aneurysm occlusion after endovascular treatment with the Pipeline embolization device. J Neurosurg.. Epub ahead of print 26 April 2019:1–8. DOI: 10.3171/2019.1.JNS183226. [DOI] [PubMed] [Google Scholar]
- 8.Enriquez-Marulanda A, Salem MM, Ascanio LC, et al. No differences in effectiveness and safety between Pipeline embolization device and stent-assisted coiling for the treatment of communicating segment internal carotid artery aneurysms. Neuroradiol J 2019; 32: 344–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Keskin F, Erdi F, Kaya B, et al. Endovascular treatment of complex intracranial aneurysms by Pipeline flow-diverter embolization device: A single-center experience. Neurol Res 2015; 37: 359–365. [DOI] [PubMed] [Google Scholar]
- 10.Brinjikji W, Kallmes DF, Kadirvel R. Mechanisms of healing in coiled intracranial aneurysms: A review of the literature. AJNR Am J Neuroradiol 2015; 36: 1216–1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Poredoš P. Interrelationship between venous and arterial thrombosis. Int Angiol 2017; 36: 295–298. [DOI] [PubMed] [Google Scholar]
- 12.Preston RJS, O’Sullivan JM, O’Donnell JS. Advances in understanding the molecular mechanisms of venous thrombosis. Br J Haematol 2019; 186: 13–23. [DOI] [PubMed] [Google Scholar]
- 13.Grover SP, Evans CE, Patel AS, et al. Assessment of venous thrombosis in animal models. Arterioscler Thromb Vasc Biol 2016; 36: 245–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lv X, Ge H, He H, et al. A systematic review of Pipeline embolization device for giant intracranial aneurysms. Neurol India 2017; 65: 35–38. [DOI] [PubMed] [Google Scholar]
- 15.Wallace AN, Madaelil TP, Kamran M, et al. Pipeline embolization of vertebrobasilar aneurysms—a multicenter case series. World Neurosurg.. 2019; 124: e460–e469. [DOI] [PubMed] [Google Scholar]
- 16.Michelozzi C, Darcourt J, Guenego A, et al. Flow diversion treatment of complex bifurcation aneurysms beyond the circle of Willis: Complications, aneurysm sac occlusion, reabsorption, recurrence, and jailed branch modification at follow-up. J Neurosurg.. 2018; 131: 1751–1762. [DOI] [PubMed] [Google Scholar]
- 17.Atallah E, Saad H, Mouchtouris N, et al. Pipeline for distal cerebral circulation aneurysms. Neurosurgery 2019; 85: E477–E484. [DOI] [PubMed] [Google Scholar]
- 18.Bender MT, Vo CD, Jiang B, et al. Embolization for salvage treatment of previously stented residual and recurrent cerebral aneurysms. Interv Neurol 2018; 7: 359–369. [DOI] [PMC free article] [PubMed] [Google Scholar]