Abstract
Background
Many original articles and case series have been published emphasizing the neuroimaging findings of congenital Zika virus (ZIKV) infection. The majority of these studies do not follow a neuroradiological methodology to describe malformations and brain abnormalities resulting from ZIKV infection. The cause-and-effect correlation between the gestational period of maternal infection and the severity of encephalic changes at birth has rarely been reported. A systematic literature review was conducted on the neuroimaging findings in children affected with microcephaly due to ZIKV.
Methods
PubMed, Cochrane Library and Web of Science were searched for full-text articles published up to July 2019. Duplicate entries were removed. Two independent reviewers performed a quality assessment of all the studies included.
Results
A total of 2214 publications were identified. Of these 2170 were excluded by analysis of titles and abstracts, resulting in the inclusion of only eight articles. Chi-square and Fisher’s exact tests were performed with a 95% confidence interval to verify the statistically significant differences in the neuroradiological findings between the cases of ZIKV infection in the first or second trimester of gestation. The studies published so far have described image abnormalities at random, without utilizing any pre-established neuroradiological criteria, and imaging modalities with different sensitivity and accuracy have been used, which jeopardizes a reliable and adequate statistical analysis.
Conclusions
Neuroimaging abnormalities are much more prevalent and severe when the infection by ZIKV is contracted in the first or second trimester of pregnancy.
Keywords: Zika virus, microcephaly, tomography, magnetic resonance
Introduction
The literature reports several original articles and case series highlighting the neuroimaging findings of congenital Zika virus (ZIKV) infection. Many of these studies do not follow a neuroradiological methodology to describe the malformations and brain abnormalities resulting from the infection. In particular, there have been no discussions on a cause-and-effect correlation between the gestational period of maternal infection and the severity of encephalic changes at birth. Neuroimaging techniques with dissimilar sensitivities and diagnostic accuracies have been employed at random to elaborate tables and descriptive results of the main neuroradiological changes occurring in newborns with congenital ZIKV infection. As a result, a specific neuroradiological gold standard that would relate to the gestational trimester of fetal infection by ZIKV is lacking.
ZIKV is a flavivirus, transmitted mainly by mosquitoes of the genus Aedes. The virus was first isolated in Uganda in 1947 and continued to be restricted to the areas of Africa and Asia for 60 years. There was an outbreak of ZIKV infection in northeastern Brazil in early 2015.1 In October 2015, an increase in the number of patients with microcephaly was reported, and this congenital malformation was found to be related to intrauterine ZIKV infection.2,3 The most frequent symptoms of ZIKV include fever, myalgia, and rash.4 ZIKV is usually diagnosed by the symptoms and biochemical findings of standard reverse transcriptase polymerase chain reaction (RT-PCR) in the blood, urine or salivary fluids. However, the diagnosis of this infection poses some difficulties due to the possible cross-reaction with other flaviviruses.5 In terms of the neuroimaging findings, a variety of cortical malformations can be identified, including parenchymal volume reduction, alterations in the gyral pattern as well in white and gray matter, ventriculomegaly, abnormalities of the corpus callosum and cerebellum, hypoplasia and/or atrophy of the brain stem, and diffuse calcifications.6 In 2016, the World Health Organization reported cases of microcephaly and neurological disorders associated with ZIKV as an international public-health emergency, thereby intensifying the need for further research in this area.7 Microcephaly may not necessarily be evident at birth and may develop after birth, along with other brain abnormalities. It is therefore essential to perform neuroimaging investigations in infants exposed to ZIKV. The objective of this study was to conduct a systematic review of literature in order to evaluate the radiological findings and to create a neuroimaging profile of children with microcephaly exposed to intrauterine ZIKV infection regarding the time of intrauterine infection.
Methods
This systematic review was performed in accordance with the methodology described in the Cochrane Handbook for Systematic Reviewers and presented by Preferred Reporting Items for Systematic Review and Meta-analyses (PRISMA) statement.8
Search strategy
A literature search was performed in PubMed, Cochrane Library, and Web of Science. The terms used for the search strategy included “Zika virus,” “microcephaly,” “newborn,” “pregnancy,” and “neuroimaging.”
Eligibility criteria
Inclusion criteria
Prospective and retrospective clinical studies with neuroimaging examinations, including computed tomography (CT) scan or magnetic resonance imaging (MRI), of individuals infected with ZIKV and microcephaly were included in the study, without any restrictions on the language or year. Reports published until July 2019 were considered. Additionally, the study included even those articles that mentioned the diagnosis of Zika infection, irrespective of whether it was confirmed or only suspected.
Exclusion criteria
Preclinical studies, in vivo studies, letters to the editor, editorials, duplicate publications, articles that did not have any data for evaluation, articles that did not address neuroimaging (CT or MRI), and studies using transfontanellar ultrasound as imaging methods were excluded.
Data extraction
Endnote version X9 software was used for data extraction. The databases were thoroughly searched, and duplicate entries were removed. Abstracts that did not provide sufficient information regarding the inclusion and exclusion criteria were selected for full-text evaluation. In the second phase, the same reviewers independently evaluated the full text of these articles and made their selection in accordance with the eligibility criteria. Two reviewers (J.M. and B.B.F.) performed the literature search and study selection independently. Disagreements were solved by consensus or a third reviewer (G.R.).
Statistical analysis
In order to verify the statistically significant differences between the neuroradiological findings of patients infected with ZIKV in the first or second trimester of gestation, chi-square and Fisher’s exact tests with 95% confidence intervals were performed.
Results
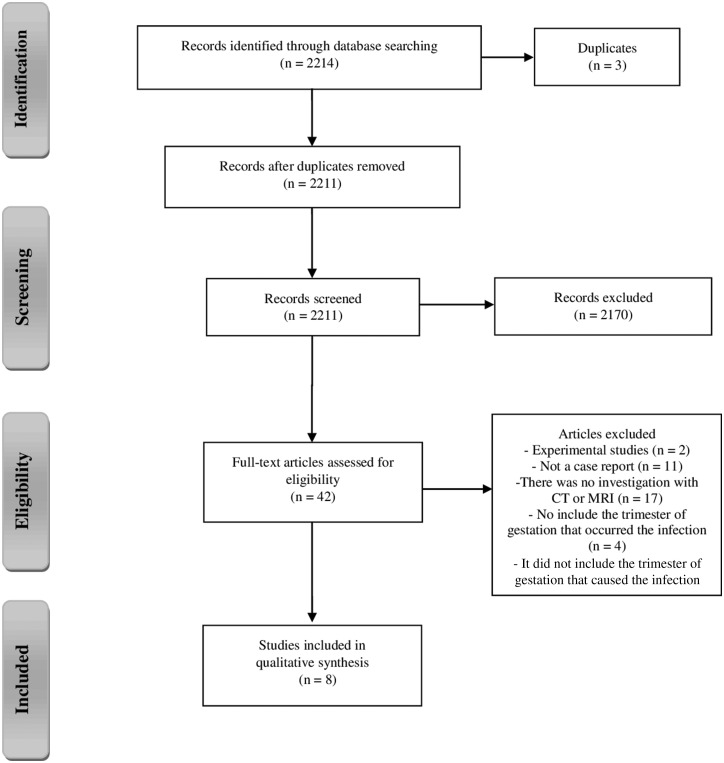
The initial database search yielded 2214 articles. After removal of duplicate entries, 2211 articles were filtered according to the inclusion criteria. Of these, 2170 were excluded following analysis of titles and abstracts, thus leaving 42 articles for full-text evaluation. Out of the rejected articles, 17 studies were excluded because they lacked CT or MRI investigation, while two were experimental studies, resulting in a total of 22 studies. Subsequently, 11 articles were excluded because they were not case reports, and four were disqualified because they did not include the trimester of gestation in which the infection occurred. Consequently, only eight articles were included in this review.9–16 The complete study flow chart is shown in Figure 1.
Figure 1.
Summary of evidence search and study selection.
This review included eight studies describing 235 cases of congenital Zika syndrome. The gestational period of ZIKV infection was reported in only 152 cases, while in the remaining 83 cases, the time of contraction of the infection was unknown (Table 1). The almost abnormal brain imaging findings (CT and/or MRI) were reviewed from 152 infants exposed to ZIKV. The infection occurred predominantly in the first trimester (n = 119) compared to the second (n = 30) and the third (n = 3). The majority of neuroimaging abnormalities occurred in the first or second trimester, and these abnormalities are described in Table 2. The most frequent findings in the first and second trimesters included decreased brain volume and increased extra-axial cerebrospinal fluid (CSF) space (81.5% and 80%, respectively), subcortical calcifications (88.2% and 93.3%, respectively), microcephaly (93.3% and 90%, respectively), ventriculomegaly (78.1% and 73.3%, respectively), malformation of cortical development (58.8% and 73.3%, respectively), basal ganglia calcifications (50.4% and 33.3%, respectively), and mega cisterna magna (50.4% and 20%, respectively). Out of the three cases in which the infection was contracted in the third trimester, the mother was infected in the seventh month of gestation in the first case, and presented with cortical calcifications and reduced cortical volume. The infection occurred in the eighth month of gestation in the second case, and neuroimaging exhibited subcortical calcifications, reduced cortical volume, and colpocephaly. In the third case, neuroimaging showed malformation of the cortical area, subcortical calcifications, periventricular calcifications, and decreased brain volume in addition to the increased extra-axial CSF space.
Table 1.
Characteristics of included studies.
| No. of cases (CT, MRI, or both) | Time of Infection (maternal symptoms) |
||||
|---|---|---|---|---|---|
| Author(s) (year) | 1st trimester | 2nd trimester | 3rd trimester | Unknown | |
| Aragao et al. (2016)9 | 23 (15, 1, 7) | 17 | 5 | — | 1 |
| Van Der Linden (2016)10 | 13 (3, 0, 10) | 4 | 2 | — | 7 |
| Aragao et al. (2017)11 | 19 (0, 19, 0) | 9 | 5 | — | 5 |
| Carvalho et al. (2017)12 | 37a | 18 | 4 | 2 | 13 |
| Castro et al. (2017)13 | 8 (0, 7, 0) | 7 | — | — | 1 |
| Pires et al. (2018)14 | 8 (8, 0, 0) | 5 | 2 | — | 1 |
| Parra-Saavedra et al. (2017)15 | 17 (0, 16, 0) | 14 | 3 | — | — |
| Pool et al. (2019)16 | 110 (81, 45, 16) | 45 | 9 | 1 | 55 |
| Total number of cases | 235 | 119 | 30 | 3 | 83 |
The study does not specify whether MRI or CT or both.
CT: computed tomography; MRI: magnetic resonance imaging.
Table 2.
Neuroimaging findings and trimester of maternal Zika virus infection.
| Infant neuroimaging findings | Trimester, n (%) |
Fisher’s exact test (p) | ||
|---|---|---|---|---|
| First (N = 119) | Second (N = 30) | OR (95% CI) | ||
| Decreased brain volume + increased extra-axial CSF space | 97 (81.5) | 24 (80.0) | 1.10 (0.32–3.22) | 0.799 |
| Subcortical calcifications | 105 (88.2) | 28 (93.3) | 0.53 (0.05–2.56) | 0.528 |
| Microcephaly | 111 (93.3) | 27 (90.0) | 1.53 (0.24–6.96) | 0.463 |
| Ventriculomegaly | 93 (78.1) | 22 (73.3) | 1.29 (0.44–3.48) | 0.628 |
| Malformation of cortical development | 70 (58.8) | 22 (73.3) | 0.52 (0.18–1.34) | 0.206 |
| Basal ganglia calcifications | 60 (50.4) | 10 (33.3) | 2.02 (0.82–5.27) | 0.105 |
| Mega cisterna magna | 60 (50.4) | 6 (20.0) | 4.03 (1.46–12.94) | 0.003 * |
| Corpus callosum abnormalities | 41 (34.4) | 9 (30.0) | 1.22 (0.48–3.32) | 0.829 |
| Brain stem hypoplasia | 48 (40.3) | 8 (26.7) | 1.85 (0.71–5.22) | 0.207 |
| Periventricular calcifications | 45 (37.8) | 7 (23.3) | 1.98 (0.74–5.94) | 0.197 |
| Cerebellum hypoplasia/atrophy | 43 (36.1) | 7 (23.3) | 1.85 (0.69–5.54) | 0.202 |
| Brain stem calcification | 22 (18.5) | 3 (10.0) | 2.03 (0.54–11.39) | 0.412 |
| Cerebellum calcification | 4 (3.3) | 3 (10.0) | 0.31 (0.05–2.28) | 0.145 |
Statistically significant.
CI: confidence interval; OR: odds ratio; CSF: cerebrospinal fluid.
In relation to the cases of contamination occurring in the second trimester of pregnancy, one outcome, mega cisterna magna (p = 0.003), had a score of p < 0.05, thus presenting to be the most recurrent symptom when there was contamination in the first trimester (Table 2).
Discussion
This systematic review evaluated changes in the brains of newborns with congenital ZIKV infection through distinct neuroimaging methods (CT and MRI exams) and tried to establish the association between the abnormalities and gestational period of infection.
The studies published so far have described image abnormalities at random, without considering any pre-established neuroradiological criteria, and have used imaging modalities with different sensitivity and accuracy, which has in turn failed to yield a reliable and adequate statistical analysis. Another limitation of the majority of such studies concerns the absence of a cause–effect relationship due to the unavailability of data describing the gestational period in which the fetus contracted the infection. In addition, the authors came across numerous neuroimaging findings only describing cerebral malformations with microcephaly, with no association between the time of evidence of the severity of outcomes and the period of contraction of ZIKV infection. The main finding of this systematic review is that the most serious and prevalent alterations in the brain were established to occur when the infection was contracted in the first trimester of gestation. In contrast, it was verified that infections occurring in the second or third trimesters of gestation presented less severe and less complex radiological changes (Table 1).
Since ZIKV interferes with the process of neuronal migration and consequent brain formation, the most prevalent radiological alterations, in both the first and second trimesters, presented to be subcortical calcifications, ventriculomegaly, microcephaly, decreased cerebral volume, and malformed cortical development. With regard to infections occurring in the third trimester, only two cases of microcephaly were described, with no other malformations.
The possible relationship between ZIKV infection in the gestational period and the aforementioned central nervous system (CNS) changes raises great global public-health concern, as this infection does not have any effective treatment to date. The period of contraction of infection of the ZIKV and the occurrence of fetal brain abnormalities that may be susceptible to viral pathogenesis, as well as the way in which it affects the appearance of brain abnormalities on imaging, are poorly understood.
The changes in the brain that are caused by the infectious agents during the gestational period differ from those occurring in children and adults after birth. This is because the former condition occurs during nervous tissue development. Manifestations of these infections differ depending on the time of contraction and are less related to the virulence of the agent. Thus, in general, infections occurring during the first or second trimester result in congenital malformations, while those occurring in the third trimester cause destructive lesions.17
The ZIKV infection causes abnormalities during neural differentiation, primarily targeting the neural stem cells (NSC). ZIKV proteins interfere with the functioning of NSCs, resulting in severe consequences such as metabolic fluxes, inhibition of cell proliferation, and cellular apoptosis.18 Neurospheres generated from human-induced pluripotent stem cells (iPSC) after three days of ZIKV infection showed morphological alterations and cell detachment, which can mainly be associated with the cellular migration processes. After six days of exposure to ZIKV, only a few neurospheres were found to survive the viral action. ZIKV can be found in membranes, mitochondria, and cellular vesicles. Other alterations caused by the ZIKV in neurospheres include apoptotic nuclei formation, characteristics of cell death, and the presence of a smooth membrane. The impact of ZIKV during embryonic neurogenesis is exhibited by the exposition of ZIKV to cerebral organoids derived from iPSC. The average growth of the organoids exposed to ZIKV in the experimental group was observed to be reduced by 40% compared to the cerebral organoids in the control group. This demonstrates that ZIKV induces cell death in human iPSC-derived NSCs, disrupts the formation of neurospheres, and decreases the growth of organoids, thus indicating that ZIKV infection may cause severe damage in models that mimic the first trimester of the brain development.19
Similar studies have shown that ZIKV infection can modulate cellular metabolism in the neuronal cells in the first stage of neurodifferentiation through P53 activation and mTOR pathway inhibition by inducing an early shift from glycolysis to OXPHOS that causes immature differentiation, apoptosis, and stem-cell exhaustion.20,21
Another important contribution of this systematic review, which may be of great significance to subsequent studies, is the discovery of numerous methodological or descriptive limitations that may have distorted the prevalence or frequency of neuroradiological findings in newborns with congenital ZIKV. One of the most important limitations noted was the absence of neuroradiological criteria for the description of brain abnormalities, which is evident by the absence of homogeneity in the findings, as two different descriptions have frequently been used to describe the same neuroradiological finding. Another relevant limitation, which certainly modified the actual frequency/prevalence of described neuroradiological abnormalities, was the equivalence between ultrasonography, CT, and MRI during description and tabulation of the results, which was done without taking into account the disparity in sensitivity and accuracy between the three methods. As a result, high frequencies and a greater diversity of brain changes were reported in the studies with a higher number of MRIs, while only a few changes/descriptions were documented in the studies with fewer MRI examinations. The difficulties encountered while assessing the relationship between gestational age at which ZIKV infection was contracted and the structural changes in the brain are associated with the lack of laboratory evidence and recording of clinical information, imaging studies with varying sensitivities and/or reports with limited details of the abnormalities, and the heterogeneity in their description in few studies. Also, in some case reports, there is no description of alterations in the midline abnormalities such as changes in the corpus callosum and cerebellar vermis (perhaps due to the predominant number of CT scans and low-quality MRIs) and no recognition of cerebral and cerebellar atrophy or enlargement of cerebrospinal fluid space. Lastly, poor experience of the radiologists in pediatric neuroimaging, many of which were interpreted by non-radiologists or general radiologists, also played a role.
The number of studies specifying radiological findings of congenital ZIKV infection is still very low. The clinical and radiological peculiarities of this pathology are in constant debate, as demonstrated in this review article. The necessity for a final description and classification of brain abnormalities caused by ZIKV infection, using appropriate neuroimaging criterion that allows a sequential cause–effect relationship with the gestational period of fetal infection, may be of great value in assessing the outcomes and prognoses of affected children.
MRI is the best method for assessing the CNS of fetuses with suspected defects.22 The absence of ionizing radiations and the ability for greater tissue differentiation allows for a better study of the CNS, thus eliminating the limitations caused by skull artifact or fetal position. Further MRI studies are warranted for a more detailed and specific evaluation of the brain abnormalities caused by congenital ZIKV infection, in addition to the studies involving a more homogenous design, with a higher number of evaluated outcomes, and studies that have two independent evaluators who are well trained to examine the images.
Conclusions
Neuroimaging abnormalities are much more prevalent and severe when the ZIKV infection occurs in the first or second trimester of pregnancy. Findings that might differentiate infections occurring in the first trimester in comparison to the second include mega cisterna magna. The most recurrent malformations seen in the infections in both the first and second trimesters of pregnancy include subcortical calcifications and microcephaly. The evidence provided in this study may help public policies in the treatment and prevention of ZIKV microcephaly worldwide.
Acknowledgements
We thank Daniel Rodrigo Marinowic, PhD, for his suggestion regarding in vitro studies, and Nathalia Bianchini Esper, MS, Bárbara Brum Fonseca, Antônio Furlanetto Cortea, and Felipe Augusto Kunzlera for text suggestions and review.
Declaration of conflicting interests
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Our study was supported by the following grants: CNPq (Conselho Nacional de Desenvolvimento Científico e Tecnológico) and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES). J.C.C. and M.L.N. were funded by CNPq (bolsa de produtividade em pesquisa). J.C.C. and M.L.N. are researchers 1A and 1D, respectively, from CNPq, Brazil.
ORCID iDs
Graciane Radaelli https://orcid.org/0000-0002-1208-362X Jaderson Costa da Costa https://orcid.org/0000-0001-6776-1515
References
- 1.Desai SK, Hartman SD, Jayarajan S, et al. Zika virus (ZIKV): a review of proposed mechanisms of transmission and associated congenital abnormalities. Am J Stem Cells 2017; 6: 13. [PMC free article] [PubMed] [Google Scholar]
- 2.Mehrjardi MZ, Carteaux G, Poretti A, et al. Neuroimage findings of postnatally acquired Zika virus infection: a pictorial essay. Jpn J Rad 2017; 35: 341–349. [DOI] [PubMed] [Google Scholar]
- 3.Nunes ML, Carlini CR, Marinowic D, et al. Microcephaly and Zika virus: a clinical and epidemiological analysis of the current outbreak in Brazil. J Pediatr 2016; 92: 230–240. [DOI] [PubMed] [Google Scholar]
- 4.De Oliveira WK, Carmo EH, Henriques CM, et al. Zika virus infection and associated neurologic disorders in Brazil. N Engl J Med 2017; 376: 1591–1593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Paiva MHS, Guedes DRD, Leal WS, et al. Sensitivity of RT-PCR method in samples shown to be positive for Zika virus by RT-qPCR in vector competence studies. Genetics Mol Biol 2017; 40: 597–599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.De Oliveria-Szejnfeld PS, Levine D, Melo ASDO. Congenital brain abnormalities and Zika virus: what the radiologist can expect to see prenatally and postnatally. Radiology 2016; 281: 203–218. [DOI] [PubMed] [Google Scholar]
- 7.De Oliveira WK, França GVA, Carmo EH, et al. Infection-related microcephaly after the 2015 and 2016 Zika virus outbreaks in Brazil: a surveillance-based analysis. Lancet 2017; 390: 861–870. [DOI] [PubMed] [Google Scholar]
- 8.Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med 2009; 151: 264–269. [DOI] [PubMed] [Google Scholar]
- 9.Aragao MDFV, Van Der Linden V, Brainer-Lima AM, et al. Clinical features and neuroimaging (CT and MRI) findings in presumed Zika virus related congenital infection and microcephaly: retrospective case series study. BMJ 2016; 353: i1901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Van Der Linden V. Description of 13 infants born during October 2015–January 2016 with congenital Zika virus infection without microcephaly at birth – Brazil. MMWR Morb Mortal Wkly Rep 2016; 65. [DOI] [PubMed] [Google Scholar]
- 11.Aragao MFVV, Holanda AC, Brainer-Lima AM, et al. Nonmicrocephalic infants with congenital Zika syndrome suspected only after neuroimage evaluation compared with those with microcephaly at birth and postnatally: how large is the Zika virus ‘iceberg’? AJNR Am J Neuroradiol 2017; 38: 1427–1434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Carvalho MDCG, Miranda-Filho DB, Van Der Linden V, et al. Sleep EEG patterns in infants with congenital Zika virus syndrome. Clin Neurophysiol 2017; 128: 204–214. [DOI] [PubMed] [Google Scholar]
- 13.Castro JDVD, Pereira LP, Dias DA, et al. Presumed Zika virus-related congenital brain malformations: the spectrum of CT and MRI findings in fetuses and newborns. Arq Neuropsiquiatr 2017; 75: 703–710. [DOI] [PubMed] [Google Scholar]
- 14.Pires P, Jungmann P, Galvão JM, et al. Neuroimage findings associated with congenital Zika virus syndrome: case series at the time of first epidemic outbreak in Pernambuco State, Brazil. Childs Nerv Syst 2018; 34: 957–963. [DOI] [PubMed] [Google Scholar]
- 15.Parra-Saavedra M, Reefhuis J, Piraquive JP, et al. Serial head and brain imaging of 17 fetuses with confirmed Zika virus infection in Colombia, South America. Obstet Gynecol 2017; 130: 207–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pool KL, Adachi K, Karnezis S, et al. Association between neonatal neuroimaging and clinical outcomes in Zika-exposed infants from Rio de Janeiro, Brazil. JAMA Netw Open 2019; 2: e198124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bale JF. Fetal infections and brain development. Clin Perinatol 2009; 36: 639–653. [DOI] [PubMed] [Google Scholar]
- 18.Rothan HA, Fang S, Mahesh M, et al. Zika virus and the metabolism of neuronal cells. Mol Neurobiol 2018, pp. 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Garcez PP, Loiola EC, Da Costa RM, et al. Zika virus impairs growth in human neurospheres and brain organoids. Science 2016; 352: 816–818. [DOI] [PubMed] [Google Scholar]
- 20.Ghouzzi VE, Bianchi FT, Molineris I, et al. ZIKA virus elicits P53 activation and genotoxic stress in human neural progenitors similar to mutations involved in severe forms of genetic microcephaly and p53. Cell Death Dis 2016; 7: e2440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liang Q, Luo Z, Zeng J, et al. Zika virus NS4A and NS4B proteins deregulate Aktm TOR signaling in human fetal neural stem cells to inhibit neurogenesis and induce autophagy. Cell Stem Cell 2016; 19: 663–671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Da Silva NA, Jr, Vassallo J, Sarian LO, et al. Magnetic resonance imaging of the fetal brain at 3 Tesla: preliminary experience from a single series. Medicine (Baltimore) 2018; 97: e12602. [DOI] [PMC free article] [PubMed] [Google Scholar]