Abstract
Background:
Diagnosing multiple sclerosis (MS) early is crucial to avoid future disability. However, potentially preventable delays in the diagnostic cascade from contact with a physician to definite diagnosis still occur and their causes are still unclear.
Objective:
To identify the possible causes of delays in the diagnostic process.
Methods:
We analyzed the data of the Swiss MS Registry. With logistic regression, we modeled the time from the first contact to the first consultation (contact-to-evaluation time, ⩽1 month/>1 month) and the evaluation-to-diagnosis time (⩽6 months/>6 months). Potential factors were health system characteristics, sociodemographic variables, first symptoms, and MS type.
Results:
We included 522 participants. Mostly, general practitioners (67%) were contacted first, without delaying the diagnosis. In contrast, first symptoms and MS type were the major contributors to delays: gait problems were associated with longer contact-to-evaluation times, depression as a concomitant symptom with longer evaluation-to-diagnosis times, and having primary progressive MS prolonged both phases. In addition, living in mountainous areas was associated with longer contact-to-evaluation times, whereas diagnosis after 2000 was associated with faster diagnoses.
Conclusion:
For a quicker diagnosis, awareness of MS as a differential diagnosis of gait disorders and the co-occurrence of depression at onset should be raised, and these symptoms should be attentively followed.
Keywords: Registries, regression analysis, time to diagnosis, delayed diagnosis, patient-reported outcomes, epidemiology
Introduction
Diagnosing a patient with multiple sclerosis (MS) is a complex and multifaceted clinical process. However, the past three decades have seen a significant reduction in the time from onset of symptoms to diagnosis.1 Probable reasons are the constantly refined diagnostic criteria,2 an increased magnetic resonance imaging (MRI) availability, and the increasing treatment options, approved in Switzerland since 1993. Nevertheless, a substantial number of patients still experience significant delays before receiving a definite diagnosis. A Swiss study by Kaufmann et al.3 found that—even in recent time periods—40% of all newly diagnosed persons with MS reported more than 2 years passing between symptom onset and the MS diagnosis. This is even more relevant in light of the mounting evidence on the benefits of early treatment initiation in relapsing remitting multiple sclerosis (RRMS) to prevent long-term disability.4–6
The reasons for delays in the diagnosis are not fully understood, and few studies have attempted more detailed investigations into the time from MS onset to diagnosis (henceforth referred to as diagnostic time).7–10 Of these studies, most were unable to investigate the delaying factors using a systematic approach and performed in highly specialized MS centers.7–9 An exception was a population-based study from Canada,10 which found that younger age at onset and having primary progressive multiple sclerosis (PPMS) were associated with longer times to diagnosis.
Health system–specific aspects and care access barriers may also play an important role in diagnostic delays. Studies from the United States found that insurance coverage, living in remote rural areas, and public transportation deficiencies can constitute significant barriers to accessing neurological care in MS patients,11 which may equally apply to the diagnostic process.
Therefore, further investigations of delaying factors should not only consider clinical aspects but also possible access barriers and the diagnostic cascade potential MS patients with first symptoms take through the healthcare system.
Using the Swiss Multiple Sclerosis Registry (SMSR), we aimed to analyze the diagnostic cascade from the moment a patient seeks care because of first symptoms until a clinically isolated syndrome (CIS) or an MS diagnosis is confirmed. In addition, we aimed to analyze which setting- and patient-specific features potentially delay the diagnosis at the different cascade steps. This knowledge could lead to an optimization of the diagnostic process and possibly to an earlier start of drug and non-drug (e.g. neurorehabilitation, lifestyle adjustments) treatment of MS patients.
Methods
The conceptual model of the diagnostic cascade
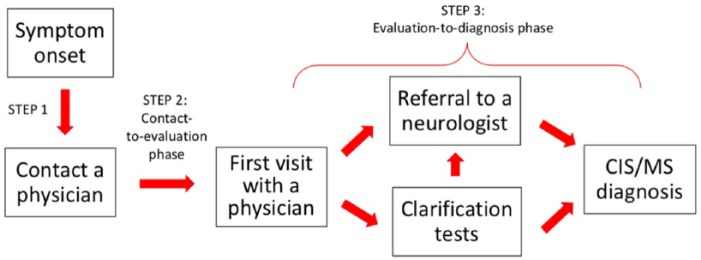
Along the lines of the diagnostic cascade described by Fernández et al.,7 we developed a conceptual model of the diagnostic steps in collaboration with MS neurologists, thereby taking into account specific features of the Swiss healthcare system (e.g. free physician choice; no mandatory first visit to the primary care provider unless the health insurance plan requires it). This concept was subsequently used to guide our analysis and was the framework for summarizing our study data. A sketch of the model is outlined in Figure 1 and includes three steps. In step 1, a person notices his or her symptoms and contacts a healthcare provider, which, in Switzerland, can be a general practitioner (GP) or directly a specialist. The contacted physician’s office then schedules a visit, which depending on symptom severity could take from a few days to months (step 2, henceforth named “contact-to-evaluation phase”). If, at the actual visit, the symptoms suggest an MS diagnosis, a neurological referral would follow (step 3, “evaluation-to-diagnosis phase”).
Figure 1.
The conceptual model of the diagnostic process.
In accordance with this conceptual model, separate data analyses were performed to investigate the factors associated with delays in steps 2 and 3, as well as to summarize the variety of observed cascades over both steps.
The data sources
The SMSR is an ongoing, Swiss-based, patient-centered, longitudinal observational study, initiated and funded by the Swiss MS Society (http://www.ClinicalTrials.gov; identifier: NCT02980640). Study recruitment is continuous and started in June 2016. Adults with CIS or confirmed MS who live or receive care in Switzerland can voluntarily participate. A wide range of measures have been implemented to motivate and enable Swiss persons with MS to participate in the SMSR (described in detail by Puhan et al.12), such as regular information through the news outlets of the Swiss Multiple Sclerosis Society in the three main national languages, presentations to health care professionals and potential participants in clinics and private practices, as well as the possibility to fill in questionnaires on paper or via a specifically designed online platform. At registration, a baseline questionnaire is completed. Afterwards, follow-up questionnaires can be answered on a semi-annual basis. The treating physician provides confirmation of the diagnosis. Details on the study design are described elsewhere.12,13
The Ethics Committee of Zurich approved the study (Study No. PB-2016-00894) and an informed consent was signed by each participant prior to study entry.12
Our analysis was restricted to patients diagnosed after 1995 and considered all data collected until 19 March 2018. The study utilized information from the first follow-up questionnaire administered 6 months post baseline. The baseline assessments provide information on age, sex, lifestyle factors, education level, living situation, year of MS diagnosis, current MS form, first symptoms, and family history.14 The 6-month follow-up questionnaire includes a comprehensive section on the diagnostic process. It covers the healthcare professional first contacted in connection with MS symptoms, details about the setting and specialty of the healthcare provider involved in the diagnosis, as well as the specialty of the healthcare provider in charge of the diagnostic tests. Moreover, for steps 2 and 3 of our conceptual model, the duration of each phase was assessed (contact-to-evaluation phase and evaluation-to-diagnosis phase, respectively).
Description of the cascade
To simplify the different cascades, we created a decision tree on the basis of our conceptual model with the following bifurcations to depict the diagnostic process steps: (1) whether a setting change occurred between the first contact with a physician’s office to the first visit (e.g. an immediate referral to a neurology clinic by a GP office), (2) whether a neurologist was present at the first visit, and (3) whether the physician at the first visit led diagnostic tests, referred to another physician, or took no action. Setting changes were defined as any changes in the care setting (private practice, hospital) and/or the specialty of the attending physician between the first contact and the first visit.
Identification of the factors associated with prolonged diagnostic times
In order to assess the influence of different factors on the diagnosis duration, we performed logistic regression models with contact-to-evaluation and evaluation-to-diagnosis durations as outcome variables.
To identify prolonged diagnostic times, the contact-to-evaluation duration was categorized into “within 1 month” and “more than 1 month”, whereas the evaluation-to-diagnosis duration was categorized into “within 6 months” and “more than 6 months”.
The main factors (variables of interest) in the regression models were the decision tree branches with some modifications due to low patient numbers as follows. For the contact-to-evaluation duration, the first two bifurcations (setting change, neurologist at first visit) were considered, and for the evaluation-to-diagnosis duration the last two bifurcations (neurologist at first visit, actions on clarification tests) were analyzed. Other fixed regression factors were sex, education level (mandatory education or apprenticeship/school leaving certificate/higher professional education/university degree), living in a mountainous area (No/Yes), MS type (RRMS start/PPMS), age at onset, time period of diagnosis (1996–2000/2001–2010/2011–2018, reflecting the release years of the diagnostic criteria), and first visit setting (private practice/hospital). Potential additional factors were living situation, rural/urban area, language region of residence, Swiss nationality, MS diagnosed in older relatives, and 20 different first symptoms. If a first symptom was rare (in less than 5% of the sample), it was discarded (Supplemental Table S2).
For each logistic regression model, the potential factors were added to the fixed factors in a stepwise approach based on Akaike information criterion (AIC).3 Missing information (ranging from 0.1% to 5% per variable) were imputed by means of the multivariate imputation by chained equations (MICE) algorithm.14 The entire analysis was performed using R, version 3.4.0.15
Results
Study population
A total of 522 participants were included (Supplemental Figure S1). Patients were mostly female with RRMS (74%), with a median age of 47 years and an Expanded Disability Status Scale (EDSS) score below 4 (81%). A detailed description of the study population is provided in Table 1.
Table 1.
Demographics, clinical information, and diagnostic process features of the included sample.
| Variables | Participants included in the regression analysesa |
|---|---|
| N | 522 |
| Age at baseline, median (IQR) | 47 (38; 54) |
| Sex—female | 384 (73.6%) |
| Disease duration at baseline (years)b, median (IQR) | 7 (3; 13) |
| Disease duration not available | 11 (2.1%) |
| MS form at baseline | |
| CIS | 9 (1.7%) |
| RRMS | 384 (73.6%) |
| PPMS | 49 (9.4%) |
| SPMS | 72 (13.8%) |
| Not available | 8 (1.5%) |
| EDSS proxy at baseline | |
| 0–3.5 | 420 (80.5%) |
| 4–6.5 | 68 (13%) |
| 7–10 | 32 (6.1%) |
| Not available | 2 (0.4%) |
| Contacted first doctor | |
| General practitioner | 352 (67.4%) |
| Ophthalmologist | 68 (13%) |
| Neurologist | 44 (8.4%) |
| Emergency room | 34 (6.5%) |
| Other specialists | 23 (4.4%) |
| Not available | 1 (0.2%) |
| Time from contact with a physician to first symptom evaluation | |
| Same day/next day | 96 (18.4%) |
| Within 1 week | 115 (22%) |
| 1–2 weeks | 75 (14.4%) |
| 2–4 weeks | 68 (13%) |
| 1–3 months | 60 (11.5%) |
| More than 3 months | 94 (18%) |
| Other | 14 (2.7%) |
| Specialization of doctor at the first visit | |
| General practitioner/internist | 308 (59%) |
| Neurologist | 124 (23.8%) |
| Other specialist (incl. ophthalmologist) | 89 (17%) |
| Not available | 1 (0.2%) |
| Place of the first visit | |
| Private practice | 349 (66.9%) |
| Hospital | 111 (21.3%) |
| Emergency room | 59 (11.3%) |
| Not available | 3 (0.6%) |
| Action on evaluation tests by the first physician | |
| Refer to other MD | 335 (64.2%) |
| Lead the tests | 146 (28%) |
| No action taken | 35 (6.7%) |
| Not available | 6 (1.1%) |
| Next steps and visits sufficiently explained | |
| Yes | 354 (67.8%) |
| Partially | 125 (23.9%) |
| No | 41 (7.9%) |
| Not available | 2 (0.4%) |
| Setting of further evaluation | |
| Outpatient | 332 (63.6%) |
| Inpatient | 136 (26.1%) |
| In- and outpatient | 47 (9%) |
| Not available | 7 (1.3%) |
| Setting of diagnosis (from confirmation of diagnosis document) | |
| Neurologist in a hospital | 267 (51.1%) |
| Neurologist in a private practice | 191 (36.6%) |
| Others (general practitioner, …) | 2 (0.6%) |
| Not available | 62 (11.9%) |
| Number of doctors visited prior to MS diagnosis | |
| One | 34 (6.5%) |
| Two | 244 (46.7%) |
| Three | 128 (24.5%) |
| More than three | 114 (21.8%) |
| Not available | 2 (0.4%) |
| Time from first symptom evaluation to MS diagnosis | |
| Less than 1 week | 87 (16.7%) |
| 1–2 weeks | 98 (18.8%) |
| 2–4 weeks | 78 (14.9%) |
| 1–3 months | 101 (19.3%) |
| 3–6 months | 48 (9.2%) |
| 6–12 months | 34 (6.5%) |
| More than 1 year | 72 (13.8%) |
| Other | 4 (0.8%) |
IQR: interquartile range; CIS: clinically isolated syndrome; RRMS: relapsing remitting multiple sclerosis; PPMS: primary progressive multiple sclerosis; SPMS: secondary progressive multiple sclerosis; EDSS: Kurtzke Expanded Disability Status Scale.
Participants at the 6-month follow-up, diagnosed since 1996 and with both dependent variables available.
The MS duration refers to the time difference between the MS diagnosis date and the date of baseline survey. Where available, the diagnosis date was obtained from the diagnosis confirmation sheet provided by the treating physician (available for 89%). For the remainder, diagnosis dates were self-reported in the baseline questionnaires.
Description of the diagnostic cascades
Table 1 presents the details of the diagnostic process. During the contact-to-evaluation phase, the majority of patients (67%) first contacted a GP because of their symptoms. A minority sought care from ophthalmologists (13%), neurologists (8%), or general emergency services (7%). For 17% of participants, the evaluation-to-diagnosis duration (step 3 in Figure 1) was shorter than a week, for another 53% it occurred within 3 months, for 16% between 3 and 12 months, and for the remaining 14% more than a year.
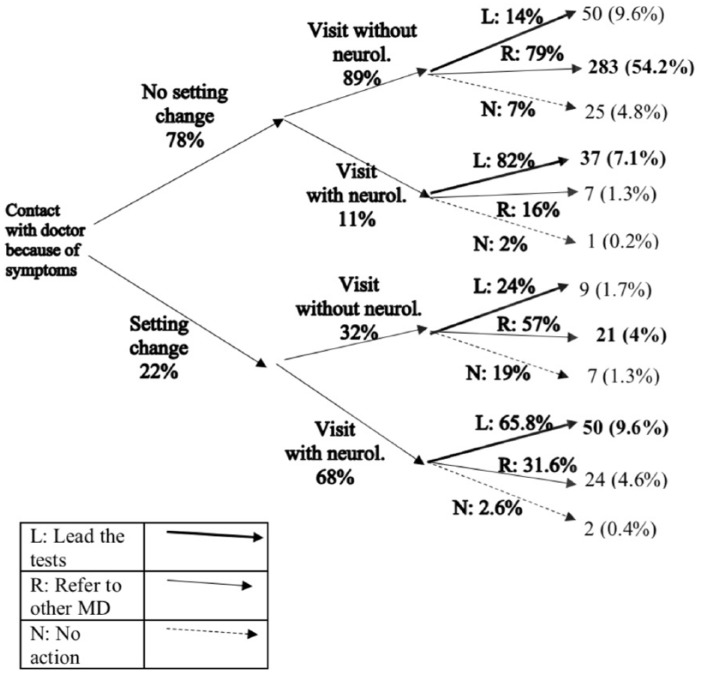
Figure 2 summarizes the first steps of the diagnostic process with a decision tree. Four out of five participants were seen by the physician they contacted (i.e. no change of setting, 78%). Of these patients, the vast majority (89%) did not see a neurologist at their first visit. By contrast, of those patients who did change the setting (22%), more than two out of three (68%) were seen by a neurologist. It is further observed from Figure 2 that, regardless of any prior setting change, neurologists were more likely to lead, perform, or organize diagnostic tests, while non-neurologists tended to refer patients to other physicians. Similar patterns can also be observed when the sample is restricted to the participants diagnosed after 2010 (Supplemental Figure S2).
Figure 2.
Decision tree, from the first contact until the decisions on the clarification tests are taken.
L: lead the tests; R: refer to other medical doctor; N: no action.
Percentages displayed next to branches illustrate what population fraction within a node entered a specific branch and sum up to 100% for each bifurcation. Numbers indicated at each tree endpoint provide the frequency of participants who have had that combination of events, as well as their share (%) of the entire sample. Numbers in bold, on the right-hand side of the figure, are the most frequent action for each node.
Factors associated with prolonged time from the first contact to symptom evaluation
Table 2 (columns 3 and 4) shows the distribution of all the considered, potential delaying factors for the contact-to-evaluation phase stratified by shorter and more prolonged duration. Persons with PPMS were more likely to wait longer for the first visit (5% in the early group, 21% in the late group), as well as those with gait problems at onset (26% early, 42% late), and those residing in a mountainous area (12% early, 20% late). The contact-to-evaluation time was more often shorter after 2000 (88% early, 77% late), or with dysarthria at onset (7% early, 2% late).
Table 2.
Stratification of all factors considered for modeling the contact-to-evaluation time (columns 3 and 4) and the evaluation-to-diagnosis time (columns 5 and 6).
| Levels | Contact-to-evaluation time: within 1 month | Contact-to-evaluation time: more than 1 month | Evaluation-to-diagnosis time: within 6 months | Evaluation-to-diagnosis time: more than 6 months | |
|---|---|---|---|---|---|
| N | 354 | 168 | 412 | 110 | |
| Diagnosis time period | 1996–2000 | 42 (11.9%) | 38 (22.6%) | 58 (14.1%) | 22 (20%) |
| 2001–2010 | 150 (42.4%) | 69 (41.1%) | 171 (41.5%) | 48 (43.6%) | |
| After 2010 | 162 (45.8%) | 61 (36.3%) | 183 (44.4%) | 40 (36.4%) | |
| Age at onset | 6–20 | 22 (6.2%) | 8 (4.8%) | 22 (5.3%) | 8 (7.3%) |
| 21–30 | 120 (33.9%) | 41 (24.4%) | 131 (31.8%) | 30 (27.3%) | |
| 31–40 | 107 (30.2%) | 50 (29.8%) | 122 (29.6%) | 35 (31.8%) | |
| 41–50 | 79 (22.3%) | 43 (25.6%) | 99 (24%) | 23 (20.9%) | |
| 51–70 | 10 (2.8%) | 16 (9.5%) | 20 (4.9%) | 6 (5.5%) | |
| NA | 16 (4.5%) | 10 (6%) | 18 (4.4%) | 8 (7.3%) | |
| PPMS | No | 336 (94.9%) | 133 (79.2%) | 379 (92%) | 90 (81.8%) |
| Yes | 17 (4.8%) | 35 (20.8%) | 32 (7.8%) | 20 (18.2%) | |
| NA | 1 (0.3%) | – | 1 (0.2%) | – | |
| Sex | Female | 267 (75.4%) | 117 (69.6%) | 304 (73.8%) | 80 (72.7%) |
| Male | 87 (24.6%) | 51 (30.4%) | 108 (26.2%) | 30 (27.3%) | |
| Decision tree—first two bifurcations | No setting change, first visit w/o neurologist | 246 (69.5%) | 115 (68.5%) | – | – |
| No setting change, first visit with neurologist | 32 (9%) | 14 (8.3%) | – | – | |
| Setting change, first visit w/o neurologist | 25 (7.1%) | 12 (7.1%) | – | – | |
| Setting change, first visit with neurologist | 51 (14.4%) | 27 (16.1%) | – | – | |
| Decision tree—last two bifurcations | First visit w/o neurologist, refer to other MD | – | – | 235 (57%) | 69 (62.7%) |
| First visit w/o neurologist, lead the tests | – | – | 49 (11.9%) | 10 (9.1%) | |
| First visit w/o neurologist, no action | – | – | 18 (4.4%) | 14 (12.7%) | |
| First visit with neurologist, lead the tests | – | – | 76 (18.4%) | 11 (10%) | |
| First visit with neurologist, no action or refer to other MD | – | – | 29 (7%) | 5 (4.5%) | |
| NA | – | – | 5 (1.2%) | 1 (0.9%) | |
| Living in mountainous area | No | 310 (87.6%) | 132 (78.6%) | 354 (85.9%) | 88 (80%) |
| Yes | 41 (11.6%) | 34 (20.2%) | 54 (13.1%) | 21 (19.1%) | |
| NA | 3 (0.8%) | 2 (1.2%) | 4 (1%) | 1 (0.9%) | |
| Place of first visit | Private practice | 226 (63.8%) | 123 (73.2%) | 267 (64.8%) | 82 (74.5%) |
| Hospital | 126 (35.6%) | 44 (26.2%) | 144 (35%) | 26 (23.6%) | |
| NA | 2 (0.6%) | 1 (0.6%) | 1 (0.2%) | 2 (1.8%) | |
| First symptoms (more than one possible) | Gait problems | 93 (26.3%) | 71 (42.3%) | 125 (30.3%) | 39 (35.5%) |
| Visual problems | 135 (38.1%) | 49 (29.2%) | 145 (35.2%) | 39 (35.5%) | |
| Bladder problems | 37 (10.5%) | 21 (12.5%) | 49 (11.9%) | 9 (8.2%) | |
| Balance problems | 90 (25.4%) | 52 (31%) | 116 (28.2%) | 26 (23.6%) | |
| Bowel problems | 24 (6.8%) | 14 (8.3%) | 29 (7%) | 9 (8.2%) | |
| Dizziness | 72 (20.3%) | 36 (21.4%) | 86 (20.9%) | 22 (20%) | |
| Dysarthria | 25 (7.1%) | 4 (2.4%) | 24 (5.8%) | 5 (4.5%) | |
| Paresthesia | 216 (61%) | 100 (59.5%) | 251 (60.9%) | 65 (59.1%) | |
| Weakness | 94 (26.6%) | 58 (34.5%) | 117 (28.4%) | 35 (31.8%) | |
| Paralysis | 87 (24.6%) | 46 (27.4%) | 105 (25.5%) | 28 (25.5%) | |
| Fatigue | 113 (31.9%) | 61 (36.3%) | 137 (33.3%) | 37 (33.6%) | |
| Pain | 41 (11.6%) | 25 (14.9%) | 52 (12.6%) | 14 (12.7%) | |
| Spasticity | 22 (6.2%) | 25 (14.9%) | 36 (8.7%) | 11 (10%) | |
| Sexual dysfunction | 17 (4.8%) | 18 (10.7%) | 27 (6.6%) | 8 (7.3%) | |
| Memory problems | 28 (7.9%) | 15 (8.9%) | 33 (8%) | 10 (9.1%) | |
| Depression | 32 (9%) | 21 (12.5%) | 37 (9%) | 16 (14.5%) | |
| Education level | Mandatory education/apprenticeship | 141 (39.8%) | 83 (49.4%) | 180 (43.7%) | 44 (40%) |
| School leaving certificate | 38 (10.7%) | 7 (4.2%) | 35 (8.5%) | 10 (9.1%) | |
| Higher professional education | 54 (15.3%) | 30 (17.9%) | 59 (14.3%) | 25 (22.7%) | |
| University degree | 105 (29.7%) | 42 (25%) | 121 (29.4%) | 26 (23.6%) | |
| NA | 16 (4.5%) | 6 (3.6%) | 17 (4.1%) | 5 (4.5%) | |
| Living situation | With partner only | 135 (38.1%) | 65 (38.7%) | 151 (36.7%) | 49 (44.5%) |
| With partner and children | 134 (37.9%) | 55 (32.7%) | 157 (38.1%) | 32 (29.1%) | |
| Alone | 55 (15.5%) | 39 (23.2%) | 73 (17.7%) | 21 (19.1%) | |
| Other | 28 (7.9%) | 9 (5.4%) | 29 (7%) | 8 (7.3%) | |
| NA | 2 (0.6%) | – | 2 (0.5%) | ||
| Language region of Switzerland | German | 302 (85.3%) | 135 (80.4%) | 340 (82.5%) | 97 (88.2%) |
| French | 38 (10.7%) | 21 (12.5%) | 51 (12.4%) | 8 (7.3%) | |
| Italian | 11 (3.1%) | 10 (6%) | 17 (4.1%) | 4 (3.6%) | |
| NA | 3 (0.8%) | 2 (1.2%) | 4 (1%) | 1 (0.9%) | |
| MS diagnosed in older relatives | Yes | 39 (11%) | 10 (6%) | 40 (9.7%) | 9 (8.2%) |
| No | 292 (82.5%) | 153 (91.1%) | 347 (84.2%) | 98 (89.1%) | |
| NA | 23 (6.5%) | 5 (3%) | 25 (6.1%) | 3 (2.7%) | |
| Swiss citizenship | Yes | 330 (93.2%) | 154 (91.7%) | 382 (92.7%) | 102 (92.7%) |
| No | 24 (6.8%) | 14 (8.3%) | 30 (7.3%) | 8 (7.3%) | |
| Typology of residence | Urban | 196 (55.4%) | 90 (53.6%) | 222 (53.9%) | 64 (58.2%) |
| Urban–rural | 99 (28%) | 41 (24.4%) | 111 (26.9%) | 29 (26.4%) | |
| Rural | 56 (15.8%) | 35 (20.8%) | 75 (18.2%) | 16 (14.5%) | |
| NA | 3 (0.8%) | 2 (1.2%) | 4 (1%) | 1 (0.9%) |
PPMS: primary progressive multiple sclerosis; NA: not available.
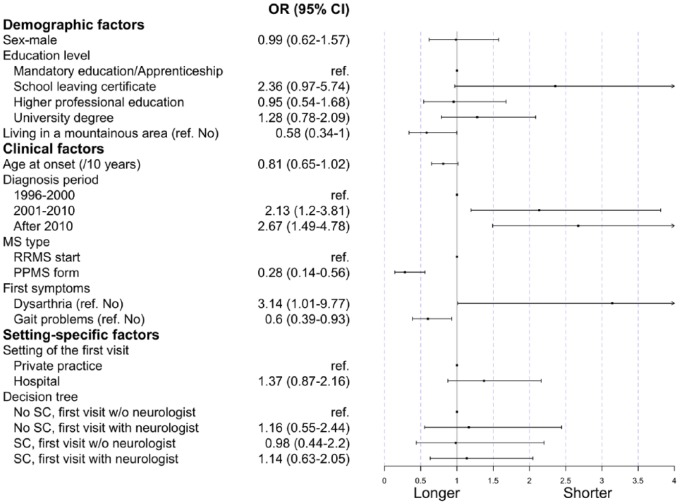
Those observations were confirmed by multivariable logistic regression analyzing the contact-to-evaluation time (Figure 3). The factors associated with a shorter contact-to-evaluation time were diagnosis after 2000 (odds ratio (OR): 2.1, 95% confidence interval (CI): [1.2; 3.8] for 2001–2010, 2.7 and [1.5; 4.8] after 2010), having a school leaving certificate (2.4, [0.97; 5.74]), and dysarthria at onset (3.14, [1.01; 9.77]).
Figure 3.
Regression coefficients of the multivariable model on the contact-to-evaluation time.
ref.: reference level; PPMS: primary progressive multiple sclerosis; RRMS: relapsing remitting multiple sclerosis; Priv. Practice: private practice; SC: setting change.
PPMS (0.28, [0.14; 0.56]), gait problems at onset (0.6, [0.39; 0.93]), and living in a mountainous area (0.58, [0.34; 1.00]) were instead associated with a longer time. Of note, the tree-derived levels describing the sequence of contacts were not associated with a prolonged time from first contact to first visit. When restricting the analysis to participants without PPMS, gait problems remained significantly associated with a longer contact-to-evaluation time (Supplemental Table S3 and Supplemental Figure S3).
Factors associated with prolonged time from symptom evaluation to MS diagnosis
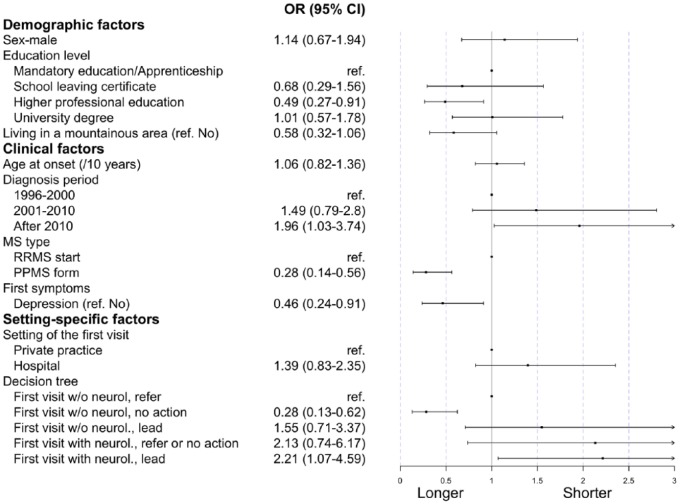
The last two columns of Table 2 describe all potential delaying factors for the evaluation-to-diagnosis phase. Shorter times were observed more often when the diagnosis was after 2010 (44% early, 36% late), the neurologist led the tests (18% early, 10% late), or the first visit occurred at a hospital (35% early, 24% late). Prolonged durations were observed with depression as a concomitant first symptom (9% early, 15% late), lack of action by a non-neurologist (4% early, 13% late), and PPMS (8% early, 18% late). These patterns also emerged in the multivariable logistic regression (Figure 4).
Figure 4.
Regression coefficients of the multivariable model on the evaluation-to-diagnosis time.
ref.: reference level; PPMS: primary progressive multiple sclerosis; RRMS: relapsing remitting multiple sclerosis; Priv. Practice: private practice; neurol.: neurologist.
Based on the different cascades illustrated in Figure 2, we further hypothesized that some cascades were more likely to lead to swifter diagnoses than others, for example, when neurologists are almost immediately involved in diagnosis. Indeed, the multivariable model indicated that the evaluation-to-diagnosis duration was statistically significantly faster when a neurologist led the clarification tests, compared to patients first seeing a GP and then being referred (OR 2.21 [1.07; 4.59]). Moreover, a diagnosis year after 2010, compared with one before 2000, was also associated with faster evaluation-to-diagnosis times (1.96, [1.03; 3.74]). By contrast, having PPMS (0.28, [0.14; 0.56]), depression as a concomitant first symptom (0.46, [0.24; 0.91]), higher professional education (0.49, [0.27; 0.91]), or seeing a (non-neurologist) physician who did not undertake further diagnostic actions (0.28, [0.13; 0.62]) were all associated with a prolonged evaluation-to-diagnosis phase.
Discussion
Using comprehensive questionnaire data from 522 Swiss MS Registry participants, we described the MS diagnosis process for persons receiving care in Switzerland. Our study analyzed in detail the steps after the first contact and identified the delaying factors.
We observed a broad variety of possibilities on how potential MS patients entered the healthcare system (Supplemental Table S1), but overall the cascade functioned as expected (Figure 2).
GPs were contacted primarily, and they usually referred patients to a neurologist who led further diagnostic tests and finally made the diagnosis. Importantly, this procedure did not prolong the overall diagnostic process compared to patients immediately contacting a neurologist, unless no action was taken. By contrast, the type of MS or first symptoms were more crucial in prolonging the process. Specifically, gait problems were associated with a longer time from the first contact to the first visit and dysarthria with a shorter one. The concomitance of depression was associated with a longer time from the first visit to the diagnosis and having PPMS was the most influential factor for a prolonged diagnosis duration and negatively influenced both phases.
To our knowledge, few studies have explored the diagnostic pathways and the role of patient-, disease-, and setting-specific aspects in MS diagnosis duration.7–10 Fernández et al.7 identified delays in several steps leading up to an MS diagnosis (onset to first visit, first visit to referral, time to perform the tests) and found the phase from the first symptoms to the first visit to be particularly critical. Kelly et al. found that approximately 78% of patients saw a neurologist within 6 weeks from onset, in accordance with UK guidelines. They also provided a list of common reasons for delay.8
Detailed examinations of factors delaying the diagnostic process were, however, largely missing. For example, Marrie et al.1,16 examined the effect of single factors, namely, onset year and comorbidities. Kingwell et al. pursued a systematic approach to identify the factors associated with the diagnostic time, but did not include sociodemographic factors, access barriers, and features of the diagnostic cascade in their assessment. Recently, Kaufmann et al.3 investigated this question using SMSR data, but without distinguishing between the different diagnostic process phases, instead modeling the whole diagnostic time at once.
This study extends these prior efforts by analyzing the different steps of the cascade in greater detail and testing a broader array of factors potentially delaying the diagnosis. Our findings may add to the literature by emphasizing the role of symptom presentation for a swift MS diagnosis. In particular, the concomitant occurrence of depression at onset (in 10% of the study population) as a delaying factor confirms the results of Marrie et al.16 There are various explanations: depression appears highly heterogeneous and is not considered a specific MS symptom, the patients themselves might not seek care due to an inability to take action, and the physician might interpret organic symptoms as psychogenic due to depression.
The delaying effect of gait problems might appear to contradict the findings of Kaufmann et al.,3 where they were associated with a quicker diagnostic process. However, in our study, gait problems were associated with a longer contact-to-evaluation phase, but not a longer evaluation-to-diagnosis phase. Gait problems often occur slowly, especially in patients with PPMS, and are therefore not urgent symptoms triggering immediate examinations, despite being distinctive first MS symptoms (Supplemental Table S3).
Furthermore, the fact that the diagnostic cascade was shorter every time the main diagnostic criteria were released (2001, 2010) extends Marrie et al.’s1 findings on a more recent timeframe.
Our finding of longer diagnostic times in patients with PPMS confirms those reported in other studies.3,10 The delaying effect of having PPMS on the time from the first evaluation to diagnosis possibly reflects the requirement of McDonald diagnostic criteria, which require 1 year of confirmed progression for this MS type. However, because delays in PPMS were also statistically significantly longer in the first phase of the diagnostic cascade (i.e. before the first evaluation), this suggests that at least a part of the delay occurs independently of the diagnostic criteria applied. For example, it is possible that the nature of symptoms in PPMS is less disruptive and urgent when compared to those that predominate in RRMS, thus already leading to delayed care seeking after the first symptom occurrence.
From a healthcare utilization perspective, specific sociodemographic factors could represent care access barriers hindering the diagnostic process.11 However, our findings revealed that most factors potentially constituting a barrier, such as lower education, living in an urban area, or having a migration background, do not lead to a prolonged diagnosis. Only living in mountainous areas was associated with longer diagnosis durations, probably due to a shortage of neurologists in those areas. But overall, our findings suggest that there is a good healthcare access, due to the Swiss mandatory healthcare insurance, a high hospital capacity,17 and diffuse hospital vicinity,18 resulting in a reasonably fast diagnostic process.19
Strengths and limitations
To better understand during which diagnostic cascade phase delays can occur, we differentiated the diagnostic process into two phases. Referring to more objective events (e.g. first contact, first visit) rather than personal interpretations (e.g. onset date) made our outcomes less prone to recall bias and subjectivity.
Moreover, the available information of the SMSR, such as education level, circumstances of life, and family history, is more comprehensive than other similar studies,20,21 and adjusting for them contributes to the findings’ robustness. In addition, having precise information on a large variety of different first symptoms allowed us to indicate which symptoms should be better monitored to prevent delays.
While some of our findings are setting specific (e.g. the distribution of primary care contacts), others are likely to generalize to other settings. For example, the diffuse nature of some of the MS symptoms associated with delays is likely also of concern in other countries.
This study presents, however, some limitations. First, self-reported data can be affected by biases, such as recall bias and underreporting. Moreover, we did not include information on comorbidities other than depression at the time of the onset that were previously found to contribute to delays.16
In addition, diagnostic delays can also occur due to financial access barriers, even in countries with social health insurance systems like Switzerland (e.g. due to high out-of-pocket expenses and upfront payments22,23). Unfortunately, we were unable to assess the actual financial situation of the participants, so we used the education level as a proxy instead.
Prospect for future research
To accelerate the diagnostic process further, future research should examine the reasons why patients may not seek immediate care after the first onset of MS symptoms. Moreover, the potential causes for initial inactivity by first healthcare providers deserve further investigation and may lie in unusual symptom combinations, lack of awareness for MS, or problems in patient–doctor communication.
Conclusion
Our study is among the first to query persons with MS on how they responded after noticing their first symptoms. This effort has provided a comprehensive picture of the patients’ first steps in the healthcare system toward an MS diagnosis after symptom onset, which was unknown until now in Switzerland. The observation that the majority of Swiss MS patients were diagnosed in a timely manner regardless of setting and specialty of first physician contact is reassuring, but still too many potentially preventable delays occur in these first steps until diagnosis initiation. For a faster diagnostic process, awareness for MS as a differential diagnosis of gait disorders should be raised, and an attentive follow-up of possible MS cases with depression as a concomitant first symptom is needed.
Supplemental Material
Supplemental material, MSJ823955_supplemental_material for How do patients enter the healthcare system after the first onset of multiple sclerosis symptoms? The influence of setting and physician specialty on speed of diagnosis by Laura Barin, Christian P Kamm, Anke Salmen, Holger Dressel, Pasquale Calabrese, Caroline Pot, Sven Schippling, Claudio Gobbi, Stefanie Müller, Andrew Chan, Stephanie Rodgers, Marco Kaufmann, Vladeta Ajdacic-Gross, Nina Steinemann, Jürg Kesselring, Milo A Puhan and Viktor von Wyl in Multiple Sclerosis Journal
Acknowledgments
The authors wish to thank the Swiss Multiple Sclerosis Society for funding the Swiss MS Registry. Moreover, we thank the study participants who not only contributed data but also were absolutely instrumental in all aspects of study design and conduct of the Swiss Multiple Sclerosis Registry. We further thank the members of the SMSR Data Center at the Epidemiology, Biostatistics and Prevention Institute of the University of Zurich. Members of the Swiss Multiple Sclerosis Registry are Bernd Anderseck, Pasquale Calabrese, Andrew Chan, Giulio Disanto, Britta Engelhardt, Claudio Gobbi, Roger Häussler, Christian P Kamm, Susanne Kägi, Jürg Kesselring (President), Jens Kuhle (Chair of Clinical and Laboratory Research Committee), Roland Kurmann, Christoph Lotter, Kurt Luyckx, Doron Merkler, Patricia Monin, Stephanie Müller, Krassen Nedeltchev, Caroline Pot, Milo A Puhan, Irene Rapold, Anke Salmen, Sven Schippling, Claude Vaney (Chair of Patient- and Population Research Committee), and Viktor von Wyl (Chair of IT and Data Committee). L.B. and C.P.K. have contributed equally.
Footnotes
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: C.G., H.D., J.K., L.B., M.K., M.A.P., N.S., S.R., V.A.-G., and V.v.W. have nothing to disclose. A.C. has received compensation for activities (speaker, boards) with Actelion, Almirall, Bayer, Biogen, Celgene, Genzyme, Merck, Novartis, Roche, and Teva, all for university research funds and research support from UCB and Genzyme. A.S. has received speaker honoraria and/or travel compensation for activities with Almirall Hermal GmbH, Biogen, Merck, Novartis, Roche, and Sanofi Genzyme, none related to this work. C.P. has received travel support and participated in the advisory board for Biogen Idec, Genzyme, Novartis, and Roche. C.P.K. has received honoraria for lectures as well as research support from Biogen, Novartis, Almirall, Bayer Schweiz AG, Teva, Merck, Sanofi Genzyme, Roche, Celgene, and the Swiss MS Society. P.C. has received honoraria for speaking at scientific meeting, serving at scientific advisory boards, and consulting activities from Abbvie, Actelion, Almirall, Bayer-Schering, Biogen Idec, EISAI, Genzyme, Lundbeck, Merck Serono, Novartis, Pfizer, Teva, and Sanofi-Aventis; his research was also supported by the Swiss Multiple Sclerosis Society, the Swiss National Research Foundation, and the SOFIA Foundation. S.M. received honoraria for travel, honoraria for lectures/consulting, and/or grants for studies from Almirall, Biogen, Celgene, Novartis, Teva, Merck Serono, Genzyme, Roche, and Bayer Schweiz AG. S.S. was supported by the Swiss National Science Foundation, the Clinical Research Priority Program of the University of Zurich, the Myelin Repair Foundation, and the Swiss Multiple Sclerosis Society and has received research grants from Novartis and Sanofi Genzyme and consultancy and speaker fees from Biogen, Merck Serono, Novartis, Roche, Sanofi Genzyme, and Teva.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the Swiss Multiple Sclerosis Society.
Supplemental Material: Supplemental material for this article is available online.
ORCID iDs: Laura Barin  https://orcid.org/0000-0001-8139-4686
https://orcid.org/0000-0001-8139-4686
Marco Kaufmann  https://orcid.org/0000-0002-4810-7734
https://orcid.org/0000-0002-4810-7734
Contributor Information
Laura Barin, Epidemiology, Biostatistics and Prevention Institute, University of Zurich, Zurich, Switzerland.
Christian P Kamm, Department of Neurology, University Hospital Bern and University of Bern, Bern, Switzerland/Neurology and Neurorehabilitation Centre, Lucerne Cantonal Hospital, Lucerne, Switzerland.
Anke Salmen, Department of Neurology, University Hospital Bern and University of Bern, Bern, Switzerland.
Holger Dressel, Epidemiology, Biostatistics and Prevention Institute, University of Zurich, Zurich, Switzerland/Division of Occupational and Environmental Medicine, University of Zurich and University Hospital Zurich, Zurich, Switzerland.
Pasquale Calabrese, Neuropsychology and Behavioral Neurology Unit, Division of Molecular and Cognitive Neuroscience, Department of Psychology, University of Basel, Basel, Switzerland.
Caroline Pot, Laboratories of Neuroimmunology, Division of Neurology and Neuroscience Research Center, Department of Clinical Neurosciences, Lausanne University Hospital, Lausanne, Switzerland.
Sven Schippling, Neuroimmunology and Multiple Sclerosis Research, Department of Neurology, University Hospital Zurich, Zurich, Switzerland/Center for Neuroscience Zurich, Federal Institute of Technology (ETH), Zurich, Switzerland.
Claudio Gobbi, Neurocenter of Southern Switzerland, Ospedale regionale di Lugano, Lugano, Switzerland.
Stefanie Müller, Department of Neurology, Cantonal Hospital St. Gallen, St. Gallen, Switzerland.
Andrew Chan, Department of Neurology, University Hospital Bern and University of Bern, Bern, Switzerland.
Stephanie Rodgers, Epidemiology, Biostatistics and Prevention Institute, University of Zurich, Zurich, Switzerland.
Marco Kaufmann, Epidemiology, Biostatistics and Prevention Institute, University of Zurich, Zurich, Switzerland.
Vladeta Ajdacic-Gross, Epidemiology, Biostatistics and Prevention Institute, University of Zurich, Zurich, Switzerland/Department of Psychiatry, Psychotherapy and Psychosomatics, Psychiatric Hospital, University of Zurich, Zurich, Switzerland.
Nina Steinemann, Epidemiology, Biostatistics and Prevention Institute, University of Zurich, Zurich, Switzerland.
Jürg Kesselring, Department of Neurology and Neurorehabilitation, Rehabilitation Centre Kliniken Valens, Valens, Switzerland.
Milo A Puhan, Epidemiology, Biostatistics and Prevention Institute, University of Zurich, Zurich, Switzerland.
Viktor von Wyl, Epidemiology, Biostatistics and Prevention Institute, University of Zurich, Zurich, Switzerland.
References
- 1. Marrie RA, Cutter G, Tyry T, et al. Changes in the ascertainment of multiple sclerosis. Neurology 2005; 65: 1066–1070. [DOI] [PubMed] [Google Scholar]
- 2. Thompson AJ, Banwell BL, Barkhof F, et al. Diagnosis of multiple sclerosis: 2017 revisions of the McDonald criteria. Lancet Neurol 2018; 17: 162–173. [DOI] [PubMed] [Google Scholar]
- 3. Kaufmann M, Kuhle J, Puhan MA, et al. Factors associated with time from first symptoms to diagnosis and treatment initiation of multiple sclerosis in Switzerland. Mult Scler J Exp Transl Clin 2018; 4: 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Comi G, Radaelli M, Soelberg Sørensen P. Evolving concepts in the treatment of relapsing multiple sclerosis. Lancet 2017; 389: 1347–1356. [DOI] [PubMed] [Google Scholar]
- 5. Chalmer TA, Baggesen LM, Nørgaard M, et al. Early versus later treatment start in multiple sclerosis: A register-based cohort study. Eur J Neurol 2018; 25: 1262-e110. [DOI] [PubMed] [Google Scholar]
- 6. Giovannoni G, Butzkueven H, Dhib-Jalbut S, et al. Brain health: Time matters in multiple sclerosis. Mult Scler Relat Dis 2016; 9: S5–S48. [DOI] [PubMed] [Google Scholar]
- 7. Fernández O, Fernandez V, Arbizu T, et al. Characteristics of multiple sclerosis at onset and delay of diagnosis and treatment in Spain (The Novo Study). J Neurol 2010; 257: 1500–1507. [DOI] [PubMed] [Google Scholar]
- 8. Kelly S, Chaila E, Kinsella K, et al. Multiple sclerosis, from referral to confirmed diagnosis: An audit of clinical practice. Mult Scler 2011; 17: 1017–1021. [DOI] [PubMed] [Google Scholar]
- 9. Adamec I, Barun B, Gabelić T, et al. Delay in the diagnosis of multiple sclerosis in Croatia. Clin Neurol Neurosur 2013; 115: S70–S72. [DOI] [PubMed] [Google Scholar]
- 10. Kingwell E, Leung AL, Roger E, et al. Factors associated with delay to medical recognition in two Canadian multiple sclerosis cohorts. J Neurol Sci 2010; 292: 57–62. [DOI] [PubMed] [Google Scholar]
- 11. Minden S, Frankel D, Hadden L, et al. Access to health care for people with multiple sclerosis. Mult Scler 2007; 13: 547–558. [DOI] [PubMed] [Google Scholar]
- 12. Puhan MA, Steinemann N, Kamm CP, et al. A digitally facilitated citizen-science driven approach accelerates participant recruitment and increases study population diversity. Swiss Med Wkly 2018; 148: w14623. [DOI] [PubMed] [Google Scholar]
- 13. Steinemann N, Kuhle J, Calabrese P, et al. The Swiss Multiple Sclerosis Registry (SMSR): Study protocol of a participatory, nationwide registry to promote epidemiological and patient-centered MS research. BMC Neurol 2018; 18: 111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Barin L, Salmen A, Disanto G, et al. The disease burden of multiple sclerosis from the individual and population perspective: Which symptoms matter most? Mult Scler Relat Dis 2018; 25: 112–121. [DOI] [PubMed] [Google Scholar]
- 15. R Core Team L. R: A language and environment for statistical computing. Vienna: R Foundation for Statistical Computing, 2017, http://www.r-project.org/ [Google Scholar]
- 16. Marrie RA, Horwitz R, Cutter G, et al. Comorbidity delays diagnosis and increases disability at diagnosis in MS. Neurology 2009; 72: 117–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Herzlinger R, Parsa-Parsi R. Consumer-driven health care lessons from Switzerland. JAMA 2004; 292: 1213–1220. [DOI] [PubMed] [Google Scholar]
- 18. Berlin C, Panczak R, Hasler R, et al. Do acute myocardial infarction and stroke mortality vary by distance to hospitals in Switzerland? Results from the Swiss National Cohort Study. BMJ Open 2016; 6: e013090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Barin L, Kaufmann M, Salmen A, et al. Patterns of care for multiple sclerosis in a setting of universal care access: A cross-sectional study from Switzerland. Mult Scler Relat Dis 2019; 28: 17–25. [DOI] [PubMed] [Google Scholar]
- 20. Calabrese P, Kobelt G, Berg J, et al. New insights into the burden and costs of multiple sclerosis in Europe: Results for Switzerland. Mult Scler 2017; 23: 192–203. [DOI] [PubMed] [Google Scholar]
- 21. Lorscheider J, Benkert P, Lienert C, et al. Comparative analysis of natalizumab versus fingolimod as second-line treatment in relapsing–remitting multiple sclerosis. Mult Scler 2018; 24: 777–785. [DOI] [PubMed] [Google Scholar]
- 22. Reinhardt UE. The Swiss health system: Regulated competition without managed care. JAMA 2004; 292: 1227–1231. [DOI] [PubMed] [Google Scholar]
- 23. Osborn R, Doty MM, Moulds D, et al. Older Americans were sicker and faced more financial barriers to health care than counterparts in other countries. Health Affair 2017; 36: 2123–2132. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, MSJ823955_supplemental_material for How do patients enter the healthcare system after the first onset of multiple sclerosis symptoms? The influence of setting and physician specialty on speed of diagnosis by Laura Barin, Christian P Kamm, Anke Salmen, Holger Dressel, Pasquale Calabrese, Caroline Pot, Sven Schippling, Claudio Gobbi, Stefanie Müller, Andrew Chan, Stephanie Rodgers, Marco Kaufmann, Vladeta Ajdacic-Gross, Nina Steinemann, Jürg Kesselring, Milo A Puhan and Viktor von Wyl in Multiple Sclerosis Journal