Abstract
Background
The purposes were to investigate the drinking patterns and hydration biomarkers among young adults with different levels of habitual total drinking fluids intake.
Methods
A cross-sectional study was conducted among 159 young adults aged 18–23 years in Baoding, China. Total drinking fluids and water from food were assessed by 7-day 24-h fluid intake questionnaire and duplicate portion method, respectively. The osmolality and electrolyte concentrations of the 24 h urine and fasting blood samples were tested. Differences in LD1 (low drinker), LD2, LD3 and HD (high drinker) groups, stratified according to the quartiles of total drinking fluids, were compared using one-way ANOVA, Kruskal-Wallis H test and chi-square test.
Results
A total of 156 participants (80 males and 76 females) completed the study. HD group had greater amounts of TWI (Total Water Intake), water from food, higher and lower contributions of total drinking fluids and water from food to TWI, respectively, than LD1, LD2 and LD3 groups (p < 0.05). Participants in HD group had higher amounts of water and water from dishes than participants in LD1, LD2 and LD3 groups (p < 0.05). No significant differences were found in the contributions of different fluids to total drinking fluids within the four groups (p > 0.05). The osmolality of urine was 59–143 mOsm/kg higher in LD1 than that in LD2, LD3 and HD group (p < 0.05). The percentage of participants in optimal hydration status increased from 12.8% in LD1 group to 56.4% in HD group (p < 0.05). HD and LD3 groups had 386~793 higher volumes of urine than that of LD1 and LD2 groups (p < 0.05). Differences were found in the concentrations of electrolytes among the four groups (p < 0.05). No significant differences were found in the plasma biomarkers (p > 0.05), with the exception of higher concentration of Mg in LD3 and HD groups than that in LD1 and LD2 groups (p < 0.05).
Conclusions
Participants with higher total drinking fluids had better drinking pattern and hydration status. Interventions should be undertaken to advise adults to have adequate total drinking fluids, in order to keep in optimal hydration status.
Trial registration
The registration number was ChiCTR-ROC-17010320, which was registered on the Chinese clinical trial registry.
Keywords: Hydration biomarkers, Drinking patterns, Total drinking fluids
Background
Water is of vital importance for the health of humans. Water accounts for almost 50–60% of the total body mass of adults [1]. Moreover, water plays important role in facilitating cellular metabolism, modulating normal osmotic pressure and regulating the temperature of the body [2]. In addition, water intake has also been associated with mood state in women [3]. Insufficient water intake may lead body into dehydration. Researches have shown that dehydration can impair the physical [4, 5] and cognitive performances [6, 7], which included vigilance attention [8], working memory [9, 10] and executive function [11]. Moreover, dehydration may also have deleterious effects om cardiovascular health [12]. Therefore, it is important for people to be in optimal hydration status.
To keep the body functioning properly, people need to consume adequate volumes of water. Many factors such as individuals’ (age, gender, etc.) and environmental conditions (temperature, humidity, etc.) influence the requirements of fluids. Consequently, the dietary reference values of adequate intake of TWI (total water intake, including total drinking fluids and water from food), such as the EFSA (European Food Safety Authority) [13], the United States [14] and China [15] were different. Yet only 40% of men and 60% of women in 13 countries drank more than the adequate intake of water from fluids set by EFSA [16]. Among children from 19 countries, 60 ± 24% of them failed to have adequate water with guidelines, suggesting that children were not consuming enough water to be adequately hydrated [17]. Moreover, in China, about 32% of adults, children and adolescents drank less fluids than the recommendation [18], which potentially may have adverse effects on their health. Unfortunately, the hydration biomarkers were not measured in the studies mentioned above. Studies reporting the hydration status among people in free-living conditions were scarce. Data of 2009 to 2012 National Health and Nutrition Examination Survey demonstrated that 29.5% of US adults aged 20–74 years were not in optimal hydration status [19]. While among elderly aged ≥60 years from NHANES 2011–2014, 29.6% and 33.3% of women and men had Sosm (osmolality of serum) levels indicative of impending dehydration, with 10.2% and 12.6% of women and men had Sosm levels indicative of dehydration [20]. In China, there was merely one study exploring hydration status among male young adults, which showed that only 35.3% of them were in optimal hydration status [21]. Research showed that the participants with low total drinking fluids excreted significantly less volume of urine than those with high total drinking fluids over each 24 h period [22]. In addition, the osmolality, specific gravity of urine among adults with high total drinking fluids were lower than those with low total drinking fluids [22]. Nevertheless, the differences in hydration biomarkers among participants with different levels of habitual total drinking fluids intake were not explored yet in China. More studies should include hydration biomarkers to investigate the hydration status of Chinese adults in the future.
Drinking patterns, defined here as the amounts and types of fluids consumed, have been associated with health [23, 24]. Water, which could reduce the total energy intake, may contribute to the regulation of body weight and help individuals reach the recommendation of TWI [25–28]. Moreover, the replacement of diet beverages with water may improve the insulin resistance of people [28]. A rising number of studies asserted that there were positive associations between SSBs (sugar-sweetened beverages) and the risk of weight gain or obesity or metabolic syndrome or obesity-related cancers and type 2 diabetes in adults, children and adolescents [29–34]. Moreover, the consumption of SSBs was also associated with short sleep in middle school students [35]. Therefore, the sources of total drinking fluids were worth exploring. However, the drinking patterns may differ among people from different countries, for instance, the Chinese tend to have tea, while the Americans prefer to drink more coffee [36]. The drinking patterns were not only different from countries, differences also existed among people from the same country. In France, the volumes of fluids such as water, were higher among adults with higher total drinking fluids intake than those with lower total drinking fluids intake [22]. However, in China, there was no study investigated the drinking patterns among participants with different levels of habitual total drinking fluids.
In reference to the volumes of water from food, the contributions of water from food to TWI were different among countries, such as in the United States [37], in European countries [38] and in Japan [39]. Among adults in China, nearly 44% of the TWI came from food [40], however, the sources of water from food among young adults have not been reported. In addition, in France, the amount of water from food were 0.55 L and 0.68 L among participants with lower total drinking fluids intake and higher total drinking fluids intake, respectively [22]. However, there was no study investigating the differences in the sources of water from food among young adults with different levels of total drinking fluids. Therefore, it remains to be determined if participants with a low habitual total drinking fluids have a different contribution of water from food and different sources of water from food comparing with those with a high habitual total drinking fluids intake in Chinese.
The objectives of this study were, firstly, to investigate the differences in drinking patterns among young adults; secondly, to explore the hydration biomarkers among young adults with different levels of habitual total drinking fluids intake in free-living conditions. This will lead to the provision of a science-based education of fluids intake for young adults.
Methods
Participants
The inclusion criteria were that participants including males and females were healthy and aged 18–23 years. The exclusion criteria were that participants with smoking, habitual alcohol consumptions (> 20 g/day) or habitual high caffeine consumptions (> 250 mg/day) or had chronic diseases or other diseases (gastrointestinal tract disease, diabetes, oral disease, kidney disease, cardiovascular disease or other chronic or metabolic diseases) were excluded from the study [41].
Sample size calculation
From a study performed among young male adults in China, the standard deviation of total drinking fluids was 468 mL [21]. Then, the SAS procedure (SAS Institute Inc., Cary, NC) was used to calculate the sample size. The δ set at 76 mL, α set at 0.05, and 10% drop-out rate was taken into account, 159 participants were needed in this study.
Study procedure
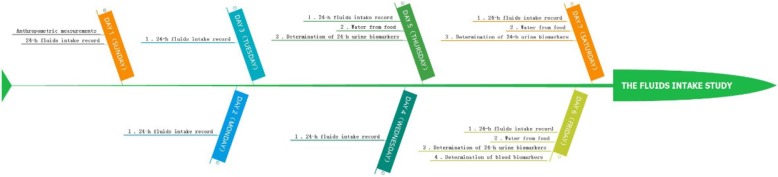
This was a cross-sectional study, which lasted for 7 consecutive days. On day 1, all participants were asked to take the anthropometric measurements including height, weight and waist circumference. After that, all participants were instructed to complete the self-designed 7-day 24-h fluid intake questionnaire to record the fluids intake from water and other beverages from day 1 to day 7. To ensure the competence and accurateness of the fluids intake, the questionnaires were checked by investigators every day. All the food the participants ate for three consecutive days (two weekdays and one weekend day, from day 5 to day 7) during the 7 consecutive days were weighed and recorded. From day 5 to day 7, 24-h urine samples including the first morning urine of three consecutive days (two weekdays and one weekend day) were collected by participants. On day 6, the fasting blood samples of all participants were collected. The temperature and humidity of indoor and outdoor was recorded each day for 7 days. The study procedure was shown in Fig. 1.
Fig. 1.
The study procedure
Measurement of Total water intake
Total water intake (mL) = Total drinking fluids (mL) + Water from food (mL).
A self-designed 7-day 24-h fluid intake questionnaire was used to assess the total drinking fluids, as showed before. Moreover, the type and amount of drinking fluid for each time was measured using a cup to the nearest of 5 mL. T According to the General standard for beverages of China (GB/T 1–789-2015) [42], all the drinking fluids were classified as below: water (plain water, tap water and bottle water), tea (fermented tea and semi-fermented tea), milk and milk products (liquid milk, yogurt and other milk products), SSBs (carbonated drinks, sports drinks, sweetened fruit juice, vegetable juice, protein drinks and other sugared drinks), alcohols(wine, beer, liquor, etc.) and other beverages [43].
In order to assess the amount of water from food, all foods the participants ate before and after were weighed for three consecutive days of the 7 days (including two weekdays and one weekend day, from day 5 to day 7). The duplicate portion method, with samples of food being collected and sent to the laboratory immediately, was used to measure the water from food. Samples of all foods were measured according to the national standard of GB 5009.3–2016 [44] and the water from fruits or other snacks was assessed according to the China Food Composition Table (2009) [45]. The water from food was classified as below: staple food (steamed bread, steamed rice, etc.), dishes (vegetables, meat, fish and eggs), porridge (millet porridge and other porridges), soup (tomato egg soup and other soups) and snacks (fruits, nuts, etc.) [43].
Temperature and humidity of the environment
The temperature and humidity of indoors and outdoors were recorded each day for 7 days (WSB-1-H2, Exasace, Zhengzhou, China).
Anthropometric measurements
Height, weight and waist circumference were measured twice with the participants wearing light clothes and barefoot [15] (HDM-300; Huaju, Zhejiang, China; Accu Measure, Greenwood Village, CO, USA). [BMI: weight (kg) / height squared (m)].
Urine biomarkers
The biomarkers of urine included the volumes, osmolality, pH, urine specific gravity (USG) and the concentrations of electrolyte.
24-h urine samples including the first morning urine were collected by participants using self-designed containers. Every urine sample was collected and tested immediately. Before being measured, all urine samples were stored at + 4°Cin refrigerator. Urine volume was measured to the nearest 0.1 g using a desktop electronic scale (YP20001, SPC, Shanghai, China). Urine osmolality was assessed with freezing point method by osmotic pressure molar concentration meter (SMC 30C; Tianhe, Tianjin, China). USG, pH, urea and creatinine were tested by automatic urinary sediment analyzer with uric dry-chemistry method (H-800; Dirui, Changchun, China). Urine electrolyte concentrations (including sodium, potassium, chloride, calcium, magnesium and phosphate) were measured by automatic biochemical analyzer with the ion-selective electrode potentiometer method (AU 5800; Beckman, Brea, CA, USA). The optimal hydration was defined when urine osmolality ≤500 mOsm/kg, middle hydration was defined as 500 mOsm/kg < urine osmolality ≤800 mOsm/kg, dehydration was defined as urine osmolality > 800 mOsm/kg [13, 46].
Plasma biomarkers
Fasting blood samples were collected to measure the osmolality and electrolyte concentrations.
Plasma osmolality was assessed with freezing point method by osmotic pressure molar concentration meter (SMC 30C; Tianhe, Tianjin, China). The electrolyte concentrations (including sodium, potassium, chloride, calcium, magnesium and phosphate) were tested by automatic biochemical analyzer with the ion-selective electrode potentiometer method (AU 5800; Beckman, Brea, CA, USA).
Statistics
The SAS 9.2 software (SAS Institute Inc., Cary, NC, USA) was used for statistical analysis. The results were reported as mean ± standard deviation (SD) if the data was normal distributed. While, median and quartile ranges (M and Q) was used to describe the data, which was not in normal distribution. Participants were divided into four groups, including LD1 (low drinker 1), LD2 (low drinker 2), LD3 (low drinker 3) and HD (high drinker) groups, according to the quartiles of total drinking fluids of participants (Q1: 324–858 mL, Q2: 859–1135 mL, Q3: 1136–1478 mL, Q4: 1479–3033 mL). One-way ANOVA, Kruskal-Wallis H test and chi-square test were used to compared the differences among the four groups. Differences between every two groups were compared using SNK (Student-Newman-Keuls) (p < 0.05). Significance levels were set at 0.05 (p < 0.05).
Results
Eventually, a total of 159 participants were recruited, and 156 (80 males and 76 females) completed the study, with 98% complete rate. Table 1 showed the characteristics of the participants. Significant differences were found in the mean height and weight when comparing LD2, LD3, HD groups with group LD1, respectively (p < 0.05), with no differences between LD2, LD3 and HD groups. There were no significant differences in age and BMI among the four groups (p > 0.05).
Table 1.
The characteristics of participants
| LD1 (n = 39) | LD2 (n = 39) | LD3 (n = 39) | HD (n = 39) | Total (n = 156) | P | |
|---|---|---|---|---|---|---|
| Age (y) | 19.6 ± 1.1 | 19.9 ± 1.0 | 19.8 ± 1.1 | 19.9 ± 1.0 | 19.8 ± 1.1 | 0.626 |
| Height (cm) | 162.6 ± 9.0a | 168.0 ± 6.5 | 166.7 ± 8.6b | 167.6 ± 8.1c | 166.2 ± 8.3 | 0.014 |
| Weight (kg) | 56.3 ± 6.2a | 63.5 ± 11.3 | 61.8 ± 13.6b | 63.8 ± 12.5c | 61.3 ± 11.5 | 0.013 |
| BMI (kg/m2) | 21.3 ± 2.0 | 22.4 ± 3.3 | 22.1 ± 4.1 | 22.6 ± 3.2 | 22.1 ± 3.3 | 0.333 |
Values are shown as the mean ± standard deviation (SD). BMI: Body Mass Index
aThere was statistically significant difference between LD1 and LD2 groups, p < 0.05; bThere was statistically significant difference between LD1 and LD3 groups, p < 0.05; cThere was statistically significant difference between LD1 and HD groups, p < 0.05
Temperature and humidity
The average indoor and outdoor temperature for the 7 days was 21.8 °C and 20.7 °C, respectively. The average indoor and outdoor humidity was 39.9% and 35.9%, respectively, see Table 2.
Table 2.
The temperature and humidity of study days
| Indoors | Outdoors | |||
|---|---|---|---|---|
| Temperature (°C) | Humidity (%) | Temperature (°C) | Humidity (%) | |
| Sunday | 19.9 | 43 | 18.1 | 37 |
| Monday | 23.0 | 48 | 22.4 | 41 |
| Tuesday | 23.3 | 31 | 24.0 | 29 |
| Wednesday | 21.5 | 48 | 17.9 | 42 |
| Thursday | 21.5 | 40 | 21.0 | 36 |
| Friday | 22.2 | 35 | 19.2 | 35 |
| Saturday | 21.2 | 34 | 22.6 | 31 |
Measurement of total water intake
TWI and water from food all increased with higher total drinking fluids among the four groups (p < 0.05). Participants in HD group had 515-1085 mL, 125–282 mL and 664–1383 mL more than those in LD1-LD3 groups in total drinking fluids, water from food and TWI (p < 0.05), respectively. Contributions of total drinking fluids to TWI were different among the four groups comparing with each other (p < 0.05), ranging from 38.7% in LD1 group to 59.1% in the HD group. The water from food accounted for 61.3–40.9% of TWI from LD1 group to HD group, which were also significantly different among the four groups (p < 0.05). Participants in HD group had a 20.4% higher and a 20.4% lower contribution of total drinking fluids and water from food to TWI than those in LD1 group, respectively (p < 0.05).
Drinking patterns
The main contributor of total drinking fluids was water in the four groups, which accounted for 77.3–85.6% and there were no significant differences among LD1, LD2, LD3 and HD groups (p > 0.05). Significant differences were found in the amounts of water in the four groups (p < 0.05), with the amounts of water increased with higher total drinking fluids. Nevertheless, there were no statistically significantly differences in the amounts of tea, milk and milk products, SSBs, alcoholic and other beverages among the four groups (p > 0.05). Moreover, there were no significant differences in the contributions of tea, milk and milk products, SSBs, alcoholic and other beverages to total drinking fluids among the four groups (p > 0.05). SSBs were the second contributor to total drinking fluids followed water in the LD1, LD2, LD3 groups, but in the HD group, milk and milk products followed water.
Water from food
Participants in HD group had 125–282 mL more water from food than those in other groups. The main contributor to water from food was dishes followed by staple food in all four groups. Participants in HD group had higher volumes of water from dishes and snacks than participants in LD1, LD2 and LD3 groups (p < 0.05). There were no statistically significant differences in the amounts of water from staple food, soup and porridge in the four groups (p > 0.05). Regarding the contributions of water from different foods, the contributions of water from snacks were different among the four groups (p < 0.05), as shown in Table 3.
Table 3.
The TWI, total drinking fluids and water from food among participants consuming different levels of total drinking fluids
| LD1 (n = 39) | LD2 (n = 39) | LD3 (n = 39) | HD (n = 39) | Total (n = 156) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| M | Q | % | M | Q | % | M | Q | % | M | Q | % | M | Q | % | |
| Total drinking fluids (mL) | 681a | 226 | 38.7%a | 967d | 114 | 45.5%d | 1251b,f | 156 | 52.0%b,f | 1766c,e | 521 | 59.1%c,e | 1135 | 620 | 50.6% |
| Water (mL) | 564a | 356 | 77.3% | 745d | 194 | 76.6% | 1082b,f | 351 | 79.5% | 1613c,e | 494 | 85.6% | 866 | 642 | 81.0% |
| Tea (mL) | 0 | 0 | 1.2% | 0 | 0 | 0.8% | 0 | 0 | 0.5% | 0 | 0 | 1.2% | 0 | 0 | 1.0% |
| Milk and milk products (mL) | 39 | 71 | 7.1% | 66 | 158 | 8.9% | 21 | 146 | 6.6% | 71 | 184 | 5.2% | 43 | 131 | 6.6% |
| SSBs (mL) | 36 | 106 | 10.3% | 57 | 113 | 10.6% | 46 | 173 | 9.2% | 16 | 71 | 5.0% | 43 | 112 | 8.0% |
| Alcohol (mL) | 0 | 0 | 0% | 0 | 0 | 0.4% | 0 | 0 | 1.0% | 0 | 0 | 0.8% | 0 | 0 | 0.7% |
| Others (mL) | 11 | 36 | 3.8% | 0 | 21 | 2.5% | 9 | 45 | 3.2% | 0 | 40 | 2.0% | 0 | 17 | 2.7% |
| Water from food (mL) | 1018a | 374 | 61.3%a | 1134b | 357 | 54.5%d | 1175b | 383 | 48.0%b,f | 1300c,e | 424 | 40.9%c,e | 1174 | 373 | 49.4% |
| Staple food (mL) | 274 | 145 | 26.4% | 338 | 131 | 26.8% | 297 | 152 | 26.3% | 323 | 162 | 25.6% | 301 | 141 | 26.3% |
| Dishes (mL) | 544 | 228 | 52.5% | 626 | 210 | 51.7% | 624b | 215 | 54.2% | 676c,e | 269 | 50.8% | 620 | 217 | 52.2% |
| Soup (mL) | 83 | 203 | 10.7% | 34 | 194 | 8.6% | 93 | 186 | 9.9% | 104 | 202 | 11.2% | 93 | 195 | 10.1% |
| Porridge (mL) | 86 | 168 | 10.1% | 132 | 202 | 12.1% | 95 | 150 | 9.0% | 92 | 259 | 10.5% | 97 | 182 | 10.4% |
| Snacks (mL) | 0 | 0 | 0.5% | 0 | 0 | 0.8% | 0f | 0 | 0.6%f | 0c,e | 0 | 1.8%c,e | 0 | 0 | 0.9% |
| Total water intake (mL) | 1735a | 467 | _ | 2142d | 467 | _ | 2454b,f | 339 | _ | 3118c,e | 709 | _ | 2342 | 844 | _ |
Values are shown as the median (M) and quartile ranges (Q)
aThere was statistically significant difference between LD1 and LD2 groups, p < 0.05; bThere was statistically significant difference between LD1 and LD3 groups, p < 0.05; cThere was statistically significant difference between LD1 and HD groups, p < 0.05; dThere was statistically significant difference between LD2 and LD3 groups, p < 0.05; eThere was statistically significant difference between LD2 and HD groups, p < 0.05; fThere was statistically significant difference between LD3 and HD groups, p < 0.05
%: Contributions of total drinking fluids and water from food to TWI; percentages of different fluids in total drinking fluids; proportions of water from different foods in water from food
There were statistical significances in the amounts of TWI, total drinking fluids and water from food (χ2 = 121.262, P<0.001; χ2 = 145.319, P<0.001; χ2 = 18.941, P<0.001) among the four groups, respectively. There were statistical significances in the contributions of total drinking fluids and water from food to TWI, respectively (F = 86.910, P = 0.000; F = 86.910, P = 0.000). There were statistical significances in the consumption of water among the four groups (χ2 = 108.966, P<0.001). The water from dishes and snacks were different in the four groups (χ2 = 12.549, P = 0.006; χ2 = 8.746, P = 0.033), but no significant differences were found in the staple food, soup and porridge (χ2 = 7.377, P = 0.061; χ2 = 3.160, P = 0.368; χ2 = 1.838, P = 0.607). The contributions of snacks to water from food among the four groups were different (χ2 = 8.331, P = 0.040)
Measurement of urine indexes
Table 4 showed that the volumes of urine increased from LD1 group, to HD group, while the osmolality, USG, and the concentrations of Na, K, Cl, Ca, Mg, phosphate, creatinine, uric acid and urea of urine were significantly different in LD1, LD2, LD3 and HD groups (p < 0.05). The percentages of participants in optimal hydration status was statistical higher in HD and LD3 groups than that in LD1, LD2 groups (p < 0.05).
Table 4.
The characteristics of 24 h urine among participants consuming different levels of total drinking fluids
| LD1 (n = 39) | LD2 (n = 39) | LD3 (n = 39) | HD (n = 39) | Total (n = 156) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| M | Q | M | Q | M | Q | M | Q | M | Q | |
| 24-h Volume (mL) | 1076 | 370 | 1256d | 500 | 1462b,f | 671 | 1869c,e | 747 | 1370 | 668 |
| 24-h urine Osmolality (mOsm/kg) | 730 | 238 | 671 | 337 | 512 | 303 | 430c,e | 280 | 587 | 309 |
| (≤500 mOsm/kg, n, %) | 5 (12.8%)* | 13 (33.3%) | 18 (46.2%)b | 22 (56.4%)c | 58 (37.2%) | |||||
| Na (mmol/L) | 200 | 123 | 171 | 95 | 175 | 101 | 148c | 110 | 175 | 101 |
| K (mmol/L) | 39.4 | 20 | 36.2 | 18.2 | 30.1b,f | 19.7 | 26.7c,e | 17.0 | 33.75 | 19.42 |
| Cl (mmol/L) | 202 | 106 | 174 | 101 | 168 | 114 | 148c | 108 | 169 | 104 |
| Mg (mmol/L) | 2.90 | 0.96 | 3.11 | 1.39 | 2.42 | 1.26 | 1.89c,e | 1.11 | 2.50 | 1.54 |
| Ca (mmol/L) | 2.47 | 2 | 2.21 | 1.59 | 2.10 | 1.25 | 1.55 | 1.06 | 2.09 | 1.54 |
| Phosphate (mmol/L) | 15.91 | 6.54 | 17.36 | 12.66 | 13.34 | 10.56 | 11.45c,e | 9.05 | 14.89 | 8.91 |
| Creatinine (mmol/L) | 10.48 | 3.45 | 10.20d | 6.82 | 8.24b | 6.59 | 7.67c | 4.97 | 8.95 | 5.17 |
| Uric acid (mmol/L) | 2.76 | 0.92 | 2.79 | 1.22 | 2.07b | 1.66 | 1.96c,e | 1.14 | 2.39 | 1.33 |
| Urea (mmol/L) | 230.30 | 108.13 | 231.50 | 162.87 | 179 | 135 | 154c,e | 117 | 200.42 | 126.95 |
| USG | 1.015 | 0.003 | 1.017 | 0.007 | 1.015 | 0.006 | 1.013c,e | 0.002 | 1.015 | 0.005 |
| pH | 6.8 | 0.5 | 6.7 | 0.3 | 6.7 | 0.3 | 6.7 | 0.3 | 6.7 | 0.3 |
Values are shown as the median (M) and quartile ranges (Q); *χ2 = 23.215, P < 0.05;
aThere was statistically significant difference between LD1 and LD2 groups, p < 0.05; bThere was statistically significant difference between LD1 and LD3 groups, p < 0.05; cThere was statistically significant difference between LD1 and HD groups, p < 0.05; dThere was statistically significant difference between LD2 and LD3 groups, p < 0.05; eThere was statistically significant difference between LD2 and HD groups, p < 0.05; fThere was statistically significant difference between LD3 and HD groups, p < 0.05
Measurement of plasma indexes
Statistically significant differences were found in the concentration of Mg among the four groups (p < 0.05). No statistically significant differences were found in plasma osmolality, the concentrations of Na, K, Cl, Ca and phosphate among participants in the four groups (p > 0.05), as shown in Table 5.
Table 5.
The characteristics of blood samples among participants consuming different levels of total drinking fluids
| LD1 (n = 39) | LD2 (n = 39) | LD3 (n = 39) | HD (n = 39) | Total (n = 156) | P | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| M | Q | X | SD | M | Q | X | SD | M | Q | X | SD | M | Q | X | SD | M | Q | X | SD | ||
| Osmolality (mOsm/kg) | 299 | 5 | 298 | 6 | 300 | 5 | 299 | 5 | 299 | 5 | 0.519 | ||||||||||
| Na (mmol/L) | 140 | 1 | 141 | 2 | 141 | 2 | 141 | 2 | 141 | 2 | 0.095 | ||||||||||
| K (mmol/L) | 4.4 | 0.5 | 4.6 | 0.4 | 4.6 | 0.6 | 4.6 | 0.6 | 4.6 | 0.5 | 0.112 | ||||||||||
| Cl (mmol/L) | 104 | 2 | 104 | 3 | 104 | 2 | 104 | 2 | 104 | 2 | 0.660 | ||||||||||
| Mg (mmol/L) | 0.90 | 0.06 | 0.90d | 0.05 | 0.92b | 0.06 | 0.92c,e | 0.08 | 0.91 | 0.07 | 0.013 | ||||||||||
| Ca (mmol/L) | 2.50 | 0.06 | 2.51 | 0.07 | 2.51 | 0.07 | 2.53 | 0.07 | 2.51 | 0.07 | 0.169 | ||||||||||
| Phosphate (mmol/L) | 1.32 | 0.13 | 1.31 | 0.16 | 1.31 | 0.17 | 1.28 | 0.18 | 1.30 | 0.16 | 0.676 | ||||||||||
Values are shown as the mean ± standard deviation (SD) and median (M) and quartile ranges (Q)
aThere was statistically significant difference between LD1 and LD2 groups, p < 0.05; bThere was statistically significant difference between LD1 and LD3 groups, p < 0.05; cThere was statistically significant difference between LD1 and HD groups, p < 0.05; dThere was statistically significant difference between LD2 and LD3 groups, p < 0.05; eThere was statistically significant difference between LD2 and HD groups, p < 0.05; fThere was statistically significant difference between LD3 and HD groups, p < 0.05
Discussion
In this study, when the total drinking fluids increased significantly, the water from food and TWI increased significantly accordingly among the four groups; the proportions of total drinking fluids in TWI were increased. Whereas, the contributions of water from food to TWI were decreased simultaneously, which were higher than the results among participants both in low and high drinkers in France [22]. The findings indicated that, participants with higher total drinking fluids may tend to prefer taking in more water from food. It meant that participants with lower total drinking fluids would not compensate from food to increase the intake of TWI. Therefore, they may have more danger to be in dehydration status than others. In addition, study performed among Europeans showed that, adults with higher TWI was associated with higher nutritional quality of the diet, assessed by several dietary indices [47]. Besides, it was also showed that high water intake remained significantly associated with higher die quality among children aged 2 to 18 years [48].
Researches demonstrated that, TWI was strongly associated with 24-h urine volume, osmolality, USG and solute concentrations [21, 49]. Moreover, urine osmolality ≤800 mOsm/kg [13, 46], was the indicator of optimal hydration status. In our study, when the total drinking fluids increased, the volume of 24-h urine increased, whereas, the 24-h osmolality, USG decreased among the four groups. Urine osmolality, USG and volume, but not plasma osmolality, responded to the changes in water intake [50]. Results of this study showed that, the 24-h urine osmolality of participants in LD1, LD2 and LD3 groups were higher than the standard, while the 24-h urine osmolality of participants in HD group was lower than the standard. Meanwhile, significantly more participants with the 24-h urine osmolality below the standard were in HD group than in other groups. Participants with lower total drinking fluids had less and more concentrated urine than participants with higher total drinking fluids, which indicated that participants with lower total drinking fluids maybe in dehydration, whereas, participants with high total drinking fluids maybe in optimal hydration status. Moreover, in France, the osmolality of urine among those with higher total drinking fluids and those with lower total drinking fluids were 371 mOsm/kg and 767 mOsm/kg, respectively [21, 22]. Blood homeostasis is well regulated by physiological compensations from the kidneys, even after 24 h of water intake deprivation [51]. Indeed, plasma osmolality was maintained at a wide range of water intakes from the study of NHANES ш [52]. Also, in our study, the plasma osmolality was 298–300 mOsm/kg with a wide range of total drinking fluids from 681 mL to 1766 mL, which indicated that plasma osmolality was not sensitive to the difference of total drinking fluids in free-living conditions, similarly to the study conducted before [14, 22, 53].
Regarding the drinking patterns, water was the main contributor of total drinking fluids among the four groups with the proportions higher than French, while the contributions of other beverages were lower than French [22]. The differences in culture and the accessibility of beverages could explain the observation. Regarding the types of beverages, SSBs have been shown to have detrimental effects on health [30, 54–56]. In our study, SSBs were the second contributor of total drinking fluids among participants in LD1, LD2 and LD3 groups, which contributed to over 10% of total drinking fluids, while the milk and milk products were the second contributor among participants in HD group. This observation suggested that participants with higher total drinking fluids may have healthier drinking behaviors. However, the consumptions of milk and milk products of participants in the four groups, which ranged from 21 mL to 71 mL, were much lower than the recommendation of China (300 mL) [57]. In China, 300 mL of milk was the main source of calcium, which could supply about 39% of the reference of the intake of calcium [15]. Therefore, more interventions should be undertaken to increase the consumptions of milk and milk products. In addition, a study demonstrated that drinking pattern based on water and milk among children was associated with better hydration including lower urine osmolality, while drinking regular soda and other drinks but not water was associated with inferior hydration [58].
In this study, water from food was also an important source of TWI, accounting for 40.9–61.3% to TWI among the four groups. Regarding the sources of water from food, dishes (such as vegetables, meat) and staple food (including steamed bread and rice) were the first and second contributor among the four groups. Moreover, participants with higher total drinking fluids had more amounts of water from dishes (such as vegetables) and snacks (for instance, the fruits) than those with lower total drinking fluids. But there were no significant differences in the contributions of water from different sources of food.
Research showed that heights were also different among the people of China and other countries due to race differences, which contributes to difference in body surface area and has corresponding effects on water requirements [59]. In this research, significant differences were found in the height and weight when comparing groups LD2, LD3, HD groups with LD1 group, respectively, but with no differences between LD2, LD3 and HD groups. However, the indexes, such as the amounts of total drinking fluids and water from food, were different between LD2, LD3 and HD groups. In addition, there were also significant differences of hydration biomarkers, for instance, the volume of urine between LD2, LD3 and HD groups. It meant that, the differences of anthropometric measurements at baseline did not influence the results of drinking patterns and hydration biomarkers among participants from different groups.
In terms of the strengths, it was the first study investigated the drinking patterns and hydration biomarkers among participants with different total drinking fluids. Moreover, 7-day 24 h fluid intake questionnaire was used to assess the total drinking fluids for seven consecutive days (two weekdays and one weekend day), which would reduce the recall bias. The duplicate portion method was used to assess the amounts of water from food, with all the foods were being weighed before and after participants ate for three consecutive days. In addition, 24 h urine was collected every time they urinated using specific container for three consecutive days (two weekdays and one weekend day), which would include incorporate factors fluctuating during weekdays and weekends. Moreover, the samples of urine collected by the participants, were stored at + 4 °C and sent to the laboratory for test immediately. All the tests were performed by professional investigators. Despite strengths mentioned above, the study employed did have limitations. This survey was performed with young adults only so extrapolation of the study findings to different age groups should be done with care. However, despite the limitation, the association between hydration status, drinking pattern and total drinking fluids intake remains meaningful. Future studies will determine the general application of our results to a wider population, including middle-aged adults and the elderly.
Conclusions
Participants with higher total drinking fluids had higher water from food and TWI than those with lower total drinking fluids. Participants with higher total drinking fluids had better hydration status and drinking pattern than those with lower total drinking fluids. Interventions should be undertaken to encourage people to drink an adequate amount of total drinking fluids, preferably water, in order to be in optimal hydration.
Acknowledgements
We would like to thank investigators from Hebei University Health Science Center for participating in this project.
Abbreviations
- LD
Low drinker
- HD
High drinker
- TWI
Total water intake
- EFSA
European Food Safety Authority
Authors’ contributions
JZ participated in the data collection of the study, the drafting of the paper, and contributed to the analysis of the data. GM was responsible for designing, monitoring the implementation of the study and the finalization of manuscript. NZ and SD designed the study and were responsible for the quality management, the control of the implementation of the study and the analysis of the data. JZ and SL was responsible for the recruitment of participants. HH, YX, HC, SL and YW were responsible for the implementation of the study. All authors read and approved the final manuscript.
Funding
This work was supported by National Natural Science Foundation of China (81673146). The National Natural Science Foundation of China did not play a role in the design of the study, the collection, analysis and interpretation of the data and in writing the manuscript.
Availability of data and materials
The datasets generated and/or analyzed during the current study are available from the corresponding author on reasonable request.
Ethics approval and consent to participate
The study protocol was approved by the Peking University Institutional Review Committee. The ethical approval project identification code is IRB00001052–16071. This study was conducted according to the guidelines of the Declaration of Helsinki. All participants signed informed consent form before participating in the study.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Jianfen Zhang, Email: ZJF@bjmu.edu.cn.
Na Zhang, Email: ziqingxuanping@126.com.
Yan Wang, Email: bwyan@163.com.
Shuxin Liang, Email: LSX393939@163.com.
Shufang Liu, Email: shufangliu@126.com.
Songming Du, Email: songmingdu097@126.com.
Yifan Xu, Email: xuyifan_1992@163.com.
Hairong He, Email: hhrhhr3@163.com.
Hao Cai, Email: caihao169@pku.edu.cn.
Guansheng Ma, Email: mags@bjmu.edu.cn.
References
- 1.Popkin BM, D’Anci KE, Rosenberg IH. Water, hydration, and health. Nutr Rev. 2010;68(8):439–458. doi: 10.1111/j.1753-4887.2010.00304.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jequier E, Constant F. Water as an essential nutrient: the physiological basis of hydration. Eur J Clin Nutr. 2010;64(2):115–123. doi: 10.1038/ejcn.2009.111. [DOI] [PubMed] [Google Scholar]
- 3.Munoz CX, Johnson EC, McKenzie AL, et al. Habitual total water intake and dimensions of mood in healthy young women. Appetite. 2015;92:81–86. doi: 10.1016/j.appet.2015.05.002. [DOI] [PubMed] [Google Scholar]
- 4.Savoie FA, Kenefick RW, Ely BR, et al. Effect of Hypohydration on muscle endurance, strength, anaerobic power and capacity and vertical jumping ability: a meta-analysis. Sports Med. 2015;45(8):1207–1227. doi: 10.1007/s40279-015-0349-0. [DOI] [PubMed] [Google Scholar]
- 5.Lewis JJ, Mark PF, Ruth MJ, et al. Does hypohydration really impair endurance performance? Methodological considerations for interpreting hydration research. Sports Med. 2019;49(suppl2):s103–s114. doi: 10.1007/s40279-019-01188-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Suhr JA, Hall J, Patterson SM, Niinisto RT. The relation of hydration status to cognitive performance in healthy older adults. Int J Psychophysiol. 2004;53(2):121–125. doi: 10.1016/j.ijpsycho.2004.03.003. [DOI] [PubMed] [Google Scholar]
- 7.Zhang N, Du SM, Zhang J, et al. Effects of dehydration and rehydration on the cognitive performances and mood among male college students in Cangzhou, China: a self-controlled trial. Int J Environ Res Public Health. 2019;16:1891. doi: 10.3390/ijerph16111891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.D’Anci KE, Mahoney CR, Vibhakar A, Kanter JH, Taylor HA. Voluntary dehydration and cognitive performance in trained college athletes. Percept Mot Skills. 2009;109(1):251–269. doi: 10.2466/pms.109.1.251-269. [DOI] [PubMed] [Google Scholar]
- 9.Ganio MS, Armstrong LE, Casa DJ, et al. Mild dehydration impairs cognitive performance and mood of men. Brit J Nutr. 2011;106(10):1535–1543. doi: 10.1017/S0007114511002005. [DOI] [PubMed] [Google Scholar]
- 10.Stachenfeld NS, Leone CA, Mitchell ES, Freese E, Harkness L. Water intake reverses dehydration associated impaired executive function in healthy young women. Physiol Behav. 2018;185:103–111. doi: 10.1016/j.physbeh.2017.12.028. [DOI] [PubMed] [Google Scholar]
- 11.Wittbrodt MT, Melinda MS. Dehydration impairs cognitive performance. Med Sci Sports Exer. 2018;50:2360–2368. doi: 10.1249/MSS.0000000000001682. [DOI] [PubMed] [Google Scholar]
- 12.Joseph CW, William BF. Hydration status and cardiovascular function. Nutrients. 2019;11:1866. doi: 10.3390/nu11081866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Authority EFS. Scientific opinion on dietary reference values for water. EFSA J. 2010;8(3):1462. [Google Scholar]
- 14.Institute of Medicine . Dietary reference intakes for water, potassium, sodium, chloride, and sulfate. Washington DC: National Academy Press; 2005. [Google Scholar]
- 15.Chinese Nutrition Society . Chinese dietary nutrients reference intakes (2013) Beijing: Science Press; 2014. [Google Scholar]
- 16.Ferreira-Pêgo C, Guelinckx I, Moreno LA, et al. Total fluid intake and its determinants: cross-sectional surveys among adults in 13 countries worldwide. Eur J Nutr. 2015;54(S2):35–43. doi: 10.1007/s00394-015-0943-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Suh HG, Kavouras SA. Water intake and hydration state in children. Eur J Nutr. 2019;58(2):475–496. doi: 10.1007/s00394-018-1869-9. [DOI] [PubMed] [Google Scholar]
- 18.Ma G, Zhang Q, Liu A, et al. Fluid intake of adults in four Chinese cities. Nutr Rev. 2012;70(Suppl 2):S105–S110. doi: 10.1111/j.1753-4887.2012.00520.x. [DOI] [PubMed] [Google Scholar]
- 19.Brooks CJ, Gortmaker SL, Long MW, Cradock AL, Kenney EL. Racial/ethnic and socioeconomic disparities in hydration status among US adults and the role of tap water and other beverage intake. Am J Public Health. 2017;107(9):1387–1394. doi: 10.2105/AJPH.2017.303923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hilary JB, Larry KW, David MA, et al. Cognitive performance in relation to hydration status and water intake among older adults, NHANES 2011–2014. Eur J Nutr. 2019. 10.1007/s00394-019-02152-9. [DOI] [PMC free article] [PubMed]
- 21.Zhang N, Du S, Tang Z, et al. Hydration, fluid intake, and related urine biomarkers among male college students in Cangzhou, China: a cross-sectional study—applications for assessing fluid intake and adequate water intake. Int J Environ Res Public Health. 2017;14(5):513. doi: 10.3390/ijerph14050513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Perrier E, Vergne S, Klein A, et al. Hydration biomarkers in free-living adults with different levels of habitual fluid consumption. Br J Nutr. 2013;109(9):1678–1687. doi: 10.1017/S0007114512003601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee KW, Shin D. A healthy beverage consumption pattern is inversely associated with the risk of obesity and metabolic abnormalities in Korean adults. J Med Food. 2018;00(0):1–11. doi: 10.1089/jmf.2017.0119. [DOI] [PubMed] [Google Scholar]
- 24.Brunkwall L, Almgren P, Hellstrand S, Orho-Melander M, Ericson U. Commonly consumed beverages associate with different lifestyle and dietary intakes. Int J Food Sci Nutr. 2018;70:1–10. doi: 10.1080/09637486.2018.1466272. [DOI] [PubMed] [Google Scholar]
- 25.Daniels MC, Popkin BM. Impact of water intake on energy intake and weight status: a systematic review. Nutr Rev. 2010;68(9):505–521. doi: 10.1111/j.1753-4887.2010.00311.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Duffey K, Poti J. Modeling the effect of replacing sugar-sweetened beverage consumption with water on energy intake, HBI score, and obesity prevalence. Nutrients. 2016;8(7):395. doi: 10.3390/nu8070395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chang T, Ravi N, Plegue MA, Sonneville KR, Davis MM. Inadequate hydration, BMI, and obesity among US adults: NHANES 2009-2012. Ann Fam Med. 2016;14(4):320–324. doi: 10.1370/afm.1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Madjd A, Taylor MA, Delavari A, et al. Effects of replacing diet beverages with water on weight loss and weight maintenance: 18-month follow-up, randomized clinical trial. Int J Obes. 2018;42:835–840. doi: 10.1038/ijo.2017.306. [DOI] [PubMed] [Google Scholar]
- 29.Hu FB. Resolved: there is sufficient scientific evidence that decreasing sugar-sweetened beverage consumption will reduce the prevalence of obesity and obesity-related diseases. Obes Rev. 2013;14(8):606–619. doi: 10.1111/obr.12040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gui Z, Zhu Y, Cai L, et al. Sugar-sweetened beverage consumption and risks of obesity and hypertension in Chinese children and adolescents: a National Cross-Sectional Analysis. Nutrients. 2017;9(12):1302. doi: 10.3390/nu9121302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hodge AM, Bassett JK, Milne RL, English DR, Giles GG. Consumption of sugar-sweetened and artificially sweetened soft drinks and risk of obesity-related cancers. Public Health Nutr. 2018;21(9):1618–1626. doi: 10.1017/S1368980017002555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ambrosini GL, Oddy WH, Huang RC, et al. Prospective associations between sugar-sweetened beverage intakes and cardiometabolic risk factors in adolescents. Am J Clin Nutr. 2013;98(2):327–334. doi: 10.3945/ajcn.112.051383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Parvin M, Emad Y, Golaleh A, et al. Consumption of sugar sweetened beverage is associated with incidence of metabolic syndrome in Tehranian children and adolescents. Nutr Metab. 2015;12(1):25. doi: 10.1186/s12986-015-0021-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Malik VS, Popkin BM, Bray GA, et al. Sugar-sweetened beverages, obesity, type 2 diabetes mellitus, and cardiovascular disease risk. Circulation. 2010;121(11):1356–1364.35. doi: 10.1161/CIRCULATIONAHA.109.876185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sampasa-Kanyinga H, Hamilton HA, Chaput JP. Sleep duration and consumption of sugar-sweetened beverages and energy drinks among adolescents. Nutrition. 2018;48:77–81. doi: 10.1016/j.nut.2017.11.013. [DOI] [PubMed] [Google Scholar]
- 36.Grigg D. The worlds of tea and coffee: patterns of consumption. Geo J. 2002;57:283–294. [Google Scholar]
- 37.Kant AK, Graubard BI, Atchison EA. Intakes of plain water, moisture in foods and beverages, and total water in the adult US population--nutritional, meal pattern, and body weight correlates: National Health and nutrition examination surveys 1999-2006. Am J Clin Nutr. 2009;90(3):655–663. doi: 10.3945/ajcn.2009.27749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Manz F, Johner SA, Wentz A, Boeing H, Remer T. Water balance throughout the adult life span in a German population. Br J Nutr. 2012;107(11):1673–1681. doi: 10.1017/S0007114511004776. [DOI] [PubMed] [Google Scholar]
- 39.Tani Y, Asakura K, Sasaki S, et al. The influence of season and air temperature on water intake by food groups in a sample of free-living Japanese adults. Eur J Clin Nutr. 2015;69(8):907–913. doi: 10.1038/ejcn.2014.290. [DOI] [PubMed] [Google Scholar]
- 40.Hu X, Zhang Q, Zhang W, et al. Water intake sources analysis of adults in four cities of China in summer. Chin J Prev Med. 2011;45(8):688–691. [PubMed] [Google Scholar]
- 41.Zhang N, Du S, Tang Z, Zheng M, Ma G. Effect of water supplementation on cognitive performances and mood among male college students in Cangzhou, China: study protocol of a randomized controlled trial. Int J Environ Res Public Health. 2017;14(9):966. doi: 10.3390/ijerph14090966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.General Administration of Quality Supervision, Inspection and Quarantine of China. General Standard for Beverages GB/T 1–789-2015. Beijing: Standardization Administration of China; 2015.
- 43.Zhang J, Zhang N, Liang S, et al. The amounts and contributions of total drinking fluids and water from food to total water intake of young adults in Baoding, China. Eur J Nutr. 2018;58:2669–2677. doi: 10.1007/s00394-018-1814-y. [DOI] [PubMed] [Google Scholar]
- 44.Standardization Administration of China . Determination of Moisture in Foods. Beijing: China Standard Press; 2016. National Food Safety Standard GB 5009.3–2016. [Google Scholar]
- 45.Institute for Nutrition and Health, Chinese Center for Disease Control and Prevention . China Food Composition. Beijing: Peking University Medicine Press; 2009. [Google Scholar]
- 46.Perrier ET, Buendia-Jimenez I, Vecchio M, et al. Twenty-four-hour urine osmolality as a physiological index of adequate water intake. Dis Markers. 2015;2015:231063. doi: 10.1155/2015/231063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gazan R, Sondey J, Maillot M, Guelinckx I, Lluch A. Drinking water intake is associated with higher diet quality among French adults. Nutrients. 2016;8(11):689. doi: 10.3390/nu8110689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Leung CW, Dimatteo SG, Gosliner WA, et al. Sugar-sweetened beverage and water intake in relation to diet quality in U.S. children. Am J Prev Med. 2018;54(3):394–402. doi: 10.1016/j.amepre.2017.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Perrier E, Rondeau P, Poupin M, et al. Relation between urinary hydration biomarkers and total fluid intake in healthy adults. Eur J Clin Nutr. 2013;67(9):939–943. doi: 10.1038/ejcn.2013.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Perrier E, Demazieres A, Girard N, et al. Circadian variation and responsiveness of hydration biomarkers to changes in daily water intake. Eur J Appl Physiol. 2013;113(8):2143–2151. doi: 10.1007/s00421-013-2649-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pross N, Demazieres A, Girard N, et al. Influence of progressive fluid restriction on mood and physiological markers of dehydration in women. Br J Nutr. 2013;109(2):313–321. doi: 10.1017/S0007114512001080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Campbell SM. Hydration needs throughout the lifespan. J Am Coll Nutr. 2007;26(5 Suppl):585S–587S. doi: 10.1080/07315724.2007.10719662. [DOI] [PubMed] [Google Scholar]
- 53.Johnson EC, Munoz CX, Jimenez L, et al. Hormonal and thirst modulated maintenance of fluid balance in young women with different levels of habitual fluid consumption. Nutrients. 2016;8(5):302. doi: 10.3390/nu8050302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Richelsen B. Sugar-sweetened beverages and cardio-metabolic disease risks. Curr Opin Clin Nutr Metab Care. 2013;16(4):478–484. doi: 10.1097/MCO.0b013e328361c53e. [DOI] [PubMed] [Google Scholar]
- 55.Malik VS, Pan A, Willett WC, Hu FB. Sugar-sweetened beverages and weight gain in children and adults: a systematic review and meta-analysis. Am J Clin Nutr. 2013;98(4):1084–1102. doi: 10.3945/ajcn.113.058362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Qin Z, Xu F, Ye Q, et al. Sugar-sweetened beverages and school students’ hypertension in urban areas of Nanjing, China. J Hum Hypertens. 2018;32:392–396. doi: 10.1038/s41371-018-0030-9. [DOI] [PubMed] [Google Scholar]
- 57.Chinese Nutrition Society . Chinese Dietary Guidelines (2016) Beijing: People’s Medical Publishing House; 2016. [Google Scholar]
- 58.Bougatsas D, Arnaoutis G, Panagiotakos DB, et al. Fluid consumption pattern and hydration among 8-14 years-old children. Eur J Clin Nutr. 2018;72(3):420. doi: 10.1038/s41430-017-0012-y. [DOI] [PubMed] [Google Scholar]
- 59.Zhang YY, Liu PY, Lu Y, et al. Race and sex differences and contribution of height: A study on bone size in healthy Caucasians and Chinese. Am J Hum Biol. 2005;17:568–575. doi: 10.1002/ajhb.20427. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated and/or analyzed during the current study are available from the corresponding author on reasonable request.