Abstract
Background
The development of paclitaxel-resistance led to the tumor relapse and treatment failure of non-small cell lung cancer. Shikonin has been demonstrated to show anti-cancer activity in many cancer types. The present study aimed to investigate the anti-cancer activity of shikonin in paclitaxel-resistant non-small cell lung cancer treatment.
Methods
MTT, clonogenic assay, apoptotic cell death analysis, western blot, qRT-PCR, gene knockdown and overexpression, xenograft experiment, immunohistochemistry were performed.
Results
Shikonin decreased paclitaxel-resistant NSCLC cell viability and inhibited the growth of xenograft tumor. Shikonin induced apoptotic cell death of paclitaxel-resistant NSCLC cell lines and suppressed the level of NEAT1 and Akt signaling of paclitaxel-resistant NSCLC cell lines and xenograft tumors. Either low dose or high dose of shikonin considerably suppressed the cell growth and induced the cell apoptotic death in NEAT1 knockdown A549/PTX cells, and p-Akt expression was decreased.
Conclusions
Shikonin could be a promising candidate for paclitaxel-resistant NSCLC treatment.
Keywords: Shikonin, Drug-resistance, NSCLC, NEAT1, Akt
Introduction
Globally, lung cancer is the most lethal malignancy and the leading cause of death. More than 85% of lung cancer patients were diagnosed as non-small cell lung cancer (NSCLC). Unfortunately, a lot of NSCLC patients are an advanced disease when the first diagnosis and unsuitable for surgery. Despite the development of immunotherapy and targeted drugs, chemotherapy remains the cornerstone of lung cancer treatment. And clinically, paclitaxel is a preferred chemotherapeutic agent and the first-line drug for advanced NSCLC (Adrianzen Herrera et al. 2019). However, the development of paclitaxel-resistance led to tumor relapse and treatment failure, which limits the application of paclitaxel. Understanding of the mechanisms of paclitaxel-resistance may improve the NSCLC treatment outcomes, but the molecular mechanisms of drug resistance are complicated. The development of drug resistance may be related to gene mutation, overactivation of by-pass signaling and aberrant expression of efflux protein. Previous studies indicated that long non-coding RNA (lncRNA) played an important role in the paclitaxel-resistance of several types of cancer. Linc01118, UCA1, Nuclear-enriched abundant transcript 1 (NEAT1) contributed paclitaxel-resistance to ovarian cancer (Shi and Wang 2018; Wang et al. 2018a; An et al. 2017), FTH1P3, H19 activated paclitaxel-resistance in breast cancer (Wang et al. 2018b; Han et al. 2018), Linc00518, Linc00473, PVT1 promoted paclitaxel-resistance of prostate cancer, colorectal cancer and glioma respectively (He et al. 2019; Wang et al. 2018c; Song et al. 2018), and NEAT1, KCNQ1OT1, ANRIL conferred paclitaxel-resistance to lung cancer (Li et al. 2019; Ren et al. 2017; Xu et al. 2017). Therefore, finding an effective and safe anti-cancer agent to overcome the paclitaxel-resistance of NSCLC and explore the underlying mechanism is necessary.
Recently, compounds derived from natural herbs have attracted attention due to their curative effect and low toxicity in cancer therapy (Jiang et al. 2014a; Jiang et al. 2014b; Zhao et al. 2015; Zhu et al. 2016; Zhao et al. 2016; Song et al. 2016; Zheng et al. 2017; Wang et al. 2017; Hong et al. 2019; Yang et al. 2018). Shikonin, a naphthoquinone, is extracted from the plant root of Lithospermum erythrorhizon. Shikonin has been demonstrated to treat inflammation, virus infection and tumor due to its anti-inflammatory, anti-bacterial, and anti-tumor properties (Wang et al. 2019). Shikonin inhibited cell proliferation, migration, and invasion of a variety of tumors through several possible molecular mechanisms. Furthermore, shikonin overcame multidrug resistance in many cancer types and mediated lncRNA related signaling pathways (Li et al. 2018; Li et al. 2017; Zhang et al. 2017a; Yang and Chen 2015). However, the anti-cancer activity and key target of shikonin on paclitaxel-resistant NSCLC are still unclear.
In this study, the therapeutic potentials of shikonin on paclitaxel-resistant NSCLC were investigated and the underlying mechanisms were further determined. As expected, shikonin inhibited the growth of paclitaxel-resistant NSCLC through suppression of NEAT1 and Akt signaling, providing a fascinating opportunity for paclitaxel-resistant NSCLC treatment.
Materials and methods
Drug, reagents and cell line
Shikonin (C16H16O5) was obtained from Sigma-Aldrich (MO, USA). MTT, apoptosis Detection Kit and p-Akt antibody were obtained from Sigma-Aldrich (MO, USA). A549 cells were obtained from the cell bank of Chinese Academy of Sciences (Shanghai, China). The paclitaxel-resistant cell line (A549/PTX) was established as previously described (Li et al. 2019). A549 cells were exposed to a high concentration of paclitaxel and the resistant clones were selected. The paclitaxel-resistant cells were cultured in the medium of RPMI-1640 with 10% fetal bovine serum in an incubator containing 5% CO2 at 37 °C.
MTT assay
Exponentially growing A549/PTX cells were seeded in 96-well plates (5000 cells/well) and cultured overnight. Cells were incubated with different concentrations of shikonin for 24 h, 48 h, and 72 h. Then MTT solution was added and incubated in the dark for 4 h. The formazan crystal was dissolved with DMSO before detecting the absorbance at 570 nm wavelength using a multi scanner auto reader.
Clonogenic assay
A549/PTX cells were trypsinized, washed, and seeded in 6-well plates (1000 cells/well). Following treating with shikonin for 48 h, the A549/PTX cells were further incubated with fresh medium for 2 weeks. Colonies were washed with PBS, fixed with methanol, and stained with crystal violet. Colonies were scored under a light microscope.
Apoptosis detection
Exponentially growing A549/PTX cells were seeded in 6-well plates (20,000 cells/well) and cultured overnight. The cells were then treated with different concentrations of shikonin for 48 h. After treatment, A549/PTX cells were harvested, washed with PBS, and stained with Annexin V-FITC and PI in the dark for 15 min at room temperature. Apoptosis was detected using a flow cytometer.
Western blot analysis
Briefly, cells were treated, then harvested and lysed. The extracted protein was quantified by BCA assay and then separated with SDS-PAGE, followed by transferring onto the PVDF membrane and blocking with 5% skimmed milk in PBS. The membrane was incubated with primary antibody against p-Akt, Akt, cleaved PARP, cleaved caspase-3(1:1000) at 4 °C overnight and secondary antibody (1:10,000) at room temperature for 1 h. Then the blots were visualized using an ECL system.
Quantitative reverse transcription-polymerase chain reaction (qRT-PCR)
Total RNA was extracted from A549/PTX cells using TRIzol reagent. RNA was reverse-transcribed with high-capacity cDNA reverse transcription kits. The cDNA was further amplified using PCR with the specific primer as follows: NEAT1: 5′-CTTCCTCCCTTTAACTTATCCATTCAC-3′(forward), 5′-CTCTTCCTCCACCATTACCAACAATAC-3′(reverse). β-actin was used for endogenous control. qRT-PCR was performed by Applied Biosystems StepOne™ Real-Time PCR System using SYBR® Premix DimerEraser Kit. The relative expression of NEAT1 was calculated using the 2-ΔΔCt method and normalized to β-actin.
NEAT1 knockdown and overexpression for transfection
NEAT1 knockdown and NEAT1 overexpression were carried out by synthesization of shRNA that specifically targeted NEAT1 (shNEAT1) and the PCR-amplified NEAT1 (NEAT1) (Genechem co., Shanghai, China). A549/PTX cells were transfected with lentiviral plasmid according to the manufacturer’s instructions.
Nude mice xenograft experiment
Four-week-old, male, weighing 20 ± 1 g BALB/c nude mice were used. The mice were kept in the pathogen-free conditioned facility. The animal experiment was approved by the ethics committee of Zhejiang Hospital (Hangzhou, China) according to the animal care guidelines. A549/PTX cells were trypsinized and suspended, then injected subcutaneously into the flank of mice to establish paclitaxel-resistant NSCLC xenograft(N = 10). The mice were intraperitoneally injected with shikonin (2 mg/kg) daily after the average volume of the tumor reached 200 mm3. The formula (0.5 × length × width2) was used to evaluate tumor growth. The mice were sacrificed after the treatment lasted for 21 days. The tumors were removed and weighed, and the samples were prepared for further analysis.
Immunohistochemistry (IHC)
Tumor samples were fixed, embedded in paraffin, and sliced up. Then slices (4 μm) were deparaffinized and then incubated with citrate buffer for antigen retrieval. The slices were incubated with primary antibody (anti-p-Akt, 1:100) overnight at 4 °C and then the second antibody for 1 h at room temperature, followed by DAB substrate incubation. The immunostaining of p-Akt was observed by light microscopy.
Statistical analysis
The data were presented as mean ± standard deviation and analyzed using GraphPad Prism 6 (CA, USA). One-way ANOVA with the SNK-q post hoc test was performed. p < 0.05 was considered a statistically significant difference.
Results
Shikonin decreased paclitaxel-resistant NSCLC cell viability and inhibited the growth of xenograft tumor
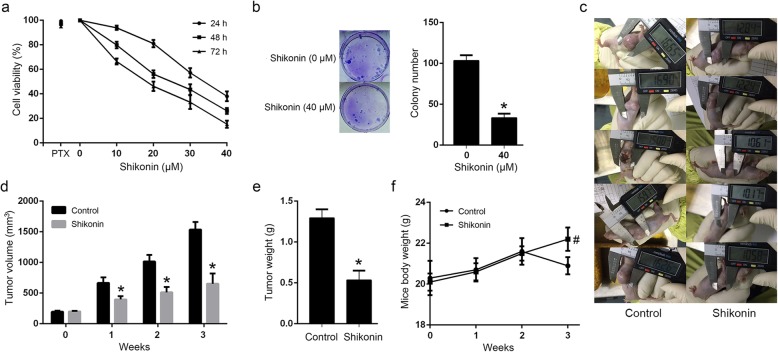
A549/PTX cell line was established and used for investigating the anti-proliferative effect of shikonin in paclitaxel-resistant NSCLC. MTT assay showed that shikonin considerably decreased cell viability in A549/PTX cells in a dose- and time-dependent manner (Fig. 1a), and colony formation assay indicated that shikonin inhibited the colony formation activity of A549/PTX cells at 48 h (Fig. 1b). Further, the A549/PTX xenograft model was established to study the anti-cancer activity of shikonin in vivo. Shikonin showed significant tumor growth inhibition than the control group. Shikonin resulted in a 57.5% tumor volume decrease and 58.9% tumor weight inhibition compared to the control group, respectively (Fig. 1c, d, e). Besides, the bodyweight decrease in the shikonin-treated mice was not observed (Fig. 1f).
Fig. 1.
Shikonin decreased paclitaxel-resistant NSCLC cell viability and inhibited the growth of xenograft tumors. a Shikonin considerably decreased cell viability in A549/PTX cells in a dose- and time-dependent manner. b shikonin inhibited the colony formation activity of A549/PTX cells at 48 h. c, d Shikonin inhibited the tumor volume of A549/PTX xenograft. e Shikonin inhibited the tumor weight of A549/PTX xenograft. f Shikonin did not decrease the mice’s body weight. *Statistically significant difference (p < 0.05). #No statistically significant difference(p > 0.05)
Shikonin induced apoptotic cell death of paclitaxel-resistant NSCLC cell lines
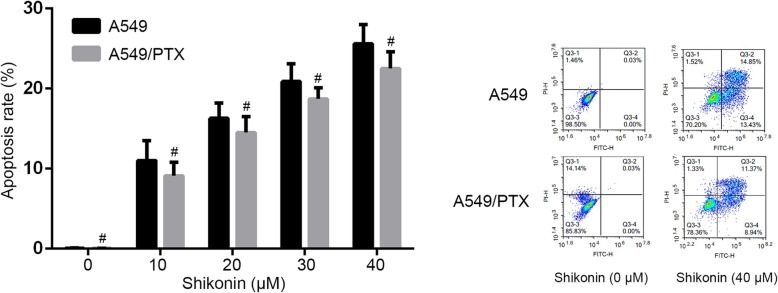
We investigated apoptotic cell death in paclitaxel-resistant A549/PTX cells after shikonin treatment. The cells were treated with shikonin for 48 h and then determined by Annexin V assay. We found that shikonin markedly increased A549/PTX cell apoptosis in a dose-dependent manner, and the apoptosis rate was similar to the A549 cells treated by shikonin (Fig. 2).
Fig. 2.
Shikonin induced apoptotic cell death of paclitaxel-resistant NSCLC cell lines in a dose-dependent manner. #No statistically significant difference(p > 0.05)
Shikonin suppressed the level of NEAT1 and Akt signaling of paclitaxel-resistant NSCLC cell lines and xenograft tumor
NEAT1 has been reported to be associated with paclitaxel-resistance in NSCLC through activation of the Akt signaling pathway (Li et al. 2019). Akt signaling activation results in apoptosis inhibition, cell proliferation, and paclitaxel-resistance in several cancer types (Chen et al. 2018; Zhang et al. 2017b). To observe the effect of shikonin on the expression of NEAT1 and Akt signaling in paclitaxel-resistant NSCLC, qRT-PCR, Western blot analysis and IHC were performed in vitro and in vivo. It showed that the level of NEAT1 expression was downregulated, and p-Akt expression was decreased after shikonin treatment compared to the control group in A549/PTX cells (Fig. 3a). In vivo, it has also been found that NEAT1 expression was downregulated, and p-Akt expression was decreased in the A549/PTX xenograft (Fig. 3b).
Fig. 3.
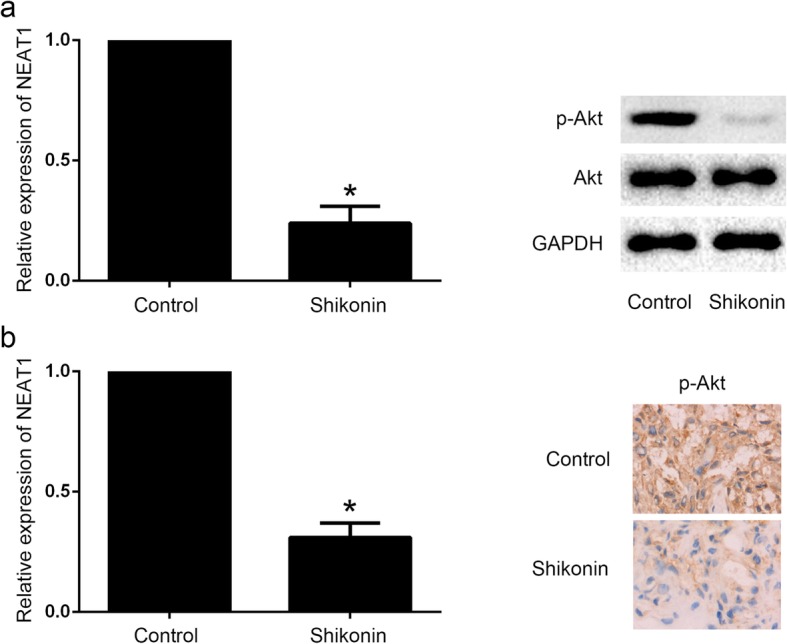
Shikonin suppressed the level of NEAT1 and Akt signaling of paclitaxel-resistant NSCLC cell lines and xenograft tumors. a The level of NEAT1 expression was downregulated, and p-Akt expression was decreased after shikonin treatment compared to the control group in A549/PTX cells. b NEAT1 expression was downregulated, and p-Akt expression was decreased in the A549/PTX xenograft. *Statistically significant difference (p < 0.05)
NEAT1 and Akt signaling downregulation was involved in the shikonin against paclitaxel-resistant NSCLC
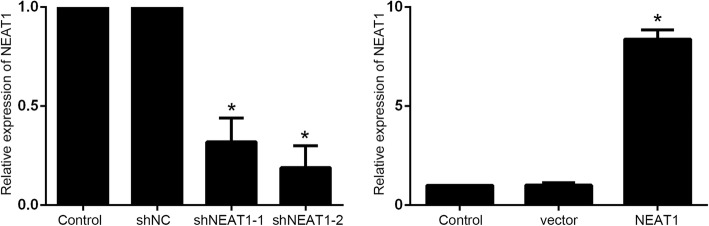
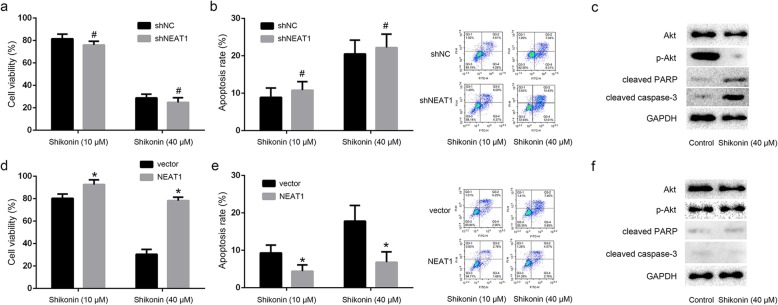
We further established the NEAT1 knockdown and NEAT1 overexpression A549/PTX cells to explore the role of NEAT1/Akt signaling in shikonin against paclitaxel-resistant NSCLC (Fig. 4). The results showed that either low dose or high dose of shikonin considerably suppressed the cell growth and induced the cell apoptotic death in NEAT1 knockdown A549/PTX cells. p-Akt expression was decreased, cleaved PARP and cleaved caspase-3 expression were increased after shikonin treatment (Fig. 5a, b, c). However, the proliferation of NEAT1 overexpression A549/PTX cells was not inhibited and apoptosis rates were not enhanced after shikonin treatment, and shikonin also could not decrease p-Akt expression and increase cleaved PARP and cleaved caspase-3 expression (Fig. 5d, e, f). These results indicated that NEAT1/Akt signaling played an important role in the shikonin against paclitaxel-resistant NSCLC.
Fig. 4.
The NEAT1 knockdown and NEAT1 overexpression A549/PTX cells were established. *Statistically significant difference (p < 0.05)
Fig. 5.
NEAT1 and Akt signaling downregulation were involved in the shikonin against paclitaxel-resistant NSCLC. a Either low dose or high dose of shikonin considerably suppressed the NEAT1 knockdown A549/PTX cell growth. b Either low dose or high dose of shikonin considerably induced the cell apoptotic death in NEAT1 knockdown A549/PTX cells. c p-Akt expression was decreased, cleaved PARP and cleaved caspase-3 expression were increased after shikonin treatment in NEAT1 knockdown A549/PTX cells. d The proliferation of NEAT1 overexpression A549/PTX cells was not inhibited after shikonin treatment. e Apoptosis rates were not enhanced after shikonin treatment. f Shikonin could not decrease p-Akt expression, also could not increase cleaved PARP and cleaved caspase-3 expression after shikonin treatment in NEAT1 knockdown A549/PTX cells. *Statistically significant difference (p < 0.05). #No statistically significant difference(p > 0.05)
Discussion
Lung cancer is the most common malignancy with poor prognosis and a low survival rate. The development of drug resistance and the lack of therapeutic options are the main obstacle for lung cancer treatment. Finding an effective therapeutic agent without adverse side effects and the acquisition of drug resistance is necessary. Shikonin has been shown to suppress tumor growth by modulating multiple signaling pathways including p21, PI3K, ERK, and p53 (Jeung et al. 2016), and overcome cancer drug resistance such as gefitinib-resistance by inhibiting TrxR and activating the EGFR proteasomal degradation pathway (Li et al. 2017). Shikonin could also enhance the antitumor effect of gefitinib in EGFR wild-type lung cancer via inhibition of PKM2/stat3/cyclinD1 signaling (Tang et al. 2018), and inhibit migration and invasion of lung cancer cells via inhibition of c-Met mediated EMT (Hsieh et al. 2017). It has been reported that shikonin could overcome cisplatin resistance, afatinib resistance, and tamoxifen resistance in several cancer types (Li et al. 2018; Zhang et al. 2017a; Wang et al. 2018d). In the present study, we explored the anti-cancer activity of shikonin on the paclitaxel-resistant NSCLC. In vitro and in vivo study, it has been found that shikonin decreased paclitaxel-resistant NSCLC cell viability and inhibited the growth of xenograft tumor without mice body weight loss. And shikonin markedly induced apoptotic cell death of paclitaxel-resistant NSCLC cells. These results demonstrated that shikonin inhibited paclitaxel-resistant NSCLC through inducing apoptosis.
Phosphoinositide 3-kinase/Akt signaling is dysregulated in most carcinomas and Akt is the key target for cancer therapy. A variety of lncRNAs have been reported to regulate Akt signaling (Dong et al. 2019), and NEAT1 could modulate Akt signaling pathway in several cancers. NEAT1 contributed to radioactive iodine resistance via PI3K/Akt signaling pathway in papillary thyroid carcinoma (Liu et al. 2019). NEAT1 promoted cell proliferation in multiple myeloma by activating PI3K/Akt pathway (Xu et al. 2018). NEAT1 regulated proliferation and invasion of cervical carcinoma by targeting Akt signaling (Guo et al. 2018). NEAT1 promoted the cell growth of prostate cancer through SRC3/IGF1R/Akt pathway (Xiong et al. 2018). NEAT1 impacted cell proliferation and apoptosis of colorectal cancer via regulation of Akt signaling (Peng et al. 2017). Besides, our previous study showed that NEAT1 mediated paclitaxel-resistance of NSCLC through activation of Akt signaling (Li et al. 2019). In the present study, we explored the molecular mechanism of NEAT1 and Akt signaling which was involved in the shikonin against paclitaxel-resistant NSCLC. It has been found that shikonin suppressed the level of NEAT1 and Akt signaling of paclitaxel-resistant NSCLC cell lines and xenograft tumors. The level of NEAT1 expression was downregulated, and p-Akt expression was decreased after shikonin treatment compared to the control group in A549/PTX cells and A549/PTX xenograft. We next established the NEAT1 knockdown and NEAT1 overexpression A549/PTX cells and found that shikonin considerably suppressed the cell growth and induced the cell apoptotic death in NEAT1 knockdown A549/PTX cells, and p-Akt expression was decreased, cleaved PARP and cleaved caspase-3 expression were increased. However, the proliferation of NEAT1 overexpression A549/PTX cells was promoted and apoptosis rates were not enhanced after shikonin treatment, and p-Akt expression was increased. These results indicated that NEAT1/Akt signaling played an important role in the shikonin against paclitaxel-resistant NSCLC.
Conclusion
Shikonin suppressed the growth of paclitaxel-resistant NSCLC by inducing apoptotic cell death through downregulating the level of NEAT1 and Akt signaling. NEAT1 and Akt signaling downregulation were involved in the shikonin against paclitaxel-resistant NSCLC. Shikonin could be a promising candidate for paclitaxel-resistant NSCLC treatment.
Acknowledgments
Not Applicable.
Abbreviations
- lncRNA
long non-coding RNA
- NEAT1
Nuclear-enriched abundant transcript 1
- NSCLC
Non-small cell lung cancer
- PTX
Paclitaxel
Authors’ contributions
HJ conceived and designed the experiments, FZ, YR, and XZ performed the experiments, ZW analyzed and interpreted the results of the experiments. All authors read and approved the final manuscript.
Funding
This study was supported by a grant from the Hangzhou health science and technology plan (Major) project (2015ZD01), Zhejiang medical and health science and technology project (2016ZDB011), Zhejiang basic public welfare research project (LGF18H160037).
Availability of data and materials
All data generated or analyzed during this study are included in this published article.
Ethics approval
The animal use protocol listed below has been reviewed and approved by the Animal Ethical and Welfare Committee.
Consent for publication
Not Applicable.
Competing interests
Authors declare that they have no competing interests, and all authors should confirm its accuracy.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Zhibing Wu, Email: wu_zhibing@163.com.
Hao Jiang, Email: zjhoncol@163.com.
References
- Adrianzen Herrera D, Ashai N, Perez-Soler R, Cheng H. Nanoparticle albumin bound-paclitaxel for treatment of advanced non-small cell lung cancer: an evaluation of the clinical evidence. Expert Opin Pharmacother. 2019;20:95–102. doi: 10.1080/14656566.2018.1546290. [DOI] [PubMed] [Google Scholar]
- An J, Lv W, Zhang Y. LncRNA NEAT1 contributes to paclitaxel resistance of ovarian cancer cells by regulating ZEB1 expression via miR-194. Onco Targets Ther. 2017;10:5377–5390. doi: 10.2147/OTT.S147586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen D, Lin X, Zhang C, Liu Z, Chen Z, Li Z, Wang J, Li B, Hu Y, Dong B, Shen L, Ji J, Gao J, Zhang X. Dual PI3K/mTOR inhibitor BEZ235 as a promising therapeutic strategy against paclitaxel-resistant gastric cancer via targeting PI3K/Akt/mTOR pathway. Cell Death Dis. 2018;9:123. doi: 10.1038/s41419-017-0132-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong P, Xiong Y, Yue J, Hanley SJB, Kobayashi N, Todo Y, Watari H. Exploring lncRNA-Mediated regulatory networks in endometrial cancer cells and the tumor microenvironment: advances and challenges. Cancers (Basel). 2019:11. 10.3390/cancers11020234. [DOI] [PMC free article] [PubMed]
- Guo HM, Yang SH, Zhao SZ, Li L, Yan MT, Fan MC. LncRNA NEAT1 regulates cervical carcinoma proliferation and invasion by targeting AKT/PI3K. Eur Rev Med Pharmacol Sci. 2018;22:4090–4097. doi: 10.26355/eurrev_201807_15400. [DOI] [PubMed] [Google Scholar]
- Han J, Han B, Wu X, Hao J, Dong X, Shen Q, Pang H. Knockdown of lncRNA H19 restores chemo-sensitivity in paclitaxel-resistant triple-negative breast cancer through triggering apoptosis and regulating Akt signaling pathway. Toxicol Appl Pharmacol. 2018;359:55–61. doi: 10.1016/j.taap.2018.09.018. [DOI] [PubMed] [Google Scholar]
- He J, Sun M, Geng H, Tian S. Long non-coding RNA Linc00518 promotes paclitaxel resistance of the human prostate cancer by sequestering miR-216b-5p. Biol Cell. 2019;111:39–50. doi: 10.1111/boc.201800054. [DOI] [PubMed] [Google Scholar]
- Hong F, Gu W, Jiang J, Liu X, Jiang H. Anticancer activity of polyphyllin I in nasopharyngeal carcinoma by modulation of lncRNA ROR and P53 signalling. J Drug Target. 2019;27:806–811. doi: 10.1080/1061186X.2018.1561887. [DOI] [PubMed] [Google Scholar]
- Hsieh YS, Liao CH, Chen WS, Pai JT, Weng MS. Shikonin inhibited migration and invasion of human lung Cancer cells via suppression of c-met-mediated epithelial-to-Mesenchymal transition. J Cell Biochem. 2017;118:4639–4651. doi: 10.1002/jcb.26128. [DOI] [PubMed] [Google Scholar]
- Jeung YJ, Kim HG, Ahn J, Lee HJ, Lee SB, Won M, Jung CR, Im JY, Kim BK, Park SK, Son MJ, Chung KS. Shikonin induces apoptosis of lung cancer cells via activation of FOXO3a/EGR1/SIRT1 signaling antagonized by p300. Biochim Biophys Acta. 2016;1863:2584–2593. doi: 10.1016/j.bbamcr.2016.07.005. [DOI] [PubMed] [Google Scholar]
- Jiang H, Zhao P, Feng J, Su D, Ma S. Effect of Paris saponin I on radiosensitivity in a gefitinib-resistant lung adenocarcinoma cell line. Oncol Lett. 2014;7:2059–2064. doi: 10.3892/ol.2014.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang H, Zhao PJ, Su D, Feng J, Ma SL. Paris saponin I induces apoptosis via increasing the Bax/Bcl-2 ratio and caspase-3 expression in gefitinib-resistant non-small cell lung cancer in vitro and in vivo. Mol Med Rep. 2014;9:2265–2272. doi: 10.3892/mmr.2014.2108. [DOI] [PubMed] [Google Scholar]
- Li B, Gu W, Zhu X. NEAT1 mediates paclitaxel-resistance of non-small cell of lung cancer through activation of Akt/mTOR signalling pathway. J Drug Target. 2019;27:1061–1067. doi: 10.1080/1061186X.2019.1585437. [DOI] [PubMed] [Google Scholar]
- Li B, Yuan Z, Jiang J, Rao Y. Anti-tumor activity of Shikonin against afatinib resistant non-small cell lung cancer via negative regulation of PI3K/Akt signaling pathway. Biosci Rep. 2018;38. 10.1042/BSR20181693. [DOI] [PMC free article] [PubMed]
- Li X, Fan XX, Jiang ZB, Loo WT, Yao XJ, Leung EL, Chow LW, Liu L. Shikonin inhibits gefitinib-resistant non-small cell lung cancer by inhibiting TrxR and activating the EGFR proteasomal degradation pathway. Pharmacol Res. 2017;115:45–55. doi: 10.1016/j.phrs.2016.11.011. [DOI] [PubMed] [Google Scholar]
- Liu C, Feng Z, Chen T, Lv J, Liu P, Jia L, Zhu J, Chen F, Yang C, Deng Z. Downregulation of NEAT1 reverses the radioactive iodine resistance of papillary thyroid carcinoma cell via miR-101-3p/FN1/PI3K-AKT signaling pathway. Cell Cycle. 2019;18:167–203. doi: 10.1080/15384101.2018.1560203. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Peng W, Wang Z, Fan H. LncRNA NEAT1 impacts cell proliferation and apoptosis of colorectal Cancer via regulation of Akt signaling. Pathol Oncol Res. 2017;23:651–656. doi: 10.1007/s12253-016-0172-4. [DOI] [PubMed] [Google Scholar]
- Ren K, Xu R, Huang J, Zhao J, Shi W. Knockdown of long non-coding RNA KCNQ1OT1 depressed chemoresistance to paclitaxel in lung adenocarcinoma. Cancer Chemother Pharmacol. 2017;80:243–250. doi: 10.1007/s00280-017-3356-z. [DOI] [PubMed] [Google Scholar]
- Shi C, Wang M. LINC01118 modulates paclitaxel resistance of epithelial ovarian Cancer by regulating miR-134/ABCC1. Med Sci Monit. 2018;24:8831–8839. doi: 10.12659/MSM.910932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song S, Du L, Jiang H, Zhu X, Li J, Xu J. Paris Saponin I sensitizes gastric Cancer cell lines to Cisplatin via cell cycle arrest and apoptosis. Med Sci Monit. 2016;22:3798–3803. doi: 10.12659/msm.898232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song T, Yan L, Cai K, Zhao T, Xu M. Downregulation of long noncoding RNA PVT1 attenuates paclitaxel resistance in glioma cells. Cancer Biomark. 2018;23:447–453. doi: 10.3233/CBM-181573. [DOI] [PubMed] [Google Scholar]
- Tang JC, Ren YG, Zhao J, Long F, Chen JY, Jiang Z. Shikonin enhances sensitization of gefitinib against wild-type EGFR non-small cell lung cancer via inhibition PKM2/stat3/cyclinD1 signal pathway. Life Sci. 2018;204:71–77. doi: 10.1016/j.lfs.2018.05.012. [DOI] [PubMed] [Google Scholar]
- Wang F, Yao X, Zhang Y, Tang J. Synthesis, biological function and evaluation of Shikonin in cancer therapy. Fitoterapia. 2019;134:329–339. doi: 10.1016/j.fitote.2019.03.005. [DOI] [PubMed] [Google Scholar]
- Wang H, Fei Z, Jiang H. Polyphyllin VII increases sensitivity to gefitinib by modulating the elevation of P21 in acquired gefitinib resistant non-small cell lung cancer. J Pharmacol Sci. 2017;134:190–196. doi: 10.1016/j.jphs.2017.06.005. [DOI] [PubMed] [Google Scholar]
- Wang J, Ye C, Liu J, Hu Y. UCA1 confers paclitaxel resistance to ovarian cancer through miR-129/ABCB1 axis. Biochem Biophys Res Commun. 2018;501:1034–1040. doi: 10.1016/j.bbrc.2018.05.104. [DOI] [PubMed] [Google Scholar]
- Wang L, Zhang X, Sheng L, Qiu C, Luo R. LINC00473 promotes the Taxol resistance via miR-15a in colorectal cancer. Biosci Rep. 2018c;38. 10.1042/BSR20180790. [DOI] [PMC free article] [PubMed]
- Wang R, Zhang T, Yang Z, Jiang C, Seng J. Long non-coding RNA FTH1P3 activates paclitaxel resistance in breast cancer through miR-206/ABCB1. J Cell Mol Med. 2018;22:4068–4075. doi: 10.1111/jcmm.13679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Hao F, Nan Y, Qu L, Na W, Jia C, Chen X. PKM2 inhibitor Shikonin overcomes the Cisplatin resistance in bladder Cancer by inducing Necroptosis. Int J Biol Sci. 2018;14:1883–1891. doi: 10.7150/ijbs.27854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong W, Huang C, Deng H, Jian C, Zen C, Ye K, Zhong Z, Zhao X, Zhu L. Oncogenic non-coding RNA NEAT1 promotes the prostate cancer cell growth through the SRC3/IGF1R/AKT pathway. Int J Biochem Cell Biol. 2018;94:125–132. doi: 10.1016/j.biocel.2017.12.005. [DOI] [PubMed] [Google Scholar]
- Xu H, Li J, Zhou ZG. NEAT1 promotes cell proliferation in multiple myeloma by activating PI3K/AKT pathway. Eur Rev Med Pharmacol Sci. 2018;22:6403–6411. doi: 10.26355/eurrev_201810_16053. [DOI] [PubMed] [Google Scholar]
- Xu R, Mao Y, Chen K, He W, Shi W, Han Y. The long noncoding RNA ANRIL acts as an oncogene and contributes to paclitaxel resistance of lung adenocarcinoma A549 cells. Oncotarget. 2017;8:39177–39184. doi: 10.18632/oncotarget.16640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang KY, Chen DL. Shikonin inhibits inflammatory response in rheumatoid arthritis synovial fibroblasts via lncRNA-NR024118. Evid Based Complement Alternat Med. 2015;2015:631737. doi: 10.1155/2015/631737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Q, Chen W, Xu Y, Lv X, Zhang M, Jiang H. Polyphyllin I modulates MALAT1/STAT3 signaling to induce apoptosis in gefitinib-resistant non-small cell lung cancer. Toxicol Appl Pharmacol. 2018;356:1–7. doi: 10.1016/j.taap.2018.07.031. [DOI] [PubMed] [Google Scholar]
- Zhang CH, Wang J, Zhang LX, Lu YH, Ji TH, Xu L, Ling LJ. Shikonin reduces tamoxifen resistance through long non-coding RNA uc.57. Oncotarget. 2017;8:88658–88669. doi: 10.18632/oncotarget.20809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, Zheng X, Meng T, You H, Dong Y, Xing J, Chen S. SET protein overexpression contributes to paclitaxel resistance in MCF-7/S cells through PI3K/Akt pathway. J Drug Target. 2017;25:255–263. doi: 10.1080/1061186X.2016.1245307. [DOI] [PubMed] [Google Scholar]
- Zhao P, Jiang H, Su D, Feng J, Ma S, Zhu X. Inhibition of cell proliferation by mild hyperthermia at 43 C with Paris Saponin I in the lung adenocarcinoma cell line PC-9. Mol Med Rep. 2015;11:327–332. doi: 10.3892/mmr.2014.2655. [DOI] [PubMed] [Google Scholar]
- Zhao PJ, Song SC, Du LW, Zhou GH, Ma SL, Li JH, Feng JG, Zhu XH, Jiang H. Paris Saponins enhance radiosensitivity in a gefitinib-resistant lung adenocarcinoma cell line by inducing apoptosis and G2/M cell cycle phase arrest. Mol Med Rep. 2016;13:2878–2884. doi: 10.3892/mmr.2016.4865. [DOI] [PubMed] [Google Scholar]
- Zheng R, Jiang H, Li J, Liu X, Xu H. Polyphyllin II restores sensitization of the resistance of PC-9/ZD cells to Gefitinib by a negative regulation of the PI3K/Akt/mTOR signaling pathway. Curr Cancer Drug Targets. 2017;17:376–385. doi: 10.2174/1568009616666161213141608. [DOI] [PubMed] [Google Scholar]
- Zhu X, Jiang H, Li J, Xu J, Fei Z. Anticancer effects of Paris Saponins by apoptosis and PI3K/AKT pathway in Gefitinib-resistant non-small cell lung Cancer. Med Sci Monit. 2016;22:1435–143‘. doi: 10.12659/msm.898558. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this published article.