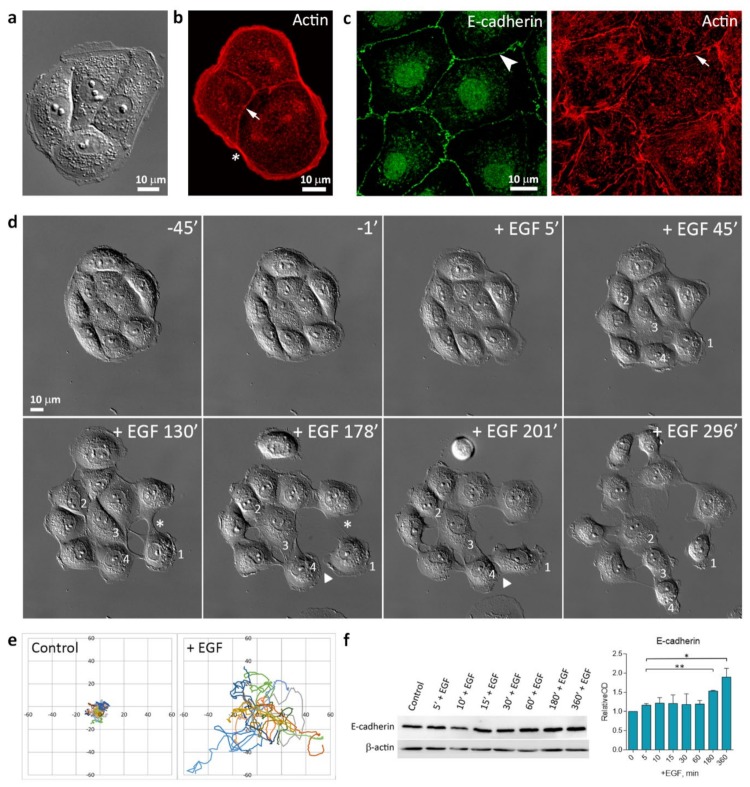
Figure 1.
IAR-20 epithelial cells undergoing epidermal growth factor (EGF)-induced epithelial-mesenchymal transition (EMT). (a) In sparse culture, control IAR-20 epithelial cells form islands. DIC-microscopy. (b) In IAR-20 cells, the actin cytoskeleton is organized into the marginal actin bundle (asterisk) and circumferential actin bundles (arrow). (c) E-cadherin-based AJs (arrowhead) in an IAR-20 monolayer exhibit linear organization and colocalize with circumferential actin bundles (arrow). (d) Scattering of IAR-20 epithelial cells in response to EGF (50 ng/mL). In the control (45 min and 1 min before treatment with EGF), cells are joined into an island with stable cell-cell contacts. Addition of EGF leads to stimulation of protrusive activity at the free cell edges (cell 1), disruption of cell-cell contacts (asterisks), and initiation of cell migration. The migratory cells can form new transient contacts with neighboring cells (arrowheads). Both individual (cell 1) and collective (cells 2, 3, and 4) migration can be observed. Selected frames from Supplementary Video S1. (e) The centroid trajectories of cells migrating for 6 h. (f) Western blot showing the expression levels of E-cadherin in IAR-20 cells treated with EGF. β-actin was used as loading control. Densitometry results are averaged across three independent experiments. Data are presented as mean ± SEM, * p < 0.05, ** p < 0.002.