Abstract
Background
While there is increasing evidence on the safety of artemisinin-based combination therapy (ACT) for the case management of malaria in early pregnancy, little is known about the association between exposure to ACT during the first trimester and the effect on fetal growth.
Methods
Data were analysed from prospective studies of pregnant women enrolled in Mozambique, Burkina Faso and Kenya designed to determine the association between anti-malarial drug exposure in the first trimester and pregnancy outcomes, including low birth weight (LBW) and small for gestational age (SGA). Exposure to anti-malarial drugs was ascertained retrospectively by record linkage using a combination of data collected from antenatal and adult outpatient clinic registries, prescription records and self-reported medication usage by the women. Site-level data synthesis (fixed effects and random effects) was conducted as well as individual-level analysis (fixed effects by site).
Results
Overall, 1915 newborns were included with 92 and 26 exposed to ACT (artemether–lumefantrine) and quinine, respectively. In Burkina Faso, Mozambique and Kenya at recruitment, the mean age (standard deviation) was 27.1 (6.6), 24.2 (6.2) and 25.7 (6.5) years, and the mean gestational age was 24.0 (6.2), 21.2 (5.7) and 17.9 (10.2) weeks, respectively. The LBW prevalence among newborns born to women exposed to ACT and quinine (QNN) during the first trimester was 10/92 (10.9%) and 7/26 (26.9%), respectively, compared to 9.5% (171/1797) among women unexposed to any anti-malarials during pregnancy. Compared to those unexposed to anti-malarials, ACT and QNN exposed women had the pooled LBW prevalence ratio (PR) of 1.13 (95% confidence interval (CI) 0.62–2.05, p-value 0.700) and 2.03 (95% CI 1.09–3.78, p-value 0.027), respectively. Compared to those unexposed to anti-malarials ACT and QNN-exposed women had the pooled SGA PR of 0.85 (95% CI 0.50–1.44, p-value 0.543) and 1.41 (95% CI 0.71–2.77, p-value 0.322), respectively. Whereas compared to ACT-exposed, the QNN-exposed had a PR of 2.14 (95% CI 0.78–5.89, p-value 0.142) for LBW and 8.60 (95% CI 1.29–57.6, p-value 0.027) for SGA. The level of between sites heterogeneity was moderate to high.
Conclusion
ACT exposure during the first trimester was not associated with an increased occurrence of LBW or SGA. However, the data suggest a higher prevalence of LBW and SGA for children born to QNN-exposed pregnancies. The findings support the use of ACT (artemether–lumefantrine) for the treatment of uncomplicated malaria during the first trimester of pregnancy.
Keywords: Low birth weight, Small for gestational age, Prospective cohort, Artemisinins, Sub-Saharan Africa, Pharmacovigilance
Background
Malaria in pregnancy is an important public health problem in malaria-endemic countries where pregnant women and their offspring have a higher risk of infection and sequelae. Malaria infection during pregnancy is associated with maternal anaemia and intra-uterine growth restriction (IUGR), leading to poor pregnancy outcomes such as low birth weight (LBW) and small for gestational age (SGA) [1, 2]. LBW is defined as a birth weight of live-born infant of less than 2500 g regardless of gestational age [3]. The SGA is the weight below the 10th percentile of weight for the gestational age. Malaria accounts for 14 to 25% of LBW in sub-Saharan Africa [4–6]. LBW is a result of a short gestational period, IUGR or a combination of both processes, and contributes globally to high neonatal and infant mortality and morbidity [1, 3]. Particularly in sub-Saharan Africa (SSA), LBW neonates are nine times more likely to die in the 1st month of life than a normal-weight baby [7, 8]. Infants who are growth-restricted experience higher rates of fetal and infant death, birth asphyxia, hypothermia, hypoglycaemia, meconium aspiration, and long-term neurological impairment [9].
Cohort studies in malaria-endemic areas show an association between malaria infection at an earlier gestational age, i.e., in the first trimester, and adverse fetal growth, pregnancy outcome, duration of pregnancy, and placental weight at term [10, 11]. Walker et al. estimated that 65.2% (95% CI 60.9–70.0) of placental malaria infections occur at the end of the first trimester, and called for targeting this period for prevention [4]. Multiple measures to prevent and treat malaria and its complications during pregnancy are recommended. These preventive measures presently include the use of long-lasting insecticide-treated nets (LLINs), administration of intermittent preventive treatment in pregnancy with sulfadoxine–pyrimethamine (IPTp-SP), and rapid diagnosis and management of malaria cases [12]. The World Health Organization (WHO) currently recommends the use of artemisinin-based combination therapy (ACT) for the treatment of uncomplicated P. falciparum malaria in pregnant women in their second or third trimester. ACT is presently only recommended by WHO in the first trimester if quinine cannot be used or in case of severe malaria where the benefit outweighs the potential risk. Quinine is recommended for uncomplicated malaria in the first trimester of pregnancy [13]. However, quinine therapy has been documented to be associated with low adherence due to tolerability (occurrence of tinnitus, hearing impairment, dizziness, and postural hypotension) and need for multiple doses (3 times a day) for 7 days [12, 14].
Findings from preclinical studies have reported that artemisinins are embryotoxic and teratogenic in multiple animal species [15–18]. In settings where malaria is endemic and ACT is highly available in the market, it is likely that a woman will be inadvertently or intentionally exposed in the first trimester because, for example, women and health care providers may be unaware of a woman’s pregnancy status at the time of prescribing an anti-malarial [19–21]. Therefore, it is imperative to assess the safety of first trimester ACT exposures for a broad range of pregnancy outcomes. The assessment of safety of anti-malarial drug use during early pregnancy (ASAP) study was a multi-country prospective cohort study of pregnant women to evaluate whether ACT exposure in early pregnancy increases the risk of miscarriage, stillbirths, congenital malformations, and LBW when compared to current therapeutic options [22]. The risk of miscarriage, stillbirths and congenital anomalies has been reported elsewhere [23, 24]. This analysis aimed to evaluate the association between ACT exposure during the first trimester of pregnancy and LBW and SGA among the offspring of pregnant women.
Methods
Study design
ASAP was a prospective cohort study of pregnant women, conducted under a single multi-centre study protocol at three SSA sites as part of Malaria in Pregnancy Consortium activities as previously published [22]. The sites were located in Asembo-Siaya County, Kenya Nanoro, Burkina Faso; and, Manhiça District, Mozambique. In all three sites, malaria transmission is intense and P. falciparum is the main species. All three ASAP sites have a health and demographic surveillance system (HDSS) [25–28]. Within their defined communities, HDSS sites ensure recording of all vital status (births, deaths and migration) and other demographic events such as pregnancy by full enumeration of the population at least twice a year using community key informants [28]. Additional recruitment and data collection strategies were employed for the ASAP study to identify pregnancies, anti-malarial exposures, determine gestational age at the time of exposure to anti-malarials, monitor pregnancy outcomes, and systematically assess infant outcomes. The emphasis was placed on identifying first-trimester pregnancies by identifying and recruiting women as early as possible in pregnancy.
Pregnant women were identified through household visits, community key informants, and at antenatal care visits in a health facility within the HDSS catchment area. All identified pregnant women were invited to the antenatal care (ANC) clinics and assessed for eligibility. Following written consent, baseline information was collected and data entered into a pregnancy register. Electronic records from outpatient and inpatient visits were recorded through the HDSS platform and linked to the study records to identify possible exposure to ACT and other anti-malarials during the first trimester of the pregnancy. For this analysis, only singleton newborns with birth weight collected within the first 7 days of life are included. Multiple methods were used for the ascertainment of gestational age, including date of last menstrual period, fundal height, ultrasound, and Ballard Score as explained elsewhere [22]. Women were encouraged to deliver at the closest health facility where systems were in place to identify and link records. Also, deliveries occurring outside the health facility were actively identified by close monitoring lists of probable delivery and home-based visits or by notification from village informants and traditional birth attendants (TBAs) whereby a study staff team assessed cases delivered at home as soon as possible.
Anti-malarial exposure group definition
The ascertainment of drug exposure included both prospective and retrospective self-reported medication usage and linkage to treatment records at local health facilities of drug prescribing and dispensing. The process of drug identification, self-reporting and record linkage with health facility data has been described elsewhere [22]. Two or more sources were required to confirm anti-malarial exposure; unconfirmed exposures were excluded. The treatments of interest were ACT or quinine during the first trimester of pregnancy, i.e., weeks 2 to 13 (inclusive) from the last menstrual period. Artemether–lumefantrine was the only ACT used in the three sites.
Outcomes
The present analysis is restricted to birth weight, LBW and SGA among live births. LBW was defined as a birth weight of < 2500 g collected within the first 24 h of life [3]. Birth weights taken between 24 and 48 h and 48 to 168 h after delivery were corrected by a factor + 2% and + 4%, respectively, to obtain the estimated weight at birth [29–32]. SGA was defined as a corrected birth weight below the 10th percentile of weight for the gestational age using the INTERGROWTH reference curves [33, 34]. Following a suggestion by the reviewers, the analysis of prematurity was conducted as a supplemental analysis. Prematurity was defined as a live birth before 37 completed weeks of gestation [35].
Statistical methods
Baseline characteristics were compared by site and by anti-malarial exposure to assess imbalances across sites and exposure groups. Frequencies were used for categorical variables and for numeric variables means and standard deviations. The prevalence of LBW and of SGA and the mean birth weight by exposure group in each site were computed. As measure of association, the mean difference (MD) was used for birth weight outcome and prevalence ratios (PR) for the prevalence of LBW and SGA. Two types of data synthesis of effect were performed. One is the site-level aggregated through the use of both fixed effects and random effects meta-analysis. Inverse variance based weights were used and for zero events study, 0.5 continuity correction was employed. To assess the heterogeneity between sites the I2 statistic was used [36].
The other data synthesis is based on patient-level data whereby linear (for MD) and log-binomial (for PR) regressions were used. All models included the site indicator as covariates. The age at recruitment, gravidity, marital status, and education level were included in the adjusted analysis. The log-binomial regressions had convergence failure and data spasticity issues. Thus, a Bayesian implementation of the log-binomial with posterior distributions approximated through Markov Chain Monte Carlo (MCMC) was performed in JAGS software [37–39]. Uninformative priors for the coefficients were set as normal distributions with 0 mean and 1000 variance, the MCMC were run with 3 chains of 100,000 iterations with 10,000 iterations as burn-in, and 50 as the thin steps. The convergence was assessed through the Gelman and Rubin’s diagnostic (Rhat < 1.2), review of the traceplots (assess the mixture of the simulations) and autocorrelation of the iterations [37, 38]. The significance level was set at 5%. Additional analyses and data preparation were performed on Stata v14 (StataCorp. 2015. Stata: Release 14. Statistical Software. College Station, TX, USA: StataCorp LP), R [40]. The INTERGROWTH-21st software was used to produce the percentiles of weights for gestation age [33].
Ethical approval
The protocol was reviewed and approved by the Ethical Review Boards of the Kenya Medical Research Institute (KEMRI), US Centers for Disease Control and Prevention (CDC), National Bioethics Committee in Mozambique, Centre Muraz Institutional Ethics committee and National Ethics committee in Burkina Faso, Liverpool School of Tropical Medicine in the UK, and the Institutional Review Board of the University of Washington.
Results
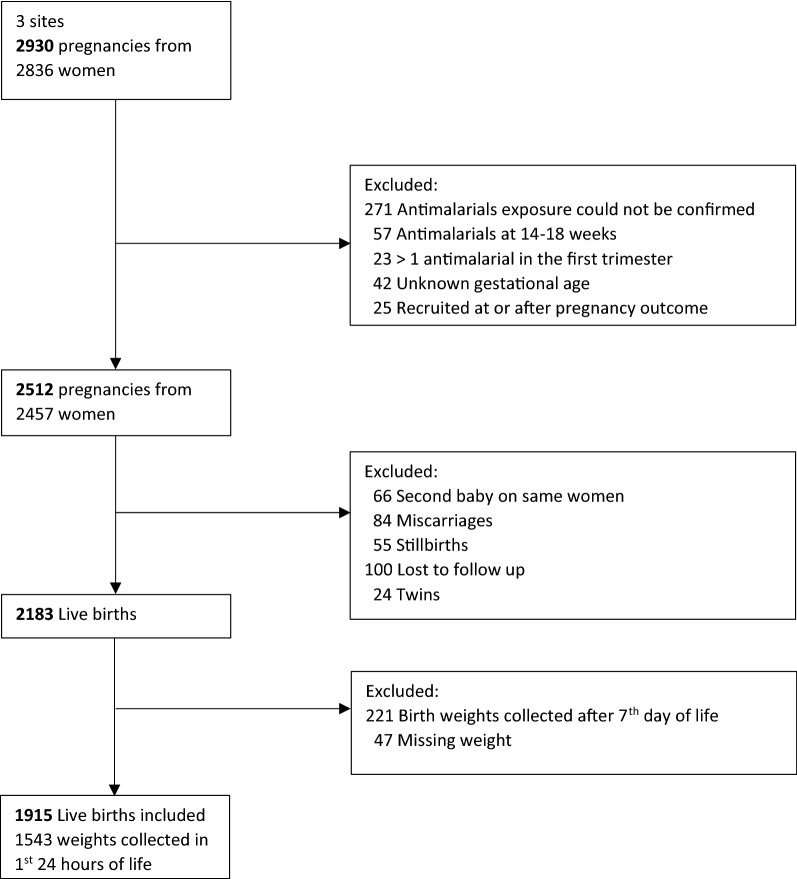
A total of 2836 recruited women representing 2930 pregnancy outcomes and 1915 live births were included in the analysis (Fig. 1). Excluded from the analysis were records that lacked confirmation of anti-malarial exposure (271), birth weights collected past 7th day of life (221), those lost to follow-up (100), either miscarriages or stillbirths (139), either twins gestation or second pregnancy follow-up (90) and other reasons (194) (Fig. 1). Each site contributed similar numbers of subjects for analysis, 656, 669 and 590 live births from Burkina Faso, Mozambique and Kenya, respectively. Of the live births included in the analysis 80.6% (1543) had birth weight collected in the first day of life with across site values of 47.8% (282/590), 92.2% (605/656), and 98.1% (656/669) in Kenya, Burkina Faso and Mozambique, respectively. The final sample in terms of anti-malarial exposure during the first trimester of pregnancy included 1797 live births not exposed to an anti-malarial, 92 exposed to ACT and 26 exposed to quinine.
Fig. 1.
Flow chart of the recruited participants in ASAP cohort included in this analysis, 2015
Baseline characteristics
Demographic characteristics among participants are reported in Tables 1 and 2 per site and exposures, respectively. The average age at recruitment of the pregnant women in Burkina Faso was 27.1 years, which is almost 2 years older than in Kenya (25.7) and 3 years older than in Mozambique (24.2). There was 30.8% primigravidae in the quinine-exposed group compared to 25.0% among those who were ACT-exposed and 21.0% among the unexposed to anti-malarials group. Approximately 21.7% of deliveries were in-home deliveries in Kenya compared to 11.7% and 5.2% in Burkina Faso and Mozambique, respectively.
Table 1.
Baseline characteristics of pregnancies included for low birth weight and small for gestational analysis per study site, ASAP cohort, 2015
| Characteristic | Burkina Faso | Mozambique | Kenya |
|---|---|---|---|
| N (%) | N (%) | N (%) | |
| Total participants | 656 (100) | 669 (100) | 590 (100) |
| Age at recruitment (years) | |||
| Range | 15.0–49.0 | 12.4–41.9 | 15.0–45.0 |
| Mean (SD) | 27.1 (6.64) | 24.2 (6.23) | 25.7 (6.48) |
| < 20 | 94 (14.3) | 207 (30.9) | 117 (19.8) |
| 20–24 | 158 (24.1) | 184 (27.5) | 160 (27.1) |
| 25–29 | 171 (26.1) | 152 (22.7) | 147 (24.9) |
| 30+ | 233 (35.5) | 126 (18.8) | 166 (28.1) |
| Gravidity | |||
| Primigravida | 113 (17.2) | 178 (26.6) | 118 (20.0) |
| 1–3 pregnancies | 307 (46.8) | 395 (59.0) | 297 (50.3) |
| 4 or more pregnancies | 236 (36.0) | 93 (13.9) | 175 (29.7) |
| Missing | 0 (0.0) | 3 (0.4) | 0 (0.0) |
| Marital status | |||
| Single | 9 (1.4) | 240 (35.9) | 125 (21.2) |
| Married or cohabiting | 647 (98.6) | 428 (64.0) | 465 (78.8) |
| Missing | 0 (0.0) | 1 (0.1) | 0 (0.0) |
| Education | |||
| Primary not completed | 0 (0.0) | 96 (14.3) | 268 (45.4) |
| Primary completed | 656 (100.0) | 330 (49.3) | 276 (46.8) |
| Secondary completed | 0 (0.0) | 241 (36.0) | 46 (7.8) |
| Missing | 0 (0.0) | 2 (0.3) | 0 (0.0) |
| HIV status | |||
| Negative | 633 (96.5) | 442 (66.1) | 445 (75.4) |
| Positive | 4 (0.6) | 164 (24.5) | 125 (21.2) |
| Missing | 19 (2.9) | 63 (9.4) | 20 (3.4) |
| Gestational age at recruitment (weeks) | |||
| Mean (SD) | 24.0 (6.15) | 21.2 (5.65) | 17.9 (10.20) |
| Median (IQR) | 24.1 (19.6–28.7) | 21.0 (17.0–25.0) | 15.9 (9.1–26.0) |
| Place of delivery | |||
| Health facility | 579 (88.3) | 611 (91.3) | 424 (71.9) |
| Home | 77 (11.7) | 35 (5.2) | 128 (21.7) |
| Other | 0 (0.0) | 23 (3.4) | 38 (6.4) |
Table 2.
Baseline characteristics by exposure level (no exposure, exposed to ACT, exposed to quinine), ASAP cohort, 2015
| Characteristic | No anti-malarial use in first trimester | Confirmed ACT use in first trimester | Confirmed quinine use in first trimester | All pregnancies |
|---|---|---|---|---|
| N (%) | N (%) | N (%) | N (%) | |
| Total | 1797 (100) | 92 (100) | 26 (100) | 1915 (100) |
| Country | ||||
| Burkina Faso | 603 (33.6) | 31 (33.7) | 22 (84.6) | 656 (34.3) |
| Mozambique | 643 (35.8) | 22 (23.9) | 4 (15.4) | 669 (34.9) |
| Kenya | 551 (30.7) | 39 (42.4) | 0 (0.0) | 590 (30.8) |
| Age at recruitment (years) | ||||
| Range | 12.4–49.0 | 15.0–45.0 | 17.0–36.0 | 12.4–49.0 |
| Mean (SD) | 25.7 (6.55) | 25.6 (6.63) | 24.7 (6.04) | 25.6 (6.54) |
| < 20 | 394 (21.9) | 17 (18.5) | 7 (26.9) | 418 (21.8) |
| 20–24 | 465 (25.9) | 30 (32.6) | 7 (26.9) | 502 (26.2) |
| 25–29 | 444 (24.7) | 19 (20.7) | 7 (26.9) | 470 (24.5) |
| 30+ | 494 (27.5) | 26 (28.3) | 5 (19.2) | 525 (27.4) |
| Gravidity | ||||
| Primigravida | 378 (21.0) | 23 (25.0) | 8 (30.8) | 409 (21.4) |
| 1–3 pregnancies | 943 (52.5) | 45 (48.9) | 11 (42.3) | 999 (52.2) |
| 4 or more pregnancies | 473 (26.3) | 24 (26.1) | 7 (26.9) | 504 (26.3) |
| Missing | 3 (0.2) | 0 (0.0) | 0 (0.0) | 3 (0.2) |
| Marital status | ||||
| Single | 354 (19.7) | 20 (21.7) | 0 (0.0) | 374 (19.5) |
| Married or cohabiting | 1442 (80.2) | 72 (78.3) | 26 (100.0) | 1540 (80.4) |
| Missing | 1 (0.1) | 0 (0.0) | 0 (0.0) | 1 (0.1) |
| Education | ||||
| Primary not completed | 348 (19.4) | 16 (17.4) | 0 (0.0) | 364 (19.0) |
| Primary completed | 1176 (65.4) | 62 (67.4) | 24 (92.3) | 1262 (65.9) |
| Secondary completed | 271 (15.1) | 14 (15.2) | 2 (7.7) | 287 (15.0) |
| Missing | 2 (0.1) | 0 (0.0) | 0 (0.0) | 2 (0.1) |
| HIV status | ||||
| Negative | 1427 (79.4) | 70 (76.1) | 23 (88.5) | 1520 (79.4) |
| Positive | 279 (15.5) | 13 (14.1) | 1 (3.8) | 293 (15.3) |
| Missing | 91 (5.1) | 9 (9.8) | 2 (7.7) | 102 (5.3) |
| Gestational age at recruitment (weeks) | ||||
| Mean (SD) | 21.3 (7.39) | 18.8 (8.72) | 16.8 (5.56) | 21.2 (7.88) |
| Gestational age at delivery (weeks) | ||||
| Mean (SD) | 38.9 (1.80) | 39.0 (1.77) | 38.8 (1.65) | 38.9 (1.79) |
SD standard deviation
Birth weight outcome
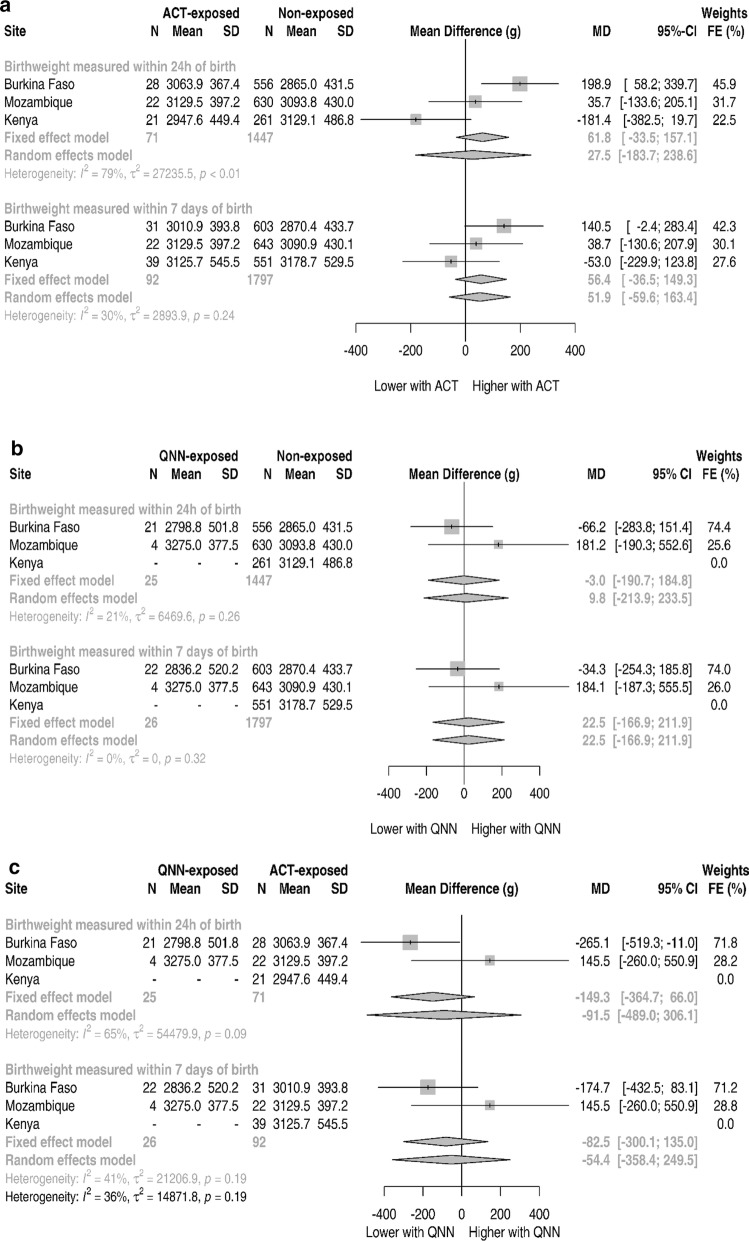
Newborns at Burkina Faso’s site had on average a weight of 2875.9 g, compared to Mozambique’s and Kenya’s site with 3093.3 g and 3175.2 g, respectively. Figure 2 and Additional file 1: Table S4 show the aggregated data synthesis of mean differences of weights. For birth weight collected in the 1st week of life, the pooled fixed effects mean-difference between ACT and non-exposed group was found to be 56.4 g (95% CI − 36.5 to 149.3, p-value 0.234) whereas for random-effects it was 51.9 g (95% CI − 59.6 to 163.4, p-value 0.362), both values are not significantly different from the null. These results did not appreciably differ from those collected only within the first 24 h of life (Fig. 2a and Additional file 1: Table S4). For the comparison between quinine and non-exposed (Fig. 2b and Additional file 1: Table S5), Kenya could not be included due to the lack of quinine exposure, the contribution of the remaining sites gave both pooled fixed and random effects of a non-significant association of 22.5 g (95% CI − 166.9 to 211.9, p-value 0.816) for birth weights collected in the first week. When examining just the birth weights collected within the first day of life the differences reduce in magnitude to just − 3.0 g and 9.8 g for fixed-effects and random-effects, respectively. Figure 2c and Additional file 1: Table S6 show the comparison between quinine and ACT exposures. All associations are in the direction of higher birth weights for ACT although not statistically significant with the fixed-effects of − 82.5 g (95% CI − 300.1 to 135.0, p-value 0.457) for birth weights collected in the first week. Except for quinine versus non-exposed comparison, the heterogeneity of all these associations is between moderate to high (I2 above 25%).
Fig. 2.
Weight at birth mean difference in grams for different sites and random-effects pooled association, ASAP study
Table 3 shows the mean difference for individual-level analysis. For the quinine versus ACT comparison using the birth weights collected within the first week of life the unadjusted mean difference was 108.0 g (95% CI − 341.2 to 125.1, p-value − 0.361) in favour of the ACT group. This MD increased slightly in favor of the ACT with a value of − 167.7 g (95% CI − 391.6 to 56.1, p-value 0.140) when adjusted for other covariates.
Table 3.
Adjusted associations for mean weight at birth
| Mean difference in g (95% CI) | ||||
|---|---|---|---|---|
| Unadjusteda | p-value | Adjustedb | p-value | |
| Birth weights measured within 24 h of birth (N) | 1543 | 1533 | ||
| No exposure | 0 (reference) | 0 (reference) | ||
| Artemisinin | 37.8 (− 67.7; 143.3) | 0.482 | 29.6 (− 62.0; 121.3) | 0.526 |
| Quinine | − 32.1 (− 207.9; 143.8) | 0.721 | − 29.0 (− 181.6; 123.6) | 0.709 |
| Birth weights measured within 7 days of birth (N) | 1915 | 1905 | ||
| No exposure | 0 (reference) | 0 (reference) | ||
| Artemisinin | 34.9 (− 62.7; 132.5) | 0.483 | 40.0 (− 47.8; 127.8) | 0.371 |
| Quinine | − 4.2 (− 185.7; 177.2) | 0.964 | − 3.6 (− 166.8; 159.6) | 0.965 |
| Quinine vs artemisinin (N) | 118 | 118 | ||
| Artemisinin | 0 (reference) | 0 (reference) | ||
| Quinine | − 108.0 (− 341.2; 125.1) | 0.361 | − 167.7 (− 391.6; 56.1) | 0.140 |
aUnadjusted regression includes dummy indicators for site
bAdjusted for site, age at recruitment, gravidity, marital status and education level
LBW outcome
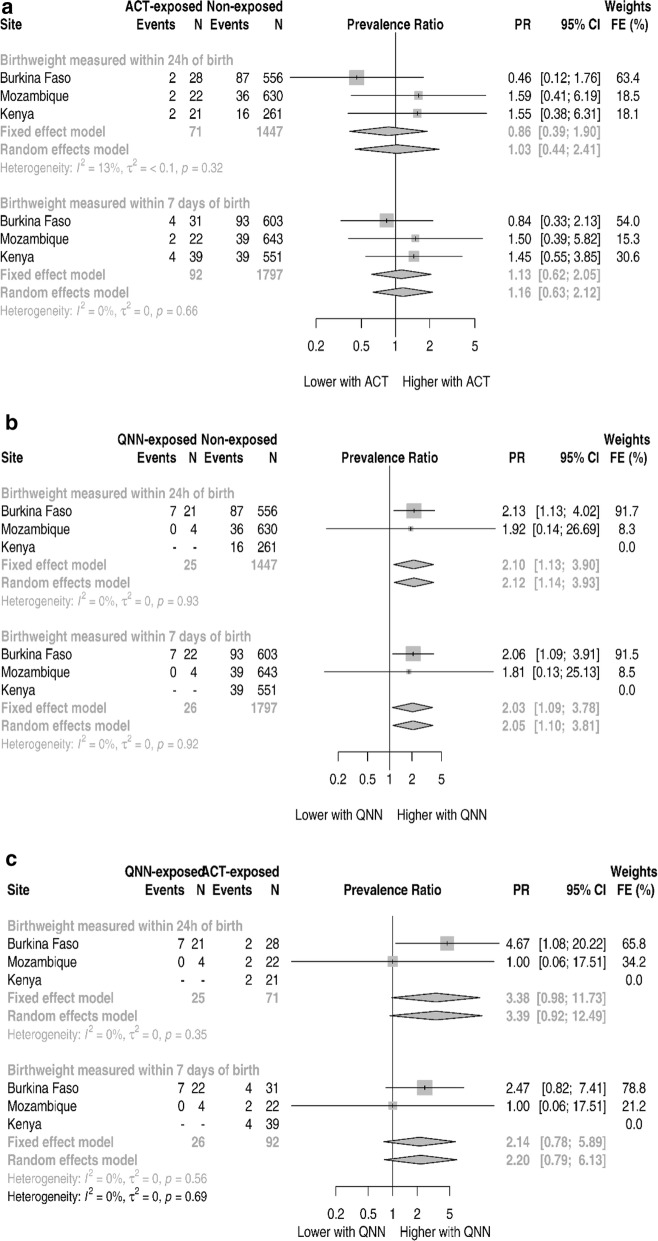
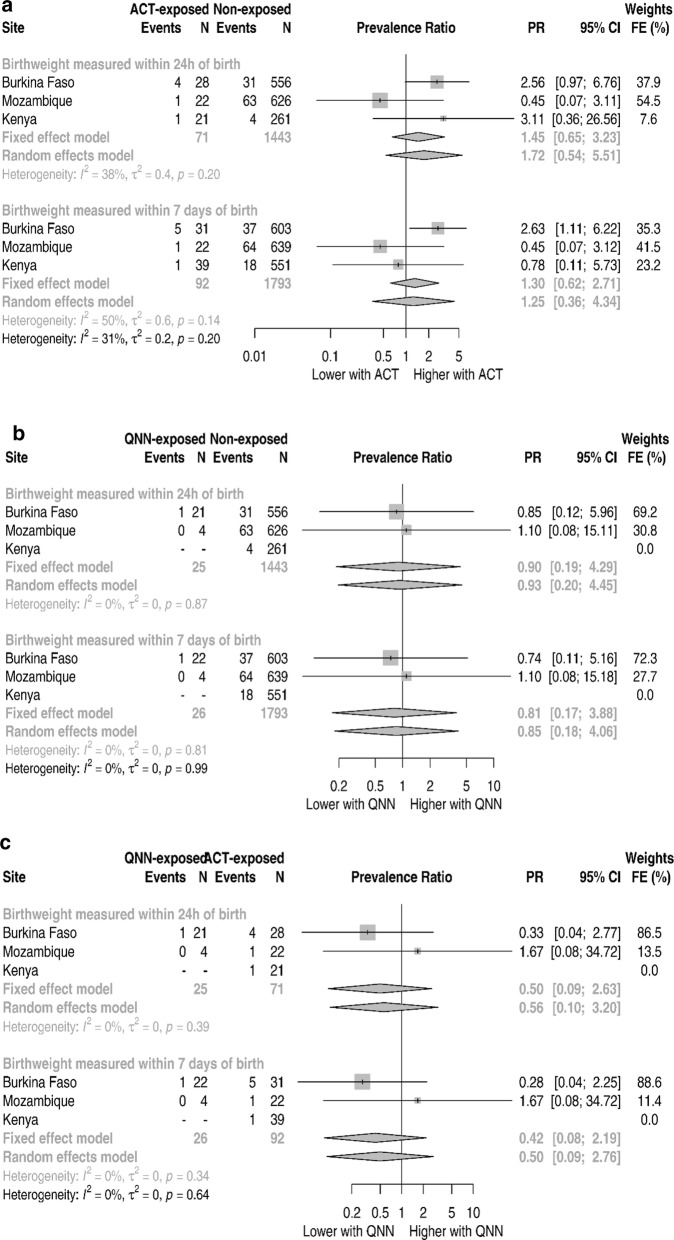
The prevalence of LBW was 15.9% (104/656), 6.1% (41/669) and 7.2% (43/590) in Burkina Faso, Mozambique and Kenya, respectively. Figure 3 shows the pooling of the site-aggregated data. Relative to non-exposed, newborns exposed to ACT (Fig. 3a and Additional file 1: Table S7) with weight collected in the first week of life had 1.13 (95% CI 0.62–2.05, p-value 0.700) times higher prevalence of LBW through the fixed-effects estimate. A similar magnitude was found through the random-effects estimate. In Kenya, there was no quinine exposure. Relative to non-exposed newborns, quinine exposed had 2.03 (95% CI 1.09–3.78, p-value 0.027) times higher prevalence of LBW through the fixed-effects estimate (Fig. 3b and Additional file 1: Table S8). A similar association was found through the random-effects. Compared to ACT, the quinine-exposed newborns had a non-significant 2.14 (95% CI 0.78–5.89, p-value 0.142) times higher prevalence of LBW through the fixed-effects analysis. Similar results were obtained on the random-effects analysis. Furthermore, this association becomes stronger, although marginally significant when restricting to weights collected within the first 24 h of birth (Fig. 3c and Additional file 1: Table S9). A small heterogeneity was observed for LBW aggregated analysis with all I2 below 15%.
Fig. 3.
ASAP site specific and pooled low birth weight prevalence ratios
Table 4 shows the results of the individual-level analysis for the LBW outcome. There were no differences in the prevalence of LBW for pregnancies treated with quinine compared to those treated with an ACT in the first trimester (unadjusted PR 2.03, 95% CI 0.73–5.86, p-value 0.172). The association reduced to 1.70 (95% CI 0.60–5.17, p-value 0.321) with adjustment for covariates.
Table 4.
Adjusted associations for low birth weight and small for gestational age
| Prevalence-ratio (95% CIc) | ||||
|---|---|---|---|---|
| Unadjusteda | p-value | Adjustedb | p-value | |
| LBW | ||||
| Birth weights measured within 24 h of birth (N) | 1543 | 1533 | ||
| No exposure | 1 (reference) | 1 (reference) | ||
| Artemisinin | 0.80 (0.31; 1.62) | 0.575 | 0.91 (0.36; 1.81) | 0.809 |
| Quinine | 1.95 (0.87; 3.42) | 0.099 | 2.17 (1.00; 3.65) | 0.049 |
| Birth weights measured within 7 days of birth (N) | 1915 | 1905 | ||
| No exposure | 1 (reference) | 1 (reference) | ||
| Artemisinin | 1.08 (0.55; 1.85) | 0.806 | 1.19 (0.61; 2.00) | 0.579 |
| Quinine | 1.87 (0.85; 3.27) | 0.109 | 2.13 (0.98; 3.59) | 0.055 |
| Quinine vs artemisinin | ||||
| Artemisinin | 1 (reference) | 1 (reference) | ||
| Quinine with no Kenya data (N = 79) | 2.03 (0.73; 5.86) | 0.172 | 1.70 (0.60; 5.17) | 0.321 |
| Quinine with Kenya data (N = 118) | 3.18 (1.02; 11.56) | 0.047 | 1.93 (0.72; 5.68) | 0.188 |
| SGA | ||||
| Birth weights measured within 24 h of birth (N) | 1520 | 1514 | ||
| No exposure | 1 (reference) | 1 (reference) | ||
| Artemisinin | 0.76 (0.37; 1.31) | 0.363 | 0.83 (0.41; 1.42) | 0.516 |
| Quinine | 1.31 (0.54; 2.43) | 0.495 | 1.19 (0.50; 2.17) | 0.660 |
| Birth weights measured within 7 days of birth (N) | 1888 | 1882 | ||
| No exposure | 1 (reference) | 1 (reference) | ||
| Artemisinin | 0.81 (0.43; 1.31) | 0.417 | 0.78 (0.43; 1.25) | 0.337 |
| Quinine | 1.29 (0.55; 2.40) | 0.514 | 1.11 (0.47; 2.02) | 0.789 |
| Quinine vs artemisinin | ||||
| Artemisinin confirmed | 1 (reference) | 1 (reference) | ||
| Quinine confirmed with no Kenya data (N = 78) | 13.75 (2.16; 409.72) | 0.003 | 13.11 (1.82; 441.93) | 0.004 |
| Quinine confirmed with Kenya data (N = 116) | 14.44 (2.18; 400.29) | 0.004 | 12.04 (1.84; 330.23) | 0.008 |
LBW low birth weight, SGA small for gestational age
aUnadjusted regression includes dummy indicators for site
bAdjusted for site, age at recruitment, gravidity, marital status and education level
c95%CI—95% credible interval based on § posterior distribution replication
Small for gestational age and prematurity
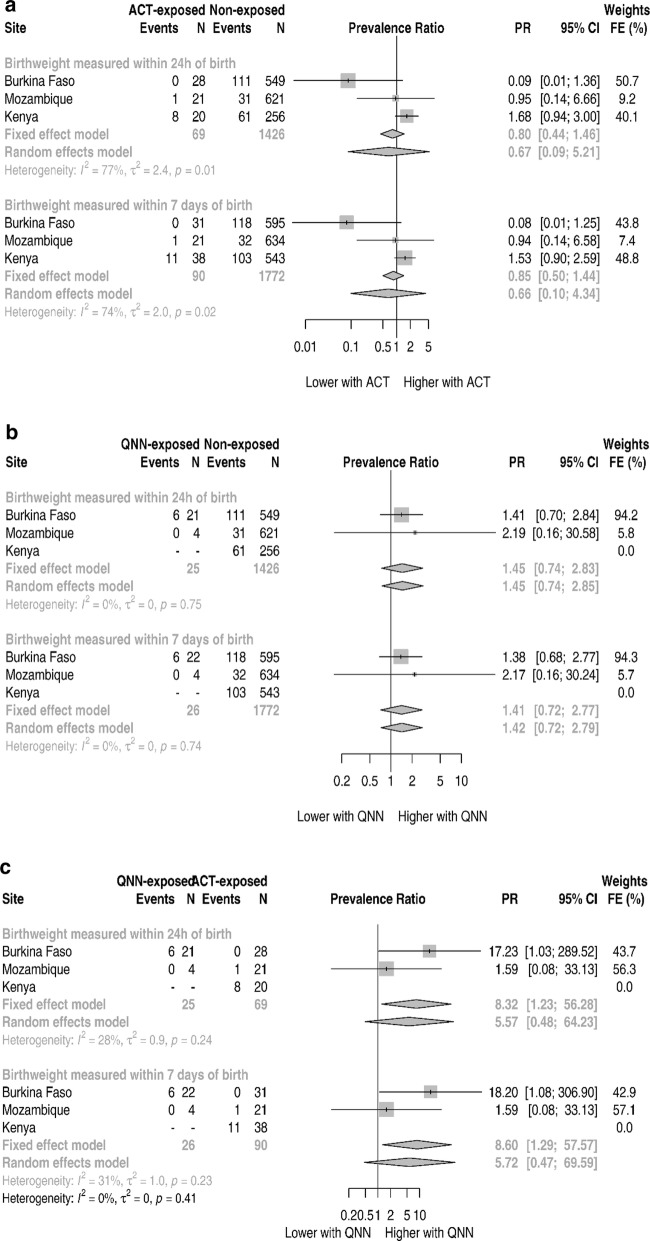
The prevalence of SGA was 19.1% (124/648), 5.0% (33/659) and 19.6% (114/581), respectively in Burkina Faso, Mozambique and Kenya. Figure 4 and Additional file 1: Tables S10–S12 show the forest plots for the site aggregated analysis for SGA prevalence ratio. Across the three sites, quinine-exposed newborns had a 41% (1.41, 95% CI 0.72–2.77, p-value 0.322) relatively higher prevalence of SGA than non-exposed under the fixed-effects pooling (Fig. 4b and Additional file 1: Table S11). The magnitude of the association increased to 8.60 (95% CI 1.29–57.57) for the quinine versus ACT comparison (Fig. 4c). Pregnancies treated with ACT during the first trimester were not associated with an increased prevalence of SGA compared with pregnancies not treated with an anti-malarial (PR 0.85, 95% CI 0.50–1.44) (Fig. 4a).
Fig. 4.
ASAP site specific and pooled small for gestation age prevalence ratios
Table 4 shows the individual level based analysis for the SGA prevalence. On the unadjusted analysis, quinine-exposed compared to ACT-exposed newborns had 13.75 times higher prevalence of SGA with a wide confidence interval (2.16–409.72) not including the null, with similar results under the adjusted analysis.
The prevalence of prematurity was 6.6% (43/656), 9.8% (65/665) and 3.2% (19/590) respectively in Burkina Faso, Mozambique and Kenya. Figure 5 and Additional file 1: Tables S13–S15 show the forest plots for the site aggregated analysis for prematurity prevalence ratio. Across the all sites, under the fixed-effects pooling none of the associations between either ACT- or quinine-exposed newborns, and prematurity reached statistical significance (Fig. 5b, c and Additional file 1: Tables S14 and S15). The individual level based analysis did not meaningfully change the results (Additional file 1: Table S16).
Fig. 5.
ASAP site specific and pooled prematurity prevalence ratios
Discussion
No evidence was found of an increased risk of LBW, SGA or prematurity among pregnancies with a confirmed exposure to ACT for malaria treatment during the first trimester of pregnancy is not associated with an increased risk of LBW, SGA and prematurity compared to newborns unexposed to anti-malarials. The findings on ACT use in early pregnancy and fetal growth add reassurance to the previously documented safety profile of ACT used during early pregnancy in many SSA countries [19, 41–43]. A meta-analysis that incorporated studies in SSA and Asia provided further evidence on the safety of ACT and the risk of miscarriages, stillbirth and congenital anomalies [24].
While infrequently used in this study, quinine exposure in the first trimester of pregnancy was associated with a twice higher prevalence of LBW when compared to the unexposed to anti-malarials group or ACT-exposed. This finding should be interpreted with caution since it may be due to small numbers in the sample or to inadequately treated malaria resulting from the known drawbacks with oral quinine related to poor tolerability characterized by tinnitus, hearing impairment, dizziness, and postural hypotension and need for multiple doses (3 times a day) for 7 days [14, 44], creating a channelling bias [45]. Furthermore, Mosha et al. reported a trend towards a protective but non-significant association on LBW of quinine exposure in the first trimester of gestation [41].
There was no evidence of an increased prevalence of SGA with exposure to ACT. This finding is consistent with Manyando et al. who reported a similar prevalence of SGA among newborns exposed to ACT compared to exposed to sulfadoxine–pyrimethamine (9.0 vs 7.7%) in a cohort in Zambia and using a more restrictive definition of SGA (weight for gestational age below the fifth percentile). Also, there was no evidence of an increased prevalence of pre-term with exposure to ACT. However, quinine is possibly associated with a higher prevalence of SGA compared to those unexposed to anti-malarials or ACT in the first trimester of gestation. Previous literature comparing these risks is limited; this is the first study to investigate this association using the recently available world reference growth curves during gestation [19, 33].
Although malaria in pregnancy is common in SSA, it can be easily confused with many other febrile diseases that may occur during pregnancy. It has been shown in Mozambique that only 27% of pregnant women presenting with fever had parasitaemia detected [46]. Moreover, there might be a high self-perceived risk of malaria among pregnant women in settings such as the ones where the study was conducted. All this may lead to the treatment of unconfirmed malaria with ACT given its higher availability [47]. Particularly, in pregnancy when early weeks of gestation are not disclosed due to cultural reasons or its unawareness, accidental anti-malarial exposure may occur more frequently than some studies have counted because only women with documented malaria are included [19, 41]. This calls for strengthening of pharmacovigilance systems in these settings.
The prevalence of SGA found in this study varied between 5.0% among infants born in Mozambique and 19.6% among infants born in Kenya. These estimates are consistent with recent estimates of SGA based on 22 birth cohort studies from the SGA-Preterm Birth working group [48]. However, a higher rate of SGA associated with P. falciparum infection was reported among pregnant women from the Thailand Myanmar border [49]. In this study, more than half of the women had their first antenatal visit during the second trimester of the pregnancy. This finding is very similar to DHS reports [50, 51]. However, Asembo is an exception given that at least a third of the recruited women had the first antenatal visit in the first trimester. Starting antenatal care early during pregnancy increases the likelihood of initiating IPTp-SP and bed nets in accordance with guidelines and thereby reducing the occurrence of malaria and need for use of quinine and ACT. HDSS procedures mandate frequent visits to a household. This increases the likelihood of detecting early pregnancies and facilitates follow-up of pregnancy outcomes. Nevertheless, cultural barriers [19] still pose challenges to the field workers to identify not yet visible pregnancies as almost half of the pregnancies were detected during the second semester.
Limitations
This study included only pregnancies ending as singleton live birth and with birth weight collected within 7 days of life in the analysis. This could lead to some bias because SGA is associated with a lower probability of survival. Thus, the inclusion in the data analysis is conditioning on the outcome. However, it is not expected to be an important source of bias because the neonatal mortality rate in these sites is small (fewer than 30 per 1000 live-births). Compared to other study sites, Asembo (Kenya) had a higher proportion of missing information of weight at birth variable, representing 12% of all pregnancies recruited (Fig. 1). The vast majority of these missing birth weights are among newborns born at home, at which evaluation on the birthday was not possible. An imputation technique was used to mitigate this problem and the results did not materially change from the non-imputed scenario. However, the used imputation assumes that all newborns in one site are similar regardless of potential unmeasured biological differences (gender and mother anthropometrics). This may have contributed to lower prevalence of LBW because newborns who died due to conditions linked to LBW did not get their weights recorded and thus they do not contribute on the imputation. However, it is not expected to have resulted in changing the overall direction of the associations. The ascertainment of the gestational age and weight at birth in the context of this study may have introduced some non-differential misclassification. While gestational age was assessed via multiple methods, as available and appropriate, including date of last menstrual period, Ballard Score, fundal height and ultrasound, there is no reason to believe that the accuracy of gestational age estimates varied by exposure status. Interviewers and study nurses were unaware of exposure status [22]. Similarly, weight at birth was recorded without knowledge of exposure status.
Regarding birth weight, some newborns who were delivered in home settings had their weight assessed post-24 h of birth. If such non-differential misclassification occurred, it would have biased the estimates toward the null [19]. Nevertheless, the direction of the associations is the same as it was for the LBW. The sample size for quinine exposure is small and no exposures to quinine in Kenya were found. In addition, the main ACT used artemether–lumefantrine. Thus, one cannot necessarily generalize these results to other ACT medicines. Furthermore, the data on the malaria episode being treated was limited and there could be a risk of confounding if there were differences in the type of underlying infections being treated by quinine and ACT. Also, the models could not be adjusted for HIV because of small counts for HIV positives.
Conclusions
No evidence was found of an increased risk of LBW, SGA or prematurity among infants born to women with confirmed first-trimester exposure to an ACT. The findings add support for the use of ACT for uncomplicated P. falciparum malaria during the first trimester of pregnancy. The existence of the HDSS platform greatly facilitated active safety surveillance of anti-malarials used during pregnancy.
Supplementary information
Additional file 1. Additional Figures S1–S3, Tables S1–S16.
Acknowledgements
We wish to thank all study participants and the study field staffs in the three countries. We acknowledge the contribution of Professor Stephen Gloyd of the University of Washington for his guidance. We thank Stephanie Kovacs for useful contributions.
Abbreviations
- ACT
Artemisinin-based combination therapy
- ASAP
Assessment of safety of antimalarial drug use during early pregnancy
- HDSS
Health and Demographic Surveillance System
- INDEPTH
International Network of Demographic Evaluation of Populations and their Health
- IPD
Individual Participant Data
- IPTp
Intermittent preventive treatment in pregnancy
- IUGR
Intrauterine growth restriction
- LBW
Low birth weight
- LLITN
Long-lasting insecticide-treated nets
- QNN
Quinine
- SSA
Sub-Saharan Africa
- SP
Sulfadoxine–pyrimethamine
- WHO
World Health Organization
Authors’ contributions
OA analysed the data under the mentorship of ES and AS with creative inputs from GC, SD and FTK. OA, ES and AS wrote the initial draft. SD, FTK and GC provided inputs for the paper writing. All authors read and approved the final manuscript.
Funding
This study was made possible thanks to the financial support of the Bill & Melinda Gates Foundation through the Malaria in Pregnancy Consortium.
Availability of data and materials
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
Ethics approval and consent to participate
All studies had institutional ethical review approvals and obtained written informed consent from all participants.
Consent for publication
Not applicable.
Competing interests
The findings and conclusions presented in this manuscript are those of the authors and do not necessarily reflect the official position of the US Centers for Disease Control and Prevention. The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Orvalho Augusto, Email: orvaquim@gmail.com.
Esperança Sevene, Email: esevene68@gmail.com.
Supplementary information
Supplementary information accompanies this paper at 10.1186/s12936-020-03210-y.
References
- 1.Briand V, Saal J, Ghafari C, Huynh B-T, Fievet N, Schmiegelow C, et al. Fetal growth restriction is associated with malaria in pregnancy: a prospective longitudinal study in Benin. J Infect Dis. 2016;214:417–425. doi: 10.1093/infdis/jiw158. [DOI] [PubMed] [Google Scholar]
- 2.McClure EM, Goldenberg RL, Dent AE, Meshnick SR. A systematic review of the impact of malaria prevention in pregnancy on low birth weight and maternal anemia. Int J Gynaecol Obstet. 2013;121:103–109. doi: 10.1016/j.ijgo.2012.12.014. [DOI] [PubMed] [Google Scholar]
- 3.Kramer MS. Determinants of low birth weight: methodological assessment and meta-analysis. Bull World Health Org. 1987;65:663–737. [PMC free article] [PubMed] [Google Scholar]
- 4.Walker PGT, Floyd J, ter Kuile F, Cairns M. Estimated impact on birth weight of scaling up intermittent preventive treatment of malaria in pregnancy given sulphadoxine–pyrimethamine resistance in Africa: a mathematical model. PLoS Med. 2017;14:e1002243. doi: 10.1371/journal.pmed.1002243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.van Eijk AM, Hill J, Noor AM, Snow RW, Ter Kuile FO. Prevalence of malaria infection in pregnant women compared with children for tracking malaria transmission in sub-Saharan Africa: a systematic review and meta-analysis. Lancet Glob Health. 2015;3:e617–e628. doi: 10.1016/S2214-109X(15)00049-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Desai M, ter Kuile FO, Nosten F, McGready R, Asamoa K, Brabin B, et al. Epidemiology and burden of malaria in pregnancy. Lancet Infect Dis. 2007;7:93–104. doi: 10.1016/S1473-3099(07)70021-X. [DOI] [PubMed] [Google Scholar]
- 7.Guyatt HL, Snow RW. Malaria in pregnancy as an indirect cause of infant mortality in sub-Saharan Africa. Trans R Soc Trop Med Hyg. 2001;95:569–576. doi: 10.1016/S0035-9203(01)90082-3. [DOI] [PubMed] [Google Scholar]
- 8.Guyatt HL, Snow RW. Impact of malaria during pregnancy on low birth weight in sub-Saharan Africa. Clin Microbiol Rev. 2004;17:760–769. doi: 10.1128/CMR.17.4.760-769.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Adams MM, Alexander GR, Kirby RS, Wingate MS. Perinatal epidemiology for public health practice. Berlin: Springer; 2009. [Google Scholar]
- 10.Huynh B-T, Fievet N, Gbaguidi G, Dechavanne S, Borgella S, Guézo-Mévo B, et al. Influence of the timing of malaria infection during pregnancy on birth weight and on maternal anemia in Benin. Am J Trop Med Hyg. 2011;85:214–220. doi: 10.4269/ajtmh.2011.11-0103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schmiegelow C, Matondo S, Minja DTR, Resende M, Pehrson C, Nielsen BB, et al. Plasmodium falciparum infection early in pregnancy has profound consequences for fetal growth. J Infect Dis. 2017;216:1601–1610. doi: 10.1093/infdis/jix530. [DOI] [PubMed] [Google Scholar]
- 12.WHO . A strategic framework for malaria prevention and control during pregnancy in the African region. Geneva: World Health Organization; 2004. [Google Scholar]
- 13.WHO . Guidelines for the treatment of malaria. 3. Geneva: World Health Organization; 2015. [PubMed] [Google Scholar]
- 14.Burger RJ, van Eijk AM, Bussink M, Hill J, ter Kuile FO. Artemisinin-based combination therapy versus quinine or other combinations for treatment of uncomplicated Plasmodium falciparum malaria in the second and third trimester of pregnancy: a systematic review and meta-analysis. Open Forum Infect Dis. 2016;3:1–11. doi: 10.1093/ofid/ofv170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen LJ, Wang MY, Sun WK, Liu MZ. Embryotoxicity and teratogenicity studies on artemether in mice, rats and rabbits. Zhongguo Yao Li Xue Bao. 1984;5:118–122. [PubMed] [Google Scholar]
- 16.Clark RL. Embryotoxicity of the artemisinin antimalarials and potential consequences for use in women in the first trimester. Reprod Toxicol. 2009;28:285–296. doi: 10.1016/j.reprotox.2009.05.002. [DOI] [PubMed] [Google Scholar]
- 17.White TE, Clark RL. Sensitive periods for developmental toxicity of orally administered artesunate in the rat. Birth Defects Res B Dev Reprod Toxicol. 2008;83:407–417. doi: 10.1002/bdrb.20157. [DOI] [PubMed] [Google Scholar]
- 18.Longo M, Zanoncelli S, Torre PD, Riflettuto M, Cocco F, Pesenti M, et al. In vivo and in vitro investigations of the effects of the antimalarial drug dihydroartemisinin (DHA) on rat embryos. Reprod Toxicol. 2006;22:797–810. doi: 10.1016/j.reprotox.2006.08.001. [DOI] [PubMed] [Google Scholar]
- 19.Manyando C, Mkandawire R, Puma L, Sinkala M, Mpabalwani E, Njunju E, et al. Safety of artemether–lumefantrine in pregnant women with malaria: results of a prospective cohort study in Zambia. Malar J. 2010;9:249. doi: 10.1186/1475-2875-9-249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hill J, D’Mello-Guyett L, Hoyt J, van Eijk AM, ter Kuile FO, Webster J. Women’s access and provider practices for the case management of malaria during pregnancy: a systematic review and meta-analysis. PLoS Med. 2014;11:e1001688. doi: 10.1371/journal.pmed.1001688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dellicour S, ter Kuile FO, Stergachis A. Pregnancy exposure registries for assessing antimalarial drug safety in pregnancy in malaria-endemic countries (Health in Action) PLoS Med. 2008;5:e187. doi: 10.1371/journal.pmed.0050187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tinto H, Sevene E, Dellicour S, Calip GS, d’Alessandro U, Macete E, et al. Assessment of the safety of antimalarial drug use during early pregnancy (ASAP): protocol for a multicenter prospective cohort study in Burkina Faso, Kenya and Mozambique. Reprod Health. 2015;12:112. doi: 10.1186/s12978-015-0101-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dellicour S, Desai M, Aol G, Oneko M, Ouma P, Bigogo G, et al. Risks of miscarriage and inadvertent exposure to artemisinin derivatives in the first trimester of pregnancy: a prospective cohort study in western Kenya. Malar J. 2015;14:461. doi: 10.1186/s12936-015-0950-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dellicour S, Krishna S, Sevene E, McGready R, Tinto H, Mosha D, et al. First- trimester artemisinin derivatives and quinine treatments and the risk of adverse pregnancy outcomes in Africa and Asia: a meta-analysis of observational studies. PLoS Med. 2017;14:e1002290. doi: 10.1371/journal.pmed.1002290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sacoor C, Nhacolo A, Nhalungo D, Aponte JJ, Bassat Q, Augusto O, et al. Profile: Manhiça Health Research Centre (Manhiça HDSS) Int J Epidemiol. 2013;42:1309–1318. doi: 10.1093/ije/dyt148. [DOI] [PubMed] [Google Scholar]
- 26.Odhiambo FO, Laserson KF, Sewe M, Hamel MJ, Feikin DR, Adazu K, et al. Profile: the KEMRI/CDC health and demographic surveillance system-western Kenya. Int J Epidemiol. 2012;41:977–987. doi: 10.1093/ije/dys108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Derra K, Rouamba E, Kazienga A, Ouedraogo S, Tahita MC, Sorgho H, et al. Profile: nanoro health and demographic surveillance system. Int J Epidemiol. 2012;41:1293–1301. doi: 10.1093/ije/dys159. [DOI] [PubMed] [Google Scholar]
- 28.Ye Y, Wamukoya M, Ezeh A, Emina JB, Sankoh O. Health and demographic surveillance systems: a step towards full civil registration and vital statistics system in sub-Sahara Africa? BMC Public Health. 2012;12:741. doi: 10.1186/1471-2458-12-741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Flaherman VJ, Schaefer EW, Kuzniewicz MW, Li SX, Walsh EM, Paul IM. Early weight loss nomograms for exclusively breastfed newborns. Pediatrics. 2015;135:e16–e23. doi: 10.1542/peds.2014-1532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Noel-Weiss J, Courant G, Woodend AK. Physiological weight loss in the breastfed neonate: a systematic review. Open Med. 2008;2:e99–e110. [PMC free article] [PubMed] [Google Scholar]
- 31.Greenwood BM, Greenwood AM, Snow RW, Byass P, Bennett S, Hatib N, et al. The effects of malaria chemoprophylaxis given by traditional birth attendants on the course and outcome of pregnancy. Trans R Soc Trop Med Hyg. 1989;83:589–594. doi: 10.1016/0035-9203(89)90362-3. [DOI] [PubMed] [Google Scholar]
- 32.D’Alessandro U, Langerock P, Bennett S, Francis N, Cham K, Greenwood BM. The impact of a national impregnated bed net programme on the outcome of pregnancy in primigravidae in The Gambia. Trans R Soc Trop Med Hyg. 1996;90:487–492. doi: 10.1016/S0035-9203(96)90289-8. [DOI] [PubMed] [Google Scholar]
- 33.Villar J, Cheikh Ismail L, Victora CG, Ohuma EO, Bertino E, Altman DG, et al. International standards for newborn weight, length, and head circumference by gestational age and sex: the newborn cross-sectional study of the INTERGROWTH-21st Project. Lancet. 2014;384:857–868. doi: 10.1016/S0140-6736(14)60932-6. [DOI] [PubMed] [Google Scholar]
- 34.Wilcox AJ. On the importance—and the unimportance—of birthweight. Int J Epidemiol. 2001;30:1233–1241. doi: 10.1093/ije/30.6.1233. [DOI] [PubMed] [Google Scholar]
- 35.Quinn J-A, Munoz FM, Gonik B, Frau L, Cutland C, Mallett-Moore T, et al. Preterm birth: case definition & guidelines for data collection, analysis, and presentation of immunisation safety data. Vaccine. 2016;34:6047–6056. doi: 10.1016/j.vaccine.2016.03.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Borenstein M, Hedges LV, Higgins JPT, Rothstein HR. A basic introduction to fixed-effect and random-effects models for meta-analysis. Res Synth Methods. 2010;1:97–111. doi: 10.1002/jrsm.12. [DOI] [PubMed] [Google Scholar]
- 37.Torman VBL, Camey SA. Bayesian models as a unified approach to estimate relative risk (or prevalence ratio) in binary and polytomous outcomes. Emerg Themes Epidemiol. 2015;12:8. doi: 10.1186/s12982-015-0030-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Williamson T, Eliasziw M, Fick G. Log-binomial models: exploring failed convergence. Emerg Themes Epidemiol. 2013;10:14. doi: 10.1186/1742-7622-10-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Plummer M. A program for analysis of Bayesian graphical models using Gibbs sampling. In: Proceedings of the 3rd international workshop on distributed statistical computing (DSC 2003). Vienna, Austria. 2003.
- 40.Raje A. R: a language and environment for statistical computing. Vienna: R Foundation for Statistical Computing; 2018. [Google Scholar]
- 41.Mosha D, Mazuguni F, Mrema S, Sevene E, Abdulla S, Genton B. Safety of artemether–lumefantrine exposure in first trimester of pregnancy: an observational cohort. Malar J. 2014;13:197. doi: 10.1186/1475-2875-13-197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nambozi M, Tinto H, Mwapasa V, Tagbor H, Kabuya J-BB, Hachizovu S, et al. Artemisinin-based combination therapy during pregnancy: outcome of pregnancy and infant mortality: a cohort study. Malar J. 2019;18:105. doi: 10.1186/s12936-019-2737-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rouamba T, Kpoda H, Valea I, Mens P, Gomes M, Tinto H, et al. Safety of antimalarial drug use during early pregnancy in Bobo Dioulasso: examining low birth weight and congenital malformations as potential adverse outcomes. Trop Med Int Health. 2017;22:Abstract 95.
- 44.Achan J, Talisuna AO, Erhart A, Yeka A, Tibenderana JK, Baliraine FN, et al. Quinine, an old anti-malarial drug in a modern world: role in the treatment of malaria. Malar J. 2011;10:144. doi: 10.1186/1475-2875-10-144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Petri H, Urquhart J. Channeling bias in the interpretation of drug effects. Stat Med. 1991;10:577. doi: 10.1002/sim.4780100409. [DOI] [PubMed] [Google Scholar]
- 46.Bardají A, Sigauque B, Bruni L, Romagosa C, Sanz S, Mabunda S, et al. Clinical malaria in African pregnant women. Malar J. 2008;7:27. doi: 10.1186/1475-2875-7-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sangaré LR, Weiss NS, Brentlinger PE, Richardson BA, Staedke SG, Kiwuwa MS, et al. Patterns of anti-malarial drug treatment among pregnant women in Uganda. Malar J. 2011;10:152. doi: 10.1186/1475-2875-10-152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lee ACC, Katz J, Blencowe H, Cousens S, Kozuki N, Vogel JP, et al. National and regional estimates of term and preterm babies born small for gestational age in 138 low-income and middle-income countries in 2010. Lancet Glob Health. 2013;1:e26–e36. doi: 10.1016/S2214-109X(13)70006-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Moore K, Simpson J, Wiladphaingern J, Aung M, Pimanpanarak M, Raksuansak J, et al. Influence of the number and timing of malaria episodes during pregnancy on prematurity and small-for-gestational-age in an area of low transmission. BMC Med. 2017;15:117. doi: 10.1186/s12916-017-0877-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ministerio da Saude—MISAU/Moçambique, Instituto Nacional de Estatística—INE/Moçambique, ICF International: Moçambique Inquérito Demográfico e de Saúde. 2011.
- 51.Kenya National Bureau of Statistics, Ministry of Health/Kenya, National AIDS Control Council/Kenya, Kenya Medical Research Institute, Population NCf, Development/Kenya: Kenya Demographic and Health Survey 2014. Rockville, MD, USA; 2015.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1. Additional Figures S1–S3, Tables S1–S16.
Data Availability Statement
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.