Abstract
Metastasis is the primary cause of cancer-related mortality. Cancer cells primarily metastasize via blood and lymphatic vessels to colonize lymph nodes and distant organs, leading to worse prognosis. Thus, strategies to limit blood and lymphatic spread of cancer have been a focal point of cancer research for several decades. Resistance to FDA-approved anti-angiogenic therapies designed to limit blood vessel growth has emerged as a significant clinical challenge. However, there are no FDA-approved drugs that target tumor lymphangiogenesis, despite the consequences of metastasis through the lymphatic system. This review highlights several of the key resistance mechanisms to anti-angiogenic therapy and potential challenges facing anti-lymphangiogenic therapy. Blood and lymphatic vessels are more than just conduits for nutrient, fluid, and cancer cell transport. Recent studies have elucidated how these vasculatures often regulate immune responses. Vessels that are abnormal or compromised by tumor cells can lead to immunosuppression. Therapies designed to improve lymphatic vessel function while limiting metastasis may represent a viable approach to enhance immunotherapy and limit cancer progression.
Keywords: lymphangiogenesis, angiogenesis, treatment resistance, metastasis
1. Introduction
Angiogenesis, the development of new blood vessels, is a feature of many solid cancers [1]. Recruitment of blood vessels is critical to support tumor growth past 1–2 mm in diameter [2]. Vascular endothelial growth factor-A (VEGF-A) is the most comprehensively studied and perhaps potent mediator of sprouting angiogenesis. Through binding to VEGFR-2 vascular endothelial growth factor receptor-2 (VEGFR-2)/human kinase insert domain receptor, VEGF-A triggers activation of VEGFR-2 and intracellular signaling mediators that promote endothelial cell proliferation, migration, and survival, as well as vascular permeability and ultimately neovascularization [3]. New blood vessels not only deliver nutrients and oxygen to growing tumors but also provide a route of cancer cell exit to distant organs [4]. Many preclinical models using anti-angiogenesis therapies blocking VEGF-A signaling have broadly prohibited or slowed tumor growth and reduced metastatic spread. There are over 20 drugs with anti-angiogenic activity approved by the FDA for cancer indications [5], with Avastin—humanized anti-VEGF-A monoclonal antibody—being the first granted approval in 2004. Hundreds of clinical trials for multiple solid cancers targeting VEGF-A alone or in combination with other therapies have been initiated. However, despite the growing list of FDA approvals, anti-angiogenesis drugs have had a modest impact on patient survival. For example, Avastin only adds a 4–5 month survival benefit in patients with advanced colorectal cancer [6]. Such discrepancies between mouse and human provide the impetus for understanding the molecular and cellular resistance mechanisms of anti-angiogenic therapy.
Lymphangiogenesis also occurs in many preclinical cancer models and in some human cancers mainly through the production of vascular endothelial growth factor-C (VEGF-C) and VEGF-D, which signal through VEGFR-2 and VEGFR-3 and drive lymphatic endothelial cell (LEC) proliferation, migration, and survival [3]. Unlike inhibiting angiogenesis, blunting lymphangiogenesis—the formation of new lymphatic vessels—has mixed results on primary tumor growth in preclinical models [7,8,9], mainly indirectly due to modulation of the anti-tumor immune response. Several drugs that inhibit VEGFR-3 have been used for cancer indications [10] and overlap exists between the targets of anti-angiogenesis drugs and molecules on lymphatic vessels [11]. Recently, an early stage clinical trial targeting VEGFR-3 was completed, but showed minimal efficacy against tumor growth [12]. However, lymphatic vessels provide a major route for cancer cell dissemination. The expression of VEGF-C and VEGF-D correlates with increased metastasis, invasion, and poor prognosis in several types of cancer [13], in part due to tumor-associated lymphangiogenesis. After invading initial lymphatic capillaries, cancer cells migrate through collecting lymphatic vessels and enter regional lymph nodes, where they form secondary tumors. A fraction of nodal metastases can then exit lymph nodes and spread to distant sites [14,15]. The majority of cancer patients die from distant metastasis [16] and thus treating metastatic disease remains a challenging clinical problem. Since most cancer cells need to access the primary or secondary tumor vasculature to metastasize, inhibiting the growth of tumor-associated vasculature remains an attractive therapeutic strategy.
Bergers and Hanahan [17] describe how tumor cells adapt to anti-angiogenic treatment or are intrinsically resistant to such therapy. Over the past decade, additional experimental data have shed light on anti-angiogenesis resistance mechanisms. Here, we review evidence that suggests targeting tumor-associated lymphatic vessels poses similar challenges as targeting tumor blood vessels.
2. Timing
Primary tumor lymphangiogenesis is an early event in cancer progression and may present a narrow window of therapeutic intervention. Unfortunately, about a third of breast, colorectal, and lung cancer patients are lymph node positive at diagnosis [18,19], missing an opportunity to block initial lymphangiogenesis in these patients. In addition to the primary site, lymphangiogenesis also occurs in metastatic organs. In many preclinical studies, lymphangiogenesis can occur in regional lymph nodes and distant organs before metastases have occurred and is thought to create a “lymphovascular niche” that creates a favorable environment for disseminated cells [20]. VEGFR-3 reporter mice revealed lymphangiogenesis in lymph nodes, liver, lungs, and spleens of tumor-bearing mice [21] before metastatic spread. High lymphatic density and lymphatic invasion in metastatic lungs was associated with poor outcome of melanoma patients [22]. Blocking pro-lymphangiogenic VEGF-C signaling after cancer cell colonization of tumor-draining lymph nodes prevented further spread to lungs [23] and inhibiting lymphangiogenesis in distant organs may prevent further dissemination of metastatic cells. However, validated biomarkers predictive of lymphangiogenesis are lacking.
3. Target
VEGF-A mediates traditional sprouting angiogenesis, while VEGF-C is a critical regulator of lymphangiogenesis. However, over 40 molecules other than VEGF have been shown to play a role in blood vessel growth [24], illustrating the complexity of inhibiting angiogenesis where alternative angiogenic pathways exist. Likewise, the formation of postnatal lymphatic vessels is not limited to VEGF-C dependent mechanisms. Many factors other than VEGF-C have been shown to stimulate lymphangiogenesis. These include other growth factors, cytokines, hormones, proteins, and peptides, many of which induce lymphangiogenesis independently of VEGFR-3 signaling [25,26]. Together, these data suggest that neutralizing VEGF-C alone may lead to drug resistance facilitated by alternative pro-lymphangiogenic molecules.
In addition to multiple growth factor pathways that are activated and promote vascular sprouting in the tumor microenvironment, molecular crosstalk exists between the blood and lymphatic endothelium. VEGFR-2, a key transducer of angiogenic signaling, is expressed on LECs as well as blood endothelial cells. Likewise, VEGFR-3 is expressed on lymphatic endothelium and also on the endothelium of tumor blood vessels [27]. In a reversal of traditional functions, VEGF-A can promote lymphangiogenesis while VEGF-C stimulates tumor angiogenesis [28]. Neutralization of VEGF-A signaling reduced lymphangiogenesis in models of inflammation and tumor growth [3,29,30]. It is unclear whether histological samples from patients treated with anti-angiogenic therapy have been comprehensively evaluated to assess an effect on tumor-associated lymphatic vessels.
4. Metastasis Independent of Lymphatic Endothelial Cell Sprouting
One mechanism that accounts for tumor resistance to anti-angiogenic therapy is blood vessel co-option, where tumors utilize pre-existing blood vessels from the surrounding tissue for nutrients. Highly vascularized tissues such as lymph nodes, where up to 10% of the organ volume is blood vessels [31], are thought to be conducive to blood vessel co-option by tumors [32]. En route to co-opting local vessels, some cancer cells exhibit a replacement pattern [33]; in this case cancer cells replace normal tissue but spare blood vessels. Peritumoral lymphatic vessel density is often significantly higher than intratumoral lymphatic vessel density [34]. In fact, intratumoral lymphatic vessels are often absent [35], suggesting that cancer growth can be destructive to intratumoral lymphatic vessels. Although lymphangiogenesis is found in pre-metastatic tumor draining lymph nodes, we measured a reduction in lymphatic vessel density within metastatic lymph nodes relative to pre-metastatic nodes [32].
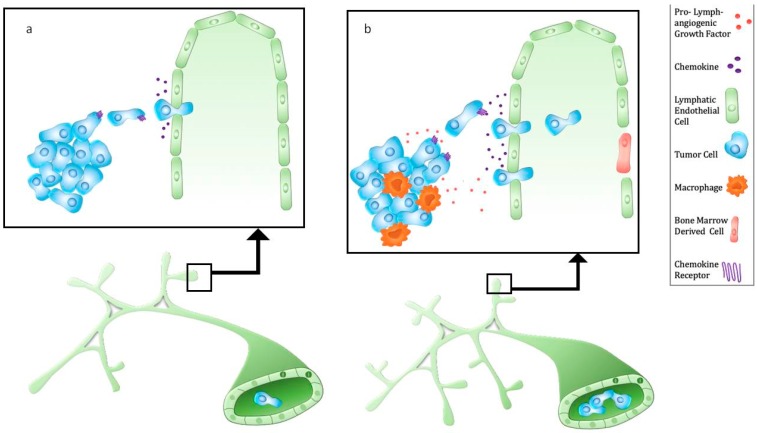
Lymphatic vessel density around the primary tumor and lymphatic vessel invasion are predictors of lymph node metastasis and poor prognosis [36]. However, tumor lymphangiogenesis may be cancer and subtype-specific [7]. Proliferating tumor-associated lymphatic vessels were identified in human squamous cell carcinoma and melanoma [37,38]. In contrast, despite human data and numerous preclinical models that show lymphangiogenesis promotes lymphatic metastasis, analyses of human breast, prostate, and esophageal cancer tissue suggest that cancer cells can invade existing lymphatic vessels (Figure 1) rather than induce proliferation [39,40,41,42,43]. The prognostic significance of lymphangiogenesis in triple negative breast cancer is unclear, yet lymphatic invasion is associated with poor prognosis [44]. Thus, there is still debate as to whether lymphangiogenesis is a pre-requisite for lymphatic metastasis. Tumors that develop in tissues with a dense lymphatic vascular network may exploit pre-existing lymphatic vessels for invasion and metastasis.
Figure 1.
Metastasis via tumor-associated lymphatic vessels. (a) Tumor cells express chemokine receptors (e.g., CXCR4, CCR7) which bind chemokines produced by lymphatic endothelium (e.g., CXCL12, CCL21). Lymphatic vessel-derived CCL21 attracts cancer cells that can enter initial lymphatic vessels through interendothelial cell gaps. (b) Cancer cells and tumor-associated macrophages secrete pro-lymphangiogenic factors such as VEGF-C, leading to an increase initial lymphatic vessel density and collecting lymphatic vessel diameter. In addition, VEGF-C upregulates CCL21 and increases lymphatic vessel permeability, resulting in enhanced lymphatic metastasis. Of note, bone marrow derived cells can closely associate with or incorporate into lymphatic vessels.
Other mechanisms of tumor blood vessel vascularization include intussusception, endothelial progenitor cell-mediated vasculogenesis, vascular mimicry by cancer cells, and differentiation of cancer stem cells into endothelial cells [1,45]. Similarly, lymphatic beds may consist of LECs derived from heterogeneous sources. Recent findings [46] show that the level of circulating myeloid derived lymphatic endothelial progenitor cells, as determined by co-staining of myeloid and LEC markers, strongly correlate with lymphatic metastasis in breast cancer patients. These progenitor cells were shown to incorporate into lymphatic vessels in human and mouse tumors (Figure 1). While there is debate concerning the extent of the contribution of bone marrow derived cells to lymphangiogenesis, these recent studies add to existing data showing that certain populations of bone marrow cells express LEC markers and can function as putative LEC progenitors in pathologic settings such as corneal and kidney transplantation, wound injury, and tumor progression [47,48,49,50,51].
5. Cancer Cells Use Existing Migratory Cues
A physiological function of lymphatic vessels is to provide chemical signals, or chemokines, for immune cell migration. In addition, many cancer cells overexpress chemokine receptors [52] that allow them to migrate toward lymphatic capillaries, employing the same chemoattraction mechanism used by dendritic cells and T cells. An example is high expression of CCR7 found in various human cancer cells [53,54,55]. C–C chemokine ligand 21 (CCL21), the ligand for CCR7, is constitutively expressed by lymphatic vessels and in secondary lymphoid organs [56] and can be upregulated in response to increased lymphatic flow and inflammatory stimuli [57]. Likewise, lymphatic vessel derived CXCL12 in physiological systems directs dendritic cell migration to lymph nodes [58]. Lymphatic vessels can attract CXCR4-expressing tumor cells to promote lymphatic metastasis [59], and CXCR4 expression is also associated with lymphatic metastasis [60]. Lymphangiogenic signaling can enhance chemokine-driven metastasis, but it appears that tumor cells can independently “hijack” host mechanisms of leukocyte trafficking to facilitate metastasis (Figure 1).
6. Myeloid Cell Recruitment
Tumor-associated macrophages (TAMs) are an abundant population in preclinical cancer models and human tumors [61]. Pro-angiogenic monocytes recruited from the bone marrow promote tumor vascularization, growth, and cause resistance to therapy [17]. TAMs are also associated with high lymphatic vessel density and lymphatic metastasis (Figure 1). Macrophages may contribute directly to the lymphatic vasculature through transdifferentiation [49], progenitor differentiation [46], and vascular mimicry [62,63] or indirectly by secretion of pro-lymphangiogenic factors [64]. VEGF-C acts as a chemotactic factor for VEGFR-3 expressing macrophages [65]. In response to paclitaxel, VEGFR-3+ macrophages were recruited to breast and lung tumors and mediated chemotherapy resistance by enhancing VEGF-C production that stimulated lymphangiogenesis [66]. Surprisingly, an anti-VEGFR-3 antibody alone did not reduce tumor volume, VEGF-C expression, or the number of lymphatic vessels in the absence of paclitaxel, suggesting that recruited macrophages mediate the lymphangiogenic response to paclitaxel.
In breast cancer models, podoplanin-expressing TAMs promote metastasis [67] by remodeling the extracellular matrix, leading to the release of VEGF-C and VEGF-D to direct lymphangiogenesis. Recently, Evans et al. identified a population of Beta-4 integrin expressing TAMs that promote tumor metastasis independent of VEGF-C and lymphangiogenesis [68]. These TAMs were closely associated with existing lymphatic vessels through β4 integrin interactions with laminin-5. TGF-β1 produced by TAMs promoted TAM attachment to LECs and also reorganized LEC architecture to favor metastasis.
VEGF-C/VEGFR-3 signaling within TAMs also drives immune tolerance within the colorectal tumor microenvironment [69]. VEGFR-3 signaling was critical for the development of pro-inflammatory macrophages, which was associated with decreased presence of CD8 T cells within colorectal tumors. Blocking VEGFR-3 decreased the presence of TAMs, but increased their antigen processing and cross-presentation, leading to decreased tumor growth.
7. Alternative Routes of Metastatic Dissemination
In addition to lymphatic transport, cancer cells at the primary tumor or distant site may take a different route to distant organs, primarily through blood vessels. It is unclear whether cancer cells preferentially disseminate from the primary site through blood or lymphatic vessels, but the frequency and site of metastatic spread is cancer-dependent [70]. Once in secondary organs, again cancer cells may further metastasize by lymphogenous or hematogenous route. Whole-exome sequencing of samples from 20 breast cancer patients suggested that nodal breast metastases did not seed distant metastasis. Instead, the authors conclude that distant organ metastases arise primarily from primary tumors, likely through a hematogenous route [71]. In another study, biopsied tissue of 17 colorectal cancer patients was sequenced to identify polyguanine repeats. The repeats allowed tracing of the evolutionary history of metastatic lymph nodes and liver metastases from the primary tumor [72]. This study found that 35% of distant metastases were seeded by metastatic lymph nodes, while 65% were seeded from the primary tumor, likely through a hematogenous route. These data show the heterogeneity in possible outcomes for cancer cells that arrive in the lymph node.
Once in lymph nodes, cancer cells can move along with lymph drainage or re-access the lymphatic system within the lymph node parenchyma [73] and may eventually enter the blood circulation through lymphovenous connections. Two recent studies suggest that cancer cells can directly enter the blood stream through blood vessels in lymph nodes. Once cancer cells exit nodes through blood vessels, they are able to colonize distant organs [14,15]. Immunohistochemical staining of human specimens show similar associations between cancer cells and blood vessels within metastatic nodes, but more work is needed to definitively track this route of dissemination in human cancer. Unlike targeting lymphangiogenesis in lung [22], inhibiting lymphangiogenesis in metastatic lymph nodes may not attenuate further metastatic spread.
8. Abnormal Tumor-Associated Lymphatic Vessels
8.1. Increased Lymphatic Permeability
Tumor-associated lymphatics are structurally and functionally abnormal, as are tumor blood vessels. The inability of lymphatic vessels to remove interstitial fluid from tumors is a contributing factor to the elevated interstitial fluid pressure and edema found in most tumors [74]. Although initial lymphatic vessels have a discontinuous basement membrane, tumor-associated lymphatic vessels become more permeable and less able to create and retain lymph (Figure 1). The discontinuities in lymphatic capillary walls not only allow transport of extracellular content both into and out of the vessel, but also allow entry by cancer cells, providing a route for metastatic dissemination. In addition to stimulating lymphangiogenesis, VEGF-C can render lymphatic vessels more permeable, as shown by dextran leakage from lymphatic vessels of mice transduced with adenoviral VEGF-C [75]. VEGF-C increased the permeability of intestinal lymphatic vessels, resulting in enhanced colorectal cancer metastasis [76]. Macrophages, which are also a source of VEGF-C, promote interendothelial gaps in LEC monolayers, providing an opportunity for enhanced cancer cell intravasation [67]. In addition, macrophages associated with LECs reduce the spread area of individual LECs, thus increasing lymphatic vessel permeability that may promote lymphatic metastasis [68]. Inflammation [77], chemotherapy [78], obesity [79], and tumor metabolites [73] have all been shown to increase lymphatic permeability. A recent study demonstrated that age can also affect lymphatic permeability. In older melanoma patients, cancer cells enter initial lymphatic capillaries but extravasate from collecting lymphatic vessels due to a loss of lymphatic vessel integrity. Consequently, melanoma cells avoid accumulation in tumor-draining lymph nodes but dissemination to distant sites is increased [80].
Few studies have sought to reverse lymphatic vessel permeability in vivo. Vascular cell adhesion molecule 1 (VCAM-1) is upregulated on LECs by tumor inflammation and was shown to regulate lymphatic vessel permeability [81]. Blocking VCAM-1 in vitro and in vivo reduced tumor-mediated lymphatic permeability and lymphatic invasion, suggesting reducing tumor-induced lymphatic vessel permeability may be a feasible approach to regulate lymphatic metastasis.
8.2. Altered Lymphatic Flow
Tumors increase both the number and size of surrounding tumor lymphatic vessels. VEGF-C and VEGF-D promote lymphangiogenesis and hyperplasia of small peritumoral lymphatic vessels, which increases the opportunity for cancer cells to enter lymphatic vessels. However, some nascent lymphatic vessels have been shown to be dysfunctional as they exhibit valve defects [82] and abnormal flow patterns. Although tumor inflammation can reduce contraction of tumor-draining collecting lymphatic vessels, lymphatic dilation due to VEGF-C and VEGF-D increases lymph flow and lymph node metastasis [82,83,84]. The impact of lymph drainage on remodeling the microenvironment and suppressing the host immune response have been described elsewhere [85]. Blocking VEGFR-2 or VEGFR-3 signaling reduced collecting lymphatic vessel dilation, which was associated with decreased lymph flow and nodal metastasis [84]. However, anti-VEGFR-3 treatment did not correct the multidirectional lymph flow pattern in peritumoral lymphatic vessels [82] and it is unclear whether immunosuppression is reversible after attenuating lymph/interstitial flow.
Transgenic mice with lymphatic insufficiency show that lymphatics are critical for drainage of extracellular fluid to prevent tumor edema [8,86,87]. Peritumoral edematous fluid contains high concentrations of cytokines that recruit leukocytes, immune-suppressive regulatory T cells, and myeloid derived suppressor cells [86]. Lymphatic vessels likely provide an exit for immune cells during inflammation, although lymphatics may also be important for initiating and modulating inflammation [86,87].
9. Immunomodulation by Lymphatics
Effector T cells home to tumors via blood vessels. Abnormal blood vessels caused by excessive VEGF-A and other pro-angiogenic factors interfere with T cell trafficking [88]. Recently, anti-angiogenesis therapy has been used to reverse the immunosuppressive microenvironment of tumors [89]. A challenge for anti-lymphangiogenic therapy is uncoupling tumor-associated lymphatic vessels from their roles in lymphatic metastasis and immune modulation. Lymphatic vessels are critical for the transport of tumor antigens by dendritic cells (DCs) to initiate anti-tumor immunity in lymph nodes and thus mice lacking dermal lymphatic vessels fail to mount adaptive immune responses against tumors [87].
Moreover, lymphangiogenesis can enhance the efficacy of cancer immunotherapy. High serum levels of VEGF-C are indicative of melanoma patients’ responses to immunotherapy, suggesting that elevated VEGF-C levels in circulation may be a biomarker for immunotherapy, despite its association with lymphatic metastasis [90]. Mechanistically, tumor-associated lymphatic vessels recruit T cells into melanoma tumors through CCL21. VEGF-C/VEGFR-3 signaling increased the number of activated T cells within primary melanoma lesions. Similarly, meningeal lymphangiogenesis induced by VEGF-C resulted in an enhanced immune response against glioblastoma tumors [91]. This response was more profoundly beneficial in combination with immune checkpoint inhibitors and VEGF-C. Thus, anti-lymphangiogenesis therapy may be incompatible with immune checkpoint blockade.
In contrast to the beneficial effects on immune surveillance, lymph from solid tumors delivers immunosuppressive molecules to attenuate anti-tumor immunity [92]. Antigen transported by lymphatic vessels can be presented by LECs, which use it to tolerize T cells [93]. Moreover, LECs actively dampen T cell mediated responses through PD-L1 expression [94]. The differential effects of lymphatic vessels on anti-tumor immunity must be considered with the use of anti-lymphangiogenic therapy.
10. Summary and Future Directions
Modulating lymphatic vessels to reduce metastatic spread has great therapeutic potential. However, the multiple functions of lymphatic vessels must be considered for targeting the process of lymphangiogenesis associated with cancer. In additional to the role of lymphatic vessels in fluid and cancer transport, evidence is emerging on how lymphatic vessels regulate innate and adaptive immunity, which may affect cancer progression. While blocking VEGFR-3 has been thought to not have an effect on existing lymphatic vessels, it was shown that VEGFR-3 is necessary for the maintenance of adult meningeal lymphatics [95]. An ongoing challenge is to identify pathways that decrease cancer cell recruitment and access to lymphatic vessels while preserving the physiological function of lymphatic vessels. Identifying and targeting pathways to normalize, or restore, tumor-associated lymphatic vessels may represent a viable therapeutic strategy. Finally, more work needs to be done to study the effects of blocking lymphangiogenesis in metastatic settings in order to address whether anti-lymphangiogenic drugs will be beneficial to patients with established metastases.
Acknowledgments
The author would like to acknowledge Ashley Doucet for her assistance with the graphical illustration and Timothy Padera for feedback.
Author Contributions
Conceptualization, writing, and editing: D.J. All authors have read and agreed to the published version of the manuscript.
Funding
The author is supported by the NIH K22CA230315 Award, Shamim and Ashraf Dahod Breast Cancer Award, and an American Cancer Society Institutional Grant to Boston University, Boston Medical Center Cancer Center.
Conflicts of Interest
The author declares no conflict of interest.
References
- 1.Donnem T., Reynolds A.R., Kuczynski E.A., Gatter K., Vermeulen P.B., Kerbel R.S., Harris A.L., Pezzella F. Non-angiogenic tumours and their influence on cancer biology. Nat. Rev. Cancer. 2018;18:323–336. doi: 10.1038/nrc.2018.14. [DOI] [PubMed] [Google Scholar]
- 2.Bielenberg D.R., Zetter B.R. The Contribution of Angiogenesis to the Process of Metastasis. Cancer J. 2015;21:267–273. doi: 10.1097/PPO.0000000000000138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jones D., Min W. An overview of lymphatic vessels and their emerging role in cardiovascular disease. J. Cardiovasc. Dis. Res. 2011;2:141–152. doi: 10.4103/0975-3583.85260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Folkman J. Role of angiogenesis in tumor growth and metastasis. Semin. Oncol. 2002;29:15–18. doi: 10.1053/sonc.2002.37263. [DOI] [PubMed] [Google Scholar]
- 5.Lugano R., Ramachandran M., Dimberg A. Tumor angiogenesis: Causes, consequences, challenges and opportunities. Cell. Mol. Life Sci. 2019;CMLS:1–26. doi: 10.1007/s00018-019-03351-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hurwitz H., Fehrenbacher L., Novotny W., Cartwright T., Hainsworth J., Heim W., Berlin J., Baron A., Griffing S., Holmgren E., et al. Bevacizumab plus Irinotecan, Fluorouracil, and Leucovorin for Metastatic Colorectal Cancer. New Engl. J. Med. 2004;350:2335–2342. doi: 10.1056/NEJMoa032691. [DOI] [PubMed] [Google Scholar]
- 7.Regan E., Sibley R.C., Cenik B.K., Silva A., Girard L., Minna J.D., Dellinger M.T. Identification of Gene Expression Differences between Lymphangiogenic and Non-Lymphangiogenic Non-Small Cell Lung Cancer Cell Lines. PLoS ONE. 2016;11:e0150963. doi: 10.1371/journal.pone.0150963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen Y., Keskin D., Sugimoto H., Kanasaki K., Phillips P.E., Bizarro L., Sharpe A., LeBleu V.S., Kalluri R. Podoplanin+ tumor lymphatics are rate limiting for breast cancer metastasis. PLoS Boil. 2018;16:e2005907. doi: 10.1371/journal.pbio.2005907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kimura T., Sugaya M., Oka T., Blauvelt A., Okochi H., Sato S. Lymphatic dysfunction attenuates tumor immunity through impaired antigen presentation. Oncotarget. 2015;6:18081–18093. doi: 10.18632/oncotarget.4018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dieterich L., Detmar M. Tumor lymphangiogenesis and new drug development. Adv. Drug Deliv. Rev. 2016;99:148–160. doi: 10.1016/j.addr.2015.12.011. [DOI] [PubMed] [Google Scholar]
- 11.Stacker S.A., Williams S.P., Karnezis T., Shayan R., Fox S., Achen M. Lymphangiogenesis and lymphatic vessel remodelling in cancer. Nat. Rev. Cancer. 2014;14:159–172. doi: 10.1038/nrc3677. [DOI] [PubMed] [Google Scholar]
- 12.Saif M.W., Knost J.A., Chiorean E.G., Kambhampati S.R.P., Yu D., Pytowski B., Qin A., Kauh J.S., O’Neil B. Phase 1 study of the anti-vascular endothelial growth factor receptor 3 monoclonal antibody LY3022856/IMC-3C5 in patients with advanced and refractory solid tumors and advanced colorectal cancer. Cancer Chemother. Pharmacol. 2016;78:815–824. doi: 10.1007/s00280-016-3134-3. [DOI] [PubMed] [Google Scholar]
- 13.Ran S., Volk L., Hall K., Flister M.J. Lymphangiogenesis and lymphatic metastasis in breast cancer. Pathophysiol. 2009;17:229–251. doi: 10.1016/j.pathophys.2009.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pereira E.R., Kedrin D., Seano G., Gautier O., Meijer E.F.J., Jones D., Chin S.-M., Kitahara S., Bouta E.M., Chang J., et al. Lymph node metastases can invade local blood vessels, exit the node, and colonize distant organs in mice. Science. 2018;359:1403–1407. doi: 10.1126/science.aal3622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brown M., Assen F.P., Leithner A., Abe J., Schachner H., Asfour G., Bago-Horvath Z., Stein J.V., Uhrin P., Sixt M., et al. Lymph node blood vessels provide exit routes for metastatic tumor cell dissemination in mice. Science. 2018;359:1408–1411. doi: 10.1126/science.aal3662. [DOI] [PubMed] [Google Scholar]
- 16.Chaffer C.L., Weinberg R.A. A Perspective on Cancer Cell Metastasis. Science. 2011;331:1559–1564. doi: 10.1126/science.1203543. [DOI] [PubMed] [Google Scholar]
- 17.Bergers G., Hanahan D. Modes of resistance to anti-angiogenic therapy. Nat. Rev. Cancer. 2008;8:592–603. doi: 10.1038/nrc2442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Steeg P.S. Tumor metastasis: Mechanistic insights and clinical challenges. Nat. Med. 2006;12:895–904. doi: 10.1038/nm1469. [DOI] [PubMed] [Google Scholar]
- 19.Sopik V., Narod S.A. The relationship between tumour size, nodal status and distant metastases: On the origins of breast cancer. Breast Cancer Res. Treat. 2018;170:647–656. doi: 10.1007/s10549-018-4796-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Alitalo A., Detmar M. Interaction of tumor cells and lymphatic vessels in cancer progression. Oncogene. 2011;31:4499–4508. doi: 10.1038/onc.2011.602. [DOI] [PubMed] [Google Scholar]
- 21.Olmeda D., Cerezo-Wallis D., Riveiro-Falkenbach E., Pennacchi P., Contreras-Alcalde M., Ibarz N., Cifdaloz M., Catena X., Calvo T.G., Cañón E., et al. Whole-body imaging of lymphovascular niches identifies pre-metastatic roles of midkine. Nature. 2017;546:676–680. doi: 10.1038/nature22977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ma Q., Dieterich L.C., Ikenberg K., Bachmann S.B., Mangana J., Proulx S.T., Amann V.C., Levesque M.P., Dummer R., Baluk P., et al. Unexpected contribution of lymphatic vessels to promotion of distant metastatic tumor spread. Sci. Adv. 2018;4:eaat4758. doi: 10.1126/sciadv.aat4758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gogineni A., Caunt M., Crow A., Lee C.V., Fuh G., Van Bruggen N., Ye W., Weimer R.M. Inhibition of VEGF-C Modulates Distal Lymphatic Remodeling and Secondary Metastasis. PLoS ONE. 2013;8:68755. doi: 10.1371/journal.pone.0068755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jain R.K. Antiangiogenesis strategies revisited: From starving tumors to alleviating hypoxia. Cancer Cell. 2014;26:605–622. doi: 10.1016/j.ccell.2014.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Corliss B.A., Azimi M.S., Munson J.M., Peirce S.M., Murfee W.L., Munson J. Macrophages: An Inflammatory Link Between Angiogenesis and Lymphangiogenesis. Microcirculation. 2016;23:95–121. doi: 10.1111/micc.12259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Karaman S., Detmar M. Mechanisms of lymphatic metastasis. J. Clin. Investig. 2014;124:922–928. doi: 10.1172/JCI71606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Partanen T.A., Alitalo K., Miettinen M. Lack of lymphatic vascular specificity of vascular endothelial growth factor receptor 3 in 185 vascular tumors. Cancer. 1999;86:2406–2412. doi: 10.1002/(SICI)1097-0142(19991201)86:11<2406::AID-CNCR31>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 28.Valtola R., Salven P., Heikkilä P., Taipale J., Joensuu H., Rehn M., Pihlajaniemi T., Weich H., DeWaal R., Alitalo K. VEGFR-3 and Its Ligand VEGF-C Are Associated with Angiogenesis in Breast Cancer. Am. J. Pathol. 1999;154:1381–1390. doi: 10.1016/S0002-9440(10)65392-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ran S., Montgomery K.E. Macrophage-Mediated Lymphangiogenesis: The Emerging Role of Macrophages as Lymphatic Endothelial Progenitors. Cancers. 2012;4:618–657. doi: 10.3390/cancers4030618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Whitehurst B., Flister M.J., Bagaitkar J., Volk L., Bivens C.M., Pickett B., Castro-Rivera E., Brekken R.A., Gerard R.D., Ran S. Anti-VEGF-A therapy reduces lymphatic vessel density and expression of VEGFR-3 in an orthotopic breast tumor model. Int. J. Cancer. 2007;121:2181–2191. doi: 10.1002/ijc.22937. [DOI] [PubMed] [Google Scholar]
- 31.Jafarnejad M., Ismail A.Z., Duarte D., Vyas C., Ghahramani A., Zawieja D.C., Celso C.L., Poologasundarampillai G., E Moore J. Quantification of the Whole Lymph Node Vasculature Based on Tomography of the Vessel Corrosion Casts. Sci. Rep. 2019;9:13380. doi: 10.1038/s41598-019-49055-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jeong H.-S., Jones D., Liao S., Wattson D.A., Cui C.H., Duda D.G., Willett C.G., Jain R.K., Padera T.P. Investigation of the Lack of Angiogenesis in the Formation of Lymph Node Metastases. J. Natl. Cancer Inst. 2015;107 doi: 10.1093/jnci/djv155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Frentzas S., Simoneau E., Bridgeman V.L., Vermeulen P.B., Foo S., Kostaras E., Nathan M.R., Wotherspoon A., Gao Z.-H., Shi Y., et al. Vessel co-option mediates resistance to anti-angiogenic therapy in liver metastases. Nat. Med. 2016;22:1294–1302. doi: 10.1038/nm.4197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sleeman J.P., Thiele W. Tumor metastasis and the lymphatic vasculature. Int. J. Cancer. 2009;125:2747–2756. doi: 10.1002/ijc.24702. [DOI] [PubMed] [Google Scholar]
- 35.Padera T.P., Rignot E., Jacobs S.S. Lymphatic Metastasis in the Absence of Functional Intratumor Lymphatics. Science. 2002;296:1883–1886. doi: 10.1126/science.1071420. [DOI] [PubMed] [Google Scholar]
- 36.Zhang S., Zhang N., Yi S., Gong M., Lu C., Cai Y., Tang X., Zou L. The relationship of lymphatic vessel density, lymphovascular invasion, and lymph node metastasis in breast cancer: A systematic review and meta-analysis. Oncotarget. 2016;8:2863–2873. doi: 10.18632/oncotarget.13752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Beasley N.J.P., Prevo R., Banerji S., Leek R.D., Moore J., Van Trappen P., Cox G., Harris A.L., Jackson D.G. Intratumoral lymphangiogenesis and lymph node metastasis in head and neck cancer. Cancer Res. 2002;62:1315–1320. [PubMed] [Google Scholar]
- 38.Straume O.R., Jackson D.G., Akslen L.A. Independent prognostic impact of lymphatic vessel density and presence of low-grade lymphangiogenesis in cutaneous melanoma. Clin. Cancer Res. 2003;9:250–256. [PubMed] [Google Scholar]
- 39.Vleugel M.M., Bos R., Van Der Groep P., E Greijer A., Shvarts A., Stel H.V., Van Der Wall E., Van Diest P.J. Lack of lymphangiogenesis during breast carcinogenesis. J. Clin. Pathol. 2004;57:746–751. doi: 10.1136/jcp.2003.014480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Williams C.S., Leek R.D., Robson A.M., Banerji S., Prevo R., Harris A.L., Jackson D.G. Absence of lymphangiogenesis and intratumoural lymph vessels in human metastatic breast cancer. J. Pathol. 2003;200:195–206. doi: 10.1002/path.1343. [DOI] [PubMed] [Google Scholar]
- 41.Van Der Schaft D.W., Pauwels P., Hulsmans S., Zimmermann M., Van De Poll-Franse L.V., Griffioen A.W. Absence of lymphangiogenesis in ductal breast cancer at the primary tumor site. Cancer Lett. 2007;254:128–136. doi: 10.1016/j.canlet.2007.03.001. [DOI] [PubMed] [Google Scholar]
- 42.Agarwal B., Saxena R., Morimiya A., Mehrotra S., Badve S.S. Lymphangiogenesis Does Not Occur in Breast Cancer. Am. J. Surg. Pathol. 2005;29:1449–1455. doi: 10.1097/01.pas.0000174269.99459.9d. [DOI] [PubMed] [Google Scholar]
- 43.Mori D., Yamasaki F., Shibaki M., Tokunaga O. Lateral peritumoral lymphatic vessel invasion can predict lymph node metastasis in esophageal squamous cell carcinoma. Mod. Pathol. 2007;20:694–700. doi: 10.1038/modpathol.3800786. [DOI] [PubMed] [Google Scholar]
- 44.Mohammed R., Ellis I., Mahmmod A.M., Hawkes E.C., Green A.R., Rakha E.A., Martin S.G. Lymphatic and blood vessels in basal and triple-negative breast cancers: Characteristics and prognostic significance. Mod. Pathol. 2011;24:774–785. doi: 10.1038/modpathol.2011.4. [DOI] [PubMed] [Google Scholar]
- 45.Carmeliet P., Jain R.K. Molecular mechanisms and clinical applications of angiogenesis. Nature. 2011;473:298–307. doi: 10.1038/nature10144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Volk-Draper L., Patel R., Bhattarai N., Yang J., Wilber A., DeNardo D., Ran S. Myeloid-Derived Lymphatic Endothelial Cell Progenitors Significantly Contribute to Lymphatic Metastasis in Clinical Breast Cancer. Am. J. Pathol. 2019;189:2269–2292. doi: 10.1016/j.ajpath.2019.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Salven P., Mustjoki S., Alitalo K., Alitalo K., Rafii S. VEGFR-3 and CD133 identify a population of CD34+ lymphatic/vascular endothelial precursor cells. Blood. 2003;101:168–172. doi: 10.1182/blood-2002-03-0755. [DOI] [PubMed] [Google Scholar]
- 48.Religa P., Cao R., Björndahl M., Zhou Z., Zhu Z., Cao Y. Presence of bone marrow–derived circulating progenitor endothelial cells in the newly formed lymphatic vessels. Blood. 2005;106:4184–4190. doi: 10.1182/blood-2005-01-0226. [DOI] [PubMed] [Google Scholar]
- 49.Maruyama K., Ii M., Cursiefen C., Jackson D.G., Keino H., Tomita M., Van Rooijen N., Takenaka H., D’Amore P.A., Stein-Streilein J., et al. Inflammation-induced lymphangiogenesis in the cornea arises from CD11b-positive macrophages. J. Clin. Investig. 2005;115:2363–2372. doi: 10.1172/JCI23874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kerjaschki D., Huttary N., Raab I., Regele H., Bojarski-Nagy K., Bartel G., Kröber S.M., Greinix H., Rosenmaier A., Karlhofer F., et al. Lymphatic endothelial progenitor cells contribute to de novo lymphangiogenesis in human renal transplants. Nat. Med. 2006;12:230–234. doi: 10.1038/nm1340. [DOI] [PubMed] [Google Scholar]
- 51.Lee J.Y., Park C., Cho Y.P., Lee E., Kim H., Kim P., Yun S.H., Yoon Y. Podoplanin-expressing cells derived from bone marrow play a crucial role in postnatal lymphatic neovascularization. Circulation. 2010;122:1413–1425. doi: 10.1161/CIRCULATIONAHA.110.941468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chow M.T., Luster A.D. Chemokines in cancer. Cancer Immunol. Res. 2014;2:1125–1131. doi: 10.1158/2326-6066.CIR-14-0160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ding Y., Shimada Y., Maeda M., Kawabe A., Kaganoi J., Komoto I., Hashimoto Y., Miyake M., Hashida H., Imamura M. Association of CC chemokine receptor 7 with lymph node metastasis of esophageal squamous cell carcinoma. Clin. Cancer Res. 2003;9:3406–3412. [PubMed] [Google Scholar]
- 54.Takeuchi H. CCL21 Chemokine Regulates Chemokine Receptor CCR7 Bearing Malignant Melanoma Cells. Clin. Cancer Res. 2004;10:2351–2358. doi: 10.1158/1078-0432.CCR-03-0195. [DOI] [PubMed] [Google Scholar]
- 55.Cabioglu N., Yazici M.S., Arun B., Broglio K., Hortobagyi G.N., Price J.E., Sahin A. CCR7 and CXCR4 as Novel Biomarkers Predicting Axillary Lymph Node Metastasis in T1 Breast Cancer. Clin. Cancer Res. 2005;11:5686–5693. doi: 10.1158/1078-0432.CCR-05-0014. [DOI] [PubMed] [Google Scholar]
- 56.Comerford I., Harata-Lee Y., Bunting M.D., Gregor C., Kara E.E., McColl S.R. A myriad of functions and complex regulation of the CCR7/CCL19/CCL21 chemokine axis in the adaptive immune system. Cytokine Growth Factor Rev. 2013;24:269–283. doi: 10.1016/j.cytogfr.2013.03.001. [DOI] [PubMed] [Google Scholar]
- 57.Farnsworth R.H., Karnezis T., Maciburko S.J., Mueller S.N., Stacker S.A. The Interplay Between Lymphatic Vessels and Chemokines. Front. Immunol. 2019;10:518. doi: 10.3389/fimmu.2019.00518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kabashima K., Shiraishi N., Sugita K., Mori T., Onoue A., Kobayashi M., Sakabe J.-I., Yoshiki R., Tamamura H., Fujii N., et al. CXCL12-CXCR4 Engagement Is Required for Migration of Cutaneous Dendritic Cells. Am. J. Pathol. 2007;171:1249–1257. doi: 10.2353/ajpath.2007.070225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kim M., Koh Y.J., Kim K.E., Koh B.I., Nam D.-H., Alitalo K., Kim I., Koh G.Y. CXCR4 Signaling Regulates Metastasis of Chemoresistant Melanoma Cells by a Lymphatic Metastatic Niche. Cancer Res. 2010;70:10411–10421. doi: 10.1158/0008-5472.CAN-10-2591. [DOI] [PubMed] [Google Scholar]
- 60.Mukherjee D., Zhao J. The Role of chemokine receptor CXCR4 in breast cancer metastasis. Am. J. Cancer Res. 2013;3:46–57. [PMC free article] [PubMed] [Google Scholar]
- 61.Noy R., Pollard J.W. Tumor-associated macrophages: From mechanisms to therapy. Immunity. 2014;41:49–61. doi: 10.1016/j.immuni.2014.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Barnett F.H., Rosenfeld M., Wood M., Kiosses W.B., Usui Y., Marchetti V., Aguilar E., Friedlander M. Macrophages form functional vascular mimicry channels in vivo. Sci. Rep. 2016;6:36659. doi: 10.1038/srep36659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Elder A.M., Tamburini B., Crump L.S., A Black S., Wessells V.M., Schedin P., Borges V.F., Lyons T.R. Semaphorin 7A promotes macrophage-mediated lymphatic remodeling during postpartum mammary gland involution and in breast cancer. Cancer Res. 2018;78:6473–6485. doi: 10.1158/0008-5472.CAN-18-1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Jones D., Pereira E.R., Padera T.P. Growth and Immune Evasion of Lymph Node Metastasis. Front. Oncol. 2018;8:36. doi: 10.3389/fonc.2018.00036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Skobe M., Hamberg L.M., Hawighorst T., Schirner M., Wolf G.L., Alitalo K., Detmar M. Concurrent Induction of Lymphangiogenesis, Angiogenesis, and Macrophage Recruitment by Vascular Endothelial Growth Factor-C in Melanoma. Am. J. Pathol. 2001;159:893–903. doi: 10.1016/S0002-9440(10)61765-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Alishekevitz D., Gingis-Velitski S., Kaidar-Person O., Gutter-Kapon L., Scherer S.D., Raviv Z., Merquiol E., Ben-Nun Y., Miller V., Rachman-Tzemah C., et al. Macrophage-Induced Lymphangiogenesis and Metastasis following Paclitaxel Chemotherapy Is Regulated by VEGFR3. Cell Rep. 2016;17:1344–1356. doi: 10.1016/j.celrep.2016.09.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bieniasz-Krzywiec P., Martín-Pérez R., Ehling M., García-Caballero M., Pinioti S., Pretto S., Kroes R., Aldeni C., Di Matteo M., Prenen H., et al. Podoplanin-Expressing Macrophages Promote Lymphangiogenesis and Lymphoinvasion in Breast Cancer. Cell Metab. 2019;30:917–936. doi: 10.1016/j.cmet.2019.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Evans R., Flores-Borja F., Nassiri S., Miranda E., Lawler K., Grigoriadis A., Monypenny J., Gillet C., Owen J., Gordon P., et al. Integrin-Mediated Macrophage Adhesion Promotes Lymphovascular Dissemination in Breast Cancer. Cell Rep. 2019;27:1967–1978.e4. doi: 10.1016/j.celrep.2019.04.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Tacconi C., Ungaro F., Correale C., Arena V., Massimino L., Detmar M., Spinelli A., Carvello M.M., Mazzone M., Oliveira A.I., et al. Activation of the VEGFC/VEGFR3 Pathway Induces Tumor Immune Escape in Colorectal Cancer. Cancer Res. 2019;79:4196–4210. doi: 10.1158/0008-5472.CAN-18-3657. [DOI] [PubMed] [Google Scholar]
- 70.DiSibio G., French S.W. Metastatic patterns of cancers: Results from a large autopsy study. Arch. Pathol. Lab. Med. 2008;132:931–939. doi: 10.5858/2008-132-931-MPOCRF. [DOI] [PubMed] [Google Scholar]
- 71.Ullah I., Karthik G.-M., Alkodsi A., Kjallquist U., Stålhammar G., Lövrot J., Martinez N.-F., Lagergren J., Hautaniemi S., Hartman J., et al. Evolutionary history of metastatic breast cancer reveals minimal seeding from axillary lymph nodes. J. Clin. Investig. 2018;128:1355–1370. doi: 10.1172/JCI96149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Naxerova K., Reiter J.G., Brachtel E., Lennerz J.K., Van De Wetering M., Rowan A., Cai T., Clevers H., Swanton C., Nowak M.A., et al. Origins of lymphatic and distant metastases in human colorectal cancer. Science. 2017;357:55–60. doi: 10.1126/science.aai8515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kerjaschki D., Bago-Horvath Z., Rudas M., Sexl V., Schneckenleithner C., Wolbank S., Bartel G., Krieger S., Kalt R., Hantusch B., et al. Lipoxygenase mediates invasion of intrametastatic lymphatic vessels and propagates lymph node metastasis of human mammary carcinoma xenografts in mouse. J. Clin. Investig. 2011;121:2000–2012. doi: 10.1172/JCI44751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Jain R.K., Tong R.T., Munn L.L. Effect of vascular normalization by antiangiogenic therapy on interstitial hypertension, peritumor edema, and lymphatic metastasis: Insights from a mathematical model. Cancer Res. 2007;67:2729–2735. doi: 10.1158/0008-5472.CAN-06-4102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Tammela T., Saaristo A., Holopainen T., Lyytikkä J., Kotronen A., Pitkonen M., Abo-Ramadan U., Ylä-Herttuala S., Petrova T.V., Alitalo K. Therapeutic differentiation and maturation of lymphatic vessels after lymph node dissection and transplantation. Nat. Med. 2007;13:1458–1466. doi: 10.1038/nm1689. [DOI] [PubMed] [Google Scholar]
- 76.Tacconi C., Correale C., Gandelli A., Spinelli A., Dejana E., D’Alessio S., Danese S. Vascular Endothelial Growth Factor C Disrupts the Endothelial Lymphatic Barrier to Promote Colorectal Cancer Invasion. Gastroenterol. 2015;148:1438–1451.e8. doi: 10.1053/j.gastro.2015.03.005. [DOI] [PubMed] [Google Scholar]
- 77.Schwager S., Detmar M. Inflammation and Lymphatic Function. Front. Immunol. 2019;10:308. doi: 10.3389/fimmu.2019.00308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zamora A., Alves M., Chollet C., Therville N., Fougeray T., Tatin F., Franchet C., Gomez-Brouchet A., Vaysse C., Martinez L.O., et al. Paclitaxel induces lymphatic endothelial cells autophagy to promote metastasis. Cell Death Dis. 2019;10:1–12. doi: 10.1038/s41419-019-2181-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Sawane M., Kajiya K., Kidoya H., Takagi M., Muramatsu F., Takakura N. Apelin Inhibits Diet-Induced Obesity by Enhancing Lymphatic and Blood Vessel Integrity. Diabetes. 2013;62:1970–1980. doi: 10.2337/db12-0604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ecker B.L., Kaur A., Douglass S.M., Webster M.R., Almeida F., Marino G., Sinnamon A.J., Neuwirth M.G., Alicea G.M., Ndoye A., et al. Age-Related Changes in HAPLN1 Increase Lymphatic Permeability and Affect Routes of Melanoma Metastasis. Cancer Discov. 2018;9:82–95. doi: 10.1158/2159-8290.CD-18-0168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Dieterich L., Kapaklikaya K., Cetintas T., Proulx S.T., Commerford C.D., Ikenberg K., Bachmann S.B., Scholl J., Detmar M. Transcriptional profiling of breast cancer-associated lymphatic vessels reveals VCAM-1 as regulator of lymphatic invasion and permeability. Int. J. Cancer. 2019;145:2804–2815. doi: 10.1002/ijc.32594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hoshida T. Imaging Steps of Lymphatic Metastasis Reveals That Vascular Endothelial Growth Factor-C Increases Metastasis by Increasing Delivery of Cancer Cells to Lymph Nodes: Therapeutic Implications. Cancer Res. 2006;66:8065–8075. doi: 10.1158/0008-5472.CAN-06-1392. [DOI] [PubMed] [Google Scholar]
- 83.Liao S., Bouta E.M., Morris L.M., Jones D., Jain R.K., Padera T.P. Inducible Nitric Oxide Synthase and CD11b+Gr1+ Cells Impair Lymphatic Contraction of Tumor-Draining Lymphatic Vessels. Lymphat. Res. Boil. 2019;17:294–300. doi: 10.1089/lrb.2018.0013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Karnezis T., Shayan R., Caesar C., Roufail S., Harris N.C., Ardipradja K., Zhang Y.F., Williams S.P., Farnsworth R.H., Chai M.G., et al. VEGF-D Promotes Tumor Metastasis by Regulating Prostaglandins Produced by the Collecting Lymphatic Endothelium. Cancer Cell. 2012;21:181–195. doi: 10.1016/j.ccr.2011.12.026. [DOI] [PubMed] [Google Scholar]
- 85.Swartz M.A., Lund A.W. Lymphatic and interstitial flow in the tumour microenvironment: Linking mechanobiology with immunity. Nat. Rev. Cancer. 2012;12:210–219. doi: 10.1038/nrc3186. [DOI] [PubMed] [Google Scholar]
- 86.Kataru R.P., Ly C.L., Shin J., Park H.J., Baik J.E., Rehal S., Ortega S., Lyden D., Mehrara B. Tumor lymphatic function regulates tumor inflammatory and immunosuppressive microenvironments. Cancer Immunol. Res. 2019;7:1345–1358. doi: 10.1158/2326-6066.CIR-18-0337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Lund A.W., Wagner M., Fankhauser M., Steinskog E.S., Broggi M.A., Spranger S., Gajewski T.F., Alitalo K., Eikesdal H.P., Wiig H., et al. Lymphatic vessels regulate immune microenvironments in human and murine melanoma. J. Clin. Investig. 2016;126:3389–3402. doi: 10.1172/JCI79434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Slaney C.Y., Kershaw M., Darcy P.K. Trafficking of T Cells into Tumors. Cancer Res. 2014;74:7168–7174. doi: 10.1158/0008-5472.CAN-14-2458. [DOI] [PubMed] [Google Scholar]
- 89.Fukumura D., Kloepper J., Amoozgar Z., Duda D.G., Jain R.K. Enhancing cancer immunotherapy using antiangiogenics: Opportunities and challenges. Nat. Rev. Clin. Oncol. 2018;15:325–340. doi: 10.1038/nrclinonc.2018.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Fankhauser M., Broggi M.A., Potin L., Bordry N., Jeanbart L., Lund A.W., Da Costa E., Hauert S., Rincon-Restrepo M., Tremblay C., et al. Tumor lymphangiogenesis promotes T cell infiltration and potentiates immunotherapy in melanoma. Sci. Transl. Med. 2017;9:eaal4712. doi: 10.1126/scitranslmed.aal4712. [DOI] [PubMed] [Google Scholar]
- 91.Song E., Mao T., Dong H., Boisserand L.S.B., Antila S., Bosenberg M., Alitalo K., Thomas J.-L., Iwasaki A. VEGF-C-driven lymphatic drainage enables immunosurveillance of brain tumours. Nature. 2020;577:689–694. doi: 10.1038/s41586-019-1912-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Pereira E.R., Jones D., Jung K., Padera T.P. The lymph node microenvironment and its role in the progression of metastatic cancer. Semin. Cell Dev. Boil. 2015;38:98–105. doi: 10.1016/j.semcdb.2015.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Hirosue S., Vokali E., Raghavan V.R., Rincon-Restrepo M., Lund A.W., Corthésy-Henrioud P., Capotosti F., Winter C.H., Hugues S., Swartz M.A. Steady-State Antigen Scavenging, Cross-Presentation, and CD8+ T Cell Priming: A New Role for Lymphatic Endothelial Cells. J. Immunol. 2014;192:5002–5011. doi: 10.4049/jimmunol.1302492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Lane R.S., Femel J., Breazeale A.P., Loo C.P., Thibault G., Kaempf A., Mori M., Tsujikawa T., Chang Y.H., Lund A.W. IFNgamma-activated dermal lymphatic vessels inhibit cytotoxic T cells in melanoma and inflamed skin. J. Exp. Med. 2018;215:3057–3074. doi: 10.1084/jem.20180654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Antila S., Karaman S., Nurmi H., Airavaara M., Voutilainen M.H., Mathivet T., Chilov D., Li Z., Koppinen T., Park J.-H., et al. Development and plasticity of meningeal lymphatic vessels. J. Exp. Med. 2017;214:3645–3667. doi: 10.1084/jem.20170391. [DOI] [PMC free article] [PubMed] [Google Scholar]