Abstract
Cardiovascular disease (CVD) is the main cause of death worldwide, and aging is its leading risk factor. Aging is much accelerated in Hutchinson–Gilford progeria syndrome (HGPS), an ultra-rare genetic disorder provoked by the ubiquitous expression of a mutant protein called progerin. HGPS patients die in their teens, primarily due to cardiovascular complications. The primary causes of age-associated CVD are endothelial dysfunction and dysregulated vascular tone; however, their contribution to progerin-induced CVD remains poorly characterized. In the present study, we found that progeroid LmnaG609G/G609G mice with ubiquitous progerin expression show both endothelial dysfunction and severe contractile impairment. To assess the relative contribution of specific vascular cell types to these anomalies, we examined LmnaLCS/LCSTie2Cretg/+ and LmnaLCS/LCSSm22αCretg/+ mice, which express progerin specifically in endothelial cells (ECs) and vascular smooth muscle cells (VSMCs), respectively. Whereas vessel contraction was impaired in mice with VSMC-specific progerin expression, we observed no endothelial dysfunction in mice with progerin expression restricted to VSMCs or ECs. Vascular tone regulation in progeroid mice was ameliorated by dietary sodium nitrite supplementation. Our results identify VSMCs as the main cell type causing contractile impairment in a mouse model of HGPS that is ameliorated by nitrite treatment.
Keywords: HGPS, progerin, VSMCs, ECs, vascular function, sodium nitrite
1. Introduction
Cardiovascular disease (CVD) is the leading cause of death worldwide, in part due to progressive aging, the main CVD risk factor [1]. A number of additional factors have been identified that increase the risk of developing CVD, either acting alone or in combination (e.g., hypercholesterolemia, diabetes, sedentary lifestyle, and smoking) [2,3,4]. However, studies on the effects of age alone (the only factor we cannot modify or treat) remain scarce due to their high research costs associated with the necessary long-term resource investment and delays in collecting results. The study of premature aging syndromes characterized by accelerated CVD thus offers a unique opportunity to investigate age-dependent drivers of CVD in the absence of other confounding risk factors [5,6].
Hutchinson–Gilford progeria syndrome (HGPS, OMIM 176670) is a rare genetic disease characterized by accelerated aging and death in adolescence [5,6,7,8,9]. HGPS children have impaired postnatal growth, lipodystrophy, alopecia, pigmented and wrinkled skin, and skeletal dysplasia. They also develop generalized atherosclerosis, arterial stiffness and calcification, electrocardiographic abnormalities, and ventricular diastolic dysfunction and die prematurely at an average age of 14.6 years mainly due to myocardial infarction, stroke, or heart failure [7,10,11]. HGPS is caused by a heterozygous de novo point mutation in the LMNA gene, which encodes the nuclear proteins lamin A and C (A-type lamins) [12,13]. This synonymous mutation activates a cryptic splice donor site that removes 150 nucleotides from exon 11, causing the synthesis of progerin [12,13]. This truncated form of prelamin A is expressed ubiquitously and acts in a dominant-negative manner, causing alterations in many essential nuclear functions, including nuclear structure, gene transcription, signal transduction, DNA damage repair, chromatin organization, mechanosensing, and proliferation [14,15]. Aside from its role in HGPS, progerin is detectable at low levels in several tissues during normal aging, including atherosclerotic coronary arteries, suggesting a role in physiological aging [11,16,17,18,19].
Homozygous Lmna-null mice lacking A-type lamins develop to term without exhibiting overt anomalies, but they develop skeletal muscle dystrophy and dilated cardiomyopathy soon after birth and are all death by the eight week [20]. Interestingly, “Lamin C-Stop” mice (LCS) expressing Lamin C but lacking lamin A are apparently normal, but are slightly heavier and longer-lived than wild-type controls [21]. Over the last decade, several animal models of HGPS have been generated and characterized [6,22]. LmnaG609G/G609G mice, which express ubiquously progerin and lamin C and lack lamin A, recapitulate the main clinical manifestations of human HGPS, such as difficulty to thrive, lipodystrophy, skeleton abnormalities, vascular calcification and stiffening, vascular smooth muscle cell (VSMC) loss, and premature death [23,24,25]. Moreover, ubiquitous or VSMC-specific progerin expression accelerates atherosclerosis in atherosclerosis-prone Apolipoprotein e-null mice (Apoe−/−), at least in part due to excessive endoplasmic reticulum stress and associated unfolded protein response [26,27].
Vasomotor function is modulated by a delicate balance between constriction and dilation, which are mainly controlled by luminal endothelial cells (ECs) and medial VSMCs [28,29,30]. The most common pathological signs of vascular aging are endothelial dysfunction and vessel stiffness, which can progress over the years to hypertension, cardiac and vessel overload, inflammation, fibrosis, atherosclerosis, and heart failure [29,30,31]. The stiffness of aged vessels correlates with changes in the mechanical properties of VSMCs, which lose their ability to contract when surrounded by an abnormally stiff extracellular matrix [32,33]. Vessel stiffness is also a pathological signature of HGPS [7,34], and progerin-expressing LmnaG609G/G609G mice exhibit altered vascular structure characterized by stiffness and inward remodeling associated with VSMC degeneration and increased collagen deposition in the medial layer [25]. In the present study, we investigated whether endothelial dysfunction and defective regulation of vascular tone characterize progeroid LmnaG609G/G609G mice. Moreover, to determine the relative contributions made by progerin-expressing ECs and VSMCs, we bred LmnaLCS/LCS Tie2Cre+/tg and LmnaLCS/LCS SM22αCre+/tg mice, which express progerin specifically in ECs and VSMCs respectively, but do not show any apparent signs of aging [25]. We also examined the potential benefit of treatment with nitrites, a physiologically important storage form of nitric oxide (NO) [35] that can partly revert vascular endothelial dysfunction and stiffness, oxidative stress, and inflammation in physiological aging [36] and ameliorates structural stiffening in progeroid mice [25].
2. Materials and Methods
2.1. Mice
All procedures with mice conformed to EU Directive 2010/63EU and Recommendation 2007/526/EC, enforced in Spanish law under Real Decreto 53/2013. Animal protocols were approved by the local ethics committees and the Animal Protection Area of the Comunidad Autónoma de Madrid (PROEX 135/14). Mice were housed at the CNIC pathogen free facility and sacrificed at 14–15 weeks of age.
Studies were carried out with males of the following genotypes (all on the C57BL/6J genetic background): LmnaG609G/G609G mice with constitutive and ubiquitous progerin and lamin C expression and lack of lamin A, obtained by crossing heterozygous LmnaG609G/+ males and females [23]; LmnaLCS/LCSSM22αCretg/+ mice with ubiquitous lamin C expression and VSMC-specific progerin expression, obtained by crossing LmnaLCS/LCS mice [23] with SM22αCre+/− mice (The Jackson Laboratory, Bar Harbor, ME, USA); LmnaLCS/LCSTie2Cretg/+ mice with ubiquituous expression of lamin C and EC-specific progerin expression, obtained by crossing LmnaLCS/LCS mice with Tie2-Cre+/− mice [37]. Controls for LmnaG609G/G609G mice were littermate wild-type mice expressing normal lamin A/C (Lmna+/+), and controls for the VSMC- and EC-specific models were LmnaLCS/LCS littermates expressing only Lamin C [23]. Specific expression of progerin in VSMCs and ECs was confirmed by immunohistochemistry in aortic sections from LmnaLCS/LCSSM22αCretg/+ and LmnaLCS/LCSTie2Cretg/+ mice [25].
2.2. Nitrite Treatment
When indicated, 6-week-old LmnaG609G/G609G mice were treated for 8 weeks with sodium nitrite (NaNO2, Sigma-Aldrich, St. Louis, MO, USA). The compound was dissolved in drinking water at a final concentration of 50 mg/L, a dose that has been reported to be safe in mice, showing no evidence of toxicological or carcinogenic effects and no effect on water consumption [38]. Consistent with these findings, we observed no adverse effects or changes in water consumption in LmnaG609G/G609G or Lmna+/+ mice treated with sodium nitrite.
2.3. Wire Myography
Animals were euthanized at 14–15 weeks of age by CO2 inhalation. Immediately after sacrifice, the thoracic and abdominal cavities were opened. Thoracic aortas were excised and immediately placed in ice-cold Krebs–Henseleit solution (KHS: 115 mM NaCl, 2.5 mM CaCl2, 4.6 mM KCl, 1.2 mM KH2PO4, 1.2 mM MgSO4, 25 mM NaHCO3, 11.1 mM glucose, and 0.01 mM EDTA), gently cleaned of fat and connective tissue, and cut into ~2 mm long segments. Aortic rings were then mounted on 2 tungsten wires in a wire myograph system (620M, Danish Myo Technology A/S, Hinnerup, Denmark) and immersed in 37 °C KHS with constant gassing (95% O2 and 5% CO2). Wire myography was performed as previously described [39]. Optimal vessel distension was determined by normalization using the Laplace Equation (Tension = [pressure × radius]/thickness) to calculate the position at which the tension was equivalent to an intraluminal pressure of 100 mmHg (L100) [39]; vessels were then set up to the optimal tension (physiological distension, 0.9 of L100).
After equilibration for 30 min, vasoconstriction was studied by exposing the aortic rings first to 120 mM KCl and then to increasing doses of phenylephrine (from 1 nm to 10 µM; Sigma-Aldrich). We assessed the contribution of vessel stiffness to the contractile function by analyzing contraction induced by 120 mM KCl before and after collagen degradation with collagenase type II (0.2% w/v, 15 min incubation; Thermo Fisher Scientific, Waltham, MA, USA). Endothelium-dependent vasodilation was assessed by examining the response to increasing doses of acetylcholine (from 0.1 nM to 10 µM; Sigma-Aldrich) in segments previously contracted with 1 µM phenylephrine. Endothelium-independent vasodilation induced by increasing doses of the NO donor diethylamine NONOate (DEA-NO) (from 0.1 nM to 10 µM; Sigma-Aldrich) was examined in segments previously contracted with 1 µM phenylephrine. Drug treatments were separated by extensive washes and a stabilization period of at least 15 min. When indicated, the contribution of NO, Prostacilin I2 (PGI2), H2O2, and O2− to endothelium-dependent vasodilation was assessed by adding the following agents to the bath 30 min before the acetylcholine dose–response curve: 0.1 mM L-NAME (NOS inhibitor), 10 µM tranylcypromine (PGI2 inhibitor), 2000 U/mL catalase (H2O2 decomposing agent), and 0.1 mM Tempol (O2− scavenger) (all from Sigma-Aldrich). All drugs were dissolved in water except for the Tempol stock solution, which was prepared in ethanol.
The results of wire myography experiments are represented as dose–response curves and as area under the curve (AUC), which provides information regarding differences in the dose–response curve as a whole and in a continuous manner.
2.4. Statistical Analysis
Results are represented as mean ± standard error of the media (SEM), and statistical differences were analyzed using the tests indicated in the figure legends.
3. Results
3.1. Impaired Vascular Function in Progeroid Mice with Ubiquitous Progerin Expression
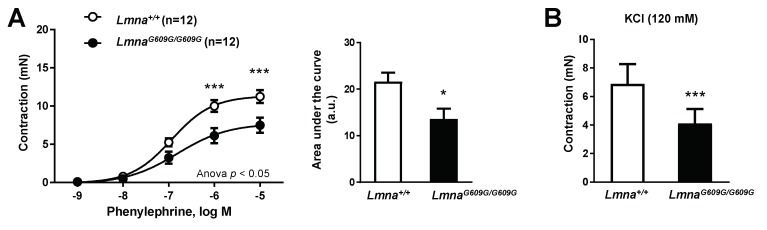
To investigate the impact of ubiquitous progerin expression on vascular function, we performed ex vivo wire myography experiments to examine contraction and dilation in thoracic aorta segments obtained from 14–15-week-old LmnaG609G/G609G mice and age-matched wild-type (Lmna+/+) littermate controls. These studies demonstrated impaired contraction of progeroid vessel segments in response to incubation with phenylephrine (Figure 1A) and KCl (Figure 1B).
Figure 1.
Defective aortic contraction in LmnaG609G/G609G mice. Thoracic aortas from 14-week-old progeroid LmnaG609G/G609G mice (ubiquitous progerin expression) and wild-type (Lmna+/+) mice were analyzed by wire myography (n = 12 for each genotype). (A) Strength of contraction induced by phenylephrine (left) and representation of the area under de curve (right; a.u.: arbitrary units). (B) Strength of contraction induced by KCl. Statistical differences were analyzed by two-way ANOVA with Bonferroni’s post-hoc test for phenylephrine data, and by two-tailed t-test for KCl data. * p < 0.05 *** p < 0.001.
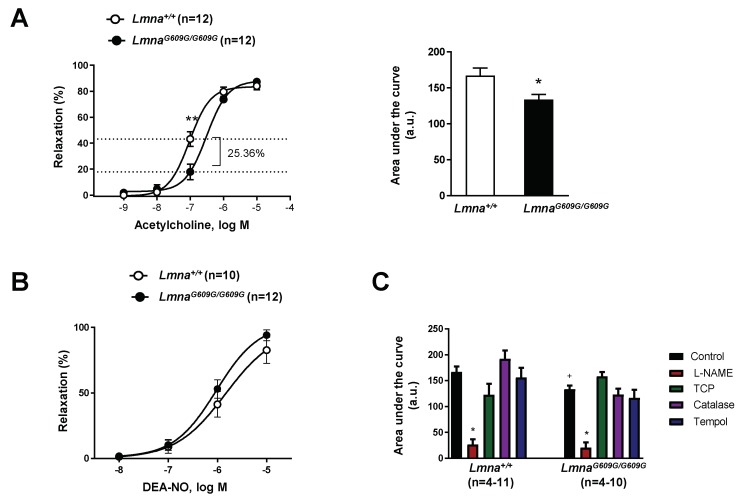
To assess endothelial function, we first exposed phenylephrine-precontrated aortas to the endothelium-dependent vasodilator acetylcholine (Figure 2A). Relaxation induced by the physiological acetylcholine dose (0.1 µM) was significantly lower in LmnaG609G/G609G vessel segments, a result also evidenced by a difference in logEC50 (−7.025 ± 0.06 in Lmna+/+ aortic rings versus −6.511 ± 0.09 in LmnaG609G/G609G aortic rings) (Figure 2A, left) and a lower AUC for acetylcholine-induced relaxation (Figure 2A, right). In contrast, there were no significant differences in the relaxation of phenylephrine-precontrated Lmna+/+ and LmnaG609G/G609G aortic rings exposed to the endothelium-independent vasodilator DEA-NO (Figure 2B). These results thus indicate that endothelial dysfunction underlies impaired vessel relaxation in mice with ubiquitous progerin expression.
Figure 2.
Defective endothelium-dependent aortic dilation in LmnaG609G/G609G mice. Thoracic aortas from 14-week-old progeroid LmnaG609G/G609G mice (ubiquitous progerin expression) and wild-type mice (Lmna+/+) were analyzed by wire myography (n = 12 for each genotype). (A) Endothelium-dependent vasodilation induced by acetylcholine (left) and representation of the area under the curve (AUC; a.u.: arbitrary units) (right). (B) Endothelium-independent vasodilation induced by the NO donor diethylamine NONOate (DEA-NO). (C) Representation of area under the curve from acetylcholine-dependent relaxation curves performed in the absence (control) or presence of L-NAME, tranylcypromine (TCP), catalase, or Tempol. Statistical differences were analyzed by two-way ANOVA with Bonferroni’s post-hoc test for acetylcholine and DEA-NO data, and by one-way ANOVA for AUC data. * p < 0.05 and ** p < 0.01 compared with corresponding control in (A) and (C); +, p < 0.05 Lmna+/+ versus LmnaG609G/G609G in (C).
We next investigated which of the main factors contributing to acetylcholine-induced endothelial relaxation were altered in LmnaG609G/G609G aorta. For this, we prepared acetylcholine-induced relaxation curves in the presence of the following agents: L-NAME (NOS inhibitor), tranylcypromine (PGI2 synthase inhibitor), catalase (H2O2 decomposing agent), and Tempol (O2− scavenger). Significant inhibition of acetylcholine-induced relaxation was observed only in L-NAME-treated Lmna+/+ and LmnaG609G/G609G aortic rings; the other drugs had no significant effect irrespective of mouse genotype (Figure 2C). These findings are consistent with the notion that NO is the main factor underlying endothelium-dependent acetylcholine-induced relaxation in vessels of both genotypes and suggest that NO deficiency may account for the endothelial dysfunction in vessels of mice with ubiquitous progerin expression.
3.2. VSMC-Specific Progerin Expression Provokes Contractile Impairment, but Neither VSMC-Specific nor EC-Specific Expression Is Sufficient to Cause Endothelial Dysfunction
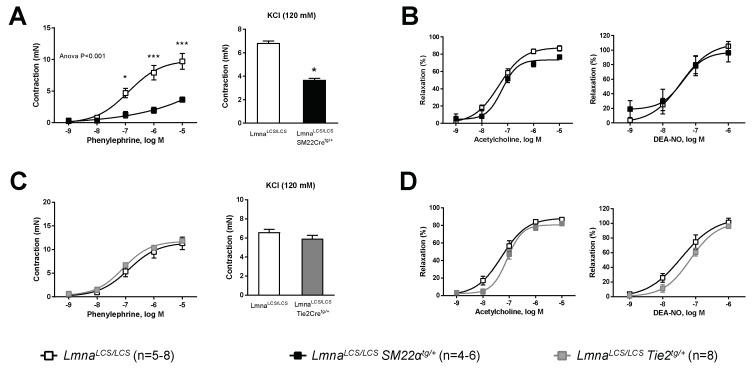
VSMCs and ECs are key cellular components of the vessel wall that play major roles in the regulation of vascular tone. To determine the relative contribution of these cell types to impaired vascular tone regulation in progeroid mice, we performed wire myography experiments in aortic rings isolated from LmnaLCS/LCSSM22αCretg/+ and LmnaLCS/LCSTie2Cretg/+ mice, which express progerin predominantly in VSMCs and ECs, respectively [25]. Controls for both models were LmnaLCS/LCS littermates that do not express progerin [23]. Contraction in response to phenylephrine or KCl was significantly lower in aortic rings from mice with VSMC-specific progerin expression (Figure 3A), like in aortas from LmnaG609G/G609G mice with ubiquous progerin expression (cf. Figure 1A). In contrast, aortas expressing progerin only in ECs contracted normally when incubated with phenylephrine or KCl (Figure 3C). Likewise, vasodilation induced by either acetylcholine or DEA-NO was not significantly different from controls in LmnaLCS/LCSSM22αCretg/+ (Figure 3B) or LmnaLCS/LCSTie2Cretg/+ aortic rings (Figure 3D). These results demonstrate that smooth muscle, not endothelium, is the main vascular cell type driving contractile impairment in progeroid mice and suggest that progeroid endothelial dysfunction requires simultaneous expression of progerin in VSMCs and ECs.
Figure 3.
Progerin expression restricted to VSMCs impairs aortic contraction, but VSMC-specific and EC-specific progerin expression is not sufficient to cause defective vessel relaxation. Wire myography was performed in thoracic aorta rings from 14-week-old mice expressing progerin specifically in VSMCs (LmnaLCS/LCSSM22αtg/+) or in ECs (LmnaLCS/LCSTie2tg/+) and in control mice without progerin expression (LmnaLCS/LCS). (A) Contraction induced by phenylephrine (left) and KCl (right) in aortic rings from LmnaLCS/LCSSM22αtg/+ mice and LmnaLCS/LCS controls. (B) Endothelium-dependent vasodilation induced by acetylcholine (left) and endothelium-independent vasodilation induced by diethylamine NONOate (DEA-NO) (right) in aortic rings from LmnaLCS/LCSSM22αtg/+ mice and LmnaLCS/LCS controls. (C) Contraction induced by phenylephrine (left) and KCl (right) in aortic rings from LmnaLCS/LCSTie2tg/+ mice and LmnaLCS/LCS controls. (D) Endothelium-dependent vasodilation induced by acetylcholine (left) and endothelium-independent vasodilation induced by DEA-NO (right) in aortic rings from LmnaLCS/LCSTie2tg/+ mice and LmnaLCS/LCS controls. Statistical differences were analyzed by two-way ANOVA with Bonferroni’s post-hoc test for acethylcholine, DEA-NO, and phenylephrine data, and by two-tailed t-test for KCl data. * p < 0.05; *** p < 0.001.
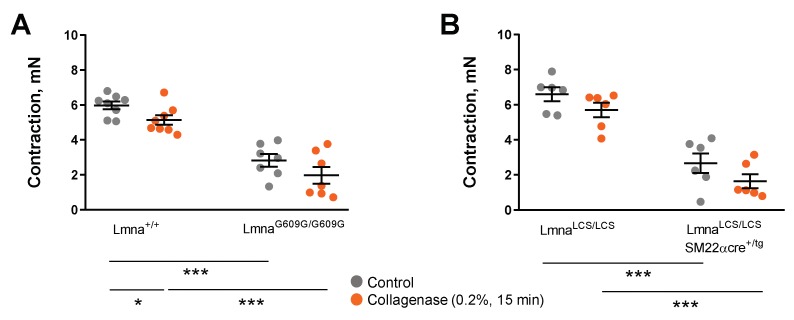
We next tested whether mechanical impediment by the stiff extracellular cell matrix might contribute to contractile impairment in progeroid mice. Since collagen deposition has been shown to cause aortic stiffness in progeroid LmnaG609G/G609G mice [25], we examined KCl-induced aortic contractions before and after collagen disruption with collagenase. Treatment with collagenase did not improve KCl-induced aortic contraction in LmnaG609G/G609G and LmnaLCS/LCSSM22αtg/+ mice (Figure 4A,B).
Figure 4.
Collagen disruption by collagenase does not rescue contractile impairment in progerin-expressing mice. Wire myography experiments to test the effect of 15 min incubation with 0.2% collagenase on contractile responses induced by 120 mM KCl in aortic rings. (A) LmnaG609G/G609G mice and wild-type controls (Lmna+/+). (B) LmnaLCS/LCSSM22αtg/+ mice and LmnaLCS/LCS controls. Statistical differences were analyzed with two-way ANOVA followed by the Sidak multiple comparison test. * p < 0.05; *** p < 0.001.
3.3. Treatment with Sodium Nitrite Improves Vascular Function in Progeroid Mice
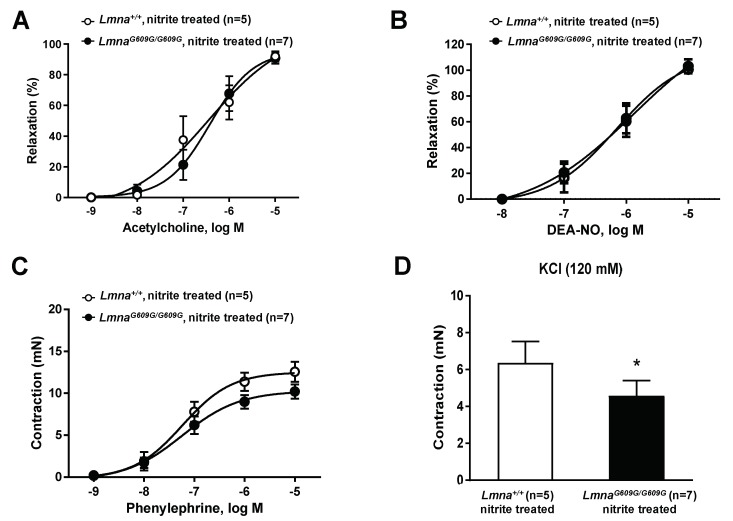
The key role of NO in progerin-induced endothelial dysfunction (Figure 2C) suggested that NO supplementation might improve vascular tone regulation in progeroid mice. We therefore treated LmnaG609G/G609G mice and Lmna+/+ controls with drinking water supplemented with sodium nitrite (see Materials and Methods). Wire myography with isolated aortic rings showed that nitrite treatment restored sensitivity to acethylcholine-induced aortic relaxation in progeroid aortic rings (Figure 5A, cf. Figure 2A). Nitrite treatment had no effect on DEA-NO-induced endothelium-independent relaxation in progerid or control mice (Figure 5B, cf. Figure 2B). Nitrite treatment also ameliorated the impaired phenylephrine-dependent contraction in aortic rings from progeroid mice (Figure 5C, cf. Figure 1A). In contrast, nitrite treatment did not normalize KCl-induced contraction, which remained significantly lower in progeroid aortic rings control aortic rings than in vessels from Lmna+/+ controls (Figure 5B, cf. Figure 1B). These results demonstrate that dietary supplementation with sodium nitrite can ameliorate defective vascular tone regulation in progeroid mice.
Figure 5.
Sodium nitrite treatment improves vascular tone regulation in progeroid mice. Wire myography in thoracic aorta rings from 14-week-old LmnaG609G/G609G and wild-type mice (Lmna+/+) treated with sodium nitrite in drinking water for 6 weeks. (A) Endothelium-dependent vasodilation induced by acetylcholine. (B) Endothelium-independent vasodilation induced by diethylamine NONOate (DEA-NO). (C) Contraction induced by phenylephrine. (D) Contraction induced by KCl. Statistical differences were analyzed by two-way ANOVA with Bonferroni’s post-hoc test for acetylcholine, DEA-NO and phenylephrine data, and by two-tailed t-test for KCl data. * p < 0.05.
4. Discussion
Aging and associated CVD constitute a major sanitary, societal, and economic challenge [1]. It is therefore of utmost importance to extend knowledge of the cellular and molecular mechanisms underlying vascular aging in order to design more effective diagnostic tools, prevention strategies, and therapies to promote healthier aging. The investigation of vascular aging in humans is challenging due to the difficulty of organizing long follow-up longitudinal studies in large population cohorts, as well as inherent complexity of post-analysis due to the concurrence with aging of other confounding cardiovascular risk factors, such as hypercholesterolemia, diabetes, hypertension, and obesity [2,3,4]. These limitations can be circumvented by studying premature aging syndromes characterized by accelerated CVD but lacking other confounding risk factors [5,6].
The HGPS mouse models generated over the last decade are excellent tools for studying cardiovascular aging over a relatively short time frame and without the presence of confounding cofactors, enabling the identification of the cell types most susceptible to progerin-induced CVD [6,22]. In the present study, we investigated vascular tone regulation in three HGPS models. We found severe contractile impairment in LmnaG609G mice with ubiquitous progerin expression, which was also observed in LmnaLCS/LCSSM22αCretg/+ with VSMC-specific progerin expression, but not in LmnaLCS/LCSTie2Cretg/+ mice with EC-specific progerin expression. These findings identify VSMCs as the main cell type targeted by progerin to impair vessel contraction, consistent with our recent studies showing that VSMC-specific progerin expression is sufficient to fully recapitulate vascular alterations observed in mice with ubiquitous progerin expression, including vessel stiffness with inward remodeling; VSMC loss; increased collagen deposition and decreased transverse waving of elastin layers in the medial layer [25]; and accelerated atherosclerosis, medial LDL retention, and plaque vulnerability (when examined in a proatherogenic Apoe−/− background) [26,27].
The defective vasoconstriction in progeroid mice reported here is in agreement with results by others showing decreased vasoconstrictor responses in normally aged mice [40,41]. We recently identified collagen deposition by VSMCs as a major contributor to vessel stiffness in LmnaG609G/G609G mice [25]. We therefore hypothesized that progerin-dependent contractile impairment could be due to mechanical impediment by a collagen stiff matrix, smooth muscle cell degeneration, or be caused by a combination of both. Treatment of aortic rings with collagenase to disrupt collagen did not improve contractile responses in LmnaG609G/G609G and LmnaLCS/LCSSM22αCretg/+ aortas, suggesting that collagen deposition is not the cause of impaired vasoconstriction in these animals. VSMC degeneration and dysfunction is therefore the most likely reason for contractile impairment, which might be the initiating cause of progerin-induced collagen deposition and defective vasoconstriction in HGPS models.
Our results show that progeroid endothelial dysfunction requires simultaneous expression of progerin in VSMCs and ECs, since progerin expression in VSMCs or ECs alone does not impair acetylcholine-dependent vessel relaxation. Moreover, vessels from mice with EC-specific progerin expression do not recapitulate the stiffness and inward remodeling observed in mice with ubiquitous or VSMC-specific progerin expression [25]. These findings indicate that the endothelial dysfunction observed in mice with ubiquitous progerin expression must be the result of systemic factors, or that it requires the combination of a dysfunctional medial and endothelial layer. However, since we have used a chemical signal to induce NO-dependent relaxation, we cannot rule out that other mechanically driven signals relevant for endothelial-dependent relaxation might be affected by EC-specific progerin expresion. Interestingly, transgenic mice with EC-specific overexpression of human progerin exhibit interstitial myocardial and perivascular fibrosis without VSMC loss [42]. Excessive collagen production in these mice is primarily derived not from ECs directly but from EC-dependent induction of a profibrotic response in the surrounding tissue [42]. HGPS patients show no alterations to flow mediated dilation [7,11], an indirect measure of endothelial function; nevertheless, the severe atherosclerosis in these patients must be preceded or accompanied by endothelial dysfunction at some point. Indeed, endothelial dysfunction and vascular stiffness might be induced independently but in parallel in HGPS, synergistically promoting vessel dysfunction and atherosclerosis as the disease progresses.
Deficient NO bioavailability is known to cause endothelial dysfunction in various CVD settings and in physiological aging [29,30]. In addition, mice overexpressing human progerin exclusively in ECs have reduced eNOS expression and NO levels [42]. We recently reported that NO supplementation by adding sodium nitrite to drinking water prevents vascular stiffness in progeroid mice [25]. In the present study, we demonstrate that NO is the main driver of endothelial-dependent vasodilation in mice with ubiquitous progerin expression and that treatment with sodium nitrite reverts endothelial dysfunction and partially ameliorates contractile impairment in progeroid mice. Nitrite supplementation increases NO bioavailability without the requirement of L-arginine and NOS [43]. The beneficial effect of sodium nitrite in phenylephrine-induced contractility we observed in progeroid aortic rings might be related to an improvement in VSMC and EC homeostasis rather than to reduced vessel stiffness, since treatment with collagenase to decrease vessel stiffness did not abrogate progerin-induced defects in contractility. Future studies should examine whether the beneficial effect of nitrites on the contraction of progeroid aortic rings can be also explained by local compensatory mechanisms, such as a shift in NO/prostaglandin/EDHF balance.
In summary, our work demonstrates for the first time the presence of vascular tone abnormalities such as a severe VSMC contractile impairment and endothelial dysfunction in a mouse model of premature aging caused by ubiquitous progerin expression. VSMCs are the main cell type involved in this contractile impairment, whereas neither VSMC-specific nor EC-specific progerin expression are sufficient to provoke endothelial dysfunction, which likely requires progerin expression in both ECs and VSMCs, and possibly also in other cell types. Our results also suggest dietary supplementation with nitrites as a novel therapy to treat CVD in HGPS patients.
Acknowledgments
We thank Carlos López-Otín for providing LmnaG609G and LmnaLCS mice, Simon Bartlett for English editing, and all the CNIC Animal Facility staff for help with animal care (with special thanks to Eva Santos).
Author Contributions
Conceptualization, L.d.C., A.S.-L., B.D. and V.A.; Data curation, L.d.C. and A.S.-L.; Formal analysis, L.d.C. and A.S.-L.; Funding acquisition, V.A.; Investigation, L.d.C., A.S.-L., C.G.-G. and M.J.A.-M.; Project administration, V.A.; Resources, V.A.; Supervision, V.A.; Validation, L.d.C. and A.S.-L.; Visualization, L.d.C.; Writing—original draft, L.d.C., A.S.-L. and V.A.; Writing—review & editing, L.d.C., A.S.-L., B.D. and V.A.; Manuscript approval, all authors. All authors have read and agreed to the published version of the manuscript.
Funding
Work in V.A.’s lab was supported by the Spanish Ministerio de Ciencia e Innovación (MCI, grant SAF2016-79490-R), with co-funding from the European Regional Development Fund (ERDF, “Una manera de hacer Europa”). L.d.C. was supported by a Jordi Soler postdoctoral contract from the Red de Investigación Cardiovascular (RETIC Program, Instituto de Salud Carlos III), and A.S.-L. was supported by a predoctoral contract from the MCI (SVP-2014-068334) and by a grant from Asociación Apadrina la Ciencia-Ford España-Ford Motor Company Fund. The CNIC is supported by the MCI, the Instituto de Salud Carlos III, and the Pro-CNIC Foundation, and is a Severo Ochoa Center of Excellence (grant SEV-2015-0505).
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.North B.J., Sinclair D.A. The Intersection between Aging and Cardiovascular Disease. Circ. Res. 2012;110:1097–1108. doi: 10.1161/CIRCRESAHA.111.246876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Strain W.D., Paldánius P.M. Diabetes, Cardiovascular Disease and the Microcirculation. Cardiovasc. Diabetol. 2018;17:57. doi: 10.1186/s12933-018-0703-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Taqueti V.R., Di Carli M.F. Coronary Microvascular Disease Pathogenic Mechanisms and Therapeutic Options: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2018;72:2625–2641. doi: 10.1016/j.jacc.2018.09.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Xing Z., Pei J., Tang L., Hu X. Traditional Cardiovascular Risk Factors and Coronary Collateral Circulation: Protocol for a Systematic Review and Meta-Analysis of Case-Control Studies. Medicine. 2018;97:e0417. doi: 10.1097/MD.0000000000010417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gordon L.B., Rothman F.G., López-Otín C., Misteli T. Progeria: A Paradigm for Translational Medicine. Cell. 2014;156:400–407. doi: 10.1016/j.cell.2013.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hamczyk M.R., del Campo L., Andrés V. Aging in the Cardiovascular System: Lessons from Hutchinson-Gilford Progeria Syndrome. Annu. Rev. Physiol. 2018;80:27–48. doi: 10.1146/annurev-physiol-021317-121454. [DOI] [PubMed] [Google Scholar]
- 7.Merideth M.A., Gordon L.B., Clauss S., Sachdev V., Smith A.C.M.M., Perry M.B., Brewer C.C., Zalewski C., Kim H.J., Solomon B., et al. Phenotype and Course of Hutchinson–Gilford Progeria Syndrome. N. Engl. J. Med. 2008;358:592–604. doi: 10.1056/NEJMoa0706898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ullrich N.J., Gordon L.B. Hutchinson–Gilford Progeria Syndrome. Handbook of Clin. Neurol. 2015;132:249–264. doi: 10.1016/B978-0-444-62702-5.00018-4. [DOI] [PubMed] [Google Scholar]
- 9.Vidak S., Foisner R. Molecular Insights into the Premature Aging Disease Progeria. Histoch. Cell Biol. 2016;145:401–417. doi: 10.1007/s00418-016-1411-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gerhard-Herman M., Smoot L.B., Wake N., Kieran M.W., Kleinman M.E., Miller D.T., Schwartzman A., Giobbie-Hurder A., Neuberg D., Gordon L.B. Mechanisms of Premature Vascular Aging in Children with Hutchinson-Gilford Progeria Syndrome. Hypertension. 2012;59:92–97. doi: 10.1161/HYPERTENSIONAHA.111.180919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Olive M., Harten I., Mitchell R., Beers J.K., Djabali K., Cao K., Erdos M.R., Blair C.D., Funke B., Smoot L.B., et al. Cardiovascular Pathology in Hutchinson-Gilford Progeria: Correlation with the Vascular Pathology of Aging. Arter. Thromb. Vasc. Biol. 2010;30:2301–2309. doi: 10.1161/ATVBAHA.110.209460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.De Sandre-Giovannoli A., Bernard R., Cau P., Navarro C.L., Amiel J., Boccaccio I., Lyonnet S., Stewart C.L., Munnich A., Le Merrer M., et al. Lamin a Truncation in Hutchinson-Gilford Progeria. Science. 2003;300:2055. doi: 10.1126/science.1084125. [DOI] [PubMed] [Google Scholar]
- 13.Eriksson M., Brown W.T., Gordon L.B., Glynn M.W., Singer J., Scott L., Erdos M.R., Robbins C.M., Moses T.Y., Berglund P., et al. Recurrent de Novo Point Mutations in Lamin A Cause Hutchinson-Gilford Progeria Syndrome. Nature. 2003;423:293–298. doi: 10.1038/nature01629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dorado B., Andrés V. A-Type Lamins and Cardiovascular Disease in Premature Aging Syndromes. Curr. Opin. Cell Biol. 2017;46:17–25. doi: 10.1016/j.ceb.2016.12.005. [DOI] [PubMed] [Google Scholar]
- 15.Gonzalez J.M., Pla D., Perez-Sala D., Andrés V. A-Type Lamins and Hutchinson-Gilford Progeria Syndrome: Pathogenesis and Therapy. Front. Biosci. 2011;3:1133–1146. doi: 10.2741/216. [DOI] [PubMed] [Google Scholar]
- 16.Ashapkin V.V., Kutueva L.I., Kurchashova S.Y., Kireev I.I. Are There Common Mechanisms Between the Hutchinson–Gilford Progeria Syndrome and Natural Aging? Front. Genet. 2019;10:455. doi: 10.3389/fgene.2019.00455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McClintock D., Ratner D., Lokuge M., Owens D.M., Gordon L.B., Collins F.S., Djabali K. The Mutant Form of Lamin A That Causes Hutchinson-Gilford Progeria Is a Biomarker of Cellular Aging in Human Skin. PLoS ONE. 2007;2:e1269. doi: 10.1371/journal.pone.0001269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rodriguez S., Coppedè F., Sagelius H., Eriksson M. Increased Expression of the Hutchinson-Gilford Progeria Syndrome Truncated Lamin A Transcript during Cell Aging. Eur. J. Hum. Genet. 2009;17:928–937. doi: 10.1038/ejhg.2008.270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Scaffidi P., Misteli T. Lamin A-Dependent Nuclear Defects in Human Aging. Science. 2006;312:1059–1063. doi: 10.1126/science.1127168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sullivan T., Escalante-Alcalde D., Bhatt H., Anver M., Bhat N., Nagashima K., Stewart C.L., Burke B. Loss of A-Type Lamin Expression Compromises Nuclear Envelope Integrity Leading to Muscular Dystrophy. J. Cell Biol. 1999;147:913–920. doi: 10.1083/jcb.147.5.913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.López-Mejía I.C., de Toledo M., Chavey C., Lapasset L., Cavelier P., Lopez-Herrera C., Chebli K., Fort P., Beranger G., Fajas L., et al. Antagonistic Functions of LMNA Isoforms in Energy Expenditure and Lifespan. EMBO Rep. 2014;15:529–539. doi: 10.1002/embr.201338126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang H., Kieckhaefer J.E., Cao K. Mouse Models of Laminopathies. Aging Cell. 2013;12:2–10. doi: 10.1111/acel.12021. [DOI] [PubMed] [Google Scholar]
- 23.Osorio F.G., Navarro C.L., Cadiñanos J., López-Mejía I.C., Quirós P.M., Bartoli C., Rivera-Torres J., Tazi J., Guzmán G., Varela I., et al. Splicing-Directed Therapy in a New Mouse Model of Human Accelerated Aging. Sci. Transl. Med. 2011;3:106ra107. doi: 10.1126/scitranslmed.3002847. [DOI] [PubMed] [Google Scholar]
- 24.Villa-Bellosta R., Rivera-Torres J., Osorio F.G., Acín-Pérez R., Enríquez J.A., López-Otín C., Andrés V. Defective Extracellular Pyrophosphate Metabolism Promotes Vascular Calcification in a Mouse Model of Hutchinson-Gilford Progeria Syndrome That Is Ameliorated on Pyrophosphate Treatment. Circulation. 2013;127:2442–2451. doi: 10.1161/CIRCULATIONAHA.112.000571. [DOI] [PubMed] [Google Scholar]
- 25.del Campo L., Sánchez-López A., Salaices M., von Kleeck R.A., Expósito E., González-Gómez C., Cussó L., Guzmán-Martínez G., Ruiz-Cabello J., Desco M., et al. Vascular Smooth Muscle Cell Specific Progerin Expression in a Mouse Model of Hutchinson–Gilford Progeria Syndrome Promotes Arterial Stiffness: Therapeutic Effect of Dietary Nitrite. Aging Cell. 2019;18:e12936. doi: 10.1111/acel.12936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hamczyk M.R., Villa-Bellosta R., Gonzalo P., Andrés-Manzano M.J., Nogales P., Bentzon J.F., López-Otín C., Andrés V. Vascular Smooth Muscle-Specific Progerin Expression Accelerates Atherosclerosis and Death in a Mouse Model of Hutchinson-Gilford Progeria Syndrome. Circulation. 2018;138:266–282. doi: 10.1161/CIRCULATIONAHA.117.030856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hamczyk M.R., Villa-Bellosta R., Quesada V., Gonzalo P., Vidak S., Nevado R.M., Andrés-Manzano M.J., Misteli T., López-Otín C., Andrés V. Progerin Accelerates Atherosclerosis by Inducing Endoplasmic Reticulum Stress in Vascular Smooth Muscle Cells. EMBO Mol. Med. 2019;11:e9736. doi: 10.15252/emmm.201809736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Levy B.I., Schiffrin E.L., Mourad J.-J., Agostini D., Vicaut E., Safar M.E., Struijker-Boudier H.A.J. Impaired Tissue Perfusion: A Pathology Common to Hypertension, Obesity, and Diabetes Mellitus. Circulation. 2008;118:968–976. doi: 10.1161/CIRCULATIONAHA.107.763730. [DOI] [PubMed] [Google Scholar]
- 29.Park K.H., Park W.J. Endothelial Dysfunction: Clinical Implications in Cardiovascular Disease and Therapeutic Approaches. J. Korean Med. Sci. 2015;30:1213–1225. doi: 10.3346/jkms.2015.30.9.1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Thijssen D.H.J., Carter S.E., Green D.J. Arterial Structure and Function in Vascular Ageing: Are You as Old as Your Arteries? J. Physiol. 2016;594:2275–2284. doi: 10.1113/JP270597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.D’Agostino R.B., Vasan R.S., Pencina M.J., Wolf P.A., Cobain M., Massaro J.M., Kannel W.B. General Cardiovascular Risk Profile for Use in Primary Care. Circulation. 2008;117:743–753. doi: 10.1161/CIRCULATIONAHA.107.699579. [DOI] [PubMed] [Google Scholar]
- 32.Lacolley P., Regnault V., Segers P., Laurent S. Vascular Smooth Muscle Cells and Arterial Stiffening: Relevance in Development, Aging, and Disease. Physiol. Rev. 2017;97:1555–1617. doi: 10.1152/physrev.00003.2017. [DOI] [PubMed] [Google Scholar]
- 33.Qiu H., Zhu Y., Sun Z., Trzeciakowski J.P., Gansner M., Depre C., Resuello R.R.G., Natividad F.F., Hunter W.C., Genin G.M., et al. Short Communication: Vascular Smooth Muscle Cell Stiffness as a Mechanism for Increased Aortic Stiffness with Aging. Circ. Res. 2010;107:615–619. doi: 10.1161/CIRCRESAHA.110.221846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Prakash A., Gordon L.B., Kleinman M.E., Gurary E.B., Massaro J., D’Agostino R.B., Kieran M.W., Gerhard-Herman M., Smoot L.B. Cardiac Abnormalities in Patients with Hutchinson-Gilford Progeria Syndrome. JAMA Cardiol. 2018;3:326–334. doi: 10.1001/jamacardio.2017.5235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lundberg J.O., Weitzberg E. Nitrite Reduction to Nitric Oxide in the Vasculature. Am. J. Physiol. Heart Circ. Physiol. 2008;295:H477–H478. doi: 10.1152/ajpheart.00611.2008. [DOI] [PubMed] [Google Scholar]
- 36.Sindler A.L., Fleenor B.S., Calvert J.W., Marshall K.D., Zigler M.L., Lefer D.J., Seals D.R. Nitrite Supplementation Reverses Vascular Endothelial Dysfunction and Large Elastic Artery Stiffness with Aging. Aging Cell. 2011;10:429–437. doi: 10.1111/j.1474-9726.2011.00679.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kisanuki Y.Y., Hammer R.E., Miyazaki J., Williams S.C., Richardson J.A., Yanagisawa M. Tie2-Cre Transgenic Mice: A New Model for Endothelial Cell-Lineage Analysis in Vivo. Dev. Biol. 2001;230:230–242. doi: 10.1006/dbio.2000.0106. [DOI] [PubMed] [Google Scholar]
- 38.National Toxicology Program Toxicology and Carcinogenesis Studies of Sodium Nitrite (CAS NO. 7632-00-0) in F344/N Rats and B6C3F1 Mice (Drinking Water Studies) Natl. Toxicol. Program. Tech. Rep. Ser. 2001;495:7–273. [PubMed] [Google Scholar]
- 39.del Campo L., Ferrer M. Methods in Mouse Atherosclerosis. Humana Press; New York, NY, USA: 2015. Wire Myography to Study Vascular Tone and Vascular Structure of Isolated Mouse Arteries; pp. 255–276. [DOI] [PubMed] [Google Scholar]
- 40.Wu H., van Thiel B.S., Bautista-Niño P.K., Reiling E., Durik M., Leijten F.P.J., Ridwan Y., Brandt R.M.C., van Steeg H., Dollé M.E.T., et al. Dietary Restriction but Not Angiotensin II Type 1 Receptor Blockade Improves DNA Damage-Related Vasodilator Dysfunction in Rapidly Aging Ercc1Δ/- Mice. Clin. Sci. 2017;131:1941–1953. doi: 10.1042/CS20170026. [DOI] [PubMed] [Google Scholar]
- 41.Durik M., Kavousi M., Van Der Pluijm I., Isaacs A., Cheng C., Verdonk K., Loot A.E., Oeseburg H., Bhaggoe U.M., Leijten F., et al. Nucleotide Excision DNA Repair Is Associated with Age-Related Vascular Dysfunction. Circulation. 2012;126:468–478. doi: 10.1161/CIRCULATIONAHA.112.104380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Osmanagic-Myers S., Kiss A., Manakanatas C., Hamza O., Sedlmayer F., Szabo P.L., Fischer I., Fichtinger P., Podesser B.K., Eriksson M., et al. Endothelial Progerin Expression Causes Cardiovascular Pathology through an Impaired Mechanoresponse. J. Clin. Investig. 2019;129:531–545. doi: 10.1172/JCI121297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sindler A.L., DeVan A.E., Fleenor B.S., Seals D.R. Inorganic Nitrite Supplementation for Healthy Arterial Aging. J. Appl. Physiol. 2014;116:463–477. doi: 10.1152/japplphysiol.01100.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]