Abstract
Much research in cognitive neuroscience supports prediction as a canonical computation of cognition across domains. Is such predictive coding implemented by feedback from higher-order domain-general circuits, or is it locally implemented in domain-specific circuits? What information sources are used to generate these predictions? This study addresses these two questions in the context of language processing. We present fMRI evidence from a naturalistic comprehension paradigm (1) that predictive coding in the brain’s response to language is domain-specific, and (2) that these predictions are sensitive both to local word co-occurrence patterns and to hierarchical structure. Using a recently developed continuous-time deconvolutional regression technique that supports data-driven hemodynamic response function discovery from continuous BOLD signal fluctuations in response to naturalistic stimuli, we found effects of prediction measures in the language network but not in the domain-general multiple-demand network, which supports executive control processes and has been previously implicated in language comprehension. Moreover, within the language network, surface-level and structural prediction effects were separable. The predictability effects in the language network were substantial, with the model capturing over 37% of explainable variance on held-out data. These findings indicate that human sentence processing mechanisms generate predictions about upcoming words using cognitive processes that are sensitive to hierarchical structure and specialized for language processing, rather than via feedback from high-level executive control mechanisms.
Keywords: language, predictive coding, multiple demand network, naturalistic, fMRI, sentence processing, syntactic structure, surprisal
Introduction
The human brain is an efficient prediction engine (James, 1890). Facilitation in processing expected information, as well as processing costs of violated expectations, have been reported in many domains. In the domain of language comprehension, various results show that listeners and readers actively predict upcoming words and structures (e.g., Kutas & Hillyard, 1984; MacDonald et al., 1994; Tanenhaus et al., 1995; Rayner et al., 2004; Frank & Bod, 2011; Smith & Levy, 2011, 2013; Staub & Benetar, 2013; Frank et al., 2015; Kuperberg & Jaeger, 2016). However, the cognitive and neural mechanisms that support predictive language processing are not well understood. Under one widely held view, predictive language processing is implemented by domain-general executive (inhibitory control and working memory) resources. This perspective receives support from numerous studies showing that prediction effects during language comprehension are absent or less pronounced for populations with reduced executive resources, such as children, older individuals, and non-native speakers (e.g., Federmeier et al., 2002; Federmeier & Kutas, 2005; Dagerman, et al., 2006; Federmeier et al., 2010; Mani & Huettig, 2012; Wlotko & Federmeier, 2012; Martin et al., 2013; Kaan, 2014; Mitsugi & Macwhinney, 2016; Gambi et al., 2018; Payne & Federmeier, 2018; cf. Dave et al., 2018; Havron et al., 2019). Furthermore, several neuroimaging studies have reported sensitivity to linguistic manipulations in what appear to be cortical regions thought to support domain-general executive function (e.g., Kaan & Swaab; 2002; Kuperberg et al., 2003; Novick et al., 2005; Rodd et al., 2005; Novais-Santos, 2007; January et al., 2009; Peelle et al., 2010; Rogalsky & Hickok, 2011; Nieuwland et al., 2012; Wild et al., 2012; McMillan et al., 2012, 2013), suggesting that such regions may also be implicated in language processing, including perhaps prediction. These results have led some to conclude that predictive coding for language is implemented by domain-general executive control resources (Linck et al., 2014; Huettig & Mani, 2016; Pickering & Gambi, 2018; Strijkers et al., 2019).
However, this interpretation is subject to several objections. First, most prior work on linguistic prediction has relied on behavioral and electrophysiological measures which are well suited for identifying global response patterns but cannot spatially localize the source of these effects in the brain to a certain functional region or network (e.g., Mather et al., 2013). Second, the (alleged) between-population differences in prediction noted above are consistent with accounts that do not directly invoke executive resources, including (1) possible qualitative differences between populations in the kind of information that is being predicted and the consequent need for population-specific norms to detect prediction effects, or (2) differences in how often predictions are correct, which may modulate the likelihood of engaging in predictive behavior (see Ryskin et al., this issue, for discussion). And third, past studies that did employ neuroimaging tools with high spatial resolution and consequently reported linguistic prediction responses – typically neural response increases for violations of linguistic structure – localized to executive control regions (e.g., Newman et al., 2001; Kuperberg et al., 2003; Nieuwland et al., 2012; Schuster et al., 2016) may have been influenced by task artifacts; indeed, some have argued that artificially constructed laboratory stimuli and tasks increase general cognitive load in comparison to naturalistic language comprehension (e.g., Blanco-Elorietta & Pylkkänen, 2017; Blank & Fedorenko, 2017; Campbell & Tyler, 2018; Wehbe et al., submitted; Diachek et al., 2019). To ensure that findings from the laboratory paradigms truly reflect the cognitive phenomenon of interest, it is important to validate them in more naturalistic experimental settings that better approximate the typical conditions of human sentence comprehension (Hasson & Honey, 2012; Hasson et al., 2018).
Despite the growing number of fMRI studies of naturalistic language comprehension (e.g., Speer et al., 2007; Yarkoni et al., 2008; Speer et al., 2009; Whitney et al., 2009; Wehbe et al., 2014; Hale et al., 2015; Henderson et al., 2015, 2016; Huth et al., 2016; Sood & Sereno, 2016; Brennan, 2016; Desai et al., 2016; de Heer et al., 2017, Dehghani et al., 2017; Bhattasali et al., 2018), only a handful have directly investigated effects of word predictability (Willems et al., 2015; Brennan et al., 2016; Henderson et al., 2016; Lopopolo et al., 2017; see Table 1 for summary), a well-established predictor of behavioral measures in naturalistic language comprehension (Demberg & Keller, 2008; Frank & Bod, 2011; Smith & Levy, 2013; van Schijndel & Schuler, 2015). These previous naturalistic studies of linguistic prediction effects in the brain – using estimates of prediction effort such as surprisal (Hale, 2001; Levy, 2008), the negative log probability of a word given its context, or entropy (Hale, 2006), an information-theoretic measure of the degree of constraint placed by the context on upcoming words – have yielded mixed results on the existence, type, and functional location of such effects. For example, of the lexicalized and unlexicalized (part-of-speech) bigram and trigram models of word surprisal explored in Brennan et al. (2016), only part-of-speech bigrams positively modulated neural responses in most regions of the functionally localized language network. Lexicalized bi- and trigrams and part-of-speech trigrams yielded generally null or negative results (16 out of 18 comparisons). By contrast, Willems et al. (2015) found lexicalized trigram effects in regions typically associated with language processing (e.g., anterior and posterior temporal lobe). In addition, Willems et al. (2015) and Lopopolo et al. (2017) found prediction effects in regions that are unlikely to be specialized for language processing, including (aggregating across both studies) the brain stem, amygdala, putamen, and hippocampus, as well as in superior frontal areas more typically associated with domain-general executive functions like self-awareness and coordination of the sensory system (Goldberg et al., 2006). It is therefore not yet clear whether predictive coding for language relies on domain-general mechanisms in addition to, or instead of, language-specific ones, especially in naturalistic contexts.
Table 1:
Previous fMRI studies of prediction effects in naturalistic sentence comprehension
| Study | # Participants | Stimulus length | HRF model | Functional localization | Out-of-sample evaluation |
|---|---|---|---|---|---|
| Willems et al., 2015 | 24 | 19 min | Canonical | No | No |
| Brennan et al., 2016 | 26 | 12 min | Canonical | Yes | No |
| Henderson et al., 2016 | 40 | 22 paragraphs | Canonical | No | No |
| Lopopolo et al., 2017 | 22 | 19 min | Canonical | No | No |
| Current study | 78 | 13.5 min (avg per participant) | Data-driven (CDR) | Yes | Yes |
In addition to questions about the functional localization of linguistic prediction, substantial prior work has also investigated the structure of the predictive model, seeking to shed light on the nature of linguistic representations in the mind. If effects from theoretical constructs like hierarchical natural language syntax can be detected in online processing measures, this would constitute evidence that such constructs are present in human mental representations and used to comprehend language. This position is widely supported by behavioral and electrophysiological experiments using constructed stimuli (see Lewis & Phillips, 2015 for review) and by some behavioral (Roark et al., 2009; Fossum & Levy, 2012; van Schijndel & Schuler, 2015; Shain et al., 2016), electrophysiological (Brennan & Hale, 2019) and neuroimaging (Brennan et al., 2016) experiments using naturalistic stimuli. However, other naturalistic studies reported null or negative syntactic effects (Frank & Bod, 2011; van Schijndel & Schuler, 2013; Shain & Schuler, 2018 contra Shain et al., 2016), or mixed syntactic results within the same set of experiments (Demberg & Keller, 2008; Henderson et al, 2016), leading some to argue that the representations used for language comprehension (in the absence of task artifacts from constructed stimuli) contain little hierarchical structure (Frank & Christiansen, 2018). Furthermore, the few naturalistic fMRI studies that have explored structural prediction effects have yielded inconsistent localization of these effects. For example, Brennan et al. (2016) found context-free grammar surprisal effects throughout the functional language network except in inferior frontal gyrus, whereas inferior frontal gyrus is the only region in which Henderson et al. (2016) found such effects.
The current study used fMRI to determine whether a signature of predictive coding during language comprehension – increased response to less predictable words, i.e. surprisal (e.g., Smith & Levy, 2013) – is primarily evident during naturalistic sentence processing in (1) the domain-specific, fronto-temporal language (LANG) network (Fedorenko et al., 2011), or (2) the domain-general, fronto-parietal multiple demand (MD) network (Duncan, 2010). The MD network supports executive functions (e.g., inhibitory control, attentional selection, conflict resolution, maintenance and manipulation of task sets) across both linguistic and non-linguistic tasks (e.g., Duncan & Owen, 2000; Fedorenko et al., 2013; Hughdahl et al., 2015; for discussion, see: Fedorenko, 2014) and has been shown to be sensitive to surprising events (Corbetta & Shulman, 2002).
On the one hand, given that the language network plausibly stores linguistic knowledge, including the statistics of language input, it might directly carry out predictive processing. Such a result would align with a growing body of cognitive neuroscience research supporting prediction as a “canonical computation” (Keller & Mrsic-Flogel, 2018) locally implemented in domain-specific circuits (Montague et al, 1996; Rao & Ballard, 1999; Alink et al., 2010; Bubic et al., 2010; Bastos et al., 2012; Wacogne et al., 2011, 2012; Singer et al., 2018). This hypothesis is also supported by prior findings of linguistic prediction effects in portions of the language network (Bonhage et al., 2015; Willems et al., 2015; Brennan et al., 2016; Henderson et al., 2016; Lopopolo et al., 2017; Matchin et al., 2018).
On the other hand, given that the MD network has been argued to encode predictive signals across domains and relay them as feedback to other regions (Strange et al., 2005; Cristescu et al., 2006; Egner et al., 2008; Wacogne et al., 2011; Chao et al., 2018), it might be recruited to predict upcoming words and structures in language. There is an extensive literature on neural signatures of prediction, such as activity associated with prediction errors, in brain regions that appear to belong to the MD network, including bilateral areas in the dorsolateral pre-frontal cortex, the inferior frontal gyrus, the anterior cingulate cortex, and the parietal lobe (for a review, see Dehaene et al., 2015; for a meta-analysis, see D’Astolfo & Rief, 2017). These areas are sensitive to rule violations in non-linguistic sequences, including hierarchically structured ones, in different sensory domains (e.g., auditory and visual; Bekinschtein et al., 2009; Ahlheim et al., 2014; Uhrug et al., 2014; Wang et al., 2015; Wang et al., 2017; Chao et al., 2018). In addition, they are recruited during learning of structured sequences in the motor domain (Bischoff-Grethe et al., 2004; Eickhoff et al., 2010). Beyond representing deterministic rules, such regions are also engaged in probabilistic predictions (Strange et al., 2005; Meyniel & Dehaene, 2017). Such predictions can be based on either inferring a generative model underlying the input sequence (Gläscher et al., 2010; Schapiro et al., 2013) or on reward contingencies (Koch et al., 2008; Zarr & Brown, 2016; Alexander & Brown, 2018; for a review, see: Rushworth & Behrens, 2008).
There are two main hypotheses in the contemporary literature that link predictive processing in the MD network with increased activity to more surprising words. First, the MD network might provide additional resources (“cognitive juice”) to various cognitive processes, including language. Under this scenario, MD regions might “come to the rescue” of the language network when processing demands are increased, which would be the case when surprisal is higher. Indeed, prior work suggests that the MD network could be recruited when language processing becomes effortful, e.g., under acoustic (Adank, 2012; Hervais-Adelman et al., 2012; Wild et al., 2012; Scott & McGettigan, 2013; Vaden et al., 2013) or syntactic (Kuperberg et al., 2003; Nieuwland et al., 2013) noise; in healthy aging (for reviews, see Wingfield & Grossman, 2006; Shafto & Tyler, 2014); during recovery from aphasia (Brownsett et al., 2014; Geranmayeh et al., 2014, 2016, 2017; Meier et al., 2016; Sims et al., 2016; Hartwigsen, 2018); and in L2 processing and multi-lingual control (e.g., Wartenburger et al., 2003; Rüschemeyer et al., 2005; Yokoyama et al., 2006; de Bruin et al., 2014; Grant, Fang, & Li, 2015; Kim et al., 2016; for reviews, see Perani & Abutalebi, 2005; Sakai, 2005; Abutalebi, 2008; Kotz, 2009; Hervais-Adelman, Moser-Mercer, Golestani, 2011; Pliatsikas & Luk, 2016). Second, the MD network, especially in the prefrontal cortex, may construct abstract representations of context, which serve as working memory for guiding behavior (Alexander & Brown, 2018). The main goal of such representations is to minimize prediction errors in other brain regions, so these representations are communicated in a top-down manner to the language network or other domain-specific networks (e.g., sensory areas). Such high-level, abstract predictive signals are potentially useful because they could perhaps “explain away” some more local prediction errors computed in the language network (e.g., in a sentence like “the cat that the dog chased on the balcony escaped”, the verb “escaped” might be unexpected based on the local context of the previous few words, but its occurrence could be explained away by a more global and abstract representation that looks farther into the past and predicts a verb for “the cat” in the main clause). In essence, then, signals from the MD network could bias representations in the language network in favor of the features that are most relevant in a given context (for a similar reasoning for sensory cortices, see Miller & Cohen, 2001; Sreenivasan et al., 2014; D’Esposito & Postle, 2015). However, these higher-level predictions are still sometimes incorrect, and when errors propagate back to the MD network, its regions would be triggered to adjust their predictive model in order to minimize future errors. This “model revision” process may register as increased neural processing (Chao et al., 2018).
Prior fMRI studies using hand-constructed sentences to probe effects of linguistic expectation have not yielded a clear answer as to the mechanisms – language-specific vs. domain-general – that support linguistic prediction. Numerous such studies have observed responses in areas of the language network to manipulations of word predictability (Kuperberg et al., 2000; Baumgaertner et al., 2002; Kiehl et al., 2002; Friederici et al., 2003; Gold et al., 2006; Obleser et al., 2007; Dien et al., 2008; Obleser et al., 2009; Bonhage et al., 2015; Schuster et al., 2016; Hartwigsen et al., 2017; Matchin et al., 2018; Schuster et al., 2019). However, many studies have also reported linguistic prediction effects in frontal, parietal, and cingulate cortical regions typically associated with the MD network (Kuperberg et al., 2000; Baumgaertner et al., 2002; Gold et al., 2006; Bonhage et al., 2015; Hartwigsen et al., 2017), as well as in other parts of the brain like the fusiform gyrus (Kuperberg et al., 2000; Gold et al., 2006) and the cerebellum (Lesage et al., 2017). Although it is certainly possible that predictive coding for language is carried out by both the LANG and the MD networks, with additional contributions from other brain areas, it is important to ensure that the foregoing results are not due to task artifacts induced by the use of artificially constructed stimuli (see Discussion), through validation of these findings in more naturalistic comprehension conditions (Hasson et al., 2018).
To distinguish the hypotheses above in a naturalistic comprehension paradigm, we searched for neural responses in LANG vs. MD regions to the contextual predictability of words as estimated by two model implementations of surprisal: a surface-level 5-gram model and a hierarchical probabilistic context-free grammar (PCFG) model. N-gram surprisal estimates are sensitive to word co-occurrence patterns but are limited in their ability to model hierarchical natural language syntax, since they contain no explicit representation of grammatical categories or syntactic composition and have limited memory for preceding words in the sentence (in our case, up to four preceding words). PCFG surprisal estimates, by contrast, are based on structured syntactic representations of the unfolding sentence but do not directly encode surface-level word co-occurrence patterns. Correlations between each of these measures and human neural responses would shed light on the relative importance assigned to these two information sources (word co-occurrences and syntactic structures) in computing predictions about upcoming words. Although surprisal is not the only extant measure of linguistic prediction (others include PCFG entropy, Roark et al., 2009; entropy reduction, Hale, 2006; and successor surprisal, Kliegl et al., 2006), surprisal has received extensive consideration in the experimental literature (e.g., Demberg and Keller, 2008; Frank & Bod, 2011; Fossum & Levy, 2012; Frank et al, 2015; van Schijndel and Schuler, 2015; Brennan et al., 2016; Henderson et al., 2016; Brennan & Hale, 2019; Shain, 2019). We did not consider these related measures in order to avoid excessive statistical comparisons.
Note that by estimating prediction effects using surprisal, we are implicitly assuming a notion of linguistic prediction as a distributed pre-activation process, following e.g. Kuperberg & Jaeger (2016), rather than as an all-or-nothing commitment to a specific upcoming word. Thus, we are investigating the degree to which the statistics of the local lexical (n-gram) and structural (PCFG) linguistic context modulate the sentence processing response, and where in the brain this modulation occurs. We leave aside questions about the underlying mechanisms by which these effects arise: e.g. the extent to which they are “active” or “passive”, or the extent to which integrative structure-building operations (e.g. composing words into syntactic constituents, constructing dependencies, etc.) underlie the observed facilitation effects (Altmann, 1998; Hale, 2014). See Discussion for elaboration on this point.
To avoid the problem of reverse inference from anatomy to function (Poldrack 2006, 2011; Figure 1), we functionally defined the LANG and MD networks in each individual participant using an independent “localizer” task (Saxe et al., 2006; Fedorenko et al., 2010), and then examined the response of those functional regions to each estimate of surprisal. Our results show significant independent effects of 5-gram and PCFG surprisal in LANG, but no such effects in MD, as well as significant differences in surprisal effect sizes between the two networks. This finding supports the hypothesis that predictive coding for language is primarily carried out by language-specialized rather than domain-general cortical circuits and exploits both surface-level and structural cues.
Figure 1:

Inter-individual variability in the mapping of function onto anatomy. Each column demonstrates variability in a different coordinate in MNI space, specified at the top (in mm). For each coordinate, sagittal T1 slices from four participants are shown, with the coordinate circled on each slice (participants differ across columns). In each case, the top two participants show a Sentences > Nonwords effect in this coordinate (colored in red-yellow), whereas the bottom two participants show the opposite, Nonwords > Sentences effect in this same coordinate (colored in green-blue). In all cases, the effect size of the circled coordinate is strong enough to be included among the participant-specific fROIs. Other voxels exhibiting strong contrast effects in the localizer task (namely, among the top 10% of voxels across the neocortical gray matter) are superimposed onto the anatomical slices, in color. Colorbars show p-values associated with each of the two localizer contrasts.
Materials and Methods
General Approach
Several features set the current study apart from prior cognitive neuroscience investigations of linguistic prediction in naturalistic stimuli.
First, we used naturalistic language stimuli rather than controlled stimuli constructed for a particular experimental goal. Naturalistic stimuli improve ecological validity compared to isolated constructed stimuli, which may introduce task artifacts that do not generalize to everyday cognition (Demberg & Keller, 2008; Hasson & Honey, 2012; Richlan et al., 2013; Schuster et al., 2016; Campbell & Tyler, 2018), and prior work indicates that naturalistic stimuli yield more reliable BOLD signals than artificial tasks (Hasson et al., 2010). Minimizing such artifacts is crucial in studies of the MD network, which is highly sensitive to task variables (Miller & Cohen, 2001; Sreenivasan et al., 2014; D’Esposito & Postle, 2015; Diachek et al., 2019).
Second, we used participant-specific functional localization to identify regions of interest constituting the LANG and MD networks (Fedorenko et al., 2010). This approach is crucial because many functional regions do not exhibit a consistent mapping onto macro-anatomical landmarks (Frost & Goebel, 2012), especially in the frontal (Amunts et al., 1999; Tomaiuolo et al., 1999), temporal (Jones and Powell, 1970; Gloor, 1997; Wise et al., 2001) and parietal (Caspers et al., 2006; Caspers et al., 2008; Scheperjans et al., 2008) lobes, which house the language and MD networks. Due to this inconsistent functional-to-anatomical mapping, a given stereotactic coordinate might belong to the language network in some participants but to the MD network in others, as is indeed the case in our sample (Figure 1) (see also Fedorenko et al., 2012a; Blank et al., 2017; Fedorenko & Blank, submitted). Such inter-individual variability severely compromises the validity of both anatomical localization (Juch et al., 2005; Poldrack, 2006; Fischl et al., 2007; Frost and Goebel, 2012; Tahmasebi et al., 2012) and group-based functional localization (Saxe et al., 2006; Fedorenko and Kanwisher, 2009): these approaches risk both decreased sensitivity (i.e. failing to identify a functional region due to insufficient spatial alignment across participants) and decreased functional resolution (i.e. mistaking two functionally distinct regions for a single region due to apparent spatial overlap across the sample). In contrast, participant-specific functional localization allows us to pool data from a given functional region across participants even in the absence of perfect anatomical alignment and is therefore better suited for the kind of questions we study here (Nieto-Castañón & Fedorenko, 2012). Both networks we probe here have been extensively functionally characterized in prior work, so responses to linguistic surprisal therein can be taken to index the engagement of linguistic processing mechanisms vs. domain-general executive mechanisms (e.g., Mather et al., 2013).
Third, we analyzed the BOLD times-series using a recently developed statistical framework – continuous-time deconvolutional regression (CDR; Shain & Schuler, 2018, 2019) – that is designed to overcome problems in hemodynamic response modeling that are presented by naturalistic experiments. The variable spacing of words in naturalistic language prevents direct application of discrete-time, data-driven techniques for hemodynamic response function (HRF) discovery, such as finite impulse response modeling (FIR) or vector autoregression. Because CDR is a parametric continuous-time deconvolutional method, it can infer the hemodynamic response directly from naturalistic time series, without distortionary preprocessing steps such as predictor interpolation (cf. Huth et al., 2016). Thus, unlike prior naturalistic fMRI studies of prediction effects in language processing (Table 1), we do not assume the shape of the HRF.
Fourth, unlike studies in Table 1, we evaluated hypotheses using non-parametric statistical tests of model fit to held-out (out-of-sample) data, an approach which builds external validity directly into the statistical test and should thereby improve replicability (e.g., Demšar, 2006).
Finally, to our knowledge, this is the largest fMRI investigation to date (78 subjects) of prediction effects in naturalistic language comprehension.
Experimental Design
Participants
Seventy-eight native English speakers (30 males), aged 18–60 (M±SD = 25.8±9, Med±SIQR = 23±3), from MIT and the surrounding Boston community participated for payment. Each participant completed a passive story comprehension task (the critical experiment) and a functional localizer task designed to identify the language and MD networks.
Sixty-nine participants (88%) were right-handed, as determined by either the Edinburgh handedness inventory (n=66) (Oldfield, 1971) or self-report (n=11) (handedness data were not collected for one participant). Eight participants were left-handed, but seven of these showed typical left-lateralized language activations, as determined by examining their activation patterns for the language localizer task (see below); the remaining participant had a right-lateralized language network. We chose to include the latter participant’s data in the analyses, to err on the conservative side, and to be able to generalize the results to the population at large (see Willems et al., 2014, for discussion).
All participants gave informed consent in accordance with the requirements of MIT’s Committee on the Use of Humans as Experimental Subjects (COUHES).
Stimuli and procedure
The localizer task and critical (story comprehension) experiment were run either in the same scanning session (67 participants) or in two separate sessions (11 participants, who have performed the localizer task while participating in other studies; see Mahowald & Fedorenko, 2016, for evidence of high stability of language localizer activations across sessions). For the critical experiment, each participant listened to one or more stories (one story: n=34; two stories: n=14; three stories: n=13; four stories: n=2; five stories: n=4; six stories: n=5; seven stories: n=1; or eight stories: n=5). In each session, participants performed a few other, unrelated tasks, with scanning sessions lasting approximately 2h.
Localizer task.
We used a single localizer task to identify functional regions of interest in both the language and MD networks, using opposite task contrasts across these networks as described below. This task, which has been described in more detail elsewhere (Fedorenko et al., 2010), consisted of reading sentences and lists of unconnected, pronounceable nonwords in a standard two-condition blocked design with a counterbalanced order across runs. Stimuli were presented one word / nonword at a time. The majority of participants (n=60) read these materials passively (and pressed a button at the end of each trial, to sustain alertness); for some participants (n=18), every trial ended with a memory probe item, and they had to indicate via a button press whether or not this probe had appeared in the preceding sentence / nonwords sequence. In addition, different participants performed versions of the task differing slightly in stimulus timing, number of blocks, etc., i.e. features that that do not affect the robustness of the contrast (e.g., Fedorenko et al., 2010; Mahowald & Fedorenko, 2016) (for experimental parameters, see Table 2). A version of this localizer is available at https://evlab.mit.edu/funcloc/download-paradigms.
Table 2:
Experimental parameters for the different versions of the localizer task.
| Version | ||||
|---|---|---|---|---|
| A | B | C | D | |
| Number of participants | 60 | 6 | 5 | 7 |
| Task (Passive Reading / Memory) | PR | M | M | M |
| Words / nonwords per trial | 12 | 12 | 8 | 12 |
| Trial duration (ms) | 6,000 | 6000 | 4,800 | 6000 |
| Fixation | 100 | 300 | 300 | 300 |
| Presentation of each word / nonword | 450 | 350 | 350 | 350 |
| Probe (M) + button press (M/PR) | 400 | 1000 | 1350 | 1000 |
| Fixation | 100 | 500 | 350 | 500 |
| Trials per block | 3 | 3 | 5 | 3 |
| Block duration (s) | 18 | 18 | 24 | 18 |
| Blocks per condition (per run) | 8 | 8 | 4 | 6 |
| Conditions | Sentences Nonwords | Sentences Nonwords | Sentences Nonwords Word-lists* | Sentences Nonwords Word-lists* |
| Fixation block duration (s) | 14 | 18 | 16 | 18 |
| Number of fixation blocks | 5 | 5 | 3 | 4 |
| Total run time (s) | 358 | 378 | 336 | 396 |
| Number of runs | 2 | 2 | 3–4 | 2–3 |
Used for the purposes of another experiment; see (Fedorenko et al., 2010).
To identify language regions, we used the contrast sentences > nonwords. This contrast targets higher-level aspects of language, to the exclusion of perceptual (speech / reading) and motor-articulatory processes (for discussion, see Fedorenko & Thompson-Schill, 2014; or Fedorenko, in press). Critically, this localizer has been extensively validated over the past decade across diverse parameters: it generalizes across task (passive reading vs. memory probe), presentation modality (visual vs. auditory), and materials (e.g., Fedorenko et al., 2010; Braze et al., 2011; Vagharchakian et al., 2012), including both coarser contrasts (e.g., between natural speech and an acoustically degraded control: Scott et al., 2017) and narrower contrasts (e.g., between lists of unconnected, real words and nonwords lists, or between sentences and lists of real words: Fedorenko et al., 2010; Blank et al., 2016). Whereas there are many potential differences (linguistic and otherwise) between the processing of sentences vs. nonwords, all regions localized with the sentences > nonwords contrast show a similar response profile: on the one hand, they exhibit sensitivity to various aspects of linguistic processing, including (but not limited to) lexical, phrasal, and sentence-level semantic and syntactic processing (e.g., Fedorenko et al., 2012b, 2018; Blank et al., 2016; Mollica et al., 2018; Blank & Fedorenko, submitted; similar patterns obtain in electrocorticographic data with high temporal resolution: Fedorenko et al., 2016). On the other hand, they show robust language-selectivity in their responses, with little or no response to non-linguistic tasks, including domain-general contrasts targeting, e.g., working memory or inhibitory control (Fedorenko et al., 2011, 2012a). In other words, the localizer shows both convergent construct validity with other linguistic contrasts and discriminant construct validity against non-linguistic contrasts. Moreover, the functional network identified by this contrast is internally synchronized yet strongly dissociated from other brain networks during naturalistic cognition (e.g., Blank et al, 2014; Paunov et al., 2019; for evidence from inter-individual effect-size differences, see: Mineroff et al., 2018), providing evidence that the localizer task is ecologically valid. Thus, a breadth of evidence demonstrates that the sentences > nonwords contrast identifies a network that is engaged in language processing and appears to be a “natural kind” in the functional architecture of the human brain.
To identify MD regions, we used the nonwords > sentences contrast, targeting regions that increase their response with the more effortful reading of nonwords compared to that of sentences. This “cognitive effort” contrast robustly engages the MD network and can reliably localize it. Moreover, it generalizes across a wide array of stimuli and tasks, both linguistic and non-linguistic including, critically, contrasts targeting executive functions such as working-memory and inhibitory control (Fedorenko et al., 2013; Mineroff et al., 2018). Supplementary Figures 1 and 2 demonstrate that the MD regions thus localized robustly respond to a difficulty (i.e. memory load) manipulation in a non-linguistic, spatial working-memory task (administered to a subset of participants in the current dataset).
Main (story comprehension) task.
Participants listened to stories from the publicly available Natural Stories Corpus (Futrell et al., 2018). These stories were adapted from existing texts (fairy tales and short stories) to be “deceptively naturalistic”: they contained an over-representation of rare words and syntactic constructions embedded in otherwise natural linguistic context. Behavioral data indicate that these stories effectively manipulate predictive processing, as self-paced reading times from an independent sample show robust effects of surprisal (Futrell et al., 2018). Stories were recorded by two native English speakers (one male, one female) at a 44.1 kHz sampling rate, ranged in length from 4m46s to 6m29s (983–1099 words), and were played over scanner-safe headphones (Sensimetrics, Malden, MA).
Following each story, some participants answered six (n=29) or twelve (n=12) comprehension questions, presented in a two-alternative forced-choice format. For all but 4 of these participants, accuracy was significantly above chance (binomial test for each participant: all ps < 0.046, uncorrected). For the remaining participants, comprehension questions were not part of the experimental design (n=30), were not collected due to equipment malfunction (n=4), or were lost (n=3). We note that BOLD time-series show indistinguishable levels of stimulus-locked activity regardless of whether comprehension questions are administered or not, at least in the networks studied here (Blank & Fedorenko, 2017).
Data acquisition and preprocessing
Data acquisition.
Structural and functional data were collected on a whole-body 3 Tesla Siemens Trio scanner with a 32-channel head coil at the Athinoula A. Martinos Imaging Center at the McGovern Institute for Brain Research at MIT. T1-weighted structural images were collected in 176 axial slices with 1mm isotropic voxels (repetition time (TR)=2,530ms; echo time (TE)=3.48ms). Functional, blood oxygenation level-dependent (BOLD) data were acquired using an EPI (echo-planar imaging) sequence with a 90° flip angle and using GRAPPA (GeneRalized Autocalibrating Partial Parallel Acquisition) with an acceleration factor of 2; the following parameters were used: thirty-one 4.4mm thick near-axial slices acquired in an interleaved order (with 10% distance factor), with an in-plane resolution of 2.1mm×2.1mm, FoV (field of view) in the phase encoding (Anterior>>Posterior) direction 200mm and matrix size 96mm×96mm, TR=2000ms and TE=30ms. The first 10s of each run were excluded to allow for steady state magnetization.
Spatial preprocessing.
Data preprocessing was carried out with SPM5 and custom MATLAB scripts. Preprocessing of anatomical data included normalization into a common space (Montreal Neurological Institute (MNI) template), resampling into 2mm isotropic voxels, and segmentation into probabilistic maps of the gray matter, white matter (WM) and cerebrospinal fluid (CSF). Note that SPM was only used for preprocessing and basic first-level modeling, aspects that have not changed much in later versions; we used an older version of SPM because data for this study are used across other projects spanning many years and hundreds of participants, and we wanted to keep the SPM version the same across all the participants. Preprocessing of functional data included motion correction, normalization, resampling into 2mm isotropic voxels, smoothing with a 4mm FWHM Gaussian kernel and high-pass filtering at 200s.
Temporal preprocessing.
Data from the story comprehension runs were additionally preprocessed using the CONN toolbox (Whitfield-Gabrieli and Nieto-Castañon, 2012) with default parameters, unless specified otherwise. Five temporal principal components of the BOLD signal time-courses from the WM were regressed out of each voxel’s time-course; signal originating in the CSF was similarly regressed out. Six principal components of the six motion parameters estimated during offline motion correction were also regressed out, as well as their first time derivative.
Participant-specific functional localization of the language and MD networks
Modeling localizer data.
A general linear model estimated the voxel-wise effect size of each condition in each experimental run of the localizer task. These effects were each modeled with a boxcar function (representing entire blocks/events) convolved with the canonical Hemodynamic Response Function (HRF). The model also included first-order temporal derivatives of these effects, as well as nuisance regressors representing entire experimental runs and offline-estimated motion parameters. The obtained beta weights were then used to compute the two functional contrasts of interest: sentences > nonwords for identifying language regions, and nonwords > sentences for identifying MD regions. These contrasts were computed only for voxels whose probability of belonging to the gray matter was greater than 1/3, based on the segmentation of the participant’s anatomical data. All other voxels were not considered further.
Defining functional regions of interest (fROIs).
For each participant, functional ROIs were defined by combining two sources of information (Fedorenko et al., 2010; Julian et al., 2012): (i) the participant’s activation map for the relevant localizer contrast (converted from beta weights to t-scores), and (ii) group-level constraints (“masks”; available for download from https://evlab.mit.edu/funcloc/download-parcels). The latter demarcated brain areas within which most or all individuals in prior studies showed activity for the localizer contrasts (Fig. 2).
Figure 2:
Defining participant-specific fROIs in the language (top) and MD (bottom) networks (only the left-hemisphere is shown). All images show approximated projections from functional volumes onto the surface of an inflated brain in common space. (A) Group-based masks used to constrain the location of fROIs. Contours of these masks are depicted in white on all brains in (B)-(D). (B) Overlap maps of localizer contrast effects (Sentence > Nonwords for the language network, Nonwords > Sentences for the MD network) across the 78 participants in the current sample (these maps were not used in the process of defining fROIs and are shown for illustration purposes). Each non gray-scale coordinate is colored according to the percentage of participants for whom that coordinate was among the top 10% of voxels showing the strongest localizer contrast effects across the nerocortical gray matter. (C) Overlap map of fROI locations. Each non gray-scale coordinate is colored according to the number of participants for whom that coordinate was included within their individual fROIs. (D) Example fROIs of three participants. Apparent overlap across language and MD fROIs within an individual is illusory and due to projection onto the cortical surface. Note that, because data were analyzed in volume (not surface) form, some parts of a given fROI that appear discontinuous in the figure (e.g., separated by a sulcus) are contiguous in volumetric space.
For the language fROIs, we used masks derived from a group-level probabilistic representation of the sentences > nonwords contrast in a set of 220 participants. These masks were similar to the masks derived from 25 participants, as originally reported in Fedorenko et al. (2010), and covered extensive portions of the left lateral frontal, temporal, and parietal cortices. In particular, six masks were used: in the inferior frontal gyrus (IFG) and its orbital part (IFGorb), middle frontal gyrus (MFG), anterior temporal cortex (AntTemp), posterior temporal cortex (PostTemp), and angular gyrus (AngG).
For the MD fROIs, we used masks derived from a group-level probabilistic representation of data from a previously validated MD-localizer task in a set of 197 participants. The task, described in detail in Fedorenko et al. (2011), contrasted hard and easy versions of a visuo-spatial working memory task (we did not use masks based on the nonwords > sentences contrast in order to maintain consistency with other current projects in our lab, and because prior work has established the similarity of the activation landscapes for these two contrasts; Fedorenko et al., 2013). These masks were constrained to be bilaterally symmetric by averaging individual hard > easy contrast maps across the two hemispheres prior to generating the group-level representation (only the group-based masks, covering large swaths of cortex, were constrained in this way; fROIs in the current study were free to vary in their location across hemispheres, within the borders of these masks). The topography of these masks largely overlapped with anatomically based masks that we had used in previous work (e.g., Fedorenko et al., 2013; Blank et al., 2014; Paunov et al., 2019). In particular, 10 masks were used in each hemisphere: in the posterior (PostPar), middle (MidPar), and anterior (AntPar) parietal cortex, precentral gyrus (PrecG), superior frontal gyrus (SFG), middle frontal gyrus (MFG) and its orbital part (MFGorb), opercular part of the inferior frontal gyrus (IFGop), the anterior cingulate cortex and pre-supplementary motor cortex (ACC/pSMA), and the insula (Insula).
These group-level masks, in the form of binary maps, were used to constrain the selection of participant-specific fROIs. In particular, for each participant, 6 language fROIs were created by (i) intersecting each language mask with each individual participant’s unthresholded t-map for the sentences > nonwords contrast; and then (ii) choosing the 10% of voxels with highest t-scores in the intersection. Similarly, 20 MD fROIs were created by intersecting each MD mask with each participant’s unthresholded t-map for the nonwords > sentences contrast and selecting the 10% of voxels with the highest t-scores within each intersection. This top-10% criterion balances the trade-off between choosing only voxels that respond robustly to the relevant contrast and having a sufficient number of voxels in each fROI of each participant. Moreover, this criterion guarantees fROIs of identical size across participants (occupying 10% of each mask). Few exceptions to this criterion were made for those cases where less than 10% of the voxels in a mask showed a t-score greater than 0; here, we only included the subset of voxels with positive t-scores in the fROI, and excluded those voxels showing effects in the opposite direction.
Prior to the critical statistical analyses, we ensured that all fROIs showed the expected functional signatures, i.e. a sentences > nonwords effect for the language fROIs, and a nonwords > sentences effect for the MD fROIs. To this end, the reliability of each contrast effect (i.e. the difference between the beta estimates of the two localizer conditions) was tested using a 2-fold across-run cross-validation: for each participant, fROIs were defined based on odd (even) run(s) and, subsequently, independent estimates of the relevant contrast effect were obtained from the left-out even (odd) run(s). These contrast effects were averaged across the two partitions (odd/even) and tested for significance across participants, via a dependent samples t-test (FDR-corrected for the number of fROIs within each network). The sentence > nonwords effect was highly reliable throughout the language network (for all six fROIs: t(77) > 9.5, p < 10−12 corrected; conservative effect size based on an independent samples test: Cohen’s d > 0.82), and the nonwords > sentences effect was highly reliable throughout the MD network (for all 20 fROIs: t(77) > 2.25, p < 0.05; conservative effect size based on an independent samples test: Cohen’s d > 0.16) (see also Supplementary Figures 1 and 2 for evidence of overlap with a spatial working memory contrast, as in Fedorenko et al., 2013).
Statistical analysis
Predictor definitions
To estimate word predictability in naturalistic data, we used an information-theoretic measure known as surprisal (Shannon, 1948; Hale, 2001): the negative log probability of a word given its context. Surprisal can be computed in many ways, depending on the choice of probability model. Three previous naturalistic fMRI studies (Willems et al., 2015; Brennan et al., 2016; Lopopolo et al., 2017) searched for surface-level n-gram surprisal effects, using words and/or parts of speech as the token-level representation. In addition, two previous naturalistic fMRI studies (Brennan et al., 2016; Henderson et al., 2016) probed structure-sensitive PCFG surprisal measures (Hale, 2001; Roark et al., 2009). As discussed in the Introduction, results from these studies failed to converge on a clear answer as to the nature and functional location of surprisal effects. In this study, we used the following surprisal estimates:
5-gram Surprisal:5-gram surprisal for each word in the stimulus set from a KenLM (Heafield et al., 2013) language model with default smoothing parameters trained on the Gigaword 3 corpus (Graff et al., 2007). 5-gram surprisal quantifies the predictability of words as the negative log probability of a word given the four words preceding it in context.
PCFG Surprisal:Lexicalized probabilistic context-free grammar surprisal computed using the incremental left-corner parser of van Schijndel et al. (2013) trained on a generalized categorial grammar (Nguyen et al., 2012) reannotation of Wall Street Journal sections 2 through 21 of the Penn Treebank (Marcus et al., 1993).
Models also included the control variables Sound Power, Repetition Time (TR) Number, Rate, Frequency, and Network, which were operationalized as follows:
Sound Power: Frame-by-frame root mean squared energy (RMSE) of the audio stimuli computed using the Librosa software library (McFee et al., 2015).
TR Number: Integer index of the current fMRI sample within the current scan.
Rate: Deconvolutional intercept. A vector of ones time-aligned with the word onsets of the audio stimuli. Rate captures influences of stimulus timing independently of stimulus properties (see e.g., Brennan et al., 2016; Shain & Schuler, 2018).
Frequency: Corpus frequency computed using a KenLM unigram model trained on Gigaword 3. For ease of comparison to surprisal, frequency is represented here on a surprisal scale (negative log probability), such that larger values index less frequent words (and thus greater expected processing cost).
Network: Numeric predictor for network ID, 0 for MD and 1 for LANG.
Models additionally included the mixed-effects random grouping factors Participant and fROI. Prior to regression, all predictors were rescaled by their standard deviations in the training set except Rate (which has no variance) and Network (which is an indicator variable). Reported effect sizes are therefore in standard units.
Continuous-time deconvolutional regression
Naturalistic language stimuli pose a challenge for established statistical methods in fMRI because the stimuli (words) (1) are variably spaced in time and (2) do not temporally align with response samples recorded by the scanner. Previous approaches to address this issue have various drawbacks. Some fMRI studies of naturalistic language processing have assumed a canonical shape for the hemodynamic response function (Boynton et al., 1994) and used it to convolve stimulus properties into response-aligned measures (Willems et al., 2015; Brennan et al., 2016; Lopopolo et al., 2017). This approach is unable to account for regional variation in the shape of the hemodynamic response, even though the canonical HRF is known to be a poor fit to some brain regions (Handwerker et al., 2004). Discrete-time methods for data-driven HRF identification such as finite impulse response modeling (FIR; Dayal et al., 1996) and vector autoregression (VAR; Sims, 1980) are widely used to overcome the limitations of the canonical HRF for fMRI research (e.g., Friston et al., 1994; Harrison et al., 2003) but are of limited use in the naturalistic setting because they assume (multiples of) a fixed time interval between stimuli that does not apply to words in naturally-occurring speech. Some studies (e.g. Huth et al., 2016) address this problem by continuously interpolating word properties, resampling the interpolated signal so that it temporally aligns with the fMRI record, and fitting FIR models using the resampled design matrix. However, this approach can be distortionary in that word properties (e.g., surprisal) are not temporally continuous.
Our study employed a recently developed continuous-time deconvolutional regression (CDR) technique that accurately infers parametric continuous-time impulse response functions – such as the HRF – from arbitrary time series (Shain & Schuler, 2018, 2019). Because CDR is data-driven, it can address the potential impact of poor fit in the canonical HRF, and because it is defined in continuous time, it eliminates the need for distortionary preprocessing steps like continuous interpolation. CDR models in this study used the following two-parameter HRF kernel based on the widely-used double-gamma canonical HRF (Lindquist et al., 2009):
where α and β are initialized to the SPM defaults of 6 and 1, respectively. More complex kernels (e.g., that fit the amplitude of the second term, rather than fixing it at 1/6) were avoided because of their potential to overfit.
The parametric continuous-time nature of CDR is similar to that of models used, for example, by Kruggel & von Camon (1999), Kruggel et al. (2000), Miezin et al. (2000), Lindquist & Wager (2007), and Lindquist et al. (2009) for nonlinear estimation of gamma-shaped HRFs. The main advantages of CDR over these approaches are that (1) it exploits the Tensorflow (Abadi et al., 2015) and Edward (Tran et al., 2016) libraries for optimizing large-scale variational Bayesian computation graphs using state of the art estimation techniques from deep learning – this study used the Adam optimizer with Nesterov momentum (Kingma & Ba, 2014; Nesterov, 1983; Dozat, 2016); (2) it supports mixed effects modeling of effect coefficients and HRF parameters; and (3) it supports parameter tying, constraining the solution space by ensuring that all predictors share a common HRF shape in a given region (with potentially differing amplitudes). Predictors in these models were given their own coefficients (which rescale h above), but the parameters α and β of h were tied across predictors, modeling the assumption of a fixed-shape blood oxygenation response to neural activity in a given cortical region.
The CDR models applied in this study assumed improper uniform priors over all parameters in the variational posterior and were optimized using a learning rate of 0.001 and stochastic minibatches of size 1024. Following standard practice from linear mixed-effects regression (Bates et al., 2014), random effects were L2-regularized toward zero at a rate of 1.0. Convergence was declared when the loss was uncorrelated with training time by t-test at the 0.5 level for at least 250 of the past 500 training epochs. For computational efficiency, predictor histories were truncated at 256 timesteps (words), which yields a maximum temporal coverage in our data of 48.34s (substantially longer than the effective influence of the canonical HRF). Prediction from the network used an exponential moving average of parameter iterates (Polyak, 1992) with a decay rate of 0.999, and models were evaluated using maximum a posteriori estimates obtained by setting all parameters in the variational posterior to their means. This approach is valid because all parameters are independent Gaussian in the CDR variational posterior (Shain & Schuler, 2018).
Model specification
The following CDR model specification was fitted to responses from each of the LANG and MD fROIs, where italics indicate predictors convolved using the fitted HRF and bold indicates predictors that were ablated for hypothesis tests:
BOLD ~ TRNumber + soundPower + Rate + Frequency + 5gram + PCFG + (TRNumber + soundPower + Rate + Frequency + 5gram + PCFG | fROI) + (1 | Participant)
The random effect by fROI indicates that the model included zero-centered by-fROI random variation in response amplitude and HRF parameters for each functional region of interest. As shown, the model also included a random intercept by participant (the data do not appear to support richer by-participant random effects, e.g. including random slopes and HRF shapes, since such models explained no held-out variance in early analyses, indicating overfitting). The above model can test whether the surprisal variables help predict neural activation in a given cortical region. However, it cannot be used to compare the magnitudes of response to surprisal across networks (Nieuwenhuis et al., 2011). Therefore, we directly tested for a difference in influence by fitting the combined responses from both LANG and MD using the following model specification with the indicator variable Network:
BOLD ~ TRNumber + soundPower + Rate + Frequency + 5gram + PCFG + Network + TRNumber:Network + soundPower:Network + Rate:Network + Frequency:Network + 5gram:Network + PCFG:Network + (1 | fROI) + (1 | Participant)
The random effects by fROI were simplified in comparison to that of the single-network models because the Network variable exactly partitions the fROIs. Thus ablated models can fully capture network differences as long as they have by-fROI random effects for surprisal. Indeed, initial tests showed virtually no difference in held-out likelihood between full and ablated combined models when those models included full by-fROI random effects despite large-magnitude estimates for the interactions with Network in the full model. Furthermore, the fitted parameters suggested that the by-fROI term was being appropriated in ablated models to capture between-network differences. In the full model, the 5-gram Surprisal estimates for 50% of LANG fROI and 45% of MD fROI were positive, while in the model with 5gram:Network ablated, 100% of LANG fROI and only 20% of MD fROI were positive, indicating that differences in response to 5-gram Surprisal had been pushed into the by-fROI random term. For this reason, we used simpler models for the combined test, despite their insensitivity to by-fROI variation in HRF shape or response amplitude.
In interactions between Network and convolved predictors, the interaction was computed following convolution but prior to rescaling with that predictor’s coefficient. Thus, the interaction term represents the offset in the estimated coefficient from the MD network to the LANG network, as is the case for binary interaction terms in linear regression models.
Finally, exact deconvolution from continuous predictors like Sound Power is not possible, since such predictors do not have an analytical form that can be integrated. Instead, we sampled sound power at fixed intervals (100ms), in which case the event-based CDR procedure reduces to a Riemann sum approximation of the continuous convolution integral. Note that the word-aligned predictors (e.g. 5-gram Surprisal) therefore have different timestamps than Sound Power, and as a result the history window spans different regions of time (up to 128 words into the past for the word-aligned predictors and up to 100ms × 128 = 12.8s of previous Sound Power samples).
Ablative statistical testing
In order to avoid confounds from (1) collinearity in the predictors and/or (2) overfitting to the training data, we followed a standard testing protocol from machine learning of evaluating differences in prediction performance on out-of-sample data using ablative non-parametric paired permutation tests for significance (Demšar, 2006). This approach can be used to assess the presence of an effect by comparing the prediction performance of a model that contains the effect against that of an ablated model that does not contain it. Specifically, given two pre-trained nested models, we computed the out-of-sample by-item likelihoods from each model over the evaluation set and constructed an empirical p value for the likelihood difference test statistic by randomly swapping by-item likelihoods n times (where n=10,000) and computing the proportion of obtained likelihood differences whose magnitude exceeded that observed between the two models. To ensure a single degree of freedom for each comparison, only fixed effects were ablated, with all random effects retained in all models.
The data partition was created by cycling TR numbers e into different bins of the partition with a different phase for each subject u:
assigning output 0 to the training set and 1 to the evaluation set. Since TR duration is 2s, this procedure splits the BOLD times series into 60 second chunks, alternating assignment of chunks into training and evaluation sets with a different phase for each participant. Partitioning in this way allowed us to (1) obtain a model of each participant, (2) cover the entire time series, and (3) sub-sample different parts of the time series for each participant during training, while at the same time suppressing correlation between the training and evaluation responses by using a relatively long period of alternation (30 TRs or 60s).
Accessibility
Access instructions for software and supplementary data needed to replicate these experiments (e.g. librosa, PyMVPA, CDR, KenLM, Gigaword 3, etc.) are given in the publications cited above. Post-processed fMRI timeseries are publicly available at the following URL: https://osf.io/eyp8q/. These experiments were not pre-registered.
Results
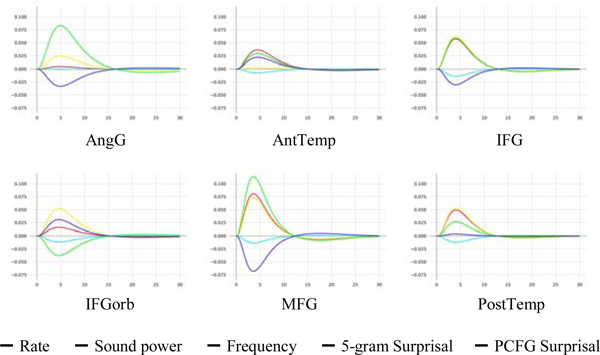
The CDR-estimated mean double-gamma hemodynamic response functions (HRFs) for the LANG and MD networks are given in Figure 3, the estimated HRFs by fROI in LANG regions are shown in Figure 4, surprisal estimates and percent variance explained by region are given in Tables 3 and 4, and population-level effect estimates (i.e. areas under the estimated HRFs) are reported in Table 5. MD estimates by region are plotted in Supplementary Figures 3 and 4; they are of little relevance because they do not generalize (Tables 4 & 6). As shown, HRF shapes resemble but deviate slightly from the canonical HRF (Boynton et al., 1996) to varying degrees in each region, highlighting both consistency with HRF estimates established by prior research as well as the potential of CDR to discover subtle differences in HRF shape between cortical regions (Handwerker et al., 2004) in naturalistic data. The models find positive effects of similar strength for both 5-gram Surprisal and PCFG Surprisal in LANG, and smaller effects of surprisal (even negative in the case of 5-gram Surprisal) in MD.
Figure 3:
Estimated overall double-gamma hemodynamic response functions (HRFs) by network
Figure 4:
Estimated language-network HRFs by fROI
Table 3: LANG surprisal estimates by fROI.
Estimates given are the area under the fitted HRF. Models explain held-out variance in all regions but AngG.
| fROI | Hemisphere | 5-gram estimate | PCFG estimate | % Held-Out Variance Explained |
|---|---|---|---|---|
| AngG | L | 0.030 | 0.156 | 0.0% |
| AntTemp | L | 0.215 | 0.017 | 5.1% |
| IFG | L | 0.287 | 0.309 | 2.2% |
| IFGorb | L | 0.010 | 0.318 | 1.3% |
| MFG | L | 0.382 | 0.346 | 2.3% |
| PostTemp | L | 0.242 | 0.258 | 6.1% |
Table 4: MD surprisal estimates by fROI.
Estimates given are the area under the fitted HRF. Models explain no held-out variance in any region except left MFGorb.
| fROI | Hemisphere | 5-gram estimate | PCFG estimate | % Held-Out Variance Explained |
|---|---|---|---|---|
| AntPar | L | 0.102 | −0.523 | 0.0% |
| IFGop | L | 0.009 | 0.141 | 0.0% |
| Insula | L | −0.200 | 0.284 | 0.0% |
| MFG | L | 0.074 | −0.026 | 0.0% |
| MFGorb | L | −0.215 | 0.252 | 0.5% |
| MidPar | L | 0.116 | −0.051 | 0.0% |
| mPFC | L | −0.125 | 0.257 | 0.0% |
| PostPar | L | 0.083 | −0.006 | 0.0% |
| PrecG | L | 0.078 | 0.048 | 0.0% |
| SFG | L | 0.180 | 0.025 | 0.0% |
| AntPar | R | 0.016 | −0.077 | 0.0% |
| IFGop | R | −0.011 | 0.075 | 0.0% |
| Insula | R | −0.185 | 0.227 | 0.0% |
| MFG | R | 0.058 | −0.006 | 0.0% |
| MFGorb | R | −0.004 | 0.019 | 0.0% |
| MidPar | R | 0.040 | −0.110 | 0.0% |
| mPFC | R | −0.321 | 0.440 | 0.0% |
| PostPar | R | −0.312 | 0.434 | 0.0% |
| PrecG | R | 0.034 | 0.118 | 0.0% |
| SFG | R | 0.066 | −0.034 | 0.0% |
Table 5:
Model effect estimates.
| Coefficient | |||
|---|---|---|---|
| Predictor | LANG | MD | Combined |
| Sound Power | −0.055 | −0.006 | −0.003 |
| TR Number | −0.148 | 0.048 | −0.005 |
| Rate | 0.242 | 0.146 | 0.048 |
| Frequency | −0.060 | −0.199 | −0.134 |
| 5-gram Surprisal | 0.209 | −0.025 | 0.003 |
| PCFG Surprisal | 0.235 | 0.097 | 0.038 |
| Network | -- | -- | −1.32 |
| Sound Power by Network | -- | -- | −0.050 |
| TR Number by Network | -- | -- | −0.008 |
| Rate by Network | -- | -- | 0.269 |
| Frequency by Network | -- | -- | 0.040 |
| 5-gram Surprisal by Network | -- | -- | 0.212 |
| PCFG Surprisal by Network | -- | -- | 0.193 |
Table 6:
Model percent variance explained compared to a “ceiling” linear model regressing against the mean response of all other participants for a particular story/fROI. “% Total” columns show absolute percent variance explained, while “% Relative” columns show percent variance explained relative to the ceiling.
| LANG | MD | Combined | ||||
|---|---|---|---|---|---|---|
| % Total | % Relative | % Total | % Relative | % Total | % Relative | |
| Ceiling | 6.18% | 100% | 1.34% | 100% | 2.63% | 100% |
| Model (train) | 3.68% | 59.5% | 0.75% | 56.0% | 1.18% | 44.9% |
| Model (evaluation) | 2.30% | 37.2% | 0.00% | 0.00% | 0.71% | 27.0% |
At the level of individual regions, the models explained held-out variance in all but one of the language fROIs (the exception was the AngG fROI). In contrast, the models explained no held-out variance in any but one MD fROI (the left MFGorb fROI). We leave these two exceptions to future research, but overall, the results demonstrate that surprisal effects are generally present throughout the language network and generally absent throughout the MD network. The differences between the individual-network models are largely replicated in the Combined model (Table 5), where main effects represent the estimated mean response in MD while interactions with Network represent the estimated difference in mean response between LANG and MD. As shown, Combined model estimates of both 5-gram:Network and PCFG:Network are positive and large-magnitude, indicating that the model estimates these variables to yield greater increases in neural activity in LANG over MD.
Table 6 reports model percent variance explained compared to a theoretical ceiling computed by regressing responses against responses from the same brain region in all other participants exposed to that stimulus. This ceiling is designed to quantify the variance that can be explained based on the stimuli alone, independently of inter-participant variation. As shown, models explain a substantial amount of the available variance in LANG. MD models explain no variance on the evaluation set, suggesting that the MD model did not learn generalizable patterns.
Because fROIs were modeled as random effects in these analyses, pairwise statistical testing of between-region differences in effect amplitude is not straightforward, and systematic investigation of regions / subnetworks within each broader functional network is left to future work. However, a qualitative examination of the by-region estimates suggests potentially interesting functional differences within the language network (Table 3). In particular, the IFG, MFG, and PostTemp fROIs all responded roughly equally to both measures of surprisal. The IFGorb fROI responded more to PCFG than 5-gram Surprisal (an unexpected finding given that this is not the language region that is traditionally most strongly associated with syntactic processing; e.g., Friederici, 2011; Blank et al., 2016). The AngG fROI showed a similar pattern, but the models did not explain held-out variance for this fROI. And the AntTemp fROI responded more to 5-gram than PCFG Surprisal (a finding which bears on debates about the functional role of this brain region in language processing, see Discussion). Although the differences in effect sizes between the two surprisals are significant in each of IFGorb, AngG, and AntTemp by Monte Carlo estimated credible intervals tests, such tests are anticonservative in CDR (Shain & Schuler, 2019). Nonetheless, they suggest that different regions of the language network might be differentially sensitive to surface-level vs. structural properties of language. The internal architecture of the language network has been long debated, and a number of proposals have been put forward (e.g., Friederici, 2011, 2012; Baggio and Hagoort, 2011; Tyler et al., 2011; Duffau et al., 2014; Ullman, 2016). However, no consensus has yet been reached about whether different regions support different aspects of language processing, and, if so, which regions support which linguistic computations (see e.g., Fedorenko et al., 2018, for discussion). Perhaps neural investigations of naturalistic language comprehension, combined with the power of the novel CDR approach and stringent statistical evaluation, can help inform this ongoing debate.
Tables 7–9 show the main finding of this study: fixed effects for 5-gram Surprisal and PCFG Surprisal significantly improve held-out likelihood in the LANG network over a model containing neither, as well as over one another. The difference in effect size between the LANG and MD networks is statistically significant, as shown by the significant likelihood improvements yielded by interactions of the surprisal variables with Network.
Table 7: LANG result.
Significance in LANG by paired permutation test of log-likelihood improvement on the evaluation set from including a fixed effect for each of 5-gram Surprisal and PCFG Surprisal, over (1) a baseline with neither fixed effect and (2) baselines containing the other fixed effect only. The Effect Estimate column shows the estimated effect size from the model containing the fixed effect (i.e. the area under the estimated HRF).
| Comparison | p | LL Improvement | Effect Estimate |
|---|---|---|---|
| 5-gram over neither | 0.0001*** | 182 | 0.307 |
| PCFG over neither | 0.0001*** | 183 | 0.352 |
| 5-gram over PCFG | 0.0001*** | 61 | 0.209 |
| PCFG over 5-gram | 0.0001*** | 61 | 0.235 |
Table 9: Combined result.
Significance in the combined data by paired permutation test of log-likelihood improvement on the evaluation set from including a fixed interaction for each of 5-gram Surprisal and PCFG Surprisal with Network, over (1) a baseline with neither fixed interaction and (2) baselines containing the other fixed interaction only. The Effect Estimate column shows the estimated interaction size from the model containing the fixed interaction (i.e. the difference in effect estimate between LANG and MD).
| Comparison | p | LL Improvement | Effect Estimate |
|---|---|---|---|
| 5-gram:Network over neither | 0.0001*** | 144 | 0.212 |
| PCFG:Network over neither | 0.0001*** | 144 | 0.193 |
| 5-gram:Network over PCFG:Network | 0.0001*** | 53 | 0.301 |
| PCFG:Network over 5-gram:Network | 0.0001*** | 53 | 0.317 |
As shown in Figure 3, the effects signs for Frequency in both networks are negative. The lack of a positive effect of Frequency is not what would be expected if word frequency modulated neural activity (Staub, 2015), but it is consistent with recent naturalistic behavioral evidence against distinct effects of frequency and predictability (Shain, 2019), as well as with previous theoretical claims that apparent frequency effects are underlyingly effects of predictability (Levy, 2008). Negative effects like these indicate suppression of the BOLD response and pose a challenge for interpretation (Harel et al., 2002). Prior work has suggested that such negative effects can arise from increased processing load elsewhere in the brain through hemodynamic factors (“vascular steal”) (Lee et al., 1995; Saad et al., 2001; Harel et al., 2002; Kannurpattie et al., 2004) and/or neuronal ones such as inhibition by an attention mechanism (Smith et al., 2000; Shmuel et al., 2002; Shmuel et al., 2006). The means by which such mechanisms might give rise to negative frequency effects in these experiments are not currently clear. Since frequency effects are not central to our present research question, we leave targeted investigation of their existence and direction to future research.
Figure 7 and Table 10 assess the generalizability of surprisal effects across participants. Figure 7 shows most by-participant improvements clustered around a positive median, without strong visual indication of large-magnitude positive outliers that might exclusively drive the effect. This intuition is quantified in Table 10. As shown, held-out likelihood improves for most participants in all comparisons. Furthermore, at least 5 of the most responsive participants in each comparison can be removed without changing the significance of the effect. Participant removal is a stringent criterion not only because it excludes the most responsive participants from consideration but also because it reduces the power of the permutation test by shrinking the evaluation set. These participant-level analyses demonstrate that surprisal effects in LANG are not merely driven by a small number of outlier participants.
Figure 7: LANG likelihood improvement by participant.
Spread of by-participant likelihood improvements in each comparison. Most improvements are positive, and effects are not driven by large positive outliers (see Table 10).
Table 10: Generality of LANG surprisal effects across participants.
Median likelihood improvement in LANG on the evaluation set by participant, percent of participants whose held-out predictions improved due to surprisal effects, and the number of participants with the largest held-out improvement whose data can be removed without changing the significance of the effect at a 0.05 level. Held-out likelihood improves for most participants in every comparison, and at least 5 of the most responsive participants can be removed in each comparison without changing the significance of the effect.
| Comparison | Median LL Improvement by Participant | % Participants Improved | Num Removable Participants |
|---|---|---|---|
| 5-gram over neither | 1.236 | 71.8% | 19 |
| PCFG over neither | 0.732 | 64.1% | 14 |
| 5-gram over PCFG | 0.335 | 61.5% | 7 |
| PCFG over 5-gram | 0.498 | 60.3% | 5 |
Discussion
The current study examined signatures of predictive processing during naturalistic story comprehension in two functionally distinct cortical networks: the domain-specific language (LANG) network, and the domain-general multiple demand (MD) network. Specifically, we tested which of these networks increased their responses with lower word predictability, operationalized using both 5-gram and probabilistic context-free grammar (PCFG) surprisal. The main results, yielded by continuous-time deconvolutional regression (CDR) analysis of surprisal effects in the two networks, are shown in Tables 7–9: in LANG, both 5-gram Surprisal and PCFG Surprisal have positive effects that yield statistically significant improvements to held-out likelihood, both over a baseline containing neither fixed effect as well as over one another. By contrast, in MD, neither surprisal effect is significant in any comparison. A direct test for a difference in surprisal effects across the two networks (Table 9) shows that the interactions of both surprisals with network are positive and statistically significant, indicating that the BOLD response to both surface-level (5-gram) and structural (PCFG) word predictability is larger in LANG than MD. These results are over a baseline that includes an effect for lexical frequency (log unigram probability), which is notable given the strong natural correlation between surprisal and frequency, both generally (Demberg & Keller, 2008) and in the current experimental materials (r = 0.78 overall). This finding suggests that the surprisal effects reported here are indeed driven by predictive coding and not merely by the cost of retrieving infrequent words. Together, these results demonstrate that predictive coding for upcoming words is primarily a canonical computation carried out by domain-specific cortical circuits, rather than by feedback from higher, domain-general executive control circuits, and that these predictions depend on both surface-level and structural information sources. Our finding of a generalized effect of PCFG Surprisal throughout the language network aligns with prior findings of evidence for linguistic prediction (e.g. Kuperberg et al., 2000; Baumgaertner et al., 2002; Friederici et al., 2003; Obleser et al., 2007) and syntactic processing (e.g., Blank et al., 2016; see Zaccarella et al., 2017 for review) in these regions, but suggests that prior evidence of linguistic prediction effects in MD (e.g. Kuperberg et al., 2000; Baumgaertner et al., 2002; Gold et al., 2006; Bonhage et al., 2015; Hartwigsen et al., 2017) may have been influenced by the use of artificially constructed linguistic stimuli and/or task artifacts.
This finding bears on an ongoing discussion in cognitive neuroscience about the compartmentalization of language processing. Early investigations of the functional organization of the brain argued for the existence of neuroanatomical modules dedicated to specific linguistic functions, from lower-level perceptual and motor components of language to higher-level ones like phonological, lexical, and combinatorial syntactic and semantic processing (Broca, 1861; Dax, 1863; Wernicke, 1874; Fodor, 1983; Petersen et al., 1988; Levelt, 1989; Pinker 1994). This position has been called into question by subsequent work stressing the distributed nature of cognition (e.g., Mesulam, 1998; Thompson-Schill et al., 2005; Blumstein & Amso, 2013), based on evidence both (1) that brain regions conventionally believed to be language-specific are also recruited for non-linguistic tasks (e.g., Dehaene et al., 1999; Stanescu-Cosson et al., 2000; Maess et al., 2001; Kaan and Swaab, 2002; Koelsch et al., 2002; Koechlin and Jubault, 2006; Hein and Knight, 2008; Blumstein, 2009; January et al., 2009), and (2) that brain regions conventionally believed to support domain-general cognitive control are also recruited for language processing, especially under difficult comprehension conditions (e.g., Kaan & Swaab; 2002; Kuperberg et al., 2003; Novick et al., 2005; Rodd et al., 2005; Novais-Santos, 2007; January et al., 2009; Peelle et al., 2010; Rogalsky & Hickok, 2011; Nieuwland et al., 2012; Wild et al., 2012; McMillan et al., 2012, 2013, Hsu & Novick, 2016). Although such results might raise doubts about the necessity and sufficiency of the putative language network for language processing, they are counterbalanced by rigorous non-replications of (1) the engagement of language regions in arithmetic, working memory, or cognitive control tasks (Fedorenko et al., 2011; Monti et al., 2012; Almaric et al., 2018), and (2) the engagement of cognitive control (MD) regions in language processing (Blank & Fedorenko, 2017; Wehbe et al., submitted). Based on this evidence, some have concluded that there does indeed exist a functionally specific cortical language network (Fedorenko, 2014; Fedorenko & Thompson-Schill, 2014; see also Hagoort, 2005; Friederici et al., 2011; Matchin et al., 2014; Rogalsky et al., 2015; Matchin, 2017, for proposals that are compatible with the idea that at least some of the language-responsive areas are specific to language) and that MD engagement in many previous studies of language processing was induced by experimental task artifacts (Campbell & Tyler, 2018; Wehbe et al., submitted; Diachek et al., 2019).
The aforementioned debate about the compartmentalization of language processing has largely focused on controlled experimental paradigms, which are prone to induce task artifacts that confound functional differentiation of neural structures. By showing strong prediction-based functional differentiation between the LANG and MD networks during naturalistic language comprehension, the present study provides evidence that predictive coding for language is primarily carried out by language-specific rather than domain-general mechanisms.
This finding also contributes to the growing literature on predictive coding in the mammalian brain, which has recently produced evidence that neurons are tuned to predict upcoming inputs but has also primarily focused on low-level perceptual processing (Rao & Ballard, 1999; Alink et al., 2010; Bubic et al. 2010; Keller & Mrsic-Flogel, 2018; Singer et al., 2018). The present study suggests that prediction extends to high-level cognitive functions like language comprehension and is similarly implemented as a domain-specific canonical computation in regions that plausibly store linguistic knowledge (e.g., Hagoort, 2005; Fedorenko, 2014).
The finding that surprisal computed by marginalizing over syntactic structures (PCFG Surprisal) modulates the LANG response independently of surface-level n-gram surprisal is evidence that participants are indeed computing such structures during incremental sentence processing (Hale, 2001; Levy, 2008; Fossum & Levy, 2012; Rasmussen & Schuler, 2018) and is inconsistent with previous arguments that the human sentence processing response is largely insensitive to such structures (Frank & Bod, 2011; Frank et al., 2012; Frank & Christiansen, 2018). At the same time, the finding that 5-gram Surprisal modulates the LANG response independently of PCFG Surprisal is evidence that the human sentence processing mechanism is sensitive to word co-occurrence patterns in ways that are not well captured by a strictly context-free parser. This suggests either (1) that the human parser is not strictly context-free (see e.g., tree-adjoining grammars, Joshi, 1985; combinatory categorial grammars, Steedman, 2000; and other context-sensitive grammar formalisms for natural language), or (2) that participants track both hierarchical structure and word co-occurrence patterns separately and simultaneously when generating predictions, and that these two kinds of processes take place in overlapping brain areas. Evaluating these hypotheses is left to future work. The lack of structured prediction effects in MD is of interest given prior proposals that ground structural effects in constraints on working memory (Abney & Johnson, 1991; Resnik, 1992; Rasmussen & Schuler, 2018). These theories view the processing of hierarchical language structures as a special case of a domain general capacity for hierarchic sequential prediction (Botvinick, 2007), which is at least consistent with the hypothesis that the resources recruited for prediction are also domain general (see e.g. Smith & Levy, 2013). However, to the extent that the memory resources used for prediction are also expected to activate in response to prediction error (e.g., by undergoing model revision, Chao et al., 2018, see Introduction), the failure to find such a signal in MD suggests that these memory resources may also be specific to the functional language network, rather than domain general (e.g., Caplan & Waters, 1999; Matchin, 2017).
Estimates at the fROI level shed light on results from prior naturalistic fMRI experiments (Willems et al., 2015; Brennan et al., 2016; Henderson et al., 2016; Lopopolo et al., 2017). We found strong effects of both surface-level and structural estimates of word predictability in roughly the union of left-hemisphere language regions for which such effects have been reported in prior work (e.g., temporal and inferior frontal regions). At the same time, we did not find clear evidence of predictive coding in regions linked with the multiple demand network, like superior frontal gyrus (cf. Lopopolo et al., 2017), in part because our use of held-out significance tests helped us avoid reporting MD surprisal effects that fail to generalize (e.g., left-hemisphere SFG, Table 4). The lack of held-out testing in earlier studies may therefore have contributed to prior findings of surprisal effects in MD regions. Finally, we obtained significant positive effects for surprisal implementations in language regions that have previously been reported null or negative (e.g., lexicalized trigrams in IFG and posterior temporal cortex or PCFG surprisal in IFG, per Brennan et al., 2016; PCFG surprisal in the temporal lobe, per Henderson et al., 2016). It is possible that the size of the present study increased sensitivity to these effects, since studies using less data are more likely to yield sign and magnitude errors (Gelman & Carlin, 2014). The picture that emerges more clearly from our results than from those of prior studies is of a predictive coding mechanism that is specific to the functional language network, generalized throughout it, and sensitive to both surface-level word co-occurrence patterns and hierarchical structure.
In focusing on prediction effects, we recognize that language comprehension involves a good deal more than simply minimizing surprise – meanings conveyed by partially-complete words and syntactic structures are rapidly and incrementally recognized, stored, and integrated into existing knowledge representations as the discourse unfolds (Tanenhaus et al., 1995; Altmann & Kamide, 1999). Numerous studies have probed the computations involved in storage, retrieval, and integration during human sentence comprehension (MacDonald et al., 1992; Kluender & Kutas, 1993; Gibson & Ko, 1998; Felser et al., 2003; Hsiao & Gibson, 2003; Aoshima et al., 2004; Grodner & Gibson, 2005; Lewis & Vasishth, 2005; Fiebach et al., 2005; Fedorenko et al., 2006, 2007; Rasmussen & Schuler, 2018), and several memory-based estimators of structural processing have been investigated across behavioral and cognitive neuroscience investigations, including embedding difference (Wu et al., 2010), the number of open nodes based on a particular parsing strategy (top-down, bottom-up, or left-corner; Nelson et al., 2016; Brennan & Pylkkänen, 2017), dependency locality costs (storage or integration cost from maintaining and retrieving syntactic dependencies; Gibson, 2000), and encoding or retrieval interference (i.e. processing costs in the ACT-R framework; Lewis and Vasishth, 2005). Effort due to memory storage and retrieval is plausibly distinct from effort due to reallocating resources between competing structural interpretations of the unfolding sentence (a standard interpretation of surprisal effects, e.g. Hale, 2001; Levy, 2008), and a complete account of human language processing will likely involve both prediction-based and integration-based computations (Levy et al., 2013; Levy & Gibson, 2013). Although these kinds of integration effects are outside the scope of the present study of predictive coding, we note that some have argued that prediction may subserve memory retrieval and therefore interact with integrative processing (Altmann, 1998). Therefore, the prediction effects reported here may, to some extent, be amenable to interpretation as effects of integration. That is, researchers who view “prediction” as a conscious lexically specific activity may view these results as evidence of conceptual pre-activation or preparedness that eases integration once a word is observed (see Ferreira & Chantavarin, 2018, for an overview of this distinction). We leave to future work a fuller investigation of this distinction and simply note that our results indicate that any such pre-activation processes appear to be restricted to the LANG network, rather than invoking the MD network, and are strongly correlated with probabilistic measures of word predictability. The fMRI dataset produced by this study will hopefully support further investigation into the interplay of memory and expectation in the language-selective and domain-general networks.
Our emphasis on structural influences on prediction, rather than sensitivity to syntactic structure more generally, is a possible explanation for one apparent discrepancy between our results and those of some previous studies. In particular, we do not find evidence of PCFG Surprisal effects in the AntTemp language fROI (although the PCFG Surprisal estimate is positive, the model explains no held-out variance in AntTemp), whereas numerous previous studies have argued for syntactic effects in left anterior temporal cortex, both using hand-constructed stimuli (Mazoyer et al., 1993; Stowe et al., 1998; Friederici et al., 2000; Vandenberghe et al., 2002; Dronkers et al., 2004; Humphries et al., 2006; Rogalsky & Hickok, 2009; Pallier et al., 2011; Brennan & Pylkkänen, 2012; Nelson et al., 2017) and naturalistic stimuli (Brennan et al., 2010; Brennan & Pylkkänen, 2017; Bhattasali et al., 2018, 2019). The role of left anterior temporal cortex in syntactic processing has been called into question by an absence of syntactic deficits in patients with anterior temporal damage (e.g., Wilson et al., 2012), and some have argued that parts of the anterior temporal lobe primarily carry out lexical and semantic processing, including perhaps semantic composition (e.g., Bemis & Pylkkänen, 2011), rather than syntactic structure building (Visser et al., 2010; Wilson et al., 2014; Lambon Ralph et al., 2017; see also Matchin et al., 2018). However, even granting that left anterior temporal cortex is implicated in syntactic processing, prior studies by and large have focused on structural measures that are arguably integrative in nature (syntactic node count, number of parser operations, etc.) or have used manipulations that are too broad to target prediction vs. integration (sentences vs. list of words or “Jabberwocky” sentences). Indeed, claims about syntactic processing in left anterior temporal cortex tend to focus on composition rather than on structured prediction. Our results thus do not preclude a role for left anterior temporal cortex in structure-building broadly construed; they simply fail to show strong evidence in this brain area of effects of structural context on word predictability. Prior studies of structured prediction effects in left anterior temporal cortex have yielded mixed results; although Brennan et al. (2016) found evidence of part-of-speech n-gram and PCFG surprisal in anterior temporal cortex over bi- and tri-gram effects, Lopopolo et al. (2017) did not find a response to part-of-speech n-gram surprisal, and the response to syntactic PCFG surprisal in Henderson et al. (2016) was too weak to achieve significance. Prediction effects based on lexical context in left anterior temporal cortex (i.e. lexical n-grams) are better attested (Willems et al., 2015; Lopopolo et al., 2017), and some have explicitly argued that left anterior temporal cortex plays a central role in lexical-semantic prediction (Lau et al., 2016). Our findings in the AntTemp fROI (large effects of 5-gram Surprisal in the AntTemp language fROI) contribute to this debate, suggesting that lexical prediction does occur in left anterior temporal cortex (among other regions) while syntactic prediction likely occurs elsewhere. Left anterior temporal cortex may therefore be an important object of study in teasing apart predictive vs. integrative processing during language comprehension, and further investigation is warranted.
In summary, our findings based on a large-scale naturalistic fMRI experiment support a view of linguistic prediction as implemented by domain-specific cortical circuits, sensitive to both surface-level and syntactic information sources, and generalized across the functional language network.
Supplementary Material
Table 8: MD result.
Significance in MD by paired permutation test of log-likelihood improvement on the evaluation set from including a fixed effect for each of 5-gram Surprisal and PCFG Surprisal, over (1) a baseline with neither fixed effect and (2) baselines containing the other fixed effect only. A p-value of 1.0 is assigned by default to comparisons in which held-out likelihood improved under ablation. The Effect Estimate column shows the estimated effect size from the model containing the fixed effect (i.e. the area under the estimated HRF).
| Comparison | p | LL Improvement | Effect Estimate |
|---|---|---|---|
| 5-gram over neither | 0.137 | 3 | 0.019 |
| PCFG over neither | 1.0 | −29 | 0.081 |
| 5-gram over PCFG | 1.0 | −8 | −0.025 |
| PCFG over 5-gram | 1.0 | −40 | 0.097 |
Highlights.
fMRI shows linguistic prediction effects using naturalistic stimuli
Prediction is implemented by language-specific, not domain-general networks
Prediction is sensitive both to word-cooccurrences and to hierarchical structure
Acknowledgments:
This research was supported by NIH award R00-HD-057522 (E.F.) and by National Science Foundation grant #1816891 (W.S.). All views expressed are those of the authors and do not necessarily reflect the views of the National Science Foundation. E.F. was additionally supported by NIH awards R01-DC-016607 and R01-DC-016950 and by a grant from the Simons Foundation via the Simons Center for the Social Brain at MIT. The authors would also like to acknowledge the Athinoula A. Martinos Imaging Center at the McGovern Institute for Brain Research at the Massachusetts Institute of Technology (MIT), and the support team (especially Steven Shannon and Atsushi Takahashi). The authors also thank Nancy Kanwisher and Ted Gibson for recording the stories, and EvLab members for help with data collection, especially Zach Mineroff and Alex Paunov.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest: The authors declare no competing financial interests.
Contributor Information
Cory Shain, The Ohio State University, 43210.
Idan Asher Blank, University of California Los Angeles, 90024; Massachusetts Institute of Technology, 02139.
Marten van Schijndel, Cornell University, 14853.
William Schuler, The Ohio State University, 43210.
Evelina Fedorenko, Massachusetts Institute of Technology, 02139; Massachusetts General Hospital, 02115; Program in Speech and Hearing Bioscience and Technology, 02115.
References
- Abadi M, Agarwal A, Barham P, Brevdo E, Chen Z, Citro C, … Zheng X (2015). TensorFlow: Large-Scale Machine Learning on Heterogeneous Distributed Systems. Retrieved from http://download.tensorflow.org/paper/whitepaper2015.pdf
- Abney SP, & Johnson M (1991). Memory Requirements and Local Ambiguities of Parsing Strategies. J. Psycholinguistic Research, 20(3), 233–250. [Google Scholar]
- Abutalebi J (2008). Neural aspects of second language representation and language control. Acta Psychologica, 128(3), 466–478. [DOI] [PubMed] [Google Scholar]
- Adank P (2012). The neural bases of difficult speech comprehension and speech production: Two activation likelihood estimation (ALE) meta-analyses. Brain and Language, 122(1), 42–54. [DOI] [PubMed] [Google Scholar]
- Ahlheim C, Stadler W, & Schubotz RI (2014). Dissociating dynamic probability and predictability in observed actions—an fMRI study. Frontiers in Human Neuroscience, 8, 273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander WH, & Brown JW (2018). Frontal cortex function as derived from hierarchical predictive coding. Scientific reports, 8(1), 3843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alink A, Schwiedrzik CM, Kohler A, Singer W, & Muckli L (2010). Stimulus predictability reduces responses in primary visual cortex. Journal of Neuroscience, 30(8), 2960–2966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altmann GTM, & Kamide Y (1999). Incremental interpretation at verbs: Restricting the domain of subsequent reference. Cognition, 73(3), 247–264. [DOI] [PubMed] [Google Scholar]
- Amunts K, Schleicher A, Bürgel U, Mohlberg H, Uylings HBM, & Zilles K (1999). Broca’s region revisited: cytoarchitecture and intersubject variability. Journal of Comparative Neurology, 412(2), 319–341. [DOI] [PubMed] [Google Scholar]
- Aoshima S, Phillips C, & Weinberg A (2004). Processing filler-gap dependencies in a head-final language. Journal of Memory and Language, 51, 23–54. [Google Scholar]
- Baggio G, & Hagoort P (2011). The balance between memory and unification in semantics: A dynamic account of the N400. Language and Cognitive Processes, 26(9), 1338–1367. [Google Scholar]
- Bastos AM, Usrey WM, Adams RA, Mangun GR, Fries P, & Friston KJ (2012). Canonical microcircuits for predictive coding. Neuron, 76(4), 695–711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bates D, Mächler M, Bolker B, & Walker S (2015). Fitting linear mixed-effects models using lme4. Journal of Statistical Software, 67(1), 1–48. 10.18637/jss.v067.i01 [DOI] [Google Scholar]
- Baumgaertner A, Weiller C, & Büchel C (2002). Event-related fMRI reveals cortical sites involved in contextual sentence integration. Neuroimage, 16(3), 736–745. [DOI] [PubMed] [Google Scholar]
- Bautista A, & Wilson SM (2016). Neural responses to grammatically and lexically degraded speech. Language, Cognition and Neuroscience, 31(4), 567–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bemis DK, & Pylkkänen L (2011). Simple composition: A magnetoencephalography investigation into the comprehension of minimal linguistic phrases. The Journal of Neuroscience, 31(8), 2801–2814. 10.1523/JNEUROSCI.5003-10.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bekinschtein TA, Dehaene S, Rohaut B, Tadel F, Cohen L, & Naccache L (2009). Neural signature of the conscious processing of auditory regularities. Proceedings of the National Academy of Sciences, 106(5), 1672–1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattasali S, Hale J, Pallier C, Brennan J, Luh W-M, & Spreng RN (2018). Differentiating Phrase Structure Parsing and Memory Retrieval in the Brain. Proceedings of the Society for Computation in Linguistics (SCiL) 2018, 74–80. [Google Scholar]
- Bischoff-Grethe A, Goedert KM, Willingham DT, & Grafton ST (2004). Neural substrates of response-based sequence learning using fMRI. Journal of Cognitive Neuroscience, 16(1), 127–138. [DOI] [PubMed] [Google Scholar]
- Blanco-Elorrieta E, & Pylkkänen L (2017). Bilingual language switching in the laboratory versus in the wild: The spatiotemporal dynamics of adaptive language control. Journal of Neuroscience, 37(37), 9022–9036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blank I, Balewski Z, Mahowald K, & Fedorenko E (2016). Syntactic processing is distributed across the language system. Neuroimage, 127, 307–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blank I & Fedorenko E (2016). Different high-level language regions integrate information over the same time-window. Poster presented at the Society for the Neurobiology of Language 8th meeting London, UK. [Google Scholar]
- Blank I, & Fedorenko E (2017). Domain-general brain regions do not track linguistic input as closely as language-selective regions. Journal of Neuroscience, 3616–3642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blank I, & Fedorenko E (submitted). No evidence for functional distinctions across fronto-temporal language regions in their temporal receptive windows. BioRxiv. 10.1101/712372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blank I, Kanwisher N, & Fedorenko E (2014). A functional dissociation between language and multiple-demand systems revealed in patterns of BOLD signal fluctuations. Journal of Neurophysiology, 112(5), 1105–1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blank IA, Kiran S, & Fedorenko E (2017). Can neuroimaging help aphasia researchers? Addressing generalizability, variability, and interpretability. Cognitive Neuropsychology, 34(6), 377–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumstein SE (2009). Auditory word recognition: Evidence from aphasia and functional neuroimaging. Language and Linguistics Compass, 3(4), 824–838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumstein SE, & Amso D (2013). Dynamic functional organization of language: insights from functional neuroimaging. Perspectives on Psychological Science, 8(1), 44–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonhage CE, Mueller JL, Friederici AD, & Fiebach CJ (2015). Combined eye tracking and fMRI reveals neural basis of linguistic predictions during sentence comprehension. Cortex, 68, 33–47. [DOI] [PubMed] [Google Scholar]
- Botvinick M (2007). Multilevel structure in behavior and in the brain: A computational model of Fuster’s hierarchy. Philosophical Transactions of the Royal Society, Series B: Biological Sciences, 362, 1615–1626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boynton GM, Engel SA, Glover GH, & Heeger DJ (1996). Linear systems analysis of functional magnetic resonance imaging in human V1. Journal of Neuroscience, 16(13), 4207–4221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braze D, Mencl WE, Tabor W, Pugh KR, Constable RT, Fulbright RK, … Shankweiler DP (2011). Unification of sentence processing via ear and eye: An fMRI study. Cortex, 47(4), 416–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brennan J (2016). Naturalistic sentence comprehension in the brain. Language and Linguistics Compass, 10(7), 299–313. [Google Scholar]
- Brennan JR, & Hale JT (2019). Hierarchical structure guides rapid linguistic predictions during naturalistic listening. PloS One, 14(1), e0207741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brennan J, Stabler EP, Van Wagenen SE, Luh W-M, & Hale JT (2016). Abstract linguistic structure correlates with temporal activity during naturalistic comprehension. Brain and Language, 157, 81–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broca P (1861). Remarks on the seat of the faculty of articulated language, following an observation of aphemia (loss of speech). Bulletin de La Société Anatomique, 6, 330–357. [Google Scholar]
- Brownsett SL, Warren JE, Geranmayeh F, Woodhead Z, Leech R, & Wise RJ (2014). Cognitive control and its impact on recovery from aphasic stroke. Brain, 137(1), 242–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bubic A, Von Cramon DY, & Schubotz RI (2010). Prediction, cognition and the brain. Frontiers in Human Neuroscience, 4, 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell KL, & Tyler LK (2018). Language-related domain-specific and domain-general systems in the human brain. Current Opinion in Behavioral Sciences, 21, 132–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caplan D, & Waters GS (1999). Verbal working memory and sentence comprehension. Behavioral and Brain Sciences, 22(1), 77–94. [DOI] [PubMed] [Google Scholar]
- Caspers S, Eickhoff SB, Geyer S, Scheperjans F, Mohlberg H, Zilles K, & Amunts K (2008). The human inferior parietal lobule in stereotaxic space. Brain Structure and Function, 212(6), 481–495. [DOI] [PubMed] [Google Scholar]
- Caspers S, Geyer S, Schleicher A, Mohlberg H, Amunts K, & Zilles K (2006). The human inferior parietal cortex: cytoarchitectonic parcellation and interindividual variability. Neuroimage, 33(2), 430–448. [DOI] [PubMed] [Google Scholar]
- Chao ZC, Takaura K, Wang L, Fujii N, & Dehaene S (2018). Large-Scale Cortical Networks for Hierarchical Prediction and Prediction Error in the Primate Brain. Neuron. [DOI] [PubMed] [Google Scholar]
- Corbetta M, & Shulman GL (2002). Control of goal-directed and stimulus-driven attention in the brain. Nature Reviews Neuroscience, 3(3), 201. [DOI] [PubMed] [Google Scholar]
- Cristescu TC, Devlin JT, & Nobre AC (2006). Orienting attention to semantic categories. Neuroimage, 33(4), 1178–1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dagerman KS, MacDonald MC, & Harm MW (2006). Aging and the use of context in ambiguity resolution: Complex changes from simple slowing. Cognitive Science, 30(2), 311–345. [DOI] [PubMed] [Google Scholar]
- D’Astolfo L, & Rief W (2017). Learning about expectation violation from prediction error paradigms–A meta-analysis on brain processes following a prediction error. Frontiers in Psychology, 8, 1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dave S, Brothers TA, Traxler MJ, Ferreira F, Henderson JM, & Swaab TY (2018). Electrophysiological evidence for preserved primacy of lexical prediction in aging. Neuropsychologia, 117, 135–147. [DOI] [PubMed] [Google Scholar]
- Dax G (1863). Observations tendant à prouver la coïncidence constante des dérangements de la parole avec une lésion de l’hémisphère gauche du cerveau. CR Acad Sci Hebd Seances Acad Sci, 61, 534. [Google Scholar]
- Dayal BS, & MacGregor JF (1996). Identification of finite impulse response models: methods and robustness issues. Industrial & Engineering Chemistry Research, 35(11), 4078–4090. [Google Scholar]
- de Bruin A, Roelofs A, Dijkstra T, & FitzPatrick I (2014). Domain-general inhibition areas of the brain are involved in language switching: FMRI evidence from trilingual speakers. NeuroImage, 90, 348–359. [DOI] [PubMed] [Google Scholar]
- de Heer WA, Huth AG, Griffiths TL, Gallant JL, & Theunissen FE (2017). The hierarchical cortical organization of human speech processing. Journal of Neuroscience, 3216–3267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dehaene S, Meyniel F, Wacongne C, Wang L, & Pallier C (2015). The neural representation of sequences: From transition probabilities to algebraic patterns and linguistic trees. Neuron, 88(1), 2–19. [DOI] [PubMed] [Google Scholar]
- Dehaene S, Spelke E, Pinel P, Stanescu R, & Tsivkin S (1999). Sources of mathematical thinking: Behavioral and brain-imaging evidence. Science, 284(5416), 970–974. [DOI] [PubMed] [Google Scholar]
- Dehghani M, Boghrati R, Man K, Hoover J, Gimbel SI, Vaswani A, … others. (2017). Decoding the neural representation of story meanings across languages. Human Brain Mapping, 38(12), 6096–6106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demberg V, & Keller F (2008). Data from eye-tracking corpora as evidence for theories of syntactic processing complexity. Cognition, 109(2), 193–210. [DOI] [PubMed] [Google Scholar]
- Demšar J (2006). Statistical comparisons of classifiers over multiple data sets. Journal of Machine Learning Research, 7(Jan), 1–30. [Google Scholar]
- Desai RH, Choi W, Lai VT, & Henderson JM (2016). Toward semantics in the wild: activation to manipulable nouns in naturalistic reading. Journal of Neuroscience, 36(14), 4050–4055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Esposito M, & Postle BR (2015). The cognitive neuroscience of working memory. Annual Review of Psychology, 66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diachek E, Blank IA, Siegelman M, & Fedorenko E (2019). The domain-general multiple demand (MD) network does not support core aspects of language comprehension: A large-scale fMRI investigation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dien J, Franklin MS, Michelson CA, Lemen LC, Adams CL, & Kiehl KA (2008). fMRI characterization of the language formulation area. Brain Research, 1229, 179–192. [DOI] [PubMed] [Google Scholar]
- Dozat T (2016). Incorporating Nesterov momentum into Adam. In ICLR Workshop. [Google Scholar]
- Dronkers NF, Wilkins DP, Van Valin RD Jr, Redfern BB, & Jaeger JJ (2004). Lesion analysis of the brain areas involved in language comprehension. Cognition, 92(1–2), 145–177. [DOI] [PubMed] [Google Scholar]
- Duffau H, Moritz-Gasser S, & Mandonnet E (2014). A re-examination of neural basis of language processing: Proposal of a dynamic hodotopical model from data provided by brain stimulation mapping during picture naming. Brain and Language, 131, 1–10. [DOI] [PubMed] [Google Scholar]
- Duncan J, & Owen AM (2000). Common regions of the human frontal lobe recruited by diverse cognitive demands. Trends in Neurosciences, 23(10), 475–483. [DOI] [PubMed] [Google Scholar]
- Egner T, Monti JMP, Trittschuh EH, Wieneke CA, Hirsch J, & Mesulam M-M (2008). Neural integration of top-down spatial and feature-based information in visual search. Journal of Neuroscience, 28(24), 6141–6151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eickhoff SB, Pomjanski W, Jakobs O, Zilles K, & Langner R (2010). Neural correlates of developing and adapting behavioral biases in speeded choice reactions—an fMRI study on predictive motor coding. Cerebral cortex, 21(5), 1178–1191. [DOI] [PubMed] [Google Scholar]
- Federmeier KD, & Kutas M (2005). Aging in context: age-related changes in context use during language comprehension. Psychophysiology, 42(2), 133–141. [DOI] [PubMed] [Google Scholar]
- Federmeier KD, Kutas M, & Schul R (2010). Age-related and individual differences in the use of prediction during language comprehension. Brain and Language, 115(3), 149–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Federmeier KD, McLennan DB, De Ochoa E, & Kutas M (2002). The impact of semantic memory organization and sentence context information on spoken language processing by younger and older adults: An ERP study. Psychophysiology, 39(2), 133–146. [DOI] [PubMed] [Google Scholar]
- Fedorenko E (2014). The role of domain-general cognitive control in language comprehension. Frontiers in Psychology, 5, 335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fedorenko E (in press). The brain network that supports high-level language processing In Gazzaniga M, Ivry RB, & Mangun GR (Eds.), Cognitive Neuroscience: The Biology of the Mind. New York: W. W. Norton and Company. [Google Scholar]
- Fedorenko E, Behr MK, & Kanwisher N (2011). Functional specificity for high-level linguistic processing in the human brain. Proceedings of the National Academy of Sciences. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fedorenko E, Blank I (submitted). Broca’s area is not a natural kind. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fedorenko E, Duncan J, & Kanwisher N (2012a). Language-selective and domain-general regions lie side by side within Broca’s area. Current Biology, 22(21), 2059–2062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fedorenko E, Duncan J, & Kanwisher N (2013). Broad domain generality in focal regions of frontal and parietal cortex. Proceedings of the National Academy of Sciences, 201315235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fedorenko E, Gibson E, & Rohde D (2006). The nature of working memory capacity in sentence comprehension: Evidence against domain-specific working memory resources. Journal of Memory and Language, 54(4), 541–553. [Google Scholar]
- Fedorenko E, Gibson E, & Rohde D (2007). The nature of working memory in linguistic, arithmetic and spatial integration processes. Journal of Memory and Language, 56(2), 246–269. [Google Scholar]
- Fedorenko E, Hsieh P-J, Nieto-Castañón A, Whitfield-Gabrieli S, & Kanwisher N (2010). New method for fMRI investigations of language: defining ROIs functionally in individual subjects. Journal of Neurophysiology, 104(2), 1177–1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fedorenko E, & Kanwisher N (2009). Neuroimaging of language: why hasn’t a clearer picture emerged? Language and Linguistics Compass, 3(4), 839–865. [Google Scholar]
- Fedorenko E, Mineroff Z, Siegelman M, & Blank I (2018). Word meanings and sentence structure recruit the same set of fronto-temporal regions during comprehension. BioRxiv. [Google Scholar]
- Fedorenko E, Nieto-Castañon A & Kanwisher N (2012b). Lexical and syntactic representations in the brain: An fMRI investigation with multi-voxel pattern analyses. Neuropsychologia, 50(4), 499–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fedorenko E, Scott TL, Brunner P, Coon WG, Pritchett B, Schalk G, & Kanwisher N (2016). Neural correlate of the construction of sentence meaning. Proceedings of the National Academy of Sciences, 113(41), E6256–E6262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fedorenko E, & Thompson-Schill SL (2014). Reworking the language network. Trends in Cognitive Sciences, 18(3), 120–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felser C, Clahsen H, & Münte TF (2003). Storage and integration in the processing of filler-gap dependencies: An ERP study of topicalization and wh-movement in German. Brain and Language, 87(3), 345–354. [DOI] [PubMed] [Google Scholar]
- Ferreira F, & Chantavarin S (2018). Integration and prediction in language processing: A synthesis of old and new. Current Directions in Psychological Science, 27(6), 443–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiebach CJ, Schlesewsky M, Lohmann G, Von Cramon DY, & Friederici AD (2005). Revisiting the role of Broca’s area in sentence processing: Syntactic integration versus syntactic working memory. Human Brain Mapping, 24(2), 79–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B, Rajendran N, Busa E, Augustinack J, Hinds O, Yeo BTT, … Zilles K (2007). Cortical folding patterns and predicting cytoarchitecture. Cerebral Cortex, 18(8), 1973–1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fodor J (1983). The modularity of mind: An essay on faculty psychology. Cambridge: MIT Press. [Google Scholar]
- Fossum V, & Levy R (2012). Sequential vs. Hierarchical Syntactic Models of Human Incremental Sentence Processing. In Proceedings of the 3rd Workshop on Cognitive Modeling and Computational Linguistics. Association for Computational Linguistics. [Google Scholar]
- Frank SL, & Bod R (2011). Insensitivity of the Human Sentence-Processing System to Hierarchical Structure. Psychological Science, 22(6), 829–834. [DOI] [PubMed] [Google Scholar]
- Frank SL, Bod R, & Christiansen MH (2012). How hierarchical is language use? Proceedings of the Royal Society B: Biological Sciences, 279(1747), 4522–4531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank SL, & Christiansen MH (2018). Hierarchical and sequential processing of language. Language, Cognition and Neuroscience, 33(9), 1213–1218. [Google Scholar]
- Frank SL, Otten LJ, Galli G, & Vigliocco G (2015). The ERP response to the amount of information conveyed by words in sentences. Brain & Language, 140, 1–11. [DOI] [PubMed] [Google Scholar]
- Friederici AD, Rueschemeyer S-A, Hahne A, & Fiebach CJ (2003). The role of left inferior frontal and superior temporal cortex in sentence comprehension: Localizing syntactic and semantic processes. Cerebral Cortex, 13(2), 170–177. [DOI] [PubMed] [Google Scholar]
- Friederici AD (2011). The brain basis of language processing: From structure to function. Physiological Reviews, 91(4), 1357–1392. [DOI] [PubMed] [Google Scholar]
- Friederici AD, Bahlmann J, Friedrich R, & Makuuchi M (2011). The neural basis of recursion and complex syntactic hierarchy. Biolinguistics, 5(1--2), 87–104. [Google Scholar]
- Friston KJ, Holmes AP, Worsley KJ, Poline J-P, Frith CD, & Frackowiak RSJ (1994). Statistical parametric maps in functional imaging: a general linear approach. Human Brain Mapping, 2(4), 189–210. [Google Scholar]
- Frost MA, & Goebel R (2012). Measuring structural--functional correspondence: spatial variability of specialised brain regions after macro-anatomical alignment. Neuroimage, 59(2), 1369–1381. [DOI] [PubMed] [Google Scholar]
- Futrell R, Gibson E, Tily HJ, Blank I, Vishnevetsky A, Piantadosi S, & Fedorenko E (2018). The Natural Stories Corpus. In Calzolari N, Choukri K, Cieri C, Declerck T, Goggi S, Hasida K, … Tokunaga T (Eds.), Proceedings of the Eleventh International Conference on Language Resources and Evaluation (LREC 2018) Paris, France: European Language Resources Association (ELRA). [Google Scholar]
- Gambi C, Gorrie F, Pickering MJ, & Rabagliati H (2018). The development of linguistic prediction: Predictions of sound and meaning in 2- to 5-year-olds. Journal of Experimental Child Psychology, 173, 351–370. [DOI] [PubMed] [Google Scholar]
- Gelman A, & Carlin J (2014). Beyond power calculations: Assessing type S (sign) and type M (magnitude) errors. Perspectives on Psychological Science, 9(6), 641–651. [DOI] [PubMed] [Google Scholar]
- Geranmayeh F, Brownsett SL, & Wise RJ (2014). Task-induced brain activity in aphasic stroke patients: What is driving recovery? Brain, 137(10), 2632–2648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geranmayeh F, Chau TW, Wise RJS, Leech R, & Hampshire A (2017). Domain-general subregions of the medial prefrontal cortex contribute to recovery of language after stroke. Brain, 140(7), 1947–1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geranmayeh F, Leech R, & Wise RJ (2016). Network dysfunction predicts speech production after left hemisphere stroke. Neurology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson E, & Ko K (1998). An integration-based theory of computational resources in sentence comprehension. In Fourth Architectures and Mechanisms in Language Processing Conference. [Google Scholar]
- Gläscher J, Daw N, Dayan P, & O’Doherty JP (2010). States versus rewards: Dissociable neural prediction error signals underlying model-based and model-free reinforcement learning. Neuron, 66(4), 585–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gloor P (1997). The temporal lobe \& limbic system. Oxford: Oxford University Press. [Google Scholar]
- Gold BT, Balota DA, Jones SJ, Powell DK, Smith CD, & Andersen AH (2006). Dissociation of automatic and strategic lexical-semantics: Functional magnetic resonance imaging evidence for differing roles of multiple frontotemporal regions. Journal of Neuroscience, 26(24), 6523–6532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg II, Harel M, & Malach R (2006). When the brain loses its self: prefrontal inactivation during sensorimotor processing. Neuron, 50(2), 329–339. [DOI] [PubMed] [Google Scholar]
- Graff D, Kong J, Chen K, & Maeda K (2007). English Gigaword Third Edition LDC2007T07. Philadelphia: Linguistic Data Consortium; Retrieved from https://catalog.ldc.upenn.edu/LDC2007T07 [Google Scholar]
- Grant AM, Fang SY, & Li P (2015). Second language lexical development and cognitive control: A longitudinal fMRI study. Brain and Language, 144, 35–47. [DOI] [PubMed] [Google Scholar]
- Grodner DJ, & Gibson E (2005). Consequences of the serial nature of linguistic input. Cognitive Science, 29, 261–291. [DOI] [PubMed] [Google Scholar]
- Hagoort P (2005). On Broca, brain, and binding: A new framework. Trends in Cognitive Sciences, 9(9), 416–423. 10.1016/j.tics.2005.07.004 [DOI] [PubMed] [Google Scholar]
- Hale J (2001). A probabilistic Earley parser as a psycholinguistic model. In Proceedings of the Second meeting of the North American Chapter of the Association for Computational Linguistics on Language technologies (pp. 1–8). 10.3115/1073336.1073357 [DOI] [Google Scholar]
- Hale J (2006). Uncertainty about the rest of the sentence. Cognitive Science, 30(4), 643–672. 10.1207/s15516709cog0000_64 [DOI] [PubMed] [Google Scholar]
- Hale J (2014). Automaton theories of human sentence comprehension CSLI Publications/Center for the Study of Language & Information. [Google Scholar]
- Hale J, Lutz D, Luh W-M, & Brennan J (2015). Modeling fMRI time courses with linguistic structure at various grain sizes. In Proceedings of the 6th workshop on cognitive modeling and computational linguistics (pp. 89–97). [Google Scholar]
- Handwerker DA, Ollinger JM, & D’Esposito M (2004). Variation of BOLD hemodynamic responses across subjects and brain regions and their effects on statistical analyses. NeuroImage, 21(4), 1639–1651. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/15050587 [DOI] [PubMed] [Google Scholar]
- Harel N, Lee S-P, Nagaoka T, Kim D-S, & Kim S-G (2002). Origin of negative blood oxygenation level—dependent fMRI signals. Journal of Cerebral Blood Flow \& Metabolism, 22(8), 908–917. [DOI] [PubMed] [Google Scholar]
- Harrison L, Penny WD, & Friston K (2003). Multivariate autoregressive modeling of fMRI time series. Neuroimage, 19(4), 1477–1491. [DOI] [PubMed] [Google Scholar]
- Hartwigsen G, Henseler I, Stockert A, Wawrzyniak M, Wendt C, Klingbeil J, … Saur D (2017). Integration demands modulate effective connectivity in a fronto-temporal network for contextual sentence integration. NeuroImage, 147, 812–824. [DOI] [PubMed] [Google Scholar]
- Hartwigsen G (2018). Flexible redistribution in cognitive networks. Trends in Cognitive Sciences, 22(8), 687–698. [DOI] [PubMed] [Google Scholar]
- Hasson U, Egidi G, Marelli M, & Willems RM (2018). Grounding the neurobiology of language in first principles: The necessity of non-language-centric explanations for language comprehension. Cognition, 180, 135–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasson U, & Honey CJ (2012). Future trends in Neuroimaging: Neural processes as expressed within real-life contexts. NeuroImage, 62(2), 1272–1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasson U, Malach R, & Heeger DJ (2010). Reliability of cortical activity during natural stimulation. Trends in Cognitive Sciences, 14(1), 40–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Havron N, de Carvalho A, Fiévet A-C, & Christophe A (2019). Three- to Four-Year-Old Children Rapidly Adapt Their Predictions and Use Them to Learn Novel Word Meanings. Child Development, 90(1), 82–90. 10.1111/cdev.13113 [DOI] [PubMed] [Google Scholar]
- Heafield K, Pouzyrevsky I, Clark JH, & Koehn P (2013). Scalable modified Kneser-Ney language model estimation. In Proceedings of the 51st Annual Meeting of the Association for Computational Linguistics (pp. 690–696). Sofia, Bulgaria. [Google Scholar]
- Hein G, & Knight RT (2008). Superior temporal sulcus—it’s my area: or is it? Journal of Cognitive Neuroscience, 20(12), 2125–2136. [DOI] [PubMed] [Google Scholar]
- Henderson JM, Choi W, Lowder MW, & Ferreira F (2016). Language structure in the brain: A fixation-related fMRI study of syntactic surprisal in reading. Neuroimage, 132, 293–300. [DOI] [PubMed] [Google Scholar]
- Henderson JM, Choi W, Luke SG, & Desai RH (2015). Neural correlates of fixation duration in natural reading: evidence from fixation-related fMRI. NeuroImage, 119, 390–397. [DOI] [PubMed] [Google Scholar]
- Hervais-Adelman AG, Carlyon RP, Johnsrude IS, & Davis MH (2012). Brain regions recruited for the effortful comprehension of noise-vocoded words. Language and Cognitive Processes, 27(7–8), 1145–1166. [Google Scholar]
- Hervais-Adelman AG, Moser-Mercer B, & Golestani N (2011). Executive control of language in the bilingual brain: Integrating the evidence from neuroimaging to neuropsychology. Frontiers in Psychology, 2, 234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huettig F, & Mani N (2016). Is prediction necessary to understand language? Probably not. Language, Cognition and Neuroscience, 31(1), 19–31. [Google Scholar]
- Hugdahl K, Raichle ME, Mitra A, & Specht K (2015). On the existence of a generalized non-specific task-dependent network. Frontiers in Human Neuroscience, 9, 430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huth AG, de Heer WA, Griffiths TL, Theunissen FE, & Gallant JL (2016). Natural speech reveals the semantic maps that tile human cerebral cortex. Nature, 532(7600), 453–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsiao F, & Gibson E (2003). Processing relative clauses in Chinese. Cognition, 90(1), 3–27. [DOI] [PubMed] [Google Scholar]
- Hsu NS, & Novick JM (2016). Dynamic engagement of cognitive control modulates recovery from misinterpretation during real-time language processing. Psychological Science, 27(4), 572–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- January D, Trueswell JC, & Thompson-Schill SL (2009). Co-localization of Stroop and syntactic ambiguity resolution in Broca’s area: Implications for the neural basis of sentence processing. Journal of Cognitive Neuroscience, 21(12), 2434–2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones EG, & Powell TPS (1970). An anatomical study of converging sensory pathways within the cerebral cortex of the monkey. Brain, 93(4), 793–820. [DOI] [PubMed] [Google Scholar]
- Joshi AK (1985). How much context sensitivity is necessary for characterizing structural descriptions: Tree adjoining grammars In Dowty LKD & Zwicky A (Eds.), Natural language parsing: Psychological, computational and theoretical perspectives (pp. 206–250). Cambridge, U.K.: Cambridge University Press. [Google Scholar]
- Juch H, Zimine I, Seghier ML, Lazeyras F, & Fasel JHD (2005). Anatomical variability of the lateral frontal lobe surface: implication for intersubject variability in language neuroimaging. Neuroimage, 24(2), 504–514. [DOI] [PubMed] [Google Scholar]
- Julian JB, Fedorenko E, Webster J, & Kanwisher N (2012). An algorithmic method for functionally defining regions of interest in the ventral visual pathway. Neuroimage, 60(4), 2357–2364. [DOI] [PubMed] [Google Scholar]
- Kaan E (2014). Predictive sentence processing in L2 and L1: What is different? Linguistic Approaches to Bilingualism, 4(2), 257–282. [Google Scholar]
- Kaan E, & Swaab TY (2002). The brain circuitry of syntactic comprehension. Trends in Cognitive Sciences, 6(8), 350–356. [DOI] [PubMed] [Google Scholar]
- Kannurpatti SS, & Biswal BB (2004). Negative functional response to sensory stimulation and its origins. Journal of Cerebral Blood Flow \& Metabolism, 24(6), 703–712. [DOI] [PubMed] [Google Scholar]
- Keller GB, & Mrsic-Flogel TD (2018). Predictive Processing: A Canonical Cortical Computation. Neuron, 100(2), 424–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiehl KA, Laurens KR, & Liddle PF (2002). Reading anomalous sentences: An event-related fMRI study of semantic processing. Neuroimage, 17(2), 842–850. [PubMed] [Google Scholar]
- Kim SY, Qi T, Feng X, Ding G, Liu L, & Cao F (2016). How does language distance between L1 and L2 affect the L2 brain network? An fMRI study of Korean–Chinese–English trilinguals. NeuroImage, 129, 25–39. [DOI] [PubMed] [Google Scholar]
- Kingma DP, & Ba J (2014). Adam: A method for stochastic optimization. CoRR, abs/1412.6. Retrieved from http://arxiv.org/abs/1412.6980 [Google Scholar]
- Kliegl R, Nuthmann A, & Engbert R (2006). Tracking the mind during reading: The influence of past, present, and future words on fixation durations. Journal of Experimental Psychology: General, 135(1), 12. [DOI] [PubMed] [Google Scholar]
- Kluender R, & Kutas M (1993). Bridging the gap: Evidence from ERPs on the processing of unbounded dependencies. Journal of Cognitive Neuroscience, 5(2), 196–214. [DOI] [PubMed] [Google Scholar]
- Koch K, Schachtzabel C, Wagner G, Reichenbach JR, Sauer H, & Schlösser R (2008). The neural correlates of reward-related trial-and-error learning: An fMRI study with a probabilistic learning task. Learning & Memory, 15(10), 728–732. [DOI] [PubMed] [Google Scholar]
- Koelsch S, Gunter TC, Cramon D. Y. v, Zysset S, Lohmann G, & Friederici AD (2002). Bach speaks: a cortical “language-network” serves the processing of music. Neuroimage, 17(2), 956–966. [PubMed] [Google Scholar]
- Kotz SA (2009). A critical review of ERP and fMRI evidence on L2 syntactic processing. Brain and Language, 109(2–3), 68–74. [DOI] [PubMed] [Google Scholar]
- Kuperberg GR, Holcomb PJ, Sitnikova T, Greve D, Dale AM, & Caplan D (2003). Distinct patterns of neural modulation during the processing of conceptual and syntactic anomalies. Journal of Cognitive Neuroscience, 15(2), 272–293. [DOI] [PubMed] [Google Scholar]
- Kuperberg GR, & Jaeger TF (2016). What do we mean by prediction in language comprehension? Language, Cognition and Neuroscience, 31(1), 32–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuperberg GR, McGuire PK, Bullmore ET, andS. Rabehesketh B. MJ, Wright IC, Lythgoe DJ, & David SC R. W. A. S. (2000). Common and Distinct Neural Substrates for Pragmatic, Semantic, and Syntactic Processing of Spoken Sentences: An fMRI Study. Journal of Cognitive Neuroscience, 12(2), 321–341. [DOI] [PubMed] [Google Scholar]
- Kutas M, & Hillyard SA (1984). Brain potentials during reading reflect word expectancy and semantic association. Nature, 307(5947), 161–163. [DOI] [PubMed] [Google Scholar]
- Kruggel F, & von Cramon DY (1999). Temporal properties of the hemodynamic response in functional MRI. Human Brain Mapping, 8(4), 259–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruggel F, Wiggins CJ, Herrmann CS, & von Cramon DY (2000). Recording of the event-related potentials during functional MRI at 3.0 Tesla field strength. Magnetic Resonance in Medicine: An Official Journal of the International Society for Magnetic Resonance in Medicine, 44(2), 277–282. [DOI] [PubMed] [Google Scholar]
- Lambon Ralph MA, Jefferies E, Patterson K, & Rogers TT (2017). The neural and computational bases of semantic cognition. Nature Reviews Neuroscience, 18(1), 42. [DOI] [PubMed] [Google Scholar]
- Lee AT, Glover GH, & Meyer CH (1995). Discrimination of large venous vessels in time-course spiral blood-oxygen-level-dependent magnetic-resonance functional neuroimaging. Magnetic Resonance in Medicine, 33(6), 745–754. [DOI] [PubMed] [Google Scholar]
- Lesage E, Hansen PC, & Miall RC (2017). Right lateral cerebellum represents linguistic predictability. Journal of Neuroscience, 37(26), 6231–6241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levelt WJM (1989). Speaking: From Intention to Articulation. Cambridge: MIT Press. [Google Scholar]
- Levy R (2008). Expectation-based syntactic comprehension. Cognition, 106(3), 1126–1177. [DOI] [PubMed] [Google Scholar]
- Lewis S, & Phillips C (2015). Aligning grammatical theories and language processing models. Journal of Psycholinguistic Research, 44(1), 27–46. [DOI] [PubMed] [Google Scholar]
- Lewis RL, & Vasishth S (2005). An activation-based model of sentence processing as skilled memory retrieval. Cognitive Science, 29(3), 375–419. [DOI] [PubMed] [Google Scholar]
- Linck JA, Osthus P, Koeth JT, & Bunting MF (2014). Working memory and second language comprehension and production: A meta-analysis. Psychonomic Bulletin \& Review, 21(4), 861–883. [DOI] [PubMed] [Google Scholar]
- Lindquist MA, Loh JM, Atlas LY, & Wager TD (2009). Modeling the hemodynamic response function in fMRI: Efficiency, bias and mis-modeling. NeuroImage, 45(1, Supplement 1), S187–S198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindquist M, & Wager T (2007). Validity and power in hemodynamic response modeling: A comparison study and a new approach. Human Brain Mapping, 28, 764–784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopopolo A, Frank SL, den Bosch A, & Willems RM (2017). Using stochastic language models (SLM) to map lexical, syntactic, and phonological information processing in the brain. PloS One, 12(5), e0177794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald MC, Just MA, & Carpenter PA (1992). Working memory constraints on the processing of syntactic ambiguity. Cognitive Psychology, 24(1), 56–98. [DOI] [PubMed] [Google Scholar]
- MacDonald MC, Pearlmutter NJ, & Seidenberg MS (1994). The lexical nature of syntactic ambiguity resolution. Psychological Review, 101(4), 676–703. [DOI] [PubMed] [Google Scholar]
- Mahowald K, & Fedorenko E (2016). Reliable individual-level neural markers of high-level language processing: A necessary precursor for relating neural variability to behavioral and genetic variability. Neuroimage, 139, 74–93. [DOI] [PubMed] [Google Scholar]
- Mani N, & Huettig F (2012). Prediction during language processing is a piece of cake—But only for skilled producers. Journal of Experimental Psychology: Human Perception and Performance, 38(4), 843. [DOI] [PubMed] [Google Scholar]
- Marcus MP, Santorini B, & Marcinkiewicz MA (1993). Building a large annotated corpus of English: The Penn Treebank. Computational Linguistics, 19(2), 313–330. [Google Scholar]
- Martin CD, Thierry G, Kuipers J-R, Boutonnet B, Foucart A, & Costa A (2013). Bilinguals reading in their second language do not predict upcoming words as native readers do. Journal of Memory and Language, 69(4), 574–588. [Google Scholar]
- Matchin W, Brodbeck C, Hammerly C, & Lau E (2018). The temporal dynamics of structure and content in sentence comprehension: Evidence from fMRI-constrained MEG. Human Brain Mapping, 40(2), 663–678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matchin W, Hammerly C, & Lau E (2017). The role of the IFG and pSTS in syntactic prediction: Evidence from a parametric study of hierarchical structure in fMRI. Cortex, 88, 106–123. [DOI] [PubMed] [Google Scholar]
- Matchin W, Sprouse J, & Hickok G (2014). A structural distance effect for backward anaphora in Broca’s area: An fMRI study. Brain and Language, 138, 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mather M, Cacioppo JT, & Kanwisher N (2013). How fMRI can inform cognitive theories. Perspectives on Psychological Science, 8(1), 108–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFee B, Raffel C, Liang D, Ellis DPW, McVicar M, Battenberg E, & Nieto O (2015). librosa: Audio and music signal analysis in python. In Proceedings of the 14th Python in Science Conference (pp. 18–25). [Google Scholar]
- McMillan CT, Clark R, Gunawardena D, Ryant N, & Grossman M (2012). fMRI evidence for strategic decision-making during resolution of pronoun reference. Neuropsychologia, 50(5), 674–687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMillan CT, Coleman D, Clark R, Liang T-W, Gross RG, & Grossman M (2013). Converging evidence for the processing costs associated with ambiguous quantifier comprehension. Frontiers in Psychology, 4, 153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meier EL, Kapse KJ, & Kiran S (2016). The Relationship between Frontotemporal Effective Connectivity during Picture Naming, Behavior, and Preserved Cortical Tissue in Chronic Aphasia. Frontiers in Human Neuroscience, 10, 109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesulam M-M (1998). From sensation to cognition. Brain: A Journal of Neurology, 121(6), 1013–1052. [DOI] [PubMed] [Google Scholar]
- Meyniel F, & Dehaene S (2017). Brain networks for confidence weighting and hierarchical inference during probabilistic learning. Proceedings of the National Academy of Sciences, 114(19), E3859–E3868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miezin FM, Maccotta L, Ollinger JM, Petersen SE, & Buckner RL (2000). Characterizing the hemodynamic response: effects of presentation rate, sampling procedure, and the possibility of ordering brain activity based on relative timing. Neuroimage, 11(6), 735–759. [DOI] [PubMed] [Google Scholar]
- Miller EK, & Cohen JD (2001). An integrative theory of prefrontal cortex function. Annual Review of Neuroscience, 24(1), 167–202. [DOI] [PubMed] [Google Scholar]
- Mineroff Z, Blank IA, Mahowald K, & Fedorenko E (2018). A robust dissociation among the language, multiple demand, and default mode networks: evidence from inter-region correlations in effect size. Neuropsychologia, 119, 501–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitsugi S, & MacWhinney B (2016). The use of case marking for predictive processing in second language Japanese. Bilingualism: Language and Cognition, 19(1), 19–35. [Google Scholar]
- Mollica F, Siegelman M, Diachek E, Piantadosi ST, Mineroff Z, Futrell R, & Fedorenko E (2018). High local mutual information drives the response in the human language network. BioRxiv, 436204. [Google Scholar]
- Montague PR, Dayan P, & Sejnowski TJ (1996). A framework for mesencephalic dopamine systems based on predictive Hebbian learning. Journal of Neuroscience, 16(5), 1936–1947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monti MM, Parsons LM, & Osherson DN (2012). Thought beyond language: Neural dissociation of algebra and natural language. Psychological Science, 23(8), 914–922. [DOI] [PubMed] [Google Scholar]
- Nesterov YE (1983). A method for solving the convex programming problem with convergence rate O(1/k^2). In Dokl. Akad. Nauk SSSR (Vol. 269, pp. 543–547). [Google Scholar]
- Newman AJ, Pancheva R, & Ozawa K (2001). An Event-Related fMRI Study of Syntactic and Semantic Violations. Journal of Psycholinguistic Research, 30(3), 339–364. [DOI] [PubMed] [Google Scholar]
- Nieto-Castañón A, & Fedorenko E (2012). Subject-specific functional localizers increase sensitivity and functional resolution of multi-subject analyses. Neuroimage, 63(3), 1646–1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieuwenhuis S, Forstmann BU, & Wagenmakers E-J (2011). Erroneous analyses of interactions in neuroscience: a problem of significance. Nature Neuroscience, 14(9), 1105. [DOI] [PubMed] [Google Scholar]
- Nieuwland MS, Martin AE, & Carreiras M (2012). Brain regions that process case: Evidence from Basque. Human Brain Mapping, 33(11), 2509–2520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen L, van Schijndel M, & Schuler W (2012). Accurate Unbounded Dependency Recovery using Generalized Categorial Grammars. In Proceedings of COLING 2012 (pp. 2125–2140). Mumbai, India. [Google Scholar]
- Novais-Santos S, Gee J, Shah M, Troiani V, Work M, & Grossman M (2007). Resolving sentence ambiguity with planning and working memory resources: Evidence from fMRI. Neuroimage, 37(1), 361–378. [DOI] [PubMed] [Google Scholar]
- Novick JM, Trueswell JC, & Thompson-Schill SL (2005). Cognitive control and parsing: Reexamining the role of Broca’s area in sentence comprehension. Cognitive, Affective, \& Behavioral Neuroscience, 5(3), 263–281. [DOI] [PubMed] [Google Scholar]
- Obleser J, & Kotz SA (2009). Expectancy constraints in degraded speech modulate the language comprehension network. Cerebral Cortex, 20(3), 633–640. [DOI] [PubMed] [Google Scholar]
- Obleser J, Wise RJS, Dresner MA, & Scott SK (2007). Functional integration across brain regions improves speech perception under adverse listening conditions. Journal of Neuroscience, 27(9), 2283–2289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldfield RC (1971). The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia, 9(1), 97–113. [DOI] [PubMed] [Google Scholar]
- Paunov A, Blank IA, & Fedorenko E (2019). Functionally distinct language and Theory of Mind networks are synchronized at rest and during language comprehension. Journal of Neurophysiology. 10.1152/jn.00619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne BR, & Federmeier KD (2018). Contextual constraints on lexico-semantic processing in aging: Evidence from single-word event-related brain potentials. Brain Research, 1687, 117–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peelle JE, Troiani V, Wingfield A, & Grossman M (2009). Neural processing during older adults’ comprehension of spoken sentences: Age differences in resource allocation and connectivity. Cerebral Cortex, 20(4), 773–782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perani D, & Abutalebi J (2005). The neural basis of first and second language processing. Current Opinion in Neurobiology, 15(2), 202–206. [DOI] [PubMed] [Google Scholar]
- Petersen SE, Fox PT, Posner MI, Mintun M, & Raichle ME (1988). Positron emission tomographic studies of the cortical anatomy of single-word processing. Nature, 331(6157), 585. [DOI] [PubMed] [Google Scholar]
- Pickering MJ, & Gambi C (2018). Predicting while comprehending language: A theory and review. Psychological Bulletin, 144(10), 1002. [DOI] [PubMed] [Google Scholar]
- Pinker S (1994). The Language Instinct: How the Mind Creates Language. New York: HarperCollins. [Google Scholar]
- Pliatsikas C, & Luk G (2016). Executive control in bilinguals: A concise review on fMRI studies. Bilingualism: Language and Cognition, 19(4), 699–705. [Google Scholar]
- Poldrack RA (2006). Can cognitive processes be inferred from neuroimaging data? Trends in Cognitive Sciences, 10(2), 59–63. [DOI] [PubMed] [Google Scholar]
- Poldrack RA (2011). Inferring mental states from neuroimaging data: from reverse inference to large-scale decoding. Neuron, 72(5), 692–697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polyak BT, & Juditsky AB (1992). Acceleration of stochastic approximation by averaging. SIAM Journal on Control and Optimization, 30(4), 838–855. [Google Scholar]
- Rao RPN, & Ballard DH (1999). Predictive coding in the visual cortex: a functional interpretation of some extra-classical receptive-field effects. Nature Neuroscience, 2(1), 79. [DOI] [PubMed] [Google Scholar]
- Rasmussen NE, & Schuler W (2018). Left-Corner Parsing With Distributed Associative Memory Produces Surprisal and Locality Effects. Cognitive Science, 42, 1009–1042. [DOI] [PubMed] [Google Scholar]
- Rayner K, Ashby J, Pollatsek A, & Reichle ED (2004). The effects of frequency and predictability on eye fixations in reading: Implications for the EZ Reader model. Journal of Experimental Psychology: Human Perception and Performance, 30(4), 720. [DOI] [PubMed] [Google Scholar]
- Resnik P (1992). Left-Corner Parsing and Psychological Plausibility. In Proceedings of COLING (pp. 191–197). Nantes, France. [Google Scholar]
- Richlan F, Gagl B, Hawelka S, Braun M, Schurz M, Kronbichler M, & Hutzler F (2013). Fixation-related fMRI analysis in the domain of reading research: using self-paced eye movements as markers for hemodynamic brain responses during visual letter string processing. Cerebral Cortex, 24(10), 2647–2656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roark B, Bachrach A, Cardenas C, & Pallier C (2009). Deriving lexical and syntactic expectation-based measures for psycholinguistic modeling via incremental top-down parsing. Proceedings of the 2009 Conference on Empirical Methods in Natural Langauge Processing, 324–333. [Google Scholar]
- Rodd JM, Davis MH, & Johnsrude IS (2005). The neural mechanisms of speech comprehension: fMRI studies of semantic ambiguity. Cerebral Cortex, 15(8), 1261–1269. [DOI] [PubMed] [Google Scholar]
- Rogalsky C, Almeida D, Sprouse J, & Hickok G (2015). Sentence processing selectivity in Broca’s area: Evident for structure but not syntactic movement. Language, Cognition and Neuroscience, 30(10), 1326–1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogalsky C, & Hickok G (2011). The role of Broca’s area in sentence comprehension. Journal of Cognitive Neuroscience, 23(7), 1664–1680. [DOI] [PubMed] [Google Scholar]
- Rüschemeyer SA, Fiebach CJ, Kempe V, & Friederici AD (2005). Processing lexical semantic and syntactic information in first and second language: fMRI evidence from German and Russian. Human Brain Mapping, 25(2), 266–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rushworth MF, & Behrens TE (2008). Choice, uncertainty and value in prefrontal and cingulate cortex. Nature Neuroscience, 11(4), 389. [DOI] [PubMed] [Google Scholar]
- Sakai KL (2005). Language acquisition and brain development. Science, 310(5749), 815–819. [DOI] [PubMed] [Google Scholar]
- Saxe R, Brett M, & Kanwisher N (2006). Divide and conquer: a defense of functional localizers. Neuroimage, 30(4), 1088–1096. [DOI] [PubMed] [Google Scholar]
- Schapiro AC, Rogers TT, Cordova NI, Turk-Browne NB, & Botvinick MM (2013). Neural representations of events arise from temporal community structure. Nature Neuroscience, 16(4), 486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheperjans F, Eickhoff SB, Hömke L, Mohlberg H, Hermann K, Amunts K, & Zilles K (2008). Probabilistic maps, morphometry, and variability of cytoarchitectonic areas in the human superior parietal cortex. Cerebral Cortex, 18(9), 2141–2157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuster S, Hawelka S, Himmelstoss NA, Richlan F, & Hutzler F (2019). The neural correlates of word position and lexical predictability during sentence reading: Evidence from fixation-related fMRI. Language, Cognition and Neuroscience, 1–12. [Google Scholar]
- Schuster S, Hawelka S, Hutzler F, Kronbichler M, & Richlan F (2016). Words in context: The effects of length, frequency, and predictability on brain responses during natural reading. Cerebral Cortex, 26(10), 3889–3904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott TL, Gallée J, & Fedorenko E (2017). A new fun and robust version of an fMRI localizer for the frontotemporal language system. Cognitive Neuroscience, 8(3), 167–176. [DOI] [PubMed] [Google Scholar]
- Scott SK, & McGettigan C (2013). The neural processing of masked speech. Hearing Research, 303, 58–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shafto MA, & Tyler LK (2014). Language in the aging brain: The network dynamics of cognitive decline and preservation. Science, 346(6209), 583–587. [DOI] [PubMed] [Google Scholar]
- Shain C (2019). Prediction is all you need: A large-scale study of the effects of word frequency and predictability in naturalistic reading. Proceedings of the 2019 Annual Conference of the North American Chapter of the Association for Computational Linguistics. [Google Scholar]
- Shain C, van Schijndel M, Futrell R, Gibson E, & Schuler W (2016). Memory access during incremental sentence processing causes reading time latency. In Proceedings of the Computational Linguistics for Linguistic Complexity Workshop (pp. 49–58). Association for Computational Linguistics. [Google Scholar]
- Shain C, & Schuler W (2018). Deconvolutional time series regression: A technique for modeling temporally diffuse effects. In Proceedings of the 2018 Conference on Empirical Methods in Natural Language Processing. [Google Scholar]
- Shain C, & Schuler W (2019). Continuous-Time Deconvolutional Regression for Psycholinguistic Modeling. PsyArXiv. [DOI] [PubMed] [Google Scholar]
- Shannon CE (1948). A Mathematical Theory of Communication. Bell System Technical Journal, 27, 379–423,623–656. [Google Scholar]
- Shmuel A, Augath M, Oeltermann A, & Logothetis NK (2006). Negative functional MRI response correlates with decreases in neuronal activity in monkey visual area V1. Nature Neuroscience, 9(4), 569. [DOI] [PubMed] [Google Scholar]
- Shmuel A, Yacoub E, Pfeuffer J, de Moortele P-F, Adriany G, Hu X, & Ugurbil K (2002). Sustained negative BOLD, blood flow and oxygen consumption response and its coupling to the positive response in the human brain. Neuron, 36(6), 1195–1210. [DOI] [PubMed] [Google Scholar]
- Sims CA (1980). Macroeconomics and reality. Econometrica: Journal of the Econometric Society, 1–48. [Google Scholar]
- Sims J, Kapse K, Glynn P, Sandberg C, & Kiran S (2016). The relationship between the amount of spared tissue, percent signal change and accuracy in language recovery in aphasia. Neuropsychologia, 84, 113–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith NJ, & Levy R (2013). The effect of word predictability on reading time is logarithmic. Cognition, 128, 302–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith AT, Singh KD, & Greenlee MW (2000). Attentional suppression of activity in the human visual cortex. Neuroreport, 11(2), 271–278. [DOI] [PubMed] [Google Scholar]
- Sood MR, & Sereno MI (2016). Areas activated during naturalistic reading comprehension overlap topological visual, auditory, and somatotomotor maps. Human Brain Mapping, 37(8), 2784–2810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speer NK, Reynolds JR, Swallow KM, & Zacks JM (2009). Reading stories activates neural representations of visual and motor experiences. Psychological Science, 20(8), 989–999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speer NK, Zacks JM, & Reynolds JR (2007). Human brain activity time-locked to narrative event boundaries. Psychological Science, 18(5), 449–455. [DOI] [PubMed] [Google Scholar]
- Sreenivasan KK, Curtis CE, & D’Esposito M (2014). Revisiting the role of persistent neural activity during working memory. Trends in Cognitive Sciences, 18(2), 82–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanescu-Cosson R, Pinel P, van de Moortele P-F, Le Bihan D, Cohen L, & Dehaene S (2000). Understanding dissociations in dyscalculia: A brain imaging study of the impact of number size on the cerebral networks for exact and approximate calculation. Brain, 123(11), 2240–2255. [DOI] [PubMed] [Google Scholar]
- Staub A (2015). The effect of lexical predictability on eye movements in reading: Critical review and theoretical interpretation. Language and Linguistics Compass, 9(8), 311–327. [Google Scholar]
- Staub A, & Benatar A (2013). Individual differences in fixation duration distributions in reading. Psychonomic Bulletin \& Review, 20(6), 1304–1311. [DOI] [PubMed] [Google Scholar]
- Steedman M (2000). The syntactic process. Cambridge, MA: MIT Press/Bradford Books. [Google Scholar]
- Strange BA, Duggins A, Penny W, Dolan RJ, & Friston KJ (2005). Information theory, novelty and hippocampal responses: Unpredicted or unpredictable? Neural Networks, 18(3), 225–230. [DOI] [PubMed] [Google Scholar]
- Strijkers K, Chanoine V, Munding D, Dubarry A-S, Trébuchon A, Badier J-M, & Alario F-X (2019). Grammatical class modulates the (left) inferior frontal gyrus within 100 milliseconds when syntactic context is predictive. Scientific Reports, 9(1), 4830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tahmasebi AM, Artiges E, Banaschewski T, Barker GJ, Bruehl R, Büchel C, … others. (2012). Creating probabilistic maps of the face network in the adolescent brain: a multicentre functional MRI study. Human Brain Mapping, 33(4), 938–957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanenhaus MK, Spivey-Knowlton MJ, Eberhard KM, & Sedivy JCE (1995). Integration of visual and linguistic information in spoken language comprehension. Science, 268, 1632–1634. [DOI] [PubMed] [Google Scholar]
- Thompson-Schill SL, Bedny M, & Goldberg RF (2005). The frontal lobes and the regulation of mental activity. Current Opinion in Neurobiology, 15(2), 219–224. [DOI] [PubMed] [Google Scholar]
- Tomaiuolo F, MacDonald JD, Caramanos Z, Posner G, Chiavaras M, Evans AC, & Petrides M (1999). Morphology, morphometry and probability mapping of the pars opercularis of the inferior frontal gyrus: an in vivo MRI analysis. European Journal of Neuroscience, 11(9), 3033–3046. [DOI] [PubMed] [Google Scholar]
- Tran D, Kucukelbir A, Dieng AB, Rudolph M, Liang D, & Blei DM (2016). Edward: A library for probabilistic modeling, inference, and criticism. ArXiv Preprint ArXiv:1610.09787. [Google Scholar]
- Tyler LK, Marslen-Wilson WD, Randall B, Wright P, Devereux BJ, Zhuang J, … Stamatakis EA (2011). Left inferior frontal cortex and syntax: Function, structure and behaviour in patients with left hemisphere damage. Brain, 134(2), 415–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhrig L, Dehaene S, & Jarraya B (2014). A hierarchy of responses to auditory regularities in the macaque brain. Journal of Neuroscience, 34(4), 1127–1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullman MT (2016). The declarative/procedural model: a neurobiological model of language learning, knowledge, and use In Neurobiology of Language (pp. 953–968). Elsevier. [Google Scholar]
- Vaden KI, Kuchinsky SE, Cute SL, Ahlstrom JB, Dubno JR, & Eckert MA (2013). The cingulo-opercular network provides word-recognition benefit. Journal of Neuroscience, 33(48), 18979–18986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vagharchakian L, Dehaene-Lambertz G, Pallier C, & Dehaene S (2012). A temporal bottleneck in the language comprehension network. Journal of Neuroscience, 32(26), 9089–9102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Schijndel M, Exley A, & Schuler W (2013). A Model of Language Processing as Hierarchic Sequential Prediction. Topics in Cognitive Science, 5(3), 522–540. 10.1111/tops.12034 [DOI] [PubMed] [Google Scholar]
- van Schijndel M, Nguyen L, & Schuler W (2013). An Analysis of Memory-based Processing Costs using Incremental Deep Syntactic Dependency Parsing. In Proc. of CMCL 2013 Association for Computational Linguistics. [Google Scholar]
- van Schijndel M, & Schuler W (2013). An Analysis of Frequency- and Memory-Based Processing Costs. In Proceedings of Human Language Technologies: The 2013 Annual Conference of the North American Chapter of the ACL. [Google Scholar]
- van Schijndel M, & Schuler W (2015). Hierarchic syntax improves reading time prediction. In Proceedings of NAACL-HLT 2015 Association for Computational Linguistics. [Google Scholar]
- Vandenberghe R, Nobre AC, & Price CJ (2002). The response of left temporal cortex to sentences. Journal of Cognitive Neuroscience, 14(4), 550–560. [DOI] [PubMed] [Google Scholar]
- Visser M, Jefferies E, & Lambon Ralph MA (2010). Semantic processing in the anterior temporal lobes: A meta-analysis of the functional neuroimaging literature. Journal of Cognitive Neuroscience, 22(6), 1083–1094. [DOI] [PubMed] [Google Scholar]
- Wacongne C, Changeux J-P, & Dehaene S (2012). A neuronal model of predictive coding accounting for the mismatch negativity. Journal of Neuroscience, 32(11), 3665–3678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wacongne C, Labyt E, van Wassenhove V, Bekinschtein T, Naccache L, & Dehaene S (2011). Evidence for a hierarchy of predictions and prediction errors in human cortex. Proceedings of the National Academy of Sciences, 108(51), 20754–20759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang R, Shen Y, Tino P, Welchman AE, & Kourtzi Z (2017). Learning predictive statistics: strategies and brain mechanisms. Journal of Neuroscience, 37(35), 8412–8427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Uhrig L, Jarraya B, & Dehaene S (2015). Representation of numerical and sequential patterns in macaque and human brains. Current Biology, 25(15), 1966–1974. [DOI] [PubMed] [Google Scholar]
- Wartenburger I, Heekeren HR, Abutalebi J, Cappa SF, Villringer A, & Perani D (2003). Early setting of grammatical processing in the bilingual brain. Neuron, 37(1), 159–170. [DOI] [PubMed] [Google Scholar]
- Wehbe L, Murphy B, Talukdar P, Fyshe A, Ramdas A, & Mitchell T (2014). Simultaneously uncovering the patterns of brain regions involved in different story reading subprocesses. PloS One, 9(11), e112575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wernicke C (1874). Der aphasische Symptomencomplex: eine psychologische Studie auf anatomischer Basis. Cohn. [Google Scholar]
- Whitfield-Gabrieli S, & Nieto-Castanon A (2012). Conn: a functional connectivity toolbox for correlated and anticorrelated brain networks. Brain Connectivity, 2(3), 125–141. [DOI] [PubMed] [Google Scholar]
- Whitney C, Huber W, Klann J, Weis S, Krach S, & Kircher T (2009). Neural correlates of narrative shifts during auditory story comprehension. Neuroimage, 47(1), 360–366. [DOI] [PubMed] [Google Scholar]
- Wild CJ, Yusuf A, Wilson DE, Peelle JE, Davis MH, & Johnsrude IS (2012). Effortful listening: The processing of degraded speech depends critically on attention. Journal of Neuroscience, 32(40), 14010–14021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willems RM, Frank SL, Nijhof AD, Hagoort P, & den Bosch A (2015). Prediction during natural language comprehension. Cerebral Cortex, 26(6), 2506–2516. [DOI] [PubMed] [Google Scholar]
- Wingfield A, & Grossman M (2006). Language and the aging brain: Patterns of neural compensation revealed by functional brain imaging. Journal of Neurophysiology, 96(6), 2830–2839. [DOI] [PubMed] [Google Scholar]
- Wise RJS, Scott SK, Blank SC, Mummery CJ, Murphy K, & Warburton EA (2001). Separate neural subsystems within Wernicke’s area. Brain, 124(1), 83–95. [DOI] [PubMed] [Google Scholar]
- Wlotko EW, & Federmeier KD (2012). Age-related changes in the impact of contextual strength on multiple aspects of sentence comprehension. Psychophysiology, 49(6), 770–785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yarkoni T, Speer NK, & Zacks JM (2008). Neural substrates of narrative comprehension and memory. Neuroimage, 41(4), 1408–1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokoyama S, Okamoto H, Miyamoto T, Yoshimoto K, Kim J, Iwata K, Jeong H, Uchida S, Ikuta N, Sassa Y, & Nakamura W, (2006). Cortical activation in the processing of passive sentences in L1 and L2: An fMRI study. Neuroimage, 30(2), 570–579. [DOI] [PubMed] [Google Scholar]
- Zarr N, & Brown JW (2016). Hierarchical error representation in medial prefrontal cortex. NeuroImage, 124, 238–247. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.