Abstract
The purpose of this study is to review the current literature on the use of hyaluronic acid (HA) specifically applied to the treatment of osteoarthritis (OA) secondary to primary inflammatory rheumatic diseases. Osteoarthritis should be carefully considered because it has potentially devastating effects on health-related quality of life. Locally injected HA seems to be an effective treatment for OA but it is not clear how to place this treatment in the context of inflammatory rheumatic disorders. To retrieve relevant articles, we conducted the search through MEDLINE, EMBASE and Cochrane Databases performing the PICO strategy. We finally selected four randomized clinical trials and six observational studies and grouped them in accordance with its main objective within three focuses: the clinical effect of HA therapy in joints without any signs of inflammation, the clinical effects of HA therapy in joints with active synovitis, and the involvement and changes of synovial fluid in the treatment of secondary OA. Our qualitative analysis clearly showed that the current literature is marked by high levels of heterogeneity and therefore difficult to interpret. Therefore, our hypothesis that viscosupplementation should be considered as a treatment for chronic moderate symptomatic OA secondary to inflammatory rheumatic diseases, and not for flares with joint swelling, cannot be definitely supported. Well-designed studies are necessary to definitively clarify the range of application of intra-articular HA injections in the treatment of inflammatory rheumatic disorders.
Keywords: Hyaluronic acid, Osteoarthritis, Qualitative synthesis, Rheumatic diseases, Rheumatology, Systematic review, Viscosupplementation
Key Summary Points
| Why carry out this study? |
| Osteoarthritis (OA) is the most frequent comorbidity with self-reported detrimental outcome in patients with systemic rheumatic disorders. |
| Locally injected hyaluronic acid (HA) seems to be an effective treatment for OA comorbidity and despite its increasing use in various conditions, doubts still exist with regard to the most successful field of application. |
| We conducted a systematic review aimed at investigating the role of HA as local treatment in OA secondary to chronic inflammatory arthritis. |
| What was learned from the study? |
| Our review clearly showed that the current literature on HA is affected by high heterogeneity. |
| Well-designed studies are necessary to definitively clarify the range of application of viscosupplementation therapy. |
Introduction
Osteoarthritis (OA) is the most frequent comorbidity with self-reported detrimental outcome in patients with systemic rheumatic disorders such as rheumatoid arthritis and other inflammatory arthritides that progressively affect joints. Osteoarthritis presence should be carefully considered because it may alters pain perception and functional status especially in long-standing patients [1]. Inflammation plays a central role in the development and progression of OA involving multiple mediators such as interleukin-1 (IL-1), interleukin-6 (IL-6), tumour necrosis factor alpha (TNFα) and metalloproteases that are responsible for degradation of proteoglycans in the extracellular matrix and reduction in the molecular weight and concentration of endogenous hyaluronic acid (HA) [2]. HA is the major component of synovial fluid and is responsible for its viscoelastic properties. As a natural component of cartilage, HA plays a crucial role in the trophic status of the cartilage and in the regulation of intra-articular environment.
Over the last decades, the introduction of disease-modifying antirheumatic drugs (DMARDs) and the acknowledgement of the importance of early diagnosis, prompt treatment and treat-to-target approach have radically changed the prognosis of these patients, preventing cartilage damage and leading to a notable decrease in total joint replacement [3, 4]. Furthermore, locally injected HA seems to be an effective treatment for OA comorbidity. Indeed, results from several in vitro and in vivo studies have indicated that exogenous HA can decrease the production of pro-inflammatory cytokines and stimulate the endogenous synthesis of HA and extracellular matrix components by synovial fibroblasts [5–9]. In all inflammatory conditions of the joint, the molecular weight of HA is reduced. Also, several clinical studies have shown that intra-articular HA relieves pain and improves function in symptomatic OA joints [9–12] and that the treatment has a positive global effect and is well tolerated. Some studies, mainly focused on the knee joint, have shown that repeated courses of HA treatment are safe and associated with total joint replacement delay for up to 3 years [13, 14].
In a meta-analysis published in 2009 [15], the authors summarised results from five trials [16–20] aimed at evaluating the efficacy of intra-articular HA injection in rheumatoid knees (Table 1). The main extracted outcomes were global pain and inflammation, global therapeutic efficacy and adverse effects. Risk ratio was used as a measure of effect size. The therapy was considered effective if it brought a moderate to remarkable benefit according to a Likert scale. The authors concluded that viscosupplementation therapy is an effective and safe alternative local treatment for the rheumatoid knee when compared to placebo as they estimated a pooled overall efficacy of 1.64 in favour of HA. The most common side effects encountered were arthralgia and joint swelling.
Table 1.
Results of the meta-analysis by Saito and Kotake [15]
| References | Patients, n | RRa of pain [95% CI] | RRa of inflammation [95% CI] | RRa of efficacy [95% CI] |
|---|---|---|---|---|
| Tanaka et al. [16] | 175 | 1.19 [0.96–1.47] | 1.40 [1.07–1.83] | 1.29 [1.07–1.55] |
| Tanaka et al. [17] | 203 | 1.83 [1.44–2.32] | 1.78 [1.37–2.32] | 1.76 [1.40–2.21] |
| Goto [18] | 20 | 2.00 [0.68–5.85] | 1.60 [0.80–3.20] | 2.12 [1.06–4.26] |
| Matuno [19] | 26 | 2.75 [1.18–6.42] | 3.50 [0.89–13.78] | 4.00 [1.46–10.93] |
| Komatubara et al. [20]b | 286 | – | – | 1.10 [0.95–1.27] |
| Pooled RR | 1.64 [1.14–2.35] | 1.61 [1.34–1.92] | 1.64 [1.14–2.35] |
aA value of risk ratio (RR) greater than 1 indicates that the observed results favours HA injections as compared to placebo
bThe study by Komatubara et al. was excluded from the estimation of RR of pain and inflammation because the authors used a different outcome measure with respect to the other included studies
Despite this body of evidence and the increasing use of HA injections in primary osteoarthritis, doubts still exist with regard to the most successful field of application [21]. First of all, viscosupplementation should be considered as a treatment for chronic moderate symptomatic OA, and not for flares with joint swelling, and therefore some poor results may be due to inappropriate use of HA injections. To better understand the place of HA local treatment in OA secondary to chronic inflammatory arthritis we conducted a systematic review of the studies that until now have accumulated evidence of safety and efficacy/effectiveness of HA in relieving pain and recovering joint functionality. As a result of the high heterogeneity of the retrieved studies (study design, treated joints, scheduling and duration of the treatment, etc.), a qualitative synthesis was performed.
Methods
Search Strategy and Eligibility Criteria
The systematic review was conducted according to the recommendations of PRISMA (Preferred Reporting Items for Systematic Review and Meta-Analysis) as conveyed in Moher’s guidelines [22]. We performed an accurate search of studies that evaluated patients suffering from OA secondary to primary inflammatory arthritis undergoing intra-articular injections of HA with the aim of assessing treatment efficacy/effectiveness. To retrieve relevant articles, we conducted the search through MEDLINE (Pubmed), EMBASE and Cochrane Database of Systematic Reviews. The search terms entered were psoriatic arthritis, rheumatoid arthritis, ankylosing spondylitis, juvenile idiopathic arthritis, spondyloarthropathies, systemic lupus erythematosus, systemic sclerosis and Sjögren’s syndrome as “population”, hyaluronic acid, hyaluronate, sodium hyaluronate, hyaluronate injection(s), viscosupplementation as “intervention” and Lequesne index, VAS, WOMAC, pain, SF-36, HAQ, effectiveness, efficacy, satisfactory as “outcome”. All relevant index and natural language terms were tailored for all databases searched. In addition, further relevant references were manually searched if needed. In particular, the latest meta-analysis and systematic reviews were additionally screened for any other relevant articles that could have been missed using the literature search strategy. Duplicate results were removed and the first screening process was done considering the title and abstract only. The inclusion/exclusion criteria used to determine which research should be retrieved are included in Table 2. A second selection was performed by fully reading the manuscript of the previously selected references. The review selection was independently performed by two reviewers (ODL and FP) whereas the discrepancies were solved by discussion with a third reviewer (AM) involved in case of no consensus could be achieved. The information about the numbers of articles generated by using search terms, the numbers of articles ruled out after the first screening and the reason for any excluded article were inserted in a PRISMA flow chart.
Table 2.
Inclusion/exclusion criteria for screening titles and abstracts
| Inclusion criteria | Exclusion criteria |
|---|---|
|
Full-text original articles English language Articles concerning OA secondary to the following diseases: psoriatic arthritis, rheumatoid arthritis, ankylosing spondylitis, juvenile idiopathic arthritis, spondyloarthropathies, systemic lupus erythematosus, systemic sclerosis and Sjögren’s syndrome Providing relevant information (e.g. response rate or other measures of effectiveness) Study design: any |
Abstract-only, letters to editor, reviews, case reports Papers that do not deal with intra-articular injection in patients with osteoarthritis secondary to inflammatory rheumatic diseases When multiple articles were based on the same study population, we included only the most complete (or recent) one |
The last part of this work focused on a critical review of the selected literature. This article is based on previously conducted studies and does not contain any studies with human participants or animals performed by any of the authors.
Quality Assessment
The articles that met all inclusion criteria were evaluated in relation to methodological quality. The Quality Assessment Tool for before–after (pre–post) studies with no control group and cross-sectional studies (https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools) was used as appraisal tool for evaluating the quality of observational studies, whereas each randomized controlled trial (RCT) was scored by the Jadad scale [23].
Results
Systematic Review Process
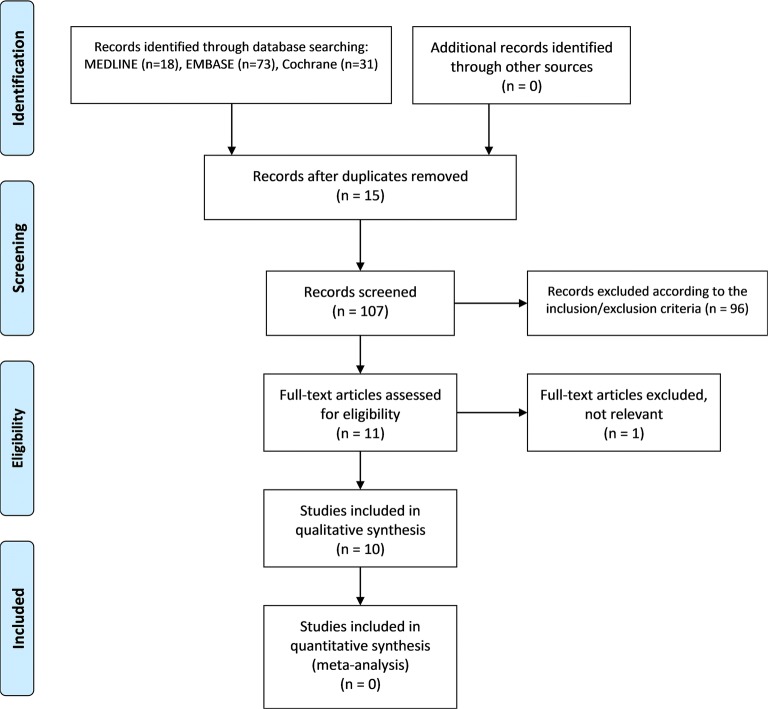
Articles were retrieved from the databases on September 24, 2019. Our search yielded 122 records, 73 from EMBASE, 18 from MEDLINE and 31 from Cochrane Library. After removal of duplicates (n = 15), 107 titles and abstracts were assessed according to inclusion/exclusion criteria. From this first screening, 11 potentially eligible articles underwent a full reading and only one text was removed as the included information was not relevant for answering our research question. It was not possible to include the five articles from the meta-analysis performed by Saito and Kotake [15] as they were all published in Japanese. Therefore, the qualitative synthesis was based on ten original articles (Fig. 1).
Fig. 1.
Flow chart showing the number of records identified and removed at each stage of the review, according to the PRISMA statement
Overall Characteristics of Selected Studies
The main characteristics of the articles are shown in Table 3. They included four RCT [24–27] and six observational studies [28–34] (five pre–post study with no control group and one cross-sectional design). In all studies, patients were affected by RA. In general, these studies differed in the primary endpoint, HA preparation and scheduling, injection site and outcome measures. Therefore, we grouped each study in accordance with its main objective within three main focuses: the clinical effect of HA therapy in joints without any signs of inflammation, the clinical effects of HA therapy in joints with active synovitis, and the involvement and changes of synovial fluid in the treatment of secondary OA (impact of the arthrocentesis and biochemical effects of exogenous HA in synovial fluid). As the included studies themselves have not clearly defined or clinically described joints with or without inflammation, we included the manuscripts in the group of joints with active synovitis when the authors did not specifically exclude patients with active disease. Indeed, in these cases the averaged values of the disease activity score-28 (DAS28) indicated a moderate to severe active rheumatic disease. On the other hand, we included the manuscript by Chou et al. [30] into the group of secondary osteoarthritis without any sign of inflammation as the authors stated that patients with RA were excluded in case of significantly inflamed osteoarthritic knees.
Table 3.
Characteristics of the final selected studies
| Study | Design | N | Joint(s) | HA | HA scheduling | Comparison | Outcome |
|---|---|---|---|---|---|---|---|
| Clinical effects in non-swollen joints | |||||||
| Chou et al. [30] | Pre–post treatment | 20 | Knee in RA | Artz | Five injections (once a week for 5 weeks) | Any | WOMAC at baseline, week 5, and week 9 (1 month after the last injection) |
| Clinical effects in joints with active synovitis | |||||||
| Wang et al. [24] | Pilot RCT | 48 | Ankle, foot in RA | Artz | Two injections (once a week for 2 weeks) | Intra-articular HA (n = 24) vs intra-articular lidocaine (2% xylocaine, n = 20) | VAS pain daily; FFI at baseline, 4 and 12 weeks after injection; CDUS score |
| Saito et al. [31] | Cross-sectional | 4725 | Knee, shoulder, elbow, wrist, ankle, fingers, toes in RA | Intra-articular HA (n = 291) vs intra-articular corticosteroid (n = 371) vs non-injection (n = 4057) | VAS pain daily; FFI. at baseline, 4 and 12 weeks after injection | ||
| Cheng and Tan [32] | Pre–post treatment | 31 | Knee, shoulder in RA | Five injections (once a week for 5 weeks) | Any | Pain, inflammation, pain on walking, morning stiffness | |
| Kopp et al. [25] | RCT | 41 | TMJ in RA | Hylartil | Three injections (biweekly for 4 weeks) | Intra-articular HA vs intra-articular glucortocoids (N = 14), saline (N = 13) | VAS pain, global evaluation, routine clinical examination according to Krough-Poulsen, CDS according to Helkimo |
| Isdale et al. [33] | Pre–post treatment, dose-ranging | 10 | Knee in RA | Healon | Three injections (once a week for 3 weeks) of 20, 40 and 60 mg | Any | Knee circumference and range of movement at baseline and weekly; daily diary of day/night pain and stiffness |
| Exogeneous hyaluronan and synovial fluid | |||||||
| Goto et al. [29] | Pre–post treatment | 25 | Knee in RA | SI-6601D | Five injections (once a week for 5 weeks) after aspiration of synovial fluid | Any | Local clinical symptoms (joint pain and inflammation, pain on walking) at baseline and 1 week after the end of the study; biochemical parameters (PGE2 and cytokines); glycosaminoglycans); systemic clinical assessment (PCR, ESR, EMS); safety assessment; pain motion and swelling and radiographical changes of the knee at arbitrary points (approximately 1–2 years) after the end of the study |
| Matsuno et al. [26] | RCT | 26 | Knee in RA | NRD101 | Five injections (once a week for 5 weeks) | 0.1% vs 0.01% HA in PBS | Pain, signs of inflammation and activities of daily living, characteristics (volume, viscosity, stringency, HA concentration, MW of HA, protein concentration, chondroitin 4,6-sulfate) of synovial fluid (before and 1 week after the end of the trial) |
| Goto et al. [34] | Pre–post treatment | 8 | Knee in RA | Artz | Six injections (biweekly for 12 weeks) after aspiration of synovial fluid | Any | Spontaneous pain, pain at motion, clinical lab determinations, volume of synovial fluid, total protein and HA concentration, intrinsic and specific viscosity, MW of HA, stringency, radiographical examination |
| Tanaka et al. [27] | RCT | 118 | Knee in RA | NRD101 | Five injections (once a week for 5 weeks) after aspiration of synovial fluid | Intra-articular HA after arthrocentesis vs intra-articular HA without arthrocentesis | Relapse of knee arthritis (no relief, recurring of symptoms and signs of arthritis, presence of joint fluid confirmed by ultrasound) |
TMJ temporomandibular joint, PBS phosphate buffered saline, WOMCAC Western Ontario and McMaster Universities Osteoarthritis Index, VAS visual analogue scale, FFI foot function index, CDUS colour Doppler ultrasound, CDS clinical dysfunction score. MW molecular weight
Overall, among the retrieved RCTs, only Tanaka [27] and Kopp [25] achieved a poorer rating score (Jadad score = 3) because the randomization method was not declared, whereas the observational studies fell within fair and good categories.
Qualitative Synthesis
Clinical Effects of Intra-Articular HA Administration in Joints Affected by Secondary Osteoarthritis Without Any Sign of Inflammation
Only one study [30] specifically covered this topic. However, it was not possible to completely rule out the inflammatory condition as patients with RA were excluded in case of significantly but only radiologically verified inflamed osteoarthritis of the knee. Briefly, HA intra-articular injections allowed an overall significant and quick improvement (after 5 weeks) of patients’ conditions in terms of pain, stiffness and physical function. Noteworthy, the authors observed a score reduction from baseline of about 50% and the effects of the local therapy were still present 1 month after the last HA injection. Nevertheless, these results are affected by a fair risk of bias because of the small sample size (n = 20) and the lack of a control group.
Clinical Effects of Intra-Articular Administration of HA in Joints with Active Synovitis
Five studies were included in this topic, two RCTs [24, 25], two uncontrolled longitudinal studies [32, 33] and one cross-sectional study [31]. Overall, 370 HA injections within eight different joints (shoulder, knee, ankle, foot, elbow, wrist, fingers and toes) were evaluated. The evaluated outcomes varied from generic scales of pain assessment such as VAS to more specific measures of joint function such as foot function index. Overall, each study resulted in improvements in joint function and pain when compared with baseline conditions, whereas the differences between HA and corticosteroid injections were not always evident. In the medical survey approach by Saito et al. [31], the authors concluded that, irrespective of joint, both HA and corticosteroids were almost equally effective in treating RA, where effectiveness was measured in terms of satisfactory rate. Similar conclusions were reached also in the RCT including patients with dysfunction of the temporomandibular joint (TMJ) [25], where HA and glucocorticoids had similar beneficial effects on both subjective and clinical signs of TMJ arthritis with a better performance of the latter in reducing lateral and posterior tenderness to palpation.
When compared to lidocaine [24], HA viscosupplementation appeared more effective in quickly reducing pain and disability with regard to ankles and feet affected by RA. No anti-inflammatory effect was seen because of the lack of reduction in synovial hypervascularization.
On the other hand, Isdale and colleagues [33] observed only a very little benefit of HA in the treatment of the knee joint of ten patients with RA compared to the group affected by primary OA, but the conclusions referred only to short-term effects.
Lastly, the study by Cheng and Tan [32] differed from the others as HA effectiveness was tested on patients with elderly-onset RA. Briefly, the authors observed longer-lasting effects, from 2 months to 3 years, confirming the positive trend of HA injections in reducing pain and disability.
Exogeneous Hyaluronan and Synovial Fluid
Biodynamic Performance
Regarding this topic, we included three studies [26, 29, 34]. In all studies except that by Matsuno et al. [26] the treatment was preceded by synovial fluid aspiration. A total of 46 patients and 47 knees were assessed before and after HA injection. Changes in some or all biochemical properties of rheumatoid synovial fluid (i.e. endogenous HA concentration, viscosity, stringency and synovial fluid volume) after intra-articular injection of HA were observed, whereas systemic inflammatory parameters (erythrocyte sedimentation rate and C-reactive protein) did not show any statistically significant improvement. A local significant reduction of the levels of prostaglandin E2 and chondroitin sulfate may be partly attributed to the improvement of synovitis parameters in the studies by Matsuno [26] and Goto [29]. The pain scores significantly decreased in all cases. Radiographical examination did not show any statistically significant improvement. Of note, only one study [26] was designed to have a control group (1% versus 0.01% HA) randomly assigned to treatment.
Impact of Arthrocentesis
The study by Tanaka [27] specifically focused on the effect of complete aspiration of the synovial fluid from the knee joint before the injection of high molecular weight hyaluronan. Patients suffering from RA and with symptoms of knee arthritis including effusion were enrolled. The study protocol envisaged a second group of patients in which the synovial fluid aspiration was not performed. The strengths of this study are the random allocation of patients to arthrocentesis or no arthrocentesis, the long-term evaluation (6 months of follow-up) and the quite large size of the sample (118 patients, 161 knees). The authors observed a higher prevalence of no relapses in the arthrocentesis group (66% versus 40% at 180 days). Predicting responders are duration of the knee arthritis (OR [95% CI] 1.07 [1.02–1.12]), C-reactive protein (OR [95% CI] 2.58 [1.11–5.95]) and radiological grade (OR [95% CI] 3.59 [1.53–8.39]) as assessed by the Larsen method.
Adverse Events
There were no adverse effects during the treatment. The only adverse event occurred after four intra-articular injections of HA and was due to the aggravation of systemic rheumatoid inflammation. Therefore, it was probably not related to the local treatment.
Discussion
In this study we performed a systematic review of the literature concerning the application of viscosupplementation in the treatment of OA in inflammatory autoimmune rheumatic disorders with the aim of investigating the current evidence of its efficacy/effectiveness.
The randomized and non-randomized studies carried out in this field are very heterogeneous and affected by some important biases (inadequate sample size; observational studies missing control groups; studies were conducted almost exclusively in Asia; subjects with different radiological degrees were not always independently analysed; outcomes were not always adequate, etc.). Furthermore, all the included manuscripts are exclusively restricted to RA and published many years ago, implicating that inclusion criteria and drug formulations could be changed over time. Therefore, the main consideration that arises from our review is the need to better clarify the correct scope of viscosupplementation in the treatment of rheumatic inflammatory disorders in a modern and well-designed setting.
The rationale of HA therapy in patients suffering from inflammatory rheumatic diseases such as RA derives from the results of several in vitro and in vivo models experiments [35]. In a hypothetical biochemical pathway, inflammatory conditions lead to the depolymerization of native HA into small fragments that may produce a range of proinflammatory responses. It is speculated that these small pieces of different sizes directly bind Toll-like receptor 4 (TLR-4) and CD44 inducing activation of several pathways that finally trigger NF-κB activation and its translocation to the nucleus, then perpetuating the tissue injury through the transcription of several detrimental intermediates. The injected HA would increase the local concentration of the synovial polysaccharide and displace the degraded HA from these receptors with consequent inhibition of TLR4 and CD44 activity and blocking of inflammation, finally allowing tissue repair. In this regard, several experimental animal models of OA in RA have elucidated the molecular and pathophysiological mechanisms of cartilage inflammation/degeneration and have demonstrated the positive effect of exogenous HA on the preservation of joint cartilage. In spite of this, in our systematic review we observed that the current literature on clinical application of viscosupplementation did not offer the same univocal message. In part, this heterogeneity can be explained because, in the majority of the cases, the therapy has been applied in patients with active synovitis. This is a critical point because intra-articular injections of both HA and corticosteroids are symptom-modifying agents and do not prevent or slow the disease progression [36]. On the other hand, only one study investigated the mechanical properties of HA but it did not offer enough evidence, missing a control group and including a very small sample size.
Furthermore, another doubtful conclusion raised from this systematic review was the lack of a substantial difference between corticosteroid and hyaluronan injection. Only two studies compared the value of these treatments, the survey by Saito et al. [31] and the manuscript by Kopp et al. [25] that concerned patients with RA involving the TMJ. However, both studies displayed crucial limitations: the former measured the effectiveness using subjective patient-reported experience, whereas the latter was affected by confounding factors influencing the outcome of treatments. Noteworthy, although the exact mechanism of action of both agents remains unknown, it is widely recognized that the viscosupplementation has a more favourable long-term profile than repeated steroids [36].
In summary, as many unresolved issues encompass the use of HA in musculoskeletal disorders, well-design studies are required to determine its most appropriate position in the framework for clinical practice.
Conclusion
This review clearly shows that the results of the current literature on HA utility in the treatment of secondary OA are based on old and heterogeneous studies, and therefore difficult to interpret. Well-designed studies are necessary to definitively clarify the range of application of viscosupplementation therapy.
Acknowledgements
Funding
No funding or sponsorship was received for this study or publication of this article.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Authorship Contributions
Substantial contributions to study conception and design: ODL, AM, IP. Substantial contributions to acquisition of data: FP, ODL, AM, AS, MDS. Substantial contributions to analysis and interpretation of data: FP, ODL. Drafting the article or revising it critically for important intellectual content: all the authors. Final approval of the version of the article to be published: all the authors.
Disclosures
Orazio De Lucia, Antonella Murgo, Francesca Pregnolato, Irene Pontikaki, Mirian De Souza, Alessandro Sinelli, Rolando Cimaz and Roberto Caporali have nothing to disclose.
Compliance with Ethics Guidelines
This article is based on previously conducted studies and does not contain any studies with human participants or animals performed by any of the authors.
Data Availability
Data sharing is not applicable to this article as no datasets were generated or analysed during the current study.
Footnotes
Enhanced Digital Features
To view enhanced digital features for this article go to 10.6084/m9.figshare.11808723.
References
- 1.Luque Ramos A, Redeker I, Hoffmann F, Callhoff J, Zink A, Albrecht K. Comorbidities in patients with rheumatoid arthritis and their association with patient-reported outcomes: results of claims data linked to questionnaire survey. J Rheumatol. 2019;46:564–571. http://www.jrheum.org/lookup/doi/10.3899/jrheum.180668. [DOI] [PubMed]
- 2.Mehana E-SE, Khafaga AF, El-Blehi SS. The role of matrix metalloproteinases in osteoarthritis pathogenesis: an updated review. Life Sci. 2019;234:116786. https://linkinghub.elsevier.com/retrieve/pii/S0024320519307131. Accessed 20 Nov 2019. [DOI] [PubMed]
- 3.Compagnoni R, Gualtierotti R, Randelli P. Total joint arthroplasty in patients with inflammatory rheumatic diseases. Adv Ther. 2018;35:1133–9. http://link.springer.com/10.1007/s12325-018-0750-9. [DOI] [PMC free article] [PubMed]
- 4.Jämsen E, Virta LJ, Hakala M, Kauppi MJ, Malmivaara A, Lehto MUK. The decline in joint replacement surgery in rheumatoid arthritis is associated with a concomitant increase in the intensity of anti-rheumatic therapy. Acta Orthop. 2013;84:331–7. http://www.tandfonline.com/doi/full/10.3109/17453674.2013.810519. [DOI] [PMC free article] [PubMed]
- 5.Shimazu A, Jikko A, Iwamoto M, et al. Effects of hyaluronic acid on the release of proteoglycan from the cell matrix in rabbit chondrocyte cultures in the presence and absence of cytokines. Arthritis Rheumatol. 1993;36:247–253. doi: 10.1002/art.1780360217. [DOI] [PubMed] [Google Scholar]
- 6.Moreland L. Intra-articular hyaluronan (hyaluronic acid) and hylans for the treatment of osteoarthritis: mechanisms of action. Arthritis Res Ther. 2003;5:54–67. doi: 10.1186/ar623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Håkansson L, Hällgren R, Venge P. Regulation of granulocyte function by hyaluronic acid. In vitro and in vivo effects on phagocytosis, locomotion, and metabolism. J Clin Investig. 1980;66:298–305. doi: 10.1172/JCI109857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nicholls MA, Fierlinger A, Niazi F, Bhandari M. The disease-modifying effects of hyaluronan in the osteoarthritic disease state. Clin Med Insights Arthritis Musculoskelet Disord. 2017;10:117954411772361. http://journals.sagepub.com/doi/10.1177/1179544117723611. [DOI] [PMC free article] [PubMed]
- 9.Maheu E, Bannuru RR, Herrero-Beaumont G, Allali F, Bard H, Migliore A. Why we should definitely include intra-articular hyaluronic acid as a therapeutic option in the management of knee osteoarthritis: results of an extensive critical literature review. Semin Arthritis Rheum. 2019;48:563–72. https://linkinghub.elsevier.com/retrieve/pii/S004901721830235X. [DOI] [PubMed]
- 10.Bellamy N, Campbell J, Welch V, Gee TL, Bourne R, Wells GA. Viscosupplementation for the treatment of osteoarthritis of the knee. Cochrane Database Syst Rev. 2006. http://doi.wiley.com/10.1002/14651858.CD005321.pub2. [DOI] [PMC free article] [PubMed]
- 11.Wang C-T, Lin J, Chang C-J, Lin Y-T, Hou S-M. Therapeutic effects of hyaluronic acid on osteoarthritis of the knee. J Bone Jt Surg. 2004;86:538–45. http://insights.ovid.com/crossref?an=00004623-200403000-00012. [DOI] [PubMed]
- 12.Quilliot J, Couderc M, Giraud C, Soubrier M, Mathieu S. Efficacy of intra-articular hyaluronic acid injection in knee osteoarthritis in everyday life. Semin Arthritis Rheum. 2019;49:e10–1. https://linkinghub.elsevier.com/retrieve/pii/S0049017219300046. [DOI] [PubMed]
- 13.Dasa V, Lim S, Heeckt P. Real-world evidence for safety and effectiveness of repeated courses of hyaluronic acid injections on the time to knee replacement surgery. Am J Orthop. 2018;47. https://www.amjorthopedics.com/article/real-world-evidence-safety-and-effectiveness-repeated-courses-hyaluronic-acid-injections. [DOI] [PubMed]
- 14.Delbarre A, Amor B, Bardoulat I, Tetafort A, Pelletier-Fleury N. Do intra-articular hyaluronic acid injections delay total knee replacement in patients with osteoarthritis—a Cox model analysis. PLoS One. 2017;12:e0187227. https://dx.plos.org/10.1371/journal.pone.0187227. [DOI] [PMC free article] [PubMed]
- 15.Saito S, Kotake S. Is there evidence in support of the use of intra-articular hyaluronate in treating rheumatoid arthritis of the knee? A meta-analysis of the published literature. Mod Rheumatol. 2009;19:493–501. doi: 10.3109/s10165-009-0189-6. [DOI] [PubMed] [Google Scholar]
- 16.Tanaka S, Souen S, Yamamoto M, Komatubara Y, Sugawara S, Matubara T. Clinical study of high molecular hyaluronic acid (NRD101) on rheumatoid arthritis. A multi-center, joint phase III comparative clinical study. Rinsho-Ryumachi. 1994;5:304–332. [Google Scholar]
- 17.Tanaka S, Souen S, Yamamoto M, Komatubara Y, Sugawara S, Matubara T. Clinical study of high molecular hyaluronic acid (NRD101) on rheumatoid arthritis. A multi-center, joint phase II clinical study. Rinsho-Ryumachi. 1994;5:279–303. [Google Scholar]
- 18.Goto M. Clinical effect of intra-articular injection of high molecular weight hyaluronic acid (NRD101) on rheumatoid arthritis and analysis of its synovial fluid. Rinsho-Ryumachi. 1994;6:33–49. [Google Scholar]
- 19.Matuno H. Effects of intra-articular therapy of hyaluronate and changes in characteristics of synovial fluid in RA patients. Rheumatology. 1996;16:154–163. [Google Scholar]
- 20.Komatubara Y, Inoue K, Souen S, Goto M, Tanaka S, Nakajima M. Dose-response study of high molecular weight hyaluronic acid (NRD101) on knee pain in rheumatoid arthritis. A multi-center comparative clinical study. Rinsho-Ryumachi. 2004;12:179–204. [Google Scholar]
- 21.Jevsevar D, Donnelly P, Brown GA, Cummins DS. Viscosupplementation for osteoarthritis of the knee. J Bone Jt Surg Am Vol. 2015;97:2047–60. https://insights.ovid.com/crossref?an=00004623-201512160-00009. [DOI] [PubMed]
- 22.Moher D, Liberati A, Tetzlaff J, Altman D. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Int J Surg. 2010;8:336–341. doi: 10.1016/j.ijsu.2010.02.007. [DOI] [PubMed] [Google Scholar]
- 23.Jadad AR, Moore RA, Carroll D, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials. 1996;17:1–12. doi: 10.1016/0197-2456(95)00134-4. [DOI] [PubMed] [Google Scholar]
- 24.Wang CC, Lee SH, Lin HY, et al. Short-term effect of ultrasound-guided low-molecular-weight hyaluronic acid injection on clinical outcomes and imaging changes in patients with rheumatoid arthritis of the ankle and foot joints. A randomized controlled pilot trial. Mod Rheumatol. 2017;27:973–980. doi: 10.1080/14397595.2016.1270496. [DOI] [PubMed] [Google Scholar]
- 25.Kopp S, Akerman S, Nilner M. Short-term effects of intra-articular sodium hyaluronate, glucocorticoid, and saline injections on rheumatoid arthritis of the temporomandibular joint. J Craniomandib Disord Facial Oral Pain. 1991;5:231–238. [PubMed] [Google Scholar]
- 26.Matsuno H, Yudoh K, Kondo M, Goto M, Kimura T. Biochemical effect of intra-articular injections of high molecular weight hyaluronate in rheumatoid arthritis patients. Inflamm Res. 1999;48:154–159. doi: 10.1007/s000110050439. [DOI] [PubMed] [Google Scholar]
- 27.Tanaka N, Sakahashi H, Sato E, Hirose K, Ishima T, Ishii S. Intra-articular injection of high molecular weight hyaluronan after arthrocentesis as treatment for rheumatoid knees with joint effusion. Rheumatol Int. 2002;22:151–154. doi: 10.1007/s00296-002-0214-y. [DOI] [PubMed] [Google Scholar]
- 28.Goto M, Hosako Y, Katayama M, Yamada T. Biochemical analysis of rheumatoid synovial fluid after serial intra-articular injection of high molecular weight sodium hyaluronate. Int J Clin Pharm Res. 1993;XIII:161–166. [PubMed] [Google Scholar]
- 29.Goto M, Hanyu T, Yoshio T, et al. Intra-articular injection of hyaluronate (SI-6601D) improves joint pain and synovial fluid prostaglandin E2 levels in rheumatoid arthritis: a multicenter clinical trial. Clin Exp Rheumatol. 2001;19:377–383. [PubMed] [Google Scholar]
- 30.Chou CL, Li HW, Lee SH, Tsai KL, Ling HY. Effect of intra-articular injection of hyaluronic acid in rheumatoid arthritis patients with knee osteoarthritis. J Chin Med Assoc. 2008;71:411–415. doi: 10.1016/S1726-4901(08)70092-3. [DOI] [PubMed] [Google Scholar]
- 31.Saito S, Momohara S, Taniguchi A, Yamanaka H. The intra-articular efficacy of hyaluronate injections in the treatment of rheumatoid arthritis. Mod Rheumatol. 2009;19:643–651. doi: 10.3109/s10165-009-0207-8. [DOI] [PubMed] [Google Scholar]
- 32.Cheng XF, Tan K. Intra-articular injections of sodium hyaluronate in treatment of eledrly-onset rheumatoid arthritis. Chin J Clin Rehabil. 2003;7:2476–2477. [Google Scholar]
- 33.Isdale AH, Hordon LD, Bird HA, Wright V. Intra-articulra hyaluronate (Healon): a dose-ranging study in rheumatoid arthritis and osteoarthritis. J Drug Dev. 1991;4:93–99. [Google Scholar]
- 34.Goto M, Hosako Y, Katayama M, Yamada T. Biochemical analysis of rheumatoid synovial fluid after serial intra-articular injection of high molecular weight sodium hyaluronate. Int J Clin Pharmacol Res. 1993;13:161–6. http://www.ncbi.nlm.nih.gov/pubmed/8225699. Accessed 29 Oct 2019. [PubMed]
- 35.Avenoso A, D’Ascola A, Scuruchi M, et al. Hyaluronan in experimental injured/inflamed cartilage: in vivo studies. Life Sci. 2018;193:132–40. https://linkinghub.elsevier.com/retrieve/pii/S0024320517305830. [DOI] [PubMed]
- 36.Bannuru RR, Osani MC, Vaysbrot EE, et al. OARSI guidelines for the non-surgical management of knee, hip, and polyarticular osteoarthritis. Osteoarthr Cartil. 2019;27:1578–1589. doi: 10.1016/j.joca.2019.06.011. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no datasets were generated or analysed during the current study.