Abstract
Introduction
This study aimed to evaluate the effect of treatment with eye drops containing citicoline and vitamin B12 on changes in function of the inner retina, morphology of the inner and outer retina, and microvascular condition in patients with type 1 diabetes (DM1) with mild signs of non-proliferative diabetic retinopathy (NPDR) during 3 years of follow-up.
Methods
A pilot study with prospective, randomized, and double-masked design was conducted to address the aims. Twenty patients with DM1 were enrolled and randomly divided into two groups: the DC group comprising patients treated with citicoline and vitamin B12 eye drops (10 patients; mean age ± standard deviation, 46.86 ± 8.78 years) and the DP group comprising those treated with placebo (10 patients; mean age ± standard deviation, 47.89 ± 7.74 years). In the DC group, one eye of each patient was treated with citicoline and vitamin B12 eye drops (OMK2®, Omikron Italia srl, Italy, 3 drops/day), while in the DP group, it was treated with placebo (eye drops containing hypromellose 0.3%, 3 drops/day) for a 3-year period. In both groups, Humphrey Matrix frequency doubling technology (FDT), spectral domain optical coherence tomography (SD-OCT) and OCT angiography (OCTA), and adaptive optics (AO) were applied at baseline and 12, 24, and 36 months of the follow-up period.
Results
In the results of follow-up evaluation, the DC and DP groups were significantly different: Significant reduction in function in terms of 10-2 FDT mean sensitivity and in morphology reflected by an increase in inner nuclear layer thickness and decrease in other plexiform layer thickness and foveal vessel density were observed in the DP group, while no such significant changes were observed in the DC group in the long term.
Conclusions
This pilot study indicated that patients with DM1 with mild signs of diabetic retinopathy (DR) who underwent treatment with citicoline and vitamin B12 eye drops for a 3-year duration achieved stabilization or decreased rate of functional impairment, neuroretinal degeneration, and microvascular damage.
Trial Registration
ClinicalTrials.gov identifier, NCT04009980.
Keywords: Citicoline, Diabetic retinopathy, FDT, OCTA, Ophthalmology, SD-OCT, Vitamin B12
Key Summary Points
| Why carry out this study? |
| Although diabetic retinopathy (DR) has been studied as a microvascular disease, degeneration of neural retinal elements, such as retinal ganglion cells (RGCs), has been found as an early event and it could worsen or participate in the development of microcirculatory abnormalities. |
| Several studies suggest that citicoline may induce an increase in function (neuroenhancement) and can prevent the neurodegeneration (neuroprotection) of RGC. |
| This double-blind, randomized, placebo-controlled, and prospective study was carried out to evaluate the effects of citicoline and vitamin B12 eye drops on function, structure, and vascular condition of central retina in patients with mild signs of DR during a period of 36 months. |
| What was learned from the study? |
| After 36 months of citicoline and vitamin B12 treatment, patients with DR showed no significant changes of macular function and of structural and vascular condition, whereas in patients with DR treated with placebo showed significant worsening of the same outcomes. |
| Data from this pilot study suggest that in patients with mild signs of DR, 3 years of treatment with citicoline and vitamin B12 eye drops induces a stabilization or slowing down of functional impairment, of the neuroretinal neurodegenerative process, and of microvascular damage. |
Introduction
Diabetic retinopathy (DR) is one of the most important causes of preventable visual impairment among patients of working age [1, 2]. Different pathogenic pathways of DR such as the patient’s glucose levels, genetic factors, and diabetes type could influence its development and progression over time.
Studies have focused on DR as a microvascular disease [3]; nevertheless, some reports indicate that generation of the neural retinal elements, such as retinal ganglion cells (RGCs) and glial cells, was as an early event that could contribute to or worsen microcirculatory abnormalities [4, 5]. Factors that can induce retinal neurodegeneration include an increase of excitoxic metabolites such as glutamate, branched chain amino acids, and homocysteine and decrease of folic acid and vitamin B12 [6].
On the basis of more recent studies, psychophysical [7], morphological [8, 9], and electrophysiological [10] abnormalities are involved in the retinal neurodegenerative process; in particular, patients with type 1 diabetes for less than 6 months’ duration showed abnormal pattern electroretinogram (PERG) responses, which suggests that functional impairment of RGCs occurs earlier in the course of DR [10]. Moreover, similar dysfunction is observed in glaucoma, in which abnormal PERG responses may precede the onset of visual filed defects [11].
Humphrey Matrix frequency doubling technology (FDT) is a psychophysical tool for evaluation of RGC function with ability to detect FDT abnormalities in the early stages of type 1 diabetes mellitus (DM1) [7].
The morphological changes of single retinal layers including thinning of the RGC layer and retinal fiber nerve layer (RNFL) were detected by spectral domain optical coherence tomography (SD-OCT) [8, 9], and these represent potential biomarkers of neurodegeneration in DR. Several studies using optical coherence tomography angiography (OCTA) indicated that parafoveal vessel density is reduced at the level of the deep capillary plexus (DCP) in patients with DM1 and in those with type 2 diabetes with absence of or mild DR [12–16], which suggests that microvascular involvement may be observed before appearance of any vascular alteration on the fundus examination. In addition, adaptive optics (AO) revealed morphological changes of the photoreceptor in patients with mild signs of DR, in particular a reduction of perifoveal photoreceptor density in vivo, increase of distance between the cones, and irregular packing arrangement compared to that of the normal cone [17–19].
On the basis of these facts, with regard to development of DR, ophthalmologists should be aware of neurodegenerative processes and challenges involved in targeting the active role of the neuronal retinal elements.
Studies using in vitro approaches indicate that cytidine-5′-diphosphocholine (citicoline) may induce a reduction in neurodegenerative processes and enhance the function of RGCs in glaucomatous patients. Recent reviews by Parisi et al. [20] and Faiq et al. [21] focused on its mechanism of action; briefly, citicoline is an essential precursor in the synthesis of phosphatidylcholine (a component of cell membranes), cardiolipin, and sphingomyelin, and inhibitor of neuronal degeneration by promotion of RGC function [21].
The effects of citicoline on RGC structure and function [21] are further supported by the role of the neurovascular unit at the level of the inner retinal layers in DR [22]. Studies indicate morpho-functional impairment of these layers in DR [21] and reduction in the levels of oxidative stress in neurogenerative processes by intake of vitamin B12 [6]. We considered these findings as the rationale for designing a pilot study to assess the potential effects of citicoline and vitamin B12 on the morpho-functional status of RGCs in patients with DM1.
The main goal of the study was to evaluate whether or not treatment with eye drops containing citicoline and vitamin B12 induces changes in the function of RGCs through FDT, in patients with DM1 with mild signs of non-proliferative diabetic retinopathy (NPDR) during a 3-year follow-up. Additional aims were to identify whether citicoline and vitamin B12 treatment influence changes of morphology of the inner and outer retina through SD-OCT and photoreceptor analysis with AO, respectively, and retinal microvascular assessment by OCTA.
Methods
Patients
Seventy patients with DM1 with mild signs of NPDR (37 male patients and 33 female patients; mean age ± standard deviation, 48.36 ± 6.34 years) were screened for enrollment in the study.
The diagnosis of mild NPDR was determined by two retinal specialists (MP and FS) through indirect ophthalmoscopy, slit lamp stereo biomicroscopy, and stereoscopic fundus photography. Mild NPDR was defined as the presence of at least one microaneurysm and/or hemorrhage in the central retina in the absence of peripheral lesions (stage 2 of the International Clinical Diabetic Retinopathy Disease Severity Scale [ICDRSS]) [23].
Inclusion criteria were patients with DM1 with mild NPDR and impairment of retinal sensitivity based on FDT findings of mean deviation (MD) P < 5%, or two locations with P < 5% and one location with P < 1% in the total, or pattern deviation plots, and best corrected visual acuity (BCVA) > 0.1 logarithm of the minimum angle of resolution (logMAR).
Exclusion criteria were hyperopia higher than + 3 diopter (D), myopia higher than − 6 D, or astigmatism higher than 2 D and BCVA < 0.1 logMAR, media opacity, previous ocular surgery, previous diagnosis of glaucoma, uveitis, other retinal disease, and ocular or systemic disease other than diabetes or mild DR; in addition, those with signs of retinopathy more advanced than mild (e.g., macular edema or any sign of proliferative DR) and poor metabolic status (hemoglobin A1c [HbA1c] > 9%) at baseline or during follow-up (see below) were excluded.
When both eyes fulfilled the inclusion criteria, the eye with worse BCVA was selected; when both eyes showed the same BCVA, the right eye was selected for analysis. As a result, 20 eyes with mild NPDR of 20 patients with DM1 (11 male patients and nine female patients; mean age ± standard deviation, 47.35 ± 8.25 years) were enrolled in the study.
Study Design
This is a prospective, interventional, randomized, double-masked, monocentric pilot study. It was conducted according to the tenets of the Declaration of Helsinki and approved by the local ethics committee (Comitato Etico Centrale IRCCS Lazio, Sezione IFO/Fondazione Bietti, Rome, Italy). The study was registered at ClinicalTrials.gov (NCT04009980). In all patients, signed informed consent forms were obtained at the time of recruitment.
Baseline
Patients with DM1 were randomly divided into two age-matched groups of 10 patients each: (a) The DC group comprised those who received treatment with citicoline and vitamin B12 eye drops (10 eyes of five male and five female patients with mean age ± standard deviation of 46.86 ± 8.78 years; mean duration of disease of 25 ± 12.82 years; mean percentage of HbA1c of 7.36 ± 0.96; and mean BCVA (Early Treatment Diabetic Retinopathy Study [ETDRS] letter score) of 87.57 ± 3.51). (b) The DP group comprised those who received placebo treatment (10 eyes of six male patients and four female patients with mean age ± standard deviation of 47.89 ± 7.74 years; mean duration of disease of 21.78 ± 9.42 years; mean percentage of HbA1c of 7.81 ± 0.79; and mean BCVA (ETDRS letter score) of 86.33 ± 5.85.
The patients with DM1 with screening by MP and PG were randomly separated into two groups by an electronically generated randomization system according to age, duration of disease, percentage of HbA1c, and similar values of FDT (mean sensitivity [MS] of 10-2, described below).
Month 0–36
During 36 months of trial, the patients in the DC group were treated with citicoline and vitamin B12 eye drops (OMK2® containing citicoline 2%, hyaluronic acid 0.2%, and cyanocobalamin 0.05%; Omikron Italia srl, Italy), 1 drop thrice daily, while those in the DP group were treated with placebo (eye drops containing hypromellose 0.3%), 1 drop thrice daily.
During the trial period, three eyes each of the DC and DP groups were lost to follow-up as a result of patients’ unavailability at the time of examination. Finally, seven eyes in the DC group and seven eyes in the DP group that completed the study were analyzed.
Compliance with eye drops treatment was assessed through a questionnaire administered by the study personnel at each visit. As expected in clinical study, self-reported adherence to treatment was high, and in all patients, rating of compliance as good to very good (regular use of eye drops in at least 80% of the trial period) was obtained.
The randomization key was opened to all investigators at the end of the follow-up.
In each patient of the DC and DP group, examinations were performed at baseline and 12, 24, and 36 months as follows.
Psychophysical Evaluation (FDT)
In both the DC and DP groups, two FDT strategies using thresholds of 24-2 and 10-2 were used, according to the method in our previous study [7]. At baseline and during follow-up, to assess test–retest variability and rule out relevant learning curve, FDT examinations were performed at two separate recording sessions within 2 weeks. Reliability criteria were defined according to fixation errors less than 33%, false positives, and false negatives. Values obtained at the second examination were submitted to statistical analysis. If the second test was deemed unreliable, a third examination was performed, which was included in the statistical analysis. In both FDT 24-2 and 10-2 strategies, the values of the following parameters were considered: mean deviation (MD), pattern standard deviation (PSD), and MS measured as the average sensitivity at all test locations divided by the total number of locations (except the fovea). MD P value of less than 5% was considered as the threshold of abnormality.
Morphological Evaluations
Spectral Domain Optical Coherence Tomography (SD-OCT) and OCT Angiography (OCTA)
In all patients of the DC and DP groups, SD-OCT and OCTA were conducted three times according to the method described in our previous studies [9, 13], and the average values for each parameter (see below) were considered for subsequent analysis (Spectralis; Heidelberg Engineering GmbH, Heidelberg, Germany; OCTA AngioVue XR Avanti; Optovue Inc., Fremont, California, USA).
In SD-OCT, the macular measurements (subfoveal, parafoveal, and total values) were obtained using in-built Spectralis mapping software, and segmentation software [9] was used for individual retinal layer thicknesses: RNFL, ganglion cell layer (GCL), inner plexiform layer (IPL), inner nuclear layer (INL), outer plexiform layer (OPL), outer nuclear layer (ONL), retinal pigment epithelium (RPE), and photoreceptor layer (PR), and central retinal (CRT).
In OCTA, en face OCT angiograms were segmented to identify the superficial capillary plexus (SCP) and DCP. The SCP slab was acquired from the internal limiting membrane (ILM) to IPL/INL (9 μm above), while the DCP slab was acquired from the IPL/INL junction (9 μm above) to the OPL/ONL junction (9 μm below), of which, the latter additionally included the intermediate capillary plexus. Moreover, parafoveal vessel density (VD), defined as the percentage of total area occupied by the vessels and microvasculature, was quantified in SCP and DCP within an annulus centered on the fovea with inner and outer ring diameters of 1 and 2.5 mm. The area of the foveal avascular zone (FAZ) was measured using the no flow function of the software. Accuracy of image segmentation in SD-OCT and OCTA was verified independently by two readers (MP and FS).
Adaptive Optics (AO)
In all patients of the DC and DP groups, AO was conducted using Rtx1 (Imagine Eyes, Orsay, France) according to the method in our studies [18, 19]. On the basis of our previous findings by AO [19], the following parameters were considered: cone density, linear dispersion index, and heterogeneity packing index.
Statistics
Sample size was estimated on the basis of a pilot study including four eyes of four patients with DM1 without signs of DR who were not included in the present study (unpublished data). Inter-individual variability is expressed as standard deviations (SD) for FDT 10-2 MS; in particular, the size of patient groups was calculated using mean value of 29.77 decibel (dB) and SD of 1.58 dB. Assuming a change of FDT 10-2 MS of about 10% with SD of 1.5 dB during the follow-up period, six patients in each group were required at α = 0.05 and β = 0.20 (power 1 − β = 0.80). On the basis of a predicted dropout rate of 40% of patients during follow-up, 10 patients per group were enrolled at baseline.
The results of the patient’s age, BCVA, and instrument measurements are expressed as mean ± SD. Normal data distribution was assessed using one-sample Kolmogorov–Smirnov test. The differences in values at each timepoint between the DC and DP groups were compared using independent-samples Mann–Whitney test. Differences in values over time were assessed using MAnova (general linear model [GLM] within factor time and between factor group) repeated measures or Friedman test, as appropriate. Intention-to-treat analysis was performed to avoid bias due to missing information. In the comparison of treatment groups, patients’ allocation after randomization was considered in the last value carried forward method.
In all analyses, P < 0.05 was considered as statistical significance. Statistical evaluation was performed using SPSS software (version 17.0; SPSS Inc, Chicago, IL).
Results
Psychophysical (FDT) Evaluation
Comparisons of the mean values of the FDT 24-2 and 10-2 parameters at baseline and 12, 24, and 36 months in the DC and DP groups are shown in Table 1.
Table 1.
Results of Humphrey Matrix frequency doubling technology 24-2 and 10-2 (mean parameter value ± standard deviation in decibel [dB]) in patients with diabetes mellitus type 1 treated with citicoline and vitamin B12 eye drops (DC group) or with placebo (DP group) at baseline and 12, 24, and 36 months of follow-up
| Baseline | 12 months | 24 months | 36 months | GLM P value* | P value (over time) | |
|---|---|---|---|---|---|---|
| 24-2 | ||||||
| Mean deviation | ||||||
| DC group | − 0.58 ± 1.68 | 0.30 ± 1.85 | 0.36 ± 1.81 | − 0.07 ± 1.94 | 0.464 | |
| DP group | − 0.03 ± 3.36 | − 0.54 ± 2.41 | − 0.09 ± 3.22 | − 0.40 ± 2.81 | 0.105 | 0.305 |
| P value (between groups)^ | 0.711 | 0.560 | 0.491 | 0.711 | ||
| Pattern standard deviation | ||||||
| DC group | 3.21 ± 0.47 | 3.00 ± 0.94 | 2.73 ± 0.78 | 3.03 ± 1.24 | 0.253 | |
| DP group | 3.02 ± 0.73 | 2.93 ± 0.47 | 2.77 ± 0.57 | 2.82 ± 0.64 | 0.169 | 0.372 |
| P value (between groups)^ | 0.368 | 0.711 | 0.751 | 0.711 | ||
| Mean sensitivity | ||||||
| DC group | 26.58 ± 1.88 | 27.47 ± 1.47 | 27.37 ± 2.34 | 27.18 ± 2.62 | 0.553 | |
| DP group | 27.61 ± 3.29 | 26.80 ± 2.66 | 26.99 ± 3.06 | 26.71 ± 3.14 | 0.170 | 0.210 |
| P value (between groups)^ | 0.711 | 0.711 | 0.634 | 0.791 | ||
| 10-2 | ||||||
| Mean deviation | ||||||
| DC group | − 0.77 ± 1.57 | − 0.27 ± 1.42 | − 1.74 ± 1.96 | − 1.84 ± 3.04 | 0.278 | |
| DP group | − 0.67 ± 2.51 | − 2.32 ± 2.83 | − 2.31 ± 3.09 | − 2.66 ± 2.74 | 0.347 | 0.055 |
| P value (between groups) ^ | 0.958 | 0.081 | 0.266 | 0.397 | ||
| Pattern standard deviation | ||||||
| DC group | 2.92 ± 0.67 | 2.70 ± 0.59 | 3.25 ± 0.91 | 3.16 ± 1.24 | 0.176 | |
| DP group | 3.12 ± 0.56 | 2.98 ± 0.66 | 3.00 ± 0.70 | 3.05 ± 0.78 | 0.593 | 0.664 |
| P value (between groups)^ | 0.427 | 0.491 | 0.525 | 0.874 | ||
| Mean sensitivity | ||||||
| DC group | 28.77 ± 2.27 | 29.66 ± 1.83 | 28.52 ± 2.41 | 28.14 ± 3.46 | 0.238 | |
| DP group | 29.76 ± 2.47 | 27.95 ± 2.90 | 27.79 ± 2.85 | 27.41 ± 2.81 | 0.018 | 0.009 |
| P value (between groups)^ | 0.791 | 0.125 | 0.427 | 0.491 | ||
Significant values are presented in bold font
*P value of the general linear model (GLM) of Friedman/MAnova repeated measures
^Mann–Whitney U
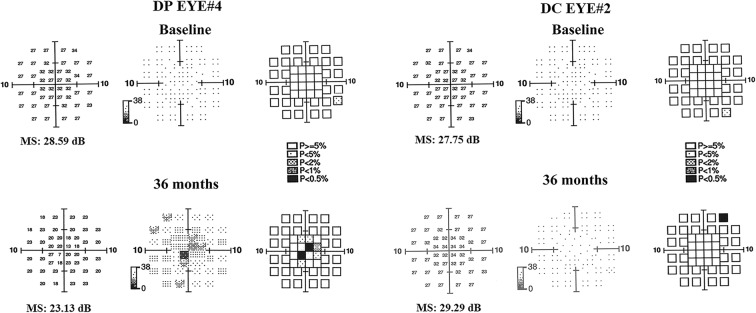
Representative findings of FDT at baseline and end of follow-up for one eye each in the DC and DP groups are shown in Fig. 1.
Fig. 1.
Examples of mean sensitivity (MS) with Humphrey Matrix frequency doubling technology (FDT) 10-2 strategy in two patients with type 1 diabetes with mild signs of non-proliferative diabetic retinopathy. One patient (DP EYE# 4) was treated with placebo (3 drops/day), and one patient (DC EYE# 2), with citicoline and vitamin B12 (3 drops/day) for 36 months of follow-up. With respect to baseline, at the end of follow-up (36 months), worsening of MS values was observed in DP EYE, while no significant changes of those in DC EYE were observed
At baseline, no statistical differences were observed for each FDT parameter between the DC and DP groups. During follow-up, no statistical differences were observed for FDT 24-2 MS, PSD, and MD between the DC and DP groups, whereas a significant difference of FDT 10-2 MS was observed between the two groups in the long-term follow-up; in particular, no statistical change of MS was observed in the DC group, while a significant decrease of 10-2 MS was observed in the DP group over time.
Morphological (SD-OCT, OCTA, and AO) Evaluations
Comparisons of the mean values of the SD-OCT and OCTA parameters at baseline and 12, 24, and 36 months in the DC and DP groups are shown in Tables 2, 3, and 4.
Table 2.
Results of spectral domain optical coherence tomography
| Baseline | 12 months | 24 months | 36 months | GLM P value* | P value (over time) | |
|---|---|---|---|---|---|---|
| CRT | ||||||
| DC group | 273.29 ± 14.95 | 275.00 ± 13.92 | 272.43 ± 14.16 | 275.71 ± 18.69 | 0.662 | |
| DP group | 271.00 ± 35.44 | 276.50 ± 30.67 | 275.38 ± 29.62 | 274.75 ± 31.98 | 0.514 | 0.536 |
| P value (between groups)^ | 0.954 | 1.000 | 1.000 | 0.728 | ||
| RNFL | ||||||
| Subfoveal | ||||||
| DC group | 12.43 ± 1.90 | 13.57 ± 3.21 | 12.29 ± 2.29 | 13.43 ± 2.07 | 0.736 | |
| DP group | 12.63 ± 3.34 | 13.00 ± 3.82 | 12.63 ± 3.66 | 13.13 ± 3.48 | 0.429 | 0.425 |
| P value (between groups)^ | 0.768 | 0.682 | 0.815 | 0.861 | ||
| Parafoveal | ||||||
| DC group | 21.86 ± 1.60 | 22.61 ± 1.82 | 21.86 ± 1.13 | 22.57 ± 1.61 | 0.050 | |
| DP group | 22.03 ± 3.42 | 21.97 ± 2.66 | 21.63 ± 2.45 | 21.72 ± 2.84 | 0.157 | 0.452 |
| P value (between groups)^ | 0.816 | 0.486 | 0.523 | 0.324 | ||
| Total | ||||||
| DC group | 26.92 ± 2.26 | 27.16 ± 1.79 | 26.57 ± 1.77 | 27.22 ± 1.54 | 0.081 | |
| DP group | 26.24 ± 4.17 | 25.79 ± 3.80 | 25.64 ± 3.70 | 26.28 ± 5.80 | 0.412 | 0.796 |
| P value (between groups)^ | 0.417 | 0.418 | 0.643 | 0.203 | ||
| GCL | ||||||
| Subfoveal | ||||||
| DC group | 13.86 ± 2.79 | 13.43 ± 2.30 | 13.71 ± 2.29 | 14.00 ± 3.32 | 0.715 | |
| DP group | 15.63 ± 6.46 | 15.13 ± 6.03 | 14.88 ± 6.01 | 15.38 ± 5.60 | 0.462 | 0.340 |
| P value (between groups)^ | 0.815 | 0.907 | 0.862 | 0.907 | ||
| Parafoveal | ||||||
| DC group | 50.39 ± 3.63 | 50.39 ± 3.58 | 49.96 ± 4.02 | 50.79 ± 3.86 | 0.729 | |
| DP group | 46.75 ± 7.60 | 47.75 ± 9.61 | 47.41 ± 9.48 | 47.72 ± 9.59 | 0.909 | 0.955 |
| P value (between groups)^ | 0.297 | 0.728 | 0.643 | 0.563 | ||
| Total | ||||||
| DC group | 40.11 ± 2.50 | 40.29 ± 2.42 | 39.98 ± 2.78 | 40.44 ± 3.09 | 0.780 | |
| DP group | 37.40 ± 5.11 | 38.44 ± 6.28 | 38.26 ± 6.22 | 38.81 ± 6.94 | 0.696 | 0.722 |
| P value (between groups)^ | 0.384 | 0.602 | 0.643 | 0.563 | ||
The central retinal thickness (CRT), retinal nerve fiber layer (RNFL), and ganglionic cell layer (GCL) thickness (mean parameter value ± standard deviation in micron) in patients with diabetes mellitus type 1 treated with citicoline and vitamin B12 eye drops (DC group) or with placebo (DP group) at baseline and 12, 24, and 36 months of follow-up. Significant values are presented in bold font
*P value of the general linear model (GLM) of Friedman/MAnova repeated measures
^Mann–Whitney U
Table 3.
Results of spectral domain optical coherence tomography
| Baseline | 12 months | 24 months | 36 months | GLM P value* | P value (over time) | |
|---|---|---|---|---|---|---|
| INL | ||||||
| Subfoveal | ||||||
| DC group | 20.14 ± 5.73 | 20.00 ± 4.47 | 20.29 ± 5.38 | 21.29 ± 6.13 | 0.929 | |
| DP group | 24.25 ± 9.04 | 23.50 ± 8.28 | 23.25 ± 6.69 | 22.75 ± 6.69 | 0.454 | 0.522 |
| P value (between groups)^ | 0.449 | 0.449 | 0.485 | 0.561 | ||
| Parafoveal | ||||||
| DC group | 39.79 ± 1.61 | 39.39 ± 2.44 | 40.25 ± 2.17 | 40.14 ± 1.39 | 0.916 | |
| DP group | 38.97 ± 3.19 | 41.31 ± 4.44 | 41.28 ± 5.35 | 42.41 ± 5.36 | 0.042 | 0.022 |
| P value (between groups)^ | 0.643 | 0.452 | 1.000 | 0.562 | ||
| Total | ||||||
| DC group | 34.29 ± 1.26 | 33.86 ± 1.59 | 34.34 ± 1.45 | 34.65 ± 1.30 | 0.770 | |
| DP group | 34.07 ± 2.92 | 35.60 ± 3.88 | 35.46 ± 4.17 | 36.60 ± 4.85 | 0.139 | 0.064 |
| P value (between groups)^ | 0.728 | 0.487 | 0.862 | 0.486 | ||
| OPL | ||||||
| Subfoveal | ||||||
| DC group | 27.00 ± 5.74 | 29.29 ± 6.47 | 28.29 ± 7.34 | 29.29 ± 6.95 | 0.619 | |
| DP group | 30.13 ± 6.36 | 26.38 ± 4.21 | 24.88 ± 3.72 | 24.63 ± 5.24 | 0.025 | 0.023 |
| P value (between groups)^ | 0.323 | 0.292 | 0.449 | 0.222 | ||
| Parafoveal | ||||||
| DC group | 34.39 ± 4.46 | 34.00 ± 4.93 | 33.61 ± 5.11 | 34.18 ± 5.55 | 0.992 | |
| DP group | 34.59 ± 5.21 | 34.06 ± 3.34 | 32.78 ± 3.76 | 33.13 ± 3.43 | 0.923 | 0.467 |
| P value (between groups)^ | 0.817 | 0.602 | 0.954 | 0.954 | ||
| Total | ||||||
| DC group | 30.71 ± 2.94 | 30.79 ± 3.24 | 30.40 ± 3.73 | 30.70 ± 3.86 | 0.997 | |
| DP group | 30.82 ± 3.65 | 30.65 ± 2.16 | 29.67 ± 2.50 | 29.71 ± 2.30 | 0.883 | 0.279 |
| P value (between groups)^ | 0.908 | 0.862 | 0.817 | 0.817 | ||
The inner nuclear layer (INL) and outer plexiform layer (OPL) thickness (mean parameter value ± standard deviation of measured in micron) in patients with diabetes mellitus type 1 treated with citicoline and vitamin B12 eye drops (DC group) or with placebo (DP groups) at baseline and 12, 24, and 36 months of follow-up. Significant values are presented in bold font
*P value of the general linear model (GLM) of Friedman/MAnova repeated measures
^Mann–Whitney U
Table 4.
Results of spectral domain optical coherence tomography
| Baseline | 12 months | 24 months | 36 months | GLM P value* | P value (over time) | |
|---|---|---|---|---|---|---|
| SCP-VD | ||||||
| Whole | ||||||
| DC group | 48.66 ± 3.65 | 49.59 ± 3.78 | 48.64 ± 4.25 | 48.49 ± 4.00 | 0.518 | |
| DP group | 49.90 ± 3.85 | 47.80 ± 4.95 | 48.47 ± 5.29 | 47.21 ± 5.62 | 0.112 | 0.062 |
| P value (between groups)^ | 0.427 | 0.560 | 0.958 | 0.634 | ||
| Foveal | ||||||
| DC group | 29.39 ± 1.87 | 28.95 ± 3.89 | 27.42 ± 2.53 | 27.10 ± 2.65 | 0.131 | |
| DP group | 31.20 ± 4.56 | 28.32 ± 7.39 | 28.46 ± 6.19 | 26.87 ± 7.55 | 0.005 | 0.017 |
| P value (between groups)^ | 0.427 | 0.711 | 0.874 | 0.491 | ||
| Parafoveal | ||||||
| DC group | 49.20 ± 4.13 | 50.17 ± 4.06 | 49.58 ± 4.58 | 49.58 ± 4.55 | 0.604 | |
| DP group | 50.07 ± 3.78 | 48.32 ± 4.93 | 49.09 ± 5.22 | 47.97 ± 5.92 | 0.191 | 0.266 |
| P value (between groups)^ | 0.634 | 0.491 | 0.874 | 0.427 | ||
| Temporal | ||||||
| DC group | 48.69 ± 3.42 | 48.92 ± 4.16 | 48.45 ± 4.57 | 48.80 ± 5.02 | 0.450 | |
| DP group | 48.88 ± 4.29 | 47.45 ± 5.22 | 48.29 ± 5.72 | 46.71 ± 6.00 | 0.358 | 0.240 |
| P value (between groups)^ | 0.958 | 0.711 | 0.958 | 0.560 | ||
| Superior | ||||||
| DC group | 49.04 ± 5.03 | 50.83 ± 4.67 | 50.82 ± 4.75 | 51.14 ± 4.42 | 0.602 | |
| DP group | 51.50 ± 3.71 | 49.87 ± 5.34 | 50.39 ± 5.59 | 48.56 ± 6.44 | 0.073 | 0.183 |
| P value (between groups)^ | 0.427 | 0.874 | 0.874 | 0.427 | ||
| Nasal | ||||||
| DC group | 48.70 ± 4.64 | 49.64 ± 3.33 | 48.11 ± 3.97 | 48.51 ± 5.44 | 0.649 | |
| DP group | 50.31 ± 3.43 | 48.16 ± 4.61 | 48.64 ± 4.83 | 48.68 ± 5.81 | 0.191 | 0.183 |
| P value (between groups)^ | 0.314 | 0.958 | 0.711 | 0.958 | ||
| Inferior | ||||||
| DC group | 50.38 ± 4.40 | 51.32 ± 5.20 | 50.94 ± 6.14 | 49.90 ± 5.71 | 0.620 | |
| DP group | 49.59 ± 4.92 | 47.82 ± 5.61 | 49.06 ± 5.55 | 47.92 ± 6.83 | 0.395 | 0.516 |
| P value (between groups)^ | 0.958 | 0.223 | 0.491 | 0.791 | ||
| FAZ | ||||||
| DC group | 0.226 ± 0.064 | 0.246 ± 0.061 | 0.244 ± 0.068 | 0.249 ± 0.069 | 0.642 | |
| DP group | 0.262 ± 0.105 | 0.270 ± 0.107 | 0.273 ± 0.113 | 0.288 ± 0.114 | 0.724 | 0.183 |
| P value (between groups)^ | 0.491 | 0.791 | 0.711 | 0.368 | ||
| DCP-VD | ||||||
| Whole | ||||||
| DC group | 55.13 ± 3.21 | 56.85 ± 1.95 | 55.02 ± 3.51 | 55.56 ± 3.67 | 0.413 | |
| DP group | 54.76 ± 4.81 | 54.28 ± 3.72 | 54.56 ± 3.63 | 53.28 ± 3.64 | 0.658 | 0.750 |
| P value (between groups)^ | 0.958 | 0.080 | 0.958 | 0.560 | ||
| Foveal | ||||||
| DC group | 29.85 ± 3.46 | 29.48 ± 5.87 | 28.08 ± 4.18 | 27.10 ± 3.81 | 0.337 | |
| DP group | 32.72 ± 6.82 | 28.33 ± 8.91 | 28.36 ± 6.62 | 25.77 ± 6.44 | 0.044 | 0.020 |
| P value (between groups)^ | 0.560 | 0.368 | 0.596 | 0.458 | ||
| Parafoveal | ||||||
| DC group | 57.29 ± 3.65 | 58.62 ± 2.72 | 57.38 ± 4.15 | 58.00 ± 4.49 | 0.617 | |
| DP group | 55.25 ± 5.24 | 55.14 ± 3.54 | 55.53 ± 3.52 | 54.47 ± 4.31 | 0.772 | 0.765 |
| P value (between groups)^ | 0.491 | 0.101 | 0.368 | 0.153 | ||
| Temporal | ||||||
| DC group | 57.86 ± 1.88 | 56.93 ± 2.66 | 56.14 ± 4.32 | 57.25 ± 5.13 | 0.511 | |
| DP group | 55.20 ± 6.04 | 54.55 ± 4.01 | 55.31 ± 4.17 | 53.81 ± 5.42 | 0.784 | 0.826 |
| P value (between groups)^ | 0.314 | 0.315 | 0.491 | 0.340 | ||
| Superior | ||||||
| DC group | 57.40 ± 6.14 | 59.75 ± 2.77 | 59.25 ± 3.88 | 60.14 ± 3.72 | 0.338 | |
| DP group | 56.21 ± 5.50 | 57.12 ± 3.96 | 56.98 ± 3.57 | 56.02 ± 5.13 | 0.645 | 0.799 |
| P value (between groups)^ | 0.874 | 0.315 | 0.223 | 0.153 | ||
| Nasal | ||||||
| DC group | 55.74 ± 3.85 | 58.17 ± 2.67 | 55.77 ± 4.39 | 56.61 ± 5.39 | 0.517 | |
| DP group | 54.38 ± 5.31 | 54.61 ± 3.30 | 54.86 ± 3.50 | 54.22 ± 4.83 | 0.483 | 0.441 |
| P value (between groups)^ | 0.958 | 0.064 | 0.634 | 0.315 | ||
| Inferior | ||||||
| DC group | 58.14 ± 4.20 | 59.63 ± 3.64 | 58.35 ± 4.77 | 58.01 ± 5.18 | 0.599 | |
| DP group | 55.20 ± 4.85 | 54.28 ± 4.63 | 54.97 ± 4.73 | 53.85 ± 5.27 | 0.722 | 0.843 |
| P value (between groups)^ | 0.634 | 0.017* | 0.315 | 0.186 | ||
Vessel density of the superficial capillary plexus (SCP-VD) and foveal avascular zone (FAZ), and the deep capillary plexus (DCP-VD) (mean value ± standard deviation in micron) in patients with diabetes mellitus type 1 treated with citicoline and vitamin B12 eye drops (DC group) or with placebo (DP Groups) at baseline and 12, 24, and 36 months of follow-up. The values of SCP-VA, DCP-VA, and FAZ were automatically generated by the device. Significant values are presented in bold font
*P value of the general linear model (GLM) of Friedman/MAnova repeated measures
^Mann–Whitney U
At baseline, no significant differences were observed for each SD-OCT, OCTA, and AO parameter between the DC and DP groups.
During follow-up, no significant differences were observed for the SD-OCT findings in terms of the CRT, RNFL and GCL thickness (subfoveal, parafoveal, and total values) between the DC and DP groups. In contrast, a significant difference in the parafoveal INL thickness was observed between the groups; in particular, no significant change of INL thickness in the DC group, while a significant increase of that in the DP group was observed over time. Moreover, a significant difference in the OPL thickness was observed between the two groups over time; the OPL thickness values were significantly reduced at 12, 24, and 36 months in the DP group, whereas the OPL values were not changed over time in the DC group.
With regard to the microvascular changes through OCTA, the foveal VD at SCP and that at DCP were different between the DC and DP groups; in particular, no significant changes were observed in the DC group, whereas a significant reduction in the foveal VD at both SCP and DCP was observed in the DP group over time. In addition, no significant changes of FAZ were observed in both groups.
With regard to the AO findings, no significant differences in the cone density, linear dispersion index, and heterogeneity packing index were observed between the DC and DP groups over time during the evaluation period.
At each follow-up timepoint, no change of HbA1c relative to baseline was observed in both DC and DP groups, indicating good overall metabolic control.
Discussion
The present pilot study highlights potential functional and morphological retinal changes in patients with DM1 with mild signs of NPDR who underwent treatment with citicoline and vitamin B12 eye drops (DC group) during 36 months follow-up period, as compared with those in a DM1 control group who underwent similar duration of treatment with placebo, which fulfills the study goals (DP group).
Our results indicate a significant difference between the DC and DP groups during 36 months follow-up: the DP group showed worsening of functional and morphological parameters, while the DC group showed stabilization of those over time.
Functional (FDT) Data
The results mainly revealed significant differences in MS of 10-2 FDT between the DC and DP groups at the end of follow-up, with worsening of its level in the DP group and no changes of that in the DC group.
The reduction of MS may be due to higher susceptibility of the magnocellular RGCs to hyperglycemia that can lead to early and selective impairment of those cells; alternatively, in the case of similar susceptibility to hyperglycemia between the magnocellular RGCs and other RGCs, lower redundancy of those cells in the entire RGCs population with no overlap of the receptive fields would allow earlier detection of losses [7]. In particular, Parravano et al. reported that the eyes of patients with DM1 showed early functional impairment of RGCs through FDT [7]. Moreover, in our study, the decrease of threshold of retinal sensitivity correlated to the reduction of RNFL in the eyes with DM1 without DR, which suggests that patients with DM1 achieve early functional and morphological retinal impairment even in the absence of retinal vasculopathy under ophthalmoscopy.
The results in the DP group reflect the natural history of disease, whereas the stability of MS after treatment with citicoline and vitamin B12 may contribute to slowing of progressive functional damage of the magnocellular RGCs.
These pieces of evidence raise a question regarding the possibility that citicoline administration through eye drops may target RGCs, and further studies are needed to clarify this point. Nevertheless, a recent study [24] on human eyes that underwent treatment with citicoline eye drops before vitreoretinal surgery for the epiretinal membranes reported a high concentration of citicoline in the vitreous body, which suggests that citicoline reached the region closer to RGCs. Collectively, our results and those in glaucomatous patients [25, 26] suggest that when citicoline is administered by eye drops, it can cause direct functional changes of the RGCs. Such functional changes may be due to the different properties of citicoline that affect neuroenhancement, neuroprotection, and neuroregeneration, and a recent review by Faiq et al. described these phenomena in detail [21].
The psychophysical FDT responses in patients with DM1 of our study who underwent treatment with citicoline were consistent with reduction in progressive visual field damage in patients with progressive glaucoma who received citicoline eye drops in another study [27].
Morphological (SD-OCT, OCTA, and AO) Data
The DC and DP groups achieved similar values through SD-OCT and OCTA at the end of follow-up, and those parameters showed worsening in the DP group but remained unchanged in the DC group.
With regard to SD-OCT findings, the DC group maintained the same levels of INL thickness and OPL thickness over time, while the DP group showed significant increase of the former and decrease of the latter. In addition, the two groups attained comparable values of the others parameters (CRT, RNFL, and GCL thickness), with no statistical differences, which may reflect an absence of worsening in the DP group and a stable condition in the DC group.
Progressive increase in INL and decrease in OPL thickness are considered as indicators of progressive changes of the retinal morphology in DR; nevertheless, we observed no changes in the HbA1c values, which indicates that these morphological changes may also occur in the case of good overall metabolic control.
Previous studies reported an increase in the INL thickness at the early stage of DR [8, 9], which may be due activation of the glia of the Müller cells [28, 29] associated with induction of diabetic macular edema. In contrast, the OPL thinning corresponds to concomitant thickening of INL, which may contribute to the pattern of fluid accumulation at OPL in diabetic patients, and as the leading factor of macular edema. Studies with a quantitative approach are required to assess the relationship between the rate of increasing INL and differences in rate of the outer retinal changes evidenced by OPL thinning.
After administration of citicoline plus vitamin B12, we observed no significant changes in both the INL and OPL thicknesses, which suggests that treatment with citicoline eye drops may prevent the retinal morphological changes related to DR. Neuroprotective effects from the structural activity of citicoline may contribute to the differences between the DC and DP groups, which is supported by another study on non-arteritic ischemic optic neuropathy in humans [30] and the review by Faiq et al. [21] on experimental models.
With regard to OCTA, the DC group showed no significant changes of VD at SCP and DCP, while the DP group showed significant reduction of that over time.
Our findings of 3-year follow-up in the DP group indicate that treatment with citicoline plus vitamin B12 inhibited progressive microvascular impairment in DR.
Several studies suggest that a reduction of VD at SCP and DCP may precede the retinal alterations through fundus examination; in particular, impairment of DCP represents a biomarker for both early diagnosis of DR and risk of progression [31]. We observed significant microvascular abnormalities in patients without other comorbidities such as hypertension and dyslipidemia, and therefore we considered that the microvascular abnormalities may be associated only with metabolic dysregulation in diabetes [32].
The finding of no significant changes of VD at SCP and DCP after treatment with citicoline and vitamin B12 can be explained by the fact that citicoline targets vascular function.
Nevertheless, studies using in vivo and in vitro models support that citicoline has a potential vascular protective effect in the brain microvascular endothelium [33], and the mechanism of its action involves protection against cell damage/apoptosis induced by calcium ionophore or hypoxia. Citicoline was an effective treatment in other neurodegenerative conditions such as Alzheimer disease, amyotrophic lateral sclerosis, stroke, and Parkinson disease [34–36], while in the case of DR, it showed potential effects in reduction of impairment of the entire neurovascular unit [22].
With regard to AO, the values of the parameters such as cone density, linear dispersion index, and heterogeneity packing index were similar between groups over time, which may be because there were no changes of morphology of the photoreceptor elements in our cohort of DC and DP patients.
Our study has some limitations. The patients with DM1 were treated with a combination of citicoline and vitamin B12, and the findings cannot be attributed to the properties of citicoline alone, since vitamin B12 [6] may have affected the results.
Conclusions
The present pilot study suggests that in patients with DM1 with mild signs of NPDR, treatment with eye drops containing citicoline and vitamin B12 for 3 years could induce stabilization or inhibit the rate of functional impairment of the neuroretinal neurodegenerative process and retinal microvascular damage; whereas, treatment with placebo achieved progressive worsening of those morphological and functional parameters.
Despite the fact that this was a pilot study including a small cohort of patients with DM1, to the best of our knowledge, it is the first clinical study on the use of available eye drops for the treatment of early stages of DR. Our preliminary results need to be confirmed by randomized clinical trials on a larger cohort of patients with DM1 with different stages of severity of DR.
Acknowledgements
We thank the participants of the study. The study was supported by the Italian Ministry of Health and Fondazione Roma.
Funding
No funding or sponsorship was received for this study or its publication. The Rapid Service Fee was funded by the authors.
Medical Writing, Editorial, and Other Assistance
The authors acknowledge Dr. Simone Armento and Ilaria Casale for their technical assistance.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Disclosures
Dr. Mariacristina Parravano received personal fees from Allergan, Novartis, and Bayer that were unrelated to the present work. Dr. Monica Varano received personal fees from Allergan, Bayer, Novartis, and SIFI that were unrelated to the present work. Drs. Fabio Scarinci, Vincenzo Parisi, Paola Giorno, Daniela Giannini, and Francesco Oddone declare no conflict of interest.
Compliance with Ethics Guidelines
The study was conducted according to the tenets of the Declaration of Helsinki and approved by the local ethics committee (Comitato Etico Centrale IRCCS Lazio, Sezione IFO/Fondazione Bietti, Rome, Italy). The study was registered at ClinicalTrials.gov (NCT04009980). In all patients, signed informed consent forms were obtained at the time of recruitment.
Data Availability
All authors had full access to all data in this study and take full responsibility for integrity of the data and accuracy of the data analysis. The datasets generated and/or analyzed are available from the corresponding author on reasonable request.
Footnotes
Enhanced Digital Features
To view enhanced digital features for this article go to 10.6084/m9.figshare.11902215.
References
- 1.Yau JW, Rogers SL, Kawasaki R, et al. Global prevalence and major risk factors of diabetic retinopathy. Diabetes Care. 2012;35:556–564. doi: 10.2337/dc11-1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Leasher JL, Bourne RR, Flaxman SR, et al. Erratum. Global estimates on the number of people blind or visually impaired by diabetic retinopathy: a meta-analysis from 1990-2010. Diabetes Care. 2016;39:1643–1649. doi: 10.2337/dc15-2171. [DOI] [PubMed] [Google Scholar]
- 3.Antonetti DA, Lieth E, Barber AJ, Gardner TW. Molecular mechanisms of vascular permeability in diabetic retinopathy. Semin Ophthalmol. 1999;14:240–248. doi: 10.3109/08820539909069543. [DOI] [PubMed] [Google Scholar]
- 4.Antonetti DA, Barber AJ, Bronson SK, et al. Diabetic retinopathy: seeing beyond glucose-induced microvascular disease. Diabetes. 2006;55:2401–2411. doi: 10.2337/db05-1635. [DOI] [PubMed] [Google Scholar]
- 5.Fletcher EL, Phipps JA, Ward MM, Puthussery T, Wilkinson-Berka JL. Neuronal and glial cell abnormality as predictors of progression of diabetic retinopathy. Curr Pharm Des. 2007;13:2699–2712. doi: 10.2174/138161207781662920. [DOI] [PubMed] [Google Scholar]
- 6.van de Lagemaat EE, de Groot L, van den Heuvel E. Vitamin B12 in relation to oxidative stress: a systematic review. Nutrients. 2019;25;11(2):482. [DOI] [PMC free article] [PubMed]
- 7.Parravano M, Oddone F, Mineo D, et al. The role of Humphrey Matrix testing in the early diagnosis of retinopathy in type 1 diabetes. Br J Ophthalmol. 2008;92:1656–1660. doi: 10.1136/bjo.2008.143057. [DOI] [PubMed] [Google Scholar]
- 8.Vujosevic S, Midena E. Retinal layers changes in human preclinical and early clinical diabetic retinopathy support early retinal neuronal and Muller cells alterations. J Diabetes Res. 2013;2013:905058. doi: 10.1155/2013/905058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Scarinci F, Picconi F, Virgili G, et al. Single retinal layer evaluation in patients with type 1 diabetes with no or early signs of diabetic retinopathy: the first hint of neurovascular crosstalk damage between neurons and capillaries? Ophthalmologica. 2017;237:223–231. doi: 10.1159/000453551. [DOI] [PubMed] [Google Scholar]
- 10.Parisi V, Uccioli L. Visual electrophysiological responses in persons with type 1 diabetes. Diabetes Metab Res Rev. 2001;17:12–18. doi: 10.1002/dmrr.177. [DOI] [PubMed] [Google Scholar]
- 11.Parisi V, Manni G, Colacino G, Bucci MG. Cytidine-5′-diphosphocholine (citicoline) improves retinal and cortical responses in patients with glaucoma. Ophthalmology. 1999;106:1126–1134. doi: 10.1016/S0161-6420(99)90269-5. [DOI] [PubMed] [Google Scholar]
- 12.Carnevali A, Sacconi R, Corbelli E, et al. Optical coherence tomography angiography analysis of retinal vascular plexuses and choriocapillaris in patients with type 1 diabetes without diabetic retinopathy. Acta Diabetol. 2017;54:695–702. doi: 10.1007/s00592-017-0996-8. [DOI] [PubMed] [Google Scholar]
- 13.Simonett JM, Scarinci F, Picconi F, et al. Early microvascular retinal changes in optical coherence tomography angiography in patients with type 1 diabetes mellitus. Acta Ophthalmol. 2017;95:e751–e755. doi: 10.1111/aos.13404. [DOI] [PubMed] [Google Scholar]
- 14.Scarinci F, Picconi F, Giorno P, et al. Deep capillary plexus impairment in patients with type 1 diabetes mellitus with no signs of diabetic retinopathy revealed using optical coherence tomography angiography. Acta Ophthalmol. 2018;96:e264–e265. doi: 10.1111/aos.13510. [DOI] [PubMed] [Google Scholar]
- 15.Vujosevic S, Muraca A, Alkabes M, et al. Early microvascular and neural changes in patients with type 1 and type 2 diabetes mellitus without clinical signs of diabetic retinopathy. Retina. 2019;39:435–445. doi: 10.1097/IAE.0000000000001990. [DOI] [PubMed] [Google Scholar]
- 16.Vujosevic S, Toma C, Villani E, et al. Early detection of microvascular changes in patients with diabetes mellitus without and with diabetic retinopathy: comparison between different swept-source OCT-A instruments. J Diabetes Res. 2019;2019:2547216. doi: 10.1155/2019/2547216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tam J, Dhamdhere KP, Tiruveedhula P, et al. Subclinical capillary changes in non-proliferative diabetic retinopathy. Optom Vis Sci. 2012;89:E692–E703. doi: 10.1097/OPX.0b013e3182548b07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lombardo M, Parravano M, Lombardo G, et al. Adaptive optics imaging of parafoveal cones in type 1 diabetes. Retina. 2014;34:546–557. doi: 10.1097/IAE.0b013e3182a10850. [DOI] [PubMed] [Google Scholar]
- 19.Lombardo M, Parravano M, Serrao S, Ziccardi L, Giannini D, Lombardo G. Investigation of adaptive optics imaging biomarkers for detecting pathological changes of the cone mosaic in patients with type 1 diabetes mellitus. PLoS One. 2016;11:e0151380. doi: 10.1371/journal.pone.0151380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Parisi V, Oddone F, Ziccardi L, Roberti G, Coppola G, Manni G. Citicoline and retinal ganglion cells: effects on morphology and function. Curr Neuropharmacol. 2018;16:919–932. doi: 10.2174/1570159X15666170703111729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Faiq MA, Wollstein G, Schuman JS, Chan KC. Cholinergic nervous system and glaucoma: from basic science to clinical applications. Prog Retin Eye Res. 2019;72:100767. doi: 10.1016/j.preteyeres.2019.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Das A, McGuire PG, Rangasamy S. Diabetic macular edema: pathophysiology and novel therapeutic targets. Ophthalmology. 2015;122:1375–1394. doi: 10.1016/j.ophtha.2015.03.024. [DOI] [PubMed] [Google Scholar]
- 23.Wilkinson CP, Ferris FL, 3rd, Klein RE, et al. Proposed international clinical diabetic retinopathy and diabetic macular edema disease severity scales. Ophthalmology. 2003;110:1677–1682. doi: 10.1016/S0161-6420(03)00475-5. [DOI] [PubMed] [Google Scholar]
- 24.Carnevale C, Manni G, Roberti G, et al. Human vitreous concentrations of citicoline following topical application of citicoline 2% ophthalmic solution. PLoS One. 2019;14:e0224982. doi: 10.1371/journal.pone.0224982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Parisi V, Centofanti M, Ziccardi L, et al. Treatment with citicoline eye drops enhances retinal function and neural conduction along the visual pathways in open angle glaucoma. Graefes Arch Clin Exp Ophthalmol. 2015;253:1327–1340. doi: 10.1007/s00417-015-3044-9. [DOI] [PubMed] [Google Scholar]
- 26.Parisi V, Oddone F, Roberti G, et al. Enhancement of retinal function and of neural conduction along the visual pathway induced by treatment with citicoline eye drops in liposomal formulation in open angle glaucoma: a pilot electrofunctional study. Adv Ther. 2019;36:987–996. doi: 10.1007/s12325-019-0897-z. [DOI] [PubMed] [Google Scholar]
- 27.Ottobelli L, Manni GL, Centofanti M, Iester M, Allevena F, Rossetti L. Citicoline oral solution in glaucoma: is there a role in slowing disease progression? Ophthalmologica. 2013;229:219–226. doi: 10.1159/000350496. [DOI] [PubMed] [Google Scholar]
- 28.Yong PH, Zong H, Medina RJ, et al. Evidence supporting a role for N-(3-formyl-3,4-dehydropiperidino)lysine accumulation in Muller glia dysfunction and death in diabetic retinopathy. Mol Vis. 2010;16:2524–2538. [PMC free article] [PubMed] [Google Scholar]
- 29.Curtis TM, Hamilton R, Yong PH, et al. Muller glial dysfunction during diabetic retinopathy in rats is linked to accumulation of advanced glycation end-products and advanced lipoxidation end-products. Diabetologia. 2011;54:690–698. doi: 10.1007/s00125-010-1971-x. [DOI] [PubMed] [Google Scholar]
- 30.Parisi V, Barbano L, Di Renzo A, Coppola G, Ziccardi L. Neuroenhancement and neuroprotection by oral solution citicoline in non-arteritic ischemic optic neuropathy as a model of neurodegeneration: a randomized pilot study. PLoS One. 2019;14:e0220435. doi: 10.1371/journal.pone.0220435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sun Z, Tang F, Wong R, et al. OCT angiography metrics predict progression of diabetic retinopathy and development of diabetic macular edema: a prospective study. Ophthalmology. 2019;126:1675–1684. doi: 10.1016/j.ophtha.2019.06.016. [DOI] [PubMed] [Google Scholar]
- 32.Song SH. Complication characteristics between young-onset type 2 versus type 1 diabetes in a UK population. BMJ Open Diabetes Res Care. 2015;3:e000044. doi: 10.1136/bmjdrc-2014-000044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Krupinski J, Abudawood M, Matou-Nasri S, et al. Citicoline induces angiogenesis improving survival of vascular/human brain microvessel endothelial cells through pathways involving ERK1/2 and insulin receptor substrate-1. Vasc Cell. 2012;4:20. doi: 10.1186/2045-824X-4-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zlokovic BV. The blood-brain barrier in health and chronic neurodegenerative disorders. Neuron. 2008;57:178–201. doi: 10.1016/j.neuron.2008.01.003. [DOI] [PubMed] [Google Scholar]
- 35.del Zoppo GJ. The neurovascular unit in the setting of stroke. J Intern Med. 2010;267:156–171. doi: 10.1111/j.1365-2796.2009.02199.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nag S, Kapadia A, Stewart DJ. Review: molecular pathogenesis of blood-brain barrier breakdown in acute brain injury. Neuropathol Appl Neurobiol. 2011;37:3–23. doi: 10.1111/j.1365-2990.2010.01138.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All authors had full access to all data in this study and take full responsibility for integrity of the data and accuracy of the data analysis. The datasets generated and/or analyzed are available from the corresponding author on reasonable request.