Abstract
Despite advances in high-throughput sequencing that have revolutionized the discovery of gene defects in rare Mendelian diseases, there are still gaps in translating individual genome variation to observed phenotypic outcomes. While we continue to improve genomics approaches to identify primary disease-causing variants, it is evident that no genetic variant acts alone. In other words, some other variants in the genome (genetic modifiers) may alleviate (suppress) or exacerbate (enhance) the severity of the disease, resulting in the variability of phenotypic outcomes. Thus, to truly understand the disease, we need to consider how the disease-causing variants interact with the rest of the genome in an individual. Here, we review the current state-of-the-field in the identification of genetic modifiers in rare Mendelian diseases and discuss the potential for future approaches that could bridge the existing gap.
Keywords: genetic modifier, mendelian disease, rare disease, GWAS, genome sequencing, genetic interaction, penetrance, expressivity, phenotypic variability, bioinformatics
1. Introduction
Diseases that follow Mendelian patterns of inheritance are known as Mendelian disorders. Approximately 80% of all rare diseases are genetic in origin, and most of these diseases are monogenic/Mendelian [1,2]. Rare diseases are individually rare, but according to the estimate, there are 400 million people all over the world suffering from around 7000 different rare diseases [1,3,4,5]. Despite being monogenic, the genetic basis of more than half of all identified Mendelian diseases remains elusive [2,4,6]. Even in cases where the causal disease gene is known, these diseases can display variable phenotypes, even in patients with the same causal mutation (e.g., siblings [7,8,9,10]), which poses diagnostic and patient management challenges [11]. Conversely, researchers have analyzed multiple human genome/exome data and found evidence of disease-causing genotypes in individuals with no reported disease presentations [12,13]. These large-scale genome-wide sequencing studies provide further information on rare disease penetrance and expressivity, as well as a potential effect of genetic modifiers.
Identifying the genetic origin of a specific phenotype is not so straightforward, as the genotypes and phenotypes may not have a linear relationship. This is partially due to genetic interactions that play a crucial role in controlling the functional dependency between genes [14] and can result in phenotypic variability. Numerous things can affect the genetic pathway to produce unexpected phenotypes. Genetic modifiers, defined as genetic variants that can modify the phenotypic outcome of the primary disease-causing variant, are one such example. They can increase (known as an enhancer) or decrease (known as a suppressor) the severity of the disease condition but may not be disease-causing themselves. Modifier variants can change a target gene’s phenotype by having a genetic, biochemical, or functional interaction with one or more target gene(s), or gene product(s) [15]. The degree of the effect of the modifiers can vary, which may result in large phenotypic variability and changes in penetrance.
Although we know very little about Mendelian rare disease modifiers, it is evident that phenotypic variability resulting from modifiers is an important aspect to be considered and researched [16,17,18,19]. For example, as discovered almost one and a half centuries ago (1882), Gaucher disease (GD), one of the rare Mendelian disorders resulting from mutations in the GBA1 gene, is known to have a wide spectrum of phenotypes even among siblings. However, despite decades of research efforts, there are major gaps in identification and understanding of genetic modifiers in Gaucher disease. Addressing these gaps for GD [8], as well as other rare diseases, is important for diagnostic, prognostic, therapeutic, and overall patient management procedures.
To completely understand this variability in the phenotypic expression of rare disorders, it is essential to look at the individual genome. The cost of Whole-Genome Sequencing (WGS) has become more economical in recent years, making it feasible to get an almost complete view (~98–99%) of an individual’s genome. WGS data allows the exploration and analysis of all the genetic variants that are present in an individual. Different bioinformatics methods have been developed to identify candidate causal variants for the disease. Because of the advances in bioinformatics methods, it is now feasible to identify causal variants for a phenotype more precisely and accurately than ever before [20,21,22], which opens up the possibility to study modifier variants at the individual level.
In this article, we review the existing challenges of genetic modifier identification with respect to rare Mendelian diseases. We summarize some experimental approaches, as well as computational methods which have been used in genetic interaction studies and discuss how this research area can benefit from further improvements.
2. Current State of Rare Disease Genetic Modifier Research
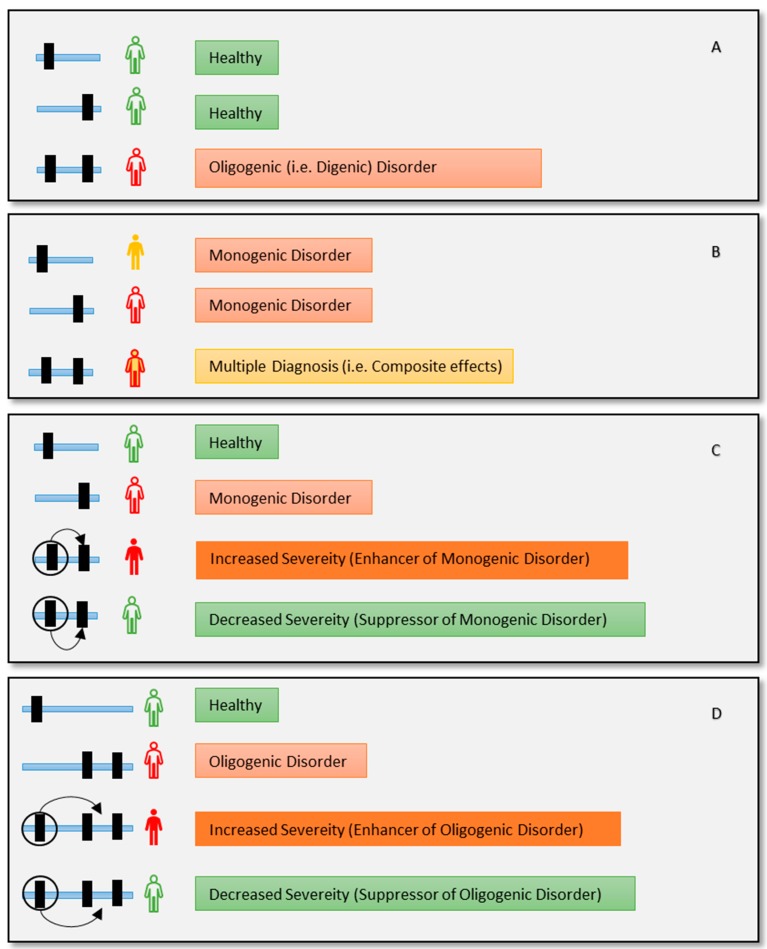
The definition of monogenic and oligogenic disorder is based on the number of the primary disease-causing genes. This does not necessarily mean that the relation between genotype and phenotype is straightforward, especially due to different phenomenon such as epistasis, genetic interactions, or modifications [23,24]. All these phenomena can be referred to as the change of phenotypic outcome of one variant by another variant [25]. However, each of these terms can mean a specific phenomenon based on its context. For example, a mono/oligogenic disease can also be modified by other mutations causing incomplete penetrance and variable expressivity [26,27,28] (Figure 1). Genetic modifier can be involved in modifying pleotropic phenotype [29,30,31]. These modifiers may result in a different/novel phenotype. Disease characterization becomes even more challenging for individuals carrying two or more monogenic disorders that may have overlapping (resulting in blended) or different (resulting in composite) phenotypes each of which may be modifiable [7,19] (Figure 1). As we are discussing the effect of genetic modifiers on monogenic disorders, it is essential to clarify why the disease is still referred to as monogenic while multiple variants are involved.
Figure 1.
Different genetic phenomena in combination with Mendelian disorders, can make disease characterization challenging. (A) This is a case of a digenic disorder. Two individually healthy variants combinedly produce the disease (e.g., Bardet-Biedl syndrome caused by BBS1 and BBS10 [35]). (B) Two different monogenic disorders may produce a blended or composite representation of both diseases (e.g., Mutation in NPL (causing sialic acid disorder) and GJB2 (causing deafness) creating composite disease phenotype for a patient [36]). (C) A case for a genetic modifier. Disease variant is modified by the modifier variant (circled) that enhances or suppresses the severity of the disease (i.e., Spinal Muscular Atrophy modified by SMN2 variants [37]). (D) An oligogenic disease can be modified by a modifier variant as well (i.e., Digenic Usher syndrome modified by PDZD7 [38]). More complex scenarios are also possible, such as multiple modifier alleles that can act independently or together (joint effect) [31].
2.1. Oligogenicity and Genetic Modifiers
Oligogenicity refers to the multigenic inheritance (involving two or more genes), regardless of modifiers [27,32,33,34]. More specifically, oligogenic diseases are caused by the co-occurrence of mutations in two or more genes. All causal variants must be present in a patient’s genome for oligogenic disease presentation; having a variant in just one gene is not sufficient for the disease manifestation [25] (Figure 1A). In contrast, it may be also possible that one individual has more than one genetic diagnosis resulting in composite or blended phenotype (Figure 1B). On the other hand, in the case of modifiers, pathogenic variant/s are enough to cause the disease (i.e., disease causing) while the other variant/s in modifier genes make the disease outcome more (enhancer) or less (suppressor) severe (Figure 1C,D). Genetic modifiers by themselves may not result in any phenotype and may be rare or common in an untargeted population.
Although some tools and public databases of oligogenic diseases also include genetic modifiers [39,40], a clear distinction between them is necessary for accurate clinical decision making. Recently, researchers have found a genetic modifier in a two-month-old girl who was diagnosed with left ventricular noncompaction (LVNC), a rare heart condition that is caused by heterozygous pathogenic variants in MYH7 and MKL2 genes [41]. Both variants were previously unknown. To find out the genetic cause, the authors performed Whole-Exome Sequencing (WES) of the proband and her family and found that the girl is carrying the same disease-causing variant combination as her father (i.e., oligogenic disease). While her father showed no significant sign of the disease at the age of 37 years, his daughter had severe symptoms at just three months of age. Their first child died as a fetus with failure in biventricular noncompaction and was carrying these same three variants. They also found that her 4-year-old sister was also carrying the causal variant combination and is an LVNC patient. To find out the source of this increased severity, the authors performed hierarchical filtering for candidate modifiers and found the existence of a modifier variant (enhancer, Figure 1D) which came from the unaffected mother. The variant was found in gene NKX2-5 with the allele frequency of 0.0009792 on gnomAD [42]. They confirmed their findings with the aid of a mouse model and induced pluripotent stem (iPS) cells.
This study shows how genetic modifiers can modulate the effect of oligogenic diseases as well (Figure 1D). It is evident that with the integration of appropriate genomic assessment, clinicians may provide more accurate patient management [36]. Furthermore, integrating genetic modifiers in genome assessments holds great promise to further help with patient management, but also to facilitate better diagnosis and prognosis of disease progression [43].
Studies have been done to develop tools to prioritize the oligogenic variants that are responsible for rare diseases. Recently, OligoPVP [39] and VarCoPP [44] were developed in an attempt to prioritize two or more variants that together cause the disease.
OligoPVP was developed based on the improved version of PhenomeNET Variant Predictor (PVP) [45]. PVP scores disease-causing variants from WGS or WES data based on its pathogenicity. The authors used six numeric and categorical features like CADD [46], DANN [47], GWAVA [48], PhenomeNET scores [49], disease inheritance mode, and genotype, along with 54 binary attributes. Together these attributes are used to train a Random Forest (RF) classifier to classify variants as causative or noncausative. Recently, the authors developed an improved version of PVP—DeepPVP [50]. It uses seven additional features. These 76 features are processed through a Deep Artificial Neural Network model to produce a score for each variant which denotes the variant’s predicted pathogenicity. OligoPVP is built on DeepPVP. OligoPVP combines and compares DeepPVP scores of two or more variants to prioritize disease-causing pairs/triplets.
VarCoPP is a more recent tool that also uses an RF classifier to classify variants. Their classifier uses 11 features and is strictly designed to process alleles in pairs. For example, there are four CADD scores among these 11 attributes. Each of these four attributes are strictly designed to process the CADD score of four alleles from a pair of genes. While VarCoPP was able to identify 20 out of 23 di-genic combinations from DIgenic diseases DAtabase (DIDA) [40] fused with 1000 Genomes Project [51] data (a >80% support score), a limitation of the tool is that it works best when the number of candidate variants is less than 150.
Of note, these two tools were trained with a small number of samples size. Both VarCopp and OligoPVP use DIDA as a source of their training variant set. DIDA is primarily focused on di-genic diseases, where two independent mutations co-occur to produce a disease phenotype (Figure 1A). The DIDA database does contain some modifier entries which might be useful for training a tool to detect genetic modifiers and the authors of OligoPVP proposed that OligoPVP may be able to identify modifier interactions. However, both OligoPVP and VarCoPP techniques and training features are mostly focused on pathogenic effects caused by a combination of multiple variants. Since modifiers do not necessarily have disease-causing properties, they may be left unrecognized by these tools. Therefore, the effectiveness of these tools in modifier discovery, especially for genetic suppressor identification, needs further assessment.
2.2. Genetic Modifier Studies in Literature
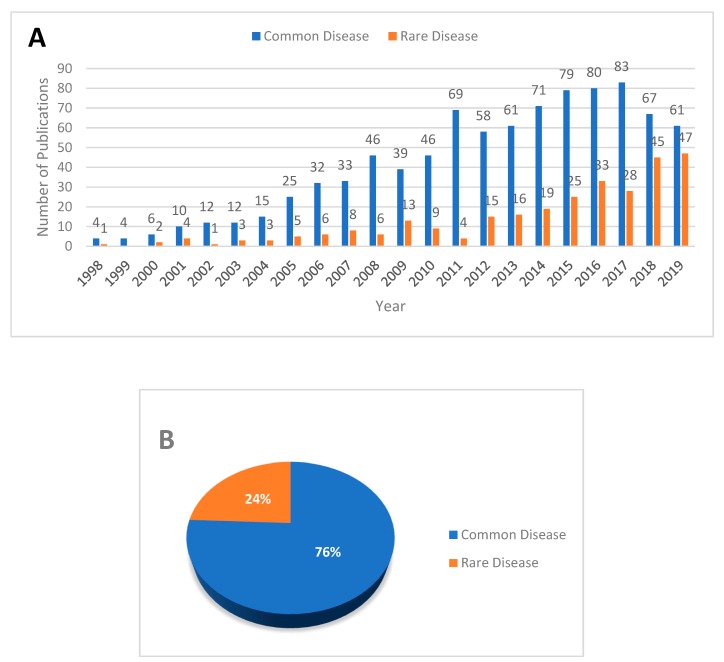
An assessment of the search result of published literature shows that interest in the genetic modifier study has substantially increased in the last decade (2010–2019: 916 records) than the previous (1998–2008: 281 records) (Figure 2). The data was collected by searching PubMed and Web of Science (WoS). The search logic and document filtration process are described in Figure S1. So far, the effect of genetic modifiers has been predominantly assessed for common diseases via genome-wide association studies (GWAS), whereas the identification of genetic modifiers in rare diseases has not been extensively studied. For instance, our assessment of the extracted literature shows that only 24% of the studies are focused on rare diseases (Figure 2B).
Figure 2.
Published literature on modifier studies from 1998 to 2019. Keyword-based literature search result was extracted from PubMed and WoS (See Figure S1). (A) Histogram showing a comparative view of published literature for each year. (B) Pie chart showing a comparison between common and rare disease studies from 846 literature records.
To explore the current knowledge on genetic modifiers of rare diseases, we explored the Online Mendelian Inheritance in Man (OMIM) database [52], which reports the curated information on Mendelian disorders. We extracted only 31 modifier entries for both common and rare disorders from OMIM. To get only rare Mendelian disease entries, we used the rare diseases list from OrphaNet [53]. In the end, we found only 24 rare Mendelian disease modifier entries (Table 1). This finding shows the scarcity of knowledge of Mendelian disease modifiers. While modifier studies are predominantly done on common disorders (Figure 2B), we find that OMIM has more modifier entries for rare diseases (24 entries) compared to common diseases (7 entries). This could be because most common diseases are often non-Mendelian complex disorders, which are not included in OMIM, as well as the nature of GWAS results.
Table 1.
Modifier gene for the rare Mendelian disorder found in the Online Mendelian Inheritance in Man (OMIM) database.
| OMIM | Modifier Gene | Disease | PhenoModifier Gene ID |
|---|---|---|---|
| 107670 | APOA2 | Hypercholesterolemia, familial | - |
| 108733 | ATP2B2 | Deafness, autosomal recessive 12 | 491 |
| 112261 | BMP2 | HFE hemochromatosis | - |
| 120353 | MMP1 | Epidermolysis bullosa dystrophica | 4312 |
| 132811 | EPHX2 | Hypercholesterolemia due to LDLR defect | - |
| 147570 | IFNG | TSC2 angiomyolipomas | 3458 |
| 155555 | MC1R | Albinism, oculocutaneous, type II | 4157 |
| 168461 | CCND1 | von Hippel-Lindau syndrome | - |
| 190180 | TGFB1 | Cystic fibrosis lung disease | 7040 |
| 600451 | AKR1C4 | 46XY sex reversal 8 | - |
| 600837 | GDNF | Pheochromocytoma | 2668 |
| 600946 | GHR | Hypercholesterolemia | - |
| 601627 | SMN2 | Spinal muscular atrophy | 6607 |
| 602421 | CFTR | Bronchiectasis with or without elevated sweat chloride 1 | 1080 |
| 603415 | SCN9A | Dravet syndrome | 6335 |
| 605204 | TOR1A | Dystonia-1, torsion | 1861 |
| 608124 | XYLT1 | Pseudoxanthoma elasticum | 64131 |
| 608125 | XYLT2 | Pseudoxanthoma elasticum | 64132 |
| 608845 | ARL6 | Bardet-Biedl syndrome 1 | - |
| 609884 | TMEM67 | Bardet-Biedl syndrome 14 | - |
| 610162 | CCDC28B | Bardet-Biedl syndrome 1 | 79140 |
| 610230 | TRMU | Deafness, mitochondrial | 55687 |
| 611089 | MTMR14 | Centronuclear myopathy | - |
| 612971 | PDZD7 | Retinal disease in Usher syndrome type | 79955 |
NeuroGeM is one of the earliest databases on genetic modifiers specific to Neurodegenerative disorders [54]. The database was built by combining knowledge gained from Drosophila melanogaster, Caenorhabditis elegans, and Saccharomyces cerevisiae resources. Recently, Sun et al. developed a manually curated database for genetic modifiers named PhenoModifier [55]. The authors sifted through publications to produce the database. They provide a web-based query platform to search modifiers by disease name, gene, or variant.
3. GWAS and Genetic Interactions
Genetic modifiers are often found in a functionally similar pathway of the target gene because of possible genetic interactions [41,56,57,58,59,60,61,62]. Hence, genetic interaction analysis plays a crucial role in identifying genetic modifiers. A range of bioinformatics tools have been developed to predict bi-locus SNP interactions from Single Nucleotide Polymorphisms (SNP) array data of large populations [63,64]. These tools are designed to capture “statistical epistasis” for the traits that are common in the population.
Historically, Bateson was the first person who introduced the term ‘epistasis’ in 1909 [65], referring to it as ‘masking’ the ‘effect’ of another independent locus. The characterization of the ‘effect’ of a locus, ‘independence of effect’ was not well defined at that time, which created confusion and debate about epistasis among researchers, especially between geneticists and biometricians who studied quantitative traits. That puzzle further expanded when R.A. Fisher first analytically showed that the Mendelian law of segregation is compatible with the biostatisticians’ segregation laws, but struggled to explain ‘dual epistacy’, or the interaction between pairs of loci [66,67]. In the early 1950s, the mystery of different types of epistasis was explored by studying different epistatic components like additive, non-additive and additive-by-additive, additive-by-dominance, and dominance-by-dominance which was explained by a quantitative model [68,69]. Later, the term “epistasis” was used by multiple disciplines like biology, biostatistics, molecular biology, evolutionary genetics, and they have used their area-specific perspective to explain epistasis [70]. Most of these efforts tried to develop a unified statistical model for epistasis, but statistical interaction often fails to describe the biological mechanism of epistasis [67,70,71,72,73,74,75]. This may be partly because statsistical methods tend to analyze interactions using additive models [31,76].
For the last 12 years, GWAS has been the primary approach to study the common genetic control of various common/complex traits in populations. To identify possible statistical epistasis (SNP interactions) from GWAS, researchers have taken different approaches such as exhaustive, stochastic, heuristic, machine learning, and neural network approaches [63,64,77].
Amongst the epistasis identification tools, Multifactor Dimensionality Reduction (MDR) is perhaps the most prominent, which uses an exhaustive method [78]. Exhaustive methods compute all n-locus (n is the number of SNPs) interactions in a brute-force fashion and search for significant interaction within the permutation space. MDR uses model-free statistical methods and is reported as capable of identifying k-way interactions (k denotes the level of interactions). GMDR-GPU [79], Model-based MDR (MB-MDR) [80] are a couple of the latest improvements over MDR. BOOST is another popular exhaustive epistatic interaction prediction tool [81], and one of the fastest among the exhaustive methods. Stochastic methods like Bayesian Epistatic Association Mapping (BEAM) determine interactions by randomly sampling the search space [82], and heuristic methods like AntEpiSeeker model the search for epistatic interactions as an ant colony optimization (ACO) procedure where each locus is represented by a certain level of pheromones [83]. All of these tools are designed to take genotype array data as input. Overall, they can handle 100,000 to ~500,000 SNPs from a few hundred to thousands of samples (balanced case-control) within a timeframe of 24 hrs to 60hrs [71,78,80,81,84]. These tools were used in a few independent studies to identify interactions between SNPs that may be associated with a particular phenotypic condition [85,86,87,88,89,90,91,92].
Over the years, researchers have been able to associate thousands of variants with certain diseases/traits through GWAS. However, pinpointing variants using the available GWAS tools is an existing challenge [93]. This shortfall becomes more obvious with challenging attempts of applying GWAS to identify SNP interactions [94,95]. As a result, to date, there is lack of reported experimental validation of the results predicted by these tools in the literature. Additionally, having interaction does not necessarily mean that it would have a modified effect on the phenotype [96]. Functional interaction between two genes also depends on the underlying pathways and their reciprocal dependence [97,98].
Furthermore, these tools are prone to the same technical shortcomings as a typical GWAS when it comes to the discovery of causal rare Mendelian disease variants. Since disease-causing variants are sporadic, and there are not enough data-samples, GWAS fails to capture the true significant alleles for rare Mendelian diseases [99]. Identification of genetic modifiers of a rare Mendelian disease thus has the same consequences because the sample size can be restricted to one or a handful of patients only [100]. Few studies tried to overcome this issue by aggregating patient data to perform GWAS [12,101,102]. Nevertheless, even if we were to collect and combine rare disease patients’ genotypes, we must ensure the privacy of genome information [99,103,104,105,106].
4. WGS Approach for Modifier Studies
WGS analysis offers a more comprehensive view of the genome than genotyping array by giving access to almost all of the genetic variants that are present in an individual (coding and non-coding regions). Thus, the exploration of the individual genetic variation is not restricted to single nucleotide variants (SNVs), as it allows the identification of structural variants (SVs) as well [2,19,107,108]. Moreover, continuous improvements in knowledge of the transcriptome, known interactions within and between different biological entities, related ontologies, and bioinformatics tools are providing a better understanding and more precise annotation of the variant effects.
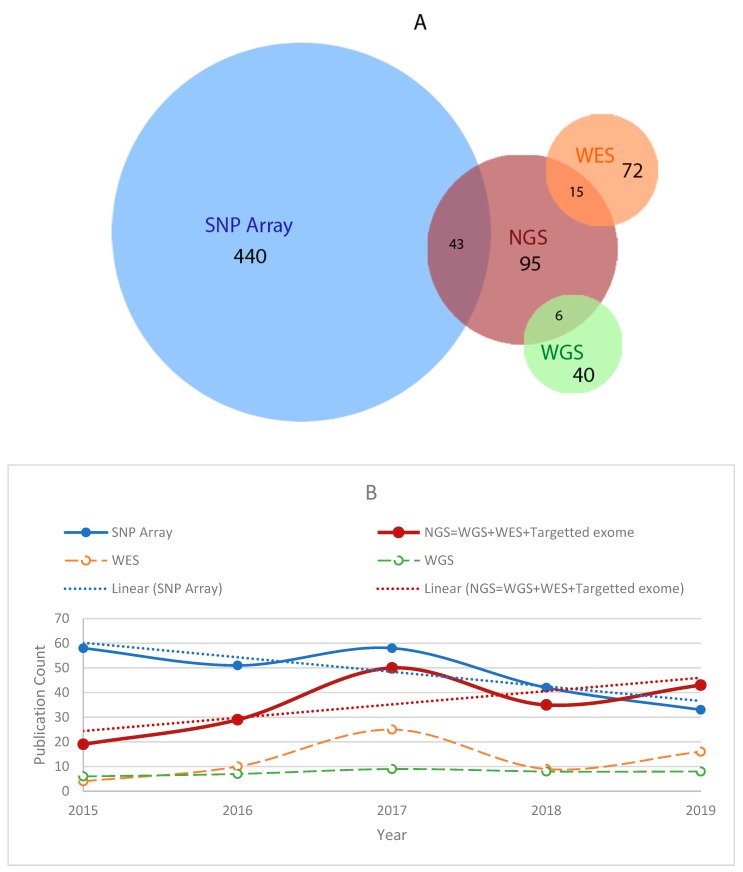
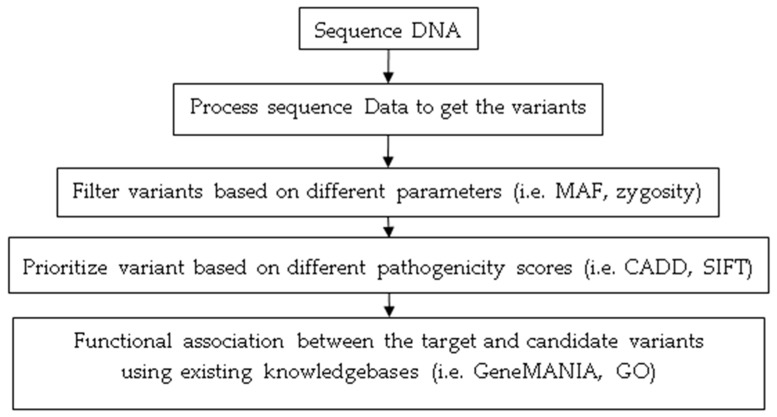
Because of these advances, it is now feasible to identify causal variants for a phenotype more precisely and accurately, opening up the prospect of studying genetic modifier variants at the individual level. As a result, WGS analysis has become a promising approach to study rare diseases genomics and modifiers, where individual genome analysis is essential [2,4,109,110]. Our literature analysis also indicates how rare disease modifier studies have risen significantly in the last five years as WGS becomes increasingly more affordable (Figure 3B). Among the total 418 studies from 2015–2019, 42% of studies used next-generation sequencing (NGS) data analysis, which includes WGS, WES, and targeted exome sequencing. To get an insight into the recent approaches of modifier studies for Mendelian disorders, we took a more in-depth look into the NGS based studies. Most of these NGS based approaches performed parameterized variant filtering, pathogenicity score-based prioritization (based on scores from pathogenicity scoring tools), and lastly, functional association to identify the modifier variant(s) (based on functionally annotated ontologies and knowledgebases) (Figure 4). These approaches often rely on manual variant prioritization from candidate variants by human. In such process, human experts infer functional similarity between the candidate variants and the target causal mutation by reviewing relevant knowledge-bases [41,111,112,113]. Incorporation of a computational method that can comprehensively analyze and prioritize modifiers from candidate modifier variants is still an open area for research.
Figure 3.
A comparative view of the usage of a SNP array and sequencing technology to study genetic modifiers. (A) Publication count between the years 1998–2019. (B) Recent (2015–2019) publications indicate increased interest in high throughput sequencing techniques. Two linear (dotted) lines are used to show the trend shift.
Figure 4.
Typical analysis steps followed to identify modifiers using NGS based approaches in recent years.
Another advantage of using WGS in a modifier study is that it allows exploration of almost all the variants in a genome and also allows comprehensive analysis. This is particularly helpful when the same gene can modulate the phenotype differently depending on the type of variant. For instance, spinal muscular atrophy (SMA) is caused by the SMN1 gene. A similar gene SMN2’s copy number acts as a dosage suppressor of SMN1. However, Prior et al. reports that it is possible to suppress SMA by having homozygous c.859G>C transversion mutation in exon 7 in the SMN2 gene [37]. The mutation replaces glycine by arginine at codon 287. This change produces an exonic splicing enhancer element and increases the amount of full-length SMN2 transcripts which is similar to the SMN2 copy-number that produces a less severe SMA phenotype.
5. Experimental Approaches to Detect Genetic Modifiers
Multiple reviews discuss the role and importance of model organisms in rare disease studies [25,114,115,116]. Most of the experimental procedures for identifying rare disease modifiers use model organisms because of the advantage of experimental reproducibility through inbred strains that have the same genetic background allowing comparative assessment to identify modifiers [117].
Among all model organisms, the single-celled Yeast has one of the most comprehensive interaction networks produced by a high-throughput reverse genetic approach [118]. On the other hand, organisms like C. elegans have the advantage of being a tractable multi-cellular organism with biological simplicity and elegant genomics. C. elegans shares extensive genome conservation with humans, which supports the translatability of the findings [119]. One particular limitation for any model organism is that not all human genes have orthologs in any given organism. For instance, 35% of human genes do not have any specific orthologs in flies [120]; however, this number decreases when moving to vertebrate models such as zebrafish or mice. In fact, zebrafish and mice offer a more robust phenotypic comparison [114,121,122]. However, a simpler organism (i.e., yeast, C. elegans) allows high-throughput screening for interaction and modifier discoveries, as reflected by the number of interactions discovered in these model organisms and compiled in BioGrid [118].
5.1. Reverse Genetic Screens
One of the most comprehensive knowledgebases of genetic interaction is available for yeast (S. cerevisiae). According to BioGrid statistics (accessed on October 7, 2019), there are 441,949 unique genetic interactions for yeast alone, which is 65% of the total known genetic interactions for all organisms combined (684,244). One of the major contributions to this yeast interaction knowledge base comes from a reverse genetic approach using synthetic gene array (SGA) screens [123] that looked for synthetic lethal interaction initially [14,29], but also recently found suppressors [117,124].
There are other experimental methods like Epistatic Mini-Array Profile (eMAP) [125] and RNA interference (RNAi) [126] to test for interactions [77,127]. In eMAP, a subset of genes is chosen (e.g., genes belonging to a given pathway or process), analyzed pairwise in an array, and then clustered to find out synthetic lethal interactions [125,128]. A more widely used approach to analyze interactions is RNAi, where instead of completely knocking out the gene function, RNAi libraries are used to knock-down the expression of a gene of interest. Multiple studies have performed RNAi on C. elegans strains to test for interactions [129,130]. However, their efficiency in terms of scale was lower than the yeast’s SGA experiments [77]. Noise in large-scale RNAi is one of the reasons for this lower efficiency; off-target effects and variable knockdown efficiencies are common RNAi complications [127,131,132].
5.2. Forward Genetic Screens
In reverse genetic approaches, researchers modify a known gene of interest and observe the phenotypic change (unknown phenotype). In contrast, the forward genetic approach tries to find out the genetic cause (unknown gene/variant) for a particular phenotype (known phenotype). With forward mutagenesis, it is possible to study the genetic modifier unbiasedly, allowing the exploration of modifiers beyond the known variants [122]. Chemical mutagens like Ethyl methanesulfonate (EMS) and N-ethyl-N-nitrosourea (ENU) are two widely used mutagens for the forward genetic screen. There is no strict rule regarding the use of each mutagen, but genetic studies in plants, fruit flies and C. elegans preferentially use EMS, whereas ENU is used in mice and zebrafish [60,114,133,134,135,136,137]. Both EMS and ENU can induce random point mutation; EMS produces G/C base pair mutations and ENU has a bias toward A/T base pairs. In high-throughput mutagenesis screens, a cocktail of EMS and ENU is also used [138].
6. Prospects and Challenges of Computational Model in Modifier Identification
Continual increases in computational power is allowing researchers to run powerful machine learning algorithms and advanced deep learning models [139,140]. Overall improvement of the core algorithms for regression, classification, and clustering analysis has significantly improved applied bioinformatics for medical science, health science, and genetics [141,142]. Even with these advancements, the resources for detection and prioritization of modifier variants remain scarce. A computational model that can identify genetic modifiers from the individual genome by aggregating known information can become a valuable resource for the diagnosis and treatment of patients.
A review on Hereditary spastic paraplegias (HSPs) [143] and another very recently on Charcot-Marie-Tooth (CMT) [27] discuss how genetic modifier studies are uncovering the ‘missing heritability’ for these rare Mendelian disorders. The authors argued that despite being a monogenic disorder, non-Mendelian factors are essential to consider to overcome the diagnostic gap and to provide better therapeutics. Genetic modifiers work through a complex interaction system, most of which are yet to be discovered [56]. In another review, Deltas C. proposed the hypothesis of the ‘Alpha effect’ of genetic modifiers in disease diagnosis and patient management [144]. According to the hypothesis, with a proper combination of diverse knowledgebases, we could potentially measure the severity or contributory role of a particular variant in an individual to a specific phenotype or disease. While identifying the negative effect of the modifiers (enhancers) will help to provide better diagnostics insight, identifying positive modifiers (suppressors) beyond diagnostic may also help researchers to develop personalized therapies.
Over the years, researchers have developed such knowledge bases and ontologies, which have facilitated the development of computational models and automation systems for bioinformatics analysis. A range of scoring and variant interpretation techniques have been developed to better understand each variant and its pathway [145]. Resources like BioGrid [118], GeneMania [146], model organism-specific knowledgebases [29,147], Gene Ontology [148,149], Phenotype Ontology [150], and pathway analysis [151] are currently helping researchers in modifier identification studies and may help to develop computational models for modifier identification in the future [2]. With all these resources available, a possible challenge to capture or measure the alpha effect or modifier effect would be identifying the appropriate feature combinations.
7. Discussion
The identification of genetic modifiers is an existing challenge, especially for rare diseases. It can be seen as a two-front challenge—experimental and computational. The experimental challenge includes establishing high-throughput forward genetic screening techniques utilizing model organisms as current mutagenesis and screening techniques often take years to identify modifiers [152,153,154]. On the other hand, the computational challenge exists in identifying genetic modifiers by comprehensively analyzing all the variants from an individual genome [155,156].
Phenotypic heterogeneity and variability is a major concern for rare Mendelian disorders, which often leads to misdiagnosis and/or delayed diagnosis [19,114,157,158]. However, naturally occurring suppressor modifiers that reduce the severity or rescue an individual from the adverse effect of a disease-causing mutation can guide researchers and clinicians to its potential therapeutics [8,117]. Phenotypic variability resulting from suppressor modifiers is less studied but presumably is a common phenomenon [117]. Therefore, future research efforts in the area of high-throughput modifier identification, in combination with experimental and computational approaches will not only help with the better diagnosis of Mendelian diseases in a time-efficient manner, but it may also provide direction to its potential therapeutics, which we are significantly lacking [159].
Acknowledgments
We thank Maroilley, Jean, Stasiuk, and Li for thoughtful comments on the manuscript.
Supplementary Materials
The following are available online at https://www.mdpi.com/2073-4425/11/3/239/s1, Figure S1: Schematic diagram of the literature review and the textual analysis process. Textual analysis is performed using NVivo 12 plus.
Author Contributions
Conceptualization, M.T.-G.; methodology and data analysis, K.M.T.H.R. and M.T.-G.; writing—original draft preparation, K.M.T.H.R.; writing—review and editing, M.T.-G.; supervision, M.T.-G.; funding acquisition, M.T.-G. and K.M.T.H.R. All authors have read and agreed to the published version of the manuscript.
Funding
The authors were supported by the Canadian Institute of Health Research, CIHR-Project grant number PJT-156068 and Alberta Graduate Excellence Scholarship (AGES).
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- 1.Baltimore M. Online Mendelian Inheritance in Man, OMIM®: OMIM Entry Statistics. [(accessed on 26 July 2019)]; Available online: https://www.omim.org/statistics/entry.
- 2.Wright C.F., FitzPatrick D.R., Firth H.V. Paediatric genomics: Diagnosing rare disease in children. Nat. Rev. Genet. 2018;19:253–268. doi: 10.1038/nrg.2017.116. [DOI] [PubMed] [Google Scholar]
- 3.WHO|Genes and Human Diseases. [(accessed on 6 December 2019)]; Available online: https://www.who.int/genomics/public/geneticdiseases/en/index2.html.
- 4.Boycott K.M., Rath A., Chong J.X., Hartley T., Alkuraya F.S., Baynam G., Brookes A.J., Brudno M., Carracedo A., den Dunnen J.T., et al. International Cooperation to Enable the Diagnosis of All Rare Genetic Diseases. Am. J. Hum. Genet. 2017;100:695–705. doi: 10.1016/j.ajhg.2017.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.De Vrueh R., Baekelandt E.R.F., De Haan J.M.H. Priority Medicines for Europe and the World 2013 Update. World Health Organization; Geneva, Switzerland: 2013. Rare Diseases (Background Paper 6.19) [Google Scholar]
- 6.Chong J.X., Buckingham K.J., Jhangiani S.N., Boehm C., Sobreira N., Smith J.D., Harrell T.M., McMillin M.J., Wiszniewski W., Gambin T., et al. The Genetic Basis of Mendelian Phenotypes: Discoveries, Challenges, and Opportunities. Am. J. Hum. Genet. 2015;97:199–215. doi: 10.1016/j.ajhg.2015.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kose M.D., Canda E., Kağnıcı M., Uçar S.K., Onay H., Yıldırım Sozmen E., Karapınar D., Özkınay F., Çoker M. Coexistence of Gaucher Disease and severe congenital neutropenia. Blood Cells Mol. Dis. 2019;76:1–6. doi: 10.1016/j.bcmd.2018.07.001. [DOI] [PubMed] [Google Scholar]
- 8.Davidson B.A., Hassan S., Garcia E.J., Tayebi N., Sidransky E. Exploring genetic modifiers of Gaucher disease: The next horizon. Hum. Mutat. 2018;39:1739–1751. doi: 10.1002/humu.23611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Silva E., Dharmaraj S., Li Y.Y., Pina A.L., Carter R.C., Loyer M., Traboulsi E., Theodossiadis G., Koenekoop R.K., Sundin O.H., et al. A missense mutation in GUCY2D acts as a genetic modifier in RPE65-related Leber congenital amaurosis. Ophthalmic Genet. 2004;25:205–217. doi: 10.1080/13816810490513451. [DOI] [PubMed] [Google Scholar]
- 10.Rudnik-Schöneborn S., Barisić N., Eggermann K., Ortiz Brüchle N., Grdan P., Zerres K. Distally pronounced infantile spinal muscular atrophy with severe axonal and demyelinating neuropathy associated with the S230L mutation of SMN1. Neuromuscul. Disord. 2016;26:132–135. doi: 10.1016/j.nmd.2015.12.003. [DOI] [PubMed] [Google Scholar]
- 11.Missaglia S., Tasca E., Angelini C., Moro L., Tavian D. Novel missense mutations in PNPLA2 causing late onset and clinical heterogeneity of neutral lipid storage disease with myopathy in three siblings. Mol. Genet. Metab. 2015;115:110–117. doi: 10.1016/j.ymgme.2015.05.001. [DOI] [PubMed] [Google Scholar]
- 12.Chen R., Shi L., Hakenberg J., Naughton B., Sklar P., Zhang J., Zhou H., Tian L., Prakash O., Lemire M., et al. Analysis of 589,306 genomes identifies individuals resilient to severe Mendelian childhood diseases. Nat. Biotechnol. 2016;34:531–538. doi: 10.1038/nbt.3514. [DOI] [PubMed] [Google Scholar]
- 13.Tarailo-Graovac M., Zhu J.Y.A., Matthews A., van Karnebeek C.D.M., Wasserman W.W. Assessment of the ExAC data set for the presence of individuals with pathogenic genotypes implicated in severe Mendelian pediatric disorders. Genet. Med. 2017;19:1300–1308. doi: 10.1038/gim.2017.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Costanzo M., Kuzmin E., van Leeuwen J., Mair B., Moffat J., Boone C., Andrews B. Global Genetic Networks and the Genotype-to-Phenotype Relationship. Cell. 2019;177:85–100. doi: 10.1016/j.cell.2019.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Domingo J., Baeza-Centurion P., Lehner B. The Causes and Consequences of Genetic Interactions (Epistasis) Annu. Rev. Genom. Hum. Genet. 2019;20:433–460. doi: 10.1146/annurev-genom-083118-014857. [DOI] [PubMed] [Google Scholar]
- 16.Veitia R.A., Caburet S., Birchler J.A. Mechanisms of Mendelian dominance. Clin. Genet. 2018;93:419–428. doi: 10.1111/cge.13107. [DOI] [PubMed] [Google Scholar]
- 17.Holmans P.A., Massey T.H., Jones L. Genetic modifiers of Mendelian disease: Huntington’s disease and the trinucleotide repeat disorders. Hum. Mol. Genet. 2017;26:R83–R90. doi: 10.1093/hmg/ddx261. [DOI] [PubMed] [Google Scholar]
- 18.Aubart M., Gazal S., Arnaud P., Benarroch L., Gross M.-S.S., Buratti J., Boland A., Meyer V., Zouali H., Hanna N., et al. Association of modifiers and other genetic factors explain Marfan syndrome clinical variability. Eur. J. Hum. Genet. 2018;26:1759–1772. doi: 10.1038/s41431-018-0164-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Maroilley T., Tarailo-Graovac M. Uncovering Missing Heritability in Rare Diseases. Genes. 2019;10:275. doi: 10.3390/genes10040275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dong C., Wei P., Jian X., Gibbs R., Boerwinkle E., Wang K., Liu X. Comparison and integration of deleteriousness prediction methods for nonsynonymous SNVs in whole exome sequencing studies. Hum. Mol. Genet. 2015;24:2125–2137. doi: 10.1093/hmg/ddu733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lappalainen T., Scott A.J., Brandt M., Hall I.M. Genomic Analysis in the Age of Human Genome Sequencing. Cell. 2019;177:70–84. doi: 10.1016/j.cell.2019.02.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Salgado D., Bellgard M.I., Desvignes J.-P., Béroud C. How to Identify Pathogenic Mutations among All Those Variations: Variant Annotation and Filtration in the Genome Sequencing Era. Hum. Mutat. 2016;37:1272–1282. doi: 10.1002/humu.23110. [DOI] [PubMed] [Google Scholar]
- 23.Schäffer A.A. Digenic inheritance in medical genetics. J. Med. Genet. 2013;50:641–652. doi: 10.1136/jmedgenet-2013-101713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dipple K.M., McCabe E.R.B. Phenotypes of patients with “Simple” mendelian disorders are complex traits: Thresholds, modifiers, and systems dynamics. Am. J. Hum. Genet. 2000;66:1729–1735. doi: 10.1086/302938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kousi M., Katsanis N. Genetic modifiers and oligogenic inheritance. Cold Spring Harb. Perspect. Med. 2015;5:1–22. doi: 10.1101/cshperspect.a017145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schacherer J. Beyond the simplicity of Mendelian inheritance. C. R. Biol. 2016;339:284–288. doi: 10.1016/j.crvi.2016.04.006. [DOI] [PubMed] [Google Scholar]
- 27.Bis-Brewer D.M., Fazal S., Züchner S. Genetic modifiers and non-Mendelian aspects of CMT. Brain Res. 2020;1726:146459. doi: 10.1016/j.brainres.2019.146459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lupski J.R., Belmont J.W., Boerwinkle E., Gibbs R.A. Clan genomics and the complex architecture of human disease. Cell. 2011;147:32–43. doi: 10.1016/j.cell.2011.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Costanzo M., VanderSluis B., Koch E.N., Baryshnikova A., Pons C., Tan G., Wang W., Usaj M.M.M., Hanchard J., Lee S.D., et al. A global genetic interaction network maps a wiring diagram of cellular function. Science. 2016;353:aaf1420. doi: 10.1126/science.aaf1420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yan D., Liu X.-Z. Modifiers of Hearing Impairment in Humans and Mice. Curr. Genom. 2010;11:269–278. doi: 10.2174/138920210791233054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Riordan J.D., Nadeau J.H. From Peas to Disease: Modifier Genes, Network Resilience, and the Genetics of Health. Am. J. Hum. Genet. 2017;101:177–191. doi: 10.1016/j.ajhg.2017.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Badano J.L., Katsanis N. Beyond mendel: An evolving view of human genetic disease transmission. Nat. Rev. Genet. 2002;3:779–789. doi: 10.1038/nrg910. [DOI] [PubMed] [Google Scholar]
- 33.Katsanis N. The continuum of causality in human genetic disorders. Genome Biol. 2016;17:233. doi: 10.1186/s13059-016-1107-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.E Samuels M. Saturation of the Human Phenome. Curr. Genom. 2010;11:482–499. doi: 10.2174/138920210793175886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stoetzel C., Laurier V., Davis E.E., Muller J., Rix S., Badano J.L., Leitch C.C., Salem N., Chouery E., Corbani S., et al. BBS10 encodes a vertebrate-specific chaperonin-like protein and is a major BBS locus. Nat. Genet. 2006;38:521–524. doi: 10.1038/ng1771. [DOI] [PubMed] [Google Scholar]
- 36.Tarailo-Graovac M., Shyr C., Ross C.J., Horvath G.A., Salvarinova R., Ye X.C., Zhang L.-H., Bhavsar A.P., Lee J.J.Y., Drögemöller B.I., et al. Exome Sequencing and the Management of Neurometabolic Disorders. N. Engl. J. Med. 2016;374:2246–2255. doi: 10.1056/NEJMoa1515792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Prior T.W., Krainer A.R., Hua Y., Swoboda K.J., Snyder P.C., Bridgeman S.J., Burghes A.H.M., Kissel J.T. A Positive Modifier of Spinal Muscular Atrophy in the SMN2 Gene. Am. J. Hum. Genet. 2009;85:408–413. doi: 10.1016/j.ajhg.2009.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ebermann I., Phillips J.B., Liebau M.C., Koenekoop R.K., Schermer B., Lopez I., Schäfer E., Roux A.F., Dafinger C., Bernd A., et al. PDZD7 is a modifier of retinal disease and a contributor to digenic Usher syndrome. J. Clin. Investig. 2010;120:1812–1823. doi: 10.1172/JCI39715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Boudellioua I., Kulmanov M., Schofield P.N., Gkoutos G.V., Hoehndorf R. OligoPVP: Phenotype-driven analysis of individual genomic information to prioritize oligogenic disease variants. Sci. Rep. 2018;8:14681. doi: 10.1038/s41598-018-32876-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gazzo A.M., Daneels D., Cilia E., Bonduelle M., Abramowicz M., Van Dooren S., Smits G., Lenaerts T. DIDA: A curated and annotated digenic diseases database. Nucleic Acids Res. 2016;44:D900–D907. doi: 10.1093/nar/gkv1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gifford C.A., Ranade S.S., Samarakoon R., Salunga H.T., Yvanka De Soysa T., Huang Y., Zhou P., Elfenbein A., Wyman S.K., Bui Y.K., et al. Oligogenic inheritance of a human heart disease involving a genetic modifier. Science. 2019;364:865–870. doi: 10.1126/science.aat5056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Karczewski K.J., Francioli L.C., Tiao G., Cummings B.B., Alföldi J., Wang Q., Collins R.L., Laricchia K.M., Ganna A., Birnbaum D.P., et al. Variation across 141,456 human exomes and genomes reveals the spectrum of loss-of-function intolerance across human protein-coding genes. BioRxiv. 2019 doi: 10.1101/531210. [DOI] [Google Scholar]
- 43.Tarailo-Graovac M., Wasserman W.W., Van Karnebeek C.D.M. Impact of next-generation sequencing on diagnosis and management of neurometabolic disorders: Current advances and future perspectives. Expert Rev. Mol. Diagn. 2017;17:307–309. doi: 10.1080/14737159.2017.1293527. [DOI] [PubMed] [Google Scholar]
- 44.Papadimitriou S., Gazzo A., Versbraegen N., Nachtegael C., Aerts J., Moreau Y., Van Dooren S., Nowe A., Smits G., Lenaerts T., et al. Predicting disease-causing variant combinations. Proc. Natl. Acad. Sci. USA. 2019;116:11878–11887. doi: 10.1073/pnas.1815601116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Boudellioua I., Mahamad Razali R.B., Kulmanov M., Hashish Y., Bajic V.B., Goncalves-Serra E., Schoenmakers N., Gkoutos G.V., Schofield P.N., Hoehndorf R. Semantic prioritization of novel causative genomic variants. PLoS Comput. Biol. 2017;13:e1005500. doi: 10.1371/journal.pcbi.1005500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rentzsch P., Witten D., Cooper G.M., Shendure J., Kircher M. CADD: Predicting the deleteriousness of variants throughout the human genome. Nucleic Acids Res. 2019;47:D886–D894. doi: 10.1093/nar/gky1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Quang D., Chen Y., Xie X. DANN: A deep learning approach for annotating the pathogenicity of genetic variants. Bioinformatics. 2015;31:761–763. doi: 10.1093/bioinformatics/btu703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ritchie G.R.S., Dunham I., Zeggini E., Flicek P. Functional annotation of noncoding sequence variants. Nat. Methods. 2014;11:294–296. doi: 10.1038/nmeth.2832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hoehndorf R., Schofield P.N., Gkoutos G.V. PhenomeNET: A whole-phenome approach to disease gene discovery. Nucleic Acids Res. 2011;39:e119. doi: 10.1093/nar/gkr538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Boudellioua I., Kulmanov M., Schofield P.N., Gkoutos G.V., Hoehndorf R. DeepPVP: Phenotype-based prioritization of causative variants using deep learning. BMC Bioinform. 2019;20:65. doi: 10.1186/s12859-019-2633-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Auton A., Abecasis G.R., Altshuler D.M., Durbin R.M., Bentley D.R., Chakravarti A., Clark A.G., Donnelly P., Eichler E.E., Flicek P., et al. A global reference for human genetic variation. Nature. 2015;526:68–74. doi: 10.1038/nature15393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Baltimore M. Online Mendelian Inheritance in Man, OMIM®. [(accessed on 26 July 2019)]; Available online: https://www.omim.org.
- 53.INSERM Orphanet: An Online Database of Rare Diseases and Orphan Drugs. [(accessed on 14 August 2019)]; Available online: http://www.orpha.net.
- 54.Na D., Rouf M., O’Kane C.J., Rubinsztein D.C., Gsponer J. NeuroGeM, a knowledgebase of genetic modifiers in neurodegenerative diseases. BMC Med. Genom. 2013;6:52. doi: 10.1186/1755-8794-6-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sun H., Guo Y., Lan X., Jia J., Cai X., Zhang G., Xie J., Liang Q., Li Y., Yu G. PhenoModifier: A genetic modifier database for elucidating the genetic basis of human phenotypic variation. Nucleic Acids Res. 2019;48:D977–D982. doi: 10.1093/nar/gkz930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kuzmin E., VanderSluis B., Wang W., Tan G., Deshpande R., Chen Y., Usaj M., Balint A., Usaj M.M., Van Leeuwen J. Systematic analysis of complex genetic interactions. Science. 2018;360:eaao1729. doi: 10.1126/science.aao1729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lee I., Lehner B., Vavouri T., Shin J., Fraser A.G., Marcotte E.M. Predicting genetic modifier loci using functional gene networks. Genome Res. 2010;20:1143–1153. doi: 10.1101/gr.102749.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Citro V., Cimmaruta C., Monticelli M., Riccio G., Mele B.H., Cubellis M.V., Andreotti G. The analysis of variants in the general population reveals that PMM2 is extremely tolerant to missense mutations and that diagnosis of PMM2-CDG can benefit from the identification of modifiers. Int. J. Mol. Sci. 2018;19:2218. doi: 10.3390/ijms19082218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lee I., Lehner B., Crombie C., Wong W., Fraser A.G., Marcotte E.M. A single gene network accurately predicts phenotypic effects of gene perturbation in Caenorhabditis elegans. Nat. Genet. 2008;40:181–188. doi: 10.1038/ng.2007.70. [DOI] [PubMed] [Google Scholar]
- 60.Enikanolaiye A., Ruston J., Zeng R., Taylor C., Shrock M., Buchovecky C.M., Shendure J., Acar E., Justice M.J. Suppressor mutations in Mecp2-null mice reveal that the DNA damage response is key to Rett syndrome pathology. BioRxiv. 2019 doi: 10.1101/gr.258400.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lim J., Hao T., Shaw C., Patel A.J., Szabó G., Rual J.F., Fisk C.J., Li N., Smolyar A., Hill D.E., et al. A Protein-Protein Interaction Network for Human Inherited Ataxias and Disorders of Purkinje Cell Degeneration. Cell. 2006;125:801–814. doi: 10.1016/j.cell.2006.03.032. [DOI] [PubMed] [Google Scholar]
- 62.Cukier H.N., Perez A.M., Collins A.L., Zhou Z., Zoghbi H.Y., Botas J. Genetic Modifiers of MeCP2 Function in Drosophila. PLoS Genet. 2008;4:e1000179. doi: 10.1371/journal.pgen.1000179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Madhukar N.S., Elemento O., Pandey G. Prediction of Genetic Interactions Using Machine Learning and Network Properties. Front. Bioeng. Biotechnol. 2015;3:172. doi: 10.3389/fbioe.2015.00172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Uppu S., Krishna A., Gopalan R.P. A Review on Methods for Detecting SNP Interactions in High-Dimensional Genomic Data. IEEE/ACM Trans. Comput. Biol. Bioinform. 2018;15:599–612. doi: 10.1109/TCBB.2016.2635125. [DOI] [PubMed] [Google Scholar]
- 65.Bateson W., Mendel G. Mendel’s Principles of Heredity. University Press; Cambridge, UK: 1909. [Google Scholar]
- 66.Fisher R.A. XV.—The Correlation between Relatives on the Supposition of Mendelian Inheritance. Trans. R. Soc. Edinb. 1919;52:399–433. doi: 10.1017/S0080456800012163. [DOI] [Google Scholar]
- 67.Phillips P.C. The language of gene interaction. Genetics. 1998;149:1167–1171. doi: 10.1093/genetics/149.3.1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Cockerham C.C. An Extension of the Concept of Partitioning Hereditary Variance for Analysis of Covariances among Relatives When Epistasis Is Present. Genetics. 1954;39:859–882. doi: 10.1093/genetics/39.6.859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kempthorne O. The correlation between relatives in a random mating population. Proc. R. Soc. Lond. B Biol. Sci. 1954;143:102–113. doi: 10.1101/SQB.1955.020.01.008. [DOI] [PubMed] [Google Scholar]
- 70.Cordell H.J. Epistasis: What it means, what it doesn’t mean, and statistical methods to detect it in humans. Hum. Mol. Genet. 2002;11:2463–2468. doi: 10.1093/hmg/11.20.2463. [DOI] [PubMed] [Google Scholar]
- 71.Cordell H.J. Detecting gene-gene interactions that underlie human diseases. Nat. Rev. Genet. 2009;10:392–404. doi: 10.1038/nrg2579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Álvarez-Castro J.M., Carlborg Ö. A unified model for functional and statistical epistasis and its application in quantitative trait loci analysis. Genetics. 2007;176:1151–1167. doi: 10.1534/genetics.106.067348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Cheverud J.M., Routman E.J. Epistasis and its contribution to genetic variance components. Genetics. 1995;139:1455–1461. doi: 10.1093/genetics/139.3.1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Moore J.H., Williams S.M. Traversing the Conceptual Divide between Biological and Statistical Epistasis: Systems Biology and a More Modern Synthesis. BioEssays. 2005;27:637–646. doi: 10.1002/bies.20236. [DOI] [PubMed] [Google Scholar]
- 75.Zeng Z.B., Wang T., Zou W. Modeling quantitative trait loci and interpretation of models. Genetics. 2005;169:1711–1725. doi: 10.1534/genetics.104.035857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Rettew D.C., Rebollo-Mesa I., Hudziak J.J., Willemsen G., Boomsma D.I. Non-additive and additive genetic effects on extraversion in 3314 Dutch adolescent twins and their parents. Behav. Genet. 2008;38:223–233. doi: 10.1007/s10519-008-9192-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Boucher B., Jenna S. Genetic interaction networks: Better understand to better predict. Front. Genet. 2013;4:290. doi: 10.3389/fgene.2013.00290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hahn L.W., Ritchie M.D., Moore J.H. Multifactor dimensionality reduction software for detecting gene-gene and gene-environment interactions. Bioinformatics. 2003;19:376–382. doi: 10.1093/bioinformatics/btf869. [DOI] [PubMed] [Google Scholar]
- 79.Zhu Z., Tong X., Zhu Z., Liang M., Cui W., Su K., Li M.D., Zhu J. Development of GMDR-GPU for Gene-Gene Interaction Analysis and Its Application to WTCCC GWAS Data for Type 2 Diabetes. PLoS ONE. 2013;8:e61943. doi: 10.1371/journal.pone.0061943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Van Lishout F., Mahachie John J.M., Gusareva E.S., Urrea V., Cleynen I., Théâtre E., Charloteaux B., Calle M.L., Wehenkel L., Steen K. Van An efficient algorithm to perform multiple testing in epistasis screening. BMC Bioinform. 2013;14:138. doi: 10.1186/1471-2105-14-138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wan X., Yang C., Yang Q., Xue H., Fan X., Tang N.L.S., Yu W. BOOST: A fast approach to detecting gene-gene interactions in genome-wide case-control studies. Am. J. Hum. Genet. 2010;87:325–340. doi: 10.1016/j.ajhg.2010.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zhang Y., Liu J.S. Bayesian inference of epistatic interactions in case-control studies. Nat. Genet. 2007;39:1167–1173. doi: 10.1038/ng2110. [DOI] [PubMed] [Google Scholar]
- 83.Wang Y., Liu X., Robbins K., Rekaya R. AntEpiSeeker: Detecting epistatic interactions for case-control studies using a two-stage ant colony optimization algorithm. BMC Res. Notes. 2010;3:117. doi: 10.1186/1756-0500-3-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Marchini J., Donnelly P., Cardon L.R. Genome-wide strategies for detecting multiple loci that influence complex diseases. Nat. Genet. 2005;37:413–417. doi: 10.1038/ng1537. [DOI] [PubMed] [Google Scholar]
- 85.Srivastava A., Mittal B., Prakash J., Srivastava P., Srivastava N., Srivastava N. Association of FTO and IRX3 genetic variants to obesity risk in north India. Ann. Hum. Biol. 2016;43:451–456. doi: 10.3109/03014460.2015.1103902. [DOI] [PubMed] [Google Scholar]
- 86.Chen G., Zhang F., Xue W., Wu R., Xu H., Wang K., Zhu J. An association study revealed substantial effects of dominance, epistasis and substance dependence co-morbidity on alcohol dependence symptom count. Addict. Biol. 2017;22:1475–1485. doi: 10.1111/adb.12402. [DOI] [PubMed] [Google Scholar]
- 87.Sun J., Song F., Wang J., Han G., Bai Z., Xie B., Feng X., Jia J., Duan Y., Lei H. Hidden risk genes with high-order intragenic epistasis in Alzheimer’s disease. J. Alzheimer’s Dis. 2014;41:1039–1056. doi: 10.3233/JAD-140054. [DOI] [PubMed] [Google Scholar]
- 88.Henckaerts L., Cleynen I., Brinar M., John J.M., Van Steen K., Rutgeerts P., Vermeire S. Genetic variation in the autophagy gene ULK1 and risk of Crohn’s disease. Inflamm. Bowel Dis. 2011;17:1392–1397. doi: 10.1002/ibd.21486. [DOI] [PubMed] [Google Scholar]
- 89.Bessonov K., Gusareva E.S., Van Steen K. A cautionary note on the impact of protocol changes for genome-wide association SNP × SNP interaction studies: An example on ankylosing spondylitis. Hum. Genet. 2015;134:761–773. doi: 10.1007/s00439-015-1560-7. [DOI] [PubMed] [Google Scholar]
- 90.Mahachie John J.M., Baurecht H., Rodríguez E., Naumann A., Wagenpfeil S., Klopp N., Mempel M., Novak N., Bieber T., Wichmann H.E., et al. Analysis of the high affinity IgE receptor genes reveals epistatic effects of FCER1A variants on eczema risk. Allergy Eur. J. Allergy Clin. Immunol. 2010;65:875–882. doi: 10.1111/j.1398-9995.2009.02297.x. [DOI] [PubMed] [Google Scholar]
- 91.Zhang Y., Tian L., Sleiman P., Ghosh S., Hakonarson H. Bayesian analysis of genome-wide inflammatory bowel disease data sets reveals new risk loci. Eur. J. Hum. Genet. 2018;26:265–274. doi: 10.1038/s41431-017-0041-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Varón-González C., Navarro N. Epistasis regulates the developmental stability of the mouse craniofacial shape. Heredity. 2018;122:501–512. doi: 10.1038/s41437-018-0140-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Edwards S.L., Beesley J., French J.D., Dunning M. Beyond GWASs: Illuminating the dark road from association to function. Am. J. Hum. Genet. 2013;93:779–797. doi: 10.1016/j.ajhg.2013.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Visscher P.M., Wray N.R., Zhang Q., Sklar P., McCarthy M.I., Brown M.A., Yang J. 10 Years of GWAS Discovery: Biology, Function, and Translation. Am. J. Hum. Genet. 2017;101:5–22. doi: 10.1016/j.ajhg.2017.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Dougherty M., Lazar J., Klein J.C., Diaz K., Gobillot T., Grunblatt E., Hasle N., Lawrence D., Maurano M., Nelson M., et al. Genome sequencing in a case of Niemann-Pick type C. Cold Spring Harb. Mol. Case Stud. 2016;2:a001222. doi: 10.1101/mcs.a001222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Vanderweele T.J. On the distinction between interaction and effect modification. Epidemiology. 2009;20:863–871. doi: 10.1097/EDE.0b013e3181ba333c. [DOI] [PubMed] [Google Scholar]
- 97.Lehner B. Molecular mechanisms of epistasis within and between genes. Trends Genet. 2011;27:323–331. doi: 10.1016/j.tig.2011.05.007. [DOI] [PubMed] [Google Scholar]
- 98.VanderSluis B., Costanzo M., Billmann M., Ward H.N., Myers C.L., Andrews B.J., Boone C. Integrating genetic and protein–protein interaction networks maps a functional wiring diagram of a cell. Curr. Opin. Microbiol. 2018;45:170–179. doi: 10.1016/j.mib.2018.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Wang S., Zhang Y., Dai W., Lauter K., Kim M., Tang Y., Xiong H., Jiang X. HEALER: Homomorphic computation of ExAct Logistic rEgRession for secure rare disease variants analysis in GWAS. Bioinformatics. 2016;32:211–218. doi: 10.1093/bioinformatics/btv563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Mitra I., Lavillaureix A., Yeh E., Traglia M., Tsang K., Bearden C.E., Rauen K.A., Weiss L.A. Reverse Pathway Genetic Approach Identifies Epistasis in Autism Spectrum Disorders. PLoS Genet. 2017;13:e1006516. doi: 10.1371/journal.pgen.1006516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Corvol H., Blackman S.M., Boelle P.-Y., Gallins P.J., Pace R.G., Stonebraker J.R., Accurso F.J., Clement A., Collaco J.M., Dang H., et al. Genome-wide association meta-analysis identifies five modifier loci of lung disease severity in cystic fibrosis. Nat. Commun. 2015;6:8382. doi: 10.1038/ncomms9382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Pottier C., Zhou X., Perkerson R.B., Baker M., Jenkins G.D., Serie D.J., Ghidoni R., Benussi L., Binetti G., López de Munain A., et al. Potential genetic modifiers of disease risk and age at onset in patients with frontotemporal lobar degeneration and GRN mutations: A genome-wide association study. Lancet Neurol. 2018;17:548–558. doi: 10.1016/S1474-4422(18)30126-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Gymrek M., McGuire A.L., Golan D., Halperin E., Erlich Y. Identifying personal genomes by surname inference. Science. 2013;339:321–324. doi: 10.1126/science.1229566. [DOI] [PubMed] [Google Scholar]
- 104.Sweeney L., Abu A., Winn J. Identifying Participants in the Personal Genome Project by Name. arXiv. 2013 doi: 10.2139/ssrn.2257732.1304.7605 [DOI] [Google Scholar]
- 105.Homer N., Szelinger S., Redman M., Duggan D., Tembe W., Muehling J., Pearson J.V., Stephan D.A., Nelson S.F., Craig D.W. Resolving Individuals Contributing Trace Amounts of DNA to Highly Complex Mixtures Using High-Density SNP Genotyping Microarrays. PLoS Genet. 2008;4:e1000167. doi: 10.1371/journal.pgen.1000167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Wang R., Li Y.F., Wang X.F., Tang H., Zhou X. Learning your identity and disease from research papers: Information leaks in genome wide association study; Proceedings of the ACM Conference on Computer and Communications Security—CCS ‘09; Chicago, IL, USA. 9–13 November 2009; New York, NY, USA: ACM Press; 2009. pp. 534–544. [Google Scholar]
- 107.Guan P., Sung W.K. Structural variation detection using next-generation sequencing data: A comparative technical review. Methods. 2016;102:36–49. doi: 10.1016/j.ymeth.2016.01.020. [DOI] [PubMed] [Google Scholar]
- 108.Rylaarsdam L., Guemez-Gamboa A. Genetic Causes and Modifiers of Autism Spectrum Disorder. Front. Cell Neurosci. 2019;13:385. doi: 10.3389/fncel.2019.00385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Bosch E., Casals F. Next-generation sequencing for rare diseases. In: Appasani K., editor. Genome-Wide Association Studies. Cambridge University Press; Cambridge, UK: 2015. pp. 231–242. [Google Scholar]
- 110.Boycott K.M., Vanstone M.R., Bulman D.E., MacKenzie A.E. Rare-disease genetics in the era of next-generation sequencing: Discovery to translation. Nat. Rev. Genet. 2013;14:681–691. doi: 10.1038/nrg3555. [DOI] [PubMed] [Google Scholar]
- 111.Abu-Duhier F., Pooranachandran V., McDonagh A.J.G., Messenger A.G., Cooper-Knock J., Bakri Y., Heath P.R., Tazi-Ahnini R. Whole Genome Sequencing in an Acrodermatitis Enteropathica Family from the Middle East. Dermatol. Res. Pract. 2018;2018:1–9. doi: 10.1155/2018/1284568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Cavaillé M., Ponelle-Chachuat F., Uhrhammer N., Viala S., Gay-Bellile M., Privat M., Bidet Y., Bignon Y.-J.J. Early Onset Multiple Primary Tumors in Atypical Presentation of Cowden Syndrome Identified by Whole-Exome-Sequencing. Front. Genet. 2018;9:353. doi: 10.3389/fgene.2018.00353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Voskarides K., Papagregoriou G., Hadjipanagi D., Petrou I., Savva I., Elia A., Athanasiou Y., Pastelli A., Kkolou M., Hadjigavriel M., et al. COL4A5 and LAMA5 variants co-inherited in familial hematuria: Digenic inheritance or genetic modifier effect? BMC Nephrol. 2018;19:114. doi: 10.1186/s12882-018-0906-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Wangler M.F., Yamamoto S., Chao H.T., Posey J.E., Westerfield M., Postlethwait J., Hieter P., Boycott K.M., Campeau P.M., Bellen H.J., et al. Model organisms facilitate rare disease diagnosis and therapeutic research. Genetics. 2017;207:9–27. doi: 10.1534/genetics.117.203067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Hmeljak J., Justice M.J. From gene to treatment: Supporting rare disease translational research through model systems. Dis. Model. Mech. 2019;12:dmm039271. doi: 10.1242/dmm.039271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Lehner B. Genotype to phenotype: Lessons from model organisms for human genetics. Nat. Rev. Genet. 2013;14:168–178. doi: 10.1038/nrg3404. [DOI] [PubMed] [Google Scholar]
- 117.van Leeuwen J., Pons C., Boone C., Andrews B.J. Mechanisms of suppression: The wiring of genetic resilience. BioEssays. 2017;39:1700042. doi: 10.1002/bies.201700042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Oughtred R., Stark C., Breitkreutz B.J., Rust J., Boucher L., Chang C., Kolas N., O’Donnell L., Leung G., McAdam R., et al. The BioGRID interaction database: 2019 update. Nucleic Acids Res. 2019;47:D529–D541. doi: 10.1093/nar/gky1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Kim W., Underwood R.S., Greenwald I., Shaye D.D. Ortholist 2: A new comparative genomic analysis of human and caenorhabditis elegans genes. Genetics. 2018;210:445–461. doi: 10.1534/genetics.118.301307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Wangler M.F., Yamamoto S., Bellen H.J. Fruit flies in biomedical research. Genetics. 2015;199:639–653. doi: 10.1534/genetics.114.171785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Leduc R.Y.M., Singh P., McDermid H.E. Genetic backgrounds and modifier genes of NTD mouse models: An opportunity for greater understanding of the multifactorial etiology of neural tube defects. Birth Defects Res. 2017;109:140–152. doi: 10.1002/bdra.23554. [DOI] [PubMed] [Google Scholar]
- 122.Hamilton B.A., Yu B.D. Modifier genes and the plasticity of genetic networks in mice. PLoS Genet. 2012;8:e1002644. doi: 10.1371/journal.pgen.1002644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Tong A.H.Y., Lesage G., Bader G.D., Ding H., Xu H., Xin X., Young J., Berriz G.F., Brost R.L., Chang M., et al. Global Mapping of the Yeast Genetic Interaction Network. Science. 2004;303:808–813. doi: 10.1126/science.1091317. [DOI] [PubMed] [Google Scholar]
- 124.van Leeuwen J., Pons C., Mellor J.C., Yamaguchi T.N., Friesen H., Koschwanez J., Ušaj M.M., Pechlaner M., Takar M., Ušaj M., et al. Exploring genetic suppression interactions on a global scale. Science. 2016;354:aag0839. doi: 10.1126/science.aag0839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Schuldiner M., Collins S.R., Thompson N.J., Denic V., Bhamidipati A., Punna T., Ihmels J., Andrews B., Boone C., Greenblatt J.F., et al. Exploration of the Function and Organization of the Yeast Early Secretory Pathway through an Epistatic Miniarray Profile. Cell. 2005;123:507–519. doi: 10.1016/j.cell.2005.08.031. [DOI] [PubMed] [Google Scholar]
- 126.Fire A., Xu S., Montgomery M.K., Kostas S.A., Driver S.E., Mello C.C. Potent and specific genetic interference by double-stranded RNA in caenorhabditis elegans. Nature. 1998;391:806–811. doi: 10.1038/35888. [DOI] [PubMed] [Google Scholar]
- 127.Phillips P.C. Epistasis-The essential role of gene interactions in the structure and evolution of genetic systems. Nat. Rev. Genet. 2008;9:855–867. doi: 10.1038/nrg2452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Collins S.R., Miller K.M., Maas N.L., Roguev A., Fillingham J., Chu C.S., Schuldiner M., Gebbia M., Recht J., Shales M., et al. Functional dissection of protein complexes involved in yeast chromosome biology using a genetic interaction map. Nature. 2007;446:806–810. doi: 10.1038/nature05649. [DOI] [PubMed] [Google Scholar]
- 129.Byrne A.B., Weirauch M.T., Wong V., Koeva M., Dixon S.J., Stuart J.M., Roy P.J. A global analysis of genetic interactions in Caenorhabditis elegans. J. Biol. 2007;6:8. doi: 10.1186/jbiol58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Lehner B., Crombie C., Tischler J., Fortunato A., Fraser A.G. Systematic mapping of genetic interactions in Caenorhabditis elegans identifies common modifiers of diverse signaling pathways. Nat. Genet. 2006;38:896–903. doi: 10.1038/ng1844. [DOI] [PubMed] [Google Scholar]
- 131.Wang X., White K.P. Large-scale genetic epistasis networks using RNAi. Nat. Methods. 2011;8:299–301. doi: 10.1038/nmeth0411-299. [DOI] [PubMed] [Google Scholar]
- 132.Jackson A.L., Linsley P.S. Recognizing and avoiding siRNA off-target effects for target identification and therapeutic application. Nat. Rev. Drug Discov. 2010;9:57–67. doi: 10.1038/nrd3010. [DOI] [PubMed] [Google Scholar]
- 133.Kutscher L.M., Shaham S. Forward and reverse mutagenesis in C. elegans. WormBook. 2014:1–26. doi: 10.1895/wormbook.1.167.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Kaufman T.C. A short history and description of Drosophila melanogaster classical genetics: Chromosome aberrations, forward genetic screens, and the nature of mutations. Genetics. 2017;206:665–689. doi: 10.1534/genetics.117.199950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Acevedo-Arozena A., Wells S., Potter P., Kelly M., Cox R.D., Brown S.D.M. ENU Mutagenesis, a Way Forward to Understand Gene Function. Annu. Rev. Genom. Hum. Genet. 2008;9:49–69. doi: 10.1146/annurev.genom.9.081307.164224. [DOI] [PubMed] [Google Scholar]
- 136.Farrell A., Coleman B.I., Benenati B., Brown K.M., Blader I.J., Marth G.T., Gubbels M.J. Whole genome profiling of spontaneous and chemically induced mutations in Toxoplasma gondii. BMC Genom. 2014;15:354. doi: 10.1186/1471-2164-15-354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Buchovecky C.M., Turley S.D., Brown H.M., Kyle S.M., McDonald J.G., Liu B., Pieper A.A., Huang W., Katz D.M., Russell D.W., et al. A suppressor screen in Mecp2 mutant mice implicates cholesterol metabolism in Rett syndrome. Nat. Genet. 2013;45:1013–1020. doi: 10.1038/ng.2714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Thompson O., Edgley M., Strasbourger P., Flibotte S., Ewing B., Adair R., Au V., Chaudhry I., Fernando L., Hutter H., et al. The million mutation project: A new approach to genetics in Caenorhabditis elegans. Genome Res. 2013;23:1749–1762. doi: 10.1101/gr.157651.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Lecun Y., Bengio Y., Hinton G. Deep Learning. Nature. 2015;521:436–444. doi: 10.1038/nature14539. [DOI] [PubMed] [Google Scholar]
- 140.Jordan M.I., Mitchell T.M. Machine learning: Trends, perspectives, and prospects. Science. 2015;349:255–260. doi: 10.1126/science.aaa8415. [DOI] [PubMed] [Google Scholar]
- 141.Shen D., Wu G., Suk H.-I. Deep Learning in Medical Image Analysis. Annu. Rev. Biomed. Eng. 2017;19:221–248. doi: 10.1146/annurev-bioeng-071516-044442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Libbrecht M.W., Noble W.S. Machine learning applications in genetics and genomics. Nat. Rev. Genet. 2015;16:321–332. doi: 10.1038/nrg3920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Bis-Brewer D.M., Züchner S. Perspectives on the genomics of HSP beyond mendelian inheritance. Front. Neurol. 2018;9:958. doi: 10.3389/fneur.2018.00958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Deltas C. Digenic inheritance and genetic modifiers. Clin. Genet. 2018;93:429–438. doi: 10.1111/cge.13150. [DOI] [PubMed] [Google Scholar]
- 145.Peña-Chilet M., Esteban-Medina M., Falco M.M., Rian K., Hidalgo M.R., Loucera C., Dopazo J. Using mechanistic models for the clinical interpretation of complex genomic variation. Sci. Rep. 2019;9:18937. doi: 10.1038/s41598-019-55454-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Mostafavi S., Ray D., Warde-Farley D., Grouios C., Morris Q. GeneMANIA: A real-time multiple association network integration algorithm for predicting gene function. Genome Biol. 2008;9:S4. doi: 10.1186/gb-2008-9-s1-s4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Lee R.Y.N., Howe K.L., Harris T.W., Arnaboldi V., Cain S., Chan J., Chen W.J., Davis P., Gao S., Grove C., et al. WormBase 2017: Molting into a new stage. Nucleic Acids Res. 2018;46:D869–D874. doi: 10.1093/nar/gkx998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Ashburner M., Ball C.A., Blake J.A., Botstein D., Butler H., Cherry J.M., Davis A.P., Dolinski K., Dwight S.S., Eppig J.T., et al. Gene ontology: Tool for the unification of biology. Nat. Genet. 2000;25:25–29. doi: 10.1038/75556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Carbon S., Douglass E., Dunn N., Good B., Harris N.L., Lewis S.E., Mungall C.J., Basu S., Chisholm R.L., Dodson R.J., et al. The Gene Ontology Resource: 20 years and still GOing strong. Nucleic Acids Res. 2019;47:D330–D338. doi: 10.1093/nar/gky1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Köhler S., Carmody L., Vasilevsky N., Jacobsen J.O.B., Danis D., Gourdine J.P., Gargano M., Harris N.L., Matentzoglu N., McMurry J.A., et al. Expansion of the Human Phenotype Ontology (HPO) knowledge base and resources. Nucleic Acids Res. 2019;47:D1018–D1027. doi: 10.1093/nar/gky1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Fabregat A., Jupe S., Matthews L., Sidiropoulos K., Gillespie M., Garapati P., Haw R., Jassal B., Korninger F., May B., et al. The Reactome Pathway Knowledgebase. Nucleic Acids Res. 2018;46:D649–D655. doi: 10.1093/nar/gkx1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Kemp C.A., Mi H.S., Addepalli M.K., Hunter G., O’Connell K. Suppressors of zyg-1 define regulators of centrosome duplication and nuclear association in Caenorhabditis elegans. Genetics. 2007;176:95–113. doi: 10.1534/genetics.107.071803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Peel N., Iyer J., Naik A., Dougherty M.P., Decker M., O’Connell K.F. Protein Phosphatase 1 Down Regulates ZYG-1 Levels to Limit Centriole Duplication. PLoS Genet. 2017;13:e1006543. doi: 10.1371/journal.pgen.1006543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Tarailo M., Kitagawa R., Rose A.M. Suppressors of spindle checkpoint defect (such) mutants identify new mdf-1/MAD1 interactors in Caenorhabditis elegans. Genetics. 2007;175:1665–1679. doi: 10.1534/genetics.106.067918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Tarailo-Graovac M., Wong T., Qin Z., Flibotte S., Taylor J., Moerman D.G., Rose A.M., Chen N. Spectrum of variations in dog-1/FANCJ and mdf-1/MAD1 defective Caenorhabditis elegans strains after long-term propagation. BMC Genom. 2015;16:210. doi: 10.1186/s12864-015-1402-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Tarailo-Graovac M., Wong T., Qin Z., Flibotte S., Taylor J., Moerman D.G., Rose A.M., Chen N. Cyclin B3 and dynein heavy chain cooperate to increase fitness in the absence of mdf-1/MAD1 in Caenorhabditis elegans. Cell Cycle. 2014;13:3089–3199. doi: 10.4161/15384101.2014.949491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Graf J., Schwitalla J.C., Albrecht P., Veltkamp R., Berlit P., Hartung H.-P., Aktas O., Kraemer M. Misdiagnoses and delay of diagnoses in Moyamoya angiopathy—A large Caucasian case series. J. Neurol. 2019;266:1153–1159. doi: 10.1007/s00415-019-09245-9. [DOI] [PubMed] [Google Scholar]
- 158.Scalco R., Morrow J., Booth S., Chatfield S., Godfrey R., Quinlivan R. Misdiagnosis and diagnostic delay in McArdle disease. Neuromuscul. Disord. 2017;27:S204. doi: 10.1016/j.nmd.2017.06.400. [DOI] [PubMed] [Google Scholar]
- 159.Nadeau J.H., Topol E.J. The genetics of health. Nat. Genet. 2006;38:1095–1098. doi: 10.1038/ng1006-1095. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.