Abstract
Adenosine is involved in a range of physiological and pathological effects through membrane-bound receptors linked to G proteins. There are four subtypes of adenosine receptors, described as A1AR, A2AAR, A2BAR, and A3AR, which are the center of cAMP signal pathway-based drug development. Several types of agonists, partial agonists or antagonists, and allosteric substances have been synthesized from these receptors as new therapeutic drug candidates. Research efforts surrounding A1AR and A2AAR are perhaps the most enticing because of their concentration and affinity; however, as a consequence of distressing conditions, both A2BAR and A3AR levels might accumulate. This review focuses on the biological features of each adenosine receptor as the basis of ligand production and describes clinical studies of adenosine receptor-associated pharmaceuticals in human diseases.
Keywords: adenosine, adenosine receptors, G protein-coupled receptors, agonists, antagonists, allosteric molecules
1. Introduction
Adenosine is a nucleoside molecule that elicits various physiological responses in tissues and organs. The pioneering identification of adenosine occurred when Drury and Szent-Gyorgyi successfully extracted an adenylic acid substance from the mammalian heart and other tissues influencing cardiac rhythm [1]. Subsequently, adenosine analogs resulted in coronary dilatation and blood flow elevation in some studies [2]. Interestingly, a small amount of caffeine diminished the effect of adenosine on the contraction of atrial muscle [3]. In fact, Sattin and Rall proposed that adenosine required a particular molecule in the cell membrane to exert its effects [4]. All these studies considered the role of adenosine-related specific receptors.
The classical autonomic neurotransmitters released from peripheral nerves were once recognized as only noradrenaline (NA) and acetylcholine (Ach). The concept of noncholinergic and nonadrenergic transmissions was introduced after 5’-adenosine triphosphate (ATP) was recognized as a purinergic neurotransmitter [5]. Next, Burnstock designed two main types of purinergic receptors, i.e., P1 and P2, which are based on agonistic and antagonistic functions [6,7]. The affinity for adenosine of P1 was stronger than that of P2 [8,9]; therefore, receptors for adenosine were classified as P1, while ATP and 5´-adenosine diphosphate (ADP) were more suitable as natural ligands for P2 [10]. Based on the latest nomenclature of the International Union of Pharmacology Committee on Receptor Nomenclature and Drug Classification (NC-IUPHAR), the receptor for adenosine is named adenosine receptor (AR), which can be subdivided into four types: A1, A2A, A2B, and A3 [11]. These ARs are activated by endogenous and exogenous adenosine or its analogs [12].
2. Adenosine: Production, Transport, and Metabolism
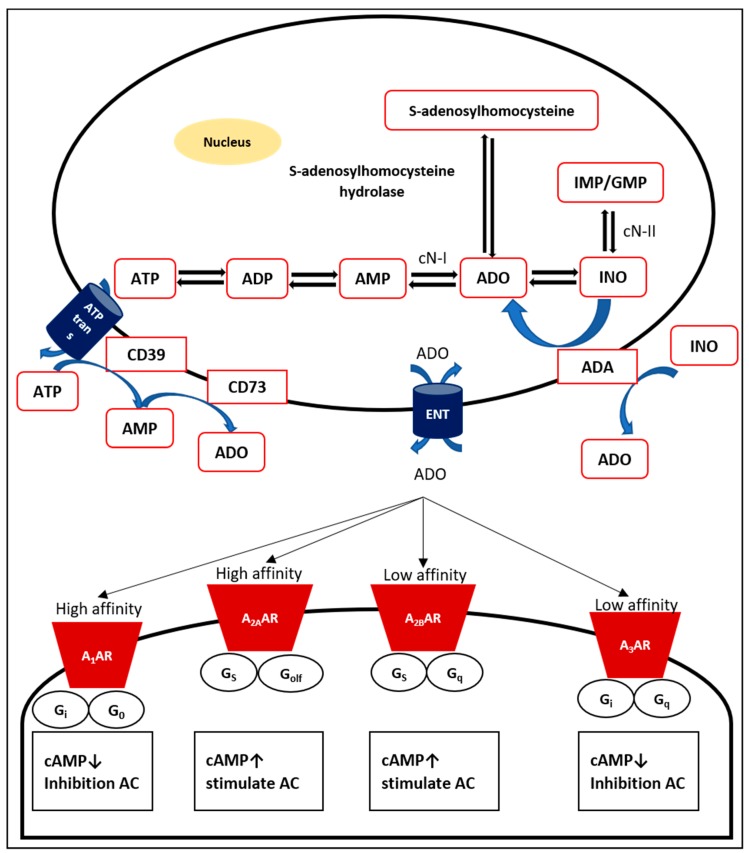
Endogenous adenosine, a natural purine nucleoside consisting of the nucleobase adenine reacted with a sugar ribose by a glycosidic linkage [13], is a normal cellular element and is continuously produced, mainly intracellularly and extracellularly [11]. Adenosine is formed via dephosphorylation of its main source, nucleotide 5´-adenosine monophosphate (AMP), via both cytosolic 5´-nucleotidase (cN)-I and the inosine monophosphate (IMP)/guanosine monophosphate (GMP)-selective cN-II [14,15]. In addition, cN-I catalyzes AMP to adenosine, while cN-II plays a dominant role in the production of inosine and guanosine from IMP and GMP, respectively [16]. Intracellular adenosine is also generated by hydrolysis of S-adenosyl-homocysteine through the enzyme S-adenosyl-L-homocysteinase hydrolase [17]. Adenosine production from AMP is relatively faster than hydrolysis of S-adenosyl-homocysteine [18]. Adenosine may be found throughout endogenous purine synthesis [19]. If there is a mismatch between the production and use of ATP, for instance, in cases of hypoxia and ischemia, adenosine as well as other purine metabolites accumulate [20].
Intracellular adenosine can be released across the plasma membrane via bidirectional, concentrative nucleoside transporters (CNTs; sodium-dependent) and equilibrative nucleoside transporters (ENTs; sodium-independent) [21]. Based upon concentration gradients, ENTs are passive bidirectional transporters that transport adenosine across the plasma membrane, while CNTs are active Na+-dependent transporters [22]. ENTs are responsible for transporting adenosine in and out of the cell and are distributed in mammalian tissues [23].
Under normal conditions, the concentration of adenosine outside the cell is relatively low [24]. Three steps are necessary to produce extracellular nucleosides. First, the main source of extracellular adenosine is particularly generated from intracellular nucleotides, such as ATP, AMP, and ADP, which are released during stress, hypoxia, inflammation, or injury [19]. Intracellular ATP is an essential fuel to drive energy-requiring processes, such as active transport, cell motility, and biosynthesis [25]. The very abundant intracellular nucleotide ATP [26] will be released through exocytosis from vesicles and membrane transport proteins [27]. Potential candidates for particular transporter channels include cystic fibrosis transmembrane conductance regulators, multiple drug resistance channels, connexin hemichannels, maxi-ion channels, stretch-activated channels, and voltage-dependent channels [23].
Moreover, adenosine is produced by inflammatory cells, including mast cells [28], leucocytes [29], neutrophils [30], and eosinophils [31]. In accordance with the concept of retaliatory metabolites, increases in the level of extracellular adenosine regulate anabolic and catabolic hormones during stress [32]. Adenosine promotes the healing process after inflammation-induced injury [33]. Remarkably, extracellular ATP and adenosine are crucial alarms for cell alertness.
Extracellular nucleotides then undergo hydrolysis to remove the phosphate groups. Nucleotide and nucleoside degradation are determined by surface-located enzymes [34]. The ectonucleotidase cluster of differentiation (CD) 39, a lymphoid cell activation antigen bearing a resemblance to ATP diphosphohydrolase (ATPdase), catalyzes the dephosphorylation of ATP and ADP to form AMP [35,36]. Other ectonucleotidases and ecto-ATPases tend to catalyze ATP more than ADP [37]. CD39 is greatly expressed by the endothelium but not in resting T and B lymphocytes, natural killer (NK) cells, neutrophils, macrophages, and monocytes [38,39]. The transformation of ATP to AMP by CD39 can be reversed by the actions of the extracellular diphosphate kinases NDP kinase and adenylate kinase [40].
The final step in the production of extracellular adenosine is the dephosphorylation of AMP. Each purine and pyrimidine nucleoside monophosphate, especially AMP, is hydrolyzed by a glycosyl phosphatidyl inositol (GPI)-anchored enzyme, ecto-5´-nucleotidase [37]. Ecto-5´-nucleotidase is also recognized as CD73, which is found on both T and B lymphocytes [41].
The conversion of AMP to adenosine by CD73 is reversible only following intracellular transport of adenosine, after which it can be changed to AMP by adenosine kinase [40]. Moreover, adenosine can also be metabolized to inosine by adenosine deaminase (ADA) [42]. Overall, adenosine extracellular production depends on the balance between (i) its release from cells, (ii) its reuptake by bidirectional adenosine transport processes, and (iii) its conversion by ectonucleotidases on the cell surface [40].
3. Adenosine Receptors
Adenosine carries out different biological effects determined by each AR on the membrane surface of specific cells or tissues (Figure 1) [43]. Initially, ARs were grouped into A1 and A2 receptors based on their effects in inhibiting and stimulating cyclic AMP (cAMP) in the brain [44]. Currently, the four receptor subtypes (A1, A2A, A2B, and A3 receptors) have been purified and successfully cloned from mammalian and nonmammalian species, particularly rats, mice, and humans [11,43]. Based upon sequence similarity and G protein-coupling specificity, A1 and A3 receptor share 49% sequence identity and preferentially couple to Gαi/o in the inhibition of adenylate cyclase (AC). In contrast, A2A and A2B receptors, which are almost 59% identical and prefer Gαs, are able to stimulate AC [45]. A1 and A2A receptors possess high affinity, while A2B and A3 receptors show relatively lower affinity.
Figure 1.
Production, transport and metabolism of adenosine. Intracellular adenosine is produced via dephosphorylation from the main source, AMP, via both cN I and cN-II and hydrolysis of S-adenosyl-homocysteine through the enzyme S-adenosyl-L-homocysteine hydrolase. The extracellular formation of adenosine is the result of enzymatic cascades consisting of ATP transport, hydrolysis of ATP and ADP by CD39 to form AMP, and dephosphorylation of AMP by CD73. Extracellular adenosine binds adenosine receptors (A1AR, A2AAR, A2BAR, and A3AR) on the surface of cells. Each AR is a GPCR that transmits the signal from adenosine by activating cAMP.
ARs are members of heteromeric guanine nucleotide-binding protein (G protein)-coupled receptor (GPCR) family A [46]. All ARs have a seven-pass transmembrane α-helical structure with an extracellular amino terminus and an intracellular carboxyl terminus, and the N-terminal domain has N-glycosylation sites that influence trafficking of the receptor to the plasma membrane [22]. GPCRs, the largest class of cell surface receptors, are activated by various kinds of ligands, including hormones, neurotransmitters, ions, odorants, and photons of light, and couple to a wide range of signaling molecules and effector systems [47].
Receptors will interact with endogenous adenosine agonists (orthosteric ligands) or with molecules (allosteric ligands) that are located far from the orthosteric site [48]. Allosteric enhancers/modulators bind adenosine receptors at a different site than agonists and stabilize the tertiary complex between the agonist, receptor, and G-protein [49]. Positive allosteric modulators (PAMs) are better therapeutic options than orthosteric agonists because PAMs are tissue-specific, while native adenosine is easily degraded, and agonist adenosine does not readily penetrate the blood–brain barrier [50]. There are several potential ways to overcome adenosine- and AR-related obstacles, including the use of partial agonists, indirect receptor targeting, allosteric enhancers, prodrugs, nonreceptor-mediated effects, neoreceptors, and conditional knockouts [49].
3.1. A1 Adenosine Receptor (A1AR)
A1AR is a glycoprotein containing a single complex carbohydrate chain [51] that was cloned from cows, dogs, rabbits, guinea pigs, and chicks [11]. The clones encode a protein of 326 amino acids with a mass of approximately 36.7 kDa [12]. A1AR binds with Gi-1, Gi-2, Gi-3, and Go but not with Gs or Gz, leading to many cellular responses, such as stimulation of K+ conductance, inhibition of Ca2+ conductance through N-type channels, stimulation of phospholipase C (PLC), and generation of Ca2+, protein kinase C (PKC) [8] and phosphoinositide 3 kinase (PI3K)/mitogen-activated protein kinase (MAPK) [52].
These receptors are distributed predominantly in the central nervous system (CNS), spinal cord, testis, kidney, and adipose tissue, whereas fewer receptors are distributed in the lung and pancreas [11]. They act on many effector or “second messenger” systems; for example, they inhibit catecholamines and histamine-stimulated AC in atrial and ventricular muscle cells of the heart [13].
All synthetic A1AR agonists are divided into specific types, i.e., A1AR agonists with an N6 substitution (N6-cyloalkyl-, N6-aylalkyl-, and N6-heterocyclic-substituted adenosine derivatives); C2 substitution, a variation of the ribose moiety (N-methanocarba analogues); and C1’-methyl and C2’-methyl substitutions, as well as AMP-579 [53].
3.2. A2A Adenosine Receptor (A2AAR)
The majority of A2AARs are distributed in the liver, heart, lung, immune systems (spleen, thymus, leucocytes, and blood platelets), and CNS [54,55]. They are coexpressed with D2 dopamine receptors (D2Rs) in γ-aminobutyric acid (GABA) striatopallidal neurons [56] but not active in striatonigral neurons that express the protachykinin and D1R genes [57]. A2AAR has been cloned from several species, including dogs, rats, humans, guinea pigs, and mice, and shows a high degree of homology among humans, mice, and rats [58]. A2AAR is a larger protein of 410–412 amino acids in length and 45 kDa [12].
A2AAR binding with Gs to activate AC results in the activation of cAMP-dependent protein kinase A (PKA) and PKC, which phosphorylates and activates various receptors, ion channels, phosphodiesterases, and phosphoproteins, such as cAMP-response element binding protein (CREB) and dopamine- and cyclic AMP-regulated phosphoprotein 32 (DARPP-32) [59]. These proteins interact with Golf in the striatum of the basal ganglia because Golf is highly expressed in the caudate-putamen, nucleus accumbens, and olfactory tubercle [60].
Five types of agonist A2AARs exist: ribose-modified adenosine derivatives, purine-modified adenosine derivatives, ribose and purine-modified adenosine derivatives, partial agonists, and agonist radioligands [61]. Inversely, their antagonists are split into xanthine and nonxanthine derivatives (adenine derivatives and related heterocyclic compounds, simplified heterocyclic compounds unrelated to adenine or xanthine, and antagonist radioligands) [62].
3.3. A2B Adenosine Receptor (A2BAR)
A2BAR has been cloned from the rat hypothalamus, human hippocampus, and mouse mast cells and is distributed in the peripheral organs, such as the bowel, bladder, lung, and vas deferens [59]. It interacts with Gs to induce the PKA signaling to increase cAMP and Gq11-mediated activation of PLC to increase the levels of 1,4,5-inositol triphosphate (IP3)/diacylglycerol (DAG), activate PKC, and elevate intracellular Ca2+ levels [63,64].
A2BARs are low-affinity receptors, and their affinity can be increased by interaction with PKC; therefore, the plasticity and versatility of A2BARs make them potential triggers of signaling in multiple signaling cascades in many physiological responses [65].
In contrast with other ARs, A2BARs have an antagonistic effect, which makes them interesting as therapeutic targets. Kalla RV et al. categorized their antagonists as xanthine-based, deazaxanthine-based, adenine-based, 2-aminopyridine-based, bipyrimidine-based, pyrimidone-based, imidazopyridine-based, pyrazine-based, and pyrazolo-triazolo-pyrimidine-based antagonists [64].
3.4. A3 Adenosine Receptor (A3AR)
The A3AR subtype was found after Zhou et al. found several cDNA sequences, one of which (R226) was highly expressed in the testis and less distributed in the lung, kidney, heart, and some parts of the CNS [66]. This confirmed a previous study that identified a cDNA encoding a novel GPCR in the rat brain [67]. A3AR has been cloned from rats, rabbits, sheep, and humans [68]. The structures of A3ARs from these organisms show some sequence homology, with a sequence homology of only 74% in rats versus sheep and humans and 85% between sheep and humans, which indicates significant interspecies differences in ligand recognition [69]. Unlike rats, human A3ARs are widespread, and the most abundant expression is found in the lung and liver [70].
A3AR coupling to Gi proteins inhibits AC and decreases cAMP accumulation and PKA activity, and A3ARs also bind with Gq proteins to stimulate PLC, resulting in increased Ca2+ levels and modulation of PKC activity [71]. In addition, they may activate the PLC pathway through the β, γ subunit [72].
A3AR agonists are classified as adenosine derivatives and xanthine-7-riboside derivatives [73]. In detail, structural manipulations of the A3AR agonist include N6-, C2-, and 5′-substitutions, or suitable combinations of these, followed by modifications involving nonadenine nucleosides and nonnucleosides [71].
4. Adenosine Receptors and Diseases
4.1. A1AR
4.1.1. A1AR in Inflammation
Activation of A1AR will generate both proinflammatory and anti-inflammatory responses [23]. By occupying A1AR, low concentrations of adenosine induce neutrophil chemotaxis and adherence to the endothelium [74] and upregulate endothelial P-selectin expression [75]. Interestingly, a high concentration of adenosine will induce antiadhesive effects via A2AR [76]. A1AR increases NK activity, induces O2− generation from eosinophils, and induces the release of thromboxane A2 and IL-6 from endothelial cells. In addition, it also induces chemotaxis of dendritic cells (DCs), suppresses vesicular major histocompatibility complex (MHC) class I cross-presentation, and increases endothelial permeability [23].
The proinflammatory effects of A1AR in monocytes include enhancing Fcγ receptor-mediated phagocytosis [77], inducing secretion of vascular endothelial growth factor (VEGF) [78], and promoting monocyte differentiation into osteoclasts [79]. Indeed, inflammation induced by an agonist of A2AR, including secretion of IL-18 mediators such as IL-12, interferon (IFN)-γ, and tumor necrosis factor (TNF)-α, was abrogated by an agonist of A1AR [80].
In contrast, A1AR is a potent anti-inflammatory mediator in various kidney, heart, liver, renal, lung, and brain injury models [59]. A1AR protected mice from septic peritonitis by attenuating the hyperacute inflammatory response [81] and prevented the worsening of murine liver ischemia-reperfusion (IR) injury by reducing necrotic and apoptotic cell death [82]. In addition, A1AR protected against kidney injury via AKT activation [83]. The different functions of A1AR during inflammation might be determined by the model of study; in addition, with deletion of A1AR, other ARs might provide protection [23].
4.1.2. A1AR in the Respiratory Systems
Adenosine regulates both proinflammatory responses and protection against lung injury through A1AR [14]. The distribution of A1AR in airway organs was low; however, its expression was found in smooth muscle tissues of asthmatic patients [84]. In fact, the expression of A1AR was upregulated in the airways of both animal models of allergic airway inflammation and human patients with asthma [85]. Specifically, A1AR induced bronchoconstriction and mucus production, evoking proinflammatory functions of monocytes and neutrophils and inducing vascular permeability [86], and might influence the severity of pulmonary inflammation and remodeling in chronic lung diseases [14].
Numerous studies have implicated a role for A1AR in respiratory diseases (Table 1). A novel A1AR antagonist, L-97-1, blocks allergic responses to house dust mites in an allergic rabbit model of asthma [87]. Regardless of the discontinuation of its study, EPI-2010, an antisense oligonucleotide targeting the A1AR promoter region, was safe and well tolerated in patients with mild asthma [88].
Table 1.
Adenosine receptor drugs in respiratory diseases.
| Receptors | Diseases | Drugs | |
|---|---|---|---|
| Selective Full Agonist | Antagonist/Partial Agonist | ||
| A1AR | COPD and asthma | L-97-1 [87] EPI-2010 [88] |
|
| Lung injury | CCPA [91] | ||
| A2AAR | COPD and asthma | CGS-21680 [109] UK371,104 (DC) [110] GW328267X (DC) [111] Regadenoson [112] Apadenoson [59] |
|
| Lung injury | CGS-21680 [113] PTX [114] |
||
| A2BAR | COPD and asthma | BAY 60-6853 [115] | CVT-6883 [116] CGS15493, WO-00125210, and ATL-907 [86] |
| Lung injury | BAY 60-6583 [117] | ||
| Fibrosis and interstitial lung diseases | GS-6201 [118] | CVT-6883 [119] QAF 805 [85] |
|
| A3AR | COPD and asthma | QAF 805 [85] | |
| Lung injury | IB-MECA [120] | ||
DC = discontinued.
In contrast, A1AR also mediated anti-inflammatory effects via macrophages in ADA-deficient mice [89] and reduced polymorphonuclear (PMN) infiltration by inhibiting the release of chemotactic cytokines and decreasing microvascular permeability in lipopolysaccharide-induced lung injury [90]. Recently, CCPA, an A1AR agonist, decreased inflammation, edema, and neutrophil chemotaxis [91]. In addition, A1AR promoted the recruitment of leukocytes to the infected lung and attenuated lung injury [92].
4.1.3. A1AR in the Cardiovascular Systems
A1AR protects the heart by downgrading inotropic effects. It binds with Gi to inhibit catecholamine-stimulated AC [93]; limit the action of β1 receptors stimulated by Gs cycling [94] via the involvement of PLC, PKC-ε, and p38 MAPK; and exclude heat shock proteins (HSP27) [95]. In addition, A1AR also inhibited norepinephrine release in the rat heart [96], providing protection in the postischemic setting [97].
Similarly, A1AR causes coronary contraction through PLC pathways [98] and increases soluble epoxide hydrolase (sEH) and cytochrome P450 A (CYP4A) [99] to protect the heart. In addition, A1AR activated the release of VEGF from monocytes [78] via the involvement of the extracellular signal-regulated kinase (ERK), c-Jun N-terminal kinase (JNK), and PI3K/AKT pathways [100]. A1AR also regulates myocardial substrate metabolism by decreasing plasma concentrations of insulin, glucose, and lactate [101], and inhibiting lipolysis [102]. Via cooperative interaction with both A2AAR and A2BAR, A1AR inhibits the necrosis cardiac cell ischemia model [103] through phosphorylation of ERK1/2 [104].
It is assumed that all ARs are involved in cardio hypertrophy and neovascularization [105]. A selective agonist of A1AR, CPA, prevented cardiac hypertrophy and heart failure (HF) in the left ventricular pressure-overload model [106]. An A1AR antagonist, BG9928, was well tolerated and significantly increased sodium excretion in patients with stable HF [107]. Conversely, rolofylline failed to protect renal function in acute heart failure (AHF) patients with volume overload and renal dysfunction [108].
Capadenoson, a new oral A1AR agonist, decreased the exercising heart rate at a comparable maximum workload in male patients with stable angina [121]. Moreover, this agonist also stimulated A2BAR, promoting cardioprotection and modulating cardiac fibrosis in heart disease [122]. Unfortunately, the clinical trial of capadenoson was discontinued [123]. A clinical trial using the partial agonist neladenoson (BAY 1067197) for the treatment of heart failure was safe without atrioventricular conduction disorders or neurological adverse effects [124]. A multiple-dose study of neladenoson in heart failure is still ongoing [123].
Taken together, A1AR activation ahead of ischemia may augment chemotaxis and neutrophil-dependent injury in cardioprotection; however, the effects of A1AR agonists during reperfusion and within both ischemic preconditioning and postconditioning settings remain controversial [125,126].
A1AR mediates negative chronotropy and dromotropy through inactivation of the inwardly rectifying K+ current (IK,Ado or IK,Ach), inhibition of the inward Ca2+ current (ICa), and activation of nitric oxide synthase (NOS) [105]. Furthermore, A1AR displays both antiarrhythmic and proarrhythmic effects [105]. High expression of A1AR results in sinus and AV node dysfunction and supraventricular arrhythmias [127].
At present, several full A1AR agonists have been used in clinical trials as antiarrhythmic agents. An optimal dose of a new A1AR selective agonist, tecadenoson (CVT-510), was successful in the conversion of paroxysmal supraventricular tachycardia (PSVT) into sinus rhythm [128], but its development for PSVT and atrial fibrillation (AF) was discontinued in 2009 [123]. Other agonists, such as selodenoson and PJ-875, have started in early phase evaluations for the treatment of AF [129]. Currently, to avoid the minor global effects of full agonists, including adverse reactions, desensitization, and arrhythmia (bradyarrhythmia or AF), partial A1AR agonists, such as CVT-2759, have been developed [130].
4.1.4. A1AR in CNS
A1AR contributes to neuroprotection and is also involved in neurodegeneration. In fact, activation of A1AR under hypoxia leads to inhibition of presynaptic Ca2+ influx-related release of transmitters [131] such as dopamine, acetylcholine, GABA, and, especially, glutamate, to generate neuroprotection [132]. Moreover, A1AR also regulates potassium current, leading to hyperpolarization of the resting membrane potential mediated via G protein-dependent activation of inwardly rectifying K+ channels (GIRKs), activating PLC, and inhibiting AC [133].
Conversely, the levels of glutamate and N-methyl-d-aspartate (NMDA), which are responsible for neuronal damage, may increase during hypoxia and ischemia [134]. It was assumed that prolonged activation of A1AR increased A2AAR expression, which generated global damage and neurodegeneration in ischemic stroke [135]. Recently, an A1AR selective agonist, NNC-21-0136, was designed for neuroprotection in stroke models [123].
By binding with A1AR, adenosine reduced neuronal activity and pain in the spinal cord and periphery by inhibiting the cAMP, PKA, and Ca2+ channels, activating K+ currents, and interacting with the PLC, IP3, DAG, and β-arrestin pathways [136]. In preclinical studies, it seems that A1AR was dominant relative to A2AAR in inhibiting nociceptive input in the dorsal spinal cord [137]. The selective A1AR agonist GR79236X significantly relieves dental pain [138].
Moreover, Cl-ENBA, a potent and highly selective A1AR agonist, amplified nociceptive thresholds in spinal glial, and microglial changes occurred in neuropathic pain [139]. MRS7469 also plays a role in pain relief or inhibition of lipolysis [140]. Further development of other agonists, such as SDZ WAG 994, GR79236, and GW-493838, as well as the allosteric enhancer T62, was discontinued [141].
The activation of A1AR is crucial for keeping epileptic foci localized [142]; therefore, A1AR may be involved in the convulsive effect of diazepam, phenobarbital, or valproate in experimental seizure models [143]. Indeed, upregulation of A1AR suppressed seizure activity, which spread within the temporal lobe in rats [144], and could powerfully and bidirectionally regulate seizure activity [145]. An antagonist, DPCPX, inhibited the ketone diet-induced seizure effect [146].
The inhibition of A1AR during pathophysiological conditions (noxious stimulation and oxygen deficiency) causes mice to be more anxious [147] and aggressive [148]. In addition, an agonist of A1AR reduced the anxiogenic effects during ethanol withdrawal [149]. In overcoming the limitations of A1AR agonists, positive allosteric modulators, such as TRR469, offer a more physiological way to treat anxiety [150]. In addition, MRS5474, a potent selective A1AR agonist, has been developed as an antidepressant drug that is without cardiovascular side effects [151].
Relating to sleep and level of arousal, A1AR was shown to control the response of the circadian clock to light [152] and regulate homeostatic sleep after prolonged wakefulness [153] via inhibition of the ascending cholinergic neurons of the basal forebrain [154]. CPA inhibited the histaminergic system and promoted non-rapid eye movement (NREM) sleep [155].
In line with the fact that synaptic plasticity is the basis for learning and memory in different brain areas [156], A1AR attenuated long-term potentiation [157] and long-term depression and depotentiation in the hippocampus [158]. The mixed dual A1AR and A2AAR antagonist ASP5854 ameliorated motor impairments through neuroprotection (A2AAR antagonism) and enhanced cognitive function (A1AR antagonism) [159].
Via both A1AR and A2AAR, adenosine influences the two neurotransmitter systems most affected by schizophrenia—the glutamatergic and dopaminergic neurotransmission systems [160]. While A1AR inhibits D1R for dopamine, A2AAR reduces D2R recognition, coupling, and signaling [161]. CPA regulates prepulse inhibition (PPI), whereas PIA/L-PIA and 2-CLA/CHA are involved in memory functions and hyperlocomotion, respectively [162].
A1AR decreases tremor and controls the spread of excitability, thereby reducing the side effects of deep brain stimulation in Parkinson’s disease (PD) [163]. However, there is little information about the clinical study of A1AR in PD.
Drugs related to A1AR prevented the development of hindlimb dystonia in Huntington’s disease (HD). Several agonists, such as CPA, CCPA, CHA, and ADAC, have been developed; however, there are physiological limitations (capacities to cross the blood–brain barrier and adverse effects) to the use of A1AR-targeted drugs [162].
4.1.5. A1AR in Metabolic and other Diseases
As mentioned earlier, A1AR agonists have potential use as antilipolytic agents. Indeed, inhibition of A1AR was associated with the enhancement of glucose-stimulated insulin, reduction of oxidative stress, and inhibition of the breakdown of triglycerides to free fatty acids (FFAs) [164].
The full A1AR agonists GR79236 and ARA are under development for the treatment of type 2 diabetes mellitus (T2DM), and their mechanisms of action includes reducing nonesterified fatty acids (NEFAs) and triglycerides (TGs) [165]. Unfortunately, the cardiovascular effects of full agonists raise a new problem; therefore, partial agonists that are capable of eliciting a greater effect in adipocytes than in the heart have been developed [166]. The A1AR partial agonist GS-9667/CVT-3619 was well tolerated in patients with T2DM and dyslipidemia, in whom it reduced plasma FFAs [167].
In addition, tecadenoson and BW-1433, an A1AR antagonist and A2BAR agonist, respectively, may lower FFA levels and improve glucose tolerance [168]. While A1AR selective agonists, such as SDZ WAG994 and RPR749, are under development for antilipolytic and hyperlipidemia, the development of other A1AR agonists, including trabodenoson and metrifudil 2 for glaucoma and glomerulonephritis, respectively, was discontinued for safety reasons [123].
4.2. A2AAR
4.2.1. A2AAR in Inflammation
A2AARs function as the most dominant anti-inflammatory effectors of extracellular adenosine through their expression on monocytes/macrophages, dendritic cells, mast cells, neutrophils, endothelial cells, eosinophils, epithelial cells, lymphocytes, NK cells, and NKT cells [169].
Activation of A2AARs inhibits neutrophil adhesion to endothelial cells, formation of reactive oxygen species, and adherence of N-formyl methionyl-leucyl-phenylalanine (fMLP)-activated neutrophils to the endothelium and downregulates endothelial cell surface proteins, including Mac-1, β2-integrin, L-selectin, vascular cell adhesion molecule-1 (VCAM-1), intracellular adhesion molecule-1 (ICAM-1), alpha 4/beta 1 integrin VLA4, and platelet cell adhesion molecule. Additionally, it decreases TNF-α, macrophage inflammatory protein (MIP)-1α/CCL3, MIP-1β/CCL4, MIP-2α/CXCL2, MIP-3α/CCL20, leukotriene LTB4, and platelet-activating factor (PAF) [23].
During innate immunity, activation of A2AAR on adaptive immune cells shifts Th1 to Th2 responses by suppressing IL-12 and increasing IL-10 [170]. In parallel, secretion of TNF-α, IL-6, and IL-8 is also diminished [171]. Interestingly, the decreases in IL-10 and IL-6 under A2AR blockade and in knockout (KO) mice resulted in an increase in survival from polymicrobial sepsis [172]. In lymphocytes, A2AR also inhibited the production of IFN-γ, IL-4, and IL-2 [173].
Furthermore, the prodrug 2-(cyclohexylethylthio)adenosine 5’-monophosphate (chet-AMP) delivered potent immunosuppression with negligible vasodilatory activity in experimental rheumatoid arthritis (RA) models [174]. In addition, a deoxyadenosine derivative, polydeoxyribonucleotide (PDRN), acted on A2AAR in relieving pain, improving the clinical signs of arthritis, and reducing histological damage in an arthritis model [175].
In contrast, other agonists resulted in poor outcomes. Regadenoson 21 (CVT-3146) for sickle cell disease was not able to produce a statistically significant reduction or clinical efficacy [176]. Clinical trials of sonedenoson (MRE-0094) and spongosine (BVT.115959) for diabetic foot ulcers and diabetic neuropathic pain, respectively, were discontinued [123].
4.2.2. A2AAR in the Respiratory System
The expression of A2AAR in the lung is distributed widely, including expression on resident macrophages, bronchial epithelial cells, mast cells, eosinophils, neutrophils, and lymphocytes [169]. Activation of A2AAR by adenosine binding affects multiple aspects of the inflammatory process, modulating neutrophil activation and degranulation, oxidative species production, adhesion molecule expression, cytokine release, and mast cell degranulation; therefore, selective A2AAR agonists have an important role in airway inflammation and neutrophil–monocyte-mediated lung tissue injury [14].
In asthmatic airways, A2AAR suppresses inflammation by reducing neutrophil adherence to the endothelium and inhibiting the fMLP-induced oxidative burst, superoxide anion generation, and LPS-induced TNF-α expression [85]. Moreover, it also inhibits histamine and tryptase release but stimulates wound healing and VEGF secretion [86]. Adversely, angiogenesis, as an important characteristic of airway remodeling in human asthma, may limit the development of such anti-asthma drugs [85].
A2AAR agonists in animal models of asthma and chronic obstructive pulmonary diseases (COPD) have resulted in good outcomes, yet subsequent clinical trials in humans have not been successful. CGS-21680 has anti-inflammatory activity in a model of allergic asthma in the Brown Norway rat [109], and UK371,104 inhibited capsaicin-induced bronchoconstriction [110]. However, an inhaled A2AAR agonist, GW328267X, in patients with nonsmoking asthma was unsuccessful in protecting against the allergen-induced early and late asthmatic reactions [111]; hence, its study and the study of UK371,104 were discontinued due to limited efficacy [177].
Recently, regadenoson has been demonstrated to be safe to use in patients with mild to moderate COPD and asthma [112]. Another agonist, apadenoson, is still in a phase I trial for asthma and COPD [59].
The selective agonist CGS-21680 also protected the lung against shock-induced injury [113]. In addition, pentoxifylline showed anti-inflammatory effects in LPS-induced lung injury through an A2AAR-dependent pathway [114].
4.2.3. A2AAR in the Cardiovascular Systems
In contrast to A1AR, activation of A2AAR prior to ischemia is not effective. Administration during or prior to reperfusion minimizes myocardial ischemia-reperfusion injury; hence, A2AAR works in ischemic postconditioning [126]. As an anti-inflammatory molecule, A2AAR protects the heart through inhibition of leukocyte-dependent inflammatory processes, angiogenesis, enhanced coronary vasodilatation during reperfusion, modified myocardial contraction [178], and increased inotropy through transient Ca2+ augmentation via a cAMP/PKA-dependent mechanism [179].
The selective A2aAR agonists CGS 21680C and ATL-193/ATL-146e protected tissue during ischemia-reperfusion injury by reducing myocardial infarct size without elevating coronary blood flow [180,181]. However, A2AAR not only protects against ischemia but also increases the occurrence of cardiac arrhythmias [182]. In fact, A2aAR indirectly alters contractility by modulating the A1AR antiadrenergic effect rather than increasing cardiac contractility directly [183]. A new study confirmed that the A2aAR agonist LASSBio-294 might be an alternative treatment for heart failure due to ischemia or hypertension [184].
Adenosine controls coronary blood flow regulation through A2aAR in both coronary smooth muscles and coronary endothelial cells [185] in a mechanisms that involves several second messengers and effectors, including p38-MAPK, IP3, NO, and K+ channels [105]. These findings led to the development of various A2aAR agonists for both diagnostic (myocardial perfusion imaging (MPI)) and therapeutic interventions. Regadenoson (CVT-3146), binodenoson (MRE-0470/WRC-0470), evodenoson (ATL-313, DE-112), sonedenoson (MRE-0094), and apadenoson (ATL-146e) have been approved by the FDA for use in MPI [123,185].
Along with being cardioprotective via inhibition of the inflammatory response, A2AAR agonists (PSB-15826, PSB-12404, and PSB-16301) also modulate platelet aggregation [186], and ATL313 regulates cholesterol homeostasis [187]. Perhaps the pharmaceutical industry will focus on the anti-inflammatory effects of A2AAR agonists against excess cholesterol accumulation to develop new cardiovascular therapies [188]. A2A receptor agonists are the focus of efforts by the pharmaceutical industry to develop new cardiovascular therapies, and pharmacological actions of the atheroprotective and anti-inflammatory drug methotrexate are mediated via release of adenosine and activation of the A2A receptor.
A list of AR ligands currently undergoing clinical trials as novel therapeutic treatments and their effects on cardiovascular and metabolic diseases is presented in Table 2.
Table 2.
Adenosine receptor drugs in cardiovascular and metabolic diseases.
| Receptors | Functions | Diseases | Drugs | ||
|---|---|---|---|---|---|
| Selective Full Agonist | Antagonist/Partial Agonist | ||||
| A1AR | Inotropic and adrenergic control | Negative | Heart failure | CPA [106] | BG9928 [107] Rolofylline [108] Capadenoson [122] Neladenoson [124] |
| Dromotropic and chronotropic control | Negative | Arrhythmia | Tecadenoson (DC)[128] Selodenoson [129] PJ-875 [129] |
CVT-2759 [130] | |
| Vascular control | Constriction | Hypertension | |||
| Ischemia | Protection | Angina | Capadenoson [121] | ||
| Adaptation | Hypertrophy | CPA [106] | |||
| Angiogenesis | |||||
| Diabetes mellitus and hyperlipidemia | GR79236 and ARA [165] SDZ WAG994 and RPR749 [123] Tecadenoson [168] |
GS-9667/CVT-3619 [167] BW-1433 [168] |
|||
| Glaucoma | Trabodenoson (DC) [123] | ||||
| Glomerulonephritis | Metrifudil 2 (DC) [123] | ||||
| A2AAR | Inotropic and adrenergic control | Positive | Heart failure | LASSBio-294 [184] | |
| Vascular control | Dilatation | Imaging | Regadenoson, binodenoson, evodenoson, sonedenoson, and apadenoson [123,185] | ||
| Anti-inflammation | Platelet aggregation | PSB-15826, PSB-12404, and PSB-16301 [186] | |||
| Cholesterol homeostasis | ATL313 [187] | ||||
| Ischemia | Protection | Angina | CGS 21680C [180] ATL-193/ATL-146e [181] |
||
| Adaptation | Hypertrophy | ||||
| Angiogenesis | |||||
| A2BAR | Vascular control | Dilatation | |||
| Anti-inflammation | Atherosclerosis and hyperlipidemia | BAY 60-6853 [189] | |||
| Ischemia | Protection | Angina | BAY 60-6583 [190] | ||
| Adaptation | Hypertrophy | ||||
| Angiogenesis | GS-6201 [191] | ||||
| Diabetes mellitus and hyperlipidemia | NECA [192] ATL-801 [193] MRS-1754 [194] |
||||
| A3AR | Vascular control | Constriction | |||
| Anti-inflammation | Diabetic kidney diseases | LJ-2698 [195] | |||
| Atherosclerosis and hyperlipidemia | LJ-1888 [196] | ||||
| Ischemia | Protection | Angina | IB-MECA [197] CI-IB-MECA [198] CP-532,903 [199] |
||
| Adaptation | Hypertrophy | ||||
| Angiogenesis | |||||
4.2.4. A2AAR in the CNS
According to physiological factors, neuromodulation of adenosine through activation of high-affinity A2AAR is important [163]. Indeed, A2AAR was associated with neurodegeneration due to its excitatory effects [135]. Presynaptic A2AAR counteracts the inhibitory effect of presynaptic A1AR on glutamate release from axon terminals, inducing glutamate release that would predispose to excitotoxic injury, yet A2AAR may also exert a neuroprotective effect by promoting vasodilation and preserving cerebral blood flow autoregulation [143]. Recently, SCH58261, an A2AAR antagonist, has proven efficacious in halting injury by inhibiting pERK1/2 in hippocampal neurons [200].
Similarly, A2AAR and A1AR of hippocampal CA1 have the opposite effect in spreading piriform cortex kindled seizures, in which A2AAR tends to have a convulsive effect [201]. In fact, the A2AAR antagonist ZM241385 had a potent anticonvulsant profile with few adverse effects [202] and suppressed subsequent recovery sleep [203]. Concerning sleep and arousal, the A2AAR agonist CGS21680 promoted both rapid eye movement (REM) and NREM sleep [133].
Activation of A2AAR increased the release of the potent anti-inflammatory cytokine IL-10, leading to suppression of pain [61]. CGS21680 produced a long-duration reversal of mechanical allodynia and thermal hyperalgesia [155]. Another agonist, spongosine (BVT.115959, CBT-1008), was effective in a clinical trial for diabetic neuropathic pain, but the production was discontinued [204].
A2AAR KO mice show increased anxiety [57], and there was an association between a polymorphism in the A2AAR gene and panic disorder [205]. In contrast, A2AAR KO mice were less sensitive to “depressant” challenges [206], which might be linked to interactions with dopaminergic transmission in the frontal cortex [207]. Recently, istradefylline became the first therapeutic agent for depression and anxiety [208].
Apart from D2R, recent studies also confirmed the involvement of the interaction between A2AAR/D3 and A2AAR/mGlu5 in schizophrenia [209]. Therefore, multiple adenosinergic targets, including A1R and A2AR, have potential as therapeutic targets for schizophrenia [210].
HD is characterized by a decrease in A2AR due to neurodegeneration of the GABA/enkephalin striatopallidal neurons [134]. Interestingly, both A2AR agonists and antagonists have shown beneficial effects [211]; thus, the potential exploitation of A2AR ligands in HD is still contentious, reflecting the complexity of A2AR regulation in this disease [132]. As in HD, there was a reduction in A2AR in regions of high density, such as the striatum, in Alzheimer’s disease (AD) [132]. Recently, the selective A2AR antagonist MSX-3 prevented the development of memory deficits in an AD mouse model without altering hippocampal and cortical gene expression [212].
A key finding in PD is the colocalization and reciprocal antagonistic interactions between A2AR and D2R [134]; hence, A2AR antagonists may not only relieve motor deficits in PD but also potentially prevent the degeneration of dopaminergic mesencephalic neurons [213]. Some PD drugs are still under development, including tozadenant/SYN115, DT1133, ZM241385, ST1535, and istradefylline; however, other drugs (preladenant, ASP5854, and vipadenant) were discontinued [61].
A2AR interacts antagonistically with D2R to counteract drug addiction-induced behavioral effects [214]. The high density of presynaptic and postsynaptic A2ARs that regulate glutamatergic transmission in the brain leads to the possibility of A2ARs as new therapeutic agents for drug addiction [163]. The adenosine agonists NECA and CGS 21680 inhibit cocaine-seeking behaviors [215].
The effects of drugs targeting A2AAR and other ARs that are currently undergoing clinical trials as novel therapeutic treatments in CNS diseases on the CNS system are listed in Table 3.
Table 3.
Adenosine receptor drugs in CNS diseases.
| Receptors | Diseases | Drugs | ||
|---|---|---|---|---|
| Selective Full Agonist | Antagonist/Partial Agonist | Allosteric Modulators | ||
| A1AR | Stroke | NNC-21-0136 [123] | ||
| Sleep | CPA [155] | |||
| Anxiety and depression | MRS5474 [151] | TRR469 [150] | ||
| Cognition and memory | ASP5854 [159] | |||
| Alzheimer’s disease | ||||
| Huntington’s diseases | CPA, CCPA, CHA and ADAC [162] | |||
| Schizophrenia | 2-CLA. NECA, CHA, CPA, PIA, L-PIA [210] | CPT, 8-CPT2, DPCPX, and MSX-3 [210] | ||
| Pain | GR79236X [138] Cl-ENBA [139] MRS7469 [140] SDZ WAG 994, GR79236, GW- 493838 (DC) [141] |
T62 (DC) [141] | ||
| Epilepsy | DPCPX [146] | |||
| A2AAR | Stroke | SCH58261 [200] | ||
| Sleep | CGS21680 [133] | |||
| Anxiety and depression | Istradefylline [208] | |||
| Cognition and memory | ASP5854 [159] | |||
| Alzheimer’s disease | MSX-3 [212] | |||
| Huntington’s diseases | ZM241385 [220] | |||
| Parkinson’s diseases | Tozadenant/SYN115, DT1133, ZM241385, ST1535, and istradefylline [61] Preladenant, ASP5854, vipadenant (DC) [61] SCH900800, and BIIB014 [221] KW-6002 [134] |
|||
| Schizophrenia | APEC, CGS21680, NECA, CV-1808, and DPMA [210] | MSX-3, DMPX, SCH58261, and ZM241385 [210] | ||
| Pain | CGS21680 [155] Spongosine (DC) [204] |
|||
| Epilepsy | ZM241385 [202] | |||
| Drug addiction | CGS21680 and NECA [215] | |||
| A3AR | Stroke | IB-MECA [222] CI-IB-MECA [223] |
LJ-1251 [72] | |
| Epilepsy | ANR235 [224] | |||
4.3. A2BAR
4.3.1. A2BAR in Inflammation
A2BAR is expressed on most inflammatory cells and has both proinflammatory and anti-inflammatory effects [177]. It generates anti-inflammatory effects by coupling with protein Gs and proinflammatory effects by coupling with protein Gq [216]. Due to its low affinity, A2BAR might require high concentrations of adenosine, for instance, during pathological conditions, to be significantly activated [217].
The proinflammatory effects of A2BAR included the induced secretion of IL-6 from macrophages and IL-1β, IL-13, IL-3, IL-8, IL-4, and VEGF from mast cells [218]. Moreover, A2BAR induced the production of IL-19 and TNF-α from bronchial epithelial cells [219].
A2BAR mediates anti-inflammatory effects in neutrophils by inhibiting adhesion to endothelial cells [23], preventing the production of TNF-α and IL-1β, as well as macrophage proliferation, and stimulating IL-10 secretion from macrophages [216]. Interestingly, A2BAR has proinflammatory and anti-inflammatory actions in mouse bone marrow-derived mast cells (BMMCs) [225].
Adenosine regulates bone metabolism and wound healing. Deletion of A2BAR inhibits periosteal development with subsequent consequences on endochondral ossification and growth plate regulation [226]. In fact, it modulated osteoblast differentiation [227]. Moreover, these receptors also regulate myocardial repair and remodeling by delaying the deactivation of myofibroblasts [228].
Receptor inhibition or knockout of A2BAR in mice suppressed intestinal inflammation and attenuated disease in murine colitis/inflammatory bowel diseases [63]. A2BAR in epithelial cells appeared to attenuate colonic inflammation through a specific barrier repair response, namely, phosphorylation of vasodilator-stimulated phosphoprotein [136]. Instead, the deletion of A2BAR or inhibition by PSB1115 exacerbated the acute inflammatory phase of dextran sodium sulfate colitis [229].
Consistent with their anti-inflammatory effects, CVT-6883 and MRS-1754 diminished the clinical symptoms of experimental autoimmune encephalitis and protected the CNS from immune damage [230]. Administration of the antagonist BAY 60-6583 for four weeks in a high-fat diet mouse model restored endocrine function and reduced inflammation [123].
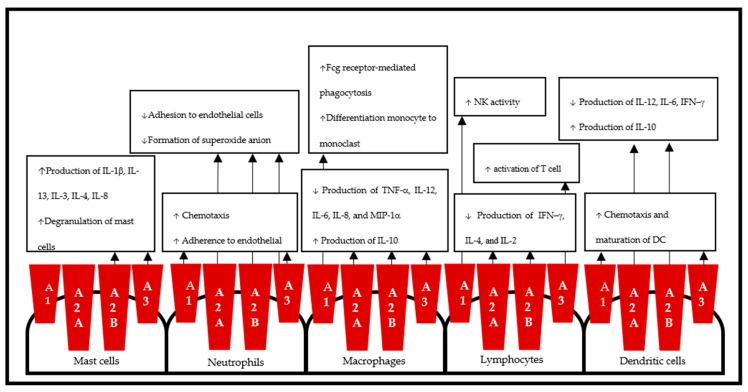
Taken together, the use of A2BAR agonists or antagonists for the treatment of the associated disease states is still controversial, and another important variable for agonists, i.e., signaling bias, needs to be considered [231]. Figure 2 illustrates the effects of AR on immune cells.
Figure 2.
Schematic representation of adenosine receptors in immune cells. Adenosine receptors carry out the proinflammatory and anti-inflammatory effects of adenosine through predominant immune cells: macrophages, neutrophils, mast cells, lymphocytes, and dendritic cells.
4.3.2. A2BAR in the Respiratory System
A2BAR binds with both Gs and Gq proteins to mediate airway reactivity, inflammation, and remodeling in asthma [85]. Activation of A2BAR in mast cells induced the release of inflammatory mediators, leading to bronchoconstriction [232]; therefore, the use of antagonist of A2BAR is based on the cellular effects downstream of A2BAR mast cells [86].
A selective A2BAR antagonist, CVT-6883, inhibited airway reactivity and inflammation in a mouse model of asthma [116]. Other compounds, including CGS15493, WO-00125210, and ATL-907, were under development in phase I and phase II trials for the treatment of asthma [86].
The role of mast cells in COPD is controversial, but there is a significant inverse correlation between the binding parameters of A2BAR and the FEV1/FVC ratio [233]. Indeed, the combination of the selective A2BAR agonist/antagonist BAY 60-6583 and dexamethasone may have clinical applications in COPD by inducing genes with anti-inflammatory activity [115]. Furthermore, some studies confirmed that adenosine interacting with A2BAR served an essential role in the pathogenesis of COPD and pulmonary fibrosis [234] by stimulating the secretion of IL-6 and the differentiation of pulmonary fibroblasts into myofibroblasts [235].
Administration of the A2BR antagonist GS-6201 or deletion of A2BR attenuated vascular remodeling and hypertension associated with interstitial lung disease [118]. Additionally, an A2BR antagonist neutralized the increase in metalloproteases and inhibitors of proteases, tissue inhibitor of metalloproteinase-1 (TIMP-1), matrix metalloproteinase (MMP)-9, and MMP-12 [119].
A2BAR is also a potential target in acute lung injury (ALI). In a ventilator-induced lung injury (VILI) mouse model, the deletion of the A2BAR gene was associated with reduced survival time and increased pulmonary albumin leakage [117]. Recently, aerosolized BAY 60-6583 was shown to attenuate pulmonary edema, improve histologic lung injury, and diminish lung inflammation in ALI [236].
4.3.3. A2BAR in Cardiovascular Systems
There is no “direct” evidence of the effect of A2BAR on ventricular myocytes [178]; however, A2BAR was shown to be required for A1AR-mediated cardioprotection [237]. In fact, adenosine levels during myocardial ischemia-reperfusion increase; therefore, low-affinity A2BAR still has cardioprotective effects upon its activation [238].
The results of studies on ischemia are conflicting. A2BAR plays an important role in ischemia preconditioning (IPC) [63]. Indeed, A2BAR attenuated myocardial infarction via hypoxia-inducible factor (HIF)-1α and the circadian rhythm protein Per2 [239]. In contrast, a previous study reported that A2BAR was not involved in the early phase of IPC but might contribute to the later stages by inducing stress-responsive genes [240]. Recently, GS-6201 was shown to attenuate cardiac remodeling by reducing caspase-1 activity after acute myocardial infarction [191], and BAY 60-6583 protected the heart against myocardial IR injury by modulating macrophage phenotype switching via the PI3K/AKT pathway [190].
A2BAR may inhibit smooth muscle cell-associated hypertension [241]. In fact, KO mice have normal blood pressure [242]. Recently, the deletion of A2BAR was shown to protect against salt-induced hypertension and stroke [243] and inhibit increases in mean arterial blood pressure [244].
A2BAR may contribute to the pathogenesis of atherosclerosis. During hypoxia, A2BAR promoted HIF-1α-induced differentiation of macrophages and foam cells (FC) in preventing atherosclerosis plaque formation [245]. Administration of BAY 60-6853 altered the lipid profile and decreased atherosclerosis in mice [189].
4.3.4. A2BAR in Metabolic Diseases
It is known that A2BAR has roles in glucose homeostasis and lipid metabolism, insulin secretion and resistance, inflammation, β-cell survival, and kidney protection [246]. The nonselective A2BAR agonist NECA, via immunomodulatory effects, inhibited diabetes and protected the pancreas [192]. Moreover, the antagonist ATL-801 increased insulin action in the liver and glucose uptake in skeletal muscle and brown adipose tissue [193].
In diabetic nephropathy, MRS-1754 inhibited high glucose via VEGF [194] and improved kidney tissue nitrite levels [247]. Tak et al. asserted that endothelial A2BAR signaling was implicated in protection from diabetic nephropathy [248].
4.3.5. A2BAR in Cancer
A2BAR is highly expressed in tumor cells and promotes tumor cell proliferation; hence, it might serve as a target for novel therapies or combination therapies for cancer [249]. A2BAR promoted tumor-inducing M2 macrophages (tumor-associated macrophages); however, it had less effect on decreasing cancer cell proliferation and metastasis and inducing proapoptotic effects [250].
The A2BAR antagonist ATL801 repressed the growth of bladder and breast tumors and reduced the metastasis of breast cancer cells [251]. Another antagonist, PSB1115, inhibited the accumulation of tumor-infiltrating myeloid-derived suppressor cells (MDSCs) and restored an efficient antitumor T cell response in a mouse model melanoma [252]. In contrast, A2BAR promoted tumor growth through VEGF and IL-8 [253,254].
Taken together, these results shown that the roles of A2BAR in cancer are complex. Gessi et al. showed that A2BAR releases angiogenic factors promoting tumor growth; however, it may exert an inhibitory signal on tumor cell proliferation [255].
4.4. A3AR
4.4.1. A3AR in Inflammation
The dual effect of A3AR in the inflammation process involves many cells that can have overlapping and opposing function [23]. Its presence in almost all inflammatory cells suggests their involvement in a number of inflammatory pathologies, spanning from wound healing and remodeling to lung injury, inflammatory bone loss, autoimmunity, and eye diseases [69].
A3AR produced a proinflammatory effect by inducing the release of histamines and other allergic mediators [256], preventing eosinophil chemotaxis [257], and inhibiting apoptosis [258]. A3AR also increased rapid inflammatory cell influx by attracting eosinophils and macrophages in the lung [259] and promoting maturation of DC and Ca2+ signaling [260].
On the other hand, A3AR inhibited LPS-stimulated release of TNF-α and nitric oxide (NO) via the NF-κβ, ERK1/2 and PI3K/AKT pathways [261,262]. Interestingly, both of the resulting modulators were found to increase neutrophil chemotaxis [263] and, in contrast, mediate inhibition of oxidative burst in human neutrophil and promyelocytic HL60 cells [264]. In addition, A3AR modulates lymphocyte T cell activation [265].
A3AR agonists suppressed the production of MIP-1α, collagen-induced arthritis [266], and TNF-α [267]. IB-MECA (CF101, piclidenoson) is safe and generally well tolerated in treating rheumatoid arthritis [268]. A recent clinical trial showed that LUF6000, an A3AR allosteric modulator, induced an anti-inflammatory effect in animal models of arthritis via deregulation of PI3K, IKK, IκB, Jak-2, and STAT-1 signaling, resulting in decreased levels of nuclear factor (NF)-κβ [269].
A3AR delivered significant protection from murine septic peritonitis primarily by attenuating the hyperacute inflammatory response in sepsis and IP lung injury [270,271]. Currently, IB-MECA is in a trial for patients with moderate to severe plaque psoriasis [272]. Furthermore, the antagonists A3AR, MRS 1292, and OT-7999 inhibited shrinkage of human nonpigmented ciliary epithelial cells and reduced mouse intraocular pressure (IOP) in an animal model of glaucoma [273,274]. A trial of IB-MECA for dry eyes was unsuccessful, yet IB-MECA was able to reduce IOP [275].
4.4.2. A3AR in the Respiratory System
A3AR plays roles in asthma, COPD, lung fibrosis, and pulmonary inflammation. Adenosine-induced airway hyperresponsiveness (AHR) in mice occurs largely through A3AR in mast cells [276] via Gi and PI3K signaling pathway-mediated accumulation of intracellular Ca2+ [277]. While the role of rodent A3AR-activated mast cells in AHR is firmly established, the mechanism of A3AR-induced release of inflammatory mediators in humans remains elusive [278]. Most A3ARs are expressed in lung eosinophils instead of mast cells, which mediate the inhibition of both degranulation and O2− release [257,279]. Indeed, A3AR has an important role in regulating lung eosinophilia and mucus production in an environment of elevated adenosine [280].
Nebulized IB-MECA directly induced lung mast cell degranulation while having no effect in A3AR KO mice [277]. Likewise, adenosine induces AHR indirectly by activating A3AR on mast cells [276]. Although A3AR is absent in human lung mast cells, it inhibits degranulation of eosinophils, so it may be useful in eosinophil-dependent allergic disorders, such as asthma and rhinitis [68]. In fact, a selective adenosine A2BAR agonist/A3AR antagonist had no significant effect on rhinorrhea, the number of sneezes, or peak nasal inspiratory flow measurements [281]. The combined A2BAR/A3AR antagonist QAF 805 failed to increase the PC20 [85].
In contrast, IB-MECA has an important role in IR-induced lung injury through the upregulation of phosphorylated ERK [271] and the nitric oxide synthase (NOS)-independent pathway [120]. Furthermore, the deletion of A3AR enhanced pulmonary inflammation by increasing eosinophil-related chemokines and cytokines [282].
Overall, A3AR is involved in both proinflammatory and anti-inflammatory responses depending on the cell type involved, although data in the recent literature appear to lean towards a protective effect [68].
4.4.3. A3AR in Cardiovascular Systems
The cardioprotective effect of A3AR is similar to the combined effects of A1AR and A2AAR. Activation of the A3AR either prior to ischemia or during reperfusion was useful in cardioprotection [125]. Administration of IB-MECA before ischemia or during reperfusion effectively reduces infarct size [197], and CI-IB-MECA (CF102, namodenoson) inhibited myocardial ischemia-reperfusion injury [198]. Additionally, CP-532,903, a highly selective agonist of A3AR, protected against ischemia-reperfusion injury [199].
It is assumed that A3AR induces cardioprotection via PKC, PI3-kinase, ERK, mitochondrial KATP channels [178], and NO [283]. Activation of A3AR limited myocardial injury in an isolated rat heart and improved survival in isolated myocytes, possibly via antiapoptotic and antinecrotic mechanisms [284]. Indeed, the basic mechanism in A3AR-associated cardioprotection remains poorly defined and may vary between species (e.g., rodents vs. humans) and protective responses (e.g., acute vs. delayed protection) [285].
Paradoxically, low-level expression in the heart provides effective protection against ischemic injury without adverse effects, whereas higher levels lead to the development of a dilated cardiomyopathy [286]. Hinze et al. first reported that A3AR induces proliferation by activating PLC and inducing the transcription factors early growth response (EGR) 2 and EGR3 [287]. A3AR acts against the protective effect of adenosine in the overloaded heart; therefore, attenuation of A3AR might be a novel approach to treat pressure overload-induced left ventricular hypertrophy and dysfunction [288]. Moreover, deletion of A3AR protects the heart against renal and cardiovascular disease [289].
A3AR induces vasoconstriction via mast cell-produced histamine and thromboxane, inhibition of cAMP in smooth muscle and aorta, and enhancement of cellular antioxidants [71]. A3AR-mediated contraction through the endothelium may play a role in cardiovascular inflammation, including hypertension and atherosclerosis, by affecting the cyclooxygenase signaling pathways [290], and is also linked with reactive oxygen species (ROS) generation via activation of nicotinamide adenine dinucleotide phosphate (NADPH) oxidase/Nox2 [291]. A3AR is involved in atherosclerosis through modulation of the hyaluronic matrix [292]. Furthermore, it induces VEGF secretion and FC synthesis in a HIF-1α-dependent manner [293].
The latest study shows that LJ-1888, a selective antagonist for A3AR, is a feasible novel candidate for the treatment of atherosclerosis and hypercholesterolemia [196]. Moreover, LJ-2698, a highly selective and species-independent A3AR antagonist, ameliorated diabetic kidney complications [195].
The role of cardioprotection by A3AR is enticing, enigmatic, and elusive [294]. Further clinical studies on cardioprotection induced by adenosine receptors need to establish the role of adenosine receptor agonists and antagonists in more clinically relevant models of myocardial ischemia [238].
4.4.4. A3AR in CNS Systems
The expression of A3AR in the brain is low and difficult to detect using sensitive in situ hybridization or receptor-labeling autoradiography methods [295], yet A3AR exists in neurotransmission [71].
Several studies have reported a neuroprotective function. Under ischemia, CA1 hippocampal A3AR might exert A1-like protective effects on neurotransmission, but severe ischemia would transform the A3 receptor-mediated effects from protective to injurious [296]. Administration of IB-MECA chronically prior to forebrain ischemia resulted in improved postischemic cerebral blood circulation, survival, and neuronal preservation, whereas a negative effect was found when given acutely [222].
In line with this, acute stimulation by CI-IB-MECA induced cell death in a concentration-dependent fashion by inhibiting cAMP production [297]. The timing of treatment also produced opposing results, as administration of IB-MECA prior to and after transient middle cerebral ischemia resulted in an increase and decrease in the infarct size, respectively [223]. The protective role of A3AR in ischemia is possibly mediated by the preservation of ischemia-sensitive microtubule-associated protein 2 (MAP-2), enhancement of the expression of glial fibrillary acidic protein [298], depression of NOS, stimulation of glial CCL2 synthesis [299], and delay of irreversible synaptic failure [300].
On the other hand, A3AR stimulated convulsion in the CA1 [301] and CA3 [302] regions of the rat hippocampus. A new antagonist, ANR235, increases the stability of IGABA in different epileptic tissues and may offer therapeutic opportunities in human epilepsy [224]. In serotonin (5-HT)-linked brain diseases, A3AR is also involved in the rapid stimulation of presynaptic serotonin transport [303] mediated by protein kinase G1 and p38 MAPK [304]. A3AR might function as a potential candidate in brain inflammation treatment by suppressing TNF-α production [262]. Further studies are mandatory for establishing the neuroprotective role of A3AR before A3AR agonists or antagonists can be used clinically.
4.4.5. A3AR in the Digestive and Renal Systems
A3AR delivers protection in inflammatory gastrointestinal diseases. IB-MECA treatment reduced oxidative damage and inflammatory mediators in the colon, including IL-1, IL-6, IL-12, MIP-1α, and MIP-2 [305]. This agonist also provides protection from CLP-induced mortality and acute organ dysfunction in murine septic peritonitis primarily by attenuating the hyperacute inflammatory response [270].
A recent study showed that A3AR had anti-inflammatory activity via inhibition of proinflammatory cytokine expression associated with the inhibition of NF-κβ signaling pathways in murine dextran sulfate sodium (DSS) colitis [306]. Blocking A3AR produced protection against ischemia- and myoglobinuria-induced renal failure [307]. Recently, selective A3AR antagonism attenuated renal ischemia-reperfusion injury [308].
4.4.6. A3AR in Cancer
Primary and metastatic solid tumors showed high expression of A3AR [309], which might be related to overexpression of NF-κβ [310]. In addition, high expression has been observed using biochemical methods in many types of cancer cells, including astrocytoma, melanoma, lymphoma, sarcoma, glioblastoma and colon, liver, pancreatic, prostate, thyroid, lung, breast, and renal carcinomas [69]. Table 4 illustrates the A3AR and A2BAR ligands that are currently undergoing clinical trials as novel therapeutic treatments in cancer.
Table 4.
Adenosine receptor drugs in cancer.
It has long been known that A3AR is involved in the regulation of the cell cycle, and both proapoptotic and antiapoptotic effects have been reported depending on the level of receptor activation [311]. In the event of tumor inhibition, adenosine plays a role in the resistance of muscle to tumor metastases [312], and its antiproliferative effect occurs mainly via A3AR-induced cell cycle arrest in the G0/G1 phase and decreases in telomeric signaling in these cells [313]. CI-IB-MECA protects the retinal ganglion by increasing survival [314]. Additionally, it mediates a tonic proliferative effect in colon tumor cells [315]. It is possible that the antiapoptotic effect is related to oxygen concentration [59].
Apart from hypoxia, HIF-1α has a role in cancer-associated angiogenesis [316]. A3AR upregulates HIF-1α protein expression in a dose-dependent and time-dependent manner via p44/p42 and p38 MAPK [317]. Moreover, an A3AR antagonist inhibited VEGF protein accumulation [318] and reduced tumor size and blood vessel formation under hypoxic conditions in glioblastoma cells [319].
IB-MECA inhibited tumor cell growth of HCT-116 human and CT-26 murine colon carcinoma cell [320] and B16-F10 melanoma cells [321]. Furthermore, other in vivo studies have shown that IB-MECA in PC-3 prostate carcinoma cells [322] and CI-IB-MECA in N1S1 rat hepatocellular carcinoma (HCC) cells [323] induced apoptosis and tumor growth inhibition via deregulation of the Wnt and NF-κβ signaling pathways.
The oral bioavailability of synthetic A3AR agonists renders them potentially useful in three different modes of treatment: as stand-alone anticancer treatments, in combination with chemotherapy to enhance its therapeutic index, and as agents inducing myeloprotection [324]. The latest study confirmed that combination use of A3AR agonists produces anticancer effects in an apoptosis-, autophagy-, and ROS-dependent manner [325].
5. Conclusions
Pharmacological interventions related to AR ligands as new drugs has been widely developed. Most ARs have roles as both proinflammatory and anti-inflammatory mediators as well as in organ protection and degeneration, and these opposing roles depend on the timing and concentration. As a consequence of being widely distributed throughout the body, adenosine activates protection for tissues and cells against injury, hypoxia, and ischemia; however, it also stimulates various adverse effects, especially in the cardiovascular and respiratory systems. Therefore, future studies should focus on the abundant amount of adenosine and reducing its harmful impact. Overall, focusing on partial agonist and allosteric enhancers might be the most appealing pharmacological angle for successful treatment in cancer, inflammation, cardiometabolic diseases, respiratory diseases, and neurovascular diseases.
Acknowledgments
We would like to express our gratitude to members of the Division of Respiratory Medicine of Kobe University Graduate School of Medicine for their helpful discussion and the Indonesian Endowment Fund for Education (LPDP) Scholarship under Beasiswa Unggulan Dosen Indonesia—Luar Negeri (BUDI-LN) batch I 2016 (Number: 20160822048989).
Author Contributions
W.I.E. and T.N. wrote the article, W.I.E., T.N., K.K., and Y.N. contributed to the concept, and critically appraised and edited the article. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Drury A.N., Szent-Györgyi A. The physiological activity of adenine compounds with especial reference to their action upon the mammalian heart. J. Physiol. 1929;68:213–237. doi: 10.1113/jphysiol.1929.sp002608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cobbin L.B., Einstein R., Maguire M.H. Studies on the coronary dilator actions of some adenosine analogues. Br. J. Pharmacol. 1974;50:25–33. doi: 10.1111/j.1476-5381.1974.tb09589.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Degubareff T., Sleator W.J. Effects of caffeine on mammalian atrial muscle, and its interaction with adenosine and calcium. J. Pharmacol. Exp. Ther. 1965;148:202–214. [PubMed] [Google Scholar]
- 4.Sattin A., Rall T.W. The effect of adenosine and adenine nucleotides on the cyclic adenosine 3′, 5′-phosphate content of guinea pig cerebral cortex slices. Mol. Pharmacol. 1970;6:13–23. [PubMed] [Google Scholar]
- 5.Burnstock G. Purinergic nerves and receptors. Prog. Biochem. Pharmacol. 1980;16:141–154. [PubMed] [Google Scholar]
- 6.Burnstock G., Verkhratsky A. Purinergic signaling. Wiley Interdiscip. Rev. Membr. Transp. Signal. 2012;1:116–125. doi: 10.1002/wmts.14. [DOI] [Google Scholar]
- 7.Burnstock G. Autonomic neurotransmitters and trophic factors. J. Auton. Nerv. Syst. 1983;7:213–217. doi: 10.1016/0165-1838(83)90074-7. [DOI] [PubMed] [Google Scholar]
- 8.Fredholm B.B., Abbracchio M.P., Burnstock G., Daly J.W., Harden T.K., Jacobson K.A., Leff P., Williams M. Nomenclature and classification of purinoceptors. Pharmacol. Rev. 1994;46:143–156. [PMC free article] [PubMed] [Google Scholar]
- 9.Krishtal O.A., Marchenko S.M., Pidoplichko V.I. Receptor for ATP in the membrane of mammalian sensory neurones. Neurosci. Lett. 1983;35:41–45. doi: 10.1016/0304-3940(83)90524-4. [DOI] [PubMed] [Google Scholar]
- 10.Fredholm B.B., Abbracchio M.P., Burnstock G., Dubyak G.R., Harden T.K., Jacobson K.A., Schwabe U., Williams M. Towards a revised nomenclature for P1 and P2 receptors. Trends Pharmacol. Sci. 1997;18:79–82. doi: 10.1016/S0165-6147(96)01038-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fredholm B.B., IJzerman A.P., Jacobson K.A., Klotz K.N., Linden J. International Union of Pharmacology. XXV. Nomenclature and classification of adenosine receptors. Pharmacol. Rev. 2001;53:527–552. [PMC free article] [PubMed] [Google Scholar]
- 12.Olah M.E., Stiles G.L. Adenosine receptor subtypes: Characterization and therapeutic regulation. Annu. Rev. Pharmacol. Toxicol. 1995;35:581–606. doi: 10.1146/annurev.pa.35.040195.003053. [DOI] [PubMed] [Google Scholar]
- 13.Shryock J.C., Belardinelli L. Adenosine and adenosine receptors in the cardiovascular system: Biochemistry, physiology, and pharmacology. Am. J. Cardiol. 1997;79:2–10. doi: 10.1016/S0002-9149(97)00256-7. [DOI] [PubMed] [Google Scholar]
- 14.Spicuzza L., Di Maria G., Polosa R. Adenosine in the airways: Implications and applications. Eur. J. Pharmacol. 2006;533:77–88. doi: 10.1016/j.ejphar.2005.12.056. [DOI] [PubMed] [Google Scholar]
- 15.Sala-Newby G.B., Skladanowski A.C., Newby A.C. The mechanism of adenosine formation in cells. Cloning of cytosolic 5′-nucleotidase-I. J. Biol. Chem. 1999;274:17789–17793. doi: 10.1074/jbc.274.25.17789. [DOI] [PubMed] [Google Scholar]
- 16.Sala-Newby G.B., Freeman N.V.E., Skladanowski A.C., Newby A.C. Distinct Roles for Recombinant Cytosolic 5′-Nucleotidase-I and -II in AMP and IMP Catabolism in COS-7 and H9c2 Rat Myoblast Cell Lines. J. Biol. Chem. 2000;275:11666–11671. doi: 10.1074/jbc.275.16.11666. [DOI] [PubMed] [Google Scholar]
- 17.Broch O.J., Ueland P.M. Regional and subcellular distribution of S-adenosylhomocysteine hydrolase in the adult rat brain. J. Neurochem. 1980;35:484–488. doi: 10.1111/j.1471-4159.1980.tb06291.x. [DOI] [PubMed] [Google Scholar]
- 18.Kroll K., Decking U.K., Dreikorn K., Schrader J. Rapid turnover of the AMP-adenosine metabolic cycle in the guinea pig heart. Circ. Res. 1993;73:846–856. doi: 10.1161/01.RES.73.5.846. [DOI] [PubMed] [Google Scholar]
- 19.Layland J., Carrick D., Lee M., Oldroyd K., Berry C. Adenosine: Physiology, pharmacology, and clinical applications. JACC Cardiovasc. Interv. 2014;7:581–591. doi: 10.1016/j.jcin.2014.02.009. [DOI] [PubMed] [Google Scholar]
- 20.Saito H., Nishimura M., Shinano H., Makita H., Tsujino I., Shibuya E., Sato F., Miyamoto K., Kawakami Y. Plasma concentration of adenosine during normoxia and moderate hypoxia in humans. Am. J. Respir. Crit. Care Med. 1999;159:1014–1018. doi: 10.1164/ajrccm.159.3.9803100. [DOI] [PubMed] [Google Scholar]
- 21.Latini S., Pedata F. Adenosine in the central nervous system: Release mechanisms and extracellular concentrations. J. Neurochem. 2001;79:463–484. doi: 10.1046/j.1471-4159.2001.00607.x. [DOI] [PubMed] [Google Scholar]
- 22.Sheth S., Brito R., Mukherjea D., Rybak L.P., Ramkumar V. Adenosine receptors: Expression, function and regulation. Int. J. Mol. Sci. 2014;15:2024–2052. doi: 10.3390/ijms15022024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Blackburn M.R., Vance C.O., Morschl E., Wilson C.N. Adenosine receptors and inflammation. In: Wilson C.N., Mustafa S.J., editors. Adenosine Receptors in Health and Disease. Springer; Berlin/Heidelberg, Germany: 2009. pp. 215–269. [DOI] [PubMed] [Google Scholar]
- 24.Polosa R., Holgate S.T. Adenosine receptors as promising therapeutic targets for drug development in chronic airway inflammation. Curr. Drug Targets. 2006;7:699–706. doi: 10.2174/138945006777435236. [DOI] [PubMed] [Google Scholar]
- 25.Yegutkin G.G. Nucleotide- and nucleoside-converting ectoenzymes: Important modulators of purinergic signalling cascade. Biochim. Biophys. Acta. 2008;1783:673–694. doi: 10.1016/j.bbamcr.2008.01.024. [DOI] [PubMed] [Google Scholar]
- 26.Eltzschig H.K. Adenosine: An old drug newly discovered. Anesthesiology. 2009;111:904–915. doi: 10.1097/ALN.0b013e3181b060f2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zimmermann H. Biochemistry, localization and functional roles of ecto-nucleotidases in the nervous system. Prog. Neurobiol. 1996;49:589–618. doi: 10.1016/0301-0082(96)00026-3. [DOI] [PubMed] [Google Scholar]
- 28.Marquardt D.L., Gruber H.E., Wasserman S.I. Adenosine release from stimulated mast cells. Proc. Natl. Acad. Sci. USA. 1984;81:6192–6196. doi: 10.1073/pnas.81.19.6192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mann J.S., Renwick A.G., Holgate S.T. Release of adenosine and its metabolites from activated human leucocytes. Clin. Sci. (Lond.) 1986;70:461–468. doi: 10.1042/cs0700461. [DOI] [PubMed] [Google Scholar]
- 30.Madara J.L., Patapoff T.W., Gillece-Castro B., Colgan S.P., Parkos C.A., Delp C., Mrsny R.J. 5′-adenosine monophosphate is the neutrophil-derived paracrine factor that elicits chloride secretion from T84 intestinal epithelial cell monolayers. J. Clin. Investig. 1993;91:2320–2325. doi: 10.1172/JCI116462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Resnick M.B., Colgan S.P., Patapoff T.W., Mrsny R.J., Awtrey C.S., Delp-Archer C., Weller P.F., Madara J.L. Activated eosinophils evoke chloride secretion in model intestinal epithelia primarily via regulated release of 5′-AMP. J. Immunol. 1993;151:5716–5723. [PubMed] [Google Scholar]
- 32.Newby A.C. Adenosine and the concept of ‘retaliatory metabolites. Trends Biochem. Sci. 1984;9:42–44. doi: 10.1016/0968-0004(84)90176-2. [DOI] [Google Scholar]
- 33.Cronstein B.N., Sitkovsky M. Adenosine and adenosine receptors in the pathogenesis and treatment of rheumatic diseases. Nat. Rev. Rheumatol. 2017;13:41–51. doi: 10.1038/nrrheum.2016.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zimmermann H. Extracellular purine metabolism. Drug Dev. Res. 1996;39:337–352. doi: 10.1002/(SICI)1098-2299(199611/12)39:3/4<337::AID-DDR15>3.0.CO;2-Z. [DOI] [Google Scholar]
- 35.Maliszewski C.R., Delespesse G.J., Schoenborn M.A., Armitage R.J., Fanslow W.C., Nakajima T., Baker E., Sutherland G.R., Poindexter K., Birks C. The CD39 lymphoid cell activation antigen. Molecular cloning and structural characterization. J. Immunol. 1994;153:3574–3583. [PubMed] [Google Scholar]
- 36.Kaczmarek E., Koziak K., Sevigny J., Siegel J.B., Anrather J., Beaudoin A.R., Bach F.H., Robson S.C. Identification and characterization of CD39/vascular ATP diphosphohydrolase. J. Biol. Chem. 1996;271:33116–33122. doi: 10.1074/jbc.271.51.33116. [DOI] [PubMed] [Google Scholar]
- 37.Zimmermann H., Braun N., Kegel B., Heine P. New insights into molecular structure and function of ectonucleotidases in the nervous system. Neurochem. Int. 1998;32:421–425. doi: 10.1016/S0197-0186(97)00126-5. [DOI] [PubMed] [Google Scholar]
- 38.Kansas G.S., Wood G.S., Tedder T.F. Expression, distribution, and biochemistry of human CD39. Role in activation-associated homotypic adhesion of lymphocytes. J. Immunol. 1991;146:2235–2244. [PubMed] [Google Scholar]
- 39.Robson S.C., Ph D., Wu Y., Ph D., Sun X., Knosalla C., Dwyer K., Ph D., Enjyoji K., Ph D. Ectonucleotidases of CD39 Family Modulate Vascular Inflammation and Thrombosis in Transplantation. Semin. Thromb. Hemost. 2005;31:217–233. doi: 10.1055/s-2005-869527. [DOI] [PubMed] [Google Scholar]
- 40.Bono M.R., Fernandez D., Flores-Santibanez F., Rosemblatt M., Sauma D. CD73 and CD39 ectonucleotidases in T cell differentiation: Beyond immunosuppression. FEBS Lett. 2015;589:3454–3460. doi: 10.1016/j.febslet.2015.07.027. [DOI] [PubMed] [Google Scholar]
- 41.Zimmermann H. 5’-Nucleotidase: Molecular structure and functional aspects. Biochem. J. 1992;285:345–365. doi: 10.1042/bj2850345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Blackburn M.R., Kellems R.E. Regulation and Function of Adenosine Deaminase in Mice. Prog. Nucleic Acid Res. Mol. Biol. 1996;55:195–226. doi: 10.1016/s0079-6603(08)60194-4. [DOI] [PubMed] [Google Scholar]
- 43.Leiva A., Guzman-Gutierrez E., Contreras-Duarte S., Fuenzalida B., Cantin C., Carvajal L., Salsoso R., Gutierrez J., Pardo F., Sobrevia L. Adenosine receptors: Modulators of lipid availability that are controlled by lipid levels. Mol. Aspects Med. 2017;55:26–44. doi: 10.1016/j.mam.2017.01.007. [DOI] [PubMed] [Google Scholar]
- 44.Van Calker D., Muller M., Hamprecht B. Adenosine regulates via two different types of receptors, the accumulation of cyclic AMP in cultured brain cells. J. Neurochem. 1979;33:999–1005. doi: 10.1111/j.1471-4159.1979.tb05236.x. [DOI] [PubMed] [Google Scholar]
- 45.Cheng R.K.Y., Segala E., Robertson N., Deflorian F., Doré A.S., Errey J.C., Fiez-Vandal C., Marshall F.H., Cooke R.M. Structures of Human A1and A2AAdenosine Receptors with Xanthines Reveal Determinants of Selectivity. Structure. 2017;25:1275–1285.e4. doi: 10.1016/j.str.2017.06.012. [DOI] [PubMed] [Google Scholar]
- 46.Piirainen H., Ashok Y., Nanekar R.T., Jaakola V.P. Structural features of adenosine receptors: From crystal to function. Biochim. Biophys. Acta Biomembr. 2011;1808:1233–1244. doi: 10.1016/j.bbamem.2010.05.021. [DOI] [PubMed] [Google Scholar]
- 47.Jeffrey Conn P., Christopoulos A., Lindsley C.W. Allosteric modulators of GPCRs: A novel approach for the treatment of CNS disorders. Nat. Rev. Drug Discov. 2009;8:41–54. doi: 10.1038/nrd2760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Christopoulos A. Allosteric binding sites on cell-surface receptors: Novel targets for drug discovery. Nat. Rev. Drug Discov. 2002;1:198–210. doi: 10.1038/nrd746. [DOI] [PubMed] [Google Scholar]
- 49.Peleli M., Fredholm B.B., Sobrevia L., Carlström M. Pharmacological targeting of adenosine receptor signaling. Mol. Aspects Med. 2017;55:4–8. doi: 10.1016/j.mam.2016.12.002. [DOI] [PubMed] [Google Scholar]
- 50.Fredholm B.B., IJzerman A.P., Jacobson K.A., Linden J., Müller C.E. International union of basic and clinical pharmacology. LXXXI. Nomenclature and classification of adenosine receptors—An update. Pharmacol. Rev. 2011;63:1–34. doi: 10.1124/pr.110.003285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Stiles G.L. Adenosine Receptors. J. Biol. Chem. 1992;10:6451–6454. [PubMed] [Google Scholar]
- 52.Haskó G., Linden J., Cronstein B., Pacher P. Adenosine receptors: Therapeutic aspects for inflammatory and immune diseases. Nat. Rev. Drug Discov. 2008;7:759–770. doi: 10.1038/nrd2638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yan L., Burbiel J.C., Maaß A., Müller C.E. Adenosine receptor agonists: From basic medicinal chemistry to clinical development. Expert Opin. Emerg. Drugs. 2003;8:537–576. doi: 10.1517/14728214.8.2.537. [DOI] [PubMed] [Google Scholar]
- 54.Fredholm B.B., Arslan G., Halldner L., Kull B., Schulte G., Wasserman W. Structure and function of adenosine receptors and their genes. Naunyn. Schmiedebergs. Arch. Pharmacol. 2000;362:364–374. doi: 10.1007/s002100000313. [DOI] [PubMed] [Google Scholar]
- 55.Fredholm B.B., Chern Y., Franco R., Sitkovsky M. Aspects of the general biology of adenosine A2A signaling. Prog. Neurobiol. 2007;83:263–276. doi: 10.1016/j.pneurobio.2007.07.005. [DOI] [PubMed] [Google Scholar]
- 56.Varani K., Vincenzi F., Tosi A., Gessi S., Casetta I., Granieri G., Fazio P., Leung E., MacLennan S., Granieri E., et al. A2A adenosine receptor overexpression and functionality, as well as TNF-α levels, correlate with motor symptoms in Parkinson’s disease. FASEB J. 2010;24:587–598. doi: 10.1096/fj.09-141044. [DOI] [PubMed] [Google Scholar]
- 57.Ledent C., Vaugeoist J.M., Schiffmann S.N., Pedrazzini T., El Yacoubi M., Vanderhaeghen J.J., Costentin J., Heath J.K., Vassart G., Parmentier M. Aggressiveness, hypoalgesia and high blood pressure in mice lacking the adenosine A2a receptor. Nature. 1997;388:674–678. doi: 10.1038/41771. [DOI] [PubMed] [Google Scholar]
- 58.Baraldi P.G., Tabrizi M.A., Gessi S., Borea P.A. Adenosine receptor antagonists: Translating medicinal chemistry and pharmacology into clinical utility. Chem. Rev. 2008;108:238–263. doi: 10.1021/cr0682195. [DOI] [PubMed] [Google Scholar]
- 59.Gessi S., Merighi S., Fazzi D., Stefanelli A., Varani K., Borea P.A. Adenosine receptor targeting in health and disease. Expert Opin. Investig. Drugs. 2011;20:1591–1609. doi: 10.1517/13543784.2011.627853. [DOI] [PubMed] [Google Scholar]
- 60.Herve D., Levi-Strauss M., Marey-Semper I., Verney C., Tassin J.P., Glowinski J., Girault J.A. G(olf) and Gs in rat basal ganglia: Possible involvement of G(olf) in the coupling of dopamine D1 receptor with adenylyl cyclase. J. Neurosci. 1993;13:2237–2248. doi: 10.1523/JNEUROSCI.13-05-02237.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.De Lera Ruiz M., Lim Y.H., Zheng J. Adenosine A2A receptor as a drug discovery target. J. Med. Chem. 2014;57:3623–3650. doi: 10.1021/jm4011669. [DOI] [PubMed] [Google Scholar]
- 62.Cristalli G., Müller C.E., Volpini R. Recent developments in Adenosine A2A receptor ligands. In: Wilson C.N., Mustafa S.J., editors. Adenosine Receptors in Health and Disease. Volume 193. Springer; Berlin/Heidelberg, Germany: 2009. pp. 59–98. [DOI] [PubMed] [Google Scholar]
- 63.Haskó G., Csóka B., Németh Z.H., Vizi E.S., Pacher P. A2B adenosine receptors in immunity and inflammation. Trends Immunol. 2009;30:263–270. doi: 10.1016/j.it.2009.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kalla R.V., Zablocki J., Tabrizi M.A., Baraldi P.G. Recent developments in A2B adenosine receptor ligands. In: Wilson C.N., Mustafa S.J., editors. Adenosine Receptors in Health and Disease. Volume 193. Springer; Berlin/Heidelberg, Germany: 2009. pp. 99–122. [DOI] [PubMed] [Google Scholar]
- 65.Cohen M.V., Yang X., Downey J.M. A 2b adenosine receptors can change their spots. Br. J. Pharmacol. 2010;159:1595–1597. doi: 10.1111/j.1476-5381.2010.00668.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zhou Q.Y., Li C., Olah M.E., Johnson R.A., Stiles G.L., Civelli O. Molecular cloning and characterization of an adenosine receptor: The A3 adenosine receptor. Proc. Natl. Acad. Sci. USA. 1992;89:7432–7436. doi: 10.1073/pnas.89.16.7432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Meyerhof W., Paust H.J., Schonrock C., Richter D. Cloning of a cDNA encoding a novel putative G-protein-coupled receptor expressed in specific rat brain regions. DNA Cell Biol. 1991;10:689–694. doi: 10.1089/dna.1991.10.689. [DOI] [PubMed] [Google Scholar]
- 68.Borea P.A., Varani K., Vincenzi F., Baraldi P.G., Tabrizi M.A., Merighi S., Gessi S. The A3 adenosine receptor: History and perspectives. Pharmacol. Rev. 2015;67:74–102. doi: 10.1124/pr.113.008540. [DOI] [PubMed] [Google Scholar]
- 69.Jacobson K.A., Merighi S., Varani K., Borea P.A., Baraldi S., Aghazadeh Tabrizi M., Romagnoli R., Baraldi P.G., Ciancetta A., Tosh D.K., et al. A3 Adenosine Receptors as Modulators of Inflammation: From Medicinal Chemistry to Therapy. Med. Res. Rev. 2018;38:1031–1072. doi: 10.1002/med.21456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Salvatore C.A., Jacobson M.A., Taylor H.E., Linden J., Johnson R.G. Molecular cloning and characterization of the human A3 adenosine receptor. Proc. Natl. Acad. Sci. USA. 1993;90:10365–10369. doi: 10.1073/pnas.90.21.10365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Baraldi P.G., Preti D., Borea P.A., Varani K. Medicinal Chemistry of A3 Adenosine Receptor Modulators: Pharmacological Activities and Therapeutic Implications. J. Med. Chem. 2012;55:5676–5703. doi: 10.1021/jm300087j. [DOI] [PubMed] [Google Scholar]
- 72.Jacobson K.A., Klutz A.M., Tosh D.K., Ivanov A.A., Preti D., Baraldi P.G. Medicinal chemistry of the A 3 adenosine receptor: Agonists, antagonists, and receptor engineering. In: Wilson C.N., Mustafa S.J., editors. Adenosine Receptors in Health and Disease. Volume 193. Springer; Berlin/Heidelberg, Germany: 2009. pp. 123–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Baraldi P.G., Cacciari B., Romagnoli R., Merighi S., Varani K., Borea P.A., Spalluto G. A(3) adenosine receptor ligands: History and perspectives. Med. Res. Rev. 2000;20:103–128. doi: 10.1002/(SICI)1098-1128(200003)20:2<103::AID-MED1>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 74.Cronstein B.N., Levin R.I., Philips M., Hirschhorn R., Abramson S.B., Weissmann G. Neutrophil adherence to endothelium is enhanced via adenosine A1 receptors and inhibited via adenosine A2 receptors. J. Immunol. 1992;148:2201–2206. [PubMed] [Google Scholar]
- 75.Zahler S., Becker B.F. Adhesion of neutrophils to cultured human endothelial cells is enhanced by stimulation of adenosine A1-receptors. Drug Dev. Res. 1998;45:350–355. doi: 10.1002/(SICI)1098-2299(199811/12)45:3/4<350::AID-DDR35>3.0.CO;2-L. [DOI] [Google Scholar]
- 76.Zahler S., Becker B.F., Raschke P., Gerlach E. Stimulation of endothelial adenosine A1 receptors enhances adhesion of neutrophils in the intact guinea pig coronary system. Cardiovasc. Res. 1994;28:1366–1372. doi: 10.1093/cvr/28.9.1366. [DOI] [PubMed] [Google Scholar]
- 77.Salmon J.E., Brogle N., Brownlie C., Edberg J.C., Kimberly R.P., Chen B.X., Erlanger B.F. Human mononuclear phagocytes express adenosine A1 receptors. A novel mechanism for differential regulation of Fc gamma receptor function. J. Immunol. 1993;151:2775–2785. [PubMed] [Google Scholar]
- 78.Clark A.N., Youkey R., Liu X., Jia L., Blatt R., Day Y.-J., Sullivan G.W., Linden J., Tucker A.L. A1 adenosine receptor activation promotes angiogenesis and release of VEGF from monocytes. Circ. Res. 2007;101:1130–1138. doi: 10.1161/CIRCRESAHA.107.150110. [DOI] [PubMed] [Google Scholar]
- 79.Kara F.M., Chitu V., Sloane J., Axelrod M., Fredholm B.B., Stanley E.R., Cronstein B.N. Adenosine A1 receptors (A1Rs) play a critical role in osteoclast formation and function. FASEB J. 2010;24:2325–2333. doi: 10.1096/fj.09-147447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Takahashi H.K., Iwagaki H., Hamano R., Wake H., Kanke T., Liu K., Yoshino T., Tanaka N., Nishibori M. Effects of adenosine on adhesion molecule expression and cytokine production in human PBMC depend on the receptor subtype activated. Br. J. Pharmacol. 2007;150:816–822. doi: 10.1038/sj.bjp.0707126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Gallos G., Ruyle T.D., Emala C.W., Lee H.T. A1 adenosine receptor knockout mice exhibit increased mortality, renal dysfunction, and hepatic injury in murine septic peritonitis. Am. J. Physiol. Ren. Physiol. 2005;289:F369–F376. doi: 10.1152/ajprenal.00470.2004. [DOI] [PubMed] [Google Scholar]
- 82.Kim J., Kim M., Song J.H., Lee H.T. Endogenous A1 adenosine receptors protect against hepatic ischemia reperfusion injury in mice. Liver Transplant. 2008;14:845–854. doi: 10.1002/lt.21432. [DOI] [PubMed] [Google Scholar]
- 83.Park S.W., Chen S.W.C., Kim M., Brown K.M., D’Agati V.D., Lee H.T. Protection against acute kidney injury via A1 adenosine receptor-mediated Akt activation reduces liver injury after liver ischemia and reperfusion in mice. J. Pharmacol. Exp. Ther. 2010;333:736–747. doi: 10.1124/jpet.110.166884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hua X., Erikson C.J., Chason K.D., Rosebrock C.N., Deshpande D.A., Penn R.B., Tilley S.L. Involvement of A1 adenosine receptors and neural pathways in adenosine-induced bronchoconstriction in mice. Am. J. Physiol. Cell. Mol. Physiol. 2007;293:L25–L32. doi: 10.1152/ajplung.00058.2007. [DOI] [PubMed] [Google Scholar]
- 85.Wilson C.N., Nadeem A., Spina D., Brown R., Page C.P., Mustafa S.J. Adenosine receptors and asthma. In: Wilson C.N., Mustafa S.J., editors. Adenosine Receptors in Health and Disease. Volume 193. Springer; Berlin/Heidelberg, Germany: 2009. pp. 329–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Brown R.A., Spina D., Page C.P. Adenosine receptors and asthma. Br. J. Pharmacol. 2008;153:S446–S456. doi: 10.1038/bjp.2008.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Obiefuna P.C.M., Batra V.K., Nadeem A., Borron P., Wilson C.N., Jamal Mustafa S. A novel A1 adenosine receptor antagonist, L-97-1 [3-[2-(4-aminophenyl)-ethyl]-8-benzyl-7-{2-ethyl-(2-hydroxy-ethyl)-amino]-ethyl} -1-propyl-3,7-dihydro-purine-2,6-dione], reduces allergic responses to house dust mite in an allergic rabbit model of asthma. J. Pharmacol. Exp. Ther. 2005;315:329–336. doi: 10.1124/jpet.105.088179. [DOI] [PubMed] [Google Scholar]
- 88.Ball H.A., Van Scott M.K., Robinson C.B. Sense and antisense: Therapeutic potential of oligonucleotides and interference RNA in asthma and allergic disorders. Clin. Rev. Allergy Immunol. 2004;27:207–217. doi: 10.1385/CRIAI:27:3:207. [DOI] [PubMed] [Google Scholar]
- 89.Chunn J.L., Young H.W., Banerjee S.K., Colasurdo G.N., Blackburn M.R. Adenosine-dependent airway inflammation and hyperresponsiveness in partially adenosine deaminase-deficient mice. J. Immunol. 2001;167:4676–4685. doi: 10.4049/jimmunol.167.8.4676. [DOI] [PubMed] [Google Scholar]
- 90.Ngamsri K.C., Wagner R., Vollmer I., Stark S., Reutershan J. Adenosine Receptor A1 Regulates Polymorphonuclear Cell Trafficking and Microvascular Permeability in Lipopolysaccharide-Induced Lung Injury. J. Immunol. 2010;185:4374–4384. doi: 10.4049/jimmunol.1000433. [DOI] [PubMed] [Google Scholar]
- 91.Fernandez L.G., Sharma A.K., Lapar D.J., Kron I.L., Laubach V.E. Adenosine A1 receptor activation attenuates lung ischemia-reperfusion injury. J. Thorac. Cardiovasc. Surg. 2013;145:1654–1659. doi: 10.1016/j.jtcvs.2013.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Aeffner F., Woods P.S., Davis I.C. Activation of A1-Adenosine Receptors Promotes Leukocyte Recruitment to the Lung and Attenuates Acute Lung Injury in Mice Infected with Influenza A/WSN/33 (H1N1) Virus. J. Virol. 2014;88:10214–10227. doi: 10.1128/JVI.01068-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Romano F.D., Dobson J.G. Adenosine modulates β-adrenergic signal transduction in guinea-pig heart ventricular membranes. J. Mol. Cell. Cardiol. 1990;22:1359–1370. doi: 10.1016/0022-2828(90)90981-7. [DOI] [PubMed] [Google Scholar]
- 94.Fenton R.A., Dobson J.G., Jr. Adenosine A1 and A2A receptor effects on G-protein cycling in β-adrenergic stimulated ventricular membranes. J. Cell. Physiol. 2007;213:785–792. doi: 10.1002/jcp.21149. [DOI] [PubMed] [Google Scholar]
- 95.Fenton R.A., Shea L.G., Doddi C., Dobson J.G., Jr. Myocardial adenosine A(1)-receptor-mediated adenoprotection involves phospholipase C, PKC-epsilon, and p38 MAPK, but not HSP27. Am. J. Physiol. Heart Circ. Physiol. 2010;298:H1671–H1678. doi: 10.1152/ajpheart.01028.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Lorbar M., Chung E.S., Nabi A., Skalova K., Fenton R.A., Dobson J.G., Meyer T.E. Receptors subtypes involved in adenosine-mediated modulation of norepinephrine release from cardiac nerve terminals. Can. J. Physiol. Pharmacol. 2004;82:1026–1031. doi: 10.1139/y04-108. [DOI] [PubMed] [Google Scholar]
- 97.Burgdorf C. Adenosine inhibits norepinephrine release in the postischemic rat heart: The mechanism of neuronal stunning. Cardiovasc. Res. 2001;49:713–720. doi: 10.1016/S0008-6363(00)00309-6. [DOI] [PubMed] [Google Scholar]
- 98.Tawfik H.E., Schnermann J., Oldenburg P.J., Mustafa S.J. Role of A1 adenosine receptors in regulation of vascular tone. Am. J. Physiol. Heart Circ. Physiol. 2005;288:H1411–H1416. doi: 10.1152/ajpheart.00684.2004. [DOI] [PubMed] [Google Scholar]
- 99.Nayeem M.A., Zeldin D.C., Boegehold M.A., Morisseau C., Marowsky A., Ponnoth D.S., Roush K.P., Falck J.R. Modulation by salt intake of the vascular response mediated through adenosine A(2A) receptor: Role of CYP epoxygenase and soluble epoxide hydrolase. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2010;299:R325–R333. doi: 10.1152/ajpregu.00823.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Shen J., Halenda S.P., Sturek M., Wilden P.A. Cell-signaling evidence for adenosine stimulation of coronary smooth muscle proliferation via the A1 adenosine receptor. Circ. Res. 2005;97:574–582. doi: 10.1161/01.RES.0000181159.83588.4b. [DOI] [PubMed] [Google Scholar]
- 101.Maeda T., Koos B.J. Adenosine A1 and A2a receptors modulate insulinemia, glycemia, and lactatemia in fetal sheep. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2009;296:R693–R701. doi: 10.1152/ajpregu.90363.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Johansson S.M., Yang J.-N., Lindgren E., Fredholm B.B. Eliminating the antilipolytic adenosine A1 receptor does not lead to compensatory changes in the antilipolytic actions of PGE2 and nicotinic acid. Acta Physiol. 2007;190:87–96. doi: 10.1111/j.1365-201X.2007.01692.x. [DOI] [PubMed] [Google Scholar]
- 103.Urmaliya V.B., Church J.E., Coupar I.M., Rose’Meyer R.B., Pouton C.W., White P.J. Cardioprotection induced by adenosine A1 receptor agonists in a cardiac cell ischemia model involves cooperative activation of adenosine A2A and A2B receptors by endogenous adenosine. J. Cardiovasc. Pharmacol. 2009;53:424–433. doi: 10.1097/FJC.0b013e3181a443e2. [DOI] [PubMed] [Google Scholar]
- 104.Urmaliya V.B., Pouton C.W., Ledent C., Short J.L., White P.J. Cooperative cardioprotection through adenosine A1 and A 2A receptor agonism in ischemia-reperfused isolated mouse heart. J. Cardiovasc. Pharmacol. 2010;56:379–388. doi: 10.1097/FJC.0b013e3181f03d05. [DOI] [PubMed] [Google Scholar]
- 105.Headrick J.P., Peart J.N., Reichelt M.E., Haseler L.J. Adenosine and its receptors in the heart: Regulation, retaliation and adaptation. Biochim. Biophys. Acta Biomembr. 2011;1808:1413–1428. doi: 10.1016/j.bbamem.2010.11.016. [DOI] [PubMed] [Google Scholar]
- 106.Liao Y., Takashima S., Asano Y., Asakura M., Ogai A., Shintani Y., Minamino T., Asanuma H., Sanada S., Kim J., et al. Activation of Adenosine A 1 Receptor Attenuates Cardiac Hypertrophy and Prevents Heart Failure in Murine Left Ventricular Pressure-Overload Model. Circ. Res. 2003;93:759–766. doi: 10.1161/01.RES.0000094744.88220.62. [DOI] [PubMed] [Google Scholar]
- 107.Greenberg B., Thomas I., Banish D., Goldman S., Havranek E., Massie B.M., Zhu Y., Ticho B., Abraham W.T. Effects of Multiple Oral Doses of an A1 Adenosine Antagonist, BG9928, in Patients With Heart Failure. Results of a Placebo-Controlled, Dose-Escalation Study. J. Am. Coll. Cardiol. 2007;50:600–606. doi: 10.1016/j.jacc.2007.03.059. [DOI] [PubMed] [Google Scholar]
- 108.Voors A.A., Dittrich H.C., Massie B.M., Delucca P., Mansoor G.A., Metra M., Cotter G., Weatherley B.D., Ponikowski P., Teerlink J.R., et al. Effects of the adenosine A1 receptor antagonist rolofylline on renal function. J. Am. Coll. Cardiol. 2011;57:1899–1907. doi: 10.1016/j.jacc.2010.11.057. [DOI] [PubMed] [Google Scholar]
- 109.Fozard J.R., Ellis K.M., Villela Dantas M.F., Tigani B., Mazzoni L. Effects of CGS 21680, a selective adenosine A2A receptor agonist, on allergic airways inflammation in the rat. Eur. J. Pharmacol. 2002;438:183–188. doi: 10.1016/S0014-2999(02)01305-5. [DOI] [PubMed] [Google Scholar]
- 110.Trevethick M.A., Mantell S.J., Stuart E.F., Barnard A., Wright K.N., Yeadon M. Treating lung inflammation with agonists of the adenosine A 2A receptor: Promises, problems and potential solutions. Br. J. Pharmacol. 2008;155:463–474. doi: 10.1038/bjp.2008.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Luijk B., Van Den Berge M., Kerstjens H.A.M., Postma D.S., Cass L., Sabin A., Lammers J.W.J. Effect of an inhaled adenosine A2A agonist on the allergen-induced late asthmatic response. Allergy. 2008;63:75–80. doi: 10.1111/j.1398-9995.2007.01557.x. [DOI] [PubMed] [Google Scholar]
- 112.Golzar Y., Doukky R. Regadenoson use in patients with chronic obstructive pulmonary disease: The state of current knowledge. Int. J. COPD. 2014;9:129–137. doi: 10.2147/COPD.S56879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Haskó G., Xu D.Z., Lu Q., Németh Z.H., Jabush J., Berezina T.L., Zaets S.B., Csóka B., Deitch E.A. Adenosine A2A receptor activation reduces lung injury in trauma/hemorrhagic shock. Crit. Care Med. 2006;34:1119–1125. doi: 10.1097/01.CCM.0000206467.19509.C6. [DOI] [PubMed] [Google Scholar]
- 114.Konrad F.M., Neudeck G., Vollmer I., Ngamsri K.C., Thiel M., Reutershan J. Protective effects of pentoxifylline in pulmonary inflammation are adenosine receptor A2A dependent. FASEB J. 2013;27:3524–3535. doi: 10.1096/fj.13-228122. [DOI] [PubMed] [Google Scholar]
- 115.Greer S., Page C.W., Joshi T., Yan D., Newton R., Giembycz M.A. Concurrent agonism of adenosine a2b and glucocorticoid receptors in human airway epithelial cells cooperatively induces genes with anti-inflammatory potential: A novel approach to treat chronic obstructive pulmonary disease. J. Pharmacol. Exp. Ther. 2013;346:473–485. doi: 10.1124/jpet.113.206284. [DOI] [PubMed] [Google Scholar]
- 116.Mustafa S.J., Nadeem A., Fan M., Zhong H., Belardinelli L., Zeng D. Effect of a specific and selective A2B adenosine receptor antagonist on adenosine agonist AMP and allergen-induced airway responsiveness and cellular influx in a mouse model of asthma. J. Pharmacol. Exp. Ther. 2007;320:1246–1251. doi: 10.1124/jpet.106.112250. [DOI] [PubMed] [Google Scholar]
- 117.Eckle T., Grenz A., Laucher S., Eltzschig H.K. A2B adenosine receptor signaling attenuates acute lung injury by enhancing alveolar fluid clearance in mice. J. Clin. Investig. 2008;118:3301–3315. doi: 10.1172/JCI34203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Karmouty-Quintana H., Zhong H., Acero L., Weng T., Melicoff E., West J.D., Hemnes A., Grenz A., Eltzschig H.K., Blackwell T.S., et al. The A2B adenosine receptor modulates pulmonary hypertension associated with interstitial lung disease. FASEB J. 2012;26:2546–2557. doi: 10.1096/fj.11-200907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Chan E.S.L., Cronstein B.N. Adenosine in fibrosis. Mod. Rheumatol. 2010;20:114–122. doi: 10.3109/s10165-009-0251-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Rivo J., Zeira E., Galun E., Matot I. Activation of A 3 adenosine receptors attenuates lung injury after in vivo reperfusion. Anesthesiology. 2004;101:1153–1159. doi: 10.1097/00000542-200411000-00015. [DOI] [PubMed] [Google Scholar]
- 121.Tendera M., Gaszewska-Zurek E., Parma Z., Ponikowski P., Jankowska E., Kawecka-Jaszcz K., Czarnecka D., Krzemińska-Pakuła M., Bednarkiewicz Z., Sosnowski M., et al. The new oral adenosine A1 receptor agonist capadenoson in male patients with stable angina. Clin. Res. Cardiol. 2012;101:585–591. doi: 10.1007/s00392-012-0430-8. [DOI] [PubMed] [Google Scholar]
- 122.Baltos J.A., Vecchio E.A., Harris M.A., Qin C.X., Ritchie R.H., Christopoulos A., White P.J., May L.T. Capadenoson, a clinically trialed partial adenosine A 1 receptor agonist, can stimulate adenosine A 2B receptor biased agonism. Biochem. Pharmacol. 2017;135:79–89. doi: 10.1016/j.bcp.2017.03.014. [DOI] [PubMed] [Google Scholar]
- 123.Jacobson K.A., Tosh D.K., Jain S., Gao Z.G. Historical and current adenosine receptor agonists in preclinical and clinical development. Front. Cell. Neurosci. 2019;13:124. doi: 10.3389/fncel.2019.00124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Voors A.A., Düngen H.D., Senni M., Nodari S., Agostoni P., Ponikowski P., Bax J.J., Butler J., Kim R.J., Dorhout B., et al. Safety and Tolerability of Neladenoson Bialanate, a Novel Oral Partial Adenosine A1 Receptor Agonist, in Patients With Chronic Heart Failure. J. Clin. Pharmacol. 2017;57:440–451. doi: 10.1002/jcph.828. [DOI] [PubMed] [Google Scholar]
- 125.Headrick J.P., Lasley R.D. Adenosine receptors and reperfusion injury of the heart. In: Wilson C.N., Mustafa S.J., editors. Adenosine Receptors in Health and Disease. Volume 193. Springer; Berlin/Heidelberg, Germany: 2009. pp. 189–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.McIntosh V.J., Lasley R.D. Adenosine receptor-mediated cardioprotection: Are all 4 subtypes required or redundant? J. Cardiovasc. Pharmacol. Ther. 2012;17:21–33. doi: 10.1177/1074248410396877. [DOI] [PubMed] [Google Scholar]
- 127.Kirchhof P., Fabritz L., Fortmüller L., Matherne G.P., Lankford A., Baba H.A., Schmitz W., Breithardt G., Neumann J., Boknik P. Altered sinus nodal and atrioventricular nodal function in freely moving mice overexpressing the A1 adenosine receptor. Am. J. Physiol. Heart Circ. Physiol. 2003;285:H145–H153. doi: 10.1152/ajpheart.01036.2002. [DOI] [PubMed] [Google Scholar]
- 128.Ellenbogen K.A., O’Neill G., Prystowsky E.N., Camm J.A., Meng L., Lieu H.D., Jerling M., Shreeniwas R., Belardinelli L., Wolff A.A., et al. Trial to evaluate the management of paroxysmal supraventricular tachycardia during an electrophysiology study with tecadenoson. Circulation. 2005;111:3202–3208. doi: 10.1161/CIRCULATIONAHA.104.510982. [DOI] [PubMed] [Google Scholar]
- 129.Mason P.K., DiMarco J.P. New pharmacological agents for arrhythmias. Circ. Arrhythmia Electrophysiol. 2009;2:588–597. doi: 10.1161/CIRCEP.109.884429. [DOI] [PubMed] [Google Scholar]
- 130.Szentmiklosi A., Galajda Z., Cseppento A., Gesztelyi R., Susan Z., Hegyi B., Nanasi P. The Janus Face of Adenosine: Antiarrhythmic and Proarrhythmic Actions. Curr. Pharm. Des. 2015;21:965–976. doi: 10.2174/1381612820666141029100346. [DOI] [PubMed] [Google Scholar]
- 131.Wu L.G., Saggau P. Presynaptic inhibition of elicited neurotransmitter release. Trends Neurosci. 1997;20:204–212. doi: 10.1016/S0166-2236(96)01015-6. [DOI] [PubMed] [Google Scholar]
- 132.Stone T.W., Ceruti S., Abbracchio M.P. Adenosine receptors and neurological disease: Neuroprotection and neurodegeneration. In: Wilson C.N., Mustafa S.J., editors. Adenosine Receptors in Health and Disease. Volume 193. Springer; Berlin/Heidelberg, Germany: 2009. pp. 535–587. [DOI] [PubMed] [Google Scholar]
- 133.Dunwiddie T.V., Masino S.A. The Role and Regulation of Adenosine in the Central Nervous System. Annu. Rev. Neurosci. 2001;24:31–55. doi: 10.1146/annurev.neuro.24.1.31. [DOI] [PubMed] [Google Scholar]
- 134.Ribeiro J.A., Sebastião A.M., De Mendonça A. Adenosine receptors in the nervous system: Pathophysiological implications. Prog. Neurobiol. 2002;68:377–392. doi: 10.1016/S0301-0082(02)00155-7. [DOI] [PubMed] [Google Scholar]
- 135.Stockwell J., Jakova E., Cayabyab F.S. Adenosine A1 and A2A receptors in the brain: Current research and their role in neurodegeneration. Molecules. 2017;22:676. doi: 10.3390/molecules22040676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Borea P.A., Gessi S., Merighi S., Varani K. Adenosine as a Multi-Signalling Guardian Angel in Human Diseases: When, Where and How Does it Exert its Protective Effects? Trends Pharmacol. Sci. 2016;37:419–434. doi: 10.1016/j.tips.2016.02.006. [DOI] [PubMed] [Google Scholar]
- 137.Dickenson A.H., Suzuki R., Reeve A.J. Adenosine as a potential analgesic target in inflammatory and neuropathic pains. CNS Drugs. 2000;13:77–85. doi: 10.2165/00023210-200013020-00001. [DOI] [Google Scholar]
- 138.Sneyd R.J., Langton J.A., Allan L.G., Peacock J.E., Rowbotham D.J. Multicentre evaluation of the adenosine agonist GR79236X in patients with dental pain after third molar extraction. Br. J. Anaesth. 2007;98:672–676. doi: 10.1093/bja/aem075. [DOI] [PubMed] [Google Scholar]
- 139.Luongo L., Petrelli R., Gatta L., Giordano C., Guida F., Vita P., Franchetti P., Grifantini M., De Novellis V., Cappellacci L., et al. 5′-Chloro-5′-deoxy-(±)-ENBA, a potent and selective adenosine A1 receptor agonist, alleviates neuropathic pain in mice through functional glial and microglial changes without affecting motor or cardiovascular functions. Molecules. 2012;17:13712–13726. doi: 10.3390/molecules171213712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Tosh D.K., Rao H., Bitant A., Salmaso V., Mannes P., Lieberman D.I., Vaughan K.L., Mattison J.A., Rothwell A.C., Auchampach J.A., et al. Design and in Vivo Characterization of A 1 Adenosine Receptor Agonists in the Native Ribose and Conformationally Constrained (N)-Methanocarba Series. J. Med. Chem. 2019;62:1502–1522. doi: 10.1021/acs.jmedchem.8b01662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Gao Z.G., Jacobson K.A. Emerging adenosine receptor agonists an update. Expert Opin. Emerg. Drugs. 2011;16:597–602. doi: 10.1517/14728214.2011.644786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Fedele D.E., Li T., Lan J.Q., Fredholm B.B., Boison D. Adenosine A1 receptors are crucial in keeping an epileptic focus localized. Exp. Neurol. 2006;200:184–190. doi: 10.1016/j.expneurol.2006.02.133. [DOI] [PubMed] [Google Scholar]
- 143.Benarroch E.E. Adenosine and its receptors: Multiple modulatory functions and potential therapeutic targets for neurologic disease. Neurology. 2008;70:231–236. doi: 10.1212/01.wnl.0000297939.18236.ec. [DOI] [PubMed] [Google Scholar]
- 144.Hargus N.J., Jennings C., Perez-Reyes E., Bertram E.H., Patel M.K. Enhanced actions of adenosine in medial entorhinal cortex layer II stellate neurons in temporal lobe epilepsy are mediated via A 1-receptor activation. Epilepsia. 2012;53:168–176. doi: 10.1111/j.1528-1167.2011.03337.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Masino S.A., Kawamura M., Ruskin D.N. Adenosine receptors and epilepsy. Current evidence and future potential. Int. Rev. Neurobiol. 2014;119:233–255. doi: 10.1016/B978-0-12-801022-8.00011-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Kovács Z., D’Agostino D.P., Dobolyi A., Ari C. Adenosine A1 Receptor Antagonism Abolished the Anti-seizure Effects of Exogenous Ketone Supplementation in Wistar Albino Glaxo Rijswijk Rats. Front. Mol. Neurosci. 2017;10:235. doi: 10.3389/fnmol.2017.00235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Johansson B., Halldner L., Dunwiddie T.V., Masino S.A., Poelchen W., Giménez-Llort L., Escorihuela R.M., Fernández-Teruel A., Wiesenfeld-Hallin Z., Xu X.J., et al. Hyperalgesia, anxiety, and decreased hypoxic neuroprotection in mice lacking the adenosine A1 receptor. Proc. Natl. Acad. Sci. USA. 2001;98:9407–9412. doi: 10.1073/pnas.161292398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Giménez-Llort L., Fernández-Teruel A., Escorihuela R.M., Fredholm B.B., Tobeña A., Pekny M., Johansson B. Mice lacking the adenosine A1 receptor are anxious and aggressive, but are normal learners with reduced muscle strength and survival rate. Eur. J. Neurosci. 2002;16:547–550. doi: 10.1046/j.1460-9568.2002.02122.x. [DOI] [PubMed] [Google Scholar]
- 149.Prediger R.D.S., Da Silva G.E., Batista L.C., Bittencourt A.L., Takahashi R.N. Activation of adenosine A1 receptors reduces anxiety-like behavior during acute ethanol withdrawal (hangover) in mice. Neuropsychopharmacology. 2006;31:2210–2220. doi: 10.1038/sj.npp.1301001. [DOI] [PubMed] [Google Scholar]
- 150.Vincenzi F., Borea P.A., Varani K. Anxiolytic properties of A1 adenosine receptor PAMs. Oncotarget. 2017;8:7216–7217. doi: 10.18632/oncotarget.13802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Serchov T., Clement H.W., Schwarz M.K., Iasevoli F., Tosh D.K., Idzko M., Jacobson K.A., de Bartolomeis A., Normann C., Biber K., et al. Increased Signaling via Adenosine A1Receptors, Sleep Deprivation, Imipramine, and Ketamine Inhibit Depressive-like Behavior via Induction of Homer1a. Neuron. 2015;87:549–562. doi: 10.1016/j.neuron.2015.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Sigworth L.A., Rea M.A. Adenosine A1 receptors regulate the response of the mouse circadian clock to light. Brain Res. 2003;960:246–251. doi: 10.1016/S0006-8993(02)03896-9. [DOI] [PubMed] [Google Scholar]
- 153.Elmenhorst D., Meyer P.T., Winz O.H., Matusch A., Ermert J., Coenen H.H., Basheer R., Haas H.L., Zilles K., Bauer A. Sleep deprivation increases A1 adenosine receptor binding in the human brain: A positron emission tomography study. J. Neurosci. 2007;27:2410–2415. doi: 10.1523/JNEUROSCI.5066-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Basheer R., Strecker R.E., Thakkar M.M., McCarley R.W. Adenosine and sleep-wake regulation. Prog. Neurobiol. 2004;73:379–396. doi: 10.1016/j.pneurobio.2004.06.004. [DOI] [PubMed] [Google Scholar]
- 155.Loram L.C., Harrison J.A., Sloane E.M., Hutchinson M.R., Sholar P., Taylor F.R., Berkelhammer D., Coats B.D., Poole S., Milligan E.D., et al. Enduring reversal of neuropathic pain by a single intrathecal injection of adenosine 2A receptor agonists: A novel therapy for neuropathic pain. J. Neurosci. 2009;29:14015–14025. doi: 10.1523/JNEUROSCI.3447-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.De Mendonça A., Ribeiro J.A. Adenosine and synaptic plasticity. Drug Dev. Res. 2001;52:283–290. doi: 10.1002/ddr.1125. [DOI] [Google Scholar]
- 157.De Mendonça A., Ribeiro J.A. Endogenous adenosine modulates long-term potentiation in the hippocampus. Neuroscience. 1994;62:385–390. doi: 10.1016/0306-4522(94)90373-5. [DOI] [PubMed] [Google Scholar]
- 158.De Mendonça A., Almeida T., Bashir Z.I., Ribeiro J.A. Endogenous adenosine attenuates long-term depression and depotentiation in the CA1 region of the rat hippocampus. Neuropharmacology. 1997;36:161–167. doi: 10.1016/S0028-3908(96)00173-6. [DOI] [PubMed] [Google Scholar]
- 159.Mihara T., Mihara K., Yarimizu J., Mitani Y., Matsuda R., Yamamoto H., Aoki S., Akahane A., Iwashita A., Matsuoka N. Pharmacological characterization of a novel, potent adenosine A1 and A2A receptor dual antagonist, 5-[5-amino-3-(4-fluorophenyl) pyrazin-2-yl]-1-isopropylpyridine-2(1H)-one (ASP5854), in models of Parkinson’s disease and cognition. J. Pharmacol. Exp. Ther. 2007;323:708–719. doi: 10.1124/jpet.107.121962. [DOI] [PubMed] [Google Scholar]
- 160.Lopes L.V., Sebastiao A.M., Ribeiro J.A. Adenosine and Related Drugs in Brain Diseases: Present and Future in Clinical Trials. Curr. Top. Med. Chem. 2011;11:1087–1101. doi: 10.2174/156802611795347591. [DOI] [PubMed] [Google Scholar]
- 161.Fuxe K., Ferré S., Genedani S., Franco R., Agnati L.F. Adenosine receptor-dopamine receptor interactions in the basal ganglia and their relevance for brain function. Physiol. Behav. 2007;92:210–217. doi: 10.1016/j.physbeh.2007.05.034. [DOI] [PubMed] [Google Scholar]
- 162.Blum D., Hourez R., Galas M.C., Popoli P., Schiffmann S.N. Adenosine receptors and Huntington’s disease: Implications for pathogenesis and therapeutics. Lancet Neurol. 2003;2:366–374. doi: 10.1016/S1474-4422(03)00411-3. [DOI] [PubMed] [Google Scholar]
- 163.Sebastião A.M., Ribeiro J.A. Adenosine receptors and the central nervous system. In: Wilson C.N., Mustafa S.J., editors. Adenosine Receptors in Health and Disease. Volume 193. Springer; Berlin/Heidelberg, Germany: 2009. pp. 471–534. [DOI] [PubMed] [Google Scholar]
- 164.Peleli M., Carlstrom M. Adenosine signaling in diabetes mellitus and associated cardiovascular and renal complications. Mol. Aspects Med. 2017;55:62–74. doi: 10.1016/j.mam.2016.12.001. [DOI] [PubMed] [Google Scholar]
- 165.Kiesman W.F., Elzein E., Zablocki J. A1 adenosine receptor antagonists, agonists, and allosteric enhancers. In: Wilson C.N., Mustafa S.J., editors. Adenosine Receptors in Health and Disease. Volume 193. Springer; Berlin/Heidelberg, Germany: 2009. pp. 25–58. [DOI] [PubMed] [Google Scholar]
- 166.Elzein E., Zablocki J. A1 adenosine receptor agonists and their potential therapeutic applications. Expert Opin. Investig. Drugs. 2008;17:1901–1910. doi: 10.1517/13543780802497284. [DOI] [PubMed] [Google Scholar]
- 167.Staehr P.M., Dhalla A.K., Zack J., Wang X., Ho Y.L., Bingham J., Belardinelli L. Reduction of free fatty acids, safety, and pharmacokinetics of oral GS-9667, an a1 adenosine receptor partial agonist. J. Clin. Pharmacol. 2013;53:385–392. doi: 10.1002/jcph.9. [DOI] [PubMed] [Google Scholar]
- 168.Dhalla A.K., Chisholm J.W., Reaven G.M., Belardinelli L. A1 adenosine receptor: Role in diabetes and obesity. In: Wilson C.N., Mustafa S.J., editors. Adenosine Receptors in Health and Disease. Volume 193. Springer; Berlin/Heidelberg, Germany: 2009. pp. 271–295. [DOI] [PubMed] [Google Scholar]
- 169.Hasko G., Pacher P. A2A receptors in inflammation and injury: Lessons learned from transgenic animals. J. Leukoc. Biol. 2008;83:447–455. doi: 10.1189/jlb.0607359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Link A.A., Kino T., Worth J.A., McGuire J.L., Crane M.L., Chrousos G.P., Wilder R.L., Elenkov I.J. Ligand-activation of the adenosine A2a receptors inhibits IL-12 production by human monocytes. J. Immunol. 2000;164:436–442. doi: 10.4049/jimmunol.164.1.436. [DOI] [PubMed] [Google Scholar]
- 171.Bouma M.G., Stad R.K., van den Wildenberg F.A., Buurman W.A. Differential regulatory effects of adenosine on cytokine release by activated human monocytes. J. Immunol. 1994;153:4159–4168. [PubMed] [Google Scholar]
- 172.Németh Z.H., Csóka B., Wilmanski J., Xu D., Lu Q., Ledent C., Deitch E.A., Pacher P., Spolarics Z., Haskó G. Adenosine A 2A Receptor Inactivation Increases Survival in Polymicrobial Sepsis. J. Immunol. 2006;176:5616–5626. doi: 10.4049/jimmunol.176.9.5616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Antonioli L., Fornai M., Blandizzi C., Pacher P., Haskó G. Adenosine signaling and the immune system: When a lot could be too much. Immunol. Lett. 2019;205:9–15. doi: 10.1016/j.imlet.2018.04.006. [DOI] [PubMed] [Google Scholar]
- 174.Flögel U., Burghoff S., Van Lent P.L.E.M., Temme S., Galbarz L., Ding Z., El-Tayeb A., Huels S., Bönner F., Borg N., et al. Selective activation of adenosine A2A receptors on immune cells by a CD73-dependent prodrug suppresses joint inflammation in experimental rheumatoid arthritis. Sci. Transl. Med. 2012;4:146ra108. doi: 10.1126/scitranslmed.3003717. [DOI] [PubMed] [Google Scholar]
- 175.Squadrito F., Bitto A., Irrera N., Pizzino G., Pallio G., Minutoli L., Altavilla D. Pharmacological Activity and Clinical Use of PDRN. Front. Pharmacol. 2017;8:224. doi: 10.3389/fphar.2017.00224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Field J.J., Majerus E., Gordeuk V.R., Gowhari M., Hoppe C., Heeney M.M., Achebe M., George A., Chu H., Sheehan B., et al. Randomized phase 2 trial of regadenoson for treatment of acute vaso-occlusive crises in sickle cell disease. Blood Adv. 2017;1:1645–1649. doi: 10.1182/bloodadvances.2017009613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Antonioli L., Csoka B., Fornai M., Colucci R., Kokai E., Blandizzi C., Hasko G. Adenosine and inflammation: what’s new on the horizon? Drug Discov. Today. 2014;19:1051–1068. doi: 10.1016/j.drudis.2014.02.010. [DOI] [PubMed] [Google Scholar]
- 178.Peart J.N., Headrick J.P. Adenosinergic cardioprotection: Multiple receptors, multiple pathways. Pharmacol. Ther. 2007;114:208–221. doi: 10.1016/j.pharmthera.2007.02.004. [DOI] [PubMed] [Google Scholar]
- 179.Dobson J.G., Shea L.G., Fenton R.A. Adenosine A2A and β-adrenergic calcium transient and contractile responses in rat ventricular myocytes. Am. J. Physiol. Heart Circ. Physiol. 2008;295:H2364–H2372. doi: 10.1152/ajpheart.00927.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Norton E.D., Jackson E.K., Turner M.B., Virmani R., Forman M.B. The effects of intravenous infusions of selective adenosine A1-receptor and A2-receptor agonists on myocardial reperfusion injury. Am. Heart J. 1992;123:332–338. doi: 10.1016/0002-8703(92)90643-A. [DOI] [PubMed] [Google Scholar]
- 181.Glover D.K., Ruiz M., Takehana K., Petruzella F.D., Rieger J.M., Macdonald T.L., Watson D.D., Linden J., Beller G.A. Cardioprotection by adenosine A2A agonists in a canine model of myocardial stunning produced by multiple episodes of transient ischemia. Am. J. Physiol. Heart Circ. Physiol. 2007;292:H3164–H3171. doi: 10.1152/ajpheart.00743.2005. [DOI] [PubMed] [Google Scholar]
- 182.Boknik P., Drzewiecki K., Eskandar J., Gergs U., Hofmann B., Treede H., Grote-Wessels S., Fabritz L., Kirchhof P., Fortmüller L., et al. Evidence for Arrhythmogenic Effects of A2A-Adenosine Receptors. Front. Pharmacol. 2019;10:1051. doi: 10.3389/fphar.2019.01051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Chandrasekera P.C., McIntosh V.J., Cao F.X., Lasley R.D. Differential effects of adenosine A2a and A2b receptors on cardiac contractility. Am. J. Physiol. Heart Circ. Physiol. 2010;299:H2082–H2089. doi: 10.1152/ajpheart.00511.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Da Silva J.S., Gabriel-Costa D., Sudo R.T., Wang H., Groban L., Ferraz E.B., Nascimento J.H.M., Fraga C.A.M., Barreiro E.J., Zapata-Sudo G. Adenosine A2A receptor agonist prevents cardiac remodeling and dysfunction in spontaneously hypertensive male rats after myocardial infarction. Drug Des. Dev. Ther. 2017;11:553–562. doi: 10.2147/DDDT.S113289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Mustafa S.J., Morrison R.R., Teng B., Pelleg A. Adenosine receptors and the heart: Role in regulation of coronary blood flow and cardiac electrophysiology. In: Wilson C., Mustafa S., editors. Adenosine Receptors in Health and Disease. Volume 193. Springer; Berlin/Heidelberg, Germany: 2009. pp. 161–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Fuentes E., Fuentes M., Caballero J., Palomo I., Hinz S., El-Tayeb A., Müller C.E. Adenosine A2A receptor agonists with potent antiplatelet activity. Platelets. 2018;29:292–300. doi: 10.1080/09537104.2017.1306043. [DOI] [PubMed] [Google Scholar]
- 187.Voloshyna I., Littlefield M.J., Kaplan L., Rieger J.M., Figler R., Reiss A.B. Adenosine A2A Receptor Agonists Regulate Cholesterol Homeostasis in Mouse Bone Marrow Derived Macrophages (BMDM) FASEB J. 2013;27 doi: 10.1096/fasebj.27.1_supplement.822.1. [DOI] [Google Scholar]
- 188.Reiss A.B., Grossfeld D., Kasselman L.J., Renna H.A., Vernice N.A., Drewes W., Konig J., Carsons S.E., DeLeon J. Adenosine and the Cardiovascular System. Am. J. Cardiovasc. Drugs. 2019;19:449–464. doi: 10.1007/s40256-019-00345-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Koupenova M., Johnston-Cox H., Vezeridis A., Gavras H., Yang D., Zannis V., Ravid K. A2b adenosine receptor regulates hyperlipidemia and atherosclerosis. Circulation. 2012;125:354–363. doi: 10.1161/CIRCULATIONAHA.111.057596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Tian Y., Piras B.A., Kron I.L., French B.A., Yang Z. Adenosine 2B Receptor Activation Reduces Myocardial Reperfusion Injury by Promoting Anti-Inflammatory Macrophages Differentiation via PI3K/Akt Pathway. Oxid. Med. Cell. Longev. 2015;2015:585297. doi: 10.1155/2015/585297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Toldo S., Zhong H., Mezzaroma E., Van Tassell B.W., Kannan H., Zeng D., Belardinelli L., Voelkel N.F., Abbate A. GS-6201, a selective blocker of the A2B adenosine receptor, attenuates cardiac remodeling after acute myocardial infarction in the mouse. J. Pharmacol. Exp. Ther. 2012;343:587–595. doi: 10.1124/jpet.111.191288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Németh Z.H., Bleich D., Csóka B., Pacher P., Mabley J.G., Himer L., Vizi E.S., Deitch E.A., Szabó C., Cronstein B.N., et al. Adenosine receptor activation ameliorates type 1 diabetes. FASEB J. 2007;21:2379–2388. doi: 10.1096/fj.07-8213com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Figler R.A., Wang G., Srinivasan S., Jung D.Y., Zhang Z., Pankow J.S., Ravid K., Fredholm B., Hedrick C.C., Rich S.S., et al. Links between Insulin resistance, adenosine A2B receptors, and inflammatory markers in mice and humans. Diabetes. 2011;60:669–679. doi: 10.2337/db10-1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Valladares D., Quezada C., Montecinos P., Concha I.I., Yañez A.J., Sobrevia L., Martín R.S. Adenosine A2B receptor mediates an increase on VEGF-A production in rat kidney glomeruli. Biochem. Biophys. Res. Commun. 2008;366:180–185. doi: 10.1016/j.bbrc.2007.11.113. [DOI] [PubMed] [Google Scholar]
- 195.Dorotea D., Cho A., Lee G., Kwon G., Lee J., Sahu P.K., Jeong L.S., Cha D.R., Ha H. Orally active, species-independent novel A3 adenosine receptor antagonist protects against kidney injury in db/db mice. Exp. Mol. Med. 2018;50:38. doi: 10.1038/s12276-018-0053-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Park J.G., Jeong S.J., Yu J., Kim G., Jeong L.S., Oh G.T. LJ-1888, a selective antagonist for the A3 adenosine receptor, ameliorates the development of atherosclerosis and hypercholesterolemia in apolipoprotein E knock-out mice. BMB Rep. 2018;51:520–525. doi: 10.5483/BMBRep.2018.51.10.098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Auchampach J.A., Ge Z.-D., Wan T.C., Moore J., Gross G.J. A3 adenosine receptor agonist IB-MECA reduces myocardial ischemia-reperfusion injury in dogs. Am. J. Physiol. Heart Circ. Physiol. 2003;285:H607–H613. doi: 10.1152/ajpheart.01001.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Ge Z.D., Peart J.N., Kreckler L.M., Wan T.C., Jacobson M.A., Gross G.J., Auchampach J.A. Cl-IB-MECA [2-chloro-N6-(3-iodobenzyl)adenosine-5′-N-methylcarboxamide] reduces ischemia/reperfusion injury in mice by activating the A3 adenosine receptor. J. Pharmacol. Exp. Ther. 2006;319:1200–1210. doi: 10.1124/jpet.106.111351. [DOI] [PubMed] [Google Scholar]
- 199.Wan T.C., Ge Z.D., Tampo A., Mio Y., Bienengraeber M.W., Tracey W.R., Gross G.J., Kwok W.M., Auchampach J.A. The A3 adenosine receptor agonist CP-532,903 [N 6-(2,5-dichlorobenzyl)-3′ -aminoadenosine-5′-N- methylcarboxamide] protects against myocardial ischemia/reperfusion injury via the sarcolemmal ATP-sensitive potassium channel. J. Pharmacol. Exp. Ther. 2008;324:234–243. doi: 10.1124/jpet.107.127480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Mohamed R.A., Agha A.M., Abdel-Rahman A.A., Nassar N.N. Role of adenosine A2A receptor in cerebral ischemia reperfusion injury: Signaling to phosphorylated extracellular signal-regulated protein kinase (pERK1/2) Neuroscience. 2016;314:145–159. doi: 10.1016/j.neuroscience.2015.11.059. [DOI] [PubMed] [Google Scholar]
- 201.Zeraati M., Mirnajafi-Zadeh J., Fathollahi Y., Namvar S., Rezvani M.E. Adenosine A1 and A2A receptors of hippocampal CA1 region have opposite effects on piriform cortex kindled seizures in rats. Seizure. 2006;15:41–48. doi: 10.1016/j.seizure.2005.10.006. [DOI] [PubMed] [Google Scholar]
- 202.Li X., Kang H., Liu X., Liu Z., Shu K., Chen X., Zhu S. Effect of adenosine A2A receptor antagonist ZM241385 on amygdala-kindled seizures and progression of amygdala kindling. J. Huazhong Univ. Sci. Technol. Med. Sci. 2012;32:257–264. doi: 10.1007/s11596-012-0046-2. [DOI] [PubMed] [Google Scholar]
- 203.Kumar S., Rai S., Hsieh K.C., McGinty D., Alam M.N., Szymusiak R. Adenosine A2A receptors regulate the activity of sleep regulatory GABAergic neurons in the preoptic hypothalamus. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2013;305:R31–R41. doi: 10.1152/ajpregu.00402.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Knezevic N.N., Cicmil N., Knezevic I., Candido K.D. Discontinued neuropathic pain therapy between 2009-2015. Expert Opin. Investig. Drugs. 2015;24:1631–1646. doi: 10.1517/13543784.2015.1099627. [DOI] [PubMed] [Google Scholar]
- 205.Hamilton S.P., Slager S.L., De Leon A.B., Heiman G.A., Klein D.F., Hodge S.E., Weissman M.M., Fyer A.J., Knowles J.A. Evidence for genetic linkage between a polymorphism in the adenosine 2A receptor and panic disorder. Neuropsychopharmacology. 2004;29:558–565. doi: 10.1038/sj.npp.1300311. [DOI] [PubMed] [Google Scholar]
- 206.Yacoubi M.E., Ledent C., Parmentier M., Bertorelli R., Ongini E., Costentin J., Vaugeois J.M. Adenosine A 2A receptor antagonists are potential antidepressants: Evidence based on pharmacology and A 2A receptor knockout mice. Br. J. Pharmacol. 2001;134:68–77. doi: 10.1038/sj.bjp.0704240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.El Yacoubi M., Costentin J., Vaugeois J.M. Adenosine A2A receptors and depression. Neurology. 2003;61:S82–S87. doi: 10.1212/01.WNL.0000095220.87550.F6. [DOI] [PubMed] [Google Scholar]
- 208.Yamada K., Kobayashi M., Kanda T. Involvement of adenosine A2A receptors in depression and anxiety. Int. Rev. Neurobiol. 2014;119:373–393. doi: 10.1016/B978-0-12-801022-8.00015-5. [DOI] [PubMed] [Google Scholar]
- 209.Wardas J. Potential role of adenosine A2A receptors in the treatment of schizophrenia. Front. Biosci. 2008;13:4071–4096. doi: 10.2741/2995. [DOI] [PubMed] [Google Scholar]
- 210.Boison D., Singer P., Shen H.-Y., Feldon J., Yee B.K. Adenosine hypothesis of schizophrenia-opportunities for pharmacotherapy. Neuropharmacology. 2012;62:1527–1543. doi: 10.1016/j.neuropharm.2011.01.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Popoli P., Blum D., Domenici M., Burnouf S., Chern Y. A Critical Evaluation of Adenosine A2A Receptors as Potentially “Druggable” Targets in Huntingtons Disease. Curr. Pharm. Des. 2008;14:1500–1511. doi: 10.2174/138161208784480117. [DOI] [PubMed] [Google Scholar]
- 212.Faivre E., Coelho J.E., Zornbach K., Malik E., Baqi Y., Schneider M., Cellai L., Carvalho K., Sebda S., Figeac M., et al. Beneficial effect of a selective adenosine A2A receptor antagonist in the APPswe/PS1dE9 mouse model of Alzheimer’s disease. Front. Mol. Neurosci. 2018;11:235. doi: 10.3389/fnmol.2018.00235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Morelli M., Carta A.R., Jenner P. Adenosine A2A receptors and Parkinson’s disease. In: Wilson C.N., Mustafa S.J., editors. Adenosine Receptors in Health and Disease. Volume 193. Springer; Berlin/Heidelberg, Germany: 2009. pp. 589–615. [DOI] [PubMed] [Google Scholar]
- 214.Ballesteros-Yáñez I., Castillo C.A., Merighi S., Gessi S. The role of adenosine receptors in psychostimulant addiction. Front. Pharmacol. 2018;8:985. doi: 10.3389/fphar.2017.00985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Knapp C.M., Foye M.M., Cottam N., Ciraulo D.A., Kornetsky C. Adenosine agonists CGS 21680 and NECA inhibit the initiation of cocaine self-administration. Pharmacol. Biochem. Behav. 2001;68:797–803. doi: 10.1016/S0091-3057(01)00486-5. [DOI] [PubMed] [Google Scholar]
- 216.Linden J. New insights into the regulation of inflammation by adenosine. J. Clin. Investig. 2006;116:1835–1837. doi: 10.1172/JCI29125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Fredholm B.B. Adenosine, an endogenous distress signal, modulates tissue damage and repair. Cell Death Differ. 2007;14:1315–1323. doi: 10.1038/sj.cdd.4402132. [DOI] [PubMed] [Google Scholar]
- 218.Ryzhov S., Zaynagetdinov R., Goldstein A.E., Novitskiy S.V., Blackburn M.R., Biaggioni I., Feoktistov I. Effect of A2B adenosine receptor gene ablation on adenosine-dependent regulation of proinflammatory cytokines. J. Pharmacol. Exp. Ther. 2008;324:694–700. doi: 10.1124/jpet.107.131540. [DOI] [PubMed] [Google Scholar]
- 219.Zhong H., Wu Y., Belardinelli L., Zeng D. A2B adenosine induce IL-19 from bronchial epithelial cells, resulting in TNF-α increase. Am. J. Respir. Cell Mol. Biol. 2006;35:587–592. doi: 10.1165/rcmb.2005-0476OC. [DOI] [PubMed] [Google Scholar]
- 220.Popoli P., Blum D., Martire A., Ledent C., Ceruti S., Abbracchio M.P. Functions, dysfunctions and possible therapeutic relevance of adenosine A2A receptors in Huntington’s disease. Prog. Neurobiol. 2007;81:331–348. doi: 10.1016/j.pneurobio.2006.12.005. [DOI] [PubMed] [Google Scholar]
- 221.Nazario L.R., da Silva R.S., Bonan C.D. Targeting adenosine signaling in Parkinson’s disease: From pharmacological to non-pharmacological approaches. Front. Neurosci. 2017;11:658. doi: 10.3389/fnins.2017.00658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Von Lubitz D.K., Lin R.C., Popik P., Carter M.F., Jacobson K.A. Adenosine A3 receptor stimulation and cerebral ischemia. Eur. J. Pharmacol. 1994;263:59–67. doi: 10.1016/0014-2999(94)90523-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Von Lubitz D.K., Simpson K.L., Lin R.C. Right Thing at a Wrong Time? Adenosine A3 Receptors and Cerebroprotection in Stroke. Ann. N. Y. Acad. Sci. 2001;939:85–96. doi: 10.1111/j.1749-6632.2001.tb03615.x. [DOI] [PubMed] [Google Scholar]
- 224.Roseti C., Palma E., Martinello K., Fucile S., Morace R., Esposito V., Cantore G., Arcella A., Giangaspero F., Aronica E., et al. Blockage of A2A and A3 adenosine receptors decreases the desensitization of human GABAA receptors microtransplanted to Xenopus oocytes. Proc. Natl. Acad. Sci. USA. 2009;106:15927–15931. doi: 10.1073/pnas.0907324106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Ryzhov S., Zaynagetdinov R., Goldstein A.E., Novitskiy S.V., Dikov M.M., Blackburn M.R., Biaggioni I., Feoktistov I. Effect of A2B Adenosine Receptor Gene Ablation on Proinflammatory Adenosine Signaling in Mast Cells. J. Immunol. 2008;180:7212–7220. doi: 10.4049/jimmunol.180.11.7212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Carroll S.H., Wigner N.A., Kulkarni N., Johnston-Cox H., Gerstenfeld L.C., Ravid K. A2B adenosine receptor promotes mesenchymal stem cell differentiation to osteoblasts and bone formation in vivo. J. Biol. Chem. 2012;287:15718–15727. doi: 10.1074/jbc.M112.344994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.He W., Mazumder A., Wilder T., Cronstein B.N. Adenosine regulates bone metabolism via A1, A2A, and A2B receptors in bone marrow cells from normal humans and patients with multiple myeloma. FASEB J. 2013;27:3446–3454. doi: 10.1096/fj.13-231233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Ryzhov S., Sung B.H., Zhang Q., Weaver A., Gumina R.J., Biaggioni I., Feoktistov I. Role of adenosine A2B receptor signaling in contribution of cardiac mesenchymal stem-like cells to myocardial scar formation. Purinergic Signal. 2014;10:477–486. doi: 10.1007/s11302-014-9410-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Frick J.S., MacManus C.F., Scully M., Glover L.E., Eltzschig H.K., Colgan S.P. Contribution of Adenosine A2B Receptors to Inflammatory Parameters of Experimental Colitis. J. Immunol. 2009;182:4957–4964. doi: 10.4049/jimmunol.0801324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Wei W., Du C., Lv J., Zhao G., Li Z., Wu Z., Haskó G., Xie X. Blocking A 2B Adenosine Receptor Alleviates Pathogenesis of Experimental Autoimmune Encephalomyelitis via Inhibition of IL-6 Production and Th17 Differentiation. J. Immunol. 2013;190:138–146. doi: 10.4049/jimmunol.1103721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Gao Z.G., Balasubramanian R., Kiselev E., Wei Q., Jacobson K.A. Probing biased/partial agonism at the G protein-coupled A2B adenosine receptor. Biochem. Pharmacol. 2014;90:297–306. doi: 10.1016/j.bcp.2014.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Van Den Berge M., Hylkema M.N., Versluis M., Postma D.S. Role of adenosine receptors in the treatment of asthma and chronic obstructive pulmonary disease: Recent developments. Drugs R D. 2007;8:13–23. doi: 10.2165/00126839-200708010-00002. [DOI] [PubMed] [Google Scholar]
- 233.Varani K., Caramori G., Vincenzi F., Adcock I., Casolari P., Leung E., Maclennan S., Gessi S., Morello S., Barnes P.J., et al. Alteration of Adenosine Receptors in Patients with Chronic Obstructive Pulmonary Disease. Am. J. Respir. Crit. Care Med. 2006;173:398–406. doi: 10.1164/rccm.200506-869OC. [DOI] [PubMed] [Google Scholar]
- 234.Cronstein B.N. Adenosine receptors and fibrosis: A translational review. F1000 Biol. Rep. 2011;3:21. doi: 10.3410/B3-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Zhong H., Belardinelli L., Maa T., Zeng D. Synergy between A2B adenosine receptors and hypoxia in activating human lung fibroblasts. Am. J. Respir. Cell Mol. Biol. 2005;32:2–8. doi: 10.1165/rcmb.2004-0103OC. [DOI] [PubMed] [Google Scholar]
- 236.Hoegl S., Brodsky K.S., Blackburn M.R., Karmouty-Quintana H., Zwissler B., Eltzschig H.K. Alveolar Epithelial A2B Adenosine Receptors in Pulmonary Protection during Acute Lung Injury. J. Immunol. 2015;195:1815–1824. doi: 10.4049/jimmunol.1401957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Zhan E., McIntosh V.J., Lasley R.D. Adenosine A2A and A2B receptors are both required for adenosine A1 receptor-mediated cardioprotection. Am. J. Physiol. Heart Circ. Physiol. 2011;301:H1183–H1189. doi: 10.1152/ajpheart.00264.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Lasley R.D. Adenosine receptor-mediated cardioprotection-current limitations and future directions. Front. Pharmacol. 2018;9:310. doi: 10.3389/fphar.2018.00310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Eltzschig H.K., Bonney S.K., Eckle T. Attenuating myocardial ischemia by targeting A2B adenosine receptors. Trends Mol. Med. 2013;19:345–354. doi: 10.1016/j.molmed.2013.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Maas J.E., Wan T.C., Figler R.A., Gross G.J., Auchampach J.A. Evidence that the acute phase of ischemic preconditioning does not require signaling by the A2B adenosine receptor. J. Mol. Cell. Cardiol. 2010;49:886–893. doi: 10.1016/j.yjmcc.2010.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Dubey R.K., Gillespie D.G., Osaka K., Suzuki F., Jackson E.K. Adenosine inhibits growth of rat aortic smooth muscle cells. Possible role of A2b receptor. Hypertension. 1996;27:786–793. doi: 10.1161/01.HYP.27.3.786. [DOI] [PubMed] [Google Scholar]
- 242.Yang D., Zhang Y., Nguyen H.G., Koupenova M., Chauhan A.K., Makitalo M., Jones M.R., St. Hilaire C., Seldin D.C., Toselli P., et al. The A2B adenosine receptor protects against inflammation and excessive vascular adhesion. J. Clin. Investig. 2006;116:1913–1923. doi: 10.1172/JCI27933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Jackson E.K., Gillespie D.G., Mi Z., Cheng D. Adenosine receptors influence hypertension in dahl salt-sensitive rats: Dependence on receptor subtype, salt diet, and sex. Hypertension. 2018;72:511–521. doi: 10.1161/HYPERTENSIONAHA.117.10765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Yadav V.R., Zhou Z., Teng B., Mustafa S.J.S. Role of A1 and A2B Adenosine receptors in Angiotensin II dependent hypertension in mice. FASEB J. 2018;32:715.2. [Google Scholar]
- 245.Eisenstein A., Patterson S., Ravid K. The Many Faces of the A2b Adenosine Receptor in Cardiovascular and Metabolic Diseases. J. Cell. Physiol. 2015;230:2891–2897. doi: 10.1002/jcp.25043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Merighi S., Borea P.A., Gessi S. Adenosine receptors and diabetes: Focus on the A2B adenosine receptor subtype. Pharmacol. Res. 2015;99:229–236. doi: 10.1016/j.phrs.2015.06.015. [DOI] [PubMed] [Google Scholar]
- 247.Patel L., Thaker A. The effects of adenosine A2B receptor inhibition on VEGF and nitric oxide axis-mediated renal function in diabetic nephropathy. Ren. Fail. 2014;36:916–924. doi: 10.3109/0886022X.2014.900404. [DOI] [PubMed] [Google Scholar]
- 248.Tak E., Ridyard D., Kim J.H., Zimmerman M., Werner T., Wang X.X., Shabeka U., Seo S.W., Christians U., Klawitter J., et al. CD73-dependent generation of adenosine and endothelial adora2b signaling attenuate diabetic nephropathy. J. Am. Soc. Nephrol. 2014;25:547–563. doi: 10.1681/ASN.2012101014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Sun Y., Huang P. Adenosine A2B receptor: From cell biology to human diseases. Front. Chem. 2016;4:37. doi: 10.3389/fchem.2016.00037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Antonioli L., Blandizzi C., Pacher P., Haskó G. Immunity, inflammation and cancer: A leading role for adenosine. Nat. Rev. Cancer. 2013;13:842–857. doi: 10.1038/nrc3613. [DOI] [PubMed] [Google Scholar]
- 251.Cekic C., Sag D., Li Y., Theodorescu D., Strieter R.M., Linden J. Adenosine A 2B Receptor Blockade Slows Growth of Bladder and Breast Tumors. J. Immunol. 2012;188:198–205. doi: 10.4049/jimmunol.1101845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Iannone R., Miele L., Maiolino P., Pinto A., Morello S. Blockade of A2b adenosine receptor reduces tumor growth and immune suppression mediated by myeloid-derived suppressor cells in a mouse model of melanoma. Neoplasia. 2013;15:1400–1409. doi: 10.1593/neo.131748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Ryzhov S., Novitskiy S.V., Zaynagetdinov R., Goldstein A.E., Carbone D.P., Biaggioni I., Dikov M.M., Feoktistov I. Host A2B receptors promote carcinoma growth. Neoplasia. 2008;10:987–995. doi: 10.1593/neo.08478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Merighi S., Simioni C., Gessi S., Varani K., Mirandola P., Tabrizi M.A., Baraldi P.G., Borea P.A. A2B and A3 adenosine receptors modulate vascular endothelial growth factor and interleukin-8 expression in human melanoma cells treated with etoposide and doxorubicin. Neoplasia. 2009;11:1064–1073. doi: 10.1593/neo.09768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Gessi S., Merighi S., Sacchetto V., Simioni C., Borea P.A. Adenosine receptors and cancer. Biochim. Biophys. Acta Biomembr. 2011;1808:1400–1412. doi: 10.1016/j.bbamem.2010.09.020. [DOI] [PubMed] [Google Scholar]
- 256.Ramkumar V., Stiles G.L., Beaven M.A., Ali H. The A3 adenosine receptor is the unique adenosine receptor which facilitates release of allergic mediators in mast cells. J. Biol. Chem. 1993;268:16887–16890. [PubMed] [Google Scholar]
- 257.Walker B.A., Jacobson M.A., Knight D.A., Salvatore C.A., Weir T., Zhou D., Bai T.R. Adenosine A3 receptor expression and function in eosinophils. Am. J. Respir. Cell Mol. Biol. 1997;16:531–537. doi: 10.1165/ajrcmb.16.5.9160835. [DOI] [PubMed] [Google Scholar]
- 258.Gao Z., Li B.S., Day Y.J., Linden J. A3 adenosine receptor activation triggers phosphorylation of protein kinase B and protects rat basophilic leukemia 2H3 mast cells from apoptosis. Mol. Pharmacol. 2001;59:76–82. doi: 10.1124/mol.59.1.76. [DOI] [PubMed] [Google Scholar]
- 259.Spruntulis L.M., Broadley K.J. A3 receptors mediate rapid inflammatory cell influx into the lungs of sensitized guinea-pigs. Clin. Exp. Allergy. 2001;31:943–951. doi: 10.1046/j.1365-2222.2001.01087.x. [DOI] [PubMed] [Google Scholar]
- 260.Fossetta J., Jackson J., Deno G., Fan X., Du X.K., Bober L., Soudé-Bermejo A., De Bouteiller O., Caux C., Lunn C., et al. Pharmacological analysis of calcium responses mediated by the human A3 adenosine receptor in monocyte-derived dendritic cells and recombinant cells. Mol. Pharmacol. 2003;63:342–350. doi: 10.1124/mol.63.2.342. [DOI] [PubMed] [Google Scholar]
- 261.Martin L., Pingle S.C., Hallam D.M., Rybak L.P., Ramkumar V. Activation of the adenosine A3 receptor in RAW 264.7 cells inhibits lipopolysaccharide-stimulated tumor necrosis factor-alpha release by reducing calcium-dependent activation of nuclear factor-kappaB and extracellular signal-regulated kinase 1/2. J. Pharmacol. Exp. Ther. 2006;316:71–78. doi: 10.1124/jpet.105.091868. [DOI] [PubMed] [Google Scholar]
- 262.Lee J.Y., Jhun B.S., Oh Y.T., Lee J.H., Choe W., Baik H.H., Ha J., Yoon K.S., Kim S.S., Kang I. Activation of adenosine A3 receptor suppresses lipopolysaccharide-induced TNF-alpha production through inhibition of PI 3-kinase/Akt and NF-kappaB activation in murine BV2 microglial cells. Neurosci. Lett. 2006;396:1–6. doi: 10.1016/j.neulet.2005.11.004. [DOI] [PubMed] [Google Scholar]
- 263.Chen Y., Corriden R., Inoue Y., Yip L., Hashiguchi N., Zinkernagel A., Nizet V., Insel P.A., Junger W.G. ATP release guides neutrophil chemotaxis via P2Y2 and A3 receptors. Science. 2006;314:1792–1795. doi: 10.1126/science.1132559. [DOI] [PubMed] [Google Scholar]
- 264.Gessi S., Varani K., Merighi S., Cattabriga E., Iannotta V., Leung E., Baraldi P.G., Borea P.A. A(3) adenosine receptors in human neutrophils and promyelocytic HL60 cells: A pharmacological and biochemical study. Mol. Pharmacol. 2002;61:415–424. doi: 10.1124/mol.61.2.415. [DOI] [PubMed] [Google Scholar]
- 265.Gessi S., Varani K., Merighi S., Cattabriga E., Avitabile A., Gavioli R., Fortini C., Leung E., Mac Lennan S., Borea P.A. Expression of A3 Adenosine Receptors in Human Lymphocytes: Up-Regulation in T Cell Activation. Mol. Pharmacol. 2004;65:711–719. doi: 10.1124/mol.65.3.711. [DOI] [PubMed] [Google Scholar]
- 266.Szabo C., Scott G.S., Virag L., Egnaczyk G., Salzman A.L., Shanley T.P., Hasko G. Suppression of macrophage inflammatory protein (MIP)-1alpha production and collagen-induced arthritis by adenosine receptor agonists. Br. J. Pharmacol. 1998;125:379–387. doi: 10.1038/sj.bjp.0702040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Baharav E., Bar-Yehuda S., Madi L., Silberman D., Rath-Wolfson L., Halpren M., Ochaion A., Weinberger A., Fishman P. Antiinflammatory effect of A3 adenosine receptor agonists in murine autoimmune arthritis models. J. Rheumatol. 2005;32:469–476. [PubMed] [Google Scholar]
- 268.Silverman M.H., Strand V., Markovits D., Nahir M., Reitblat T., Molad Y., Rosner I., Rozenbaum M., Mader R., Adawi M., et al. Clinical evidence for utilization of the A3 adenosine receptor as a target to treat rheumatoid arthritis: Data from a phase II clinical trial. J. Rheumatol. 2008;35:41–48. [PubMed] [Google Scholar]
- 269.Cohen S., Barer F., Bar-Yehuda S., Ijzerman A.P., Jacobson K.A., Fishman P. A3 adenosine receptor allosteric modulator induces an anti-inflammatory effect: In vivo studies and molecular mechanism of action. Mediat. Inflamm. 2014;3:708746. doi: 10.1155/2014/708746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Lee H.T., Kim M., Joo J.D., Gallos G., Chen J.F., Emala C.W. A3 adenosine receptor activation decreases mortality and renal and hepatic injury in murine septic peritonitis. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2006;291:R959–R969. doi: 10.1152/ajpregu.00034.2006. [DOI] [PubMed] [Google Scholar]
- 271.Matot I., Weiniger C.F., Zeira E., Galun E., Joshi B.V., Jacobson K.A. A3 adenosine receptors and mitogen-activated protein kinases in lung injury following in vivo reperfusion. Crit. Care. 2006;10:R65. doi: 10.1186/cc4893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.David M., Akerman L., Ziv M., Kadurina M., Gospodinov D., Pavlotsky F., Yankova R., Kouzeva V., Ramon M., Silverman M.H., et al. Treatment of plaque-type psoriasis with oral CF101: Data from an exploratory randomized phase 2 clinical trial. J. Eur. Acad. Dermatol. Venereol. 2012;26:361–367. doi: 10.1111/j.1468-3083.2011.04078.x. [DOI] [PubMed] [Google Scholar]
- 273.Yang H., Avila M.Y., Peterson-Yantorno K., Coca-Prados M., Stone R.A., Jacobson K.A., Civan M.M. The cross-species A3 adenosine-receptor antagonist MRS 1292 inhibits adenosine-triggered human nonpigmented ciliary epithelial cell fluid release and reduces mouse intraocular pressure. Curr. Eye Res. 2005;30:747–754. doi: 10.1080/02713680590953147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Okamura T., Kurogi Y., Hashimoto K., Sato S., Nishikawa H., Kiryu K., Nagao Y. Structure-activity relationships of adenosine A3 receptor ligands: New potential therapy for the treatment of glaucoma. Bioorg. Med. Chem. Lett. 2004;14:3775–3779. doi: 10.1016/j.bmcl.2004.04.099. [DOI] [PubMed] [Google Scholar]
- 275.Jacobson K.A., Civan M.M. Ocular Purine Receptors as Drug Targets in the Eye. J. Ocul. Pharmacol. Ther. 2016;32:534–547. doi: 10.1089/jop.2016.0090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Hua X., Chason K.D., Fredholm B.B., Deshpande D.A., Penn R.B., Tilley S.L. Adenosine induces airway hyperresponsiveness through activation of A3 receptors on mast cells. J. Allergy Clin. Immunol. 2008;122:107–113. doi: 10.1016/j.jaci.2008.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Zhong H., Shlykov S.G., Molina J.G., Sanborn B.M., Jacobson M.A., Tilley S.L., Blackburn M.R. Activation of murine lung mast cells by the adenosine A3 receptor. J. Immunol. 2003;171:338–345. doi: 10.4049/jimmunol.171.1.338. [DOI] [PubMed] [Google Scholar]
- 278.Rudich N., Ravid K., Sagi-Eisenberg R. Mast cell adenosine receptors function: A focus on the A3 adenosine receptor and inflammation. Front. Immunol. 2012;3:134. doi: 10.3389/fimmu.2012.00134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Ezeamuzie C.I., Philips E. Adenosine A3 receptors on human eosinophils mediate inhibition of degranulation and superoxide anion release. Br. J. Pharmacol. 1999;127:188–194. doi: 10.1038/sj.bjp.0702476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Young H.W.J., Molina J.G., Dimina D., Zhong H., Jacobson M., Chan L.N.L., Chan T.S., Lee J.J., Blackburn M.R. A3 adenosine receptor signaling contributes to airway inflammation and mucus production in adenosine deaminase-deficient mice. J. Immunol. 2004;173:1380–1389. doi: 10.4049/jimmunol.173.2.1380. [DOI] [PubMed] [Google Scholar]
- 281.Rimmer J., Peake H.L., Santos C.M.C., Lean M., Bardin P., Robson R., Haumann B., Loehrer F., Handel M.L. Targeting adenosine receptors in the treatment of allergic rhinitis: A randomized, double-blind, placebo-controlled study. Clin. Exp. Allergy. 2007;37:8–14. doi: 10.1111/j.1365-2222.2006.02546.x. [DOI] [PubMed] [Google Scholar]
- 282.Morschl E., Molina J.G., Volmer J.B., Mohsenin A., Pero R.S., Hong J.S., Kheradmand F., Lee J.J., Blackburn M.R. A3 Adenosine Receptor Signaling Influences Pulmonary Inflammation and Fibrosis. Am. J. Respir. Cell Mol. Biol. 2008;39:697–705. doi: 10.1165/rcmb.2007-0419OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Hussain A., Karjian P., Maddock H. The role of nitric oxide in A3 adenosine receptor-mediated cardioprotection. Auton. Autacoid Pharmacol. 2009;29:97–104. doi: 10.1111/j.1474-8673.2009.00438.x. [DOI] [PubMed] [Google Scholar]
- 284.Maddock H.L., Mocanu M.M., Yellon D.M. Adenosine A(3) receptor activation protects the myocardium from reperfusion/reoxygenation injury. Am. J. Physiol. Heart Circ. Physiol. 2002;283:H1307–H1313. doi: 10.1152/ajpheart.00851.2001. [DOI] [PubMed] [Google Scholar]
- 285.Headrick J.P., Peart J. A3 adenosine receptor-mediated protection of the ischemic heart. Vascul. Pharmacol. 2005;42:271–279. doi: 10.1016/j.vph.2005.02.009. [DOI] [PubMed] [Google Scholar]
- 286.Black R.G.J., Guo Y., Ge Z.D., Murphree S.S., Prabhu S.D., Jones W.K., Bolli R., Auchampach J.A. Gene dosage-dependent effects of cardiac-specific overexpression of the A3 adenosine receptor. Circ. Res. 2002;91:165–172. doi: 10.1161/01.RES.0000028007.91385.EE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Hinze A.V., Mayer P., Harst A., von Kugelgen I. Adenosine A(3) receptor-induced proliferation of primary human coronary smooth muscle cells involving the induction of early growth response genes. J. Mol. Cell. Cardiol. 2012;53:639–645. doi: 10.1016/j.yjmcc.2012.08.003. [DOI] [PubMed] [Google Scholar]
- 288.Lu Z., Fassett J., Xu X., Hu X., Zhu G., French J., Zhang P., Schnermann J., Bache R.J., Chen Y. Adenosine A3 receptor deficiency exerts unanticipated protective effects on the pressure-overloaded left ventricle. Circulation. 2008;118:1713–1721. doi: 10.1161/CIRCULATIONAHA.108.788307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Yang T., Zollbrecht C., Winerdal M.E., Zhuge Z., Zhang X.M., Terrando N., Checa A., Sallstrom J., Wheelock C.E., Winqvist O., et al. Genetic Abrogation of Adenosine A3 Receptor Prevents Uninephrectomy and High Salt-Induced Hypertension. J. Am. Heart Assoc. 2016;5:e003868. doi: 10.1161/JAHA.116.003868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Ansari H.R., Nadeem A., Tilley S.L., Mustafa S.J. Involvement of COX-1 in A3 adenosine receptor-mediated contraction through endothelium in mice aorta. Am. J. Physiol. Heart Circ. Physiol. 2007;293:H3448–H3455. doi: 10.1152/ajpheart.00764.2007. [DOI] [PubMed] [Google Scholar]
- 291.El-Awady M.S., Ansari H.R., Fil D., Tilley S.L., Mustafa S.J. NADPH oxidase pathway is involved in aortic contraction induced by A3 adenosine receptor in mice. J. Pharmacol. Exp. Ther. 2011;338:711–717. doi: 10.1124/jpet.111.180828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Grandoch M., Hoffmann J., Rock K., Wenzel F., Oberhuber A., Schelzig H., Fischer J.W. Novel effects of adenosine receptors on pericellular hyaluronan matrix: Implications for human smooth muscle cell phenotype and interactions with monocytes during atherosclerosis. Basic Res. Cardiol. 2013;108:340. doi: 10.1007/s00395-013-0340-6. [DOI] [PubMed] [Google Scholar]
- 293.Gessi S., Fogli E., Sacchetto V., Merighi S., Varani K., Preti D., Leung E., Maclennan S., Borea P.A. Adenosine modulates HIF-1{alpha}, VEGF, IL-8, and foam cell formation in a human model of hypoxic foam cells. Arterioscler. Thromb. Vasc. Biol. 2010;30:90–97. doi: 10.1161/ATVBAHA.109.194902. [DOI] [PubMed] [Google Scholar]
- 294.Rothermel B.A., Hill J.A. Adenosine A3 receptor and cardioprotection: Enticing, enigmatic, elusive. Circulation. 2008;118:1691–1693. doi: 10.1161/CIRCULATIONAHA.108.810101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Rivkees S.A., Thevananther S., Hao H. Are A3 adenosine receptors expressed in the brain? Neuroreport. 2000;11:1025–1030. doi: 10.1097/00001756-200004070-00026. [DOI] [PubMed] [Google Scholar]
- 296.Pugliese A.M., Coppi E., Volpini R., Cristalli G., Corradetti R., Jeong L.S., Jacobson K.A., Pedata F. Role of adenosine A3 receptors on CA1 hippocampal neurotransmission during oxygen-glucose deprivation episodes of different duration. Biochem. Pharmacol. 2007;74:768–779. doi: 10.1016/j.bcp.2007.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Sei Y., von Lubitz D.K.J.E., Abbracchio M.P., Ji X., Jacobson K.A. Adenosine A3 receptor agonist-induced neurotoxicity in rat cerebellar granule neurons. Drug Dev. Res. 1997;40:267–273. doi: 10.1002/(SICI)1098-2299(199703)40:3<267::AID-DDR7>3.0.CO;2-I. [DOI] [Google Scholar]
- 298.Von Lubitz D.K., Lin R.C., Boyd M., Bischofberger N., Jacobson K.A. Chronic administration of adenosine A3 receptor agonist and cerebral ischemia: Neuronal and glial effects. Eur. J. Pharmacol. 1999;367:157–163. doi: 10.1016/S0014-2999(98)00977-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Wittendorp M.C., Boddeke H.W.G.M., Biber K. Adenosine A3 receptor-induced CCL2 synthesis in cultured mouse astrocytes. Glia. 2004;46:410–418. doi: 10.1002/glia.20016. [DOI] [PubMed] [Google Scholar]
- 300.Pugliese A.M., Coppi E., Spalluto G., Corradetti R., Pedata F. A3 adenosine receptor antagonists delay irreversible synaptic failure caused by oxygen and glucose deprivation in the rat CA1 hippocampus in vitro. Br. J. Pharmacol. 2006;147:524–532. doi: 10.1038/sj.bjp.0706646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Etherington L.A.V., Frenguelli B.G. Endogenous adenosine modulates epileptiform activity in rat hippocampus in a receptor subtype-dependent manner. Eur. J. Neurosci. 2004;19:2539–2550. doi: 10.1111/j.0953-816X.2004.03355.x. [DOI] [PubMed] [Google Scholar]
- 302.Laudadio M.A., Psarropoulou C. The A3 adenosine receptor agonist 2-Cl-IB-MECA facilitates epileptiform discharges in the CA3 area of immature rat hippocampal slices. Epilepsy Res. 2004;59:83–94. doi: 10.1016/j.eplepsyres.2004.03.005. [DOI] [PubMed] [Google Scholar]
- 303.Zhu C.B., Steiner J.A., Munn J.L., Daws L.C., Hewlett W.A., Blakely R.D. Rapid stimulation of presynaptic serotonin transport by A(3) adenosine receptors. J. Pharmacol. Exp. Ther. 2007;322:332–340. doi: 10.1124/jpet.107.121665. [DOI] [PubMed] [Google Scholar]
- 304.Zhu C.B., Lindler K.M., Campbell N.G., Sutcliffe J.S., Hewlett W.A., Blakely R.D. Colocalization and regulated physical association of presynaptic serotonin transporters with A(3) adenosine receptors. Mol. Pharmacol. 2011;80:458–465. doi: 10.1124/mol.111.071399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Mabley J., Soriano F., Pacher P., Haskó G., Marton A., Wallace R., Salzman A., Szabó C. The adenosine A3 receptor agonist, N6-(3-iodobenzyl)-adenosine-5′-N-methyluronamide, is protective in two murine models of colitis. Eur. J. Pharmacol. 2003;466:323–329. doi: 10.1016/S0014-2999(03)01570-X. [DOI] [PubMed] [Google Scholar]
- 306.Ren T., Tian T., Feng X., Ye S., Wang H., Wu W., Qiu Y., Yu C., He Y., Zeng J., et al. An adenosine A3 receptor agonist inhibits DSS-induced colitis in mice through modulation of the NF-κB signaling pathway. Sci. Rep. 2015;5:9047. doi: 10.1038/srep09047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Lee H.T., Ota-Setlik A., Xu H., D’Agati V.D., Jacobson M.A., Emala C.W. A 3 adenosine receptor knockout mice are protected against ischemia- and myoglobinuria-induced renal failure. Am. J. Physiol. Renal. Physiol. 2003;284:F267–F273. doi: 10.1152/ajprenal.00271.2002. [DOI] [PubMed] [Google Scholar]
- 308.Yap S.C., Thomas Lee H. Adenosine and protection from acute kidney injury. Curr. Opin. Nephrol. Hypertens. 2012;21:24–32. doi: 10.1097/MNH.0b013e32834d2ec9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Madi L., Ochaion A., Rath-Wolfson L., Bar-Yehuda S., Erlanger A., Ohana G., Harish A., Merimski O., Barer F., Fishman P. The A3 adenosine receptor is highly expressed in tumor versus normal cells: Potential target for tumor growth inhibition. Clin. Cancer Res. 2004;10:4472–4479. doi: 10.1158/1078-0432.CCR-03-0651. [DOI] [PubMed] [Google Scholar]
- 310.Fishman P., Bar-Yehuda S., Synowitz M., Powell J.D., Klotz K.N., Gessi S., Borea P.A. Adenosine receptors and cancer. In: Wilson C.N., Musta S.J., editors. Adenosine Receptors in Health and Disease. Springer; Berlin/Heidelberg, Germany: 2009. pp. 399–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311.Gessi S., Merighi S., Varani K., Leung E., Mac Lennan S., Borea P.A. The A3 adenosine receptor: An enigmatic player in cell biology. Pharmacol. Ther. 2008;117:123–140. doi: 10.1016/j.pharmthera.2007.09.002. [DOI] [PubMed] [Google Scholar]
- 312.Fishman P., Bar-Yehuda S., Vagman L. Adenosine and other low molecular weight factors released by muscle cells inhibit tumor cell growth. Cancer Res. 1998;58:3181–3187. [PubMed] [Google Scholar]
- 313.Fishman P., Bar-Yehuda S., Ohana G., Pathak S., Wasserman L., Barer F., Multani A.S. Adenosine acts as an inhibitor of lymphoma cell growth: A major role for the A3 adenosine receptor. Eur. J. Cancer. 2000;36:1452–1458. doi: 10.1016/S0959-8049(00)00130-1. [DOI] [PubMed] [Google Scholar]
- 314.Zhang X., Zhang M., Laties A.M., Mitchell C.H. Balance of purines may determine life or death of retinal ganglion cells as A3 adenosine receptors prevent loss following P2X7 receptor stimulation. J. Neurochem. 2006;98:566–575. doi: 10.1111/j.1471-4159.2006.03900.x. [DOI] [PubMed] [Google Scholar]
- 315.Gessi S., Merighi S., Varani K., Cattabriga E., Benini A., Mirandola P., Leung E., Mac Lennan S., Feo C., Baraldi S., et al. Adenosine receptors in colon carcinoma tissues and colon tumoral cell lines: Focus on the A(3) adenosine subtype. J. Cell. Physiol. 2007;211:826–836. doi: 10.1002/jcp.20994. [DOI] [PubMed] [Google Scholar]
- 316.Powis G., Kirkpatrick L. Hypoxia inducible factor-1alpha as a cancer drug target. Mol. Cancer Ther. 2004;3:647–654. [PubMed] [Google Scholar]
- 317.Merighi S., Benini A., Mirandola P., Gessi S., Varani K., Leung E., MacLennan S., Baraldi P.G., Borea P.A. A3 adenosine receptors modulate hypoxia-inducible factor-1α expression in human A375 melanoma cells. Neoplasia. 2005;7:894–903. doi: 10.1593/neo.05334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318.Merighi S., Benini A., Mirandola P., Gessi S., Varani K., Leung E., Maclennan S., Borea P.A. Adenosine modulates vascular endothelial growth factor expression via hypoxia-inducible factor-1 in human glioblastoma cells. Biochem. Pharmacol. 2006;72:19–31. doi: 10.1016/j.bcp.2006.03.020. [DOI] [PubMed] [Google Scholar]
- 319.Rocha R., Torres Á., Ojeda K., Uribe D., Rocha D., Erices J., Niechi I., Ehrenfeld P., San Martín R., Quezada C. The Adenosine A₃ Receptor Regulates Differentiation of Glioblastoma Stem-Like Cells to Endothelial Cells under Hypoxia. Int. J. Mol. Sci. 2018;19:1228. doi: 10.3390/ijms19041228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 320.Ohana G., Bar-Yehuda S., Arich A., Madi L., Dreznick Z., Rath-Wolfson L., Silberman D., Slosman G., Fishman P. Inhibition of primary colon carcinoma growth and liver metastasis by the A3 adenosine receptor agonist CF101. Br. J. Cancer. 2003;89:1552–1558. doi: 10.1038/sj.bjc.6601315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 321.Madi L., Bar-Yehuda S., Barer F., Ardon E., Ochaion A., Fishman P. A3 adenosine receptor activation in melanoma cells: Association between receptor fate and tumor growth inhibition. J. Biol. Chem. 2003;278:42121–42130. doi: 10.1074/jbc.M301243200. [DOI] [PubMed] [Google Scholar]
- 322.Fishman P., Bar-Yehuda S., Ardon E., Rath-Wolfson L., Barrer F., Ochaion A., Madi L. Targeting the A3 adenosine receptor for cancer therapy: Inhibition of prostate carcinoma cell growth by A3AR agonist. Anticancer Res. 2003;23:2077–2083. [PubMed] [Google Scholar]
- 323.Bar-Yehuda S., Stemmer S.M., Madi L., Castel D., Ochaion A., Cohen S., Barer F., Zabutti A., Perez-Liz G., Del Valle L., et al. The A3 adenosine receptor agonist CF102 induces apoptosis of hepatocellular carcinoma via de-regulation of the Wnt and NF-κB signal transduction pathways. Int. J. Oncol. 2008;33:287–295. [PubMed] [Google Scholar]
- 324.Fishman P., Bar-Yehuda S., Madi L., Cohn I. A3 adenosine receptor as a target for cancer therapy. Anti-Cancer Drugs. 2002;13:437–443. doi: 10.1097/00001813-200206000-00001. [DOI] [PubMed] [Google Scholar]
- 325.Marucci G., Santinelli C., Buccioni M., Navia A.M., Lambertucci C., Zhurina A., Yli-Harja O., Volpini R., Kandhavelu M. Anticancer activity study of A3 adenosine receptor agonists. Life Sci. 2018;205:155–163. doi: 10.1016/j.lfs.2018.05.028. [DOI] [PubMed] [Google Scholar]