Abstract
Observational epidemiological studies indicate that endometriosis and migraine co-occur within individuals more than expected by chance. However, the aetiology and biological mechanisms underlying their comorbidity remain unknown. Here we examined the relationship between endometriosis and migraine using genome-wide association study (GWAS) data. Single nucleotide polymorphism (SNP) effect concordance analysis found a significant concordance of SNP risk effects across endometriosis and migraine GWAS. Linkage disequilibrium score regression analysis found a positive and highly significant genetic correlation (rG = 0.38, P = 2.30 × 10−25) between endometriosis and migraine. A meta-analysis of endometriosis and migraine GWAS data did not reveal novel genome-wide significant SNPs, and Mendelian randomisation analysis found no evidence for a causal relationship between the two traits. However, gene-based analyses identified two novel loci for migraine. Also, we found significant enrichment of genes nominally associated (Pgene < 0.05) with both traits (Pbinomial-test = 9.83 × 10−6). Combining gene-based p-values across endometriosis and migraine, three genes, two (TRIM32 and SLC35G6) of which are at novel loci, were genome-wide significant. Genes having Pgene < 0.1 for both endometriosis and migraine (Pbinomial-test = 1.85 ×10−°3) were significantly enriched for biological pathways, including interleukin-1 receptor binding, focal adhesion-PI3K-Akt-mTOR-signaling, MAPK and TNF-α signalling. Our findings further confirm the comorbidity of endometriosis and migraine and indicate a non-causal relationship between the two traits, with shared genetically-controlled biological mechanisms underlying the co-occurrence of the two disorders.
Keywords: causality, comorbidity, endometriosis, gene-based association study, genetic overlap, GWAS, Mendelian randomisation, migraine, molecular genetics, pathway enrichment study
1. Introduction
Endometriosis is one of the leading gynaecological disorders affecting 6%–10% of women of reproductive age and 35%–50% of women with infertility worldwide [1,2]. The disorder is defined by the presence of endometrial tissue in extra-uterine locations and characterised by varying degrees of pelvic, menstrual, abdominal, bowel and lower-back pain as well as infertility [1,2]. With an estimated global prevalence of 14.7%, migraine, on the other hand, is the most disabling neurologic disorder and the third most common illness worldwide [3,4]. Like endometriosis, women in their reproductive and most productive years are more commonly affected with migraine [5,6]. A typical migraine presents with a recurrent, unilateral and episodic headache of moderate to severe intensity [7]. Both endometriosis and migraine portend substantial morbidity with wide-ranging socioeconomic burdens to sufferers, their families, relationships, and the society at large [8,9,10,11]. Notably, the diagnosis of the two disorders is challenging, due to a lack of diagnostic markers, which often results in missed or delayed diagnosis. Also, the aetiology and pathogenesis of endometriosis and migraine remain relatively obscure, and there are currently no known curative treatments for them.
While endometriosis and migraine appear to have clear-cut distinctions—anatomically, as well as in terms of clinical diagnosis and disease classification—some shared epidemiological characteristics or similarities suggest a comorbid relationship between them. For instance, similar to endometriosis, which almost exclusively affects women [12], migraine has a substantially higher prevalence in women (15%‒18%) compared to men (6%), and women of reproductive age also experience a longer duration of migraine attacks with greater disability [13,14,15,16]. The two disorders share similar risk factors in women including early menarche, menorrhagia, and involvement of the menstrual cycle in their pathogenesis [17,18,19]. Indeed, increased exposure to menstruation is a known risk factor for endometriosis just as menstrual migraine and menstrually-related migraine (with prevalence varying from 4%–70%) are common subtypes of migraine in women [20,21,22,23,24]. Furthermore, Danazol (a synthetic androgen for managing endometriosis) has been reported to reduce the frequency of migraine attacks [25]. In addition to their shared similarities and risk factors, the comorbidity (co-occurrence of two or more conditions in the same individual) of endometriosis with migraine has been consistently reported by observational epidemiological studies [5,6,18,26,27,28,29].
As far back as 1975, for example, a clinic-based study had reported a higher prevalence of headache (84%) among women diagnosed with endometriosis compared to the control (60%, P = 0.007) [26]. Interestingly, 28% of the endometriosis cases described their headaches as migraine-like compared to only 18% (P = 0.023) in control [26]. In a related case-control study, over two times higher prevalence of migraine was found in endometriosis (38.3%) compared to the control (15.1%, P < 0.001) [27]. A study investigating the cost implications of endometriosis in the United States similarly found a three-fold greater prevalence of migraine in endometriosis compared to the general population [28]. More recently, adolescents with surgically confirmed endometriosis had over two-fold greater prevalence (69.3% vs 30.7%) and nearly five-fold increased odds of migraine (adjusted Odds Ratio [AOR] = 4.77; 95% CI: 2.53–9.02) compared to their counterparts with no endometriosis [30]. Also, a recent French case-control study similarly found a higher prevalence of endometriosis in migraine cases (35.2%) compared to controls (17.4%, P = 0.003) [5].
The consistent and growing evidence on the endometriosis–migraine comorbid relationship, notwithstanding, some questions remain unanswered. First, is the endometriosis–migraine comorbidity reported in observational studies a true association, or could the findings be due to the confounding effects, biases, or otherwise false-positive results of the traditional observational studies? Second, is there a causal relationship between endometriosis and migraine? Third, are there some shared genetic variants, susceptibility loci, genes and biological pathways between the two disorders? Last, what biological mechanism(s) may underlie possible endometriosis–migraine comorbidity?
Using a twin-based study approach, Nyholt et al. [13] examined the genetic influences and comorbidity of migraine and endometriosis and reported that additive genetic factors accounting for 69% (95% CI: 60%–77%) of the phenotypic variance in migraine also account for 17% (95% CI: 8%–27%) of the variance in endometriosis (i.e., bivariate heritability of 17%)—suggesting shared genetic influences completely explain their co-occurrence within individuals. Additional bivariate heritability analyses utilising direction-of-causation twin models did not support endometriosis as the cause of migraine or vice versa; however, given the sample size and similar heritability for endometriosis and migraine, these analyses lacked power [13].
To date, genome-wide association studies (GWAS) have identified 19 independent single nucleotide polymorphisms (SNPs) for endometriosis [31] and 44 for migraine [32]. However, molecular genetic studies of the association between endometriosis and migraine, including causality and shared genetic risk variants and loci are currently lacking. Lastly, biological pathways driving possible endometriosis and migraine comorbidity remain poorly understood. The present study, thus, aims to assess the molecular genetic overlap, causal relationship and shared pathways between endometriosis and migraine using GWAS data.
2. Materials and Methods
2.1. Data Sources and Study Samples
We utilise GWAS meta-analysis summary statistics from the International Endogene Consortium (IEC, endometriosis GWAS data) [31] and the International Headache Genetics Consortium (IHGC, migraine GWAS data) for analysis in the present study. Summary statistics data sourced from the United Kingdom Biobank (UKBB, migraine GWAS data) were used in testing the reproducibility of our findings for SNP-level genetic overlap and correlation studies.
2.1.1. IEC Endometriosis GWAS Data
The ‘IEC endometriosis’ GWAS summary statistics utilised in this study represent the largest endometriosis genetic study published to date [31]. The data combined 11 separate GWAS case-control datasets (QIMRHCS, deCODE, LEUVEN, OX, 23andMe, NHS2-dbGaP, WGHS, iPSYCH, BBJ, Adachi-6, and Adachi-500K) consisting of 17,054 cases of endometriosis (all stages of endometriosis) and 191,858 controls (n = 208,912). A total of 6,979,035 SNPs passed quality control in six or more (at least 50%) of the studies and those were included in a fixed-effect meta-analysis [31]. Study participants in the GWAS were of European (approximately 93%) and Japanese ancestries (from Australia, Belgium, Denmark, Iceland, Japan, the UK, and the USA). Endometriosis was surgically confirmed (using the revised American Fertility Society system [33]) in cases from QIMRHCS, OX, deCODE and LEUVEN studies, while cases from other studies were self-reported or their diagnosis was based on combined self-report and surgical records [31]. Similar quality control procedures were used in each of the GWAS. A detailed description of these GWAS, the quality control and the analyses carried out have previously been published [31].
2.1.2. IHGC Migraine GWAS Data
Our migraine data were sourced from the 2016 IHGC (http://www.headachegenetics.org) migraine GWAS, which meta-analysed migraine summary statistics from 22 GWAS (obtained from six tertiary headache clinics and 27 population-based cohorts) [32]. A total of 59,674 migraine cases and 316,078 controls were included in the meta-analysis, and all participants were unrelated individuals of European ancestry [32]. Diagnosis of migraine was through self-reported questionnaires or clinical interview, and, in line with the criteria of the International Classification of Headache Disorder (ICHD) [34]. Standard protocols for quality control were included and a common 1000 Genomes Project [35] reference panel (Phase I, v3) was used in imputing missing genotypes into each of the samples. Logistic regression analysis was conducted on the imputed genotypes in each of the GWAS for association analysis [32].
To account for possible population stratification and other confounders, an adjustment was made for the top ten principal components, sex and other covariates where necessary [32]. The GWAMA program [36] was used to perform a combined fixed-effect meta-analysis. SNPs were filtered based on imputation quality and other metrics [32]. A detailed and more comprehensive description of the ‘IHGC migraine’ GWAS sample has previously been published [32]. The data utilised in the present study were restricted to 29,208 cases and 172,931 controls (n = 202,139) with a total of 8,935,979 SNPs, following the exclusion of the 23andMe GWAS sample (30,465 migraine cases and 143,147 controls). The 23andMe GWAS sample was excluded to ensure there was no sample overlap between the ‘IEC endometriosis’ GWAS data (which comprise 23andMe GWAS data) and the ‘IHGC migraine’ GWAS data.
2.1.3. United Kingdom (UK) Biobank Data
The UK Biobank is a large, population-based cohort study that was established in the United Kingdom in the year 2006. A total of 500,000 volunteers aged 40–69 years were recruited for the study between 2006 and 2010 with the aim of investigating the genetic and environmental determinants of health and diseases [37]. Extensive genotype and phenotype data, including biological samples, physical measurements, health and lifestyle information, multimodal imaging, and genome-wide genotyping have been collected from these study participants [37]. Also, a variety of their health-related outcomes are being followed up [37]. Anonymised data from the study are made available to researchers via an application process [37]. We utilised UK Biobank GWAS summary statistics for migraine sourced from the Neale Lab, which performed linear regression analysis controlling for 10 principal components of ancestry, in a sample of 337,159 unrelated individuals of “White British” ancestry, comprising 10,007 self-reported migraine cases and 327,152 controls (https://nealelab.github.io/UKBB_ldsc/h2_summary_20002_1265.html, downloaded 12/03/2018). GWAS summary statistics were available for 10,894,597 SNPs.
2.2. SNP Effect Concordance Analysis (SECA)
We assessed the genetic overlap between the ‘IEC endometriosis’ GWAS data and the ‘IHGC migraine’ GWAS data using SECA (https://sites.google.com/site/qutsgel/software/seca-local-version) [38]. SECA utilises GWAS summary statistics data and tests whether the direction of single nucleotide polymorphism (SNPs) are positively correlated across GWAS results thereby facilitating the assessment of genetic overlap between traits [38]. We formatted our datasets appropriately so that SECA requirements were met [38]. Thereafter, the ‘IEC endometriosis’ GWAS data was assigned, for SECA analysis, as dataset 1 and the ‘IHGC migraine’ GWAS as dataset 2. SECA first aligns the SNP effects across dataset 1 and dataset 2 to the same effect allele, and, subsequently extracts a subset of independent SNPs by utilising a ‘p-value informed’ SNP clumping, accounting for linkage disequilibrium (LD) between SNPs.
For each of the ‘IEC endometriosis’ and ‘IHGC migraine’ GWAS datasets, SECA partitions the extracted SNPs into 12 p-value subsets which ranges from 0.01 to 1 (P ≤ 0.01, 0.05, 0.1, 0.2, 0.3. 0.4, 0.5, 0.6, 0.7, 0.8, 0.9, 1.0). The p-value partitioning yields 144 subsets of SNPs from all possible combinations of dataset 1 (P1, 12 SNP subsets) with dataset 2 (P2, 12 SNP subsets). SECA performs two tests: a binomial test to assess the presence of excess SNP subsets associated between the two datasets, and, the Fisher exact test for the concordance in the direction of effect of the individual SNPs across datasets 1 and dataset 2 [38].
Our SECA analysis was restricted to SNPs that are most strongly associated with dataset 1; hence, we swapped ‘IEC endometriosis’ GWAS data as dataset 2 and ‘IHGC migraine’ GWAS data as dataset 1 in an analogous analysis. This ability to condition on one of the GWAS datasets (not possible using the linkage disequilibrium score regression method can help determine whether an observed genetic overlap is driven similarly by both datasets, or driven predominantly by one dataset. We estimated LD using the 1000G Phase I v3 CEU genotype data and LD pruning prioritised SNPs with smaller p-values (P1) in dataset 1. Also, we tested the reproducibility of our study using independent migraine summary statistics GWAS data from the UKBiobank.
2.3. Linkage Disequilibrium Score Regression (LDSC)
We estimated the SNP-based heritability and cross-trait genetic correlation for endometriosis and migraine using the LDSC software (https://github.com/bulik/ldsc). The ‘IEC endometriosis’ and ‘IHGC migraine’ GWAS data were utilised in the analysis. These datasets were formatted using the ‘munge_sumstats.py’ script in line with the LDSC documentation (https://github.com/bulik/ldsc/wiki/Heritability-and-Genetic-Correlation). We performed univariate LDSC analyses to estimate SNP-based liability heritability (h2SNP) using the ‘IEC endometriosis’ (sample prevalence = 8.2%, population prevalence = 8% [31]) and the ‘IHGC migraine’ (sample prevalence = 14.5%, population prevalence = 15% [39])’ GWAS data. Also, to estimate the genetic correlation (rG) between the two traits, we conducted a bivariate cross-trait LDSC analysis utilising the ‘IEC endometriosis’ GWAS data and the ‘IHGC migraine’ GWAS data. This analysis complements our SECA-based study in assessing the genetic overlap between endometriosis and migraine. We constrained the intercept to one for ‘IEC endometriosis’ GWAS data (both in heritability and cross-trait LDSC correlation analysis) because the estimated intercept (without constraining) was not significantly different from one. We also constrained the genetic covariance intercept to zero (in the cross-trait LDSC correlation analysis) given there was no sample overlap between the two datasets. The intercept for all migraine data was significantly different from one, hence, their estimated intercepts (obtained without constraining) were retained in the model. In all the LDSC analyses, we calculated the LD scores based on the European 1000 Genomes Project haplotype reference data (Phase I, v3). Last, we repeated the above analysis procedures using the ‘IEC endometriosis’ GWAS data and the migraine GWAS data from the UKBB (‘UKBB migraine’ GWAS data).
2.4. Cross-Disorder Meta-Analysis of Endometriosis and Migraine
We conducted a cross-disorder meta-analysis of the ‘IEC endometriosis’ and the ‘IHGC migraine’ GWAS summary statistics data to identify possible genetic variants and loci shared by both endometriosis and migraine. The inverse variance-weighted fixed effect (FE) and ‘Han and Eskin’s random effect’ (RE2) models, implemented in METASOFT (http://genetics.cs.ucla.edu/meta/), were utilised in the meta-analysis. We accounted for possible between-study heterogeneity using RE2—a modified random effect model. Unlike the traditional random effect (RE) model, which is highly conservative, RE2 has greater power under heterogeneity [39,40,41]. We included a total of 411,051 participants in the analysis, and meta-analysed the 6,904,914 SNPs overlapping the two GWAS. We aimed at identifying novel cross-disorder genome-wide significantly enriched (P < 5 × 10−8)) SNPs and loci associated with both endometriosis and migraine.
2.5. Mendelian Randomisation (MR)
To assess the causal relationship between endometriosis and migraine, we performed a two-sample Mendelian Randomization analysis (“TwoSampleMR”) [42] utilising genome-wide significant (P < 5 × 10−8) SNPs associated with ‘IEC endometriosis’ summary statistics data. Randomised controlled trials (RCTs) are considered the most reliable evidence for drawing causal inferences. However, due to limitations such as substantial costs, non-availability of appropriate interventions and controls and certain ethical constraints [43,44], conducting an RCT may not always be feasible. MR analysis mimics the design of an RCT thereby providing an alternative approach to assessing and estimating the causal relationship between an exposure and outcome variables [45].
MR method is anchored on the principle of Mendel’s law of inheritance—gene segregation and natural randomisation at gamete formation which is comparable to the experimental randomisation in RCTs. The method is supported by the understanding that genotypes are naturally fixed at conception and generally not subject to confounding effects or bias of reverse causation [45]. MR analysis, thus, exploits the presence of specific genetic variants associated with the variable of interest as proxies for assessing causality with the outcome of interest. The effect of the genetic variants (instrumental variables, IVs) on the outcome is expected to be through the exposure variable (vertical pleiotropy). Although not without limitations—possible violations of some of its assumptions—MR analysis is increasingly being used as an unbiased causality detection, and, where possible, estimation method [45].
In the present study, we performed “TwoSampleMR” analyses [42]. First, we extracted a total of 338 SNPs associated with endometriosis, in the ‘IEC endometriosis’ GWAS data, at a genome-wide significance level (P < 5 × 10−8). We assigned endometriosis as the exposure variable and migraine (‘IHGC migraine’ GWAS data) as the outcome variable. Following LD clumping (r2 < 0.001; to ensure the independence of the extracted SNPs), 11 genome-wide significant SNPs associated in the ‘IEC endometriosis’ GWAS were retained as our IVs. Second, we extracted SNP effects from the outcome (‘IHGC migraine’ GWAS) data. To ensure that the SNP effects on exposure and outcome data correspond to the same allele, we carried out harmonisation of both the exposure and the outcome variables.
Last, we conducted a “TwoSampleMR” analysis using the inverse variance weighted (IVW) method. IVW estimates are essentially the weighted average of the individual Wald-type ratios for each of the IVs. The IVW method assumes the absence of horizontal pleiotropy or a balance of same among the IVs. We conducted sensitivity analyses to address a possible violation of this assumption using the weighted median (which provides valid causal estimates even if up to 50% of the IVs have pleiotropic effect) [46], and the MR-Egger method (which corrects pleiotropy and provides valid causal estimates even if all the IVs are invalid) [47]. We implemented the “TwoSampleMR” [42] analysis methods in the R statistical package following a well-established protocol (https://mrcieu.github.io/TwoSampleMR/).
MR analyses are based on three fundamental assumptions [48]. First, is that a robust association exists between the selected genetic variants (IVs) and the exposure variable [48]. This assumption can easily be validated, and we utilised only the genome-wide significant (P < 5 × 10−8) SNPs associated with endometriosis thereby satisfying the assumption. Second, is that the IVs are not associated with potential confounders [48]. We acknowledge that this assumption is difficult to prove, however, to reduce chances of violating it, we ensured that our IVs were independent. Also, we assessed the association between the IVs and age at menarche, age at menopause, menorrhagia, oestrogen level, as well as oral contraceptives use—all of which are possible risk factors for endometriosis and migraine [17,18,19]. This assessment was carried out using PhenoScanner v2 [49] (http://www.phenoscanner.medschl.cam.ac.uk, accessed on 2nd September 2019), at P < 1 × 10−05 (suggestive genome-wide significance level). Our IVs were not associated with any of these traits, except “rs74485684” which we found to be associated with ‘length of menstrual cycle’ and ‘excessive, frequent and irregular menstruation’ (Supplementary Table S1). To address possible pleiotropy implied by this finding, we carried out a ‘leave-one-out’ MR analysis.
The third assumption, which is also difficult to validate, is that the IVs do not affect the outcome through any alternative pathway other than the exposure variable, that is, there is no horizontal pleiotropy [48]. We conducted a test for horizontal pleiotropy as well as used alternative MR approaches including MR-Egger, and weighted median, to minimise the possibility of breaching this assumption.
2.6. Gene-Based Association Study
Gene-based analysis examines associations between a trait of interest and all SNPs while accounting for LD and allelic heterogeneity between the SNPs [50]. Compared to SNP-level studies (in which individual SNPs are assessed), gene-based studies are more powerful in gaining mechanistic insights into the biology of complex traits [50] given that, as the basic functional units of the human genome, they are more closely related to biological mechanisms than SNPs. Thus, to identify genes associated with endometriosis and migraine as well as further assess the molecular genetic overlap between the two traits, we conducted gene-based tests using Vegas2 software (https://vegas2.qimrberghofer.edu.au/) [51]. Vegas2 is user-friendly, computationally tractable, and has been used extensively in studies [31,51]. We utilised the ‘IEC endometriosis’ and ‘IHGC migraine’ GWAS data for the Vegas2 gene-based analyses.
Prior to conducting Vegas2 analyses, we extracted all SNPs from each of the two GWAS data. Following the exclusion of SNPs with no rsIDs, a total of 6,978,534 SNPs from the ‘IEC endometriosis’ GWAS and 8,175,736 SNPs from the ‘IHGC migraine’ GWAS were available for analysis. However, to ensure equivalent gene-based tests were performed for both disorders, we restricted Vegas2 analyses to a total of 6,904,914 SNPs overlapping the ‘IEC endometriosis’ and ‘IHGC migraine’ GWAS. We utilised the following Vegas2 options: Use SNPs from = ‘1000G EUROPEAN’; Select Sub-population from = ‘ALL EUROPEAN’; Use Gene definition from = ‘+/− 0 kb outside gene’; and Chromosome = ‘All’. Importantly, rather than the default ‘Top-x% test with top 100 per cent’ test, we specified the ‘Best-SNP test’. These analysis procedures were carried out separately for the ‘IEC endometriosis’ and ‘IHGC migraine’ GWAS data. We extracted nominally significant genes (at P < 0.1, P < 0.05, and P < 0.01) from Vegas2 outputs for each of the two traits and assessed those for overlapping genes between endometriosis and migraine. We also estimated gene-based Fisher’s combined p-values (FCP) for association (at Pgene < 0.1) across endometriosis and migraine to assess genes overlapping the two traits at a genome-wide level of significance.
Due to the presence of ‘LD between the most significant SNP (‘Best-SNP’) assigned to each gene, gene-based association results could be correlated across neighbouring genes’ [52]. Hence, we estimated the effective number of independent genes (independent gene-based tests) by examining the LD between the ‘Best-SNP’ assigned to each gene. Briefly, we estimated the effective number of independent gene-based tests in both the endometriosis and migraine datasets utilising the ‘genetic type 1 error calculator’ (GEC) software [53]. This analysis adjusts for multiple testing corrections taking into account correlation due to LD which may exist across neighbouring genes in our gene-based results. ‘Best-SNPs’ from the endometriosis and migraine Vegas2 results were processed as input files for GEC analysis [53]. GEC first partitions input SNPs into LD blocks with the assumption that LD blocks are independent (r2LD < 0.1), and thereafter estimates the effective number of independent SNPs (hence, the independent gene-based tests) in the LD blocks.
2.7. Overlapping Genes and Statistics Tests
To allow for differences in power across the endometriosis and migraine GWA studies, we generated gene sets with gene-based association p-values less than three nominal p-value thresholds (Pgene < 0.1, Pgene < 0.05, and Pgene < 0.01). For each gene set, estimates of the effective number of independent gene-based tests were calculated by GEC [53]. We assigned endometriosis as the ‘discovery’ set and migraine as the ‘target’ set to test whether the proportion of overlapping genes was more than expected by chance. The observed number of overlapping genes was defined as ‘the effective number of independent genes with p-values less than the threshold in both the discovery and target sets’ [52]. The observed proportion of overlapping genes was ‘calculated as the observed effective number of independent overlapping genes divided by the effective number of independent genes with a p-value less than the threshold in the discovery set’ [52]. The expected proportion of overlapping genes was calculated as the effective number of independent genes with a p-value less than the threshold in the target set divided by the total effective number of independent genes in the target set. The statistical significance of whether the number of overlapping genes was more than expected by chance was calculated using one-sided exact binomial test. We also report the raw number of genes in the gene sets to highlight the importance of estimating the effective number of independent genes.
2.8. Pathway-Based Functional Enrichment Analyses
To further elucidate potential biological mechanisms underlying the co-occurrence of endometriosis and migraine, we conducted pathway-based functional enrichment analyses. The protocols proposed by Reimand and colleagues [54] for enrichment analysis (using the g:Gost tool in g:Profiler [55]), visualisation (using Enrichmentmap [56]) and interpretation (using auto annotate [54]) of enriched pathways were adopted in this study. The g:Gost tool, implemented in g:Profiler, performs statistical enrichment analysis and automates the functional annotation of user-inputted genes based on their molecular, cellular and biological functions [54,55], thereby identifying over-represented (significantly enriched) biological pathways for the trait(s) of interest [54,55]. We utilised the web-based (http://biit.cs.ut.ee/gprofiler/) version of the tool, which is user-friendly. Notably, g:Gost’s databases, including Gene Ontology, WikiPathways and Human Phenotype Ontology (for human disease phenotypes), are updated on a regular basis [55]. Regulatory motifs matches (TRANSFAC), miRNA targets (miRTarBase), Human Protein Atlas (for tissue specificity), CORUM (for protein complexes) and Biological pathways (Kyoto Encyclopedia of Genes [KEGG], as well as Reactome) are also included in the g:Gost tool of g:Profiler [55].
In the present study, we utilised the g:Gost tool of g:Profiler (accessed 1st October 2019) to perform pathway-based functional enrichment analysis [54,55] using genes overlapping endometriosis and migraine at Pgene < 0.1 [52]. We employed the ‘g:SCS algorithm’ recommended for multiple testing correction in the g:Gost analysis, and restricted our results to only significantly enriched pathways at Padj < 0.05 (adjusted p-value for multiple testing correction [54]). Also, the size of the functional category (term size) was restricted to within 5 and 350 values (minimum and maximum) as recommended [54]. Several of the pathways enriched in g:Gost tool may be redundant. Therefore, we utilised the ‘Enrichmentmap’ application to produce ‘enrichment maps’ by collapsing related versions of over-represented pathways (g:Gost results) into simplified biological themes—thus, eliminating redundancy and enhancing the visualisation of enriched pathways [54,56]. We also utilised the ‘auto annotate’ application, to promote the interpretation of enriched pathways by organising ‘enrichment maps’ into clusters [54]. We implemented both ‘Enrichmentmap’ and ‘auto annotate’ tools via the Cytoscape environment [54,57].
3. Results
3.1. SECA: Genetic Overlap between Endometriosis and Migraine
SECA reveals significant concordance of SNP effects across the endometriosis and migraine GWAS, indicating that a strong molecular genetic overlap exists between the two traits. All 144 SNP subsets produced Fisher’s exact tests with at least nominally significant concordance effects (OR > 1 and P ≤ 0.05) between the ‘IEC endometriosis’ GWAS data (dataset 1) and ‘IHGC migraine’ GWAS data (dataset 2) [PFsig-permuted = 9.99 × 10−04; 95%CI: 5.12 × 10−05 – 5.64 × 10−03]. The most statistically significant concordance test was produced by SNP subsets with ‘IEC endometriosis’ GWAS Passoc ≤ 0.2 and the ‘IHGC migraine’ GWAS Passoc ≤ 0.6 (ORFT = 1.36; PFTmin-permuted = 1.66 × 10−32). Moreover, a total of 59,188 independent SNPs was shared by both endometriosis and migraine (SNP subsets with P1 = P2 = 1), out of which 30,790 (52%) showed concordance effect (Table 1). The test of association between the two traits (for SNP subsets with P1 = P2 = 1) was positive and highly significant statistically (OR = 1.18, Fisher’s p-value [two-sided] = 8.77 × 10−23). Importantly, SNP effect concordance increased as one conditioned on SNPs with smaller p-values. For example, the risk increasing alleles were concordant (OR = 1.92, Fisher’s p-value [two-sided] = 2.23 × 10−09) for 804 (58%) of the 1,383 independent endometriosis and migraine SNPs with nominally significant p-values (P1 = P2 < 0.05) [Table 1]. The proportion of concordance further increased to 66% (OR = 3.61, Fisher’s p-value [two-sided] = 7.20 × 10−04) for the 128 independent endometriosis and migraine SNPs with p-values (P1 and P2) < 0.01 (Table 1).
Table 1.
SNP effect concordance analysis (SECA) results for the test of genetic concordance between endometriosis and migraine.
| P1 | P2 | Concordant SNPs | Discordant SNPs | Total SNPs | Proportion of Concordance | OR | P |
|---|---|---|---|---|---|---|---|
| 1 | 1 | 30790 | 28398 | 59188 | 0.52 | 1.18 | 8.77 × 10−23 |
| 0.9 | 0.9 | 27540 | 25213 | 52753 | 0.52 | 1.19 | 4.11 × 10−24 |
| 0.8 | 0.8 | 24222 | 21971 | 46193 | 0.52 | 1.22 | 1.30 × 10−25 |
| 0.7 | 0.7 | 20842 | 18665 | 39507 | 0.53 | 1.25 | 6.60 × 10−28 |
| 0.6 | 0.6 | 17474 | 15458 | 32932 | 0.53 | 1.28 | 1.23 × 10−28 |
| 0.5 | 0.5 | 14218 | 12383 | 26601 | 0.53 | 1.32 | 2.69 × 10−29 |
| 0.4 | 0.4 | 10973 | 9415 | 20388 | 0.54 | 1.36 | 1.31 × 10−27 |
| 0.3 | 0.3 | 7804 | 6510 | 14314 | 0.55 | 1.44 | 3.21 × 10−27 |
| 0.2 | 0.2 | 4771 | 3855 | 8626 | 0.55 | 1.53 | 6.92 × 10−23 |
| 0.1 | 0.1 | 1946 | 1496 | 3442 | 0.57 | 1.69 | 2.06 × 10−14 |
| 0.05 | 0.05 | 804 | 579 | 1383 | 0.58 | 1.92 | 2.23 × 10−09 |
| 0.01 | 0.01 | 85 | 43 | 128 | 0.66 | 3.61 | 7.20 × 10−04 |
P1: International Endogene Consortium (IEC) Endometriosis data p-value; P2: International Headache Genetics Consortium (IHGC) migraine data p-value; SNP: single nucleotide polymorphism; OR: odds ratio for the effect direction concordance association test for endometriosis and migraine; P: Fisher’s exact p-value for the effect direction concordance association test between endometriosis and migraine.
In an analogous analysis where the ‘IHGC migraine’ and ‘IEC endometriosis’ GWAS dataset order was reversed (designated dataset 1 and dataset 2, respectively), the number of SNP subsets with significant effect concordance remained unchanged at 144 (PFsig-permuted = 9.99 × 10−04; 95%CI: 5.12 × 10−05 – 5.64 × 10−03) and produced a similar pattern of results as before. The subset of SNPs producing the most statistically significant concordance test was Passoc ≤ 0.6 for the ‘IHGC migraine’ and Passoc ≤ 0.6 for the ‘IEC endometriosis’ GWAS (ORFT = 1.27; PFTmin-permuted = 1.35 × 10−26).
We replicated SECA analysis using another independent migraine GWAS data from the UKBiobank. Our results confirmed significant SNP effect concordance between the ‘IEC endometriosis’ GWAS data and the ‘UKBiobank migraine’ GWAS data with 85 SNP subsets showing significant concordance of effect direction (PFsig-permuted = 4.0 × 10−03; 95%CI: 1.56 × 10 −03 – 1.02 × 10 −02). The most significant test was for SNP subsets with ‘IEC endometriosis’ GWAS Passoc ≤ 0.1 and ‘UKBiobank migraine’ GWAS Passoc ≤ 0.2 (ORFT = 1.20; PFTmin-permuted = 4.83 × 10−04). An analogous concordance test where the ‘UKBiobank migraine’ and ‘IEC endometriosis’ GWAS dataset order was reversed (designated dataset 1 and dataset 2, respectively) similarly indicated significant effect concordance with 119 SNP subsets producing Fisher’s exact tests with at least nominally significant concordance effects (PFsig-permuted = 9.99 × 10−04; 95%CI: 5.12 × 10−05 – 5.64 × 10−03) between the two datasets. The most statistically significant subset being SNPs with ‘UKBiobank migraine’ GWAS Passoc ≤ 0.1 and ‘IEC endometriosis’ GWAS Passoc ≤ 0.05 (ORFT = 1.48; PFTmin-permuted = 1.10 × 10−04).
3.2. LD Score Regression Results for Endometriosis-Migraine
Our univariate LDSC analysis estimated SNP-based liability heritability (h2SNP) of 11.44% (95%CI: 10.73%–12.15%) and 8.99% (95%CI: 8.23%–9.75%), for the ‘IEC endometriosis’ and ‘IHGC migraine’ GWAS, respectively (Table 2). Cross-trait bivariate LD score regression analysis revealed a moderate, positive and highly significant genetic correlation between endometriosis and migraine (rG = 0.38, P = 2.30 × 10−25). Using the ‘UKBiobank migraine’ GWAS data, we estimated a h2SNP of 16.87% (96%CI: 15.07%–18.67%, Table 2) for migraine and a statistically significant positive correlation between the ‘IEC endometriosis’ and ‘UKBiobank migraine’ GWAS data’ (rG = 0.14, P = 1.60 × 10−3).
Table 2.
Linkage disequilibrium (LD) score regression summary.
| SNP-based Heritability | ||||||
| Phentype | Dataset source | Valid SNPs in analysis | Liability scale h2SNP (95% CI) | Intercept (se) | ||
| Endometriosis | IEC | 1,157,235 | 11.44% (10.73–12.15%) | Constrained to 1 | ||
| Migraine | IHGC | 1,173,223 | 8.99% (8.23–9.75%) | 1.0232 (0.008) | ||
| Migraine | UKBB | 1,177,705 | 16.87% (15.07–18.67%) | 1.0122 (0.007) | ||
| SNP-based Genetic Correlation | ||||||
|
Phenotype 1
(data source) |
Phenotype 2
(data source) |
SNPs with valid alleles |
rG (se)
[p-value] |
Phenotype 1
Intercept |
Phenotype 2
Intercept |
Gencov
Intercept (se) |
| Endometriosis (IEC) | Migraine (IHGC) |
1,154,255 |
0.38 (0.0364) [2.30 × 10−25] |
Constrained to 1 | 1.0214 (specified) | Constrained to 0 |
| Endometriosis (IEC) | Migraine (UKBB) | 1,152,558 |
0.14 (0.0438) 1.60 × 10−°3 |
Constrained to 1 | 1.0136 (specified) | Constrained to 0 |
IEC: International Endogene Consortium, IHGC: International Headache Genetics Consortium, UKBB: United Kingdom BioBank, SNP: single nucleotide polymorphism, h2SNP: SNP-based heritability, CI: confidence interval, se: standard error.
3.3. SNPs Associated with Endometriosis and Migraine
Based on the results of our RE2 meta-analysis model (RE2 selected due to the presence of heterogeneity), 13 SNPs at one locus, associated with both endometriosis and migraine, were enriched to genome-wide signficance (PSNPs < 5 × 10−8) in our meta-analysis of the ‘IEC endometriosis’ and the ‘IHGC migraine’ GWAS data (Supplementary Table S2). The 13 SNPs (rs11031005, rs11031006, rs11031040, rs11031047, rs12223987, rs12278989, rs3858429, rs4071558, rs4071559, rs4071563, rs75525300, rs7929660, rs7947350) are at a locus (the 11p14.1 locus) which has previously been reported to be genome-wide significantly associated with endometriosis (with rs74485684 as the index SNP) [31]. Indeed, all 13 SNPs are in strong LD with the endometriosis lead SNP (rs74485684), 12 having r2 > 0.8, and the remaining one, rs12223987, having r2 = 0.694. An additional 47 independent SNPs loci associated with both endometriosis and migraine showed evidence of genome-wide suggestive (P < 1 × 10−5) association (Supplementary Table S3).
3.4. Mendelian Randomisation (MR)
Table 3 presents the results of the individual Wald-type ratio, IVW MR as well as the various sensitivity analyses—summarising the association of our IVs with endometriosis and migraine. Given the large sample size of our ‘IEC endometriosis’ GWAS data, the robust association between our IVs and endometriosis and the approximate F-statistics greater than 30, our IVs are strong and are not expected to suffer from weak instrument bias [58].
Table 3.
Instrumental variables, Mendelian randomisation (MR) results and sensitivity analyses.
| SNPs | EA | OA | Beta (endo) | SE (endo) | P(endo) | Beta (migr) | SE (migr) | P(migr) | Beta (endo-migr) | F-Stat * | SE (endo-migr) | P (endo-migr) |
| rs10167914 | A | G | −0.11 | 0.02 | 1.10 × 10−°9 | 0.01 | 0.01 | 0.27 | −0.11 | 37.27 | 0.10 | 0.27 |
| rs11674184 | T | G | 0.12 | 0.01 | 2.67 × 10−17 | −0.02 | 0.01 | 0.16 | −0.13 | 71.40 | 0.09 | 0.16 |
| rs12037376 | A | G | 0.15 | 0.02 | 8.87 × 10−17 | 0.01 | 0.01 | 0.35 | −0.09 | 68.97 | 0.10 | 0.35 |
| rs12700667 | A | G | 0.10 | 0.02 | 9.08 × 10−1° | 0.01 | 0.01 | 0.6 | −0.07 | 37.64 | 0.13 | 0.61 |
| rs1537377 | T | C | −0.09 | 0.01 | 1.33 × 10−1° | 0.00 | 0.01 | 0.83 | 0.03 | 41.53 | 0.12 | 0.83 |
| rs1903068 | A | G | 0.10 | 0.01 | 1.04 × 10−11 | 0.01 | 0.01 | 0.32 | 0.11 | 46.28 | 0.11 | 0.32 |
| rs4762326 | T | C | 0.08 | 0.01 | 2.20 × 10−°9 | −0.01 | 0.01 | 0.31 | 0.14 | 35.55 | 0.13 | 0.31 |
| rs6546324 | A | C | 0.08 | 0.01 | 3.01 × 10−°8 | −0.01 | 0.01 | 0.42 | 0.11 | 30.56 | 0.14 | 0.42 |
| rs71575922 | C | G | −0.11 | 0.02 | 2.02 × 10−°8 | −0.01 | 0.01 | 0.35 | −0.12 | 31.41 | 0.13 | 0.35 |
| rs74485684 | T | C | 0.11 | 0.02 | 2.00 × 10−°8 | 0.04 | 0.01 | 0.01 | 0.36 | 31.55 | 0.13 | 0.01 |
| rs760794 | T | C | 0.09 | 0.01 | 1.79 × 10−1° | −0.02 | 0.01 | 0.07 | −0.22 | 40.43 | 0.12 | 0.07 |
| Methods | Number of SNPs | Beta | SE | P | ||||||||
| All—IVW | 11 | −0.02 | 0.05 | 0.67 | ||||||||
| All—MR Egger | 11 | −0.25 | 0.27 | 0.38 | ||||||||
| All—Simple mode | 11 | 0.08 | 0.12 | 0.50 | ||||||||
| All—Weighted mode | 11 | −0.11 | 0.07 | 0.14 | ||||||||
| All—Weighted median | 11 | −0.09 | 0.05 | 0.10 | ||||||||
SNP: single nucleotide polymorphism, endo: endometriosis, migr: migraine, EA: effect allele, OA: other allele, Endo-migr: endometriosis as exposure and migraine as the outcome variable, Beta: effect size in standard deviation unit, SE: standard error, P: p-value, IVW: inverse variance weighted; * we estimated approximate F statistics values using t-statistics = Beta/SE, which is the t distribution with N-1 degrees of freedom (N is our sample size). The square of the t statistic represents approximate F statistics with degrees of freedom = 1. Thus, approximate F-statistics = (Beta_endo/SE_endo)2.
Nevertheless, we found evidence for marginally significant heterogeneity among the IVs (Cochran’s Q statistics for IVW = 18.39, degree of freedom [df] = 10, P = 0.049, and Q’ statistics for MR Egger = 16.99, df = 9, P = 0.049). Under the null hypothesis of no heterogeneity, we expect that the value of Q and Q′ will be same as their corresponding df (10 and 9, respectively). This is not the case. However, the difference between Q and its df (18.39 – 10 = 8.39) for IVW and (Q’, 16.99 – 9 = 7.99) for MR Egger are small, indicating (alongside the borderline significant p-value) that the heterogeneity was not substantial. In addition, we did not find MR Egger a better fit for our data than the IVW model since the difference Q − Q′ = 1.4 is not sufficiently extreme under a distribution. Our selected instruments reportedly explained about 1.75% variance in endometriosis [31].
Combining all the 11 endometriosis SNPs (IVs), MR analysis did not find evidence for a causal relationship between endometriosis and migraine based on the IVW method ([OR = 0.98, 95%CI: 0.89 – 1.07, P = 0.667] per standard deviation increase in endometriosis risk). Our results for sensitivity analyses using the MR-Egger (OR = 0.78, 95%CI: 0.46 – 1. 32, P = 0.381), and the weighted median (OR = 0.92, 95%CI: 0.83 – 1.02, P = 0.098) agree with that of the IVW method. Furthermore, the MR-Egger intercept (representing the average estimate of the pleiotropic effects of a SNP) was 0.0232 (SE: 0.027, P: 0.413). This intercept was not significantly different from zero, indicating that there was no evidence of directional (unbalanced horizontal) pleiotropy. However, our results for ‘single SNPs MR’ analysis identify rs74485684 to be statistically significant as endometriosis genetic variant with risk-increasing effect on migraine (OR= 1.43, 95%CI: 1.11 – 1.83, P = 0.006). This SNP was nominally associated with migraine (Table 3) as well as, ‘length of menstrual cycle’ and ‘excessive, frequent and irregular menstruation’ (Supplementary Table S1). The results of MR excluding the SNP (data not shown) did not make any difference to our previous finding, supporting the evidence of no causal association between our exposure and outcome variables.
Compared to endometriosis, a greater number of genome-wide significant SNPs have been identified for migraine [31,32]. Consequently, we conducted a “TwoSampleMR” utilising independent genome-wide significant SNPs from migraine GWAS as IVs, migraine as the exposure variable, and endometriosis as the outcome variable, reversing the direction of the datasets (data not shown). We note, however, that the causal effects of migraine on endometriosis may be difficult to explain conceptually. Regardless, the results for this analysis also did not provide evidence for a causal association between migraine and endometriosis.
3.5. Gene-Based Analysis for Endometriosis and Migraine
Our gene-based association analyses identified 1,749 and 1,871 genes nominally significant (Pgene < 0.05) in the ‘IEC endometriosis’ and ‘IHGC migraine’ GWAS gene-level association results, respectively (Supplementary Tables S4 and S5). A Bonferroni adjustment using the largest estimated total effective number of genes (17,104) produced a genome-wide, gene-based threshold of 2.92 × 10−6 (0.05/17,104). At this threshold, nine genes (ARL14EP, VEZT, CDC42, LINC00339, WNT4, GREB1, IL1A, FGD6, KDR) were genome-wide significant (Supplementary Table S6) in the gene-based analysis for the ‘IEC endometriosis’ GWAS, all of which have previously been reported for endometriosis (assessed using PhenoScanner v2 [49] (http://www.phenoscanner.medschl.cam.ac.uk, on 30 September 2019). Similarly, for migraine, a total of 17 genes (PLCE1, PLCE1-AS1, MRVI1, LRP1, STAT6, MEF2D, PRDM16, MROH2A, TRPM8, POC5, FHL5, KCNK5, PHACTR1, UFL1, TMEM91, MSL3P1, ANKDD1B) were genome-wide significant (Supplementary Table S6) in the gene-based analysis (at 2.92 × 10−6 threshold). Following an assessment in PhenoScanner v2 (accessed on 22nd September 2019), five of the 17 genes (PLCE1-AS1, MROH2A, POC5, TMEM91, ANKDD1B) have not previously been reported for migraine. Of the five new migraine genes, two (MROH2A on chromosome 2q37.1, and PLCE1-AS1 on chromosome 10q23.33) were located at previously reported migraine loci. The remaining three genes are located at two loci not previously identified for migraine (POC5 and ANKDD1B on chromosome 5q13.3, and TMEM91 on chromosome 19q13.2), thus, representing novel loci for migraine risks.
We assessed overlapping genes between endometriosis and migraine using gene-based test outputs, and our results revealed a total of 17 (at Pgene < 0.01), 196 (at Pgene < 0.05), and 493 (at Pgene < 0.1) significantly enriched genes shared by the two traits (Supplementary Tables S7–S9, respectively). Moreover, following FCP estimation for overlapping genes at Pgene < 0.1, three genes, ARL14EP (on chromosome 11p14.1), TRIM32 (on chromosome 9q33.1), and SLC35G6 (on chromosome 17p13.1) were enriched to a genome-wide significant level based on their combined p-value (Table 4). Two of these three genes (TRIM32, and SLC35G6) were not genome-wide significant in endometriosis or migraine; rather they attained genome-wide significance following the combination of the respective gene association p-values for the two traits indicating evidence of their involvement in the two disorders (and possibly their comorbid state). ARL14EP, on the other hand, was genome-wide significant for endometriosis only but attained a more genome-wide significance status following the estimation of FCP using both endometriosis and migraine gene p-values.
Table 4.
Genome-wide significant genes overlapping endometriosis and migraine in gene-based association analyses.
| S/N | Chro | Gene | Start Position | Stop Position | IEC Endometriosis | IHGC Migraine | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Gene p-Value | Top SNP | Top SNP p-Value | Gene p-Value | Top SNP | Top SNP p-Value | FCP | |||||
| 1 | 11 | ARL14EP | 30344648 | 30359165 | 1.00 × 10−06 | rs4071559 | 5.60 × 10−08 | 5.54 × 10−02 | rs4071559 | 5.97 × 10−03 | 9.81 × 10−07 |
| 2 | 9 | TRIM32 | 119449580 | 119463579 | 2.76 × 10−02 | rs11793648 | 3.14 × 10−03 | 5.00 × 10−06 | rs76973802 | 7.15 × 10−07 | 2.32 × 10−06 |
| 3 | 17 | SLC35G6 | 7384720 | 7386383 | 1.59 × 10−03 | rs9891297 | 3.09 × 10−04 | 9.40 × 10−05 | rs8065577 | 2.21 × 10−05 | 2.50 × 10−06 |
Chr: chromosomes; FCP: Fisher’s combined p-value; IEC: International Endogene Consortium; IHGC: International Headache Genetic Consortium.
Lastly, the exact binomial test confirms that significant gene-based genetic overlap exists between endometriosis and migraine at p-value thresholds of P < 0.1 and P < 0.05 (Table 5). For example, at gene-based p-value < 0.05, the observed proportion of genes overlapping the two traits (12%) was significantly higher than the expected proportion (8.6%) [Pbinomial-test = 9.83 ×10−06]. These results indicate that the observed gene-based genetic overlap between endometriosis and migraine was more than expected by chance implying that at the least, a proportion of the identified overlapping genes are truly associated with both endometriosis and migraine.
Table 5.
Summary of gene-level association analyses for endometriosis and depression under three p-value thresholds.
| The effective number of genes in Endometriosis and migraine | |||||||||||
| Disorder | Total genes | P value < 0.1 | P < 0.05 | P < 0.01 | |||||||
| Raw c | Effective d | Raw c | Effective d | Proportion e | Raw c | Effective d | Proportion e | Raw c | Effective d | Proportion e | |
| Endometriosis a | 20473 | 17104 | 2966 | 2433 | 0.142 | 1749 | 1430 | 0.084 | 481 | 386 | 0.023 |
| Migraine b | 20473 | 17046 | 3239 | 2579 | 0.151 | 1871 | 1467 | 0.086 | 587 | 450 | 0.026 |
| Number of overlapping genes and binomial test results for gene-based association | |||||||||||
| Discovery | Targets | Overlapping genes | Proportion of overlap | Binomial test P-value | |||||||
| Raw | Effective | Expected | Observed | ||||||||
| P value < 0.01 | |||||||||||
| Endometriosis | Migraine | 17 | 15 | 450/17046 = 0.026 | 15/386 = 0.039 | 0.08259 | |||||
| P value < 0.05 | |||||||||||
| Endometriosis | Migraine | 196 | 171 | 1467/17046 = 0.086 | 171/1430 = 0.120 | 9.83 × 10−06 | |||||
| P value < 0.1 | |||||||||||
| Endometriosis | Migraine | 493 | 420 | 2579/17046 = 0.151 | 420/2433 = 0.173 | 1.85 × 10−03 | |||||
a Endometriosis data from International Endogene Consortium, b migraine data from International Headache Genetic Consortium (IHGC), c raw number of genes, d effective number of independent genes, e proportion of total effective number of genes.
3.6. Functional Enrichment Analyses
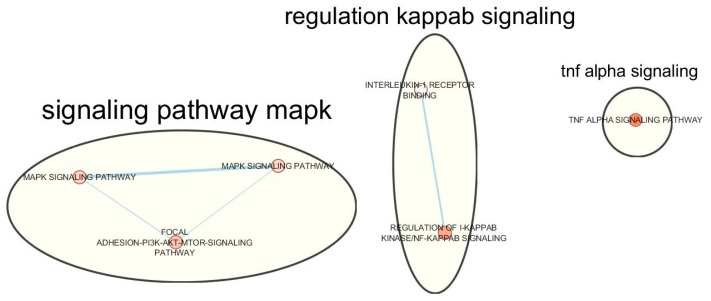
Functional enrichment analysis identifies six significantly enriched biological pathways for the 493 genes overlapping endometriosis and migraine at Pgene < 0.1. Table 6 presents a summary of these pathways. Clusters were generated following enrichment mapping and auto-annotation thereby collapsing the identified pathways into three main biological themes and clusters: mitogen-activated protein kinase (MAPK) signalling pathway, regulation of kappa-light-chain-enhancer of activated B cells (kappaB) signalling and tumor necrosis factor (TNF) alpha signalling pathway (Figure 1).
Table 6.
Significantly enriched ordered pathways for overlapping endometriosis-migraine genes.
| Term ID for pathway | Pathway term name | Adjusted p-value | Genes |
| Source: Gene Ontology (Molecular function) | |||
| Interleukin-1 receptor binding | GO: 0005149 | 9.19 × 10−03 | IL36RN, IL37, IL36B, IL1B, IL1F10 |
| Source: Gene Ontology (Biological process) | |||
| Regulation of I-kappaB kinase and NF-kappaB signalling | GO: 0043122 | 1.90 × 10−02 | TRIM32, IL36RN, IL37, TMED4, IL36B, IL1B, RNF31, IKBKB, SHISA5, TANK, PARK2, IL1F10, ZDHHC17, GSTP1, DAB2IP, SLC35B2, TRIM13 |
| Source: Biological pathways (Kyoto Encyclopedia of Genes [KEGG]) | |||
| MAPK signalling pathway | KEGG: 04010 | 1.40 × 10−02 | IL1B, FGF18, NGF, IKBKB, MAP2K5, PTPN5, PDGFC, MAPK9, NRAS, PPP3CA, CACNA1E, FGF17, MAP2K6, FGF9, MET, RPS6KA4, FGFR4 |
| Source: Biological pathways (WikiPathways) | |||
| MAPK Signalling Pathway | WP: WP382 | 1.3 × 10−02 | FGF11, IL1B, FGF18, NGF, IKBKB, MAP2K5, PTPN5, MAPK9, NRAS, PPP3CA, CACNA1E, FGF17, MAP2K6, FGF9, RPS6KA4, FGFR4 |
| Focal Adhesion-PI3K-Akt-mTOR-signaling pathway | WP: WP3932 | 1.54 × 10−02 | FGF11, ITGB5, CREB5, PFKFB4, PPP2CA, DDIT4, FGF18, NGF, IKBKB, PTK2, PDGFC, SLC2A4, NRAS, CREB3L2, FGF17, FGF9, MET, FGFR4 |
| TNF alpha Signalling Pathway | WP: WP231 | 2.3 × 10−02 | PPP2CA, RFK, IKBKB, PTPRCAP, MAPK9, NSMAF, NRAS, TANK, MAP2K6 |
Figure 1.
Clustered biological themes for overlapping endometriosis–migraine genes.
4. Discussion
Several observational epidemiological studies have reported the comorbidity of endometriosis with migraine. For the first time, however, we present a comprehensive assessment of the molecular genetic overlap, causal relationship as well as shared genes and biological pathways between the two disorders. SECA reveals the existence of a strong and significant genetic overlap between endometriosis and migraine. For instance, the proportion of nominally significant (P < 0.05) independent SNPs with concordant risk allele effects for endometriosis and migraine (58%) was higher than expected under the null hypothesis of no association (Pconcordant = 2.23 × 10−9). Bivariate LDSC analysis estimates a moderate, positive and highly significant genetic correlation between endometriosis and migraine (rG = 0.38, P = 2.30 × 10−25). Notably, we reproduced these significant findings using a second independent migraine GWAS dataset from the UKBB (rG = 0.14, P = 1.60 × 10−3). The weaker genetic correlation observed in the latter is most likely due to the smaller sample size (migraine cases) and the broader ‘self-reported migraine’ phenotype in the ‘UKBB migraine’ GWAS.
Our finding of significant genetic overlap and correlation between endometriosis and migraine indicates the presence of shared genetic components between the two disorders and confirms their comorbidity. This means that endometriosis patients share a non-negligible proportion of genetic risk variants with migraine patients. The SNP-based heritability estimated for endometriosis and migraine were lower than those reported from the twin-based studies due to the imperfect tagging of causal variants by common SNPs, in particular, if the causal variants are rare [59]. However, our findings compare favourably with those of a previous twin-based study which concluded that common genetic influences explain the comorbidity of migraine and endometriosis [13].
Although a meta-analysis of migraine and endometriosis GWAS produced a number of SNPs with genome-wide significant p-values, no novel risk loci were identified as all 13 SNPs reported were in strong LD with a previously reported risk locus for endometriosis on 11p14.1. Our finding, nonetheless, indicates the potential involvement of the locus in both disorders, and possibly, in their comorbid state. In addition to endometriosis, the 11p14.1 locus comprising FSHB has been associated with several female hormone-related traits including age at menarche and menopause, short menstrual cycle, polycystic ovarian syndrome, and increased risk of dizygotic twinning [31,60,61,62]. Thus, the locus may influence risk for both endometriosis and migraine via more frequent (menstrual-related) hormonal fluctuations in women as the same variants at this locus are associated with shorter and more frequent menstrual cycles and influencing oestradiol release, which have both been implicated in migraine risk [31,60]. We identified an additional 47 independent SNPs loci enriched to genome-wide suggestive (P < 1 × 10−5) association which should be prioritised in future studies. Meta-analysing more powerful GWAS data (with larger sample sizes) for endometriosis and migraine will identify more robust SNPs and novel risk loci shared by the two disorders.
Results for ‘single SNPs MR’ analysis showed evidence that one of the 11 endometriosis genome-wide significant SNPs, rs74485684, had a statistically significant risk-increasing effect on migraine. The rs74485684 SNP is located on chromosome 11p14.1 near FSHB gene, a locus significantly enriched for both endometriosis and migraine in our meta-analysis. The results for our PhenoScanner analysis, however, indicate a significant and strong association between rs74485684 SNP and some traits namely ‘length of menstrual cycle’ and ‘excessive, frequent and irregular menstruation’. There is evidence that the named traits represent important risk factors for both endometriosis and migraine [13,14,15,16,17,18,19] which may have confounded our MR analysis—i.e., a violation of the second assumption of MR analysis [48]. This observation would negate a causal relationship of the endometriosis SNP (rs74485684) on migraine but lend support for a ‘shared genetic risk factor mechanism of association’ in the comorbidity of the two disorders.
Combining all 11 endometriosis risk SNPs, MR analysis did not provide evidence of a causal relationship between endometriosis and migraine. We note, however, that the variance in endometriosis explained by the combined multi-allelic instrument is rather small (less than 2%) indicating that our MR estimates were biased towards the null [63]. Thus, we cannot completely rule out the possibility of causal effects of endometriosis on migraine. Future studies should revisit the MR analysis when more genome-wide significant SNPs associated with endometriosis are available. Although we do not have evidence of a causal relationship between endometriosis and migraine, some other mechanisms of association may explain their co-occurrence. For example, observational studies have identified some epidemiological similarities for both endometriosis and migraine [13,14,15,16,17,18,19], suggesting a ‘shared risk factor mechanism of association’. The results of our genetic overlap analyses support this position—identifying shared genetic risk factors for the co-occurrence of endometriosis and migraine.
Moving beyond the SNP-level study, we conducted gene-based analyses thereby furthering our assessment of the genetic overlap between endometriosis and migraine. Considered the basic physical and functional unit of the human genome, genes exhibit a closer relationship with biological mechanisms than SNPs. Moreover, gene-based analyses have the ability to account for LD and allelic heterogeneity while examining the association between a trait of interest and multiple co-located SNPs [64]. Thus, gene-based methods can provide a more robust and interpretable approach to understanding the biology of complex traits [64]. Like the SNP-based analysis, we found a significant gene-level genetic overlap between endometriosis and migraine with a total of 196 significantly enriched genes nominally associated (Pgene < 0.05) with both traits (Pbinomial-test = 9.83 × 10−6). Three overlapping genes, ARL14EP (on chromosome 11p14.1), TRIM32 (on chromosome 9q33.1), and SLC35G6 (on chromosome 17p13.1), were genome-wide significant based on their combined gene association p-values. We note, nonetheless, that these results are based on a statistical association of variants in and directly flanking each gene and do not strictly functionally implicate the genes. The 11p14.1 locus harbouring the ARL14EP gene has previously been associated with endometriosis [31], and implicated in our cross-disorder meta-analysis as well as the ‘single SNPs MR’ analysis (present study). However, the roles of the gene in migraine as well as in the comorbidity of endometriosis and migraine remain to be elucidated. ARL14EP is well expressed in thyroid and adrenal glands, brain, endometrium, lymph nodes, ovary, and many other tissues. More targeted studies are now warranted for a clearer understanding of the gene and its relationship with both endometriosis and migraine.
The remaining two genome-wide significant genes, TRIM32 and SLC35G6, have not been previously reported for endometriosis or migraine, neither are they located at or near established loci for any of the two disorders; hence, they represent two novel genes and susceptibility loci for the two traits. The SLC35G6 gene is well expressed in the testis, lowly expressed in the endometrium and adrenal gland; however, information about its biological functions is limited; hence, further investigation into the gene and its involvement in endometriosis and migraine is necessitated. Conversely, TRIM32 is a protein-coding gene consisting of a ‘RING, B-box, coiled-coil and six C-terminal NHL domains’ [65]. Being a ubiquitously expressed E3 ligase, the gene targets several proteins for degradation through ubiquitination [65]. TRIM32 has broad substrate specificity and has been associated with several biological activities including the regulation of microRNA, tumorigenesis, development and differentiation, as well as innate immunity. Moreover, TRIM32 has been linked with certain disorders such as Bardet–Biedl syndrome (mutation in ‘the B-box’ domain of the gene) [66,67], and limb-girdle muscular dystrophy (mutations in the ’C-terminal NHL domain’ of the gene) [68]. Endometrium, adrenal gland and the brain are among the three leading sites of TRIM32’s expression, lending greater support for its potential involvement in endometriosis and migraine. More targeted studies are required to elucidate TRIM32’s exact role in the two traits.
To explain the pathogenesis of co-occurring endometriosis and migraine, some authors have suggested a number of possible biological mechanisms including the roles of elevated levels of circulating prostaglandins [27], hormonal fluctuations [13], and impaired regulation of nitric oxide synthesis [69,70]. The involvement of hormone fluctuations is especially favoured by the fact that both endometriosis and migraine share risk factors consistent with the hormone-based regulation of the menstrual cycle such as early menarche, and menorrhagia [17,18,19]. The results of our meta-analysis, ‘single SNPs MR’ and, partly, overlapping genes assessment, potentially support a role for sex hormones activities in the pathogenesis of the two disorders. Following functional enrichment analysis, we also found significantly enriched biological pathways shared by both traits that may differ in some respects from the aforementioned mechanisms. For ease of interpretation as well as to eliminate possible redundancy, we carried out enrichment mapping thereby collapsing the over-represented pathways into three simplified biological themes and clusters. The first cluster of biological pathways, MAPK signalling pathway, comprises ‘focal adhesion-PI3K-Akt-mTOR-signaling’ and ‘MAPK signalling’. MAPK and ‘PI3K-Akt-mTOR’ are expressed differently, however, there is evidence that both are activated by steroid hormones and growth factors [71], supporting a role for sex hormones in the pathogenesis of endometriosis and migraine.
‘Focal adhesion-PI3K-Akt-mTOR’ is a signalling cascade made up of ‘focal adhesion’ (or cell-matrix adhesions), ‘phosphatidylinositide 3 kinases’ (PI3K), ‘protein kinase B’ (AKT), and ‘mammalian target of rapamycin’ (mTOR). Besides their structural role of mediating the molecular contact between intra- and extra-cellular spaces [63], focal adhesions relay signals between cells and the extracellular matrix, consequence upon which a range of cellular responses—cell growth, differentiation and movement—are initiated [63]. Protein kinases and phosphatases—two opposing but complementary groups of cells signalling proteins—as well as integrins, constitute essential parts of focal adhesive molecules [72,73]. While kinases and phosphatases co-regulate protein phosphorylation, a biological process that is critical to several cellular functions, integrins sense the environment and subsequently evoke responses resulting in the regulation of cell motility and shapes [72,73]. Also, through a complex interplay of its core components—PI3K stimulation, AKT phosphorylation, mTOR activation—the ‘PI3K-Akt-mTOR’ pathway facilitates several cellular processes including cell proliferation, metabolism, angiogenesis, and apoptosis [74]. There is evidence implicating these mechanisms in the biology of endometriosis and migraine [71,75].
For example, the role of kinases, particularly, MAPK, is well supported in the causal pathway of endometriosis, and arguably migraine [64,65,66,67]. Altered peritoneal microenvironment caused by endometriotic lesions is believed to activate kinase signalling pathways which may result in kinase-dependent growth or proliferation of endometriotic lesions [68]. In the case of migraine, activation of MAPK is suggested to mediate the synthesis and the release of calcitonin gene-related peptide (CGRP) which has long been implicated in the pathophysiology of migraine [66,69]. Indeed, recently approved monoclonal antibodies (mAbs) targeting CGRP or its receptor have lately been developed, representing a first major breakthrough for migraine-specific treatments in 30 years [76,77,78]. Furthermore, overexpression of ‘PI3K-Akt-mTOR’ has been noted in endometriosis and certain types of cancers (ovarian, breast and urothelial), and therapeutic agents targeting its core components have been developed [71,79].
‘Interleukin-1 receptor binding’ and ‘regulation of I-kappaB kinase and NF-kappaB signalling’, converged to a second biological cluster of pathways, regulation of kappaB signaling, following enrichment mapping and auto-annotation. Nuclear factor- kappaB (‘NF-kappaB’) is a transcription factor regulating inflammatory responses and mediating several functions of both the adaptive and innate immunity [80,81]. In addition to participating in the regulation of inflammatory processes, ‘NF-kappaB’ plays an important role in the expression of certain pro-inflammatory genes such as those involved in coding for cytokines [81]. Interleukin 1, on the other hand, is a pro-inflammatory cytokine whose activities are mediated through interleukin-1 receptor binding [82,83]. There are two types of this receptor: interleukin-1 receptor I which mainly transmits inflammatory signals, and interleukin-1 receptor II which although transmits no signals may suppress the effects of interleukin-1 by competing for its active binding sites [82,84]. Interleukin 1 not only mediates innate immune reactions, it also activates ‘NF-kappaB’ inflammatory pathways [83,84]. Thus, in line with previous studies [83,85], our study suggests that inflammatory processes and immune system dysfunction, mediated by the deregulation of cytokines and the ‘NF-kappaB’ factor [81], maybe relevant in the causal pathways of endometriosis and migraine.
Lastly, the tumour necrosis factor-alpha (TNF-α) signalling pathway was significantly enriched as one of the biological mechanisms underpinning endometriosis and migraine in the present study. Primarily produced by activated macrophages, T helper type 1 cells and natural killer cells, TNF-α, is among the most studied member of the TNF family [86,87]. The protein acts commonly alongside interleukin-1 and similarly activates the ‘NF-kappaB’ inflammatory pathways [86,87]. Consistent evidence indicates that women with endometriosis have a higher level of TNF-α in their peritoneal fluid and endometrium [83,88]. Also, the size of endometriotic lesions has been reported to be positively correlated with the concentration of TNF-α [83,88]. Therefore, our finding agrees with previous studies which have recognised the role of TNF in the pathogenesis of endometriosis [83,88]. In contrast, contradictory evidence for the role of TNF-α in migraine has been reported [89,90]. Hence, the present study provides important support for TNF-α in both endometriosis and migraine pathogenesis.
Strengths and Limitations
Major strengths of this study include our use of multiple statistical methods in analysing well-powered world-leading datasets to provide a comprehensive assessment of the relationship between endometriosis and migraine at the molecular genetic level. Furthermore, being based on genotype data, these analyses are generally not susceptible to potential confounding effects often associated with observational studies, thus providing strong and reliable evidence in support of our findings. For example, unlike in the traditional observational studies where the confounding effects of lifestyles or environmental factors are highly likely, genotypes are known to be well established and fixed at conception and should not be confounded by lifestyles or environments. Also, given that the inheritance of genotype precedes exposure to environmental factors, and, hence, disease onset later on in the offspring, the possibility of reverse causality is avoided in our study, lending credence to our findings. Limitations of our study mainly relate to those specific to the analysis methods. For example, sample overlap may confound LDSC and MR analyses. However, we ensured the independence of our samples and used a range of recommended approaches to minimise a possible violation of the MR assumptions. Lastly, several of the significantly enriched mechanisms in the pathway-based analyses are prone to redundancy. To minimise this limitation, however, we performed enrichment mapping and auto-annotation to collapse related pathways to simplified biological themes and clusters, thereby, enhancing the visualisation and interpretation of the significantly enriched biological pathways.
5. Conclusions
Our findings further confirm the comorbidity of endometriosis and migraine and indicate a non-causal relationship between the two traits, with shared genetically controlled biological mechanisms underlying the co-occurrence of the two disorders. After combining gene-based p-values across endometriosis and migraine GWAS, we found that three genes (ARL14EP, TRIM32, and SLC35G6), were genome-wide significant. Two of these genes (TRIM32, and SLC35G6) have not previously been reported for endometriosis or migraine, nor were they located on or near previously identified loci for any of the two traits—indicating that they represent novel genes and susceptibility loci for both endometriosis and migraine. Our functional enrichment analyses reveal some genetically controlled biological pathways underlying endometriosis and migraine including interleukin-1 receptor binding, focal adhesion-PI3K-Akt-mTOR-signaling, MAPK and TNF alpha signalling. Biological mechanisms related to sex hormone activities, protein adhesion and phosphorylation as well as inflammatory and immune system dysfunction, among others, are implicated by these pathways. Our study further supports the importance of a concurrent screening for migraine in patients presenting with or being investigated for endometriosis. Clinicians, thus, would need to start exercising a heightened suspicion for migraine in endometriosis patients. Shared genes and biological pathways identified in the present study could serve as potential therapeutic targets for endometriosis and migraine and perhaps the comorbid state of the two traits. However, further molecular and functional studies are needed for a targeted investigation into their roles in both disorders. Future analyses utilising results from more powerful GWAS are expected to improve the power to identify more robust SNPs and loci, as well as genes for endometriosis, migraine and the co-occurrence of the two disorders.
Acknowledgments
We acknowledge all the study participants in the 11 individual endometriosis studies that provided an opportunity for the current study. We also thank many hospital directors and staff, gynaecologists, general practitioners and pathology services in Australia who helped with confirmation of diagnoses. We would like to thank the research participants and employees of 23andMe for making this work possible. We thank the subjects of the Icelandic deCODE study for their participation. We thank the research staff and clinicians for providing diagnostic confirmation for the OX data set. We would like to express our gratitude to the staff and members of the Biobank Japan and Laboratory for Statistical Analysis, RIKEN Center for Integrative Medical Sciences for their outstanding assistance. A full list of the investigators who contributed to the generation of these data is available from http://www.wtccc.org.uk. We thank IHGC and all study participants in the migraine studies for making this study possible. We gratefully appreciate the UKBB for providing free access to their migraine GWAS summary data (sourced through the Neale Lab). Last but not least, we thank members of the Statistical and Genomic Epidemiology Laboratory (SGEL) at Queensland University of Technology, Brisbane (Anita, Ammarah, Hamzeh, Linduni, Rafiqul, and Sana), for their support and encouragement towards the success of this work.
List of Members of Consortia and Institutions
International Endogene Consortium
Yadav Sapkota2, Kosuke Yoshihara5, Mette Nyegaard6,7 Valgerdur Steinthorsdottir10, Andrew P. Morris11,12, Amelie Fassbender13,14, Nilufer Rahmioglu12, Immaculata De Vivo15,16, Julie E. Buring15,17, Futao Zhang8, Todd L. Edwards18, Sarah Jones19, Dorien13,14, Danie¨lle Peterse13,14, Kathryn M. Rexrode15,17, Paul M. Ridker15,17, Andrew J. Schork20,21, Stuart MacGregor22, Nicholas G. Martin22, Christian M. Becker23, Sosuke Adachi5, Takayuki Enomoto5, Atsushi Takahashi24, Yoichiro Kamatani24, Koichi Matsuda25, Michiaki Kubo24, Gudmar Thorleifsson10, Reynir T. Geirsson26,27, Unnur Thorsteinsdottir10,27, Leanne M. Wallace 22,8, Jian Yang8, Digna R. Velez Edwards28, Mette Nyegaard6,7, Siew-Kee Low24, Krina T. Zondervan 12,23, Stacey A. Missmer15,16, Thomas D’Hooghe 13,14,29, Kari Stefansson10,27, Joyce Y. Tung30, Grant W. Montgomery8, Daniel I. Chasman9, and Dale R. Nyholt1.
The 23andMe Research Team
Michelle Agee30, Babak Alipanahi30, Adam Auton30, Robert K. Bell30, Katarzyna Bryc30, Sarah L. Elson30, Pierre Fontanillas30, Nicholas A. Furlotte30, Karen E. Huber30, Aaron Kleinman30, Nadia K. Litterman30, Matthew H. McIntyre30, Joanna L. Mountain30, Elizabeth S. Noblin30, Carrie A.M. Northover30, StevenJ. Pitts30, J. Fah Sathirapongsasuti30, Olga V. Sazonova30, Janie F. Shelton30, Suyash Shringarpure30, Chao Tian30, Vladimir Vacic30 & Catherine H. Wilson30.
International Headache Genetic Consortium (IHGC)
Padhraig Gormley31−34, Verneri Anttila32,33,35, Bendik S Winsvold 36−38, Priit Palta39, Tonu Esko32,40,41, Tune H Pers32,41−43, Kai-How Farh32,35,44, Ester Cuenca-Leon31−33,45, Mikko Muona39,46−48, Nicholas A Furlotte30, Tobias Kurth49,9, Andres Ingason10, George McMahon50, Lannie Ligthart51, Gisela M Terwindt52, Mikko Kallela53, Tobias M Freilinger54,55, Caroline Ran56, Scott G Gordon22, Anine H Stam52, Stacy Steinberg10, Guntram Borck57, Markku Koiranen58, Lydia Quaye59, Hieab H H Adams 6°,61, Terho Lehtimäki62, Antti-Pekka Sarin39, Juho Wedenoja63, David A Hinds30, Julie E Buring9,64, Markus Schürks65, Paul M Ridker9,64, Maria Gudlaug Hrafnsdottir66, Hreinn Stefansson10, Susan M Ring50, Jouke-Jan Hottenga51, Brenda W J H Penninx67, Markus Färkkilä53, Ville Artto53, Mari Kaunisto39, Salli Vepsäläinen53, Rainer Malik55, Andrew C Heath68, Pamela A F Madden68, Nicholas G Martin22, Grant W Montgomery8, Mitja I Kurki31−33,39, 69, Mart Kals40, Reedik Mägi40, Kalle Pärn40, Eija Hämäläinen39, Hailiang Huang32,33,35, Andrea E Byrnes32,33,35, Lude Franke 70, Jie Huang34, Evie Stergiakouli50, Phil H Lee31−33, Cynthia Sandor71, Caleb Webber71, Zameel Cader72,73, Bertram Muller-Myhsok 74,75, Stefan Schreiber76, Thomas Meitinger77,78, Johan G Eriksson 79,8°, Veikko Salomaa80, Kauko Heikkilä81, Elizabeth Loehrer60, 82, Andre G Uitterlinden83, Albert Hofman60, Cornelia M van Duijn 60, Lynn Cherkas59, Linda M Pedersen36, Audun Stubhaug84,85, Christopher S Nielsen 84,86, Minna Männikkö 58, Evelin Mihailov40, Lili Milani 40, Hartmut Göbel87, Ann-Louise Esserlind88, Anne Francke Christensen88, Thomas Folkmann Hansen89, Thomas Werge90,91,7, Jaakko Kaprio39,63,92, Arpo J Aromaa80, Olli Raitakari93,94, M Arfan Ikram 60,61,95, Tim Spector59, Marjo-Riitta Järvelin58, 96−98, Andres Metspalu 40, Christian Kubisch99, David P Strachan100, Michel D Ferrari 52, Andrea C Belin56, Martin Dichgans55,75, Maija Wessman39,46, Arn M J M van den Maagdenberg52,101, John-Anker Zwart 36−38, Dorret I Boomsma51, George Davey Smith 50, Kari Stefansson 10,102, Nicholas Eriksson30, Mark J Daly 32,33,35, Benjamin M Neale 32,33,35, Jes Olesen88, Daniel I Chasman9, Dale R Nyholt1 and Aarno Palotie 31−35, 103.
Affiliations
1School of Biomedical Sciences, Faculty of Health, and Institute of Health and Biomedical Innovation, Queensland University of Technology, Brisbane, Queensland, Australia. 2Department of Epidemiology and Cancer Control, St. Jude Children’s Research Hospital, Memphis, Tennessee 38105, USA. 323andMe, Inc., 899 W. Evelyn Avenue, Mountain View, California 94041, USA. 4School of Pharmacy and Biomedical Sciences, University of Central Lancashire, Preston PR1 2HE, United Kingdom. 5Department of Obstetrics and Gynecology, Niigata University Graduate School of Medical and Dental Sciences, Niigata 950-2181, Japan. 6Department of Biomedicine - Human Genetics, Aarhus University, DK-8000 Aarhus, Denmark. 7iPSYCH, The Lundbeck Foundation Initiative for Integrative Psychiatric Research, DK-2100 Copenhagen, Denmark. 8Institute for Molecular Bioscience, The University of Queensland, Brisbane, Queensland 4072, Australia. 9Divisions of Preventive Medicine, Department of Medicine, Brigham and Women’s Hospital, Harvard Medical School, Boston, MA, USA. 10deCODE Genetics/Amgen, 101 Reykjavik, Iceland. 11Department of Biostatistics, University of Liverpool, Liverpool L69 3GL, UK. 12Wellcome Trust Centre for Human Genetics, University of Oxford, Oxford OX3 7BN, UK.13KULeuven, Department of Development and Regeneration, Organ systems, 3000 Leuven, Belgium. 14Department of Obstetrics and Gynaecology, Leuven University Fertility Centre, University Hospital Leuven, 3000 Leuven, Belgium. 15Harvard T.H. Chan School of Public Health, Boston, Massachusetts 02115, USA. 16Channing Division of Network Medicine, Department of Medicine, Brigham and Women’s Hospital and Harvard Medical School, Boston, Massachusetts 02115, USA. 17Division of Preventive Medicine, Brigham and Women’s Hospital, Boston, Massachusetts 02215, USA. 18Institute of Medicine and Public Health, Vanderbilt University Medical Center, Nashville, Tennessee 37203, USA. 19Vanderbilt Genetics Institute, Division of Epidemiology, Institute of Medicine and Public Health, Department of Medicine, Vanderbilt University Medical Center, Nashville, Tennessee 37203, USA. 20Cognitive Science Department, University of California, San Diego, La Jolla, California 92093, USA. 21Institute of Biological Psychiatry, Mental Health Centre Sct. Hans, Copenhagen University Hospital, DK-2100 Copenhagen, Denmark. 22Department of Genetics and Computational Biology, QIMR Berghofer Medical Research Institute, Brisbane, Queensland 4006, Australia. 23Endometriosis CaRe Centre, Nuffield Dept of Obstetrics & Gynaecology, University of Oxford, John Radcliffe Hospital, Oxford OX3 9DU, UK. 24Center for Integrative Medical Sciences, RIKEN, Yokohama 230-0045, Japan. 25Institute of Medical Sciences, The University of Tokyo, Tokyo 108-8639, Japan. 26Department of Obstetrics and Gynecology, Landspitali University Hospital, 101 Reykjavik, Iceland. 27Faculty of Medicine, School of Health Sciences, University of Iceland, 101 Reykjavik, Iceland. 28Vanderbilt Genetics Institute, Vanderbilt Epidemiology Center, Institute of Medicine and Public Health, Department of Obstetrics and Gynecology, Vanderbilt University Medical Center, Nashville, Tennessee 37203, USA. 29Global Medical Affairs Fertility, Research and Development, Merck KGaA, Darmstadt, Germany. 3023andMe, Inc., 899 W. Evelyn Avenue, Mountain View, California 94041, USA. 31Psychiatric and Neurodevelopmental Genetics Unit, Massachusetts General Hospital and Harvard Medical School, Boston, Massachusetts, USA. 32Medical and Population Genetics Program, Broad Institute of MIT and Harvard, Cambridge, Massachusetts, USA. 33Stanley Center for Psychiatric Research, Broad Institute of MIT and Harvard, Cambridge, Massachusetts, USA. 34Wellcome Trust Sanger Institute, Wellcome Trust Genome Campus, Hinxton, UK. 35Analytic and Translational Genetics Unit, Massachusetts General Hospital and Harvard Medical School, Boston, Massachusetts, USA. 36FORMI, Oslo University Hospital, Oslo, Norway. 37Department of Neurology, Oslo University Hospital, Oslo, Norway. 38Institute of Clinical Medicine, University of Oslo, Oslo, Norway. 39Institute for Molecular Medicine Finland (FIMM), University of Helsinki, Helsinki, Finland. 40Estonian Genome Center, University of Tartu, Tartu, Estonia. 41Division of Endocrinology, Boston Children’s Hospital, Boston, Massachusetts, USA. 42Department of Epidemiology Research, Statens Serum Institut, Copenhagen, Denmark. 43Novo Nordisk Foundation Center for Basic Metabolic Research, University of Copenhagen, Copenhagen, Denmark. 44Illumina, San Diego, California, USA. 45Pediatric Neurology, Vall d’Hebron Research Institute, Barcelona, Spain. 46Folkhälsan Institute of Genetics, Helsinki, Finland. 47Neuroscience Center, University of Helsinki, Helsinki, Finland. 48Molecular Neurology Research Program, Research Programs Unit, University of Helsinki, Helsinki, Finland. 49Institute of Public Health, Charité–Universitätsmedizin Berlin, Berlin, Germany. 50Medical Research Council (MRC) Integrative Epidemiology Unit, University of Bristol, Bristol, UK. 51Department of Biological Psychology, Vrije Universiteit, Amsterdam, the Netherlands. 52Department of Neurology, Leiden University Medical Centre, Leiden, the Netherlands. 53Department of Neurology, Helsinki University Central Hospital, Helsinki, Finland. 54Department of Neurology and Epileptology, Hertie-Institute for Clinical Brain Research, University of Tuebingen, Tuebingen, Germany. 55Institute for Stroke and Dementia Research, Klinikum der Universität München, Ludwig-Maximilians-Universität München, Munich, Germany. 56Department of Neuroscience, Karolinska Institutet, Stockholm, Sweden. 57Institute of Human Genetics, Ulm University, Ulm, Germany. 58Center for Life Course Epidemiology and Systems Medicine, University of Oulu, Oulu, Finland. 59Department of Twin Research and Genetic Epidemiology, King’s College London, London, UK. 60Department of Epidemiology, Erasmus University Medical Center, Rotterdam, the Netherlands. 61Department of Radiology, Erasmus University Medical Center, Rotterdam, the Netherlands. 62Department of Clinical Chemistry, Fimlab Laboratories, School of Medicine, University of Tampere, Tampere, Finland. 63Department of Public Health, University of Helsinki, Helsinki, Finland. 64Harvard Medical School, Boston, Massachusetts, USA. 65Department of Neurology, University Duisburg–Essen, Essen, Germany. 66Landspitali University Hospital, Reykjavik, Iceland. 67Department of Psychiatry, VU University Medical Centre, Amsterdam, the Netherlands. 68Department of Psychiatry, Washington University School of Medicine, St. Louis, Missouri, USA. 69Department of Neurosurgery, NeuroCenter, Kuopio University Hospital, Kuopio, Finland. 70Department of Genetics, University Medical Center Groningen, University of Groningen, Groningen, the Netherlands. 71MRC Functional Genomics Unit, Department of Physiology, Anatomy & Genetics, Oxford University, Oxford, UK. 72Nuffield Department of Clinical Neuroscience, University of Oxford, Oxford, UK. 73Oxford Headache Centre, John Radcliffe Hospital, Oxford, UK. 74Max Planck Institute of Psychiatry, Munich, Germany. 75Munich Cluster for Systems Neurology (SyNergy), Munich, Germany. 76Institute of Clinical Molecular Biology, Christian Albrechts University, Kiel, Germany. 77Institute of Human Genetics, Helmholtz Zentrum München, Neuherberg, Germany. 78Institute of Human Genetics, Technische Universität München, Munich, Germany. 79Department of General Practice and Primary Health Care, University of Helsinki and Helsinki University Hospital, Helsinki, Finland. 80National Institute for Health and Welfare, Helsinki, Finland. 81Institute of Clinical Medicine, University of Helsinki, Helsinki, Finland. 82Department of Environmental Health, Harvard T.H. Chan School of Public Health, Boston, Massachusetts, USA. 83Department of Internal Medicine, Erasmus University Medical Center, Rotterdam, the Netherlands. 84Department of Pain Management and Research, Oslo University Hospital, Oslo, Norway. 85Medical Faculty, University of Oslo, Oslo, Norway. 86Department of Ageing and Health, Norwegian Institute of Public Health, Oslo, Norway. 87Kiel Pain and Headache Center, Kiel, Germany. 88Danish Headache Center, Department of Neurology, Rigshospitalet, Glostrup Hospital, University of Copenhagen, Copenhagen, Denmark. 89Institute of Biological Psychiatry, Mental Health Center Sct. Hans, University of Copenhagen, Roskilde, Denmark. 90Institute of Biological Psychiatry, MHC Sct. Hans, Mental Health Services Copenhagen, Copenhagen, Denmark. 91Institute of Clinical Sciences, Faculty of Medicine and Health Sciences, University of Copenhagen, Copenhagen, Denmark. 92Department of Health, National Institute for Health and Welfare, Helsinki, Finland. 93Research Centre of Applied and Preventive Cardiovascular Medicine, University of Turku, Turku, Finland. 94Department of Clinical Physiology and Nuclear Medicine, Turku University Hospital, Turku, Finland. 95Department of Neurology, Erasmus University Medical Center, Rotterdam, the Netherlands. 96Department of Epidemiology and Biostatistics, MRC Health Protection Agency (HPE) Centre for Environment and Health, School of Public Health, Imperial College London, London, UK. 97Biocenter Oulu, University of Oulu, Oulu, Finland. 98Unit of Primary Care, Oulu University Hospital, Oulu, Finland. 99Institute of Human Genetics, University Medical Center Hamburg-Eppendorf, Hamburg, Germany. 100Population Health Research Institute, St George’s, University of London, London, UK. 101Department of Human Genetics, Leiden University Medical Centre, Leiden, the Netherlands. 102Faculty of Medicine, University of Iceland, Reykjavik, Iceland. 103Department of Neurology, Massachusetts General Hospital, Boston, Massachusetts, USA.
Supplementary Materials
The following are available online at https://www.mdpi.com/2073-4425/11/3/268/s1, Supplementary Table S1: PhenoScanner Results for Mendelian Randomisation instrumental variables, Supplementary Table S2: Meta-analysis of the IEC Endometriosis GWAS data and the IHGC migraine GWAS data Supplementary Table S3: Summary statistics of SNPs utilised as instrumental variables and their association with migraine risks, Supplementary Table S4: Nominally significant Vegas2-generated genes for endometriosis, Supplementary Table S5: Nominally significant Vegas2-generated genes for migraine, Supplementary Table S6: Genome-wide significant genes associated with endometriosis and migraine in gene-based study, Supplementary Table S7: Nominally significant overlapping genes (at Pgene < 0.01) between endometriosis and migraine GWAS data following gene-based analysis (VEGAS2 method), Supplementary Table S8: Nominally significant overlapping genes (at Pgene < 0.05) between endometriosis and migraine GWAS data following gene-based analysis (VEGAS2 method), Supplementary Table S9: Nominally significant overlapping genes (at Pgene < 0.1) between endometriosis and migraine GWAS data following gene-based analysis (VEGAS2 method).
Author Contributions
Conceptualization, E.O.A., Y.S., L.R.G., G.W.M. and Dale R Nyholt; Data curation, Y.S., International Endogene Consortium (IEC), 23andMe research team, International Headache Genetics Consortium (IHGC), Kosuke Yoshihara, Mette Nyegaard, G.W.M. and Daniel I Chasman; Formal analysis, E.O.A. and Dale R Nyholt; Funding acquisition, E.O.A., L.R.G., G.W.M., Daniel I Chasman and Dale R Nyholt; Investigation, E.O.A.; Methodology, E.O.A., Y.S., International Endogene Consortium (IEC), 23andMe research team, International Headache Genetics Consortium (IHGC), Asa Auta, Mette Nyegaard, L.R.G., G.W.M., Daniel I Chasman and Dale R Nyholt; Project administration, E.O.A., Asa Auta, L.R.G., G.W.M., Daniel I Chasman and Dale R Nyholt; Resources, E.O.A., Y.S., International Endogene Consortium (IEC), 23andMe research team, International Headache Genetics Consortium (IHGC), Mette Nyegaard, L.R.G., G.W.M., D.I.C. and D.R.N.; Software, E.O.A., K.Y. and D.R.N.; Supervision, L.R.G. and D.R.N.; Writing—original draft, E.O.A.; Writing—review & editing, E.O.A., Y.S., International Endogene Consortium (IEC), 23andMe research team, International Headache Genetics Consortium (IHGC), Asa Auta, K.Y., Mette Nyegaard, L.R.G., G.W.M., D.I.C. and D.R.N.. All authors read and agreed on the final draft. All authors have read and agreed to the published version of the manuscript.
Funding
EOA was supported by Queensland University of Technology Postgraduate Research Awards (QUTPRA). The QIMR study was supported by grants from the National Health and Medical Research Council (NHMRC) of Australia (241,944, 339,462, 389,927, 389,875, 389,891, 389,892, 389,938, 443,036, 442,915, 442,981, 496,610, 496,739, 552,485, 552,498, 1,026,033 and 1,050,208), the Cooperative Research Centre for Discovery of Genes for Common Human Diseases (CRC), Cerylid Biosciences (Melbourne) and donations from N. Hawkins and S. Hawkins. Analyses of the QIMRHCS and OX GWAS were supported by the Wellcome Trust (WT084766/Z/08/Z) and makes use of WTCCC2 control data generated by the Wellcome Trust Case-Control Consortium (awards 076113 and 085475). The iPSYCH study was funded by The Lundbeck Foundation, Denmark (R102-A9118, R155-2014-1724), and the research has been conducted using the Danish National Biobank resource supported by the Novo Nordisk Foundation. The Japanese GWA study was funded by the BioBank Japan project, which is supported by the Ministry of Education, Culture, Sports, Sciences and Technology of Japanese government.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Giudice L.C. Endometriosis. N. Engl. J. Med. 2010;362:2389–2398. doi: 10.1056/NEJMcp1000274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zondervan K.T., Becker C.M., Koga K., Missmer S.A., Taylor R.N., Viganò P. Endometriosis. Nat. Rev. Dis. Primers. 2018;4:9. doi: 10.1038/s41572-018-0008-5. [DOI] [PubMed] [Google Scholar]
- 3.Vos T., Barber R.M., Bell B., Bertozzi-Villa A., Biryukov S., Bolliger I., Charlson F., Davis A., Degenhardt L., Dicker D. Global, regional, and national incidence, prevalence, and years lived with disability for 301 acute and chronic diseases and injuries in 188 countries, 1990–2013: A systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2015;386:743–800. doi: 10.1016/S0140-6736(15)60692-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yeh W.Z., Blizzard L., Taylor B.V. What is the actual prevalence of migraine? Brain Behav. 2018;8:e00950. doi: 10.1002/brb3.950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Maitrot-Mantelet L., Hugon-Rodin J., Vatel M., Marcellin L., Santulli P., Chapron C., Plu-Bureau G. Migraine in relation with endometriosis phenotypes: Results from a French case-control study. Cephalalgia: Int. J. Head. 2019 doi: 10.1177/0333102419893965. [DOI] [PubMed] [Google Scholar]
- 6.Tanos V., Raad E.A., Berry K.E., Toney Z.A. Review of migraine incidence and management in obstetrics and gynaecology. Eur. J. Obstetr. Gynecol. Reprod. Biol. 2019;240:248–255. doi: 10.1016/j.ejogrb.2019.07.021. [DOI] [PubMed] [Google Scholar]
- 7.Rothrock J.F. What is migraine? Head.: J. Head Face Pain. 2008;48:330. doi: 10.1111/j.1526-4610.2007.01025.x. [DOI] [Google Scholar]
- 8.Nnoaham K.E., Hummelshoj L., Webster P., d’Hooghe T., de Cicco N.F., de Cicco N.C., Jenkinson C., Kennedy S.H., Zondervan K.T., World Endometriosis Research Foundation Global Study of Women's Health consortium Reprint of: Impact of endometriosis on quality of life and work productivity: A multicenter study across ten countries. Fertil. Steril. 2019;112 doi: 10.1016/j.fertnstert.2019.08.082. [DOI] [PubMed] [Google Scholar]
- 9.Agosti R. Migraine burden of disease: From the patient’s experience to a socio-economic view. Head.: J. Head Face Pain. 2018;58:17–32. doi: 10.1111/head.13301. [DOI] [PubMed] [Google Scholar]
- 10.Buse D.C., Fanning K.M., Reed M.L., Murray S., Dumas P.K., Adams A.M., Lipton R.B. Life with migraine: Effects on relationships, career, and finances from the chronic migraine epidemiology and outcomes (CaMEO) study. Head.: J. Head Face Pain. 2019;59:1286–1299. doi: 10.1111/head.13613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hållstam A., Stålnacke B.M., Svensén C., Löfgren M. Living with painful endometriosis – A struggle for coherence. A qualitative study. Sex. Reprod. Healthcare. 2018;17:97–102. doi: 10.1016/j.srhc.2018.06.002. [DOI] [PubMed] [Google Scholar]
- 12.Rei C., Williams T., Feloney M. Endometriosis in a man as a rare source of abdominal pain: A case report and review of the literature. Case Rep. Obstetr. Gynecol. 2018;2018:2083121. doi: 10.1155/2018/2083121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nyholt D.R., Gillespie N.G., Merikangas K.R., Treloar S.A., Martin N.G., Montgomery G.W. Common genetic influences underlie comorbidity of migraine and endometriosis. Genet. Epidemiol. 2009;33:105–113. doi: 10.1002/gepi.20361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rasmussen B.K. Migraine and tension-type headache in a general population: Precipitating factors, female hormones, sleep pattern and relation to lifestyle. Pain. 1993;53:65–72. doi: 10.1016/0304-3959(93)90057-V. [DOI] [PubMed] [Google Scholar]
- 15.Wang S.J., Fuh J.L., Young Y.H., Lu S.R., Shia B.C. Prevalence of migraine in Taipei, Taiwan: A population-based survey. Cephalalgia. 2000;20:566–572. doi: 10.1046/j.1468-2982.2000.00085.x. [DOI] [PubMed] [Google Scholar]
- 16.Vetvik K.G., MacGregor E.A. Sex differences in the epidemiology, clinical features, and pathophysiology of migraine. Lancet Neurol. 2017;16:76–87. doi: 10.1016/S1474-4422(16)30293-9. [DOI] [PubMed] [Google Scholar]
- 17.Aegidius K., Zwart J.A., Hagen K., Dyb G., Holmen T., Stovner L. Increased headache prevalence in female adolescents and adult women with early menarche. The Head-HUNT Studies. Eur. J. Neurol. 2011;18:321–328. doi: 10.1111/j.1468-1331.2010.03143.x. [DOI] [PubMed] [Google Scholar]
- 18.Tietjen G.E., Conway A., Utley C., Gunning W.T., Herial N.A. Migraine is associated with menorrhagia and endometriosis. Head.: J. Head Face Pain. 2006;46:422–428. doi: 10.1111/j.1526-4610.2006.00290.x. [DOI] [PubMed] [Google Scholar]
- 19.James A. More than menorrhagia: A review of the obstetric and gynaecological manifestations of bleeding disorders. Haemophilia. 2005;11:295–307. doi: 10.1111/j.1365-2516.2005.01108.x. [DOI] [PubMed] [Google Scholar]
- 20.Vercellini P., Viganò P., Somigliana E., Fedele L. Endometriosis: Pathogenesis and treatment. Nat. Rev. Endocrinol. 2014;10:261. doi: 10.1038/nrendo.2013.255. [DOI] [PubMed] [Google Scholar]
- 21.Matalliotakis I.M., Cakmak H., Fragouli Y.G., Goumenou A.G., Mahutte N.G., Arici A. Epidemiological characteristics in women with and without endometriosis in the Yale series. Arch. Gynecol. Obstetr. 2008;277:389–393. doi: 10.1007/s00404-007-0479-1. [DOI] [PubMed] [Google Scholar]
- 22.Missmer S.A., Hankinson S.E., Spiegelman D., Barbieri R.L., Marshall L.M., Hunter D.J. Incidence of laparoscopically confirmed endometriosis by demographic, anthropometric, and lifestyle factors. Am. J. Epidemiol. 2004;160:784–796. doi: 10.1093/aje/kwh275. [DOI] [PubMed] [Google Scholar]
- 23.Kvisvik E.V., Stovner L.J., Helde G., Bovim G., Linde M. Headache and migraine during pregnancy and puerperium: The MIGRA-study. J. Head. Pain. 2011;12:443–451. doi: 10.1007/s10194-011-0329-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Petrovski B.É., Vetvik K.G., Lundqvist C., Eberhard-Gran M. Characteristics of menstrual versus non-menstrual migraine during pregnancy: A longitudinal population-based study. J. Head. Pain. 2018;19:27. doi: 10.1186/s10194-018-0853-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lichten E.M., Bennett R.S., Whitty A.J., Daoud Y. Efficacy of danazol in the control of hormonal migraine. J. Reprod. Med. 1991;36:419–424. [PubMed] [Google Scholar]
- 26.Tervilä L., Marttila P. Headache as a Symptom of Endometriosis Externa. Ann. Chirurgiae Gynaecol. Fenniae. 1975;64:239–241. [PubMed] [Google Scholar]
- 27.Ferrero S., Pretta S., Bertoldi S., Anserini P., Remorgida V., Del Sette M., Gandolfo C., Ragni N. Increased frequency of migraine among women with endometriosis. Hum. Reprod. 2004;19:2927–2932. doi: 10.1093/humrep/deh537. [DOI] [PubMed] [Google Scholar]
- 28.Mirkin D., Murphy-Barron C., Iwasaki K. Actuarial analysis of private payer administrative claims data for women with endometriosis. J. Manag. Care Pharm. 2007;13:262–272. doi: 10.18553/jmcp.2007.13.3.262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Karamustafaoglu Balci B., Kabakci Z., Guzey D.Y., Avci B., Guler M., Attar E. Association between endometriosis, headache, and migraine. J. Endometr. Pelvic Pain Disord. 2019;11:19–24. doi: 10.1177/2284026518818975. [DOI] [Google Scholar]
- 30.Miller J.A., Missmer S.A., Vitonis A.F., Sarda V., Laufer M.R., DiVasta A.D. Prevalence of migraines in adolescents with endometriosis. Fertil. Steril. 2018;109:685–690. doi: 10.1016/j.fertnstert.2017.12.016. [DOI] [PubMed] [Google Scholar]
- 31.Sapkota Y., Steinthorsdottir V., Morris A.P., Fassbender A., Rahmioglu N., De Vivo I., Buring J.E., Zhang F., Edwards T.L., Jones S., et al. Meta-analysis identifies five novel loci associated with endometriosis highlighting key genes involved in hormone metabolism. Nat. Commun. 2017;8:15539. doi: 10.1038/ncomms15539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gormley P., Anttila V., Winsvold B.S., Palta P., Esko T., Pers T.H., Farh K.H., Cuenca-Leon E., Muona M., Furlotte N.A., et al. Meta-analysis of 375,000 individuals identifies 38 susceptibility loci for migraine. Nat. Genet. 2016;48:856–866. doi: 10.1038/ng.3598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Canis M., Donnez J., Guzick D., Halme J., Rock J., Schenken R., Vernon M. Revised american society for reproductive medicine classification of endometriosis: 1996. Fertil. Steril. 1997;67:817–821. doi: 10.1016/s0015-0282(97)81391-x. [DOI] [PubMed] [Google Scholar]
- 34.Headache Classification Committee of the International Headache Society (IHS) The International Classification of Headache Disorders, 3rd edition (beta version) Cephalalgia. 2013;33:629–808. doi: 10.1177/0333102413485658. [DOI] [PubMed] [Google Scholar]
- 35.1000 Genomes Project Consortium. Abecasis G.R., Auton A., Brooks L.D., DePristo M.A., Durbin R.M., Handsaker R.E., Kang H.M., Marth G.T., McVean G.A. An integrated map of genetic variation from 1092 human genomes. Nature. 2012;491:56–65. doi: 10.1038/nature11632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Magi R., Morris A.P. GWAMA: Software for genome-wide association meta-analysis. BMC Bioinform. 2010;11:288. doi: 10.1186/1471-2105-11-288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sudlow C., Gallacher J., Allen N., Beral V., Burton P., Danesh J., Downey P., Elliott P., Green J., Landray M. UK biobank: An open access resource for identifying the causes of a wide range of complex diseases of middle and old age. PLoS Med. 2015;12:e1001779. doi: 10.1371/journal.pmed.1001779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nyholt D.R. SECA: SNP effect concordance analysis using genome-wide association summary results. Bioinformatics. 2014;30:2086–2088. doi: 10.1093/bioinformatics/btu171. [DOI] [PubMed] [Google Scholar]
- 39.Yang Y., Zhao H., Boomsma D.I., Ligthart L., Belin A.C., Smith G.D., Esko T., Freilinger T.M., Hansen T.F., Ikram M.A. Molecular genetic overlap between migraine and major depressive disorder. Eur. J. Hum. Genet. 2018;26:1202. doi: 10.1038/s41431-018-0150-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sapkota Y., Low S.-K., Attia J., Gordon S.D., Henders A.K., Holliday E.G., MacGregor S., Martin N.G., McEvoy M., Morris A.P., et al. Association between endometriosis and the interleukin 1A (IL1A) locus. Hum. Reprod. 2015;30:239–248. doi: 10.1093/humrep/deu267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Han B., Eskin E. Random-effects model aimed at discovering associations in meta-analysis of genome-wide association studies. Am. J. Hum. Genet. 2011;88:586–598. doi: 10.1016/j.ajhg.2011.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hemani G., Zheng J., Elsworth B., Wade K.H., Haberland V., Baird D., Laurin C., Burgess S., Bowden J., Langdon R. The MR-Base platform supports systematic causal inference across the human phenome. Elife. 2018;7:e34408. doi: 10.7554/eLife.34408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Stone G.W., Pocock S.J. Randomized trials, statistics, and clinical inference. J. Am. Coll. Cardiol. 2010;55:428–431. doi: 10.1016/j.jacc.2009.06.066. [DOI] [PubMed] [Google Scholar]
- 44.Liberopoulos G., Trikalinos N.A., Ioannidis J.P. The elderly were under-represented in osteoarthritis clinical trials. J. Clin. Epidemiol. 2009;62:1218–1223. doi: 10.1016/j.jclinepi.2008.12.009. [DOI] [PubMed] [Google Scholar]
- 45.Zheng J., Baird D., Borges M.C., Bowden J., Hemani G., Haycock P., Evans D.M., Smith G.D. Recent Developments in Mendelian Randomization Studies. Curr. Epidemiol. Rep. 2017;4:330–345. doi: 10.1007/s40471-017-0128-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bowden J., Davey Smith G., Haycock P.C., Burgess S. Consistent estimation in Mendelian randomization with some invalid instruments using a weighted median estimator. Genet. Epidemiol. 2016;40:304–314. doi: 10.1002/gepi.21965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bowden J., Davey Smith G., Burgess S. Mendelian randomization with invalid instruments: Effect estimation and bias detection through Egger regression. Int. J. Epidemiol. 2015;44:512–525. doi: 10.1093/ije/dyv080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Davies N.M., Holmes M.V., Smith G.D. Reading Mendelian randomisation studies: A guide, glossary, and checklist for clinicians. BMJ. 2018;362:k601. doi: 10.1136/bmj.k601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Staley J.R., Blackshaw J., Kamat M.A., Ellis S., Surendran P., Sun B.B., Paul D.S., Freitag D., Burgess S., Danesh J., et al. PhenoScanner: A database of human genotype–phenotype associations. Bioinformatics. 2016;32:3207–3209. doi: 10.1093/bioinformatics/btw373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Liu J.Z., McRae A.F., Nyholt D.R., Medland S.E., Wray N.R., Brown K.M., Hayward N.K., Montgomery G.W., Visscher P.M., Martin N.G., et al. A Versatile Gene-Based Test for Genome-wide Association Studies. Am. J. Hum. Genet. 2010;87:139–145. doi: 10.1016/j.ajhg.2010.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mishra A., Macgregor S. VEGAS2: Software for More Flexible Gene-Based Testing. Twin Res. Hum. Genet. 2014;18:86–91. doi: 10.1017/thg.2014.79. [DOI] [PubMed] [Google Scholar]
- 52.Zhao H., Eising E., De Vries B., Vijfhuizen L.S., Consortium I.H.G., Anttila V., Winsvold B.S., Kurth T., Stefansson H., Kallela M. Gene-based pleiotropy across migraine with aura and migraine without aura patient groups. Cephalalgia. 2016;36:648–657. doi: 10.1177/0333102415591497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Li M.-X., Yeung J.M., Cherny S.S., Sham P.C. Evaluating the effective numbers of independent tests and significant p-value thresholds in commercial genotyping arrays and public imputation reference datasets. Hum. Genet. 2012;131:747–756. doi: 10.1007/s00439-011-1118-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Reimand J., Isserlin R., Voisin V., Kucera M., Tannus-Lopes C., Rostamianfar A., Wadi L., Meyer M., Wong J., Xu C. Pathway enrichment analysis and visualization of omics data using g: Profiler, GSEA, Cytoscape and EnrichmentMap. Nat. Protocols. 2019:1. doi: 10.1038/s41596-018-0103-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Reimand J., Arak T., Adler P., Kolberg L., Reisberg S., Peterson H., Vilo J. g:Profiler-a web server for functional interpretation of gene lists (2016 update) Nucleic Acids Res. 2016;44:W83–W89. doi: 10.1093/nar/gkw199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Merico D., Isserlin R., Stueker O., Emili A., Bader G.D. Enrichment map: A network-based method for gene-set enrichment visualization and interpretation. PLoS ONE. 2010;5:e13984. doi: 10.1371/journal.pone.0013984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Shannon P., Markiel A., Ozier O., Baliga N.S., Wang J.T., Ramage D., Amin N., Schwikowski B., Ideker T. Cytoscape: A software environment for integrated models of biomolecular interaction networks. Genome Res. 2003;13:2498–2504. doi: 10.1101/gr.1239303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Burgess S., Butterworth A., Thompson S.G. Mendelian randomization analysis with multiple genetic variants using summarized data. Genet. Epidemiol. 2013;37:658–665. doi: 10.1002/gepi.21758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wainschtein P., Jain D.P., Yengo L., Zheng Z., Cupples L.A., Shadyab A.H., McKnight B., Shoemaker B.M., Mitchell B.D., Psaty B.M. Recovery of trait heritability from whole genome sequence data. BioRxiv. 2019:588020. doi: 10.1530/ey.16.14.15. [DOI] [Google Scholar]
- 60.Mbarek H., Steinberg S., Nyholt D.R., Gordon S.D., Miller M.B., McRae A.F., Hottenga J.J., Day F.R., Willemsen G., de Geus E.J., et al. Identification of Common Genetic Variants Influencing Spontaneous Dizygotic Twinning and Female Fertility. Am. J. Hum. Genet. 2016;98:898–908. doi: 10.1016/j.ajhg.2016.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ruth K.S., Beaumont R.N., Tyrrell J., Jones S.E., Tuke M.A., Yaghootkar H., Wood A.R., Freathy R.M., Weedon M.N., Frayling T.M. Genetic evidence that lower circulating FSH levels lengthen menstrual cycle, increase age at menopause and impact female reproductive health. Hum. Reprod. 2016;31:473–481. doi: 10.1093/humrep/dev318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ruth K.S., Campbell P.J., Chew S., Lim E.M., Hadlow N., Stuckey B.G., Brown S.J., Feenstra B., Joseph J., Surdulescu G.L. Genome-wide association study with 1000 genomes imputation identifies signals for nine sex hormone-related phenotypes. Eur. J. Hum. Genet. 2016;24:284. doi: 10.1038/ejhg.2015.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Haycock P.C., Burgess S., Wade K.H., Bowden J., Relton C., Davey Smith G. Best (but oft-forgotten) practices: The design, analysis, and interpretation of Mendelian randomization studies. Am. J. Clin. Nutr. 2016;103:965–978. doi: 10.3945/ajcn.115.118216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zhao H., Nyholt D.R. Gene-based analyses reveal novel genetic overlap and allelic heterogeneity across five major psychiatric disorders. Hum. Genet. 2017;136:263–274. doi: 10.1007/s00439-016-1755-6. [DOI] [PubMed] [Google Scholar]
- 65.Joazeiro C.A., Weissman A.M. RING finger proteins: Mediators of ubiquitin ligase activity. Cell. 2000;102:549–552. doi: 10.1016/S0092-8674(00)00077-5. [DOI] [PubMed] [Google Scholar]
- 66.Blacque O., Leroux M. Bardet-Biedl syndrome: An emerging pathomechanism of intracellular transport. Cell. Mol. Life Sci. CMLS. 2006;63:2145–2161. doi: 10.1007/s00018-006-6180-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Chiang A.P., Beck J.S., Yen H.-J., Tayeh M.K., Scheetz T.E., Swiderski R.E., Nishimura D.Y., Braun T.A., Kim K.-Y.A., Huang J. Homozygosity mapping with SNP arrays identifies TRIM32, an E3 ubiquitin ligase, as a Bardet–Biedl syndrome gene (BBS11) Proc. Natl. Acad. Sci. USA. 2006;103:6287–6292. doi: 10.1073/pnas.0600158103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Neri M., Selvatici R., Scotton C., Trabanelli C., Armaroli A., De Grandis D., Levy N., Gualandi F., Ferlini A. A patient with limb girdle muscular dystrophy carries a TRIM32 deletion, detected by a novel CGH array, in compound heterozygosis with a nonsense mutation. Neuromusc. Disord. 2013;23:478–482. doi: 10.1016/j.nmd.2013.02.003. [DOI] [PubMed] [Google Scholar]
- 69.Buzzi M.G., Moskowitz M.A. The pathophysiology of migraine: Year 2005. J. Head. Pain. 2005;6:105. doi: 10.1007/s10194-005-0165-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Alpay Z., Saed G.M., Diamond M.P. Female infertility and free radicals: Potential role in adhesions and endometriosis. J. Soc. Gynecol. Investig. 2006;13:390–398. doi: 10.1016/j.jsgi.2006.05.002. [DOI] [PubMed] [Google Scholar]
- 71.Makker A., Goel M.M., Das V., Agarwal A. PI3K-Akt-mTOR and MAPK signaling pathways in polycystic ovarian syndrome, uterine leiomyomas and endometriosis: An update. Gynecol. Endocrinol. 2012;28:175–181. doi: 10.3109/09513590.2011.583955. [DOI] [PubMed] [Google Scholar]
- 72.Tonks N.K. Protein tyrosine phosphatases: From genes, to function, to disease. Nat. Rev. Mol. Cell Biol. 2006;7:833–846. doi: 10.1038/nrm2039. [DOI] [PubMed] [Google Scholar]
- 73.Danen E.H. Madame Curie Bioscience Database. Landes Bioscience; Austin, TX, USA: 2013. Integrins: An overview of structural and functional aspects. [Google Scholar]
- 74.Karar J., Maity A. PI3K/AKT/mTOR pathway in angiogenesis. Front Mol. Neurosci. 2011;4:51. doi: 10.3389/fnmol.2011.00051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Liu Y.Y., Jiao Z.Y., Li W., Tian Q. PI3K/AKT signaling pathway activation in a rat model of migraine. Mol. Med. Rep. 2017;16:4849–4854. doi: 10.3892/mmr.2017.7191. [DOI] [PubMed] [Google Scholar]
- 76.Goadsby P.J., Reuter U., Hallström Y., Broessner G., Bonner J.H., Zhang F., Sapra S., Picard H., Mikol D.D., Lenz R.A. A controlled trial of erenumab for episodic migraine. N. Engl. J. Med. 2017;377:2123–2132. doi: 10.1056/NEJMoa1705848. [DOI] [PubMed] [Google Scholar]
- 77.Silberstein S.D., Dodick D.W., Bigal M.E., Yeung P.P., Goadsby P.J., Blankenbiller T., Grozinski-Wolff M., Yang R., Ma Y., Aycardi E. Fremanezumab for the preventive treatment of chronic migraine. N. Engl. J. Med. 2017;377:2113–2122. doi: 10.1056/NEJMoa1709038. [DOI] [PubMed] [Google Scholar]
- 78.Cady R., Lipton R.B. Qualitative change in migraine prevention? Head.: J. Head Face Pain. 2018;58:1092–1095. doi: 10.1111/head.13354. [DOI] [PubMed] [Google Scholar]
- 79.Slomovitz B.M., Coleman R.L. The PI3K/AKT/mTOR pathway as a therapeutic target in endometrial cancer. Clin. Cancer Res. 2012;18:5856–5864. doi: 10.1158/1078-0432.CCR-12-0662. [DOI] [PubMed] [Google Scholar]
- 80.Shih R.-H., Wang C.-Y., Yang C.-M. NF-kappaB Signaling Pathways in Neurological Inflammation: A Mini Review. Front. Mol. Neurosci. 2015;8 doi: 10.3389/fnmol.2015.00077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Liu T., Zhang L., Joo D., Sun S.-C. NF-κB signaling in inflammation. Sign. Transd. Target. Ther. 2017;2:17023. doi: 10.1038/sigtrans.2017.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zhang J.-M., An J. Cytokines, inflammation, and pain. Int. Anesthesiol. Clin. 2007;45:27–37. doi: 10.1097/AIA.0b013e318034194e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ahn S.H., Monsanto S.P., Miller C., Singh S.S., Thomas R., Tayade C. Pathophysiology and Immune Dysfunction in Endometriosis. BioMed. Res. Int. 2015;2015:12. doi: 10.1155/2015/795976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Weber A., Wasiliew P., Kracht M. Interleukin-1 (IL-1) Pathway. Sci. Signal. 2010;3 doi: 10.1126/scisignal.3105cm1. [DOI] [PubMed] [Google Scholar]
- 85.Capobianco A., Rovere-Querini P. Endometriosis, a disease of the macrophage. Front. Immunol. 2013;4:9. doi: 10.3389/fimmu.2013.00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Idriss H.T., Naismith J.H. TNFα and the TNF receptor superfamily: Structure-function relationship (s) Microsc. Res. Tech. 2000;50:184–195. doi: 10.1002/1097-0029(20000801)50:3<184::AID-JEMT2>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 87.Bouwmeester T., Bauch A., Ruffner H., Angrand P.-O., Bergamini G., Croughton K., Cruciat C., Eberhard D., Gagneur J., Ghidelli S., et al. A physical and functional map of the human TNF-α/NF-κB signal transduction pathway. Nat. Cell Biol. 2004;6:97–105. doi: 10.1038/ncb1086. [DOI] [PubMed] [Google Scholar]
- 88.Pizzo A., Salmeri F.M., Ardita F.V., Sofo V., Tripepi M., Marsico S. Behaviour of cytokine levels in serum and peritoneal fluid of women with endometriosis. Gynecol. Obstetr. Investing. 2002;54:82–87. doi: 10.1159/000067717. [DOI] [PubMed] [Google Scholar]
- 89.Schürks M., Rist P.M., Zee R.Y., Chasman D.I., Kurth T. Tumour necrosis factor gene polymorphisms and migraine: A systematic review and meta-analysis. Cephalalgia. 2011;31:1381–1404. doi: 10.1177/0333102411419022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Gu L., Yan Y., Long J., Su L., Hu Y., Chen Q., Xie J., Wu G. The TNF-α-308G/A polymorphism is associated with migraine risk: A meta-analysis. Exp. Therapeut. Med. 2012;3:1082–1086. doi: 10.3892/etm.2012.533. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.