Abstract
Cholangiocarcinoma (CCA) is a highly invasive and metastatic form of carcinoma with bleak prognosis due to limited therapies, frequent relapse, and chemotherapy resistance. There is an urgent need to identify the molecular regulators of CCA in order to develop novel therapeutics and advance diseases diagnosis. Many cellular proteins including histones may undergo a series of enzyme-mediated post-translational modifications including acetylation, methylation, phosphorylation, sumoylation, and crotonylation. Histone deacetylases (HDACs) play an important role in regulating epigenetic maintenance and modifications of their targets, which in turn exert critical impacts on chromatin structure, gene expression, and stability of proteins. As such, HDACs constitute a group of potential therapeutic targets for CCA. The aim of this review was to summarize the role that HDACs perform in regulating epigenetic changes, tumor development, and their potential as therapeutic targets for CCA.
Keywords: cholangiocarcinoma, hepatobiliary cancers, HDACs, HDACi
1. Introduction
Cholangiocarcinoma (CCA) is a malignancy arising from cholangiocytes, the epithelial cells of the biliary tree. It is the part of the adenocarcinomas family of tumors also known as bile duct cancer. CCA is a less common malignancy in the liver than hepatocellular carcinoma (HCC), accountable for 10–20% of all liver cancer [1]. CCA is categorized into different groups on the basis of its topography in the liver and bile ducts. If the tumors arise in the liver, it is classified as intrahepatic cholangiocarcinoma (iCCA), and when found outside the liver it is categorized as distal cholangiocarcinoma (dCCA). However, when tumor occurs in the bile duct junctions, it is considered as perihilar cholangiocarcionma (pCCA). Among these, cancer at the perihilar and distal regions are very common, whereas intrahepatic CCA is reported only in 10% of cases [2]. Characteristically, CCA demonstrates non-specific symptoms until a late stage. Inadequate knowledge of the risk factors and correct screening methods make CCA difficult to diagnose at early stages. As a result of frequent late diagnosis, CCA is considered among the deadliest form of cancers, with unfortunate rates of 5-year survival (approximately 5%) in patients [1,3].
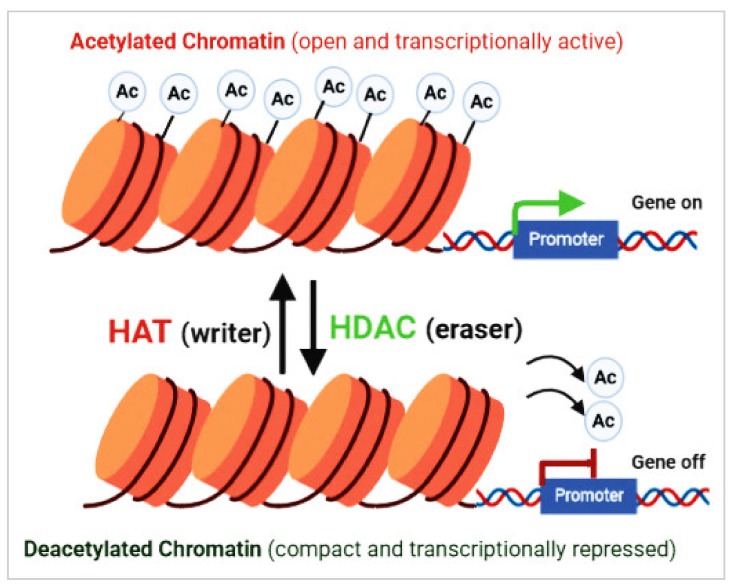
Epigenetic modifications, such as acetylation and deacetylation of histones and other cellular proteins, play a vital role in the tumorigenesis and cancer progression. Among these, the importance of histone deacetylase (HDAC)-mediated epigenetic changes in the pathogenesis of CCA has been highlighted [4]. Histone acetylation is a reversible post-translational modification that plays a main role in structure/function of chromatin and in regulating eukaryotic gene expression. Histone/protein acetylation is regulated by the functions of HDACs and histone acetyltransferase (HAT) enzymes [4,5]. HDACs are able to remove acetyl groups from histones and non-histone proteins, and are categorized into two major families, including zinc-dependent and NAD+-dependent HDACs (Table 1). HATs catalyze the relocation of an acetyl group from acetyl coenzyme A (acetyl-CoA) to proteins in lysine residues (Figure 1) [4,5]. The equilibrium between histone acetylation and deacetylation is oftentimes deregulated in cancer, causing impaired expression of tumor suppressor genes. HDAC inhibitors (HDACis) are a family of synthetic and natural compounds that differ in their target specificities and activities. HDACis are divided into four main groups on the basis of their structure, including cyclic peptides, benzamides, hydroxamic acids, and short chain fatty acids [4,6,7]. HDACis can significantly affect cancer cells, inducing cell death, cycle growth arrest, angiogenesis reduction, and the immune system modulation [4,7]. This current review mainly focuses on the action of HDACs in the pathogenesis of cancer, including CCA and the therapeutic role of HDACis.
Table 1.
Different classes of HDACs, their co-factors, and their cellular locations.
| Co-Factor | Class | Members | Location |
|---|---|---|---|
| Zn+2-dependent | HDAC I | HDAC1 | Nucleus |
| HDAC2 | Nucleus | ||
| HDAC3 | Nucleus | ||
| HDAC8 | Nucleus | ||
| HDAC II | HDAC4 | Nucleus/cytoplasm | |
| HDAC5 | Nucleus/cytoplasm | ||
| HDAC7 | Nucleus/cytoplasm | ||
| HDAC9 | Nucleus/cytoplasm | ||
| HDAC6 | Cytoplasm | ||
| HDAC10 | Cytoplasm | ||
| HDAC IV | HDAC11 | Nucleus | |
| NAD+-dependent | HDAC III | SIRT1 | Nucleus/cytoplasm |
| SIRT2 | Nucleus | ||
| SIRT3 | Mitochondria | ||
| SIRT4 | Mitochondria | ||
| SIRT5 | Mitochondria | ||
| SIRT6 | Nucleus | ||
| SIRT7 | Nucleus |
Figure 1.
Acetylation and deacetylation of nucleosomal histones and other cellular proteins that play an important role in the modulation of chromatin arrangement and gene expression, as well as in the regulation of protein stability and cellular function. Histone acetyltransferases (HATs) and histone deacetylases (HDACs) are two contrasting classes of enzymes that closely regulate histone acetylation and deacetylation.
2. Classification of Histone Deacetylases
Approximately 18 HDACs have been identified in humans, which have been further classified into four groups on the basis of their homology with yeast HDACs and co-factors. HDAC classes I, II, and IV require a zinc molecule (Zn+2) as a cofactor in their active site. As a result, the Zn2+ binding HDACis can inhibit these HDACs [8], whereas sirtuins (SIRTs), a class III HDAC, require NAD+ as a cofactor rather than Zn2+, and are homologous to Sir2 protein in yeast, with similar functions. As a result, Zn+2-binding HDACis cannot inhibit class III HDACs (Table 2) [9]. The role of sirtuin proteins in carcinogenesis is dubious, as some SIRT proteins have shown roles as tumor suppressors and/or as oncoproteins [10].
Table 2.
Targets of different HDAC inhibitors (HDACis). SAHA—suberoylanilide hydroxamic acid, TSA—trichostatin A, SCFA—short chain fatty acids.
| HDAC Inhibitors | Activity | Target HDACs |
|---|---|---|
| SAHA | Hydroxamates | I, II, IV |
| TSA | Hydroxamates | I, II, IV |
| Ky2 | Hydroxamates | I |
| Apicidine | Depsipeptides | I |
| FK228 | Depsipeptides | I |
| VPA | SCFA | I, II |
| Butyrate | SCFA | I, IIa |
| 4-BP | SCFA | I, IIa |
| MS-275 | Benzamides | I |
| Cl-994 | Benzamides | I |
| MGCD0103 | Benzamides | I, IV |
| LAQ842 | Other | I, II, IV |
| MY192 | Other | I |
| PXD101 | Other | I, II, IV |
| LBX589 | Other | I, II, IV |
| MPT0G009 | Other | I, II, IV |
| ACY1215 | Other | II |
HDACs 1, 2, 3, and 8 are class I HDACs. They are the most abundant class of HDACs present in the nucleus. Class II HDACs can travel between the cytoplasm and nucleus and are larger than the other two Zn+2-dependent classes of HDACs, on the basis of domain organization and sequence. HDAC class II is further subdivided into class IIa (HDACs 4, 5, 7, and 9) and class IIb (HDACs 6 and 8), with the latter being characterized as having two deacetylase domains [8]. Class IV has only one HDAC member (HDAC11) [11]. The classification and functions of HDACs are shown in Table 1.
Sirtuins are NAD+-dependent class III protein deacetylases, found in bacteria to humans [10,12]. Even though they have diversified functions through evolution, their main functions is to sense changes in redox states of the cell due to intracellular and extracellular stress (i.e., metabolic, oxidative, or genotoxic) and to orchestrate a suitable response [12]. Fluctuations in cellular energy can be sensed by sirtuins by using the NAD+ as a cofactor for its enzymatic activity. Even though most of the sirtuins have a wide range of substrates including both histone and nonhistone proteins, some can strictly target to only histones, whereas others have only specificity to nonhistone proteins (Table 1) [12]. Sirtuins include seven members (Sirt1-7) in mammalian cells that may vary extensively in their activity, localization, and functions. Sirt1, 6, and 7 are mainly located in the nucleus, Sirt2 is cytoplasmic, Sirt3 is mitochondrial, whereas Sirt4 and Sirt5 are primarily present in mitochondria [10,13]. Sirtuins can perform numerous functions, including cell survival during stress, metabolic homeostasis, chromatin regulation, and cell differentiation [14,15]. In line with this, sirtuins play an important role at both the cellular and organism levels. They have been associated with diabetes, obesity, cancer, cardiovascular diseases, and also help in viral replication [12,13,16,17], among other disorders.
Histone acetyltransferases (HATs) comprise two main types including A-type (nuclear) and B-type (cytoplasmic). Type-A HATs have various families classified into at least three distinct groups based on structural and functional similarities and, including the N-acetyltransferase family, GCN5-related family, family of Moz-Ybf2/Sas3-Sas2-Tip60, and the family of p300/CREB-binding protein (CBP/CREBBP) [18,19]. HAT type-B is highly conserved and has sequence homology with the scHat1 protein [18].
3. Histone Deacetylases and Cancer
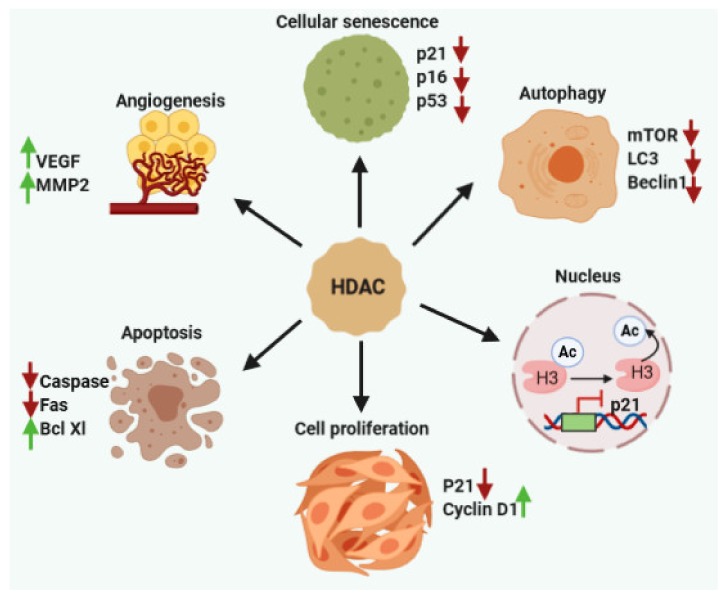
According to previous studies, dysfunctions of HDAC enzymes and altered level of acetylation are linked to various cancers including CCA [20]. HDACs play a vital role in the epigenetic regulation of gene transcription and expression through their effects on the chromatin compaction state (Figure 1) and affecting the stability of other cellular target proteins [21]. In recent times, HDACs have developed as potential therapeutic targets as these can alter the aberrant epigenetic conditions associated with cancer development. The overexpression of HDACs have been reported in various solid and hematological cancers [22], affecting diverse cellular mechanisms such as proliferation, cell death, metastasis, autophagy, metabolism, and ciliary expression, as described below (Figure 2).
Figure 2.
HDACs help in the progression of cancer cell growth and spreading via regulating cell proliferation, angiogenesis, apoptosis, metastasis, autophagy, and senescence.
3.1. Cell Proliferation
HDACs are known to deacetylate a wide variety of proteins, including the proteins that regulate cell cycle. The cell cycle consists of four phases including G1, S, G2, and M phases. The changes in S phase and M phase are important for genomic integrity [23]. Retinoblastoma protein (pRb) interacts with the E2F members to drive cell cycle progression and apoptosis. pRb can repress E2F-mediated transcription of cell cycle proteins by recruiting HDAC1 to the E2F-responsive promoters [24]. Moreover, HDAC10 has been reported to regulate the cell cycle via modulation of cyclin A2 overexpression, which further rescues G2/M transition arrest in HDAC10 knockdown cells. HDAC10 can regulate cyclin A2 expression by histone deacetylation near let-7 promoter, subsequently repressing transcription [25].
In addition to the transcriptional regulation of cell cycle-linked genes, HDACs also regulate cell cycle progress in transcription-independent manners. HDAC3 is a critical regulator of cell proliferation via a transcription-independent manner, which is recruited by AKAP95 and HA95 along with Aurora B. Deacetylation of histones by HDAC3 induce the Aurora kinase B-mediated maximal phosphorylation of histone H3 on Ser10, leading to progression of cell cycle [26]. In the same way, HDAC3 and HDAC6 induced the cell proliferation and cell survival in CCA cells [27,28].
3.2. Cell Death
HDACs have been reported to control apoptosis in various cancer cells via altering expression of apoptotic proteins. Inhibition of HDAC2 leads to the inhibition of tumor cell growth and initiation of apoptosis via activation of p53 and Bax in human lung cancer cells [29]. HDAC3 can down-regulate the expression of PUMA in gastric cancer cells. However, treatment with HDACi promotes PUMA expression through increasing the p53 binding to the PUMA promoter [30]. Aberrant expression of HDAC2 was reported in cancer cells, and treatment with HDAC2 inhibitors reduced cell motility, cell invasion, and cell growth, and induced cell death in gastric cancer cells [31]. In addition, HDAC2 was found to positively regulate Aurora A kinase, which induces pancreatic cancer cell growth and inhibits the cells death via inducing ciliary loss [32].
p53 is a tumor suppressor gene that can induce cell death in the transformed cells. Acetylation of p53 and its role in regulating tumor suppression have been widely explored [33]. When HDACs such as SIRT1 and SIRT2 remove acetyl groups form C-terminal lysines of p53, MDM2 ubiquitinates p53 and leads to its degradation, thus inhibiting p53 level in the cells [34]. Lower levels of p53 in cells may enhance the cell growth and inhibit apoptosis.
3.3. Metastasis
Cancer cell invasion and metastasis is an important process in the cancer spreading, and studies have confirmed the important role of HDACs in cancer cell metastasis in a variety of cancers.
Epithelial-cadherin (CDH1) is an epithelial cell marker that is depleted during metastasis. In pancreatic cancer cells, HDAC1 is recruited to the CDH1 promoter, resulting in deacetylation of histone 3 and histone 4 proteins in the nucleus and depletion in E-cadherin, which consequently helps in the epithelial–mesenchymal transition (EMT) [35]. EMT has been recently described as having a prominent role if CCA development; therefore, the regulation of this process by HDACs warrant further investigation [36]. The snail/HDAC1/HDAC2 regulatory axis is essential for EZH2-induced CDH1 inhibition in nasopharyngeal carcinoma cells [37]. ZEB1 regulates the recruitment of HDAC1 and HDAC2 to the CHD1 promoter in human pancreatic cancer cells [38]. SIRT1 also prompts cancer cell migration and metastasis in vivo and in vitro by combining with ZEB1 to inhibit CDH1 expression in prostate cancer cells [39]. Sun et al. also revealed a vital role of SIRT1 and MMP8 interaction in silencing of CDH1 and E-cadherin in prostate cancer cells [40].
3.4. Autophagy
The role of autophagy has been extensively studied in the development, maintenance, and progression of cancer cells. Autophagy is not only important for intracellular dynamics, but it has also been observed to control the interaction between the cells and the change in surrounding environment.
Just as autophagy has complex role in cancer development, so too is the effect of autophagy in different members of the Zn+2-dependent HDAC enzymes [41]. HDACs such as HDAC6 [28] and HDAC10 induce the deacetylation of cytoplasmic proteins in CCA cells; they have been directly involved in the autophagy process by modulation of key autophagy proteins such as LC3-II and Beclin1 [42]. Cells depleted in class I HDACs were observed with increased autophagic flux, demonstrated by increased autophagosomal proteins such as LC3-II, Beclin1, and ATG5 [43]. On the contrary, HDAC1- and HDAC2-deficient mice had obstructed autophagosome formation [44]. HDAC4 knockdown led to the induction of autophagy, with augmented LC3-II, Beclin-1, and ATG7 levels in the cells [45]. Similarly, HDAC5 downregulation in breast cancer cells led to the increased LC3-II and autophagic flux [46]. According to most studies, depletion of HDACs such as class I and IIa isozymes is linked with higher expression of autophagy regulators involved in the various cell functions. Because autophagy can improve cancer cell viability, simultaneously targeting autophagy might improve the therapeutic effects of HDACi against cancer.
3.5. Metabolism
Increased level of HDAC3 was noticed alongside decreased level of pyruvate, subsequently protecting CCA cells from apoptosis. HDAC3 synergistically increased expression of LDHA and PKM2 levels, resulting in low levels of pyruvate, as well as poor survival reports in CCA patients. The authors also found that decreasing levels of pyruvate supported cell proliferation in CCA cells. Elevated HDAC3 activity also stabilized c-MYC protein levels through deacetylation at K323, which further contributed to the low pyruvate levels [47]. In agreement, Yin et al. 2017 also reported that HDAC3 have oncogenic effects in CCA cells by inhibiting apoptosis and contributing to the cell proliferation. In addition, high expression of HDAC3 and HDAC6 was observed in CCA patients’ tissues, linked to low survival [27,28,48].
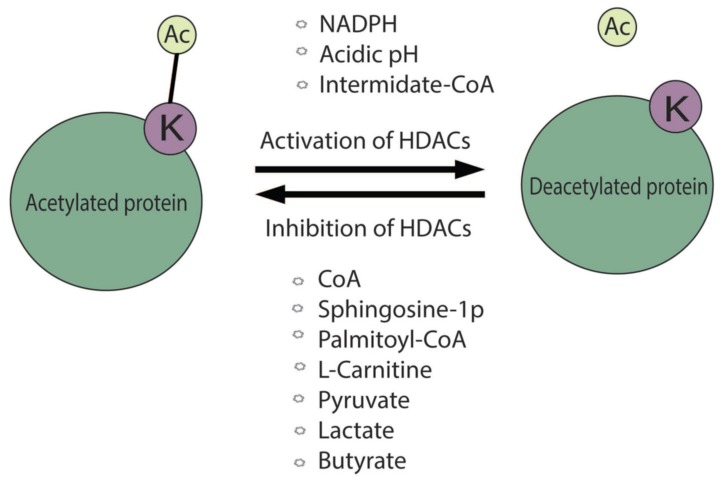
Several metabolites, produced from various intracellular biochemical reactions, may directly control HDAC activities (Figure 3). The cellular activity of recombinant HDAC1 and HDAC2 complexes were found to be increased with the addition of NADPH and CoA-derivates [49]. In particular, molecules generated from the glycolysis and/or involved in the fatty acid and sterol biosynthesis, including acetyl-CoA, butyryl-CoA, isobutyryl-CoA, succinyl-CoA, malonyl-CoA, methylmalonyl-CoA, and methylcrotonyl-CoA, may increase HDAC activity. In contrast, HDAC1 and HDAC2 activities were inhibited by the long chain fatty acid derivatives such as palmitoyl-CoA and the free CoA [49]. Further studies on the role of metabolic regulation in HDAC activity were observed by a bioactive lipid sphingosine-1 phosphate, formed during nuclear sphingolipid metabolism involved in β-oxidation of fatty acid, which could inhibit HDAC activity by binding to its active site [50,51].
Figure 3.
The role of metabolites affecting HDAC activity. In normal conditions, HDACs leads to the removal of the acetyl group from lysine from the target proteins. This reaction is modulated by the described metabolites or physical factors via the activation or inhibition function of HDAC activities.
Interestingly, pyruvate and lactate could act as inhibitors of HDACs in different cancer cells. Pyruvate and lactate significantly inhibited HDAC I and HDAC III activities, as comparable to the known HDACIs such as butyrate and trichostatin A (TSA) [52,53].
In a study by McBrian et al., the authors showed that low level of intracellular pH helps in HDAC activity and significantly decreases histone acetylation. Consequently, it releases the free acetate anions in order to prevent further decrease in the pH, suggestive of a relationship between the acidic environment in cancer cells and decreased levels of histone acetylation [54].
Butyrate, a short chain fatty acid that is produced by bacterial fermentation of polysaccharides, can inhibit HDACs [55]. Interestingly, butyrate specially targets cancer cells, whereas it stimulates the growth in normal cells [56]. Further, the researchers validated that the different inhibitory effects in normal and cancer cells is due to differences in the energy metabolism. In non-cancerous cells, the oxidative metabolism is active, which metabolizes butyrate through the tricarboxylic acid (TCA) cycle producing acetyl-CoA that also may favor HAT activity and cell proliferation. On the other hand, cancer cells are characterized as glycolytic in nature, and therefore uses a moderately small amount of butyrate through the TCA cycle, enabling butyrate to accumulate in the cell, acting as an HDAC inhibitor [56]. In addition, β-hydroxybutyrate (βOHB), another source of energy for cells, produced during prolonged physical activities and starvation, has also been found to be an inhibitor of HDAC (HDAC1, HDAC3, and HDAC4) activities [57].
3.6. Ciliary Assembly
The primary cilium is a sensory organelle expressed in almost every cell of the body, including cholangiocytes [58,59]. Several studies have reported that primary cilia can serve as antennae, which have mechanical and chemical sensory capabilities to communicate with the extracellular environment. As the primary cilia act as sensory organelles of the cells, deficiencies in primary cilia formation may lead to various disorders in humans, or ciliopathies. Disorders linked with genetic mutations in ciliary proteins often lead to abnormality in cilia formation. Ciliary dysfunction has been associated with intellectual disabilities, Bardet–Biedl syndrome, polycystic kidney disease, obesity, oral facial syndrome, cancer, and others [58,60,61]. Specifically, decreased or distorted primary cilia in cholangiocytes have important clinical implications and are associated with many biliary diseases, including CCA [62,63].
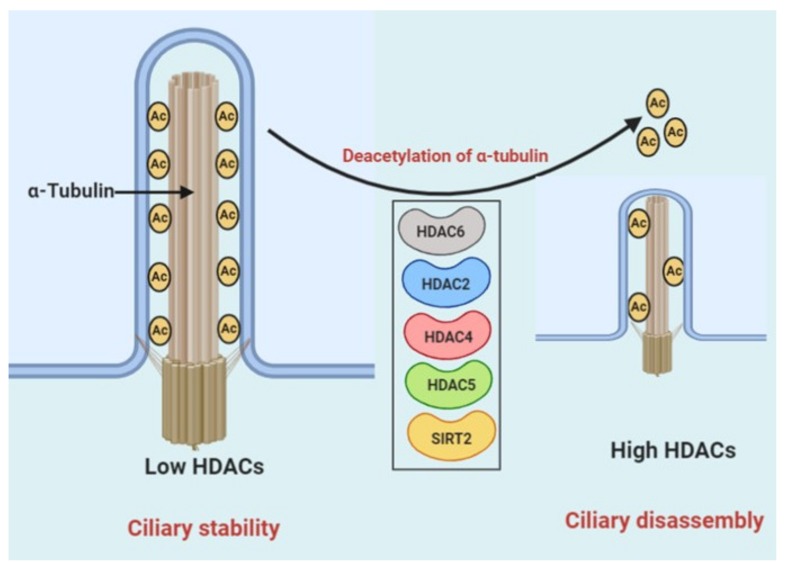
HDAC6 is known to inhibit ciliary assembly in CCA cells via deacetylating the tubulin protein in the ciliary axoneme [48,64]. Previously, we reported that HDAC6 expression was noticed as being upregulated in CCA cells [28] due to a dysregulation of miRNA-433 and miRNA-22, which induces the expression of HDAC6, consequently prompting ciliary loss and cell growth in normal cholangiocytes [65]. Interestingly, exposure to cigarette smoke induced HDAC6-mediated deacetylase activity, which led to autophagy-mediated cilia shortening in epithelial cells of mouse models [66]. Additionally, in pancreatic ductal adenocarcinoma cells, HDAC2 has been reported as a regulator of primary cilium formation, which reduces the expression of Aurora A kinase and thereby stimulates disassembly of primary cilia [32]. In zebrafish, HDAC4 and HDAC5 modulate cilia formation during kidney morphogenesis [4]. In another study in zebrafish, decreased cilia numbers and uncharacteristic centrosome amplification and polyploidy were observed with overexpression of SIRT2 in Kupffer’s vesicle. Reduction of SIRT2 inhibited the irregular amplification of centrosome and polyploidy associated with loss of polycystin-1 (PC1) [67] (Figure 4).
Figure 4.
Working model for the role of HDACs in ciliary disassembly. Ciliary disassembly is mediated through deacetylation of α-tubulin in the ciliary axoneme mediated by HDACs. Removal of acetyl groups from α-tubulin destabilizes axoneme microtubules, promoting ciliary resorption.
HDACs Inhibitors (HDACis) and CCA
HDACis can inhibit the HDAC activities, increase the aggregation of histone acetylation in autosomes, and stimulate gene expression [4]. Many previous studies have shown that HDACis can exert anticancer effects through several mechanisms inducing cell cycle arrest, induction of apoptosis, and autophagy in cancer cells [66,67].
The FDA has approved a number of HDACis such as vorinostat and romidepsin, which can target chromatin remodeling. These inhibitors have increased therapeutic efficacy in hepatobiliary cancer including CCA. HDACi (MS-275) treatment effectively inhibits the cell growth in EGI-1 and TFK-1 CCA cells by inducing cell cycle arrest and apoptosis [22]. Apoptosis was accompanied by the activation of caspase-3, Bax, and downregulation of Bcl-2. Cell cycle arrest is mostly inhibited at the G1/S checkpoint, which is associated with the induction of p21Waf/CIP1, an inhibitor of cyclin-dependent kinase [44,45]. MI192 has been shown to inhibit the deacetylase activity of HDAC3 in CCA, leading to reduced cancer cell growth in vitro and vivo [27]. HDAC3 was noticed as being upregulated in CCA cells as compared to adjacent normal cells. Taken together, HDAC3 was inhibited by MI192 and prompted apoptosis in human CCA cells [27].
Furthermore, application of cisplatin is related with drug resistance, implying the need for urgent development of improved combination therapies. In clinical application of HDAC inhibitors, including suberoylanilide hydroxamic acid (SAHA) and trichostatin A (TSA), synergistically induced anticancer effects occurred in CCA cell growth with cisplatin. Cisplatin, when combined with TSA or SAHA, synergistically led to cell growth inhibition and apoptosis in CCA cell lines such as KKU-M214 and KKU-100 [68]. The expression of HDAC1 and 2 was upregulated in the CCA cell lines. In other studies, the combination of 5-flourouracil and HDACis such as SAHA and TSA inhibited the cell growth in CCA cells [69] and administration of HDACi CG200745, along with other chemotherapeutic agents including cisplatin, 5-fluorouracil, and oxaliplatin, and decreased CCA cell viability via modulating the Hippo signaling pathway by inducing expression of miR-509-3p [70].
HDAC6 is another isoform of HDACs that is considered as enhancing CCA cell growth [58].
Inhibitors of HDAC6 such as ACY1215 and tubastatin-A significantly inhibited CCA cell growth in vitro and in vivo [28,71,72].
In gallbladder cancer, SAHA treatment inhibits cell proliferation by activating tumor suppressor gene p21 in the cells. Treatment of SAHA also caused significant decline in cell number in carcinoma cells when used synergistically with EZH2 siRNA; conversely, the expression of EZH2 and HDAC1/2 were decreased by SAHA treatment in TGBC2TKB cells [73]. The combination of SAHA and TSA reduces the cell growth and induces apoptosis in gallbladder carcinoma SGC-996 cells. Furthermore, they downregulate the phosphorylation of Akt and mTOR and inhibit the expressions of c-Myc, cyclin D1, and Bmi1 [74]. Some experimental studies have demonstrated that HDACi PCI-24781 have an anticancer effect on human gallbladder carcinoma cell and in mice. This effect is associated with downregulation of erbB2 protein activity and increased levels of tubulin and histone acetylation. PCI-24781 also resulted in effective inhibition of gemcitabine-resistant gallbladder carcinoma cell growth [75].
Drugs, including clofibratem, imexon, gefitinib, ciprofloxacin, and dexamethasone, have the capability to induce the formation of primary cilia in cancer cells [76]. These drugs also inhibit cell growth and may induce apoptosis. Because overexpression of HDAC6 in cholangiocytes induces ciliary loss; HDAC6 is a novel therapeutic target for ciliopathy-related disorders. HDAC6-mediated deacetylation of α-tubulin disrupts the stability of the axoneme leading to ciliary loss [7,10]. A previous study demonstrated that high expression of HDAC6 induced loss of primary cilia in CCA and polycystic liver disease (PLD) [28,72]. Consequently, the application of HDAC6 inhibitors to block the malignant phenotype in CCA cells could be a promising therapeutic strategy. CCA growth was significantly inhibited in in vitro and in vivo models by HDAC6 inhibitors via inducing re-expression of cilia [28]. Thus, the restoration of the primary cilia may be a potential therapeutic approach for CCA, and HDAC6 inhibitors may be important agents for the treatment of other ciliopathy-related diseases [77].
4. Natural Compounds Targeting HDACs
Natural compounds provide potent and pleiotropic ranges of drugs. Various HDACis discovered thus far are of natural origin. HDACi such as FK322, a cyclic peptide isolated from Chromobacterium violaceum, as well as TSA from Streptomyces hygroscopicus, selectively prevent HDAC1 and 2 activities. Trapoxin A and depudecin are also naturally occurring HDACis extracted from a fungus. Some marine organisms are also the source of potent natural HDACis, such as largazole and azumamides [6,78].
Other natural HDAC inhibitors such as KK4 and ICG15042, isolated from peanut skin extract, inhibited the growth of CCA cell via apoptosis induction and cell proliferation inhibition [79].
Epigallocatechin-3-gallate (EGCG) is a potent cancer chemopreventive and therapeutic agent isolated from the tea polyphenols, and is the major green tea polyphenol extract [80,81]. EGCG has been reported to have anticancer properties via inhibition of the HDAC activities in various cancers including lung, cervical, and melanoma [79,80,81,82,83,84].
Butyrate is a short chain fatty acid produced by the gut microbiota during fermentation of the dietary fibers, and has been shown to affect post-translational modifications of histones by inhibiting HDAC class III [85,86]. Butyrate has been reported to inhibit growth in hepatocellular carcinoma [14,87,88], lung cancer [89], breast cancer [90], pancreatic cancer [91,92], and colon cancer cells via increasing the acetylation of histones [56,93]. However, the role of butyrate in CCA has not been studied thus far.
5. Conclusions
Previous studies in the last decade have validated the fact that HDACs can play a critical role in cancer development by controlling acetylation of histone and non-histone proteins. As an eraser of acetylation from proteins, HDACs have been found to enhance oncogenic functions, suggestive of a crucial attractive therapeutic target against CCA. HDACs may play a main role in carcinogenesis through many pathways, and HDACis can prevent HDAC activity and work as an anticancer strategy by affecting multiple mechanisms inducing cell growth inhibition, cell cycle arrest, apoptosis, and restoration of cilia formation in CCA cells. These compounds can enhance histone acetylation levels, by which they control the expressions of genes that are involved in various biological pathways in cancer cells. Together, a good understanding of the HDAC- and HDACi-mediated molecular mechanism and pathway could lead to a new window toward developing a new therapeutic target against CCA.
Author Contributions
Conceptualization, K.P. and S.G.; Writing—Original Draft, K.P.; Writing—Reviewing and Editing, E.P., S.R. and S.G.; Supervision, S.G. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by National Institutes of Health grant R01CA183764 (to S.A.G.).
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Rizvi S., Khan S.A., Hallemeier C.L., Kelley R.K., Gores G.J. Cholangiocarcinoma—Evolving concepts and therapeutic strategies. Nat. Rev. Clin. Oncol. 2018;15:95–111. doi: 10.1038/nrclinonc.2017.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Blechacz B., Gores G.J. Cholangiocarcinoma: Advances in Pathogenesis, Diagnosis, and Treatment. Hepatology. 2008;48:308–321. doi: 10.1002/hep.22310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rizvi S., Borad M.J., Patel T., Gores G.J. Cholangiocarcinoma: Molecular pathways and therapeutic opportunities. Semin. Liver Dis. 2014;34:456–464. doi: 10.1055/s-0034-1394144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sanaei M., Kavoosi F. Histone Deacetylases and Histone Deacetylase Inhibitors: Molecular Mechanisms of Action in Various Cancers. Adv. Biomed. Res. 2019;8:63. doi: 10.4103/abr.abr_142_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shahbazian M.D., Grunstein M. Functions of site-specific histone acetylation and deacetylation. Annu. Rev. Biochem. 2007;76:75–100. doi: 10.1146/annurev.biochem.76.052705.162114. [DOI] [PubMed] [Google Scholar]
- 6.Singh A.K., Bishayee A., Pandey A.K. Targeting Histone Deacetylases with Natural and Synthetic Agents: An Emerging Anticancer Strategy. Nutrients. 2018;10:731. doi: 10.3390/nu10060731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Eckschlager T., Plch J., Stiborova M., Hrabeta J. Histone Deacetylase Inhibitors as Anticancer Drugs. Int. J. Mol. Sci. 2017;18:1414. doi: 10.3390/ijms18071414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li Y., Wang F., Chen X., Wang J., Zhao Y., Li Y., He B. Zinc-dependent Deacetylase (HDAC) Inhibitors with Different Zinc Binding Groups. Curr. Top. Med. Chem. 2019;19:223–241. doi: 10.2174/1568026619666190122144949. [DOI] [PubMed] [Google Scholar]
- 9.Kim B., Ratnayake R., Lee H., Shi G., Zeller S.L., Li C., Luesch H., Hong J. Synthesis and biological evaluation of largazole zinc-binding group analogs. Bioorg. Med. Chem. 2017;25:3077–3086. doi: 10.1016/j.bmc.2017.03.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen B., Zang W., Wang J., Huang Y., He Y., Yan L., Liu J., Zheng W. The chemical biology of sirtuins. Chem. Soc. Rev. 2015;44:5246–5264. doi: 10.1039/C4CS00373J. [DOI] [PubMed] [Google Scholar]
- 11.Chiaradonna F., Cirulli C., Palorini R., Votta G., Alberghina L. New Insights into the Connection Between Histone Deacetylases, Cell Metabolism, and Cancer. Antioxid. Redox Signal. 2014;23:30–50. doi: 10.1089/ars.2014.5854. [DOI] [PubMed] [Google Scholar]
- 12.Kida Y., Goligorsky M.S. Sirtuins, Cell Senescence, and Vascular Aging. Can. J. Cardiol. 2016;32:634–641. doi: 10.1016/j.cjca.2015.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Carafa V., Nebbioso A., Altucci L. Sirtuins and disease: The road ahead. Front. Pharmacol. 2012;3:4. doi: 10.3389/fphar.2012.00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pant K., Saraya A., Venugopal S.K. Oxidative stress plays a key role in butyrate-mediated autophagy via Akt/mTOR pathway in hepatoma cells. Chem. Biol. Interact. 2017;273:99–106. doi: 10.1016/j.cbi.2017.06.001. [DOI] [PubMed] [Google Scholar]
- 15.Wang Y., He J., Liao M., Hu M., Li W., Ouyang H., Wang X., Ye T., Zhang Y., Ouyang L. An overview of Sirtuins as potential therapeutic target: Structure, function and modulators. Eur. J. Med. Chem. 2019;161:48–77. doi: 10.1016/j.ejmech.2018.10.028. [DOI] [PubMed] [Google Scholar]
- 16.Pant K., Mishra A.K., Pradhan S.M., Nayak B., Das P., Shalimar D., Saraya A., Venugopal S.K. Butyrate inhibits HBV replication and HBV-induced hepatoma cell proliferation via modulating SIRT-1/Ac-p53 regulatory axis. Mol. Carcinog. 2019;58:524–532. doi: 10.1002/mc.22946. [DOI] [PubMed] [Google Scholar]
- 17.De Matteis S., Granato A.M., Napolitano R., Molinari C., Valgiusti M., Santini D., Foschi F.G., Ercolani G., Vespasiani Gentilucci U., Faloppi L., et al. Interplay Between SIRT-3, Metabolism and Its Tumor Suppressor Role in Hepatocellular Carcinoma. Dig. Dis. Sci. 2017;62:1872–1880. doi: 10.1007/s10620-017-4615-x. [DOI] [PubMed] [Google Scholar]
- 18.Zhao S., Zhang X., Li H. Beyond histone acetylation-writing and erasing histone acylations. Curr. Opin. Struct. Biol. 2018;53:169–177. doi: 10.1016/j.sbi.2018.10.001. [DOI] [PubMed] [Google Scholar]
- 19.Seto E., Yoshida M. Erasers of histone acetylation: The histone deacetylase enzymes. Cold Spring Harb. Perspect. Biol. 2014;6:a018713. doi: 10.1101/cshperspect.a018713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Peng L., Seto E. Deacetylation of nonhistone proteins by HDACs and the implications in cancer. Handb. Exp. Pharmacol. 2011;206:39–56. doi: 10.1007/978-3-642-21631-2_3. [DOI] [PubMed] [Google Scholar]
- 21.Ropero S., Esteller M. The role of histone deacetylases (HDACs) in human cancer. Mol. Oncol. 2007;1:19–25. doi: 10.1016/j.molonc.2007.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shankar S., Srivastava R.K. Histone deacetylase inhibitors: Mechanisms and clinical significance in cancer: HDAC inhibitor-induced apoptosis. Adv. Exp. Med. Biol. 2008;615:261–298. doi: 10.1007/978-1-4020-6554-5_13. [DOI] [PubMed] [Google Scholar]
- 23.Telles E., Seto E. Modulation of Cell Cycle Regulators by HDACs. Front. Biosci. 2012;4:831–839. doi: 10.2741/s303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brehm A., Miska E.A., McCance D.J., Reid J.L., Bannister A.J., Kouzarides T. Retinoblastoma protein recruits histone deacetylase to repress transcription. Nature. 1998;391:597–601. doi: 10.1038/35404. [DOI] [PubMed] [Google Scholar]
- 25.Li Y., Peng L., Seto E. Histone Deacetylase 10 Regulates the Cell Cycle G2/M Phase Transition via a Novel Let-7–HMGA2–Cyclin A2 Pathway. Mol. Cell. Biol. 2015;35:3547–3565. doi: 10.1128/MCB.00400-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li Y., Kao G.D., Garcia B.A., Shabanowitz J., Hunt D.F., Qin J., Phelan C., Lazar M.A. A novel histone deacetylase pathway regulates mitosis by modulating Aurora B kinase activity. Genes Dev. 2006;20:2566–2579. doi: 10.1101/gad.1455006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yin Y., Zhang M., Dorfman R.G., Li Y., Zhao Z., Pan Y., Zhou Q., Huang S., Zhao S., Yao Y., et al. Histone deacetylase 3 overexpression in human cholangiocarcinoma and promotion of cell growth via apoptosis inhibition. Cell Death Dis. 2017;8:e2856. doi: 10.1038/cddis.2016.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gradilone S.A., Radtke B.N., Bogert P.S., Huang B.Q., Gajdos G.B., LaRusso N.F. HDAC6 inhibition restores ciliary expression and decreases tumor growth. Cancer Res. 2013;73:2259–2270. doi: 10.1158/0008-5472.CAN-12-2938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jung K.H., Noh J.H., Kim J.K., Eun J.W., Bae H.J., Xie H.J., Chang Y.G., Kim M.G., Park H., Lee J.Y., et al. HDAC2 overexpression confers oncogenic potential to human lung cancer cells by deregulating expression of apoptosis and cell cycle proteins. J. Cell. Biochem. 2012;113:2167–2177. doi: 10.1002/jcb.24090. [DOI] [PubMed] [Google Scholar]
- 30.Feng L., Pan M., Sun J., Lu H., Shen Q., Zhang S., Jiang T., Liu L., Jin W., Chen Y., et al. Histone deacetylase 3 inhibits expression of PUMA in gastric cancer cells. J. Mol. Med. 2013;91:49–58. doi: 10.1007/s00109-012-0932-x. [DOI] [PubMed] [Google Scholar]
- 31.Kim J.K., Noh J.H., Eun J.W., Jung K.H., Bae H.J., Shen Q., Kim M.G., Chang Y.G., Kim S.-J., Park W.S., et al. Targeted inactivation of HDAC2 restores p16INK4a activity and exerts antitumor effects on human gastric cancer. Mol. Cancer Res. MCR. 2013;11:62–73. doi: 10.1158/1541-7786.MCR-12-0332. [DOI] [PubMed] [Google Scholar]
- 32.Kobayashi T., Nakazono K., Tokuda M., Mashima Y., Dynlacht B.D., Itoh H. HDAC2 promotes loss of primary cilia in pancreatic ductal adenocarcinoma. EMBO Rep. 2017;18:334–343. doi: 10.15252/embr.201541922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dai C., Gu W. p53 post-translational modification: Deregulated in tumorigenesis. Trends Mol. Med. 2010;16:528–536. doi: 10.1016/j.molmed.2010.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lee J.T., Gu W. SIRT1: Regulator of p53 Deacetylation. Genes Cancer. 2013;4:112–117. doi: 10.1177/1947601913484496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Von Burstin J., Eser S., Paul M.C., Seidler B., Brandl M., Messer M., von Werder A., Schmidt A., Mages J., Pagel P., et al. E-cadherin regulates metastasis of pancreatic cancer in vivo and is suppressed by a SNAIL/HDAC1/HDAC2 repressor complex. Gastroenterology. 2009;137:361–371. doi: 10.1053/j.gastro.2009.04.004. [DOI] [PubMed] [Google Scholar]
- 36.Vaquero J., Guedj N., Clapéron A., Ho-Bouldoires T.H.N., Paradis V., Fouassier L. Epithelial-mesenchymal transition in cholangiocarcinoma: From clinical evidence to regulatory networks. J. Hepatol. 2017;66:424–441. doi: 10.1016/j.jhep.2016.09.010. [DOI] [PubMed] [Google Scholar]
- 37.Tong Z.-T., Cai M.-Y., Wang X.-G., Kong L.-L., Mai S.-J., Liu Y.-H., Zhang H.-B., Liao Y.-J., Zheng F., Zhu W., et al. EZH2 supports nasopharyngeal carcinoma cell aggressiveness by forming a co-repressor complex with HDAC1/HDAC2 and Snail to inhibit E-cadherin. Oncogene. 2012;31:583–594. doi: 10.1038/onc.2011.254. [DOI] [PubMed] [Google Scholar]
- 38.Aghdassi A., Sendler M., Guenther A., Mayerle J., Behn C.-O., Heidecke C.-D., Friess H., Büchler M., Evert M., Lerch M.M., et al. Recruitment of histone deacetylases HDAC1 and HDAC2 by the transcriptional repressor ZEB1 downregulates E-cadherin expression in pancreatic cancer. Gut. 2012;61:439–448. doi: 10.1136/gutjnl-2011-300060. [DOI] [PubMed] [Google Scholar]
- 39.Byles V., Zhu L., Lovaas J.D., Chmilewski L.K., Wang J., Faller D.V., Dai Y. SIRT1 induces EMT by cooperating with EMT transcription factors and enhances prostate cancer cell migration and metastasis. Oncogene. 2012;31:4619–4629. doi: 10.1038/onc.2011.612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sun L., Kokura K., Izumi V., Koomen J.M., Seto E., Chen J., Fang J. MPP8 and SIRT1 crosstalk in E-cadherin gene silencing and epithelial-mesenchymal transition. EMBO Rep. 2015;16:689–699. doi: 10.15252/embr.201439792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mrakovcic M., Kleinheinz J., Fröhlich L.F. Histone Deacetylase Inhibitor-Induced Autophagy in Tumor Cells: Implications for p53. Int. J. Mol. Sci. 2017;18:1883. doi: 10.3390/ijms18091883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Koeneke E., Witt O., Oehme I. HDAC Family Members Intertwined in the Regulation of Autophagy: A Druggable Vulnerability in Aggressive Tumor Entities. Cells. 2015;4:135–168. doi: 10.3390/cells4020135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schipper H., Alla V., Meier C., Nettelbeck D.M., Herchenröder O., Pützer B.M. Eradication of metastatic melanoma through cooperative expression of RNA-based HDAC1 inhibitor and p73 by oncolytic adenovirus. Oncotarget. 2014;5:5893–5907. doi: 10.18632/oncotarget.1839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Moresi V., Carrer M., Grueter C.E., Rifki O.F., Shelton J.M., Richardson J.A., Bassel-Duby R., Olson E.N. Histone deacetylases 1 and 2 regulate autophagy flux and skeletal muscle homeostasis in mice. Proc. Natl. Acad. Sci. USA. 2012;109:1649–1654. doi: 10.1073/pnas.1121159109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kang Z.-H., Wang C.-Y., Zhang W.-L., Zhang J.-T., Yuan C.-H., Zhao P.-W., Lin Y.-Y., Hong S., Li C.-Y., Wang L. Histone deacetylase HDAC4 promotes gastric cancer SGC-7901 cells progression via p21 repression. PLoS ONE. 2014;9:e98894. doi: 10.1371/journal.pone.0098894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Peixoto P., Castronovo V., Matheus N., Polese C., Peulen O., Gonzalez A., Boxus M., Verdin E., Thiry M., Dequiedt F., et al. HDAC5 is required for maintenance of pericentric heterochromatin, and controls cell-cycle progression and survival of human cancer cells. Cell Death Differ. 2012;19:1239–1252. doi: 10.1038/cdd.2012.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Xu L., Wang L., Zhou L., Dorfman R.G., Pan Y., Tang D., Wang Y., Yin Y., Jiang C., Zou X., et al. The SIRT2/cMYC Pathway Inhibits Peroxidation-Related Apoptosis In Cholangiocarcinoma Through Metabolic Reprogramming. Neoplasia. 2019;21:429–441. doi: 10.1016/j.neo.2019.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hubbert C., Guardiola A., Shao R., Kawaguchi Y., Ito A., Nixon A., Yoshida M., Wang X.-F., Yao T.-P. HDAC6 is a microtubule-associated deacetylase. Nature. 2002;417:455–458. doi: 10.1038/417455a. [DOI] [PubMed] [Google Scholar]
- 49.Vogelauer M., Krall A.S., McBrian M.A., Li J.-Y., Kurdistani S.K. Stimulation of Histone Deacetylase Activity by Metabolites of Intermediary Metabolism. J. Biol. Chem. 2012;287:32006–32016. doi: 10.1074/jbc.M112.362467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Huang H., Liu N., Guo H., Liao S., Li X., Yang C., Liu S., Song W., Liu C., Guan L., et al. L-Carnitine Is an Endogenous HDAC Inhibitor Selectively Inhibiting Cancer Cell Growth In Vivo and In Vitro. PLoS ONE. 2012;7:e49062. doi: 10.1371/journal.pone.0049062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hait N.C., Allegood J., Maceyka M., Strub G.M., Harikumar K.B., Singh S.K., Luo C., Marmorstein R., Kordula T., Milstien S., et al. Regulation of Histone Acetylation in the Nucleus by Sphingosine-1-Phosphate. Science. 2009;325:1254–1257. doi: 10.1126/science.1176709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Thangaraju M., Carswell K.N., Prasad P.D., Ganapathy V. Colon cancer cells maintain low levels of pyruvate to avoid cell death caused by inhibition of HDAC1/HDAC3. Biochem. J. 2009;417:379–389. doi: 10.1042/BJ20081132. [DOI] [PubMed] [Google Scholar]
- 53.Latham T., Mackay L., Sproul D., Karim M., Culley J., Harrison D.J., Hayward L., Langridge-Smith P., Gilbert N., Ramsahoye B.H. Lactate, a product of glycolytic metabolism, inhibits histone deacetylase activity and promotes changes in gene expression. Nucleic Acids Res. 2012;40:4794–4803. doi: 10.1093/nar/gks066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.McBrian M.A., Behbahan I.S., Ferrari R., Su T., Huang T.-W., Li K., Hong C.S., Christofk H.R., Vogelauer M., Seligson D.B., et al. Histone Acetylation Regulates Intracellular pH. Mol. Cell. 2013;49:310–321. doi: 10.1016/j.molcel.2012.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Joensuu T., Mester J. Inhibition of cell cycle progression by sodium butyrate in normal rat kidney fibroblasts is altered by expression of the adenovirus 5 early 1A gene. Biosci. Rep. 1994;14:291–300. doi: 10.1007/BF01199053. [DOI] [PubMed] [Google Scholar]
- 56.Donohoe D.R., Collins L.B., Wali A., Bigler R., Sun W., Bultman S.J. The Warburg Effect Dictates the Mechanism of Butyrate-Mediated Histone Acetylation and Cell Proliferation. Mol. Cell. 2012;48:612–626. doi: 10.1016/j.molcel.2012.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Shimazu T., Hirschey M.D., Newman J., He W., Shirakawa K., Moan N.L., Grueter C.A., Lim H., Saunders L.R., Stevens R.D., et al. Suppression of Oxidative Stress by β-Hydroxybutyrate, an Endogenous Histone Deacetylase Inhibitor. Science. 2013;339:211–214. doi: 10.1126/science.1227166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gradilone S.A., Pisarello M.J.L., LaRusso N.F. Primary Cilia in Tumor Biology: The Primary Cilium as a Therapeutic Target in Cholangiocarcinoma. Curr. Drug Targets. 2017;18:958–963. doi: 10.2174/1389450116666150223162737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Higgins M., Obaidi I., McMorrow T. Primary cilia and their role in cancer. Oncol. Lett. 2019;17:3041–3047. doi: 10.3892/ol.2019.9942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Liu H., Kiseleva A.A., Golemis E.A. Ciliary signalling in cancer. Nat. Rev. Cancer. 2018;18:511–524. doi: 10.1038/s41568-018-0023-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Nishimura Y., Kasahara K., Shiromizu T., Watanabe M., Inagaki M. Primary Cilia as Signaling Hubs in Health and Disease. Adv. Sci. 2019;6:1801138. doi: 10.1002/advs.201801138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mansini A.P., Peixoto E., Thelen K.M., Gaspari C., Jin S., Gradilone S.A. The cholangiocyte primary cilium in health and disease. Biochim. Biophys. Acta Mol. Basis Dis. 2018;1864:1245–1253. doi: 10.1016/j.bbadis.2017.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Masyuk T.V., Masyuk A.I., LaRusso N.F. Therapeutic Targets in Polycystic Liver Disease. Curr. Drug Targets. 2017;18:950–957. doi: 10.2174/1389450116666150427161743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ran J., Yang Y., Li D., Liu M., Zhou J. Deacetylation of α-tubulin and cortactin is required for HDAC6 to trigger ciliary disassembly. Sci. Rep. 2015;5:1–13. doi: 10.1038/srep12917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mansini A.P., Lorenzo Pisarello M.J., Thelen K.M., Cruz-Reyes M., Peixoto E., Jin S., Howard B.N., Trussoni C.E., Gajdos G.B., LaRusso N.F., et al. MicroRNA (miR)-433 and miR-22 dysregulations induce histone-deacetylase-6 overexpression and ciliary loss in cholangiocarcinoma. Hepatology. 2018;68:561–573. doi: 10.1002/hep.29832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lam H.C., Cloonan S.M., Bhashyam A.R., Haspel J.A., Singh A., Sathirapongsasuti J.F., Cervo M., Yao H., Chung A.L., Mizumura K., et al. Histone deacetylase 6-mediated selective autophagy regulates COPD-associated cilia dysfunction. J. Clin. Invest. 2013;123:5212–5230. doi: 10.1172/JCI69636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Luchenko V.L., Litman T., Chakraborty A.R., Heffner A., Devor C., Wilkerson J., Stein W., Robey R.W., Bangiolo L., Levens D., et al. Histone deacetylase inhibitor-mediated cell death is distinct from its global effect on chromatin. Mol. Oncol. 2014;8:1379–1392. doi: 10.1016/j.molonc.2014.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Asgar M.A., Senawong G., Sripa B., Senawong T. Synergistic anticancer effects of cisplatin and histone deacetylase inhibitors (SAHA and TSA) on cholangiocarcinoma cell lines. Int. J. Oncol. 2016;48:409–420. doi: 10.3892/ijo.2015.3240. [DOI] [PubMed] [Google Scholar]
- 69.Sriraksa R., Limpaiboon T. Histone deacetylases and their inhibitors as potential therapeutic drugs for cholangiocarcinoma—Cell line findings. Asian Pac. J. Cancer Prev. 2013;14:2503–2508. doi: 10.7314/APJCP.2013.14.4.2503. [DOI] [PubMed] [Google Scholar]
- 70.Jung D.E., Park S.B., Kim K., Kim C., Song S.Y. CG200745, an HDAC inhibitor, induces anti-tumour effects in cholangiocarcinoma cell lines via miRNAs targeting the Hippo pathway. Sci. Rep. 2017;7:1–13. doi: 10.1038/s41598-017-11094-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mansini A.P., Peixoto E., Jin S., Richard S., Gradilone S.A. The Chemosensory Function of Primary Cilia Regulates Cholangiocyte Migration, Invasion, and Tumor Growth. Hepatology. 2019;69:1582–1598. doi: 10.1002/hep.30308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Gradilone S.A., Habringer S., Masyuk T.V., Howard B.N., Masyuk A.I., LaRusso N.F. HDAC6 Is Overexpressed in Cystic Cholangiocytes and Its Inhibition Reduces Cystogenesis. Am. J. Pathol. 2014;184:600–608. doi: 10.1016/j.ajpath.2013.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Yamaguchi J., Sasaki M., Sato Y., Itatsu K., Harada K., Zen Y., Ikeda H., Nimura Y., Nagino M., Nakanuma Y. Histone deacetylase inhibitor (SAHA) and repression of EZH2 synergistically inhibit proliferation of gallbladder carcinoma. Cancer Sci. 2010;101:355–362. doi: 10.1111/j.1349-7006.2009.01387.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zhang P., Guo Z., Wu Y., Hu R., Du J., He X., Jiao X., Zhu X. Histone Deacetylase Inhibitors Inhibit the Proliferation of Gallbladder Carcinoma Cells by Suppressing AKT/mTOR Signaling. PLoS ONE. 2015;10:e0136193. doi: 10.1371/journal.pone.0136193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kitamura T., Connolly K., Ruffino L., Ajiki T., Lueckgen A., Di Giovanni J., Kiguchi K. The therapeutic effect of histone deacetylase inhibitor PCI-24781 on gallbladder carcinoma in BK5.erbB2 mice. J. Hepatol. 2012;57:84–91. doi: 10.1016/j.jhep.2012.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Khan N.A., Willemarck N., Talebi A., Marchand A., Binda M.M., Dehairs J., Rueda-Rincon N., Daniels V.W., Bagadi M., Raj D.B.T.G., et al. Identification of drugs that restore primary cilium expression in cancer cells. Oncotarget. 2016;7:9975–9992. doi: 10.18632/oncotarget.7198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lorenzo Pisarello M., Masyuk T.V., Gradilone S.A., Masyuk A.I., Ding J.F., Lee P.-Y., LaRusso N.F. Combination of a Histone Deacetylase 6 Inhibitor and a Somatostatin Receptor Agonist Synergistically Reduces Hepatorenal Cystogenesis in an Animal Model of Polycystic Liver Disease. Am. J. Pathol. 2018;188:981–994. doi: 10.1016/j.ajpath.2017.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Bassett S.A., Barnett M.P.G. The role of dietary histone deacetylases (HDACs) inhibitors in health and disease. Nutrients. 2014;6:4273–4301. doi: 10.3390/nu6104273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Saenglee S., Senawong G., Jogloy S., Sripa B., Senawong T. Peanut testa extracts possessing histone deacetylase inhibitory activity induce apoptosis in cholangiocarcinoma cells. Biomed. Pharmacother. 2018;98:233–241. doi: 10.1016/j.biopha.2017.12.054. [DOI] [PubMed] [Google Scholar]
- 80.Chen D., Milacic V., Chen M.S., Wan S.B., Lam W.H., Huo C., Landis-Piwowar K.R., Cui Q.C., Wali A., Chan T.H., et al. Tea polyphenols, their biological effects and potential molecular targets. Histol. Histopathol. 2008;23:487–496. doi: 10.14670/hh-23.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Chen D., Wan S.B., Yang H., Yuan J., Chan T.H., Dou Q.P. EGCG, green tea polyphenols and their synthetic analogs and prodrugs for human cancer prevention and treatment. Adv. Clin. Chem. 2011;53:155–177. doi: 10.1016/b978-0-12-385855-9.00007-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hu Q., Chang X., Yan R., Rong C., Yang C., Cheng S., Gu X., Yao H., Hou X., Mo Y., et al. (-)-Epigallocatechin-3-gallate induces cancer cell apoptosis via acetylation of amyloid precursor protein. Med. Oncol. 2015;32:390. doi: 10.1007/s12032-014-0390-0. [DOI] [PubMed] [Google Scholar]
- 83.Khan M.A., Hussain A., Sundaram M.K., Alalami U., Gunasekera D., Ramesh L., Hamza A., Quraishi U. (-)-Epigallocatechin-3-gallate reverses the expression of various tumor-suppressor genes by inhibiting DNA methyltransferases and histone deacetylases in human cervical cancer cells. Oncol. Rep. 2015;33:1976–1984. doi: 10.3892/or.2015.3802. [DOI] [PubMed] [Google Scholar]
- 84.Nihal M., Ahmad N., Mukhtar H., Wood G.S. Anti-proliferative and proapoptotic effects of (-)-epigallocatechin-3-gallate on human melanoma: Possible implications for the chemoprevention of melanoma. Int. J. Cancer. 2005;114:513–521. doi: 10.1002/ijc.20785. [DOI] [PubMed] [Google Scholar]
- 85.Delage B., Dashwood R.H. Dietary Manipulation of Histone Structure and Function. Annu. Rev. Nutr. 2008;28:347–366. doi: 10.1146/annurev.nutr.28.061807.155354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Morrison D.J., Preston T. Formation of short chain fatty acids by the gut microbiota and their impact on human metabolism. Gut Microb. 2016;7:189–200. doi: 10.1080/19490976.2015.1134082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Pant K., Yadav A.K., Gupta P., Islam R., Saraya A., Venugopal S.K. Butyrate induces ROS-mediated apoptosis by modulating miR-22/SIRT-1 pathway in hepatic cancer cells. Redox Biol. 2017;12:340–349. doi: 10.1016/j.redox.2017.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wang H.-G., Huang X.-D., Shen P., Li L.-R., Xue H.-T., Ji G.-Z. Anticancer effects of sodium butyrate on hepatocellular carcinoma cells in vitro. Int. J. Mol. Med. 2013;31:967–974. doi: 10.3892/ijmm.2013.1285. [DOI] [PubMed] [Google Scholar]
- 89.Amoêdo N.D., Rodrigues M.F., Pezzuto P., Galina A., da Costa R.M., de Almeida F.C.L., El-Bacha T., Rumjanek F.D. Energy metabolism in H460 lung cancer cells: Effects of histone deacetylase inhibitors. PLoS ONE. 2011;6:e22264. doi: 10.1371/journal.pone.0022264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Chopin V., Toillon R.-A., Jouy N., Bourhis X.L. P21 WAF1/CIP1 is dispensable for G1 arrest, but indispensable for apoptosis induced by sodium butyrate in MCF-7 breast cancer cells. Oncogene. 2004;23:21–29. doi: 10.1038/sj.onc.1207020. [DOI] [PubMed] [Google Scholar]
- 91.Farrow B., Rychahou P., O’Connor K.L., Evers B.M. Butyrate inhibits pancreatic cancer invasion. J. Gastrointest. Surg. 2003;7:864–870. doi: 10.1007/s11605-003-0031-y. [DOI] [PubMed] [Google Scholar]
- 92.Natoni F., Diolordi L., Santoni C., Gilardini Montani M.S. Sodium butyrate sensitises human pancreatic cancer cells to both the intrinsic and the extrinsic apoptotic pathways. Biochim. Biophys. Acta. 2005;1745:318–329. doi: 10.1016/j.bbamcr.2005.07.003. [DOI] [PubMed] [Google Scholar]
- 93.Donohoe D.R., Holley D., Collins L.B., Montgomery S.A., Whitmore A.C., Hillhouse A., Curry K.P., Renner S.W., Greenwalt A., Ryan E.P., et al. A gnotobiotic mouse model demonstrates that dietary fiber protects against colorectal tumorigenesis in a microbiota- and butyrate-dependent manner. Cancer Discov. 2014;4:1387–1397. doi: 10.1158/2159-8290.CD-14-0501. [DOI] [PMC free article] [PubMed] [Google Scholar]