A synthetic maize auxin response circuit recapitulated in Saccharomyces cerevisiae reveals an auxin signaling network with functional homology to the Arabidopsis circuit.
Abstract
Auxin plays a key role across all land plants in growth and developmental processes. Although auxin signaling function has diverged and expanded, differences in the molecular functions of signaling components have largely been characterized in Arabidopsis (Arabidopsis thaliana). Here, we used the nuclear Auxin Response Circuit recapitulated in yeast (Saccharomyces cerevisiae) system to functionally annotate maize (Zea mays) auxin signaling components, focusing on genes expressed during the development of ear and tassel inflorescences. All 16 maize auxin/indole-3-acetic acid repressor proteins were degraded in response to auxin with rates that depended on both receptor and repressor identities. When fused to the maize TOPLESS homolog RAMOSA1 ENHANCER LOCUS2, maize auxin/indole-3-acetic acids were able to repress AUXIN RESPONSE FACTOR transcriptional activity. A complete auxin response circuit comprising all maize components, including the ZmAFB2/3 b1 maize AUXIN SIGNALING F-BOX (AFB) receptor, was fully functional. The ZmAFB2/3 b1 auxin receptor was more sensitive to hormone than AtAFB2 and allowed for rapid circuit activation upon auxin addition. These results validate the conserved role of predicted auxin response genes in maize as well as provide evidence that a synthetic approach can facilitate broader comparative studies across the wide range of species with sequenced genomes.
Auxin is an ancient molecule, and its role as a phytohormone dates back to the earliest diverging land plants (Mutte et al., 2018). In recent years, mounting evidence has emerged for both transcriptional and nontranscriptional cellular auxin response pathways that paint an increasingly complex picture of how auxin signals are detected and transmitted in plant cells. (Leyser, 2018). The gene families involved in the best characterized nuclear auxin response pathway show evidence of expansion that parallels increasing complexity in plant form. For example, the moss Physcomitrella patens has three members of the auxin/indole-3-acetic acid (Aux/IAA) gene family (Prigge et al., 2016), while the eudicot Arabidopsis (Arabidopsis thaliana) has 29 and the monocot maize (Zea mays) has 34 (Ludwig et al., 2013; Luo et al., 2018; Matthes et al., 2019). The retention of large gene families involved in this nuclear auxin response following genome duplications, in combination with the central role of auxin in plant development, has led to the hypothesis that functional diversification in this auxin response circuit underpins much of the structural and functional innovations during land plant evolution (Mutte et al., 2018). Connecting natural variation to functional divergence remains a major challenge. This problem becomes even more complicated if selection is operating on the amplitude or dynamics of a network rather than the function of any one component, a premise that itself has been quite difficult to test.
The nuclear auxin response is among the best understood signaling pathways in plants and thus is an excellent model to tackle questions about functional divergence in gene families within a single species and in network functions across multiple species. This nuclear auxin response circuit consists of three modules: (1) activation of auxin-responsive genes by AUXIN RESPONSE FACTOR (ARF) transcription factors; (2) repression of ARFs in the absence of auxin by recruitment of TOPLESS (TPL)/TOPLESS-RELATED (TPR) corepressors via Aux/IAA proteins; and (3) degradation of Aux/IAA proteins via their auxin-induced association with TRANSPORT INHIBITOR RESPONSE1 (TIR1)/AUXIN SIGNALING F-BOX (AFB) receptors (Leyser, 2018). There is some evidence to support a model where distinct combinations of these proteins acting in different cell types lead to the variety of cellular auxin responses observed across plant tissues (Vernoux et al., 2011; Rademacher et al., 2012; Bargmann et al., 2013). However, coexpression of components, feedback regulation, and interactions with other signaling pathways (Piya et al., 2014) have made it virtually impossible to conclusively support or refute this model.
The wealth of genetic, genomic, and biochemical tools in Arabidopsis have made it possible to rapidly build a strong foundational understanding of auxin response. However, to both explore the extent of shared auxin signaling properties across plants and fully interrogate the connection between natural variation in protein sequences and functional innovations in plant development, this nuclear auxin response must be examined in more species. Understanding differences between eudicots and monocots is of particular interest, as the molecular mechanism that explains the differential impacts of widely used auxinic herbicides remains a mystery, where eudicot weeds are killed while grasses are often unaffected (McSteen, 2010). In recent years, functional studies in maize have made inroads in delineating auxin response components. For example, the maize Aux/IAA protein ROOTLESS WITH UNDETECTABLE MERISTEMS1 (RUM1) is known to be functionally important in the development of the root in the embryo as well as in root branching in seedlings (von Behrens et al., 2011). BIF1 and BIF4 are Aux/IAAs known to have important functions in the development of maize inflorescences (Galli et al., 2015). Mutations in REL2, a functional homolog of TPL in Arabidopsis, can rescue TPL mutants in Arabidopsis and have pleiotropic auxin developmental effects in maize, rice (Oryza sativa), and Arabidopsis (Gallavotti et al., 2010; Yoshida et al., 2012; Liu et al., 2019). Maize ARFs show similar preferences for auxin response elements to Arabidopsis ARFs (Galli et al., 2018), and the specificity of activator ARFs within maize is not explained by differences in their promoter preferences (Lanctot et al., 2020).
Auxin response circuits built in yeast (Saccharomyces cerevisiae) cells (ARCSc) make it possible to analyze individual and combinatorial functions of Aux/IAA, TIR1/AFB, ARF, and TPL/TPR proteins (Havens et al., 2012; Pierre-Jerome et al., 2014). This heterologous system has several advantages, including precise control over the amount and duration of auxin input, highly quantitative fluorescence outputs, and the ability to study auxin signaling modules with defined connectivity and in the absence of other plant signaling pathways. Studies using Arabidopsis components in ARCSc have confirmed and extended previous studies showing that Aux/IAAs exhibit a range of tunable auxin-induced degradation rates (Ramos et al., 2001; Dreher et al., 2006; Calderón Villalobos et al., 2012; Havens et al., 2012; Moss et al., 2015) and that this variation in Aux/IAA degradation is central to controlling auxin transcriptional response dynamics and the rate of developmental events in planta (Havens et al., 2012; Pierre-Jerome et al., 2014; Guseman et al., 2015; Moss et al., 2015). The yeast system has also enabled functional characterization of genetic natural variation in auxin receptors (Wright et al., 2017) as well as functional annotation of two putative maize auxin repressor proteins (Galli et al., 2015) and several maize class A activator ARFs (Lanctot et al., 2020).
Here, we have used the ARCSc system to functionally annotate additional maize auxin signaling components, focusing on genes that are expressed during inflorescence development. The maize Aux/IAAs (ZmIAAs) tested degraded in response to auxin with rates that depended on both receptor and repressor identity. ZmIAAs were able to repress ARF-mediated transcription when fused to a truncated form of the maize TPL homolog REL2. The maize auxin receptor ZmAFB2/3 b1 was more sensitive to auxin than was observed with the fastest acting Arabidopsis receptor, AtAFB2, allowing for more rapid degradation of Aux/IAAs. Finally, a complete auxin response circuit composed of all maize components was fully functional, allowing very sensitive activation of a transcriptional reporter following the addition of auxin. These results validate the conserved role of predicted nuclear auxin response genes in maize as well as provide evidence that a synthetic approach can facilitate broader comparative studies that incorporate the wide range of species with sequenced genomes.
RESULTS
ZmIAAs Are Functional in Auxin Degradation and Repression Modules
The maize B73 genome contains 34 Aux/IAA repressor genes, 16 of which were selected for further analysis here because they are expressed in developing maize inflorescences (Davidson et al., 2011; Bolduc et al., 2012; Eveland et al., 2014) and represent members of six of the eight Aux/IAA clades found in monocots (there is a ninth clade of IAAs in Arabidopsis; Matthes et al., 2019). Out of 13 ZmIAAs that were tested by in situ hybridizations, six gave detectable and specific expression patterns in immature tassel inflorescence meristems and spikelet meristems (Fig. 1, A and B). In general, most of these ZmIAA genes revealed highly restricted expression patterns marking emerging primordia in inflorescence meristems (ZmIAA2, ZmIAA5, ZmIAA9, ZmIAA14, and ZmIAA28) as well as vasculature (ZmIAA5 and ZmIAA28), and all showed strong expression in spikelet meristems (Fig. 1B). These expression patterns strongly resembled those previously reported for BIF1 and BIF4 (Galli et al., 2015) and suggest a high degree of functional redundancy in this family of transcriptional regulators. However, it is not known whether auxin-dependent degradation dynamics or repression strengths may reveal differences in molecular function among individual Aux/IAAs. To assess the auxin sensitivity of each ZmIAA, we fused these inflorescence-expressed ZmIAAs to YFP and coexpressed them with an Arabidopsis auxin receptor in yeast (Fig. 1C). The F-box domain of these receptors interacts with the yeast S-Phase Kinase-Associated Protein1 (SKP1), Cullin, F-box protein (SCF) complex, allowing for the ubiquitination of Aux/IAAs and subsequent degradation by the yeast 26S proteasome. All ZmIAA proteins were expressed in yeast cells and degraded in response to auxin treatment (Fig. 1D). The ARCSc degradation module also allowed us to measure functional differences in auxin-induced degradation rates by measuring changes in fluorescence following auxin exposure (Havens et al., 2012). We selected three ZmIAAs that were expressed at similar levels and showed different auxin sensitivities (ZmIAA8, ZmIAA12, and BIF1) and measured auxin-induced degradation dynamics (Fig. 1, E–G). As seen previously for AtIAAs (Dreher et al., 2006; Havens et al., 2012), ZmIAA degradation rates varied between different ZmIAAs coexpressed with AtTIR1 (BIF1, ZmIAA8, and ZmIAA12 half-lives being 49.8, 29.7, and 33.8 min, respectively; Fig. 1, E and F) and were generally more rapid in cells expressing AtAFB2 (11.6, 15.1, and 18.8 min, respectively; Fig. 1, E and G). Previous studies demonstrated that residue substitutions within the core of the highly conserved degron can slow the rate of Aux/IAA degradation (Ramos et al., 2001; Guseman et al., 2015). However, none of the ZmIAAs expressed in developing inflorescence had variations in the core degron other than V > I in ZmIAA12 and ZmIAA21 (Supplemental Fig. S1A). When paired with AtAFB2, auxin-induced degradation of ZmIAA12 was not the slowest (Fig. 1E).
Figure 1.
Auxin repressors expressed in maize inflorescence exhibited variable auxin-induced degradation dynamics. A and B, Expression of maize Aux/IAA genes by mRNA in situ hybridizations in immature tassels. Expression patterns are shown in inflorescence meristems (A) and spikelet meristems (B). Gray arrowheads indicate vasculature, and green arrowheads indicate axillary meristems. Bars = 100 μm and 50 μm in the inset image. C, The nuclear auxin degradation module in yeast consists of ZmIAAs (purple) tagged with YFP and coexpressed with an Arabidopsis auxin receptor (green). The F-box domain of the receptor facilitates complex formation with the yeast SCF ubiquitin (Ub) ligase machinery (gray). When yeast are exposed to auxin, shown as a black circle, the hormone acts as molecular glue that brings the coreceptor complex together and leads to ubiquitination and proteasomal degradation of the YFP-tagged ZmIAA. This results in a decrease in fluorescence over time. D, The 16 ZmIAAs coexpressed with Arabidopsis TIR1 were degraded in response to auxin. Fluorescence measurements were obtained 2 h post-auxin exposure on a flow cytometer. Data from two replicates are shown; error bars represent se. AU, Absorbance units. E and G, ZmIAAs degrade at different rates that are dependent upon both repressor (E) and receptor (G) identities. Yeast strains expressing YFP-tagged ZmIAAs and either Arabidopsis TIR1 or AFB2 auxin receptor were exposed to 1 μm auxin or mock treatment (95% [v/v] ethanol) at 0 min, and fluorescence measurements were acquired on a flow cytometer. Data from two replicates are shown. F, YFP:ZmIAA degradation half-lives were calculated from cytometry data in E and G and are presented with 95% confidence intervals.
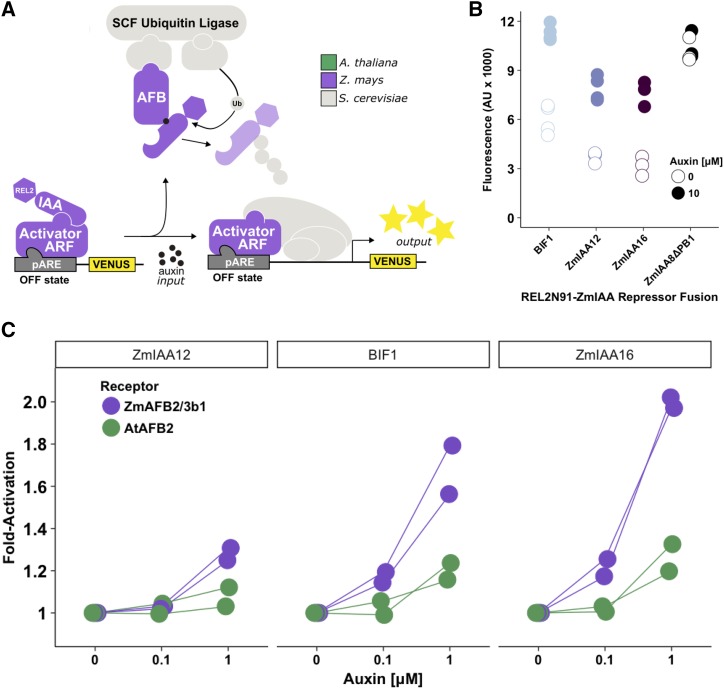
In addition to degradation in response to auxin, the other major function of Aux/IAAs is repression of ARF-mediated transcriptional regulation, a process that is facilitated by TPL/TPR corepressors (Causier et al., 2012). The maize genome has four TPL-like corepressors, the REL2/REL2-like family, of which REL2 has been shown to have pleiotropic phenotypes associated with meristem maintenance and initiation in maize (Liu et al., 2019). In the Arabidopsis ARCSc (AtARCSc), an N-terminal fusion of the first 100 amino acids of TPL (TPLN100) was functional if directly fused to IAAs, facilitating transcriptional repression of ARFs (Pierre-Jerome et al., 2014). We confirmed that REL2 can also confer repression of ZmIAAs by fusing ZmIAA8 to either TPLN100 or REL2N91, a fragment of REL2 that is structurally analogous to TPL100 (Fig. 2A; Supplemental Fig. S1B). Based on new structural information, we designed REL2N91 to include only the first five helices that encompass the LisH and CTLH domains (Martin-Arevalillo et al., 2017). The ARCSc strains used for repression assays contained Arabidopsis AFB2 and ARF19, which was the strongest and fastest activating ARF in AtARCSc (Pierre-Jerome et al., 2014). Each corepressor conferred a similar degree of repression, and in the presence of auxin that repression was relieved and transcription was activated to similar degrees (Fig. 2B). Both the degree of repression and auxin-induced activation dynamics varied greatly across ZmIAAs, and repression level did not necessarily predict activation level (Fig. 2C; Supplemental Fig. S1C). Two ZmIAAs were unable to repress the nuclear auxin response (Supplemental Fig. S1D), possibly due to poor expression or inability to interact with ARF19.
Figure 2.
The TPL homolog REL2 enabled ZmIAAs to repress ARFs. A, The auxin repression module in yeast consisted of ZmIAA repressors fused to N-terminal fragments of either the Arabidopsis TPL or maize REL2 corepressors; these were coexpressed with an auxin receptor (Arabidopsis AFB2) and activator transcription factor (Arabidopsis ARF19). Auxin-induced derepression of ARF19 results in activation of the auxin response element-containing promoter (pARE) and expression of VENUS fluorescent protein. B, The N-terminal 91 amino acids of maize REL2 assist ZmIAA8 in conferring transcriptional repression on AtARF19, and this repression is relieved upon the addition of auxin. The REL2N91 fragment was directly compared with the analogous Arabidopsis TPLN100. The AtARF19_H170A mutant is unable to bind DNA, so the auxin response stays off. Strains labeled none contain a ZmIAA8 that has not been fused to a corepressor. C, ZmIAAs fused to REL2N91 exhibited different patterns of auxin-responsive gene activation, independent of their basal repression strength and degradation rate. Two ZmIAAs were unable to repress the auxin response (Supplemental Fig. S1D). All fluorescence measurements in B and C were made 5 h post-treatment. Data from two replicates are shown; error bars represent se. AU, Absorbance units; WT, wild type.
Maize Auxin Receptor ZmAFB2/3 b1 Is More Auxin Sensitive Than AtAFB2
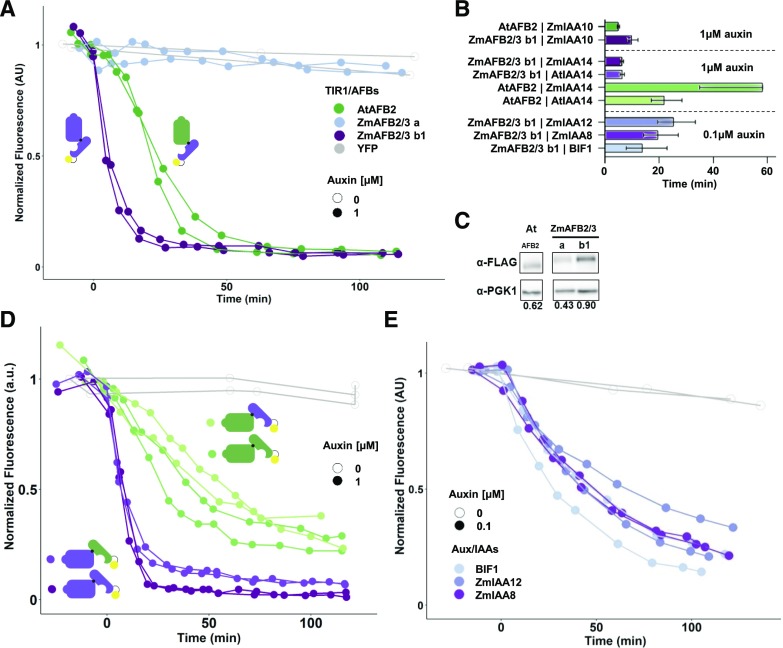
In the maize genome, there are eight members of the TIR1/AFB auxin receptor family (Matthes et al., 2019), four of which appear to be related to the Arabidopsis AFB2/3 clade. Two of these AFB2/3-like maize auxin receptors (ZmAFB2/3 a and b1) were highly expressed in inflorescence meristems and so were tested for activity within an ARCSc degradation module (Bolduc et al., 2012; Eveland et al., 2014). The ZmAFB2/3 b1 receptor was the only one able to induce the degradation of ZmIAAs in the presence of auxin (Fig. 3A). This receptor is also the most highly expressed in developing inflorescences (Eveland et al., 2014). Alignments and pairwise comparisons between ZmAFB2/3 b1 and Arabidopsis auxin receptors showed that they share 60% to 67% sequence similarity across both the F-box and LRR domains (Supplemental Fig. S2, A–D). Auxin-induced degradation carried out by ZmAFB2/3 b1 was even faster than AtAFB2, with ZmIAA10 half-lives of 4.9 and 9.9 min, respectively (Fig. 3, A and B). We next assessed the expression of the auxin receptors in the yeast strains and found that all were expressed and accumulated to similar levels (Fig. 3C; Supplemental Fig. S2, E and F), suggesting that differences in expression level are not likely to explain differences in degradation module activity. Furthermore, the amount of AtAFB2 receptor accumulation in yeast (within a twofold range) was previously shown to have no effect on auxin-induced repressor degradation rate (Havens et al., 2012). Hypermorphic variations like those described in AtAFB2 natural variants might explain the increased activity of ZmAFB2/3 b1 (Wright et al., 2017). Although the known AtAFB2 hypermorphic variant D176E was not present in ZmAFB2/3 b1, the T491R substitution is at a known hypermorphic site (T491V variant found in AtAFB2) and might explain differences in activity (Supplemental Fig. S2A).
Figure 3.
The maize auxin receptor ZmAFB2/3 b1 exhibited a higher basal activity compared with the Arabidopsis ortholog. A, ZmAFB2/3 b1 was sensitive to auxin and exhibited faster degradation than AtAFB2. ZmAFB2/3 a was not sensitive to auxin. Yeast coexpressing an Arabidopsis or maize auxin receptor and YFP-ZmIAA10 were treated with 1 μm auxin or mock (0), and fluorescence was measured on a flow cytometer. B, ZmIAA half-lives were calculated with 95% confidence intervals for data shown in A, D, and E. C, The auxin receptor expression level in yeast does not necessarily correlate with functionality. Yeast lysates were probed with anti-FLAG (for receptors) or anti-PGK1 (loading control) antibodies. Fold expression values shown below the bands were calculated by using ImageJ to quantify the intensity of each band and dividing the intensity of the receptor band by the intensity of the PGK1 band. D, ZmAFB2/3 b1 always exhibited faster IAA degradation in response to auxin, whether paired with an Arabidopsis or a maize Aux/IAA. All combinations of ZmAFB2/3 b1 or AtAFB2 paired with AtIAA14 or ZmIAA14 were tested within an auxin degradation module. E, When paired with ZmAFB2/3 b1, three maize Aux/IAAs (BIF1, ZmIAA8, and ZmIAA12) exhibited different orders of degradation speed than when paired with AtAFB2 (Fig. 1). AU, Absorbance units.
The auxin sensitivity of ZmAFB2/3 b1 and AtAFB2 was next tested with Aux/IAAs from each species to determine which component was primarily responsible for driving differences in speed of the degradation module. The maize receptor was faster than AtAFB2 when paired with either a maize or an Arabidopsis Aux/IAA (Fig. 3, B and D). We also noticed that preauxin Aux/IAA accumulation levels were lower in strains expressing ZmAFB2/3 b1 compared with AtAFB2 (Supplemental Fig. S3A). We hypothesized that the maize receptor might be interacting with the repressor independently of auxin. Treatment with auxinole, a compound shown to block the interaction of rice TIR1 (OsTIR1) and auxin repressors (Yesbolatova et al., 2019), resulted in greater accumulation of Aux/IAAs, particularly in yeast strains expressing ZmAFB2/3 b1 (Supplemental Fig. S3A). This suggested that at least some of the increased auxin sensitivity of ZmAFB2/3 b1 may be due to auxin-independent degradation. Auxin sensitivity analyses showed that ZmAFB2/3 b1 is responsive to auxin over 2 orders of magnitude (0.01–1 μm; Supplemental Fig. S3B). We next assessed whether ZmAFB2/3 b1 degradation modules were functional with each of the 16 ZmIAAs. All ZmIAAs were degraded by ZmAFB2/3 b1 (Fig. 3E; Supplemental Fig. S3C). Furthermore, some ZmIAAs exhibited different patterns of auxin-induced degradation dynamics than were observed when coexpressed with AtAFB2: for example, BIF1 paired with ZmAFB2/3 b1 degraded faster (13.9 min) than ZmIAA8 (19.7 min) or ZmIAA12 (25.4 min) when paired with AtAFB2 (Figs. 1D and 3E; note also the 10-fold difference in auxin sensitivities). This is reminiscent of the different patterns of AtIAA degradation rates observed for Arabidopsis AFB2 and TIR1 auxin receptors (Havens et al., 2012). The auxin repressors that possess V > I variation in the core degron (ZmIAA12 and ZmIAA21; Supplemental Fig. S1A) exhibited consistently slow degradation rates compared to most other ZmIAAs when paired with ZmAFB2/3 b1 (Fig. 3E; Supplemental Fig. S3C), in contrast with the AtAFB2 data (Fig. 1E). Thus, maize auxin degradation modules constituted in yeast are more sensitive to hormone, and degradation dynamics are dependent upon both receptor and repressor identity.
The Maize Auxin Response Circuit Is Tuned to Respond to Low Auxin Concentrations
Having determined that maize receptors, repressors, and corepressors are functional in varying combinations, the final step was to express them with a maize auxin transcription factor to reconstitute an entire maize auxin response circuit (ZmARCSc; Fig. 4A). ZmARF27 is orthologous to AtARF19, the ARF used in the AtARCSc, and is expressed strongly in immature tassel (Eveland et al., 2014; Matthes et al., 2019). ZmARCSc yeast strains containing various ZmIAAs were able to repress ZmARF27 transcriptional activity to varying degrees (Fig. 4B), conferring as strong or stronger repression on ZmARF27 than on AtARF19 (Supplemental Fig. S4A). The ZmARCSc strains were all derepressed by the addition of a high concentration of auxin (Fig. 4B; Supplemental Fig. S4A). Given that ZmAFB2/3 b1 is more sensitive to auxin than AtAFB2 in auxin degradation modules (Fig. 3), we hypothesized that this would also result in ZmARCSc transcriptional responses activating at lower doses of auxin. For each ZmARCSc strain tested, the transcriptional response was activated at lower auxin levels compared with strains in which the ZmAFB2/3 b1 was replaced with AtAFB2 (Fig. 4C; Supplemental Fig. S4B). Thus, the maize auxin response circuit is exquisitely sensitive to auxin hormone levels, and response dynamics can be tuned by altering ZmIAA identity.
Figure 4.
A maize auxin response circuit featuring ZmAFB2/3 b1 is functional and more sensitive to auxin than a circuit with AtAFB2. A, An auxin response circuit in yeast utilizing maize components (ZmARCSc) was assembled as shown with ZmARF27. B, The ZmARCSc is functional and responds to auxin. Data represent three to four replicates of fluorescence measurements taken 5 h following auxin hormone treatment. The ZmIAA8 strain with a deleted PB1 domain represents an always-on state. AU, Absorbance units. C, The ZmAFB2/3 b1 auxin receptor confers higher auxin sensitivity than AtAFB2 in ZmARCSc yeast strains. Strains shown in B and their AtAFB2 cognates were treated with 0, 0.1, or 1 μm auxin for 5 h before fluorescence measurements. Data are plotted as fold untreated.
DISCUSSION
The synthetic auxin response circuit used in this study is impressively modular, with the ability to mix and match components from distantly related plant species. Maize ZmIAAs expressed in developing inflorescence tissues degraded in response to auxin, with variable degradation rates that depended on both ZmIAA and receptor identity (Fig. 1). Furthermore, these ZmIAAs were able to repress transcription when paired with the maize corepressor REL2 (Fig. 2). Maize auxin degradation modules were more sensitive to auxin than orthologous Arabidopsis degradation modules in yeast (Fig. 3). Finally, a maize auxin response circuit featuring ZmARF27 was fully functional and more sensitive to auxin than circuits containing an Arabidopsis receptor (Fig. 4). This work represents a broadly useful strategy for employing synthetic biology approaches to functionally annotate and characterize genetic diversity within conserved signaling networks.
The decreasing costs of genome sequencing have led to a dramatic increase in genetic natural variation information available. However, functional annotation of this new genetic information is lagging behind. The ARCSc platform allows for rapid functional validation and characterization of large gene families, as seen previously for auxin signaling modules within Arabidopsis (Havens et al., 2012; Pierre-Jerome et al., 2014, 2016; Moss et al., 2015; Wright et al., 2017) and now for maize (Figs. 1 and 2). Of particular note, the yeast system enabled multidimensional functional annotation (degradation and repression behaviors) of 16 different Aux/IAAs expressed in developing maize inflorescences. We observed variable degradation and repression behaviors across the ZmIAAs studied, providing evidence of biochemical differences that introduce another layer of complexity on top of known phylogenetic diversity (Ludwig et al., 2013) and tissue-specific expression patterns (Matthes et al., 2019). In agreement with previous studies (von Behrens et al., 2011; Ludwig et al., 2013; Zhang et al., 2016), ZmIAA2, ZmIAA10/RUM1, ZmIAA20/BIF4, and ZmIAA29/RUL1 were functional within auxin degradation modules (Figs. 1 and 3) and capable of repressing transcription (Fig. 2). Furthermore, by showing that ZmIAAs can repress the auxin circuit with the assistance of REL2, we provide an additional piece of evidence to support existing genetic and biochemical data demonstrating that REL2 is a functional homolog of TPL (Liu et al., 2019). Thus, our functional annotation and characterization of maize auxin signaling modules is in agreement with the few existing genetic and biochemical studies on maize Aux/IAAs and REL2 and extends functional characterization to many previously uninvestigated maize auxin signaling components for which there are no known mutants. The recent recapitulation of the Arabidopsis abscisic acid hormone signaling pathway in yeast underscores the feasibility of performing similar functional annotation efforts for putative orthologous proteins across many plant signaling pathways (Ruschhaupt et al., 2019). Admittedly these minimal signaling modules do not encompass the full complexity present in vivo, although the ARCSc has been used to investigate competition between corepressors and the functional role of IAA and ARF PB1 domain oligomerization (Pierre-Jerome et al., 2014, 2016) as well as ARF promoter architecture preferences (Lanctot et al., 2020).
The ARCSc system is a valuable platform for continued dissecting of the differences in auxin response between monocots and dicots. For example, ARCSc should be useful in elucidating the mechanisms of auxinic herbicide tolerance and resistance from the perspectives of chemical biology, evolution, and genetic engineering; however, further work will be needed to overcome the observed limitation that 2,4-dichlorophenoxyacetic acid and other auxinic herbicides are actively transported out of yeast cells (Cabrito et al., 2011). In monocots, responses to most auxinic herbicides are much weaker than in eudicots, which die when these herbicides are applied to them (McSteen, 2010). Auxin metabolism and transport diverge between monocots and eudicots in ways that could contribute to a divergence in their auxin response (Zhao et al., 2013; Lu et al., 2015; He et al., 2018, 2019). Monocot differences in auxin signaling and reception components might also contribute to these different responses. Because maize and Arabidopsis both have similar numbers of members in nuclear auxin signaling gene families (TIR1/AFBs, Aux/IAAs, ARFs, etc.), we believe that any auxin response differences present at this level would more likely be driven by differences in protein function than by differences in pathway protein stoichiometries. Our results bring up exciting new questions about the role of auxin in maize. In the yeast system, ZmAFB2/3 b1 is more sensitive to lower auxin dosages than AtAFB2 and can lead to Aux/IAA turnover even in the absence of auxin (Fig. 3). Analysis of relative auxin levels during tassel and ear development, perhaps using the DII-VENUS reporter (Mir et al., 2017), could be used to validate these findings in planta and provide possible clues to the impact of increased sensitivity on feedback within the auxin system and/or on the dynamics of downstream responses.
Understanding how hormone signaling protein functions have changed (or remained similar) throughout evolution can help connect protein function to growth and developmental processes and inform the design of synthetic proteins. ARCSc has revealed the functional ramifications of the evolution of auxin signaling components within Arabidopsis, allowing for the functional comparison of gene variants across accessions (Wright et al., 2017). By allowing for quantitative comparisons between Arabidopsis and maize auxin signaling pathways, ARCSc is a promising tool to study the evolution of molecular function more broadly across distantly related plant species, in this case diverging 250 million years ago. It also provides the ability to directly and quickly study multiple functional protein interactions to help elucidate the evolution of widespread developmental phenomena such as inflorescence development. From a synthetic biology perspective, platforms like ARCSc can enable the rapid design, characterization, and comparison of highly divergent proteins for use in engineering synthetic signaling machinery within plants or other biological systems.
The types of experiments and analyses described here that utilize a synthetic hormone signaling system in yeast are readily carried out by novice scientists in research laboratories and in course settings. Many of the experiments described in this article were piloted or executed by 45 undergraduate students at a primarily undergraduate institution, Whitman College. Most of these students were participants in several semesters of course-based undergraduate research experiences. This type of undergraduate-focused synthetic biology research experience offers an exciting way to acquaint young scientists with plant synthetic biology and could be readily adapted to focus on the standardization and characterization of parts for use in synthetic biology, an area of continuing concern (Decoene et al., 2018).
MATERIALS AND METHODS
In Situ Hybridizations in Maize Inflorescences
For mRNA in situ hybridizations, 0.2- to 0.4-cm tassel primordia from the maize (Zea mays) B73 inbred line were dissected and fixed in cold paraformaldehyde acetic acid solution as described previously (Galli et al., 2015). Hybridizations were carried out at 59°C. Antisense in situ probes for all 13 Aux/IAA genes (IAA2, IAA4, IAA5, IAA8, IAA9, IAA10, IAA12, IAA14, IAA16, IAA21, IAA25, IAA28, and IAA29) were synthesized by in vitro transcription (T7 RNA polymerase; Promega) of the entire or partial coding sequences cloned in pENTR vector or pGEM T-easy (Promega) and digested with respective endonuclease enzymes. The vectors and primers used for probe design are listed in Supplemental Table S1.
Plasmid Construction
Plasmid and primer design was performed in Benchling. Maize Aux/IAA and ARF sequences were obtained from the Grassius Database or synthesized by Integrated DNA Technologies. The maize REL2 fragment was cloned from plasmids generated by Liu et al. (2019). The maize TIR1/AFB gene sequences were obtained from Paula McSteen. These sequences were ordered from Integrated DNA Technologies with codon optimization for Saccharomyces cerevisiae and then cloned into pCR-BLUNT plasmid using the Zero Blunt TOPO PCR Cloning Kit (James et al., 2000). The ZmIAA genes were inserted into pGP4GY plasmids (Havens et al., 2012) via Gateway cloning (Life Technologies) or by Gibson cloning (Gibson et al., 2009). ZmARF27 was cloned into the pGP8G vector by Gibson cloning. Auxin receptors (TIR1 and AFB2s) were cloned into pGP8GF plasmid containing a 3X-FLAG-6X-HIS tandem affinity purification tag. Each PCR amplification was performed using Q5 High-Fidelity DNA Polymerase (New England Biolabs), and the products were purified using the EZNA Cycle Pure Kit or the NEB Monarch PCR & DNA Cleanup Kit and confirmed by sequencing (MCLab or Genewiz). All plasmids used in this research are listed in Supplemental Table S2, and a subset of these have been made available at Addgene.
Yeast Methods
The yeast S. cerevisiae were was grown in either yeast peptone dextrose (YPD) or Synthetic Complete (SC) medium made according to standard recipes and supplemented with 80 mg mL−1 adenine. Yeast transformation of linearized plasmids was performed using a standard lithium acetate protocol (Gietz and Woods, 2002). Matings were performed by coinoculation of MATa and MATɑ strains at low density in YPD medium with shaking overnight at 30°C. All yeast transformations and matings were streaked onto SC plates lacking appropriate auxotrophic compounds for selection, followed by isostreaking onto yeast peptone adenine dextrose (YPAD) plates prior to glycerol stocking. The full yeast strain list is included in Supplemental Table S3.
Auxin Degradation Module Assays
Auxin degradation module assays were carried out as previously described (Pierre-Jerome et al., 2017). In brief, yeast colonies from YPAD plates were used to inoculate 3 mL of SC medium in culture tubes. Cell density (in events μL−1) was estimated by flow cytometry, and cultures were diluted into 4 mL of fresh SC medium in culture tubes such that cells were in log-phase growth 16 h later and for the duration of the assay (typically in the range of 0.5 to 2.5 events μL−1 was sufficient). All growth was at 30°C in an incubated shaker at 250 rpm. Fluorescence measurements were taken on the flow cytometer prior to the addition of auxin to establish a baseline. Auxin (indicated concentrations, IAA) or mock (95% [v/v] ethanol) treatments were added, and cultures were returned to the incubator. Fluorescence measurements were acquired at 10-min intervals for the first 1 h after auxin addition and every 15 min thereafter until the fluorescence level in most strains had plateaued (approximately 2 h). Control strains were measured every 1 h for the duration of the experiment. For auxinole assays, yeast were grown overnight in 2 mL of SC medium and diluted 1:200 into fresh SC medium containing auxinole (50 μm in ethanol) or mock (ethanol); fluorescence measurement was taken 6 h following treatment.
Repression/Activation Module Assays
Auxin repression/activation module assays were carried out as previously described (Pierre-Jerome et al., 2017). Briefly, yeast cells inoculated from colonies on a YPAD plate were grown overnight in culture tubes containing 2 mL of SC medium at 30°C in an incubated shaker at 250 rpm. In the morning, the cells were diluted 1:200 into 2 mL of fresh SC medium in culture tubes and returned to the incubator. After 2 h, auxin (indicated concentrations) or mock treatment (95% [v/v] ethanol) were added and strains continued to incubate at 30°C for 5 h. Fluorescence measurements were taken on a flow cytometer.
Flow Cytometry Settings and Flow Set Data Analysis
Fluorescence measurements were taken with a BD Accuri C6 flow cytometer and CSampler plate adapter using an excitation wavelength of 488 and an emission detection filter at 533 nm. A total of 10,000 to 20,000 events above a 400,000 FSC-H threshold (to exclude debris) were collected for each sample, and data were exported as FCS 3.0 files for processing using the flowCore R software package and custom R scripts (Havens et al., 2012; Pierre-Jerome et al., 2017). These scripts enable gating for live, diploid, singlet yeast cells; for time-course data, they also facilitate the subtraction of background fluorescence and fold initial normalization. Data from at least two independent replicates were combined and plotted in R. Half-life values with 95% confidence intervals were calculated by importing fold-initialized auxin degradation time series data to GraphPad Prism 8 and using nonlinear regression analysis (plateau followed by one-phase decay with X0 set to the time of auxin addition). All data can be found in Flow Repository.
Immunoblotting
Yeast were grown overnight in 5 mL of liquid YPD medium at 30°C with shaking at 225 rpm. The next morning, cultures were diluted to an OD of 0.2 into fresh YPD medium and grown to an OD of ∼1. Cells were then pelleted and resuspended in 1% (w/v) SDS, 8 m urea, 10 mm MOPS, pH 6.8, 10 mm EDTA, and 0.01% (w/v) bromophenol blue buffer with 1 mm phenylmethylsulfonyl fluoride. Cell pellets were lysed by glass bead disruption in lysis buffer containing protease inhibitors. Aliquots of 7 µL of yeast proteins in buffer were loaded onto a 10% SDS-PAGE gel (Bio-Rad). Anti-FLAG antibody (Cell Signaling Technology) was used to probe for TIR1/AFBs-3X-FLAG-6X-HIS, and anti-PGK1 antibody (Invitrogen) was used as a loading control.
Protein Sequence Alignments
All protein sequence alignments were performed using ClustalW in MegaX software. Alignments were visualized using CLC Workbench.
Accession Numbers
All names and accession numbers of major genes and proteins used in our experiments are listed in Supplemental Table S2.
Supplemental Data
The following supplemental materials are available.
Supplemental Figure S1. The N-terminal fragment of REL2 fused to ZmIAAs repressed transcription.
Supplemental Figure S2. The maize auxin AFB2/3 b1 receptor protein is orthologous to those in other species and expressed at similar levels in yeast.
Supplemental Figure S3. ZmAFB2/3 b1 degraded ZmIAAs more readily in the absence of auxin and was more sensitive to auxin.
Supplemental Figure S4. Auxin response circuit behavior depended on the identity of the ARF, TIR1/AFB, and Aux/IAA.
Supplemental Table S1. Vectors and primers used for Aux/IAA cloning and expression analysis.
Supplemental Table S2. Gene identifiers and plasmids used in yeast transformations.
Supplemental Table S3. Yeast strains used in this study.
Acknowledgments
We thank members of the Nemhauser and Imaizumi labs for helpful discussion and guidance on experimental design and execution, Alex Leydon for assistance with REL2N91 designs, Paula McSteen and Norman Best for providing maize receptor sequences and for advice on the article, and especially the students in B.L.M.’s Synthetic Cell Biology classes, Tristan Cates, Kristina Jackson, Kathleen Daly-Jensen, Hannah Klaeser, and Alex Koriath, for initial experimental work.
Footnotes
This work was supported by the National Science Foundation (grant no. IOS-1546873 to J.L.N., A.G., and B.L.M. and grant no. IOS-1456950 to A.G.), the M.J. Murdock Charitable Trust (to B.M.), Whitman College (to B.M.), and the Howard Hughes Medical Institute (Gilliam Fellowship no. GT11355 to R.R.B.).
Articles can be viewed without a subscription.
References
- Bargmann BOR, Vanneste S, Krouk G, Nawy T, Efroni I, Shani E, Choe G, Friml J, Bergmann DC, Estelle M, et al. (2013) A map of cell type-specific auxin responses. Mol Syst Biol 9: 688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolduc N, Yilmaz A, Mejia-Guerra MK, Morohashi K, O’Connor D, Grotewold E, Hake S(2012) Unraveling the KNOTTED1 regulatory network in maize meristems. Genes Dev 26: 1685–1690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabrito TR, Teixeira MC, Singh A, Prasad R, Sá-Correia I(2011) The yeast ABC transporter Pdr18 (ORF YNR070w) controls plasma membrane sterol composition, playing a role in multidrug resistance. Biochem J 440: 195–202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calderón Villalobos LIA, Lee S, De Oliveira C, Ivetac A, Brandt W, Armitage L, Sheard LB, Tan X, Parry G, Mao H, et al. (2012) A combinatorial TIR1/AFB-Aux/IAA co-receptor system for differential sensing of auxin. Nat Chem Biol 8: 477–485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Causier B, Ashworth M, Guo W, Davies B(2012) The TOPLESS interactome: A framework for gene repression in Arabidopsis. Plant Physiol 158: 423–438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson RM, Hansey CN, Gowda M, Childs KL, Lin H, Vaillancourt B, Sekhon RS, de Leon N, Kaeppler SM, Jiang N, et al. (2011) Utility of RNA sequencing for analysis of maize reproductive transcriptomes. Plant Genome 4: 191–203 [Google Scholar]
- Decoene T, De Paepe B, Maertens J, Coussement P, Peters G, De Maeseneire SL, De Mey M(2018) Standardization in synthetic biology: An engineering discipline coming of age. Crit Rev Biotechnol 38: 647–656 [DOI] [PubMed] [Google Scholar]
- Dreher KA, Brown J, Saw RE, Callis J(2006) The Arabidopsis Aux/IAA protein family has diversified in degradation and auxin responsiveness. Plant Cell 18: 699–714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eveland AL, Goldshmidt A, Pautler M, Morohashi K, Liseron-Monfils C, Lewis MW, Kumari S, Hiraga S, Yang F, Unger-Wallace E, et al. (2014) Regulatory modules controlling maize inflorescence architecture. Genome Res 24: 431–443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallavotti A, Long JA, Stanfield S, Yang X, Jackson D, Vollbrecht E, Schmidt RJ(2010) The control of axillary meristem fate in the maize ramosa pathway. Development 137: 2849–2856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galli M, Khakhar A, Lu Z, Chen Z, Sen S, Joshi T, Nemhauser JL, Schmitz RJ, Gallavotti A(2018) The DNA binding landscape of the maize AUXIN RESPONSE FACTOR family. Nat Commun 9: 4526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galli M, Liu Q, Moss BL, Malcomber S, Li W, Gaines C, Federici S, Roshkovan J, Meeley R, Nemhauser JL, et al. (2015) Auxin signaling modules regulate maize inflorescence architecture. Proc Natl Acad Sci USA 112: 13372–13377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson DG, Young L, Chuang RY, Venter JC, Hutchison CA III, Smith HO(2009) Enzymatic assembly of DNA molecules up to several hundred kilobases. Nat Methods 6: 343–345 [DOI] [PubMed] [Google Scholar]
- Gietz RD, Woods RA(2002) Transformation of yeast by lithium acetate/single-stranded carrier DNA/polyethylene glycol method. Methods Enzymol 350: 87–96 [DOI] [PubMed] [Google Scholar]
- Guseman JM, Hellmuth A, Lanctot A, Feldman TP, Moss BL, Klavins E, Calderón Villalobos LI, Nemhauser JL(2015) Auxin-induced degradation dynamics set the pace for lateral root development. Development 142: 905–909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Havens KA, Guseman JM, Jang SS, Pierre-Jerome E, Bolten N, Klavins E, Nemhauser JL(2012) A synthetic approach reveals extensive tunability of auxin signaling. Plant Physiol 160: 135–142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Q, Yang L, Hu W, Zhang J, Xing Y(2018) Overexpression of an auxin receptor OsAFB6 significantly enhanced grain yield by increasing cytokinin and decreasing auxin concentrations in rice panicle. Sci Rep 8: 14051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Y, Yan L, Ge C, Yao XF, Han X, Wang R, Xiong L, Jiang L, Liu CM, Zhao Y(2019) PINOID is required for formation of the stigma and style in rice. Plant Physiol 180: 926–936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- James KD, Hughes MA, Williams PA(2000) Cloning and expression of ntnD, encoding a novel NAD(P)+-independent 4-nitrobenzyl alcohol dehydrogenase from Pseudomonas sp. Strain TW3. J Bacteriol 182: 3136–3141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanctot A, Taylor-Teeples M, Oki EA, Nemhauser J (2020) Specificity in auxin responses is not explained by the promoter preferences of activator ARFs. Plant Physiol 182: 1533–1536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leyser O.(2018) Auxin signaling. Plant Physiol 176: 465–479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Galli M, Camehl I, Gallavotti A(2019) RAMOSA1 ENHANCER LOCUS2-mediated transcriptional repression regulates vegetative and reproductive architecture. Plant Physiol 179: 348–363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu G, Coneva V, Casaretto JA, Ying S, Mahmood K, Liu F, Nambara E, Bi YM, Rothstein SJ(2015) OsPIN5b modulates rice (Oryza sativa) plant architecture and yield by changing auxin homeostasis, transport and distribution. Plant J 83: 913–925 [DOI] [PubMed] [Google Scholar]
- Ludwig Y, Zhang Y, Hochholdinger F(2013) The maize (Zea mays L.) AUXIN/INDOLE-3-ACETIC ACID gene family: Phylogeny, synteny, and unique root-type and tissue-specific expression patterns during development. PLoS ONE 8: e78859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo J, Zhou JJ, Zhang JZ(2018) Aux/IAA gene family in plants: Molecular structure, regulation, and function. Int J Mol Sci 19: E259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin-Arevalillo R, Nanao MH, Larrieu A, Vinos-Poyo T, Mast D, Galvan-Ampudia C, Brunoud G, Vernoux T, Dumas R, Parcy F(2017) Structure of the Arabidopsis TOPLESS corepressor provides insight into the evolution of transcriptional repression. Proc Natl Acad Sci USA 114: 8107–8112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthes MS, Best NB, Robil JM, Malcomber S, Gallavotti A, McSteen P(2019) Auxin EvoDevo: Conservation and diversification of genes regulating auxin biosynthesis, transport, and signaling. Mol Plant 12: 298–320 [DOI] [PubMed] [Google Scholar]
- McSteen P.(2010) Auxin and monocot development. Cold Spring Harb Perspect Biol 2: a001479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mir R, Aranda LZ, Biaocchi T, Luo A, Sylvester AW, Rasmussen CG(2017) A DII domain-based auxin reporter uncovers low auxin signaling during telophase and early G1. Plant Physiol 173: 863–871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss BL, Mao H, Guseman JM, Hinds TR, Hellmuth A, Kovenock M, Noorassa A, Lanctot A, Villalobos LIAC, Zheng N, et al. (2015) Rate motifs tune auxin/indole-3-acetic acid degradation dynamics. Plant Physiol 169: 803–813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mutte SK, Kato H, Rothfels C, Melkonian M, Wong GKS, Weijers D(2018) Origin and evolution of the nuclear auxin response system. eLife 7: e33399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierre-Jerome E, Jang SS, Havens KA, Nemhauser JL, Klavins E(2014) Recapitulation of the forward nuclear auxin response pathway in yeast. Proc Natl Acad Sci USA 111: 9407–9412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierre-Jerome E, Moss BL, Lanctot A, Hageman A, Nemhauser JL(2016) Functional analysis of molecular interactions in synthetic auxin response circuits. Proc Natl Acad Sci USA 113: 11354–11359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierre-Jerome E, Wright RC, Nemhauser JL(2017) Characterizing auxin response circuits in Saccharomyces cerevisiae by flow cytometry. Methods Mol Biol 1497: 271–281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piya S, Shrestha SK, Binder B, Stewart CN Jr., Hewezi T(2014) Protein-protein interaction and gene co-expression maps of ARFs and Aux/IAAs in Arabidopsis. Front Plant Sci 5: 744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prigge MJ, Greenham K, Zhang Y, Santner A, Castillejo C, Mutka AM, O’Malley RC, Ecker JR, Kunkel BN, Estelle M(2016) The Arabidopsis auxin receptor F-box proteins AFB4 and AFB5 are required for response to the synthetic auxin picloram. G3 (Bethesda) 6: 1383–1390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rademacher EH, Lokerse AS, Schlereth A, Llavata-Peris CI, Bayer M, Kientz M, Freire Rios A, Borst JW, Lukowitz W, Jürgens G, et al. (2012) Different auxin response machineries control distinct cell fates in the early plant embryo. Dev Cell 22: 211–222 [DOI] [PubMed] [Google Scholar]
- Ramos JA, Zenser N, Leyser O, Callis J(2001) Rapid degradation of auxin/indoleacetic acid proteins requires conserved amino acids of domain II and is proteasome dependent. Plant Cell 13: 2349–2360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruschhaupt M, Mergner J, Mucha S, Papacek M, Doch I, Tischer SV, Hemmler D, Chiasson D, Edel KH, Kudla J, et al. (2019) Rebuilding core abscisic acid signaling pathways of Arabidopsis in yeast. EMBO J 38: e101859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vernoux T, Brunoud G, Farcot E, Morin V, Van den Daele H, Legrand J, Oliva M, Das P, Larrieu A, Wells D, et al. (2011) The auxin signalling network translates dynamic input into robust patterning at the shoot apex. Mol Syst Biol 7: 508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Behrens I, Komatsu M, Zhang Y, Berendzen KW, Niu X, Sakai H, Taramino G, Hochholdinger F(2011) Rootless with undetectable meristem 1 encodes a monocot-specific AUX/IAA protein that controls embryonic seminal and post-embryonic lateral root initiation in maize. Plant J 66: 341–353 [DOI] [PubMed] [Google Scholar]
- Wright RC, Zahler ML, Gerben SR, Nemhauser JL(2017) Insights into the evolution and function of auxin signaling F-box proteins in Arabidopsis thaliana through synthetic analysis of natural variants. Genetics 207: 583–591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yesbolatova A, Natsume T, Hayashi KI, Kanemaki MT(2019) Generation of conditional auxin-inducible degron (AID) cells and tight control of degron-fused proteins using the degradation inhibitor auxinole. Methods 164–165: 73–80 [DOI] [PubMed] [Google Scholar]
- Yoshida A, Ohmori Y, Kitano H, Taguchi-Shiobara F, Hirano HY(2012) Aberrant spikelet and panicle1, encoding a TOPLESS-related transcriptional co-repressor, is involved in the regulation of meristem fate in rice. Plant J 70: 327–339 [DOI] [PubMed] [Google Scholar]
- Zhang Y, Marcon C, Tai H, von Behrens I, Ludwig Y, Hey S, Berendzen KW, Hochholdinger F(2016) Conserved and unique features of the homeologous maize Aux/IAA proteins ROOTLESS WITH UNDETECTABLE MERISTEM 1 and RUM1-like 1. J Exp Bot 67: 1137–1147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Z, Zhang Y, Liu X, Zhang X, Liu S, Yu X, Ren Y, Zheng X, Zhou K, Jiang L, et al. (2013) A role for a dioxygenase in auxin metabolism and reproductive development in rice. Dev Cell 27: 113–122 [DOI] [PubMed] [Google Scholar]