GA-mediated cell proliferation in rice involves down-regulation of a microRNA by the degradation of DELLA protein SLENDER RICE1, thus modulating GROWTH-REGULATING FACTOR expression.
Abstract
GAs play key roles in controlling cell proliferation through the GIBBERELLIN INSENSITIVE DWARF1/DELLA-mediated pathway. However, how DELLA proteins affect downstream pathways is not well understood. Therefore, discovering the signaling events downstream of DELLAs is key to better understanding the roles of GAs in plant development. Here, we discovered that miR396 is regulated by SLENDER RICE1 (SLR1) in controlling cell proliferation. The positive response of rice (Oryza sativa) GROWTH-REGULATING FACTORs (OsGRFs) to GAs was found to be caused by a negative response of miR396 to GAs. miR396 acts downstream of SLR1 and upstream of GA-induced cell-cycle genes. Rice INDETERMINATE DOMAIN2 (OsIDD2) directly binds the promoter of OsmiR396a and can interact with SLR1 in vivo and in vitro. Rice lines overexpressing miR396a (miR396OE) or OsIDD2 (OsIDD2OE) displayed dwarfism resulting from higher abundance of miR396 RNA. However, the stem elongation of OsIDD2OE plants could be significantly stimulated by applying exogenous GA3, while that of miR396OE plants could not. Rice with OsIDD2 knocked down by RNA interference showed a slr1-like phenotype, in which the expression of miR396 was inhibited while its targets were enhanced. The protein levels of OsIDD2 were unaffected by GA in wild-type and OsIDD2OE plants, implying that OsIDD2 promotes the expression of miR396 and likely requires the coactivator of SLR1. Taken together, these results provided a close link between SLR1/OsIDD2 and GRFs via a negative regulator, miR396, and thus highlighted a molecular mechanism of GA-mediated cell proliferation in rice.
The height of crops is an important agronomic trait drawing attention from breeders and agronomists around the world. Numerous dwarf mutants were shown to be deficient in the biosynthesis or perception of GAs (Matsuo et al., 1997; Van De Velde et al., 2017). GAs are one of the most important plant hormones that control diverse aspects of plant growth and development, including stem elongation, seed germination, leaf expansion, flowering, pollen maturation, and seed development (Thomas and Sun, 2004; Yamaguchi, 2008). We know a lot about GA perception and how this leads to the degradation of DELLAs but have less information on how DELLAs restrict growth, especially cell proliferation (Davière and Achard, 2013; Van De Velde et al., 2017).
During the past two decades, three key components that play vital roles in the upstream of GA signaling pathways have been well identified: GIBBERELLIN-INSENSITIVE DWARF1 (GID1), a soluble receptor of GAs in rice (Oryza sativa; Ueguchi-Tanaka et al., 2005); GID2, a subunit of Skp1-Cullin-F box protein (SCF) E3 ubiquitin ligase involved in the degradation of DELLA proteins in rice (Sasaki et al., 2003; Ueguchi-Tanaka et al., 2008); and DELLA proteins, a signaling hub in various plant species (Peng et al., 1997, 1999; Silverstone et al., 1998; Ikeda et al., 2001; Chandler et al., 2002; Itoh et al., 2002; Van De Velde et al., 2017). Rice has only one DELLA protein, SLENDER RICE1 (SLR1), whereas Arabidopsis (Arabidopsis thaliana) has five (Sun and Gubler, 2004).
With the progress from many studies on various mutants related to the above three components, the outline of upstream GA signaling cascades has been gradually emerging. In the absence of GAs, DELLA proteins repress various GA responses in plants. However, the inhibitory effects of DELLA proteins are removed through the degradation of DELLA proteins via the 26S-proteasome pathway in the presence of GAs, resulting in various GA-dependent responses (Hirano et al., 2008). Several studies in Arabidopsis demonstrated that DELLA proteins can interact with a list of DELLA-interacting proteins (DIPs) such as transcription factors, transcriptional regulators, chromatin-remodeling complexes, and cochaperones. DELLA proteins interact with DIPs mainly via transactivation or sequestration to regulate gene expression (Van De Velde et al., 2017). However, insight into the DELLA-regulatory mechanism of downstream responses in cereal crops is very limited, especially on cell proliferation (Davière and Achard, 2013; Van De Velde et al., 2017). Therefore, elucidating the signaling events downstream of DELLA proteins is key to better understanding how GAs control plant development.
Rice GROWTH-REGULATING FACTOR1 (OsGRF1) was originally identified as a GA-responsive gene expressed mainly in the intercalary meristem of internodes in rice (van der Knaap and Kende, 1995). The GRF family, comprising nine members in Arabidopsis and 12 members in rice, has been identified as a conserved plant-specific transcription factor family in many plant species (Kim et al., 2003; Choi et al., 2004; Omidbakhshfard et al., 2015). Subcellular localization of OsGRF1 protein was found to be in the nucleus (van der Knaap et al., 2000), and the C terminus of OsGRF1 protein was shown to have transactivation function (Choi et al., 2004). Heterologous overexpression of OsGRF1 in Arabidopsis resulted in a dwarf phenotype (van der Knaap et al., 2000), suggesting that GRFs are involved in stem growth. Some studies have uncovered the diverse functions of GRFs in other aspects of plant development and growth (Omidbakhshfard et al., 2015), including the regulation of GA biosynthesis and stem elongation (Kuijt et al., 2014; Diao et al., 2018). GRFs biochemically interact with another group of transcriptional coactivators, GRF-INTERACTING FACTORS (GIFs), which are also highly conserved in land plants (Kim and Kende, 2004; Horiguchi et al., 2005; Lee et al., 2009, 2014). The expression of most GRF genes is GA inducible in rice (Choi et al., 2004), and the mechanism of this response to GAs still remains unclear. GAs promote plant elongation by influencing both cell proliferation and cell elongation (Sauter and Kende, 1992; Sauter, 1997). Some studies suggested that OsGRF1 might be involved in mediating an early event in GA-promoted entry into the cell cycle (van der Knaap and Kende, 1995; van der Knaap et al., 2000). However, the precise correlation between GRFs and cell cycle-related functions is still unclear.
Using computational and experimental means, miR396, whose target genes are GRFs, was initially identified in Arabidopsis and rice (Jones-Rhoades and Bartel, 2004; Sunkar et al., 2005; Wu et al., 2009; Xue et al., 2009). The miR396 family is a highly conserved plant microRNA family found in all land plants (Axtell and Bartel, 2005). Some reports have shown that miR396 is involved in various aspects of plant growth and development (Liu et al., 2009, 2014; Gao et al., 2010; Rodriguez et al., 2010, 2015, 2016; Wang et al., 2011; Hewezi and Baum, 2012; Bazin et al., 2013; Casadevall et al., 2013; Mecchia et al., 2013). As a regulatory molecule, the roles of miR396 depend on the function of its targets as well as the way in which it regulates its targets.
Here, we report that SLR1 controls the expression of OsmiR396 and OsGRFs via transactivation with INDETERMINATE DOMAIN2 (OsIDD2). Transgenic lines overexpressing miR396a or OsIDD2 were dwarf. However, the stem length and the RNA levels of OsmiR396 and OsGRFs could be regulated by applying exogenous GA in OsIDD2-overexpressing plants but not in miR396a-overexpressing plants. Based on the analyses of gene expression and morphological features of cells in related mutants, miR396 was found to be negatively regulated by GA during cell proliferation. Our results suggested a model for the new roles of miR396/GRFs in GA signal transduction that control stem elongation in rice.
RESULTS
miR396 Targets Most GRFs in Rice
Members of the OsmiR396 family, comprising eight members in rice, can be classified into five groups with very slight differences between each other (Supplemental Fig. S1A). Eleven of 12 OsGRFs, except OsGRF11, were found to have a region highly complementary to OsmiR396a in mRNA sequences (Supplemental Fig. S1B). The degree of complementarity between miR396a and GRFs in rice is higher than that of Arabidopsis (Supplemental Figs. S1A and S2A). 5′-RACE analysis verified that all targets of OsmiR396 are accurately cleaved at the site between the 10th and 11th bases of OsmiR396a (Supplemental Fig. S1B), suggesting that miR396 regulates its targets mainly through cleavage of its target RNA site, similar to most other plant microRNAs (Tang et al., 2003). However, no cleavage sites for OsGRF11 were detected.
OsmiR396 acted on regions with highly similar amino acid sequences, located within the WRC domain (a conserved region containing the amino acid sequence WRC). The corresponding WRC of OsGRF11 is substantially different (Supplemental Fig. S1C). OsGRF11 was also shown to be a unique member by phylogenetic analysis (Supplemental Fig. S1D). Using transient expression in onion (Allium cepa) epidermal cells, OsGRF11 was also found to localize in the nucleus (Supplemental Fig. S3).
To investigate individual expression dynamics in detail, the promoter of OsGRF9 was isolated and fused to the coding region of GUS to generate OsGRF9 promoter-GUS-expressing plants. GUS activity assays of transgenic plants revealed that OsGRF9 mainly accumulates in embryo cells of germinating seeds (Supplemental Fig. S4C), and its expression pattern displayed a dynamic fluctuation during seed germination (Supplemental Fig. S4D). The GUS activity started to increase at the beginning of germination and reached the peak at day 4 of germination. After day 4, the GUS activity gradually decreased (Supplemental Fig. S4D), suggesting a possible role of OsGRF9 in regulating seed germination. In addition, reverse transcription quantitative PCR (RT-qPCR) showed that OsGRF1 and OsGRF2 highly accumulated in the young leaves and shoot tips containing the shoot apical meristem (SAM), but OsGRF3 to OsGRF6 were highly expressed in both root and shoot tips (Supplemental Fig. S4, A and B), suggesting that different members of OsGRFs may have unique expression preferences. However, the expression pattern of the fused OsGRF9 promoter with GUS did not reflect the regulation of miR396 on OsGRF9.
Overexpression of miR396 Leads to Dwarfism and Reduced Cell Proliferation
OsmiR396 targets most OsGRF members and provides an opportunity to study the overall function of GRF family members in rice. To explore the roles of miR396 and its targets in rice, we generated transgenic lines overexpressing miR396a driven by the Ubiquitin1 (Ubi1) promoter, a very efficient constitutive promoter in monocots (Supplemental Fig. S2C). The precursor of OsmiR396a with a length of 193 bp containing the stem-loop structure (Supplemental Fig. S2B) was chosen for overexpression.
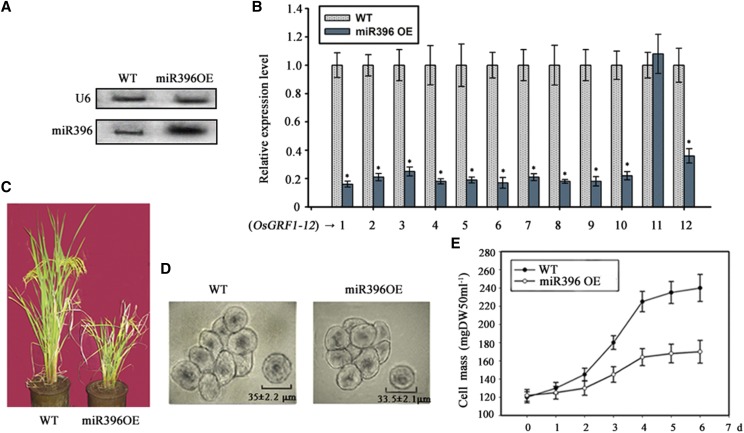
In the T0 generation, several transgenic lines displayed dwarfism with different heights. Then the homozygous lines were selected on hygromycin in the T2 generation and their growth traits were further investigated. The prominent phenotype of the transgenic plants was dwarf along with other characteristics such as delayed germination and growth, smaller root volumes, as well as smaller leaf size (Supplemental Fig. S5, A–C; Supplemental Table S1). The wild-type seeds usually need 3 d to germinate under suitable conditions, while the transgenic seeds needed 1 week or more. The roots of 3-week-old seedlings of OsmiR396a-overexpressing plants (miR396OE) were thinner and smaller than those of the wild type (Supplemental Table S1). Four weeks after germination, the height of the wild type was about 30 cm while that of the miR396OE plants was only about 14 cm (Supplemental Fig. S5B). In addition, the emergence of tillers in miR396OE plants was 14 d later than that of the wild type. The final height of miR396OE plants was only ∼55 cm, about half that of the wild type (Fig. 1C; Supplemental Table S1). The lifetime of miR396OE plants was 15 to 25 d longer than that of the wild type. Interestingly, the number of tillers exhibited no significant difference between the wild type and OsmiR396a-overexpressing plants (Supplemental Table S1). As a whole, the flowers and seeds of miR396OE lines were morphologically similar to those of the wild type. The phenotype of miR396OE plants was further confirmed by a new report in which overexpression of miR396a also led to dwarfism (Diao et al., 2018). By northern blotting for miR396 and RT-qPCR for GRFs, Figure 1, A and B, show that the RNA abundance of miR396 was higher but that of its targets were lower in the miR396OE lines. However, OsGRF11 mRNA abundance did not change significantly in the miR396OE lines (Fig. 1B).
Figure 1.
Phenotype of transgenic plants overexpressing OsmiR396a. A, RNA levels of OsmiR396 in the wild type (WT) and miR396OE. Expression levels of miR396 were detected by northern blotting, and U6 was used as a loading control. B, The RNA levels of OsGRFs in 2-week-old seedlings of the wild type and miR396OE lines were analyzed by RT-qPCR. Asterisks indicate significant difference at P ≤ 0.05 compared with the wild type by Student’s t test (n = 3; means ± sd). C, The height of adult plants of the wild type and miR396OE at the filling grain stage. D, The morphology and size of the cells in a suspension-cultured system of the wild type and miR396OE (n = 7; means ± sd). Bars = 50 µm. E, The growth curve of the biomass of the suspension-cultured cells. The suspension-cultured cells were harvested, dried, and weighed at the indicated times. DW, Dry weight. The data shown are means of three biological repetitions ± se.
Plant height is determined by both cell division and elongation in the stem. To investigate the activities of cell division and elongation in the miR396OE lines, we measured cell size in the rice stem as well as the suspension-cultured cells of the miR396OE lines. The image of tissues sliced from the elongation zone of the top-most internode showed that the cell sizes of the miR396OE lines were slightly smaller than those of the wild type (Supplemental Fig. S5D). Calculations from the image of sliced tissues indicated that the average longitudinal diameter of cells from miR396OE lines was about 82% that of the wild type and the average transverse diameter was about 90% that of the wild type. This smaller difference of cell sizes does not explain the larger difference of stem length between the two genotypes, suggesting that the reduced cell number is the main cause for the dwarfism of the OmiR396OE lines. Moreover, the observation from suspension-cultured cells showed that the cells of the miR396OE lines and the wild type were similar in size (Fig. 1D). Plant cell suspension systems are usually made up of numerous lumps in which dozens of cells clump together, and very few dissociated cells can be observed in a suspension-cultured system. After 6 d of being cultured, the biomass increment of suspension-cultured cells of miR396OE lines was significantly lower than that of the wild type (Fig. 1E), implying that cell division in the suspension-cultured cells of miR396OE lines was inhibited. These results suggested that the activities of cell proliferation are likely the limiting factor in controlling plant height in the miR396OE lines.
OsmiR396 Negatively Responds to GA and Contributes to GA-Induced Stem Elongation
It is well known that most OsGRFs are a class of GA-induced genes and that GAs play key roles in promoting stem elongation (van der Knaap and Kende, 1995; van der Knaap et al., 2000; Choi et al., 2004; Yamaguchi, 2008). However, results from overexpression of OsmiR396 may shed more light on the function of GRFs while providing less information on the function of miR396 in regulating GRF levels. The opposite expression pattern between OsGRFs and miR396 (Fig. 1, A and B) suggested that OsGRFs and OsmiR396 may have opposite responses to GAs. To investigate how OsmiR396 and all OsGRF members respond to GAs, 2-week-old seedlings of the wild type and miR396OE lines were chosen for detecting transcripts of OsmiR396 and OsGRF after GA3 treatment. As shown in Figure 2A, the RNA abundance of miR396 was down-regulated and the mRNA abundance of OsGRFs (targets) was up-regulated in the wild type after applying GA3 (Fig. 2A). However, no response to GA3 of miR396/targets occurred in the miR396OE lines (Fig. 2B). These results suggested that the positive response of OsGRFs (targets of miR396) to GA is caused by the negative response of miR396 to GA. Besides, OsGRF11, which is not the target of miR396, did not respond to GA3 in either the miR396OE lines or the wild type (Fig. 2, A and B). A number of studies have shown that GA treatment can significantly stimulate stem elongation and cell proliferation in rice (Sauter, 1997; Spielmeyer et al., 2002; Sakamoto et al., 2004; Ueguchi-Tanaka et al., 2005; Li et al., 2011). To investigate the effect of exogenous GA3 on the growth of the OsmiR396a-overexpressing lines, 10-d-old seedlings of the wild type and miR396OE lines were sprayed with 50 μm GA3 at 1-d intervals for 25 d. Figure 2C shows that the stem elongation of the wild type could be significantly stimulated by application of GA3 but not in the miR396OE lines, suggesting that GA-mediated stem elongation is likely through OsmiR396/OsGRFs.
Figure 2.
Effects of GA on stem elongation as well as RNA levels of OsmiR396 and OsGRFs. A and B, Responses of OsmiR396 and OsGRFs to GA in the wild type (WT; A) and miR396OE (B). Two-week-old seedlings were incubated into N6 solution with or without 50 µm GA3 for 24 h, and total RNA extracted from the seedlings of the wild type (A) and miR396OE (B) was used for gene expression. Expression levels of miR396 were detected by northern blotting. U6 was used as a loading control; OsGRFs were analyzed by RT-qPCR. Asterisks indicate significant difference at P ≤ 0.05 compared with no GA3 treatment by Student’s t test (n = 3; means ± sd). C, Effect of GA3 on stem elongation. Ten-day-old seedlings of the wild type and miR396OE were sprayed with 50 µm GA3 solution containing 0.02% (v/v) Tween 20 at 1-d intervals for 25 d (n = 7; means ± sd).
To further determine the correlation between the RNA levels of OsmiR396 and GA concentrations, 2-week-old seedlings of the wild type were exposed to different GA3 concentrations. After a 24-h treatment, the minimal concentration of GA3 for the alteration of the OsmiR396 level was 0.1 µmol L−1 or more, and higher concentrations of GA3 led to more alteration of RNA levels of miR396 and OsGRF1 (Fig. 3A), suggesting that the expression of miR396/targets is regulated by GA in a concentration-dependent manner.
Figure 3.
Response of OsmiR396 and OsGRF1 to GA concentrations. A, RNA levels of OsmiR396 and OsGRF1 in the wild type under different exogenous GA3 concentrations. Two-week-old seedlings of the wild type were exposed to the designated GA3 concentrations for 24 h. B, RNA levels of OsmiR396 and OsGRF1 in SAM and intercalary meristems of different internodes of 8-week-old rice. The RNA levels of OsmiR396 were detected by northern blotting, and U6 was used as a loading control; mRNA levels of OsGRF1 were analyzed by RT-qPCR. Asterisks indicate significant difference at P ≤ 0.05 compared with no GA3 treatment (A) or the lowest tested internode (B) by Student’s t test (n = 3; means ± sd).
Unlike the SAM of dicotyledonous plants from which stem, leaves, and floral organs originate, the intercalary meristem and SAM of monocotyledonous plants contribute mainly to stem elongation (Sauter et al., 1995; van der Knaap et al., 2000). Therefore, investigating the dynamics of miR396/OsGRFs in the SAM and intercalary meristems of different internodes may provide insight on how miR396/OsGRFs control stem elongation. As shown in Figure 3B, from the SAM to the interior intercalary meristems of the lower internodes, the RNA levels of miR396 increased gradually and that of OsGRF1 showed an opposite trend (Fig. 3B). Overall, the RNA abundance of OsGRF1 positively correlated with the growth rate of the place where it was emerging, whereas miR396 had a negative correlation (Fig. 3B).
OsmiR396 and OsGRFs Act between SLR1 and Cell Cycle-Related Genes
Even though the negative response of SLR1 (DELLA protein) to GA (Ikeda et al., 2001; Itoh et al., 2002) is reminiscent of the way in which miR396 responds to GA (Figs. 2A and 3A), a link between SLR1 and OsmiR396 is not known. To investigate the relationship between SLR1 and OsmiR396, we detected miR396 levels in slr1 mutants and mRNA levels of SLR1 in the miR396OE lines. Our results showed that OsmiR396 levels were drastically reduced and the mRNA abundance of OsGRF1 was increased in 2-week-old slr1 mutant seedlings (Fig. 4A). By contrast, no apparent difference in RNA abundance of SLR1 was observed between the wild type and OsmiR396a-overexpressing lines (Fig. 4B). To further determine this relationship, two key mutants, gid1 and elongated uppermost internode (eui), were used for detecting the RNA abundance of OsmiR396 and OsGRF1. GID1, a receptor of biologically active GAs, mediates the degradation of SLR1 in the presence of biologically active GAs. So the level of SLR1 is stable and can avoid being degraded in the gid1 mutant (Ueguchi-Tanaka et al., 2005, 2007). EUI, a GA-deactivating enzyme, helps to maintain higher levels of inactive GAs. So the level of SLR1 is reduced in the eui mutant due to higher levels of biologically active GAs (Zhu et al., 2006). Figure 4C shows that the OsmiR396 levels were higher while the mRNA levels of OsGRF1 were lower in the gid1 mutant than in the wild type. By contrast, opposite patterns of RNA accumulation of OsmiR396 and OsGRF1 were observed in the eui mutant (Fig. 4C). These results suggested that OsmiR396 acts downstream of SLR1 and is positively regulated by SLR1.
Figure 4.
The relationship of RNA abundance between OsmiR396 and SLR1 as well as CycOs1/CycOs2. A, The RNA levels of OsmiR396 and OsGRF1 in the wild type (WT) and slr1. miR396 was detected by northern blotting, and U6 was used as a loading control; OsGRF1 was analyzed by RT-qPCR. Asterisks indicate significant difference at P ≤ 0.05 compared with the wild type by Student’s t test (n = 3; means ± sd). B, The mRNA levels of SLR1 were analyzed by RT-qPCR in the wild type and miR396OE. C, The RNA levels of OsmiR396 and OsGRF1 in the wild type, gid1, and eui. miR396 was detected by northern blotting, and U6 was used as a loading control; OsGRF1 was analyzed by RT-qPCR. Asterisks indicate significant difference at P ≤ 0.05 compared with the wild type by Student’s t test (n = 3; means ± sd). D, The mRNA levels of cycOs1 and cycOs2 were analyzed by RT-qPCR in both the wild type and miR396OE with or without GA3 (50 µm) treatment (24 h). Asterisks indicate significant difference at P ≤ 0.05 compared with the wild type without treatment by Student’s t test (n = 3; means ± sd). E, Time course of response to GA (50 µm) of miR396, OsGRF1, cycOs1, and cycOs2. miR396 was detected by northern blotting, and U6 was used as a loading control. OsGRF1, cycOs1, and cycOs2 were analyzed by RT-qPCR. Asterisks indicate significant difference at P ≤ 0.05 compared with no GA3 treatment by Student’s t test (n = 3; means ± sd).
The above inferences from the reduced cell number in miR396OE lines (Fig. 1, D and E; Supplemental Fig. S5D) suggested that some cell cycle-related genes might also be suppressed in the miR396OE lines. It has been reported that some cell cycle-related genes such as cyclin Oryza sativa1 (cycOs1), cycOs2, and cyclin-dependent-kinases Oryza sativa2 are GA induced (Sauter et al., 1995; Sauter, 1997), even though it remains unknown whether these cell cycle-related genes link with OsmiR396/OsGRFs. To investigate the relationships between OsmiR396/OsGRFs and these cell cycle-related genes, 2-week-old seedlings of the wild type and miR396OE lines were used for detecting the mRNA levels of cycOs1 and cycOs2 by RT-qPCR. Compared with that of the wild type, the mRNA levels of both cycOs1 and cycOs2 were significantly reduced in the miR396OE lines (Fig. 4D), suggesting that cycOs1 and cycOs2 act downstream of GRFs. After 24 h of GA3 treatment, the mRNA levels of cycOs1 and cycOs2 increased drastically in the wild type whereas no apparent alteration was observed in the miR396OE lines (Fig. 4D), suggesting that the response of cycOs1 and cycOs2 to GA is likely through OsmiR396/OsGRFs. Moreover, a time-course analysis showed that the RNA levels of both OsmiR396 and OsGRF1 could rapidly respond (∼1 h) to exogenous GA3 with a synchronously opposite pace. However, compared with that of miR396/OsGRF1, the responses to GAs of CycOs1 and CycOs2 genes were delayed with a longer lag time of more than 8 h (Fig. 4E), further indicating that CycOs1 and CycOs2 act downstream of OsmiR396/OsGRFs. The long span between the responses to GA of miR396/GRFs and cycOs1/2 implied that other factor(s) might trigger the start of the cell cycle, because some evidence showed that enhanced cell division activity started ∼4 h after GA treatment (Sauter and Kende, 1992).
OsIDD2 Directly Promotes the Expression of miR396
Our results suggested that miR396 acts downstream of SLR1. However, the absence of a DNA-binding domain in DELLA proteins impeded the conclusion that SLR1 directly controls the expression of miR396. To identify gene(s) that directly control(s) the expression of miR396, we performed a yeast one-hybrid screen. A cDNA library derived from various tissues of rice was used as prey and the HISTONE3 reporter gene constructs with an ∼1-kb putative promoter sequence upstream of the predicted fold-back structure of the OsmiR396a precursor was used as bait. Given that miR396 prefers to accumulate in relatively older tissues, the cDNA library was expressly constructed with mRNA extracted from tissues including the basal roots, the shoots of basal internodes, and the older leaves. A total of ∼1,500,000 transformants were screened, and we obtained six positive clones (Fig. 5A). After sequencing analysis, all these identified clones showed homology to the rice gene encoding OsIDD2 (LOC_Os01g09850). Among these six candidates, two members were well matched with the full length of OsIDD2 protein, and the other four members contained partial N-terminal fragments with different lengths, hinting that the N-terminal end is needed for DNA binding. In vitro electrophoretic mobility shift assays (EMSAs) further confirmed that OsIDD2 fused with glutathione S-transferase (GST) can bind to the fragments of the miR396a promoter within a length of ∼1 kb (Fig. 5B). Using subfragments of different regions of the OsmiR396a promoter for EMSAs, the DNA-binding cis-elements were found to be localized at the region ∼500 bp upstream of the putative TATA box of the OsmiR396a promoter (Fig. 5B).
Figure 5.
The interaction between OsIDD2 and the promoter of miR396a. A, Interaction between OsIDD2 and the promoter of OsmiR396a in yeast one-hybrid (Y1H) assay. –LU, Medium lacking Leu and Ura; −LUH, medium lacking Leu, Ura, and His; OsIDD2, clones that contained full or partial cDNA of OsIDD2 fused with the activating domain (AD) of GAL4 in vector pGADT7; Vec, clones transformed with empty vector of pGADT7 without fused protein. B, Interaction between OsIDD2 and the promoter of OsmiR396a in EMSAs. Left, A 1,134-bp fragment upstream of the putative precursor of OsmiR396a was tested for interaction with GST, GST-OsIDD2; middle, the region between −1,134 and −539 bp upstream of the putative precursor was tested for interaction with GST, GST-OsIDD2; right, the fragment of 538 bp upstream of the putative precursor was tested for interaction with GST, GST-OsIDD2. C, Comparison of phenotypes between OsIDD2OE and miR396OE. WT, Wild type. D, Effect of GA3 on plant height of OsIDD2OE and miR396OE. Ten-day-old seedlings were sprayed with distilled water containing 0.02% (v/v) Tween 20 with or without 50 µm GA3 at 1-d intervals for 25 d (n = 7; means ± sd). E, The RNA levels of miR396, OsIDD2, and OsGRF1 in 2-week-old seedlings of the wild type and OsIDD2OE with or without GA3 (50 µm) treatment for 24 h. miR396 was detected by northern blotting, and U6 was used as a loading control. OsGRF1 and OsIDD2 were analyzed by RT-qPCR. Asterisks indicate significant difference at P ≤ 0.05 compared with the wild type by Student’s t test (n = 3; means ± sd). F, Effect of GA3 (50 µm) on the expression levels of OsIDD2 analyzed by RT-qPCR in 2-week-old seedlings of the wild type.
The IDD family of proteins, conserved in all land plants, comprises dozens of members with four zinc finger motifs (Colasanti et al., 1998, 2006). This family of transcriptional regulators has been reported to control diverse processes at different stages of plant development (Coelho et al., 2018), notably in GA signal transduction (Feurtado et al., 2011; Fukazawa et al., 2014; Yoshida et al., 2014). To explore the biologic roles of OsIDD2, we generated transgenic rice overexpressing OsIDD2 driven by the Ubi1 promoter (OsIDD2OE). A number of positive lines in the T0 generation displayed dwarfism and were propagated to the T2 generation, and homozygous lines were selected for further studies. The phenotype of the OsIDD2OE lines was similar to that of the miR396OE lines (Fig. 5C; Supplemental Table S1). As expected, the mRNA level of OsIDD2 was much higher in the OsIDD2OE lines in the T2 generation than that of the wild type (Fig. 5E). The RNA level of miR396 was higher and that of OsGRF1 was lower in the OsIDD2OE lines (Fig. 5E). Compared with the miR396OE lines, in which the stem extension was not significantly affected by applying the exogenous GA3 (Fig. 3C), the stem length of the OsIDD2OE lines was markedly stimulated by applying GA3 (Fig. 5D). The average increment of stem length in the OsIDD2OE lines was 18 cm after 25 d of GA3 treatment, whereas the stem elongation was not affected by GA3 in the miR396OE lines (Fig. 5D). Surprisingly, the mRNA levels of OsIDD2 were not affected while those of miR396/OsGRF1 were markedly affected by applying GA3 in both the OsIDD2OE lines and the wild type (Figs. 2A and 5, E and F), suggesting that OsIDD2, driving the expression of miR396, likely requires other factor(s) whose activity is regulated by GA.
To further investigate whether the expression of OsmiR396 is controlled by OsIDD2, we knocked down the expression of OsIDD2 by RNA interference (RNAi) technology. A specific fragment of DNA comprising 150 bp between the regions encoding zinc fingers and the TRDFLG motif was used in order to specifically inhibit the expression of OsIDD2. Several RNAi transgenic lines (OsIDD2RNAi) were about 50% taller than the wild type in the T2 generation. Northern blotting and RT-qPCR showed that the expression of OsIDD2 and OsmiR396 was greatly reduced and the expression of OsGRF1 was increased in the T2 lines examined when compared with the wild type (Fig. 6A). The height of the wild-type seedlings treated with GA3 reached the length of the OsIDD2RNAi lines (Fig. 6B). However, GA treatment had no apparent effect on the stem elongation and expression of OsIDD2, OsmiR396, and OsGRF1 in the OsIDD2RNAi lines (Fig. 6, A, B, and D). To further investigate the roles of endogenous GAs on stem elongation caused by OsIDD2 silencing, a GA biosynthesis inhibitor, uniconazole (Izumi et al., 1984; Mitsunaga and Yamaguchi, 1993), was used. As shown in Figure 6C, the height of the wild-type seedlings was significantly reduced while that of the lines with OsIDD2 silencing was not affected after nearly 10 d of treatment (Fig. 6C).
Figure 6.
Down-regulation of OsIDD2 gene in rice results in a slender phenotype and a reduced level of OsmiR396. A, RNA levels of OsmiR396, OsIDD2, and OsGRF1 in the wild type (WT) and OsIDD2RNAi with or without GA3 treatment. Total RNA was extracted from 2-week-old seedlings and was analyzed by northern blotting for miR396 and RT-qPCR for OsIDD2 and OsGRF1. U6 was used as a loading control for miR396. Asterisks indicate significant difference at P ≤ 0.05 compared with the wild type by Student’s t test (n = 3; means ± sd). B, The height of 7-d-old seedlings of the wild-type and OsIDD2RNAi. Shoot elongation is shown for the wild type treated with (2) or without (1) 50 μm GA3 and OsIDD2RNAi with (4) or without (3) GA3 treatment. C, The length of 10-d-old seedlings of the wild type and OsIDD2RNAi lines. Shoot elongation is shown for the wild type treated with (2) or without (1) 6.9 μm uniconazole (Unico) and OsIDD2RNAi with (4) or without (3) uniconazole treatment. D, Effect of GA3 on plant height of the wild type and OsIDD2RNAi lines. Ten-day-old seedlings were sprayed with distilled water containing 0.02% (v/v) Tween 20 with or without 50 µm GA3 at 1-d intervals for 25 d. The data shown are means of seven biological repetitions ± se.
OsIDD2 Physically Interacts with SLR1 and Is Not Affected by GAs
Evidence that miR396 was positively regulated by both SLR1 (Fig. 4, A and C) and OsIDD2 (Figs. 5E and 6A) prompted us to speculate that OsIDD2 interacts with SLR1. To test this assumption, we performed a yeast two-hybrid (Y2H) assay. To avoid the strong transcriptional activity of DELLA that would lead to false positives (Feurtado et al., 2011; Fukazawa et al., 2014; Yoshida et al., 2014), we expressed both SLR1 and OsIDD2 as bait and prey with two weak promoters. Y2H assays clearly demonstrated that SLR1 and OsIDD2 proteins interacted with each other in vivo (Fig. 7A). This interaction was further validated by a GST pull-down assay. SLR1 was fused with GST as bait and captured on an immobilized affinity ligand. By western blotting using an antibody of OsIDD2, the two proteins were shown to interact with each other in vitro (Fig. 7B). The interaction between SLR1 and OsIDD2 was further confirmed by a coimmunoprecipitation (Co-IP) assay in vivo (Fig. 7C). In addition to zinc fingers, which define the IDD family, most members of this family also contain two other conserved motifs, MSTALLQKAA and TRDFLG (Colasanti et al., 2006), which are localized at the region near the C terminus of OsIDD2 (Fig. 7E). To determine which region of OsIDD2 is key for the interaction with SLR1, various truncated versions of OsIDD2 lacking different regions were generated (Fig. 7E) and Y2H assays were performed. The results showed that the middle region, which localizes between zinc fingers and MSTALLQKAA motifs that have no apparent conserved features, is required for this interaction (Fig. 7E). Zinc finger motifs of IDD proteins are necessary for DNA binding (Kozaki et al., 2004), and the relatively conserved regions of MSTALLQKAA and TRDFLG were shown to be unnecessary for the interaction between proteins. This discovery was consistent with previous observations that AtIDD3, AtIDD4, and AtIDD5 can interact with the DELLA protein REPRESSOR OF ga1-3 in Arabidopsis (Yoshida et al., 2014), but these three members do not have MSTALLQKAA and TRDFLG motifs (Colasanti et al., 2006). To test the effect of GAs on the stability of OsIDD2, western-blot analysis was performed in both the OsIDD2OE lines and the wild type under GA3 treatment. As shown in Figure 7D, the levels of OsIDD2 were not affected by applying exogenous GA3 in both genotypes.
Figure 7.
The interaction between OsIDD2 and SLR1. A, Interactions between OsIDD2 and SLR1 in Y2H assay. OsIDD2, OsIDD2-activating domain (AD) + BD; SLR1, SLR1-BD + AD; SLR1+OsIDD2, SLR1-BD + OsIDD2-AD; –TL, medium lacking Trp and Leu; –TLHA, medium lacking Trp, Leu, His, and adenine; Vec, empty vectors not fused with bait and prey protein in one clone together, pGADT7 (AD of GAL4) + pGBT9 (BD of GAL4). B, Interaction between SLR1 and OsIDD2 in vitro analyzed by pull-down assays. GST and GST-SLR1 proteins incubated with recombinant 6×His-OsIDD2 proteins were bound to Glutathione Sepharose and then eluted and analyzed by immunoblotting with anti-OsIDD2 antibody. C, Interaction between SLR1 and OsIDD2 in vivo analyzed by Co-IP assay. Tobacco leaves coexpressing 35S:GST-SLR1 and 35S:OsIDD2 or only 35S:OsIDD2 were used to immunoprecipitate with anti-GST antibody, and the immunoblot was probed with anti-OsIDD2 antibody. D, Effects of GA on OsIDD2 levels in the wild type (WT) and OsIDD2OE. Total protein was extracted from 2-week-old seedlings with and without GA3 (50 µm) treatment of 24 h and was immunoblotted by anti-OsIDD2 (top). Actin was immunoblotted by anti-actin as a control (bottom). E, The domain of OsIDD2 binding with SLR1 was determined by Y2H assay. The left schematic diagram represents the full length or different truncations of OsIDD2 used for Y2H assays. Yeast was transformed with a recombination of the indicated plasmids, in which truncated or full-length OsIDD2 was fused with BD and SLR1 was fused with AD of GAL4 protein. Subsequently, β-galactosidase activity was measured. Data are means ± sd; n = 3. aa, Amino acids.
DISCUSSION
Coordination of SLR1, OsIDD2, OsmiR396, and OsGRFs Is Critical for GA-Mediated Cell Proliferation
Deep conservation of miR396/GRFs and the GRFs’ coactivator, GIFs, in various plants (Sunkar et al., 2005; Omidbakhshfard et al., 2015) indicates that they likely play important roles in higher plants. OsmiR396 targeting most members of OsGRFs could provide a potential strategy to gain insight into the overall roles of OsGRFs in rice. Here, we show that the transgenic rice plants overexpressing OsmiR396a exhibited a dwarf phenotype and a delayed growth rate. However, the transgenic lines could ultimately produce tillers, flowers, and seeds similar to the wild type, implying that the roles of miR396/OsGRFs are mainly involved in controlling the growth rate rather than cell differentiation. The micro difference of cell sizes in stem and suspension cells between wild-type and miR396OE plants indicated that the suppressed cell proliferation is likely the main cause for the dwarfism of the transgenic plants (Fig. 1, D and E; Supplemental Fig. S5D). Furthermore, suppressed OsGRFs in the miR396OE plants consequently led to the suppressed expression of some cell cycle-related genes (cycOs1 and cycOs2; Figs. 1A and 4D).
GAs play key roles in stimulating cell proliferation and elongation in rice (Sauter, 1997; Spielmeyer et al., 2002; Sakamoto et al., 2004; Ueguchi-Tanaka et al., 2005; Li et al., 2011). Earlier studies demonstrated that the expression of most OsGRFs is induced by GA (Choi et al., 2004), and here, we verified that this induction is caused by lower RNA abundance of miR396 (Figs. 2, A and B, and 3A). In addition, the influence of SLR1 on miR396/OsGRFs as well as some cell cycle-related genes was observed to be in the same pathway. The RNA abundance of miR396 was lower in the slr1 and eui mutants (DELLA is reduced in both mutants) and higher in the gid mutant (in which DELLA is stable) than in the wild type (Fig. 4, A and C), but the RNA abundance of SLR1 was nearly at the same level in OsmiR396a-overexpressing plants and the wild type (Fig. 4B), indicating that miR396 acts downstream of SLR1. Moreover, the observation that the mRNA levels of GA-induced cycOs1 and cycOs2 were lower and could not be restored by applied GA3 in miR396OE plants implied that these cell cycle-related genes act downstream of miR396/OsGRFs and that their response to GA is mediated by miR396/OsGRFs (Fig. 4D). Furthermore, we discovered that OsIDD2 directly binds the promoter of OsmiR396a and can physically interact with SLR1. Overexpression of OsIDD2 also resulted in a phenotype similar to that of miR396OE plants resulting from higher OsmiR396 levels (Fig. 5, C and E). By contrast, OsIDD2 silencing caused a slr1-like phenotype and a lower OsmiR396 level (Fig. 6, A–C). From the observations that both stem length and the RNA level of miR396 could be regulated but OsIDD2 could not at protein/mRNA levels by applying GA3 in the OsIDD2OE lines and the wild type (Figs. 5, D–F, and 7D), we proposed that OsIDD2, promoting the expression of miR396, requires the coactivator of SLR1.
Therefore, we proposed a working model for OsmiR396/GRFs in regulating cell proliferation under the influence of GA. When GA is absent, OsIDD2 and higher levels of SLR1 together promote miR396 expression, resulting in a suppressed expression of OsGRFs as well as GA-mediated cell cycle-related genes, thus leading to inhibited stem elongation. However, when GA is present, SLR1 is degraded by the GID1/GA/SLR1-mediated SCFGID2/SLY1 complex. The degradation of SLR1 protein in turn disrupts the interaction between SLR1 and OsIDD2 and consequently inhibits the promotion of OsIDD2/SLR1 to miR396; thus, the mRNA levels of OsGRFs rise as well as those of GA-mediated cell cycle-related genes, ultimately leading to GA-stimulated cell proliferation.
Regulation of OsGRFs by OsmiR396 Is Dependent on GA Concentration
GAs are involved in various aspects of plant growth and development. Earlier studies have revealed that GAs are mainly present in actively growing and elongating tissues, such as shoot apices, young leaves, and flowers (Jones and Phillips, 1966). In rice, OsGA20ox2 (Oryza sativa Gibberellin20 oxidase-2) and OsGA3ox2 are expressed in rapidly elongating or dividing tissues (Kaneko et al., 2003). These results suggested that, in many cases, bioactive GAs are produced at the sites of their action. In rice internodes, OsGRF1 transcripts were only detected in the intercalary meristem, which is the primary site of GA action (Sauter et al., 1995). In this study, we found that the RNA level of miR396 was negatively dependent on the concentration of exogenous GA3 in rice (Fig. 3A). Although the concentrations of endogenous bioactive GAs were not measured in this study, the fact that the RNA abundance of OsmiR396 is much lower while the abundance of its targets is much higher in rapidly growing and young tissues (Fig. 3B) suggested that the RNA level of miR396 may be regulated by endogenous GA concentrations. Our results are consistent with a previous report that showed that Arabidopsis miR396 levels in older parts of leaves are higher than that in leaf primordia (Rodriguez et al., 2010). Some studies showed that the expression of miR396 is also enhanced by various types of abiotic stresses, including low temperature, high salinity, and drought stress (Hewezi and Baum, 2012; Omidbakhshfard et al., 2015), but various stresses reportedly induce changes in GA metabolism too, resulting in the stabilization of DELLAs that are responsible for controlling cell proliferation (Claeys et al., 2012).
Function of IDD Protein Members
Compared with other genes involved in this study, OsIDD members likely have divergent functions in plant development (Coelho et al., 2018). As a family of transcription factors, IDD genes are defined by encoding four zinc finger motifs utilized for binding DNA (Colasanti et al., 2006). However, the length and sequences of the amino acid spacer between ZF1 (Zinc Finger1) and ZF2 are not consistent among the majority of members, indicating that the target cis-elements used for binding DNA are variable for different members of IDD (Colasanti et al., 2006). So far, diverse genes such as AtGA20ox2 and GID1b (Fukazawa et al., 2014), the GA-positive regulator SCARECROW-LIKE3 and SCARECROW (Yoshida et al., 2014; Kobayashi et al., 2017), ammonium-mediated gene (Xuan et al., 2013), auxin biosynthesis-related gene (Cui et al., 2013), and Suc synthase genes (Seo et al., 2011) have been reported to be directly targeted by different IDDs. IDDs are reportedly involved in various aspects of plant growth and development, including flowering transition, root development, nitrogen and starch metabolism, sugar homeostasis, seed germination, and leaf development (Coelho et al., 2018). A number of AtIDDs have been shown to be capable of interacting with DELLA proteins via transactivation to regulate downstream genes in Arabidopsis (Feurtado et al., 2011; Fukazawa et al., 2014; Yoshida et al., 2014), but no DELLA-DIP interactions have been reported in cereals. Here, we suggest that OsIDD2 interacting with SLR1 may promote the expression of miR396 in controlling cell proliferation in rice. Recently, another study also showed a similar phenotype of transgenic rice overexpressing OsIDD2 to our miR396OE lines (Huang et al., 2018), further confirming the involvement of OsIDD2 in stem elongation.
MATERIALS AND METHODS
Plant Material and Growth Conditions
Rice (Oryza sativa) genotype Nipponbare was used as the wild type and is the genetic background for all transgenic lines in this study. Rice seeds were immersed in a water bath for 24 h at room temperature and were germinated under moist conditions (seeds were covered with two layers of moistened cheesecloth) at 37°C for another 30 h. The germinated seeds were either grown in petri dishes (diameter, 90 mm) containing CHU (N6) liquid medium, which is defined to improve the formation, growth, and differentiation of callus in rice, under a 16/8-h photoperiod at ∼200 μmol m−2 s−1 at 28°C or grown in a field under natural long-day conditions.
For RNA analysis under GA3 treatment, whole 2-week-old seedlings were first incubated into N6 liquid medium for 8 h and then transferred into another N6 solution containing the designated concentrations of GA3 and 0.02% (v/v) Tween 20 and incubated for the designated times. Then total RNA was extracted from seedlings for analysis of gene expression. To measure the influence of GA on plant height, 10-d-old seedlings of the wild type and transgenic lines growing in pots were sprayed with distilled water (with or without 50 µm GA3) containing 0.02% (v/v) Tween 20 at 1-d intervals for 25 d. For uniconazole treatment, sterilized seeds were soaked in water with or without 6.9 µm uniconazole for 24 h and then placed in distilled water for another 24 h.
Sequence Alignment and Phylogenetic Analysis
A phylogenetic tree and multiple amino acid sequence alignments were created by the DNAMAN (DNA 5.22; Lynnon Biosoft) program with default parameters as follows: gap penalty at 3, K-tuple at 1, gap open penalty at 10, and gap extension at 0.2. The complementarity between miR396 and its target genes was performed by Omiga 2.0 (Oxford Molecular).
5′-RACE Analysis
5′-RACE was performed on poly(A)-selected total RNA extracted from 2-week-old rice seedlings using the GeneRacer Kit (Invitrogen), as described by Kasschau et al. (2003). For every round of PCR, we used 3′ gene-specific primers designed about 100 bp away from the predicted miR396-binding site and the 5′ GeneRacer nested primer. PCR products were separated by agarose gel electrophoresis, and distinct bands of the appropriate size for microRNA-mediated cleavage were purified, cloned, and sequenced.
Northern-Blot Analysis
Total RNA was extracted from seedlings or various tissues using TRIzol reagent (Invitrogen). The RNA levels of OsmiR396 and U6 were determined according to the procedure described by Sunkar et al. (2006). The probes for OsmiR396 and U6 were DNA oligonucleotides (antisense to miR396: 5′-CAGTTCAAGAAAGCTGTGGAA-3′ and 5′-GTTCAAGAAAGCCTGTGGA-3′, the former can completely match with OsmiR396a-b and the latter can completely match with OsmiR396c-h; antisense to U6 RNA: 5′-ATTTCTCGATTTGTGCGTGTC-3′) synthesized by ShengGong, which match with either miR396 or a partial sequence of U6 RNA. Total RNA (about 50 µg) was loaded onto 15% (w/v) polyacrylamide Tris-Boric acid-EDTA (TBE)-urea gels and ran at 150 V in 0.5× TBE for 1.5 to 2 h. Small RNAs were electroblotted to a Hybond N+ membrane (Amersham Pharmacia Biotech) for 30 min at 24 V in 0.5× TBE buffer. Membranes were hybridized overnight at room temperature with 100 ng mL−1 probes, which were labeled with [γ-32P]ATP at the 5′ terminus by using phosphatase and T4 polynucleotide kinase. For probing miR396, equal molar concentrations of the two probes were mixed together. Membranes were washed twice for 5 min each with 2× SSC and 0.1% (w/v) SDS at room temperature and twice for 15 min each with 2× SSC and 0.1% (w/v) SDS at 50°C.
Construction of Expression Vector and Generation of Transgenic Lines
To generate transgenic rice overexpressing OsmiR396 and OsIDD2, the two genes were cloned and constructed into an expression vector of pCAMBIA1301, which has a constitutive ubiquitin promoter, Ubi1 (Supplemental Fig. S2C). OsIDD2 was cloned by RT-PCR, and the precursor of OsmiR393a with 193 bp forming a stem loop containing miR396 (Supplemental Fig. S2B) was amplified by PCR using genomic DNA as a template. To generate OsIDD2-silencing lines, both forward and reverse directions of a specific DNA fragment comprising 150 bp were inserted into pCAMBIA1301. To generate the ProOsGRF9-GUS constructs, the promoter of OsGRF9 was cloned by PCR from genomic DNA and then the 35S promoter that drove the expression of GUS in pCAMBIA1301 was replaced by the promoter of OsGRF9. All the above expression constructs were introduced into rice calli by Agrobacterium tumefaciens (EHA105) transformation, and the related vectors and primers are listed in Supplemental Table S2.
RT-qPCR
For RT-qPCR analysis, 2 μg of total RNA was reverse transcribed in a total volume of 20 μL with 0.5 mg of oligo(dT)15, 0.75 mm deoxynucleoside triphosphate, 10 mm dithiothreitol (DTT), and 100 units of SuperScript II RNase H2 reverse transcriptase (Invitrogen). PCR was performed in a total volume of 20 μL with 1 μL of the RT reactions including a CFX real-time PCR instrument (Bio-Rad) and SYBR Green mixture (Roche), 0.2 mm gene-specific primers, and 1 unit of Taq Polymerase (TaKaRa). A total of 28 to 30 cycles was performed. The expression levels of the samples were normalized by OsUbiquitin. The primers used in qPCR are listed in Supplemental Table S2.
Observation of Cells and Creation of Suspension-Cultured Cells
For the creation of suspension cells, the rice calli derived from sterilized seeds of the wild type and miR396OE lines had been grown on N6 culture medium (solid). Four weeks later, 1 g of the fresh calli was incubated into 500 mL of AA medium (Toriyama and Hinata, 1985) and biomass was measured at given intervals. The suspension cells were observed and recorded using a microscope. To observe tissue cells, the cells in the elongation zone of the top-most internode of wild-type and miR396OE plants were cut along the longitudinal direction and fixed with formalin alcohol acetic acid solution. After dehydration, the image of cells embedded in paraffin was sliced and photographed using a microscope.
Nuclear Localization of OsGRF11
The entire coding region of OsGRF11 except for the last five amino acids was amplified by PCR (primers with NocI and SpeI; Supplemental Table S2) and then was inserted into pCAMBIA1304, which was digested by NocI and SpeI, yielding the p35S:OsGRF11-GFP constructs. Onion (Allium cepa) epidermal cells were transformed and nuclear localization of GFP was assayed by the method of Varagona et al. (1992).
Y1H/Y2H Assays
Yeast (Saccharomyces cerevisiae) one-hybrid/two-hybrid screening was performed as previously described (Mitsuda et al., 2010) with some modifications. The library used for the Y1H assay was constructed into pGADT7 by All-Direct way (Bio Gene) using cDNA derived from various tissues (especially relatively older sections) including the roots in the basal region of 4-week-old seedlings, the shoots of basal internodes of 4-week-old seedlings, and the older leaves in basal shoots of 4-week-old seedlings, in which the RNA levels of miR396 were higher than in other tissues (Fig. 3B). To maximize the coverage of the cDNA library, total RNA over 200 μg was extracted from the above-mentioned tissues and mRNA was separated and homogenized before being constructed into pGADT7. The promoter of OsmiR396a with a length of 1,134 bp upstream of the predicted precursor was cloned into pHIS2 as bait. The original Y1H strains were transformed with a 4-μg library and plated onto medium lacking His but containing 15 mm 3-amino-1,2,4-triazole. Then the larger positive colonies were subjected to a colony-lift β-galactosidase assay to validate the selection. For Y2H assays, SLR1 fused with BD of GAL4 was constructed into pGBT9 driven by a 396-bp partial ADH1 (AT1G77120) promoter whose transcription ability is weaker than that of the full-length promoter. Then the open reading frame and different truncations of OsIDD2 fused with the AD of GAL4 were cloned into pGADT7 in which the ADH1 promoter was also replaced by a 396-bp fragment as laid in pGBT9. The positive colonies on medium lacking His and adenine were selected, and the β-galactosidase assay was performed as described for Y1H. The related primers are shown in Supplemental Table S2.
Pull-Down, Co-IP, and Western-Blot Assays
Pull-down and Co-IP assays were performed as described (Zhang et al., 2009). For the pull-down assay, SLR1 fused to GST was purified using glutathione beads (GE Healthcare). The beads bound with GST-SLR1 and GST were washed with phosphate buffered saline buffer. His-tagged OsIDD2 was constructed into pET28 and expressed in Escherichia coli BL21, and the proteins were purified using Ni+ resin. Then the glutathione beads were incubated with 6× His-OsIDD2 in pull-down buffer (20 mm Tris-HCl, pH 7.5, 100 mm NaCl, 1 mm EDTA, and 1 mm DTT). The mixture was rotated for 1 h, and the beads were washed five times with wash buffer (20 mm Tris-HCl, pH 7.5, 300 mm NaCl, 0.1% [v/v] Nonidet P-40, 1 mm EDTA, and 1 mm DTT). The proteins were eluted from the beads by boiling in 50 μL of 2× SDS buffer and loaded onto a 10% SDS-PAGE gel. Gel blots were analyzed using an anti-GST antibody dilution and an anti-OsIDD2 antibody dilution. For Co-IP, tobacco (Nicotiana tabacum) leaves coexpressing 35S:GST-SLR1 and 35S:OsIDD2 or only 35S:OsIDD2 were ground in immunoprecipitation buffer (20 mm HEPES, pH 7.5, 40 mm KCl, 1 mm EDTA, and 1% [v/v] Triton X-100), filtered, and centrifuged. The supernatant (1 mL) was incubated with anti-GST coupled to Protein A-Sepharose beads for 30 min. Beads were washed five times with wash buffer (20 mm HEPES, pH 7.5, 40 mm KCl, and 0.1% [v/v] Triton X-100), and bound proteins were eluted with 2× SDS buffer, run onto an SDS-PAGE gel, and probed by anti-OsIDD2 antibody.
For western-blot analysis of OsIDD2, whole 2-week-old seedlings of the wild type and OsIDD2OE with/without GA3 treatment were immersed into liquid nitrogen. Total protein was extracted by SDS sample buffer and boiled for 10 min. Then the extracted proteins were separated by SDS-PAGE and immunoblotted with anti-OsIDD2 antibody at 1:1,000 dilution.
To prepare the antibodies of OsIDD2, the 6×His-OsIDD2 constructed into pET28 was expressed and used as antigen to produce antibodies in rabbits (purchased from Junhui Biotech). Primer information is provided in Supplemental Table S2.
Gel Retardation Assay
The gel retardation assays were performed following the procedure described previously (Fukazawa et al., 2010). Recombinant protein 6×His-OsIDD2 was expressed and purified by using Ni+ resin (Novagen). The primers for different fragments of the double-stranded OsmiR396a promoter used for the gel mobility shift assays are listed in Supplemental Table S2. The fragments of promoter were annealed and then labeled with [α-32P]dCTP. Binding mixtures contained 50 fmol of labeled probe and 1 mg of purified recombinant OsIDD2. The binding buffer contained 20 mm Tris-HCl, pH 7.5, 3 mm MgCl2, 50 mm KCl, 1 mm EDTA, 10% (v/v) glycerol, and 2 mm ZnCl2. Reactions were incubated at 4°C for 30 min and loaded onto 4% (w/v) polyacrylamide gels with the current switched on (10 V cm–1), and 0.5× TBE was used as a running buffer. The gels were dried on Whatman DE81 paper on top of Whatman 3MM paper and autoradiographed. The related primers are shown in Supplemental Table S2.
GUS Staining and Activity Assays
For histochemical analysis, maturing seeds were cut longitudinally with a razor blade at an interval of every 1 d after sowing into water, and then the longitudinal face was stained with 5-bromo-4-chloro-3-indolyl glucuronide as described previously (Ulmasov et al., 1997). Fluorometric assays of GUS activities were performed as described by Jefferson (1987).
Accession Numbers
Sequence data from this article can be found in the GenBank/EMBL databases or rice annotation project database (rap-db) under the following accession numbers: OsGRF1 (BK004856; Os02g0776900), OsGRF2 (BK004857; Os06g0204800), OsGRF3 (BK004858; Os04g0600900), OsGRF4 (BK004859; Os02g0701300), OsGRF5 (BK004860), OsGRF6 (BK004861; Os03g0729500), OsGRF7 (BK004862; Os12g0484900), OsGRF8 (BK004863; Os11g0551900), OsGRF9 (BK004878; Os03g0674700), OsGRF10 (BK004879; Os02g0678800), OsGRF11 (BK004880; Os07g0467500), OsGRF12 (BK004881; Os04g0574500), SLR1 (Os03g0707600), cycOs1 (X82035; Os04g0563700), cycOs2 (X82036; Os06g0726800), OsIDD2 (Os01g09850; Os01g019500), AtGRF1 (At2g22840), and OsmiR396a (MI0001046).
Supplemental Data
The following supplemental materials are available.
Supplemental Figure S1. Sequence comparisons of OsmiR396 and OsGRFs.
Supplemental Figure S2. Sequence analysis and diagram of the T-DNA in the expression vector.
Supplemental Figure S3. The subcellular localization of OsGRF11.
Supplemental Figure S4. The expression of some OsGRFs.
Supplemental Figure S5. Phenotype of transgenic plants overexpressing OsmiR396a.
Supplemental Table S1. Comparison of the phenotypes of the wild type and the miR396OE and OsIDD2OE lines.
Supplemental Table S2. Primers used in this work.
Acknowledgments
We thank Dr. Jin-Gui Chen for his suggestions and revision of this article. We thank Dr. Yu Zhao for her contribution of the slr1 mutant and Dr. Xiao-Feng Cui for his contribution of eui and gid1 mutants.
Footnotes
This work was supported by the National Natural Science Foundation of China (grant no. 31271623).
References
- Axtell MJ, Bartel DP (2005) Antiquity of microRNAs and their targets in land plants. Plant Cell 17: 1658–1673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bazin J, Khan GA, Combier JP, Bustos-Sanmamed P, Debernardi JM, Rodriguez R, Sorin C, Palatnik J, Hartmann C, Crespi M, et al. (2013) miR396 affects mycorrhization and root meristem activity in the legume Medicago truncatula. Plant J 74: 920–934 [DOI] [PubMed] [Google Scholar]
- Casadevall R, Rodriguez RE, Debernardi JM, Palatnik JF, Casati P (2013) Repression of growth regulating factors by the microRNA396 inhibits cell proliferation by UV-B radiation in Arabidopsis leaves. Plant Cell 25: 3570–3583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandler PM, Marion-Poll A, Ellis M, Gubler F (2002) Mutants at the Slender1 locus of barley cv Himalaya: Molecular and physiological characterization. Plant Physiol 129: 181–190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi D, Kim JH, Kende H (2004) Whole genome analysis of the OsGRF gene family encoding plant-specific putative transcription activators in rice (Oryza sativa L.). Plant Cell Physiol 45: 897–904 [DOI] [PubMed] [Google Scholar]
- Claeys H, Skirycz A, Maleux K, Inzé D (2012) DELLA signaling mediates stress-induced cell differentiation in Arabidopsis leaves through modulation of anaphase-promoting complex/cyclosome activity. Plant Physiol 159: 739–747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coelho CP, Huang P, Lee DY, Brutnell TP (2018) Making roots, shoots, and seeds: IDD gene family diversification in plants. Trends Plant Sci 23: 66–78 [DOI] [PubMed] [Google Scholar]
- Colasanti J, Tremblay R, Wong AY, Coneva V, Kozaki A, Mable BK (2006) The maize INDETERMINATE1 flowering time regulator defines a highly conserved zinc finger protein family in higher plants. BMC Genomics 7: 158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colasanti J, Yuan Z, Sundaresan V (1998) The indeterminate gene encodes a zinc finger protein and regulates a leaf-generated signal required for the transition to flowering in maize. Cell 93: 593–603 [DOI] [PubMed] [Google Scholar]
- Cui D, Zhao J, Jing Y, Fan M, Liu J, Wang Z, Xin W, Hu Y (2013) The Arabidopsis IDD14, IDD15, and IDD16 cooperatively regulate lateral organ morphogenesis and gravitropism by promoting auxin biosynthesis and transport. PLoS Genet 9: e1003759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davière JM, Achard P (2013) Gibberellin signaling in plants. Development 140: 1147–1151 [DOI] [PubMed] [Google Scholar]
- Diao Z, Yu M, Bu S, Duan Y, Zhang L, Wu W (2018) Functional characterization of OsmiR396a in rice (Oryza sativa L.). Plant Growth Regul 85: 351–361 [Google Scholar]
- Feurtado JA, Huang D, Wicki-Stordeur L, Hemstock LE, Potentier MS, Tsang EW, Cutler AJ (2011) The Arabidopsis C2H2 zinc finger INDETERMINATE DOMAIN1/ENHYDROUS promotes the transition to germination by regulating light and hormonal signaling during seed maturation. Plant Cell 23: 1772–1794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukazawa J, Nakata M, Ito T, Yamaguchi S, Takahashi Y (2010) The transcription factor RSG regulates negative feedback of NtGA20ox1 encoding GA 20-oxidase. Plant J 62: 1035–1045 [DOI] [PubMed] [Google Scholar]
- Fukazawa J, Teramura H, Murakoshi S, Nasuno K, Nishida N, Ito T, Yoshida M, Kamiya Y, Yamaguchi S, Takahashi Y (2014) DELLAs function as coactivators of GAI-ASSOCIATED FACTOR1 in regulation of gibberellin homeostasis and signaling in Arabidopsis. Plant Cell 26: 2920–2938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao P, Bai X, Yang L, Lv D, Li Y, Cai H, Ji W, Guo D, Zhu Y (2010) Over-expression of osa-MIR396c decreases salt and alkali stress tolerance. Planta 231: 991–1001 [DOI] [PubMed] [Google Scholar]
- Hewezi T, Baum TJ (2012) Complex feedback regulations govern the expression of miRNA396 and its GRF target genes. Plant Signal Behav 7: 749–751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirano K, Ueguchi-Tanaka M, Matsuoka M (2008) GID1-mediated gibberellin signaling in plants. Trends Plant Sci 13: 192–199 [DOI] [PubMed] [Google Scholar]
- Horiguchi G, Kim GT, Tsukaya H (2005) The transcription factor AtGRF5 and the transcription coactivator AN3 regulate cell proliferation in leaf primordia of Arabidopsis thaliana. Plant J 43: 68–78 [DOI] [PubMed] [Google Scholar]
- Huang P, Yoshida H, Yano K, Kinoshita S, Kawai K, Koketsu E, Hattori M, Takehara S, Huang J, Hirano K, et al. (2018) OsIDD2, a zinc finger and INDETERMINATE DOMAIN protein, regulates secondary cell wall formation. J Integr Plant Biol 60: 130–143 [DOI] [PubMed] [Google Scholar]
- Ikeda A, Ueguchi-Tanaka M, Sonoda Y, Kitano H, Koshioka M, Futsuhara Y, Matsuoka M, Yamaguchi J (2001) slender rice, a constitutive gibberellin response mutant, is caused by a null mutation of the SLR1 gene, an ortholog of the height-regulating gene GAI/RGA/RHT/D8. Plant Cell 13: 999–1010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh H, Ueguchi-Tanaka M, Sato Y, Ashikari M, Matsuoka M (2002) The gibberellin signaling pathway is regulated by the appearance and disappearance of SLENDER RICE1 in nuclei. Plant Cell 14: 57–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izumi K, Yamaguchi I, Wada A, Oshio H, Takahashi N (1984) Effects of a new plant growth retardant (E)-1-(4-chlorophenyl)-4,4-dimethyl-2-(1,2,4-triazol-1-yl)-1-penten-3-ol (S-3307) on the growth and gibberellin content of rice plants. Plant Cell Physiol 25: 611–617 [Google Scholar]
- Jefferson RA. (1987) Assay for chimeric genes in plants: The GUS fusion system. Plant Mol Biol Rep 5: 387–405 [Google Scholar]
- Jones RL, Phillips ID (1966) Effect of CCC on the gibberellin content of excised sunflower organs. Planta 72: 53–59 [DOI] [PubMed] [Google Scholar]
- Jones-Rhoades MW, Bartel DP (2004) Computational identification of plant microRNAs and their targets, including a stress-induced miRNA. Mol Cell 14: 787–799 [DOI] [PubMed] [Google Scholar]
- Kaneko M, Itoh H, Inukai Y, Sakamoto T, Ueguchi-Tanaka M, Ashikari M, Matsuoka M (2003) Where do gibberellin biosynthesis and gibberellin signaling occur in rice plants? Plant J 35: 104–115 [DOI] [PubMed] [Google Scholar]
- Kasschau KD, Xie Z, Allen E, Llave C, Chapman EJ, Krizan KA, Carrington JC (2003) P1/HC-Pro, a viral suppressor of RNA silencing, interferes with Arabidopsis development and miRNA unction. Dev Cell 4: 205–217 [DOI] [PubMed] [Google Scholar]
- Kim JH, Choi D, Kende H (2003) The AtGRF family of putative transcription factors is involved in leaf and cotyledon growth in Arabidopsis. Plant J 36: 94–104 [DOI] [PubMed] [Google Scholar]
- Kim JH, Kende H (2004) A transcriptional coactivator, AtGIF1, is involved in regulating leaf growth and morphology in Arabidopsis. Proc Natl Acad Sci USA 101: 13374–13379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi A, Miura S, Kozaki A (2017) INDETERMINATE DOMAIN PROTEIN binding sequences in the 5′-untranslated region and promoter of the SCARECROW gene play crucial and distinct roles in regulating SCARECROW expression in roots and leaves. Plant Mol Biol 94: 1–13 [DOI] [PubMed] [Google Scholar]
- Kozaki A, Hake S, Colasanti J (2004) The maize ID1 flowering time regulator is a zinc finger protein with novel DNA binding properties. Nucleic Acids Res 32: 1710–1720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuijt SJ, Greco R, Agalou A, Shao J, ’t Hoen CC, Overnäs E, Osnato M, Curiale S, Meynard D, van Gulik R, et al. (2014) Interaction between the GROWTH-REGULATING FACTOR and KNOTTED1-LIKE HOMEOBOX families of transcription factors. Plant Physiol 164: 1952–1966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee BH, Ko JH, Lee S, Lee Y, Pak JH, Kim JH (2009) The Arabidopsis GRF-INTERACTING FACTOR gene family performs an overlapping function in determining organ size as well as multiple developmental properties. Plant Physiol 151: 655–668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee BH, Wynn AN, Franks RG, Hwang YS, Lim J, Kim JH (2014) The Arabidopsis thaliana GRF-INTERACTING FACTOR gene family plays an essential role in control of male and female reproductive development. Dev Biol 386: 12–24 [DOI] [PubMed] [Google Scholar]
- Li J, Jiang J, Qian Q, Xu Y, Zhang C, Xiao J, Du C, Luo W, Zou G, Chen M, et al. (2011) Mutation of rice BC12/GDD1, which encodes a kinesin-like protein that binds to a GA biosynthesis gene promoter, leads to dwarfism with impaired cell elongation. Plant Cell 23: 628–640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu D, Song Y, Chen Z, Yu D (2009) Ectopic expression of miR396 suppresses GRF target gene expression and alters leaf growth in Arabidopsis. Physiol Plant 136: 223–236 [DOI] [PubMed] [Google Scholar]
- Liu H, Guo S, Xu Y, Li C, Zhang Z, Zhang D, Xu S, Zhang C, Chong K (2014) OsmiR396d-regulated OsGRFs function in floral organogenesis in rice through binding to their targets OsJMJ706 and OsCR4. Plant Physiol 165: 160–174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuo T, Futsuhara Y, Kikuchi F, Yamaguchi H (1997) Science of the Rice Plant. Nobunkyo, Tokyo [Google Scholar]
- Mecchia MA, Debernardi JM, Rodriguez RE, Schommer C, Palatnik JF (2013) MicroRNA miR396 and RDR6 synergistically regulate leaf development. Mech Dev 130: 2–13 [DOI] [PubMed] [Google Scholar]
- Mitsuda N, Ikeda M, Takada S, Takiguchi Y, Kondou Y, Yoshizumi T, Fujita M, Shinozaki K, Matsui M, Ohme-Takagi M (2010) Efficient yeast one-/two-hybrid screening using a library composed only of transcription factors in Arabidopsis thaliana. Plant Cell Physiol 51: 2145–2151 [DOI] [PubMed] [Google Scholar]
- Mitsunaga S, Yamaguchi J (1993) Production of α-amylase is repressed by uniconazole, an inhibitor of the biosynthesis of gibberellin, in a dwarf mutant of rice, Waito-C. Plant Cell Physiol 34: 243–249 [Google Scholar]
- Omidbakhshfard MA, Proost S, Fujikura U, Mueller-Roeber B (2015) Growth-Regulating Factors (GRFs): A small transcription factor family with important functions in plant biology. Mol Plant 8: 998–1010 [DOI] [PubMed] [Google Scholar]
- Peng J, Carol P, Richards DE, King KE, Cowling RJ, Murphy GP, Harberd NP (1997) The Arabidopsis GAI gene defines a signaling pathway that negatively regulates gibberellin responses. Genes Dev 11: 3194–3205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng J, Richards DE, Hartley NM, Murphy GP, Devos KM, Flintham JE, Beales J, Fish LJ, Worland AJ, Pelica F, et al. (1999) ‘Green Revolution’ genes encode mutant gibberellin response modulators. Nature 400: 256–261 [DOI] [PubMed] [Google Scholar]
- Rodriguez RE, Ercoli MF, Debernardi JM, Breakfield NW, Mecchia MA, Sabatini M, Cools T, De Veylder L, Benfey PN, Palatnik JF (2015) MicroRNA miR396 regulates the switch between stem cells and transit-amplifying cells in Arabidopsis roots. Plant Cell 27: 3354–3366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez RE, Mecchia MA, Debernardi JM, Schommer C, Weigel D, Palatnik JF (2010) Control of cell proliferation in Arabidopsis thaliana by microRNA miR396. Development 137: 103–112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez RE, Schommer C, Palatnik JF (2016) Control of cell proliferation by microRNAs in plants. Curr Opin Plant Biol 34: 68–76 [DOI] [PubMed] [Google Scholar]
- Sakamoto T, Miura K, Itoh H, Tatsumi T, Ueguchi-Tanaka M, Ishiyama K, Kobayashi M, Agrawal GK, Takeda S, Abe K, et al. (2004) An overview of gibberellin metabolism enzyme genes and their related mutants in rice. Plant Physiol 134: 1642–1653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki A, Itoh H, Gomi K, Ueguchi-Tanaka M, Ishiyama K, Kobayashi M, Jeong DH, An G, Kitano H, Ashikari M, et al. (2003) Accumulation of phosphorylated repressor for gibberellin signaling in an F-box mutant. Science 299: 1896–1898 [DOI] [PubMed] [Google Scholar]
- Sauter M. (1997) Differential expression of a CAK (cdc2-activating kinase)-like protein kinase, cyclins and cdc2 genes from rice during the cell cycle and in response to gibberellin. Plant J 11: 181–190 [DOI] [PubMed] [Google Scholar]
- Sauter M, Kende H (1992) Gibberellin-induced growth and regulation of the cell division cycle in deepwater rice. Planta 188: 362–368 [DOI] [PubMed] [Google Scholar]
- Sauter M, Mekhedov SL, Kende H (1995) Gibberellin promotes histone H1 kinase activity and the expression of cdc2 and cyclin genes during the induction of rapid growth in deepwater rice internodes. Plant J 7: 623–632 [DOI] [PubMed] [Google Scholar]
- Seo PJ, Ryu J, Kang SK, Park CM (2011) Modulation of sugar metabolism by an INDETERMINATE DOMAIN transcription factor contributes to photoperiodic flowering in Arabidopsis. Plant J 65: 418–429 [DOI] [PubMed] [Google Scholar]
- Silverstone AL, Ciampaglio CN, Sun T (1998) The Arabidopsis RGA gene encodes a transcriptional regulator repressing the gibberellin signal transduction pathway. Plant Cell 10: 155–169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spielmeyer W, Ellis MH, Chandler PM (2002) Semidwarf (sd-1), “green revolution” rice, contains a defective gibberellin 20-oxidase gene. Proc Natl Acad Sci USA 99: 9043–9048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun TP, Gubler F (2004) Molecular mechanism of gibberellin signaling in plants. Annu Rev Plant Biol 55: 197–223 [DOI] [PubMed] [Google Scholar]
- Sunkar R, Girke T, Jain PK, Zhu JK (2005) Cloning and characterization of microRNAs from rice. Plant Cell 17: 1397–1411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sunkar R, Kapoor A, Zhu JK (2006) Posttranscriptional induction of two Cu/Zn superoxide dismutase genes in Arabidopsis is mediated by downregulation of miR398 and important for oxidative stress tolerance. Plant Cell 18: 2051–2065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang G, Reinhart BJ, Bartel DP, Zamore PD (2003) A biochemical framework for RNA silencing in plants. Genes Dev 17: 49–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas SG, Sun TP (2004) Update on gibberellin signaling. A tale of the tall and the short. Plant Physiol 135: 668–676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toriyama K, Hinata K (1985) Cell suspension and protoplast culture in rice. Plant Sci 41: 179–183 [Google Scholar]
- Ueguchi-Tanaka M, Ashikari M, Nakajima M, Itoh H, Katoh E, Kobayashi M, Chow TY, Hsing YI, Kitano H, Yamaguchi I, et al. (2005) GIBBERELLIN INSENSITIVE DWARF1 encodes a soluble receptor for gibberellin. Nature 437: 693–698 [DOI] [PubMed] [Google Scholar]
- Ueguchi-Tanaka M, Hirano K, Hasegawa Y, Kitano H, Matsuoka M (2008) Release of the repressive activity of rice DELLA protein SLR1 by gibberellin does not require SLR1 degradation in the gid2 mutant. Plant Cell 20: 2437–2446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueguchi-Tanaka M, Nakajima M, Motoyuki A, Matsuoka M (2007) Gibberellin receptor and its role in gibberellin signaling in plants. Annu Rev Plant Biol 58: 183–198 [DOI] [PubMed] [Google Scholar]
- Ulmasov T, Murfett J, Hagen G, Guilfoyle TJ (1997) Aux/IAA proteins repress expression of reporter genes containing natural and highly active synthetic auxin response elements. Plant Cell 9: 1963–1971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van De Velde K, Ruelens P, Geuten K, Rohde A, Van Der Straeten D (2017) Exploiting DELLA signaling in cereals. Trends Plant Sci 22: 880–893 [DOI] [PubMed] [Google Scholar]
- van der Knaap E, Kende H (1995) Identification of a gibberellin-induced gene in deepwater rice using differential display of mRNA. Plant Mol Biol 28: 589–592 [DOI] [PubMed] [Google Scholar]
- van der Knaap E, Kim JH, Kende H (2000) A novel gibberellin-induced gene from rice and its potential regulatory role in stem growth. Plant Physiol 122: 695–704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varagona MJ, Schmidt RJ, Raikhel NV (1992) Nuclear localization signal(s) required for nuclear targeting of the maize regulatory protein Opaque-2. Plant Cell 4: 1213–1227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Gu X, Xu D, Wang W, Wang H, Zeng M, Chang Z, Huang H, Cui X (2011) miR396-targeted AtGRF transcription factors are required for coordination of cell division and differentiation during leaf development in Arabidopsis. J Exp Bot 62: 761–773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu L, Zhang Q, Zhou H, Ni F, Wu X, Qi Y (2009) Rice microRNA effector complexes and targets. Plant Cell 21: 3421–3435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xuan YH, Priatama RA, Huang J, Je BI, Liu JM, Park SJ, Piao HL, Son DY, Lee JJ, Park SH, et al. (2013) Indeterminate domain 10 regulates ammonium-mediated gene expression in rice roots. New Phytol 197: 791–804 [DOI] [PubMed] [Google Scholar]
- Xue LJ, Zhang JJ, Xue HW (2009) Characterization and expression profiles of miRNAs in rice seeds. Nucleic Acids Res 37: 916–930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi S. (2008) Gibberellin metabolism and its regulation. Annu Rev Plant Biol 59: 225–251 [DOI] [PubMed] [Google Scholar]
- Yoshida H, Hirano K, Sato T, Mitsuda N, Nomoto M, Maeo K, Koketsu E, Mitani R, Kawamura M, Ishiguro S, et al. (2014) DELLA protein functions as a transcriptional activator through the DNA binding of the indeterminate domain family proteins. Proc Natl Acad Sci USA 111: 7861–7866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang LY, Bai MY, Wu J, Zhu JY, Wang H, Zhang Z, Wang W, Sun Y, Zhao J, Sun X, et al. (2009) Antagonistic HLH/bHLH transcription factors mediate brassinosteroid regulation of cell elongation and plant development in rice and Arabidopsis. Plant Cell 21: 3767–3780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y, Nomura T, Xu Y, Zhang Y, Peng Y, Mao B, Hanada A, Zhou H, Wang R, Li P, et al. (2006) ELONGATED UPPERMOST INTERNODE encodes a cytochrome P450 monooxygenase that epoxidizes gibberellins in a novel deactivation reaction in rice. Plant Cell 18: 442–456 [DOI] [PMC free article] [PubMed] [Google Scholar]