Abstract
Serial membranes that operate through a common compartment, the cytosol for transport at the plasma membrane and tonoplast, are intrinsically connected and communicate through this pool of solutes.
Dear Editor,
A very substantial body of data now exists for ion transport at both the plasma membrane and the tonoplast of several plant cell models, notably for guard cells, root hairs, and epidermal cells of several species to name a few (Grierson et al., 2014; Jezek and Blatt, 2017; Wang et al., 2019). This knowledge has its foundation in detailed electrophysiological and flux studies that have provided quantitative biophysical and kinetic information. Our understanding has expanded through molecular biology to include the genetic identities of many transporters that operate at both membranes. Among these studies, it is common to deconstruct the mechanics and genetics of transport and to characterize each transporter in isolation. In many cases, this work has included the cloning and heterologous expression of specific transport gene products and their analysis under voltage clamp and by radiotracer flux measurements.
Where such studies often struggle is in phenotypic analysis in vivo to associate a genetic lesion with a function for the transport gene of interest. We suggest that, in focusing on single gene products, the deconstructionist approach can lose sight of the unique feature of transport, namely its physiological integration and apparent communication with other transport processes in situ, what is often referred to as emergent properties arising from transport interactions. This communication is particularly evident when the transporter in question moves charge across the membrane. Although less often appreciated, similar considerations apply to transport across serial membranes when these operate on common pools of solutes within an enclosed and finite compartment. Both situations are common to plants. Communication is especially important between serial membranes, for example between the plasma membrane and tonoplast, when the consequences of manipulating transport at one membrane affects the cytosolic contents and, hence, transport across the other membrane. In these circumstances, quantitative mathematical modeling is frequently essential to gain true insight into the communication between membranes and their transport processes.
Within a single membrane, there exist connections between different transporters that ensure their fundamental interdependency. Notably, voltage exerts a dominant control on charge-carrying transport, acting both as a driving force for transport and as a product of charge flux across the membrane (Jezek and Blatt, 2017). Because physical laws require that net charge flux across a membrane is zero in the steady state, the transport of each ionic species is necessarily joined to the transport of all other ions that affect voltage across the same membrane. Only by imposing the circuit of a voltage clamp is this interconnection between transporters bypassed. In short, manipulations affecting charge flux through any one transporter will necessarily impact on all other charge-carrying transporters in the same membrane, often with unforeseen consequences. As one example, the interdependency between H+-coupled K+ transport and the primary H+-ATPase (Blatt and Slayman, 1987; Gibrat et al., 1990; Maathuis and Sanders, 1994) was ultimately a key factor in explaining why more than a decade of research failed to uncover a hypothetical H+/K+ exchange ATPase in plants (Leonard and Hotchkiss, 1976), proposed to operate in a manner analogous to the mammalian Na+/K+-ATPase.
Similar considerations apply to communication between the plant plasma membrane and tonoplast. Because these serial membranes operate through a common and enclosed compartment, the cytosol, the transport activities of both are connected through this single pool of solutes. Analogies may be drawn here to transport interactions across the apoplast between cells, for example as highlighted in the plant vasculature (Gajdanowicz et al., 2011) and fungal symbiosis (Dreyer et al., 2019). Some caution in drawing such analogies is advisable, however, as the nature of the apoplast, being semiopen, will moderate interactions between membranes and thus precludes a direct comparison with the enclosed cytosolic compartment within a cell.
We focus here on communication between the serial membranes of the plasma membrane and tonoplast and how alterations in transport at one are a predictable consequence of manipulations affecting transport across the other membrane. The examples are for guard cells and the ensuing stomatal phenotypes, but the concepts apply equally to transport in other plant cell types. We use two studies (Wang et al., 2012, 2017) to address the mechanics of how Cl− flux mediated by the SLAC1 Cl− channel at the plasma membrane affects transport across the tonoplast. The physiology of the SLAC1 channel in guard cells ensures that it mediates Cl− efflux for stomatal closure and, as expected, the slac1 null mutation suppresses stomatal closure and greatly slows stomatal kinetics. Surprisingly, the mutant also greatly slows the kinetics of stomatal opening (Vahisalu et al., 2008; Wang et al., 2012, 2017), an effect that we now know arises because the mutation indirectly suppresses the activity of the K+ channels that mediate K+ uptake (Wang et al., 2012, 2017).
How does the slac1 mutation influence transport at the tonoplast? Direct access to the vacuolar membrane in vivo is not practicable. However, it is possible to assess the consequences for solute contents and to examine the underlying mechanisms that can explain these phenomena through simulation. We used the OnGuard platform (Chen et al., 2012; Hills et al., 2012; Wang et al., 2012) to explore the connections between these membranes. OnGuard2 (freely available at www.psrg.org.uk) incorporates all of the quantitative detail for transport and the relevant metabolic activities in guard cells to reproduce the characteristics of solute flux, stomatal aperture, and conductance known in the literature, and it has yielded a number of unexpected predictions, many now validated experimentally. For comparison with guard cells of wild-type Arabidopsis (Arabidopsis thaliana) in OnGuard2, simulations of the slac1 mutant were generated by setting to zero the ohmic (voltage-independent) Cl− conductance and the dominant fraction of the voltage-gated Cl− conductance, which, combined, normally represent the characteristics of SLAC1 (Wang et al., 2017). Negi et al. (2008) reported that the slac1 mutant accumulates osmotically active solutes, not only K+ and Cl− but also substantial amounts of organic anions. The simulations carried out by Wang et al. (2012, 2017) similarly yielded accumulations of K+, Cl−, and organic anions, the latter subsumed as malate in the OnGuard platform.
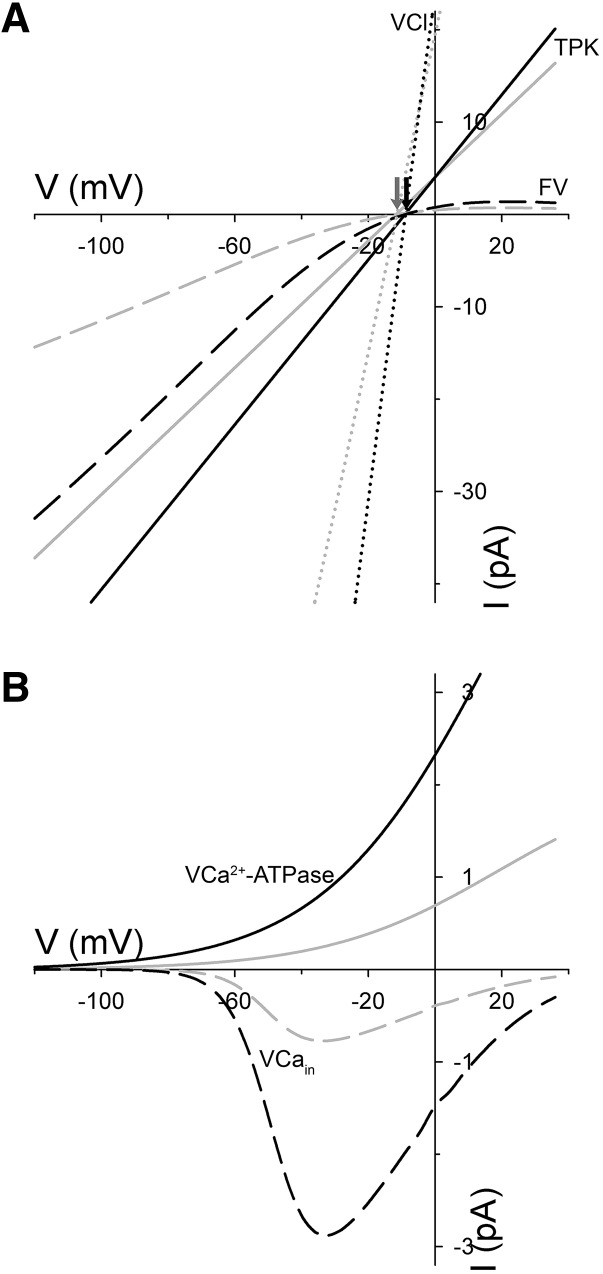
It is not surprising that eliminating a major pathway for anion efflux should result in its accumulation in the cytosol and vacuole through its buildup in the cytosol and transinhibition of efflux across the tonoplast. However, a comparison of the fluxes through each of the major tonoplast transporters is instructive. These data are available in the supplemental figures of Wang et al. (2012). The simulations predict an early increase in daytime K+ flux through the tonoplast TPK1 and FV K+ channels (see supplemental figure S7 of Wang et al., 2012), thereby accounting for the overall accumulation of this cation in the guard cell, as reported previously by Negi et al. (2008); they also show a daytime reversal of Cl− flux through the tonoplast Cl− channels (VCl). Additional to these outputs, a detailed analysis here yields a number of other predictions highlighting the apparent communication between plasma membrane and tonoplast. It predicts, counterintuitively, roughly threefold increases in net Ca2+ transport by the tonoplast Ca2+-ATPases (VCa2+-ATPase) and the Ca2+ channels (VCain), as is clearly evident in supplemental figure S6 of Wang et al. (2012) and a substantial increase in the activity of both sets of transporters (Fig. 1). These changes arise from the enhanced Ca2+ influx with plasma membrane hyperpolarization in the slac1 mutant and stimulation of endomembrane Ca2+ release and recycling (Wang et al., 2012). The juxtaposition of these two Ca2+ fluxes accounts for the overall lower total Ca2+ levels in the vacuole as well as the elevated cytosol-free [Ca2+] ([Ca2+]i) reported both in simulation and as validated through experimental measurements (Wang et al., 2012, 2017).
Figure 1.
Current-voltage (IV) curves predicted for the major tonoplast K+ and Cl− channels and for the tonoplast Ca2+ ATPases and channels in guard cells of wild-type (gray lines) and slac1 (black lines) Arabidopsis. Data were extracted from OnGuard2 simulations, as described, at 8 h into the daylight period, which corresponded to the maximum diurnal stomatal conductance in each case. The curves are plotted separately here for clarity. A, IV curves predicted for the TPK1 (solid lines) and FV (dashed lines) K+ channels and for the VCl Cl− channel (dotted lines). The free-running tonoplast voltages are indicated by the arrows (wild type, gray; slac1, black). Note the substantial increase in the conductances (slopes) for the current of each channel type in the slac1 mutant. B, IV curves predicted for the VCain Ca2+ channels (dashed lines) and for the VCa2+-ATPase (solid lines). The identity of the VCain remains unknown and, in the OnGuard platform, the VCa2+-ATPase subsumes the characteristics of all endomembrane Ca2+-ATPases (Chen et al., 2012; Hills et al., 2012; Wang et al., 2012, 2017). Again, the analysis yields a substantial increase in the conductances and amplitudes of both currents in the slac1 mutant.
OnGuard2 yields a number of other predictions that, although still to be tested experimentally, gain credibility from the accuracy of simulations to date in predicting experimental observations. Notable among these, in simulation the effect of the slac1 mutant is to increase the activity of the tonoplast FV, TPK, and VCl channels as would be resolved under voltage clamp (Fig. 1). The effect on all three currents is substantially greater than might be expected for the overall K+ and Cl− fluxes (see supplemental figure S7 of Wang et al., 2012) and is seemingly counterintuitive. However, the effects on these transporters is a natural consequence of changes in [Ca2+]i and cytosolic pH: all three channels are [Ca2+]i sensitive and the K+ channels are also subject to the elevated cytosolic pH that is characteristic of the slac1 mutant (Allen and Sanders, 1996; Wang et al., 2012, 2017). In short, the simulation predicts, in the slac1 mutant, an enhanced capacity for K+ and Cl− flux, even if this capacity is kinetically restricted by charge balance and the free-running voltage across the tonoplast.
Each of these predictions, and other outputs of OnGuard2, highlight the emergent properties of transport communication within and, especially, between the membranes of the guard cells. This communication is a natural consequence of a system of nonlinear biological processes that share substrates and products across each membrane and within cellular compartments. It arises from the membrane voltage that is shared between all charge-carrying transporters on any one membrane, as well as the common pool of ionic substrates shared between the membranes and enclosed by them. Between the plasma membrane and tonoplast, it arises from the common pool of cytosolic solutes that contribute to transport across both membranes.
We stress that there is nothing unusual about this network of interactive communication or the component transport processes. However, the intrinsic nonlinearities in flux behavior of each transporter ensure that the consequences of experimental manipulations are beyond intuitive understanding. Thus, in vivo, the consequence of manipulating a single transporter at a membrane is rarely (if ever) restricted to this one process, the distributions of the transported species alone, or, in plants, solely to the target membrane. Distinguishing between the primary effects of a mutation and off-target effects clearly benefits in these circumstances from quantitative mathematical modeling.
Footnotes
This work was supported by the National Research Council of Thailand (Ph.D. studentship to W.H.), the Biotechnology and Biological Sciences Research Council (grant nos. BB/N006909/1, BB/M001601/1, BB/N01832X/1, and BB/P011586/1 to M.R.B.), and the European Union (grant no. 678168 to M.R.B.).
Articles can be viewed without a subscription.
References
- Allen GJ, Sanders D (1996) Control of ionic currents in guard cell vacuoles by cytosolic and luminal calcium. Plant J 10: 1055–1069 [DOI] [PubMed] [Google Scholar]
- Blatt MR, Slayman CL (1987) Role of “active” potassium transport in the regulation of cytoplasmic pH by nonanimal cells. Proc Natl Acad Sci USA 84: 2737–2741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen ZH, Hills A, Bätz U, Amtmann A, Lew VL, Blatt MR (2012) Systems dynamic modeling of the stomatal guard cell predicts emergent behaviors in transport, signaling, and volume control. Plant Physiol 159: 1235–1251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreyer I, Spitz O, Kanonenberg K, Montag K, Handrich MR, Ahmad S, Schott-Verdugo S, Navarro-Retamal C, Rubio-Meléndez ME, Gomez-Porras JL, et al. (2019) Nutrient exchange in arbuscular mycorrhizal symbiosis from a thermodynamic point of view. New Phytol 222: 1043–1053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gajdanowicz P, Michard E, Sandmann M, Rocha M, Corrêa LGG, Ramírez-Aguilar SJ, Gomez-Porras JL, González W, Thibaud JB, van Dongen JT, et al. (2011) Potassium (K+) gradients serve as a mobile energy source in plant vascular tissues. Proc Natl Acad Sci USA 108: 864–869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibrat R, Grouzis JP, Rigaud J, Grignon C (1990) Potassium stimulation of corn root plasmalemma ATPase. II. H+-pumping in native and reconstituted vesicles with purified ATPase. Plant Physiol 93: 1183–1189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grierson C, Nielsen E, Ketelaarc T, Schiefelbein J (2014) Root hairs. The Arabidopsis Book 12: e0172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hills A, Chen ZH, Amtmann A, Blatt MR, Lew VL (2012) OnGuard, a computational platform for quantitative kinetic modeling of guard cell physiology. Plant Physiol 159: 1026–1042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jezek M, Blatt MR (2017) The membrane transport system of the guard cell and its integration for stomatal dynamics. Plant Physiol 174: 487–519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonard RT, Hotchkiss CW (1976) Cation-stimulated adenosine triphosphatase activity and cation transport in corn roots. Plant Physiol 58: 331–335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maathuis FJM, Sanders D (1994) Mechanism of high-affinity potassium uptake in roots of Arabidopsis thaliana. Proc Natl Acad Sci USA 91: 9272–9276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negi J, Matsuda O, Nagasawa T, Oba Y, Takahashi H, Kawai-Yamada M, Uchimiya H, Hashimoto M, Iba K (2008) CO2 regulator SLAC1 and its homologues are essential for anion homeostasis in plant cells. Nature 452: 483–486 [DOI] [PubMed] [Google Scholar]
- Vahisalu T, Kollist H, Wang YF, Nishimura N, Chan WY, Valerio G, Lamminmäki A, Brosché M, Moldau H, Desikan R, et al. (2008) SLAC1 is required for plant guard cell S-type anion channel function in stomatal signalling. Nature 452: 487–491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Guo MY, Thibaud JB, Véry AA, Sentenac H (2019) A repertoire of cationic and anionic conductances at the plasma membrane of Medicago truncatula root hairs. Plant J 98: 418–433 [DOI] [PubMed] [Google Scholar]
- Wang Y, Hills A, Vialet-Chabrand S, Papanatsiou M, Griffiths H, Rogers S, Lawson T, Lew VL, Blatt MR (2017) Unexpected connections between humidity and ion transport discovered using a model to bridge guard cell-to-leaf scales. Plant Cell 29: 2921–2939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Papanatsiou M, Eisenach C, Karnik R, Williams M, Hills A, Lew VL, Blatt MR (2012) Systems dynamic modeling of a guard cell Cl− channel mutant uncovers an emergent homeostatic network regulating stomatal transpiration. Plant Physiol 160: 1956–1967 [DOI] [PMC free article] [PubMed] [Google Scholar]