Two plastid fatty acid exporters from Arabidopsis, FAX2 and FAX4, mediate FA export from plastids in seed embryos and thus regulate triacylglycerol accumulation in seeds.
Abstract
Triacylglycerols (TAGs) are the major storage form of seed oil in oilseed plants. They are biosynthesized de novo in seed plastids and then transported into the endoplasmic reticulum. However, the transport mechanism for plastid fatty acids in developing seeds remains unknown. Here, we isolated two novel plastid fatty acid exporters (FATTYACID EXPORT 2 [FAX2] and FAX4, respectively) specifically abundant in seed embryos during the seed-filling stage in Arabidopsis (Arabidopsis thaliana). FAX2 and FAX4 were both localized to the chloroplast membrane. FAX2 and FAX4 loss-of-function mutations caused deficiencies in embryo and cotyledon development. Seeds of fax2fax4 double mutants exhibited significantly reduced TAG contents but elevated levels of plastid lipid contents compared with those of wild-type plants. By contrast, overexpression of FAX2 or FAX4 enhanced TAG deposition. Seed-feeding experiments showed that the two FAX proteins transported 14C-plastid fatty acids and 13C-oleic acids for TAG biosynthesis during the seed-filling stage. Together, our data demonstrate that FAX2 and FAX4 play critical roles in transporting plastid fatty acids for TAG biosynthesis during seed embryo development. These two transporters may have broad application for increasing oil yield in oilseed crops.
Plant oils are necessary for providing unsaturated fatty acids (FAs) in the diet to promote human health. Triacylglycerols (TAGs) are the majority form of storage lipids in seeds of oilseed plants, including Arabidopsis (Arabidopsis thaliana), Brassica napus, and Canarium album (Li-Beisson et al., 2013). In these oilseed plants, TAGs are synthesized mainly in the embryo and contribute up to 50% of the seed dry weight. Therefore, the characterization of TAG biosynthesis in the seeds of oil plants is vital for increasing seed oil yield.
In plants, TAG biosynthesis requires the transport of plastid-synthesized FAs from the plastids to the endoplasmic reticulum (ER). In Arabidopsis, ∼62% of plastid free FAs are exported to the cytosol through the eukaryotic pathway (Browse et al., 1986; Somerville and Browse, 1991). It is well demonstrated that in Arabidopsis leaves, free FAs are exported by FATTY ACID EXPORT1 (FAX1), which is localized at the plastid inner envelope. The exported toxic free FAs are used for vectorial acylation driven by the plastid outer envelope-localized long-chain acyl-CoA synthetase 9 (LACS9). Then, the esterified acyl-CoAs are shuttled from the plastid/cytosol to the plastid envelope-ER contact site by acyl-CoA binding proteins (Leung et al., 2006; Benning, 2009; Xiao et al., 2009; Meng et al., 2014; Xie et al., 2015). The acyl-CoAs are further imported into the ER by ATP-binding cassette transporter 9 (ABCA9), which is localized at the ER membrane of seed cells (Kim et al., 2009, 2013).The ER-localized acyl-CoAs are further incorporated into TAG biosynthesis (Li-Beisson et al., 2013). Overexpression of ABCA9 enhanced TAG contents in seeds (Kim et al., 2009, 2013).Therefore, regulation of the transcription levels of membrane protein genes involved in TAG biosynthesis should be one option to increase seed storage lipid content in oilseed plants.
TAGs are usually synthesized in three organs, the seeds, leaves, and flower buds. To identify the transporters that export free FAs from plastids for TAG synthesis, we focused on FAX proteins in Arabidopsis. Previous studies showed that overexpression of AtFAX1 increased ER-derived TAGs in flowers and leaves but not in seeds (Li et al., 2015). In Arabidopsis, at least seven genes are predicted to encode FAX transporters, implying that other FAX members may mediate TAG synthesis in seeds. Among these FAX members, FAX2 and FAX4 are predicted to localize at plastids, and their transcripts are abundant in seeds (Li et al., 2015, 2016). We speculated that these FAX proteins may mediate plastid FA export specifically in seeds during the seed oil biosynthesis stage. Here, we characterized the function of FAX2 and FAX4 in seed TAG biosynthesis. Our current study provides an important genetic engineering strategy to improve seed lipid yield for oil plants.
RESULTS
fax2 and fax4 Mutants Exhibit Abnormal Seed Size Phenotypes
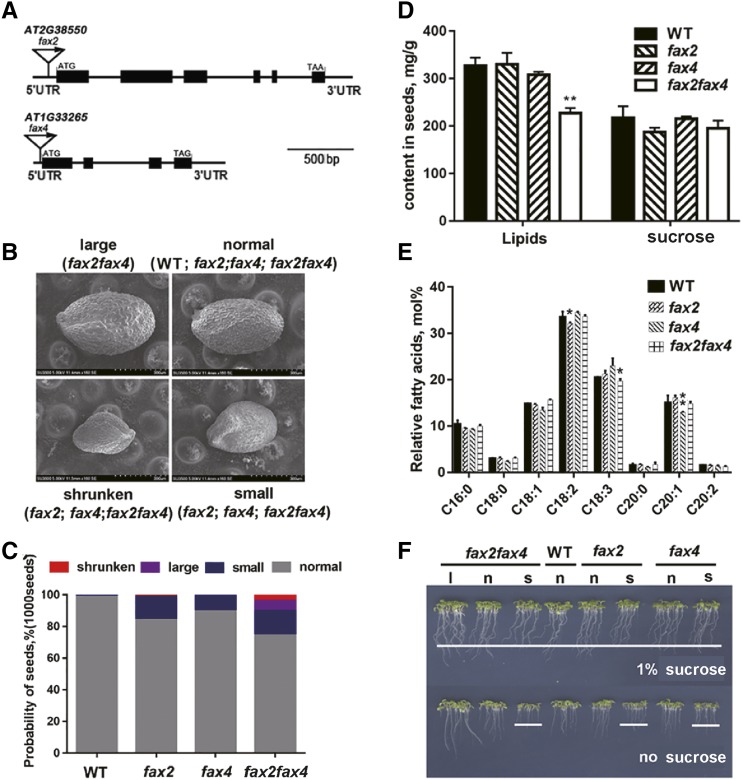
To identify the potential FAX transporters involved in free FA export from plastids during seed development, we characterized the expression levels of these FAXs, which are predicted to encode proteins with chloroplast-targeting peptides, in developing seeds of Arabidopsis. The phylogenetic tree reveals that these FAX proteins are classified into two major clades, and that AtFAX1, AtFAX2, and AtFAX4 belong to the same group (Supplemental Fig. S1, A and D). AtFAX2 (At2g38550) and AtFAX4 (At1g33265) are highly expressed during the early stage of seed development (Supplemental Fig. S1B). We grew transfer DNA insertion mutants of AtFAX2 (fax2), AtFAX4 (fax4), and double mutant fax2fax4 under normal conditions (Supplemental Fig. S1, A and C). Many dry seeds of the mutants were abnormal compared with those of wild-type plants (ecotype Columbia of Arabidopsis [Col-0]; Fig. 1B; Supplemental Fig. S2A). The seeds were classified into four groups: normal, large, shrunken, and small, according to the area of seeds (Fig. 1B; Supplemental Fig. S2B). Only 74% of fax2fax4, 83% of fax2, and 89% of fax4 seeds were normal compared with wild-type seeds (Fig. 1C; Supplemental Fig. S2B). Furthermore, fax2fax4 seeds were more varied in size than wild-type seeds when measured using ImageJ (Supplemental Fig. S2B). The complementation lines of FAX2 and FAX4 (AtFAX2-comp and AtFAX4-comp, respectively) showed the same size seeds as in wild-type plants (Supplemental Fig. S3, A and B).
Figure 1.
Characterization of fax2 and fax4 mutants. A, Schematic representation of the FAX2 and FAX4 genes. Two transfer DNA insertion sites in the 5′ untranslated (UTR) regions are indicated by triangles. The mutants are SALK_135883 (fax2) and SAIL_671_D03(fax4). B, The four seed classification groups of fax2fax4 mutants according to seed area. Large seeds are shown only in the fax2fax4 mutant. Shrunken and small seeds are found in the fax2, fax4, and fax2fax4 mutants. WT, wild type. C, Probability of the four classified seed groups in 1,000 seeds of each mutant and the wild type. D, Total lipid and sucrose weight per seed in fax2, fax4, and fax2fax4 mutants and the wild type (mean ± sd of n = 3–5 seeds). E, FA composition of TAG in mature seeds of fax2, fax4, fax2fax4, and the wild type (mean ± sd of n = 3–5 seeds). *P <0.05 and **P < 0.01, Student’s t test. F, Abnormal shrunken and small seeds (s) in each mutant line are delayed in seedling growth on one-half strength Murashige and Skoog medium with and without Suc. l, Large seeds; n, normal seeds.
To characterize the potential roles of FAX2 and FAX4, we detected the contents of total lipids and Suc in seeds. The lipid contents in the fax2fax4 mutant plants were significantly reduced relative to those in the fax2 and fax4 mutant and wild-type plants, but the Suc content did not differ (Fig. 1D). The seed FA composition of TAG was comparable in the mutants and wild type (Fig. 1E), which indicates that neither fax2 nor fax4 affects the relative probability of FA composition in TAG of seeds. To demonstrate that the genetic seed lipid phenotype of the mutant was caused by FAX2 or FAX4, we also detected the seed TAG and FA composition in the different complementation lines of FAX2 or FAX4. All transgenic lines produced normal seeds and showed similar or even increased TAG content compared with the wild type (Supplemental Fig. S3, C and D). In oilseed plants, early seedling growth usually depends on storage lipids in the absence of Suc (Cernac et al., 2006; Kim et al., 2013). When different abnormal and normal seeds from the mutant and wild-type plants were grown on one-half strength Murashige and Skoog medium with or without Suc, we observed that the growth of small and shrunken seeds in the mutants was defective in the absence of Suc but normal in the medium with Suc (Fig. 1F). These results indicate that the shrunken and small seeds in fax2, fax4, and fax2fax4 mutants might be defective in the accumulation or mobilization of storage lipids.
FAX2 and FAX4 Are Localized at the Chloroplast Membrane
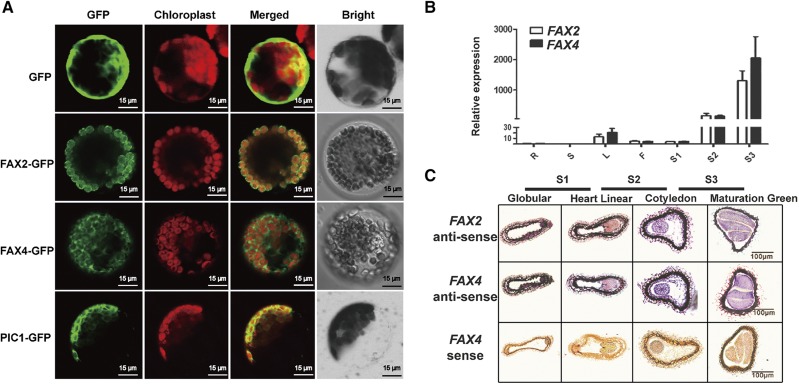
To determine whether FAX2 and FAX4 are involved in lipid biosynthesis, we first detected their subcellular localization using transgenic plants expressing FAX2-GFP or FAX4-GFP under the CaMV 35S promoter (Pro-35S::FAX2-GFP and Pro-35S::FAX4-GFP, respectively). Permease in Chloroplasts1 (PIC1), which is a chloroplast inner envelope protein, was used as a positive control (Duy et al., 2007).Green fluorescence signals were detected around the chloroplast in the mesophyll protoplasts isolated (Fig. 2A), which was consistent with the prediction by ARAMEMNON (http://aramemnon.uni-koeln.de/) that the FAX2 and FAX4 protein sequences contain chloroplast transit peptides at the N terminus. The results showed that both FAX2 and FAX4 are localized at the chloroplast membrane, similar to PIC1 and FAX1 (Li et al., 2015).
Figure 2.
Subcellular localization of FAX2 and FAX4 and their transcript levels in different tissues of Arabidopsis. A, GFP signals and chlorophyll fluorescence signal from the protoplast of the Pro-35s::FAX2 and Pro-35s::FAX4 gDNA-GFP transgenic Arabidopsis. PIC1-GFP was used as a chloroplast inner envelope marker. B, Relative expression of FAX2 and FAX4 transcripts in tissues of Arabidopsis roots (R), stems (S), leaves (L), flowers (F), siliques from 4–6 DAF (S1), siliques from 10–12 DAF (S2), and siliques from 16–18 DAF (S3). Student’s t test (mean ± sd of n = 3–4 tissue samples). C, RNA in situ hybridization of FAX2 and FAX4 in siliques at different seed development stages. The siliques of Col-0 were cross-sectioned for hybridization with antisense and sense probes of FAX2 and FAX4.
To test the possibility that FAX2 and FAX4 are physical partners at the chloroplast envelope in the seeds, we characterized the interaction between FAX2 and FAX4 using a mating-based split ubiquitin system and bimolecular fluorescence complementation. In the mating-based split ubiquitin system analysis, the coding sequences (CDSs) of both proteins and the sequences without chloroplast transit peptides were inserted into PBT3-N and PPR3-N vectors, respectively. No signal was detected in the synthetic dropout medium without Trp/Leu/Ade/His when these constructs containing FAX2 and FAX4 were incubated together (Supplemental Fig. S4A). Similarly, no interaction signal was observed in the protoplast when these two proteins were cotransformed in the bimolecular fluorescence complementation assay (Supplemental Fig. S4B). These results reveal that FAX2 and FAX4 do not physically interact, although both are localized at the chloroplast envelope.
FAX2 and FAX4 Are Expressed Specifically in the Embryo of Seeds
We determined the spatial and temporal expression patterns of FAX2 and FAX4 in different tissues of Arabidopsis by reverse-transcription quantitative PCR (RT-qPCR). FAX2 and FAX4 transcripts accumulated slightly in the root, stem, leaf, flower, and silique from 4 to 6 d after flowering (DAF), whereas high expression levels were detected in the siliques at 10–12 DAF, and even higher levels in the older siliques, such as at 16–18 DAF (Fig. 2B). RNA in situ hybridization further showed that FAX2 and FAX4 were preferentially expressed in the embryos of developing seeds (Fig. 2C). RT-qPCR analysis also revealed that amplification of the transcripts of both FAX2 and FAX4 was significantly higher in the embryo than at the endosperm (Supplemental Fig. S5). These findings indicate that FAX2 and FAX4 may be involved in seed development, especially storage lipid biosynthesis.
FAX2 and FAX4 Are Required for Storage Lipid Biosynthesis During Seed-Filling Stages
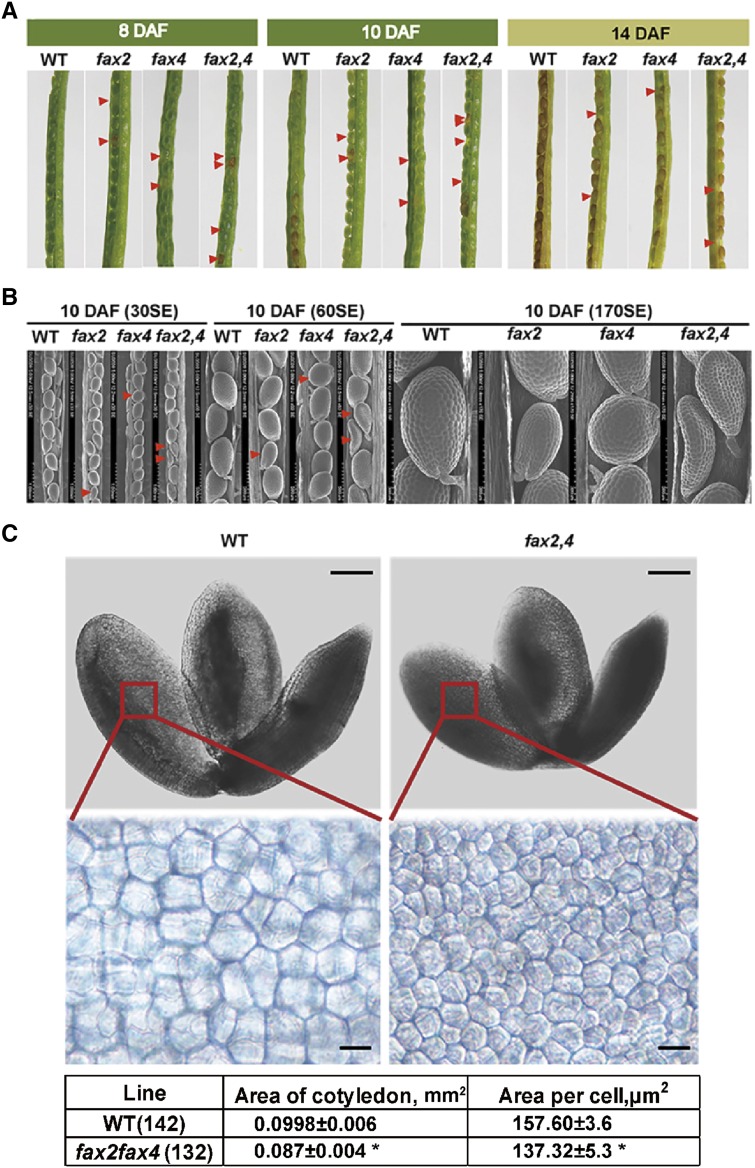
Given that both FAX2 and FAX4 transcripts mainly accumulate during the seed development process, we investigated the seed morphology of fax2, fax4, fax2fax4, and the wild type at 8, 10, and 14 DAF. Approximately 13% of fax2, 13% of fax4, and 25% of fax2fax4 seeds were shrunken and/or smaller at the early stage of seed development (Fig. 3A). We further detected the seed morphology at 10 DAF using scanning electron microscopy (SEM) and found that defective seeds in the fax2fax4 double mutant were more shrunken and smaller than those in the single mutants (Fig. 3B). The area of the cotyledon and the area of cotyledon cells of the fax2fax4 mutant were smaller than those of the wild type (Fig. 3C). These results coincided with the spatial and temporal expression of FAX2 and FAX4, supporting our hypothesis that the abnormal seed development in these mutants is caused by knock-out of FAX2 and/or FAX4.
Figure 3.
Detection of the abnormal seeds of fax2 and fax4mutants. A, Seeds of fax2, fax4, and fax2fax4 mutants and wild-type (WT) plants at different seed development stages, including 8, 10, and 14 DAF. Red arrowheads indicate defective seeds. B, Detection of abnormal seeds of the mutants and wild type at 10 DAF using SEM. Secondary electron size (SE). C, The fax2fax4 double mutant produces abnormal seeds. Shown are mature embryos isolated from mature dry seeds of the wild type and the fax2fax4 knock-out line after imbibition for 1 h (top) and cotyledon cells from the boxed areas in A (bottom). Scale bars = 100 μm (top) and 10 μm (bottom).The table lists the surface areas of cotyledons and of individual cells of cotyledons (132 from fax2fax4 and 142 from the wild type) measured from images of the embryos. *P < 0.05, Student’s t test.
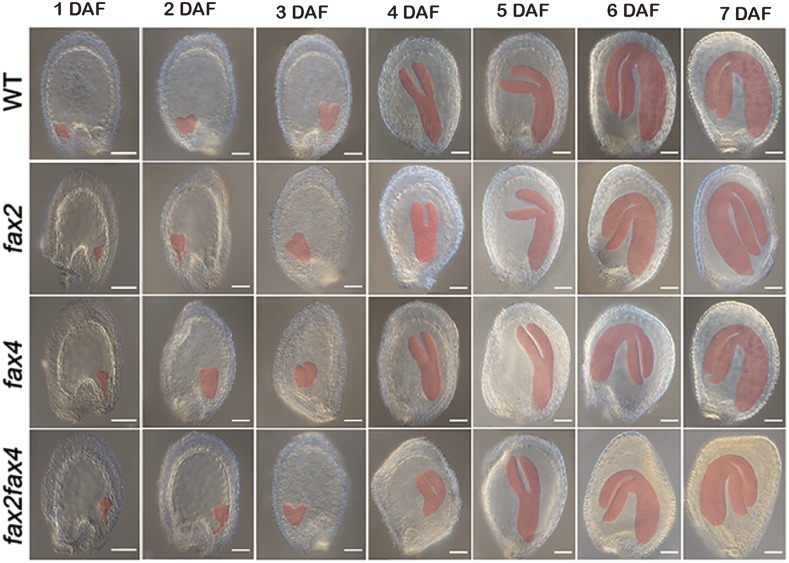
In seed plants, seed formation is an important process involving the coordinated growth of the embryo, endosperm, and seed coat, and embryo maturation (6–20 DAF) determines the size and storage components of seeds (Baud et al., 2003, 2008). We examined the development of the embryo and endosperm in fax2, fax4, fax2fax4, and wild-type plants. The embryo development of the mutants was distinctly delayed compared with that of the wild type. At 3 DAF, the majority of wild-type embryos reached the early heart stage, whereas the mutants reached the globular stage (Fig. 4). At 4 DAF, the embryos and cotyledons of the fax2fax4 mutant were significantly smaller and more shrunken than those of the wild type. Similar phenotypes were also observed in the fax2 and fax4 single mutants. This phenotypic alteration in the mutants was maintained at 6–7 DAF (Fig. 4). These results showed that FAX2 and FAX4 are required for the proper development of the embryo and cotyledon, where the majority of storage lipids are synthesized, indicating that FAX2 and FAX4 may affect storage lipid biosynthesis during the seed-filling stage.
Figure 4.
Seed embryo development is delayed to different degrees in fax2, fax4, and fax2fax4 mutants. The figure shows whole-mount seeds observed with differential interference contrast optics at different developmental stages of embryogenesis in the wild type (WT) and fax2, fax4, and fax2fax4 mutants. Scale bars = 50 μm.
fax2fax4 Mutant Seeds Have Reduced TAG Content
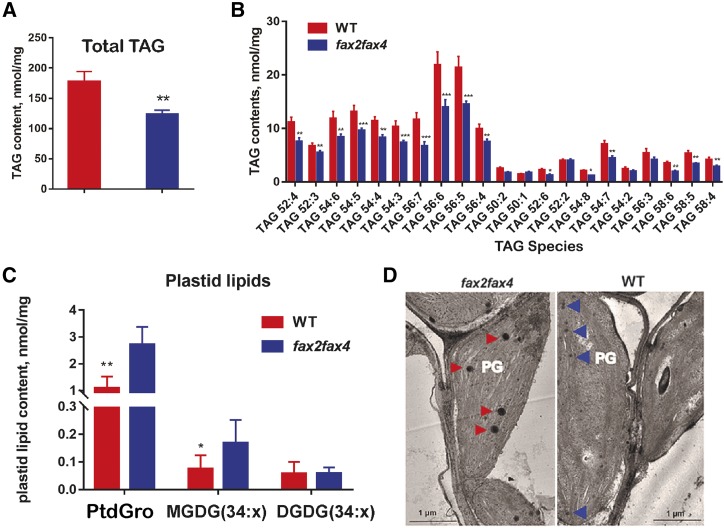
We further determined whether the fax2fax4 seeds had decreased content of TAGs, which comprise the majority of lipids in seeds, compared with the wild type. The total TAG content in dry seeds of fax2fax4 mutants was ∼30% less than in the wild type (Fig. 5A). Most of the TAG biosynthesis in the fax2fax4 seeds was reduced compared to that in the wild type. Fold changes were highest for low-abundance TAGs (e.g. 1.6- and 1.8-fold reduction for TAG54:7 and TAG58:6, respectively, in the fax2fax4 seeds [Fig. 5B]). A massive reduction in highly abundant TAGs was also observed in fax2fax4 seeds (Fig. 5B). More importantly, the largest reduction, which had the most impact on the total TAG amount, was in the highly abundant species, such as TAG56:5 and TAG56:6, in the fax2fax4 seeds (Fig. 5B). However, when the relative TAG molecule content (mol %) of these highly regulated TAG species was calculated, almost no difference was detected between the mutant and the wild type (data not shown), indicating that the relative abundance of TAG molecular species was not affected by FAX2 and FAX4.
Figure 5.
TAG contents in fax2fax4 and the wild type (WT). A and B, Total TAG contents (A) and TAG species contents (B) in the fax2fax4 mutant and the wild type. *P < 0.05, **P < 0.01, and ***P < 0.001, Student’s t test (mean ± sd of n = 4–6 samples). C, The plastid-localized lipid contents in the fax2fax4 mutant and the wild type. Ptd-Gro, Phosphatidyl-glycerol. *P < 0.05 and **P < 0.01, Student’s t test (mean ± sd of n = 4–6 samples). D, PG lipid droplets (red arrowheads) were detected in the fax2fax4 mutant and the wild type by transmission electron microscopy.
Glycolipid biosynthesis consists of two pathways: (1) the prokaryotic pathway, occurring in the plastid stroma; and (2) the eukaryotic pathway, where plastid FAs export to the cytosol and synthesized phosphatidylcholine and phosphatidic acid, and are further imported into the plastid (Li-Beisson et al., 2013; Li et al., 2016). The knockout of AtFAX1 prevented the eukaryotic pathway, while it accelerated the prokaryotic pathway, which increased plastid-derived glycolipid accumulation (Li et al., 2015). In this study, we hypothesized that the plastid-derived glycolipid contents in 10–14 DAF seeds should be increased in the fax2fax4 mutant line. We determined the plastid-derived 34:x glycolipid contents, including monogalactosyldiacylglycerol (MGDG), digalactosyldiacylglycerol (DGDG), and phosphatidylglycerol, and found that more plastid-derived lipids had accumulated in the fax2fax4 seeds compared to the wild type (Fig. 5C). We further investigated the cytological difference in developing seeds between fax2fax4 mutants and wild-type plants using transmission electron microscopy. The fax2fax4 chloroplasts clearly accumulated larger plastoglobule (PG) lipid droplets than did the wild-type chloroplasts (Fig. 5D). Overall, the reduction in TAG contents in the fax2fax4 seeds supports the idea that FAX2 and FAX4 are involved in chloroplast-localized FA transport for TAG biosynthesis in the embryos of developing seeds of Arabidopsis.
FAX2 and FAX4 Mediate Chloroplast-Localized FA Transport for TAG Biosynthesis
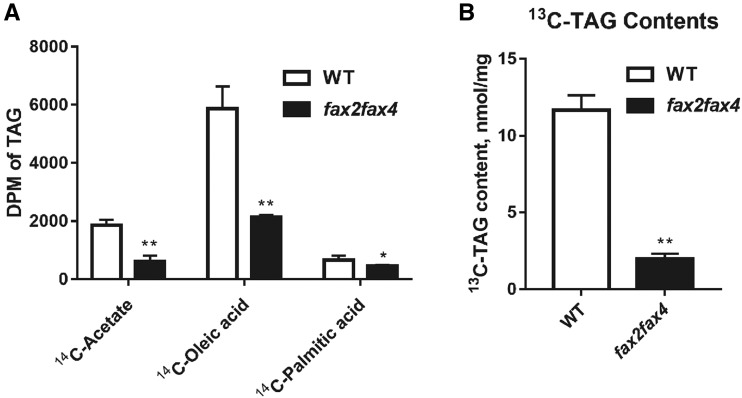
The plastid de novo synthesized FAs are exported mainly as palmitic acid (C16:0), oleic acid (C18:1), and stearic acid (C18:0), which were derived from acetate. Among these FAs, C18:1 is the major free FA form (Browse et al., 1986). Palmitic acid is the chloroplast-localized secondary high-abundance free FA (Browse et al., 1986). To assess the transport specificity of FAX2 and FAX4 for FAs, which are exported from chloroplasts for TAG biosynthesis in vivo, we separately fed 14C-acetate, 14C-oleic acid, and14C-palmitic acid (C16:0) to 10-DAF developing seeds isolated from fax2, fax4, fax2fax4, and wild-type siliques and detected the radioactivity of the isolated TAG fraction. Acetate can be converted into acetyl-CoA and malonyl-CoA for further FA biosynthesis in plastids (Kim et al., 2013; Li-Beisson et al., 2013). With feeding of 14C-acetate, we found that the 14C-labeled TAG content was significantly reduced in the fax2fax4 seeds compared to the wild type (Fig. 6A; Supplemental Fig. S6A). After feeding with 14C-oleic acid, the amount of radioactivity incorporated into TAG in the fax2fax4 seeds was ∼40% of that in wild-type seeds (Fig. 6A). The amount of labeled TAGs in the fax2 and fax4 seeds was also lower than in the wild type (Supplemental Fig. S6B). With the feeding of 14C-palmitic acid, the amount of radioactivity incorporated into TAGs in fax2fax4 mutants was weakly decreased compared with that in wild-type seeds (Fig. 6A).
Figure 6.
Reduced TAG biosynthesis in developing seeds of the fax2fax4 mutant. A, Incorporation of radioactivity from 14C-acetate (50 mCi/mmol), 14C-oleic acid (50 mCi/mmol), and 14C-palmitic acid (50 mCi/mmol) into TAG was detected using developing seeds at 10 DAF. DPM, Disintegrations per minute. B, Total TAG content of 10-DAF developing seeds fed with 13C-oleic acid for 18 h. At least three replicates were averaged, and the sd is shown. *P < 0.05 and **P < 0.01, Student’s t test (mean ± sd of n = 3–5).
To further determine the difference in TAG content between fax2, fax4, fax2fax4, and wild-type seeds, we fed 13C-oleic acid to 10-DAF developing seeds from fax2, fax4, fax2fax4, and wild-type siliques. The total TAGs and amounts of TAG species were detected by a reverse-phase HPLC electrospray ionization tandem mass spectrometry (MS/MS) approach described previously (Lam et al., 2014; Jiang et al., 2017; Li et al., 2019). The 13C-labeled TAG content was significantly lower in seeds of fax2, fax4, and fax2fax4 mutants than in wild-type seeds (Fig. 6B; Supplemental Fig. S6C). These results indicated that both FAX2 and FAX4 are chloroplast FAXs that specifically mediate free FA efflux for acyl-CoA and further supply FA substrates for TAG biosynthesis in the embryos of developing seeds in Arabidopsis.
Overexpression of FAX2and FAX4 Elevates Seed Oil Content
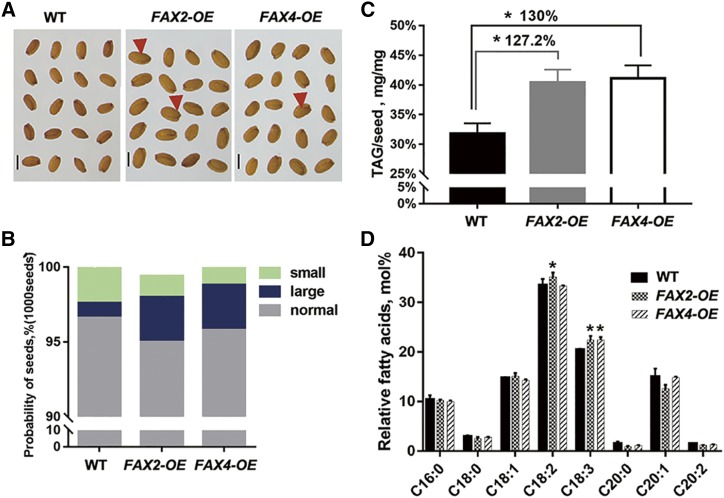
To investigate whether FAX proteins mediate free FA transport from plastids and improve lipid synthesis during seed development, we generated transgenic Arabidopsis plants overexpressing FAX2 and FAX4, respectively (Fig. 7). The FAX2- or FAX4-overexpressing plants produced seeds that were enlarged compared to wild-type seeds (Fig. 7, A and B; Supplemental Fig. S7). Biochemical analyses revealed that the lipid content per seed, especially C18:2 and C18:3 unsaturated FA compositions, in the FAX2- or FAX4-overexpressing lines was up to ∼127% to 148% of that in the wild type (Fig. 7, C and D). Therefore, we conclude that overexpression of FAX2 and FAX4 is capable of increasing the seed lipid yield in oilseed plants.
Figure 7.
FAX2- and FAX4-overexpressing plants show enlarged seeds with elevated lipid content. A, Enlarged seeds of transgenic plants overexpressing FAX2 or FAX4 (FAX2-OE and FAX4-OE, respectively) as compared to those of the wild type (WT). Scale bar = 200 μm. B, Probability of the four classified seed groups in 1,000 seeds of the two overexpressing plants and the wild type. C, TAG content in seeds of overexpressing plants. D, FA composition of overexpressing and wild-type seeds. *P < 0.05, Student’s t test (mean ± sd of n = 6 seeds).
DISCUSSION
TAG biosynthesis in seeds is critical to improving the quality and quantity of storage lipids in oilseed plants. FA synthesis provides substrates for TAG biosynthesis during seed filling. However, in contrast to other eukaryotes, de novo FA synthesis in plants occurs in the plastid (Li-Beisson et al., 2013). In previous studies, genetic engineering strategies have been reported to increase seed oil content by overexpressing transporter, enzyme, and transcription factor genes specifically involved in TAG biosynthesis (Roesler et al., 1997; Thelen and Ohlrogge, 2002; Masaki et al., 2005; Baud et al., 2007; Maeo et al., 2009; Baud et al., 2010). Although FAX1 has been demonstrated to mediate plastid FA export for vegetative TAG biosynthesis in flowers and leaves of Arabidopsis, the mechanism of plastid FA export for oil accumulation in seeds remains unknown. Here, we showed that two plastid envelope-localized proteins, FAX2 and FAX4, are required for FA transport from plastids to the ER for TAG biosynthesis during seed filling.
We propose that FAX2 and FAX4 function as two separate FA transporters specifically for TAG biosynthesis in the embryo of seed development, mainly based on the following evidence: (1) both FAX2 and FAX4 are localized at the plastid envelope (Fig. 2A), as we predicted; (2) transcripts of both FAX2 and FAX4 are specifically expressed in the seed embryos during the seed-filling stage (Fig. 2, B and C), during which TAGs are rapidly synthesized; (3) the abnormal phenotypes of seed embryos of fax2, fax4, and fax2fax4 are correlated with the defects in FAX2 and/or FAX4 (Figs. 3 and 4); (4) TAG contents are reduced by 30% in fax2fax4 double mutant seeds compared with wild-type seeds (Fig. 5); (5) the plastid-localized lipid droplets in fax2fax4 double mutants are distinctly increased relative to the number in the wild type (Fig. 5, C and D); and (6) TAG synthesis is reduced in fax2, fax4, and fax2fax4 seeds, as demonstrated by feeding experiments in growing seeds with radioactively labeled 14C-acetate, 14C-oleic acid, radioactively labeled 14C-palmitic acid, or stable-isotope-labeled 13C-oleic acid (Fig. 6, A and B; Supplemental Fig. S6). Oleic and palmitic acid, which are known as the most abundant FAs, were synthesized in plastids and then exported to the cytosol and further to the ER for TAG synthesis. Therefore, palmitic and oleic acid were used in 14C-labeling experiments. Although 14C-linoleic acid was also used in the radioactive labeling experiment, no difference was detected between mutant and wild-type seeds (Supplemental Fig. S6). We hypothesized that plastid-derived linoleic acid would account for very low abundance and in the experiment, we detected a very weak radioactive signal derived from 14C-linoleic acid. 13C labeling is not a very sensitive method comparing with 14C labeling, and therefore, only oleic acid was used in the 13C labeling experiment. In a previous study, it was demonstrated that FAX1 is involved in TAG biosynthesis in vegetative tissues of Arabidopsis, but plants generally produce TAGs in developing embryos (Penfield et al., 2006). Our findings support a role for FAX2 and FAX4 in seed TAG biosynthesis. We hypothesized a working model for FAX2 and FAX4 during seed embryo TAG biosynthesis. When both FAX2 and FAX4 were knocked out, plastid FA export of the fax2fax4 seed embryo was affected, resulting in inefficient TAG biosynthesis.
Although we demonstrated that FAX2 and FAX4 mediate FA export for TAG accumulation in seed embryos, the growth, development, and seed quantity of the mutants are not obviously different from those in the wild type (Fig. 1). However, the TAG content of fax2fax4 seeds was decreased by ∼30% relative to that of wild-type seeds (Fig. 5A). Many TAG molecular species were also significantly decreased in fax2fax4seeds. When content (mol %) of relative TAG molecules was calculated, however, very little difference was detected between the fax2fax4 mutant and the wild type (Supplemental Fig. S8). These findings indicated that the relative abundance of TAG molecular species was stable in both the fax2fax4 mutant and the wild type. Other putative transporters might possess functions similar to those of FAX2 and FAX4 in TAG biosynthesis in seeds. Furthermore, ABCA9 has been described as a FA transporter, as demonstrated by a 14C-acetate, 14C-oleic acid seed-feeding experiment in abca9 mutants (Kim et al., 2013). We hypothesize that putative ABCA9, FAX2, and FAX4 homologs may perform the same functions for FA export at the plastid membrane. The exported FAs are used not only for TAG synthesis but also for synthesis of surface lipids, which constitute 1% of seed storage lipids, and membrane lipids, which account for 5% of seed storage lipids (Ohlrogge and Browse, 1995; Li et al., 2006; Molina et al., 2006; Beisson et al., 2007). In addition, half of the exported FAs are returned for plastid-intrinsic lipid assembly by the eukaryotic pathway (Browse et al., 1986). Therefore, FAX2 and FAX4 contribute to part of plastid FA export for TAG biosynthesis in seed embryos.
Recently, we also identified two homologous genes of Arabidopsis FAX1, CrFAX1 and CrFAX2, in the green alga Chlamydomonas reinhardtii. The overexpression of both CrFAXs increased TAG content by 38% (Li et al., 2019). We found that embryo cells from FAX4 overexpression lines are larger and have higher TAG levels than the corresponding wild-type seeds (Fig. 7). However, FAX4 overexpression did not change the number of seeds per silique. Interestingly, there was no difference in protein and starch contents between FAX4-overexpressing and wild-type seeds. Therefore, our findings suggest that FAX2 and FAX4 are important for their potential to specifically increase seed oil production without reducing storage protein and starch in oilseed crops.
In summary, we conclude that FAX2 and FAX4 are plastid-localized FA exporters for TAG biosynthesis in seed embryos. This discovery provides an important strategy to boost seed oil yield without reducing storage protein and starch content in seeds and without reducing shoot biomass. These two transporters would be very valuable in improving oil production and increasing carbon and energy storage in crop plants.
MATERIALS AND METHODS
Plant Growth Conditions
Arabidopsis (Arabidopsis thaliana) ecotype Columbia-0 (Col-0) was used as the wild type. The mutants used in the current study, including fax2 (SALK_135883) and fax4 (SAIL_671_D03), were obtained from ABRC (Ohio State University). The double mutant fax2fax4 was screened from the F3 generation following artificial pollination between fax2 and fax4. Seeds of wild-type and mutant plants were surface sterilized and plated on one-half strength Murashige and Skoog medium with or without 1% (w/v) Suc. To generate complementation lines and overexpressing lines of FAX2 and FAX4 under the CaMV 35S promoter, the coding sequences of FAX2 and FAX4 were subcloned into the pCambia1301M vector, a binary expression vector (Xu et al., 2019). The constructs were transformed into Agrobacterium tumefaciens GC3101, which were then transfected into fax2 or fax4 mutants (FAX2-comp and FAX4-comp, respectively) and Col-0 (FAX2-OE and FAX4-OE, respectively) plants (Li et al., 2015). The plates were cold-treated at 4°Cin the dark for 2 d and then grown under sterile conditions with a 16 h/8 h day/night cycle at ∼20°C to 22°C for 2 weeks. For further analysis, the plants were transferred to soil and grown in an incubator with a 16 h/8 h day/night cycle at ∼20°C to 22°C.
Phylogenetic Analysis
A phylogenetic tree was constructed using the neighbor-joining method, and a bootstrap test was performed using 1,000 iterations in MEGA 6.0 according to a previous study (Li et al., 2015).
Seed Lipid and Suc Analysis
For FA composition and oil content measurement, total lipids were extracted using a method described previously (Kim et al., 2013; Lu et al., 2018, 2019). Dried seeds (3–5 mg/sample) were added to methanol with 5%(v/v) H2SO4 and 0.01% (v/v) butylated hydroxyl toluene and incubated for 90 min in an 85°C water bath for transmethylation. FA methyl esters (FAMEs) were extracted with hexane. FAMEs were quantified using gas chromatography supplied with a hydrogen flame ionization detector and a SUPELCOWAX capillary column with a helium carrier at 20 mL/min. The oven temperature was maintained at 170°C for 1 min and then increased in steps to 210°C, raising the temperature by 3°C/min. FAMEs from TAGs were identified by comparing their retention times with known standards. Heptadecanoic acid (C17:0) was used as the internal standard to quantify the amounts of individual FAs. FA composition was calculated as mole percent. Total Suc quantification was performed according to previous methods (Kim et al., 2013).
Subcellular Localization Analysis
Primers containing enzyme cleavage sites were designed to amplify the coding sequences (CDSs) of candidate genes (Supplemental Table S1). Then, the CDSs of FAX2, FAX4, and PIC1 were inserted into the pAN580 vector, in which CaMV35S was the promoter and GFP was the reporter gene. We examined the subcellular location of genes by PEG/Ca2+-mediated protoplast transformation. We cultured Arabidopsis seedlings in nutrient soil. When the seedlings had grown for 20 d, young leaves were selected for experimentation. Cellulase R10 and Macerozyme R10 were applied to separate the mesophyll cell protoplast in the dark at 23°C, and 10–20 μg of plasmids, protoplast, and PEG/Ca2+ solution were cocultured for transformation. The protoplast after transformation was incubated overnight. Then, the green fluorescence signal and chlorophyll fluorescence were observed by laser scanning confocal microscopy (Leica, SP8), and the chloroplast in each cell was used as the control.
RT-qPCR Analysis
Total RNA was extracted, and complementary DNA (cDNA) was synthesized as described previously (Li et al., 2019). Diluted cDNA (2 μL) was used for RT-qPCR analysis according to the manufacturer’s instructions on an ABI Prism 7900 Sequence Detection system. Gene-specific primers are listed in Supplemental Table S1. The housekeeping gene ACTIN was used as the internal reference. At least three biological replicates were performed for each gene.
RNA In Situ Hybridization
For probe preparation, the cDNAs of FAX2 or FAX4 were amplified for FAX2 and FAX4 detection, respectively. The two probes were labeled according to the DIG RNA Labeling Kit (Roche). Section pretreatment, hybridization, and immunological detection were performed as previously described (Xu et al., 2019).
SEM and Image Processing
Seeds of the wild type and fax2fax4 mutant were collected at three developmental stages: 8, 10, and 14 DAF. The seeds in the same siliques were detected using a stereoscopic microscope (SteREO Discovery V20, Zeiss) and a SEM (SU3500, Hitachi).
Cytological Analysis of Developing Seeds
Green embryos were dissected from wild-type and fax2fax4 seeds and fixed in freshly prepared FAA (10% [v/v] formalin, 5% [v/v] acetic acid, and 45% [v/v] ethanol) at 4°C for 2 h. The embryos were dehydrated in 30%, 50%, 75%, 95%, and 100% (v/v) ethanol for 30 min each at 4°C. After dehydration, methyl salicylate was used for transparency treatment. ImageJ software (https://imagej.nih.gov/ij/) was used to quantify the cotyledon area as well as the average size of epidermal cells in the central region of the cotyledons.
Differential Interference Contrast Analysis of Cotyledon Cells
Seeds of wild-type and fax2fax4 mutant were collected at various developmental stages and fixed in formaldehyde-acetic acid overnight at 4°C. The samples were then stepwise dehydrated with different concentrations of ethanol, and methyl salicylate was used to render them transparent. The development of cotyledon cells in the decolored seeds was observed by differential interference contrast optics of the Olympus BX53.
13C Labeling and Measurement of Plastid Lipids and TAG Contents in Seeds
When the siliques of Arabidopsis plants had grown for 12–16 d, we peeled the green siliques under a microscope, and all of the intact seeds from five siliques were collected as the sample. The weights of all the samples were measured first. 13C-oleic acid (Absin Biotechnology) was dissolved in methyl alcohol at a concentration of 1 mg/mL. The intact seeds were dipped into reagent (500 μL) containing 13C-oleic acid for 18 h. And then samples were collected and rinsed three times with reagent without isotope oleic acid to wash off the isotope on the surface of seeds. Butylated hydroxyl toluene and isopropanol were applied at 75°C for 20 min to deactivate lipid-hydrolyzing enzymes. Chloroform, methyl alcohol, and 300 mm ammonium acetate at a volume ratio of 30:41.5:3.5 were used to extract the single phase containing FA, and 1 m KCl was essential in the experiment. The Termovap sample concentrator was used to dry all the samples before storage at −80°C. Phospholipids and galactolipids were analyzed by a shotgun lipidomics method described by a Triple Quad 4000 liquid chromatography (LC) MS/MS (Welti et al., 2002). TAGs were quantitatively analyzed by a modified version of the reverse-phase HPLC electrospray ionization MS/MS approach, as described previously (Lam et al., 2014; Lu et al., 2018). LC-multiple reaction monitoring (MRM) scans of TAG species were used to determine the species of TAG (Lam et al., 2014; Lu et al., 2018). Briefly, separation of the aforementioned lipid extracts was carried out on a Phenomenex Kinetex 2.6 μ-C18 column using an isocratic mobile phase of chloroform:methanol:0.1 m ammonium acetate (100:100:4). Neutral-loss MS/MS scans of FAs were used to determine the levels of TAGs. TAGs were quantified against the relative amounts of TAG-14:0/14:0/14:0-d5, TAG-15:0/15:0/15:0-d29, and TAG-18:0/18:0/18:0-d5 internal standards (CDN Isotopes). MGDGs and DGDGs were separated using Phenomenex Kinetex 2.6 μ-C18 column (internal diameter 4.6 × 100 mm) using an isocratic mobile phase of chloroform:methanol:2% 50 mm sodium acetate (49:49:2) at a flow rate of 160 μL/min for 25 min, as previously described (Lu et al., 2018). Individual MGDG and DGDG molecular species were quantitated by referencing to MGDG-16:0/18:0 and DGDG-18:0/18:0 internal standards (Matreya). 13C-labeled TAGs were detected by a method described previously (Jiang et al., 2017). Lipid standards were purchased from Avanti Polar Lipids, and the LIPID Metabolites and the Pathways Strategy.
14C-Labeling Assay Monitoring the Incorporation of Precursors into TAG
Fifty Arabidopsis seeds from the wild type and fax2, fax4, and fax2fax4 mutants at 10 DAF were collected and transferred to 200 μL of 20 mm MES buffer (pH 5.8) as previously described (Kim et al., 2013). Then, 0.5 μCi of 14C-acetate (50 mCi/mmol), 0.5 μCi of 14C-oleic acid (50 mCi/mmol), and 0.5 μCi of 14C-palmitic acid (50 mCi/mmol) were added separately to the solution. The labeled seeds were incubated for 18 h in the dark with rotation at 100 rpm. Next, the seeds were washed with ice-cold water three times and homogenized in 50 μL of chloroform:methanol:formic acid (10:10:1 [v:v:v]). The organic and aqueous phases were then divided by adding 12.5 μL of solution containing 1 m KCl and 0.2 m H3PO4. After centrifugation at 16,000g for 5 min, the total lipids in the lower layer were separated and added to a silica thin-layer chromatography plate. The TAGs were separated after a 1–2 h incubation of the thin-layer chromatography plate in hexane:diethylether:acetic acid (80:30:1 [v:v:v]) buffer in a glass incubator. After dyeing with iodine pellets, the TAG signal was shown on the silica plate and then scraped from the plate and mixed with the scintillation mixture. Radioactivity was measured by scintillation counting. At least six replicates were performed for each FA fed.
Statistical Analysis
Significant values were determined using Student’s t test with *P < 0.05 and **P < 0.01. All data were statistically analyzed using SPSS 18.0 and GraphPad Prism 5 with at least three biological replicates.
Data Availability
The authors declare that all data supporting the findings of this study are available within the manuscript and its supplementary files or available from the corresponding author on request.
Accession Numbers
Sequence data from this article can be found in the GenBank/EMBL data libraries under accession numbers: At2g38550 (AtFAX2) and At1g33265 (AtFAX4).
Supplemental Data
The following supplemental information is available.
Supplemental Figure S1. Sequence alignment and transcript levels of FAX1, FAX2, and FAX4 in seeds.
Supplemental Figure S2. Seed size distribution of the four classified seed groups in fax2fax4 mutants.
Supplemental Figure S3. The seeds of FAX2 and FAX4 complementation lines show no difference in seed lipids and seed size compared with the wild type.
Supplemental Figure S4. Physical interaction between AtFAX2 and AtFAX4.
Supplemental Figure S5. Transcript levels of FAX1, FAX2, and FAX4 in seeds and siliques during seed-filling stages.
Supplemental Figure S6. TAG biosynthesis is reduced in developing seeds of fax2, fax4, and fax2fax4 mutants.
Supplemental Figure S7. Seed size distribution of the FAX2 and FAX4 complementation and overexpression lines.
Supplemental Figure S8. Content (mol %) of relative TAG molecules in wild-type and fax2fax4 mutant seeds.
Supplemental Table S1. Primers used in this study.
Acknowledgments
The authors thank Xiaoyan Zhu for performing subcellular localization and Yunfeng Li for microscopy detection.
Footnotes
This work was supported by the National Key R & D Program of China (2018YFD0200903 and 2017YFD0200203-4), the National Natural Science Foundation of China (31870587 and 31800505), and the Fundamental Research Funds for the Central Universities (XDJK2020B065 and XDJK2018AA005).
References
- Baud S, Dubreucq B, Miquel M, Rochat C, Lepiniec L (2008) Storage reserve accumulation in Arabidopsis: Metabolic and developmental control of seed filling. TheArabidopsis Book 6: e0113, doi:10.1199/tab.0113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baud S, Feria Bourrellier AB, Azzopardi M, Berger A, Dechorgnat J, Daniel-Vedele F, Lepiniec L, Miquel M, Rochat C, Hodges M, et al. (2010) PII is induced by WRINKLED1 and fine-tunes fatty acid composition in seeds of Arabidopsis thaliana. Plant J 64: 291–303 [DOI] [PubMed] [Google Scholar]
- Baud S, Guyon V, Kronenberger J, Wuillème S, Miquel M, Caboche M, Lepiniec L, Rochat C (2003) Multifunctional acetyl-CoA carboxylase 1 is essential for very long chain fatty acid elongation and embryo development in Arabidopsis. Plant J 33: 75–86 [DOI] [PubMed] [Google Scholar]
- Baud S, Mendoza MS, To A, Harscoët E, Lepiniec L, Dubreucq B (2007) WRINKLED1 specifies the regulatory action of LEAFY COTYLEDON2 towards fatty acid metabolism during seed maturation in Arabidopsis. Plant J 50: 825–838 [DOI] [PubMed] [Google Scholar]
- Beisson F, Li Y, Bonaventure G, Pollard M, Ohlrogge JB (2007) The acyltransferase GPAT5 is required for the synthesis of suberin in seed coat and root of Arabidopsis. Plant Cell 19: 351–368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benning C. (2009) Mechanisms of lipid transport involved in organelle biogenesis in plant cells. Annu Rev Cell Dev Biol 25: 71–91 [DOI] [PubMed] [Google Scholar]
- Browse J, Warwick N, Somerville CR, Slack CR (1986) Fluxes through the prokaryotic and eukaryotic pathways of lipid synthesis in the “16:3” plant Arabidopsis thaliana. Biochem J 235: 25–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cernac A, Andre C, Hoffmann-Benning S, Benning C (2006) WRI1 is required for seed germination and seedling establishment. Plant Physiol 141: 745–757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duy D, Wanner G, Meda AR, von Wirén N, Soll J, Philippar K (2007) PIC1, an ancient permease in Arabidopsis chloroplasts, mediates iron transport. Plant Cell 19: 986–1006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Y, Wang W, Xie Q, Liu N, Liu L, Wang D, Zhang X, Yang C, Chen X, Tang D, et al. (2017) Plants transfer lipids to sustain colonization by mutualistic mycorrhizal and parasitic fungi. Science 356: 1172–1175 [DOI] [PubMed] [Google Scholar]
- Kim C, Lee KP, Baruah A, Nater M, Göbel C, Feussner I, Apel K (2009) 1O2-mediated retrograde signaling during late embryogenesis predetermines plastid differentiation in seedlings by recruiting abscisic acid. Proc Natl Acad Sci USA 106: 9920–9924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S, Yamaoka Y, Ono H, Kim H, Shim D, Maeshima M, Martinoia E, Cahoon EB, Nishida I, Lee Y (2013) AtABCA9 transporter supplies fatty acids for lipid synthesis to the endoplasmic reticulum. Proc Natl Acad Sci USA 110: 773–778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam SM, Tong L, Duan X, Petznick A, Wenk MR, Shui G (2014) Extensive characterization of human tear fluid collected using different techniques unravels the presence of novel lipid amphiphiles. J Lipid Res 55: 289–298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung KC, Li HY, Xiao S, Tse MH, Chye ML (2006) Arabidopsis ACBP3 is an extracellularly targeted acyl-CoA-binding protein. Planta 223: 871–881 [DOI] [PubMed] [Google Scholar]
- Li-Beisson Y, Shorrosh B, Beisson F, Andersson MX, Arondel V, Bates PD, Baud S, Bird D, Debono A, Durrett TP, et al. (2013) Acyl-lipid metabolism. Arabidopsis Book 11: e0161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li N, Gügel IL, Giavalisco P, Zeisler V, Schreiber L, Soll J, Philippar K (2015) FAX1, a novel membrane protein mediating plastid fatty acid export. PLoS Biol 13: e1002053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li N, Xu C, Li-Beisson Y, Philippar K (2016) Fatty acid and lipid transport in plant cells. Trends Plant Sci 21: 145–158 [DOI] [PubMed] [Google Scholar]
- Li N, Zhang Y, Meng H, Li S, Wang S, Xiao Z, Chang P, Zhang X, Li Q, Guo L, et al. (2019) Characterization of Fatty Acid Exporters involved in fatty acid transport for oil accumulation in the green alga Chlamydomonas reinhardtii. Biotechnol Biofuels 12: 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Beisson F, Pollard M, Ohlrogge J (2006) Oil content of Arabidopsis seeds: The influence of seed anatomy, light and plant-to-plant variation. Phytochemistry 67: 904–915 [DOI] [PubMed] [Google Scholar]
- Lu S, Sturtevant D, Aziz M, Jin C, Li Q, Chapman KD, Guo L (2018) Spatial analysis of lipid metabolites and expressed genes reveals tissue-specific heterogeneity of lipid metabolism in high- and low-oil Brassica napus L. seeds. Plant J 94: 915–932 [DOI] [PubMed] [Google Scholar]
- Maeo K, Tokuda T, Ayame A, Mitsui N, Kawai T, Tsukagoshi H, Ishiguro S, Nakamura K (2009) An AP2-type transcription factor, WRINKLED1, of Arabidopsis thaliana binds to the AW-box sequence conserved among proximal upstream regions of genes involved in fatty acid synthesis. Plant J 60: 476–487 [DOI] [PubMed] [Google Scholar]
- Masaki T, Mitsui N, Tsukagoshi H, Nishii T, Morikami A, Nakamura K (2005) ACTIVATOR of Spomin::LUC1/WRINKLED1 of Arabidopsis thaliana transactivates sugar-inducible promoters. Plant Cell Physiol 46: 547–556 [DOI] [PubMed] [Google Scholar]
- Meng W, Hsiao AS, Gao C, Jiang L, Chye ML (2014) Subcellular localization of rice acyl-CoA-binding proteins (ACBPs) indicates that OsACBP6:GFP is targeted to the peroxisomes. New Phytol 203: 469–482 [DOI] [PubMed] [Google Scholar]
- Molina I, Bonaventure G, Ohlrogge J, Pollard M (2006) The lipid polyester composition of Arabidopsis thaliana and Brassica napus seeds. Phytochemistry 67: 2597–2610 [DOI] [PubMed] [Google Scholar]
- Ohlrogge J, Browse J (1995) Lipid biosynthesis. Plant Cell 7: 957–970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penfield S, Li Y, Gilday AD, Graham S, Graham IA (2006) Arabidopsis ABA INSENSITIVE4 regulates lipid mobilization in the embryo and reveals repression of seed germination by the endosperm. Plant Cell 18: 1887–1899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roesler K, Shintani D, Savage L, Boddupalli S, Ohlrogge J (1997) Targeting of the Arabidopsis homomeric acetyl-coenzyme A carboxylase to plastids of rapeseeds. Plant Physiol 113: 75–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somerville C, Browse J (1991) Plant lipids: Metabolism, mutants, and membranes. Science 252: 80–87 [DOI] [PubMed] [Google Scholar]
- Thelen JJ, Ohlrogge JB (2002) Metabolic engineering of fatty acid biosynthesis in plants. Metab Eng 4: 12–21 [DOI] [PubMed] [Google Scholar]
- Xiao S, Chen QF, Chye ML (2009) Light-regulated Arabidopsis ACBP4 and ACBP5 encode cytosolic acyl-CoA-binding proteins that bind phosphatidylcholine and oleoyl-CoA ester. Plant Physiol Biochem 47: 926–933 [DOI] [PubMed] [Google Scholar]
- Xie LJ, Yu LJ, Chen QF, Wang FZ, Huang L, Xia FN, Zhu TR, Wu JX, Yin J, Liao B, et al. (2015) Arabidopsis acyl-CoA-binding protein ACBP3 participates in plant response to hypoxia by modulating very-long-chain fatty acid metabolism. Plant J 81: 53–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu C, Shen Y, He F, Fu X, Yu H, Lu W, Li Y, Li C, Fan D, Wang HC, et al. (2019) Auxin-mediated Aux/IAA-ARF-HB signaling cascade regulates secondary xylem development in Populus. New Phytol 222: 752–767 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The authors declare that all data supporting the findings of this study are available within the manuscript and its supplementary files or available from the corresponding author on request.