Stuctural aspects of plant hormone sensing mechanisms by Ub ligases add to our current understanding of the emerging field of strigolactone signaling.
Abstract
Hormonal cues regulate many aspects of plant growth and development, facilitating the plant’s ability to systemically respond to environmental changes. Elucidating the molecular mechanisms governing these signaling pathways is crucial to understanding how plants function. Structural and functional biology methods have been essential in decoding plant genetic findings and revealing precise molecular actions at the protein level. Past studies of plant hormone signaling have uncovered mechanisms that involve highly coordinated protein turnover to elicit immediate cellular responses. Many phytohormone signaling pathways rely on the ubiquitin (Ub) proteasome system, specifically E3 Ub ligases, for perception and initiation of signaling transduction. In this review, we highlight structural aspects of plant hormone-sensing mechanisms by Ub ligases and discuss our current understanding of the emerging field of strigolactone signaling.
Historically, the study of plants has greatly advanced human knowledge. Numerous scientific landmarks were first discovered in plants including the laws of genetic heredity, cytogenetics, RNA interference, transposable elements, and the identification of the first virus (Mendel, 1865; Vines, 1880; Beijerinck, 1898; McClintock, 1984; Napoli et al., 1990; van der Krol et al., 1990) Similarly, proteins extracted from plant tissues laid the foundations of protein crystallography. In 1926, James Batcheller Sumner used Jack beans (Canavalia ensiformis) to isolate and crystalize, for the first time, the protein Urease (Sumner, 1926). In addition to revealing the first protein crystals, Sumner’s work also provided the very first evidence that enzymes are proteins. In the era of x-ray crystallography and high-resolution single particle electron microscopy, determining three-dimensional protein structures in atomic detail has become the ultimate tool to decrypt the molecular mechanism of biological pathways. Despite the advances made in structural biology methodologies, the percentages of plant protein structures deposited in Protein Data Bank (PDB; Berman et al., 2000) have remained remarkably low compared with other kingdoms of life . Nevertheless, the past decade has witnessed groundbreaking structure-function studies in plants. These studies have had a significant impact on our understanding of fundamental biological processes, including photomorphogenesis, immune responses, and, in particular, phytohormone signaling pathways.
Plants maintain the ability to respond to environmental changes by altering growth patterns and varying developmental outcomes. This plasticity is attributed to the ability of plants to translate the environmental input into a systemic signal using a diverse range of molecular instruments, orchestrated by phytohormones. Many important genetic studies have identified the distinct key players of hormonal signaling cascades. The recent integration of structural biology with plant genetics has uncovered molecular mechanisms through which a small molecule can facilitate protein-protein interactions and trigger the transduction of a signal into a developmental outcome.
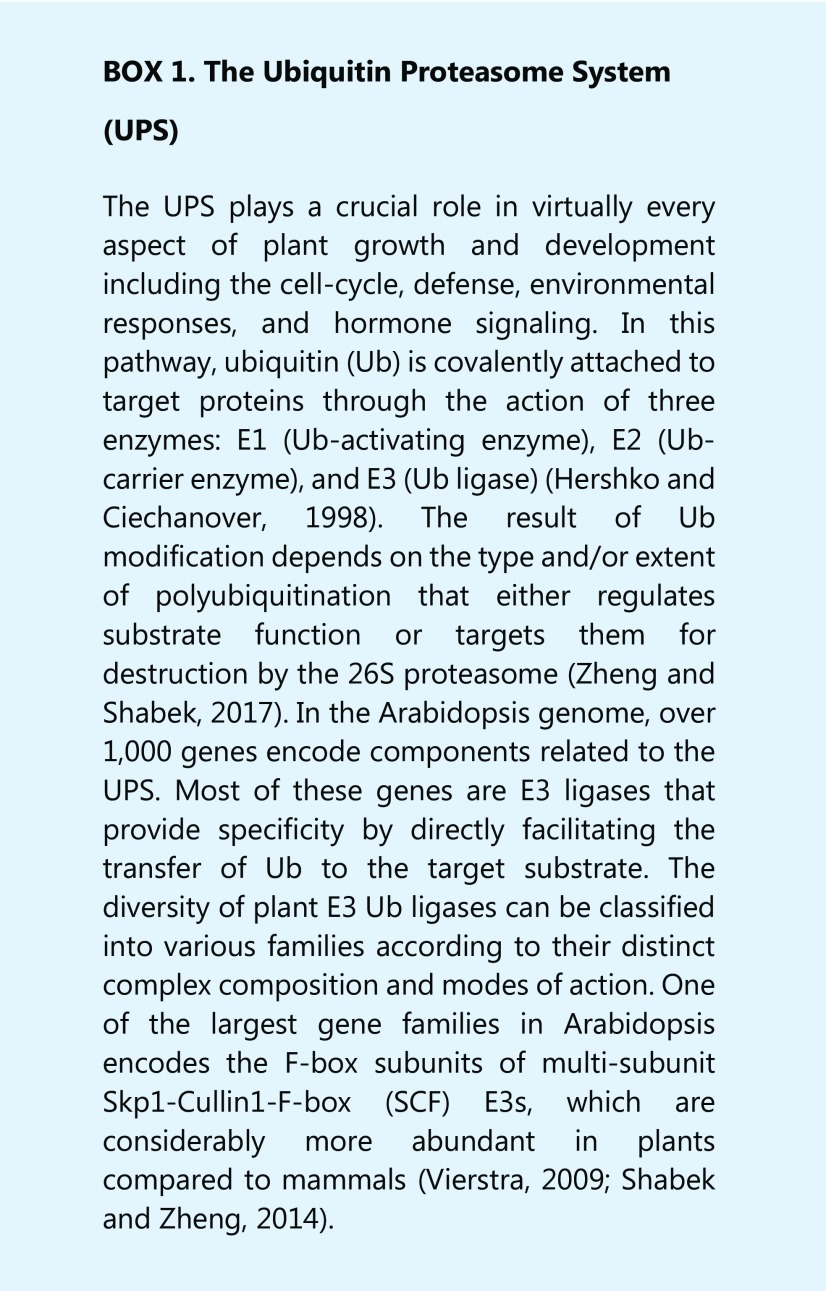
Phytohormones comprise a set of structurally unrelated small organic compounds. Notably, most phytohormone signaling pathways are tightly regulated by a highly coordinated intracellular protein degradation machine known as the ubiquitin-proteasome system (UPS; see Box 1). The specificity of UPS is conferred by the action of a family of E3 ubiquitin ligase enzymes that target specific proteins for destruction in a timely manner (Hershko and Ciechanover, 1998; Zheng and Shabek, 2017). Plants have used this machinery multiple times across evolution to achieve time-dependent activation of a signaling pathway upon phytohormone perception. In this review, we focus on and outline the structural aspects of plant hormone perception by the UPS, and detail the contribution of these findings to our understanding of signaling at the molecular level. We also discuss the current view and recent advances in the emerging field of strigolactone (SL) signaling.
PERCEPTION AND SIGNALING BY UBIQUITIN LIGASES
The first line of phytohormone perception involves distinct intracellular protein receptors that evolved to sense and respond to extremely low concentrations of these naturally occurring chemical signals. By leveraging the structurally diverse low molecular weight of these small molecules, phytohormones were proposed to facilitate selective protein-protein interaction and subsequently initiate a sequence of signaling cascades. Notably, Cullin-RING ligases, one of the prevalent E3 ligase superfamilies, were found to function as sensing centers in plant hormone perception and signaling (Vierstra, 2009; Shabek and Zheng, 2014). Cullin1, one of the CUL1–4 subtypes, serves as a large scaffold module with a C-terminal portion that interacts with the E2-recruiting RING domain (RBX1) and an N-terminal portion that binds the interchangeable substrate-receptor F-box protein via SKP1/ASK1 to form the functional ligase multisubunit SCF (Skp1-Cullin1-F-box; see Box 1 and Fig. 1). To better illustrate the molecular basis of phytohormone signaling cascade, we divided the receptors into two main modes of actions: direct interaction enhancers (molecular glues) and allosteric effectors. The perception mechanism of auxin and jasmonates (JAs) operates as molecular glue as the presence of these phytohormones enhances the interactions between the F-box receptors and their target proteins. On the other hand, GAs and SLs act as allosteric effectors by inducing conformational changes in their receptors to regulate downstream interactions with F-box proteins. This review focuses on the contribution of E3 ligases as direct or allosteric receptors; however, a dominant role of the UPS in downstream parts of the signaling cascade was documented for several other hormones, such as ethylene, cytokinin (Kim et al., 2013; Lee and Seo, 2015; Chen et al., 2018), and multiple components of abscisic acid signaling, as reviewed in Yu et al. (2016).
Figure 1.
Structural models of direct phytohormone perception and target recognition by E3 Ub ligase receptors. SCFTIR1 (A) and SCFCOI1 (B) mediate AUX/IAA and JAZ ubiquitination and degradation, respectively. Auxin and Ja-Ile (Jasmonic Acid – Ile) are directly perceived by F-box receptors (TIR1 and COI1, respectively), and this results in an altered interface that recruits substrate for ubiquitination and degradation. In the SCF (SKP1-CUL1-F-box) complex, Cullin1 serves as a scaffold that binds RBX1 (RING protein, required for E2-Ub recruitment) via its C terminus and ASK1/SKP1 (F-box adaptor protein) via its N terminus. In (i), molecular surface of TIR1 (PDB: 2P1Q) and COI1 (PDB: 3OGL) crystal structures are modeled with CUL1-RBX1 (PDB: 1LDK). In (ii), phytohormone recognition interface between F-box LRR and the target substrate recognition element (degron) is shown. Detailed amino acid side chain interactions with auxin (A) and JA (B) are shown in the small framed windows. In (iii), a model of phytohormone signaling mechanism is shown. All colored texts are consistent with colored structural elements. Ubiquitination is denoted U.
AUXINS AND JAS AS “MOLECULAR GLUES”
Auxin and JAs were the first hormones shown to be perceived by the F-box proteins TRANSPORT INHIBITOR RESPONSE 1 (TIR1; Ruegger et al., 1998; Dharmasiri et al., 2005; Kepinski and Leyser, 2005) and CORONATINE INSENSITIVE1 (COI1; Feys et al., 1994; Xie et al., 1998; Katsir et al., 2008), respectively. Extensive genetic and biochemical studies before any structural insight on the pathways showed that auxin and JAs induce the rapid degradation of distinct families of corepressors Auxin/INDOLE-3-ACETIC ACID (Aux/IAA; Abel et al., 1994; Gray et al., 2001; Tiwari et al., 2001) and JASMONATE ZIM DOMAIN (JAZ; Chini et al., 2007; Yan et al., 2007), which is followed by subsequent transcriptional activation of hormone-responsive genes.
The groundbreaking revelation of TIR1-Aux/IAA (Tan et al., 2007) and COI1-JAZ (Sheard et al., 2010) crystal structures illuminated the molecular basis for the perception of these phytohormones. These structural studies uncovered a new mechanism of ligand perception in which hormones act as “molecular glue” in macromolecular assembly. In the case of auxin, auxin docking to the bottom of TIR1 (via a side chain carboxyl group and an indole ring) creates a modified surface that stabilizes its interaction with Aux/IAAs, leading to their polyubiquitination and degradation. TIR1 and COI1 are structurally similar to horseshoe-shaped Leu Rich Repeat (LRR) domains followed by F-box domain bound to ASK1 adaptor (Fig. 1). The top surfaces of the TIR1- and COI1-LRR domains form a shallow pocket that binds both the hormone and the target substrate (Aux/IAA or JAZ). A short recognition motif (degron) within Aux/IAAs directly engages with auxin-loaded TIR1 and sandwiches auxin in the middle (Fig. 1A). Similarly, JAZ degrons interact with COI1 to ensure high-affinity hormone binding. The JAZ degron covers the opening where JA-Ile binds and traps the hormone in the pocket (Fig. 1B). Interestingly, both structures revealed the presence of secondary small molecule metabolites at the hormone perception site, inositol hexakisphosphate (InsP6) in TIR1 and inositol pentakisphosphate (InspP5) in COI1. These cofactors directly interact with the hormone-binding pocket and potentiate the hormone-receptor-substrate interaction.
The structural studies of the auxin and JA pathways were instrumental in improving our understanding of hormonal cues and transcriptional regulation. Aux/IAAs and JAZs participate in transcriptional repressive complexes containing transcription factors like AUXIN RESPONSE FACTOR (ARFs) for auxin and MYC for JA, adaptor proteins like NOVEL INTERACTOR OF JAZ (NINJA) for JAs, and corepressors like TOPLESS (TPL) for both phytohormones (Lorenzo et al., 2004; Szemenyei et al., 2008; Pauwels et al., 2010). Crystal data of the NINJA-TPL interaction uncovered the molecular basis of protein interactions with TPL through their ethylene response factor–associated amphiphilic repression (EAR) motifs (Ke et al., 2015). Crystallographic data of ARFs alone (Boer et al., 2014) or complexed with Aux/IAAs (Nanao et al., 2014) revealed the structural foundations of oligomerization of auxin transcriptional repressors, whereas the structure of MYC3 (Zhang et al., 2015) was the first illustration of noncomplexed MYC transcription activation domain. These are selected examples that illustrate the value of adding crystallographic data to the fields of plant development and physiology. Efforts to understand transcriptional repressive complexes at the biochemical and structural level of other hormones are necessary to gain a full appreciation of the complexity of hormone-dependent transcriptional regulation.
GAs and SLs as allosteric activators
Unlike TIR1 and COI1, the GA and SL receptors are not F-box proteins themselves. The GA receptor GIBBERELLIN INSENSITIVE DWARF 1 (GID1) and the SL receptor DWARF 14 (D14) are α/β hydrolases that associate with F-box proteins in a hormone-dependent manner to recognize substrate proteins for ubiquitination and degradation. In contrast with the “molecular glue” mechanism, GAs and SLs induce conformational changes in their receptors that enable them to interact with their respective F-box proteins (Fig. 2). GID1 is a soluble protein (Ueguchi-Tanaka et al., 2005) that has structural similarity to hormone-sensitive lipases involved in lipid metabolism in animals (Yeaman, 2004). Perception of GA by GID1 induces conformational changes in the enzyme that enable it to interact with the SCF-type E3 Ub ligase AtSLEEPY1 (SLY1)/AtSNEEZY (SNZ)/OsGID2 (SCFGID2/SLY1) to ubiquitinate and degrade DELLA transcriptional regulators, including GIBBERELLIN INSENSITIVE (GAI1; Peng et al., 1997; Fu et al., 2002; McGinnis et al., 2003; Sasaki et al., 2003; Ueguchi-Tanaka et al., 2007).
Figure 2.
Structural models of allosteric regulation of phytohormone perception and signaling by E3 Ub ligases. A, Crystal structure of GA perception complex (based on PDB: 2ZSI) contains GA3, GID1A, and the DELLA domain of GAI. In (i), molecular surface of GID1A N-terminal extension (N-Ex, labeled in violet) and its core (labeled in blue) are shown. Helices of GAI DELLA (labeled green) are represented as cylinders. In (ii), there is recognition interface between GID1-GA and DELLA, shown in detail by ribbon representation. The inset represents detailed amino acid side chain interfaces. In (iii), a model for GA perception and signaling is shown. GA binding induces a conformational change in the N-Ex of GID1 and promotes an interaction with the DELLA domain. Subsequently, GID1-GA-DELLA can be recognized and ubiquitinated (U) by SCFSLY. B, (i) and (ii), molecular surface representation of ASK1-D3 complex and CTH (light blue) dislodged. D14, the SL receptor (labeled in green; SL in magenta), is bound to D3-CTH (light blue) in an open conformation and can perceive SL. Crystals structures are modified from Shabek et al. (2018; based on PDB: 6BRO and 6BRT). In (iii) and (iv), molecular surface representation of ASK1-D3-D14 complex in a closed conformation (modified from Yao et al. (2016); based on PDB: 5HZG). C, Model of SL signaling. In (i), D3/MAX2 binds D14, the SL receptor, via D3′s CTH. In (ii), this interaction results in an allosteric inhibition of SL hydrolysis, allowing sufficient time to recruit and ubiquitinate (U) D53/SMXL’s transcriptional repressors. In (iii), targeting of D53 through SCFD3 triggers a conformational change in D3 and D14 that results in SL hydrolysis by D14 and a butenolide-bound intermediate (CLIM, small magenta triangle). D14 is subsequently ubiquitinated by D3 and recycled and/or degraded to reset the signal.
The mechanism by which GA is recognized by GID1 was elucidated by the crystal structures of GA-bound GID1 in both free and DELLA-associated forms (Murase et al., 2008; Shimada et al., 2008). GAI1 is a monomeric protein composed of one α/β core hydrolase (albeit with a nonfunctional catalytic triad; Ueguchi-Tanaka et al., 2005) and a unique N-terminal extension that folds back over the GA-bound pocket upon hormone perception and covers it like a lid (Fig. 2A). This process creates binding surfaces for the N-terminal DELLA domain of GAI1. Binding of this region to GID1 induces its coil-to-helix conformational transition, which in turn affects the structure of the C-terminal GRAS domain of the same protein. Mediated by the GRAS domain, the GID1-GA-DELLA complex is then recognized by the SCFGID2/SLY1 for ubiquitination and degradation of the DELLAs by the UPS. The structure of a GRAS domain has been recently solved in the non-DELLA protein SCARECROW-LIKE 7 (SCL7; Li et al., 2016). However, determining the structure of the GID1-GA-DELLA complex with SCFGID2/SLY1 will further our understanding of the GA signaling core regulatory mechanism.
The most recently identified phytohormone, SL, represents yet another new perception and signaling paradigm wherein both the F-box protein, namely MORE AXILLARY BRANCHES2 / DWARF3 (MAX2/D3), and the SL receptor α/β-hydrolase, namely, have multiple functional states. Similar to GA perception, SL does not function as molecular glue in the interface between the receptor and the E3 ligase. Unlike GID1, the D14 is an active hydrolase that slowly metabolizes SL into nonbioactive products. Here, D14 forms a SL-dependent complex with SCF-MAX2/D3 and recruits DWARF53 (D53; rice [Oryza sativa]) or SUPPRESSOR OF MAX2 LIKE (SMXL6/7/8; Arabidopsis [Arabidopsis thaliana]) for ubiquitination and rapid proteasomal degradation. The dynamic state of SL perception, hydrolysis, and signaling is biologically intriguing yet perplexing. The following part of this review will discuss our current understanding of SL perception and regulation.
SL PERCEPTION BY THE UPS AND DOWNSTREAM SIGNALING
SLs were first identified as germination stimulants of the parasitic witchweed Striga hermonthica, following their isolation from cotton (Gossypium hirsutum) root exudates in 1966 (Cook et al., 1966). Since 1966, researchers have characterized a variety of SLs as well as a diversity of functions of the SL molecule(s). SLs function exogenously to initiate symbiosis with mycorrhizal fungi and incidentally stimulate germination of parasitic plants (Akiyama et al., 2005). Whereas SLs function endogenously and regulate many aspects of growth and development, they are notoriously known to inhibit shoot branching (Gomez-Roldan et al., 2008; Umehara et al., 2008). Before the SL receptor was identified, MAX2 was first recognized as a protein involved in SL-related pathways (Stirnberg et al., 2002; Stirnberg et al., 2007; Gomez-Roldan et al., 2008). Mutant max2 plants presented phenotypes similar to SL synthesis mutants (Stirnberg et al., 2002; Booker et al., 2005; Ishikawa et al., 2005; Gomez-Roldan et al., 2008); however, unlike these mutants, max2 phenotypes could not be rescued with SL treatment, pointing to MAX2 involvement in SL signaling (Umehara et al., 2008). MAX2 encodes an F-box E3 ubiquitin ligase that targets its substrates for selective protein degradation. Thus, at the birth of the field it was apparent that like auxin, JA, salicylic acid, and GA signaling pathways, the SL signaling cascade was dependent on regulated turnover via the UPS.
Later, the SL receptor protein was first identified via its mutant characterized by an increased shoot branching phenotype (Arite et al., 2009). Multiple groups provided evidence that an α/β hydrolase such as D14 was responsible, and in fact later showed that D14 is the receptor of SL (Arite et al., 2009; Hamiaux et al., 2012; Waters et al., 2012; de Saint Germain et al., 2016). The SL receptor is remarkable because it serves as both a receptor as well as an enzyme, capable of hydrolyzing its ligand SL (Hamiaux et al., 2012; Waters et al., 2012; Zhao et al., 2013; Abe et al., 2014; Waters et al., 2015; de Saint Germain et al., 2016; Shahul Hameed et al., 2018; Yao et al., 2018; Bürger et al., 2019). The sought-after degradation targets of the MAX2-D14 complex were characterized in 2013 using the rice mutant d53 that shows increased branching and SL-insensitivity phenotypes. D53 encodes a protein that shares a similar secondary structure composition to proteins of the class I Clp ATPase family (Jiang et al., 2013; Zhou et al., 2013). D53/SMXLs contain a transcriptional repressor EAR motif and are rapidly degraded in response to D14-MAX2-dependent SL treatment (Zhou et al., 2013; Soundappan et al., 2015; Wang et al., 2015; Liang et al., 2016). Unlike in other phytohormone signaling cascades, the exact function and the specific transcriptional targets of D53/SMXLs remain largely unknown (Song et al., 2017).
SL perception and hydrolysis by D14
Structural biology has become a promising tool in understanding the function, specificity, and complexity of the D14-SL perception mechanism. The crystal structures of D14 have been determined for Arabidopsis, rice (Kagiyama et al., 2013; Zhao et al., 2013), striga (Xu et al., 2018), and petunia (Petunia hybrida; Hamiaux et al., 2012). These structures reveal a common α/β hydrolase fold with a deep pocket that is formed by a V-shaped lid composed of four α-helices. The D14 pocket contains a canonical Ser catalytic triad that is conserved across plant species, indicating that the protein hydrolase activity was maintained throughout evolution (Bythell-Douglas et al., 2017). Notably, the pocket is widely open to solvent, and several crystal structures have claimed to capture synthetic SL analogs or byproducts bound to D14 (Jiang et al., 2013; Nakamura et al., 2013; Zhao et al., 2013; Zhao et al., 2015; Takeuchi et al., 2018). Whereas it has been biochemically demonstrated that the binding pocket can accommodate SL for hydrolysis, it is less clear whether any of the D14 structures are able to capture the ligand at atomic resolution. Because of the low occupancy and poor electron density of SL analogs in all D14 crystal structures, the precise position and topography of SL has remained considerably elusive (Carlsson et al., 2018).
One of the major questions in the SL field is centered on SL hydrolysis and its necessity to propagate SL signaling. Much of the consensus was driven by the idea that hydrolysis of SL conferred a conformational change in D14, thus providing an interfacing ability with SCF-MAX2 Ub ligase (de Saint Germain et al., 2016; Yao et al., 2016). However, the requirement of hydrolysis was challenged by a recent study showing that D14 catalysis mutants are able to bind SL, but not hydrolyze it, and rescue the d14 mutant phenotype in a SL-dependent manner. This implies that SL binding (not hydrolysis) is necessary for initiating the signaling cascade (Seto et al., 2019). Additionally, the distinct states of intact SL binding and SL hydrolysis may confer different signals. In this model, one state is important for D14-MAX2 to create an interface for SMXLs, and another for the release or degradation of D14 and/or deactivation of the SL molecule. Nonetheless, in all these cases, it is the Ub ligase MAX2 that directs the rate of SL hydrolysis, the interactions, and the proteasomal degradation of D14 and SMXLs.
SL regulation by the Ub ligase SCF-MAX2/D3
Protein structures containing the complex of D14-D3 (D3, rice ortholog of MAX2) indicate an even more intriguing level of complexity and regulation at the level of the Ub ligase. It was shown that D14 undergoes a great conformational change when complexed with D3, presumably after SL hydrolysis (Yao et al., 2016). This finding was strongly supported by the identification of a SL-hydrolysis covalently linked intermediate molecule (CLIM), and by the fact that D14 is a single turnover enzyme (or very slowly releases SL products in vitro; de Saint Germain et al., 2016; Yao et al., 2016). Given that D14 is also targeted for degradation by MAX2 (Chevalier et al., 2014), it is unclear whether the D14CLIM-D3 complex recapitulates the effective interface to recruit SMXLs or simply targets the receptor to reset signaling.
A recent study reported another mode of action that is centered around the conformational state of the Ub ligase (Shabek et al., 2018). It has been shown that MAX2/D3 exists in multiple functional states highlighted by a flexible, highly conserved C-terminal alpha helix domain (CTH). The CTH, which is the last LRR repeat within the horseshoe structure, can be either open (dislodged) or closed (engaged). The dislodged D3-CTH can directly bind D14-SL, and this interaction results in allosteric inhibition of SL hydrolysis (Fig. 2B). It was also proposed that the dislodged form provides an interface to enable the recruitment of D53/SMXLs and their subsequent ubiquitination and degradation (Shabek et al., 2018). Before D53/SMXLs are released to the proteasome, the transient interaction with D3 alters D14 inhibition and slowly restores SL hydrolysis. The restored activity can successively reset the SL signal by both depleting SL and degrading the D14 receptor until the next environmental cue. Taken together, the mode of perception and signaling cascade of SL is likely dynamic and complex and may differ molecularly from other characterized phytohormone signaling pathways.
CONCLUDING REMARKS
The study of phytohormone perception and signaling cascades illustrates the importance of complementary genetic and structural biology methods. Integration of data from both disciplines overcomes their individual limitations and leads to a holistic understanding of molecular processes. In this review, we described various structural studies outlining how plants utilize the UPS to perceive hormonal triggers and activate signaling cascades. Hormone perception followed by protein complex formation is a dynamic, multifaceted process, and an x-ray crystallography approach may recapitulate a single energetically favored state of the protein machinery. Thus, comprehensive understanding of the molecular action of Ub ligase complexes in plant hormone biology remains a great challenge that is technically difficult to recapitulate at atomic resolution. For example, considering the gaseous phytohormone ethylene, the structure function of its perception mechanism is currently unresolved, although its signaling components are well known. The contribution of structural biology to plant biology has been immense and likely to provide answers to many unresolved questions (see Outstanding Questions). Whereas x-ray crystallography has remained the favorable approach to determine Ub ligase function in hormone signaling, this method relies heavily on minimum protein flexibility and maximum ability to be organized in a rigid and specific pattern. This notion can explain why most published structures were recapitulated using only portion of the complex components (AUX/IAA, JAZ, SLY). The emerging method of single particle cryo-electron microscopy holds promise to resolve the long-standing challenges of dynamic full-length complete protein complexes in plant hormone biology.
Acknowledgments
We apologize to those colleagues whose work was not cited due to space constraints.
Footnotes
This work was supported by United States - Israel Binational Agricultural Research and Development Fund (BARD; Vaadia-BARD Postdoctoral Fellowship Award FI-559-2017 to L.T) and University of California Davis College of Biological Sciences Start-Up Funds to N.S.
Articles can be viewed without a subscription.
References
- Abe S, Sado A, Tanaka K, Kisugi T, Asami K, Ota S, Kim HI, Yoneyama K, Xie X, Ohnishi T, et al. (2014) Carlactone is converted to carlactonoic acid by MAX1 in Arabidopsis and its methyl ester can directly interact with AtD14 in vitro. Proc Natl Acad Sci USA 111: 18084–18089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abel S, Oeller PW, Theologis A (1994) Early auxin-induced genes encode short-lived nuclear proteins. Proc Natl Acad Sci 91: 326–330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akiyama K, Matsuzaki K, Hayashi H (2005) Plant sesquiterpenes induce hyphal branching in arbuscular mycorrhizal fungi. Nature 435: 824–827 [DOI] [PubMed] [Google Scholar]
- Arite T, Umehara M, Ishikawa S, Hanada A, Maekawa M, Yamaguchi S, Kyozuka J (2009) D14, a strigolactone-insensitive mutant of rice, shows an accelerated outgrowth of tillers. Plant Cell Physiol 50: 1416–1424 [DOI] [PubMed] [Google Scholar]
- Beijerinck MW. (1898) Über ein contagium vivum fluidum als Ursache der Fleckenkrankheit der Tabaksblätter. Verh. K. Akad. Wet, Amsterdam [Google Scholar]
- Berman HM, Westbrook J, Feng Z, Gilliland G, Bhat TN, Weissig H, Shindyalov IN, Bourne PE (2000) The protein data bank. Nucleic Acids Res 28: 235–242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boer DR, Freire-Rios A, van den Berg WAM, Saaki T, Manfield IW, Kepinski S, López-Vidrieo I, Franco-Zorrilla JM, de Vries SC, Solano R, et al. (2014) Structural basis for DNA binding specificity by the auxin-dependent ARF transcription factors. Cell 156: 577–589 [DOI] [PubMed] [Google Scholar]
- Booker J, Sieberer T, Wright W, Williamson L, Willett B, Stirnberg P, Turnbull C, Srinivasan M, Goddard P, Leyser O (2005) MAX1 encodes a cytochrome P450 family member that acts downstream of MAX3/4 to produce a carotenoid-derived branch-inhibiting hormone. Dev Cell 8: 443–449 [DOI] [PubMed] [Google Scholar]
- Bürger M, Mashiguchi K, Lee HJ, Nakano M, Takemoto K, Seto Y, Yamaguchi S, Chory J (2019) Structural basis of Karrikin and non-natural Strigolactone perception in Physcomitrella patens. Cell Reports 26: 855–865.e5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bythell-Douglas R, Rothfels CJ, Stevenson DWD, Graham SW, Wong GK-S, Nelson DC, Bennett T (2017) Evolution of strigolactone receptors by gradual neo-functionalization of KAI2 paralogues. BMC Biol 15: 52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlsson GH, Hasse D, Cardinale F, Prandi C, Andersson I (2018) The elusive ligand complexes of the DWARF14 strigolactone receptor. J Exp Bot 69: 2345–2354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H, Ma B, Zhou Y, He SJ, Tang SY, Lu X, Xie Q, Chen SY, Zhang JS (2018) E3 ubiquitin ligase SOR1 regulates ethylene response in rice root by modulating stability of Aux/IAA protein. Proc Natl Acad Sci 115: 4513–4518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chevalier F, Nieminen K, Sánchez-Ferrero JC, Rodríguez ML, Chagoyen M, Hardtke CS, Cubas P (2014) Strigolactone promotes degradation of DWARF14, an α/β hydrolase essential for strigolactone signaling in Arabidopsis. Plant Cell 26: 1134–1150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chini A, Fonseca S, Fernández G, Adie B, Chico JM, Lorenzo O, García-Casado G, López-Vidriero I, Lozano FM, Ponce MR, et al. (2007) The JAZ family of repressors is the missing link in jasmonate signalling. Nature 448: 666–671 [DOI] [PubMed] [Google Scholar]
- Cook CE, Whichard LP, Turner B, Wall ME, Egley GH (1966) Germination of witchweed (Striga lutea Lour.): Isolation and properties of a potent stimulant. Science 154: 1189–1190 [DOI] [PubMed] [Google Scholar]
- de Saint Germain A, Clavé G, Badet-Denisot M-A, Pillot J-P, Cornu D, Le Caer J-P, Burger M, Pelissier F, Retailleau P, Turnbull C, et al. (2016) An histidine covalent receptor and butenolide complex mediates strigolactone perception. Nat Chem Biol 12: 787–794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dharmasiri N, Dharmasiri S, Estelle M (2005) The F-box protein TIR1 is an auxin receptor. Nature 435: 441–445 [DOI] [PubMed] [Google Scholar]
- Feys B, Benedetti CE, Penfold CN, Turner JG (1994) Arabidopsis mutants selected for resistance to the phytotoxin coronatine are male sterile, insensitive to methyl jasmonate, and resistant to a bacterial pathogen. Plant Cell 6: 751–759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu X, Richards DE, Ait-Ali T, Hynes LW, Ougham H, Peng J, Harberd NP (2002) Gibberellin-mediated proteasome-dependent degradation of the barley DELLA protein SLN1 repressor. Plant Cell 14: 3191–3200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez-Roldan V, Fermas S, Brewer PB, Puech-Pagès V, Dun EA, Pillot J-P, Letisse F, Matusova R, Danoun S, Portais J-C, et al. (2008) Strigolactone inhibition of shoot branching. Nature 455: 189–194 [DOI] [PubMed] [Google Scholar]
- Gray WM, Kepinski S, Rouse D, Leyser O, Estelle M (2001) Auxin regulates SCF(TIR1)-dependent degradation of AUX/IAA proteins. Nature 414: 271–276 [DOI] [PubMed] [Google Scholar]
- Hamiaux C, Drummond RSM, Janssen BJ, Ledger SE, Cooney JM, Newcomb RD, Snowden KC (2012) DAD2 is an α/β hydrolase likely to be involved in the perception of the plant branching hormone, strigolactone. Curr Biol 22: 2032–2036 [DOI] [PubMed] [Google Scholar]
- Hershko A, Ciechanover A (1998) The ubiquitin system. Annu Rev Biochem 67: 425–479 [DOI] [PubMed] [Google Scholar]
- Ishikawa S, Maekawa M, Arite T, Onishi K, Takamure I, Kyozuka J (2005) Suppression of tiller bud activity in tillering dwarf mutants of rice. Plant Cell Physiol 46: 79–86 [DOI] [PubMed] [Google Scholar]
- Jiang L, Liu X, Xiong G, Liu H, Chen F, Wang L, Meng X, Liu G, Yu H, Yuan Y, et al. (2013) DWARF 53 acts as a repressor of strigolactone signalling in rice. Nature 504: 401–405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kagiyama M, Hirano Y, Mori T, Kim S-Y, Kyozuka J, Seto Y, Yamaguchi S, Hakoshima T (2013) Structures of D14 and D14L in the strigolactone and karrikin signaling pathways. Genes Cells 18: 147–160 [DOI] [PubMed] [Google Scholar]
- Katsir L, Schilmiller AL, Staswick PE, He SY, Howe GA (2008) COI1 is a critical component of a receptor for jasmonate and the bacterial virulence factor coronatine. Proc Natl Acad Sci USA 105: 7100–7105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ke J, Ma H, Gu X, Thelen A, Brunzelle JS, Li J, Xu HE, Melcher K (2015) Structural basis for recognition of diverse transcriptional repressors by the TOPLESS family of corepressors. Sci Adv 1: e1500107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kepinski S, Leyser O (2005) The Arabidopsis F-box protein TIR1 is an auxin receptor. Nature 435: 446–451 [DOI] [PubMed] [Google Scholar]
- Kim HJ, Chiang Y-H, Kieber JJ, Schaller GE (2013) SCF(KMD) controls cytokinin signaling by regulating the degradation of type-B response regulators. Proc Natl Acad Sci USA 110: 10028–10033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee K, Seo PJ (2015) The E3 ubiquitin ligase HOS1 is involved in ethylene regulation of leaf expansion in Arabidopsis. Plant Signal Behav 10: e1003755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, Zhao Y, Zhao Z, Wu X, Sun L, Liu Q, Wu Y (2016) Crystal structure of the GRAS domain of SCARECROW-LIKE7 in Oryza sativa. Plant Cell 28: 1025–1034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang Y, Ward S, Li P, Bennett T, Leyser O (2016) SMAX1-LIKE7 signals from the nucleus to regulate shoot development in Arabidopsis via partially EAR motif-independent mechanisms. Plant Cell 28: 1581–1601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenzo O, Chico JM, Sánchez-Serrano JJ, Solano R (2004) JASMONATE-INSENSITIVE1 encodes a MYC transcription factor essential to discriminate between different jasmonate-regulated defense responses in Arabidopsis. Plant Cell 16: 1938–1950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClintock B. (1984) The significance of responses of the genome to challenge. Science 226: 792–801 [DOI] [PubMed] [Google Scholar]
- McGinnis KM, Thomas SG, Soule JD, Strader LC, Zale JM, Sun TP, Steber CM (2003) The Arabidopsis SLEEPY1 gene encodes a putative F-box subunit of an SCF E3 ubiquitin ligase. Plant Cell 15: 1120–1130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendel G. (1865) Experiments in plant hybridization. In Proceedings of the Natural History Society of Brno, Volume IV (Biodiversity Heritage Library; ), pp.3–47. [Google Scholar]
- Murase K, Hirano Y, Sun TP, Hakoshima T (2008) Gibberellin-induced DELLA recognition by the gibberellin receptor GID1. Nature 456: 459–463 [DOI] [PubMed] [Google Scholar]
- Nakamura H, Xue Y-L, Miyakawa T, Hou F, Qin H-M, Fukui K, Shi X, Ito E, Ito S, Park S-H, et al. (2013) Molecular mechanism of strigolactone perception by DWARF14. Nat Commun 4: 2613. [DOI] [PubMed] [Google Scholar]
- Nanao MH, Vinos-Poyo T, Brunoud G, Thévenon E, Mazzoleni M, Mast D, Lainé S, Wang S, Hagen G, Li H, et al. (2014) Structural basis for oligomerization of auxin transcriptional regulators. Nat Commun 5: 3617. [DOI] [PubMed] [Google Scholar]
- Napoli C, Lemieux C, Jorgensen R (1990) Introduction of a chimeric chalcone synthase gene into petunia results in reversible co-suppression of homologous genes in trans. Plant Cell 2: 279–289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pauwels L, Barbero GF, Geerinck J, Tilleman S, Grunewald W, Pérez AC, Chico JM, Bossche RV, Sewell J, Gil E, et al. (2010) NINJA connects the co-repressor TOPLESS to jasmonate signalling. Nature 464: 788–791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng J, Carol P, Richards DE, King KE, Cowling RJ, Murphy GP, Harberd NP (1997) The Arabidopsis GAI gene defines a signaling pathway that negatively regulates gibberellin responses. Genes Dev 11: 3194–3205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruegger M, Dewey E, Gray WM, Hobbie L, Turner J, Estelle M (1998) The TIR1 protein of Arabidopsis functions in auxin response and is related to human SKP2 and yeast grr1p. Genes Dev 12: 198–207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki A, Itoh H, Gomi K, Ueguchi-Tanaka M, Ishiyama K, Kobayashi M, Jeong D-H, An G, Kitano H, Ashikari M, Matsuoka M (2003) Accumulation of phosphorylated repressor for gibberellin signaling in an F-box mutant. Science 299: 1896–1898 [DOI] [PubMed] [Google Scholar]
- Seto Y, Yasui R, Kameoka H, Tamiru M, Cao M, Terauchi R, Sakurada A, Hirano R, Kisugi T, Hanada A, et al. (2019) Strigolactone perception and deactivation by a hydrolase receptor DWARF14. Nat Commun 10: 191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shabek N, Ticchiarelli F, Mao H, Hinds TR, Leyser O, Zheng N (2018) Structural plasticity of D3-D14 ubiquitin ligase in strigolactone signalling. Nature 563: 652–656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shabek N, Zheng N (2014) Plant ubiquitin ligases as signaling hubs. Nat Struct Mol Biol 21: 293–296 [DOI] [PubMed] [Google Scholar]
- Shahul Hameed U, Haider I, Jamil M, Kountche BA, Guo X, Zarban RA, Kim D, Al-Babili S, Arold ST (2018) Structural basis for specific inhibition of the highly sensitive ShHTL7 receptor. EMBO Rep 19: e45619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheard LB, Tan X, Mao H, Withers J, Ben-Nissan G, Hinds TR, Kobayashi Y, Hsu FF, Sharon M, Browse J, et al. (2010) Jasmonate perception by inositol-phosphate-potentiated COI1-JAZ co-receptor. Nature 468: 400–405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimada A, Ueguchi-Tanaka M, Nakatsu T, Nakajima M, Naoe Y, Ohmiya H, Kato H, Matsuoka M (2008) Structural basis for gibberellin recognition by its receptor GID1. Nature 456: 520–523 [DOI] [PubMed] [Google Scholar]
- Song X, Lu Z, Yu H, Shao G, Xiong J, Meng X, Jing Y, Liu G, Xiong G, Duan J, et al. (2017) IPA1 functions as a downstream transcription factor repressed by D53 in strigolactone signaling in rice. Cell Res 27: 1128–1141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soundappan I, Bennett T, Morffy N, Liang Y, Stanga JP, Abbas A, Leyser O, Nelson DC (2015) SMAX1-LIKE/D53 family members enable distinct MAX2-dependent responses to Strigolactones and Karrikins in Arabidopsis. Plant Cell 27: 3143–3159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stirnberg P, Furner IJ, Ottoline Leyser HM (2007) MAX2 participates in an SCF complex which acts locally at the node to suppress shoot branching. Plant J 50: 80–94 [DOI] [PubMed] [Google Scholar]
- Stirnberg P, van De Sande K, Leyser HMO (2002) MAX1 and MAX2 control shoot lateral branching in Arabidopsis. Development 129: 1131–1141 [DOI] [PubMed] [Google Scholar]
- Sumner JB. (1926) The isolation and crystallization of the enzyme urease. J Biol Chem 69: 435–441 [Google Scholar]
- Szemenyei H, Hannon M, Long JA (2008) TOPLESS mediates auxin-dependent transcriptional repression during Arabidopsis embryogenesis. Science 319: 1384–1386 [DOI] [PubMed] [Google Scholar]
- Takeuchi J, Jiang K, Hirabayashi K, Imamura Y, Wu Y, Xu Y, Miyakawa T, Nakamura H, Tanokura M, Asami T (2018) Rationally designed strigolactone analogs as antagonists of the D14 receptor. Plant Cell Physiol 59: 1545–1554 [DOI] [PubMed] [Google Scholar]
- Tan X, Calderon-Villalobos LIA, Sharon M, Zheng C, Robinson CV, Estelle M, Zheng N (2007) Mechanism of auxin perception by the TIR1 ubiquitin ligase. Nature 446: 640–645 [DOI] [PubMed] [Google Scholar]
- Tiwari SB, Wang XJ, Hagen G, Guilfoyle TJ (2001) AUX/IAA proteins are active repressors, and their stability and activity are modulated by auxin. Plant Cell 13: 2809–2822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueguchi-Tanaka M, Ashikari M, Nakajima M, Itoh H, Katoh E, Kobayashi M, Chow TY, Hsing YI, Kitano H, Yamaguchi I, Matsuoka M (2005) GIBBERELLIN INSENSITIVE DWARF1 encodes a soluble receptor for gibberellin. Nature 437: 693–698 [DOI] [PubMed] [Google Scholar]
- Ueguchi-Tanaka M, Nakajima M, Katoh E, Ohmiya H, Asano K, Saji S, Hongyu X, Ashikari M, Kitano H, Yamaguchi I, Matsuoka M (2007) Molecular interactions of a soluble gibberellin receptor, GID1, with a rice DELLA protein, SLR1, and gibberellin. Plant Cell 19: 2140–2155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umehara M, Hanada A, Yoshida S, Akiyama K, Arite T, Takeda-Kamiya N, Magome H, Kamiya Y, Shirasu K, Yoneyama K, Kyozuka J, Yamaguchi S (2008) Inhibition of shoot branching by new terpenoid plant hormones. Nature 455: 195–200 [DOI] [PubMed] [Google Scholar]
- van der Krol AR, Mur LA, Beld M, Mol JN, Stuitje AR (1990) Flavonoid genes in petunia: Addition of a limited number of gene copies may lead to a suppression of gene expression. Plant Cell 2: 291–299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vierstra RD. (2009) The ubiquitin-26S proteasome system at the nexus of plant biology. Nat Rev Mol Cell Biol 10: 385–397 [DOI] [PubMed] [Google Scholar]
- Vines SH. (1880) The works of Carl Von Nägeli. Nature 23: 78–80 [Google Scholar]
- Wang L, Wang B, Jiang L, Liu X, Li X, Lu Z, Meng X, Wang Y, Smith SM, Li J (2015) Strigolactone signaling in Arabidopsis regulates shoot development by targeting D53-Like SMXL repressor proteins for ubiquitination and degradation. Plant Cell 27: 3128–3142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waters MT, Nelson DC, Scaffidi A, Flematti GR, Sun YK, Dixon KW, Smith SM (2012) Specialisation within the DWARF14 protein family confers distinct responses to karrikins and strigolactones in Arabidopsis. Development 139: 1285–1295 [DOI] [PubMed] [Google Scholar]
- Waters MT, Scaffidi A, Moulin SLY, Sun YK, Flematti GR, Smith SM (2015) A Selaginella moellendorffii Ortholog of KARRIKIN INSENSITIVE2 functions in Arabidopsis development but cannot mediate responses to karrikins or strigolactones. Plant Cell 27: 1925–1944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie DX, Feys BF, James S, Nieto-Rostro M, Turner JG (1998) COI1: An Arabidopsis gene required for jasmonate-regulated defense and fertility. Science 280: 1091–1094 [DOI] [PubMed] [Google Scholar]
- Xu Y, Miyakawa T, Nosaki S, Nakamura A, Lyu Y, Nakamura H, Ohto U, Ishida H, Shimizu T, Asami T, Tanokura M (2018) Structural analysis of HTL and D14 proteins reveals the basis for ligand selectivity in Striga. Nat Commun 9: 3947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan Y, Stolz S, Chételat A, Reymond P, Pagni M, Dubugnon L, Farmer EE (2007) A downstream mediator in the growth repression limb of the jasmonate pathway. Plant Cell 19: 2470–2483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao R, Chen L, Xie D (2018) Irreversible strigolactone recognition: A non-canonical mechanism for hormone perception. Curr Opin Plant Biol 45(Pt A): 155–161 [DOI] [PubMed] [Google Scholar]
- Yao R, Ming Z, Yan L, Li S, Wang F, Ma S, Yu C, Yang M, Chen L, Chen L, et al. (2016) DWARF14 is a non-canonical hormone receptor for strigolactone. Nature 536: 469–473 [DOI] [PubMed] [Google Scholar]
- Yeaman SJ. (2004) Hormone-sensitive lipase--new roles for an old enzyme. Biochem J 379: 11–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu F, Wu Y, Xie Q (2016) Ubiquitin-proteasome system in ABA signaling: From perception to action. Mol Plant 9: 21–33 [DOI] [PubMed] [Google Scholar]
- Zhang F, Yao J, Ke J, Zhang L, Lam VQ, Xin X-F, Zhou XE, Chen J, Brunzelle J, Griffin PR, et al. (2015) Structural basis of JAZ repression of MYC transcription factors in jasmonate signalling. Nature 525: 269–273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao L-H, Zhou XE, Wu Z-S, Yi W, Xu Y, Li S, Xu T-H, Liu Y, Chen R-Z, Kovach A, et al. (2013) Crystal structures of two phytohormone signal-transducing α/β hydrolases: Karrikin-signaling KAI2 and strigolactone-signaling DWARF14. Cell Res 23: 436–439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao L-H, Zhou XE, Yi W, Wu Z, Liu Y, Kang Y, Hou L, de Waal PW, Li S, Jiang Y, et al. (2015) Destabilization of strigolactone receptor DWARF14 by binding of ligand and E3-ligase signaling effector DWARF3. Cell Res 25: 1219–1236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng N, Shabek N (2017) Ubiquitin ligases: Structure, function, and regulation. Annu Rev Biochem 86: 129–157 [DOI] [PubMed] [Google Scholar]
- Zhou F, Lin Q, Zhu L, Ren Y, Zhou K, Shabek N, Wu F, Mao H, Dong W, Gan L, et al. (2013) D14-SCF(D3)-dependent degradation of D53 regulates strigolactone signalling. Nature 504: 406–410 [DOI] [PMC free article] [PubMed] [Google Scholar]