TERMINAL FLOWER1 acts in the shoot apical meristem as a mobile cell-non-autonomous transcriptional cofactor that associates with DNA to regulate meristem indeterminacy and flowering.
Abstract
The floral transition is a critical step in the life cycle of flowering plants, and several mechanisms control this finely orchestrated process. TERMINAL FLOWER1 (TFL1) is a floral repressor and close relative of the florigen, FLOWERING LOCUS T (FT). During the floral transition, TFL1 expression is up-regulated in the inflorescence apex to maintain the indeterminate growth of the shoot apical meristem (SAM). Both TFL1 and FT are mobile proteins, but they move in different ways. FT moves from the leaves to the SAM, while TFL1 appears to move within the SAM. The importance of TFL1 movement for its function in the regulation of flowering time and shoot indeterminacy and its molecular function are still largely unclear. Our results using Arabidopsis (Arabidopsis thaliana) indicate that TFL1 moves from its place of expression in the center of the SAM to the meristem layer L1 and that the movement in the SAM is required for the regulation of the floral transition. Chromatin immunoprecipitation sequencing and RNA sequencing demonstrated that TFL1 functions as a cotranscription factor that associates with and regulates the expression of hundreds of genes. These newly identified direct TFL1 targets provide the possibility to discover new roles for TFL1 in the regulation of floral transition and inflorescence development.
Plants have to tightly control the switch from vegetative to reproductive growth to ensure reproductive success. Several factors, such as sugars, hormones, temperature, quality of the light, and the length of the day (photoperiod), can influence flowering time. In Arabidopsis (Arabidopsis thaliana), photoperiod is mainly perceived in the leaves, where the exposure to inductive long-day (LD) conditions promotes the expression of the main florigen, FLOWERING LOCUS T (FT), in the phloem companion cells (An et al., 2004; Corbesier et al., 2007). FT protein is then loaded in the sieve elements and transported to the shoot apical meristem (SAM), where it regulates the switch to reproductive development (Corbesier et al., 2007; Jaeger and Wigge, 2007; Mathieu et al., 2007). At the SAM, FT has been proposed to act as a transcription cofactor in a floral activation complex (FAC), that includes 14-3-3 proteins and the basic leucine zipper (bZIP) transcription factor FD (Pnueli et al., 2001; Abe et al., 2005; Wigge et al., 2005; Hanano and Goto, 2011; Taoka et al., 2011).
FT is a member of the phosphatidylethanolamine-binding protein (PEBP) family, which in Arabidopsis is composed of six members that fall into three major clades: MOTHER OF FT (MFT)-like, FT-like, and TERMINAL FLOWER1 (TFL1)-like (Karlgren et al., 2011). MFT, which forms the oldest branch of the PEBP family, is expressed in gametophytes and acts during seed germination (Xi et al., 2010; Wang et al., 2015). TWIN SISTER OF FT is the gene most closely related to FT, and the two proteins have been shown to act in part redundantly to promote flowering in response to LD (Yamaguchi et al., 2005; Fornara et al., 2010; Wang et al., 2015). The main representative of the third PEBP protein subgroup is TFL1, and it inhibits flowering as do the two other members of the clade, ARABIDOPSIS THALIANA CENTRORADIALIS homolog (ATC) and BROTHER OF FT (BFT; Mimida et al., 2001; Yoo et al., 2010). Both ATC and BFT have been shown to interact with FD and to repress APETALA1 (AP1) expression (Huang et al., 2012; Ryu et al., 2014). Furthermore, similar to FT, ATC can move over a long distance from leaves to the SAM (Huang et al., 2012).
TFL1 is the best characterized member of its clade and a key regulator of inflorescence development and flowering time. The primary sequences of TFL1 and FT are highly similar, but the two proteins have been suggested to have opposite molecular functions, acting as a repressor and activator of flowering, respectively (Ahn et al., 2006; Hanano and Goto, 2011). Mutants at the TFL1 locus flower earlier compared with the wild type, both in terms of days to flowering and number of leaves (Shannon and Meeks-Wagner, 1991). Its role as a flowering time regulator is further confirmed by the late-flowering phenotype of 35Spro::TFL1 plants (Ratcliffe et al., 1998).
TFL1 is also involved in the maintenance of the SAM, allowing indeterminate growth of the inflorescence, so that in tfl1 mutants the inflorescence SAM is converted into a terminal floral meristem (Shannon and Meeks-Wagner, 1991). During the vegetative phase, when new rosette leaves are produced, TFL1 is only expressed at low levels in the center of the SAM (Bradley et al., 1997; Ratcliffe et al., 1999; Conti and Bradley, 2007). At the time of the switch from the vegetative to the reproductive phase, the SAM is converted into an inflorescence meristem that starts to produce cauline leaves. At this stage, TFL1 expression is strongly up-regulated, first in axillary meristems and soon after in the SAM (Bradley et al., 1997; Conti and Bradley, 2007). Later during the development of the inflorescence, TFL1 and floral meristem identity genes are expressed in distinct domains in the main shoot apex. TFL1 mRNA is detected predominantly in the inner part of the central zone of the SAM, while LEAFY (LFY), AP1, and CAULIFLOWER (CAL) mRNAs accumulate at the periphery of the apex where new lateral floral meristems originate (Ratcliffe et al., 1998, 1999; Baumann et al., 2015). This spatial separation between TFL1 and the floral meristem identity genes is essential to the maintenance of the inflorescence. For example, in tfl1 mutants, the formation of the terminal floral meristem is enhanced by the ectopic expression of LFY in the meristem (Baumann et al., 2015). On the other hand, strong lfy and ap1 mutants have floral meristems converted into inflorescence meristems (Schultz and Haughn, 1991; Weigel et al., 1992; Bowman et al., 1993). It is worth noting that TFL1 and LFY (or AP1) mutually inhibit each other (Ratcliffe et al., 1999). As a consequence, TFL1 and the floral meristem identity genes are expressed in specific domains of the SAM, facilitating flower development while at the same time ensuring indeterminate growth of the plant during the reproductive phase.
The molecular function of TFL1 is still elusive. The protein has been reported to be mobile within the SAM, suggesting that it might regulate meristem development in a non-cell-autonomous manner (Conti and Bradley, 2007). Specifically, it has been shown that the TFL1 protein can move from the center toward the periphery of the SAM, where it prevents the expression of floral meristem genes (Conti and Bradley, 2007). However, whether this protein movement is critical for TFL1 function has not been addressed. At the subcellular level, TFL1 has been detected in the nucleus and cytoplasm (Conti and Bradley, 2007; Hanano and Goto, 2011). In the cytoplasm, TFL1 has been suggested to participate in endomembrane trafficking of proteins to storage vesicles (Sohn et al., 2007). In the nucleus, TFL1 has been shown to interact with the bZIP transcription factor FD, giving rise to the hypothesis that TFL1 and FT might compete for the formation of the FAC (Abe et al., 2005; Wigge et al., 2005; Hanano and Goto, 2011; Taoka et al., 2011; Kaneko-Suzuki et al., 2018). Accordingly, a TFL1-containing FAC that could either be transcriptionally inactive or actively repress the formation of flowers would be formed in the center of the SAM, whereas the formation of an FT-containing FAC would actively promote floral initiation at the periphery of the inflorescence meristem. In agreement with this hypothesis, it has been shown that TFL1 can be converted into a transcriptional activator when fused with the strong VP16 activation domain (Hanano and Goto, 2011). However, direct evidence that TFL1 functions as a transcription cofactor and the genome-wide set of its targets are still missing.
Here, we expressed TFL1-Venus fluorescent protein fusion proteins under the control of TFL1 regulatory elements (gTFL1), already described by Serrano-Mislata et al. (2016), to investigate the role of protein movement in the SAM during the floral transition. We observed that the movement of TFL1 from the central meristem toward the periphery of the SAM is necessary for TFL1 function and contributes to the regulation of the switch from vegetative to reproductive growth. Furthermore, confocal imaging demonstrated that TFL1 movement in the SAM can occur in either direction, inward or outward. To assess whether TFL1 associates with DNA and can function in a transcriptional complex, we performed chromatin immunoprecipitation sequencing (ChIP-seq) using a gTFL1-1xVenus reporter line to identify loci bound by TFL1 at the genome-wide scale. In combination with results obtained by RNA sequencing (RNA-seq) using a 35Spro::TFL1-GR line, in which TFL1 has been fused to the glucocorticoid receptor (GR) domain, we identified 115 direct targets of TFL1, which, among others, includes the major floral meristem identity gene LFY. In addition, we found that G-box motifs were highly enriched among the TFL1 target sites and that many of the bound regions were shared with regions previously reported to be bound by FD (Collani et al., 2019). Nevertheless, many loci specifically bound and regulated by TFL1 were not bound by FD, indicating that TFL1 participates in different transcriptional complexes at the apex that might regulate diverse aspects of the floral transition and inflorescence development.
RESULTS
Movement in the SAM Is Required for TFL1 Function
TFL1 in Arabidopsis has been shown to move from its place of expression in the center toward the periphery of the SAM (Conti and Bradley, 2007). To test if the protein movement within the SAM is actually required for its function, we first established a genomic TFL1 rescue construct (hereafter named gTFL1), consisting of the 592-bp upstream promoter sequence, all exons and introns, as well as 3,396 bp of the downstream sequence (Fig. 1A). The gTFL1 fragment, which is very similar to one used by Serrano-Mislata et al. (2016), contains all regulatory elements essential for correct temporal and spatial expression of TFL1. However, gTFL1 lacks the cis-regulatory region V (located at +3.3–3.6 kb from the stop codon) that has been reported to harbor an enhancer element that boosts TFL1 expression (Serrano-Mislata et al., 2016). The gTFL1 construct restored flowering time to almost that of the wild type (Supplemental Fig. S1), indicating that the cloned gTFL1 sequence contains the essential cis-regulatory elements for the correct expression of the transgene.
Figure 1.
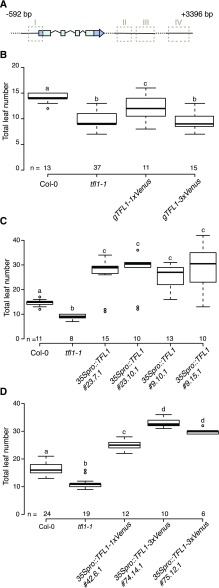
C-terminal Venus-tagged TFL1 protein is functional. A, Schematic representation of the gTFL1 sequence used in this study. Dark blue boxes indicate 5′ and 3′ untranslated regions, light blue boxes are exons, and junctions are introns. Dashed boxes indicate regulatory regions as described by Serrano-Mislata et al. (2016). B, Flowering time distribution of T1 plants carrying the gTFL1 transgene fused in frame to either 1xVenus or 3xVenus compared with Col-0 and the tfl1-1 mutant. C, Flowering time distribution of homozygous T4 plants constitutively expressing the TFL1 coding sequence (CDS) under the control of the 35S promoter. Data for four lines originating from two independent T1 plants are shown. D, Flowering time distribution of homozygous T4 plants constitutively expressing the TFL1 CDS fused to a single or triple C-terminal Venus tag under the control of the 35S promoter. Data for three different lines originating from independent T1 plants are shown. Plants were grown under LD conditions. Numbers (n) on the x axis indicate the number of plants. Boxes span the first (Q1) to third (Q3) quartiles, and thick black lines indicate the median value (Q2) for each group; the bottom whisker corresponds to Q1 − 1.5 × IQR (interquartile range), and the top whisker corresponds to Q3 + 1.5 × IQR. Groups with different letters are significantly different from each other after Tukey-Kramer correction (P < 0.05).
Reasoning that the ability of TFL1 to move from cell to cell would be influenced by the size of the protein, we next generated fusion proteins in which gTFL1 was tagged in frame with either a single or three copies of the fluorescent proteins Venus (gTFL1-1xVenus or gTFL1-3xVenus) and GFP (gTFL1-1xGFP or gTFL1-3xGFP) at its C terminus. The constructs were transformed into the tfl1-1 mutant, and flowering time of T1 plants was scored. Only gTFL1-1xVenus plants (T1) showed significant rescue of the early-flowering phenotype of the tfl1-1 mutant, whereas most of the gTFL1-3xVenus plants were still early flowering (Fig. 1B). gTFL1-1xGFP suppressed the formation of a terminal flower and restored indeterminate growth of the apical inflorescence in more than 50% of the T1 lines (Table 1; Supplemental Fig. S2). In contrast, indeterminate growth was observed in less than 10% of the T1 gTFL1-3xGFP lines (Table 1; Supplemental Fig. S2). Together, these findings suggest that either TFL1 movement was impaired by the large 3xVenus and 3xGFP tags, thereby preventing it from rescuing the tfl1-1 mutant, or alternatively that the 3x-tagged proteins had lost their activity. To distinguish between these two possibilities, we expressed the unmodified and the tagged versions of TFL1 from the constitutive 35S promoter in Columbia-0 (Col-0). As expected, independent stably transformed T4 homozygous lines carrying 35Spro::TFL1 flowered very late when compared with Col-0 (Fig. 1C; Supplemental Fig. S3, A–C), confirming previous results (Ratcliffe et al., 1998; Hanano and Goto, 2011). Importantly, late flowering was also observed in the 35Spro::TFL1-1xVenus and 35Spro::TFL1-3xVenus lines (Fig. 1D; Supplemental Fig. S3, D–F), indicating that the presence of C-terminal 1xVenus or 3xVenus tags did not affect the ability of TFL1 to repress flowering and that the TFL1 protein retained activity irrespective of the size of the tag. The most parsimonious explanation for these findings is that the 3xVenus (and 3xGFP) tag impairs TFL1 protein movement in the SAM and that this movement is important for normal TFL1 function in the control of the floral transition.
Table 1. Inflorescence phenotypes of Col-0, tfl1-1, and transgenic T1 lines.
The numbers of individuals with determinate and indeterminate inflorescence were determined in LD-grown Col-0, tfl1-1, and independent T1 lines in the tfl1-1 background transformed with various TFL1 constructs. See also Supplemental Figure S2.
| Genotype | Inflorescence Phenotype | |
|---|---|---|
| Determinate | Indeterminate | |
| Col-0 | 0 | 19 |
| tfl1-1 | 20 | 0 |
| gTFL1-1xGFP/tfl1-1 | 17 | 21 |
| gTFL1-3xGFP/tfl1-1 | 22 | 2 |
| gTFL1-3xGFP-NLS/tfl1-1 | 46 | 0 |
| ML1pro::TFL1/tfl1-1 | 50 | 1 |
| ML1pro::TFL1-NLS/tfl1-1 | 49 | 2 |
| ML1pro::TFL1-1xGFP/tfl1-1 | 49 | 2 |
| ML1pro::TFL1-1xGFP-NLS/tfl1-1 | 52 | 5 |
TFL1-1xVenus Is Detected in the Epidermal Meristem Layer
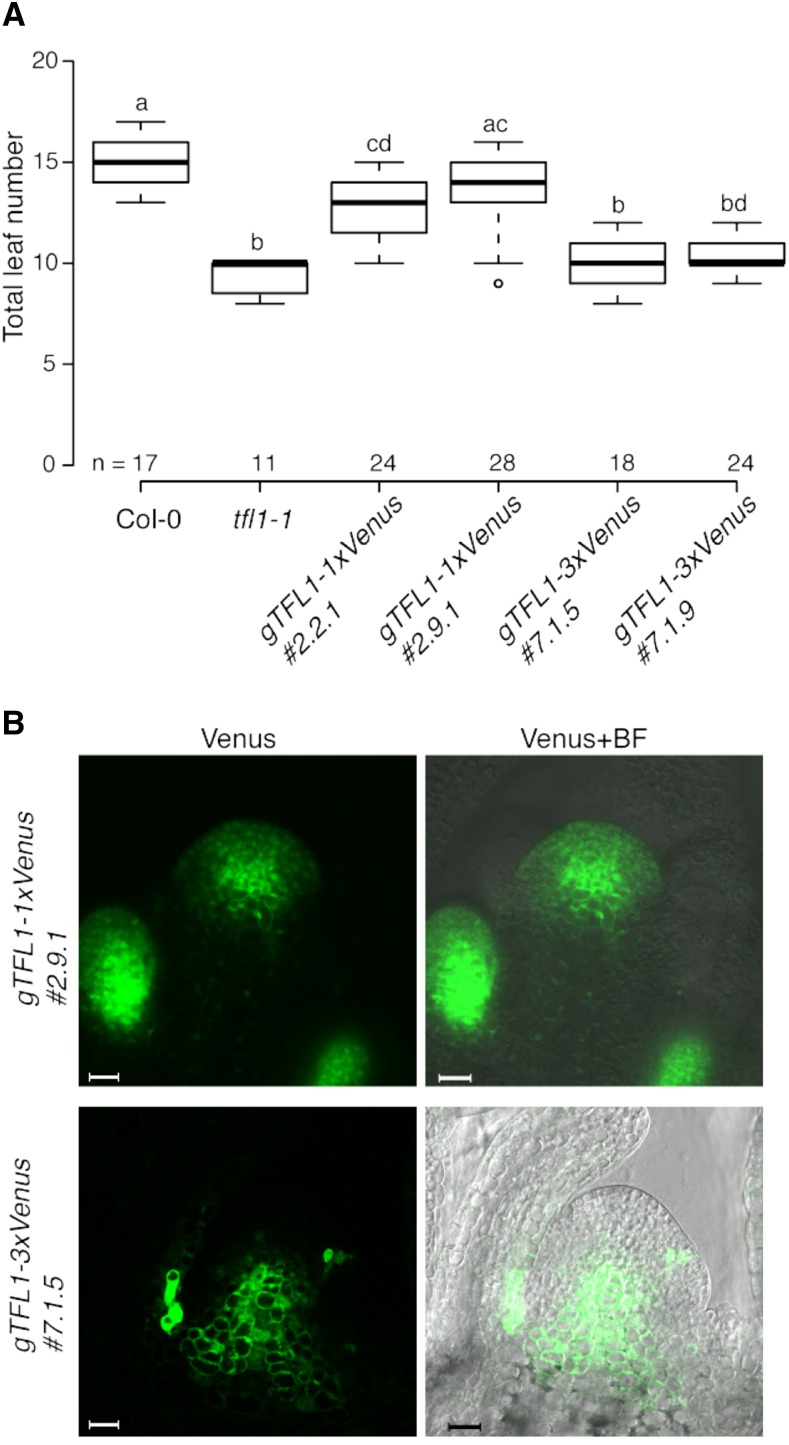
To visualize the localization of TFL1 in vivo more precisely, we established homozygous single and triple Venus-tagged T4 lines. Two selected gTFL1-1xVenus lines largely complemented the early-flowering phenotype of the tfl1-1 mutant, both in terms of total leaves and days to flowering, and displayed indeterminate growth in 100% of the plants (Fig. 2A; Supplemental Fig. S4). Next, we investigated the distribution of TFL1-1xVenus protein in the SAM by confocal microscopy. For this, plants were grown under inductive LD conditions and images were taken before and after the floral transition. In 13-d-old plants, before the transition to flowering, TFL1-1xVenus was detectable only in the axillary meristems (Supplemental Fig. S5A). However, 2 d later (15 d after sowing [DAS]), when floral transition was occurring, TFL1-1xVenus appeared also in the SAM, where it was detected in the central zone and in more peripheral regions, including the epidermal layer (L1; Fig. 2B; Supplemental Figs. S5, A and B, and S6A). Transverse sections of apices collected 15 DAS showed enrichment of TFL1-1xVenus especially in the adaxial regions of the axillary meristems (Supplemental Fig. S5B). In contrast to gTFL1-1xVenus, gTFL1-3xVenus failed to rescue the tfl1-1 mutant phenotype, and the plants transitioned to flowering relatively early at approximately 13 DAS (Fig. 2A; Supplemental Fig. S4). In addition, growth of all established T4 gTFL1-3xVenus lines was determinate. At the time of floral transition, 13 DAS, TFL1-3xVenus was detectable only in the inner zone but absent from the peripheral region of the meristem (Fig. 2B; Supplemental Fig. S6A). The distribution of TFL1-3xVenus resembles that of the GUS reporter line 0.6-G-3.3 described by Serrano-Mislata et al. (2016), which is interesting as the large GUS protein is thought to act largely cell autonomously (Kim et al., 2003).
Figure 2.
TFL1 movement in the SAM is required for the regulation of flowering. A, Flowering time distribution of Col-0, tfl1-1, and homozygous T4 plants expressing fusion proteins under the control of the native gTFL1 regulatory elements. Data for two different lines carrying either 1xVenus- or 3xVenus-tagged proteins are shown. Plants were grown under LD conditions. Numbers (n) on the x axis indicate the number of plants. Boxes span the first (Q1) to third (Q3) quartiles, and thick black lines indicate the median value (Q2) for each group; the bottom whisker corresponds to Q1 − 1.5 × IQR (interquartile range), and the top whisker corresponds to Q3 + 1.5 × IQR. Groups with different letters are significantly different from each other after Tukey-Kramer correction (P < 0.05). B, Confocal microscopy images of lines expressing the mobile (1xVenus tagged; top) or the immobile (3xVenus tagged; bottom) form of TFL1. Images of longitudinal sections of the main shoot apex were taken at the time of floral transition, 15 and 13 d after sowing, respectively. Bars = 20 μm. BF, Bright field.
To verify that the observed differences in the distribution of 1xVenus- and 3xVenus-tagged TFL1 protein were not due to differences in gene expression, we performed reverse transcription quantitative PCR (RT-qPCR) on the transgenes in dissected apices collected from gTFL1-1xVenus and gTFL1-3xVenus plants. The results clearly showed that gTFL1-1xVenus and gTFL1-3xVenus were expressed at comparable levels in all lines (Supplemental Fig. S6B). Taken together, the flowering time and protein localization results demonstrated that the presence of TFL1 only in the central zone of the meristem is apparently not sufficient to rescue the early-flowering phenotype of the tfl1-1 mutant. Furthermore, these findings provided indirect evidence for the importance of the movement of TFL1 toward the proximal cell layers of the SAM.
Expression of TFL1 in the Epidermis Partially Rescues the tfl1-1 Early-Flowering Phenotype
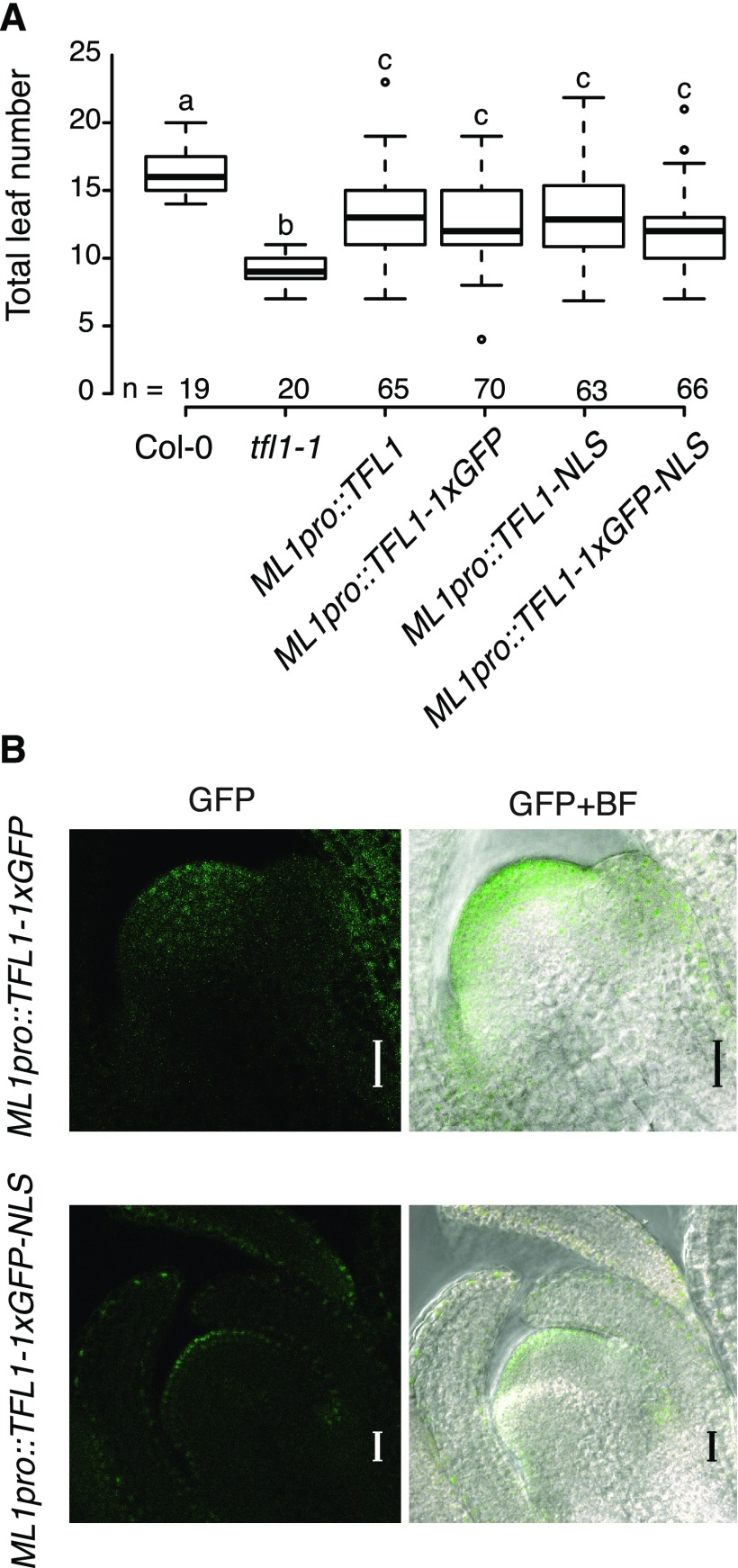
To test whether the presence of TFL1 in the epidermal layer of the SAM is important for its function, we expressed TFL1 under the control of the MERISTEM LAYER1 (ML1) promoter. T1 plants carrying ML1pro::TFL1 showed a partial rescue of the early-flowering phenotype of the tfl1-1 mutant (Fig. 3A; Supplemental Fig. S7). However, the terminal flower phenotype was suppressed only in one out of 51 T1 plants examined (Table 1), indicating that TFL1 expression in the L1 is insufficient to fully complement the mutant.
Figure 3.
Expression of TFL1 in the epidermis partially rescues the early-flowering phenotype of the tfl1-1 mutant. A, Flowering time distribution of T1 plants expressing the TFL1 CDS under the control of the epidermis-specific ML1 promoter, without any tag, tagged with either GFP or an NLS, or both. Data from Col-0 and the tfl1-1 mutant plants serve as controls. Plants were grown under LD conditions. Numbers (n) on the x axis indicate the number of independent T1 plants. Boxes span the first (Q1) to third (Q3) quartiles, and thick black lines indicate the median value (Q2) for each group; the bottom whisker corresponds to Q1 − 1.5 × IQR (interquartile range), and the top whisker corresponds to Q3 + 1.5 × IQR. Groups with different letters are significantly different from each other after Tukey-Kramer correction (P < 0.05). B, Confocal microscopy images taken during the floral transition of T2 lines expressing TFL1-1xGFP under the control of the ML1 promoter, without (top) or with (bottom) a C-terminal NLS. Images of longitudinal sections of the main shoot apex were taken 15 d after sowing. Bars = 20 μm. BF, Bright field.
To visualize the protein and its localization in the SAM, we tagged TFL1 at its C terminus with GFP and expressed the fusion protein under the control of the ML1 promoter. Similar to the lines expressing the untagged TFL1 protein, the ML1pro::TFL1-1xGFP plants displayed a partial rescue of the early flowering of tfl1-1, but most individuals were still determinate (Fig. 3A; Table 1; Supplemental Fig. S7). Interestingly, TFL1-1xGFP could be detected several cell layers inward from the L1, reaching the L2 and L3 layers (Fig. 3B). This indicates that movement of TFL1 is most likely not directional and the protein moves away from the L1 cells in which the transgene is expressed. However, the finding that TFL1-1xGFP moves toward the center of the SAM makes it impossible to conclude if localization in the L1 is required for TFL1 function.
To prevent the movement of TFL1 from the L1, we next added a strong nuclear localization signal (NLS) to TFL1 and expressed the resulting TFL1-NLS construct from the ML1 promoter. The nucleus-localized TFL1-NLS protein rescued the early-flowering phenotype of the tfl1-1 mutant to a similar extent to the untagged and GFP-tagged TFL1 proteins (Fig. 3A; Supplemental Fig. S7) but had little effect on the formation of the terminal flower (Table 1). To verify that the NLS was functional, we generated an ML1pro::TFL1-1xGFP-NLS construct. As expected, the GFP fluorescent signal was largely confined to the L1 layer, localized to the nucleus, and rescued the early-flowering phenotype of the tfl1-1 mutant similarly to the untagged TFL1 protein (Fig. 3), suggesting that TFL1 works in the nucleus and regulates flowering time at least in part in the L1.
Genome-Wide Targets of TFL1
TFL1 has been shown to interact with transcription factors and, when fused to the strong transcription activation domain VP16, is capable of inducing the expression of floral genes (Hanano and Goto, 2011), suggesting that TFL1 might be involved in transcriptional regulation. However, as there is no indication that it can directly bind DNA, TFL1 likely functions as a transcriptional cofactor. To identify potential target genes of TFL1 and to gain insight into the transcriptional networks in which TFL1 might be involved, we adopted a ChIP-seq approach.
To identify regions in the genome bound by TFL1-containing protein complexes, we sampled apices (including the SAM, young organ primordia, and the closest axillary meristems) of 15-d-old gTFL1-1xVenus #2.9.1 plants, as we had previously established that this line shows rescue of the early-flowering phenotype of the tfl1-1 mutant and the TFL1-1xVenus protein also was detectable in the SAM at this time point (Fig. 2).
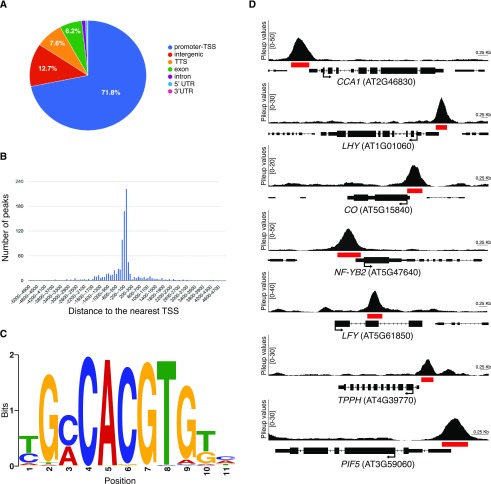
ChIP-seq identified 1,891, 1,752, and 1,140 regions enriched for TFL1 in the three biological replicates, of which 971 peaks were shared between all the replicates (Supplemental Data S1). Subsequent analyses were performed on this set of 971 high-confidence peaks that correspond to 952 unique genes, since some loci contained more than one region enriched for TFL1. Annotation of the peak regions showed that the majority (71.8%) mapped in promoter regions (Fig. 4, A and B), lending support to the idea that TFL1 acts as a cofactor in transcriptional complexes. De novo motif analysis using Multiple EM for Motif Elicitation (MEME)-ChIP (Machanick and Bailey, 2011) revealed a G-box (CACGTG) as the most highly overrepresented binding sites in the sequences underlying the peaks (Fig. 4C; Supplemental Fig. S8). Gene Ontology (GO) analysis showed that TFL1 is enriched on genes involved in cellular and metabolic processes, response to stimuli, and single-organism processes (Supplemental Fig. S9A).
Figure 4.
Identification of TFL1 targets at the SAM during the floral transition. A, Annotation of the locations of high-confidence peaks found in three biological replicates in the gTFL1-1xVenus line by ChIP-seq according to genome features. UTR, Untranslated region. B, Distribution of the distance of the center of the 971 peaks shared between the three biological ChIP-seq replicates to the nearest transcription start site (TSS). C, Motif of the most highly enriched TFL1 complex-binding site identified by de novo prediction. D, Examples of peaks from the gTFL1-1xVenus line for selected loci. Pileup values shown on the y axis were calculated with MACS2. A red box under each peak indicates the region of significant enrichment as determined by MACS2. Black arrows indicate the positions of the translation start site (ATG) and the direction of gene transcription.
Among the genes that showed strong enrichment of TFL1 were several important regulators of the circadian clock, flowering time, and floral development. For example, we detected enrichment of TFL1 on the promoters of CIRCADIAN CLOCK ASSOCIATED1 (CCA1) and LATE ELONGATED HYPOCOTYL (LHY), which are components of the core circadian oscillator in Arabidopsis (Fig. 4D; Alabadí et al., 2001; Mizoguchi et al., 2002). We also observed enrichment of TFL1 on the core promoter of CONSTANS (CO; Fig. 4D), a key player in the photoperiod pathway that is circadian regulated and induces flowering in response to inductive daylength (Suárez-López et al., 2001). Interestingly, TFL1 was also recruited to the promoter of NUCLEAR FACTOR-YB2 (NF-YB2; Fig. 4D), which has been shown to interact with CO and induce flowering by promoting FT expression in the leaf (Kumimoto et al., 2008, 2010). TFL1 was also strongly enriched on the second exon of the floral meristem identity gene LFY and the promoters of TREHALOSE-6-PHOSPHATE PHOSPHATASE H (TPPH) and PHYTOCHROME-INTERACTING FACTOR5 (PIF5; Fig. 4D), involved in sugar metabolism and in metabolic signaling to the circadian clock, respectively (Vandesteene et al., 2012; Shor et al., 2017). Taken together, these results demonstrate that TFL1-containing protein complexes bind directly to and might regulate the expression of important flowering time and plant development genes.
Genes Regulated by TFL1 in the Main Apex at the Floral Transition
To determine which of the bound genes were transcriptionally regulated by TFL1, we transformed the tfl1-1 mutant with a construct carrying TFL1 fused to the GR domain under the control of the 35S constitutive promoter (35Spro::TFL1-GR). In the absence of a ligand, GR fusion proteins are efficiently retained in the cytoplasm and only imported into the nucleus after application of a steroid hormone ligand such as dexamethasone (DEX). Constitutively expressed GR fusion proteins have been widely used to identify targets of transcription factors in plants (Gómez-Mena et al., 2005; Kaufmann et al., 2010; Reymond et al., 2012). As our ChIP-seq results indicated that TFL1 was associated with DNA, we reasoned that a TFL1-GR fusion protein would be retained in the cytoplasm, thereby preventing it from executing its nuclear function. Expression of TFL1-GR from the 35S promoter was thus predicted to delay flowering in a DEX-inducible manner, which could be used in a transcriptomic approach to identify TFL1-regulated genes.
To test whether the TFL1-GR fusion protein was functional, plants were grown under noninductive short-day (SD) conditions for 3 weeks and then shifted to LD to induce flowering. DEX or mock solution at 30 µm was directly applied to the shoot apex every 72 h from the time plants had been shifted to LD until they had bolted. Col-0 plants started to bolt 15 d after the shift to LD, independently of whether they had been treated with DEX or mock solution (Supplemental Fig. S10A). In contrast, bolting was substantially delayed in two 35Spro::TFL1-GR tfl1-1 (T4) lines (#4.7 and #7.9) after application of DEX, while mock-treated plants flowered very early, as expected for a tfl1-1 mutant (Supplemental Fig. S10A). Line #7.9, in particular, displayed a homogenous delay in flowering time, with 50% of the plants flowering 22 d after the shift to LD versus 15 d of Col-0 and 7 d of the same #7.9 line mock treated (Supplemental Fig. S10, A and B). In this line, continuous application of DEX prevented flowering in approximately 20% of the individuals tested at the end of the experiment, 55 d after the shift to LD and the beginning of the DEX treatments. These initial analyses indicated that the TFL1-GR system was functional and that plants responded to DEX treatment as expected. In addition, these analyses provide further indirect evidence that TFL1 needs to enter the nucleus in order to regulate flowering time.
To determine the optimal time of DEX treatments for subsequent transcriptome analyses of TFL1 induction, we applied 30 µm DEX 4, 5, or 6 d after shifting to LD at Zeitgeber time (ZT) +2. While application of DEX 4 and 5 d after the shift essentially blocked the induction of LFY and AP1 expression completely, application after 6 d allowed for an initial expression of these two genes that was subsequently repressed by TFL1-GR (Supplemental Fig. S10, C and D). Further experiments demonstrated that mock- and DEX-treated plants showed strong differences in AP1 and LFY expression 24 h after the treatment (Supplemental Fig. S10, E and F). Based on these initial results, we performed a transcriptome-wide RNA-seq expression analysis using RNA isolated from apices of 35Spro::TFL1-GR (#7.9) plants 24 h after they had been treated with either 30 µm DEX or mock solution at ZT+2 6 d after the shift to LD, following the initial cultivation in SD for 21 d.
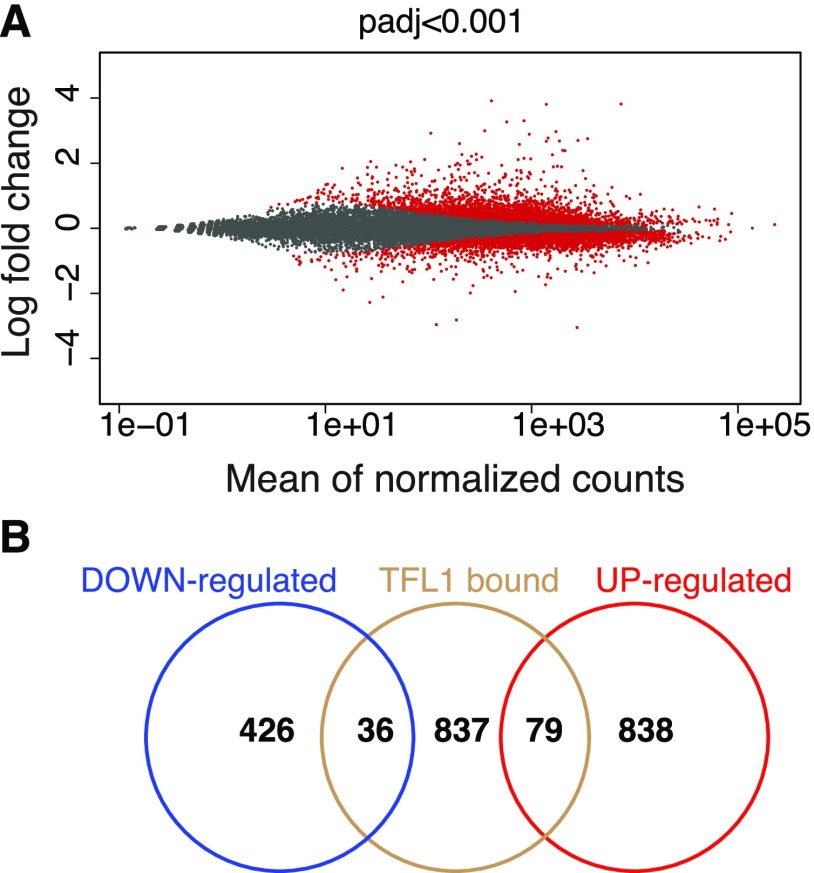
In total, this RNA-seq experiment identified 1,379 genes that were differentially expressed between mock- and DEX-treated samples (false discovery rate < 0.001, fold change > 1.5; Fig. 5A; Supplemental Data S2). Of these, 462 were down-regulated and 917 were up-regulated in response to DEX treatment (Supplemental Data S2). It is worth noting that among the genes significantly down-regulated by TFL1-GR, we could detect the floral identity genes LFY, CAL, and FRUITFULL. Overall, GO analyses showed that categories related to cellular and metabolic processes were overrepresented among the genes differentially expressed by induction of TFL1-GR (Supplemental Fig. S9B).
Figure 5.
Identification of TFL1-regulated genes by RNA-seq. A, Scatterplot of significantly differentially expressed genes (red) in 35Spro::TFL1-GR plants in response to DEX treatment. B, Three-set Venn diagram depicting the overlap of TFL1 targets identified by ChIP-seq (TFL1 bound) and differentially expressed genes identified by RNA-seq (down-regulated and up-regulated).
Intersection of RNA-Seq and ChIP-Seq Data Sets
To identify which of the 1,379 differentially expressed genes were also bound by TFL1-containing protein complexes, we intersected data from the RNA-seq and ChIP-seq experiments. Of the 952 genes that were enriched for TFL1-1xVenus, 115 (12%) were also differentially expressed (Fig. 5B; Supplemental Data S3). Of these, 36 genes were down-regulated and 79 were up-regulated at the SAM upon induction of TFL1-GR by DEX (Fig. 5B; Supplemental Data S3).
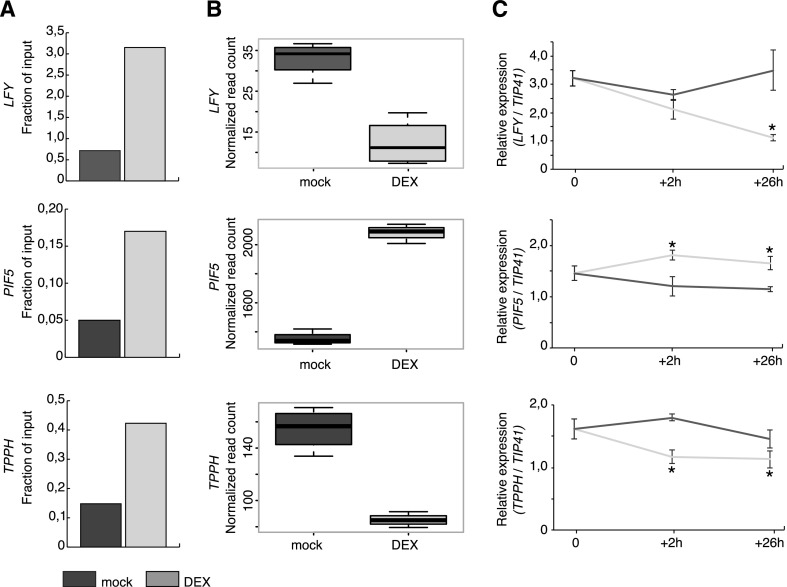
These 115 genes comprise the core set of high-confidence TFL1 targets. However, as the RNA-seq and ChIP-seq experiments were performed using different experimental systems and plant lines, we confirmed our results in an independent experiment. To this end, we performed ChIP on the DEX-inducible 35Spro::TFL1-GR line using an anti-GR antibody and tested enrichment on three target genes by qPCR. These genes were selected based on their known role in regulating the floral transition (LFY) or for their involvement in light (PIF5) or trehalose (TPPH) signaling. Consistent with our previous ChIP-seq results, ChIP-PCR detected enrichment of TFL1-GR on the genes tested in DEX-treated compared with mock-treated plants (Fig. 6A; Supplemental Fig. S11). We also examined the expression of these genes in the 35Spro::TFL1-GR line 2 and 26 h after mock and DEX treatments (Fig. 6C). Interestingly, already 2 h after treatment, we could detect significant changes in the expression level of TPPH and PIF5. Importantly, and in agreement with our RNA-seq analyses, the expression of LFY and TPPH was down-regulated whereas that of PIF5 was significantly up-regulated in DEX-treated samples in our RT-qPCR analysis (Fig. 6, B and C).
Figure 6.
Validation of direct TFL1 targets. A, Quantification of the enrichment of TFL1-GR on the LFY, PIF5, and TPPH genes in 35Spro::TFL1-GR plants by ChIP. Fractions of input in mock-treated (dark gray) and DEX-treated (light gray) plants are shown for one biological replicate. Data for additional biological replicates are shown in Supplemental Figure S11. B, Expression of LFY, PIF5, and TPPH in 35Spro::TFL1-GR plants in response to DEX treatment. Expression estimates (read counts) were extracted from RNA-seq data. Reads are normalized for sequencing depth. Error bars show sd. C, Expression of LFY, PIF5, and TPPH in DEX- and mock-treated 35Spro::TFL1-GR plants quantified by RT-qPCR 2 and 26 h after DEX application. Error bars show sd. Asterisks indicate significant differences between mock- and DEX-treated plants (P < 0.05, Student’s t test).
TFL1 Is an Important Interactor of FD at the SAM
It has previously been shown that TFL1 physically interacts with the bZIP transcription factor FD, an important regulator of flowering time at the SAM (Wigge et al., 2005; Hanano and Goto, 2011). More recently, it was demonstrated in vitro that this protein complex can bind G-box sequences (Collani et al., 2019). The same study has shown by ChIP-seq that genome-wide G-boxes are the most strongly enriched binding site of FD at the SAM. As a G-box is also the most highly overrepresented binding site in our TFL1 ChIP-seq analyses, we compared the list of TFL1 and FD targets. In total, we obtained 358 peak regions that were shared between the 971 targets of TFL1 identified in this study and the 595 high-confidence FD targets (Collani et al., 2019; Supplemental Data S4; Supplemental Fig. S12A). The large proportion of shared peaks between gTFL1-1xVenus and pFD::GFP-FD strongly suggests that FD is an important interactor of TFL1 at the SAM. Among the genes bound by both TFL1 and FD are important regulators involved in LD photoperiodism and flowering (CCA1, LHY, and NF-YB2), in development of the reproductive shoot system (HOMEODOMAIN GLABROUS5, MONOPTEROS, SEUSS-LIKE1, and CYCLING DOF FACTOR5), and in the jasmonate pathway (JASMONATE-ZIM-DOMAIN PROTEIN3 [JAZ3], JAZ6, MYC2, ETHYLENE-RESPONSIVE ELEMENT-BINDING FACTOR4, UBIQUITIN-SPECIFIC PROTEASE13, and JASMONATE RESISTANT1).
However, we also found 613 unique peaks for TFL1 and 237 unique peaks for FD (Supplemental Data S5 and S6). Since we detected a considerable number of peaks specific for TFL1, we can assume that TFL1 binds DNA also through interactions with other DNA-binding proteins (Supplemental Data S5; Supplemental Fig. S12B). For example, TFL1, but not FD, binds to genes that are involved in Fru-1,6-bisphosphate metabolism (CHLOROPLASTIC FRUCTOSE-1,6-BISPHOSPHATASE1 and CYTOSOLIC FRUCTOSE-1,6-BISPHOSPHATASE) and in the trehalose pathway (TPPA, TPPE, TPPJ, TPPH, and TREHALOSE-6-PHOSPHATE SYNTHASE6 [TPS6]). Which transcription factors, besides FD, interact with TFL1 is currently not known. However, given the strong overrepresentation of G-box motifs among the genes bound specifically by TFL1 (not shared with FD), these yet unknown interaction partners most likely include DNA-binding proteins with a preference for a G-box, such as other bZIP or basic helix-loop-helix (bHLH) proteins.
DISCUSSION
Movement of TFL1 within the Shoot Meristem
All multicellular living beings rely on mobile, long-distance and cell-to-cell signals to coordinate growth and development of different parts of the organism. The production of signaling molecules is temporally and spatially tightly controlled, and they are usually present in cells at low concentration. Furthermore, mobile signaling molecules are usually small molecules to facilitate efficient translocation. Common signal molecules include hormones, RNAs, small peptides, and transcription factors (Busch and Benfey, 2010; Van Norman et al., 2011). In plants, signaling molecules can move either through the apoplastic space between cells or symplastically from cell to cell through plasmodesmata. A prominent example of long-distance signaling is the transport of the FT protein, a florigen that is produced in leaf phloem companion cells and transported through the sieve elements to induce flowering at the SAM (Srikanth and Schmid, 2011). Interestingly, TFL1, a protein that is closely related to FT but acts as a repressor of flowering, has been suggested to move within the SAM (Conti and Bradley, 2007). This notion is based on the observation that TFL1 mRNA is expressed in the central part of the SAM, but the protein has a wider distribution, reaching the outer layers of the apex (Conti and Bradley, 2007). However, the relevance of TFL1 protein movement for its function in SAM development and flowering time regulation has not been addressed in detail.
Here, we first showed that the ability of TFL1 to move within the SAM is essential for complementing the early-flowering phenotype of the tfl1-1 mutant (Fig. 1B). When tagged with a single copy of the Venus fluorescent protein under the control of the genomic TFL1 sequences (gTLF1-1xVenus), the fusion protein was detected most strongly in the center of the SAM, but it also reached the epidermal L1 layer of the SAM and largely rescued the early-flowering phenotype of the tfl1-1 mutant (Fig. 2; Supplemental Fig. S6A). However, rescue of tfl1-1 early flowering by gTFL1 was not always complete, possibly because our genomic constructs lack the block V TFL1 cis-regulatory region, which has been reported to contain an enhancer element that contributes to the expression level of TFL1, without being required for correct temporal and spatial expression. Furthermore, when we expressed TFL1 under the control of the epidermis-specific ML1 promoter, 44% of the T1 plants (ML1pro::TFL1) flowered similar to the wild type, with more than 14 total leaves (Fig. 3A; Supplemental Fig. S7). Taken together, these results suggest that TFL1 acts at least in part in the apical cells of the tunica during the floral transition and provide evidence that movement within the SAM contributes to TFL1 function.
It seems possible that TFL1 interacts in the tunica with transcription factors, such as FD, to prevent the switch from vegetative to reproductive development, thereby maintaining the inflorescence meristem in an undifferentiated state. In this context, it is also worthwhile to note that ATC, a protein that is closely related to TFL1, has been shown to influence flowering time specifically when expressed under the control of the ML1 promoter under SD conditions (Huang et al., 2012).
The ML1 promoter is expressed in all epidermal cells, but its activity increases at the shoot apex during the transition to flowering (Sessions et al., 1999). However, even at the inflorescence meristem, activity of the ML1 promoter is thought to be weaker than that of the 35S promoter. Furthermore, the 35S promoter drives strong expression in the vegetative SAM as well, and across the entire apex, not only in the L1 cells. This could explain why lines expressing TFL1 under the control of the ML1 promoter, while flowering later than the tfl1 mutant, were nevertheless not as late flowering as the 35Spro::TFL1 lines (Figs. 1C and 3A).
When expressed from the ML1 promoter, the TFL1-1xGFP signal accumulated in the L1 layer but could also be detected in L2 and to some extent even in L3 cells (Fig. 3B). The finding that the TFL1 protein can move in either direction in the SAM suggests that movement occurs via passive diffusion, following a concentration gradient, rather than by active directional transport. This is not entirely surprising, as it has previously been shown that small proteins such as GFP (27 kD), which has a similar size to TFL1 (20 kD), can move relatively freely at the apex (Crawford and Zambryski, 2000; Wu et al., 2003; Kim et al., 2005). However, this is in contrast to a previous report that TFL1 movement was impaired in lfy mutants, in which the protein accumulates in the central region of the SAM where its mRNA is expressed (Conti and Bradley, 2007). One possible explanation for this discrepancy is that LFY could regulate the size exclusion limit of plasmodesmata in the SAM, which would indirectly affect the movement of macromolecules between cells. Alternatively, it seems possible that LFY facilitates TFL1 movement through direct protein interactions or by regulating the expression of an unknown factor that is required for TFL1 movement.
It should be noted that the homeodomain protein WUSCHEL, which is expressed in the organizing center of the SAM, has been shown to move upward toward the stem cell population of the tip of the shoot meristem (Yadav et al., 2011; Daum et al., 2014), suggesting that protein movement within the SAM might be more common than originally thought.
TFL1 Movement Affects Meristem Determinacy
Another interesting outcome of our experiments relates to the regulation of meristem indeterminacy by TFL1. T1 plants expressing the mobile TFL1-1xGFP or immobile TFL1-3xGFP variants under the control of either its own regulatory elements or the ML1 promoter, either in the presence or absence of NLS signal (Table 1), displayed substantial differences in shoot determinacy. Indeterminate growth of the inflorescences was restored in approximately 50% of T1 plants expressing the gTFL1-1xGFP protein. All the other combinations showed a determinate growth of the main inflorescence, as in the tfl1-1 mutant. These results suggest that TFL1 regulates shoot indeterminacy outside the TFL1 expression domain, as TFL1 variants that are prevented from moving by the large 3xGFP tag and/or nuclear localization (due to the addition of an NLS) are incapable of rescuing the determinate growth of the tfl1-1 mutant. An alternative explanation is that, to regulate the indeterminate growth of the shoot, TFL1 is required at the same time both in the center of the SAM and in the tunica.
In contrast to the effect of TFL1 on flowering time, expression of TFL1 from the ML1 promoter was not sufficient to rescue the determinate growth of the tfl1-1 mutant. This suggests that the function of TFL1 in the regulation of indeterminate growth is not limited to the L1 layer. Interestingly, the MADS domain transcription factors AGAMOUS and SEPALLATA3, two important regulators of floral development, have been reported to convert the inflorescence meristem to a terminal flower when expressed from a constitutive promoter, but they had no effect on the growth habit when expression was restricted to the L1 (Mizukami and Ma, 1992; Honma and Goto, 2001; Urbanus et al., 2010). In summary, our findings indicate that TFL1 movement is crucial in the regulation of inflorescence meristem indeterminacy, although the precise region in which TFL1 activity is required remains to be determined.
TFL1 Is a Transcriptional Coregulator of Flowering-Related Genes
The subcellular localization and molecular function of TFL1 have been discussed controversially. However, it had been shown that a translational fusion between TFL1 and the strong transcription activation domain VP16 induced the expression of genes that are believed to be normally repressed by TFL1, suggesting that the protein might function as a transcriptional corepressor (Hanano and Goto, 2011). Our ChIP-seq and RNA-seq results support the idea that TFL1 acts as a transcriptional cofactor in the apex during the floral transition, identifying 115 putative direct targets that are both bound and transcriptionally regulated by a TFL1-containing protein complex. TFL1 has been reported to have transcriptional repressor activity based on the phenotype of SRDX-tagged plants (Hanano and Goto, 2011). Consistent with this previous study, application of DEX to our inducible TFL1-GR lines also resulted in late flowering, confirming the negative effect of TFL1 on this process (Supplemental Fig. S10, A and B). It is thus surprising that our RNA-seq analysis identified more up-regulated than down-regulated genes in the 35Spro::TFL1-GR line in response to DEX treatment (Fig. 5B). However, as we sampled apices 24 h after DEX application, it is likely that a substantial part of the differentially expressed genes are regulated by TFL1 indirectly. Furthermore, in our RNA-seq analyses, DEX was applied to 35Spro::TFL1-GR plants 6 d after the plants had been shifted from SD to LD. At this time point, the transition to flowering has started; however, expression of the early flower meristem and floral homeotic genes LFY and AP1 can still be efficiently suppressed by DEX application (Supplemental Fig. S10, C and D). Nevertheless, we cannot completely rule out the possibility that other genes that are normally repressed by TFL1 were not responding to the DEX treatment, which might explain the relatively large number of up-regulated transcripts in our analysis. However, given that the 35S::TFL1-SRDX lines closely resemble 35S::TFL1 overexpression plants (Hanano and Goto, 2011), the most parsimonious explanation still remains that TFL1 acts as a transcriptional corepressor of flowering time genes.
As there is no evidence that TFL1 can directly interact with DNA, binding is most likely facilitated through transcription factors. In this context, it is worthwhile to note that among the TFL1-bound regions, G-box motifs are highly overrepresented. This suggests that TFL1 interacts with DNA through transcription factors that bind to G-box motifs, such as bZIP and bHLH proteins (Sibéril et al., 2001; Jones, 2004).
One bZIP transcription factor that has been shown to physically interact in complex with TFL1 is FD, an important regulator of flowering time in Arabidopsis (Abe et al., 2005; Wigge et al., 2005). Interestingly, comparison of TFL1-bound regions with direct FD targets identified 358 shared peaks, confirming that TFL1 is an important FD interactor (Collani et al., 2019). Both TFL1 and FD are involved in floral transition at the SAM and bind genes that regulate auxin-mediated signaling and response to stimuli. However, the two proteins clearly have partially separate roles in the regulation of development. For example, binding of FD was enriched on genes involved in maintenance of the inflorescence meristem and flower and carpel development, suggesting that FD but not TFL1 might have a yet unreported role in fruit and seed development. Similarly, TFL1 also appears to have FD-independent functions, as we identified 613 peaks unique to TFL1, suggesting that it can form complexes with proteins other than FD. One likely candidate that might mediate TFL1 binding to DNA is FD PARALOG (AtbZIP27), which has been shown to interact with TFL1 (Wigge et al., 2005) and mutation of which enhances the late-flowering phenotype of the fd-2 mutant (Jaeger et al., 2013). Among these TFL1-specific targets were many genes involved in the regulation of carbohydrate metabolic processes, whose impact on SAM organization at the floral transition has not yet been explored in detail, and response to different wavelengths of light.
Interestingly, LFY, which has long been known to be genetically regulated by TFL1 (Ratcliffe et al., 1998), is among the direct TFL1-specific target genes (Fig. 6). ChIP-seq identified a G-box in the second exon of LFY (Fig. 4D), which showed strong enrichment for TFL1 but not for FD binding. This suggests that at the apex FD can induce LFY expression only indirectly, most likely by up-regulating the expression of SPL genes and SUPPRESSOR OF OVEREXPRESSION OF CO1 (Jung et al., 2012). To the best of our knowledge, no positive regulators that directly bind to the second exon of LFY have been reported, indicating that the regulation by TFL1 is mediated by a yet unknown transcription factor. Importantly, LFY is able to directly bind both FD and TFL1 cis-regulatory regions (Moyroud et al., 2011). This suggests the existence of a tight feedback loop between these genes that likely contributes to a fast and stable regulation of the floral transition and meristem identity.
Another interesting case is the up-regulation of PIF5 by TFL1, independently from FD (Figs. 4D and 6). The role of PIF5, and even more so PIF4, as a positive regulator of cell elongation in the hypocotyl has already been documented (Niwa et al., 2009; Kunihiro et al., 2011; Choi and Oh, 2016). However, the role of PIFs at the SAM has not yet been investigated in detail. Recently, it was reported that misexpression of PIFs from the FD promoter had only a minor effect on flowering time (Galvão et al., 2015). During the floral transition, the SAM first increases in size due to a proliferation of cells in the meristem, followed by the elongation of internodes during bolting (Jacqmard et al., 2003). As the growth of the apical region of the stem is in part due to an elongation of cells of the rib meristem, we wonder whether PIFs could be involved in regulating these morphological changes during the floral transition. In this context, it is worth mentioning that PIFs belong to the bHLH class of transcription factors that bind G-box consensus sequences on the DNA (Leivar and Quail, 2011). These findings suggest that TFL1-PIFs might interact in a protein complex; however, further experiments will be required to test this hypothesis.
Finally, several genes related to trehalose 6-phosphate were differentially expressed in response to TFL1 induction according to our RNA-seq experiments. As trehalose 6-phosphate is a floral promoter, changes in the expression of TPS and TPP genes at the apex under LD conditions could affect the floral transition (Wahl et al., 2013). Furthermore, in maize (Zea mays), the gene RAMOSA3, which encodes a TPP, regulates identity and determinacy of the axillary meristems (Satoh-Nagasawa et al., 2006). It would thus be extremely interesting to elucidate if a similar mechanism involving TFL1 and some TPPs could be active in the regulation of the inflorescence architecture also in Arabidopsis.
CONCLUSION
In summary, we found that movement of TFL1 in the SAM is critical for its function in the regulation of flowering time and inflorescence development in Arabidopsis. Furthermore, TFL1 movement from the inner parts of the meristem to the L1 layer is probably not a directional process. In the SAM, TFL1 is strongly enriched at G-box motifs, suggesting that it is preferentially recruited to DNA through interaction with bZIP and possibly bHLH transcription factors. Our results demonstrate that TFL1 acts as a transcription cofactor at the SAM during the floral transition, and we identified a genome-wide list of putative TFL1 targets.
MATERIALS AND METHODS
Plant Materials and Growth Conditions
Arabidopsis (Arabidopsis thaliana) accession Col-0 was used as the wild type. The tfl1-1 mutation and phenotype in the Col-0 background have been described previously (Shannon and Meeks-Wagner, 1991). Plants were grown on soil at 65% relative humidity under wide-spectrum fluorescent lights with a fluence rate of 125 to 175 μmol m−2 s−1. For flowering time studies and ChIP experiments, seeds were stratified for 3 d in 0.1% (w/v) agar in the dark at 4°C and directly planted on soil. Plants were grown on soil under LD (16 h of light and 8 h of night) at 22°C during the day and 18°C during the night. Plants intended for measuring flowering time were grown in a randomized design to minimize positional effects. Flowering time was scored as rosette leaves, cauline leaves, and total leaf number, as well as days to flowering, which is defined as the number of days from sowing until the emergence of the first visible flower buds. For RNA-seq experiments, plants were first grown under SD conditions (8 h of light and 16 h of night) at 21°C during the day and 19°C during the night for 3 weeks and then shifted to LD.
DNA Vectors and Plant Transformation
Sequences were amplified by PCR from cDNA or genomic DNA and cloned into pGREEN vectors by using either a classical restriction enzyme cloning approach or the GreenGate system (Lampropoulos et al., 2013). To generate the 35Spro::TFL1-GR line, the CDS of TFL1 was cloned into the pGreen0229 plasmid containing a 35Spro::GR construct (Yu et al., 2004). Final constructs were transformed into Agrobacterium tumefaciens (strain GV3101 pMP90 pSoup) by electroporation (Gene Pulser Xcell system). Arabidopsis plants of accession Col-0 and the tfl1-1 mutant were transformed by the floral dip method. BASTA selection (0.1%, v/v) was used for screening the transgenic lines on soil. Lists of the PCR primers used for cloning and the plant binary vectors generated in this study can be found in Supplemental Tables S1 and S2.
DEX Treatment
To induce the translocation of TFL1-GR to the nucleus, 35Spro::TFL1-GR plants were treated after their shift to LD conditions with a solution containing either 30 µm DEX, 0.03% (v/v) ethanol, and 0.01% (v/v) Silwet L-77 or a solution with an equal composition but without DEX (mock solution) by applying a single drop directly to the shoot apex at ZT+2.
Establishing and Verifying Experimental Conditions for RNA-Seq
To establish the experimental conditions and test the effect of DEX treatment on LFY and AP1 expression, 12 manually dissected apices were collected for three biological replicates each. Total RNA was extracted using the RNeasy Mini Kit (Qiagen) and treated with DNaseI (Ambion) following the manufacturer’s instructions. Then, 1 µg of RNA was reverse transcribed into single-stranded cDNA using SuperScriptII (Invitrogen) in a 20-µL reaction mixture. The resulting cDNA was diluted to a final volume of 90 µL and 5 μL of each sample was used in qPCR. qPCR reactions were performed on a Fast II system (Applied Biosystems) using SYBR Green (Takara). The primers used for the quantitative analysis are described in Supplemental Table S1. qPCR was performed on three independent biological replicates, and TIP41 was used as a reference gene. Relative expression was calculated based on the ddCt method and normalized to the T0 control (mock) sample.
RNA-Seq and Validation
For RNA-seq experiments, 35Spro::TFL1-GR plants were grown for 3 weeks under SD before being shifted to LD. DEX or mock solution was applied directly to the shoot apex at ZT+2 6 d after the shift to LD, and samples were collected 24 h later. Approximately 60 manually dissected apices were collected for each of three replicates per condition. Total RNA was extracted using the RNeasy Mini Kit (Qiagen) and treated with DNaseI (Ambion) following the manufacturer’s instructions. For RNA-seq analysis, the quality of the resulting RNA was checked on an Agilent 2100 Bioanalyzer instrument using the RNA6000 nano kit. Strand-specific RNA libraries were constructed using the TruSeq stranded mRNA kit (Illumina). Libraries were sequenced on a NextSeq500 platform (Illumina) using the High Output 75 cycles kit v2.0 (Illumina) to produce 75-nucleotide single-end reads. Libraries were sequenced at the genomics core facility at Servicio Central de Soporte a la Investigación of the University of Valencia, Spain. All RNA-seq data are available from the European Nucleotide Archive under accession number PRJEB29016.
For validation of the RNA-seq results by RT-qPCR, 35Spro::TFL1-GR plants were grown 3 weeks under SD conditions and then shifted to LD. DEX or mock solution was applied at ZT0 6 d after the shift to LD, and samples were collected 2 and 26 h later. RNA extraction and RT-qPCR were performed as described above.
ChIP-Seq and ChIP-PCR
For ChIP-seq experiments, approximately 300 mg of manually dissected apices (gTFL1-1xVenus and Col-0) from 15-d-old plants grown on soil under LD were harvested. Samples were first prefixed under vacuum for 30 min in 2 mm ethyleneglycol-bis-succinimidyl-succinate solution and then postfixed for an additional 30 min after the addition of formaldehyde to a final concentration of 1% (v/v). ChIP was performed as previously described (Kaufmann et al., 2010). Anti-GFP from Abcam (ab290) was used for immunoprecipitation of the Venus-tagged protein complex. ChIP-seq libraries were prepared by Novogene Bioinformatics Technology using the NEB Next Ultra II DNA Library Prep Kit, selecting fragments between 100 and 350 bp. Libraries were sequenced on an Illumina HiSeq3000 system using the 50-bp single-end kit. All ChIP-seq data are available from the European Nucleotide Archive under accession number PRJEB29016.
For the ChIP-PCR experiment using the anti-GR antibody (Abcam, ab3580), 35Spro::TFL1-GR and Col-0 plants were grown for 3 weeks under SD conditions and then transferred to LD. After 5 d in LD, plants were treated with one drop of 30 µm DEX or mock directly applied to the shoot apex. Approximately 300 mg of apices were manually dissected 24 h after DEX application, and ChIP was performed as described above. For ChIP-PCR, a 1:3 dilution of immunoprecipitated DNA was analyzed by qPCR, performed on a CFX96 Touch Real-Time PCR Detection System (Bio-Rad) using LightCycler 480 SYBR Green I Master (Roche). Oligonucleotides used as primers for qPCR are listed in Supplemental Table S1.
ChIP-Seq and RNA-Seq Analyses
ChIP-seq raw data were aligned to the Arabidopsis genome (TAIR10 release) using bwa (Li and Durbin, 2010). Peaks were called with MACS2 (Zhang et al., 2008) using default parameters, and mapped reads from Col-0 were used as a control. Overlapping peaks were retrieved using the R package DiffBind using default parameters (Ross-Innes et al., 2012; Stark and Brown, 2013). De novo motif analysis using MEME-ChIP (Machanick and Bailey, 2011) was used for identifying the binding site.
For RNA-seq analyses, ribosomal RNA sequences were filtered out using SortMeRNA (Kopylova et al., 2012). Adapters were trimmed from the remaining reads using Trimmomatic (Bolger et al., 2014). The trimmed sequences were then aligned against the Arabidopsis transcriptome (TAIR10) using STAR (Dobin et al., 2013) and reads were counted with HTSeqCount (Anders et al., 2015). DESeq2 with default parameters was used to perform differential expression analysis (Love et al., 2014).
Confocal Laser Scanning Microscopy
To detect Venus- and GFP-tagged proteins, manually dissected apices were collected from plants grown under LD conditions. Samples were fixed as previously described (Gregis et al., 2009). Sections between 70 and 90 μm thick were obtained using a Leica vibratome (VT1000S). Confocal laser scanning microscopy was performed using a Zeiss LSM 780 microscope (25× magnification).
Statistical Analyses
Flowering time data were analyzed by one-way ANOVA with posthoc Tukey’s honestly significant difference based on Tukey-Kramer correction (P < 0.05). Statistical significance calculations for the RT-qPCR data shown in Figure 6C were performed with two-tailed Student’s t test using three biological replicates with at least 12 plants per replicate. Differences in expression were deemed significant at P < 0.05. Normality of the data was determined with the Shapiro-Wilk test.
Accession Numbers
RNA-seq and ChIP-seq data were deposited at the European Nucleotide Archive under project identifier PRJEB29016.
Supplemental Data
The following supplemental materials are available.
Supplemental Figure S1. Flowering time of gTFL1 lines.
Supplemental Figure S2. gTFL1-1xGFP but not gTFL1-3xGFP complements the flowering time and shoot meristem defects of the tfl1-1 mutant.
Supplemental Figure S3. Flowering time data for stable (T4) overexpressor lines.
Supplemental Figure S4. Flowering time data for stable (T4) tagged gTFL1 lines.
Supplemental Figure S5. Localization of gTFL1-1xVenus protein at the shoot apex.
Supplemental Figure S6. Localization of gTFL1-1xVenus and gTFL1-3xVenus protein in different T4 lines.
Supplemental Figure S7. Flowering time data for T1 lines expressing tagged and untagged TFL1 from the ML1 promoter.
Supplemental Figure S8. MEME output for the three most strongly enriched motifs in TFL1-bound regions.
Supplemental Figure S9. GO analysis output for differentially bound (ChIP-seq) and expressed (RNA-seq) genes.
Supplemental Figure S10. Characterization of 35Spro::TFL1-GR lines.
Supplemental Figure S11. Validation of TFL1 target genes by ChIP-PCR using 35Spro::TFL1-GR (#7.9).
Supplemental Figure S12. GO analysis output for genes bound by FD and TFL1 or specifically by TFL1.
Supplemental Table S1. List of primers used in this study.
Supplemental Table S2. List of plasmids used in this study.
Supplemental Data S1. List of the 971 TFL1-bound peaks identified in seedlings expressing gTFL1-1xVenus in tfl1-1.
Supplemental Data S2. List of genes regulated by TFL1-GR.
Supplemental Data S3. List of the 115 bound and differentially expressed genes.
Supplemental Data S4. List of the 358 TFL1- and FD-bound peaks.
Supplemental Data S5. List of the 613 TFL1-bound peaks not shared with FD.
Supplemental Data S6. List of the 237 FD-bound peaks not shared with TFL1.
Footnotes
This work was supported by the Spanish Ministerio de Ciencia Innovación y Universidades (grant no. BIO2015-64307-R to F.M.) and FEDER (grant no. PGC2018-099232-B-I00 to F.M.) and by the Knut and Alice Wallenberg Foundation (grant no. KAW 2016.0025 to M.Sc.). Additional support was provided by the Umeå Plant Science Centre (UPSC) through VINNOVA. M.Si. was supported by an Formación de Personal Universitario (FPU) contract from the Spanish Ministerio de Educación, Cultura y Deporte, and C.M. was supported by a Santiago Grisolía fellowship from the Generalitat Valenciana.
Articles can be viewed without a subscription.
References
- Abe M, Kobayashi Y, Yamamoto S, Daimon Y, Yamaguchi A, Ikeda Y, Ichinoki H, Notaguchi M, Goto K, Araki T (2005) FD, a bZIP protein mediating signals from the floral pathway integrator FT at the shoot apex. Science 309: 1052–1056 [DOI] [PubMed] [Google Scholar]
- Ahn JH, Miller D, Winter VJ, Banfield MJ, Lee JH, Yoo SY, Henz SR, Brady RL, Weigel D (2006) A divergent external loop confers antagonistic activity on floral regulators FT and TFL1. EMBO J 25: 605–614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alabadí D, Oyama T, Yanovsky MJ, Harmon FG, Más P, Kay SA (2001) Reciprocal regulation between TOC1 and LHY/CCA1 within the Arabidopsis circadian clock. Science 293: 880–883 [DOI] [PubMed] [Google Scholar]
- An H, Roussot C, Suárez-López P, Corbesier L, Vincent C, Piñeiro M, Hepworth S, Mouradov A, Justin S, Turnbull C, et al. (2004) CONSTANS acts in the phloem to regulate a systemic signal that induces photoperiodic flowering of Arabidopsis. Development 131: 3615–3626 [DOI] [PubMed] [Google Scholar]
- Anders S, Pyl PT, Huber W (2015) HTSeq: A Python framework to work with high-throughput sequencing data. Bioinformatics 31: 166–169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumann K, Venail J, Berbel A, Domenech MJ, Money T, Conti L, Hanzawa Y, Madueno F, Bradley D (2015) Changing the spatial pattern of TFL1 expression reveals its key role in the shoot meristem in controlling Arabidopsis flowering architecture. J Exp Bot 66: 4769–4780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolger AM, Lohse M, Usadel B (2014) Trimmomatic: A flexible trimmer for Illumina sequence data. Bioinformatics 30: 2114–2120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowman JL, Alvarez J, Weigel D, Meyerowitz EM, Smyth DR (1993) Control of flower development in Arabidopsis thaliana by APETALA1 and interacting genes. Development 119: 721 [Google Scholar]
- Bradley D, Ratcliffe O, Vincent C, Carpenter R, Coen E (1997) Inflorescence commitment and architecture in Arabidopsis. Science 275: 80–83 [DOI] [PubMed] [Google Scholar]
- Busch W, Benfey PN (2010) Information processing without brains: The power of intercellular regulators in plants. Development 137: 1215–1226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi H, Oh E (2016) PIF4 integrates multiple environmental and hormonal signals for plant growth regulation in Arabidopsis. Mol Cells 39: 587–593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collani S, Neumann M, Yant L, Schmid M (2019) FT modulates genome-wide DNA-binding of the bZIP transcription factor FD. Plant Physiol 180: 367–380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conti L, Bradley D (2007) TERMINAL FLOWER1 is a mobile signal controlling Arabidopsis architecture. Plant Cell 19: 767–778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbesier L, Vincent C, Jang S, Fornara F, Fan Q, Searle I, Giakountis A, Farrona S, Gissot L, Turnbull C, et al. (2007) FT protein movement contributes to long-distance signaling in floral induction of Arabidopsis. Science 316: 1030–1033 [DOI] [PubMed] [Google Scholar]
- Crawford KM, Zambryski PC (2000) Subcellular localization determines the availability of non-targeted proteins to plasmodesmatal transport. Curr Biol 10: 1032–1040 [DOI] [PubMed] [Google Scholar]
- Daum G, Medzihradszky A, Suzaki T, Lohmann JU (2014) A mechanistic framework for noncell autonomous stem cell induction in Arabidopsis. Proc Natl Acad Sci USA 111: 14619–14624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobin A, Davis CA, Schlesinger F, Drenkow J, Zaleski C, Jha S, Batut P, Chaisson M, Gingeras TR (2013) STAR: Ultrafast universal RNA-seq aligner. Bioinformatics 29: 15–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fornara F, de Montaigu A, Coupland G (2010) SnapShot: Control of flowering in Arabidopsis. Cell 141: 550.e1-550.e2 [DOI] [PubMed] [Google Scholar]
- Galvão VC, Collani S, Horrer D, Schmid M (2015) Gibberellic acid signaling is required for ambient temperature-mediated induction of flowering in Arabidopsis thaliana. Plant J 84: 949–962 [DOI] [PubMed] [Google Scholar]
- Gómez-Mena C, de Folter S, Costa MMR, Angenent GC, Sablowski R (2005) Transcriptional program controlled by the floral homeotic gene AGAMOUS during early organogenesis. Development 132: 429–438 [DOI] [PubMed] [Google Scholar]
- Gregis V, Sessa A, Dorca-Fornell C, Kater MM (2009) The Arabidopsis floral meristem identity genes AP1, AGL24 and SVP directly repress class B and C floral homeotic genes. Plant J 60: 626–637 [DOI] [PubMed] [Google Scholar]
- Hanano S, Goto K (2011) Arabidopsis TERMINAL FLOWER1 is involved in the regulation of flowering time and inflorescence development through transcriptional repression. Plant Cell 23: 3172–3184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honma T, Goto K (2001) Complexes of MADS-box proteins are sufficient to convert leaves into floral organs. Nature 409: 525–529 [DOI] [PubMed] [Google Scholar]
- Huang NC, Jane WN, Chen J, Yu TS (2012) Arabidopsis thaliana CENTRORADIALIS homologue (ATC) acts systemically to inhibit floral initiation in Arabidopsis. Plant J 72: 175–184 [DOI] [PubMed] [Google Scholar]
- Jacqmard A, Gadisseur I, Bernier G (2003) Cell division and morphological changes in the shoot apex of Arabidopsis thaliana during floral transition. Ann Bot 91: 571–576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaeger KE, Pullen N, Lamzin S, Morris RJ, Wigge PA (2013) Interlocking feedback loops govern the dynamic behavior of the floral transition in Arabidopsis. Plant Cell 25: 820–833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaeger KE, Wigge PA (2007) FT protein acts as a long-range signal in Arabidopsis. Curr Biol 17: 1050–1054 [DOI] [PubMed] [Google Scholar]
- Jones S. (2004) An overview of the basic helix-loop-helix proteins. Genome Biol 5: 226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung JH, Ju Y, Seo PJ, Lee JH, Park CM (2012) The SOC1-SPL module integrates photoperiod and gibberellic acid signals to control flowering time in Arabidopsis. Plant J 69: 577–588 [DOI] [PubMed] [Google Scholar]
- Kaneko-Suzuki M, Kurihara-Ishikawa R, Okushita-Terakawa C, Kojima C, Nagano-Fujiwara M, Ohki I, Tsuji H, Shimamoto K, Taoka KI (2018) TFL1-like proteins in rice antagonize rice FT-like protein in inflorescence development by competition for complex formation with 14-3-3 and FD. Plant Cell Physiol 59: 458–468 [DOI] [PubMed] [Google Scholar]
- Karlgren A, Gyllenstrand N, Källman T, Sundström JF, Moore D, Lascoux M, Lagercrantz U (2011) Evolution of the PEBP gene family in plants: Functional diversification in seed plant evolution. Plant Physiol 156: 1967–1977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufmann K, Muiño JM, Østerås M, Farinelli L, Krajewski P, Angenent GC (2010) Chromatin immunoprecipitation (ChIP) of plant transcription factors followed by sequencing (ChIP-SEQ) or hybridization to whole genome arrays (ChIP-CHIP). Nat Protoc 5: 457–472 [DOI] [PubMed] [Google Scholar]
- Kim I, Cho E, Crawford K, Hempel FD, Zambryski PC (2005) Cell-to-cell movement of GFP during embryogenesis and early seedling development in Arabidopsis. Proc Natl Acad Sci USA 102: 2227–2231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JY, Yuan Z, Jackson D (2003) Developmental regulation and significance of KNOX protein trafficking in Arabidopsis. Development 130: 4351–4362 [DOI] [PubMed] [Google Scholar]
- Kopylova E, Noé L, Touzet H (2012) SortMeRNA: Fast and accurate filtering of ribosomal RNAs in metatranscriptomic data. Bioinformatics 28: 3211–3217 [DOI] [PubMed] [Google Scholar]
- Kumimoto RW, Adam L, Hymus GJ, Repetti PP, Reuber TL, Marion CM, Hempel FD, Ratcliffe OJ (2008) The Nuclear Factor Y subunits NF-YB2 and NF-YB3 play additive roles in the promotion of flowering by inductive long-day photoperiods in Arabidopsis. Planta 228: 709–723 [DOI] [PubMed] [Google Scholar]
- Kumimoto RW, Zhang Y, Siefers N, Holt BF III (2010) NF-YC3, NF-YC4 and NF-YC9 are required for CONSTANS-mediated, photoperiod-dependent flowering in Arabidopsis thaliana. Plant J 63: 379–391 [DOI] [PubMed] [Google Scholar]
- Kunihiro A, Yamashino T, Nakamichi N, Niwa Y, Nakanishi H, Mizuno T (2011) Phytochrome-interacting factor 4 and 5 (PIF4 and PIF5) activate the homeobox ATHB2 and auxin-inducible IAA29 genes in the coincidence mechanism underlying photoperiodic control of plant growth of Arabidopsis thaliana. Plant Cell Physiol 52: 1315–1329 [DOI] [PubMed] [Google Scholar]
- Lampropoulos A, Sutikovic Z, Wenzl C, Maegele I, Lohmann JU, Forner J (2013) GreenGate: A novel, versatile, and efficient cloning system for plant transgenesis. PLoS ONE 8: e83043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leivar P, Quail PH (2011) PIFs: Pivotal components in a cellular signaling hub. Trends Plant Sci 16: 19–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Durbin R (2010) Fast and accurate long-read alignment with Burrows-Wheeler transform. Bioinformatics 26: 589–595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Love MI, Huber W, Anders S (2014) Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol 15: 550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machanick P, Bailey TL (2011) MEME-ChIP: Motif analysis of large DNA datasets. Bioinformatics 27: 1696–1697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathieu J, Warthmann N, Küttner F, Schmid M (2007) Export of FT protein from phloem companion cells is sufficient for floral induction in Arabidopsis. Curr Biol 17: 1055–1060 [DOI] [PubMed] [Google Scholar]
- Mimida N, Goto K, Kobayashi Y, Araki T, Ahn JH, Weigel D, Murata M, Motoyoshi F, Sakamoto W (2001) Functional divergence of the TFL1-like gene family in Arabidopsis revealed by characterization of a novel homologue. Genes Cells 6: 327–336 [DOI] [PubMed] [Google Scholar]
- Mizoguchi T, Wheatley K, Hanzawa Y, Wright L, Mizoguchi M, Song HR, Carré IA, Coupland G (2002) LHY and CCA1 are partially redundant genes required to maintain circadian rhythms in Arabidopsis. Dev Cell 2: 629–641 [DOI] [PubMed] [Google Scholar]
- Mizukami Y, Ma H (1992) Ectopic expression of the floral homeotic gene AGAMOUS in transgenic Arabidopsis plants alters floral organ identity. Cell 71: 119–131 [DOI] [PubMed] [Google Scholar]
- Moyroud E, Minguet EG, Ott F, Yant L, Posé D, Monniaux M, Blanchet S, Bastien O, Thévenon E, Weigel D, et al. (2011) Prediction of regulatory interactions from genome sequences using a biophysical model for the Arabidopsis LEAFY transcription factor. Plant Cell 23: 1293–1306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niwa Y, Yamashino T, Mizuno T (2009) The circadian clock regulates the photoperiodic response of hypocotyl elongation through a coincidence mechanism in Arabidopsis thaliana. Plant Cell Physiol 50: 838–854 [DOI] [PubMed] [Google Scholar]
- Pnueli L, Gutfinger T, Hareven D, Ben-Naim O, Ron N, Adir N, Lifschitz E (2001) Tomato SP-interacting proteins define a conserved signaling system that regulates shoot architecture and flowering. Plant Cell 13: 2687–2702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratcliffe OJ, Amaya I, Vincent CA, Rothstein S, Carpenter R, Coen ES, Bradley DJ (1998) A common mechanism controls the life cycle and architecture of plants. Development 125: 1609–1615 [DOI] [PubMed] [Google Scholar]
- Ratcliffe OJ, Bradley DJ, Coen ES (1999) Separation of shoot and floral identity in Arabidopsis. Development 126: 1109–1120 [DOI] [PubMed] [Google Scholar]
- Reymond MC, Brunoud G, Chauvet A, Martínez-Garcia JF, Martin-Magniette ML, Monéger F, Scutt CP (2012) A light-regulated genetic module was recruited to carpel development in Arabidopsis following a structural change to SPATULA. Plant Cell 24: 2812–2825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross-Innes CS, Stark R, Teschendorff AE, Holmes KA, Ali HR, Dunning MJ, Brown GD, Gojis O, Ellis IO, Green AR, et al. (2012) Differential oestrogen receptor binding is associated with clinical outcome in breast cancer. Nature 481: 389–393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryu JY, Lee HJ, Seo PJ, Jung JH, Ahn JH, Park CM (2014) The Arabidopsis floral repressor BFT delays flowering by competing with FT for FD binding under high salinity. Mol Plant 7: 377–387 [DOI] [PubMed] [Google Scholar]
- Satoh-Nagasawa N, Nagasawa N, Malcomber S, Sakai H, Jackson D (2006) A trehalose metabolic enzyme controls inflorescence architecture in maize. Nature 441: 227–230 [DOI] [PubMed] [Google Scholar]
- Schultz EA, Haughn GW (1991) LEAFY, a homeotic gene that regulates inflorescence development in Arabidopsis. Plant Cell 3: 771–781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serrano-Mislata A, Fernández-Nohales P, Doménech MJ, Hanzawa Y, Bradley D, Madueño F (2016) Separate elements of the TERMINAL FLOWER 1 cis-regulatory region integrate pathways to control flowering time and shoot meristem identity. Development 143: 3315–3327 [DOI] [PubMed] [Google Scholar]
- Sessions A, Weigel D, Yanofsky MF (1999) The Arabidopsis thaliana MERISTEM LAYER 1 promoter specifies epidermal expression in meristems and young primordia. Plant J 20: 259–263 [DOI] [PubMed] [Google Scholar]
- Shannon S, Meeks-Wagner DR (1991) A mutation in the Arabidopsis TFL1 gene affects inflorescence meristem development. Plant Cell 3: 877–892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shor E, Paik I, Kangisser S, Green R, Huq E (2017) PHYTOCHROME INTERACTING FACTORS mediate metabolic control of the circadian system in Arabidopsis. New Phytol 215: 217–228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sibéril Y, Doireau P, Gantet P (2001) Plant bZIP G-box binding factors: Modular structure and activation mechanisms. Eur J Biochem 268: 5655–5666 [DOI] [PubMed] [Google Scholar]
- Sohn EJ, Rojas-Pierce M, Pan S, Carter C, Serrano-Mislata A, Madueño F, Rojo E, Surpin M, Raikhel NV (2007) The shoot meristem identity gene TFL1 is involved in flower development and trafficking to the protein storage vacuole. Proc Natl Acad Sci USA 104: 18801–18806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srikanth A, Schmid M (2011) Regulation of flowering time: All roads lead to Rome. Cell Mol Life Sci 68: 2013–2037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stark R, Brown G (2013). DiffBind: Differential binding analysis of ChIP-Seq peak data. http://bioconductor.org/packages/release/bioc/vignettes/DiffBind/inst/doc/DiffBind.pdf (December 1, 2018)
- Suárez-López P, Wheatley K, Robson F, Onouchi H, Valverde F, Coupland G (2001) CONSTANS mediates between the circadian clock and the control of flowering in Arabidopsis. Nature 410: 1116–1120 [DOI] [PubMed] [Google Scholar]
- Taoka K, Ohki I, Tsuji H, Furuita K, Hayashi K, Yanase T, Yamaguchi M, Nakashima C, Purwestri YA, Tamaki S, et al. (2011) 14-3-3 proteins act as intracellular receptors for rice Hd3a florigen. Nature 476: 332–335 [DOI] [PubMed] [Google Scholar]
- Urbanus SL, Martinelli AP, Dinh QD, Aizza LCB, Dornelas MC, Angenent GC, Immink RGH (2010) Intercellular transport of epidermis-expressed MADS domain transcription factors and their effect on plant morphology and floral transition. Plant J 63: 60–72 [DOI] [PubMed] [Google Scholar]
- Vandesteene L, López-Galvis L, Vanneste K, Feil R, Maere S, Lammens W, Rolland F, Lunn JE, Avonce N, Beeckman T, et al. (2012) Expansive evolution of the trehalose-6-phosphate phosphatase gene family in Arabidopsis. Plant Physiol 160: 884–896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Norman JM, Breakfield NW, Benfey PN (2011) Intercellular communication during plant development. Plant Cell 23: 855–864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahl V, Ponnu J, Schlereth A, Arrivault S, Langenecker T, Franke A, Feil R, Lunn JE, Stitt M, Schmid M (2013) Regulation of flowering by trehalose-6-phosphate signaling in Arabidopsis thaliana. Science 339: 704–707 [DOI] [PubMed] [Google Scholar]
- Wang Z, Zhou Z, Liu Y, Liu T, Li Q, Ji Y, Li C, Fang C, Wang M, Wu M, et al. (2015) Functional evolution of phosphatidylethanolamine binding proteins in soybean and Arabidopsis. Plant Cell 27: 323–336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weigel D, Alvarez J, Smyth DR, Yanofsky MF, Meyerowitz EM (1992) LEAFY controls floral meristem identity in Arabidopsis. Cell 69: 843–859 [DOI] [PubMed] [Google Scholar]
- Wigge PA, Kim MC, Jaeger KE, Busch W, Schmid M, Lohmann JU, Weigel D (2005) Integration of spatial and temporal information during floral induction in Arabidopsis. Science 309: 1056–1059 [DOI] [PubMed] [Google Scholar]
- Wu X, Dinneny JR, Crawford KM, Rhee Y, Citovsky V, Zambryski PC, Weigel D (2003) Modes of intercellular transcription factor movement in the Arabidopsis apex. Development 130: 3735–3745 [DOI] [PubMed] [Google Scholar]
- Xi W, Liu C, Hou X, Yu H (2010) MOTHER OF FT AND TFL1 regulates seed germination through a negative feedback loop modulating ABA signaling in Arabidopsis. Plant Cell 22: 1733–1748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yadav RK, Perales M, Gruel J, Girke T, Jönsson H, Reddy GV (2011) WUSCHEL protein movement mediates stem cell homeostasis in the Arabidopsis shoot apex. Genes Dev 25: 2025–2030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi A, Kobayashi Y, Goto K, Abe M, Araki T (2005) TWIN SISTER OF FT (TSF) acts as a floral pathway integrator redundantly with FT. Plant Cell Physiol 46: 1175–1189 [DOI] [PubMed] [Google Scholar]
- Yoo SJ, Chung KS, Jung SH, Yoo SY, Lee JS, Ahn JH (2010) BROTHER OF FT AND TFL1 (BFT) has TFL1-like activity and functions redundantly with TFL1 in inflorescence meristem development in Arabidopsis. Plant J 63: 241–253 [DOI] [PubMed] [Google Scholar]
- Yu H, Ito T, Zhao Y, Peng J, Kumar P, Meyerowitz EM (2004) Floral homeotic genes are targets of gibberellin signaling in flower development. Proc Natl Acad Sci USA 101: 7827–7832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Liu T, Meyer CA, Eeckhoute J, Johnson DS, Bernstein BE, Nusbaum C, Myers RM, Brown M, Li W, et al. (2008) Model-based analysis of ChIP-Seq (MACS). Genome Biol 9: R137. [DOI] [PMC free article] [PubMed] [Google Scholar]