Recent significant advances allow a more complete understanding of the many functions for TOR signaling in plant responses to different nutrient deficiencies and abiotic stresses.
Abstract
Target of Rapamycin (TOR) is an atypical Ser/Thr protein kinase that is evolutionally conserved among yeasts, plants, and mammals. In plants, TOR signaling functions as a central hub to integrate different kinds of nutrient, energy, hormone, and environmental signals. TOR thereby orchestrates every stage of plant life, from embryogenesis, meristem activation, root, and leaf growth to flowering, senescence, and life span determination. Besides its essential role in the control of plant growth and development, recent research has also shed light on its multifaceted roles in plant environmental stress responses. Here, we review recent findings on the involvement of TOR signaling in plant adaptation to nutrient deficiency and various abiotic stresses. We also discuss the mechanisms underlying how plants cope with such unfavorable conditions via TOR–abscisic acid crosstalk and TOR-mediated autophagy, both of which play crucial roles in plant stress responses. Until now, little was known about the upstream regulators and downstream effectors of TOR in plant stress responses. We propose that the Snf1-related protein kinase–TOR axis plays a role in sensing various stress signals, and predict the key downstream effectors based on recent high-throughput proteomic analyses.
Plants are challenged throughout their life cycles by various types of environmental stresses, such as nutrient deficiencies, extreme temperatures, drought, and high salinity. To deal with such unfavorable growth conditions, plants have evolved elaborate and efficient stress perception and signal transduction systems. Furthermore, plant stress responses are always accompanied by extensive transcriptional, translational, and metabolic changes to redirect energy and nutrient resources for stress adaptation. Increasing evidence has revealed an essential role of target of rapamycin (TOR), a master regulator of energy maintenance and metabolic homeostasis in all eukaryotic organisms, in plant stress responses and stress adaptation.
TOR was first identified in budding yeast through genetic mutant screens for resistance to rapamycin, a chemical molecule produced by the bacterium Streptomyces hygroscopicus (Heitman et al., 1991). Subsequent studies identified TOR genes in almost all eukaryotes, including animals and plants (Kunz et al., 1993; Menand et al., 2002; Sabatini et al., 1994). TOR is an atypical Ser/Thr protein kinase, resembling phosphatidylinositol lipid kinases, that is both structurally and functionally conserved among all eukaryotes (Xiong and Sheen, 2014). TOR exerts its function in complex forms. In mammals and yeasts, TOR forms at least two structurally and functionally distinct protein complexes (TORCs) with both shared (LST8) and distinct (Raptor in TORC1; Rictor and mSIN1 in TORC2) TOR-interacting partners. In plants, the precise compositions of the TOR kinase complexes have not been characterized. TOR, Raptor, and LST8 (but neither Rictor nor mSIN1) gene orthologs could be identified in all available plant genomes, indicating that only classical TORC1 exists in plants. One copy of TOR, two copies of Raptor (RaptorA and RaptorB), and two copies of LST8 (LST8-1 and LST8-2) exist in the Arabidopsis (Arabidopsis thaliana) genome, although LST8-2 might be a pseudogene due to its undetectable transcript level (Anderson et al., 2005; Deprost et al., 2005; Moreau et al., 2012). Gain-of-function and loss-of-function analyses have revealed that Arabidopsis TOR, Raptor, and LST8 are all essential for regulating multiple aspects of plant growth, development, and stress adaptation (Anderson et al., 2005; Deprost et al., 2005; Ren et al., 2011; Moreau et al., 2012; Xiong et al., 2013). It is worth noting that, based on these functional analyses, TOR appears to regulate a much broader spectrum of biological functions than Raptor or LST8. For example, the null tor mutant is embryo-lethal, while the raptora/b double mutant exhibits normal embryonic development but is arrested during seedling development, and the lst8-1 mutant only exhibits modest dwarf growth and early senescence phenotypes. Interestingly, although most eukaryotes have only one copy of the TOR gene, two TOR genes have been identified in three polyploids (Glycine max, Populus trichocarpa, and Brassica rapa) and four TOR genes have been identified in allotetraploid cotton (Gossypium hirsutum; Song et al., 2019). Sessile plants might possess unique TOR complexes with plant-specific components that serve as a functional equivalent of TORC2 or may even have plant-specialized functions for adaptation to constant environmental challenges.
In plants, TOR functions as a central hub that integrates signals, including nutrient, hormone, light, energy, and other environmental cues to orchestrate growth and development. TOR modulates a myriad of cellular activities, including cell division, cell expansion, transcription, mRNA translation, ribosome biogenesis, metabolism, nutrient assimilation and transport, and signaling via multiple partners and effectors in complex signaling networks, which have been extensively discussed in several excellent recent reviews (Shi et al., 2018; Jamsheer K et al., 2019; Ryabova et al., 2019; Wu et al., 2019). Besides its essential role in the control of plant growth and development, recent research also suggests an indispensable role for TOR in plant environmental stress responses. Plants with TOR dysfunctions behave as if they are stressed, even in the absence of a stressor. Transcriptome and metabolomics analyses in lst8-1 and the conditionally inducible tor-es, amiR-tor mutant revealed a broad regulation of plant stress- and autophagy-related genes, and diverse plant metabolic pathways modulating myo-inositol, raffinose, and galactinol, which usually accumulate under stress conditions such as high light, nutrient starvation, cold, drought, and high salt (Moreau et al., 2012; Caldana et al., 2013; Xiong et al., 2013). Downregulated TOR signaling by chemical inhibitor AZD-8055 also activates genes involved in stress hormone (e.g. ethylene, jasmonic acid, and abscisic acid [ABA]) signaling pathways (Dong et al., 2015). Intriguingly, modulating TOR expression can cause either stress-sensitive or stress-tolerant phenotypes depending on the type of stress encountered, further supporting the multifaceted roles of TOR in plant responses to abiotic stress (Deprost et al., 2007; Bakshi et al., 2017; Wang et al., 2017; Dong et al., 2019). Here, we focus on recent advances that enable a more thorough understanding of TOR’s many functions in plant responses to different nutrient deficiencies and various abiotic stresses, and discuss potential upstream regulators and downstream effectors of TOR.
TOR SIGNALING IN NUTRIENT SENSING AND DEFICIENCY
Plants obtain different kinds of nutrients from above-ground photosynthesis and below-ground soil nutrient assimilation. The ability to sense, assimilate, transport, and utilize various nutrients between sink and source organs is vital for plant survival and growth. TOR is a core component in plant nutrient sensing and communication networks.
In plants, Glc derived from photosynthesis in leaf sources provides carbon-based energy and building blocks (Sheen, 2014; Li and Sheen, 2016). Depletion of Glc completely blocks the kinase activity of TOR, and increases the expression of sets of autophagy- and protein degradation-related genes, indicating that recycling processes are activated to overcome the nutrient-deficient conditions (Xiong and Sheen, 2012; Xiong et al., 2013). Glc can quickly reactivate TOR activity via the glycolysis–mitochondria–electron transport chain energy relay, as chemical inhibitors targeting the first step of glycolysis and different steps of the electron transport chain completely prevent TOR activation by Glc. Thus, sugar-mediated TOR can sense the cellular metabolic and bioenergetic status to manipulate energy signaling in plants. Glc-activated TOR then phosphorylates and activates transcription factor E2Fa/E2Fb to promote root growth and true leaf formation by enhancing cell division activity in the root meristem and shoot apex, respectively (Xiong and Sheen, 2012; Xiong et al., 2013; Li et al., 2017). Interestingly, in the shoot apex, Glc alone is not enough to activate cell proliferation; the Rho-like small GTPase ROP2 was shown to bind to and activate TOR in a synergistic action along with Glc and auxin signaling (Xiong and Sheen, 2012; Xiong et al., 2013; Li et al., 2017). TOR also mediates crosstalk between sugar signaling and brassinosteroid signaling. Glc-activated TOR can inhibit autophagy to stabilize BZR1, which is a positive regulator in brassinosteroid signaling, to promote cell growth in hypocotyls (Zhang et al., 2016).
Sulfur is another important nutrient for plants. Sulfur assimilation begins with SO42− that is absorbed by sulfate transporters in the roots and transformed into adenosine 5′-phosphosulfate (APS), SO32−, and S2−, which are catalyzed by ATP sulfurylase, APS reductase (APR), and sulfite reductase (SIR), respectively (Jobe et al., 2019). S2− then reacts with o-acetyl-Ser to produce Cys, which serves as the donor for either protein synthesis or sulfur-containing compounds including glutathione (GSH) and various glucosinolates. Recently, the relationship between TOR and sulfur signaling has become evident. In the sir1-1 mutant, which could not produce S2−, TOR activity is abolished, and Glc content is significantly lower than that in wild-type Arabidopsis (Dong et al., 2017). Interestingly, exogenous supply of Glc or grafting the wild-type shoot onto the sir1-1 root rescues TOR activity, cell division in root apical meristem, and the growth arrest phenotype in the sir1-1 mutant (Dong et al., 2017), suggesting that sulfur availability does not affect TOR signaling independently, but acts through Glc energy signaling. Moreover, reducing GSH synthesis by inhibiting Glu-Cys ligase activity partially restores the dwarf phenotype and increases TOR activity in the sir1-1mutant, suggesting that reallocation of sulfur flux from GSH biosynthesis to protein translation can promote plant growth via the regulation of TOR (Speiser et al., 2018). In addition, Malinovsky et al. (2017) reported that a distinct plant defense-related glucosinolate, 3-hydroxypropylglucosinolate, can function like a TOR inhibitor to block Glc-TOR–promoted root meristem activation and root elongation. Thus, the direction of sulfur flux and its derived metabolites appear to serve key roles in balancing plant growth and stress responses via TOR regulation in response to environmental cues.
Organic nitrogen-containing molecules (amino acids) are key upstream signals for mammalian TOR activation. A very recent study showed that the accumulation of branched-chain amino acids could also upregulate TOR activity in Arabidopsis, causing reorganization of the actin cytoskeleton and actin-associated endomembranes (Cao et al., 2019). Although amino acid sensors for Leu, Arg, and Gln have been discovered in mammalian systems in the past decades (Saxton and Sabatini, 2017), no orthologs have been identified in plant genomes. Plants obtain organic nitrogen through nitrogen assimilation. Plants take in nitrate/ammonium from the soil and convert these compounds to Gln, and then into other amino acids via the Gln synthetase/Gln-2-oxoglutarate aminotransferase cycle (Krapp, 2015). It has been reported that Arabidopsis seedlings overexpressing TOR are hypersensitive to high nitrate inhibition of root growth (Deprost et al., 2007). Recent studies showed that TOR is inhibited in nitrogen-deprived seedlings, and that resupply of either nitrate, ammonium, or amino acids quickly reactivates TOR (Liu et al., 2018). However, nitrogen starvation is often associated with higher level of sugars. It remains to be examined whether inhibition of TOR by nitrogen starvation, like sulfur deprivation, is related to metabolic and energy generation processes, or if plants have evolved unique nitrogen-sensing systems for TOR activation.
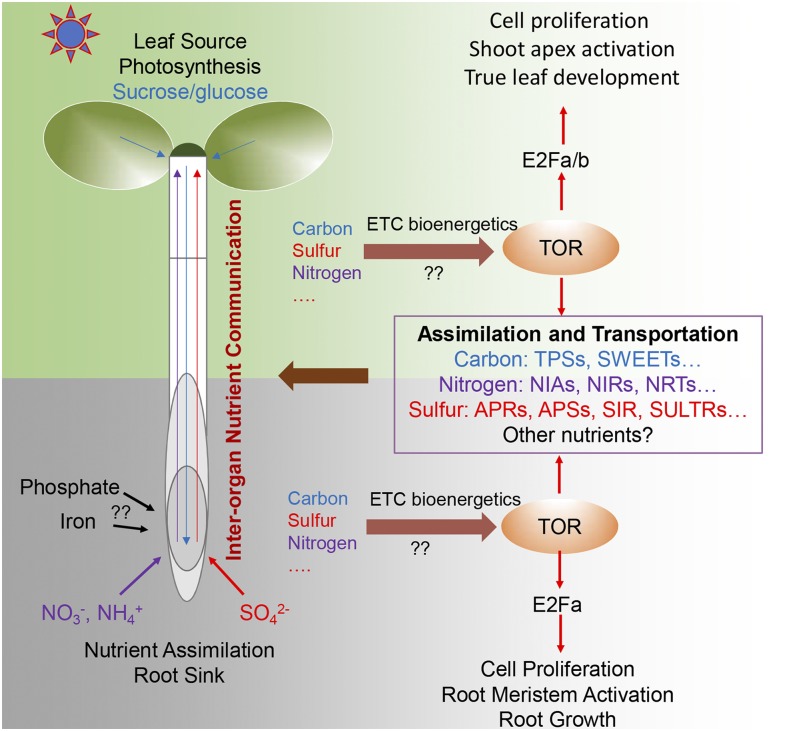
A direct link between other essential inorganic nutrients and TOR is also being established. Couso et al. (2020) reported that in Chlamydomonas reinhardtii, phosphorus deprivation negatively affected LST8 protein stability, resulting in a downregulation of TORC1 activity. Interestingly, in addition to the direct influence of carbon, nitrogen, sulfur, and phosphorus availability on TOR kinase activity, genome-wide transcriptional profiling has revealed that Glc-TOR signaling activates transcription of genes involved in sulfur assimilation and transport including APS1, APS3, APK1, APK2, APR1, APR2, APR3, SIR, SULTR1.2, SULTR2.2, SULTR3.5, and SULTR4.2, as well as genes involved in nitrogen assimilation and transport, including NIA1, NIA2, NIR1, NRT1.1, NRT1.2, NRT1.5, NRT2.2, and NRT 3.1 (Xiong et al., 2013). Therefore, there is a reciprocal positive feedback regulation loop among Glc, sulfur, and nitrogen signaling, and TOR may function as a central hub that orchestrates nutrient acquisition, shuttling, and communication between above-ground and below-ground tissues (Fig. 1).
Figure 1.
TOR signaling networks mediate nutrient interorgan dialogues to drive plant growth. Plant obtain carbon, nitrogen, sulfur, phosphate, and other micronutrients from above-ground photosynthesis and below-ground soil nutrient assimilation. There is a reciprocal positive feedback regulation loop among Glc, sulfur, and nitrogen signaling, and TOR functions as a central hub that orchestrates nutrient acquisition, shuttling, and communication between interorgan coordination. ETC, electron transport chain; NIA, nitrate reductase; NIR, nitrite reductase; NRT, nitrate transporter; SULTR, sulfate transporter; SWEET, Suc transporter; TPS, trehalose-6-phosphate synthase.
TOR SIGNALING IN ABIOTIC STRESSES
Advancing research has shown that TOR plays multifaceted roles in the plant response to various kinds of abiotic stress, and may function as either a positive or a negative regulator depending on the type and duration of stress encountered.
Temperature is a major factor in plant metabolism and growth. Wang et al. (2017) showed that Arabidopsis TOR activity is quickly abolished by cold treatment at time points as early as 10 min, but recovers after 2 h of treatment. Furthermore, cold treatment compromises enhanced anthocyanin accumulation in the inducible tor-es mutant under normal temperature, indicating that TOR is likely to be a negative regulator in cold acclimation. Because inhibition of translation is essential for cold tolerance, inactive TOR might decrease translation in plants to prepare them for unfavorable cold conditions (Wang et al., 2017). However, another independent study suggested that TOR seems to positively regulate the plant cold response (Dong et al., 2019). Depletion of AtTHADA (which codes for AtTHADA, the plant protein ortholog of the cold response regulator HsTHADA in humans) lowers energy status, decreases TOR activity, and causes growth arrest in Arabidopsis (Dong et al., 2019). Meanwhile, the Atthada mutant and TOR-RNAi (35-7) lines are hypersensitive to cold conditions (Dong et al., 2019). The differences between these studies might be caused by different silencing efficiencies in different TOR-RNAi lines or by different growth conditions, further indicating the complexity and dynamic nature of the TOR-regulated cold response.
In addition to cold stress, TOR is involved in high temperature tolerance. Exogenous application of Glc, overexpression of TOR, and overexpression of E2Fa all result in higher heat shock gene expression and seedling survival rates after recovery from heat stress treatment. Downregulation of TOR, downregulation of E2Fa, and treatment with the TOR inhibitor AZD-8055 or Torin1 lead to decreased seedling survival (Sharma et al., 2019). HIKESHI-LIKE PROTEIN1 (AtHLP1) is an ortholog of HsHikeshi, which imports HSP70 into the nucleus to promote thermo-tolerance in humans (Kose et al., 2012; Koizumi et al., 2014; Sharma et al., 2019). Glc-TOR-activated E2Fa directly binds to the promoter of AtHLP1 to activate AtHLP1. AtHLP1 binds directly to the promoters of many heat shock genes, which in turn leads to histone acetylation and H3K4me3 accumulation to activate and maintain thermo-memory, eventually enhancing thermo-tolerance (Sharma et al., 2019). Interestingly, proHLP1::GUS exhibits strong GUS induction in the proliferation zone of the shoot apex after 24 h of heat stress recovery in the presence of Glc. These results suggest that cell proliferation in the shoot apex must be coordinated with internal and external cues to maintain growth and survival, and is mediated by Glc-TOR energy signaling.
TOR positively regulates the plant response to drought and osmotic stresses. In Arabidopsis, TOR overexpression lines have a longer primary root than control lines exposed to a high concentration of potassium chloride (Deprost et al., 2007). Ectopic expression of Arabidopsis TOR gene in rice (Oryza indica) enhances water-use efficiency, growth, and yield under water-limiting conditions (Bakshi et al., 2017). These transgenic rice lines also show seed germination insensitivity to ABA treatment (Bakshi et al., 2017, 2019). These observations suggest that constitutive TOR expression might alleviate the effect of drought or osmotic stress on plant growth.
In contrast, TOR negatively regulates the plant response to oxidative stress and DNA/RNA damage. Maf1 is a conserved repressor of RNA polymerase III, which is responsible for synthesizing small RNAs, 5S ribosomal RNA, and tRNAs. Maf1’s activity is mediated by phosphorylation/dephosphorylation, and dephosphorylation of Maf1 promotes its repressor activity. Both oxidative stress or DNA/RNA damage and TOR silencing stimulate Maf1 dephosphorylation (Ahn et al., 2019). It is very likely that these stresses inhibit TOR activity to enhance the dephosphorylation of Maf1 and activate its repressor function. In this way, plants may slow down protein synthesis and cell growth or division to overcome these environmental stresses.
CROSSTALK BETWEEN TOR SIGNALING AND ABA SIGNALING
The phytohormone ABA plays a key role in integrating a wide range of stress signals and controlling downstream stress responses. Upon stress, ABA accumulates rapidly and binds to its intracellular PYR/PYL/RCAR receptors. The ABA-receptor complex binds to and inhibits the clade A PP2C protein phosphatases. PP2C inhibition releases the activity of Snf1-related protein kinase 2s (SnRK2s), which phosphorylate downstream targets to mediate protective responses such as stomatal closure and the expression of ABA-responsive genes (Chen et al., 2020).
TOR signaling has been found to regulate ABA biosynthesis and distribution. ABA content is decreased in raptorb seedlings, lst8-1 seedlings, and seedlings treated with the TOR inhibitor AZD-8055 (Kravchenko et al., 2015). Some genes that encode critical enzymes in ABA biosynthesis, such as NECD3 and AOO3, show decreased expression in the raptorb mutant (Kravchenko et al., 2015). However, the ABA content of raptorb mutant seeds is elevated (Salem et al., 2017), suggesting that TOR may also be involved in the distribution of ABA.
TOR signaling and ABA signaling converge on two Protein Phosphatase 2A (PP2A)-associated proteins, TAP46 and TIP41 (Ahn et al., 2011; Hu et al., 2014; Punzo et al., 2018a, 2018b). TAP46 is directly phosphorylated by TOR kinase, and functions as a positive effector in TOR signaling (Ahn et al., 2011). Meanwhile, TAP46 negatively regulates the phosphatase activity of PP2A, prevents it from dephosphorylating ABI5 (thereby stabilizes ABI5), and finally enhances ABA sensitivity in plants (Hu et al., 2014). TIP41 interacts with the catalytic subunit of PP2A and negatively regulates ABA sensitivity (Punzo et al., 2018a, 2018b). TIP41 is also involved in TOR signaling. The tip41 mutants display growth retardation, similar to the phenotype caused by TOR silencing, and are hypersensitive to the TOR inhibitor AZD-8055 (Punzo et al., 2018a, 2018b). Recent large-scale genetic screens for insensitivity to the TOR inhibitor AZD-8055 identified two important mediators of ABA signaling, YAK1 and ABI4, as the key downstream regulators of TOR signaling to control root growth, meristem activation, and seed germination (Li et al., 2015; Kim et al., 2016; Barrada et al., 2019).
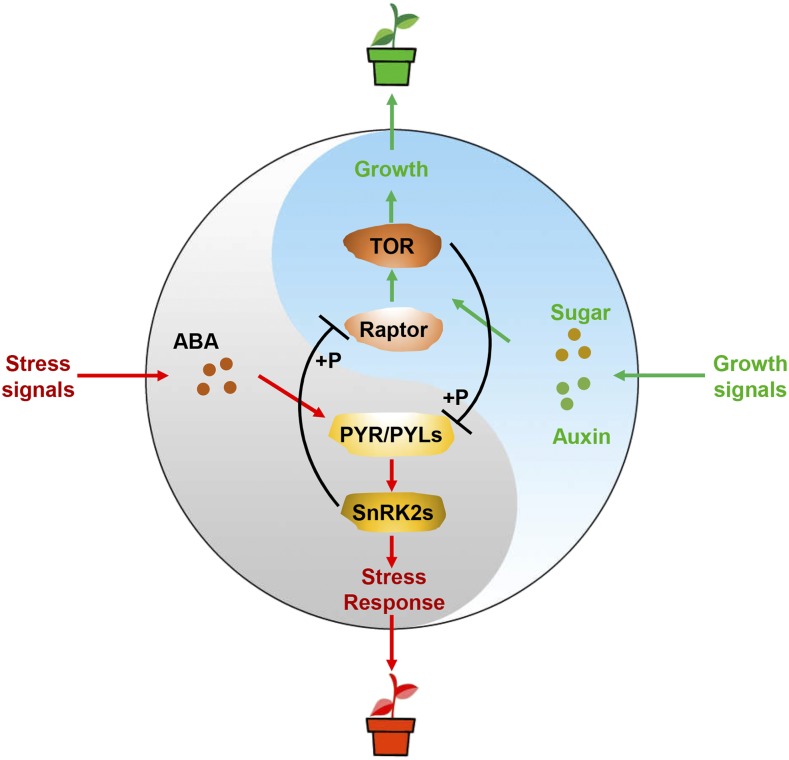
Upon sensing environmental stresses, plants usually transiently sacrifice growth and activate protective stress responses. Recently, a reciprocal negative crosstalk between TOR and ABA signaling has been shown to regulate such a trade-off between plant growth and stress adaptation (Fig. 2; Wang et al., 2018). In unstressed Arabidopsis, TOR phosphorylates ABA receptors at a highly conserved Ser, corresponding to Ser-119 in PYL1, to compromise ABA signaling by abolishing PYL binding activity to ABA, thereby inhibiting PP2C phosphatase. Expression of phosphor-mimicking PYL1S119D in multiple ABA receptor mutants does not complement the ABA-insensitive phenotype (Wang et al., 2018). The raptorb and lst8-1 mutants display hypersensitivity to exogenous ABA application (Salem et al., 2017; Wang et al., 2018). On the other hand, ABA also antagonizes TOR signaling. ABA-activated SnRK2s directly interact and phosphorylate RaptorB. This phosphorylation triggers the disassociation of RaptorB from the TOR complex, and thereby inhibits TOR’s kinase activity (Wang et al., 2018). Therefore, under nutrient-rich conditions, active TOR inhibits ABA signaling to direct resources to growth, whereas under stress conditions, ABA signaling is activated, and ABA-activated SnRK2 inhibits TOR activity to sacrifice growth for survival during stress. Importantly, the Ser residue corresponding to Ser-119 in PYL1 is highly conserved across all 121 PYLs from 12 different plant species, suggesting that this phosphor-regulatory feedback loop is a conserved mechanism that land plants utilize to optimize the balance of growth and stress responses. Strikingly, several PYLs (PYL5 to PYL12) in Arabidopsis can bind to and inhibit PP2Cs even in the absence of ABA (Hao et al., 2011; Fujii and Zhu, 2012), while the phosphor-mimicking mutation of the TOR phosphorylation site within PYL10 abolishes this ABA-independent interaction with PP2Cs (Wang et al., 2018). Therefore, TOR might also inhibit the activation of the ABA-independent PYLs under nonstress conditions to promote growth and development.
Figure 2.
A Tai-Chi model of the phospho-reciprocal regulation of the TOR kinase and ABA signaling to balance plant growth and stress response. Under growth-promoting conditions, active TOR phosphorylates ABA receptors PYR/PYLs to inhibit ABA signaling, and directs resources toward growth; under stress conditions, ABA-activated SnRK2s phosphorylate Raptor to decrease TOR activity, and sacrifice growth for survival during stress.
TOR NEGATIVELY REGULATES AUTOPHAGY
Autophagy is a process in which harmful or unwanted cellular components are delivered into lytic vacuoles to be recycled (Zhuang et al., 2018; Signorelli et al., 2019). Autophagy promotes plant resistance to nutrient deficiency, salt stress, drought stress, oxidative stress, and endoplasmic reticulum stress (Pu et al., 2017a). TOR is one of the key negative regulators of autophagy. Downregulation of TOR expression or kinase activity leads to constitutive activation of autophagy (Liu and Bassham, 2010). However, TOR antagonizes some, but not all, of the abiotic stress-triggered autophagy process (Liu and Bassham, 2010; Pu et al., 2017a, 2017b; Soto-Burgos and Bassham, 2017). Nutrient deficiency, salt stress, and drought stress all induce autophagy through TOR kinase, as overexpression of TOR under these condition significantly reduces the autophagy caused by these stresses (Pu et al., 2017a, 2017b; Soto-Burgos and Bassham, 2017). However, oxidative stress and ER stress trigger autophagy in a TOR-independent manner (Pu et al., 2017a, 2017b; Soto-Burgos and Bassham, 2017).
The autophagy-related1 (ATG1)/ATG13/ATG17 kinase complex plays an essential role in the onset of autophagy, and is the direct TORC1 substrate in mammals and yeast. In Arabidopsis, there are three ATG1 and two ATG13 homologs; their roles in the regulation of autophagy in response to nutrient starvation have been uncovered (Suttangkakul et al., 2011). Son et al. (2018) found that ATG13 contains a motif that could be phosphorylated by TOR kinase, and that deletion of this TOR-recognized motif in ATG13 enhances autophagy in Arabidopsis protoplasts. Recent high-throughput phosphoproteomics analysis using Arabidopsis suspension cell culture also revealed that ATG1s and ATG13s are direct TOR substrates. These studies reinforce that TOR-regulated phosphorylation of the ATG1/ATG13/ATG17 complex is essential for inhibiting autophagy in plants.
The upstream signals of TOR signaling also regulate autophagy. As a growth hormone, auxin stimulates TOR activity through a physical interaction between TOR and auxin-activated ROP2 to promote the activation of shoot apex cell proliferation (Schepetilnikov et al., 2013; Li et al., 2017). Interestingly, auxin also acts upstream of TOR in the regulation of autophagy. As mentioned above, nutrient deficiency, salt stress, and drought stress induce autophagy via TOR signaling, but the addition of auxin prevents the autophagy phenomenon induced by these stress conditions (Pu et al., 2017a). Meanwhile, auxin has no effect on oxidative or ER stress-induced autophagy, indicating that auxin specifically affects TOR-dependent autophagy (Pu et al., 2017a).
UPSTREAM REGULATORS OF PLANT TOR-STRESS SIGNALING
In contrast with the significant progress made in discovering the various molecular functions of TOR signaling in plant stress responses, the upstream regulators of TOR remain poorly understood. Plants possess a family of unique Rho-like small GTPases with 11 members that function as central hubs in signaling networks (Nagawa et al., 2010). As mentioned above, ROP2/3/6 has been shown to bind to and activate TOR stimulated by auxin signaling (Schepetilnikov et al., 2013; Li et al., 2017). Whether other ROPs are involved in stress sensing and regulation in TOR signaling remains a worthwhile question to be studied.
SnRKs are a group of kinases that play vital roles in a wide range of plant stress responses. Plants contain three SnRK families: SnRK1s, SnRK2s, and SnRK3s (Halford and Hey, 2009). Increasing evidence suggests that part of the SnRK-regulated stress response is achieved by the SnRKs-TOR module.
SnRK1 complex functions as a conserved energy sensor, which is activated under low energy conditions and is repressed under energy-rich conditions. In yeast and animal cells, nutrient starvations stimulate SNF1/AMPK, which repress TOR activity by phosphorylating Raptor proteins to suppresses cell growth and biosynthetic processes (Gwinn et al., 2008). In Arabidopsis, KIN10/11 protein kinases provide catalytic activities in the SnRK1 complex, and act antagonistically to TOR in the regulation of convergent primary sugar-responsive genes (Baena-González et al., 2007; Xiong et al., 2013; Li and Sheen, 2016), indicating that KIN10/11 functions upstream of TOR to regulate energy starvation processes. Furthermore, it was reported that KIN10 interacts with and phosphorylates Raptor in the TOR complex, providing a biochemical basis for the SnRK1-TOR regulation module (Nukarinen et al., 2016). Notably, KIN10 also functions upstream of TOR to activate autophagy (Pu et al., 2017b; Soto-Burgos and Bassham, 2017).
The SnRK2s are a group of plant-specific Ser/Thr kinases with 10 members (Kulik et al., 2011). SnRK2.2, SnRK2.3, and SnRK2.6 are key regulators in ABA signaling, where all 10 members are essential for osmotic stress responses (Zhu, 2016). As discussed above, ABA-dependent SnRK2.6 phosphorylates RaptorB and dissociates it from the TOR complex (Fig. 2). In this way, SnRK2s shut down TOR-promoted growth and enhance stress adaptation responses (Wang et al., 2018). Osmotic stresses also repress TOR activity (Wang et al., 2018), and PYR1/PYLs/RCARs could interact with SnRK2s to inhibit activation of SnRK2s upon osmotic stress condition (Zhao et al., 2018). Whether TOR phosphorylation of PYLs regulates osmotic stress-induced SnRK2 activation or vice versa is not known yet.
SnRK3 is also known as Calcineurin B-like protein-interacting protein kinase (CIPK; Manik et al., 2015). Arabidopsis has 26 CIPKs in total (Kolukisaoglu et al., 2004). The majority of stresses trigger rapid, transient Ca2+ signatures; and consequently, as a Ca2+ sensor, the Calcineurin B-like protein-CIPK module participates broadly in various kinds of stress responses, especially in ion homeostasis (Liu et al., 2000; Zhu, 2016; Sardar et al., 2017). Interestingly, the expression of SnRK3.24 (CIPK5) is downregulated after long-term TOR inhibition (Dong et al., 2015), and the cipk5 mutant exhibits decreased TOR activity (Meteignier et al., 2017), suggesting that SnRK3s might, like KIN10 and SnRK2s, phosphorylate Raptor to regulate TOR activity and signaling.
DOWNSTREAM EFFECTORS OF PLANT TOR-STRESS SIGNALING
TOR is a core merging point in the plant stress signaling network. However, until now, only a very limited number of TOR substrates or TOR-regulated proteins have been identified. Very recently, van Leene et al. (2019) performed quantitative phosphoproteomics and interactome analysis using Arabidopsis cell cultures with or without AZD8055 treatment. A total of 83 TOR-regulated phosphoproteins and 215 proteins interacting with the TOR complex (TOR, LST8-1, RaptorA, or RaptorB) were identified (van Leene et al., 2019). Some of these proteins may be direct TOR substrates. We performed a literature search to examine the biological functions of these proteins, and found that 19% of TOR-regulated phosphoproteins and 20% of TOR complex interacting proteins participate in various stress responses (Table 1). These TOR signaling-related targets include VirE2-Interacting Protein1 involved in osmotic and sulfate deprivation response, General Control Nonderepressible5 affecting histone acetylation under cold and salt stress, ATG1/13 for autophagy induction, and La-related protein1 involved in the heat stress-triggered mRNA degradation process (Pitzschke et al., 2009; Merret et al., 2013; Qi et al., 2017; Son et al., 2018; Zheng et al., 2019). These putative TOR substrates provide valuable directions for future studies of TOR-regulated stress responses.
Table 1. TOR-regulated stress-related proteins.
| Protein | AGI No.a | Related Plant Stress Responses | Methods |
|---|---|---|---|
| VIP1 | At1g43700 | Wound, cold, heavy metal, salt, osmotic, oxidative, and mechanical stress; sulfur deficiency (Pitzsche et al., 2009; Wu et al., 2010; Tsugama et al., 2012, 2018) | Phosphoproteomics |
| OZF1 | At2g19810 | Sugar and nitrogen deficiency; oxidative, drought, salt, and osmotic stress (Contento et al., 2004; Peng et al., 2007; Huang et al., 2011; Lee et al., 2012; Ding et al., 2013) | Phosphoproteomics |
| ATG1c | At2g37840 | Autophagy-related stress (Qi et al., 2017) | Phosphoproteomics |
| BAM1 | At3g23920 | Drought, osmotic, salt, and heat stress (Simpson et al., 2003; Monroe et al., 2014; Prasch et al., 2015; Liu et al., 2019b) | Phosphoproteomics |
| At3g26730 | At3g26730 | ABA-related stress (Bang et al., 2008) | Phosphoproteomics |
| ATG13 | At3g49590 | Autophagy-related stress (Son et al., 2018) | Phosphoproteomics |
| ATG1b | At3g53930 | Autophagy-related stress (Qi et al., 2017) | Phosphoproteomics |
| GCN5 | At3g54610 | Cold and salt stress (Pavangadkar et al., 2010; Zheng et al., 2019) | Phosphoproteomics |
| EIF4G | At3g60240 | Heat stress (Wu et al., 2013) | Phosphoproteomics |
| ATG1a | At3g61960 | Autophagy-related stress (Qi et al., 2017) | Phosphoproteomics |
| ATHD1 | At4g38130 | Salt, drought, and heat stress (Ueda et al., 2018) | Phosphoproteomics |
| LARP1a | At5g21160 | Heat stress (Merret et al., 2013) | Phosphoproteomics |
| SGS3 | At5g23570 | Heat stress (Liu et al., 2019a) | Phosphoproteomics |
| YAK1 | At5g35980 | ABA-related and drought stress (Kim et al., 2016) | Phosphoproteomics |
| PLDRP1 | At5g39570 | Drought and salt stress (Ufer et al., 2017) | Phosphoproteomics |
| PAH2 | At5g42870 | Phosphorus depletion (Nakamura et al., 2009) | Phosphoproteomics |
| AKS2 | At1g05805 | ABA-related stress (Takahashi et al., 2013) | Interactome |
| PFD4 | At1g08780 | ABA-related and cold stress (Kurup et al., 2000; Perea-Resa et al., 2017) | Interactome |
| KINβγ | At1g09020 | Sugar deficiency (Emanuelle et al., 2015) | Interactome |
| FHY2 | At1g09570 | UV and cold stress (Rusaczonek et al., 2015) | Interactome |
| HOP1 | At1g12270 | Heat stress (Fernández-Bautista et al., 2018) | Interactome |
| HSP70B | At1g16030 | Heat stress (Sung et al., 2001) | Interactome |
| CAT1 | At1g20630 | Drought stress (Hsieh et al., 2002; Xing et al., 2008) | Interactome |
| CPK11 | At1g35670 | ABA-related stress (Zhu et al., 2007) | Interactome |
| TUA2 | At1g50010 | Wounding, osmotic, and cold stress (Testerink et al., 2004) | Interactome |
| FYPP1 | At1g50370 | ABA-related stress (Dai et al., 2013) | Interactome |
| MKK4 | At1g51660 | Wounding and osmotic stress (Li et al., 2018) | Interactome |
| CPN60B | At1g55490 | Cold stress (Goulas et al., 2006) | Interactome |
| PP2A-1 | At1g59830 | ABA-related stress (Punzo et al., 2018b) | Interactome |
| HOP2 | At1g62740 | Heat stress (Fernández-Bautista et al., 2018) | Interactome |
| PP2A | At1g69960 | ABA-related and salt stress (Hu et al., 2017) | Interactome |
| PP5 | At2g42810 | Heat stress (Park et al., 2011) | Interactome |
| KIN10 | At3g01090 | Autophagy-related and ABA-related stress; low-energy, carbon, and phosphorus deficiency (Hamasaki et al., 2019) | Interactome |
| S6K1 | At3g08730 | Cold, salt, and osmotic stress (Mahfouz et al., 2006) | Interactome |
| HSP70 | At3g09440 | Cold and heat stress (Sharma et al., 2007) | Interactome |
| 2CPA | At3g11630 | Cold and oxidative stress (Goulas et al., 2006; Pulido et al., 2010; Juszczak et al., 2016) | Interactome |
| HSC70-4 | At3g12580 | Heat, salt, osmotic, and oxidative stress (Montero-Barrientos et al., 2010) | Interactome |
| KIN11 | At3g29160 | Sugar deficiency (Baena-González et al., 2007; Sheen, 2014) | Interactome |
| ATJ3 | At3g44110 | Salt and osmotic stress (Salas-Muñoz et al., 2016) | Interactome |
| ATG13 | At3g49590 | Autophagy-related stress (Son et al., 2018) | Interactome |
| ATG1b | At3g53930 | Autophagy-related stress (Qi et al., 2017) | Interactome |
| FER3 | At3g56090 | Oxidative stress (Ravet et al., 2009) | Interactome |
| MPK4 | At4g01370 | Salt and heat stress (Andrási et al., 2019) | Interactome |
| GRXS17 | At4g04950 | Cold, heat, and drought stress (Wu et al., 2017) | Interactome |
| TUA6 | At4g14960 | Salt stress (Dinneny et al., 2008) | Interactome |
| ATPDX1 | At5g01410 | Chilling, drought, salt, osmotic, and ozone stress (Denslow et al., 2007) | Interactome |
| HSP70-1 | At5g02500 | Cold, heat, salt, osmotic, and heavy metal stress (Lee and Schöffl, 1996; Zhang et al., 2003; Leng et al., 2017) | Interactome |
| UBP12 | At5g06600 | UV stress (Al Khateeb et al., 2019) | Interactome |
| TSN1 | At5g07350 | Heat and salt stress (Gutierrez-Beltran et al., 2015) | Interactome |
| GDH2 | At5g07440 | Salt stress (Jiang et al., 2007) | Interactome |
| ASN3 | At5g10240 | Nitrogen deficiency (Bi et al., 2007) | Interactome |
| GDH1 | At5g18170 | Low oxygen stress (Sarry et al., 2006) | Interactome |
| ATJ2 | At5g22060 | Heat and cold stress (Li et al., 2005) | Interactome |
| PFD5 | At5g23290 | Salt stress (Rodríguez-Milla and Salinas, 2009) | Interactome |
| YAK1 | At5g35980 | ABA-related and drought stress (Kim et al., 2016) | Interactome |
| PFD3 | At5g49510 | Salt stress (Rodríguez-Milla and Salinas, 2009) | Interactome |
| TAP46_2A | At5g53000 | Cold stress (Harris et al., 1999) | Interactome |
| ATG101 | At5g66930 | Autophagy-related stress (Li et al., 2014) | Interactome |
AGI, Arabidopsis Gene-Initiative Identifier
CONCLUSION
During the last decade, our knowledge of plant TOR signaling has increased significantly. It is now clear that TOR acts as a master regulator to sense and transduce nutrient, energetic, hormonal, metabolic, and environmental stress inputs into physiological, molecular, and developmental responses for growth and stress adaptation. Despite the great wealth of information that has become available, several questions still remain to be answered, and many others are emerging (see Outstanding Questions). In addition to its well-known roles in regulation of protein translation, it will be fruitful to dissect how TOR signaling represses a vast spectrum of primary target gene pathways in stress and immune responses. As a protein kinase, the phosphorylation of Thr-449 in the TOR-substrate protein ribosomal S6 kinase1 is used as a conserved indicator of endogenous TOR activity. Developing tissue-specific and fluorescence-visualized TOR kinase activity markers will help to quantitatively measure TOR activity and specific signaling output in different organs, e.g. sink and source tissues, thereby facilitating a more accurate interpretation of the different or even opposite phenotypes when TOR signaling is perturbed under various environmental conditions.
Footnotes
This work was supported by the Recruitment Program of Global Experts, People’s Republic of China, the National Natural Science Foundation of China (grant no. 31870269 to Y.X. and grant no. 31771358 to P.W.), Strategic Priority Research Program of the Chinese Academy of Sciences (grant no. XDB27040106 to P.W.), and the Basic Forestry and Proteomics Research Centre, Haixia Institute of Science and Technology, Fujian Agriculture and Forestry University.
Articles can be viewed without a subscription.
References
- Ahn CS, Han JA, Lee HS, Lee S, Pai HS (2011) The PP2A regulatory subunit Tap46, a component of the TOR signaling pathway, modulates growth and metabolism in plants. Plant Cell 23: 185–209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahn CS, Lee D-H, Pai H-S (2019) Characterization of Maf1 in Arabidopsis: Function under stress conditions and regulation by the TOR signaling pathway. Planta 249: 527–542 [DOI] [PubMed] [Google Scholar]
- Al Khateeb WM, Sher AA, Marcus JM, Schroeder DF (2019) UVSSA, UBP12, and RDO2/TFIIS contribute to Arabidopsis UV tolerance. Front Plant Sci 10: 516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson GH, Veit B, Hanson MR (2005) The Arabidopsis AtRaptor genes are essential for post-embryonic plant growth. BMC Biol 3: 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrási N, Rigó G, Zsigmond L, Pérez-Salamó I, Papdi C, Klement E, Pettko-Szandtner A, Baba AI, Ayaydin F, Dasari R, et al. (2019) The mitogen-activated protein kinase 4-phosphorylated heat shock factor A4A regulates responses to combined salt and heat stresses. J Exp Bot 70: 4903–4918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baena-González E, Rolland F, Thevelein JM, Sheen J (2007) A central integrator of transcription networks in plant stress and energy signalling. Nature 448: 938–942 [DOI] [PubMed] [Google Scholar]
- Bakshi A, Moin M, Kumar MU, Reddy ABM, Ren M, Datla R, Siddiq EA, Kirti PB (2017) Ectopic expression of Arabidopsis Target of Rapamycin (AtTOR) improves water-use efficiency and yield potential in rice. Sci Rep 7: 42835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakshi A, Moin M, Madhav MS, Kirti PB (2019) Target of Rapamycin, a master regulator of multiple signalling pathways and a potential candidate gene for crop improvement. Plant Biol (Stuttg) 21: 190–205 [DOI] [PubMed] [Google Scholar]
- Bang WY, Kim SW, Jeong IS, Koiwa H, Bahk JD (2008) The C-terminal region (640–967) of Arabidopsis CPL1 interacts with the abiotic stress- and ABA-responsive transcription factors. Biochem Biophys Res Commun 372: 907–912 [DOI] [PubMed] [Google Scholar]
- Barrada A, Djendli M, Desnos T, Mercier R, Robaglia C, Montané MH, Menand B (2019) A TOR-YAK1 signaling axis controls cell cycle, meristem activity and plant growth in Arabidopsis. Development 146: dev171298. [DOI] [PubMed] [Google Scholar]
- Bi YM, Wang RL, Zhu T, Rothstein SJ (2007) Global transcription profiling reveals differential responses to chronic nitrogen stress and putative nitrogen regulatory components in Arabidopsis. BMC Genomics 8: 281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldana C, Li Y, Leisse A, Zhang Y, Bartholomaeus L, Fernie AR, Willmitzer L, Giavalisco P (2013) Systemic analysis of inducible Target of Rapamycin mutants reveal a general metabolic switch controlling growth in Arabidopsis thaliana. Plant J 73: 897–909 [DOI] [PubMed] [Google Scholar]
- Cao P, Kim S-J, Xing A, Schenck CA, Liu L, Jiang N, Wang J, Last RL, Brandizzi F (2019) Homeostasis of branched-chain amino acids is critical for the activity of TOR signaling in Arabidopsis. eLife 8: e50747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen K, Li GJ, Bressan RA, Song CP, Zhu JK, Zhao Y (2020) Abscisic acid dynamics, signaling and functions in plants. J Integr Plant Biol 62: 25–54 [DOI] [PubMed] [Google Scholar]
- Contento AL, Kim SJ, Bassham DC (2004) Transcriptome profiling of the response of Arabidopsis suspension culture cells to Suc starvation. Plant Physiol 135: 2330–2347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couso I, Perez-Perez ME, Ford MM, Martinez Force E, Hicks LM, Umen JG, Crespo JL (2020) Phosphorus availability regulates TORC1 signaling via LST8 in Chlamydomonas. Plant Cell 32: 69–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai M, Xue Q, McCray T, Margavage K, Chen F, Lee JH, Nezames CD, Guo L, Terzaghi W, Wan J, et al. (2013) The PP6 phosphatase regulates ABI5 phosphorylation and abscisic acid signaling in Arabidopsis. Plant Cell 25: 517–534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denslow SA, Rueschhoff EE, Daub ME (2007) Regulation of the Arabidopsis thaliana vitamin B6 biosynthesis genes by abiotic stress. Plant Physiol Biochem 45: 152–161 [DOI] [PubMed] [Google Scholar]
- Deprost D, Truong HN, Robaglia C, Meyer C (2005) An Arabidopsis homolog of RAPTOR/KOG1 is essential for early embryo development. Biochem Biophys Res Commun 326: 844–850 [DOI] [PubMed] [Google Scholar]
- Deprost D, Yao L, Sormani R, Moreau M, Leterreux G, Nicolaï M, Bedu M, Robaglia C, Meyer C (2007) The Arabidopsis TOR kinase links plant growth, yield, stress resistance and mRNA translation. EMBO Rep 8: 864–870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding Y, Liu N, Virlouvet L, Riethoven JJ, Fromm M, Avramova Z (2013) Four distinct types of dehydration stress memory genes in Arabidopsis thaliana. BMC Plant Biol 13: 229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinneny JR, Long TA, Wang JYJ, Jung JW, Mace D, Pointer S, Barron C, Brady SM, Schiefelbein J, Benfey PN (2008) Cell identity mediates the response of Arabidopsis roots to abiotic stress. Science 320: 942–945 [DOI] [PubMed] [Google Scholar]
- Dong P, Xiong F, Que Y, Wang K, Yu L, Li Z, Ren M (2015) Expression profiling and functional analysis reveals that TOR is a key player in regulating photosynthesis and phytohormone signaling pathways in Arabidopsis. Front Plant Sci 6: 677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong Y, Silbermann M, Speiser A, Forieri I, Linster E, Poschet G, Allboje Samami A, Wanatabe M, Sticht C, Teleman AA, et al. (2017) Sulfur availability regulates plant growth via glucose-TOR signaling. Nat Commun 8: 1174. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Dong Y, Teleman AA, Jedmowski C, Wirtz M, Hell R (2019) The Arabidopsis THADA homologue modulates TOR activity and cold acclimation. Plant Biol (Stuttg) 21(Suppl 1): 77–83 [DOI] [PubMed] [Google Scholar]
- Emanuelle S, Hossain MI, Moller IE, Pedersen HL, van de Meene AM, Doblin MS, Koay A, Oakhill JS, Scott JW, Willats WGT, et al. (2015) SnRK1 from Arabidopsis thaliana is an atypical AMPK. Plant J 82: 183–192 [DOI] [PubMed] [Google Scholar]
- Fernández-Bautista N, Fernández-Calvino L, Muñoz A, Toribio R, Mock HP, Castellano MM (2018) HOP family plays a major role in long-term acquired thermotolerance in Arabidopsis. Plant Cell Environ 41: 1852–1869 [DOI] [PubMed] [Google Scholar]
- Fujii H, Zhu JK (2012) Osmotic stress signaling via protein kinases. Cell Mol Life Sci 69: 3165–3173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goulas E, Schubert M, Kieselbach T, Kleczkowski LA, Gardeström P, Schröder W, Hurry V (2006) The chloroplast lumen and stromal proteomes of Arabidopsis thaliana show differential sensitivity to short- and long-term exposure to low temperature. Plant J 47: 720–734 [DOI] [PubMed] [Google Scholar]
- Gutierrez-Beltran E, Moschou PN, Smertenko AP, Bozhkov PV (2015) Tudor staphylococcal nuclease links formation of stress granules and processing bodies with mRNA catabolism in Arabidopsis. Plant Cell 27: 926–943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gwinn DM, Shackelford DB, Egan DF, Mihaylova MM, Mery A, Vasquez DS, Turk BE, Shaw RJ (2008) AMPK phosphorylation of raptor mediates a metabolic checkpoint. Mol Cell 30: 214–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halford NG, Hey SJ (2009) Snf1-related protein kinases (SnRKs) act within an intricate network that links metabolic and stress signalling in plants. Biochem J 419: 247–259 [DOI] [PubMed] [Google Scholar]
- Hamasaki H, Kurihara Y, Kuromori T, Kusano H, Nagata N, Yamamoto YY, Shimada H, Matsui M (2019) SnRK1 kinase and the NAC transcription factor SOG1 are components of a novel signaling pathway mediating the low energy response triggered by ATP depletion. Front Plant Sci 10: 503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao Q, Yin P, Li W, Wang L, Yan C, Lin Z, Wu JZ, Wang J, Yan SF, Yan N (2011) The molecular basis of ABA-independent inhibition of PP2Cs by a subclass of PYL proteins. Mol Cell 42: 662–672 [DOI] [PubMed] [Google Scholar]
- Harris DM, Myrick TL, Rundle SJ (1999) The Arabidopsis homolog of yeast TAP42 and mammalian α4 binds to the catalytic subunit of protein phosphatase 2A and is induced by chilling. Plant Physiol 121: 609–617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heitman J, Movva NR, Hiestand PC, Hall MN (1991) FK 506-binding protein proline rotamase is a target for the immunosuppressive agent FK 506 in Saccharomyces cerevisiae. Proc Natl Acad Sci USA 88: 1948–1952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh TH, Lee JT, Charng YY, Chan MT (2002) Tomato plants ectopically expressing Arabidopsis CBF1 show enhanced resistance to water deficit stress. Plant Physiol 130: 618–626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu R, Zhu Y, Shen G, Zhang H (2014) TAP46 plays a positive role in the ABSCISIC ACID INSENSITIVE5-regulated gene expression in Arabidopsis. Plant Physiol 164: 721–734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu R, Zhu Y, Wei J, Chen J, Shi H, Shen G, Zhang H (2017) Overexpression of PP2A-C5 that encodes the catalytic subunit 5 of protein phosphatase 2A in Arabidopsis confers better root and shoot development under salt conditions. Plant Cell Environ 40: 150–164 [DOI] [PubMed] [Google Scholar]
- Huang P, Chung MS, Ju HW, Na HS, Lee DJ, Cheong HS, Kim CS (2011) Physiological characterization of the Arabidopsis thaliana oxidation-related zinc finger 1, a plasma membrane protein involved in oxidative stress. J Plant Res 124: 699–705 [DOI] [PubMed] [Google Scholar]
- Jamsheer K M, Jindal S, Laxmi A (2019) Evolution of TOR-SnRK dynamics in green plants and its integration with phytohormone signaling networks. J Exp Bot 70: 2239–2259 [DOI] [PubMed] [Google Scholar]
- Jiang Y, Yang B, Harris NS, Deyholos MK (2007) Comparative proteomic analysis of NaCl stress-responsive proteins in Arabidopsis roots. J Exp Bot 58: 3591–3607 [DOI] [PubMed] [Google Scholar]
- Jobe TO, Zenzen I, Rahimzadeh Karvansara P, Kopriva S (2019) Integration of sulfate assimilation with carbon and nitrogen metabolism in transition from C3 to C4 photosynthesis. J Exp Bot 70: 4211–4221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juszczak I, Cvetkovic J, Zuther E, Hincha DK, Baier M (2016) Natural variation of cold deacclimation correlates with variation of cold-acclimation of the plastid antioxidant system in Arabidopsis thaliana accessions. Front Plant Sci 7: 305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim D, Ntui VO, Xiong L (2016) Arabidopsis YAK1 regulates abscisic acid response and drought resistance. FEBS Lett 590: 2201–2209 [DOI] [PubMed] [Google Scholar]
- Koizumi S, Ohama N, Mizoi J, Shinozaki K, Yamaguchi-Shinozaki K (2014) Functional analysis of the Hikeshi-like protein and its interaction with HSP70 in Arabidopsis. Biochem Biophys Res Commun 450: 396–400 [DOI] [PubMed] [Google Scholar]
- Kolukisaoglu U, Weinl S, Blazevic D, Batistic O, Kudla J (2004) Calcium sensors and their interacting protein kinases: Genomics of the Arabidopsis and rice CBL-CIPK signaling networks. Plant Physiol 134: 43–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kose S, Furuta M, Imamoto N (2012) Hikeshi, a nuclear import carrier for Hsp70s, protects cells from heat shock-induced nuclear damage. Cell 149: 578–589 [DOI] [PubMed] [Google Scholar]
- Krapp A. (2015) Plant nitrogen assimilation and its regulation: A complex puzzle with missing pieces. Curr Opin Plant Biol 25: 115–122 [DOI] [PubMed] [Google Scholar]
- Kravchenko A, Citerne S, Jéhanno I, Bersimbaev RI, Veit B, Meyer C, Leprince AS (2015) Mutations in the Arabidopsis Lst8 and Raptor genes encoding partners of the TOR complex, or inhibition of TOR activity decreases abscisic acid (ABA) synthesis. Biochem Biophys Res Commun 467: 992–997 [DOI] [PubMed] [Google Scholar]
- Kulik A, Wawer I, Krzywińska E, Bucholc M, Dobrowolska G (2011) SnRK2 protein kinases—key regulators of plant response to abiotic stresses. OMICS 15: 859–872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunz J, Henriquez R, Schneider U, Deuter-Reinhard M, Movva NR, Hall MN (1993) Target of Rapamycin in yeast, TOR2, is an essential phosphatidylinositol kinase homolog required for G1 progression. Cell 73: 585–596 [DOI] [PubMed] [Google Scholar]
- Kurup S, Jones HD, Holdsworth MJ (2000) Interactions of the developmental regulator ABI3 with proteins identified from developing Arabidopsis seeds. Plant J 21: 143–155 [DOI] [PubMed] [Google Scholar]
- Lee JH, Schöffl F (1996) An Hsp70 antisense gene affects the expression of HSP70/HSC70, the regulation of HSF, and the acquisition of thermotolerance in transgenic Arabidopsis thaliana. Mol Gen Genet 252: 11–19 [DOI] [PubMed] [Google Scholar]
- Lee SJ, Jung HJ, Kang H, Kim SY (2012) Arabidopsis zinc finger proteins AtC3H49/AtTZF3 and AtC3H20/AtTZF2 are involved in ABA and JA responses. Plant Cell Physiol 53: 673–686 [DOI] [PubMed] [Google Scholar]
- Leng L, Liang Q, Jiang J, Zhang C, Hao Y, Wang X, Su W (2017) A subclass of HSP70s regulate development and abiotic stress responses in Arabidopsis thaliana. J Plant Res 130: 349–363 [DOI] [PubMed] [Google Scholar]
- Li F, Chung T, Vierstra RD (2014) AUTOPHAGY-RELATED11 plays a critical role in general autophagy- and senescence-induced mitophagy in Arabidopsis. Plant Cell 26: 788–807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li GL, Li B, Liu HT, Zhou RG (2005) The responses of AtJ2 and AtJ3 gene expression to environmental stresses in Arabidopsis. J Plant Phys Mol Biol 31: 47. [PubMed] [Google Scholar]
- Li L, Sheen J (2016) Dynamic and diverse sugar signaling. Curr Opin Plant Biol 33: 116–125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Song Y, Wang K, Dong P, Zhang X, Li F, Li Z, Ren M (2015) TOR-inhibitor insensitive-1 (TRIN1) regulates cotyledons greening in Arabidopsis. Front Plant Sci 6: 861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, Han X, Yang L, Deng X, Wu H, Zhang M, Liu Y, Zhang S, Xu J (2018) Mitogen-activated protein kinases and calcium-dependent protein kinases are involved in wounding-induced ethylene biosynthesis in Arabidopsis thaliana. Plant Cell Environ 41: 134–147 [DOI] [PubMed] [Google Scholar]
- Li X, Cai W, Liu Y, Li H, Fu L, Liu Z, Xu L, Liu H, Xu T, Xiong Y (2017) Differential TOR activation and cell proliferation in Arabidopsis root and shoot apexes. Proc Natl Acad Sci USA 114: 2765–2770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Feng L, Gu X, Deng X, Qiu Q, Li Q, Zhang Y, Wang M, Deng Y, Wang E, et al. (2019a) An H3K27me3 demethylase-HSFA2 regulatory loop orchestrates transgenerational thermomemory in Arabidopsis. Cell Res 29: 379–390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Ishitani M, Halfter U, Kim CS, Zhu JK (2000) The Arabidopsis thaliana SOS2 gene encodes a protein kinase that is required for salt tolerance. Proc Natl Acad Sci USA 97: 3730–3734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu K, Zou W, Gao X, Wang X, Yu Q, Ge L (2019b) Young seedlings adapt to stress by retaining starch and retarding growth through ABA-dependent and -independent pathways in Arabidopsis. Biochem Biophys Res Commun 515: 699–705 [DOI] [PubMed] [Google Scholar]
- Liu Y, Bassham DC (2010) TOR is a negative regulator of autophagy in Arabidopsis thaliana. PLoS One 5: e11883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Wang Y, Xiong Y (2018) Nitrogen–TOR signaling in shoot apex activation. In: Target of rapamycin (TOR) signaling in photo- synthetic organisms. EMBO Workshop. Programme and Abstract Book, 96. Heidelberg, Germany, EMBO Press
- Mahfouz MM, Kim S, Delauney AJ, Verma DPS (2006) Arabidopsis TARGET OF RAPAMYCIN interacts with RAPTOR, which regulates the activity of S6 kinase in response to osmotic stress signals. Plant Cell 18: 477–490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malinovsky FG, Thomsen MF, Nintemann SJ, Jagd LM, Bourgine B, Burow M, Kliebenstein DJ (2017) An evolutionarily young defense metabolite influences the root growth of plants via the ancient TOR signaling pathway. eLife 6: 1–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manik SM, Shi S, Mao J, Dong L, Su Y, Wang Q, Liu H (2015) The calcium sensor CBL-CIPK is involved in plant’s response to abiotic stresses. Int J Genomics 2015: 493191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menand B, Desnos T, Nussaume L, Berger F, Bouchez D, Meyer C, Robaglia C (2002) Expression and disruption of the Arabidopsis TOR (Target of Rapamycin) gene. Proc Natl Acad Sci USA 99: 6422–6427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merret R, Descombin J, Juan YT, Favory JJ, Carpentier MC, Chaparro C, Charng YY, Deragon JM, Bousquet-Antonelli C (2013) XRN4 and LARP1 are required for a heat-triggered mRNA decay pathway involved in plant acclimation and survival during thermal stress. Cell Reports 5: 1279–1293 [DOI] [PubMed] [Google Scholar]
- Meteignier L-V, El Oirdi M, Cohen M, Barff T, Matteau D, Lucier J-F, Rodrigue S, Jacques P-E, Yoshioka K, Moffett P (2017) Translatome analysis of an NB-LRR immune response identifies important contributors to plant immunity in Arabidopsis. J Exp Bot 68: 2333–2344 [DOI] [PubMed] [Google Scholar]
- Monroe JD, Storm AR, Badley EM, Lehman MD, Platt SM, Saunders LK, Schmitz JM, Torres CE (2014) β-Amylase1 and β-amylase3 are plastidic starch hydrolases in Arabidopsis that seem to be adapted for different thermal, pH, and stress conditions. Plant Physiol 166: 1748–1763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montero-Barrientos M, Hermosa R, Cardoza RE, Gutiérrez S, Nicolás C, Monte E (2010) Transgenic expression of the Trichoderma harzianum hsp70 gene increases Arabidopsis resistance to heat and other abiotic stresses. J Plant Physiol 167: 659–665 [DOI] [PubMed] [Google Scholar]
- Moreau M, Azzopardi M, Clément G, Dobrenel T, Marchive C, Renne C, Martin-Magniette ML, Taconnat L, Renou JP, Robaglia C, et al. (2012) Mutations in the Arabidopsis homolog of LST8/GβL, a partner of the Target of Rapamycin kinase, impair plant growth, flowering, and metabolic adaptation to long days. Plant Cell 24: 463–481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagawa S, Xu T, Yang Z (2010) RHO GTPase in plants: Conservation and invention of regulators and effectors. Small GTPases 1: 78–88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura Y, Koizumi R, Shui G, Shimojima M, Wenk MR, Ito T, Ohta H (2009) Arabidopsis lipins mediate eukaryotic pathway of lipid metabolism and cope critically with phosphate starvation. Proc Natl Acad Sci USA 106: 20978–20983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nukarinen E, Nägele T, Pedrotti L, Wurzinger B, Mair A, Landgraf R, Börnke F, Hanson J, Teige M, Baena-Gonzalez E, et al. (2016) Quantitative phosphoproteomics reveals the role of the AMPK plant ortholog SnRK1 as a metabolic master regulator under energy deprivation. Sci Rep 6: 31697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park JH, Lee SY, Kim WY, Jung YJ, Chae HB, Jung HS, Kang CH, Shin MR, Kim SY, Su’udi M, et al. (2011) Heat-induced chaperone activity of serine/threonine protein phosphatase 5 enhances thermotolerance in Arabidopsis thaliana. New Phytol 191: 692–705 [DOI] [PubMed] [Google Scholar]
- Pavangadkar K, Thomashow MF, Triezenberg SJ (2010) Histone dynamics and roles of histone acetyltransferases during cold-induced gene regulation in Arabidopsis. Plant Mol Biol 74: 183–200 [DOI] [PubMed] [Google Scholar]
- Peng M, Bi YM, Zhu T, Rothstein SJ (2007) Genome-wide analysis of Arabidopsis responsive transcriptome to nitrogen limitation and its regulation by the ubiquitin ligase gene NLA. Plant Mol Biol 65: 775–797 [DOI] [PubMed] [Google Scholar]
- Perea-Resa C, Rodríguez-Milla MA, Iniesto E, Rubio V, Salinas J (2017) Prefoldins negatively regulate cold acclimation in Arabidopsis thaliana by promoting nuclear proteasome-mediated HY5 degradation. Mol Plant 10: 791–804 [DOI] [PubMed] [Google Scholar]
- Pitzschke A, Djamei A, Teige M, Hirt H (2009) VIP1 response elements mediate mitogen-activated protein kinase 3-induced stress gene expression. Proc Natl Acad Sci USA 106: 18414–18419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasch CM, Ott KV, Bauer H, Ache P, Hedrich R, Sonnewald U (2015) ß-amylase1 mutant Arabidopsis plants show improved drought tolerance due to reduced starch breakdown in guard cells. J Exp Bot 66: 6059–6067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pu Y, Luo X, Bassham DC (2017a) TOR-dependent and -independent pathways regulate autophagy in Arabidopsis thaliana. Front Plant Sci 8: 1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pu Y, Soto-Burgos J, Bassham DC (2017b) Regulation of autophagy through SnRK1 and TOR signaling pathways. Plant Signal Behav 12: e1395128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pulido P, Spínola MC, Kirchsteiger K, Guinea M, Pascual MB, Sahrawy M, Sandalio LM, Dietz KJ, González M, Cejudo FJ (2010) Functional analysis of the pathways for 2-Cys peroxiredoxin reduction in Arabidopsis thaliana chloroplasts. J Exp Bot 61: 4043–4054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Punzo P, Ruggiero A, Grillo S, Batelli G (2018a) TIP41 network analysis and mutant phenotypes predict interactions between the TOR and ABA pathways. Plant Signal Behav 13: e1537698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Punzo P, Ruggiero A, Possenti M, Nurcato R, Costa A, Morelli G, Grillo S, Batelli G (2018b) The PP2A-interactor TIP41 modulates ABA responses in Arabidopsis thaliana. Plant J 94: 991–1009 [DOI] [PubMed] [Google Scholar]
- Qi H, Xia FN, Xie LJ, Yu LJ, Chen QF, Zhuang XH, Wang Q, Li F, Jiang L, Xie Q, et al. (2017) TRAF family proteins regulate autophagy dynamics by modulating AUTOPHAGY PROTEIN6 stability in Arabidopsis. Plant Cell 29: 890–911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravet K, Touraine B, Boucherez J, Briat JF, Gaymard F, Cellier F (2009) Ferritins control interaction between iron homeostasis and oxidative stress in Arabidopsis. Plant J 57: 400–412 [DOI] [PubMed] [Google Scholar]
- Ren M, Qiu S, Venglat P, Xiang D, Feng L, Selvaraj G, Datla R (2011) Target of Rapamycin regulates development and ribosomal RNA expression through kinase domain in Arabidopsis. Plant Physiol 155: 1367–1382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodríguez-Milla MA, Salinas J (2009) Prefoldins 3 and 5 play an essential role in Arabidopsis tolerance to salt stress. Mol Plant 2: 526–534 [DOI] [PubMed] [Google Scholar]
- Rusaczonek A, Czarnocka W, Kacprzak S, Witoń D, Ślesak I, Szechyńska-Hebda M, Gawroński P, Karpiński S (2015) Role of phytochromes A and B in the regulation of cell death and acclimatory responses to UV stress in Arabidopsis thaliana. J Exp Bot 66: 6679–6695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryabova LA, Robaglia C, Meyer C (2019) Target of Rapamycin kinase: Central regulatory hub for plant growth and metabolism. J Exp Bot 70: 2211–2216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabatini DM, Erdjument-Bromage H, Lui M, Tempst P, Snyder SH (1994) RAFT1: A mammalian protein that binds to FKBP12 in a rapamycin-dependent fashion and is homologous to yeast TORs. Cell 78: 35–43 [DOI] [PubMed] [Google Scholar]
- Salas-Muñoz S, Rodríguez-Hernández AA, Ortega-Amaro MA, Salazar-Badillo FB, Jiménez-Bremont JF (2016) Arabidopsis AtDjA3 null mutant shows increased sensitivity to abscisic acid, salt, and osmotic stress in germination and post-germination stages. Front Plant Sci 7: 220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salem MA, Li Y, Wiszniewski A, Giavalisco P (2017) Regulatory-associated protein of TOR (RAPTOR) alters the hormonal and metabolic composition of Arabidopsis seeds, controlling seed morphology, viability and germination potential. Plant J 92: 525–545 [DOI] [PubMed] [Google Scholar]
- Sardar A, Nandi AK, Chattopadhyay D (2017) CBL-interacting protein kinase 6 negatively regulates immune response to Pseudomonas syringae in Arabidopsis. J Exp Bot 68: 3573–3584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarry JE, Kuhn L, Le Lay P, Garin J, Bourguignon J (2006) Dynamics of Arabidopsis thaliana soluble proteome in response to different nutrient culture conditions. Electrophoresis 27: 495–507 [DOI] [PubMed] [Google Scholar]
- Saxton RA, Sabatini DM (2017) mTOR Signaling in Growth, Metabolism, and Disease. Cell 169: 361–371 [DOI] [PubMed] [Google Scholar]
- Schepetilnikov M, Dimitrova M, Mancera-Martínez E, Geldreich A, Keller M, Ryabova LA (2013) TOR and S6K1 promote translation reinitiation of uORF-containing mRNAs via phosphorylation of eIF3h. EMBO J 32: 1087–1102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma M, Banday ZZ, Shukla BN, Laxmi A (2019) Glucose-regulated HLP1 acts as a key molecule in governing thermomemory. Plant Physiol 180: 1081–1100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma N, Cram D, Huebert T, Zhou N, Parkin IAP (2007) Exploiting the wild crucifer Thlaspi arvense to identify conserved and novel genes expressed during a plant’s response to cold stress. Plant Mol Biol 63: 171–184 [DOI] [PubMed] [Google Scholar]
- Sheen J. (2014) Master regulators in plant glucose signaling networks. J Plant Biol 57: 67–79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi L, Wu Y, Sheen J (2018) TOR signaling in plants: Conservation and innovation. Development 145: dev160887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Signorelli S, Tarkowski Ł, van den Ende W, Bassham DC (2019) Linking autophagy to abiotic and biotic stress responses. Trends Plant Sci 24: 413–430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson SD, Nakashima K, Narusaka Y, Seki M, Shinozaki K, Yamaguchi-Shinozaki K (2003) Two different novel cis-acting elements of erd1, a clpA homologous Arabidopsis gene function in induction by dehydration stress and dark-induced senescence. Plant J 33: 259–270 [DOI] [PubMed] [Google Scholar]
- Son O, Kim S, Kim D, Hur YS, Kim J, Cheon CI (2018) Involvement of TOR signaling motif in the regulation of plant autophagy. Biochem Biophys Res Commun 501: 643–647 [DOI] [PubMed] [Google Scholar]
- Song Y, Li L, Yang Z, Zhao G, Zhang X, Wang L, Zheng L, Zhuo F, Yin H, Ge X, et al. (2019) Target of Rapamycin (TOR) regulates the expression of lncRNAs in response to abiotic stresses in cotton. Front Genet 9: 690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soto-Burgos J, Bassham DC (2017) SnRK1 activates autophagy via the TOR signaling pathway in Arabidopsis thaliana. PLoS One 12: e0182591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speiser A, Silbermann M, Dong Y, Haberland S, Uslu VV, Wang S, Bangash SAK, Reichelt M, Meyer AJ, Wirtz M, et al. (2018) Sulfur partitioning between glutathione and protein synthesis determines plant growth. Plant Physiol 177: 927–937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sung DY, Vierling E, Guy CL (2001) Comprehensive expression profile analysis of the Arabidopsis Hsp70 gene family. Plant Physiol 126: 789–800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suttangkakul A, Li F, Chung T, Vierstra RD (2011) The ATG1/ATG13 protein kinase complex is both a regulator and a target of autophagic recycling in Arabidopsis. Plant Cell 23: 3761–3779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi Y, Ebisu Y, Kinoshita T, Doi M, Okuma E, Murata Y, Shimazaki K (2013) bHLH transcription factors that facilitate K+ uptake during stomatal opening are repressed by abscisic acid through phosphorylation. Sci Signal 6: ra48. [DOI] [PubMed] [Google Scholar]
- Testerink C, Dekker HL, Lim ZY, Johns MK, Holmes AB, Koster CG, Ktistakis NT, Munnik T (2004) Isolation and identification of phosphatidic acid targets from plants. Plant J 39: 527–536 [DOI] [PubMed] [Google Scholar]
- Tsugama D, Liu S, Fujino K, Takano T (2018) Possible inhibition of Arabidopsis VIP1-mediated mechanosensory signaling by streptomycin. Plant Signal Behav 13: e1521236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsugama D, Liu S, Takano T (2012) A bZIP protein, VIP1, is a regulator of osmosensory signaling in Arabidopsis. Plant Physiol 159: 144–155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueda M, Matsui A, Nakamura T, Abe T, Sunaoshi Y, Shimada H, Seki M (2018) Versatility of HDA19-deficiency in increasing the tolerance of Arabidopsis to different environmental stresses. Plant Signal Behav 13: e1475808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ufer G, Gertzmann A, Gasulla F, Röhrig H, Bartels D (2017) Identification and characterization of the phosphatidic acid-binding A. thaliana phosphoprotein PLDrp1 that is regulated by PLDα1 in a stress-dependent manner. Plant J 92: 276–290 [DOI] [PubMed] [Google Scholar]
- van Leene J, Han C, Gadeyne A, Eeckhout D, Matthijs C, Cannoot B, de Winne N, Persiau G, van de Slijke E, van de Cotte B, et al. (2019) Capturing the phosphorylation and protein interaction landscape of the plant TOR kinase. Nat Plants 5: 316–327 [DOI] [PubMed] [Google Scholar]
- Wang L, Li H, Zhao C, Li S, Kong L, Wu W, Kong W, Liu Y, Wei Y, Zhu JK, Zhang H (2017) The inhibition of protein translation mediated by AtGCN1 is essential for cold tolerance in Arabidopsis thaliana. Plant Cell Environ 40: 56–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang P, Zhao Y, Li Z, Hsu CC, Liu X, Fu L, Hou YJ, Du Y, Xie S, Zhang C, et al. (2018) Reciprocal regulation of the TOR kinase and ABA receptor balances plant growth and stress response. Mol Cell 69: 100–112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Q, Hu Y, Sprague SA, Kakeshpour T, Park J, Nakata PA, Cheng N, Hirschi KD, White FF, Park S (2017) Expression of a monothiol glutaredoxin, AtGRXS17, in tomato (Solanum lycopersicum) enhances drought tolerance. Biochem Biophys Res Commun 491: 1034–1039 [DOI] [PubMed] [Google Scholar]
- Wu TY, Juan YT, Hsu YH, Wu SH, Liao HT, Fung RWM, Charng YY (2013) Interplay between heat shock proteins HSP101 and HSA32 prolongs heat acclimation memory posttranscriptionally in Arabidopsis. Plant Physiol 161: 2075–2084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y, Shi L, Li L, Fu L, Liu Y, Xiong Y, Sheen J (2019) Integration of nutrient, energy, light, and hormone signalling via TOR in plants. J Exp Bot 70: 2227–2238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y, Zhao Q, Gao L, Yu XM, Fang P, Oliver DJ, Xiang CB (2010) Isolation and characterization of low-sulphur-tolerant mutants of Arabidopsis. J Exp Bot 61: 3407–3422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xing Y, Jia W, Zhang J (2008) AtMKK1 mediates ABA-induced CAT1 expression and H2O2 production via AtMPK6-coupled signaling in Arabidopsis. Plant J 54: 440–451 [DOI] [PubMed] [Google Scholar]
- Xiong Y, McCormack M, Li L, Hall Q, Xiang C, Sheen J (2013) Glucose-TOR signalling reprograms the transcriptome and activates meristems. Nature 496: 181–186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong Y, Sheen J (2012) Rapamycin and glucose Target of Rapamycin (TOR) protein signaling in plants. J Biol Chem 287: 2836–2842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong Y, Sheen J (2014) The role of Target of Rapamycin signaling networks in plant growth and metabolism. Plant Physiol 164: 499–512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Lohmann C, Prändl R, Schöffl F (2003) Heat stress-dependent DNA binding of Arabidopsis heat shock transcription factor HSF1 to heat shock gene promoters in Arabidopsis suspension culture cells in vivo. Biol Chem 384: 959–963 [DOI] [PubMed] [Google Scholar]
- Zhang Z, Zhu JY, Roh J, Marchive C, Kim SK, Meyer C, Sun Y, Wang W, Wang ZY (2016) TOR signaling promotes accumulation of BZR1 to balance growth with carbon availability in Arabidopsis. Curr Biol 26: 1854–1860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y, Zhang Z, Gao J, Wang P, Hu T, Wang Z, Hou YJ, Wan Y, Liu W, Xie S, et al. (2018) Arabidopsis duodecuple mutant of PYL ABA receptors reveals PYL repression of ABA-independent SnRK2 activity. Cell Reports 23: 3340–3351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng M, Liu X, Lin J, Liu X, Wang Z, Xin M, Yao Y, Peng H, Zhou DX, Ni Z, et al. (2019) Histone acetyltransferase GCN5 contributes to cell wall integrity and salt stress tolerance by altering the expression of cellulose synthesis genes. Plant J 97: 587–602 [DOI] [PubMed] [Google Scholar]
- Zhu JK. (2016) Abiotic stress signaling and responses in plants. Cell 167: 313–324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu SY, Yu XC, Wang XJ, Zhao R, Li Y, Fan RC, Shang Y, Du SY, Wang XF, Wu FQ, et al. (2007) Two calcium-dependent protein kinases, CPK4 and CPK11, regulate abscisic acid signal transduction in Arabidopsis. Plant Cell 19: 3019–3036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhuang X, Chung KP, Luo M, Jiang L (2018) Autophagosome biogenesis and the endoplasmic reticulum: A plant perspective. Trends Plant Sci 23: 677–692 [DOI] [PubMed] [Google Scholar]