Abstract
Sekizaisou, a red-wood mulberry variety used in traditional sericulture, is a naturally occurring lignin mutant.
Dear Editor,
Lignin is a major polymer in the vascular plant secondary-thickened cell wall. This aromatic polymer provides hydrophobicity and strength to the cell wall, thereby facilitating vascular conductivity and upward growth. Mutations in lignin biosynthetic genes induce qualitative and/or quantitative alterations of the polymer (Boerjan et al., 2003). Such mutations are sometimes accompanied by changes in the color of the xylem cell walls. Although the color change is an indicator of lignin alteration, assessing color differences is extremely difficult based on only external appearance, especially in woody plant species, because xylem is hidden by bark. This may be one of the reasons why the discovery of naturally occurring lignin mutants in trees is quite rare (Ralph et al., 1997; Marroni et al., 2011; Vanholme et al., 2013). Here, we report a naturally occurring mulberry (Morus alba), ‘Sekizaisou’, which is deficient in a gene encoding CINNAMYL ALCOHOL DEHYDROGENASE (CAD), which catalyzes the last step in lignin monomer biosynthesis.
Mulberry trees (Morus sp.) are grown widely for their fruit and silk production. Their leaves contain all the essential chemical components for silkworm (Bombyx mori) growth and development. According to Yoshimura and Saito (1924), a mulberry tree with drooping branches (Supplemental Fig. S1), reminiscent of those in weeping willow, and unusual red-colored wood was discovered around 1912 by a farmer in bushland on Okushiri Island, northern Japan. It was locally called “Kusuri-guwa (medicine mulberry)” or “Murasaki-guwa (purple mulberry)” a century ago and was believed by people on the island to be a medicinal herb (Hotta, 1937). In 1950, the striking appearance of this tree’s red-colored wood was immortalized in a painting published in a book written by Hotta (1950; Fig. 1A). Grafted plantlets of cv Sekizaisou were established in Tokyo in 1922, and the propagules have been preserved by sequential vegetative propagation ever since (Supplemental Materials and Methods). The tree was named cv Sekizaisou (“Sekizai” means “red colored wood” and “sou” means “mulberry” in Japanese) by Yoshimura and Saito (1924). The leaves of this tree were used as feed for sericulture on Okushiri Island from 1916 until around the 1930s, because of their positive effects on silkworm growth and silk cocoon quality compared with those from other local mulberry cultivars planted on the island (Yoshimura and Saito, 1924).
Figure 1.
Morphological characteristics of cv Sekizaisou. A, A hand-made painting of debarked branching stems of cv Sekizaisou (left) and a control mulberry (right) published by Hotta (1950). B and C, cv Sekizaisou plantlets growing in the experimental field in early summer and midsummer, respectively. The creeping growth habit can be observed for the branches originating from the bottom part of the stumps (white arrows in B). Unlike other cultivars growing upright, such as the plants growing behind cv Sekizaisou, most of the branches of cv Sekizaisou drooped (white arrows in C), and supporting poles were necessary to maintain upward growth of the main stems. D, Debarked branches of cv Sekizaisou (left) and cv Nezumigaeshi (right) prepared from plants in midsummer. E, Longitudinal sections of top (right) and bottom (left) parts of branches of cv Sekizaisou. The red color of the inner xylem, close to the pith parenchyma, was lighter than that of the outer xylem. X and P indicate xylem and parenchyma tissues, respectively. F, Debarked stems of cv Sekizaisou (left) and cv Nezumigaeshi (right) after air-drying for 3 d. The xylem tissue of cv Sekizaisou turned brown. G, Wood powder of extractive-free wood from cv Sekizaisou (left) and cv Nezumigaeshi (right). The resultant brown pigment in air-dried xylem could not be removed from the powder upon sequential extraction with hot water and organic solvents.
F1 progenies derived from crosses between cvs Sekizaisou (seed parent) and Kokusou 21 (pollen parent) had neither drooping stems nor red-colored xylem (Supplemental Fig. S2). This suggested that a homozygous recessive gene caused the morphological characteristics and the red-colored wood. The characteristics that typify cv Sekizaisou resemble those of mutants and transgenic plants with altered lignin structure (Fig. 1; Supplemental Fig. S2).
Lignin is derived from the polymerization of the monolignols p-coumaryl, coniferyl, and sinapyl alcohols, called monolignols, producing p-hydroxyphenyl (H), guaiacyl (G), and syringyl (S) units in the polymer (Fig. 2), respectively (Boerjan et al., 2003; Vanholme et al., 2019). The last step in monolignol biosynthesis, the reduction of an aldehyde to an alcohol, is catalyzed by CAD (Fig. 3). Plants with reduced CAD activity typically have a brown- or red-colored xylem (Baucher et al., 1996; MacKay et al., 1997; Pilate et al., 2002), and have often insufficient strength to allow the plants to stand fully upright (Sibout et al., 2005). These observations suggested that cv Sekizaisou may be a natural mutant with altered lignin structure due a mutation in CAD.
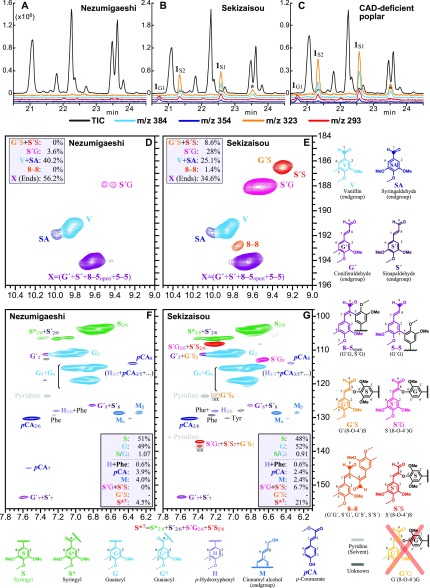
Figure 2.
GC-MS and HSQC analysis. Partial GC-MS traces showing the detection of the diagnostic indene markers released by thioacidolysis of lignins in cv Nezumigaeshi (A), cv Sekizaisou (B), and CAD-deficient transgenic poplar (C). Peaks labeled 1G1, 1S2, and 1S1 correspond to the indenes whose structures are shown in Supplemental Figures S3 and S4. Total-ion (TIC) and selected-ion (m/z 293, 323, 354, and 384) chromatograms are indicated with different colors. The selected-ion chromatograms are shown at 100-fold magnification, and the y axis zero-point is offset so that the chromatograms are more clearly resolved and viewed. Representative chromatograms are shown for 3 independent biological samples. D to G, Two-dimensional heteronuclear single-quantum coherence NMR spectra of cellulolytic enzyme lignins from cv Nezumigaeshi (D and F) and cv Sekizaisou (E and G). The spectra show the aldehyde (D and E) and aromatic (F and G) structures in both lignins. The quantification values shown are for relative comparisons of the lignin components determined from NMR contour volume-integrals. Correlation peaks are colored to match those of the structures; Phe is from Phe (in proteins), and Tyr is from Tyr (in proteins); the H2/6 correlation peak overlaps with a Phe peak so cannot be properly quantified.
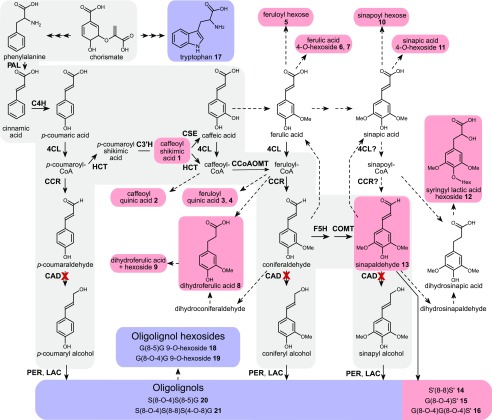
Figure 3.
Metabolic pathway based on phenolic profiling. Methanol-soluble phenolics differentially accumulated between Sekizaisou and other cultivars. The molecular structures of the individual metabolites can be found in Supplemental Figure S6 and Supplemental Table S2. The canonical monolignol biosynthetic pathway is shown with a gray background. As no near-isogenic line of cv Sekizaisou can be provided at present, the methanol-soluble phenolics from a single plant of each of 51 different mulberry cultivars (Supplemental Tables S3) that had been cultivated in the same experimental field and belonged to M. alba were profiled as controls. More and less abundant metabolites in cv Sekizaisou compared with the other cultivars are represented with red and blue backgrounds, respectively. The dashed arrows indicate one or more proposed conversions. C4H, cinnamate 4-hydroxylase; 4CL, 4-coumaric acid:CoA ligase; C3′H, p-coumaroyl quinate/shikimate 3′-hydroxylase; CAD, cinnamyl alcohol dehydrogenase; CCR, cinnamoyl-CoA reductase; COMT, caffeate O-methyltransferase; CSE, caffeoyl shikimate esterase; CCoAOMT, caffeoyl-CoA O-methyltransferase; F5H, ferulate 5-hydroxylase; HCT, hydroxycinnamoyl-CoA:shikimate hydroxycinnamoyl transferase; LAC, laccase; PAL, Phe ammonia lyase; PER, peroxidase.
We evaluated the lignin amount and composition of cv Sekizaisou along with six other cultivars that served as controls (Supplemental Table S1). The lignin content of the extractive-free wood was determined via the acetyl bromide protocol and appeared significantly lower in cv Sekizaisou (14.9% [w/w]) than in the other examined cultivars (20.1% to 22.3%; Supplemental Table S1). The lignin monomeric composition was determined by the cleavage of the ether linkages via thioacidolysis, followed by the detection of the released monomers via gas chromatography (GC)–flame-ionization detection (FID). Conventional G and S thioacidolysis-released monomers (2G and 2S, respectively) were detected in all samples. The S/G ratio was lower in cv Sekizaisou (0.78) than in the other examined cultivars (0.99–1.19; Supplemental Table S1), indicative of the fact that lignin biosynthesis is altered in cv Sekizaisou.
A deficiency in CAD activity typically induces the substantial incorporation of its substrates, coniferaldehyde, and sinapaldehyde, into the lignin polymer, giving rise to various substituted coniferaldehyde (G´) and sinapaldehyde (S´) units in the lignin (Fig. 2). Thioacidolysis-released indenes (1G and 1S; Kim et al., 2002; Lapierre et al., 2004), which are indicative of the incorporation of coniferaldehyde and sinapaldehyde into the lignin, may be detected via GC-FID, but GC-mass spectrometry (MS) is required for proper authentication (Supplemental Materials and Methods). Therefore, thioacidolysis products of wood from cv Sekizaisou were analyzed via GC-MS and compared with those of cv Nezumigaeshi used as a control mulberry cultivar, and to those of a transgenic CAD-deficient poplar (Populus tremula × Populus alba) used as a positive control for the incorporation of p-hydroxycinnamaldehydes into the lignin (line hpCAD19; Van Acker et al., 2017). The thioacidolysis-released indenes 1G (m/z 293 and 354) and 1S (m/z 293, 323 and 384), were observed in samples from 'Sekizaisou' and the CAD-deficient poplar (denoted as 1G1, 1S1, 1S2, and with an asterisk in Fig. 2, B and C), but not in those from 'Nezumigaeshi' (Fig. 2A; for mass spectra and structures of the indenes see Supplemental Figs. S3 and S4). Although the peak eluting at 23.5 min, and which is indicated with an asterisk in Figure 2, B and C, could not be identified, these data indicate that the lignin from cv Sekizaisou contains increased levels of sinapaldehyde and, to a lesser extent, also coniferaldehyde, as observed in other CAD-deficient plants (Kim et al., 2002; Lapierre et al., 2004; Van Acker et al., 2017).
To further elucidate the nature of the lignin, 1H–13C heteronuclear single-quantum correlation spectroscopy (HSQC) was performed on the lignin isolated from cv Sekizaisou and from the control cv Nezumigaeshi (Fig. 2, D–G). The aldehyde spectral region (Fig. 2, D and E) is the most diagnostic feature for the incorporation of the various hydroxycinnamaldehydes. As in the lignin of other angiosperms, a peak corresponding to S´(8–O–4)G structures, derived from 8–O–4 cross-coupled sinapaldehyde in the polymer, could be detected at a low level in cv Nezumigaeshi (Fig. 2D). Contrarily, in cv Sekizaisou (Fig. 2E), it was evident that coniferaldehyde and sinapaldehyde were integrally coupled at much higher levels into the lignin polymer via 8-coupling into G´(8–O–4)S (from coniferaldehyde), and S´(8–O–4)G and S´(8–O–4)S (from sinapaldehyde) structures. As the coniferaldehyde was found to be unable to couple with G units in previous studies, such as in synthetic lignin oligomers (Kim et al., 2000) and CAD-deficient tobacco (Nicotiana tabacum; Kim et al., 2003), and later reported in CAD-deficient poplar (Van Acker et al., 2017), we assumed that there are no G´(8–O–4)G-structures in the lignin of cv Sekizaisou. A cross-peak from homodimerization via 8–8-coupling was also detected, indicating that a sufficient number of aldehyde monomers was exported into the cell wall that their homodimerization could initiate polymerization, as also reported in CAD-deficient tobacco (Kim et al., 2003) and poplar (Van Acker et al., 2017).
To examine the effect of the putative mutation in CAD on soluble phenolics, including low Mr lignin precursors, methanol extracts from stems of field-cultivated mulberry plants were analyzed by using UHPLC/MS (Fig. 3; Supplemental Fig. S6; Supplemental Tables S2, S3, and S4). As no near-isogenic lines of cv Sekizaisou are available, the methanol-soluble phenolics from a single plant of each of 51 different mulberry cultivars belonging to M. alba were profiled as controls. A list of 208 compounds (166 more and 42 less abundant) with significantly different signal intensities between the control (average for 51 cultivars) and Sekizaisou was obtained; of these, 21 compounds (16 more and 5 less abundant) could be structurally characterized.
Most of the more abundant metabolites that could be structurally characterized were phenylpropanoids from the canonical monolignol pathway (compounds 1 and 13; Fig. 3; Supplemental Table 2) and from pathways branching therefrom. As in a previous study of the transgenic poplar with suppressed CAD activity (Van Acker et al., 2017), the abundance of syringyl lactic acid hexoside (compound 12) and an oxidatively coupled homodimer of sinapaldehyde, S´(8–8)S´ (compound 14) were significantly higher in ‘Sekizaisou’ compared with the controls. In addition, a dimer (compound 15) and a trimer (compound 16) derived from sinapaldehyde were also more abundant in cv Sekizaisou. By contrast, a trimer and a tetramer from the canonical monolignols, in addition to two such dimer hexosides, were less abundant in cv Sekizaisou. The aromatic amino acid, Trp, was also lower in abundance in cv Sekizaisou. A lower abundance of Trp has not been reported before in other lignin mutants. Collectively, these data strongly suggested that cv Sekizaisou is indeed a CAD-deficient mulberry plant.
As reported by Yoshimura and Saito (1924), the leaves of cv Sekizaisou had been used as feed for silkworms for local sericulture on Okushiri Island. Caffeoyl quinic acid (chlorogenic acid), for which the biosynthetic pathway overlaps with that of monolignols, is known as a growth-promoting factor for the silkworm (Kato and Yamada, 1964). Caffeoyl quinic acid (compound 2) also significantly accumulated in stems of cv Sekizaisou compared with the control (Fig. 3; Supplemental Table S2). Future studies will determine whether leaves from cv Sekizaisou have a positive effect on the feeding quality compared with its controls.
To investigate whether the observed morphological and molecular phenotypes of cv Sekizaisou were truly caused by a deficiency in CAD, the genomes of cv Sekizaisou and the control cv Nezumigaeshi were sequenced by using the HiSeq X Ten (Illumina) platform (Supplemental Materials and Methods). By using a BLAST search, we found three putative CAD gene sequences in the draft genome of cv Sekizaisou (gene identifcations, 2117-RA, 2718-RA, and 9780-RA). Of these, 9780-RA was the closest ortholog to MnCAD1 (98.6% coding sequence identity), the CAD gene that was predicted to be involved in lignin biosynthesis in Morus notabilis (Fig. 4B; Supplemental Fig. S5; He et al., 2013). We therefore designated 9780-RA as Sekizai-CAD1. The coding regions of the other two CAD homologs, 2117-RA and 2718-RA, exhibited only 56.5% coding sequence identity with MnCAD1, respectively. To further confirm the nucleotide sequence of 9780-RA, we performed PCR of this region, followed by sequencing of the amplicon (Supplemental Table S5).
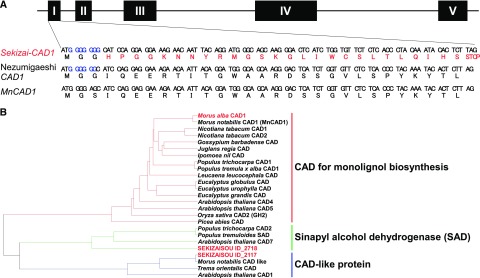
Figure 4.
Identification of CAD1 by draft genome sequencing, de novo assembly, and subsequent BLAST analysis. A, The gene structure of CAD1 in cv Sekizaisou (Sekizai-CAD1), cv Nezumigaeshi, and M. notabilis (MnCAD1). The genes include five exons (black boxes) and four introns (black lines). Nucleotide sequences of first exons (top line) and the deduced amino acid sequences (bottom line) of the genes are shown. After the first codon (ATG) in the first exon, a guanine hexad (blue) was detected in the sequence of Sekizai-CAD1, whereas both CAD1 alleles in cv Nezumigaeshi contain a guanine pentad. Amino acid sequences deduced from the first 29 codons of CAD1 in cv Nezumigaeshi and M. notabilis were identical, except for the residues encoded by the third codon. The guanine insertion into the first, second, or third codon of the first exon in Sekizai-CAD1 causes a frameshift mutation and subsequent premature stop codon. B, The phylogenetic tree of CAD sequences in different plant species. The amino acid sequence encoded by Sekizai-CAD1 was identical to those (319 amino acids) encoded by both entire CAD1 alleles in cv Nezumigaeshi if the inserted guanine residue was removed from the Sekizai-CAD1 sequence. The phylogenetic tree was constructed by using the entire CAD1 sequence of cv Nezumigaeshi as the CAD1 sequence of all M. alba cultivars.
Based on the sequencing results, the Sekizai-CAD1 locus in cv Sekizaisou is homozygous (accession no., LC476972) and the CAD1 locus in cv Nezumigaeshi is heterozygous (LC476973 and LC476974). When the sequence of Sekizai-CAD1 was compared with the MnCAD1 and CAD1 sequences from cv Nezumigaeshi, a guanine insertion was detected close to the predicted translation start codon of Sekizai-CAD1 (Fig. 4; Supplemental Fig. S5). This mutation was also confirmed by direct sequencing of the first-strand cDNA derived from the reverse transcripts of Sekizai-CAD1 mRNA. This insertion caused a frameshift and, consequently, a premature stop codon in the putative first exon of Sekizai-CAD1. A frameshift mutation in CAD has been also reported in the pine cad-null mutant in which wood color was brown (Ralph et al., 1997; Gill et al., 2003). Sekizai-CAD1 transcripts of similar size as CAD1 transcripts in cv Nezumigaeshi were detected by reverse transcription-PCR (Supplemental Fig. S5), indicating that the mutant gene is still actively transcribed. The sequencing results are in line with a homozygous recessive allele in cv Sekizaisou, as predicted from the phenotypes of the F1 progenies derived from crosses between cvs Sekizaisou and Kokusou 21 (Supplemental Fig. S2). To the best of our knowledge, this is the first report on a homozygous mutation in Morus with an abnormal phenotype and the first report on an angiosperm lignin mutant, even though the mutation had not been recognized until now.
Accession Numbers
DNA sequences of CAD1 from cvs Sekizaisou and Nezumigaeshi have been deposited in DDBJ, ENA, and GenBank with the following accession numbers: LC476972 (Sekizai-CAD1), LC476973 (allele 1 in cv Nezumigaeshi), and LC476974 (allele 2 in cv Nezumigaeshi).
Supplemental Data
The following supplemental materials are available.
Supplemental Materials and Methods. Detailed information on materials and methods.
Supplemental Figure S1. An ancient photograph of cultivated Sekizaisou.
Supplemental Figure S2. cv Sekizaisou and its F1 progenies.
Supplemental Figure S3. Structures of the main thioacidolysis products.
Supplemental Figure S4. Mass spectra of lignin-derived indenes.
Supplemental Figure S5. Exon-intron structure of CAD1 genes in Morus sp.
Supplemental Figure S6. Molecular structures of differentially accumulated metabolites.
Supplemental Table S1. Lignin content and monomeric composition.
Supplemental Table S2. Methanol-soluble phenolics differentially accumulated between cv Sekizaisou and other cultivars.
Supplemental Table S3. List of cultivars used for metabolomic analysis.
Supplemental Table S4. Nucleotide sequences of PCR and sequencing primers.
Supplemental Table S5. Full list of peaks detected in the cv Sekizaisou and other cultivars.
Acknowledgments
We thank Dr. Gilles Pilate and Dr. Annabelle Déjardin (Institut National de la Recherche Agronomique) for providing the transgenic poplar plants, and National Agriculture and Food Research Organization Genebank for the distribution of mulberry cultivars. We would also like to express our gratitude to Shinta Inagaki, Dr. Hiroaki Yamanouchi, Dr. Akemi Shimizu, and Takeshi Yokoyama (Okushiri Board of Education) for providing the information on cv Sekizaisou. Dr. Yasuhiko Matsushita is acknowledged for technical support in analysis of the genome sequences.
Footnotes
This work was supported by the Japan Society for the Promotion of Science (JSPS) (JP18K45678 to S.K.), Japan Science and Technology Agency (JST) (JPMJAL1107 to N.M.), and DOE | Office of Under Secretary for Science (DE-SC0018409).
Articles can be viewed without a subscription.
References
- Baucher M, Chabbert B, Pilate G, Van Doorsselaere J, Tollier MT, Petit-Conil M, Cornu D, Monties B, Van Montagu M, Inze D, Jouanin L, Boerjan W(1996) Red xylem and higher lignin extractability by down-regulating a cinnamyl alcohol dehydrogenase in poplar. Plant Physiol 112: 1479–1490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boerjan W, Ralph J, Baucher M(2003) Lignin biosynthesis. Annu Rev Plant Biol 54: 519–546 [DOI] [PubMed] [Google Scholar]
- Gill GP, Brown GR, Neale DB(2003) A sequence mutation in the cinnamyl alcohol dehydrogenase gene associated with altered lignification in loblolly pine. Plant Biotechnol J 1: 253–258 [DOI] [PubMed] [Google Scholar]
- He N, Zhang C, Qi X, Zhao S, Tao Y, Yang G, Lee TH, Wang X, Cai Q, Li D, et al. (2013) Draft genome sequence of the mulberry tree Morus notabilis. Nat Commun 4: 2445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotta T. (1937) Hosan kuwazoku bunrui no saiginmi (Re-classification of Morus genus originated in Japan). Sangyo Shinpo 45: 64–69 (in Japanese) [Google Scholar]
- Hotta T.(1950) Kuwa (Mulberry). Heibonsha, Tokyo, Japan [Google Scholar]
- Kato M, Yamada H(1964) Chlorogenic acid as a growth factor of silkworm. J Silkworm 15–16: 85–92 [Google Scholar]
- Kim H, Ralph J, Lu F, Pilate G, Leplé JC, Pollet B, Lapierre C(2002) Identification of the structure and origin of thioacidolysis marker compounds for cinnamyl alcohol dehydrogenase deficiency in angiosperms. J Biol Chem 277: 47412–47419 [DOI] [PubMed] [Google Scholar]
- Kim H, Ralph J, Lu F, Ralph SA, Boudet AM, MacKay JJ, Sederoff RR, Ito T, Kawai S, Ohashi H, Higuchi T(2003) NMR analysis of lignins in CAD-deficient plants. Part 1. Incorporation of hydroxycinnamaldehydes and hydroxybenzaldehydes into lignins. Org Biomol Chem 1: 268–281 [DOI] [PubMed] [Google Scholar]
- Kim H, Ralph J, Yahiaoui N, Pean M, Boudet AM(2000) Cross-coupling of hydroxycinnamyl aldehydes into lignins. Org Lett 2: 2197–2200 [DOI] [PubMed] [Google Scholar]
- Lapierre C, Pilate G, Pollet B, Mila I, Leplé JC, Jouanin L, Kim H, Ralph J(2004) Signatures of cinnamyl alcohol dehydrogenase deficiency in poplar lignins. Phytochemistry 65: 313–321 [DOI] [PubMed] [Google Scholar]
- MacKay JJ, O’Malley DM, Presnell T, Booker FL, Campbell MM, Whetten RW, Sederoff RR(1997) Inheritance, gene expression, and lignin characterization in a mutant pine deficient in cinnamyl alcohol dehydrogenase. Proc Natl Acad Sci USA 94: 8255–8260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marroni F, Pinosio S, Di Centa E, Jurman I, Boerjan W, Felice N, Cattonaro F, Morgante M(2011) Large-scale detection of rare variants via pooled multiplexed next-generation sequencing: Towards next-generation Ecotilling. Plant J 67: 736–745 [DOI] [PubMed] [Google Scholar]
- Pilate G, Guiney E, Holt K, Petit-Conil M, Lapierre C, Leplé JC, Pollet B, Mila I, Webster EA, Marstorp HG, et al. (2002) Field and pulping performances of transgenic trees with altered lignification. Nat Biotechnol 20: 607–612 [DOI] [PubMed] [Google Scholar]
- Ralph J, MacKay JJ, Hatfield RD, O’Malley DM, Whetten RW, Sederoff RR(1997) Abnormal lignin in a loblolly pine mutant. Science 277: 235–239 [DOI] [PubMed] [Google Scholar]
- Sibout R, Eudes A, Mouille G, Pollet B, Lapierre C, Jouanin L, Séguin A(2005) CINNAMYL ALCOHOL DEHYDROGENASE-C and -D are the primary genes involved in lignin biosynthesis in the floral stem of Arabidopsis. Plant Cell 17: 2059–2076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Acker R, Déjardin A, Desmet S, Hoengenaert L, Vanholme R, Morreel K, Laurans F, Kim H, Santoro N, Foster C, et al. (2017) Different routes for conifer- and sinapaldehyde and higher saccharification upon deficiency in the dehydrogenase CAD1. Plant Physiol 175: 1018–1039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanholme B, Cesarino I, Goeminne G, Kim H, Marroni F, Van Acker R, Vanholme R, Morreel K, Ivens B, Pinosio S, et al. (2013) Breeding with rare defective alleles (BRDA): A natural Populus nigra HCT mutant with modified lignin as a case study. New Phytol 198: 765–776 [DOI] [PubMed] [Google Scholar]
- Vanholme R, De Meester B, Ralph J, Boerjan W(2019) Lignin biosynthesis and its integration into metabolism. Curr Opin Biotechnol 56: 230–239 [DOI] [PubMed] [Google Scholar]
- Yoshimura M, Saito K (1924) Kuwa no ichihinsyu sekizaisou ni tsuite (A mulberry cultivar, Sekizaisou). Sakurakai Zasshi 15: 107–111 (in Japanese) [Google Scholar]