Receptor-like kinases regulate different stages of symbiosis establishment and development.
Abstract
Plant receptor-like kinases (RLKs) control the initiation, development, and maintenance of symbioses with beneficial mycorrhizal fungi and nitrogen-fixing bacteria. Carbohydrate perception activates symbiosis signaling via Lysin-motif RLKs and subsequently the common symbiosis signaling pathway. As the receptors activated are often also immune receptors in multiple species, exactly how carbohydrate identities avoid immune activation and drive symbiotic outcome is still not fully understood. This may involve the coincident detection of additional signaling molecules that provide specificity. Because of the metabolic costs of supporting symbionts, the level of symbiosis development is fine-tuned by a range of local and mobile signals that are activated by various RLKs. Beyond early, precontact symbiotic signaling, signal exchanges ensue throughout infection, nutrient exchange, and turnover of symbiosis. Here, we review the latest understanding of plant symbiosis signaling from the perspective of RLK-mediated pathways.
Plants interact with a plethora of other organisms, and these symbioses range from detrimental to beneficial in outcomes and from transient to persistent in terms of stability. For durable relationships where symbiosis is reestablished every generation, the ability to recruit, maintain, and terminate symbiotic relationships involves genetically encoded components that sense and orchestrate signaling, metabolic, and developmental changes in both parties to achieve mutually beneficial outcomes. Among these components, receptor-like kinases (RLKs) transduce external and endogenous signals and activate the corresponding signaling processes.
Here, we focus on the roles of plant RLKs in initiating, establishing, limiting, and maintaining distinct steps of symbiotic interactions, specifically in root endosymbioses where the genetic underpinnings are well understood. Box 1 provides a primer on the functions and stages of the two plant symbioses with arbuscular mycorrhizal fungi (AMF) and with nitrogen-fixing bacteria. At each stage, we highlight emerging themes and the range of pathways identified. With this, we aim to complement the suite of recent reviews of plant symbioses from the perspective of transcriptional regulation (Diédhiou and Diouf, 2018; Pimprikar and Gutjahr, 2018), phytohormone regulation (Fonouni-Farde et al., 2016; Liu et al., 2018; Müller and Harrison, 2019), nutrient exchange (Udvardi and Poole, 2013; Chiu and Paszkowski, 2019), and evolution (Martin et al., 2017; Strullu-Derrien et al., 2018).
THE COMMON SYMBIOSIS SIGNALING PATHWAY
From the diverse microorganisms in the soil, plant hosts are able to recruit beneficial symbionts into the root. Both AMF and nitrogen-fixing bacteria require a core signaling pathway that orchestrates the signaling and developmental changes necessary for symbiont accommodation. Elucidation of plant symbiotic signaling pathways began with forward genetic screens in model legumes such as Medicago truncatula (Medicago) and Lotus japonicus (Lotus). Several mutants defective in nodulation were also defective in arbuscular mycorrhizal symbiosis (AMS), leading to the concept of a common symbiosis signaling pathway (CSSP). RLK activation upon ligand recognition converges on the CSSP to activate symbiosis signaling. This ancient toolkit evolved for AMS and was hypothesized to be coopted by the nodulating clade for root nodule symbiosis (RNS; Parniske, 2000, 2008; Oldroyd, 2013). The CSSP begins with SYMBIOSIS RECEPTOR KINASE (SYMRK/DMI2/NORK), a malectin domain-leucine-rich repeat (MLD-LRR) RLK that activates perinuclear calcium oscillations (hereafter Ca2+ oscillations) involving Ca2+ channels on the nuclear envelope (e.g. DOESN’T MAKE INFECTION1 [DMI1]/CASTOR/POLLUX; Kim et al., 2019). These Ca2+ oscillations activate a CALCIUM AND CALMODULIN-DEPENDENT KINASE (CCaMK/DMI3) that interacts with and phosphorylates CYCLOPS/INTERACTING PROTEIN OF DMI3 (IPD3), a transcriptional regulator, launching symbiosis signaling. In addition, the nuclear pore complex components of NUCLEOPORIN85 (NUP85), NUP133, and NENA in Lotus are also CSSP components upstream of Ca2+ oscillations, but their symbiotic defects are temperature sensitive (permissive at lower temperatures). Readers are directed to previous articles (Parniske, 2008; Oldroyd, 2013; MacLean et al., 2017) for comprehensive reviews of the CSSP.
Nonetheless, it is important to point out that compared with strong nodulation defects, intraradical colonization as well as arbuscule development is possible in CSSP mutants, as is observed in Lotus symrk and ccamk mutants (Demchenko et al., 2004; Parniske, 2008), that CSSP-independent signaling pathways occur (Kosuta et al., 2003; Kistner et al., 2005; Gutjahr et al., 2008; Camps et al., 2015), and that species-specific differences exist for mutant phenotypes. CYCLOPS is a case in point, where strong AM phenotypes in Lotus and rice (Oryza sativa) contrast starkly against reduced but successful arbuscule development in Medicago ipd3/ipd3-like double mutants (Lindsay et al., 2019). Collectively, these and other data show that CYCLOPS is sufficient but not necessary for symbiosis signaling (Pimprikar et al., 2016). The clearest demonstration of a CSSP-independent pathway comes from Lotus LACK OF SYMBIONT ACCOMODATION (LjLAN), which encodes for a MEDIATOR complex protein and regulates both AMS and RNS while displaying normal Ca2+ oscillations to Nod factor. Ljlan is impaired in infection thread formation but low numbers of intercellular infection lead to some nodule development, whereas Ljlan/cyclops was completely defective in nodule formation (Suzaki et al., 2019).
The existence of CSSP puts symbiosis evolution into perspective and offers the tantalizing possibility of engineering nitrogen-fixing symbiosis into crops by identifying components and regulatory mechanisms that evolved to enable RNS (Mus et al., 2016). Yet the CSSP also raises an unresolved conundrum: how do rhizobia bacteria and AMF both activate CSSP but yet specify different symbiotic outcomes in their host? Bifurcating downstream transcriptional regulation (e.g. by transcription factors NODULATION SIGNALING PROTEIN1 [NSP1]/REQUIRED FOR ARBUSCULAR MYCORRHIZA1, previously suggested to specify RNS/AMS, respectively [Oldroyd, 2013]) is now revised with evidence demonstrating a role for NSP1 in AMS (Liu et al., 2011; Delaux et al., 2013; Takeda et al., 2013; van Zeijl et al., 2015; Floss et al., 2017), leaving the specificity determinants unresolved.
LYSIN-MOTIF RLKS ACTIVATE THE CSSP
Symbiosis signaling initiates with microbial carbohydrate perception by plant plasma membrane (PM)-localized RLKs, the very first step of symbiotic scrutiny. In the symbiosis between rhizobia bacteria and legumes or Parasponia, this step is especially stringent, as lipochitooligosaccharides (LCOs, also known as Nod factors) from compatible rhizobia bind to and activate their cognate RLKs. These Lysin-motif (LysM)-RLKs contain three LysM domains in the extracellular domain capable of binding oligosaccharides containing β1,4-glycosidic bonds. Forward genetic screens for nodulation-defective mutants and subsequent biochemical characterization identified Lotus NOD FACTOR RECEPTOR1 (LjNFR1) and LjNFR5 to be necessary for directly binding and perceiving Mesorhizobium loti Nod factors and activating the downstream signaling cascades for infection and nodule organogenesis (Madsen et al., 2003; Radutoiu et al., 2003; Broghammer et al., 2012). LjNFR1 and LjNFR5 bind Nod factors at nanomolar ranges, concentrations at which physiological responses are triggered. Loss of either LjNFR1 or LjNFR5 abolished infection or nodule organogenesis. Medicago LYSM DOMAIN RECEPTOR KINASE3 (MtLYK3) and NOD FACTOR PERCEPTION (MtNFP) are similarly required for nodulation. However, Mtlyk3-RNAi or mutants still retain some Nod factor responses, notably Ca2+ oscillations. MtLYK3 therefore has been proposed to be an entry receptor that regulates rhizobia infection (Catoira et al., 2000; Wais et al., 2000; Amor et al., 2003; Limpens et al., 2003; Smit et al., 2007). This model also holds true for pea (Pisum sativum), where PsSYM10 (LjNFR5/MtNFP homolog; Madsen et al., 2003) as well as PsSYM37 and PsK1 (MtLYK3 homologs; Zhukov et al., 2008; Kirienko et al., 2018, 2019) are involved in the perception of Rhizobium leguminosarum. Figure 1 highlight the roles of the LysM-RLKs involved as well as a phylogenetic relationship of some of these receptors with their most recent gene names. Importantly, the transfer of LjNFR1 and LjNFR5 into Medicago enabled the Lotus symbiont M. loti to infect and develop nodules on Medicago (a nonhost; Radutoiu et al., 2007), demonstrating that the perception of specifically decorated LCOs by its cognate LysM-RLKs in a receptor complex may determine symbiosis specificity in RNS.
Figure 1.
LysM-RLKs in symbioses and beyond.A, LysM Receptors with characterised roles in symbiosis or immunity.Nod-factors activate receptor complexes that comprise both active kinases (blue) and pseudokinases (red), with homologues identified in Lotus, Medicagoand Pea. Only the pseudokinase LysM-RLK is known for P. andersonii, a species of the only non-legume genus that engages in root nodule symbiosis with Rhizobia. A similar receptor complex may be employed for AMF perception; indeed, numerous active kinases have been identified to activate symbiosis signaling in response to AMF, whereas the identities of pseudokinases are less clear. Knockouts of NFR5/NFP pseudokinases have wild-type colonization, and only have a quantitative contribution in the case of Mtnfp/cerk1 double mutants. However, PaNFP/SlLYK10 may be required for full arbuscule development. Some receptors (e.g. MtLYR3)have been characterized biochemically to be a high-affinity LCO receptor, but their symbiotic roles are not reported, or potentially redundant (e.g. LjLYS11) and are excluded in (A). While CERK1/LYK1/LYK9 orthologs are required for both immunity and AMS in some species, some species-specific differences exists (e.g. for tomato, Lotus). And while Nod-factors (LCOs) and short-chain chitin oligomers (CO4 and CO5) are non-immunogenic (with respect to reactive oxygen species [ROS] production and MAPK activation) symbiosis signaling elicitors; peptidoglycan and long-chain chitin oligomers (CO8) can activate both immune and symbiosis signaling. Note that superscript numbers indicate: 1, MtLYK3 is not equivalent to LjNFR1 sensu stricto, since Mtlyk3 retains some nod-factor responses, especially Ca2+-oscillations whereas all nod-factor responses are abolished in Ljnfr1; 2, RNA-silencing was used, hence non-specific silencing of other LysM-RLKs cannot be ruled out in RNAi lines except in SlLYK12/1/10 where it was examined; and 3, Different AMS phenotypes in different reports. Ljnfr1 and Mtlyk3 had weak phenotypes at early-timepoints and at low spore inoculums but not in two other separate studies. B, Unrooted maximum likelihood phylogenetic tree of full length LysM-RLKs containing active kinases with clear, characterized roles in immunity and/or symbiosis. The two most recent gene names are included, as different publications may refer to same genes with different names. For each node, bootstrap values are shown as percentages based on 1000 repetitions. Note that branch lengths are not proportional to the number of substitutions per site. Species prefixes are: At, Arabidopsis thaliana; Lj, Lotus japonicus; Ma, Musa acuminata; Mt, Medicago truncatula; Os, Oryza sativa; Pa, Parasponia andersonii; Ph, Petunia hybrida; Ps, Pisum sativum; Sl, Solanum lycopersicum.
In addition to LjNFR1/5, a new Nod factor receptor was recently described. LjNFRe is closely related to LjNFR1 and is hypothesized to ensure robust epidermal Nod factor signaling at the susceptible zone of Lotus roots (Murakami et al., 2018). Ljnfre mutants develop fewer nodules, consistent with its role in inducing NODULE INCEPTION (LjNIN) expression in the epidermis. Like LjNFR1, LjNFRe has an active kinase domain, binding affinity for LCOs, and also phosphorylates and requires NFR5 for activating epidermal NIN expression. Furthermore, replacing the kinase domain activation segments of LjNFRe with that of LjNFR1 is sufficient to change the signaling output to that of NFR1, enabling cortical NIN induction and nodule development, showing that the activation segment determines signaling specificity between NFR1 and NFRe (Murakami et al., 2018).
Together, these Nod factor signaling components present a paradigm whereby a LysM-RLK with functional, active kinase domain (LjNFR1/MtLYK3) forms a complex with an RLK with an inactive, pseudokinase domain (LjNFR5/MtNFP), probably requiring the former to transphosphorylate the latter to generate a signaling response (Madsen et al., 2011; Antolín-Llovera et al., 2014; Murakami et al., 2018). These two types also correspond to the two LysM-RLK clades present in land plants. Why a pseudokinase is necessary for signaling remains to be fully appreciated, but the additional involvement of LjSYMRK in phosphorylating LjNFR5 may point to the formation of heterotypic complexes or different separable, distinct receptor complexes during RNS as well as AMS.
In contrast to RNS, where Nod factor perception explains most of host-symbiont specificity, the nature, range, and exact suite of ligand(s) for AMS are hitherto elusive. AMF produce simple LCOs and short-chain chitin oligomers (CO4 and CO5), both of which elicit symbiotic outputs via the CSSP, often shown by activation of Ca2+ oscillations and increased lateral root formation (Maillet et al., 2011; Genre et al., 2013; Sun et al., 2015). Exogenous application of LCOs increased root length colonization by AMF, consistent with its role as a positive regulator of symbiosis (Maillet et al., 2011). However, apart from LCOs and CO4 and CO5, the elicitors capable of activating Ca2+ oscillations and symbiotic gene expression may be wider than expected. Feng et al. (2019) demonstrated that CO8 and peptidoglycan (PGN), typically considered pathogen-associated molecular patterns, are also capable of eliciting symbiotic signaling outputs. Coordinated perception of LCO and CO8/PGN attenuated the immunity outputs of ROS production and MAPK activation and synergistically boosted symbiotic gene expression, thereby suggesting that AMS might involve coordinated perception of microbe-associated molecular patterns via LysM-RLKs and activating symbiosis signaling via SYMRK. Thus, redundancy at both ligand and receptors could explain (1) the lack of LysM-RLK mutants where symbiosis with AMF is abolished and (2) the broad host range of AMF.
While the exact nature of signaling molecules remains to be identified, it is clear that carbohydrate perception by LysM-RLKs activates symbiosis signaling in AMS. The requirement for LysM-RLKs has recently been elucidated in various plant species. First demonstrated in rice, similar work in pea, Medicago, tomato (Solanum lycopersicum), and banana (Musa acuminata) all revealed that the LysM-RLK CHITIN ELICITOR RECEPTOR KINASE1 (CERK1; or equivalent homologs) is involved in signal perception of AMF-derived chitinaceous ligands, since cerk1 null and/or cerk1-RNAi had reduced colonization (Miyata et al., 2014; Zhang et al., 2015, 2019; Leppyanen et al., 2017; Chiu et al., 2018; Liao et al., 2018; Feng et al., 2019; Gibelin-Viala et al., 2019). Importantly, symbiosis is not abolished, showing that CERK1 is necessary but not essential for AM symbiosis. In all cases (except for pea, where microscopy data are lacking), arbuscule development appears normal.
Furthermore, in rice, where the LysM-RLK family is less expanded vis-à-vis the Fabaceae, Oscerk1 mutants lack detectable epidermal Ca2+ oscillations in response to CO4 and germinated spore exudates, a cocktail of signal molecules from naïve AMF spores (Carotenuto et al., 2017). Nevertheless, intraradical colonization and arbuscule formation in Oscerk1 mutants (Miyata et al., 2014; Zhang et al., 2015; Chiu et al., 2018) suggest that either symbiosis development can be achieved independent of Ca2+ oscillations or that yet unobserved oscillations occur under prolonged signal exchange employing redundant LysM-RLKs or SYMRK.
As such, in contrast to their essential role for symbiotic scrutiny in most RNS, the role of LCOs in AM symbiosis is at present equivocal. Null mutants of Ljnfr5, Mtnfp displayed wild-type levels of colonization (Maillet et al., 2011; Zhang et al., 2015; Feng et al., 2019). Contradictory observations were made for Ljnfr1 or Mtlyk3 mutants, which either showed transiently reduced colonization (Zhang et al., 2015) or no difference relative to the wild type (Takeda et al., 2011; Feng et al., 2019). Normal colonization in mutants of LCO receptors may be due to the genetic redundancy present in the Fabaceae, where the receptors have expanded. For instance, LjLYS11 encodes an LCO receptor closely related to LjNFR5 that is induced during AM colonization. Yet, even triple mutants of Ljnfr1 nfr5 lys11 in Lotus had no observable defects in AM colonization (Rasmussen et al., 2016). Also, whereas silencing Parasponia andersonii NFP (PaNFP) did not prevent fungal colonization, it blocked arbuscule development and decreased nodulation frequency (Op den Camp et al., 2011). However, it cannot be excluded that closely related LysM-RLKs may be silenced as well. Whether PaNFP directly binds to or mediates LCO perception has yet to be shown. In rice, where the LysM-RLK family is not neofunctionalized for RNS, knockout of the LjNFR5/MtNFP ortholog, OsNFR5/RLK2/MYR1, produced contrasting phenotypes. Homologous recombination mutants showed normal AM symbiosis or Ca2+-spiking responses to germinated spore exudates (Miyata et al., 2016; Carotenuto et al., 2017). However, CRISPR-edited mutants of the same gene had reduced colonization levels under low spore inoculum strength. Under high inoculation strength, osnfr5/rlk2/myr1 have wild-type-like colonization levels (He et al., 2019), suggesting that the CO4-binding function of OsNFR5/MYR1 is dispensable for symbiosis.
On the other hand, LCO receptors of two solanaceaeous species, petunia (Petunia hybrida) and tomato, have been recently identified to be involved in AM symbiosis. Virus-induced gene silencing of the tomato ortholog (SlLYK10) delayed fungal colonization and resulted in abnormal arbuscule development (Buendia et al., 2016). Similarly, in a missense allele of Sllyk10-1, and in Phlyk10-1, a transposon-insertion mutant, the mutation/loss of LCO receptor quantitatively reduced AM colonization and full arbuscule development in both species (Girardin et al., 2019). Importantly, both SlLYK10 and PhLYK10 can restore RNS in Mtnfp and Ljnfr5 mutants (Girardin et al., 2019). This is consistent with the notion that the evolution of RNS recruited existing components involved in AMS.
Therefore, evidence so far suggests that LCO perception by LysM-RLKs contributes to, but may not be essential for, AMS. This is supported by the observation that reduction in AM colonization is further diminished in the Mtcerk1 nfp double mutant relative to Mtcerk1 mutants (Feng et al., 2019). Whether LCO perception by LysM-RLKs is necessary, or fully dispensable, for AM symbiosis remains to be ascertained.
Overall, carbohydrate signaling during AMS appears to be more complex than expected, as symbiont-encoded proteins can also affect ligand binding and receptor activation. Fungus-secreted LysM effectors previously described to be involved in subverting host defense responses during chitin-triggered immunity have been recently identified in AMF as well (Schmitz et al., 2019; Zeng et al., 2020). Rhizophagus irregularis SECRETED LYSM EFFECTOR (RiSLM) was capable of binding both COs and LCOs. Recombinant RiSLM reduced chitin-triggered ROS production but allowed the activation of symbiosis signaling. Consistent with its role as a positive regulator, host-induced gene silencing of RiSLM reduced AM colonization, although overexpression of RiSLM in Medicago did not increase colonization (Zeng et al., 2020).
Whereas mutants of LysM-RLK show reduced symbiosis, AMS is fully abolished when the α/β-hydrolase receptor DWARF14-LIKE (D14L) is mutated (Gutjahr et al., 2015; Liu et al., 2019). How D14L, the ancient paralog of the strigolactone receptor D14, fits with the CSSP in AM signaling remains to be demonstrated. Also unknown is the identity of the karrikin-like D14L ligand, of plant or fungal origin. Nonetheless, the essential function of D14L for AMS in rice is conserved in petunia (Gutjahr et al., 2015; Liu et al., 2019), suggesting that the critical role of D14L in symbiotic scrutiny is similar in monocotyledonous and dicotyledonous plant species.
MULTIFACETED LYSM RECEPTORS HAVE ROLES BEYOND SYMBIOSIS
An enigma with the chitin receptor is its role in activating both defense and symbiotic responses in response to fungal elicitors. This is the case in rice, Medicago, and pea (Miyata et al., 2014; Leppyanen et al., 2017; Gibelin-Viala et al., 2019). Like its Arabidopsis (Arabidopsis thaliana) counterpart, CERK1 is required for activating immunity outputs (ROS production and MAPK activation; Miya et al., 2007; Wan et al., 2008) in both roots and shoots, but it also mediates AM symbiosis in the roots. Nonetheless, ROS and MAPK assays are limited in their specificity, as they are also triggered by damage, development, and early AMF perception. When exposed to pathogens, Oscerk1/Mtcerk1 mutants are more susceptible to both fungal (rice blast, Magnaporthe oryzae; Kouzai et al., 2014a) and also oomycete (in the case of Mtcerk1) pathogens (Bozsoki et al., 2017; Gibelin-Viala et al., 2019). Yet immunity and symbiosis appear to be separable in other species instead, showing that species-specific differences and subfunctionalization of chitin receptors exist (Fig. 1). For example, in Ljcerk6 mutants, where chitin-induced responses were abolished, AM colonization was not affected (Bozsoki et al., 2017). Similarly, in tomato, whereas silencing SlLYK12 impaired AM colonization, silencing SlLYK1/Bti9 impaired ROS production and resistance to Pseudomonas syringae (Zeng et al., 2012; Liao et al., 2018).
Symbiosis versus immunity signaling could be determined at the level of receptor/coreceptor complex composition. For instance, OsCEBiP, the high-affinity receptor for CO8, is not involved in AM symbiosis but in basal resistance against M. oryzae (Kaku et al., 2006; Kishimoto et al., 2010; Kouzai et al., 2014b). However, the fact that CO8 and CERK1 signaling can activate both defense and symbiotic signaling means that while exclusively pathogenic elicitors may activate distinct immune receptor complexes, other elicitors may activate overlapping pathways for immunity and symbiosis via a shared coreceptor and require other microbe-associated molecular pattern detection and/or effector detection for signal integration before a full immune or symbiosis response is mounted. This is consistent with observations that AMF transiently activates early defense responses that are attenuated (Harrison and Dixon, 1993; Kapulnik et al., 1996; García-Garrido and Ocampo, 2002; Liu et al., 2003; Campos-Soriano et al., 2010). It has been proposed that the changing ratio of immune-modulating CO4 and CO5/LCOs relative to immunogenic CO7-8 may be relevant (Schmitz and Harrison, 2014), but the supporting evidence is lacking.
On top of symbiosis signaling and chitin-triggered defenses, OsCERK1 is also required for increased root-branching responses, specifically of large lateral roots, the preferred root type for AMF colonization (Chiu et al., 2018). The ultimate explanation for this response is perhaps that symbiont perception elicits an overall increase in symbiotic interfaces available. Lateral root branching has been used as a biological assay for Myc/Nod factors. In rice, this developmental response is however independent of CSSP and also independent of the karrikin receptor D14L that is necessary for presymbiotic dialogue and symbiotic accommodation (Gutjahr et al., 2009, 2015; Chiu et al., 2018). The developmental and intraradical colonization steps are thus genetically separable, at least in a monocot.
LCO PERCEPTION AND CSSP MAY NOT BE SPECIFIC TO ENDOSYMBIOSIS SIGNALING
As Nod factors and potentially Myc factors, LCOs have been regarded as a nonimmunogenic symbiotic signal that connects membrane perception to CSSP for endosymbiont accommodation. Before discussing signaling events downstream of LysM-RLKs, it is important to highlight that many nonendosymbionts produce similar signaling molecules that activate CSSP. Therefore, how specificity between different organisms in the rhizosphere is achieved is still unresolved.
LCO production is not an endosymbiont-exclusive trait, as they have recently been demonstrated to also be produced by ectomycorrhizal (ECM) fungi, another ecologically important plant-fungus symbiosis (Cope et al., 2019) but fundamentally not an endosymbiotic association. The fungus Laccaria bicolor produces LCOs and elicits perinuclear Ca2+ oscillations in Poplar trichorcarpa via PtCASTOR/POLLUX. Both castor/pollux-RNAi and ccamk-RNAi show slightly reduced ECM formation (Cope et al., 2019). However, a cautious interpretation is that interfering with CSSP affects both LCO and CO signaling, so whether ECM symbiosis requires LCO recognition is not yet demonstrated. As alluded to by this and earlier work, CSSP components are missing in some host species (e.g. Norway spruce [Picea abies]) and hence is likely not a conserved pathway required for ECM associations (Garcia et al., 2015; Cope et al., 2019). Moreover, compounds with nearly identical chemical and biological properties to LCOs have been reported to be produced by nitrogen-fixing maize (Zea mays) root endophytes of Bacillus spp. (Lian et al., 2001) and nematodes (Weerasinghe et al., 2005). Finally, the LCO perception machinery is involved in other biotic interactions where Mtnfp mutants were more susceptible to oomycete and fungal infections (Rey et al., 2013). Collectively, it appears that LCO production and perception in the rhizosphere may extend beyond AMF and rhizobia.
Downstream of LCO perception, Ca2+ oscillations are also not exclusively activated by fungal or rhizobial symbionts, as glucan-chitosaccharide fractions from Aphanomyces euteiches were reported to elicit perinuclear Ca2+ oscillations independent of MtNFP, MtDMI1, and MtDMI2 (Nars et al., 2013). However, the robustness of Ca2+ oscillations in the traces, especially for the mutants, appears to be relatively low. More recently, it was demonstrated that endophytic Fusarium solani and Serendipita indica, as well as pathogenic Fusarium oxysporum, are capable of eliciting Ca2+ oscillations (Skiada et al., 2020). Clearly, beyond symbiotic AM/ECM fungi and rhizobia, nuclear Ca2+ oscillations can also be activated by other microorganisms.
In addition, the loss of LysM-RLK or CSSP signaling could either suppress (Skiada et al., 2020) or promote colonization by nonendosymbionts (Fernandez-Aparicio et al., 2010). There is also no obvious trend whether this correlates with the intimacy of the pathogen lifestyle. However, the small quantitative nature of these effects relative to those of CSSP mutations on AMS/RNS suggests that endophytes/pathogens rely less on the CSSP for host access. Further support is provided by M. oryzae, which leads an extended biotrophic lifestyle in rice roots but is still capable of proliferating in roots of rice CSSP mutants and d14l mutants (Marcel et al., 2010; Gutjahr et al., 2015), where AMF accommodation is abolished.
Recently, next-generation sequencing has enabled the profiling of bacterial and fungal communities in the root microbiome (Zgadzaj et al., 2016; Thiergart et al., 2019; Xue et al., 2019). As demonstrated for Lotus mutants, the use of microbiome profiling, especially in complex soils or controlled mesocosms, may allow a more refined understanding of whether the CSSP/LysM-RLK/autoregulatory mutants become more or less susceptible to pathogens/biotrophs of interest when challenged in a nearly native context.
Moving out of the model legumes, LCOs are not necessary for symbiosis signaling. In the rhizobia-legume symbiosis between photosynthetic Bradyrhizobium spp. and Aeschynomene sensitiva and Aeschynomene indica, nodules can develop in both stems and roots of the tropical tree without involving LCOs (Giraud et al., 2007) but still requiring the CSSP (Fabre et al., 2015). Similarly, in the actinorrhizal species Casuarina glauca, the unknown elicitors of Nod factor-independent signaling have been characterized to be heat stable, hydrophilic, and chitinase resistant (Chabaud et al., 2016). For Nod factor-independent signaling, the type III secretion system of Bradyrhizobium spp. has been shown to be required for successful symbiosis development by delivering effectors that manipulate host processes (Okazaki et al., 2016; Miwa and Okazaki, 2017).
RLK INTERACTIONS DOWNSTREAM OF LIGAND PERCEPTION
After ligand binding, downstream signaling events occur to allow symbiont infection of the host. During RNS, concomitant production of lateral organs (nodules) is also required for infection and functional nitrogen fixation. Intimate association is only achieved several days after the initial signal exchanges, so the spatiotemporal regulation of RLKs and their effect on transcriptional networks are instrumental to understanding symbiosis. To date, a few downstream components have been identified (Fig. 2), but there is not yet an overarching view of how these components link the RLKs to transcriptional regulation via the CSSP (Fig. 2).
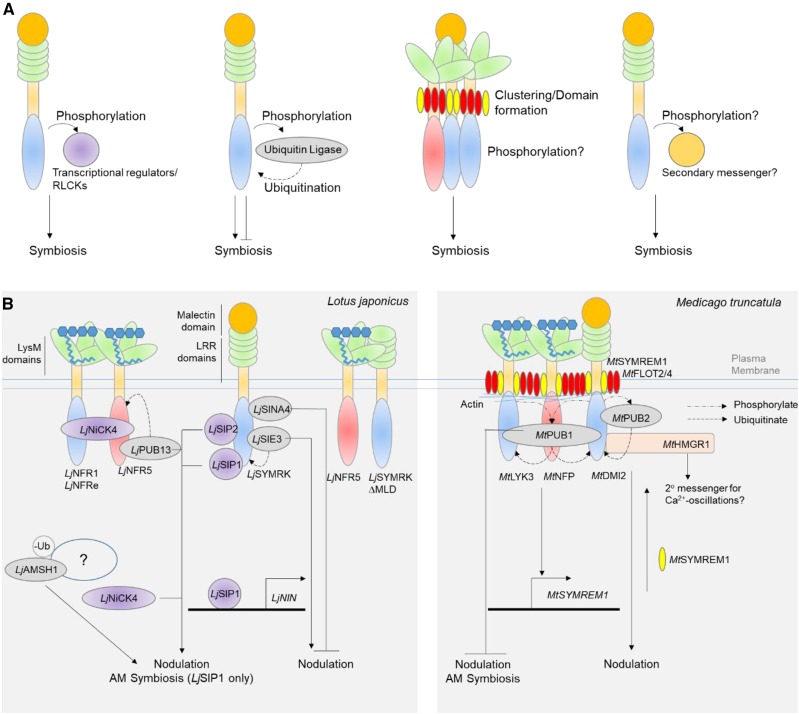
Figure 2.
Posttranslational modifications and interactions of LysM-RLKs determine symbiotic outcome. A, General themes of receptor regulation and activation towards symbiosis signaling. B, Known regulators of receptors. Signal cascades downstream of RLKs is best understood in Lotus japonicus (Lj)and Medicago truncatula (Mt). Nod-factor perception by LjNFR1/5/e complexes activate phosphorylation cascades and transduces signals to LjNiCK4, which then accumulates in the nucleus, positively regulating nodule organogenesis via an unknown mechanism. SYMRK activation can also directly activate transcription (e.g. via LjSIP1 to activate LjNIN expression) or suppress MAPK signalling (via LjSIP2). RLKs are also regulated by ubiquitination (such regulators denoted in gray), which may target RLKs to degradation via the 26S proteasome (e.g. MtPUB2), or alter RLK interactions/localization (e.g. LjSINA4, MtPUB1). Equally, they can be either positive or negative regulators of symbioses. Lotus PUB13, SINA4, and SIE3 and Medicago PUB1 and PUB2 are E3 ubiquitin ligases, but can either promote or suppress symbiosis. Deubiquitination of K63-linked ubiquitin chains by LjAMSH1 is also required to coordinate infection and nodule organogenesis; although the differentially ubiquitinated targets are not clear. In Medicago, MtDMI2 was proposed to activate MtHMGR1 and hence the mevalonate pathway to produce a secondary messenger linking plasma membrane activation to perinuclear Ca2+-oscillations; however genetic data is still elusive. Cell biology and genetics also revealed that creation of nanodomains as potential signaling clusters are instrumental for infection. MtFLOT2/4 and actin are primary nucleation sites, stabilising MtLYK3 while symbiosis-induced MtSYMREM1can further recruit MtNFP, DMI2 to the cluster. With the numerous possible downstream signals, how each of these pathways are activated in space and time and controlled during symbiosis will be important to fully understand the signaling processes in both RNS and AMS.
The localization and stability of LysM-RLKs is altered upon ligand perception, which is important for allowing rhizobia infection to proceed. One of the emerging insights of receptor biology is their compartmentalization at the PM into nanodomains/microdomains (Ott, 2017). RLKs in immunity signaling, such as Arabidopsis FLAGELLIN SENSING2 and BRASSINOSTEROID INSENSITIVE1, have been localized to submicron protein/lipid assemblies and are hypothesized to nucleate other signaling components that change in size and composition dynamically, especially upon stimuli (Bücherl et al., 2017). These membrane nanodomains therefore could partition RLKs into functionally distinct signaling units. Medicago MtLYK3, MtNFP, and MtDMI2 all are able to interact with SYMBIOTIC REMORIN1 (MtSYMREM1), a molecular scaffold protein (Tóth et al., 2012; Liang et al., 2018). Prior to Nod factor or rhizobia application, MtLYK3 has high lateral mobility at the PM, after which it becomes immobilized in nanodomains labeled by MtFLOTILLIN4 (MtFLOT4; Haney et al., 2011). MtSYMREM1 mediates the immobilization and stabilization of MtLYK3, reduces its endocytosis, and increases its dwell time in the domain. This is hypothesized to recruit additional signaling components that determine infection and control symbiosis output. Genetic evidence implicates MtFLOT4 and MtSYMREM1 for successful infection, and cell biology reveals MtFLOT4/2 and actin to serve as a primary core of the nanodomain and MtSYMREM1 as a symbiotically induced secondary component (Liang et al., 2018). One possible member recruited for signaling downstream of LCO perception may be Lotus RHIZOBIAL INFECTION RECEPTOR-LIKE KINASE1 (LjRINRK1), an atypical receptor kinase first identified from a mutant screen for infection thread defects. The full induction of early infection genes, including LjNIN, is independent of LjRINRK1 kinase activity and may point to a possible role in a larger receptor complex in coordinating Nod factor signaling (Li et al., 2019).
Downstream of LysM-RLKs, multiple signaling pathways emerge from SYMRK, ranging from the activation of the mevalonate pathway for a putative secondary messenger for activating Ca2+ oscillations (Kevei et al., 2007; Venkateshwaran et al., 2015), inhibition of MAPKK activity (Chen et al., 2012), and importantly to activation of LjNIN (Zhu et al., 2008; Wang et al., 2013), a transcriptional regulator that coordinates both infection and nodule organogenesis and recently was demonstrated to have recruited the lateral root development pathway for nodule development (Schiessl et al., 2019). Because overexpression of LjNFR5, LjNFR1, or especially LjSYMRK is sufficient for spontaneous nodule formation in the absence of symbiont (Ried et al., 2014), it is unsurprising that there is a multitude of regulatory mechanisms on the receptors to ensure appropriate activation only when the symbiont is present. One notable mechanism is via the cleavage of its MLD. When expressed in tobacco (Nicotiana tabacum) leaves, truncated LjSYMRK(∆MLD) had increased affinity for LjNFR5, whereas the LRR domain regulates its turnover (Antolín-Llovera et al., 2014). Whether overexpression of LjNFR1/LjNFR5/LjSYMRK or ectodomain cleavage/turnover of LjSYMRK also affects AMS, or is specific to RNS, remains to be addressed.
Nod factor receptors and SYMRK have also been revealed to be under tight posttranslational regulation by ubiquitination via Plant U-Box (PUB) proteins. There is an increasing body of work on the regulation of RLKs, specifically MtLYK3, LjSYMRK/MtDMI2, and LjNFR5, by various PUBs. LjSYMRK, for example, is targeted by Lotus SEVEN IN ABSENTIA4 for degradation, negatively regulating RNS but not AMS (Den Herder et al., 2012). LjCERBERUS, an E3 ubiquitin ligase, as well the deubiquitinating enzyme ASSOCIATED MOLECULE WITH THE SH3 DOMAIN OF STAM (AMSH1) are also involved in both infection and nodule organogenesis processes (Yano et al., 2009; Małolepszy et al., 2015). As a posttranslational modification, changes in the ubiquitination of RLKs or other signaling components by PUBs/E3 ligases may target them for degradation by the 26S proteasome. But as seen in the case with MtPUB1, ubiquitination may not always lead to degradation but possibly altered localization via endocytosis/vesicle trafficking or altered protein-protein interactions (Mbengue et al., 2010; Vernié et al., 2016). The importance of ubiquitination as well as the signaling components modified in both symbioses still require a more complete analysis.
Downstream of membrane perception, the phosphorylation cascades are also not well understood. Recently, a receptor-like cytoplasmic kinase (RLCK) was identified biochemically to act downstream of LjNFR5. Lotus NFR5-INTERACTING CYTOPLASMIC KINASE4 (LjNiCK4) phosphorylates LjNFR1 and LjNFR5 in vitro and relocates to the nucleus upon Nod factor perception to positively regulate nodule organogenesis (Wong et al., 2019). LjNiCK4 adds to the growing theme of RLK-RLCK combinations in other signaling contexts (e.g. immunity and stress response; Liang and Zhou, 2018).
Overall, complex signaling activation and regulation occurs for receptors, especially for SYMRK, but how this receptor functions to specify fungal and bacterial symbioses remains to be fully dissected. Keeping Nod factor-independent RNS in mind, the effector ErnA is delivered to activate nodule development, and ectopic expression in plant roots generated numerous lateral meristem-like structures along the root length (Teulet et al., 2019). This could be a result of ErnA interacting with RLK/SYMRK and the CSSP or of activating lateral organogenesis independently.
During RNS, symbiont recognition also activates the expression of other LysM-RLKs for additional symbiotic scrutiny by the plant host. Nod factor perception by LjNFR1/5 activates CSSP and induces the expression of another LysM-RLK, Lotus EXOPOLYSACCHARIDE RECEPTOR3 (LjEPR3; Kawaharada et al., 2015, 2017). The surface exopolysaccharides (EPS) of rhizobia have been long studied for their roles in symbiosis. Like Nod factors, EPS have strain-specific characteristics but are heteropolymers of eight to nine sugar residues. The LjEPR3 extracellular domain directly binds EPS, and Ljepr3 mutants show that EPS recognition is necessary for intracellular infection progression in epidermis, cortex, and into nodule primordia (Kawaharada et al., 2015, 2017).
The continuation of both bacterial Nod gene expression and plant expression of LjNFR1/NFR5/EPR3 in cortex cells of developing nodule primordia (but not mature ones), observations of Ca2+ oscillations ahead of the growing infection thread (Sieberer et al., 2012), together suggest that Nod factor perception and CSSP activation are involved beyond presymbiotic stages, through to infection and nodule development. This insight is also demonstrated using promoters that enrich for epidermal or cortical expression (Rival et al., 2012; Hayashi et al., 2014). Beyond RNS, the role of EPR3-type LysM-RLKs remains enigmatic, as recent phylogenetic and phylogenomic surveys of LysM-RLKs identified the LjEPR3/MtLYK10 clade to be conserved in plant species capable of engaging in AMS, suggesting a possible function during AMS (Bravo et al., 2016; Buendia et al., 2018). The likely rice ortholog, OsLYK1, is induced by AMF and expressed in arbusculated cells, but Oslyk1 mutants showed no defects in AMS (Roth et al., 2018). Thus, whether the various mechanisms of symbiotic scrutiny that are described for RNS also exist in AMS remains unknown.
RLKS AND SIGNALING NETWORKS ARE REQUIRED IN LATE STAGES OF SYMBIOSIS
Apart from vesicle trafficking and nutrient exchange in sustaining AM symbiosis development (for review, see Harrison and Ivanov, 2017; Chiu and Paszkowski, 2019), we now also appreciate the existence of RLK-mediated signaling in symbiosis maintenance via novel and unknown signaling cascades at the periarbuscular membrane. Rice ARBUSCULE RLK1 (OsARK1)/Medicago KINASE3 encodes a Ser/Thr RLK present in AM host species (Bravo et al., 2016; Roth et al., 2018). Localized to the periarbuscular membrane, OsARK1 controls processes after arbuscule development and is required for sustaining fungal fitness during progressing symbiosis, as reflected by the compromised vesicle formation in Osark1 mutants (Roth et al., 2018). Vesicles emerge as lipid storage bodies and could be crucial for subsequent rounds of infection affected in Osark1 mutants. Interestingly, the Osark1 phenotype is rescued in the presence of a common mycorrhizal network where AMF are supported by wild-type plants, suggesting that OsARK1 is required for fungal vigor (Roth et al., 2018). The OsARK1 signaling mechanisms are still unknown. Whether kinase activity is required for its symbiotic role, and whether it is required to suppress defense signaling, also remain to be shown.
On the other hand, immune suppression is required for the full development of functional nitrogen-fixing symbiosis, especially when bacteria in infection threads are released into nodules. In many nonfixing nodulating mutants, strong immune responses lead to white, necrotic, nonfixing nodules, highlighting the need for immune suppression for continued symbiosis. Medicago SYMBIOTIC CYSTEINE-RICH RLK (MtSymCRK) is the only Cys-rich RLK known to have a symbiotic function so far (Berrabah et al., 2014), whereas the other members of the RLK family have known roles in defense, abiotic stress response, and programmed cell death. Recently, it was demonstrated that MtSymCRK works through ethylene signaling (Berrabah et al., 2018) as well as the CDPK-Rboh signaling axis to regulate immune responses in nodules (Yu et al., 2018).
Secreted, Cys-rich peptides provide another mechanism for exercising host-symbiont compatibility, especially for symbiotic function (N fixation). These peptides are typically regarded as antimicrobial defense peptides (defensins). Nodule Cys-rich (NCR) peptides constitute an expanded, large gene family in inverted repeat-lacking clade legumes. The small, white, nonfixing nodules in mutants lacking specific NCRs point to an essential function in developing functional RNS (Horváth et al., 2015; Kim et al., 2015). The majority of NCRs are targeted to the bacteria, where they are proposed to act in ensuring terminal differentiation of the symbionts into bacteroids. At the same time, rhizobial bacteria are capable of degrading NCRs, illustrating a molecular dialogue at late symbiotic stages (Price et al., 2015). Excessive levels of NCRs are detrimental to RNS; thus quantity, combination of NCRs, and bacterial strains are important determinants of symbiotic outcome (Pan and Wang, 2017). However, other mechanisms of scrutiny at the stage of N fixation probably exist, as NCRs appear to have arisen in inverted repeat-lacking clade legumes with indeterminate nodules (where the meristem is maintained and bacteroids are terminally differentiated), independently in dalbergoid legumes (e.g. Aeschynomene spp.; Czernic et al., 2015), and not in legumes with determinate nodules such as soybean (Glycine max), where phytoalexin accumulation and hypersensitive response appear to play a role (Parniske et al., 1990; Mergaert et al., 2003). The existence of symbiotic scrutiny up to the late stages of symbiosis is perhaps driven by the need to monitor and terminate nutritional exchanges.
Taken together, immune modulation for symbiosis is a recurring requirement from precontact signal exchange to intimate endosymbiotic accommodation.
PEPTIDE LIGANDS AND COGNATE RLKS ENFORCE HOST CONTROL OF THE EXTENT OF SYMBIOSIS DEVELOPMENT
Because symbiosis with microorganisms comes at a cost mostly but not limited to carbon, the extent of microbial proliferation in situ needs to be balanced to the nutrient demands of the plant. To achieve this, signal perception by RLKs also activates negative regulatory mechanisms, which act systemically via another set of RLKs (Fig. 3).
Figure 3.
Auto-regulation of nodulation and mycorrhizal symbioses share overlapping signalling pathways.Nod-factors activate a RWP-RK transcriptional regulator LjNIN which positively regulates nodule organogenesis, but also initiates long-distance negative regulation by activating LjCLE-RS transcription. Nitrate sufficiency induces nuclear accumulation of paralogous NLPs LjNRSYM1 and MtNLP4to transcriptionally activate overlapping sets of CLE-RS (red). How AMS activate CLE-RS transcription is currently unknown. In both cases, CLE peptides are transported to the shoot where they activate by direct binding (shown for LjCLE-RS2 to LjHAR1) or via possible co-receptors to activate CLAVATA1-like RLKs (LjHAR1/MtSUNN1/GmNARK/BdFON) MtCORYNE as well as LjCLV2/PsSYM28, which initiate less understood signaling cascades to generate a shoot-to-root signal. LjKLAVIER (KLV) is another LRR-RLK involved. LjHAR1 activation reduces miR2111 levels, allowing TOO MUCH LOVE (TML) in the roots to negatively regulate RNS. LjHAR1 also activates shoot cytokinin production, another shoot-to-root signal. Signals downstream of HAR1/SUNN/NARK/FON to inhibit AMS are unclear, except for its effect on reducing strigolactone (SL) biosynthesis and export in the roots. In parallel, C-terminal encoded peptides (e.g. MtCEP1) also acts systemically on shoot MtCRA2 to positively regulate RNS via an unknown shoot-to-root signal, but this require common symbiosis signaling pathway and ethylene receptor ETHYLENE INSENSITIVE 2. Species prefix as in Figure 1 except: Bd, Brachypodium distachyon.AON, autoregulation of nodulation; AOM, autoregulation of mycorrhizal symbiosis.
Autoregulatory mechanisms involve systemic peptide transport and are common to both rhizobia-legume symbiosis and AMS. RWP-RK-type transcriptional regulators of the NIN/NIN-like protein (NLP) family, named after the conserved amino acid residues in the clade, activate the expression of root-derived CLAVATA3/EMBRYO SURROUNDING REGION (CLE) peptides. Nod factor perception activates LjNIN, which transcriptionally induces LjCLE-RS1,2. In the shoot, they activate the CLAVATA1-like LRR-RLKs Medicago SUPER NUMERIC NODULES1/Lotus HYPERNODULATION ABERRANT ROOT1/soybean NODULE AUTOREGULATION RECEPTOR KINASE1 (MtSUNN1/LjHAR1/GmNARK1) that repress nodule formation in the roots (Krusell et al., 2002, 2011; Nishimura et al., 2002; Searle et al., 2003). Ligand-receptor binding of the glycosylated LjCLE-RS2 peptide to LjHAR1 has also been demonstrated (Okamoto et al., 2013). Similar to the regulation of shoot apical meristem in Arabidopsis, autoregulation of nodulation also requires Medicago and pea CLV2 and Medicago CORYNE, and in Lotus, another LRR-RLK, KLAVIER, is also involved (Miyazawa et al., 2010; Krusell et al., 2011; Crook et al., 2016), as summarized in Figure 3. In addition, signaling via MtSUNN1/LjHAR1/GmNARK1 also integrates the plant nitrate status (Jeudy et al., 2010; Reid et al., 2011; Okamoto and Kawaguchi, 2015). Using a forward genetics screen for loss of nitrate-suppression of nodulation in Lotus or a reverse genetic screen for NLP mutants in Medicago, Lotus NITRATE UNRESPONSIVE SYMBIOSIS1/NLP4 and MtNLP1 were identified to undergo nitrate-triggered nuclear accumulation, inducing their downstream CLE-RS peptides (Lin et al., 2018; Nishida et al., 2018). Together, nitrate status and rhizobia presence work via RWP-RK regulators (NLPs) to induce specific and overlapping sets of CLE peptides that activate shoot CLV1-like RLKs (Nishida and Suzaki, 2018).
One of the consequences of LjHAR1 activation is the down-regulation of miR2111 production, a mobile shoot-to-root signal delivered via the phloem. Overexpressing miR2111 resulted in hypernodulation (Tsikou et al., 2018), as it targets Lotus TML, a Kelch F-box protein that negatively regulates nodulation (Magori et al., 2009; Takahara et al., 2013). In uninfected roots, low TML levels maintain susceptibility for rhizobia. In symbiotic plants, root-derived CLE peptides activate LjHAR1 in the shoot, which reduces miR2111 abundance in shoots and roots, allowing LjTML to negatively regulate RNS (Tsikou et al., 2018). Another consequence of LjHAR1 signaling is shoot-derived cytokinins, which also inhibit nodulation (Sasaki et al., 2014).
Meanwhile, the CLE-SUNN signaling module also regulates AMS. As in RNS, plant phosphate status and AM colonization induce root-derived CLE peptides that negatively regulate AM symbiosis. Overexpression of Pi-induced MtCLE33, or AM-induced MtCLE53, reduced colonization levels by inhibiting the expression of genes involved in SL biosynthesis and transport, leading to reduced SL levels (Müller et al., 2019). Accordingly, the reduced AM colonization could be overcome by exogenous application of the synthetic SL analog GR24. MtSUNN1 is required for CLE-mediated signaling responses and is also involved in the suppression of AM symbiosis under phosphate sufficiency (Müller et al., 2019). Whether MtCLE33/53 directly bind to MtSUNN1 remains to be demonstrated, but a common mode of systemic regulation is emerging from these recent findings (for review, see Kereszt et al., 2018; Müller and Harrison, 2019). As the CLE-SUNN/HAR mechanism for autoregulation of AM colonization is present in Brachypodium distachyon (Müller et al., 2019) and because tomato CLV2 is also involved in autoregulation of AM colonization (Wang et al., 2018), it is tempting to speculate that this host-control mechanism may predate the evolution of RNS, and like the CSSP, this signaling pathway may have been coopted in the evolution of RNS to exert host control over the extent of symbiosis.
Independent of LjHAR1/MtSUNN/GmNARK, another peptide-LRR-RLK pathway exists in parallel, regulating nodulation and root development. C-terminally encoded peptides (CEPs) undergo extensive posttranslational modifications and have proposed roles in regulating plant development and abiotic and biotic responses (Taleski et al., 2018). MtCEP1 inhibits lateral root emergence but promotes nodule formation via separate pathways downstream of its putative receptor, Medicago COMPACT ROOT ARCHITECTURE2 (MtCRA2). MtCRA2 acts locally to limit lateral root development, but its systemic activity positively regulates nodule formation. And while MtCEP1-MtCRA2 regulation of lateral root development is independent of CSSP and Medicago ETHYLENE INSENSITIVE2, the regulation of nodule development requires these signaling components, suggesting that two different pathways may exist downstream of MtCRA2 (Mohd-Radzman et al., 2016; Laffont et al., 2019). Moreover, the expression of CEP peptides is also differentially regulated during AMS (de Bang et al., 2017), but their role in regulating AMS awaits further investigation.
CONCLUSION AND PERSPECTIVES
To perceive and engage with symbionts, plant roots recognize bacterial or fungal carbohydrate molecules and orchestrate a series of signal exchanges before a mutually beneficial symbiosis is established. Because of the rich understanding of the host signaling mechanisms relative to the symbiont, this review focused on the roles of plant RLKs in recruiting and scrutinizing endosymbionts from precontact to termination of the association. It is still unclear how host-symbiont specificity, especially for AMS, can be encoded in a rhizosphere where very similar carbohydrate molecules are likely to be produced by different organisms. To date, the lack of genetically tractable AMF restricts our understanding of the biosynthesis and relative importance of important fungal signaling molecules, be they LCOs, COs, or others. The most parsimonious hypothesis, based on the recent discoveries, might be that the combinatorial and/or sequential perception of signaling molecules, including but not restricted to carbohydrates, activates a set of host receptors for symbiosis signaling. The present challenge (see Outstanding Questions) is to identify the range of signals for AMF to explain its broad host range and to explain how plants integrate the signals downstream of perception and receptor activation. Moreover, although multiple classes of RLKs have been identified to regulate various stages of symbiosis, there is a lack of understanding on their signaling mechanisms. At the level of receptor complexes, membrane receptor domains, and phosphorylation/signal transduction cascade(s), we anticipate future discoveries to reveal shared and diverging modes of receptor activities in the two most studied plant root endosymbioses.
Footnotes
This work was supported by a Gates Cambridge Scholarship to C.H.C., the Biotechnology and Biological Sciences Research Council (grant no. BB/P003419/1 to U.P.), and the Bill and Melinda Gates Foundation (grant no. OPP1028264 to U.P.).
Articles can be viewed without a subscription.
References
- Amor BB, Shaw SL, Oldroyd GED, Maillet F, Penmetsa RV, Cook D, Long SR, Dénarié J, Gough C(2003) The NFP locus of Medicago truncatula controls an early step of Nod factor signal transduction upstream of a rapid calcium flux and root hair deformation. Plant J 34: 495–506 [DOI] [PubMed] [Google Scholar]
- Antolín-Llovera M, Ried MK, Parniske M(2014) Cleavage of the SYMBIOSIS RECEPTOR-LIKE KINASE ectodomain promotes complex formation with Nod factor receptor 5. Curr Biol 24: 422–427 [DOI] [PubMed] [Google Scholar]
- Berrabah F, Balliau T, Aït-Salem EH, George J, Zivy M, Ratet P, Gourion B(2018) Control of the ethylene signaling pathway prevents plant defenses during intracellular accommodation of the rhizobia. New Phytol 219: 310–323 [DOI] [PubMed] [Google Scholar]
- Berrabah F, Bourcy M, Eschstruth A, Cayrel A, Guefrachi I, Mergaert P, Wen J, Jean V, Mysore KS, Gourion B, et al. (2014) A nonRD receptor-like kinase prevents nodule early senescence and defense-like reactions during symbiosis. New Phytol 203: 1305–1314 [DOI] [PubMed] [Google Scholar]
- Bozsoki Z, Cheng J, Feng F, Gysel K, Vinther M, Andersen KR, Oldroyd G, Blaise M, Radutoiu S, Stougaard J(2017) Receptor-mediated chitin perception in legume roots is functionally separable from Nod factor perception. Proc Natl Acad Sci USA 114: E8118–E8127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bravo A, York T, Pumplin N, Mueller LA, Harrison MJ(2016) Genes conserved for arbuscular mycorrhizal symbiosis identified through phylogenomics. Nat Plants 2: 15208. [DOI] [PubMed] [Google Scholar]
- Broghammer A, Krusell L, Blaise M, Sauer J, Sullivan JT, Maolanon N, Vinther M, Lorentzen A, Madsen EB, Jensen KJ, et al. (2012) Legume receptors perceive the rhizobial lipochitin oligosaccharide signal molecules by direct binding. Proc Natl Acad Sci USA 109: 13859–13864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bücherl CA, Jarsch IK, Schudoma C, Segonzac C, Mbengue M, Robatzek S, MacLean D, Ott T, Zipfel C(2017) Plant immune and growth receptors share common signalling components but localise to distinct plasma membrane nanodomains. eLife 6: e25114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buendia L, Girardin A, Wang T, Cottret L, Lefebvre B(2018) LysM receptor-like kinase and LysM receptor-like protein families: An update on phylogeny and functional characterization. Front Plant Sci 9: 1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buendia L, Wang T, Girardin A, Lefebvre B(2016) The LysM receptor-like kinase SlLYK10 regulates the arbuscular mycorrhizal symbiosis in tomato. New Phytol 210: 184–195 [DOI] [PubMed] [Google Scholar]
- Campos-Soriano L, García-Garrido JM, San Segundo B(2010) Activation of basal defense mechanisms of rice plants by Glomus intraradices does not affect the arbuscular mycorrhizal symbiosis. New Phytol 188: 597–614 [DOI] [PubMed] [Google Scholar]
- Camps C, Jardinaud MF, Rengel D, Carrère S, Hervé C, Debellé F, Gamas P, Bensmihen S, Gough C(2015) Combined genetic and transcriptomic analysis reveals three major signalling pathways activated by Myc-LCOs in Medicago truncatula. New Phytol 208: 224–240 [DOI] [PubMed] [Google Scholar]
- Carotenuto G, Chabaud M, Miyata K, Capozzi M, Takeda N, Kaku H, Shibuya N, Nakagawa T, Barker DG, Genre A(2017) The rice LysM receptor-like kinase OsCERK1 is required for the perception of short-chain chitin oligomers in arbuscular mycorrhizal signaling. New Phytol 214: 1440–1446 [DOI] [PubMed] [Google Scholar]
- Catoira R, Galera C, de Billy F, Penmetsa RV, Journet EP, Maillet F, Rosenberg C, Cook D, Gough C, Dénarié J(2000) Four genes of Medicago truncatula controlling components of a nod factor transduction pathway. Plant Cell 12: 1647–1666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chabaud M, Gherbi H, Pirolles E, Vaissayre V, Fournier J, Moukouanga D, Franche C, Bogusz D, Tisa LS, Barker DG, et al. (2016) Chitinase-resistant hydrophilic symbiotic factors secreted by Frankia activate both Ca2+ spiking and NIN gene expression in the actinorhizal plant Casuarina glauca. New Phytol 209: 86–93 [DOI] [PubMed] [Google Scholar]
- Chen T, Zhu H, Ke D, Cai K, Wang C, Gou H, Hong Z, Zhang Z(2012) A MAP kinase kinase interacts with SymRK and regulates nodule organogenesis in Lotus japonicus. Plant Cell 24: 823–838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu CH, Choi J, Paszkowski U(2018) Independent signalling cues underpin arbuscular mycorrhizal symbiosis and large lateral root induction in rice. New Phytol 217: 552–557 [DOI] [PubMed] [Google Scholar]
- Chiu CH, Paszkowski U(2019) Mechanisms and impact of symbiotic phosphate acquisition. Cold Spring Harb Perspect Biol 11: a034603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cope KR, Bascaules A, Irving TB, Venkateshwaran M, Maeda J, Garcia K, Rush TA, Ma C, Labbé J, Jawdy S, et al. (2019) The ectomycorrhizal fungus Laccaria bicolor produces lipochitooligosaccharides and uses the common symbiosis pathway to colonize Populus roots. Plant Cell 31: 2386–2410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crook AD, Schnabel EL, Frugoli JA(2016) The systemic nodule number regulation kinase SUNN in Medicago truncatula interacts with MtCLV2 and MtCRN. Plant J 88: 108–119 [DOI] [PubMed] [Google Scholar]
- Czernic P, Gully D, Cartieaux F, Moulin L, Guefrachi I, Patrel D, Pierre O, Fardoux J, Chaintreuil C, Nguyen P, et al. (2015) Convergent evolution of endosymbiont differentiation in dalbergioid and inverted repeat-lacking clade legumes mediated by nodule-specific cysteine-rich peptides. Plant Physiol 169: 1254–1265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Bang TC, Lundquist PK, Dai X, Boschiero C, Zhuang Z, Pant P, Torres-Jerez I, Roy S, Nogales J, Veerappan V, et al. (2017) Genome-wide identification of Medicago peptides involved in macronutrient responses and nodulation. Plant Physiol 175: 1669–1689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delaux PM, Bécard G, Combier JP(2013) NSP1 is a component of the Myc signaling pathway. New Phytol 199: 59–65 [DOI] [PubMed] [Google Scholar]
- Demchenko K, Winzer T, Stougaard J, Parniske M, Pawlowski K(2004) Distinct roles of Lotus japonicus SYMRK and SYM15 in root colonization and arbuscule formation. New Phytol 163: 381–392 [DOI] [PubMed] [Google Scholar]
- Den Herder G, Yoshida S, Antolín-Llovera M, Ried MK, Parniske M(2012) Lotus japonicus E3 ligase SEVEN IN ABSENTIA4 destabilizes the symbiosis receptor-like kinase SYMRK and negatively regulates rhizobial infection. Plant Cell 24: 1691–1707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diédhiou I, Diouf D(2018) Transcription factors network in root endosymbiosis establishment and development. World J Microbiol Biotechnol 34: 37. [DOI] [PubMed] [Google Scholar]
- Fabre S, Gully D, Poitout A, Patrel D, Arrighi JF, Giraud E, Czernic P, Cartieaux F, Cirad FC(2015) Nod factor-independent nodulation in Aeschynomene evenia required the common plant-microbe symbiotic toolkit. Plant Physiol 169: 2654–2664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng F, Sun J, Radhakrishnan GV, Lee T, Bozsóki Z, Fort S, Gavrin A, Gysel K, Thygesen MB, Andersen KR, et al. (2019) A combination of chitooligosaccharide and lipochitooligosaccharide recognition promotes arbuscular mycorrhizal associations in Medicago truncatula. Nat Commun 10: 5047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Aparicio M, Rispail N, Prats E, Morandi D, García-Garrido JM, Dumas-Gaudot E, Duc G, Rubiales D(2010) Parasitic plant infection is partially controlled through symbiotic pathways. Weed Res 50: 76–82 [Google Scholar]
- Floss DS, Gomez SK, Park HJ, MacLean AM, Müller LM, Bhattarai KK, Lévesque-Tremblay V, Maldonado-Mendoza IE, Harrison MJ(2017) A transcriptional program for arbuscule degeneration during AM symbiosis is regulated by MYB1. Curr Biol 27: 1206–1212 [DOI] [PubMed] [Google Scholar]
- Fonouni-Farde C, Diet A, Frugier F(2016) Root development and endosymbioses: DELLAs lead the orchestra. Trends Plant Sci 21: 898–900 [DOI] [PubMed] [Google Scholar]
- Garcia K, Delaux PM, Cope KR, Ané JM(2015) Molecular signals required for the establishment and maintenance of ectomycorrhizal symbioses. New Phytol 208: 79–87 [DOI] [PubMed] [Google Scholar]
- García-Garrido JM, Ocampo JA(2002) Regulation of the plant defence response in arbuscular mycorrhizal symbiosis. J Exp Bot 53: 1377–1386 [PubMed] [Google Scholar]
- Genre A, Chabaud M, Balzergue C, Puech-Pagès V, Novero M, Rey T, Fournier J, Rochange S, Bécard G, Bonfante P, et al. (2013) Short-chain chitin oligomers from arbuscular mycorrhizal fungi trigger nuclear Ca2+ spiking in Medicago truncatula roots and their production is enhanced by strigolactone. New Phytol 198: 190–202 [DOI] [PubMed] [Google Scholar]
- Gibelin-Viala C, Amblard E, Puech-Pages V, Bonhomme M, Garcia M, Bascaules-Bedin A, Fliegmann J, Wen J, Mysore KS, le Signor C, et al. (2019) The Medicago truncatula LysM receptor-like kinase LYK9 plays a dual role in immunity and the arbuscular mycorrhizal symbiosis. New Phytol 223: 1516–1529 [DOI] [PubMed] [Google Scholar]
- Girardin A, Wang T, Ding Y, Keller J, Buendia L, Gaston M, Ribeyre C, Gasciolli V, Auriac MC, Vernié T, et al. (2019) LCO receptors involved in arbuscular mycorrhiza are functional for rhizobia perception in legumes. Curr Biol 29: 4249–4259.e5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giraud E, Moulin L, Vallenet D, Barbe V, Cytryn E, Avarre JC, Jaubert M, Simon D, Cartieaux F, Prin Y, et al. (2007) Legumes symbioses: Absence of Nod genes in photosynthetic bradyrhizobia. Science 316: 1307–1312 [DOI] [PubMed] [Google Scholar]
- Gutjahr C, Banba M, Croset V, An K, Miyao A, An G, Hirochika H, Imaizumi-Anraku H, Paszkowski U(2008) Arbuscular mycorrhiza-specific signaling in rice transcends the common symbiosis signaling pathway. Plant Cell 20: 2989–3005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutjahr C, Casieri L, Paszkowski U(2009) Glomus intraradices induces changes in root system architecture of rice independently of common symbiosis signaling. New Phytol 182: 829–837 [DOI] [PubMed] [Google Scholar]
- Gutjahr C, Gobbato E, Choi J, Riemann M, Johnston MG, Summers W, Carbonnel S, Mansfield C, Yang SY, Nadal M, et al. (2015) Rice perception of symbiotic arbuscular mycorrhizal fungi requires the karrikin receptor complex. Science 350: 1521–1524 [DOI] [PubMed] [Google Scholar]
- Haney CH, Riely BK, Tricoli DM, Cook DR, Ehrhardt DW, Long SR(2011) Symbiotic rhizobia bacteria trigger a change in localization and dynamics of the Medicago truncatula receptor kinase LYK3. Plant Cell 23: 2774–2787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison MJ, Dixon RA(1993) Isoflavonoid accumulation and expression of defense gene transcripts during the establishment of vesicular-arbuscular mycorrhizal associations in roots of Medicago truncatula. Mol Plant Microbe Interact 6: 643–654 [Google Scholar]
- Harrison MJ, Ivanov S(2017) Exocytosis for endosymbiosis: Membrane trafficking pathways for development of symbiotic membrane compartments. Curr Opin Plant Biol 38: 101–108 [DOI] [PubMed] [Google Scholar]
- Hayashi T, Shimoda Y, Sato S, Tabata S, Imaizumi-Anraku H, Hayashi M(2014) Rhizobial infection does not require cortical expression of upstream common symbiosis genes responsible for the induction of Ca2+ spiking. Plant J 77: 146–159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He J, Zhang C, Dai H, Liu H, Zhang X, Yang J, Chen X, Zhu Y, Wang D, Qi X, et al. (2019) A LysM receptor heteromer mediates perception of arbuscular mycorrhizal symbiotic signal in rice. Mol Plant 12: 1561–1576 [DOI] [PubMed] [Google Scholar]
- Horváth B, Domonkos Á, Kereszt A, Szűcs A, Ábrahám E, Ayaydin F, Bóka K, Chen Y, Chen R, Murray JD, et al. (2015) Loss of the nodule-specific cysteine rich peptide, NCR169, abolishes symbiotic nitrogen fixation in the Medicago truncatula dnf7 mutant. Proc Natl Acad Sci USA 112: 15232–15237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeudy C, Ruffel S, Freixes S, Tillard P, Santoni AL, Morel S, Journet EP, Duc G, Gojon A, Lepetit M, et al. (2010) Adaptation of Medicago truncatula to nitrogen limitation is modulated via local and systemic nodule developmental responses. New Phytol 185: 817–828 [DOI] [PubMed] [Google Scholar]
- Kaku H, Nishizawa Y, Ishii-Minami N, Akimoto-Tomiyama C, Dohmae N, Takio K, Minami E, Shibuya N(2006) Plant cells recognize chitin fragments for defense signaling through a plasma membrane receptor. Proc Natl Acad Sci USA 103: 11086–11091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapulnik Y, Volpin H, Itzhaki H, Ganon D, Galili S, David R, Shaul O, Elad Y, Chet I, Okon Y(1996) Suppression of defence responses in mycorrhizal alfalfa and tobacco roots. New Phytol 133: 59–64 [Google Scholar]
- Kawaharada Y, Kelly S, Nielsen MW, Hjuler CT, Gysel K, Muszyński A, Carlson RW, Thygesen MB, Sandal N, Asmussen MH, et al. (2015) Receptor-mediated exopolysaccharide perception controls bacterial infection. Nature 523: 308–312 [DOI] [PubMed] [Google Scholar]
- Kawaharada Y, Nielsen MW, Kelly S, James EK, Andersen KR, Rasmussen SR, Füchtbauer W, Madsen LH, Heckmann AB, Radutoiu S, et al. (2017) Differential regulation of the Epr3 receptor coordinates membrane-restricted rhizobial colonization of root nodule primordia. Nat Commun 8: 14534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kereszt A, Mergaert P, Montiel J, Endre G, Kondorosi É(2018) Impact of plant peptides on symbiotic nodule development and functioning. Front Plant Sci 9: 1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kevei Z, Lougnon G, Mergaert P, Horváth GV, Kereszt A, Jayaraman D, Zaman N, Marcel F, Regulski K, Kiss GB, et al. (2007) 3-Hydroxy-3-methylglutaryl coenzyme A reductase 1 interacts with NORK and is crucial for nodulation in Medicago truncatula. Plant Cell 19: 3974–3989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim M, Chen Y, Xi J, Waters C, Chen R, Wang D(2015) An antimicrobial peptide essential for bacterial survival in the nitrogen-fixing symbiosis. Proc Natl Acad Sci USA 112: 15238–15243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S, Zeng W, Bernard S, Liao J, Venkateshwaran M, Ane JM, Jiang Y(2019) Ca2+-regulated Ca2+ channels with an RCK gating ring control plant symbiotic associations. Nat Commun 10: 3703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirienko AN, Porozov YB, Malkov NV, Akhtemova GA, Le Signor C, Thompson R, Saffray C, Dalmais M, Bendahmane A, Tikhonovich IA, et al. (2018) Role of a receptor-like kinase K1 in pea Rhizobium symbiosis development. Planta 248: 1101–1120 [DOI] [PubMed] [Google Scholar]
- Kirienko AN, Vishnevskaya NA, Kitaeva AB, Shtark OY, Kozyulina PY, Thompson R, Dalmais M, Bendahmane A, Tikhonovich IA, Dolgikh EA(2019) Structural variations in LysM domains of LysM-RLK PsK1 may result in a different effect on pea-rhizobial symbiosis development. Int J Mol Sci 20: E1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishimoto K, Kouzai Y, Kaku H, Shibuya N, Minami E, Nishizawa Y(2010) Perception of the chitin oligosaccharides contributes to disease resistance to blast fungus Magnaporthe oryzae in rice. Plant J 64: 343–354 [DOI] [PubMed] [Google Scholar]
- Kistner C, Winzer T, Pitzschke A, Mulder L, Sato S, Kaneko T, Tabata S, Sandal N, Stougaard J, Webb KJ, et al. (2005) Seven Lotus japonicus genes required for transcriptional reprogramming of the root during fungal and bacterial symbiosis. Plant Cell 17: 2217–2229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosuta S, Chabaud M, Lougnon G, Gough C, Dénarié J, Barker DG, Bécard G(2003) A diffusible factor from arbuscular mycorrhizal fungi induces symbiosis-specific MtENOD11 expression in roots of Medicago truncatula. Plant Physiol 131: 952–962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kouzai Y, Mochizuki S, Nakajima K, Desaki Y, Hayafune M, Miyazaki H, Yokotani N, Ozawa K, Minami E, Kaku H, et al. (2014a) Targeted gene disruption of OsCERK1 reveals its indispensable role in chitin perception and involvement in the peptidoglycan response and immunity in rice. Mol Plant Microbe Interact 27: 975–982 [DOI] [PubMed] [Google Scholar]
- Kouzai Y, Nakajima K, Hayafune M, Ozawa K, Kaku H, Shibuya N, Minami E, Nishizawa Y(2014b) CEBiP is the major chitin oligomer-binding protein in rice and plays a main role in the perception of chitin oligomers. Plant Mol Biol 84: 519–528 [DOI] [PubMed] [Google Scholar]
- Krusell L, Madsen LH, Sato S, Aubert G, Genua A, Szczyglowski K, Duc G, Kaneko T, Tabata S, de Bruijn F, et al. (2002) Shoot control of root development and nodulation is mediated by a receptor-like kinase. Nature 420: 422–426 [DOI] [PubMed] [Google Scholar]
- Krusell L, Sato N, Fukuhara I, Koch BEV, Grossmann C, Okamoto S, Oka-Kira E, Otsubo Y, Aubert G, Nakagawa T, et al. (2011) The Clavata2 genes of pea and Lotus japonicus affect autoregulation of nodulation. Plant J 65: 861–871 [DOI] [PubMed] [Google Scholar]
- Laffont C, Huault E, Gautrat P, Endre G, Kalo P, Bourion V, Duc G, Frugier F(2019) Independent regulation of symbiotic nodulation by the SUNN negative and CRA2 positive systemic pathways. Plant Physiol 180: 559–570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leppyanen IV, Shakhnazarova VY, Shtark OY, Vishnevskaya NA, Tikhonovich IA, Dolgikh EA(2017) Receptor-like kinase LYK9 in Pisum sativum L. is the CERK1-like receptor that controls both plant immunity and AM symbiosis development. Int J Mol Sci 19: E8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Zheng Z, Kong X, Xu J, Qiu L, Sun J, Reid D, Jin H, Andersen SU, Oldroyd GED, et al. (2019) Atypical receptor kinase RINRK1 required for rhizobial infection but not nodule development in Lotus japonicus. Plant Physiol 181: 804–816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lian B, Prithiviraj B, Souleimanov A, Smith DL(2001) Evidence for the production of chemical compounds analogous to nod factor by the silicate bacterium Bacillus circulans GY92. Microbiol Res 156: 289–292 [DOI] [PubMed] [Google Scholar]
- Liang P, Stratil TF, Popp C, Marín M, Folgmann J, Mysore KS, Wen J, Ott T(2018) Symbiotic root infections in Medicago truncatula require remorin-mediated receptor stabilization in membrane nanodomains. Proc Natl Acad Sci USA 115: 5289–5294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang X, Zhou JM(2018) Receptor-like cytoplasmic kinases: Central players in plant receptor kinase-mediated signaling. Annu Rev Plant Biol 69: 267–299 [DOI] [PubMed] [Google Scholar]
- Liao D, Sun X, Wang N, Song F, Liang Y(2018) Tomato LysM receptor-like kinase SlLYK12 is involved in arbuscular mycorrhizal symbiosis. Front Plant Sci 9: 1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Limpens E, Franken C, Smit P, Willemse J, Bisseling T, Geurts R(2003) LysM domain receptor kinases regulating rhizobial Nod factor-induced infection. Science 302: 630–633 [DOI] [PubMed] [Google Scholar]
- Lin JS, Li X, Luo Z, Mysore KS, Wen J, Xie F(2018) NIN interacts with NLPs to mediate nitrate inhibition of nodulation in Medicago truncatula. Nat Plants 4: 942–952 [DOI] [PubMed] [Google Scholar]
- Lindsay PL, Williams BN, MacLean A, Harrison MJ(2019) A phosphate-dependent requirement for transcription factors IPD3 and IPD3L during arbuscular mycorrhizal symbiosis in Medicago truncatula. Mol Plant Microbe Interact 32: 1277–1290 [DOI] [PubMed] [Google Scholar]
- Liu G, Stirnemann M, Gübeli C, Egloff S, Courty PE, Aubry S, Vandenbussche M, Morel P, Reinhardt D, Martinoia E, et al. (2019) Strigolactones play an important role in shaping exodermal morphology via a KAI2-dependent pathway. iScience 17: 144–154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H, Zhang C, Yang J, Yu N, Wang E(2018) Hormone modulation of legume-rhizobial symbiosis. J Integr Plant Biol 60: 632–648 [DOI] [PubMed] [Google Scholar]
- Liu J, Blaylock LA, Endre G, Cho J, Town CD, VandenBosch KA, Harrison MJ(2003) Transcript profiling coupled with spatial expression analyses reveals genes involved in distinct developmental stages of an arbuscular mycorrhizal symbiosis. Plant Cell 15: 2106–2123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu W, Kohlen W, Lillo A, Op den Camp R, Ivanov S, Hartog M, Limpens E, Jamil M, Smaczniak C, Kaufmann K, et al. (2011) Strigolactone biosynthesis in Medicago truncatula and rice requires the symbiotic GRAS-type transcription factors NSP1 and NSP2. Plant Cell 23: 3853–3865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLean AM, Bravo A, Harrison MJ(2017) Plant signaling and metabolic pathways enabling arbuscular mycorrhizal symbiosis. Plant Cell 29: 2319–2335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madsen EB, Antolín-Llovera M, Grossmann C, Ye J, Vieweg S, Broghammer A, Krusell L, Radutoiu S, Jensen ON, Stougaard J, et al. (2011) Autophosphorylation is essential for the in vivo function of the Lotus japonicus Nod factor receptor 1 and receptor-mediated signalling in cooperation with Nod factor receptor 5. Plant J 65: 404–417 [DOI] [PubMed] [Google Scholar]
- Madsen EB, Madsen LH, Radutoiu S, Olbryt M, Rakwalska M, Szczyglowski K, Sato S, Kaneko T, Tabata S, Sandal N, et al. (2003) A receptor kinase gene of the LysM type is involved in legume perception of rhizobial signals. Nature 425: 637–640 [DOI] [PubMed] [Google Scholar]
- Magori S, Oka-Kira E, Shibata S, Umehara Y, Kouchi H, Hase Y, Tanaka A, Sato S, Tabata S, Kawaguchi M(2009) Too much love, a root regulator associated with the long-distance control of nodulation in Lotus japonicus. Mol Plant Microbe Interact 22: 259–268 [DOI] [PubMed] [Google Scholar]
- Maillet F, Poinsot V, André O, Puech-Pagès V, Haouy A, Gueunier M, Cromer L, Giraudet D, Formey D, Niebel A, et al. (2011) Fungal lipochitooligosaccharide symbiotic signals in arbuscular mycorrhiza. Nature 469: 58–63 [DOI] [PubMed] [Google Scholar]
- Małolepszy A, Urbański DF, James EK, Sandal N, Isono E, Stougaard J, Andersen SU(2015) The deubiquitinating enzyme AMSH1 is required for rhizobial infection and nodule organogenesis in Lotus japonicus. Plant J 83: 719–731 [DOI] [PubMed] [Google Scholar]
- Marcel S, Sawers R, Oakeley E, Angliker H, Paszkowski U(2010) Tissue-adapted invasion strategies of the rice blast fungus Magnaporthe oryzae. Plant Cell 22: 3177–3187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin FM, Uroz S, Barker DG(2017) Ancestral alliances: Plant mutualistic symbioses with fungi and bacteria. Science 356: eaad4501. [DOI] [PubMed] [Google Scholar]
- Mbengue M, Camut S, de Carvalho-Niebel F, Deslandes L, Froidure S, Klaus-Heisen D, Moreau S, Rivas S, Timmers T, Hervé C, et al. (2010) The Medicago truncatula E3 ubiquitin ligase PUB1 interacts with the LYK3 symbiotic receptor and negatively regulates infection and nodulation. Plant Cell 22: 3474–3488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mergaert P, Nikovics K, Kelemen Z, Maunoury N, Vaubert D, Kondorosi A, Kondorosi E(2003) A novel family in Medicago truncatula consisting of more than 300 nodule-specific genes coding for small, secreted polypeptides with conserved cysteine motifs. Plant Physiol 132: 161–173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miwa H, Okazaki S(2017) How effectors promote beneficial interactions. Curr Opin Plant Biol 38: 148–154 [DOI] [PubMed] [Google Scholar]
- Miya A, Albert P, Shinya T, Desaki Y, Ichimura K, Shirasu K, Narusaka Y, Kawakami N, Kaku H, Shibuya N(2007) CERK1, a LysM receptor kinase, is essential for chitin elicitor signaling in Arabidopsis. Proc Natl Acad Sci USA 104: 19613–19618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyata K, Hayafune M, Kobae Y, Kaku H, Nishizawa Y, Masuda Y, Shibuya N, Nakagawa T(2016) Evaluation of the role of the LysM receptor-like kinase, OsNFR5/OsRLK2 for AM symbiosis in rice. Plant Cell Physiol 57: 2283–2290 [DOI] [PubMed] [Google Scholar]
- Miyata K, Kozaki T, Kouzai Y, Ozawa K, Ishii K, Asamizu E, Okabe Y, Umehara Y, Miyamoto A, Kobae Y, et al. (2014) The bifunctional plant receptor, OsCERK1, regulates both chitin-triggered immunity and arbuscular mycorrhizal symbiosis in rice. Plant Cell Physiol 55: 1864–1872 [DOI] [PubMed] [Google Scholar]
- Miyazawa H, Oka-Kira E, Sato N, Takahashi H, Wu GJ, Sato S, Hayashi M, Betsuyaku S, Nakazono M, Tabata S, et al. (2010) The receptor-like kinase KLAVIER mediates systemic regulation of nodulation and non-symbiotic shoot development in Lotus japonicus. Development 137: 4317–4325 [DOI] [PubMed] [Google Scholar]
- Mohd-Radzman NA, Laffont C, Ivanovici A, Patel N, Reid D, Stougaard J, Frugier F, Imin N, Djordjevic MA(2016) Different pathways act downstream of the CEP peptide receptor CRA2 to regulate lateral root and nodule development. Plant Physiol 171: 2536–2548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller LM, Flokova K, Schnabel E, Sun X, Fei Z, Frugoli J, Bouwmeester HJ, Harrison MJ(2019) A CLE-SUNN module regulates strigolactone content and fungal colonization in arbuscular mycorrhiza. Nat Plants 5: 933–939 [DOI] [PubMed] [Google Scholar]
- Müller LM, Harrison MJ(2019) Phytohormones, miRNAs, and peptide signals integrate plant phosphorus status with arbuscular mycorrhizal symbiosis. Curr Opin Plant Biol 50: 132–139 [DOI] [PubMed] [Google Scholar]
- Murakami E, Cheng J, Gysel K, Bozsoki Z, Kawaharada Y, Hjuler CT, Sørensen KK, Tao K, Kelly S, Venice F, et al. (2018) Epidermal LysM receptor ensures robust symbiotic signalling in Lotus japonicus. eLife 7: e33506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mus F, Crook MB, Garcia K, Garcia Costas A, Geddes BA, Kouri ED, Paramasivan P, Ryu MH, Oldroyd GED, Poole PS, et al. (2016) Symbiotic nitrogen fixation and the challenges to its extension to nonlegumes. Appl Environ Microbiol 82: 3698–3710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nars A, Lafitte C, Chabaud M, Drouillard S, Mélida H, Danoun S, Le Costaouëc T, Rey T, Benedetti J, Bulone V, et al. (2013) Aphanomyces euteiches cell wall fractions containing novel glucan-chitosaccharides induce defense genes and nuclear calcium oscillations in the plant host Medicago truncatula. PLoS ONE 8: e75039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishida H, Suzaki T(2018) Nitrate-mediated control of root nodule symbiosis. Curr Opin Plant Biol 44: 129–136 [DOI] [PubMed] [Google Scholar]
- Nishida H, Tanaka S, Handa Y, Ito M, Sakamoto Y, Matsunaga S, Betsuyaku S, Miura K, Soyano T, Kawaguchi M, et al. (2018) A NIN-LIKE PROTEIN mediates nitrate-induced control of root nodule symbiosis in Lotus japonicus. Nat Commun 9: 499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimura R, Hayashi M, Wu GJ, Kouchi H, Imaizumi-Anraku H, Murakami Y, Kawasaki S, Akao S, Ohmori M, Nagasawa M, et al. (2002) HAR1 mediates systemic regulation of symbiotic organ development. Nature 420: 426–429 [DOI] [PubMed] [Google Scholar]
- Okamoto S, Kawaguchi M(2015) Shoot HAR1 mediates nitrate inhibition of nodulation in Lotus japonicus. Plant Signal Behav 10: e1000138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamoto S, Shinohara H, Mori T, Matsubayashi Y, Kawaguchi M(2013) Root-derived CLE glycopeptides control nodulation by direct binding to HAR1 receptor kinase. Nat Commun 4: 2191. [DOI] [PubMed] [Google Scholar]
- Okazaki S, Tittabutr P, Teulet A, Thouin J, Fardoux J, Chaintreuil C, Gully D, Arrighi JF, Furuta N, Miwa H, et al. (2016) Rhizobium-legume symbiosis in the absence of Nod factors: Two possible scenarios with or without the T3SS. ISME J 10: 64–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldroyd GED.(2013) Speak, friend, and enter: Signalling systems that promote beneficial symbiotic associations in plants. Nat Rev Microbiol 11: 252–263 [DOI] [PubMed] [Google Scholar]
- Op den Camp R, Streng A, De Mita S, Cao Q, Polone E, Liu W, Ammiraju JSS, Kudrna D, Wing R, Untergasser A, et al. (2011) LysM-type mycorrhizal receptor recruited for Rhizobium symbiosis in nonlegume Parasponia. Science 331: 909–912 [DOI] [PubMed] [Google Scholar]
- Ott T.(2017) Membrane nanodomains and microdomains in plant-microbe interactions. Curr Opin Plant Biol 40: 82–88 [DOI] [PubMed] [Google Scholar]
- Pan H, Wang D(2017) Nodule cysteine-rich peptides maintain a working balance during nitrogen-fixing symbiosis. Nat Plants 3: 17048. [DOI] [PubMed] [Google Scholar]
- Parniske M.(2000) Intracellular accommodation of microbes by plants: A common developmental program for symbiosis and disease? Curr Opin Plant Biol 3: 320–328 [DOI] [PubMed] [Google Scholar]
- Parniske M.(2008) Arbuscular mycorrhiza: The mother of plant root endosymbioses. Nat Rev Microbiol 6: 763–775 [DOI] [PubMed] [Google Scholar]
- Parniske M, Zimmermann C, Cregan PB, Werner D(1990) Hypersensitive reaction of nodule cells in the Glycine sp./Bradyrhizobium japonicum symbiosis occurs at the genotype-specific level. Bot Acta 103: 143–148 [Google Scholar]
- Pimprikar P, Carbonnel S, Paries M, Katzer K, Klingl V, Bohmer MJ, Karl L, Floss DS, Harrison MJ, Parniske M, et al. (2016) A CCaMK-CYCLOPS-DELLA complex activates transcription of RAM1 to regulate arbuscule branching. Curr Biol 26: 987–998 [DOI] [PubMed] [Google Scholar]
- Pimprikar P, Gutjahr C(2018) Transcriptional regulation of arbuscular mycorrhiza development. Plant Cell Physiol 59: 673–690 [DOI] [PubMed] [Google Scholar]
- Price PA, Tanner HR, Dillon BA, Shabab M, Walker GC, Griffitts JS(2015) Rhizobial peptidase HrrP cleaves host-encoded signaling peptides and mediates symbiotic compatibility. Proc Natl Acad Sci USA 112: 15244–15249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radutoiu S, Madsen LH, Madsen EB, Felle HH, Umehara Y, Grønlund M, Sato S, Nakamura Y, Tabata S, Sandal N, et al. (2003) Plant recognition of symbiotic bacteria requires two LysM receptor-like kinases. Nature 425: 585–592 [DOI] [PubMed] [Google Scholar]
- Radutoiu S, Madsen LH, Madsen EB, Jurkiewicz A, Fukai E, Quistgaard EMH, Albrektsen AS, James EK, Thirup S, Stougaard J(2007) LysM domains mediate lipochitin-oligosaccharide recognition and Nfr genes extend the symbiotic host range. EMBO J 26: 3923–3935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen SR, Füchtbauer W, Novero M, Volpe V, Malkov N, Genre A, Bonfante P, Stougaard J, Radutoiu S(2016) Intraradical colonization by arbuscular mycorrhizal fungi triggers induction of a lipochitooligosaccharide receptor. Sci Rep 6: 29733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid DE, Ferguson BJ, Gresshoff PM(2011) Inoculation- and nitrate-induced CLE peptides of soybean control NARK-dependent nodule formation. Mol Plant Microbe Interact 24: 606–618 [DOI] [PubMed] [Google Scholar]
- Rey T, Nars A, Bonhomme M, Bottin A, Huguet S, Balzergue S, Jardinaud MF, Bono JJ, Cullimore J, Dumas B, et al. (2013) NFP, a LysM protein controlling Nod factor perception, also intervenes in Medicago truncatula resistance to pathogens. New Phytol 198: 875–886 [DOI] [PubMed] [Google Scholar]
- Ried MK, Antolín-Llovera M, Parniske M(2014) Spontaneous symbiotic reprogramming of plant roots triggered by receptor-like kinases. eLife 3: 1–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rival P, de Billy F, Bono JJ, Gough C, Rosenberg C, Bensmihen S(2012) Epidermal and cortical roles of NFP and DMI3 in coordinating early steps of nodulation in Medicago truncatula. Development 139: 3383–3391 [DOI] [PubMed] [Google Scholar]
- Roth R, Chiapello M, Montero H, Gehrig P, Grossmann J, O’Holleran K, Hartken D, Walters F, Yang SY, Hillmer S, et al. (2018) A rice serine/threonine receptor-like kinase regulates arbuscular mycorrhizal symbiosis at the peri-arbuscular membrane. Nat Commun 9: 4677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki T, Suzaki T, Soyano T, Kojima M, Sakakibara H, Kawaguchi M(2014) Shoot-derived cytokinins systemically regulate root nodulation. Nat Commun 5: 4983. [DOI] [PubMed] [Google Scholar]
- Schiessl K, Lilley JLS, Lee T, Tamvakis I, Kohlen W, Bailey PC, Thomas A, Luptak J, Ramakrishnan K, Carpenter MD, et al. (2019) NODULE INCEPTION recruits the lateral root developmental program for symbiotic nodule organogenesis in Medicago truncatula. Curr Biol 29: 3657–3668.e5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitz AM, Harrison MJ(2014) Signaling events during initiation of arbuscular mycorrhizal symbiosis. J Integr Plant Biol 56: 250–261 [DOI] [PubMed] [Google Scholar]
- Schmitz AM, Pawlowska TE, Harrison MJ(2019) A short LysM protein with high molecular diversity from an arbuscular mycorrhizal fungus, Rhizophagus irregularis. Mycoscience 60: 63–70 [Google Scholar]
- Searle IR, Men AE, Laniya TS, Buzas DM, Iturbe-Ormaetxe I, Carroll BJ, Gresshoff PM(2003) Long-distance signaling in nodulation directed by a CLAVATA1-like receptor kinase. Science 299: 109–112 [DOI] [PubMed] [Google Scholar]
- Sieberer BJ, Chabaud M, Fournier J, Timmers ACJ, Barker DG(2012) A switch in Ca2+ spiking signature is concomitant with endosymbiotic microbe entry into cortical root cells of Medicago truncatula. Plant J 69: 822–830 [DOI] [PubMed] [Google Scholar]
- Skiada V, Avramidou M, Bonfante P, Genre A, Papadopoulou K (2020) An endophytic Fusarium‐legume association is partially dependent on the common symbiotic signalling pathway. New Phytol doi:10.1111/nph.16457. [DOI] [PubMed] [Google Scholar]
- Smit P, Limpens E, Geurts R, Fedorova E, Dolgikh E, Gough C, Bisseling T(2007) Medicago LYK3, an entry receptor in rhizobial nodulation factor signaling. Plant Physiol 145: 183–191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strullu-Derrien C, Selosse MA, Kenrick P, Martin FM(2018) The origin and evolution of mycorrhizal symbioses: From palaeomycology to phylogenomics. New Phytol 220: 1012–1030 [DOI] [PubMed] [Google Scholar]
- Sun J, Miller JB, Granqvist E, Wiley-Kalil A, Gobbato E, Maillet F, Cottaz S, Samain E, Venkateshwaran M, Fort S, et al. (2015) Activation of symbiosis signaling by arbuscular mycorrhizal fungi in legumes and rice. Plant Cell 27: 823–838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzaki T, Takeda N, Nishida H, Hoshino M, Ito M, Misawa F, Handa Y, Miura K, Kawaguchi M(2019) LACK OF SYMBIONT ACCOMMODATION controls intracellular symbiont accommodation in root nodule and arbuscular mycorrhizal symbiosis in Lotus japonicus. PLoS Genet 15: e1007865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahara M, Magori S, Soyano T, Okamoto S, Yoshida C, Yano K, Sato S, Tabata S, Yamaguchi K, Shigenobu S, et al. (2013) Too much love, a novel Kelch repeat-containing F-box protein, functions in the long-distance regulation of the legume-Rhizobium symbiosis. Plant Cell Physiol 54: 433–447 [DOI] [PubMed] [Google Scholar]
- Takeda N, Haage K, Sato S, Tabata S, Parniske M(2011) Activation of a Lotus japonicus subtilase gene during arbuscular mycorrhiza is dependent on the common symbiosis genes and two cis-active promoter regions. Mol Plant Microbe Interact 24: 662–670 [DOI] [PubMed] [Google Scholar]
- Takeda N, Tsuzuki S, Suzaki T, Parniske M, Kawaguchi M(2013) CERBERUS and NSP1 of Lotus japonicus are common symbiosis genes that modulate arbuscular mycorrhiza development. Plant Cell Physiol 54: 1711–1723 [DOI] [PubMed] [Google Scholar]
- Taleski M, Imin N, Djordjevic MA(2018) CEP peptide hormones: Key players in orchestrating nitrogen-demand signalling, root nodulation, and lateral root development. J Exp Bot 69: 1829–1836 [DOI] [PubMed] [Google Scholar]
- Teulet A, Busset N, Fardoux J, Gully D, Chaintreuil C, Cartieaux F, Jauneau A, Comorge V, Okazaki S, Kaneko T, et al. (2019) The rhizobial type III effector ErnA confers the ability to form nodules in legumes. Proc Natl Acad Sci USA 116: 21758–21768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiergart T, Zgadzaj R, Bozsóki Z, Garrido-Oter R, Radutoiu S, Schulze-Lefert P(2019) Lotus japonicus symbiosis genes impact microbial interactions between symbionts and multikingdom commensal communities. MBio 10: e01833-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tóth K, Stratil TF, Madsen EB, Ye J, Popp C, Antolín-Llovera M, Grossmann C, Jensen ON, Schüssler A, Parniske M, et al. (2012) Functional domain analysis of the Remorin protein LjSYMREM1 in Lotus japonicus. PLoS ONE 7: e30817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsikou D, Yan Z, Holt DB, Abel NB, Reid DE, Madsen LH, Bhasin H, Sexauer M, Stougaard J, Markmann K(2018) Systemic control of legume susceptibility to rhizobial infection by a mobile microRNA. Science 362: 233–236 [DOI] [PubMed] [Google Scholar]
- Udvardi M, Poole PS(2013) Transport and metabolism in legume-rhizobia symbioses. Annu Rev Plant Biol 64: 781–805 [DOI] [PubMed] [Google Scholar]
- van Zeijl A, Liu W, Xiao TT, Kohlen W, Yang WC, Bisseling T, Geurts R(2015) The strigolactone biosynthesis gene DWARF27 is co-opted in rhizobium symbiosis. BMC Plant Biol 15: 260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venkateshwaran M, Jayaraman D, Chabaud M, Genre A, Balloon AJ, Maeda J, Forshey K, Den Os D, Kwiecien NW, Coon JJ, et al. (2015) A role for the mevalonate pathway in early plant symbiotic signaling. Proc Natl Acad Sci USA 112: 9781–9786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vernié T, Camut S, Camps C, Rembliere C, de Carvalho-Niebel F, Mbengue M, Timmers T, Gasciolli V, Thompson R, le Signor C, et al. (2016) PUB1 interacts with the receptor kinase DMI2 and negatively regulates rhizobial and arbuscular mycorrhizal symbioses through its ubiquitination activity in Medicago truncatula. Plant Physiol 170: 2312–2324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wais RJ, Galera C, Oldroyd G, Catoira R, Penmetsa RV, Cook D, Gough C, Denarié J, Long SR(2000) Genetic analysis of calcium spiking responses in nodulation mutants of Medicago truncatula. Proc Natl Acad Sci USA 97: 13407–13412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan J, Zhang XC, Neece D, Ramonell KM, Clough S, Kim SY, Stacey MG, Stacey G(2008) A LysM receptor-like kinase plays a critical role in chitin signaling and fungal resistance in Arabidopsis. Plant Cell 20: 471–481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C, Reid JB, Foo E(2018) The art of self-control: Autoregulation of plant-microbe symbioses. Front Plant Sci 9: 988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C, Zhu H, Jin L, Chen T, Wang L, Kang H, Hong Z, Zhang Z(2013) Splice variants of the SIP1 transcripts play a role in nodule organogenesis in Lotus japonicus. Plant Mol Biol 82: 97–111 [DOI] [PubMed] [Google Scholar]
- Weerasinghe RR, Bird DMK, Allen NS(2005) Root-knot nematodes and bacterial Nod factors elicit common signal transduction events in Lotus japonicus. Proc Natl Acad Sci USA 102: 3147–3152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong JEMM, Nadzieja M, Madsen LH, Bücherl CA, Dam S, Sandal NN, Couto D, Derbyshire P, Uldum-Berentsen M, Schroeder S, et al. (2019) A Lotus japonicus cytoplasmic kinase connects Nod factor perception by the NFR5 LysM receptor to nodulation. Proc Natl Acad Sci USA 116: 14339–14348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue L, Almario J, Fabiańska I, Saridis G, Bucher M(2019) Dysfunction in the arbuscular mycorrhizal symbiosis has consistent but small effects on the establishment of the fungal microbiota in Lotus japonicus. New Phytol 224: 409–420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yano K, Shibata S, Chen WL, Sato S, Kaneko T, Jurkiewicz A, Sandal N, Banba M, Imaizumi-Anraku H, Kojima T, et al. (2009) CERBERUS, a novel U-box protein containing WD-40 repeats, is required for formation of the infection thread and nodule development in the legume-Rhizobium symbiosis. Plant J 60: 168–180 [DOI] [PubMed] [Google Scholar]
- Yu H, Xiao A, Dong R, Fan Y, Zhang X, Liu C, Wang C, Zhu H, Duanmu D, Cao Y, et al. (2018) Suppression of innate immunity mediated by the CDPK-Rboh complex is required for rhizobial colonization in Medicago truncatula nodules. New Phytol 220: 425–434 [DOI] [PubMed] [Google Scholar]
- Zeng L, Velásquez AC, Munkvold KR, Zhang J, Martin GB(2012) A tomato LysM receptor-like kinase promotes immunity and its kinase activity is inhibited by AvrPtoB. Plant J 69: 92–103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng T, Rodriguez-Moreno L, Mansurkhodzaev A, Wang P, van den Berg W, Gasciolli V, Cottaz S, Fort S, Thomma BPHJ, Bono JJ, et al. (2020) A lysin motif effector subverts chitin-triggered immunity to facilitate arbuscular mycorrhizal symbiosis. New Phytol 225: 448–460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zgadzaj R, Garrido-Oter R, Jensen DB, Koprivova A, Schulze-Lefert P, Radutoiu S(2016) Root nodule symbiosis in Lotus japonicus drives the establishment of distinctive rhizosphere, root, and nodule bacterial communities. Proc Natl Acad Sci USA 113: E7996–E8005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Yuan L, Staehelin C, Li Y, Ruan J, Liang Z, Xie Z, Wang W, Xie J, Huang S(2019) The LYSIN MOTIF-CONTAINING RECEPTOR-LIKE KINASE 1 protein of banana is required for perception of pathogenic and symbiotic signals. New Phytol 223: 1530–1546 [DOI] [PubMed] [Google Scholar]
- Zhang X, Dong W, Sun J, Feng F, Deng Y, He Z, Oldroyd GED, Wang E(2015) The receptor kinase CERK1 has dual functions in symbiosis and immunity signalling. Plant J 81: 258–267 [DOI] [PubMed] [Google Scholar]
- Zhu H, Chen T, Zhu M, Fang Q, Kang H, Hong Z, Zhang Z(2008) A novel ARID DNA-binding protein interacts with SymRK and is expressed during early nodule development in Lotus japonicus. Plant Physiol 148: 337–347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhukov V, Radutoiu S, Madsen LH, Rychagova T, Ovchinnikova E, Borisov A, Tikhonovich I, Stougaard J(2008) The pea Sym37 receptor kinase gene controls infection-thread initiation and nodule development. Mol Plant Microbe Interact 21: 1600–1608 [DOI] [PubMed] [Google Scholar]