Abstract
Background -
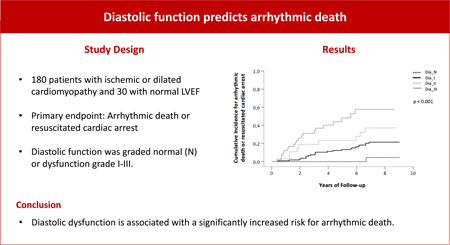
Patients with ischemic or dilated cardiomyopathy and reduced left ventricular ejection fraction (LVEF) face a high risk for ventricular arrhythmias. Exact grading of diastolic function might improve risk stratification for arrhythmic death.
Methods -
We prospectively enrolled 120 patients with ischemic, 60 patients with dilated cardiomyopathy and 30 patients with normal LVEF. Diastolic function was graded normal (N) or dysfunction grade I-III. Primary outcome parameter was arrhythmic death (AD) or resuscitated cardiac arrest (RCA).
Results -
Normal diastolic function was found in 23 (11%) patients, dysfunction grade I in 107 (51%), grade II in 31 (14.8%) and grade III in 49 (23.3%) patients, respectively. After an average follow-up of 7.0±2.6 years, AD or RCA was observed in 28 (13.3%) and 33 (15.7%) patients, respectively. Non-arrhythmic death was found in 41 (19.5%) patients. On Kaplan-Meier analysis, patients with dysfunction grade III had the highest risk for AD or RCA (p<0.001). This finding was independent from the degree of LVEF dysfunction and was observed in patients with LVEF≤35% (p=0.001) and with LVEF>35% (p=0.014). Non-arrhythmic mortality was highest in patients with dysfunction grade III. This was true for patients with LVEF≤35% (p=0.009) or >35% (p<0.001). In an adjusted model for relevant confounding factors, grade III dysfunction was associated with a 3.5-fold increased risk for AD or RCA in the overall study population (HR=3.52, p<0.001).
Conclusions -
Diastolic dysfunction is associated with a high risk for AD or RCA regardless if LVEF is ≤35% or >35%. Diastolic function grading might improve risk stratification for AD.
Keywords: sudden cardiac death, diastolic dysfunction, heart failure, arrhythmic death
Journal Subject Term: Arrhythmias, Sudden Cardiac Death, Heart Failure
Graphical Abstract
Introduction
Patients with ischemic cardiomyopathy (ICM) or dilated cardiomyopathy (DCM) and reduced left ventricular ejection fraction (LVEF) carry a high risk for sudden cardiac death, especially arrhythmic death (AD).1–3 Current guidelines recommend primary prevention with an implantable cardioverter-defibrillator (ICD) in patients with severely reduced LVEF of ≤35%.4,5 However, prediction of arrhythmic death solely based on LVEF has significant limitations.6,7 Patients with a LVEF >35% have a lower relative risk, but the absolute number of ADs in these patient group is high.8–11 Many potential predictors and techniques have been proposed to improve risk stratification for AD.7 So far, there is only limited data on the role of diastolic dysfunction in the prediction of AD.12 The present study tests the hypothesis that severe diastolic dysfunction is associated with an increased risk for AD.
Methods
Study Design
The data that support the findings of this study are available from the corresponding author upon reasonable request.
This prospective observational, observer-blind study was approved and monitored by the local ethics committee. All participants gave written consent. Screening, enrollment and follow-up after 3 and 10 years were conducted at the Medical University of Vienna, Austria. Test scoring, interpretation and statistical data processing underwent a blinded assessment at the Vanderbilt Autonomic Dysfunction Center, Nashville, USA. After enrollment, optimal medical treatment was established. Test results were not disclosed to participants or their physicians. Antiarrhythmic drug therapy and ICD implantation were not guided by the study.
Study population
A total of 120 patients with ICM, 60 patients with DCM and 30 patients with normal LVEF were included in the study. Patients were eligible to participate if they had recently undergone coronary angiography with ventriculography as standard use of care at the physician’s discretion due to typical symptoms, an abnormal echocardiogram or abnormal magnetic resonance imaging. Patients were excluded from participation if they had a history of sustained ventricular arrhythmia or permanent atrial fibrillation or if they were dependent on ventricular pacing. The treatment of patients was not guided by this study.
Outcome parameters
The primary endpoint of the study was time to arrhythmic death (AD) or resuscitated cardiac arrest (RCA). For statistical analysis, only the first event (AD or RCA) was taken. Non-arrhythmic death was a secondary endpoint. Deaths were categorized applying an adapted form of the Hinkle classification:13 Detailed information on the circumstances of death and abruptness of loss of consciousness were gathered from witnesses, reports from emergency service and records from treating hospital and physician. Family members were interviewed for activities and the condition in the time before death. Autopsy reports were obtained from all study participants. Appropriate ICD therapy without ventricular tachycardia (VT) acceleration that failed to save the patient’s life was classified as AD. An RCA was ventricular fibrillation or VT >240 bpm (beats per minute) leading to syncope before ICD therapy and multiple slower VT episodes (electrical storm) leading to syncope and ICD discharge without ICD therapy related acceleration. All other ICD therapies due to VT <240bpm were not taken as surrogate for AD. All ICD devices were programmed to allow maximum possible detection duration.
Assessment of diastolic function
Doppler measures of diastolic function were performed according to recommendations 14 and averaged over 3 cardiac cycles in sinus rhythm only. Trans-mitral pulsed-wave Doppler velocities were recorded at rest and during Valsalva maneuver from an apical 4-chamber view with a Doppler sample of 2 mm placed between the tips of the mitral leaflets. Diastolic function was graded blinded to baseline data or outcomes as normal, impaired relaxation (grade I), pseudo-normal pattern (grade II), or as restrictive pattern (grade III), using offline Doppler measurements of the mitral inflow, mitral inflow during Valsalva maneuver, and tissue Doppler imaging of the mitral annulus. Valsalva manoeuver and E/Ératio (E early mitral valve flow velocity, É left ventricular annular early lengthening velocity) were used to distinguish between normal and pseudo-normal filling pattern. For a normal pattern, the reference ranges used were as follows: ratio of early (E) to late (A) diastolic filling velocities in the mitral inflow recording between 1 and 2; deceleration time (DT) of early filling between 150ms and 220ms; ratio of systolic to diastolic peak velocity mitral E/A ratio during Valsalva manoeuver >1 with a reduction compared to baseline of <0.5 of the absolute ratio. Grade I diastolic dysfunction was consistent with an E/A ratio <1, DT>220 ms, and no substantial change in E/A ratio during Valsalva. Grade II diastolic dysfunction resembled the normal configuration with respect to the mitral inflow but with ≥2 of the following features: mitral E/A ratio <1 during Valsalva manoeuver with a reduction of at least 0.5 of the absolute ratio, and E/Ératio by tissue Doppler imaging of >15.14 Grade III diastolic dysfunction was characterized by an E/A ratio of >2, a DT <150, and E/É>15. In case of 15 > E/É > 8, additional investigations were required to confirm the diagnosis: E/A<0.5 (A late mitral valve flow velocity), DT >280ms and LAVI >40ml/m2 (left atrial volume index).
Statistical Analysis
Categorical variables are presented as numbers and percentage and continuous variables are presented as mean ± standard deviation. Chi-square test was used for comparison of categorical variables, Kruskal-Wallis test was used for comparison of continuous variables. Kaplan Meier analysis was performed to detect differences in arrhythmic and non-arrhythmic mortality in the overall study population and in patients stratified according to their LVEF: ≤35% and >35%. Log-rank test was used to determine statistical significance. Uni- and multivariable Cox regression analysis was performed to determine the effect of diastolic dysfunction on the risk for arrhythmic death. All variables with a p-value of <0.10 in the univariable analysis were included in the multivariable analysis. The univariable Cox regression model was fit for the variables age, gender, LVEF ≤35%, hypertension, diabetes mellitus, chronic kidney disease, implanted cardioverter-defibrillator, implanted cardiac resynchronization-therapy pacemaker, underlying heart disease, body-mass index, history of syncope, treatment with ACE/ARB, beta-blockers, diuretics, amiodarone or sotalol, New York Heart association (NYHA) classification, QRS duration ≥ 0.12 seconds and history of paroxysmal atrial fibrillation. Statistical Analysis was performed using the software package SPSS, Version 25.0, SPSS Inc, Chicago, IL, USA with a significance level of a two-sided p-value of ≤ 0.05.
Results
The overall study population included 210 patients, of whom 23 (11.0%) patients had normal diastolic function, 107 (51.0%) patients had dysfunction grade I, 31 (14.8%) patients had dysfunction grade II and 49 (23.3%) had dysfunction grade III. Mean LVEF of the study population was 38.0 ± 14.7%. Baseline clinical characteristics of the study population stratified according to the grades of diastolic dysfunction are presented in Table 1. All patients completed the predefined follow-up visits. The shorter follow-up duration of the patient group with grade III diastolic dysfunction is a result of the higher mortality rate in this group.
Table 1.
Baseline Clinical Characteristics according to the grades of diastolic dysfunction*
| DIA_N | DIA_I | DIA_II | DIA_III | p - value | |
|---|---|---|---|---|---|
| No. of patients | 23 | 107 | 31 | 49 | |
| Age (m ± SD) | 52.8 ± 13.0 | 59.5 ± 9.1 | 59.4 ± 8.5 | 58.9 ± 9.9 | 0.196 |
| Follow-up (years) | 8.6 ± 1.1 | 7.1 ± 1.7 | 5.7 ± 2.9 | 3.9 ± 2.4 | < 0.001 |
| Female (%) | 9 (39.1) | 18 (16.8) | 4 (12.9) | 6 (12.2) | 0.032 |
| BMI | 24.2 ± 4.4 | 28.3 ± 4.3 | 27.3 ± 4.0 | 28.8 ± 5.0 | 0.001 |
| ICM | 3 (13.0) | 60 (56.1) | 20 (64.5) | 37 (75.5) | < 0.001 |
| DCM | 1 (4.3) | 36 (33.6) | 11 (35.5) | 12 (24.5) | 0.028 |
| Hypertension | 14 (60.7) | 93 (86.9) | 28 (90.3) | 45 (91.8) | 0.003 |
| DM II | 2 (8.7) | 30 (28.0) | 11 (35.5) | 20 (40.8) | 0.039 |
| CKD | 0 (0) | 23 (21.5) | 10 (32.2) | 16 (32.7) | 0.012 |
| Syncope | 2 (8.7) | 24 (22.4) | 5 (16.1) | 7 (14.3) | 0.349 |
| ICD | 2 (8.7) | 45 (42.1) | 13 (41.9) | 20 (40.8) | 0.023 |
| CRT P | 1 (4.3) | 16 (15.0) | 2 (6.4) | 5 (10.2) | 0.350 |
| LVEF ≤35 | 1 (4.3) | 54 (50.5) | 25 (80.6) | 37 (75.5) | < 0.001 |
| ACE/ARB | 15 (65.2) | 99 (92.5) | 31 (100) | 47 (96.0) | < 0.001 |
| Beta blocker | 12 (52.2) | 96 (89.7) | 26 (83.9) | 45 (91.8) | < 0.001 |
| Diuretics | 4 (17.4) | 68 (63.6) | 21 (67.7) | 35 (71.4) | < 0.001 |
| Amiodaron | 2 (8.7) | 19 (17.8) | 3 (9.7) | 8 (16.3) | 0.557 |
| Sotalol | 1 (4.3) | 4 (3.7) | 3 (9.7) | 0 (0.0) | 0.181 |
| NYHA I | 10 (43.5) | 40 (37.4) | 9 (29.0) | 11 (22.4) | 0.193 |
| NYHA II | 13 (56.5) | 55 (51.4) | 16 (51.6) | 20 (40.8) | 0.543 |
| NYHA III | 0 (0) | 12 (11.2) | 6 (19.4) | 18 (36.7) | < 0.001 |
| QRS >0.12 s | 0 (0) | 27 (25.2) | 16 (51.6) | 23 (46.9) | < 0.001 |
n (%); ACE = angiotensin converting enzyme inhibitors, ARB = angiotensin II receptor blockers, BMI = body mass index, CKD = chronic kidney disease, CRT P = cardiac resynchronization therapy pacemaker, DCM = dilated cardiomyopathy, DIA_N = normal diastolic function, DIA_I = impaired relaxation, DIA_II = pseudo-normal pattern, DIA_III = restrictive pattern, DM II = diabetes mellitus 2, ICD = implantable cardioverter/defibrillator, ICM = ischemic cardiomyopathy, LVEF = left ventricular ejection fraction
Endpoints
An overview of the primary and secondary endpoints is provided in Figure S1 in the supplemental material. During an average follow-up of 7.0 ± 2.6 years, 69 patients died in the overall study population. Of these deaths, 28 were classified as AD and 41 died a non-arrhythmic death. Out of the 28 participants that died an arrhythmic death, 13 patients had an ICD implanted, but the device could not abort an electrical storm. RCA occurred in 33 patients as the first event. Of these individuals with RCA, 8 died later during follow-up an AD. In patients with normal diastolic function, no patient died of an AD, but 8 patients with dysfunction grade I, 2 patients with dysfunction grade II and 18 patients with dysfunction grade III died of an AD.
Diastolic dysfunction and the association to AD or RCA
Kaplan Meier estimates of the time to AD or RCA for the overall study population are shown in Figure 1. Patients with dysfunction grade III had the significantly highest risk for AD or RCA compared with patients with dysfunction grade II, I or normal diastolic function (p<0.001, Fig. 1). After 8 years of follow-up, the risk for AD or RCA was 4.5% in patients with normal diastolic function, 21.5% in patients with dysfunction grade I, 37.2% in patients with dysfunction grade II and 57.7% in patients with dysfunction grade III. Uni- and multivariable Cox Regression analysis (Table S1 in the supplemental material) was performed to identify confounding variables for AD or RCA. Relevant confounding variables in the multivariable analysis were hypertension (hazard ratio (HR)=2.82, 95%CI: 0.84–9.46, p=0.093), implantation of an ICD (HR=3.44, 95% CI:1.69–7.01, p=0.001), treatment with diuretics (HR=1.74, 95%CI: 0.84–3.57, p=0.134) and QRS duration >0.12s (HR=2.37, 95%CI: 1.28–4.39, p=0.006). In an additional model adjusted for these confounding factors (Table S2 in the supplemental material), patients with dysfunction grade III had a HR of 3.52 for AD or RCA (95%CI:2.00–6.22, p<0.001), whereas patients with dysfunction grade I had a significantly lower risk for AD or RCA (HR=0.41, 95%CI: 0.23–0.71, p=0.002). Normal diastolic function (HR=0.42, 95%CI: 0.06–3.21; p=0.405) or grade II diastolic dysfunction (HR=1.03, 95%CI: 0.50–2.16; p=0.930) did not significantly influence the risk for AD or RCA. Ischemic cardiomyopathy (HR=1.00, 95%CI: 0.47–2.16, p=0.994) and dilated cardiomyopathy (HR=0.91, 95%CI:0.49–1.70, p=0.764) were non-significant confounding variables for AD or RCA in the multivariable Cox regression.
Figure 1:
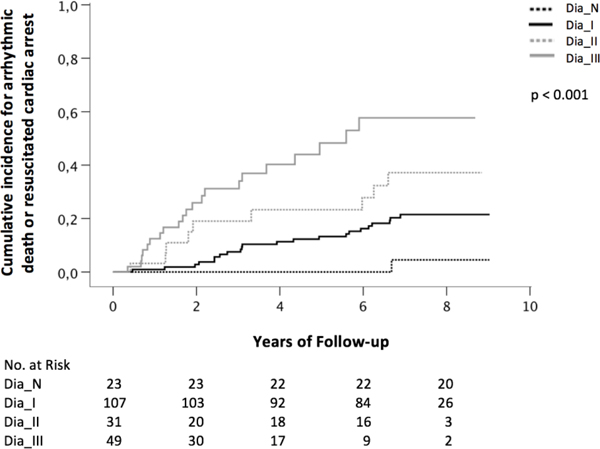
Kaplan Meier curves showing the association between diastolic function and arrhythmic death or resuscitated cardiac arrest in the overall study population (Dia_N normal diastolic function, Dia_I diastolic dysfunction grade one, Dia_II diastolic dysfunction grade two, Dia_III diastolic dysfunction grade three)
Stratification to LVEF
Patients were stratified in two groups according to their left ventricular function. Overall, 117 (55.7%) patients were categorized in the group with LVEF ≤35% and 93 (44.3%) patients in the group with LVEF >35%. Kaplan Meier analysis showed in the group with LVEF ≤35% that patients with dysfunction grade III had a significantly higher risk for AD or RCA than patients with normal diastolic function or with dysfunction grade I or II (p=0.001, Fig. 2a). This was also true in the group of patients with LVEF >35% (p=0.014, Fig. 2b). The risk for non-arrhythmic death was highest in patients with dysfunction grade III in the group with LVEF ≤35% (p=0.009, Fig. 3a) and in the group with LVEF >35% (p<0.001, Fig. 3b). In the latter, 78% of patients with dysfunction grade III died after 8 years of follow-up.
Figure 2:
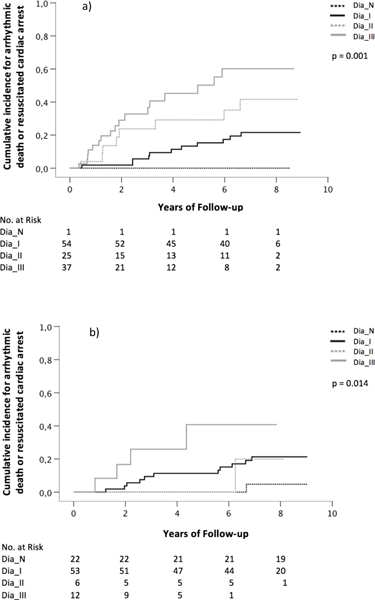
Kaplan Meier curves showing the association between diastolic function and arrhythmic death or resuscitated cardiac arrest in patients with a) LVEF ≤35% and b) LVEF >35% (Dia_N normal diastolic function, Dia_I diastolic dysfunction grade one, Dia_II diastolic dysfunction grade two, Dia_III diastolic dysfunction grade three)
Figure 3:
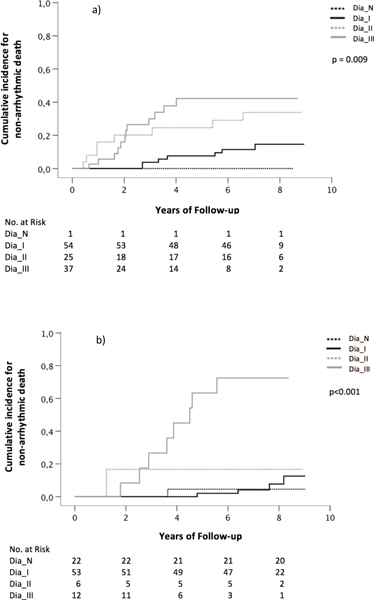
Kaplan Meier curves showing the association between diastolic function and non-arrhythmic death in patients with a) LVEF ≤35% and b) LVEF >35% (Dia_N normal diastolic function, Dia_I diastolic dysfunction grade one, Dia_II diastolic dysfunction grade two, Dia_III diastolic dysfunction grade three)
Discussion
The underlying study reports two major findings. First, diastolic dysfunction is associated with a significantly increased risk for AD or RCA in patients with ischemic or dilated cardiomyopathy. Second, this association is regardless of the degree of LVEF reduction. Patients with severe diastolic dysfunction have a significantly higher risk for AD or RCA regardless if LVEF is ≤35% or >35%. Diastolic function might be an important tool in risk stratification for arrhythmic death.
Diastolic dysfunction is associated with increased myocardial fibrosis and ventricular stiffness.15,16 Scars and fibrotic tissue in the ventricular myocardium increase the risk for cardiovascular mortality and contribute to potential reentry mechanisms for ventricular tachyarrhythmia.17–19 This might explain the high number of AD or RCA in patients with diastolic dysfunction grade III in the present study. After eight years of follow-up, the risk for AD or RCA was 58% in patients with dysfunction grade III, compared to 37% and 22% in patients with dysfunction grade II or I, respectively. Patients with normal diastolic function had a risk of 5% for AD or RCA only. To the best of our knowledge, this is the first study that prospectively evaluated the association between diastolic function and the risk for AD or RCA during long term follow-up.
Current Guidelines on the prevention of sudden cardiac death recommend primary preventive ICD implantation in symptomatic patients (NYHA classification II-III) with a LVEF ≤35% in ischemic and non-ischemic heart disease.4,5 This recommendation is based on landmark studies like MADIT II1 and SCD-HeFT2 that demonstrated the benefit of primary preventive ICD implantation in patients with LVEF <30% and ≤35%, respectively. Nevertheless, risk stratification according to LVEF has considerable limitations in sensitivity and specificity.7,20 Importantly, the absolute number of sudden cardiac death is highest in patients with only moderately or preserved LVEF7–9, but these patients are missed by risk stratification solely based on LVEF7. In addition to this, a considerable number of patients that receives an ICD never experiences a potentially life-threatening arrhythmic event21,22.
The present study strongly indicates that diastolic function grading might improve risk stratification for AD. After eight years of follow-up, the cumulative probability of AD or RCA in the group with LVEF ≤35% was 60%, 42% and 22% in patients with dysfunction grade III, II or I, respectively. Risk of AD or RCA was significantly different (p=0.001) between diastolic dysfunction grades and was highest in patients with grade III dysfunction. These patients might represent a population that particularly benefits from primary preventive ICD implantation. Importantly, risk for AD or RCA was also high in patients with LVEF >35%. After eight years of follow-up, the probability of AD or RCA in the group with LVEF >35% was 41% in patients with dysfunction grade III and 21% in patients with dysfunction grade I or II. This result indicates that diastolic function grading might improve risk stratification for AD in patients with LVEF>35%. Potentially, high-risk patients can be identified in this group that might benefit from primary preventive ICD implantation. This finding warrants further investigations in prospective and randomized trials.
Non-arrhythmic mortality was significantly higher in patients with diastolic dysfunction grade III compared to patients with normal diastolic function or dysfunction grade I or II. This was true for the group of patients with LVEF ≤35% and with LVEF >35%. This finding might be particularly relevant for heart failure patients with mid-range LVEF (40–50%, HFmrEF) that may have properties of systolic and diastolic impairment.23 Treatment options in these patients are scarce and focus on the control of cardiovascular comorbidities.24 However, diastolic function grading might identify high-risk patients in this cohort.
Study limitations
This is a non-randomized prospective, observational study to determine the role of diastolic function grading in the prediction of AD. It is still possible that some events, classified as RCA, might not have led to death. Another limitation is the small sample size, which does not allow making firm conclusions. Therefore, any finding should be labeled as pilot study results and further confirmation of results and discussed interpretations in large randomized trials is warrant.
Conclusion
Diastolic dysfunction is associated with a high risk for AD or RCA. This finding is independent from the degree of LVEF reduction and is observed in patients with LVEF ≤35% and in patients with LVEF >35%. Diastolic function grading might contribute to an improved risk stratification for AD or RCA.
Supplementary Material
What is known:
Patients with structural heart disease and reduced left ventricular ejection fraction (LVEF) carry a high risk for ventricular arrhythmias. Nevertheless, risk stratification for arrhythmic death based on LVEF reduction has important shortcomings in sensitivity and specificity.
What the study adds:
Diastolic dysfunction is associated with a significantly increased risk for arrhythmic death in patients with ischemic cardiomyopathy and patients with non-ischemic, dilated cardiomyopathy.
This association is independent of the degree of LVEF reduction and is present in patients with LVEF ≤35% and in patients with LVEF >35%.
Acknowledgments
Sources of Funding. Research reported in this publication was partly supported by the National Center for Advancing Translational Science of the National Institute of Health (NIH) under Award Number UL1TR000445, NIH 5P01 HL56693 and R01 HL142583 and is solely the responsibility of the authors and does not necessarily represent official views of the NIH. All authors had access to all of the data in the study and take responsibility for the integrity and the accuracy of the data analysis.
Non-standard Abbreviations and Acronyms
- A
late mitral valve flow velocity
- ACE
angiotensin converting enzyme
- AD
arrhythmic death
- ARB
angiotensin receptor blocker
- bpm
beats per minute
- CI
confidence interval
- CRT
cardiac resynchronization therapy
- DCM
dilated cardiomyopathy
- DT
deceleration time
- E
early mitral valve flow velocity
- E′
left ventricular annular early lengthening velocity
- HFmrEF
heart failure with mid-range ejection fraction
- HR
hazard ratio
- ICD
implantable cardioverter-defibrillator
- ICM
ischemic cardiomyopathy
- LAVI
left atrial volume index
- LVEF
left ventricular ejection fraction
- NYHA
New York heart association
- RCA
resuscitated cardiac arrest
- VT
ventricular tachycardia
Footnotes
Disclosures. None
References:
- 1.Moss AJ, Zareba W, Hall WJ, Klein H, Wilber DJ, Cannom DS, Daubert JP, Higgins SL, Brown MW, Andrews ML. Prophylactic implantation of a defibrillator in patients with myocardial infarction and reduced ejection fraction. N Engl J Med. 2002;346:877–883. [DOI] [PubMed] [Google Scholar]
- 2.Bardy GH, Lee KL, Mark DB, Poole JE, Packer DL, Boineau R, Domanski M, Troutman C, Anderson J, Johnson G, et al. Amiodarone or an implantable cardioverter-defibrillator for congestive heart failure. N Engl J Med. 2005;352:225–237. [DOI] [PubMed] [Google Scholar]
- 3.Kadish A, Dyer A, Daubert JP, Quigg R, Estes NA, Anderson KP, Calkins H, Hoch D, Goldberger J, Shalaby A, et al. Prophylactic defibrillator implantation in patients with nonischemic dilated cardiomyopathy. N Engl J Med. 2004;350:2151–2158. [DOI] [PubMed] [Google Scholar]
- 4.Priori SG, Blomstrom-Lundqvist C, Mazzanti A, Blom N, Borggrefe M, Camm J, Elliott PM, Fitzsimons D, Hatala R, Hindricks G, et al. 2015 ESC Guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: The Task Force for the Management of Patients with Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death of the European Society of Cardiology (ESC). Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC). Eur Heart J. 2015;36:2793–2867. [DOI] [PubMed] [Google Scholar]
- 5.Al-Khatib SM, Stevenson WG, Ackerman MJ, Bryant WJ, Callans DJ, Curtis AB, Deal BJ, Dickfeld T, Field ME, Fonarow GC, et al. 2017 AHA/ACC/HRS Guideline for Management of Patients With Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death. Circulation. 2018;138:e272–e391. [DOI] [PubMed] [Google Scholar]
- 6.Yap YG, Duong T, Bland JM, Malik M, Torp-Pedersen C, Kober L, Gallagher MM, Camm AJ. Optimising the dichotomy limit for left ventricular ejection fraction in selecting patients for defibrillator therapy after myocardial infarction. Heart. 2007;93:832–836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dagres N, Hindricks G. Risk stratification after myocardial infarction: is left ventricular ejection fraction enough to prevent sudden cardiac death? Eur Heart J. 2013;34:1964–1971. [DOI] [PubMed] [Google Scholar]
- 8.Gorgels AP, Gijsbers C, de Vreede-Swagemakers J, Lousberg A, Wellens HJ. Out-of-hospital cardiac arrest--the relevance of heart failure. The Maastricht Circulatory Arrest Registry. Eur Heart J. 2003;24:1204–1209. [DOI] [PubMed] [Google Scholar]
- 9.Makikallio TH, Barthel P, Schneider R, Bauer A, Tapanainen JM, Tulppo MP, Schmidt G, Huikuri HV. Prediction of sudden cardiac death after acute myocardial infarction: role of Holter monitoring in the modern treatment era. Eur Heart J. 2005;26:762–769. [DOI] [PubMed] [Google Scholar]
- 10.Pezawas T, Diedrich A, Robertson D, Winker R, Richter B, Wang L, Schmidinger H. Risk of arrhythmic death in ischemic heart disease: a prospective, controlled, observer-blind risk stratification over 10 years. Eur J Clin Invest. 2017;47:231–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pezawas T, Diedrich A, Winker R, Robertson D, Richter B, Wang L, Byrne DW, Schmidinger H. Multiple autonomic and repolarization investigation of sudden cardiac death in dilated cardiomyopathy and controls. Circ Arrhythm Electrophysiol. 2014;7:1101–1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Al-Khatib SM, Shaw LK, O’Connor C, Kong M, Califf RM. Incidence and predictors of sudden cardiac death in patients with diastolic heart failure. J Cardiovasc Electrophysiol. 2007;18:1231–1235. [DOI] [PubMed] [Google Scholar]
- 13.Hinkle LE Jr., Thaler HT. Clinical classification of cardiac deaths. Circulation. 1982;65:457–464. [DOI] [PubMed] [Google Scholar]
- 14.Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA, Picard MH, Roman MJ, Seward J, Shanewise JS, et al. Recommendations for chamber quantification: a report from the American Society of Echocardiography’s Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr. 2005;18:1440–1463. [DOI] [PubMed] [Google Scholar]
- 15.Martos R, Baugh J, Ledwidge M, O’Loughlin C, Conlon C, Patle A, Donnelly SC, McDonald K. Diastolic heart failure: evidence of increased myocardial collagen turnover linked to diastolic dysfunction. Circulation. 2007;115:888–895. [DOI] [PubMed] [Google Scholar]
- 16.Zile MR, Baicu CF, Gaasch WH. Diastolic heart failure--abnormalities in active relaxation and passive stiffness of the left ventricle. N Engl J Med. 2004;350:1953–1959. [DOI] [PubMed] [Google Scholar]
- 17.Almehmadi F, Joncas SX, Nevis I, Zahrani M, Bokhari M, Stirrat J, Fine NM, Yee R, White JA. Prevalence of myocardial fibrosis patterns in patients with systolic dysfunction: prognostic significance for the prediction of sudden cardiac arrest or appropriate implantable cardiac defibrillator therapy. Circ Cardiovasc Imaging. 2014;7:593–600. [DOI] [PubMed] [Google Scholar]
- 18.Piers SR, Tao Q, de Riva Silva M, Siebelink HM, Schalij MJ, van der Geest RJ, Zeppenfeld K. CMR-based identification of critical isthmus sites of ischemic and nonischemic ventricular tachycardia. JACC Cardiovasc Imaging. 2014;7:774–784. [DOI] [PubMed] [Google Scholar]
- 19.Dickfeld T, Tian J, Ahmad G, Jimenez A, Turgeman A, Kuk R, Peters M, Saliaris A, Saba M, Shorofsky S, et al. MRI-Guided ventricular tachycardia ablation: integration of late gadolinium-enhanced 3D scar in patients with implantable cardioverter-defibrillators. Circ Arrhythm Electrophysiol. 2011;4:172–184. [DOI] [PubMed] [Google Scholar]
- 20.Bailey JJ, Berson AS, Handelsman H, Hodges M. Utility of current risk stratification tests for predicting major arrhythmic events after myocardial infarction. J Am Coll Cardiol. 2001;38:1902–1911. [DOI] [PubMed] [Google Scholar]
- 21.Greenberg H, Case RB, Moss AJ, Brown MW, Carroll ER, Andrews ML. Analysis of mortality events in the Multicenter Automatic Defibrillator Implantation Trial (MADIT-II). J Am Coll Cardiol. 2004;43:1459–1465. [DOI] [PubMed] [Google Scholar]
- 22.Burger AL, Stojkovic S, Schmidinger H, Ristl R, Pezawas T. Defensive Implantable Cardioverter-Defibrillator Programming Is Safe and Reduces Inappropriate Therapy- Comparison of 3 Programming Strategies in 1,471 Patients. Circ J. 2018;82:2976–2982. [DOI] [PubMed] [Google Scholar]
- 23.He KL, Burkhoff D, Leng WX, Liang ZR, Fan L, Wang J, Maurer MS. Comparison of ventricular structure and function in Chinese patients with heart failure and ejection fractions >55% versus 40% to 55% versus <40%. Am J Cardiol. 2009;103:845–851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JG, Coats AJ, Falk V, Gonzalez-Juanatey JR, Harjola VP, Jankowska EA, et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail. 2016;18:891–975. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


