Abstract
The immune system is central to our interactions with the world in which we live and importantly dictates our response to potential allergens, toxins, and pathogens to which we are constantly exposed. Understanding the mechanisms that underlie protective host immune responses against microbial pathogens is vital for the development of improved treatment and vaccination strategies against infections. To that end, inherited immunodeficiencies that manifest with susceptibility to bacterial, viral, and/or fungal infections have provided fundamental insights into the indispensable contribution of key immune pathways in host defense against various pathogens. In this mini-review, we summarize the findings from a series of recent publications in which inherited immunodeficiencies have helped illuminate the interplay of human immunity and resistance to infection.
Keywords: immunodeficiency, inherited, monogenic, infection, immunology, infectious disease
Introduction
Insights through the observation and study of primary immunodeficiency disorders (PIDs) provide a unique in vivo lens through which our understanding of human immunology can be advanced. Herein, we discuss recent discoveries that have furthered our collective understanding of the host immune response in protection from the constant environmental threats posed by bacteria, viruses, and fungi. We focus on interleukin (IL)-6 receptor-alpha (IL-6Rα) deficiency and dominant activating Rac family small GTPase 2( RAC2) mutations leading to bacterial infection susceptibility, EROS deficiency and its impact on innate immunity, interferon (IFN) regulatory factor 4 (IRF4) haploinsufficiency causing susceptibility to Whipple’s disease (WD), defects in IL-12 receptor subunit beta 2 (IL-12RB2), IL-23 receptor (IL-23R), RAR-related orphan receptor C (RORC), Janus kinase 1 (JAK1), and tyrosine kinase 2 (TYK2) contributing to mycobacterial disease, deficiency in IFNα receptor-1 (IFNAR1), melanoma differentiation associated protein-5 (MDA5), IRF9, or IL-18 binding protein (IL18-BP) and gain-of-function (GOF) mutations in NLR family pyrin domain containing 1 ( NLRP1) leading to viral infection susceptibility, and fungal infection susceptibility in the setting of caspase recruitment domain-containing protein 9 (CARD9) deficiency, zinc finger protein 341 (ZNF341) deficiency, and c-Jun N-terminal kinase 1 (JNK1) haploinsufficiency.
Recent insights gained from primary immunodeficiency disorders manifesting with bacterial infection susceptibility
IL-6 signaling: a critical role in control of bacteria
Prompt recognition of bacterial pathogens is paramount in the defense against infection, and, among the mechanisms that initiate the immune cascade, IL-6 plays a central role. As a pleiotropic pro-inflammatory cytokine, IL-6 leads to the initiation of the acute-phase response by driving the recruitment of innate immune cells and pathogen phagocytosis while also promoting adaptive immune responses through mediating B cell survival and maturation, T cell proliferation, and the lineage commitment of T follicular, T H17, and T H22 helper cells 1– 3. Classical cell-membrane signaling of IL-6 is mediated through a hexameric complex composed of two IL-6 molecules, two IL-6Rα receptors, and two transmembrane glycoprotein 130 (gp130) proteins through which signal transduction is propagated via JAK and signal transducer and activator of transcription (STAT) interactions 4– 6. Iatrogenic, acquired, and inherited defects have provided evidence of the consequences of impaired IL-6 signaling through their impact on infection susceptibility. Severe staphylococcal infections have been described in patients treated with the anti-IL-6R monoclonal antibody tocilizumab and in spontaneously acquired autoantibodies against circulating IL-6 7– 9. Furthermore, inherited homozygous mutations in IL6ST, encoding the gp130 co-receptor, predispose to recurrent severe staphylococcal and streptococcal infections 10 as well as elevated serum IgE and dental and cranio-skeletal manifestations reminiscent of hyper-IgE syndrome (HIES) caused by dominant-negative mutations in STAT3 11, 12. It was not until recently that inherited deficiency in IL-6Rα was identified. Specifically, Spencer et al. reported two patients with severe recurrent infections with defective acute-phase response, elevated IgE, atopic dermatitis, and peripheral eosinophilia caused by pathogenic variants in IL6R 13. An additional four patients from two unrelated families with IL6R mutations were also reported sharing a similar clinical phenotype with severe bacterial infectious complications caused by Streptococcus pneumoniae, Staphylococcus aureus, Haemophilus influenzae, and Neisseria meningiditis 14 ( Table 1). Mechanistically, shared among all of these IL-6 pathway functional defects is absent or delayed production of acute-phase reactants including C-reactive protein, which plays a crucial role in the protection against bacterial pathogens including Streptococci , Klebsiella pneumoniae, and Neisseria meningitidis through opsonization and phagocytosis by macrophages and neutrophils 1, 15– 17.
Table 1. Recently described primary immunodeficiencies associated with increased risk of infection.
| Gene/
protein |
Variant(s) | Inheritance
model |
Clinical manifestations | Laboratory findings | Associated pathogens |
|---|---|---|---|---|---|
| Host response to bacteria | |||||
|
IL6R/IL-
6RA |
G183Efs*7;I279N | AR, LOF | Atopic dermatitis,
respiratory infections, skin abscesses, recurrent skin infections |
Variably low IgG, IgA
↑ IgE ↓ CRP |
Streptococcus
pneumoniae, Staphylococcus aureus, Haemophilus influenzae, Neisseria meningitidis |
|
IRF4
/IRF4 |
R98W | AD,
haploinsufficiency |
Whipple’s disease,
chronic Tropheryma whipplei carriage |
NR | T. whipplei |
|
RAC2
/RAC2 |
E62K; P34H | AD, GOF | Recurrent sinopulmonary
infections, bronchiectasis, cellulitis, lymphadenitis, B cell lymphoma, littoral cell angioma |
Neutropenia,
lymphopenia, variable hypogammaglobulinemia, increased DHR |
N. meningitidis, varicella
zoster, HPV, herpes simplex |
|
CYBC1/
EROS |
c.127G>A;
Y2X |
AR, LOF | Short stature, skin/soft tissue
infections, lymphadenitis, recurrent sinopulmonary infections, granulomatous inflammation, AIHA, HLH, CGD, IBD |
Impaired DHR,
lymphopenia |
BCG,
Burkholderia
cepacia, Legionella, Candida albicans, Clostridium difficile, Mycobacterium tuberculosis |
| Host response to mycobacteria | |||||
|
IL12RB2/
IL-12Rβ1 |
Q138X | AR, LOF | Lymphadenitis, pulmonary
tuberculosis |
Increased naïve CD4
+
and CD8 + T cells |
BCG, M. tuberculosis |
|
IL23R/IL-
23R |
C115Y | AR, LOF | Lymphadenitis,
disseminated BCG infection |
Increased naïve CD4
+
and CD8 + T cells |
BCG |
|
TYK2/
TYK2 |
P1104A | AR, LOF | Primary tuberculosis | Increased naïve CD4
+
and CD8 + T cells, reduced effector memory T cells |
M. tuberculosis, NTM |
|
RORC
/RORγ/ RORγt |
Q308X; Q420X | AR, LOF | Thymic hypoplasia, CMC,
disseminated BCG infection, tuberculosis, eczematous dermatitis, recurrent skin infections |
CD4
+ and CD8
+T cell
lymphopenia ↓IFN-γ production ↓ IL-17A/F, IL-21, IL-22 production |
BCG,
M. tuberculosis,
C. albicans |
|
JAK1/
JAK1 |
P733L; P832S | AR, LOF | Recurrent sinopulmonary
infections, osteomyelitis, developmental delay, urothelial carcinoma, skin infections, flat warts, crusted scabies |
Reduced naive CD4
+ and
CD8 + T cells, Impaired PHA response Impaired IFN-γ, IFN-α, IL- 2, IL-4, IL-10, and IL-27 responses ↓IFN-γ production ↑ IL-6 production |
NTM,
Sarcoptes scabiei,
HPV |
| Host response to viruses | |||||
|
IFNAR1/
IFNAR1 |
V225fs;
W261X, V225_P23 2del |
AR, LOF | Disseminated vaccine-
strain measles, yellow fever vaccine-associated viscerotropic disease |
Impaired type I IFN
responses |
Vaccine-strain measles,
vaccine-strain yellow fever |
|
IFIH1/
MDA5 |
K365E | AR, LOF | Recurrent viral respiratory
infections |
Reduced IFN-β, IFN-λ
transcription |
HRV, influenza B,
influenza A, coronavirus, RSV, parainfluenza |
| IRF9/IRF9 | E166Lfs*80,
D331N |
AR, LOF | Recurrent viral infections,
severe influenza infection |
Variable mild
lymphopenia, variable hypogammaglobulinemia, impaired type I IFN responses |
Influenza A |
|
IL18BP/IL-
18 BP |
c.508-19_528del | AR, LOF | HAV-induced acute liver
failure |
↑ IL-18 | HAV |
|
NLRP1/
NLRP1 |
T755N | AR, GOF | Recurrent respiratory
papillomatosis, palmar and plantar warts |
↑ IL-18
↑TNF-α |
HPV |
| TLR3/TLR3 | P554S; P680L | AD, LOF | Acute respiratory distress
syndrome |
↓IFN-β and IFN-λ from
fibroblasts |
Influenza A, RSV |
| Host response to fungi | |||||
|
CARD9/
CARD9 |
NA | AR, LOF | Mucocutaneous and CNS
fungal infections |
↓ CNS neutrophil
recruitment due to impaired IL-1β/CXCL1 microglial production ↓ neutrophil killing against unopsonized Candida yeasts Impaired IL-17 responses |
C. albicans, Aspergillus
species, agents of dermatophytosis and phaeohyphomycosis |
|
ZNF341/
ZNF341 |
R302X;
K355fs; Y542X; Q195X |
AR, LOF | CMC, staphylococcal
skin infections, respiratory infections, bronchiectasis, severe atopic dermatitis, food and environmental allergy, connective tissue abnormalities |
Low NK Cells
Low memory B cells Low central memory CD4 + and CD8 + T cells Low IgM, IgA ↑ IgE |
S. aureus, C. albicans,
Candida glabrata |
|
MAPK8/
JNK1 |
c.311+1G>A | AD,
haploinsufficiency |
Systemic connective tissue
disorder, CMC, recurrent bacterial skin infections, urinary tract infections |
Impaired IL-17A/F
responses |
C. albicans,
Staphylococcal species |
AD, autosomal dominant; AIHA, autoimmune hemolytic anemia; AR, autosomal recessive; BCG, Bacillus Calmette-Guérin; CARD9, caspase recruitment domain-containing protein 9; CGD, chronic granulomatous disease; CMC, chronic mucocutaneus candidiasis; CNS, central nervous system; CRP, C-reactive protein; DHR, dihydrorhodamine test; GOF, gain-of-function; HAV, Hepatitis A virus; HLH, hemophagocytic lymphohistiocytosis; HPV, human papilloma virus; HRV, human rhinovirus; IBD, inflammatory bowel disease; IFN, interferon; IFNAR1, interferon alpha receptor-1; Ig, immunoglobulin; IL, interleukin; IL-6RA, interleukin-6 receptor-alpha; IL-12Rβ1, interleukin-12 receptor subunit beta 1; IL-18 BP, interleukin-18 binding protein; IL-23R, interleukin-23 receptor; IRF, interferon regulatory factor; JAK1, Janus kinase 1; JNK1, c-Jun N-terminal kinase 1; LOF, loss-of-function; MDA5, melanoma differentiation associated protein-5; NA, not applicable; NK, natural killer; NLRP1, NLR family pyrin domain containing 1; NR, not reported; NTM, non-tuberculous mycobacteria; PHA, phytohemagglutinin; RAC2, Rac family small GTPase 2; ROR, RAR-related orphan receptor; RSV, respiratory syncytial virus; TNF, tumor necrosis factor; TLR3, Toll-like receptor-3; ZNF341, zinc finger protein 341.
As the IL-6 signaling pathway has been implicated in autoimmune and inflammatory diseases such as rheumatoid arthritis, giant cell arteritis, Castleman’s disease, and cytokine release syndrome in the setting of CAR T cell therapy for malignancies, anti-IL-6R therapies have become increasingly more common 18. As such, surveillance for bacterial infectious complications is warranted in the setting of anti-IL-6 biological therapies.
Whipple’s disease: 111 years later
Described initially by George Hoyt Whipple 19, WD is a chronic infection manifested by malabsorptive diarrhea, weight loss, and arthritis caused by the Gram-positive bacillus Tropheryma whipplei 20. Despite T. whipplei being ubiquitous in the soil and water, WD is rare, with an estimated incidence of 1–3 cases per 1 million people 21. Interestingly, asymptomatic carriage is relatively frequent, ranging from ~4–31% depending on the geographical area 22, 23. The vast majority of infected individuals suffer from self-limited illness, suggesting that an impaired immunological response may play a role in those with chronic or invasive forms of the disease 24– 27.
Through the investigation of four related patients with WD, Guérin et al. reported a rare loss-of-function (LOF) mutation in IRF4 causing haploinsufficiency 28. It is important to note that a total of 12 individuals were found to be heterozygous for the p.R98W variant in IRF4 ( Table 1), including the four WD patients, five chronic T. whipplei carriers, two non-carriers, and one relative who was not tested for T. whipplei, thereby indicating incomplete penetrance. IRF4 is an important transcription factor for the development of immune cells as well as T H2, T H9, and T H17 responses ( Figure 1); however, its role in the control of bacterial infection has been less clear 29, 30. In vitro studies of patient cells demonstrated that the mutant IRF4 allele failed to bind DNA or induce transcription. Additional transcriptional analysis of T. whipplei-infected peripheral blood mononuclear cells (PBMCs) did not detect specific pathway enrichment; however, Mycobacterium bovis–Bacillus Calmette-Guérin (BCG)-infected PBMCs demonstrated downregulation of STAT1 and IFN-γ-associated pathways. These results suggest that IRF4 haploinsufficiency may predispose to T. whipplei via impaired T H1 helper cell mechanisms. Despite the potential impact on the IFN-γ axis, patients with IRF4 haploinsufficiency appear to be selectively susceptible to WD without susceptibility to other intracellular pathogens that rely on intact IFN-γ/STAT1 signaling (e.g. mycobacteria, Salmonella, etc.), implying that other parallel and/or compensatory immune mechanisms may play a role. Additional work is needed to further delineate the cell-specific mechanistic role of IRF4 in host defense against WD.
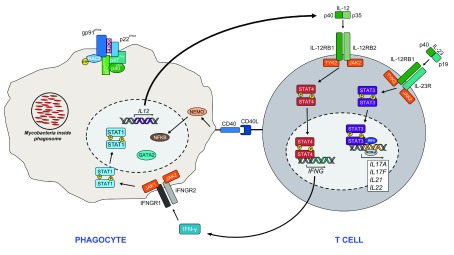
Figure 1. The IFN-γ/IL-12, IL-23, IL-17 axis and NADPH complex in host defense against pathogens.
Mycobacteria are recognized and phagocytosed, leading to IL-12 (p40/p35) secretion by macrophages. Binding of IL-12 to its receptor on the surface of T H1 (or NK) cells activates the downstream JAK2 and TYK2 signaling cascade, resulting in STAT4 phosphorylation and dimerization. STAT4 homodimers translocate to the nucleus and induce the transcription of IFN-γ. Phosphorylated STAT1 homodimers translocate to the nucleus, resulting in the transcription of host defense genes. Similarly, IL-23 comprising the p40 and p19 subunits binds to the IL-23 receptor, leading to JAK2/TYK2 activation and STAT3 phosphorylation, dimerization, and nuclear translocation, where it associates with IRF4 and RORγt in the transcription of IL17- , IL21- , and IL22-related genes. The membrane-bound gp91 phox and p22 phox heterodimer (cytochrome b558) is stabilized by the transmembrane chaperone protein EROS. The remaining cytosolic components, p67 phox, p47 phox, and p40 phox, along with RAC2-GTP, associate to form the activated NADPH complex. Secondary signal from CD40/CD40L signaling activates the NF-κB pathway. GATA2, GATA binding factor 2; gp, glycoprotein; IFN, interferon; IFNG, interferon gamma; IFNGR, interferon gamma receptor; IL, interleukin; IL-12RB, IL-12 receptor subunit beta; IRF4, interferon regulatory factor 4; JAK2, Janus kinase 2; NAPDH, nicotinamide adenine dinucleotide; NEMO, nuclear factor-κB essential modulator; NF-κB, nuclear factor-κB; NK, natural killer; RAC2, Rac family small GTPase 2;RORγt, RAR-related orphan receptor γt; STAT, signal transducer and activator of transcription; T H1, T helper type 1; TYK2, tyrosine kinase 2. This figure is adapted and amended with permissions, from Figure 3 in Abers, A, Lionakis, M. Chronic mucocutaneous candidiasis and invasive fungal infection susceptibility. In: Sullivan, K.E, Stiehm, E.R Stiehm's Immune Deficiencies-Inborn Errors of Immunit. p1–44. 2nd ed. Copyright Elsevier Science & Technology. 2020 37.
This figure is adapted and amended with permissions, from Figure 3 in Abers, A, Lionakis, M. Chronic mucocutaneous candidiasis and invasive fungal infection susceptibility. In: Sullivan, K.E, Stiehm, E.R Stiehm's Immune Deficiencies-Inborn Errors of Immunit. p1–44. 2nd ed. © Academic Press
EROS, NADPH oxidase, and innate immunity
The generation of reactive oxygen species (ROS) via the nicotinamide adenine dinucleotide (NADPH) complex is an essential component of innate host defense. Mutations in four of the genes encoding the protein components of the NADPH complex (gp91 phox, p22 phox, p67 phox, and p47 phox) cause chronic granulomatous disease (CGD) manifested by an impaired phagocyte respiratory burst resulting in infectious susceptibility to bacteria and fungi 31, 32. Instead, inherited defects affecting the fifth protein, p40 phox, result in severe inflammatory complications with modest infection susceptibility 33. A newly described transmembrane protein, EROS, has also been identified to play a key role in ROS production through stabilization of the gp91 phox and p22 phox heterodimer, cytochrome 588b 34 ( Figure 1). A cohort of eight individuals with a homozygous null mutation in CYBC1 encoding EROS demonstrated a CGD-like disease with severe infections and associated colitis 35. Patients demonstrated absent cytochrome 588b surface expression and severely reduced neutrophil oxidative burst. Interestingly, Thomas et al. 36 reported a Saudi Arabian boy with biallelic mutations affecting CYBC1 who, unlike the previously reported patients, suffered from recurrent sinopulmonary infections, autoimmune hemolytic anemia, and hemophagocytic lymphohistiocytosis (HLH) ( Table 1). Evaluation of the patient’s PBMCs showed absent EROS protein, while neutrophils demonstrated defective respiratory burst following stimulation with either phorbol 12-myristate 13-acetate (PMA) or zymosan. These cases provide additional evidence for EROS deficiency manifesting as a CGD-like disease but, given the heterogeneous clinical phenotype, also raise questions for additional roles that EROS may play in infection susceptibility and inflammation.
Dominant activating RAC2 mutations and bacterial lung disease
RAC2 is a guanine nucleotide-binding protein belonging to the rho guanosine triphosphatase (GTPase) family. It is expressed in the hematopoietic compartment, where it promotes cell migration, adhesion, and oxygen radical production 38, 39 ( Figure 1). Human RAC2 LOF mutations have been described to result in neutrophil defects 40, severe combined immunodeficiency 41, and a common variable immunodeficiency (CVID) clinical phenotype 42. Recently, RAC2 dominant activating mutations were discovered in which patients suffered from recurrent bacterial sinopulmonary infections and bronchiectasis 43, 44. Patients exhibited lymphopenia possibly as a result of increased apoptosis 43 while neutrophils demonstrated excessive superoxide production ( Table 1), aberrant n-formyl methionyl-leucyl-phenylalanine (fMLF)-mediated chemotaxis, impaired macropinocytosis, and compromised actin remodeling in vitro 44 . This combined immunodeficiency underscores the importance of both neutrophil and lymphocyte function in the protection against bacterial lung disease.
Teasing apart cellular signaling in the host response to mycobacteria
IFN-γ is critical for the control of intracellular pathogens including mycobacteria, Salmonella, Listeria, endemic fungi, and others 45, 46. Disorders of mendelian susceptibility to mycobacterial disease (MSMD) comprise 11 different genes to date, resulting in 21 different genetic diseases 47. Of these rare inherited disorders, autosomal-recessive (AR) IL-12 receptor β1 (IL-12Rβ1) deficiency accounts for the majority of cases 48. The IL12RB1 gene encodes for IL-12Rβ1, which mediates IL-12 and IL-23 signaling via dimerization with IL-12Rβ2 and IL-23R components to form IL-12 and IL-23 receptors, respectively 49, 50 ( Figure 1). Similarly, mutations in IL12B encoding the IL-12 p40 subunit common to both IL-12 and IL-23 exhibit a clinical phenocopy of IL-12Rβ1 deficiency 51. Unlike most other MSMDs, a subset of patients with either of these two PIDs also carry susceptibility to chronic mucocutaneous candidiasis (CMC), thought to originate from compromised signaling via the IL-23/IL-17 pathway that is important for T H17 cell development and mucosal immunity 52. As both IL-12 and IL-23 signaling are impaired by these disorders, the individual contribution of each of these cytokines in the immune response to mycobacteria and mucosal candidiasis had remained unclear.
Through the identification of AR LOF mutations in either IL12RB2 or IL23R affecting separate kindreds with MSMD without CMC, Martínez-Barricarte et al. underscored the importance of both IL-12 and IL-23 signaling pathways and their effects on IFN-γ mediated immunity 53 ( Table 1). Compared to healthy controls, patients deficient in IL-12Rβ2 demonstrated fewer memory T H1 cells and impaired T H1 differentiation in vitro, while IL-23R-deficient patient T cells showed impaired T H17 differentiation in vitro. The authors further investigated the impact of IL-12 and IL-23 on IFN-γ cellular responses by isolating several lymphoid cell types (i.e. B, CD4 + T, CD8 + T, γδ + T, and natural killer [NK] cells, type-1 innate lymphocyte cells [ILC1s], type-2 ILCs [ILC2s], type-3 ILCs [ILC3s], NKT cells, and mucosal-associated invariant T [MAIT] cells) from healthy donors. In response to IL-12 stimulation, B, T, γδ +, and NK cells and ILC1s and ILC2s produced IFN-γ, whereas only NKT and MAIT cells preferentially produced IFN-γ in response to IL-23. Interestingly, ILC3s produced IFN-γ upon either IL-12 or IL-23 stimulation. Taken together, these recent findings demonstrate the cooperative effect of these cytokines among a panoply of cell types to promote IFN-γ-mediated protection from mycobacterial infection.
Like IL-12Rβ1 deficiency, AR TYK2 deficiency demonstrates impaired IFN-γ-mediated immunity through defective IL-12 and IL-23 signaling, resulting in intracellular bacterial (and/or viral) infections 54, 55 ( Figure 1). Recently, a common TYK2 mutation, P1104A, with a homozygous allele frequency of ~1/600 Europeans, has been identified 56 ( Table 1). Unlike complete TYK2 deficiency, TYK2 P1104A exclusively impairs IL-23 responses due to compromised catalytic activity, thereby disrupting IFN-γ production and conferring risk of mycobacterial infection 56. In individuals of European ancestry, TYK2 P1104A may underlie a genetic basis for ~1% of tuberculosis cases; these findings indicate that inherited risk alleles may underpin infection susceptibility at the population level 57.
RORC in the control of mycobacteria and Candida
RORC encodes the transcription factors RORγ and RORγt. RORγ is ubiquitously expressed, whereas the RORγt isoform is restricted to lymphocytes where it regulates IL-17-related gene transcription ( Figure 1) and T H17 T cell development 58, 59. Recently, biallelic mutations in RORC affecting seven individuals from three unrelated kindreds with CMC and atypical mycobacterial infections have been described 60. Patient cells demonstrated impaired IL-17A, IL-17F, and IL-22 responses, likely accounting for mucosal susceptibility to Candida albicans. In the evaluation of the etiology of mycobacterial vulnerability, PBMCs exposed to BCG and IL-12 demonstrated reduced IFN-γ secretion as a result of selectively impaired γδ and CCR6 +CXCR3 +CD4+ αβ T cell subsets. These findings show that RORC plays an integral role in both IL-17 and type II IFN pathways.
JAK1: a complex immunodeficiency
The JAK–STAT pathway is central to many signaling cascades. Of the four JAKs, JAK1 is involved in multiple signaling pathways including IL-2, IL-4, IL-7, IL-9, IL-15, IL-21, and IL-27 as well as the IL-6 and IL-10 families of cytokines 61. As a result of JAK1’s widespread function, its deletion has been shown to be lethal in mice 62. To date, only a single patient with partial JAK1 deficiency has been reported ( Table 1), resulting in a combined immunodeficiency complicated by atypical mycobacterial osteomyelitis, sinopulmonary and skin infections, flat warts, and severe crusted scabies 63. Beyond infectious complications, the patient demonstrated developmental delay and an early death as a result of metastatic urothelial cancer at age 23. Functional studies demonstrated impaired JAK–STAT phosphorylation leading to compromised responses to multiple cytokines including IL-2 and type I and type II IFNs. These broad signaling defects are likely responsible for the T cell lymphopenia and viral and mycobacterial susceptibility, respectively.
Recent insights gained from primary immunodeficiency disorders manifesting with viral infection susceptibility
Novel primary immunodeficiency disorders of defective type I and III interferon signaling
Patients with germline defects in antiviral immunity have been shown to suffer from disseminated infection when exposed to live viral vaccine strains 64. Deficiencies in STAT1 65, STAT2 66, 67, or IFNAR2 68 have been reported to predispose to severe infection from the live-attenuated measles, mumps, and rubella (MMR) vaccination strain. Shared among these disorders are important elements in the host antiviral response as mediated through type I (and type III) IFNs. Type I IFN signaling occurs via binding of the IFNAR, composed of two subunits, IFNAR1 and IFNAR2. IFNAR then activates JAK1 and TYK2, which phosphorylate STAT1 and STAT2. The resulting STAT1/STAT2 heterodimers translocate to the nucleus, where they assemble with IRF9 to form the IFN-stimulated gene factor 3 (ISGF3) complex, leading to the downstream transcription of IFN-stimulated genes (ISGs) responsible for antiviral host defense 69.
In a recent report, Hernandez et al. describe two patients with AR splice defects in IFNAR1 who experienced severe infectious complications following vaccination with MMR and yellow fever vaccine strains, respectively 70. Patient cells demonstrated lack of IFNAR1 expression, unresponsiveness to type I IFN stimulation ( Table 1), and inability to control viral replication of measles and yellow fever viruses in vitro.
Novel advances in the understanding of IFN signaling have also been illuminated by the study of three patients with AR LOF IRF9 mutations. A 5-year-old girl manifested severe influenza A virus (IAV) pneumonitis 71, and a 10-year-old boy suffered from recurrent viral infections resulting in neurological impairment and bronchiectasis. A third patient, the 6-month-old sister of the 10-year-old boy, was healthy at the time of the report, potentially suggestive of incomplete penetrance 72 ( Table 1). Patient cells demonstrated normal STAT1 and STAT2 phosphorylation but were unable to form the necessary ISGF3 complex, resulting in impaired induction of ISGs and failure to control viral replication in vitro.
Additional genetic evidence of influenza virus susceptibility comes from the recent description of three children with autosomal-dominant (AD) Toll-like receptor-3 (TLR3) deficiency complicated by IAV-induced acute respiratory distress syndrome 73. TLR3 acts as an intracellular sensor of double-stranded DNA (dsDNA), and its activation stimulates type I and III IFN production 74 ( Table 1). Dominant-negative TLR3 mutations were initially recognized to cause defective intrinsic immunity of the central nervous system (CNS), impairing host IFN response to herpes simplex virus-1 (HSV-1) and underlying HSV encephalitis (HSE) in otherwise healthy children 75. As previously described for dominant-negative TLR3 deficiency 75, leukocytes from the patients with AD TLR3 deficiency demonstrated normal type I/III IFN activation. However, like patients with IRF9 71 or IRF7 deficiency 76, fibroblasts and induced pluripotent stem cell-derived pulmonary epithelial cells (iPSC-PECs) failed to control IAV replication. Importantly, the phenotype could be rescued in iPSC-PECs when treated with IFN-α or IFN-λ1, highlighting the importance of intrinsic type I and III IFN immunity for viral control in the lung 73.
In addition to influenza, enteroviruses such as human rhinovirus (HRV) are major causes of respiratory viral infections, particularly in young children and immunocompromised adults 77. In the induction of protection against these viruses, MDA5 senses long fragments of cytosolic dsDNA, leading to the production of type I IFNs and pro-inflammatory IL-1 family of cytokines 78, 79. Several LOF mutations in IFH1, encoding MDA5, have been reported in patients suffering from severe respiratory viral infections 80– 82 ( Table 1). These mutations impaired downstream IFN signaling and led to defective control of HRV replication in vitro 80, 81.
Taken together, these data underscore the complex interplay found in host defense against a variety of viral pathogens and the crucial role that type I and III IFN signaling plays in the antiviral response.
Novel defects of hyperactive immunity leading to viral infection-related morbidity
Recurrent respiratory papillomatosis (RRP) is a rare disease resulting in recurrent benign papillomas of the respiratory mucosa in otherwise healthy children and is typically caused by chronic infection with human papilloma virus (HPV) serotypes 6 or 11 83. Thus far, an immunologic etiology for this disease has remained unknown. Drutman et al. reported two brothers with juvenile-onset RRP who harbor a private homozygous NLRP1 mutation resulting in a GOF as demonstrated by increased IL-1β secretion and spontaneous inflammasome activation 84 ( Table 1). Interestingly, HPV6 or HPV11 was not detected in either patient, presumably because of an exaggerated antiviral response from inflammasome activation and inflammatory cytokine secretion. The cause of papilloma formation remains to be elucidated but may be secondary to the overproduction of inflammatory IL-1 family cytokines resulting in increased keratinocyte growth factor expression-mediated proliferation and papilloma formation 85, 86.
Less than one percent of children with hepatitis A viral (HAV) infection develop acute liver failure (ALF), and although liver transplantation has significantly improved outcomes, mortality remains greater than 10% 87– 89. HAV replication alone does not result in hepatocellular injury; instead, non-HAV-specific “bystander-activated” CD8 + T cells have been implicated in hepatocyte cytotoxicity 90. The mechanisms remain unclear, but evidence suggests that co-stimulation by type I IFNs and IL-18 results in potent T cell activation and IFN-γ secretion 91, 92. IL-18BP is important in the negative regulation of IFN-γ-mediated immune responses by binding IL-18 and preventing interaction with the IL-18 receptor. Belkaya et al. reported a child who succumbed to HAV-induced ALF as a result of AR IL-18BP deficiency, demonstrating that the mutant IL-18BP failed to block IL-18 activity 93 ( Table 1). Additionally, they provided important in vitro evidence that IL-18 mediates hepatotoxic effects via NK cell activation and cytotoxicity and that treatment with IL-18BP rescues the phenotype, indicating that recombinant IL-18BP may have therapeutic value in patients with ALF 93.
These reports emphasize the concept that unrestrained immune activation following viral infection may lead to serious clinical ramifications in patients with defects in immunoregulation or persistent inflammatory mediator production.
Recent insights gained from PIDs manifesting with fungal infection susceptibility
The mechanism of CARD9-dependent protection against central nervous system fungal invasion
CARD9 deficiency has been known to result in fungal-specific infection susceptibility with a predilection for mucocutaneous tissues and the CNS 94, 95. The CNS-targeted susceptibility to Candida infection was recently elucidated. Specifically, CARD9 deficiency results in impaired recruitment of neutrophils into the Candida-infected CNS in mice and humans. The resultant CNS neutropenia, which is detrimental for control of fungal CNS invasion, is not due to peripheral neutropenia or impaired neutrophil-intrinsic chemotaxis but instead is caused by defective induction of critical pro-inflammatory molecules in the fungal-infected CNS that abrogate neutrophil trafficking from the blood into the CNS 96. A recent follow-up study by Drummond et al. shed further light onto the cellular and molecular basis of the CNS neutrophil trafficking defect of CARD9 deficiency 97. Specifically, it was shown that, in response to the fungal peptide toxin Candidalysin, CARD9 is critical in CNS-resident microglia for the production of IL-1β via both transcriptional regulation and inflammasome activation, which in turn activates the production of neutrophil-recruiting CXCL1 by CNS-resident microglia and astrocytes to promote fungal control in the CNS. Taken together, two-step tissue-specific “hits” are operational in CARD9 deficiency; impaired IL-17 responses at the mucosal level allow for Candida translocation into the blood and from there to the CNS, where impaired IL-1β/CXCL1-dependent crosstalk between resident microglia and recruited neutrophils results in CNS fungal persistence.
Fungal mucosal immunity and connective tissue disorders
CMC is manifested by superficial infections of the skin, nails, and mucous membranes caused by Candida species. As IL-17 is essential for antifungal mucosal immunity, inherited disorders underlying vulnerability to CMC are associated with impaired IL-17 signaling. Indeed, mutations in genes of the IL-17 signaling pathway, namely IL-17RA 98, IL-17RC 99, IL-17F 98, and TRAF3IP2 100, have been described in humans. Patients with AR AIRE deficiency resulting in autoimmune polyendocrinopathy-candidiasis-ectodermal dystrophy (APECED) 101, 102 carry neutralizing autoantibodies against IL-17F and IL-22 103, 104. Other genetic disorders that lead to impaired T H17 development include dominant-negative STAT3 mutations underlying Job’s syndrome 105, DOCK8 deficiency 106, the recently described deficiency in ZNF341 ( Table 1), a transcription factor that binds to the STAT3 promoter and results in defective STAT3 transcription and activity 107, 108, RORC deficiency 60, and STAT1 GOF 109, 110.
In a recent report, Li et al. described a three-generational family with AD CMC and an atypical connective tissue disorder (CTD) due to mitogen-activated protein kinase-8 (MAPK8) haploinsufficiency 111. MAPK8 encodes JNK1, a central component in the MAPK signaling pathway important in the transduction of cellular responses within many contexts 112, including transforming growth factor beta (TGF-β) signaling 113 and the IL-17A response pathway downstream of ACT1 100. Evaluation of patient cells demonstrated reduced activation following IL-17A/F cytokine stimulation, impaired T H17 differentiation, and reduced proportion of T H17 cells in vitro 111. Moreover, patient fibroblasts showed impaired JNK1-mediated phosphorylation of activator protein-1 (AP-1) following TGF-β stimulation. These findings suggest that JNK1 haploinsufficiency results in combined impairment of IL-17 and TGF-β cellular responses, resulting in CMC and CTD.
Conclusion
Research of PIDs provides an exciting opportunity to advance our understanding of human health. As technology continues to rapidly advance, so too does our sophistication in detecting subtle and once-unrecognized defects in the human immune response. As illustrated in the reviewed works above, certain defects may lend to a narrow range of infectious susceptibly often as a result of redundant host defense mechanisms selected throughout our evolution. Furthermore, with the increased access and availability to molecular sequencing platforms, the number of novel and interesting variants is expected to increase at an exponential rate. Evaluating these coding, non-coding, and epigenetic variants will further our understanding of the function and regulation of the human immune system. Deciphering the intricate interplay of hematopoietic and non-hematopoietic cells, organ-specific immunity, and environmental factors that influence infection susceptibility should help inform improved therapeutic and vaccination strategies in vulnerable patients.
Editorial Note on the Review Process
F1000 Faculty Reviews are commissioned from members of the prestigious F1000 Faculty and are edited as a service to readers. In order to make these reviews as comprehensive and accessible as possible, the referees provide input before publication and only the final, revised version is published. The referees who approved the final version are listed with their names and affiliations but without their reports on earlier versions (any comments will already have been addressed in the published version).
The referees who approved this article are:
David M. Lowe, Institute of Immunity and Transplantation, University College London, London, UK
James Verbsky, Division of Rheumatology, Department of Pediatrics, Medical College of Wisconsin, Milwaukee, WI, USA
Funding Statement
This work was supported by the Division of Intramural Research at National Institute of Allergy and Infectious Diseases (NIAID), National Institutes of Health (NIH). The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government.
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
[version 1; peer review: 2 approved]
References
- 1. Rose-John S, Winthrop K, Calabrese L: The role of IL-6 in host defence against infections: immunobiology and clinical implications. Nat Rev Rheumatol. 2017;13(7):399–409. 10.1038/nrrheum.2017.83 [DOI] [PubMed] [Google Scholar]
- 2. Korn T, Bettelli E, Oukka M, et al. : IL-17 and Th17 Cells. Annu Rev Immunol. 2009;27:485–517. 10.1146/annurev.immunol.021908.132710 [DOI] [PubMed] [Google Scholar]
- 3. Hunter CA, Jones SA: IL-6 as a keystone cytokine in health and disease. Nat Immunol. 2015;16(5):448–57. 10.1038/ni.3153 [DOI] [PubMed] [Google Scholar]
- 4. Boulanger MJ, Chow D, Brevnova EE, et al. : Hexameric structure and assembly of the interleukin-6/IL-6 alpha-receptor/gp130 complex. Science. 2003;300(5628):2101–4. 10.1126/science.1083901 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 5. Taga T, Kishimoto T: Gp130 and the interleukin-6 family of cytokines. Annu Rev Immunol. 1997;15:797–819. 10.1146/annurev.immunol.15.1.797 [DOI] [PubMed] [Google Scholar]
- 6. O’Shea JJ, Plenge R: JAK and STAT signaling molecules in immunoregulation and immune-mediated disease. Immunity. 2012;36(4):542–50. 10.1016/j.immuni.2012.03.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Nguyen MT, Pødenphant J, Ravn P: Three cases of severely disseminated Staphylococcus aureus infection in patients treated with tocilizumab. BMJ Case Rep. 2013;2013: pii: bcr2012007413. 10.1136/bcr-2012-007413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Puel A, Casanova JL: The nature of human IL-6. J Exp Med. 2019;216(9):1969–71. 10.1084/jem.20191002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bloomfield M, Parackova Z, Cabelova T, et al. : Anti-IL6 Autoantibodies in an Infant With CRP-Less Septic Shock. Front Immunol. 2019;10: 2629. 10.3389/fimmu.2019.02629 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 10. Schwerd T, Twigg SRF, Aschenbrenner D, et al. : A biallelic mutation in IL6ST encoding the GP130 co-receptor causes immunodeficiency and craniosynostosis. J Exp Med. 2017;214(9):2547–62. 10.1084/jem.20161810 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 11. Holland SM, DeLeo FR, Elloumi HZ, et al. : STAT3 mutations in the hyper-IgE syndrome. N Engl J Med. 2007;357(16):1608–19. 10.1056/NEJMoa073687 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 12. Minegishi Y, Saito M, Tsuchiya S, et al. : Dominant-negative mutations in the DNA-binding domain of STAT3 cause hyper-IgE syndrome. Nature. 2007;448(7157):1058–62. 10.1038/nature06096 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 13. Spencer S, Köstel Bal S, Egner W, et al. : Loss of the interleukin-6 receptor causes immunodeficiency, atopy, and abnormal inflammatory responses. J Exp Med. 2019;216(9):1986–98. 10.1084/jem.20190344 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 14. Nahum A, Sharfe N, Broides A, et al. : Defining the biological responses of IL-6 by the study of a novel IL-6 receptor chain immunodeficiency. J Allergy Clin Immunol. 2020;145(3):1011–1015.e6. 10.1016/j.jaci.2019.11.015 [DOI] [PubMed] [Google Scholar]
- 15. Simons JP, Loeffler JM, Al-Shawi R, et al. : C-reactive protein is essential for innate resistance to pneumococcal infection. Immunology. 2014;142(3):414–20. 10.1111/imm.12266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Casey R, Newcombe J, McFadden J, et al. : The acute-phase reactant C-reactive protein binds to phosphorylcholine-expressing Neisseria meningitidis and increases uptake by human phagocytes. Infect Immun. 2008;76(3):1298–304. 10.1128/IAI.00741-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Påhlman LI, Mörgelin M, Kasetty G, et al. : Antimicrobial activity of fibrinogen and fibrinogen-derived peptides--a novel link between coagulation and innate immunity. Thromb Haemost. 2013;109(5):930–9. 10.1160/TH12-10-0739 [DOI] [PubMed] [Google Scholar]
- 18. Tanaka T, Narazaki M, Kishimoto T: Interleukin (IL-6) Immunotherapy. Cold Spring Harb Perspect Biol. 2018;10(8): pii: a028456. 10.1101/cshperspect.a028456 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 19. Whipple GH: A hitherto undescribed disease characterized anatomically by deposits of fat and fatty acids in the intestinal and mesenteric lymphatic tissues. Bull Johns Hopkins Hosp. 1907;18(198):382–391. Reference Source [Google Scholar]
- 20. Fenollar F, Puéchal X, Raoult D: Whipple’s disease. N Engl J Med. 2007;356(1):55–66. 10.1056/NEJMra062477 [DOI] [PubMed] [Google Scholar]
- 21. Antunes C, Gossman WG: Whipple Disease.In: StatPearls. Treasure Island (FL): StatPearls Publishing;2017; 2872296 [Google Scholar]
- 22. Fenollar F, Trani M, Davoust B, et al. : Prevalence of asymptomatic Tropheryma whipplei carriage among humans and nonhuman primates. J Infect Dis. 2008;197(6):880–7. 10.1086/528693 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 23. Marth T, Moos V, Müller C, et al. : Tropheryma whipplei infection and Whipple’s disease. Lancet Infect Dis. 2016;16(3):e13–22. 10.1016/S1473-3099(15)00537-X [DOI] [PubMed] [Google Scholar]
- 24. Marth T, Neurath M, Cuccherini BA, et al. : Defects of monocyte interleukin 12 production and humoral immunity in Whipple’s disease. Gastroenterology. 1997;113(2):442–8. 10.1053/gast.1997.v113.pm9247462 [DOI] [PubMed] [Google Scholar]
- 25. Kalt A, Schneider T, Ring S, et al. : Decreased levels of interleukin-12p40 in the serum of patients with Whipple’s disease. Int J Colorectal Dis. 2006;21(2):114–20. 10.1007/s00384-005-0778-6 [DOI] [PubMed] [Google Scholar]
- 26. Moos V, Schmidt C, Geelhaar A, et al. : Impaired immune functions of monocytes and macrophages in Whipple’s disease. Gastroenterology. 2010;138(1):210–20. 10.1053/j.gastro.2009.07.066 [DOI] [PubMed] [Google Scholar]
- 27. Schinnerling K, Moos V, Geelhaar A, et al. : Regulatory T cells in patients with Whipple’s disease. J Immunol. 2011;187(8):4061–7. 10.4049/jimmunol.1101349 [DOI] [PubMed] [Google Scholar]
- 28. Guérin A, Kerner G, Marr N, et al. : IRF4 haploinsufficiency in a family with Whipple’s disease. eLife. 2018;7: pii: e32340. 10.7554/eLife.32340 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 29. Nam S, Lim JS: Essential role of interferon regulatory factor 4 (IRF4) in immune cell development. Arch Pharm Res. 2016;39(11):1548–55. 10.1007/s12272-016-0854-1 [DOI] [PubMed] [Google Scholar]
- 30. Biswas PS, Bhagat G, Pernis AB: IRF4 and its regulators: evolving insights into the pathogenesis of inflammatory arthritis? Immunol Rev. 2010;233(1):79–96. 10.1111/j.0105-2896.2009.00864.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Segal BH, Leto TL, Gallin JI, et al. : Genetic, biochemical, and clinical features of chronic granulomatous disease. Medicine (Baltimore). 2000;79(3):170–200. 10.1097/00005792-200005000-00004 [DOI] [PubMed] [Google Scholar]
- 32. Panday A, Sahoo MK, Osorio D, et al. : NADPH oxidases: an overview from structure to innate immunity-associated pathologies. Cell Mol Immunol. 2015;12(1):5–23. 10.1038/cmi.2014.89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. van de Geer A, Nieto-Patlán A, Kuhns DB, et al. : Inherited p40phox deficiency differs from classic chronic granulomatous disease. J Clin Invest. 2018;128(9):3957–3975. 10.1172/JCI97116 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 34. Thomas DC, Clare S, Sowerby JM, et al. : Eros is a novel transmembrane protein that controls the phagocyte respiratory burst and is essential for innate immunity. J Exp Med. 2017;214(4):1111–28. 10.1084/jem.20161382 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 35. Arnadottir GA, Norddahl GL, Gudmundsdottir S, et al. : A homozygous loss-of-function mutation leading to CYBC1 deficiency causes chronic granulomatous disease. Nat Commun. 2018;9(1):4447. 10.1038/s41467-018-06964-x [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 36. Thomas DC, Charbonnier LM, Schejtman A, et al. : EROS/CYBC1 mutations: Decreased NADPH oxidase function and chronic granulomatous disease. J Allergy Clin Immunol. 2019;143(2):782–785.e1. 10.1016/j.jaci.2018.09.019 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 37. Abers A, Lionakis M: Chronic mucocutaneous candidiasis and invasive fungal infection susceptibility.In: Sullivan, K.E, Stiehm, E.R Stiehm's Immune Deficiencies-Inborn Errors of Immunity.2nd ed. Elsevier Science & Technology.2020;1–44. Reference Source [Google Scholar]
- 38. Yang FC, Atkinson SJ, Gu Y, et al. : Rac and Cdc42 GTPases control hematopoietic stem cell shape, adhesion, migration, and mobilization. Proc Natl Acad Sci USA. 2001;98(10):5614–8. 10.1073/pnas.101546898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Knaus UG, Heyworth PG, Evans T, et al. : Regulation of phagocyte oxygen radical production by the GTP-binding protein Rac 2. Science. 1991;254(5037):1512–5. 10.1126/science.1660188 [DOI] [PubMed] [Google Scholar]
- 40. Ambruso DR, Knall C, Abell AN, et al. : Human neutrophil immunodeficiency syndrome is associated with an inhibitory Rac2 mutation. Proc Natl Acad Sci USA. 2000;97(9):4654–9. 10.1073/pnas.080074897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Accetta D, Syverson G, Bonacci B, et al. : Human phagocyte defect caused by a Rac2 mutation detected by means of neonatal screening for T-cell lymphopenia. J Allergy Clin Immunol. 2011;127(2):535–538.e1–2. 10.1016/j.jaci.2010.10.013 [DOI] [PubMed] [Google Scholar]
- 42. Alkhairy OK, Rezaei N, Graham RR, et al. : RAC2 loss-of-function mutation in 2 siblings with characteristics of common variable immunodeficiency. J Allergy Clin Immunol. 2015;135(5):1380–4.e1–5. 10.1016/j.jaci.2014.10.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Lougaris V, Chou J, Beano A, et al. : A monoallelic activating mutation in RAC2 resulting in a combined immunodeficiency. J Allergy Clin Immunol. 2019;143(4):1649–1653.e3. 10.1016/j.jaci.2019.01.001 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 44. Hsu AP, Donkó A, Arrington ME, et al. : Dominant activating RAC2 mutation with lymphopenia, immunodeficiency, and cytoskeletal defects. Blood. 2019;133(18):1 977–88. 10.1182/blood-2018-11-886028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. MacLennan C, Fieschi C, Lammas DA, et al. : Interleukin (IL)-12 and IL-23 are key cytokines for immunity against Salmonella in humans. J Infect Dis. 2004;190(10):1755–7. 10.1086/425021 [DOI] [PubMed] [Google Scholar]
- 46. Roesler J, Kofink B, Wendisch J, et al. : Listeria monocytogenes and recurrent mycobacterial infections in a child with complete interferon-gamma-receptor (IFNgammaR1) deficiency: mutational analysis and evaluation of therapeutic options. Exp Hematol. 1999;27(9):1368–74. 10.1016/s0301-472x(99)00077-6 [DOI] [PubMed] [Google Scholar]
- 47. Rosain J, Kong XF, Martinez-Barricarte R, et al. : Mendelian susceptibility to mycobacterial disease: 2014-2018 update. Immunol Cell Biol. 2019;97(4):360–7. 10.1111/imcb.12210 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 48. Bustamante J, Boisson-Dupuis S, Abel L, et al. : Mendelian susceptibility to mycobacterial disease: genetic, immunological, and clinical features of inborn errors of IFN-γ immunity. Semin Immunol. 2014;26(6):454–70. 10.1016/j.smim.2014.09.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Presky DH, Yang H, Minetti LJ, et al. : A functional interleukin 12 receptor complex is composed of two beta-type cytokine receptor subunits. Proc Natl Acad Sci U S A. 1996;93(24):14002–7. 10.1073/pnas.93.24.14002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Parham C, Chirica M, Timans J, et al. : A receptor for the heterodimeric cytokine IL-23 is composed of IL-12Rbeta1 and a novel cytokine receptor subunit, IL-23R. J Immunol. 2002;168(11):5699–708. 10.4049/jimmunol.168.11.5699 [DOI] [PubMed] [Google Scholar]
- 51. Picard C, Fieschi C, Altare F, et al. : Inherited interleukin-12 deficiency: IL12B genotype and clinical phenotype of 13 patients from six kindreds. Am J Hum Genet. 2002;70(2):336–48. 10.1086/338625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Ouederni M, Sanal O, Ikinciogullari A, et al. : Clinical features of Candidiasis in patients with inherited interleukin 12 receptor β1 deficiency. Clin Infect Dis. 2014;58(2):204–13. 10.1093/cid/cit722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Martínez-Barricarte R, Markle JG, Ma CS, et al. : Human IFN-γ immunity to mycobacteria is governed by both IL-12 and IL-23. Sci Immunol. 2018;3(30): pii: eaau6759. 10.1126/sciimmunol.aau6759 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 54. Minegishi Y, Saito M, Morio T, et al. : Human tyrosine kinase 2 deficiency reveals its requisite roles in multiple cytokine signals involved in innate and acquired immunity. Immunity. 2006;25(5):745–55. 10.1016/j.immuni.2006.09.009 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 55. Kreins AY, Ciancanelli MJ, Okada S, et al. : Human TYK2 deficiency: Mycobacterial and viral infections without hyper-IgE syndrome. J Exp Med. 2015;212(10):1641–62. 10.1084/jem.20140280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Boisson-Dupuis S, Ramirez-Alejo N, Li Z, et al. : Tuberculosis and impaired IL-23-dependent IFN-γ immunity in humans homozygous for a common TYK2 missense variant. Sci Immunol. 2018;3(30): pii: eaau8714. 10.1126/sciimmunol.aau8714 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 57. Kerner G, Ramirez-Alejo N, Seeleuthner Y, et al. : Homozygosity for TYK2 P1104A underlies tuberculosis in about 1% of patients in a cohort of European ancestry. Proc Natl Acad Sci U S A. 2019;116(21):10430–4. 10.1073/pnas.1903561116 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 58. Yang XO, Pappu BP, Nurieva R, et al. : T helper 17 lineage differentiation is programmed by orphan nuclear receptors ROR alpha and ROR gamma. Immunity. 2008;28(1):29–39. 10.1016/j.immuni.2007.11.016 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 59. Ivanov II, McKenzie BS, Zhou L, et al. : The orphan nuclear receptor RORgammat directs the differentiation program of proinflammatory IL-17 + T helper cells. Cell. 2006;126(6):1121–33. 10.1016/j.cell.2006.07.035 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 60. Okada S, Markle JG, Deenick EK, et al. : IMMUNODEFICIENCIES. Impairment of immunity to Candida and Mycobacterium in humans with bi-allelic RORC mutations. Science. 2015;349(6248):606–13. 10.1126/science.aaa4282 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 61. O’Shea JJ, Schwartz DM, Villarino AV, et al. : The JAK-STAT pathway: impact on human disease and therapeutic intervention. Annu Rev Med. 2015;66:311–28. 10.1146/annurev-med-051113-024537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Leonard WJ, O’Shea JJ: Jaks and STATs: biological implications. Annu Rev Immunol. 1998;16:293–322. 10.1146/annurev.immunol.16.1.293 [DOI] [PubMed] [Google Scholar]
- 63. Eletto D, Burns SO, Angulo I, et al. : Biallelic JAK1 mutations in immunodeficient patient with mycobacterial infection. Nat Commun. 2016;7:13992. 10.1038/ncomms13992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Pöyhönen L, Bustamante J, Casanova JL, et al. : Life-Threatening Infections Due to Live-Attenuated Vaccines: Early Manifestations of Inborn Errors of Immunity. J Clin Immunol. 2019;39(4):376–90. 10.1007/s10875-019-00642-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Burns C, Cheung A, Stark Z, et al. : A novel presentation of homozygous loss-of-function STAT-1 mutation in an infant with hyperinflammation-A case report and review of the literature. J Allergy Clin Immunol Pract. 2016;4(4):777–9. 10.1016/j.jaip.2016.02.015 [DOI] [PubMed] [Google Scholar]
- 66. Hambleton S, Goodbourn S, Young DF, et al. : STAT2 deficiency and susceptibility to viral illness in humans. Proc Natl Acad Sci U S A. 2013;110(8):3053–8. 10.1073/pnas.1220098110 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 67. Moens L, Van Eyck L, Jochmans D, et al. : A novel kindred with inherited STAT2 deficiency and severe viral illness. J Allergy Clin Immunol. 2017;139(6):1995–1997.e9. 10.1016/j.jaci.2016.10.033 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 68. Duncan CJ, Mohamad SM, Young DF, et al. : Human IFNAR2 deficiency: Lessons for antiviral immunity. Sci Transl Med. 2015;7(307):307ra154. 10.1126/scitranslmed.aac4227 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 69. Schreiber G, Piehler J: The molecular basis for functional plasticity in type I interferon signaling. Trends Immunol. 2015;36(3):139–49. 10.1016/j.it.2015.01.002 [DOI] [PubMed] [Google Scholar]
- 70. Hernandez N, Bucciol G, Moens L, et al. : Inherited IFNAR1 deficiency in otherwise healthy patients with adverse reaction to measles and yellow fever live vaccines. J Exp Med. 2019;216(9):2057–70. 10.1084/jem.20182295 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 71. Hernandez N, Melki I, Jing H, et al. : Life-threatening influenza pneumonitis in a child with inherited IRF9 deficiency. J Exp Med. 2018;215(10):2567–85. 10.1084/jem.20180628 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 72. Bravo García-Morato M, Calvo Apalategi A, Bravo-Gallego LY, et al. : Impaired control of multiple viral infections in a family with complete IRF9 deficiency. J Allergy Clin Immunol. 2019;144(1):309–312.e10. 10.1016/j.jaci.2019.02.019 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 73. Lim HK, Huang SXL, Chen J, et al. : Severe influenza pneumonitis in children with inherited TLR3 deficiency. J Exp Med. 2019;216(9):2038–56. 10.1084/jem.20181621 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 74. Ioannidis I, Ye F, McNally B, et al. : Toll-like receptor expression and induction of type I and type III interferons in primary airway epithelial cells. J Virol. 2013;87(6):3261–70. 10.1128/JVI.01956-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Zhang SY, Jouanguy E, Ugolini S, et al. : TLR3 deficiency in patients with herpes simplex encephalitis. Science. 2007;317(5844):1522–7. 10.1126/science.1139522 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 76. Ciancanelli MJ, Huang SX, Luthra P, et al. : Infectious disease. Life-threatening influenza and impaired interferon amplification in human IRF7 deficiency. Science. 2015;348(6233):448–53. 10.1126/science.aaa1578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Walter JM, Wunderink RG: Severe Respiratory Viral Infections: New Evidence and Changing Paradigms. Infect Dis Clin North Am. 2017;31(3):455–74. 10.1016/j.idc.2017.05.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Schoggins JW, Wilson SJ, Panis M, et al. : A diverse range of gene products are effectors of the type I interferon antiviral response. Nature. 2011;472(7344):481–5. 10.1038/nature09907 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 79. Chow KT, Gale M, Jr, Loo YM: RIG-I and Other RNA Sensors in Antiviral Immunity. Annu Rev Immunol. 2018;36:667–94. 10.1146/annurev-immunol-042617-053309 [DOI] [PubMed] [Google Scholar]
- 80. Lamborn IT, Jing H, Zhang Y, et al. : Recurrent rhinovirus infections in a child with inherited MDA5 deficiency. J Exp Med. 2017;214(7):1949–72. 10.1084/jem.20161759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Asgari S, Schlapbach LJ, Anchisi S, et al. : Severe viral respiratory infections in children with IFIH1 loss-of-function mutations. Proc Natl Acad Sci U S A. 2017;114(31):8342–7. 10.1073/pnas.1704259114 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 82. Zaki M, Thoenes M, Kawalia A, et al. : Recurrent and Prolonged Infections in a Child with a Homozygous IFIH1 Nonsense Mutation. Front Genet. 2017;8:130. 10.3389/fgene.2017.00130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Ivancic R, Iqbal H, deSilva B, et al. : Current and future management of recurrent respiratory papillomatosis. Laryngoscope Investig Otolaryngol. 2018;3(1):22–34. 10.1002/lio2.132 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 84. Drutman SB, Haerynck F, Zhong FL, et al. : Homozygous NLRP1 gain-of-function mutation in siblings with a syndromic form of recurrent respiratory papillomatosis. Proc Natl Acad Sci U S A. 2019;116(38):19055–63. 10.1073/pnas.1906184116 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 85. Maas-Szabowski N, Stark HJ, Fusenig NE: Keratinocyte growth regulation in defined organotypic cultures through IL-1-induced keratinocyte growth factor expression in resting fibroblasts. J Invest Dermatol. 2000;114(6):1075–84. 10.1046/j.1523-1747.2000.00987.x [DOI] [PubMed] [Google Scholar]
- 86. Zhong FL, Mamaï O, Sborgi L, et al. : Germline NLRP1 Mutations Cause Skin Inflammatory and Cancer Susceptibility Syndromes via Inflammasome Activation. Cell. 2016;167(1):187–202.e17. 10.1016/j.cell.2016.09.001 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 87. Moreira-Silva SF, Frauches DO, Almeida AL, et al. : Acute liver failure in children: observations in Vitória, Espírito Santo State, Brazil. Rev Soc Bras Med Trop. 2002;35(5):483–6. 10.1590/s0037-86822002000500010 [DOI] [PubMed] [Google Scholar]
- 88. Squires RH, Jr, Shneider BL, Bucuvalas J, et al. : Acute liver failure in children: the first 348 patients in the pediatric acute liver failure study group. J Pediatr. 2006;148(5):652–8. 10.1016/j.jpeds.2005.12.051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Ng VL, Li R, Loomes KM, et al. : Outcomes of Children With and Without Hepatic Encephalopathy From the Pediatric Acute Liver Failure Study Group. J Pediatr Gastroenterol Nutr. 2016;63(3):357–64. 10.1097/MPG.0000000000001178 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 90. Kim J, Chang DY, Lee HW, et al. : Innate-like Cytotoxic Function of Bystander-Activated CD8+ T Cells Is Associated with Liver Injury in Acute Hepatitis A. Immunity. 2018;48(1):161–173.e5. 10.1016/j.immuni.2017.11.025 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 91. Freeman BE, Hammarlund E, Raué HP, et al. : Regulation of innate CD8 + T-cell activation mediated by cytokines. Proc Natl Acad Sci U S A. 2012;109(25):9971–6. 10.1073/pnas.1203543109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Kim TS, Shin EC: The activation of bystander CD8 + T cells and their roles in viral infection. Exp Mol Med. 2019;51(12):1–9. 10.1038/s12276-019-0316-1 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 93. Belkaya S, Michailidis E, Korol CB, et al. : Inherited IL-18BP deficiency in human fulminant viral hepatitis. J Exp Med. 2019;216(8):1777–90. 10.1084/jem.20190669 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 94. Corvilain E, Casanova JL, Puel A: Inherited CARD9 Deficiency: Invasive Disease Caused by Ascomycete Fungi in Previously Healthy Children and Adults. J Clin Immunol. 2018;38(6):656–93. 10.1007/s10875-018-0539-2 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 95. Drummond RA, Franco LM, Lionakis MS: Human CARD9: A Critical Molecule of Fungal Immune Surveillance. Front Immunol. 2018;9:1836. 10.3389/fimmu.2018.01836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Drummond RA, Collar AL, Swamydas M, et al. : CARD9-Dependent Neutrophil Recruitment Protects against Fungal Invasion of the Central Nervous System. PLoS Pathog. 2015;11(12):e1005293. 10.1371/journal.ppat.1005293 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 97. Drummond RA, Swamydas M, Oikonomou V, et al. : CARD9 + microglia promote antifungal immunity via IL-1β- and CXCL1-mediated neutrophil recruitment. Nat Immunol. 2019;20(5):559–70. 10.1038/s41590-019-0377-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Puel A, Cypowyj S, Bustamante J, et al. : Chronic mucocutaneous candidiasis in humans with inborn errors of interleukin-17 immunity. Science. 2011;332(6025):65–8. 10.1126/science.1200439 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 99. Ling Y, Cypowyj S, Aytekin C, et al. : Inherited IL-17RC deficiency in patients with chronic mucocutaneous candidiasis. J Exp Med. 2015;212(5):619–31. 10.1084/jem.20141065 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 100. Boisson B, Wang C, Pedergnana V, et al. : An ACT1 mutation selectively abolishes interleukin-17 responses in humans with chronic mucocutaneous candidiasis. Immunity. 2013;39(4):676–86. 10.1016/j.immuni.2013.09.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Constantine GM, Lionakis MS: Lessons from primary immunodeficiencies: Autoimmune regulator and autoimmune polyendocrinopathy-candidiasis-ectodermal dystrophy. Immunol Rev. 2019;287(1):103–20. 10.1111/imr.12714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Ferre EMN, Rose SR, Rosenzweig SD, et al. : Redefined clinical features and diagnostic criteria in autoimmune polyendocrinopathy-candidiasis-ectodermal dystrophy. JCI Insight. 2016;1(13): pii: e88782. 10.1172/jci.insight.88782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Kisand K, Bøe Wolff AS, Podkrajsek KT, et al. : Chronic mucocutaneous candidiasis in APECED or thymoma patients correlates with autoimmunity to Th17-associated cytokines. J Exp Med. 2010;207(2):299–308. 10.1084/jem.20091669 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 104. Puel A, Döffinger R, Natividad A, et al. : Autoantibodies against IL-17A, IL-17F, and IL-22 in patients with chronic mucocutaneous candidiasis and autoimmune polyendocrine syndrome type I. J Exp Med. 2010;207(2):291–7. 10.1084/jem.20091983 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 105. Milner JD, Brenchley JM, Laurence A, et al. : Impaired T( H)17 cell differentiation in subjects with autosomal dominant hyper-IgE syndrome. Nature. 2008;452(7188):773–6. 10.1038/nature06764 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 106. Tangye SG, Pillay B, Randall KL, et al. : Dedicator of cytokinesis 8-deficient CD4 + T cells are biased to a T H2 effector fate at the expense of T H1 and T H17 cells. J Allergy Clin Immunol. 2017;139(3):933–49. 10.1016/j.jaci.2016.07.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Frey-Jakobs S, Hartberger JM, Fliegauf M, et al. : ZNF341 controls STAT3 expression and thereby immunocompetence. Sci Immunol. 2018;3(24): pii: eaat4941. 10.1126/sciimmunol.aat4941 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 108. Béziat V, Li J, Lin JX, et al. : A recessive form of hyper-IgE syndrome by disruption of ZNF341-dependent STAT3 transcription and activity. Sci Immunol. 2018;3(24): pii: eaat4956. 10.1126/sciimmunol.aat4956 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 109. van de Veerdonk FL, Plantinga TS, Hoischen A, et al. : STAT1 mutations in autosomal dominant chronic mucocutaneous candidiasis. N Engl J Med. 2011;365(1):54–61. 10.1056/NEJMoa1100102 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 110. Liu L, Okada S, Kong XF, et al. : Gain-of-function human STAT1 mutations impair IL-17 immunity and underlie chronic mucocutaneous candidiasis. J Exp Med. 2011;208(8):1635–48. 10.1084/jem.20110958 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 111. Li J, Ritelli M, Ma CS, et al. : Chronic mucocutaneous candidiasis and connective tissue disorder in humans with impaired JNK1-dependent responses to IL-17A/F and TGF-β. Sci Immunol. 2019;4(41): pii: eaax7965. 10.1126/sciimmunol.aax7965 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 112. Zhang W, Liu HT: MAPK signal pathways in the regulation of cell proliferation in mammalian cells. Cell Res. 2002;12(1):9–18. 10.1038/sj.cr.7290105 [DOI] [PubMed] [Google Scholar]
- 113. Zhang YE: Non-Smad Signaling Pathways of the TGF-β Family. Cold Spring Harb Perspect Biol. 2017;9(2): pii: a022129. 10.1101/cshperspect.a022129 [DOI] [PMC free article] [PubMed] [Google Scholar]