Abstract
Background
A novel coronavirus disease (COVID-19) in Wuhan has caused an outbreak and become a major public health issue in China and great concern from international community. Myocarditis and myocardial injury were suspected and may even be considered as one of the leading causes for death of COVID-19 patients. Therefore, we focused on the condition of the heart, and sought to provide firsthand evidence for whether myocarditis and myocardial injury were caused by COVID-19.
Methods
We enrolled patients with confirmed diagnosis of COVID-19 retrospectively and collected heart-related clinical data, mainly including cardiac imaging findings, laboratory results and clinical outcomes. Serial tests of cardiac markers were traced for the analysis of potential myocardial injury/myocarditis.
Results
112 COVID-19 patients were enrolled in our study. There was evidence of myocardial injury in COVID-19 patients and 14 (12.5%) patients had presented abnormalities similar to myocarditis. Most of patients had normal levels of troponin at admission, that in 42 (37.5%) patients increased during hospitalization, especially in those that died. Troponin levels were significantly increased in the week preceding the death. 15 (13.4%) patients have presented signs of pulmonary hypertension. Typical signs of myocarditis were absent on echocardiography and electrocardiogram.
Conclusions
The clinical evidence in our study suggested that myocardial injury is more likely related to systemic consequences rather than direct damage by the 2019 novel coronavirus. The elevation in cardiac markers was probably due to secondary and systemic consequences and can be considered as the warning sign for recent adverse clinical outcomes of the patients.
Keywords: COVID-19, Novel coronavirus, Myocardial injury, Cardiac marker, Myocarditis
Highlights
-
•
The evidence from clinical standpoint and front-line data in Wuhan for COVID-19.
-
•
The novel coronavirus in COVID-19 less likely caused myocardial injury directly.
-
•
Elevation in cardiac markers is the warning sign of adverse outcomes for COVID-19.
1. Introduction
A novel coronavirus in Wuhan has infected clusters in December 2019 and caused an outbreak of severe pneumonia with worldwide human-to-human transmission [[1], [2], [3]]. As of February 29, 2020, there were 79,824 confirmed cases and 2870 deaths in China [4]. It has become a major public health issue in China and great concern from the international community. The World Health Organization has officially named the novel coronavirus disease as Corona Virus Disease 2019 (COVID-19) and declared a public emergency of international concern [5]. Currently, new confirmed cases and deaths caused by COVID-19 still increased daily and it has spread all over China and over 170 countries and territories around the world.
Besides focusing on the severe condition of pneumonia, the novel coronavirus may attack many important organs and cause multiple organ failure with cytokine storm [6,7]. The heart is one of these most important organs and it is highly suspicious that viral myocarditis and myocardial injury could be also involved and may even be considered as one of the leading causes for the death of COVID-19 patients. However, currently the evidence is rare regard to viral myocarditis and myocardial injury, and the knowledge of this disease is still limited.
Thus, as doctors working at the front-line in Wuhan and providing medical service for COVID-19 patients, we sought to investigate the clinical diagnosis and treatment of COVID-19 patients, focusing on the condition of the heart, and to provide firsthand information that whether there is sufficient evidence for viral myocarditis and myocardial injury caused by COVID-19.
2. Method
2.1. Study design and participants
We performed a retrospective study at Renmin Hospital of Wuhan University, which is one of the major tertiary teaching hospitals and government authorized hospitals for COVID-19 patients. All patients were enrolled with symptom onset from January 6 to February 20, 2020 and confirmed the diagnosis of COVID-19 infections during hospitalization in Renmin Hospital of Wuhan University. Our study was approved by Ethics Committee, Renmin Hospital of Wuhan University (WDRY2020-K031) and registered with the official website of China Clinical Trial Registration Center (ChiCTR2000030017).
2.2. Diagnosis and grading of COVID-19
All patients enrolled in the study were diagnosed and graded according to the interim guidance from World Health Organization [8] and National Health Commission of China [9]. The diagnosis of COVID-19 was confirmed as positive result for nasopharyngeal swab and respiratory pathogen nucleic acid test with high-throughput sequencing or real-time reverse transcriptase polymerase chain reaction (RT-PCR) [10]. All the patients have undergone chest computed tomographic (CT) scans and confirmed as viral pneumonia with characteristic changes [11,12].
According to the signs and symptoms, imaging findings and laboratory results, COVID-19 patients can be divided into four types as mild, moderate, severe and critical types. Patients in severe type should meet one of the three criteria: respiratory distress and respiratory rate higher than 30 times per minute; fingertip blood oxygen saturation <93% at rest; partial arterial oxygen pressure (PaO2)/fraction of inspiration oxygen (FiO2) <300 mmHg. Patients with one of the three conditions are considered as critical type: respiratory failure, requiring mechanical ventilation; shock; multiple organ failure, requiring intensive care management. We divided the patients in non-severe group (mild and moderate types) and severe group (severe and critical types).
2.3. Data collection
The clinical data of patients were collected from electronic medical records, including demographics, clinical symptoms and signs, co-existing conditions, imaging findings, laboratory results, treatment and clinical outcomes.
Related clinical information was recorded and analyzed. According to American Heart Association [13], myocarditis related abnormalities define as: triple elevation in cardiac Troponin I (over 0.12 ng/mL) plus abnormalities on echocardiography and/or electrocardiogram (ECG). The abnormalities on echocardiography define as reduced left ventricular ejection fraction (LVEF) (<50%), or segmental wall motion abnormality, or left ventricular wall thickening (>10 mm) and/or presence of pericardial effusion (≥5 mm); the abnormalities on ECG define as ST segment elevation/ST-T changes.
According to the guidelines of American Society of Echocardiography [14] and European Society of Cardiology [15], signs of pulmonary hypertension defines as peak tricuspid regurgitation velocity >2.8 m/s with echocardiographic signs including the changes of ventricles as right ventricle/left ventricle basal diameter ratio >1.0; or pulmonary changes as pulmonary acceleration time <105 msec and/or mid-systolic notching; or signs of the increase of right atrial pressure as inferior cava diameter >21 mm with decreased inspiratory collapse (<50% with a sniff or <20% with quiet inspiration). At least two different echocardiographic signs above should be present to determine higher probability of pulmonary hypertension.
Based on clinical demand, echocardiography, ECG and cardiac markers test and other laboratory tests may be performed at least one time or several times for each patient during hospitalization. The imaging findings and the laboratory results were recorded for the reflection of COVID-19 patients' condition during treatment and clinical management. In addition, the results of series cardiac marker and N-terminal pro brain natriuretic peptide (NT-pro BNP) tests were traced for the analysis of potential myocardial injury, myocarditis and cardiac dysfunction.
The composite endpoint of our study was the admission to intensive care unit (ICU), or mechanical ventilation, or extracorporeal membrane oxygenation (ECMO), or death. Clinical outcomes of the patients were followed up to March 11, 2020. Patients with one of the following situations should be considered as responding to treatment in hospital: continuously decreased temperature; improved respiratory symptoms; CT shows gradual absorption of pulmonary inflammation; high-throughput sequencing or RT-PCR result for respiratory pathogen nucleic acid test becomes negative.
2.4. Statistical analysis
The statistical analysis was performed with SPSS version 22.0 (IBM, Illinois, USA). Binary and ranked variables were described as counts and percentages. Continuous variables were expressed as the means and standard deviations or median, and interquartile range (IQR) values as appropriate. Continuous parameters were compared by independent-samples t-test when the data were normally distributed or otherwise the Mann-Whitney test, while binary and ranked data were analyzed by Wilcoxon rank sum test. Cox proportional hazard ratio analysis of cardiac imaging findings and laboratory results was performed for the risk prediction of the death of COVID-19 patients adjusted with demographics, clinical symptoms and signs and co-existing conditions. Statistical significance for all analysis were defined as two-tailed p value <0.05.
3. Results
3.1. Demographic and clinical characteristic
The demographics and baseline clinical characteristics of COVID-19 patients were summarized in Table 1 . There were 112 hospitalized patients with confirmed diagnosis of COVID-19 in our study population. The median age was 65.0 years (IQR: 49.0–70.8; range: 24.0–92.0 years), and 57 (50.9%) were men.
Table 1.
Clinical characteristics of patients with COVID-19.
| Clinical characteristics | All patients (n = 112) |
Disease severity |
Composite endpoint |
||||
|---|---|---|---|---|---|---|---|
| Non-severe (n = 45) |
Severe (n = 67) |
p Value | Yes (n = 31) |
No (n = 81) |
p Value | ||
| Age, years | 65.0(49.0–70.8) | 56.0(39.0–67.0) | 68.0(57.0–77.0) | <0.01 | 63.0(50.0–70.0) | 66.0(48.5–71.0) | 0.94 |
| Male sex, n (%) | 57(50.9%) | 19(42.2%) | 38(56.7%) | 0.13 | 19(61.3%) | 38(46.9%) | 0.17 |
| Signs and symptoms | |||||||
| Fever, n (%) | 98(87.5%) | 36(80.0%) | 62(92.5%) | 0.05 | 31(100.0%) | 67(82.7%) | 0.01 |
| Temperature on admission, °C | 36.9 ± 0.8 | 36.5 ± 0.6 | 37.1 ± 0.9 | <0.01 | 37.1 ± 0.9 | 36.8 ± 0.7 | 0.06 |
| Highest Temperature, °C | 38.3 ± 1.0 | 38.0 ± 1.0 | 38.5 ± 0.9 | 0.01 | 38.9 ± 0.8 | 38.1 ± 0.9 | <0.01 |
| Cough, n (%) | 79(70.5%) | 32(71.1%) | 47(70.1%) | 0.91 | 24(77.4%) | 55(67.9%) | 0.32 |
| Shortness of breath, n (%) | 63(56.3%) | 13(28.9%) | 50(74.6%) | <0.01 | 30(96.8%) | 33(40.7%) | <0.01 |
| Chest pain/tightness, n (%) | 73(65.2%) | 14(31.1%) | 59(88.1%) | <0.01 | 31(100.0%) | 42(51.9%) | <0.01 |
| Respiratory rates, bpm | 25.5 ± 8.2 | 20.2 ± 2.3 | 29.1 ± 8.8 | <0.01 | 33.8 ± 9.1 | 22.3 ± 5.1 | <0.01 |
| Systolic blood pressure, mmHg | 130.0 ± 26.7 | 131.0 ± 18.3 | 129.3 ± 31.3 | 0.76 | 117.7 ± 32.6 | 134.7 ± 22.6 | 0.01 |
| Diastolic blood pressure, mmHg | 74.3 ± 14.8 | 78.6 ± 10.5 | 71.5 ± 16.6 | 0.01 | 68.0 ± 21.1 | 76.8 ± 10.8 | 0.03 |
| Heart rates, bpm | 92.8 ± 23.6 | 91.6 ± 18.0 | 93.6 ± 26.8 | 0.66 | 98.3 ± 33.7 | 90.7 ± 18.2 | 0.24 |
| Body mass index>28 kg/m2, n (%) | 41(36.6%) | 13(28.9%) | 28(41.8%) | 0.17 | 15(48.4%) | 26(32.1%) | 0.11 |
| Blood saturation of Oxygen, % | 87.3 ± 16.4 | 97.3 ± 1.5 | 80.9 ± 16.2 | <0.01 | 73.6 ± 19.5 | 92.6 ± 11.4 | <0.01 |
| Co-existing conditions | |||||||
| Chronic obstructive pulmonary disease, n (%) | 4(3.6%) | 1(2.2%) | 3(4.5%) | 0.53 | 1(3.2%) | 3(3.7%) | 0.90 |
| Hypertension, n (%) | 36(32.1%) | 12(26.7%) | 24(35.8%) | 0.31 | 12(38.7%) | 24(29.6%) | 0.36 |
| Diabetes, n (%) | 19(17.0%) | 5(11.1%) | 14(20.9%) | 0.18 | 7(22.6%) | 12(14.8%) | 0.33 |
| Coronary heart disease, n (%) | 15(13.4%) | 4(8.9%) | 11(16.4%) | 0.25 | 6(19.4%) | 9(11.1%) | 0.25 |
| Atrial fibrillation, n (%) | 4(3.6%) | 2(4.4%) | 2(3.0%) | 0.68 | 1(3.2%) | 3(3.7%) | 0.90 |
Each value represents the median (interquartile range), mean ± SD or the number (%).
Of the 112 patients, 58 (51.8%) had one or more co-existing conditions, and hypertension (36 [32.1%]), diabetes (19 [17.0%]) and coronary heart disease (CHD) (15 [13.4%]) were the most common co-existing conditions. The most common heart-related symptoms were fever (98 [87.5%]), cough (79 [70.5%]), chest pain/tightness (73 [65.2%]) and shortness of breath (63 [56.3%]).
67 (59.8%) patients were identified in severe group. Compared with patients in non-severe group, there were more patients in severe group with significantly older age (median age, 68.0 years [IQR: 57.0–77.0] vs 56.0 years [IQR: 39.0–67.0], p < 0.01). There were 28 (41.8%) and 13 (28.9%) patients with body mass index >28Kg/m2 in severe and non-severe groups, respectively. There were no significant differences between patients in severe and non-severe groups with co-existing conditions.
3.2. Cardiac imaging findings and laboratory results
Echocardiography has presented that cardiac chamber sizes, LVEF (representing left ventricular systolic function) and tricuspid anterior plane systolic excursion (representing right ventricular systolic function) were within the normal ranges [14,16] and there were no significant differences of cardiac chamber sizes between patients in non-severe and severe groups (summarized in Table 2 ). There were 6 (5.4%) patients with LVEF <50% and no patients had LVEF <40%. The maximum depth of pericardial effusion was 6.2 ± 1.1 mm and there were more patients in severe group with this small amount of pericardial effusion (19 [28.3%] vs 3 [6.7%], p < 0.01). 15 (13.4%) patients have presented the signs of pulmonary hypertension however categorized as low probability (peak tricuspid regurgitation velocity < 2.8 m/s or no presence of at least two echocardiographic signs) [15].
Table 2.
Imaging and laboratory findings of patients with COVID-19.
| Imaging and laboratory findings | All patients (n = 112) |
Disease severity |
Composite endpoint |
||||
|---|---|---|---|---|---|---|---|
| Non-severe (n = 45) |
Severe (n = 67) |
p Value | Yes (n = 31) |
No (n = 81) |
p Value | ||
| aPossible myocarditis, n (%) | 14(12.5%) | 1(2.2%) | 13(19.4%) | <0.01 | 12(38.7%) | 2(2.5%) | <0.01 |
| Echocardiography | |||||||
| Left atrium (mm) | 33.8 ± 4.2 | 33.0 ± 4.0 | 34.3 ± 4.3 | 0.12 | 33.5 ± 3.8 | 33.9 ± 4.3 | 0.62 |
| Left ventricle (mm) | 44.4 ± 3.7 | 44.1 ± 3.3 | 44.6 ± 4.0 | 0.42 | 45.3 ± 3.5 | 44.1 ± 3.8 | 0.14 |
| Right atrium (mm) | 35.8 ± 4.5 | 35.8 ± 4.8 | 35.8 ± 4.4 | 0.91 | 35.4 ± 4.9 | 35.9 ± 4.4 | 0.57 |
| Right ventricle (mm) | 21.3 ± 2.2 | 21.3 ± 2.3 | 21.3 ± 2.2 | 0.97 | 21.4 ± 2.0 | 21.3 ± 2.3 | 0.85 |
| Wall thickness (mm) | 9.5 ± 0.9 | 9.5 ± 0.6 | 9.5 ± 1.1 | 0.69 | 9.5 ± 0.6 | 9.5 ± 1.0 | 0.78 |
| Wall thickness ≥ 10 mm, n (%) | 3(2.7%) | 1(2.2%) | 2(3.0%) | 0.81 | 0(0.0%) | 3(3.7%) | 0.28 |
| Segmental wall motion abnormality, n (%) | 5(4.5%) | 0(0.0%) | 5(7.5%) | 0.06 | 4(12.9%) | 1(1.2%) | 0.01 |
| LVEF (%) | 60.0 ± 5.6 | 62.0 ± 5.5 | 58.5 ± 5.4 | <0.01 | 57.7 ± 7.2 | 60.8 ± 4.8 | 0.01 |
| LVEF<50%, n (%) | 6(5.4%) | 1(2.2%) | 5(7.5%) | 0.25 | 5(16.1%) | 1(1.2%) | <0.01 |
| TAPSE (mm) | 20.0 ± 2.3 | 20.8 ± 2.2 | 19.4 ± 2.3 | <0.01 | 19.2 ± 2.6 | 20.3 ± 2.2 | 0.03 |
| TAPSE<16 mm, n (%) | 4(3.6%) | 0(0.0%) | 4(6.0%) | 0.09 | 4(12.9%) | 0(0.0%) | <0.01 |
| Signs of pulmonary hypertension, n (%) | 15(13.4%) | 1(2.2%) | 14(20.9%) | <0.01 | 11(35.5%) | 4(4.9%) | <0.01 |
| PE ≥ 5 mm, n (%) | 22(19.6%) | 3(6.7%) | 19(28.4%) | <0.01 | 13(41.9%) | 9(11.1%) | <0.01 |
| Depth of PE (mm) | 6.2 ± 1.1 | 6.3 ± 1.2 | 6.2 ± 1.1 | 0.80 | 6.0 ± 1.1 | 6.4 ± 1.0 | 0.34 |
| Electrocardiogram | |||||||
| Tachycardia, n (%) | 33(29.5%) | 11(24.4%) | 22(32.8%) | 0.34 | 16(51.6%) | 17(21.0%) | <0.01 |
| ST segment elevation/ST-T changes, n (%) | 22(19.6%) | 7(15.6%) | 15(22.4%) | 0.37 | 8(25.8%) | 14(17.3%) | 0.31 |
| Laboratory tests | |||||||
| Hemoglobin, g/L (130–175 g/L) |
103.0(87.0–115.0) | 112.0(100.0–128.0) | 98.0(78.0–105.0) | <0.01 | 86.0(73.8–104.0) | 105.0(93.3–116.5) | <0.01 |
| C-reactive protein level, mg/L (0–10 mg/L) |
82.7(11.9–174.7) | 15.1(5.0–83.4) | 132.6(65.2–200.0) | <0.01 | 200.0(173.0–200.0) | 45.0(5.0–98.3) | <0.01 |
| Procalcitonin level, ng/mL (<0.1 ng/mL) |
0.1(0.0–1.2) | 0.1(0.0–0.3) | 0.5(0.1–1.7) | <0.01 | 1.5(0.7–3.8) | 0.1(0.0–0.3) | <0.01 |
| D-dimer, mg/L (0–0.55 mg/L) |
4.0(1.0–19.0) | 0.7(0.4–1.7) | 11.9(3.8–52.4) | <0.01 | 48.8(17.9–79.3) | 1.5(0.7–7.0) | <0.01 |
| Lactose dehydrogenase, U/L (120–250 U/L) |
346.0(215.0–550.0) | 201.0(181.0–282.0) | 476.0(344.0–770.0) | <0.01 | 721.0(498.0–1247.5) | 255.5(200.0–427.3) | <0.01 |
| Creatinine kinase, U/L (50–310 U/L) |
28.0(18.0–94.0) | 33.0(24.5–57.5) | 25.0(17.0–191.5) | 0.41 | 29.5(12.0–424.0) | 28.0(18.0–58.3) | 0.61 |
| Creatinine kinase MB, ng/mL (0–5 ng/mL) |
1.9(0.7–3.5) | 1.1(0.6–2.1) | 2.2(1.6–6.7) | <0.01 | 3.9(2.1–10.1) | 1.3(0.7–2.3) | <0.01 |
| Cardiac troponin I, ng/mL (<0.04 ng/mL) |
0.01(0.00–0.14) | 0.00(0.00–0.01) | 0.10(0.01–0.77) | <0.01 | 0.56(0.09–2.69) | 0.00(0.00–0.03) | <0.01 |
| Cardiac troponin I > 0.04 ng/mL, n (%) | 42(37.5%) | 3(6.7%) | 39(58.2%) | <0.01 | 26(83.9%) | 16(19.8%) | <0.01 |
| Cardiac troponin I > 0.12 ng/mL, n (%) | 32(28.6%) | 1(2.2%) | 31(46.3%) | <0.01 | 23(74.2%) | 9(11.1%) | <0.01 |
| NT-pro BNP, ng/L (0–1800 ng/L) |
430.1(100.6–2859.3) | 101.9(34.0–363.8) | 1142.0(388.3–5956.5) | <0.01 | 2887.5(881.8–11,866.8) | 301.2(80.6–995.0) | <0.01 |
Each value represents the median (interquartile range), mean ± SD or the number (%). The abnormalities on echocardiography define as reduced left ventricular ejection fraction (LVEF) (<50%), or segmental wall motion abnormality, or left ventricular wall thickening (>10 mm) and/or presence of pericardial effusion (≥5 mm); the abnormalities on ECG define as ST segment elevation/ST-T changes. LVEF: left ventricular ejection fraction. TAPSE: tricuspid annular plane systolic excursion. PE: pericardial effusion. NT-pro BNP: N-terminal pro-brain natriuretic peptide.
Myocarditis related abnormalities define as: triple elevation in hypersensitive cardiac Troponin I (over 0.12 ng/mL) plus abnormalities on echocardiography and/or electrocardiogram (ECG).
Cardiac troponin I were elevated (over 0.04 ng/mL) in 42 (37.5%) patients and triple elevated (over 0.12 ng/mL) in 32 (28.6%) patients during hospitalization. Compared with non-severe group, the peak cardiac troponin I level (0.00 ng/L [IQR: 0.00–0.01] vs 0.10 ng/L [IQR: 0.01–0.77], p < 0.01) and peak NT-pro BNP level (101.9 ng/L [IQR: 34.0–363.8] vs 1142.0 ng/L [IQR: 388.3–5956.5], p < 0.01) were significantly higher in severe group. There were also significant differences of lactose dehydrogenase and creatinine kinase between non-severe and severe groups (summarized in Table 2).
According to the definition, 14 (12.5%) COVID-19 patients possibly had myocarditis. The characteristics of these patients were summarized in Table 3 and Supplementary Table 1. All the patients possibly with myocarditis had elevation in creatine kinase MB and NT-pro BNP. Of these patients, 10 (8.9%) and 2 (1.8%) had the abnormalities on echocardiography and electrocardiogram, respectively, and 2 (1.8%) had both the abnormalities. 6 patients were removed among the possible acute myocarditis because 5 patients had pre-existing cardiac disorders and 1 had an acute inferior myocardial infarction 4 days after hospitalization. For these 5 patients with pre-existing cardiac disorders, 4 patients were with history of CHD and heart failure and 3 of them presented reduced LVEF and segmental wall motion abnormality while the other one presented reduced LVEF; another patient with history of hypertrophic cardiomyopathy (HCM) has presented wall thickening. The patient with myocardial infarction after hospitalization presented reduced LVEF and segmental wall motion abnormality. Besides that, the abnormalities on echocardiography were mainly the presence of a small amount of pericardial effusion.
Table 3.
Clinical characteristics of COVID-19 patients with possible myocarditis.
| Clinical characteristics | Possible myocarditis |
||
|---|---|---|---|
| Yes (n = 14) |
No (n = 98) |
p Value | |
| Age, years (range) | 74.0(57.5–80.8) | 64.5(48.3–69.0) | 0.03 |
| Male sex, n (%) | 10(71.4%) | 47(48.0%) | 0.10 |
| Signs and symptoms | |||
| Fever, n (%) | 14(100.0%) | 84(85.7%) | 0.13 |
| Temperature on admission, °C | 36.9 ± 0.5 | 36.9 ± 0.8 | 0.78 |
| Highest temperature, °C | 38.8 ± 0.8 | 38.2 ± 1.0 | 0.03 |
| Cough, n (%) | 13(92.8%) | 66(67.3%) | 0.05 |
| Shortness of breath, n (%) | 13(92.8%) | 50(51.0%) | <0.01 |
| Chest pain/tightness, n (%) | 13(92.8%) | 60(61.2%) | 0.02 |
| Respiratory rates, bpm | 33.1 ± 9.9 | 24.3 ± 7.2 | <0.01 |
| Systolic blood pressure, mmHg | 136.9 ± 43.9 | 128.8 ± 22.8 | 0.27 |
| Diastolic blood pressure, mmHg | 72.9 ± 22.3 | 74.6 ± 13.3 | 0.68 |
| Heart rates, bpm | 94.7 ± 34.0 | 92.5 ± 21.6 | 0.73 |
| Body mass index >28 kg/m2, n (%) | 8(57.1%) | 33(33.7%) | 0.09 |
| Blood saturation of Oxygen, % | 78.3 ± 21.4 | 88.9 ± 13.0 | 0.01 |
| Co-existing conditions | |||
| Chronic obstructive pulmonary disease, n (%) | 1(7.1%) | 3(3.1%) | 0.44 |
| Hypertension, n (%) | 6(42.9%) | 30(30.6%) | 0.36 |
| Diabetes, n (%) | 4(28.6%) | 15(15.3%) | 0.22 |
| Coronary heart disease, n (%) | 3(21.4%) | 12(12.2%) | 0.35 |
| Atrial fibrillation, n (%) | 1(7.1%) | 3(3.1%) | 0.44 |
Each value represents the median (interquartile range), mean ± SD or the number (%).
3.3. Clinical outcomes
In our study, 37 (33.0%) patients recovered and discharged with median duration (from onset to discharge) of 32 days (IQR: 22.0–38.0). 31 (27.7%) patients have reached the composite endpoint. Of these patients, 26 (23.2%) were admitted to ICU; 28 (25.0%) and 3 (2.7%) required mechanical ventilation and ECMO, respectively. 14 (12.5%) patients died during hospitalization (summarized in Table 4 ) and the median time from onset to death was 23.0 days (IQR: 20.0–28.0; range: 15–39 days).
Table 4.
Cardiac findings of 14 patients died from COVID-19.
| Patient/gender | Age/time from onset to death/co-existing conditions | Cardiac markers First test vs peak within the week preceding death |
Electrocardiogram | Echocardiography | |||||
|---|---|---|---|---|---|---|---|---|---|
| CK-MB (ng/mL) |
Troponin I (ng/mL) |
NT-pro BNP (ng/L) |
|||||||
| 1/F | 62Y/15D hypertension |
3.87 | 129.39 | 0.02 | 0.90 | 1331.0 | 1039.0 | Tachycardia; left axis deviation |
LA dilation; PE: 6 mm LVEF:60%; TAPSE = 18 mm |
| 2/M | 69Y/17D hypertension |
1.35 | 6.58 | 0.02 | 4.23 | 20,109.0 | 770.3 | Left axis deviation; abnormal ST-T changes |
LA dilation; LVEF:58%; TAPSE = 20 mm |
| 3/F | 57Y/20D NA |
2.13 | 1.36 | 0.13 | 1.95 | 11,723.0 | 1461.0 | Normal sinus rhythm | LVEF:64%; TAPSE = 21 mm |
| 4/M | 92Y/39D CHD |
3.84 | 7.52 | 0.11 | 33.94 | 17,830.0 | 11,966.0 | Tachycardia; abnormal ST-T changes |
Cardiac dilatation; LVEF:40%; TAPSE = 14 mm |
| 5/M | 86Y/26D hypertension, CHD |
9.19 | 72.58 | 0.79 | 0.89 | 15,966.0 | 35,000.0 | Left axis deviation; abnormal ST-T changes | LV wall thickening; PE:7 mm; LVEF:42%; TAPSE = 16 mm |
| 6/Fa | 61Y/26D hypertension |
0.47 | 3.32 | 0.01 | 0.88 | 35,000.0 | 33,313.0 | Q wave in II, III and aVF leads | RWMA in LV inferior wall; LVEF:43%; TAPSE = 18 mm |
| 7/F | 80Y/20D CHD |
2.70 | 9.64 | 0.00 | 8.02 | 35,000.0 | 5950.0 | Abnormal ST-T changes | PE:6 mm; LVEF:55%; TAPSE = 17 mm |
| 8/M | 78Y/22D NA |
32.95 | 32.95 | 0.13 | 7.96 | 2944.0 | 338.2 | Normal sinus rhythm | LVEF:56%; TAPSE = 19 mm |
| 9/M | 81Y/22D COPD |
2.30 | 6.86 | 0.01 | 0.76 | 9001.0 | 1545.0 | Tachycardia; abnormal ST-T changes |
RA and RV enlargement; pulmonary hypertension;LVEF:55%; TAPSE = 15 mm |
| 10/M | 45Y/32D diabetes |
6.92 | 5.47 | 2.28 | 3.24 | 19,474.0 | 2271.0 | Abnormal ST segment | LVEF:58%; TAPSE = 18 mm |
| 11/M | 39Y/28D NA |
0.46 | 26.68 | 0.01 | 7.10 | 1043.0 | 169.4 | Normal sinus rhythm | LVEF:66%; TAPSE = 23 mm |
| 12/M | 82Y/19D CHD |
0.71 | 0.66 | 0.00 | 0.63 | 24,337.0 | 1496.0 | Abnormal ST-T changes | Cardiac dilatation; LVEF:45%; TAPSE = 16 mm |
| 13/F | 77Y/24D hypertension |
11.30 | 8.31 | 0.06 | 2.60 | 35,000.0 | 27,365.0 | Left axis deviation; abnormal ST-T changes | LA dilation; LVEF:56%; TAPSE = 18 mm |
| 14/M | 65Y/33D NA |
7.55 | 16.77 | 0.70 | 3.58 | 28,892.0 | 882.5 | Abnormal ST-T changes | LVEF:60%; TAPSE = 19 mm |
CHD: coronary heart disease; COPD: chronic obstructive pulmonary disease; NA: no significant abnormalities; CK-MB: creatinine kinase MB; NT-pro BNP: N-terminal pro-brain natriuretic peptide; LA: left atrium. LV: left ventricle; RA: right atrium; RV: right ventricle; LVEF: left ventricular ejection fraction; TAPSE: tricuspid annular plane systolic excursion; RWMA: regional wall motion abnormalities; PE: pericardial effusion.
This patient had an acute inferior myocardial infarction four days after hospital admission.
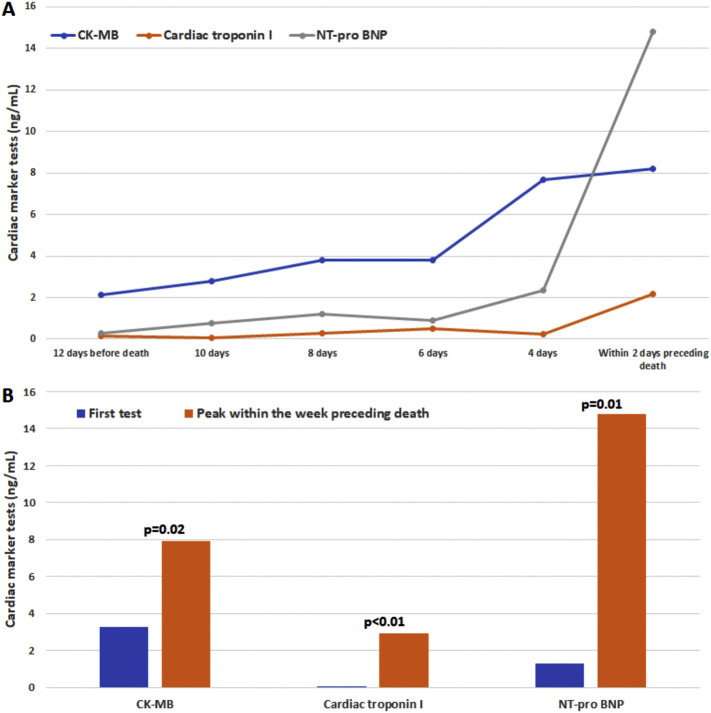
Of note, peak cardiac troponin I level (0.00 ng/L [IQR: 0.00–0.03] vs 0.56 ng/L [IQR: 0.09–2.69], p < 0.01) and peak NT-pro BNP level (301.2 ng/L [IQR: 80.6–995.0] vs 2887.5 ng/L [IQR: 881.8–11,866.8], p < 0.01) were higher for patients who have reached the composite endpoint. In addition, for the risk of death in patients with COVID-19, cox proportional hazard ratio analysis showed that peak cardiac troponin I level and peak NT-pro BNP level have presented relatively higher hazard ratios (8.9 [95%CI: 1.9–40.6, p < 0.01] and 1.2 [95%CI: 1.1–1.3, p < 0.01], respectively), while there was no statistical significance for the hazard ratio analysis of cardiac troponin I level at first test (p = 0.10). For all the patients who died during hospitalization, cardiac markers were elevated before death (Supplementary Fig. 1) and cardiac troponin I was peaked within a week preceding death with the median time of 2 days (IQR: 0.0–5.5; range: 0.0–7.0 days).
Supplementary Fig. 1.
The changes of cardiac markers over time in patients with COVID-19 and died during hospitalization.
(A) The changes over time before death. (B) The comparisons between the first test and peak within the week preceding death. CK-MB: Creatinine kinase MB; NT-pro BNP: N-terminal pro brain natriuretic peptide.
4. Discussion
With short-term follow-ups and front-line clinical data analysis, our study has revealed that there was evidence of myocardial injury in COVID-19 patients during hospitalization and 14 (12.5%) patients had presented abnormalities similar to myocarditis, especially the elevation in cardiac troponin I. We have discovered the characteristic change over time for cardiac troponin I: Most of patients had normal levels of troponin at admission, that in 42 (37.5%) patients increased during hospitalization, especially in those that died. Troponin levels were significantly increased in the week preceding the death. In the absence of typical signs on echocardiography and ECG, though the clinical evidence in our study can't exclude myocardial injury and myocarditis directly caused by the 2019 novel coronavirus, we consider that the elevation in cardiac troponin I was more likely related to systemic disorders and could be the warning sign for the death of patients with COVID-19, which should be paid more attention to in clinical practice.
Recently, the novel coronavirus in 2019 has been discovered as the seventh member of enveloped RNA coronavirus [17,18] and resembled the severe acute respiratory syndrome coronavirus (SARS-CoV) which caused an outbreak in 2003. Previous studies [[19], [20], [21]] have revealed that other types of coronavirus including SARS-CoV can cause myocardial inflammation and damage via human angiotensin converting enzyme II as receptor. Thus, it is presumed that the novel coronavirus may also lead to myocardial injury and myocarditis in patients with COVID-19. In addition, cytokine storm [6,7,22] is another speculated mechanism that could be caused by coronavirus, presenting as indiscriminate attack of overreacting immune system on many organs including the heart. As the extensively and rapidly spreading of COVID-19, possible myocardial inflammation and injury have always been highly concerned and suspected. Finding evidence that the virus directly attacks the heart has become an urgent demand currently.
However, the pathological findings at present of the first reported COVID-19 case by autopsy [23] presented that only a few interstitial inflammatory has infiltrated the heart tissue, suggesting that the novel coronavirus infection might not directly impair the heart. From the clinical standpoint, our study has also discovered that the speculation of myocarditis caused by COVID-19 lacked solid evidence. Except for the patients with pre-existing cardiac disorders and another patient with acute myocardial infarction 4 days after hospitalization, echocardiography didn't present typical signs of myocarditis such as segmental wall motion abnormality, reduced LVEF or wall thickening for the patients with COVID-19 during hospitalization. The only presentation that could speculate myocarditis was the presence of pericardial effusion in some patients, however there was only a small amount of pericardial effusion, which was not specific for myocardial injury but more inclined to systemic causes. In addition, some patients have presented the signs of pulmonary hypertension and it was more likely because of the severe conditions in lungs and acute respiratory distress syndrome. ECG manifestations were also nonspecific for the patients. ST-T changes is common and lacks specificity in older patients, especially those with co-existing cardiac conditions. Tachycardia was more common in patients reached the composite endpoint, which is more likely due to other reasons, such as severe hypoxia. For most of the time during hospitalization, the levels of cardiac troponin I and NT-pro BNP have not elevated. Even the condition of lungs became extremely severe, the changes of the heart were still not obvious for patients with COVID-19, indicating that the novel coronavirus has not presented direct aggression on the heart and it was less likely to be considered as the primary cause for myocardial injury.
Meanwhile, it is worth noting that the elevation in cardiac troponin I and NT-pro BNP peaked within one-week preceding death of the patients. Cardiac troponin I is a sensitive marker for myocardial injury [24] and NT-pro BNP is an optimal biomarker for heart failure [25]. Myocardial injury and heart failure presented before death may be attributed to other reasons, like severe hypoxia induced myocardial ischemia, management of mechanical ventilation or ECMO, multiple organ failure requiring kidney or liver replacement therapies, severe water and electrolyte unbalance or irreversible metabolic acidosis and coagulation dysfunction, which would cause severe systemic disorders in patients with COVID-19. All these conditions may have influences on the heart and cause secondary myocardial injury and heart failure. Though this was not the presence of the novel coronavirus directly attacking the heart, the elevation in cardiac markers should be considered as a warning sign, highly suggesting for recent adverse clinical outcomes of patients with COVID-19. Once the conditions of patients have become severe, such as the decline of blood oxygen saturation, the cardiac markers should be tested and monitored in order to improve clinical management and outcomes for these patients.
5. Limitation
Our study has several limitations. First, cardiac magnetic resonance and myocardial biopsy were unavailable in this clinical setting to confirm myocardial injury and myocarditis. The exclusion of potential myocardial inflammation and injury caused by COVID-19 requires more evidence from the demonstration of pathophysiological mechanisms. Second, we have followed up the patients for over two months, however 61 patients (54.5%) are still hospitalized and the median time (from onset to the end of follow-up) of these patients were 46 days (IQR: 39.5–49.0; range: 24–66 days). Thus, the relationship between the study parameters and clinical outcomes remains to be further validated. Third, echocardiography, ECG and all the laboratory tests were based on clinical demand. Differences in the timing of the examinations and tests may lead to differences in the results.
6. Conclusions
Our findings will facilitate understanding of COVID-19 and improve clinical strategies and management against the disease. From the clinical standpoint and front-line data analysis in our study, though there was evidence of myocardial injury and 12.5% COVID-19 patients had cardiac abnormalities similar to myocarditis, the characteristic changes of cardiac troponin I over time and the absence of typical signs on echocardiography and ECG have suggested that myocardial injury is more likely related to systemic consequences rather than direct damage by the 2019 novel coronavirus. The elevation in cardiac markers was probably due to secondary and systemic causes and can be considered as the warning sign for recent adverse clinical outcomes of the patients.
The following are the supplementary data related to this article.
Cardiac findings of COVID-19 patients with and without elevation in cardiac troponin I.
Grant supporting this paper
This work was supported by National Natural Science Foundation of China (Grant Numbers: 81971624 and 81901759).
CRediT authorship contribution statement
Qing Deng:Investigation, Methodology.Bo Hu:Data curation, Writing - original draft.Yao Zhang:Investigation, Formal analysis.Hao Wang:Formal analysis.Xiaoyang Zhou:Data curation.Wei Hu:Investigation.Yuting Cheng:Investigation.Jie Yan:Investigation, Data curation.Haiqin Ping:Investigation, Data curation.Qing Zhou:Methodology, Data curation.
Declaration of competing interest
The authors report no relationships that could be construed as a conflict of interest.
Footnotes
All the authors take responsibility for all aspects of the reliability and freedom from bias of the data presented and their discussed interpretation.
References
- 1.Wu J.T., Leung K., Leung G.M. 2020. Nowcasting and Forecasting the Potential Domestic and International Spread of the 2019-nCoV Outbreak Originating in Wuhan, China: A Modelling Study, Lancet. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chan J.F., Yuan S., Kok K.H., To K.K., Chu H., Yang J., Xing F., Liu J., Yip C.C., Poon R.W., Tsoi H.W., Lo S.K., Chan K.H., Poon V.K., Chan W.M., Ip J.D., Cai J.P., Cheng V.C., Chen H., Hui C.K., Yuen K.Y. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: a study of a family cluster. Lancet. 2020;395:514–523. doi: 10.1016/S0140-6736(20)30154-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li Q., Guan X., Wu P., Wang X., Zhou L., Tong Y., Ren R., Leung K.S.M., Lau E.H.Y., Wong J.Y., Xing X., Xiang N., Wu Y., Li C., Chen Q., Li D., Liu T., Zhao J., Li M., Tu W., Chen C., Jin L., Yang R., Wang Q., Zhou S., Wang R., Liu H., Luo Y., Liu Y., Shao G., Li H., Tao Z., Yang Y., Deng Z., Liu B., Ma Z., Zhang Y., Shi G., Lam T.T.Y., Wu J.T.K., Gao G.F., Cowling B.J., Yang B., Leung G.M., Feng Z. Early transmission dynamics in wuhan, china, of novel coronavirus-infected pneumonia. N. Engl. J. Med. 2020;382(13):1199–1207. doi: 10.1056/NEJMoa2001316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Update on New Coronavirus Pneumonia as at 24:00 Chinese Standard Time on February 29, 2020. National Health Commision of the People's Republic of China.
- 5.WHO main website.
- 6.Tisoncik J.R., Korth M.J., Simmons C.P., Farrar J., Martin T.R., Katze M.G. Into the eye of the cytokine storm. Microbiol. Mol. Biol. Rev. 2012;76:16–32. doi: 10.1128/MMBR.05015-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Channappanavar R., Perlman S. Pathogenic human coronavirus infections: causes and consequences of cytokine storm and immunopathology. Semin. Immunopathol. 2017;39:529–539. doi: 10.1007/s00281-017-0629-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Clinical Management of Severe Acute Respiratory Infection When Novel Coronavirus (nCoV) Infection is Suspected: Interim Guidance. World Health Organization.
- 9.Interim Guidance for Novel Coronavirus Pneumonia (Trial Implementation of Sixth Edition). National Health Commision of the People's Republic of China.
- 10.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., Zhang L., Fan G., Xu J., Gu X., Cheng Z., Yu T., Xia J., Wei Y., Wu W., Xie X., Yin W., Li H., Liu M., Xiao Y., Gao H., Guo L., Xie J., Wang G., Jiang R., Gao Z., Jin Q., Wang J., Cao B. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chung M., Bernheim A., Mei X., Zhang N., Huang M., Zeng X., Cui J., Xu W., Yang Y., Fayad Z.A., Jacobi A., Li K., Li S., Shan H. CT imaging features of 2019 novel coronavirus (2019-nCoV) Radiology. 2020;200230 doi: 10.1148/radiol.2020200230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lei J., Li J., Li X., Qi X. CT imaging of the 2019 novel coronavirus (2019-nCoV) pneumonia. Radiology. 2020;200236 doi: 10.1148/radiol.2020200236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kociol R.D., Cooper L.T., Fang J.C., Moslehi J.J., Pang P.S., Sabe M.A., Shah R.V., Sims D.B., Thiene G., Vardeny O., F. American Heart Association Heart, C Transplantation Committee of the Council on Clinical, recognition and initial management of fulminant myocarditis: a scientific statement from the American Heart Association. Circulation. 2020;141:e69–e92. doi: 10.1161/CIR.0000000000000745. [DOI] [PubMed] [Google Scholar]
- 14.Rudski L.G., Lai W.W., Afilalo J., Hua L., Handschumacher M.D., Chandrasekaran K., Solomon S.D., Louie E.K., Schiller N.B. Guidelines for the echocardiographic assessment of the right heart in adults: a report from the American Society of Echocardiography endorsed by the European Association of Echocardiography, a registered branch of the European Society of Cardiology, and the Canadian Society of Echocardiography. J. Am. Soc. Echocardiogr. 2010;23:685–713. doi: 10.1016/j.echo.2010.05.010. (quiz 786-688) [DOI] [PubMed] [Google Scholar]
- 15.Galie N., Humbert M., Vachiery J.L., Gibbs S., Lang I., Torbicki A., Simonneau G., Peacock A., Vonk Noordegraaf A., Beghetti M., Ghofrani A., Gomez Sanchez M.A., Hansmann G., Klepetko W., Lancellotti P., Matucci M., McDonagh T., Pierard L.A., Trindade P.T., Zompatori M., Hoeper M., E.S.C.S.D. Group 2015 ESC/ERS guidelines for the diagnosis and treatment of pulmonary hypertension: the joint task force for the diagnosis and treatment of pulmonary hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT) Eur. Heart J. 2016;37:67–119. doi: 10.1093/eurheartj/ehv317. [DOI] [PubMed] [Google Scholar]
- 16.Lang R.M., Badano L.P., Mor-Avi V., Afilalo J., Armstrong A., Ernande L., Flachskampf F.A., Foster E., Goldstein S.A., Kuznetsova T., Lancellotti P., Muraru D., Picard M.H., Rietzschel E.R., Rudski L., Spencer K.T., Tsang W., Voigt J.U. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J. Am. Soc. Echocardiogr. 2015;28:1–39. doi: 10.1016/j.echo.2014.10.003. (e14) [DOI] [PubMed] [Google Scholar]
- 17.Lu R., Zhao X., Li J., Niu P., Yang B., Wu H., Wang W., Song H., Huang B., Zhu N., Bi Y., Ma X., Zhan F., Wang L., Hu T., Zhou H., Hu Z., Zhou W., Zhao L., Chen J., Meng Y., Wang J., Lin Y., Yuan J., Xie Z., Ma J., Liu W.J., Wang D., Xu W., Holmes E.C., Gao G.F., Wu G., Chen W., Shi W., Tan W. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet. 2020;395(10224):565–574. doi: 10.1016/S0140-6736(20)30251-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhu N., Zhang D., Wang W., Li X., Yang B., Song J., Zhao X., Huang B., Shi W., Lu R., Niu P., Zhan F., Ma X., Wang D., Xu W., Wu G., Gao G.F., Tan W., I. China Novel Coronavirus, T. Research A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382(8):727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Oudit G.Y., Kassiri Z., Jiang C., Liu P.P., Poutanen S.M., Penninger J.M., Butany J. SARS-coronavirus modulation of myocardial ACE2 expression and inflammation in patients with SARS. Eur. J. Clin. Investig. 2009;39:618–625. doi: 10.1111/j.1365-2362.2009.02153.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Crackower M.A., Sarao R., Oudit G.Y., Yagil C., Kozieradzki I., Scanga S.E., Oliveira-dos-Santos A.J., da Costa J., Zhang L., Pei Y., Scholey J., Ferrario C.M., Manoukian A.S., Chappell M.C., Backx P.H., Yagil Y., Penninger J.M. Angiotensin-converting enzyme 2 is an essential regulator of heart function. Nature. 2002;417:822–828. doi: 10.1038/nature00786. [DOI] [PubMed] [Google Scholar]
- 21.Ge X.Y., Li J.L., Yang X.L., Chmura A.A., Zhu G., Epstein J.H., Mazet J.K., Hu B., Zhang W., Peng C., Zhang Y.J., Luo C.M., Tan B., Wang N., Zhu Y., Crameri G., Zhang S.Y., Wang L.F., Daszak P., Shi Z.L. Isolation and characterization of a bat SARS-like coronavirus that uses the ACE2 receptor. Nature. 2013;503:535–538. doi: 10.1038/nature12711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wong J.P., Viswanathan S., Wang M., Sun L.Q., Clark G.C., D’Elia R.V. Current and future developments in the treatment of virus-induced hypercytokinemia. Future Med. Chem. 2017;9:169–178. doi: 10.4155/fmc-2016-0181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xu Z., Shi L., Wang Y., Zhang J., Huang L., Zhang C., Liu S., Zhao P., Liu H., Zhu L., Tai Y., Bai C., Gao T., Song J., Xia P., Dong J., Zhao J., Wang F.S. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med. 2020;8(4):420–422. doi: 10.1016/S2213-2600(20)30076-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kimenai D.M., Martens R.J.H., Kooman J.P., Stehouwer C.D.A., Tan F.E.S., Schaper N.C., Dagnelie P.C., Schram M.T., van der Kallen C.J.H., Sep S.J.S., van Suijlen J.D.E., Kroon A.A., Bekers O., van Dieijen-Visser M.P., Henry R.M.A., Meex S.J.R. Troponin I and T in relation to cardiac injury detected with electrocardiography in a population-based cohort - the Maastricht study. Sci. Rep. 2017;7:6610. doi: 10.1038/s41598-017-06978-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rorth R., Jhund P.S., Yilmaz M.B., Kristensen S.L., Welsh P., Desai A.S., Kober L., Prescott M.F., Rouleau J.L., Solomon S.D., Swedberg K., Zile M.R., Packer M., McMurray J.J.V. Comparison of BNP and NT-proBNP in patients with heart failure and reduced ejection fraction. Circ Heart Fail. 2020;13 doi: 10.1161/CIRCHEARTFAILURE.119.006541. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Cardiac findings of COVID-19 patients with and without elevation in cardiac troponin I.