Abstract
Background
Febrile neutropenia is a frequent adverse event experienced by people with cancer who are undergoing chemotherapy, and is a potentially life‐threatening situation. The current treatment is supportive care plus antibiotics. Colony‐stimulating factors (CSFs), such as granulocyte‐CSF (G‐CSF) and granulocyte‐macrophage CSF (GM‐CSF), are cytokines that stimulate and accelerate the production of one or more cell lines in the bone marrow. Clinical trials have addressed the question of whether the addition of a CSF to antibiotics could improve outcomes in individuals diagnosed with febrile neutropenia. However, the results of these trials are conflicting.
Objectives
To evaluate the safety and efficacy of adding G‐CSF or GM‐CSF to standard treatment (antibiotics) when treating chemotherapy‐induced febrile neutropenia in individuals diagnosed with cancer.
Search methods
We conducted the search in March 2014 and covered the major electronic databases: the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, Embase, LILACS, and SCI. We contacted experts in hematology and oncology and also scanned the citations from the relevant articles. In addition, we also searched for economic evaluations via MEDLINE(R) In‐Process & Other Non‐Indexed Citations, Embase, CENTRAL and NHS Economic Evaluation Database in May 2015 to support a Brief Economic Commentary (BEC).
Selection criteria
We searched for randomized controlled trials (RCTs) and economic evaluations that compared CSF plus antibiotics versus antibiotics alone for the treatment of chemotherapy‐induced febrile neutropenia in adults and children.
Data collection and analysis
We used the standard methodological procedures expected by The Cochrane Collaboration. We performed meta‐analysis of the selected studies using Review Manager 5 software.
Main results
Fourteen RCTs (15 comparisons) including a total of 1553 participants addressing the role of CSF plus antibiotics in febrile neutropenia were included. Overall mortality was not improved by the use of CSF plus antibiotics versus antibiotics alone (hazard ratio (HR) 0.74 (95% confidence interval (CI) 0.47 to 1.16) P = 0.19; 13 RCTs; 1335 participants; low quality evidence). A similar finding was seen for infection‐related mortality (HR 0.75 (95% CI 0.47 to 1.20) P = 0.23; 10 RCTs; 897 participants; low quality evidence). Individuals who received CSF plus antibiotics were less likely to be hospitalized for more than 10 days (risk ratio (RR) 0.65 (95% CI 0.44 to 0.95) P = 0.03; 8 RCTs; 1221 participants; low quality evidence) and had more number of participants with a more faster neutrophil recovery (RR 0.52 (95% CI 0.34 to 0.81) P = 0.004; 5 RCTs; 794 participants; moderate quality evidence) than those treated with antibiotics alone. Similarly, participants receiving CSF plus antibiotics had shorter duration of neutropenia (standardized mean difference (SMD) ‐1.70 (95% CI ‐2.65 to ‐0.76) P = 0.0004; 9 RCTs; 1135 participants; moderate quality evidence), faster recovery from fever (SMD ‐0.49 (95% CI ‐0.90 to ‐0.09) P value = 0.02; 9 RCTs; 966 participants; moderate quality evidence) and shorter duration of antibiotics use (SMD ‐1.50 (95% CI ‐2.83 to ‐0.18) P = 0.03; 3 RCTs; 457 participants; low quality evidence) compared with participants receiving antibiotics alone. We found no significant difference in the incidence of deep venous thromboembolism (RR 1.68 (95% CI 0.72 to 3.93) P = 0.23; 4 RCTs; 389 participants; low quality evidence) in individuals treated with CSF plus antibiotics compared with those treated with antibiotics alone. We found higher incidence of bone or joint pain or flu‐like symptoms (RR 1.59 (95% CI 1.04 to 2.42) P = 0.03; 6 RCTs; 622 participants; low quality evidence) in individuals treated with CSF plus antibiotics compared with those treated with antibiotics alone. Overall, the methodological quality of studies was moderate to low across different outcomes. The main reasons to downgrade the quality of evidence were inconsistency across the included studies and imprecision of results. No full economic evaluations were identified. Several of the included RCTs identified economic benefits regarding a reduction in overall length of stay attributable to the use of CSF plus antibiotics, however they fell short of undertaking a full economic evaluation.
Authors' conclusions
The use of a CSF plus antibiotics in individuals with chemotherapy‐induced febrile neutropenia had no effect on overall mortality, but reduced the amount of time participants spent in hospital and improved their ability to achieve neutrophil recovery. It was not clear whether CSF plus antibiotics had an effect on infection‐related mortality. Participants receiving CSFs had shorter duration of neutropenia, faster recovery from fever and shorter duration of antibiotics use. The current scarcity of relevant economic evaluations highlights an evidence gap and the need for further research fully explore the cost‐effectiveness of these treatment alternatives.
Plain language summary
Does administering colony‐stimulating factors plus antibiotics in people with fever and low white cell count reduce hospitalization?
Background: People undergoing chemotherapy often experience febrile neutropenia, characterized by a high temperature combined with a low white blood cell count. Febrile neutropenia is a potentially life‐threatening condition and requires prompt medical intervention. The standard treatment for febrile neutropenia includes supportive care of fluids, electrolytes (any substance that contains free ions that can conduct electricity given either intravenously or orally) and antibiotics given either orally or intravenously.Review question: Whether colony‐stimulating factors (CSFs) (hormones that stimulate the production of white blood cells) added to antibiotics are better than antibiotics alone in the treatment of febrile neutropenia caused by cancer chemotherapy? Literaturesearch date: March 2014 and May 2015 for the economic evaluation. Main findings: We identified 14 randomized controlled trials (RCTs) enrolling a total of 1553 participants. Six trials were funded by industry alone and 3 trials were jointly funded by industry and public sources and only 1 trial was funded by public sources alone. Our study shows that although CSFs do not appear to improve survival, they shorten the amount of time a person spends in hospital and increase their chances that white blood cells will recover to normal levels. It is not clear whether the use of a CSF reduces the number of people who die due to infection. Our study shows that CSFs shorten the time taken for the white blood cells to return to normal levels, recovery from fever and stopping antibiotics. The number of patients suffering from treatment related harms such as blood clots in the veins were similar between patients receiving CSF and antibiotics and antibiotics alone. The number of patients experiencing bone or joint pain or flu‐like symptoms was higher among individuals receiving CSF and antibiotics compared with individuals receiving antibiotics alone. No economic evaluations were identified. Several of the included RCTs identified economic benefits regarding a reduction in overall length of stay attributable to the use of CSF plus antibiotics, however they fell short of undertaking a full economic evaluation. Quality of the evidence: The overall methodological quality of included studies was moderate to low.
Summary of findings
Summary of findings 1. Benefits and harms of CSF plus antibiotics versus antibiotics alone.
| CSF plus ATB compared to ATB alone for chemotherapy induced febrile neutropenia | |||||
| Population: people with chemotherapy‐induced febrile neutropenia Settings: in‐patient/hospital Intervention: CSF plus ATB Comparison: ATB alone | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | |
| Assumed risk | Corresponding risk | ||||
| ATB alone | CSF + ATB | ||||
| Overall mortality | Study population | HR 0.74 (0.47 to 1.16) | 1335 (13) | ⊕⊕⊝⊝ low1,2,3 | |
| 70 per 1000 | 52 per 1000 (33 to 80) | ||||
| Moderate | |||||
| 29 per 1000 | 22 per 1000 (14 to 34) | ||||
| Infection‐related mortality | Study population | RR 0.75 (0.47 to 1.20) | 897 (10) | ⊕⊕⊝⊝ low1,2,3 | |
| 56 per 1000 | 43 per 1000 (27 to 67) | ||||
| Moderate | |||||
| 22 per 1000 | 17 per 1000 (10 to 26) | ||||
| People hospitalized for > 10 days | Study population | RR 0.65 (0.44 to 0.95) | 1087 (7) | ⊕⊕⊝⊝ low3,4 | |
| 349 per 1000 | 227 per 1000 (153 to 331) | ||||
| Moderate | |||||
| 338 per 1000 | 220 per 1000 (149 to 321) | ||||
| Duration of grade IV neutropenia (lower values signify better outcomes) | The mean duration of grade IV neutropenia in the intervention groups was 1.70 standard deviations lower (2.65 to 0.76 lower) |
SMD ‐1.70 (‐2.65 to ‐0.76) |
1135 (9) | ⊕⊕⊝⊝ low3,5 | |
|
Time to recovery from fever (lower values signify better outcomes) |
The mean time to recovery from fever in the intervention groups was 0.49 standard deviations lower (0.9 to 0.09 lower) |
SMD ‐0.49 (‐0.9 to ‐0.09) |
966 (9) | ⊕⊕⊝⊝ low3,6 | |
|
Time to withdrawal from ATB (lower values signify better outcomes) |
The mean time to withdrawal from ATB in the intervention groups was 1.5 standard deviations lower (2.83 to 0.18 lower) |
SMD ‐1.5 (‐2.83 to ‐0.18) |
457 (3) | ⊕⊕⊝⊝ low3,7 | |
| Deep vein thrombosis | Study population | RR 1.68 (0.72 to 3.93) | 389 (4) | ⊕⊕⊝⊝ low1,3 | |
| 26 per 1000 | 43 per 1000 (18 to 101) | ||||
| Moderate | |||||
| 5 per 1000 | 8 per 1000 (4 to 20) | ||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). ATB: antibiotics; CI: confidence interval; HR: hazard ratio; RR: risk ratio; SMD: standardized mean difference | |||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | |||||
1 Seven of the included articles described an adequate method of randomization (Anaissie 1996; Aviles 1996; Garcia‐Carbonero 2001; Lopez‐Hernandez 2000; Riikonen 1994; Vellenga 1996; Yoshida 1999) and five reported adequate concealment of the sequence of allocation (Aviles 1996; Garcia‐Carbonero 2001; Maher 1994; Mayordomo 1995; Mitchell 1997). Seven trials were placebo controlled (Arnberg 1998; Biesma 1990; Maher 1994; Mayordomo 1995; Mitchell 1997; Riikonen 1994; Vellenga 1996). A sample size was preplanned in seven (Garcia‐Carbonero 2001; Maher 1994; Mayordomo 1995; Mitchell 1997; Ravaud 1998; Vellenga 1996; Rodriguez 2005), but the planned number was not reached in one trial (Ravaud 1998). Hence we downgraded the quality of evidence by 1 for the risk of bias (also see footnote # 2 below). 2 Of all the included trials only that by Aviles et al (Aviles 1996) appeared to show a benefit of CSF plus antibiotics compared with antibiotics alone for the outcome of overall mortality. It is important to note that 33% (15 of 45) of the events for this outcome in the control group were reported in this trial. These 15 deaths represent 33% (15 of 45) of the total of deaths in the control group among all trials. Similarly, there were 15 infection‐related deaths among the 471 participants randomized to the intervention group and 25 among 426 randomized to the control group. However, it is important to note that 60% (15 of 25) of the events in the control group were reported in one study (Aviles 1996). 3 A majority of the individual randomized controlled trials and the pooled estimates have wide CIs. We downgraded the quality of evidence by 1 for the observed imprecision.
4 Substantial heterogeneity was detected (P = 0.0009, I2 = 70%). As planned, we explored the possible causes of heterogeneity to determine if it was appropriate to pool the trials. All trials but one trial (Yoshida 1999) had a point estimate that favored the intervention group. This trial enrolled only people with hematological malignancies. We explored the impact of type of malignancy on number of participants hospitalized for more than 10 days (Subgroup analysis and investigation of heterogeneity: type of malignancy). The trials enrolling people with mixed cancers favored the use of CSF plus antibiotics (RR 0.64, 95% CI 0.45 to 0.89) compared with trial enrolling people with only hematological malignancies (RR 1.17, 95% CI 0.81 to 1.70) (test of interaction P = 0.02) for the outcome of number of participants hospitalized for more than 10 days (Analysis 5.1). We also noticed that only two trials reached statistical significance (Maher 1994; Mayordomo 1995). By inspecting the forest plot, we could detect that trial by Mayordomo et al (Mayordomo 1995) and Maher et al (Maher 1994) indicated a much stronger effect than that detected in all other trials. We therefore repeated our analysis, excluding these trials (Maher 1994; Mayordomo 1995). The exclusion resulted in a substantial reduction in the statistical heterogeneity (I2 = 48%; P = 0.10) and the significance of the effect of CSF plus antibiotics on this outcome disappeared (RR 0.85, 95% CI = 0.61 to 1.17; P = 0.32). Hence we downgraded the quality of evidence by 1 for the observed inconsistency.
5 Considerable heterogeneity was detected (P value < 0.00001, I2 = 98%). In a subgroup analysis according to CSF type, G‐CSF showed a statistically significantly stronger beneficial effect than GM‐CSF for the outcome of duration of grade IV neutropenia (Analysis 3.3). Nonetheless, we downgraded the quality of evidence by 1 for the observed inconsistency.
6 Considerable heterogeneity was detected (P value < 0.00001, I2 = 89%). We downgraded the quality of evidence by 1 for the observed inconsistency.
7 Considerable heterogeneity was detected (P value < 0.00001, I2 = 97%). We downgraded the quality of evidence by 1 for the observed inconsistency. We noted that in all of these trials the mean/median duration of ATB use was similar (mean of 5 days) among patients receiving CSF. However, the high precision observed around the effect size in the trial by Garcio‐Carbonero et al (Garcia‐Carbonero 2001) compared with other two trial estimates was potentially leading to the substantial heterogeneity. The heterogeneity disappeared completely once we removed this trial from the analysis (P value = 0.72, I2 = 0%) and the overall effect still showed benefit with the use of CSF plus ATB compared with ATB alone.
Background
Description of the condition
Febrile neutropenia is a relatively frequent event in people with cancer receiving chemotherapy. It is a potentially life‐threatening condition and requires prompt medical intervention (Pizzo 1999). Febrile neutropenia is one of the most concerning complications of cancer chemotherapy and is a major cause of morbidity, healthcare resource use and compromised efficacy resulting from delays and dose reductions in chemotherapy. Mortality from febrile neutropenia has diminished steadily but remains significant. Overall mortality rates are around 5% in people with solid tumours (1% in low‐risk people) and as high as 11% in people diagnosed with some hematological malignancies. Prognosis is worst in people with proven bacteremia, with mortality rates of 18% and 5% reported in people with Gram‐negative and Gram‐positive bacteremia, respectively. Elderly people are at a higher risk of febrile neutropenia following chemotherapy, with worse morbidity and mortality rates (de Naurois 2010).
Chemotherapy‐induced febrile neutropenia is a potentially life‐threatening complication for which the incidence and mortality varies according to cancer type and chemotherapy regimen. The economic burden of the complication has been estimated in the literature, however, estimated costs are variable. One study suggests that for solid tumour patients within a routine oncology hospital setting the average cost per episode is $3855* (2013 US$) (*adjusted from 2007 GBP to 2014 US$ using CCEMG‐EPPI cost conversion tool (http://eppi.ioe.ac.uk/costconversion/default.aspx) (Schelenz 2012) whilst a review of cost‐of‐illness studies in lymphoma patients experiencing febrile neutropenia found large variation in the cost estimates ranging from $5819 to $34,756 (2013 US$) per episode (Wang 2015)
Description of the intervention
The standard treatment for febrile neutropenia includes supportive care plus broad‐spectrum antibiotics (Pizzo 1999). There is no consensus in the literature as to which antibiotics or combination of antibiotics is ideal for the treatment for febrile neutropenia (Giamerellou 2001). Hematopoietic growth‐stimulating factors are a class of cytokines that regulate the proliferation, differentiation, and functions of hematopoietic cells (Griffin 2001). More than 20 different types of stimulating factor have been identified (Griffin 2001) and many have been tested in clinical studies for different applications (Griffin 2001; Segal 2001). Among them, granulocyte colony‐stimulating factor (G‐CSF) and granulocyte‐macrophage colony‐stimulating factor (GM‐CSF) have been studied in people with cancer because of their potential effect on neutropenia.
How the intervention might work
G‐CSF regulates the production of the neutrophil lineage. The administration of G‐CSF to humans results in a dose‐dependent increase in circulating neutrophils (Griffin 2001; Petros 2001), due mainly to a reduction in transit time from stem cell to mature neutrophil (Griffin 2001). GM‐CSF stimulates the growth of granulocyte, macrophage and eosinophil colonies (Griffin 2001; Petros 2001). The administration of GM‐CSF to humans results in a dose‐dependent increase in blood neutrophils, eosinophils, macrophages and sometimes lymphocytes (Griffin 2001; Petros 2001). Different types of G‐CSF and GM‐CSF have been tested in clinical trials and are available. Among the most used G‐CSFs are filgrastim and lenograstim, and among the most used GM‐CSFs are sargramostim and molgramostim. Both G‐CSFs and GM‐CSFs have been demonstrated to be effective in reducing the incidence of febrile neutropenia when given immediately after chemotherapy (Freyer 1998; Lyman 2002) and as supportive therapy in people undergoing bone marrow transplantation (Griffin 2001; Petros 2001). The known effect of G‐CSF and GM‐CSF in increasing the number of circulating neutrophils provided the background for clinical studies designed to assess their role as adjunct therapy to antibiotics in people diagnosed with febrile neutropenia.
Why it is important to do this review
The results of randomized controlled trials (RCTs) addressing the role of CSFs in the management of febrile neutropenia have not been clear and conflicting findings have been published. Whereas two studies found no significant effect of CSFs in the prevention of prolonged hospitalization (Anaissie 1996; Maher 1994), Another reported CSFs to have a significant impact on length of hospitalization (Riikonen 1994). Time to recovery from fever was favorably affected by CSF in two studies (Mayordomo 1995; Ravaud 1998) but not in another (Vellenga 1996). Also, different results regarding the use of CSFs have been reported in people classified as having a low or high baseline risk of developing a life‐threatening complication (Ravaud 1998). Individually, these studies included fewer than 220 participants, and this factor, in addition to reported low rates of clinical events such as death, means that they may have been underpowered to detect a difference between the treated groups. Conflicting results obtained from small studies demands the conduct of a systematic review of the literature (Egger 2001) in order to extend the totality of evidence to allow informed medical decision. Accordingly, in 2000, we conducted and published a Cochrane systematic review addressing the role of CSF plus antibiotics versus antibiotics alone in individuals with chemotherapy‐induced febrile neutropenia (Clark 2000). However, since the publication of this review, further RCTs in this area have been published. This gave us an impetus to update our previous systematic review.
Objectives
To evaluate the safety and efficacy of adding G‐CSF or GM‐CSF to standard treatment (antibiotics) when treating chemotherapy‐induced febrile neutropenia in individuals diagnosed with cancer.
Methods
Criteria for considering studies for this review
Types of studies
We included all randomized controlled trials (RCTs) with a parallel design that compared the use of a CSF plus antibiotics versus antibiotics alone for the treatment of individuals with established chemotherapy‐induced febrile neutropenia. We also looked for full economic evaluations.
Types of participants
Individuals undergoing chemotherapy for cancer who experienced neutropenia (absolute neutrophil count (ANC) less than 1 x 109/L (1000/mm3)) and fever (body temperature higher than 38.5°C on one occasion or higher than 38°C on two or more occasions).
Types of interventions
Intervention group: G‐CSF or GM‐CSF plus antibiotics
Control group: antibiotics plus no further treatment or placebo
Types of outcome measures
Primary outcomes
Overall mortality
Infection‐related mortality
Secondary outcomes
Number of people hospitalized for more than 10 days
Time to neutrophil recovery (number of people with neutropenia (ANC < 1000/mm3) for more than 5 to 10 days)
Duration (measured in days) of grade IV neutropenia (ANC < 500/mm3)
Time to recovery from fever
Time to withdrawal from antibiotics
Time to defervescence (the abatement of a fever due to a decrease in body temperature)
Treatment‐related harms, including deep vein thrombosis (DVT) and bone, joint pain or flu like symptoms
Search methods for identification of studies
Electronic searches
We performed a wide search on the main computerized databases of interest. For the original review, the search dates were: the Cochrane Central Register of Controlled Trials (CENTRAL) (Issue 1, 2003), MEDLINE (1966 to 2002), EMBASE (1974 to 2001), LILACS (1980 to 2003), CANCERLIT (1975 to 2002), and SCI (1974 to 2001).
For this update, we extended the searches to: CENTRAL (Issue 3 of 12, March 2014; searched 25 March 2014), MEDLINE (2002 to 2014; searched 25 March 2014), EMBASE (2001 to 2014), LILACS (2014), CANCERLIT (2002 to 2004; now incorporated into MEDLINE), and SCI (2001 to 2014).
For MEDLINE, we used the methodological search strategy for RCTs (Dickersin 1994) recommended by The Cochrane Collaboration (Higgins 2011a). For EMBASE, we used adaptations of this same strategy and for LILACS we used the methodological search strategy reported by Castro 1999. We performed an additional search of the SCI database to look for studies that had cited the included studies (see Appendix 1; Appendix 2; Appendix 3; Appendix 4; Appendix 5; Appendix 6 for the respective search strategies).
The search strategies used have been developed and executed by the author team.
For economic evaluations we used the non‐methodological portion of the original search strategies in combination with a economic evaluation filter (Appendix 7) for all databases except NHS EED since this database only contains economic evaluation citations.The following databases were searched: Ovid MEDLINE(R) In‐Process & Other Non‐Indexed Citations and Ovid MEDLINE(R)1946 to May 2015, Embase 1974 to 2015 Week 24, EBM Reviews ‐ Cochrane Central Register of Controlled Trials May 2015 and EBM Reviews ‐ NHS Economic Evaluation Database 2nd Quarter 2015.
Searching other resources
We scanned all the references of relevant articles and retrieved all additional articles of potential interest for further analysis. We consulted experts in oncology and hematology about ongoing studies or studies that have not yet been published. We also scanned the personal collections of articles of two of the authors (GL and BD).
Data collection and analysis
Selection of studies
Two review authors (OACC and RM) independently scanned the retrieved titles and abstracts of all studies for their eligibility for inclusion in the systematic review. We resolved any disagreements in the selection of studies by consensus (Higgins 2011b). At every stage of searching and screening, we documented the overall number of studies identified, excluded, and included, with reasons, according to the preferred reporting items for systematic reviews and meta‐analyses (PRISMA) guidelines, which we also used to create a flow diagram (Moher 2009).
Data extraction and management
Two review authors (OACC and RM) independently extracted data using a standardized data extraction form. From each relevant trial, we retrieved data on the selected clinical outcomes, methodological characteristics, and types of participants in each study. Specifically we extracted data on the following.
Clinical outcomes
Mortality ‐ overall and infection related
Number of people hospitalized for more than 10 days
Time to neutrophil recovery (number of people with neutropenia (ANC < 1000/mm3) for more than 5 to 10 days)
Duration of grade IV neutropenia (Data reported in individual studies as either median days to recovery to ANC > 500/mm3 or median days of grade IV neutropenia: ANC < 500/mm3)
Time to recovery from fever
Time to withdrawal from antibiotics
Time to defervescence (the abatement of a fever due to a decrease in body temperature)
Treatment‐related harms, including deep DVT and bone, joint pain or flu like symptoms
Additional data
General information on the study: authors, date of publication, title, publication type (full text, abstract, unpublished), number of centers involved, and funding source
Study characteristics: inclusion/exclusion criteria, length of follow up, diagnostic criteria for neutropenia (ANC), criteria for hospital discharge
Participant characteristics: age, adults versus children, gender, number of participants recruited/allocated/evaluated, participants lost to follow up, type of tumour (solid versus hematological)
-
Intervention: detailed description of both the intervention and standard treatment in terms of:
type of CSF used with dosage and duration;
type of antibiotics used with dosage and duration
Studies reported data for the outcome of time to neutrophil recovery in the form of number of participants with neutropenia for more than 5 to 10 days. Accordingly, we extracted data in the form of number of participants with neutropenia for 5 to 10 days in both the experimental and control arms and have reported risk ratio (RR) and 95% confidence intervals (CIs).
Studies reported data for the outcome of duration of grade IV neutropenia in the form of median days to recovery to ANC > 500/mm3 or median days of grade IV neutropenia: ANC < 500/mm3. Accordingly, we extracted data in the form of mean and standard deviation of duration of neutropenia in both the experimental and control arms and have reported standardized mean difference (SMD) and 95%CIs.
When time‐to‐event data were not available for direct extraction, we extracted data according to the methods described by Tierney et al (Tierney 2007). These methods allow the calculation of the hazard ratio (HR) and associated statistics using indirect calculation of the variance and the number of observed minus expected events, based on parameters reported in the included studies (e.g. P values, log‐rank statistics, or survival curves).
We extracted details regarding the main methodological dimensions empirically linked to bias (Egger 2001) and two review authors assessed the methodological quality of each selected trial. We gave special attention to the generation of randomization sequence, allocation concealment, blinding, use of intention‐to‐treat (ITT) versus per‐protocol analyses, and source of funding. We used these data in sensitivity analyses to test the robustness of our findings.
Data management
Two review authors manually extracted data from publications into a standardized data extraction form. A third review author validated the extracted data. We resolved all disagreements at each step by consensus or, where necessary, by consulting a third reviewer. One review author entered data into Review Manager (Review Manager 5.3) and another checked the entries for accuracy, consistency, and completeness. Senior review authors randomly reviewed 15% of the data for accuracy.
Assessment of risk of bias in included studies
Two review authors independently assessed all eligible studies for their risk of bias (assessment of methodological quality) using methods suggested in Chapter 8 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011c). The summary judgment of the review authors comprised an answer for each risk criterion, based on the following three‐point scale:
yes (low risk of bias: plausible bias unlikely to seriously alter the results if all criteria were met);
no (high risk of bias: plausible bias that seriously weakens confidence in the results if one or more criteria were not met);
unclear (uncertain risk of bias: plausible bias that raises some doubt about the results if one or more criteria were assessed as unclear).
The following items were included in the assessment of risk of bias:
sequence generation (whether allocation sequence was adequately generated);
allocation concealment (whether allocation was adequately concealed);
masking/blinding (whether the knowledge of the allocated intervention was adequately prevented during the study; i.e. we extracted data regarding who (participants, personnel, outcome assessors, data analysts) was blinded);
incomplete outcome data (whether incomplete outcome data were adequately addressed);
selective outcome reporting (whether reports of the study were free of selective outcome reporting);
other sources of bias (whether reports of the study included pre specification of the expected difference in the primary outcome (delta), alpha error, beta error or sample size calculation);
ITT analysis (whether ITT analysis was undertaken in the study).
In addition, we assessed whether domains related to random error and sample size were specified a priori in each trial.
Measures of treatment effect
For dichotomous outcomes (e.g. treatment‐related harms), data were summarized as RR with 95% CIs for each trial
For time‐to‐event outcome (e.g. overall mortality), data were summarized as HRs and 95% CIs
For continuous outcomes (e.g. time to withdrawal from antibiotics), data were summarized as means and standard deviations for each trial. Where data were reported as median and range, we converted these data into mean and standard deviation using the method by Hozo et al (Hozo 2005).
Unit of analysis issues
The unit of analysis was a study from which aggregate data were extracted as follows: for dichotomous variables, the number of participants in the 'CSF plus antibiotics' arm (intervention group) and the number of participants in the 'antibiotics alone' arm (control group). For continuous variables, the means, standard deviations, and the numbers of participants in the intervention and control groups were used. For studies with multiple intervention groups, we included each pair‐wise comparison separately. Hence, for dichotomous outcomes, both the number of events and the total number of participants were divided for the control arm (ATB alone). Similarly, for time‐to‐event outcomes, the number of events and the total number of participants were divided for the control arm and the variance and number of observed minus expected events were calculated separately for each comparison. For continuous outcomes, the total number of participants was divided for the control arm, and means and standard deviations were calculated separately for each comparison (Higgins 2011d).
Dealing with missing data
Where necessary outcome data were not available from the primary literature we requested missing data or complementary information from the first or corresponding authors of such publications.
Assessment of heterogeneity
We assessed heterogeneity among trials and between subgroups using a Chi2 test with a P value of < 0.10 as the level of significance. The degree of heterogeneity among trials and between subgroups was also assessed using the I² statistic. We used the following guide to the interpretation of the I² statistic: I² = 0% to 40%: heterogeneity that might not be important; I² = 30% to 60%: moderate heterogeneity; I² = 50% to 90%: substantial heterogeneity), I² = 75% to 100%: considerable heterogeneity (Higgins 2011a). The importance of the observed value of I2 depends on (i) the magnitude and direction of effects and (ii) the strength of evidence for the heterogeneity (e.g. a P value from the Chi2 test, or the I2 statistic). When heterogeneity was detected, we searched intensively for a possible explanation.
Assessment of reporting biases
We assessed the possibility of publication bias by generating a funnel plot and by conducting a linear regression test using a P value of < 0.1 as the level of significance (Egger 1997; Higgins 2011a).
Data synthesis
We conducted meta analyses using Review Manager 5.3 software by the Cochrane Collaboration (Review Manager 5.3). For the outcomes of number of participants hospitalized for more than 10 days, time to neutrophil recovery (reported as number of people with neutropenia (ANC < 1000/mm3) for more than 5 to 10 days), DVT and bone and joint pain, or flu‐like symptoms we calculated the RRs with 95% CIs and pooled the data using the Mantel–Haenszel random‐effects model. For the outcomes of overall mortality and infection‐related mortality we calculated the observed minus expected log‐rank statistics plus the variance, according to the methods described by Tierney et al (Tierney 2007), and presented the results (pooled using a random effects model and inverse variance method) as HRs (Tierney 2007). For the outcomes of duration of grade IV neutropenia, time to recovery from fever and time to withdrawal from antibiotics we calculated the standardized mean differences with 95% CIs and pooled the data using the random‐effects model and inverse variance method. We provided a 'Summary of findings' table, produced using Grading of Recommendations Assessment, Development and Evaluation (GRADE) software (GRADEpro; Guyatt 2011; Guyatt 2011a; Guyatt 2011b; Guyatt 2011c; Guyatt 2011d; Guyatt 2011e).
Subgroup analysis and investigation of heterogeneity
We performed subgroup analyses on the following clinical characteristics:
age group: use of CSF in children versus adults;
type of CSF used;
criteria for hospital discharge and
type of tumor: mix versus hematological versus solid tumors.
Sensitivity analysis
We assessed the robustness of our results by conducting a sensitivity analysis with respect to the methodological quality of the included RCTs.
Results
Description of studies
Fourteen RCTs addressing the role of CSF in chemotherapy‐induced febrile neutropenia, involving a total of 1553 participants, were included in the final analysis. See '(Characteristics of included studies)' and '(Characteristics of excluded studies)' tables for details. No full economic evaluations were identified.
Randomization of participants versus episodes of febrile neutropenia
A particular difficulty with trials of febrile neutropenia is the problem of re‐randomization (Paesmans 1998). This practice has the potential to bias the results of clinical trials because of the possible dependence of outcomes on previous events. People who have already developed one episode of febrile neutropenia are expected to be more prone to develop another, which in turn may violate the assumption that all events must be independent of each other to allow proper analysis of a trial as well as the pooling of data in meta‐analysis (Hozo 2005a). Five included trials (Anaissie 1996; Lopez‐Hernandez 2000; Mitchell 1997; Riikonen 1994; Rodriguez 2005) allowed people to be entered in the study and randomized more than once. These trials analysed 419 episodes of febrile neutropenia and are responsible for about one‐quarter of the total number of febrile neutropenia episodes and people included in this systematic review. It was impossible to extract data from these trials according to the number of people. As the practice of re‐randomization is allowed by the Immunocompromised Host Society (IHS 1990), we included these trials in our analyses.
Results of the search
For this update we extended our search from the end of the search date in the original review to March 2014. We identified 1007 papers investigating G‐CSF or GM‐CSF in chemotherapy‐induced febrile neutropenia in our updated search of electronic databases. We did not identify any studies through other search methods. We selected and retrieved 12 articles for full‐text analysis. Of those identified, we excluded 11 for various reasons. We had excluded 21 studies in the original review process. Hence the total number of excluded studies from both reviews is 32 (Characteristics of excluded studies). Thus, in addition to the 13 studies included in the original review, we included 1 new study (Rodriguez 2005), bringing the included total to 14 (see Figure 1 for details).
1.
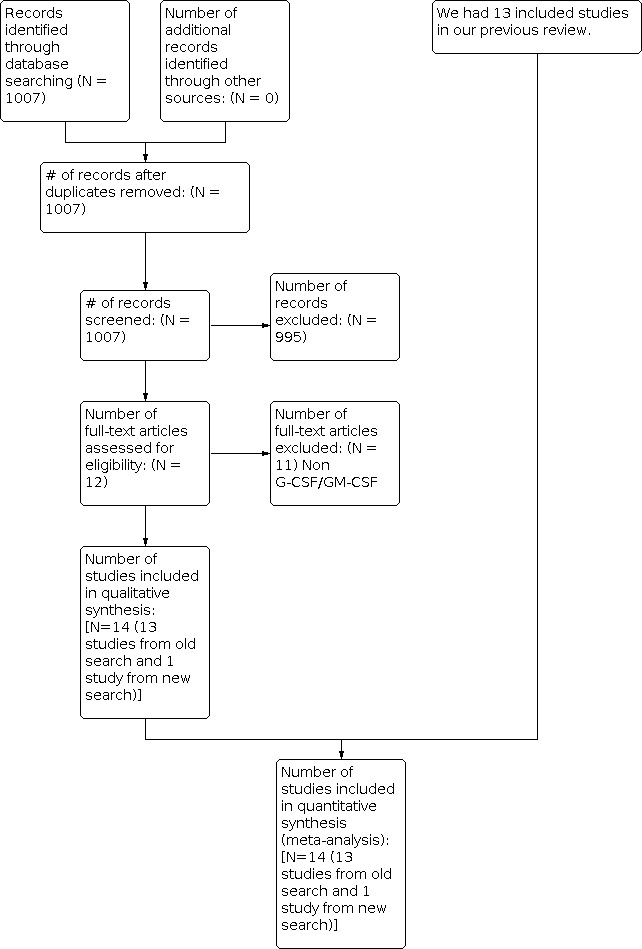
Study flow diagram.
Included studies
Seven articles described the effects of G‐CSF (Aviles 1996; Garcia‐Carbonero 2001; Lopez‐Hernandez 2000; Maher 1994; Mitchell 1997; Rodriguez 2005Yoshida 1999), six described the effects of GM‐CSF (Anaissie 1996; Arnberg 1998; Biesma 1990; Ravaud 1998; Riikonen 1994; Vellenga 1996) and one was a three‐arm study in which participants were randomized to G‐CSF, GM‐CSF or placebo (Mayordomo 1995). Six articles included people with an ANC less than 1 x 109/L (Anaissie 1996; Arnberg 1998; Biesma 1990; Maher 1994; Ravaud 1998; Yoshida 1999), six included people with an ANC less than 0.5 x 109/L (Garcia‐Carbonero 2001; Lopez‐Hernandez 2000; Mayordomo 1995; Mitchell 1997; Rodriguez 2005; Vellenga 1996), one included those with an ANC less than 0.2 x 109/L (Riikonen 1994) and one included participants with an ANC less than 0.1 x 109/L (Aviles 1996). Ten articles enrolled adults (Anaissie 1996; Arnberg 1998; Aviles 1996; Biesma 1990; Garcia‐Carbonero 2001; Maher 1994; Mayordomo 1995; Ravaud 1998; Vellenga 1996; Yoshida 1999), three enrolled children (Mitchell 1997; Riikonen 1994; Rodriguez 2005) and one included both (Lopez‐Hernandez 2000). Three articles enrolled participants with hematological malignancies only (Aviles 1996; Lopez‐Hernandez 2000; Yoshida 1999), one solid tumors only (Ravaud 1998) and ten articles included people with either type of malignancy (Anaissie 1996; Arnberg 1998; Biesma 1990; Garcia‐Carbonero 2001; Maher 1994; Mayordomo 1995; Mitchell 1997; Riikonen 1994; Rodriguez 2005; Vellenga 1996).
Excluded studies
A total of 21 studies were excluded after full text review in the original version of this review. Eight of these 21 excluded studies were duplicate reports (Bodey 1994; Garcia‐Carb 1999a; Garcia‐Carb 1999b; Mayordomo 1992; Mayordomo 1993; Ravaud 1995; Uyl‐de Groot 1997; Vellenga 1996b). In this update we excluded 11 studies after the full text review. The study by Montalar was published in abstract form only and no information on outcomes was available (Montalar 1998). We tried to contact the authors of this abstract by e‐mail, but received no answer. Therefore, this trial was excluded from our analysis. We excluded the trial by (Timmer‐Bonte 2005) as it is a randomized trial addressing the prophylactic role of G‐CSF.
Risk of bias in included studies
Results of the 'Risk of bias' assessments are presented in (Figure 2). The overall methodological quality of included studies for the majority of outcomes we judged to be low according to GRADE methodology (Table 1).
2.

Allocation
Three of the included studies described an adequate method of randomization (Anaissie 1996; Garcia‐Carbonero 2001; Lopez‐Hernandez 2000) and five reported adequate concealment of the sequence of allocation (Aviles 1996; Garcia‐Carbonero 2001; Maher 1994; Mayordomo 1995; Mitchell 1997).
Blinding
Six trials were blinded (Arnberg 1998; Biesma 1990; Maher 1994; Mitchell 1997; Riikonen 1994; Vellenga 1996). Eight trials did not employ blinding in the conduct of the trial (Anaissie 1996; Aviles 1996; Garcia‐Carbonero 2001; Lopez‐Hernandez 2000; Mayordomo 1995; Ravaud 1998; Rodriguez 2005; Yoshida 1999).
Incomplete outcome data
An ITT analysis was performed in ten trials (Anaissie 1996; Arnberg 1998; Garcia‐Carbonero 2001; Maher 1994; Mayordomo 1995; Mitchell 1997; Ravaud 1998; Vellenga 1996; Yoshida 1999; Rodriguez 2005).
Selective reporting
We did not have access to the trial protocols therefore we were unable to investigate the potential for selective reporting bias based only on trial publications.
Other potential sources of bias
Seven trials were placebo controlled (Arnberg 1998; Biesma 1990; Maher 1994; Mayordomo 1995; Mitchell 1997; Riikonen 1994; Vellenga 1996). A sample size was preplanned in seven (Garcia‐Carbonero 2001; Maher 1994; Mayordomo 1995; Mitchell 1997; Ravaud 1998; Vellenga 1996; Rodriguez 2005), but the planned number was not reached in one trial (Ravaud 1998).
Seven trials were multicentric studies (Garcia‐Carbonero 2001; Maher 1994; Mitchell 1997; Ravaud 1998; Riikonen 1994; Vellenga 1996; Yoshida 1999).
We noted that one of the studies reported a much higher rate of mortality in the control group than in the intervention group (Aviles 1996). Because of this, and because of a lack of description of important baseline characteristics of the people in both groups, concerns regarding the comparability of the study arms in this trial (Aviles 1996) have been raised in the literature (Rubenstein 2000).
Effects of interventions
See: Table 1
Our analysis included 14 trials (15 comparisons) involving a total of 1553 participants, 797 of whom were randomized to the intervention group and 756 to the control group. Not all trials provided data on all endpoints.
Overall mortality
Data on overall mortality could be extracted from 13 trials (14 comparisons) with 1335 participants. The meta‐analysis showed a statistically non significant trend of a benefit favoring the intervention group (HR 0.74, 95% CI 0.47 to 1.16; P = 0.19) (Analysis 1.1). No heterogeneity was detected in the analysis (P = 0.53, I2 = 0%). There were 35 deaths among 688 participants randomized to the intervention group and 45 among 647 randomized to the control group. However, it is important to note that 33% (15 of 45) of the events in the control group were reported in one study (Aviles 1996).
1.1. Analysis.

Comparison 1: Benefits and harms ‐ CSF + antibiotics vs antibiotics alone, Outcome 1: Overall mortality
Infection‐related mortality
We could extract data on infection‐related mortality from 10 trials (11 comparisons) with 897 participants. The meta‐analysis showed a statistically non‐significant trend in favor of CSF use (HR 0.75, 95% CI 0.47 to 1.20; P = 0.23) (Analysis 1.2). No heterogeneity was detected (P = 0.33, I2 = 12%). There were 15 infection‐related deaths among the 471 participants randomized to the intervention group and 25 among 426 randomized to the control group. However, it is important to note that 60% (15 of 25) of the events in the control group were reported in one study (Aviles 1996).
1.2. Analysis.

Comparison 1: Benefits and harms ‐ CSF + antibiotics vs antibiotics alone, Outcome 2: Infection related mortality
Participants hospitalized for more than 10 days
Data regarding the number of participants hospitalized for more than 10 days were extracted from eight trials (nine comparisons) that included 1221 participants. The pooled analysis showed a benefit in favor of the intervention group (RR 0.65, 95% CI 0.44 to 0.95; P = 0.03) (Analysis 1.3). Substantial heterogeneity was detected (P = 0.002, I2 = 69%).
1.3. Analysis.

Comparison 1: Benefits and harms ‐ CSF + antibiotics vs antibiotics alone, Outcome 3: Patients with hospitalization for greater than10 days
Time to neutrophil recovery (number of participants with neutropenia (ANC < 1000/mm3) for more than 5 to 10 days)
Data regarding the number of participants with neutropenia (ANC < 1000/mm3) for more than 5 days were extracted from five trials (six comparisons) with a total of 794 participants. The pooled analysis revealed a significant effect of CSF plus antibiotics versus the antibiotics alone (RR 0.52, 95% CI 0.34 to 0.81; P = 0.004) for the outcome of time to neutrophil recovery (Analysis 1.4). There was substantial heterogeneity among these trials (P = 0.006, I2 = 70%).
1.4. Analysis.

Comparison 1: Benefits and harms ‐ CSF + antibiotics vs antibiotics alone, Outcome 4: Time to neutrophil recovery
Duration of grade IV neutropenia
Data were extracted from nine trials (10 comparisons), with a total of 1135 participants, for the outcome of duration of grade IV neutropenia. A significant effect of CSF plus antibiotics was detected compared with the control group (SMD = ‐1.70, 95% CI ‐2.65 to ‐0.76; P = 0.0004) (Analysis 1.5). There was considerable heterogeneity among these trials (P < 0.00001, I2 = 98%).
1.5. Analysis.

Comparison 1: Benefits and harms ‐ CSF + antibiotics vs antibiotics alone, Outcome 5: Duration of grade IV neutropenia
Time to recovery from fever
Data were extracted from nine trials (10 comparisons), with a total of 966 participants, for the outcome of time to recovery from fever. A significant effect of CSF plus antibiotics was detected compared with the control group (SMD ‐0.49, 95% CI ‐0.90 to ‐0.09; P = 0.02) (Analysis 1.6). There was considerable heterogeneity among these trials (P < 0.00001, I2 = 89%).
1.6. Analysis.

Comparison 1: Benefits and harms ‐ CSF + antibiotics vs antibiotics alone, Outcome 6: Time to recovering from fever
Time to withdrawal from antibiotics
Data were extracted from three trials, with a total of 457 participants, for the outcome of time to withdrawal from antibiotics. A significant effect of CSF plus antibiotics was detected compared with antibiotics alone (SMD ‐1.50, 95% CI ‐2.83 to ‐0.18; P = 0.03) (Analysis 1.7). There was considerable heterogeneity among these trials (P < 0.00001, I2 = 97%).
1.7. Analysis.

Comparison 1: Benefits and harms ‐ CSF + antibiotics vs antibiotics alone, Outcome 7: Time to withdrawal from antibiotics
Treatment‐related harms
Treatment‐related harms were poorly reported in the included studies. We could only extract data related to DVT, and bone pain, joint pain and flu‐like symptoms from the included studies. Moreover, there was significant variation in the methods used by the authors to report treatment‐related harms.
Deep vein thrombosis
The number of participants developing DVT could be extracted from four studies with 389 participants. There were 9 cases of DVT among 194 participants randomized to the intervention group and 5 among 195 controls. The difference between the groups was not statistically significant (RR 1.68, 95% CI 0.72 to 3.93; P = 0.23) (Analysis 1.8). No heterogeneity was detected (P = 0.62, I2 = 0%).
1.8. Analysis.

Comparison 1: Benefits and harms ‐ CSF + antibiotics vs antibiotics alone, Outcome 8: Deep vein thrombosis
Bone and joint pain, and flu‐like symptoms
Data on these outcomes could be extracted from six studies (seven comparisons) with 622 participants. Forty‐nine participants developed these symptoms from 328 participants randomized to the intervention group versus 25 of 294 randomized to the control group. The difference between the groups were statistically significant (RR 1.59, 95% CI 1.04 to 2.42; P = 0.03) indicating higher incidence of these events with use of CSF plus antibiotics compared with use of antibiotics alone (Analysis 1.9). No statistically significant heterogeneity was detected (P = 0.52, I2 = 0%).
1.9. Analysis.

Comparison 1: Benefits and harms ‐ CSF + antibiotics vs antibiotics alone, Outcome 9: Bone and joint pain or flu‐like symptoms
Subgroup analyses
Study population
Our results did not change based on the study population (adults only versus children versus mixed) for any of the outcomes (Analysis 2.1; Analysis 2.3; Analysis 2.4). We noticed a statistically significant subgroup differences between trials with adult population (RR 0.45 (95% CI 0.29 to 0.70); 4 RCTs; 608 participants) (Garcia‐Carbonero 2001; Maher 1994; Mayordomo 1995; Ravaud 1998) versus trial enrolling only children (RR 0.80 (95% CI 0.66 to 0.97); 1 RCT; 186 participants) (Mitchell 1997) for the outcome of time to neutrophil recovery with a stronger benefit for adults (test of interaction P = 0.02) (Analysis 2.2). However, both trials (enrolling adults and children) favored use of antibiotics and CSF compared with antibiotics alone, but a stronger effect was seen among adult population.
2.1. Analysis.

Comparison 2: Subgroup analysis ‐ Study population (children vs. adults), Outcome 1: Patients with hospitalization for greater than10 days
2.3. Analysis.

Comparison 2: Subgroup analysis ‐ Study population (children vs. adults), Outcome 3: Duration of grade IV neutropenia
2.4. Analysis.

Comparison 2: Subgroup analysis ‐ Study population (children vs. adults), Outcome 4: Time to recovering from fever
2.2. Analysis.

Comparison 2: Subgroup analysis ‐ Study population (children vs. adults), Outcome 2: Time to neutrophil recovery
Type of CSF
Our results did not change based on the type of CSF (G‐CSF and GM‐CSF) for any of the outcomes (Analysis 3.1; Analysis 3.2; Analysis 3.4; Analysis 3.5). We noticed a statistically significant subgroup differences between trials with G‐CSF (SMD ‐2.73 (95% CI ‐4.43 to ‐1.04); 5 RCTs; 784 participants) (Aviles 1996; Garcia‐Carbonero 2001; Maher 1994; Mayordomo 1995; Mitchell 1997) versus trials with GM‐CSF (SMD ‐0.67 (95% CI ‐1.12 to ‐0.22); 5 RCTs; 351 participants) (Biesma 1990; Mayordomo 1995; Ravaud 1998; Riikonen 1994; Vellenga 1996) for the outcome of duration of grade IV neutropenia (ANC < 500/mm3) with stronger benefit with G‐CSF (test of interaction P = 0.02) (Analysis 3.3). However, both CSF types (G‐CSF and GM‐CSF) plus antibiotics were statistically significantly superior to antibiotics alone for this outcome but a stronger effect was observed with G‐CSF use.
3.1. Analysis.

Comparison 3: Subgroup analysis ‐ Type of CSF (G‐CSF vs. GM‐CSF), Outcome 1: Patients with hospitalization for greater than 10 days
3.2. Analysis.

Comparison 3: Subgroup analysis ‐ Type of CSF (G‐CSF vs. GM‐CSF), Outcome 2: Time to neutrophil recovery
3.4. Analysis.

Comparison 3: Subgroup analysis ‐ Type of CSF (G‐CSF vs. GM‐CSF), Outcome 4: Time to recovering from fever
3.5. Analysis.

Comparison 3: Subgroup analysis ‐ Type of CSF (G‐CSF vs. GM‐CSF), Outcome 5: Time to withdrawal from antibiotics
3.3. Analysis.

Comparison 3: Subgroup analysis ‐ Type of CSF (G‐CSF vs. GM‐CSF), Outcome 3: Duration of grade IV neutropenia
Hospital discharge criteria
We also tested a possible impact of criteria for discharge from hospital on various outcomes. The criteria of discharge after 24 hours of resolution of fever was employed by one study (Ravaud 1998). Three studies used the criteria of discharge after 48 hours of resolution of fever (Biesma 1990; Garcia‐Carbonero 2001; Mayordomo 1995). Three studies used the criteria of discharge after 72 hours of resolution of fever (Mitchell 1997; Riikonen 1994; Vellenga 1996). One study used the criteria of discharge after 96 hours of resolution of fever (Maher 1994). The study by Rodriguez et al used criteria of discharge after at least 48 to 72 hours of resolution of fever (Rodriguez 2005). Four studies did not report the hospital discharge criteria (Anaissie 1996; Arnberg 1998; Aviles 1996; Lopez‐Hernandez 2000). Different criteria required for hospital discharge (time since defervescence) had no effect on any endpoint (Analysis 4.1; Analysis 4.3; Analysis 4.4). We noticed a statistically significant subgroup differences between these trials based on hospital discharge criteria ((discharge after 24 hrs of resolution of fever: RR 0.54 (95% CI 0.35 to 0.84); 1 RCT; 68 participants); discharge after 48 hrs of resolution of fever: RR 0.14 (95% CI 0.04 to 0.48); 2 RCTs; 324 participants); discharge after 72 hrs of resolution of fever: RR 0.80 (95% CI 0.66 to 0.97); 1 RCT; 186 participants); discharge after 96 hrs of resolution of fever: RR 0.52 (95% CI 0.37 to 0.74); 1 RCT; 216 participants)] for the outcome of time to neutrophil recovery with the strongest benefit with discharge after 48 hours of resolution of fever (test of interaction P = 0.006) (Analysis 4.2). However, all these trials trials with varying hospital discharge criterions (discharge after 24, 48, 72, 96hours post fever resolution) favored use of antibiotics and CSF compared with antibiotics alone.
4.1. Analysis.

Comparison 4: Subgroup analysis ‐ Hospital discharge criteria (fever resolution), Outcome 1: Patients with hospitalization for greater than 10 days
4.3. Analysis.

Comparison 4: Subgroup analysis ‐ Hospital discharge criteria (fever resolution), Outcome 3: Duration of grade IV neutropenia
4.4. Analysis.

Comparison 4: Subgroup analysis ‐ Hospital discharge criteria (fever resolution), Outcome 4: Time to recovering from fever
4.2. Analysis.

Comparison 4: Subgroup analysis ‐ Hospital discharge criteria (fever resolution), Outcome 2: Time to neutrophil recovery
Type of malignancy
The trials enrolling participants with mixed cancers (Anaissie 1996; Garcia‐Carbonero 2001; Maher 1994; Mayordomo 1995; Mitchell 1997; Riikonen 1994) favored the use of CSF plus antibiotics (RR 0.64 (95% CI 0.45 to 0.89); 6 RCTs; 884 participants) compared with trial (Yoshida 1999) enrolling participants with only hematological malignancies (RR 1.17 (95% CI 0.81 to 1.70); 1 RCT; 203 participants) (test of interaction P = 0.02) for the outcome of number of participants hospitalized for more than 10 days (Analysis 5.1). We noticed a statistically significant subgroup differences between trials based on type of malignancy [(mix tumors: SMD ‐1.50 (95% CI ‐2.50 to ‐0.50); 7 RCTs; 948 participants); (hematological malignancies: SMD ‐4.55 (95% CI ‐5.24 to ‐3.86); 1 RCT; 119 participants); (solid tumors: SMD ‐0.53 (95% CI ‐1.01 to ‐0.04); 1 RCT; 68 participants) for the outcome of duration of grade IV neutropenia (ANC < 500/mm3) with stronger benefit for people with hematological malignancies (test of interaction P < 0.00001) (Analysis 5.3). However, all of these trials enrolling participants with various different kinds of cancers (mix versus hematological malignancies versus solid tumors) favored use of antibiotics and CSF compared with antibiotics alone. Our results did not change based on the type of malignancy for any of the other outcomes with significant heterogeneity (Analysis 5.2; Analysis 5.4).
5.1. Analysis.

Comparison 5: Subgroup analysis ‐ Type of malignancy, Outcome 1: Patients with hospitalisation for greater than10 days
5.3. Analysis.

Comparison 5: Subgroup analysis ‐ Type of malignancy, Outcome 3: Duration of grade IV neutropenia
5.2. Analysis.

Comparison 5: Subgroup analysis ‐ Type of malignancy, Outcome 2: Time to neutrophil recovery
5.4. Analysis.

Comparison 5: Subgroup analysis ‐ Type of malignancy, Outcome 4: Time to recovering from fever
Sensitivity analyses
Our results did not change based on the methodological quality of studies for any outcomes. We have shown the analysis for the outcome of participants hospitalized for more than 10 days for allocation concealment (Analysis 6.1) and blinding (Analysis 6.2) for illustration purposes.
6.1. Analysis.

Comparison 6: Sensitivity analysis ‐ Patients hospitalized for more than 10 days by allocation concealment and blinding, Outcome 1: Adequacy of allocation concealment
6.2. Analysis.

Comparison 6: Sensitivity analysis ‐ Patients hospitalized for more than 10 days by allocation concealment and blinding, Outcome 2: Blinding
Discussion
Summary of main results
This systematic review summarized the totality of the evidence for the use of CSFs plus antibiotics in the treatment of chemotherapy‐induced febrile neutropenia among people with cancer. Overall and infection‐related mortality were not influenced by the addition of CSF to antibiotics. Participants treated with a CSF and antibiotics were less likely to be hospitalized for more than 10 days and had more participants with a faster neutrophil recovery than those treated with antibiotics alone. Similarly, people receiving CSF and antibiotics had shorter duration of neutropenia, more rapid recovery from fever and shorter duration of antibiotics use compared with people receiving antibiotics alone. The addition of CSF to antibiotics was well tolerated by participants: we found no significant difference in the incidence of DVT in individuals treated with CSF and antibiotics compared with those treated with antibiotics alone. However, we noticed a higher incidence of bone or joint pain, or flu‐like symptoms among participants receiving CSF and antibiotics compared with those receiving antibiotics alone.
In our meta‐analysis, a CSF plus antibiotics was found to reduce the number of participants hospitalized for more than 10 days, a finding sustained in subgroup and sensitivity analyses. In particular, when meta‐analysis was restricted to trials with an adequate allocation concealment and to double‐blind trials, the two most powerful ways of avoiding bias, the result was unchanged (Mhaskar 2012). The use of different criteria for hospital discharge also had no effect on this outcome. This benefit of CSF in shortening the duration of hospitalization has the potential to further influence current clinical practice. Shortening hospitalization translates into a reduction in costs, but this has to be weighed against the cost of CSF; hence, an cost effectiveness analysis should be performed in the light of this finding. A shorter duration in hospital may also translate into a better quality of life for the individual (Smeenk 1998); however, we did not perform a formal analysis of quality of life.
A link between use of CSFs and an increase in the number of participants achieving neutrophil recovery was expected, but this is the first time that such an increase has been linked to clinical benefit, as defined by a shorter duration of hospitalization. Our findings show the benefit of using CSF in reducing the duration of fever and time to withdrawal from antibiotics. This has the potential to influence the decision about the use of CSFs, since the antibiotics used can be associated with substantial costs. Median time to antibiotic withdrawal was statistically significantly shorter in the intervention group than in the control group in all trials, representing a clinical benefit. Treatment‐related harms, such as bone and joint pain, flu‐like syndromes, were common and in some reports intense, but were not life‐threatening.
In summary, this systematic review highlights the beneficial effect of a CSF added to antibiotics in terms of reducing the duration of hospitalization and expediting neutrophil recovery, compared with antibiotics alone, in participants diagnosed with chemotherapy‐induced febrile neutropenia. We think it would be highly desirable that individual patient data meta‐analyses is carried out in order to further explore the impact of CSF on overall and infection‐related mortality among this group of individuals.
To augment the main clinical effectiveness systematic review we sought to identify economic evaluations, to support a Brief Economic Commentary, that have compared the use of a CSF plus antibiotics versus antibiotics alone for the treatment of individuals with established chemotherapy‐induced febrile neutropenia. Systematic supplemental searches were undertaken to identify evaluations relevant to this question. Results were screened using the review inclusion criteria, less study design, however, no full economic evaluations were identified. Several of the included RCTs identified economic benefits regarding a reduction in overall length of stay attributable to the use of CSF plus antibiotics, however they fell short of undertaking a full economic evaluation.
Overall completeness and applicability of evidence
Our analysis included 14 trials (15 comparisons) with a total of 1553 participants, 797 of whom were randomized to receive CSF plus antibiotics and 756 to antibiotics alone. For this update we conducted a comprehensive search of the literature without any language restrictions and we believe that there is low risk of potential publication bias and we found no evidence of this via the funnel plots. The conclusions we reach in this analysis have direct application to clinical practice for people diagnosed with chemotherapy‐induced febrile neutropenia. Our findings clearly demonstrate the superiority of supplementing antibiotics with a CSF compared with the use of antibiotics alone, with regard to reducing the duration of hospitalization and expediting neutrophil recovery.
Quality of the evidence
We assessed the quality of the included trials according to previously described quality domains (Figure 2). The majority of included trials were not free of selection bias. Most included studies reported analyses according to the ITT principle, but some were not blinded and had high risks of performance and detection bias. Majority of the included trials were free of other biases. We considered the overall quality of evidence for a majority of the outcomes to be moderate to low across various outcomes according to the The Grading of Recommendations Assessment, Development and Evaluation (GRADE) criteria (Table 1). The main reasons to downgrade the quality of evidence were inconsistency across the included studies and imprecision of results.
We noted via a sub‐group analysis that participants diagnosed with hematological malignancies appeared to statistically non‐significantly benefit more from the addition of CSF to antibiotics than those with solid and mix tumors for the outcome of overall mortality (Analysis 5.5). However, this possible beneficial effect of CSF on mortality in people diagnosed with hematological malignancies was highly influenced by the results of one trial (Aviles 1996). In this study, three times more participants died in the control group than in the intervention group (Aviles 1996). This difference between groups is much higher than that seen in other, similar studies, and concerns regarding the comparability of the two groups investigated in this trial have been raised (Rubenstein 2000). Also in this trial by Aviles et al all deaths were considered to be due to infection (Aviles 1996). Hence, we recommend caution in drawing a definitive conclusion about a possible beneficial effect of CSF on the outcome of overall mortality in individuals with hematological tumors. We noticed substantial heterogeneity for the outcome of number of participants hospitalized for more than 10 days. We explored the possible causes of heterogeneity to determine whether it was appropriate to pool the trials for this outcome. All trials but one trial (Yoshida 1999) had a point estimate that favored the intervention group. This trial enrolled only people with hematological malignancies. We explored the impact of type of malignancy on number of participants hospitalized for more than 10 days (Subgroup analysis and investigation of heterogeneity: type of malignancy). We also noticed that only two reached statistical significance (Maher 1994; Mayordomo 1995). By inspecting the forest plot, we could detect that trial by Mayordomo et al (Mayordomo 1995) and Maher et al (Maher 1994) indicated a much stronger effect than that detected in all other trials. We therefore repeated our analysis, excluding these trials (Maher 1994; Mayordomo 1995). The exclusion resulted in a substantial reduction in the statistical heterogeneity (I2 = 48%; P = 0.10) and the significance of the effect of CSF plus antibiotics on this outcome disappeared (RR 0.85, 95% CI = 0.61 to 1.17; P = 0.32). All but two studies (Maher 1994; Mayordomo 1995) favored the use of CSF plus antibiotics over the use of antibiotics alone, and the heterogeneity detected was mainly due to a superior effect of CSF plus antibiotics detected in these trials. Hence, our interpretation of these results is that CSF plus antibiotics significantly reduce the number of participants hospitalized for more than 10 days compared to the use of antibiotics alone, but that the magnitude of this effect cannot be precisely estimated using the currently available data. We also noticed considerable heterogeneity for the outcome of time to withdrawal from antibiotics. We noted that in all of the trials for this outcome the mean/median duration of ATB use was similar (mean of 5 days) among patients receiving CSF(Garcia‐Carbonero 2001; Mitchell 1997; Ravaud 1998). However, the high precision observed around the effect size in the trial by Garcio‐Carbonero et al (Garcia‐Carbonero 2001) compared with other two trial estimates was potentially leading to the substantial heterogeneity. The heterogeneity disappeared completely once we removed this trial from the analysis (P value = 0.72, I2 = 0%) and the overall effect still showed benefit with the use of CSF plus ATB compared with ATB alone.
5.5. Analysis.

Comparison 5: Subgroup analysis ‐ Type of malignancy, Outcome 5: Overall mortality
Potential biases in the review process
We did not find any methodological issues in the preparation of the review that could put it at risk for bias.
Agreements and disagreements with other studies or reviews
In 2002, Berghmans et al published a systematic review that addressed the question of the addition of CSF to antibiotics in the treatment of individuals with febrile neutropenia (Berghmans 2002). This systematic review identified 11 trials and performed a meta‐analysis of mortality that included nine trials. The RR for mortality was 0.71 (95% CI 0.44 to 1.15). This result is similar to our findings. However, Berghmans et al failed to extract and include mortality data from two studies (Biesma 1990; Ravaud 1998). In addition, Berghmans et al used a very restricted search strategy (Berghmans 2002): it included only articles in English, published up to 1998, and did not search relevant abstracts and conference proceedings. These restrictions related to the search methods may have led to the failure to include three studies satisfying the study inclusion criteria (Garcia‐Carbonero 2001; Lopez‐Hernandez 2000; Montalar 1998). Although the pooled results for mortality in Berghmans et al do not differ from ours, our analysis is broader (in that we included additional endpoints) and up‐to‐date (we included the trial by Rodriguez 2005) compared with the systematic review by Berghmans et al (Berghmans 2002).
Authors' conclusions
Implications for practice.
The use of CSF plus antibiotics in people with chemotherapy‐induced febrile neutropenia was not found to improve overall survival compared with antibiotics alone but did reduce the time participants spent in hospital and the duration neutropenia, time to recovering from fever and time to withdrawal from antibiotics. The people receiving CSF plus antibiotics also had a faster neutrophil recovery compared with people receiving antibiotics alone. The incidence of adverse events with CSF in addition to antibiotics appear to be similar compared with antibiotics alone. The impact of CSF plus antibiotics on infection‐related mortality was not clear.
Implications for research.
Following these results, it is unlikely that future trials will feature a no‐treatment control group. To assess the effect of CSF plus antibiotics on mortality therefore, an individual patient data analysis is highly desirable. The current scarcity of relevant economic evaluations highlights an evidence gap and the need for further research fully explore the cost‐effectiveness of these treatment alternatives.
What's new
| Date | Event | Description |
|---|---|---|
| 5 January 2022 | Amended | No longer for update as any future update will require the development of a new protocol reflecting current Cochrane methodological criteria. |
History
Protocol first published: Issue 2, 2002 Review first published: Issue 3, 2003
| Date | Event | Description |
|---|---|---|
| 27 July 2016 | Amended | Brief Economic Commentary added. |
| 30 October 2014 | Amended | Minor text amendment |
| 23 April 2013 | New search has been performed | Review text updated |
| 23 April 2013 | New citation required but conclusions have not changed | New search identified one additional study for inclusion. Conclusions unchanged. |
| 13 October 2008 | Amended | Converted to new review format. |
| 12 July 2000 | New citation required and conclusions have changed | Substantive amendment |
Acknowledgements
We would like to thank Dr Chris Williams for his enthusiastic support at the very beginning of this study; Jane Dennis and Machiko Miyakoshi for translating a Japanese article; and Yemi Agboola for the cooperative effort in locating the studies. We would like to thank Mrs Coralia Vázquez‐Otero (from the USF Center for EBM) for translating an article that was published in Spanish.
We would also like to thank the Cochrane Gynaecological, Neuro‐oncology and Orphan Cancers Editorial Team for their contribution to the editorial process for the original review and this update.
We would also like to thank Dawn Craig and Luke Vale (Institute of Health & Society, Newcastle University) and Ian Shemilt (Economic and Evidence Synthesis Methodology, EPPI‐Centre, University College London) for their help in preparing the Brief Economic Commentary.
This project was supported by the National Institute for Health Research, via Cochrane Infrastructure to the Cochrane Gynaecological, Neuro‐oncology and Orphan Cancer Group. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS or the Department of Health.
Appendices
Appendix 1. Search strategy CENTRAL
"CSF" AND febrile neutropenia
Appendix 2. Search strategy MEDLINE
To the methodological search strategy of each database we add the specific terms pertinent to this review (see below). Search strategy: #1 (Methodological search strategy) # 2 explode COLONY‐STIMULATING‐FACTORS / all subheadings #3 CSF #4 #2 OR #3 #5 explode FEVER / all subheadings #6 FEVER* OR FEBR* #7 #5 OR #6 #8 #4 AND #7 #9 #1 AND #8
Appendix 3. Search strategy EMBASE
COLONY STIMULATING FACTORS AND febrile neutropenia
Appendix 4. Search strategy CANCERLIT
COLONY STIMULATING FACTORS AND febrile neutropenia
Appendix 5. Search strategy LILACS
tw:(tw:( (tw:(colony stimulating factors)) AND neutropenia) AND (instance:"regional")) AND (instance:"regional")
Appendix 6. Search strategy SCI
COLONY STIMULATING FACTORS AND febrile neutropenia (Limits: content type: Journal)
Appendix 7. Economic evaluation search filter
1. Economics/ 2. exp "costs and cost analysis"/ 3. Economics, Dental/ 4. exp economics, hospital/ 5. Economics, Medical/ 6. Economics, Nursing/ 7. Economics, Pharmaceutical/ 8. (economic$ or cost or costs or costly or costing or price or prices or pricing or pharmacoeconomic$ or cost‐effectiveness).ti,ab. 9. (expenditure$ not energy).ti,ab. 10. value for money.ti,ab. 11. budget$.ti,ab. 12. (fiscal or funding or financial or finance).tw. 13. (unit adj cost$).mp. 14. Ec.fs. 15. or/1‐14 16. ((energy or oxygen) adj cost).ti,ab. 17. (metabolic adj cost).ti,ab. 18. ((energy or oxygen) adj expenditure).ti,ab. 19. or/16‐18 20. 15 not 19 21. letter.pt. 22. editorial.pt. 23. historical article.pt. 24. or/21‐23 25. 20 not 24 26. exp animals/ not humans/ 27. 25 not 26
Data and analyses
Comparison 1. Benefits and harms ‐ CSF + antibiotics vs antibiotics alone.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 Overall mortality | 13 | 1335 | Hazard Ratio (IV, Random, 95% CI) | 0.74 [0.47, 1.16] |
| 1.2 Infection related mortality | 10 | 897 | Hazard Ratio (IV, Random, 95% CI) | 0.75 [0.47, 1.20] |
| 1.3 Patients with hospitalization for greater than10 days | 7 | 1087 | Risk Ratio (M‐H, Random, 95% CI) | 0.65 [0.44, 0.95] |
| 1.4 Time to neutrophil recovery | 5 | 794 | Risk Ratio (M‐H, Random, 95% CI) | 0.52 [0.34, 0.81] |
| 1.5 Duration of grade IV neutropenia | 9 | 1135 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.70 [‐2.65, ‐0.76] |
| 1.6 Time to recovering from fever | 9 | 966 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.49 [‐0.90, ‐0.09] |
| 1.7 Time to withdrawal from antibiotics | 3 | 457 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.50 [‐2.83, ‐0.18] |
| 1.8 Deep vein thrombosis | 4 | 389 | Risk Ratio (M‐H, Random, 95% CI) | 1.68 [0.72, 3.93] |
| 1.9 Bone and joint pain or flu‐like symptoms | 6 | 622 | Risk Ratio (M‐H, Random, 95% CI) | 1.59 [1.04, 2.42] |
Comparison 2. Subgroup analysis ‐ Study population (children vs. adults).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 2.1 Patients with hospitalization for greater than10 days | 7 | 1087 | Risk Ratio (M‐H, Random, 95% CI) | 0.65 [0.44, 0.95] |
| 2.1.1 Adults | 5 | 843 | Risk Ratio (M‐H, Random, 95% CI) | 0.65 [0.41, 1.03] |
| 2.1.2 Children | 2 | 244 | Risk Ratio (M‐H, Random, 95% CI) | 0.61 [0.24, 1.53] |
| 2.2 Time to neutrophil recovery | 5 | 794 | Risk Ratio (M‐H, Random, 95% CI) | 0.52 [0.34, 0.81] |
| 2.2.1 Adults | 4 | 608 | Risk Ratio (M‐H, Random, 95% CI) | 0.45 [0.29, 0.70] |
| 2.2.2 Children | 1 | 186 | Risk Ratio (M‐H, Random, 95% CI) | 0.80 [0.66, 0.97] |
| 2.3 Duration of grade IV neutropenia | 9 | 1135 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.70 [‐2.65, ‐0.76] |
| 2.3.1 Adults | 7 | 891 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.65 [‐2.75, ‐0.55] |
| 2.3.2 Children | 2 | 244 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.93 [‐3.87, 0.02] |
| 2.4 Time to recovering from fever | 9 | 966 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.49 [‐0.90, ‐0.09] |
| 2.4.1 Adults | 6 | 682 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.56 [‐1.15, 0.03] |
| 2.4.2 Children | 2 | 244 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.21 [‐0.46, 0.04] |
| 2.4.3 Adults and Children | 1 | 40 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.69 [‐1.33, ‐0.05] |
Comparison 3. Subgroup analysis ‐ Type of CSF (G‐CSF vs. GM‐CSF).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 3.1 Patients with hospitalization for greater than 10 days | 7 | 1087 | Risk Ratio (M‐H, Random, 95% CI) | 0.65 [0.44, 0.95] |
| 3.1.1 G‐CSF | 5 | 868 | Risk Ratio (M‐H, Random, 95% CI) | 0.79 [0.51, 1.23] |
| 3.1.2 GM‐CSF | 3 | 219 | Risk Ratio (M‐H, Random, 95% CI) | 0.40 [0.18, 0.92] |
| 3.2 Time to neutrophil recovery | 5 | 794 | Risk Ratio (M‐H, Random, 95% CI) | 0.52 [0.34, 0.81] |
| 3.2.1 G‐CSF | 4 | 665 | Risk Ratio (M‐H, Random, 95% CI) | 0.54 [0.31, 0.94] |
| 3.2.2 GM‐CSF | 2 | 129 | Risk Ratio (M‐H, Random, 95% CI) | 0.28 [0.04, 2.21] |
| 3.3 Duration of grade IV neutropenia | 9 | 1135 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.70 [‐2.65, ‐0.76] |
| 3.3.1 G‐CSF | 5 | 784 | Std. Mean Difference (IV, Random, 95% CI) | ‐2.73 [‐4.43, ‐1.04] |
| 3.3.2 GM‐CSF | 5 | 351 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.67 [‐1.12, ‐0.22] |
| 3.4 Time to recovering from fever | 9 | 966 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.49 [‐0.90, ‐0.09] |
| 3.4.1 G‐CSF | 5 | 621 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.78 [‐1.47, ‐0.08] |
| 3.4.2 GM‐CSF | 5 | 345 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.20 [‐0.58, 0.18] |
| 3.5 Time to withdrawal from antibiotics | 3 | 457 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.50 [‐2.83, ‐0.18] |
| 3.5.1 G‐CSF | 2 | 389 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.86 [‐3.80, 0.07] |
| 3.5.2 GM‐CSF | 1 | 68 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.77 [‐1.27, ‐0.28] |
Comparison 4. Subgroup analysis ‐ Hospital discharge criteria (fever resolution).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 4.1 Patients with hospitalization for greater than 10 days | 5 | 784 | Risk Ratio (M‐H, Random, 95% CI) | 0.53 [0.32, 0.88] |
| 4.1.1 Discharge after 48h of resolution of fever | 2 | 324 | Risk Ratio (M‐H, Random, 95% CI) | 0.26 [0.04, 1.57] |
| 4.1.2 Discharge after 72 hours of the resolution of fever | 2 | 244 | Risk Ratio (M‐H, Random, 95% CI) | 0.61 [0.24, 1.53] |
| 4.1.3 Discharge after 96 hours of the resolution of fever | 1 | 216 | Risk Ratio (M‐H, Random, 95% CI) | 0.57 [0.39, 0.81] |
| 4.2 Time to neutrophil recovery | 5 | 794 | Risk Ratio (M‐H, Random, 95% CI) | 0.52 [0.34, 0.81] |
| 4.2.1 Discharge after 24h of resolution of fever | 1 | 68 | Risk Ratio (M‐H, Random, 95% CI) | 0.54 [0.35, 0.84] |
| 4.2.2 Discharge after 48h of resolution of fever | 2 | 324 | Risk Ratio (M‐H, Random, 95% CI) | 0.14 [0.04, 0.48] |
| 4.2.3 Discharge after 72h of resolution of fever | 1 | 186 | Risk Ratio (M‐H, Random, 95% CI) | 0.80 [0.66, 0.97] |
| 4.2.4 Discharge after 96h of resolution of fever | 1 | 216 | Risk Ratio (M‐H, Random, 95% CI) | 0.52 [0.37, 0.74] |
| 4.3 Duration of grade IV neutropenia | 8 | 1016 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.39 [‐2.29, ‐0.50] |
| 4.3.1 Discharge after 24 hours of resolution of fever | 1 | 68 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.53 [‐1.01, ‐0.04] |
| 4.3.2 Discharge after 48 hours of resolution of fever | 3 | 354 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.87 [‐3.66, ‐0.07] |
| 4.3.3 Discharge after 72 hours of resolution of fever | 3 | 378 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.38 [‐3.04, 0.28] |
| 4.3.4 Discharge after 96 hours of resolution of fever | 1 | 216 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.44 [‐0.71, ‐0.17] |
| 4.4 Time to recovering from fever | 7 | 807 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.27 [‐0.55, 0.01] |
| 4.4.1 Discharge after 24 hours of resolution of fever | 1 | 68 | Std. Mean Difference (IV, Random, 95% CI) | 0.00 [‐0.48, 0.48] |
| 4.4.2 Discharge after 48 hours of resolution of fever | 2 | 145 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.72 [‐1.41, ‐0.02] |
| 4.4.3 Discharge after 72 hours of resolution of fever | 3 | 378 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.14 [‐0.34, 0.07] |
| 4.4.4 Discharge after 96 hours of resolution of fever | 1 | 216 | Std. Mean Difference (IV, Random, 95% CI) | 0.00 [‐0.27, 0.27] |
Comparison 5. Subgroup analysis ‐ Type of malignancy.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 5.1 Patients with hospitalisation for greater than10 days | 7 | 1087 | Risk Ratio (M‐H, Random, 95% CI) | 0.65 [0.44, 0.95] |
| 5.1.1 Mix | 6 | 884 | Risk Ratio (M‐H, Random, 95% CI) | 0.58 [0.39, 0.87] |
| 5.1.2 Hematological malignancies | 1 | 203 | Risk Ratio (M‐H, Random, 95% CI) | 1.17 [0.81, 1.70] |
| 5.2 Time to neutrophil recovery | 5 | 794 | Risk Ratio (M‐H, Random, 95% CI) | 0.52 [0.34, 0.81] |
| 5.2.1 Mix | 4 | 726 | Risk Ratio (M‐H, Random, 95% CI) | 0.48 [0.26, 0.87] |
| 5.2.2 Solid tumors | 1 | 68 | Risk Ratio (M‐H, Random, 95% CI) | 0.54 [0.35, 0.84] |
| 5.3 Duration of grade IV neutropenia | 9 | 1135 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.70 [‐2.65, ‐0.76] |
| 5.3.1 Mix | 7 | 948 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.50 [‐2.50, ‐0.50] |
| 5.3.2 Hematological malignancies | 1 | 119 | Std. Mean Difference (IV, Random, 95% CI) | ‐4.55 [‐5.24, ‐3.86] |
| 5.3.3 Solid tumors | 1 | 68 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.53 [‐1.01, ‐0.04] |
| 5.4 Time to recovering from fever | 9 | 966 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.49 [‐0.90, ‐0.09] |
| 5.4.1 Mix | 6 | 739 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.31 [‐0.62, 0.00] |
| 5.4.2 Hematological malignancies | 2 | 159 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.32 [‐2.53, ‐0.12] |
| 5.4.3 Solid tumors | 1 | 68 | Std. Mean Difference (IV, Random, 95% CI) | 0.00 [‐0.48, 0.48] |
| 5.5 Overall mortality | 13 | 1335 | Hazard Ratio (IV, Random, 95% CI) | 0.74 [0.47, 1.16] |
| 5.5.1 Mix | 10 | 1108 | Hazard Ratio (IV, Random, 95% CI) | 1.03 [0.60, 1.75] |
| 5.5.2 Hematological malignancies | 2 | 159 | Hazard Ratio (IV, Random, 95% CI) | 0.32 [0.13, 0.78] |
| 5.5.3 Solid tumors | 1 | 68 | Hazard Ratio (IV, Random, 95% CI) | 0.14 [0.00, 6.82] |
Comparison 6. Sensitivity analysis ‐ Patients hospitalized for more than 10 days by allocation concealment and blinding.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 6.1 Adequacy of allocation concealment | 7 | 1087 | Risk Ratio (M‐H, Random, 95% CI) | 0.65 [0.44, 0.95] |
| 6.1.1 Adequate allocation concealment | 4 | 726 | Risk Ratio (M‐H, Random, 95% CI) | 0.57 [0.32, 1.00] |
| 6.1.2 Inadequate allocation concealment | 3 | 361 | Risk Ratio (M‐H, Random, 95% CI) | 0.73 [0.41, 1.31] |
| 6.2 Blinding | 7 | 1087 | Risk Ratio (M‐H, Random, 95% CI) | 0.65 [0.44, 0.95] |
| 6.2.1 Blinded studies | 3 | 460 | Risk Ratio (M‐H, Random, 95% CI) | 0.61 [0.39, 0.93] |
| 6.2.2 Non‐Blinded studies | 4 | 627 | Risk Ratio (M‐H, Random, 95% CI) | 0.65 [0.36, 1.16] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Anaissie 1996.
| Study characteristics | ||
| Methods | Not placebo‐controlled, not blinded, industry‐sponsored, single‐center trial | |
| Participants | Adults; mixed tumors; ANC < 1 x 109/L | |
| Interventions | GM‐CSF (Sandoz) 3 mcg/kg IV ATB: Ticarcilin plus Netilmicin |
|
| Outcomes | Overall mortality Infection‐related mortality People with hospitalization for greater than 10 days | |
| Notes | Hospital discharge criteria: Not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated sequence of numbers was used |
| Allocation concealment (selection bias) | Unclear risk | We were not able to assess the adequacy of allocation concealment based on the information provided in the article |
| Blinding (performance bias and detection bias) All outcomes | High risk | There was no blinding |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | There was no blinding |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | There was no blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | ITT analysis used, Withdrawals are described |
| Other bias | High risk | Prespecified values of sample size, alpha and beta errors were not provided |
Arnberg 1998.
| Study characteristics | ||
| Methods | Placebo‐controlled, not blinded, industry and public‐sponsored, single‐center trial | |
| Participants | Adults; mixed tumors; ANC < 1 x 109/L | |
| Interventions | GM‐CSF (not specified) 5.5 mcg/kg SC ATB: Many different antibiotics |
|
| Outcomes | Overall mortality Bone and joint pain or flu‐like symptoms Deep vein thrombosis | |
| Notes | Hospital discharge criteria: not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | We were not able to assess the adequacy of method of sequence generation based on the information provided in the article |
| Allocation concealment (selection bias) | Unclear risk | We were not able to assess the adequacy of allocation concealment based on the information provided in the article |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double blinding was employed |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | We were not able to assess who were blinded based on the information provided in the article |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | We were not able to assess the outcomes for which blinding was employed based on the information provided in the article |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | ITT analysis used, withdrawals are described |
| Other bias | Unclear risk | Prespecified values of sample size, alpha and beta errors were not provided |
Aviles 1996.
| Study characteristics | ||
| Methods | Not placebo‐controlled, not blinded, unclear sponsorship, single‐center trial | |
| Participants | Adults; hematologic tumors; ANC < 0.1 x 109/L | |
| Interventions | G‐CSF (not specified) 5 mcg/kg SC ATB: Amikacin plus Ceftazidime |
|
| Outcomes | Overall mortality Infection‐related mortality | |
| Notes | Hospital discharge criteria: not reported Duration of grade IV neutropenia reported as days to absolute granulocyte recovery to > 0.5 x 109/L |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | We were not able to assess the adequacy of method of sequence generation based on the information provided in the article |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes were used |
| Blinding (performance bias and detection bias) All outcomes | High risk | There was no blinding |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | There was no blinding |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | There was no blinding |
| Incomplete outcome data (attrition bias) All outcomes | High risk | ITT analysis was not used, withdrawals are described |
| Other bias | High risk | Prespecified values of sample size, alpha and beta errors were not provided |
Biesma 1990.
| Study characteristics | ||
| Methods | Placebo‐controlled, industry‐sponsored, single‐center trial | |
| Participants | Adults; mixed tumors; ANC < 1 x 109/L | |
| Interventions | GM‐CSF (not specified) 2.8 mcg/kg IV ATB: Tobramicin plus Cefuroxime |
|
| Outcomes | Overall mortality Infection‐related mortality Deep vein thrombosis | |
| Notes | Hospital discharge criteria: afebrile for at least 48 hours Duration of grade IV neutropenia reported as days to absolute granulocyte recovery to > 0.5 x 109/L |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Description of method of sequence generation not provided |
| Allocation concealment (selection bias) | High risk | Description of methods to conceal allocation not provided |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double blinding was employed |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | We were not able to assess who were blinded based on the information provided in the article |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | We were not able to assess the outcomes for which blinding was employed based on the information provided in the article |
| Incomplete outcome data (attrition bias) All outcomes | High risk | ITT analysis was not used, withdrawals are described |
| Other bias | High risk | Prespecified values of sample size, alpha and beta errors were not provided |
Garcia‐Carbonero 2001.
| Study characteristics | ||
| Methods | Not placebo‐controlled, industry‐ and public‐sponsored, multicenter trial | |
| Participants | Adults; mixed tumors; ANC < 0.5 x 109/L | |
| Interventions | G‐CSF (not specified) 5 mcg/kg SC ATB: Amikacin plus Ceftazidime |
|
| Outcomes | Overall mortality Infection‐related mortality People with hospitalization for greater than 10 days Time to neutrophil recovery Deep vein thrombosis | |
| Notes | Hospital discharge criteria: afebrile for at least 48 hours; ANC at least 1000/mm3 Duration of grade IV neutropenia reported as days to ANC > 500mm3 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Sequence was computer generated |
| Allocation concealment (selection bias) | Low risk | Participants were randomly assigned by the consecutive drawing of sequentially numbered, opaque, sealed envelopes |
| Blinding (performance bias and detection bias) All outcomes | High risk | There was no blinding |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | There was no blinding |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | There was no blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | ITT analysis used, withdrawals are described |
| Other bias | Low risk | Prespecified values of sample size, alpha and beta errors were provided |
Lopez‐Hernandez 2000.
| Study characteristics | ||
| Methods | Not placebo‐controlled, unclear funding, single‐center trial | |
| Participants | Adults and children; hematological tumors; ANC < 0.5 x 109/L | |
| Interventions | G‐CSF (filgrastim) 5 mcg/kg SC ATB: Amikacin plus Ceftriaxone |
|
| Outcomes | Overall mortality Infection‐related mortality | |
| Notes | Hospital discharge criteria: not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random sequence was computer generated |
| Allocation concealment (selection bias) | Unclear risk | We were not able to assess the adequacy of allocation concealment based on the information provided in the article |
| Blinding (performance bias and detection bias) All outcomes | High risk | There was no blinding |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | There was no blinding |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | There was no blinding |
| Incomplete outcome data (attrition bias) All outcomes | High risk | ITT analysis not used, Withdrawals are described |
| Other bias | High risk | Prespecified values of sample size, alpha and beta errors were not provided |
Maher 1994.
| Study characteristics | ||
| Methods | Placebo‐controlled, industry‐funded, multicenter trial | |
| Participants | Adults; mixed tumors; ANC < 1 x 109/L | |
| Interventions | G‐CSF (filgrastim) 12 mcg/kg SC ATB: Piperacilin plus Tobramicin |
|
| Outcomes | Overall mortality People with hospitalization for greater than 10 days Bone and joint pain or flu‐like symptoms Time to neutrophil recovery | |
| Notes | Hospital discharge criteria: afebrile for at least 96 hours; ANC at least 500/mm3 Duration of grade IV neutropenia reported as median number of days of neutropenia ANC < 0.5 x 109/L |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Description of method of sequence generation not provided |
| Allocation concealment (selection bias) | Low risk | The randomization code was maintained by the Biostatistical Department of Amgen, Inc., and was not broken until all collected data had been verified by Amgen and its designee, Biomedicus, and had been audited by a separate department at Amgen |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double blinding was employed |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | We were not able to assess who were blinded based on the information provided in the article |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | We were not able to assess the outcomes for which blinding was employed based on the information provided in the article |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | ITT analysis used, withdrawals are described |
| Other bias | Low risk | Prespecified values of sample size, alpha and beta errors were provided |
Mayordomo 1995.
| Study characteristics | ||
| Methods | Placebo‐controlled, industry‐funded, single‐center trial | |
| Participants | Adults; mixed tumors; ANC < 0.5 x 109/L | |
| Interventions | G‐CSF (filgrastim) 5 mcg/kg IV
OR
GM‐CSF (molgramostim) 5 mcg/kg IV ATB: Amikacin + Ceftazidime |
|
| Outcomes | Overall mortality Infection‐related mortality People with hospitalization for greater than 10 days Bone and joint pain or flu‐like symptoms Time to neutrophil recovery | |
| Notes | Hospital discharge criteria: afebrile for at least 48 hours; ANC at least 1000/mm3 Duration of grade IV neutropenia reported as median number of days of neutropenia ANC < 0.5 x 109/L |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Description of method of sequence generation not provided |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes were used |
| Blinding (performance bias and detection bias) All outcomes | High risk | There was no blinding |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | There was no blinding |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | There was no blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | ITT analysis used, withdrawals are described |
| Other bias | Low risk | Prespecified values of sample size, alpha and beta errors were provided |
Mitchell 1997.
| Study characteristics | ||
| Methods | Placebo‐controlled, industry‐funded, multicenter trial | |
| Participants | Children; mixed tumors; ANC < 0.5 x 109/L | |
| Interventions | G‐CSF (filgrastim) 5 mcg/kg IV ATB: Gentamicin plus Piperacilin plus Flucloxacilin OR Gentamicin plus Imipenen‐Cilastatin |
|
| Outcomes | Overall mortality People with hospitalization for greater than 10 days Time to neutrophil recovery | |
| Notes | Hospital discharge criteria: afebrile for at least 72 hours; ANC at least 200/mm3 Duration of grade IV neutropenia reported as days to absolute granulocyte recovery to > 0.5 x 109/L |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Description of method of sequence generation not provided |
| Allocation concealment (selection bias) | Low risk | The study drug and placebo were delivered to the ward in identically labeled syringes, and the staff and investigators were unaware of treatment allocation until the study had been completed. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double blinding was employed |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Staff and investigators were unaware of treatment allocation until the study had been completed |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | We were not able to assess the outcomes for which blinding was employed based on the information provided in the article |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | ITT analysis used, withdrawals are described |
| Other bias | Low risk | Prespecified values of sample size, alpha and beta errors were provided |
Ravaud 1998.
| Study characteristics | ||
| Methods | Not placebo‐controlled, industry‐funded, multicenter trial | |
| Participants | Adults; solid tumors; ANC < 1 x 109/L | |
| Interventions | GM‐CSF (molgramostim) 5 mcg/kg SC ATB: Amikacin plus Ceftriaxone OR Piperacilin plus Ofloxacin for people receiving Cisplatin |
|
| Outcomes | Overall mortality Infection‐related mortality Bone and joint pain or flu‐like symptoms Time to neutrophil recovery | |
| Notes | Hospital discharge criteria: afebrile for at least 24 hours; ANC at least 1000/mm3 Duration of grade IV neutropenia reported as median number of days of neutropenia ANC < 0.5 x 109/L |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Description of method of sequence generation is not provided |
| Allocation concealment (selection bias) | Unclear risk | We were not able to assess the adequacy of allocation concealment based on the information provided in the article |
| Blinding (performance bias and detection bias) All outcomes | High risk | There was no blinding |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | There was no blinding |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | There was no blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | ITT analysis used, withdrawals are described |
| Other bias | Low risk | Prespecified values of sample size, alpha and beta errors were provided |
Riikonen 1994.
| Study characteristics | ||
| Methods | Placebo‐controlled, public‐funded, multi‐center trial | |
| Participants | Children, mixed tumors; ANC < 0.2 x 109/L | |
| Interventions | GM‐CSF (Sandoz) 5 mcg/kg IV ATB: Imipenen‐Cilastatin |
|
| Outcomes | Overall mortality Infection‐related mortality Duration of hospitalization Bone and joint pain or flu‐like symptoms | |
| Notes | Hospital discharge criteria: afebrile for at least 72 hours; ANC at least 500/mm3 Duration of grade IV neutropenia reported as days to absolute granulocyte recovery to > 0.5 x 109/L |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | We were not able to assess the adequacy of method of sequence generation based on the information provided in the article |
| Allocation concealment (selection bias) | Unclear risk | We were not able to assess the adequacy of allocation concealment based on the information provided in the article |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double blinding was employed |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | We were not able to assess who were blinded based on the information provided in the article |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | We were not able to assess the outcomes for which blinding was employed based on the information provided in the article |
| Incomplete outcome data (attrition bias) All outcomes | High risk | ITT analysis not used, withdrawals are described |
| Other bias | Low risk | Prespecified values of sample size, alpha and beta errors were provided |
Rodriguez 2005.
| Study characteristics | ||
| Methods | Not‐placebo controlled, unclear funding, single‐center trial | |
| Participants | Children, mixed tumors, ANC ≤ 0.5 x 109/L | |
| Interventions | G‐CSF (Neupogen (Filgrastim), Roche) 5 mcg/kg/day IV ATB: Cloxacillin / Cefotaxim / Amikacin. Vancomycin for people with previous infection due to Gram‐positive cocci resistant to Oxacilllin. Metronidazol for people with diarrhea |
|
| Outcomes | Overall mortality
Infection‐related mortality Duration of hospitalization |
|
| Notes | Hospital discharge criteria: afebrile for at least 48 to 72 hours | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | We were not able to assess the adequacy of method of sequence generation based on the information provided in the article |
| Allocation concealment (selection bias) | Unclear risk | We were not able to assess the adequacy of allocation concealment based on the information provided in the article |
| Blinding (performance bias and detection bias) All outcomes | High risk | There was no blinding |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | There was no blinding |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | There was no blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | ITT analysis used, withdrawals are not described |
| Other bias | Low risk | Prespecified values of sample size, alpha and beta errors were provided |
Vellenga 1996.
| Study characteristics | ||
| Methods | Placebo‐controlled, public‐ and industry‐funded, multicenter trial | |
| Participants | Adults; mixed tumors; ANC < 0.5 x 109/L | |
| Interventions | GM‐CSF (Sandoz) 5 mcg/kg SC ATB: Imipenem, cefuroxime in combination with an aminoglycoside, Augmentin in combination with an aminoglycoside, and Ceftazidime |
|
| Outcomes | Overall mortality Infection‐related mortality Duration of hospitalization Deep vein thrombosis Bone and joint pain or flu‐like symptoms | |
| Notes | Hospital discharge criteria: afebrile for at least 72 hours; ANC at least 1000/mm3 Duration of grade IV neutropenia reported as median number of days of neutropenia ANC < 0.5 x 109/L |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Description of method of sequence generation is not provided |
| Allocation concealment (selection bias) | Unclear risk | Description of method of allocation concealment is not provided |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double blinding was employed |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | We were not able to assess who were blinded based on the information provided in the article |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | We were not able to assess the outcomes for which blinding was employed based on the information provided in the article |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | ITT analysis used, withdrawals are described |
| Other bias | Low risk | Prespecified values of sample size, alpha and beta errors were provided |
Yoshida 1999.
| Study characteristics | ||
| Methods | Not placebo‐controlled, unclear funding, multicenter trial | |
| Participants | Adults; hematological tumors; ANC < 1 x 109/L | |
| Interventions | G‐CSF (filgrastim or lenograstim) variable doses, IV ATB: Flomoxef Sodium plus Tobramicin |
|
| Outcomes | Duration of hospitalization | |
| Notes | Hospital discharge criteria: not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Description of method of sequence generation is not provided |
| Allocation concealment (selection bias) | High risk | Description of method of allocation concealment is not provided |
| Blinding (performance bias and detection bias) All outcomes | High risk | There was no blinding |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | There was no blinding |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | There was no blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | ITT analysis used, withdrawals are described |
| Other bias | High risk | Prespecified values of sample size, alpha and beta errors were not provided |
ATB = Antibiotics; ITT = intention to treat analysis; ANC = absolute neutrophil count; G = granulocyte; M = macrophage; CSF = colony‐stimulating factor; IV = intravenous; SC = subcutaneous
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Balcerska 1995 | Not randomized |
| Beveridge 1998 | Studied non‐febrile people and did not have a no‐therapy group |
| Bodey 1994 | Duplicate publication of Anaissie 1996 |
| Feng 1998 | Cross‐over study |
| Fengyi 1998 | Cross‐over study |
| Garcia‐Carb 1999a | Duplicate publication of Garcia‐Carbonero 2001 |
| Garcia‐Carb 1999b | Duplicate publication of Garcia‐Carbonero 2001 |
| Gebbia 1994 | Included non‐neutropenic participants. The treatment began immediately after chemotherapy |
| Gunay 1998 | Not randomized |
| Herrmann 1990 | Not randomized |
| Kaku 1993 | People who had developed febrile neutropenia were randomized to receive CSF only after the next cycle of chemotherapy |
| Kawa 1999 | Randomized participants were to receive CSF before or after neutropenia developed |
| Kotake 1999 | Not randomized |
| Mayordomo 1992 | Duplicate publication of Garcia‐Carbonero 2001 |
| Mayordomo 1993 | Duplicate publication of Garcia‐Carbonero 2001 |
| Michon 1998 | Did not included people with febrile neutropenia |
| Montalar 1998 | Data not extractable |
| Moriyama 1993 | Not randomized |
| Motoyoshi 1986 | Cross‐over trial |
| Nakajima 1995 | Also included people who had documented infection but were not neutropenic |
| Ohno 1997 | Included non‐neutropenic people. The treatment began immediately after chemotherapy |
| Oshita 2000 | Participants were randomized to receive CSF after the development of monocytopenia or leukopenia |
| Ravaud 1995 | Duplicate of Ravaud 1998 |
| Schroder 1999 | Not randomized |
| Soda 1996 | Randomized participants to receive CSF before or after the neutropenia developed |
| Timmer‐Bonte 2005 | A randomized trial addressing the prophylactic role of G‐CSF |
| Torrecillas 1998 | Participants were randomized to duration of CSF use |
| Uyl‐de Groot 1997 | Duplicate publication of Vellenga 1996 |
| van Pelt 1997 | Not neutropenic participants |
| Vellenga 1996b | Duplicate publication of Vellenga 1996 |
| Yalcin 1996 | Not randomized |
| Yamazaki 1989 | Not neutropenic participants |
Differences between protocol and review
None
Contributions of authors
For this update: RM performed the search for articles, selected articles, extracted and analyzed data, wrote the first and final draft of the manuscript. OACC, TAEB and LMP performed the search for articles, selected articles, extracted and analyzed data and approved the final version. GL designed the study and approved the final version. OACC and RM performed the search for articles, selected articles, extracted data, and approved the final version. BD designed the study, wrote the protocol, performed the search for articles, selected articles, extracted and analyzed data, wrote the manuscript and approved the final version.
All authors contributed to the analysis and interpretation of data and results.
Sources of support
Internal sources
H Lee Moffitt Cancer Center, USA
Center for Evidence based Medicine and Health Outcomes Research, University of South Florida, USA
External sources
No sources of support provided
Declarations of interest
OACC ‐ None known BD ‐ None known TEAB ‐ None known GL ‐ PI on research grant to the Fred Hutchinson Cancer Research Center, Seattle, WA, USA RM ‐ None known LMP ‐ None known
Edited (no change to conclusions)
References
References to studies included in this review
Anaissie 1996 {published data only}
- Anaissie EJ, Vartivarian S, Bodey GP, Legrand C, Kantarjian H, Abi-Said D, et al. Randomized comparison between antibiotics alone and antibiotics plus granulocyte-macrophage colony-stimulating factor (Escherichia coli-derived in cancer patients with fever and neutropenia. The American Journal of Medicine 1996;100(1):17-23. [DOI] [PubMed] [Google Scholar]
Arnberg 1998 {published data only}
- Arnberg H, Letocha H, Nou F, Westlin JF, Nilsson S. GM-CSF in chemotherapy-induced febrile neutropenia - a double-blind randomized study. Anticancer Research 1998;18(2B):1255-60. [PubMed] [Google Scholar]
Aviles 1996 {published data only}
- Aviles A, Guzman R, Garcia EL, Talavera A, Diaz-Maqueo JC. Results of a randomized trial of granulocyte colony-stimulating factor in patients with infection and severe granulocytopenia. Anticancer Drugs 1996;7(4):392-7. [DOI] [PubMed] [Google Scholar]
Biesma 1990 {published data only}
- Biesma B, Vries EG, Willemse PH, Sluiter WJ, Postmus PE, Limburg PC, et al. Efficacy and tolerability of recombinant human granulocyte-macrophage colony-stimulating factor in patients with chemotherapy-related leukopenia and fever. European Journal of Cancer 1990;26(9):932-6. [DOI] [PubMed] [Google Scholar]
Garcia‐Carbonero 2001 {published data only}
- Garcia-Carbonero R, Mayordomo JI, Tornamira MV, Lopez-Brea M, Rueda A, Guillem V, et al. Granulocyte colony-stimulating factor in the treatment of high-risk febrile neutropenia: a multicenter randomized trial. Journal of the National Cancer Institute 2001;93(1):31-8. [DOI] [PubMed] [Google Scholar]
Lopez‐Hernandez 2000 {published data only}
- Lopez-Hernandez MA, Jimenez-Alvarado R, Borbolla-Escoboza R, Diego Flores-Chapa J, Alvarado-Ibarra M, Gonzalez-Avante M, et al. Granulocyte colony-stimulating factor in the treatment of febrile neutropenia. Gaceta Médica de México 2000;136(2):99-105. [PubMed] [Google Scholar]
Maher 1994 {published data only}
- Maher DW, Lieschke GJ, Green M, Bishop J, Stuart-Harris R, Wolf M, et al. Filgrastim in patients with chemotherapy-induced febrile neutropenia. A double-blind, placebo-controlled trial. Annals of Internal Medicine 1994;121(7):492-501. [DOI] [PubMed] [Google Scholar]
Mayordomo 1995 {published data only}
- Mayordomo JI, Rivera F, Diaz-Puente MT, Lianes P, Colomer R, Lopez-Brea M, et al. Improving treatment of chemotherapy-induced neutropenic fever by administration of colony-stimulating factors. Journal of the National Cancer Institute 1995;87(11):803-8. [DOI] [PubMed] [Google Scholar]
Mitchell 1997 {published data only}
- Mitchell PL, Morland B, Stevens MC, Dick G, Easlea D, Meyer LC, et al. Granulocyte colony-stimulating factor in established febrile neutropenia: a randomized study of pediatric patients. Journal of Clinical Oncology 1997;15(3):1163-70. [DOI] [PubMed] [Google Scholar]
Ravaud 1998 {published data only}
- Ravaud A, Chevreau C, Cany L, Houyau P, Dohollou N, Roche H, et al. Granulocyte-macrophage colony-stimulating factor in patients with neutropenic fever is potent after low-risk but not after high-risk neutropenic chemotherapy regimens: results of a randomized phase III trial. Journal of Clinical Oncology 1998;16(9):2930-6. [DOI] [PubMed] [Google Scholar]
Riikonen 1994 {published data only}
- Riikonen P, Saarinen UM, Makipernaa A, Hovi L, Komulainen A, Pihkala J, Jalanko H. Recombinant human granulocyte-macrophage colony-stimulating factor in the treatment of febrile neutropenia: a double blind placebo-controlled study in children. Pediatric Infectious Disease Journal 1994;13(3):197-202. [DOI] [PubMed] [Google Scholar]
Rodriguez 2005 {published data only}
- Rodriguez ZN, Tordecilla CJ, Campbell BM, Joannon SP, Rizzardini LC, Soto AV, et al. Usefulness of G-CSF in pediatric high risk cancer patients with fever and neutropenia [Utilidad del factor estimulador de colonias de granulocitos (G-CSF) en episodios de neutropenia febril de alto riesgo en ninos con cancer.]. Revista Chilena de Infectologia 2005;22(3):223-7. [PMID: ] [DOI] [PubMed] [Google Scholar]
Vellenga 1996 {published data only}
- Vellenga E, Uyl-de Groot CA, Wit R, Keizer HJ, Lowenberg B, ten Haaft MA, et al. Randomized placebo-controlled trial of granulocyte-macrophage colony-stimulating factor in patients with chemotherapy-related febrile neutropenia. Journal of Clinical Oncology 1996;14(2):619-27. [DOI] [PubMed] [Google Scholar]
Yoshida 1999 {published data only}
- Yoshida M, Karasawa M, Naruse T, Fukuda M, Hirashima K, Oh H, et al. Effect of granulocyte-colony stimulating factor on empiric therapy with flomoxef sodium and tobramycin in febrile neutropenic patients with hematological malignancies. Kan-etsu Hematological Disease and Infection Study Group. International Journal of Hematology 1999;69(2):81-8. [PubMed] [Google Scholar]
References to studies excluded from this review
Balcerska 1995 {published data only}
- Balcerska A, Ploszynska A, Polczynska K, Maciejka-Kapuscinska L, Wlazlowski M, Drozynska E, et al. The effect of cytokines G-CSF and GM-CSF in therapy of childhood malignancies [Ocena Skutecznosci Czynnikow Wzrostu G-CSF I GM-CSF W Leczeniu Wspomagajacym Choroby Nowotworowej U Dzieci]. Annales Academiae Medicae Gedanensis 1995;25:117-24. [Google Scholar]
Beveridge 1998 {published data only}
- Beveridge RA, Miller JA, Kales AN, Binder RA, Robert NJ, Harvey JH, et al. A comparison of efficacy of sargramostim (yeast-derived RhuGM-CSF) and filgrastim (bacteria-derived RhuG-CSF) in the therapeutic setting of chemotherapy-induced myelosuppression. Cancer Investigation 1998;16(6):366-73. [DOI] [PubMed] [Google Scholar]
Bodey 1994 {published data only}
- Bodey GP, Anaissie E, Gutterman J, Vadhan-Raj S. Role of granulocyte-macrophage colony-stimulating factor as adjuvant treatment in neutropenic patients with bacterial and fungal infection. European Journal of Clinical Microbiology & Infectious Diseases 1994;13 Suppl 2:S18-22. [DOI] [PubMed] [Google Scholar]
Feng 1998 {published data only}
- Feng F, Zhou L. Randomized controlled study of leucomax (recombinant human granulocyte-macrophage colony stimulating factor, rhGM-CSF) in the treatment of cancer chemotherapy-induced leucopenia. Zhonghua Zhong Liu Za Zhi [Chinese Journal of Oncology] 1998;20(6):451-3. [PubMed] [Google Scholar]
Fengyi 1998 {published data only}
- Fengyi F, Liqiang Z. Randomized controlled study of leucomax (recombinant human granulocyte- macrophage colony stimulating factor, rhGM-CSF) in the treatment of cancer chemotherapy-induced leucopenia. Zhonghua Zhong Liu Za Zhi [Chinese Journal of Oncology] 1998;20(6):451-3. [PubMed] [Google Scholar]
Garcia‐Carb 1999a {published data only}
- Garcia-Carbonero R, Mayordomo JI, Tornamira MV, Lopez-Brea M, Rueda A, Guillem V, et al. Filgrastim in the treatment of high-risk febrile neutropenia: results of a multicenter randomized phase III trial. Proceedings of the Annual Meeting of the American Society of Clinical Oncology 1999;18:Abstract #2253. [Google Scholar]
Garcia‐Carb 1999b {published data only}
- Garcia-Carbonero R, Mayordomo JI, Tornamira MV, Lopez-Brea M, Rueda A, Guillem V, et al. Randomized comparison of broad spectrum antibiotics with or without filgrastim in the treatment of patients with high-risk fever and grade IV neutropenia. European Journal of Cancer 1999;35(Supplement 4):360. [Google Scholar]
Gebbia 1994 {published data only}
- Gebbia V, Valenza R, Testa A, Cannata G, Borsellino N, Gebbia N. A prospective randomized trial of thymopentin versus granulocyte--colony stimulating factor with or without thymopentin in the prevention of febrile episodes in cancer patients undergoing highly cytotoxic chemotherapy. Anticancer Research 1994;14(2B):731-4. [PubMed] [Google Scholar]
Gunay 1998 {published data only}
- Gunay U, Tanritanir A, Meral A, Sevinir BB, Hacimollaoglu MK. The effects of granulocyte colony-stimulating factor (G-CSF) and intravenous immunoglobulin (IVIG) on the treatment of neutropenic fever in children.. Cocuk Sagligi Ve Hastaliklari Dergisi 1998;41(4):433-44. [Google Scholar]
Herrmann 1990 {published data only}
- Herrmann F, Schulz G, Wieser M, Kolbe K, Nicolay U, Noack M, et al. Effect of granulocyte-macrophage colony-stimulating factor on neutropenia and related morbidity induced by myelotoxic chemotherapy. The American Journal of Medicine 1990;88(6):619-24. [DOI] [PubMed] [Google Scholar]
Kaku 1993 {published data only}
- Kaku K, Takahashi M, Moriyama Y, Nakahata T, Masaoka T, Yoshida Y, et al. Recombinant human granulocyte-macrophage colony-stimulating factor (rhGM-CSF) after chemotherapy in patients with non-Hodgkin's lymphoma; a placebo-controlled double blind phase III trial. Leukemia and Lymphoma 1993;11(3-4):229-38. [DOI] [PubMed] [Google Scholar]
Kawa 1999 {published data only}
- Kawa K, Keiko Y, Dong PY, Sako M, Tanaka H, Hara J, et al. A multi-institutional study of optimal use of recombinant granulocyte colony stimulating factor (lenograstim) in pediatric malignancies - III. Biotherapy 1999;13(4):361-7. [Google Scholar]
Kotake 1999 {published data only}
- Kotake T, Usami M, Miki T, Togashi M, Akaza H, Kubota Y, et al. Effect of recombinant human granulocyte colony stimulating factor (lenograstim) on chemotherapy induced neutropenia in patients with urothelial cancer. International Journal of Urology 1999;6(2):61-7. [DOI] [PubMed] [Google Scholar]
Mayordomo 1992 {published data only}
- Mayordomo JI, Rivera F, Diaz-Puente MT, Lianes MP, Lopez-Brea M, López E. Progress report of a randomized trial on the value of adding G-CSF or GM-CSF to standard antibiotic therapy in the treatment of febrile neutropenia. Annals of Oncology 1992;3(Supplement 5: Growth factors / Oncogenes / Tumour suppressor):2. [Google Scholar]
Mayordomo 1993 {published data only}
- Mayordomo JI, Rivera F, Diaz Puente MT, Lianes MP, Lopez-Brea M, Lopez E, et al. Decreasing morbidity and cost of treating febrile neutropenia by adding G-CSF and GM-CSF to standard antibiotic therapy: results of a randomized trial. In: Proceedings of the Annual Meeting of the American Society of Clinical Oncology. Vol. 12. 1993:Abstract # 1510.
Michon 1998 {published data only}
- Michon JM, Hartmann O, Bouffet E, Meresse V, Coze C, Rubie H, et al. An open-label, multicentre, randomised phase 2 study of recombinant human granulocyte colony-stimulating factor (filgrastim) as an adjunct to combination chemotherapy in paediatric patients with metastatic neuroblastoma. European Journal of Cancer 1998;34(7):1063-9. [DOI] [PubMed] [Google Scholar]
Montalar 1998 {published data only}
- Montalar J, Santaballa A, Oltra A, Segura A, Aparicio A, Pastor M. Ofloxacin with or without stimulating colony-factors in the treatment of neutropenic fever. Annals of Oncology 1998;9(Supplement 4 palliative and supportive care):151 abstract #724. [Google Scholar]
Moriyama 1993 {published data only}
- Moriyama Y, Takahashi M, Kaku K, Yoshida Y, Masaoka T, Nakanishi S, et al. Effect of granulocyte-macrophage colony-stimulating factor on chemotherapy-induced granulocytopenia in patients with malignancies. Acta Haematologica 1993;89(2):70-5. [DOI] [PubMed] [Google Scholar]
Motoyoshi 1986 {published data only}
- Motoyoshi K, Takaku F, Maekawa T, Miura Y, Kimura K, Furusawa S, et al. Protective effect of partially purified human urinary colony-stimulating factor on granulocytopenia after antitumor chemotherapy. Experimental Hematology 1986;14(11):1069-75. [PubMed] [Google Scholar]
Nakajima 1995 {published data only}
- Nakajima H, Ikeda Y, Hirashima K, Toyama K, Okuma M, Saito H, et al. A randomized controlled study of rG.CSF in patients with neutropenia after induction therapy for acute myelogenous leukemia. (rG.CSF Clinical Study Group). Rinsho Ketsueki [The Japanese Journal of Clinical Hematology] 1995;36(6):597-605. [PubMed] [Google Scholar]
Ohno 1997 {published data only}
- Ohno R, Miyawaki S, Hatake K, Kuriyama K, Saito K, Kanamaru A, et al. Human urinary macrophage colony-stimulating factor reduces the incidence and duration of febrile neutropenia and shortens the period required to finish three courses of intensive consolidation therapy in acute myeloid leukemia: a double-blind controlled study. Journal of Clinical Oncology 1997;15(8):2954-65. [DOI] [PubMed] [Google Scholar]
Oshita 2000 {published data only}
- Oshita F, Yamada K, Nomura I, Tanaka G, Ikehara M, Noda K. Prophylactic administration of granulocyte colony-stimulating factor when monocytopenia appears lessens neutropenia caused by chemotherapy for lung cancer. American Journal of Clinical Oncology 2000;23(3):278-82. [DOI] [PubMed] [Google Scholar]
Ravaud 1995 {published data only}
- Ravaud A, Chevreau C, Bonichon F, Mihura J, Bui BN, Tabah I. A phase III trial of recombinant granulocyte-macrophage colony stimulating factor (GM-CSF) as corrective treatment in patients (pts) with neutropenic fever following antineoplastic chemotherapy (CT): results of an intermediate analysis. In: Proceedings of the Annual Meeting of the American Society of Clinical Oncology. 1995:Abstract A716.
Schroder 1999 {published data only}
- Schroder CP, Vries EG, Mulder NH, Willemse PH, Sleijfer DT, Hospers GA, et al. Prevention of febrile leucopenia after chemotherapy in high-risk breast cancer patients: no significant difference between granulocyte-colony stimulating growth factor or ciprofloxacin plus amphotericin B. The Journal of Antimicrobial Chemotherapy 1999;43(5):741-3. [DOI] [PubMed] [Google Scholar]
Soda 1996 {published data only}
- Soda H, Oka M, Fukuda M, Kinoshita A, Sakamoto A, Araki J, et al. Optimal schedule for administering granulocyte colony-stimulating factor in chemotherapy-induced neutropenia in non-small-cell lung cancer. Cancer Chemotherapy and Pharmacology 1996;38(1):9-12. [DOI] [PubMed] [Google Scholar]
Timmer‐Bonte 2005 {published data only}
- Timmer-Bonte JN, Boo TM, Smit HJ, Biesma B, Wilschut FA, Cheragwandi SA, et al. Prevention of chemotherapy-induced febrile neutropenia by prophylactic antibiotics plus or minus granulocyte colony-stimulating factor in small-cell lung cancer: a Dutch randomized phase III study. Journal of Clinical Oncology 2005;23(31):7974-84. [PMID: ] [DOI] [PubMed] [Google Scholar]
Torrecillas 1998 {published data only}
- Torrecillas L, Cervantes G, Zamora R, Acosta A, Cepeda F, Cortés P, et al. GM-CSF discontinuation safe level for patients with chemotherapy induced febrile or afebrile neutropenia. In: Proceedings of the Annual Meeting of the American Society of Clinical Oncology. 1998:Abstract #304.
Uyl‐de Groot 1997 {published data only}
- Uyl-de Groot CA, Vellenga E, Vries EG, Lowenberg B, Stoter GJ, Rutten FF. Treatment costs and quality of life with granulocyte-macrophage colony-stimulating factor in patients with antineoplastic therapy-related febrile neutropenia. Results of a randomised placebo-controlled trial. Pharmacoeconomics 1997;12(3):351-60. [DOI] [PubMed] [Google Scholar]
van Pelt 1997 {published data only}
- Pelt LJ, Craen AJ, Langeveld NE, Weening RS. Granulocyte-macrophage colony-stimulating factor (GM-CSF) ameliorates chemotherapy-induced neutropenia in children with solid tumors. Pediatric Hematology and Oncology 1997;14(6):539-45. [DOI] [PubMed] [Google Scholar]
Vellenga 1996b {published data only}
- Vellenga E, Uyl-De Groot C, Stoter G, Lowenberg B, De Vries E. Faster recovery of leukocytes due to haemopoietic growth factor in patients with chemotherapy-related granulocytopenia and fever, but no shortened hospital stay. Nederlands Tijdschrift voor Geneeskunde 1996;140(32):1650-5. [Google Scholar]
Yalcin 1996 {published data only}
- Yalcin S, Guler N, Kansu E, Ertenli I, Gullu I, Barista I, et al. Granulocyte-colony stimulating factor (G-CSF) administration for chemotherapy-induced neutropenia. Hematology 1996;1(2):155-61. [DOI] [PubMed] [Google Scholar]
Yamazaki 1989 {published data only}
- Yamazaki K, Kumamoto Y, Tsukamoto T, Hirose T. Prophylaxis of fever during leukocytopenia by anticancer chemotherapy. Chemotherapy 1989;37(6):838-47. [Google Scholar]
Additional references
Berghmans 2002
- Berghmans T, Paesmans M, Lafitte JJ, Mascaux C, Meert AP, Jacquy C, et al. Therapeutic use of granulocyte and granulocyte-macrophage colony-stimulating factors in febrile neutropenic cancer patients. A systematic review of the literature with meta-analysis. Supportive Care in Cancer 2002;10(3):181-8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Castro 1999
- Castro AA, Clark OA, Atallah AN. Optimal search strategy for clinical trials in the Latin American and Caribbean Health Science Literature database (LILACS database): update. Sao Paulo Medical Journal 1999;117(3):138-9. [DOI] [PubMed] [Google Scholar]
de Naurois 2010
- Naurois J, Novitzky-Basso I, Gill MJ, Marti FM, Cullen MH, Roila F, et al. Management of febrile neutropenia: ESMO Clinical Practice Guidelines. Annals of Oncology 2010;21 Suppl 5:v252-v6. [DOI] [PubMed] [Google Scholar]
Dickersin 1994
- Dickersin K, Scherer R, Lefebvre C. Identifying relevant studies for systematic reviews. BMJ 1994;309(6964):1286-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
Egger 1997
- Egger M, Smith GD, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997;315(7109):629-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
Egger 2001
- Egger M, Smith GD, Altman D. Systematic Reviews in Health Care. 2nd edition. London: BMJ Books, 2001. [Google Scholar]
Freyer 1998
- Freyer G, Ligneau B, Trillet-Lenoir V. Colony-stimulating factors in the prevention of solid tumors induced by chemotherapy in patients with febrile neutropenia. International Journal of Antimicrobial Agents 1998;10(1):3-9. [DOI] [PubMed] [Google Scholar]
Giamerellou 2001
- Giamarellou H, Antoniadou A. Infectious complications of febrile leukopenia. Infectious Disease Clinics of North America 2001;15(2):457-82. [DOI] [PubMed] [Google Scholar]
GRADEpro [Computer program]
- GRADEpro. Version 3.2 for Windows. Jan Brozek, Andrew Oxman, Holger Schünemann, 2008.
Griffin 2001
- Griffin JD. Hematopoietic growth factors. In: DeVitaJr VT, Hellman S, Rosenberg SA, editors(s). Cancer: Principles and Practice of Oncology. 6th edition. Philadelphia, PA: Lippincott Williams & Wilkins, 2001. [Google Scholar]
Guyatt 2011
- Guyatt GH, Oxman AD, Kunz R, Woodcock J, Brozek J, Helfand M, et al. GRADE guidelines: 7. Rating the quality of evidence - inconsistency. Journal of Clinical Epidemiology 2011;64(12):1294-302. [PMID: ] [DOI] [PubMed] [Google Scholar]
Guyatt 2011a
- Guyatt GH, Oxman AD, Kunz R, Woodcock J, Brozek J, Helfand M, et al. GRADE guidelines: 8. Rating the quality of evidence - indirectness. Journal of Clinical Epidemiology 2011;64(12):1303-10. [PMID: ] [DOI] [PubMed] [Google Scholar]
Guyatt 2011b
- Guyatt G, Oxman AD, Kunz R, Brozek J, Alonso-Coello P, Rind D, et al. GRADE guidelines: 6. Rating the quality of evidence - imprecision. Journal of Clinical Epidemiology 2011;64(12):1283-93. [PMID: ] [DOI] [PubMed] [Google Scholar]
Guyatt 2011c
- Guyatt GH, Oxman AD, Montori V, Vist G, Kunz R, Brozek J, et al. GRADE guidelines: 5. Rating the quality of evidence - publication bias. Journal of Clinical Epidemiology 2011;64(12):1277-82. [PMID: ] [DOI] [PubMed] [Google Scholar]
Guyatt 2011d
- Guyatt GH, Oxman AD, Vist G, Kunz R, Brozek J, Alonso-Coello P, et al. GRADE guidelines: 4. Rating the quality of evidence - risk of bias. Journal of Clinical Epidemiology 2011;64(4):407-15. [PMID: ] [DOI] [PubMed] [Google Scholar]
Guyatt 2011e
- Guyatt G, Oxman AD, Akl E, Kunz R, Vist G, Brozek J, et al. GRADE guidelines: 1. Introduction-GRADE evidence profiles and summary of findings tables. Journal of Clinical Epidemiology 2011;64(4):383-94. [PMID: ] [DOI] [PubMed] [Google Scholar]
Higgins 2011a
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011.. Available from www.cochrane-handbook.org.
Higgins 2011b
- Higgins JPT, Deeks JJ. Chapter 7: Selecting studies and collecting data. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane-handbook.org.
Higgins 2011c
- Higgins JPT, Altman DG, Sterne JAC. Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane-handbook.org.
Higgins 2011d
- Higgins JPT, Altman DG, Sterne JAC. Chapter 16.5.4: Special topics in statistics. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane-handbook.org.
Hozo 2005
- Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Medical Research Methodology 2005;5:13. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hozo 2005a
- Hozo I, Djulbegovic B, Clark O, Lyman GH, . Use of re-randomized data in meta-analysis. BMC Medical Research Methodology 2005;10(5):17. [DOI] [PMC free article] [PubMed] [Google Scholar]
IHS 1990
- Immunocompromised Host Society. The design, analysis, and reporting of clinical trials on the empirical antibiotic management of the neutropenic patient. Report of a consensus panel. The Journal of Infectious Diseases 1990;161(3):397-401. [DOI] [PubMed] [Google Scholar]
Lyman 2002
- Lyman GH, Kuderer NM, Djulbegovic B. Prophylactic granulocyte colony-stimulating factor in patients receiving dose-intensive cancer chemotherapy: a meta-analysis. The American Journal of Medicine 2002;112(5):406-11. [DOI] [PubMed] [Google Scholar]
Mhaskar 2012
- Mhaskar Rahul, Djulbegovic Benjamin, Magazin Anja, Soares Heloisa P, Kumar Ambuj. Published methodological quality of randomized controlled trials does not reflect the actual quality assessed in protocols. Journal of Clinical Epidemiology 2012;65(6):602-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Moher 2009
- Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Journal of Clinical Epidemiology 2009;62(10):1006-12. [DOI] [PubMed] [Google Scholar]
Paesmans 1998
- Paesmans M. Statistical considerations in clinical trials testing empiric antibiotic regimens in patients with febrile neutropenia. Supportive Care in Cancer 1998;6(5):438-43. [DOI] [PubMed] [Google Scholar]
Petros 2001
- Petros WP. Colony-stimulating factors. In: Chabner BA, Longo DL, editors(s). Cancer Chemotherapy and Biotherapy - Principles and Practice. 5th edition. Lippincott Williams & Wilkins, 2001. [Google Scholar]
Pizzo 1999
- Pizzo PA. Fever in immunocompromised patients. The New England Journal of Medicine 1999;341(12):893-900. [DOI] [PubMed] [Google Scholar]
Review Manager 5.3
- Review Manager (RevMan) [Computer program]. Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration 2014.
Rubenstein 2000
- Rubenstein EB. Colony stimulating factors in patients with fever and neutropenia. International Journal of Antimicrobial Agents 2000;16(2):117-21. [DOI] [PubMed] [Google Scholar]
Schelenz 2012
- Schelenz S, Giles D, Abdallah S. Epidemiology, management and economic impact of febrile neutropenia in oncology patients receiving routine care at a regional UK cancer centre. Annals of Oncology 2012;23(7):1889-93. [DOI] [PubMed] [Google Scholar]
Segal 2001
- Segal BH, Walsh TJ, Holland SM. Infections in the cancer patient. In: DeVitaJr VT, Hellman S, Rosenberg SA, editors(s). Cancer: Principles and Practice of Oncology. 6th edition. Philadelphia, PA: Lippincott Williams & Wilkins, 2001. [Google Scholar]
Smeenk 1998
- Smeenk FW, Haastregt JC, Witte LP, Crebolder HF. Effectiveness of home care programmes for patients with incurable cancer on their quality of life and time spent in hospital: systematic review. BMJ 1998;316(7149):1939-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
Tierney 2007
- Tierney JF, Stewart LA, Ghersi D, Burdett S, Sydes MR. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials 2007;8:16. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Wang 2015
- Wang XJ, Lopez SE, Chan A Economic burden of chemotherapy-induced febrile neutropenia in patients with lymphoma: A systematic review. Economic burden of chemotherapy-induced febrile neutropenia in patients with lymphoma: A systematic review. Critical Reviews in Oncology / Hematology 2015;94(2):201-12. [DOI] [PubMed] [Google Scholar]


