Abstract
While both baseline regional cerebral oxygen saturation (rSO2) and intraoperative rSO2 decreases have prognostic importance in cardiac surgery, evidence is limited in patients who received interventions to correct rSO2 decreases. The primary aim was to examine the association between rSO2 values (both baseline rSO2 and intraoperative decrease in rSO2) with the composite of morbidity endpoints. We retrospectively analyzed 356 cardiac surgical patients having continuously recorded data of intraoperative rSO2 values. Per institutional guidelines, patients received interventions to restore the rSO2 value to ≥80% of the baseline value. Analyzed rSO2 variables included baseline value, and area under the threshold below an absolute value of 50% (AUT50). Their association with outcome was analyzed with multivariable logistic regression. AUT50 (odds ratio, 1.05; 95% confidence interval; 1.01–1.08; p = 0.015) was shown to be an independent risk factor (along with age, chronic kidney disease, and cardiopulmonary bypass time) of adverse outcomes. In cardiac surgical patients who received interventions to correct decreases in rSO2, increased severity of intraoperative decrease in rSO2 as reflected by AUT below an absolute value of 50% was associated with a composite of adverse outcomes, implicating the importance of cerebral oximetry to monitor the brain as an index organ.
Keywords: cardiac surgery, cerebral desaturation, morbidity, regional cerebral oxygen saturation
1. Introduction
Cerebral oximetry based on near-infrared spectroscopy measures regional tissue oxygen saturation (rSO2) at the watershed zone of the anterior and middle cerebral arteries [1]. Due to its non-invasiveness and the practicability of providing real-time rSO2 even during non-pulsatile perfusion, it has gained popularity as a key monitor in cardiac surgery over the past two decades.
Earlier studies were mainly focused on detecting adverse neurocognitive outcome and often showed conflicting results [2,3,4]. These inconsistencies were to be expected to some extent, considering the inability of cerebral oximetry to adequately assess the middle and posterior cerebral circulation. In addition, the relatively low incidence of stroke and diversity in the diagnosis of cognitive dysfunction posed significant challenges for studies in this regard [4,5].
With its increasing application in cardiac surgery, another use for cerebral oximetry has arisen—i.e., viewing the brain as an index organ for overall hemodynamic stability [6]. This seems logical considering that cerebral oximetry measures tissue oxygen saturation at the ischemia-vulnerable watershed zone while our physiologic response aims to provide cerebral perfusion even at the cost of systemic hypoperfusion [6]. In addition, approximately 70% of the measured oxygen saturation comes from the hemoglobin (Hb) of the venous bed, indicating that a decrease in rSO2 would more likely be the consequence of increased oxygen extraction, reflecting significant hemodynamic compromise assuming a constant cerebral metabolic rate [7].
In this context, two major areas of studies exist regarding the potential role of cerebral oximetry to monitor the brain as an index organ. First, studies elucidated the association between significant intraoperative reductions in rSO2 and adverse outcome, and whether interventions to correct these would improve prognosis [2,8]. Second, compelling evidence has also indicated the importance of low baseline rSO2 value concerning its association with adverse outcome [9]. However, being a potentially modifiable risk factor, efforts to correct decrease in rSO2 might alter the prognostic importance of baseline rSO2, whereas intraoperative decrease in rSO2 despite these efforts may show a meaningful association with adverse outcome. Yet, evidence to that regard is scarce.
In this retrospective review, the primary aim was to study the association between the rSO2 values (both baseline rSO2 and decrease in intraoperative rSO2) with overall outcome in cardiac surgical patients who received interventions based on cerebral oximetry monitoring. The secondary aim was to evaluate the variables that were associated with the rSO2 value that showed significant association with adverse outcomes.
2. Materials and Methods
2.1. Patients
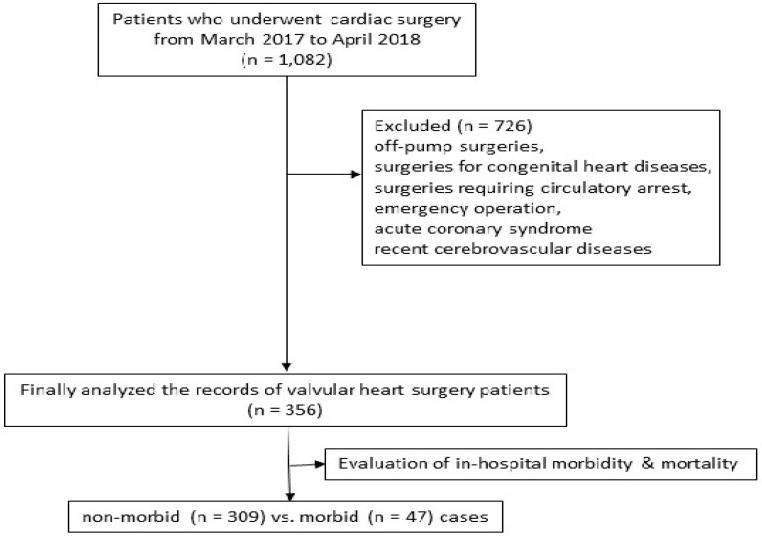
After obtaining approval (20 December 2018) from the Institutional Review Board, we reviewed the medical records of patients ≥20 years who underwent cardiac surgery at the Severance Cardiovascular Hospital, Seoul, Korea, from 20 March 2017 to 10 April 2018. Of the 1082 records initially identified, we excluded patients having off-pump surgery, congenital heart disease operations, using hypothermic circulatory arrest, emergency operations, as well as patients with acute coronary syndrome, recent myocardial infarction, cerebrovascular disease that required surgery, and cerebrovascular events within the preceding one month. Accordingly, the records of 356 valvular heart surgery patients were analyzed (Figure 1).
Figure 1.
Flow chart.
2.2. rSO2 Measurement and Study Endpoints
The rSO2 was measured using the INVOSTM Cerebral/Somatic Oximeter 5100 (Covidien, Dublin, Ireland) with bi-hemispheric near-infrared spectroscopy sensors. The baseline rSO2 value was obtained in the supine position in room air before anesthesia, and measurement was continued until intensive care unit (ICU) transfer. For the analysis of rSO2 values, after simultaneously obtaining both left and right rSO2 values, the lower value was selected for analysis. Excel 2016 (Microsoft Office, Redmond, WA, USA) was used to calculate the cumulative area under the threshold (AUT) of rSO2 values (80% of baseline value; and below an absolute value of 50%) [10]. AUT was calculated using the following formula: AUT (%∙min) = SUM {[rSO2threshold – (rSO2TN + rSO2TN-1)/2] * 0.5}, where, rSO2TN and rSO2TN-1 are two consecutive rSO2 values, and 0.5 min represents the time interval between two consecutive rSO2 values. If the average value of the last two consecutive values [(rSO2TN + rSO2TN-1)/2] was below zero, the average was excluded from the SUM. Therefore, AUT should reflect the severity of decrease in rSO2 as it has both the magnitude and time components. Minimal and maximal rSO2 values and maximal degree of desaturation were also determined.
The primary aim of the study was to evaluate the association between rSO2 values (both baseline rSO2 and intraoperative decrease in rSO2) with a composite of morbidity endpoints. The composite of morbidity endpoints included in-hospital mortality combined with postoperative morbidity endpoints as defined by the Society of Thoracic Surgery (STS) database registry [11]. These included permanent stroke, renal failure (serum creatinine [Cr] ≥4.0 mg/dL with increase of ≥0.5 mg/dL or 3 times most recent preoperative creatinine level, newly required dialysis), prolonged ventilation (>24 h), deep sternal wound infection, and any cause of reoperation in the absence of an evident surgical bleeding focus, which were checked during the postoperative hospitalization.
The secondary aim was to evaluate the factors including the hemodynamic and arterial-blood gas variables, temperature, and Hb, that were associated with either low baseline rSO2 value or significant decreases in intraoperative rSO2, depending on their association with adverse outcomes.
2.3. Perioperative Data Assessment
Preoperative data assessment included demographic data, comorbidities (hypertension, diabetes mellitus, congestive heart failure [New York Heart Association classification 3 or 4], myocardial infarction, chronic kidney disease [CKD, glomerular filtration rate under 60 mL/min/1.73 m2 for more than 3 months], cerebrovascular disease, chronic obstructive lung disease), medications, European System for Cardiac Operative Risk Evaluation (EuroSCORE), left ventricular ejection fraction (LVEF), and Hb and Cr levels.
Intraoperative data assessment included the duration of cardiopulmonary bypass (CPB) and aortic cross clamp, fluid balance, packed erythrocytes (pRBCs) transfusion, urine output, and the use of norepinephrine, vasopressin, milrinone, and dobutamine. Hemodynamic and blood gas variables including mean arterial pressure (MAP), cardiac index (CI), mixed venous oxygen saturation (SvO2), central venous pressure (CVP), mean pulmonary arterial pressure (mPAP), PaO2, PaCO2, and Hb concentrations were recorded at predetermined time points (before anesthetic induction [baseline], 15 min after anesthetic induction, 15 min after starting CPB, 20 min after weaning from CPB, and after sternal closure). Postoperative data assessment included fluid balance, transfusion requirement, chest tube drainage for 24 h, as well as the use of norepinephrine, vasopressin, milrinone, and dobutamine.
2.4. Perioperative Management Including Interventions Guided by Decrease in rSO2
All patients were managed according to standardized institutional anesthetic and CPB practice guidelines. Briefly, standard monitoring included bispectral index (BIS; A-200 Bispectral Index® score monitor; Aspect Medical System Inc., Norwood, MA, USA), rSO2, pulmonary artery catheter, and transesophageal echocardiography. Anesthesia was provided using sufentanil and sevoflurane and BIS was maintained at 40–60. MAP was maintained at 60–80 mmHg using norepinephrine first, and if the target MAP could not be maintained with escalating doses of norepinephrine (maximum of 0.3 μg/kg/min), vasopressin was added (2.4–4 unit/h). Milrinone was used in cases of LVEF <30%, right ventricular dysfunction, or pulmonary hypertension. During CPB, non-pulsatile perfusion was utilized at 2.0–2.5 L/min/m2 using a tepid temperature (32–34 °C) and alpha-stat management. pRBCs were transfused when Hb was <7.0 g/dL during CPB or <8.0 g/dL otherwise.
All patients received interventions according to a modified institutional guideline based on the algorithm of Denault et al. [12] when significant decrease in rSO2 occurred (defined as a 20% reduction from baseline values), which were as follows: checking neck and cannulae position, increasing end-tidal CO2 to approx. 40 mmHg (in the absence of pulmonary hypertension), increasing the dose of norepinephrine or addition of vasopressin to increase the MAP above 65 mmHg up to 80 mmHg, if necessary. In addition, milrinone and/or dobutamine to maintain a SvO2 of ≥60% and CI > 2.0 L/min/m2 (increase of flow rate to 2.5 L/min/m2 during CPB) was administered, while efforts to optimize preload was done using mini-fluid boluses in 100 mL aliquots. Lastly, when all interventions failed to restore the rSO2 value above 80% of the baseline, pRBCs transfusion was considered at Hb of 8–9 g/dL.
2.5. Statistical Analysis
Statistical analyses were performed using SPSS version 17 (SPSS, Inc., Chicago, IL, USA) and SAS (version 9.4, SAS Inc., Cary, NC, USA) and R package (version 3.4.3. http://www.R-project.org). Results were shown as mean (standard deviation [SD]) or number of patients (%). Sample size was not determined a priori.
For intergroup comparisons, continuous data were tested for normality using the Kolmogorov–Smirnov test and analyzed using the Mann–Whitney U test or independent t-test, as required. Categorical data were analyzed using Fisher’s exact test or the chi-square test. Intergroup comparisons of the serially assessed data, including hemodynamic and laboratory variables, were done using linear mixed models with an unstructured covariance matrix. The model included group, time, and group-by-time as fixed effects. When the interaction of group, time, and group-by-time of the variables showed statistical significance, post hoc analysis was carried out with Bonferroni correction for the adjustment of multiple comparisons.
For analysis of the primary endpoint, to confirm independent risk factors of the composite of morbidity endpoints, multivariable logistic regression analysis was performed. First, well known risk factors were chosen a priori to minimize the introduction selection bias, which included age, CKD, congestive heart failure, and CPB duration in order to abide the rule of ten [13,14,15]. In terms of the variables of interest, rSO2, they were tested for their probability on the composite of morbidity endpoints using the area under the receiver operating characteristic (AUROC) curve. Then, rSO2 variables with the highest AUROC were introduced to the regression analysis, which were baseline and AUT 50. An ROC curve was constructed for the identified rSO2 variables with prognostic importance.
For analysis of the secondary outcome, Pearson’s correlation analysis was used to verify the correlation between the factors influencing rSO2 values (perfusion pressure, Hb, PaCO2, CI, mPAP) and rSO2. The Pearson’s correlation coefficient (r) was demonstrated. In addition, intergroup analysis of the hemodynamic and arterial-blood gas analysis variables was performed between patients who exhibited AUT50 greater than 113.2 min·% or not. A p < 0.05 was deemed as statistically significant.
3. Results
The composite of morbidity endpoints occurred in 47 of the 356 (13.2%) patients that were analyzed. In those patients, permanent stroke occurred in 5 patients (1.4%), reoperation in 7 patients (1.9%), renal failure in 8 patients (2.2%), mechanical ventilation >24 h in 29 patients (8.1%), deep sternal wound infection in 4 patients (1.1%), and mortality in 1 patient (0.3%).
Patients who exhibited a composite of morbidity endpoints had a higher incidence of preoperative CKD, higher incidence of heart failure, higher EuroSCORE, lower LVEF, lower Hb and albumin levels, higher Cr levels, longer CPB time, higher incidences of intraoperative pRBCs transfusion, and milrinone and vasopressin requirements compared to those without morbidities (Table 1 and Table 2).
Table 1.
Patients’ characteristics and preoperative data.
| Non-Morbid (n = 309) |
Morbid (n = 47) |
p-Value | |
|---|---|---|---|
| Age (years) | 61.8 ± 13.3 | 67.9 ± 11.9 | 0.002 |
| Female sex (n) | 147 (48) | 26 (55) | 0.322 |
| Body surface area (m2) | 1.68 ± 0.20 | 1.64 ± 0.19 | 0.140 |
| Preoperative morbidity (n) | |||
| Hypertension | 144 (47) | 33 (70) | 0.003 |
| Diabetes mellitus | 53 (17) | 11 (23) | 0.298 |
| Congestive heart failure | 57 (18) | 15 (32) | 0.032 |
| CKD | 27 (9) | 17 (36) | <0.001 |
| COPD | 11 (4) | 1 (2) | 0.612 |
| Cerebrovascular attack | 34 (11) | 5 (11) | 0.941 |
| EuroSCORE | 5.6 ± 3.2 | 8.0 ± 3.0 | <0.001 |
| LVEF (%) | 61.4 ± 12.2 | 57.3 ± 15.7 | 0.040 |
| Preoperative medication (n) | |||
| Beta blocker | 119 (39) | 14 (30) | 0.468 |
| Calcium channel blocker | 78 (25) | 17 (36) | 0.115 |
| ACE-I/ARB | 144 (47) | 23 (49) | 0.765 |
| Diuretics | 218 (71) | 34 (72) | 0.801 |
| Preoperative laboratory value | |||
| Hemoglobin (g/dL) | 12.8 ± 1.9 | 11.8 ± 2.3 | 0.001 |
| Serum creatinine (mg/dL) | 0.90 ± 0.71 | 1.53 ± 1.87 | <0.001 |
| Albumin (g/dL) | 3.97 ± 0.45 | 3.69 ± 0.63 | <0.001 |
| Operations (n) | 0.662 | ||
| Mitral valve replacement | 110 (36) | 15 (32) | |
| Aortic valve replacement | 142 (46) | 20 (43) | |
| Double valve replacement | 40 (13) | 8 (17) | |
| Others | 16 (4) | 4 (9) | |
Values are mean ± standard deviation, or number of patients (%). CKD, chronic kidney disease; COPD, chronic obstructive pulmonary disease; EuroSCORE, European system for cardiac operative risk evaluation; LVEF, left ventricular ejection fraction; ACE-I, angiotensin converting enzyme inhibitor; ARB, angiotensin receptor blocker.
Table 2.
Intraoperative data.
| Non-Morbid (n = 309) |
Morbid (n = 47) |
p-Value | |
|---|---|---|---|
| ACC duration (min) | 78.7 ± 39.4 | 91.0 ± 46.5 | 0.090 |
| CPB duration (min) | 109.8 ± 47.6 | 127.5 ± 51.1 | 0.031 |
| Total infused fluid (mL) | 1090.2 ± 375.6 | 1192.6 ± 471.2 | 0.165 |
| pRBCs transfusion (n) | 125 (41) | 32 (68) | <0.001 |
| Total urine output (mL) | 937.8 ± 519.4 | 731.5 ± 602.9 | 0.030 |
| Norepinephrine (n) | 307 (99) | 47 (100) | 0.580 |
| Milrinone (n) | 99 (32) | 22 (47) | 0.046 |
| Vasopressin (n) | 215 (70) | 41 (87) | 0.012 |
| Dobutamine (n) | 5 (2) | 2 (4) | 0.227 |
Values are mean ± standard deviation, or number of patients (%). ACC, aorta cross clamping; CPB, cardiopulmonary bypass, pRBCs, packed red blood cell.
Representative rSO2 values are shown in Table 3. All of the assessed rSO2 values including the baseline, AUT < 80% of baseline rSO2, and AUT < absolute value of 50% were significantly lower in the group with morbidity compared with the non-morbidity group, except for the percentage of maximal decrease from baseline rSO2 recovery to more than 80% of the baseline value at the end of the surgery (which occurred in 67% of patients). Subgroup analysis between patients whose rSO2 value was recovered to more than 80% of the baseline value at the end of the surgery and those who did not revealed no significant differences in the composite of postoperative morbidity endpoints (data not shown).
Table 3.
Representative values of regional cerebral oxygen saturation.
| Total (n = 356) |
Non-Morbid (n = 309) |
Morbid (n = 47) |
p-Value | |
|---|---|---|---|---|
| Baseline rSO2 (%) | 58.0 ± 10.1 | 58.7 ± 9.7 | 52.8 ± 11.5 | 0.002 |
| Maximal rSO2 (%) | 70.8 ± 9.1 | 71.7 ± 8.7 | 65.0 ± 9.3 | <0.001 |
| Minimal rSO2 (%) | 37.6 ± 9.9 | 38.4 ± 9.6 | 32.7 ± 10.4 | 0.001 |
| AUT 80base (min%) | 355.2 ± 537.4 | 328.2 ± 479.1 | 532.4 ± 809.4 | 0.015 |
| AUT 50 (min%) | 678.7 ± 1046.5 | 566.6 ± 895.7 | 1415.6 ± 1562.0 | <0.001 |
| Complete recovery (n) | 72 (20) | 57 (19) | 15 (32) | 0.034 |
| 80% recovery (n) | 240 (67) | 208 (68) | 32 (68) | 0.988 |
| Maximal decrease (%) | 34.7 ± 14.6 | 34.2 ± 14.1 | 37.3 ± 17.4 | 0.176 |
Values are mean ± standard deviation or number of patients (%). rSO2, regional cerebral oxygen saturation; AUT 80base, area under the threshold below 80% of baseline rSO2; AUT50, area under the threshold below an absolute value of 50% of rSO2; complete recovery, restoration of rSO2 to the baseline value at the time of sternal closure; 80% recovery; restoration of rSO2 over the 80% of baseline value at the time of sternal closure. Maximal decrease; maximal decrease of rSO2 compared to the baseline value.
In the multivariable logistic regression analysis for finding risk factors of the composite of morbidity endpoints, age, preoperative CKD, AUT of rSO2 below an absolute value of 50%, and duration of CPB were identified as independent risk factors, whereas baseline rSO2 and AUT below 80% of the baseline rSO2 were not (Table 4). The cut-off value of AUT 50 to predict the composite of morbidity endpoints was 113.2 min·% with an AUROC of 0.697 (95% confidence interval, 0.607–0.787; p < 0.001) with 80.9% sensitivity and 40.8% specificity.
Table 4.
Logistic regression analysis for finding risk factors of composite of morbidity end points.
| Univariable | Multivariable | |||||
|---|---|---|---|---|---|---|
| OR | 95% CI | p-Value | OR | 95% CI | p-Value | |
| Age (years) | 1.04 | 1.01–1.08 | 0.004 | 1.05 | 1.02–1.09 | 0.004 |
| Chronic kidney disease (n) | 5.92 | 2.90–12.09 | <0.001 | 4.92 | 2.13–11.36 | <0.001 |
| Congestive heart failure (n) | 2.07 | 1.05–4.08 | 0.035 | 1.41 | 0.65–3.09 | 0.386 |
| Baseline rSO2 (%) | 0.95 | 0.92–0.98 | <0.001 | 1.02 | 0.97–1.07 | 0.424 |
| AUT 50 (min%) | 1.06 | 1.03–1.08 | <0.001 | 1.05 | 1.01–1.08 | 0.015 |
| CPB duration (min) | 1.93 | 1.09–3.39 | 0.023 | 1.01 | 1.00–1.02 | 0.014 |
OR, odds ratio; 95% CI, 95% confidence interval; rSO2, regional cerebral oxygen saturation; AUT50, area under the threshold below an absolute value of 50% of regional cerebral oxygen saturation; CPB; cardiopulmonary bypass.
In Pearson correlation analysis, changes in MAP (r = 0.126; p < 0.001), Hb (r = 0.432; p < 0.001), CI (r = 0.110; p < 0.001), and CVP (r = −0.115; p < 0.001) were statistically significantly, but weakly correlated with changes in rSO2. In further analyses to find variables related to decrease in rSO2, there were no significant differences in MAP, CI, PaO2, CVP, and body temperature between patients who exhibited AUT of greater than 113.2 min·% below an absolute value of 50% (high AUT 50) or not (low AUT 50), while Hb levels were significantly lower and PaCO2 levels were significantly higher in the high AUT50 group throughout surgery (Table 5). Patients in the high AUT 50 group received more intraoperative pRBCs transfusion (58% versus 22%; p < 0.001) and had higher vasopressin requirements (77% versus 64%; p = 0.01) than patients in the low AUT 50 group.
Table 5.
Analysis of variables related to rSO2 desaturation.
| Variables | Group | Time | ||||
|---|---|---|---|---|---|---|
| Baseline | Ind 15 min | CPB 15 min | PostCPB | Sternal Closure | ||
| MAP (mmHg) |
High AUT50 | 90.9 ± 16.4 | 75.0 ± 10.5 | 63.7 ± 11.3 | 67.6 ± 9.9 | 74.9 ± 11.7 |
| Low AUT50 | 89.1 ± 15.0 | 74.9 ± 10.5 | 61.9 ± 13.0 | 68.9 ± 10.2 | 77.0 ± 12.8 | |
| CI (L/min/m2) |
High AUT50 | 2.1 ± 0.7 | 2.5 ± 0.7 | 2.2 ± 0.6 | ||
| Low AUT50 | 2.2 ± 0.8 | 2.6 ± 0.9 | 2.2 ± 0.7 | |||
| CVP (mmHg) | High AUT50 | 10.5 ± 3.6 | 10.6 ± 2.6 | 11.5 ± 2.8 * | ||
| Low AUT50 | 9.8 ± 3.0 | 10.1 ± 2.8 | 10.6 ± 2.2 | |||
| PaCO2 (mmHg) | High AUT50 | 34.2 ± 3.4 * | 32.9 ± 3.9 | 34.4 ± 3.9 | 36.9 ± 3.5 * | |
| Low AUT50 | 33.5 ± 3.0 | 33.1 ± 3.8 | 33.8 ± 3.6 | 35.7 ± 3.3 | ||
| PaO2 (mmHg) | High AUT50 | 176.0 ± 44.5 | 329.0 ± 57.6 | 185.2 ± 41.8 | 167.7 ± 42.9 | |
| Low AUT50 | 179.0 ± 39.9 | 326.5 ± 59.4 | 185.6 ± 37.9 | 171.7 ± 44.6 | ||
| Temperature (°C) | High AUT50 | 36.1 ± 0.6 | 32.6 ± 2.7 | 36.4 ± 0.8 | 36.4 ± 0.5 | |
| Low AUT50 | 36.0 ± 0.6 | 32.2 ± 3.1 | 36.4 ± 0.4 | 36.4 ± 0.5 | ||
| Hemoglobin (g/dL) | High AUT50 | 12.2 ± 2.3 * | 11.5 ± 1.7 * | 7.8 ± 1.2 * | 8.2 ± 1.0 * | 9.6 ± 1.0 * |
| Low AUT50 | 13.2 ± 1.8 | 12.3 ± 1.5 | 8.4 ± 1.5 | 8.8 ± 1.2 | 10.0 ± 1.1 | |
Values are mean (standard deviation). High and low AUT50, Patients who exhibited area under the threshold of regional cerebral oxygen saturation below and absolute value of 50% greater than 113.2 min% or not; Baseline, before anesthetic induction; Ind 15 min, 15 min after anesthetic induction; CPB 15 min, 15 min after staring cardiopulmonary bypass (CPB); PostCPB, 20 min after weaning from CPB; MAP, mean arterial pressure; CI, cardiac index; CVP, central venous pressure, * p < 0.05 vs. low AUT50.
4. Discussion
In this retrospective inquiry into the impact of baseline rSO2 and intraoperative decrease in rSO2 on a composite of morbidity endpoints in cardiac surgical patients who received interventions to correct decrease in rSO2 of more than 20% from baseline value, the AUT of rSO2 below an absolute value of 50% was identified as an independent risk factor along with age, preoperative CKD, and duration of CPB.
Cumulating evidence indicated the value of rSO2 in reflecting overall hemodynamic status and thus, a relationship between a low rSO2 value and systemically impaired tissue perfusion [8]. Indeed, studies have shown promising results supporting this concept of monitoring the brain as an index organ and have depicted associations of low baseline rSO2 values or prolonged intraoperative rSO2 desaturation with an increased risk of overall postoperative morbidity after cardiac surgery [6]. rSO2 has also been shown to predict abnormal cardiac function better than hemodynamic variables derived from a pulmonary artery catheter [16]. Although the evidence is not conclusive, further studies have shown the beneficial influence of interventions to correct intraoperative decrease in rSO2 on outcome [8,17]. As these corrective interventions mainly target improved perfusion [12], they have gained increased acceptance in our daily clinical practice. Consequently, by being a potentially modifiable risk factor, the value of baseline rSO2 as an aid for accurate risk stratification may be altered by intraoperative interventions to correct its decrease. In addition, evidence is also limited in terms of the predictive value of intraoperative decrease in rSO2 on outcome when being exposed to corrective interventions.
As our findings show, various assessed rSO2 values including baseline, minimal (the lowest measured value), AUT of 80% of the baseline, and AUT below an absolute value of 50%, were all significantly different between the morbid and non-morbid groups, indicating the prognostic importance of cerebral oximetry monitoring. However, the prognostic value of low baseline rSO2 was not present after adjusting for potential confounders of adverse outcome. In contrast, the severity of intraoperative decrease in rSO2 below an absolute value of 50%, expressed as AUT 50, was revealed to be an independent risk factor of adverse outcome together with advanced age, preoperative CKD, and prolonged CPB time. The assessed major STS morbidity endpoints are well validated surrogate measures of overall outcome [11]. In the current study, prolonged ventilation was most prevalent among the assessed morbidity endpoints. As with any other morbidity endpoints, prolonged ventilation is attributable to multiple, complex factors that cannot be properly delved in the current study. However, common to all morbidity endpoints, it is well-known that lung injury after cardiac surgery is related to the degree of hypoperfusion or ischemia-reperfusion injury during CPB [18]. In addition, hemodynamic instability would play a major role in prolonged ventilation as well, supporting the role of monitoring the brain as an index organ.
In previous studies, evidence was more solid in terms of the prognostic importance of low baseline rSO2 values for a hard, clinical endpoint, mortality, when compared to those of intraoperative decrease in rSO2. This evidence includes a prospective, observational study involving more than 1100 cardiac surgical patients, showing a close correlation between baseline rSO2 value and mortality, which was even more predictive than the EuroSCORE in high-risk patients [9]. Another retrospective study involving more than 2000 cardiac surgical patients also showed that a baseline rSO2 value was a simple and useful predictor of mortality [19]. It is difficult to deduct any plausible explanation for the discrepancy with our result as only one of the previous studies was observational [9], while the other retrospective study stated that rSO2 values were actively treated without any specific information regarding the cut-off value upon which treatment was initiated [19]. Yet, the discrepancy may be attributable to the fact that all patients received intraoperative interventions to correct the decrease in rSO2 value below a specific point (20% reduction from baseline) in the current study. Also, it may be related to the low mean baseline rSO2 values of the current study (58% versus >62%) when compared to other studies or to the suggested normal range of approximately 70% [9,20,21]. This would simply indicate a poorer baseline cardiovascular functional reserve, and thus a generally more guarded prognosis in the patients we studied.
Likewise, the low baseline values may have affected the results that showed only an intraoperative decrease in rSO2 below an absolute value of 50% was a meaningful predictor of outcome, whereas desaturation to 80% of the baseline value was not. The currently used cerebral oximetry device (INVOSTM Cerebral/Somatic Oximeter 5100) uses 2 wavelengths to detect rSO2 and accordingly, the U.S. Food and Drug Administration recommends its use only as a trend monitor. Thus, a decrease to 80% of the baseline rSO2 value was a commonly adopted threshold for interventions in many previous studies [17,22]. Yet, in patients with low baseline values, as in our studied population, 80% of the baseline would be below the meaningful cut-off found in our study, an absolute value of 50%. Various cut-off values were previously suggested to be related to adverse outcomes and were recommended as potential intervention thresholds, including the manufacturer’s suggestion of 40% (absolute value) [20]. As our results indicate, a cut-off of an absolute value of 50% may be an appropriate target for intervention in cardiac surgical patients with limited cardiovascular functional reserves, as reflected by low baseline rSO2 values in whom a 20% decrease from baseline would be even lower.
Variables that are associated with changes in intraoperative rSO2 and that are commonly targeted for cerebral oximetry-based interventions such as MAP, CI, CVP, and Hb, all showed significant correlation, reaffirming the importance of the brain as an index organ for overall hemodynamic status. In addition, patients in the high AUT 50 group received more pRBCs transfusion and vasopressin, and had higher PaCO2 levels and lower Hb levels compared to the low AUT 50 group, indicating that they apparently received more interventions to increase rSO2. Still, only 67% of the patients showed restoration of rSO2 value above 80% of the baseline value and considerable numbers of patients with AUT 50 were present in the morbid group despite corrective interventions. Studies have shown the lower limit of cerebral autoregulation to be higher than 90 mmHg in some cardiac surgical patients [23]. Additionally, cumulating evidence favors the use of restrictive transfusion strategies in cardiac surgery with Hb thresholds of 7–8 g/dL [24], while cerebral oximetry has been shown to be a useful monitor to safely implement restrictive transfusion [25]. Nonetheless, it remains questionable whether increasing the MAP to near or above 90 mmHg or giving pRBCs transfusions at Hb levels of above 9 g/dL to correct decrease in rSO2 would result in improved outcomes considering the inherent technical limitations related to cerebral oximetry and its well-known high sensitivity but low specificity in detecting adverse outcome [6]. This could also be observed in our ROC analysis, showing the predictive power of AUT 50 on a composite of morbidity endpoints at 80.9% of sensitivity and 40.8% of specificity.
The current study is linked to limitations intrinsic to its retrospective nature and the technical aspects of the cerebral oximetry (INVOSTM Cerebral/Somatic Oximeter 5100) including the approximate depth of measurement of 2 cm [26], while the distance from the skin to the frontal cerebral cortex could be longer in elderly patients with cortical brain atrophy [27]. Second, although interventions to restore the rSO2 values were guided by our given institutional protocol, we were unable to check the rate of non-adherence to the protocol. Third, composite of morbidity endpoints could only be observed in 47 patients, which have limited comprehensive introduction of variables known to affect outcome and thus, limiting the statistical power of the study.
Still, the strength of the current study lies in that it provides primary evidence regarding the prognostic value of baseline rSO2 as well as intraoperative rSO2 desaturation on overall outcome in cardiac surgical patients who received interventions to correct intraoperative decrease in rSO2 using a comprehensive rSO2 data set collected at 30-s intervals.
In conclusion, in cardiac surgical patients who received interventions according to cerebral oximetry monitoring, increased severity of intraoperative decrease in rSO2 as reflected by AUT below an absolute value of 50% was found to be an independent risk factor of dismal prognosis, implicating an important ancillary role of cerebral oximetry to monitor the brain as an index organ.
Author Contributions
Conceptualization, Y.Y.J., J.-K.S. and Y.L.K.; Methodology, Y.Y.J.; Formal analysis, Y.Y.J. and S.S. (Sarah Soh); Investigation, S.S. (Sarah Soh) and S.S. (Sungmin Suh); Data curation, S.S. (Sungmin Suh); Writing-original draft preparation, Y.Y.J.; Writing-review and editing, J.-K.S. and Y.L.K.; Supervision, Y.L.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Ghosh A., Elwell C., Smith M. Review article: Cerebral near-infrared spectroscopy in adults: A work in progress. Anesth. Analg. 2012;115:1373–1383. doi: 10.1213/ANE.0b013e31826dd6a6. [DOI] [PubMed] [Google Scholar]
- 2.Yao F.S., Tseng C.C., Ho C.Y., Levin S.K., Illner P. Cerebral oxygen desaturation is associated with early postoperative neuropsychological dysfunction in patients undergoing cardiac surgery. J. Cardiothorac. Vasc. Anesth. 2004;18:552–558. doi: 10.1053/j.jvca.2004.07.007. [DOI] [PubMed] [Google Scholar]
- 3.Zheng F., Sheinberg R., Yee M.S., Ono M., Zheng Y., Hogue C.W. Cerebral near-infrared spectroscopy monitoring and neurologic outcomes in adult cardiac surgery patients: A systematic review. Anesth. Analg. 2013;116:663–676. doi: 10.1213/ANE.0b013e318277a255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang H., Meng L.Z., Lyon R., Wang D.X. Monitoring cerebral ischemia during cerebrovascular surgery. J. Biomed. Res. 2017;31:279–282. doi: 10.7555/JBR.31.20150106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Evered L., Silbert B., Knopman D.S., Scott D.A., DeKosky S.T., Rasmussen L.S., Oh E.S., Crosby G., Berger M., Eckenhoff R.G., et al. Recommendations for the nomenclature of cognitive change associated with anaesthesia and surgery-2018. Anesthesiology. 2018;129:872–879. doi: 10.1097/ALN.0000000000002334. [DOI] [PubMed] [Google Scholar]
- 6.Murkin J.M. Cerebral oximetry: Monitoring the brain as the index organ. Anesthesiology. 2011;114:12–13. doi: 10.1097/ALN.0b013e3181fef5d2. [DOI] [PubMed] [Google Scholar]
- 7.Vranken N.P.A., Weerwind P.W., Sutedja N.A., Ševerdija E.E., Barenbrug P.J.C., Maessen J.G. Cerebral oximetry and autoregulation during cardiopulmonary bypass: A review. J. Extra Corpor. Technol. 2017;49:182–191. [PMC free article] [PubMed] [Google Scholar]
- 8.Murkin J.M., Adams S.J., Novick R.J., Quantz M., Bainbridge D., Iglesias I., Cleland A., Schaefer B., Irwin B., Fox S. Monitoring brain oxygen saturation during coronary bypass surgery: A randomized, prospective study. Anesth. Analg. 2007;104:51–58. doi: 10.1213/01.ane.0000246814.29362.f4. [DOI] [PubMed] [Google Scholar]
- 9.Heringlake M., Garbers C., Käbler J.H., Anderson I., Heinze H., Schön J., Berger K.U., Dibbelt L., Sievers H.H., Hanke T. Preoperative cerebral oxygen saturation and clinical outcomes in cardiac surgery. Anesthesiology. 2011;114:58–69. doi: 10.1097/ALN.0b013e3181fef34e. [DOI] [PubMed] [Google Scholar]
- 10.de Tournay-Jetté E., Dupuis G., Bherer L., Deschamps A., Cartier R., Denault A. The relationship between cerebral oxygen saturation changes and postoperative cognitive dysfunction in elderly patients after coronary artery bypass graft surgery. J. Cardiothorac. Vasc. Anesth. 2011;25:95–104. doi: 10.1053/j.jvca.2010.03.019. [DOI] [PubMed] [Google Scholar]
- 11.O’Brien S.M., Feng L., He X., Xian Y., Jacobs J.P., Badhwar V., Kurlansky P.A., Furnary A.P., Cleveland J.C., Jr., Lobdell K.W., et al. The society of thoracic surgeons 2018 adult cardiac surgery risk models: Part 2-Statistical methods and results. Ann. Thorac. Surg. 2018;105:1419–1428. doi: 10.1016/j.athoracsur.2018.03.003. [DOI] [PubMed] [Google Scholar]
- 12.Denault A., Deschamps A., Murkin J.M. A proposed algorithm for the intraoperative use of cerebral near-infrared spectroscopy. Semin. Cardiothorac. Vasc. Anesth. 2007;11:274–281. doi: 10.1177/1089253207311685. [DOI] [PubMed] [Google Scholar]
- 13.Lee T.H., Marcantonio E.R., Mangione C.M., Thomas E.J., Polanczyk C.A., Cook E.F., Sugarbaker D.J., Donaldson M.C., Poss R., Ho K.K., et al. Derivation and prospective validation of a simple index for prediction of cardiac risk of major noncardiac surgery. Circulation. 1999;100:1043–1049. doi: 10.1161/01.CIR.100.10.1043. [DOI] [PubMed] [Google Scholar]
- 14.Salis S., Mazzanti V.V., Merli G., Salvi L., Tedesco C.C., Veglia F., Sisillo E. Cardiopulmonary bypass duration is an independent predictor of morbidity and mortality after cardiac surgery. J. Cardiothorac. Vasc. Anesth. 2008;22:814–822. doi: 10.1053/j.jvca.2008.08.004. [DOI] [PubMed] [Google Scholar]
- 15.Najafi M. Serum creatinine role in predicting outcome after cardiac surgery beyond acute kidney injury. World J. Cardiol. 2014;6:1006–1021. doi: 10.4330/wjc.v6.i9.1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Paquet C., Deschamps A., Denault A.Y., Couture P., Carrier M., Babin D., Levesque S., Piquette D., Lambert J., Tardif J.C. Baseline regional cerebral oxygen saturation correlates with left ventricular systolic and diastolic function. J. Cardiothorac. Vasc. Anesth. 2008;22:840–846. doi: 10.1053/j.jvca.2008.02.013. [DOI] [PubMed] [Google Scholar]
- 17.Colak Z., Borojevic M., Bogovic A., Ivancan V., Biocina B., Majeric-Kogler V. Influence of intraoperative cerebral oximetry monitoring on neurocognitive function after coronary artery bypass surgery: A randomized, prospective study. Eur. J. Cardiothorac. Surg. 2015;47:447–454. doi: 10.1093/ejcts/ezu193. [DOI] [PubMed] [Google Scholar]
- 18.Huffmyer J.L., Groves D.S. Pulmonary complications of cardiopulmonary bypass. Best Pract. Res. Clin. Anaesthesiol. 2015;29:163–175. doi: 10.1016/j.bpa.2015.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sun X., Ellis J., Corso P.J., Hill P.C., Lowery R., Chen F., Lindsay J. Mortality predicted by preinduction cerebral oxygen saturation after cardiac operation. Ann. Thorac. Surg. 2014;98:91–96. doi: 10.1016/j.athoracsur.2014.03.025. [DOI] [PubMed] [Google Scholar]
- 20.Murkin J.M., Arango M. Near-infrared spectroscopy as an index of brain and tissue oxygenation. Br. J. Anaesth. 2009;103(Suppl. 1):i3–i13. doi: 10.1093/bja/aep299. [DOI] [PubMed] [Google Scholar]
- 21.Schoen J., Meyerrose J., Paarmann H., Heringlake M., Hueppe M., Berger K.U. Preoperative regional cerebral oxygen saturation is a predictor of postoperative delirium in on-pump cardiac surgery patients: A prospective observational trial. Crit. Care. 2011;15:R218. doi: 10.1186/cc10454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Deschamps A., Hall R., Grocott H., Mazer C.D., Choi P.T., Turgeon A.F., de Medicis E., Bussières J.S., Hudson C., Syed S., et al. Cerebral oximetry monitoring to maintain normal cerebral oxygen saturation during high-risk cardiac surgery: A randomized controlled feasibility trial. Anesthesiology. 2016;124:826–836. doi: 10.1097/ALN.0000000000001029. [DOI] [PubMed] [Google Scholar]
- 23.Joshi B., Ono M., Brown C., Brady K., Easley R.B., Yenokyan G., Gottesman R.F., Hogue C.W. Predicting the limits of cerebral autoregulation during cardiopulmonary bypass. Anesth. Analg. 2012;114:503–510. doi: 10.1213/ANE.0b013e31823d292a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mazer C.D., Whitlock R.P., Fergusson D.A., Hall J., Belley-Cote E., Connolly K., Khanykin B., Gregory A.J., de Médicis É., McGuinness S., et al. Restrictive or liberal red-cell transfusion for cardiac surgery. NEJM. 2017;377:2133–2144. doi: 10.1056/NEJMoa1711818. [DOI] [PubMed] [Google Scholar]
- 25.Serraino G.F., Murphy G.J. Effects of cerebral near-infrared spectroscopy on the outcome of patients undergoing cardiac surgery: A systematic review of randomised trials. BMJ Open. 2017;7:e016613. doi: 10.1136/bmjopen-2017-016613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yu Y., Lu Y., Meng L., Han R. Monitoring cerebral ischemia using cerebral oximetry: Pros and cons. J. Biomed. Res. 2016;30:1–4. doi: 10.7555/JBR.30.20150096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Provencher D., Hennebelle M., Cunnane S.C., Bérubé-Lauzière Y., Whittingstall K. Cortical thinning in healthy aging correlates with larger motor-evoked EEG desynchronization. Front. Aging Neurosci. 2016;8:63. doi: 10.3389/fnagi.2016.00063. [DOI] [PMC free article] [PubMed] [Google Scholar]