Abstract
Development of gastric cancer is often preceded by chronic inflammation, but the immune cellular mechanisms underlying this process are unclear. Here we demonstrated that an inflammasome molecule, absent in melanoma 2 (Aim2), was upregulated in patients with gastric cancer and in spasmolytic polypeptide-expressing metaplasia of chronically Helicobacter felis–infected stomachs in mice. However, we found that Aim2 was not necessary for inflammasome function during gastritis. In contrast, Aim2 deficiency led to an increase in gastric CD8+ T cell frequency, which exacerbated metaplasia. These gastric CD8+ T cells from Aim2–/– mice were found to have lost their homing receptor expression (sphingosine-1-phosphate receptor 1 [S1PR1] and CD62L), a feature of tissue-resident memory T cells. The process was not mediated by Aim2-dependent regulation of IFN-β or by dendritic cell–intrinsic Aim2. Rather, Aim2 deficiency contributed to an increased production of CXCL16 by B cells, which could suppress S1PR1 and CD62L in CD8+ T cells. This study describes a potentially novel function of Aim2 that regulates CD8+ T cell infiltration and retention within chronically inflamed solid organ tissue. This function operates independent of the inflammasome, IFN-β, or dendritic cells. We provide evidence that B cells can contribute to this mechanism via CXCL16.
Keywords: Gastroenterology
Keywords: Gastric cancer
Aim2 regulates stomach immunopathology independent of its traditionally described functions in regulating the inflammasome or IFN-γ signaling.
Introduction
It is well established that a prolonged latency period of chronic inflammation precedes the development of gastrointestinal cancers (1, 2), including gastric cancer (3, 4). However, the mechanisms of how chronic inflammation contributes to gastric cancer development remain unclear (5). Differentiated gastric adenocarcinoma arises from a series of sequential developments comprising chronic inflammation, atrophy of acid-secreting parietal cells, expansion of mucus-secreting neck cells and chief cell transdifferentiation, and dysplasia, leading to neoplasia (3). The development of metaplastic lesions in the stomach is important in that patients who acquire them have an increased risk of developing differentiated gastric adenocarcinoma (6). Two types of metaplastic lesions of the stomach have been identified in human patients as precursors to gastric adenocarcinoma: (a) intestinal metaplasia and (b) pseudopyloric metaplasia, also defined as spasmolytic peptide-expressing metaplasia (SPEM) (7). Parietal cell atrophy has been previously defined as a necessary step toward the development of SPEM (8–10). However, recent reports indicate that the loss of parietal cells is not sufficient (11) and requires the contribution of the inflammatory milieu to trigger SPEM (12).
We observed that absent in melanoma 2 (Aim2), an inflammatory molecule and inflammasome component (13–15), was upregulated in mouse models of SPEM. We therefore used chronic Helicobacter felis (H. felis) infection to study the role of Aim2 in this context. Because Aim2 mediates IL-1β and IL-18 cleavage and bioactivity (13–17), we expected Aim2 deficiency to ameliorate gastric immunopathology during H. felis infection. However, in contrast to our anticipated results, Aim2-deficient mice had markedly pronounced gastritis compared with controls. This suggested that Aim2 was necessary for restraining metaplasia via a potentially novel function that is likely independent of the inflammasome, which is the focus of this study.
Results
Aim2 deletion exacerbates SPEM.
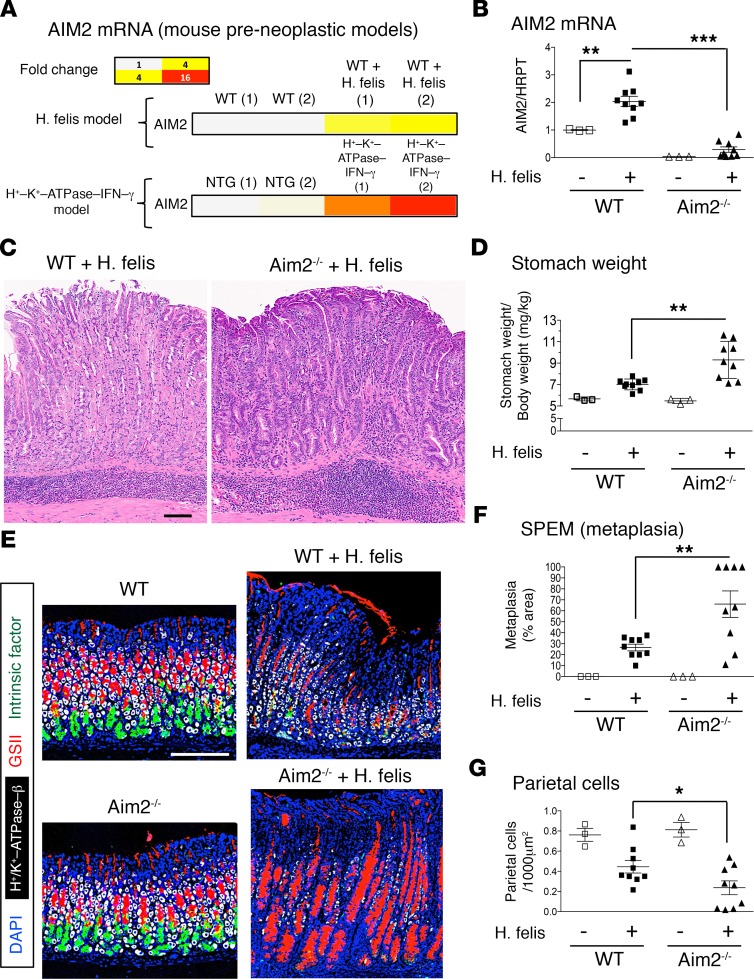
We identified Aim2 in a screen of upregulated gastric genes in 2 mouse models of SPEM: (a) chronic (6-month) H. felis infection and (b) gastric tissue-specific overexpression of IFN-γ (H+/K+–ATPase–IFN-γ) (18) (Figure 1A). The upregulation of Aim2 in gastric tissue of chronically H. felis–infected mice at 6 months was corroborated by real-time quantitative PCR (RT-qPCR) (Figure 1B). To study the role of Aim2 in SPEM, we used mice deficient in Aim2 (B6.129P2-Aim2Gt(CSG445)Byg/J; Aim2–/–) and chronically infected them with H. felis. We confirmed that Aim2 deletion abrogated Aim2 expression in the stomach (Figure 1B). Aim2 deficiency worsened gastric immunopathology (Figure 1C), increased stomach weight (Figure 1D), exacerbated SPEM (Figure 1, E and F), and enhanced parietal cell atrophy (Figure 1G). All the latter characteristics epitomize features of enhanced gastric preneoplastic development in Aim2–/– mice.
Figure 1. Aim2 is induced in chronic (6-month) gastric inflammation, and its deficiency exacerbates SPEM.
(A) Microarray heatmap of gastric Aim2 mRNA expression in 6-month H. felis–infected stomach and gastric-tissue specific IFN-γ–overexpressing mice. NTG, nontransgenic. (B) RT-qPCR of Aim2 mRNA expression in 6-month H. felis–infected WT versus Aim2–/– stomachs relative to uninfected. HPRT, hypoxanthine phosphoribosyltransferase. (C) Representative H&E image of 6-month H. felis–infected WT versus Aim2–/– gastric mucosa. Scale bar: 100 μm. (D) Stomach weight, normalized to total body weight, of 6-month H. felis–infected WT versus Aim2–/– mouse stomachs relative to uninfected. (E) Representative immunofluorescence image for quantifying SPEM, by labeling gastric mucous neck cells (Griffonia simplicifolia II; GSII; shown in red), chief cells (intrinsic factor, shown in green), parietal cells (H+/K+-ATPase, shown in gray), and cell nuclei (DAPI, shown in blue). Scale bar: 200 μm (all images). (F) Morphometric analysis showing SPEM quantification by percentage area. SPEM was quantified by GSII and trefoil factor 2 coexpression in ×20 field views. (G) Morphometric analysis of parietal cell number per 1000 μm2 of gastric mucosal tissue. Each data point represents 1 mouse. Error bars represent mean ± SEM. *P < 0.05; **P < 0.01; ***P < 0.001. Data were compared using one-way ANOVA with Dunnet’s (parametric) multiple comparison tests.
Aim2 lacks a significant effect on gastric inflammasome activity.
Because Aim2 functions as a component of the inflammasome (13–15), we investigated whether Aim2 was mediating its observed effects by regulating IL-1β and IL-18 secretion. We found gastric explants from 6-month infected Aim2–/– stomachs did not show a significant effect on the secretion of IL-1β or IL-18 (Supplemental Figure 1, A and B; supplemental material available online with this article; https://doi.org/10.1172/jci.insight.94035DS1). Hence, these observations did not provide mechanistic insight into how the loss of Aim2 led to increased SPEM. This prompted us to dissect the mechanism of how Aim2 inhibits SPEM further.
Aim2 is not required for the expression of the inhibitory Fcγreceptor. Because a previous report showed that Aim2 is necessary for the expression of the inhibitory Fcγ receptor (FcγRIIB) (19), we investigated a possible alternative mechanism for Aim2 via this protein. FcγRIIB is a lupus susceptibility protein that suppresses antibody production in B cells (20) and blocks maturation of dendritic cells (21). However, we did not observe a significant effect of Aim2 deficiency on FcγRIIB expression in the inflamed stomach (Supplemental Figure 1C).
Aim2 deficiency increases gastric CD8+ T cell frequency in the chronically inflamed stomach.
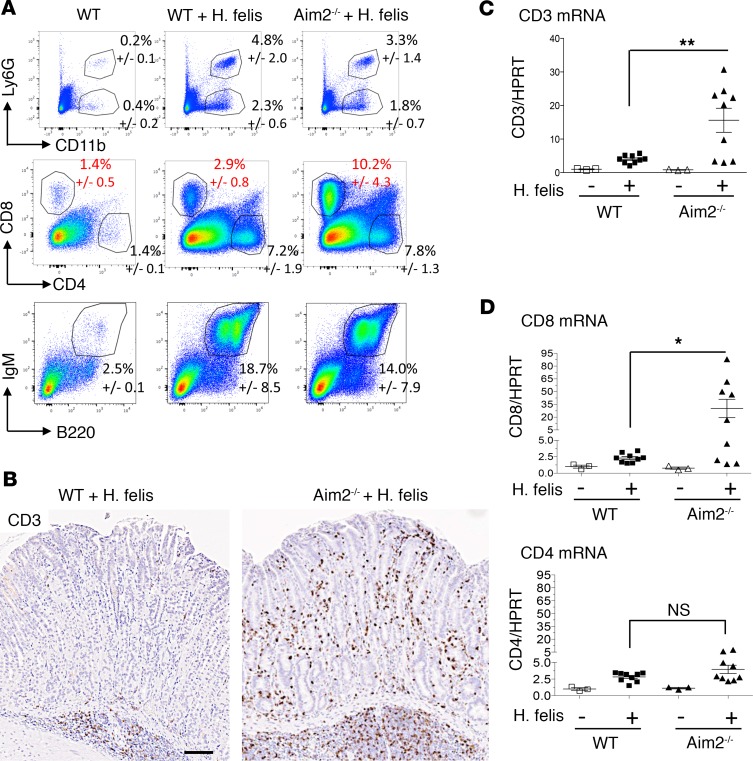
Because the mechanism of Aim2 in gastric pathology remained unclear, we screened several gastric immune populations from chronically infected Aim2–/– stomachs versus WT stomachs. Previous reports have documented several immune subtypes that increase during chronic gastric inflammation and SPEM (22–28). These include CD11b+Ly6G+ myeloid-derived suppressor cells (MDSCs) (22–24), CD11b+Ly6G– myeloid cells (22), CD4+ and CD8+ T cells (25–27), and B220+IgM+ B cells (28). We therefore screened these populations in our system (Figure 2A). In chronically infected WT stomachs, we observed a significant increase in all these populations relative to uninfected WT stomachs (Figure 2A). However, gastric CD8+ T cells were the only population whose frequency was affected by Aim2 deficiency in that they were dramatically increased in 6-month H. felis–infected Aim2–/– mice (Figure 2A, red font, percentages; mean gastric CD8+ in WT = 2.9%; mean gastric CD8+ in Aim2–/– = 10.2; P < 0.05). Aim2 deficiency did not affect gastric CD8+ T cell frequency at baseline without infection (not shown). Neither MDSC nor B cell populations showed a significant difference between infected WT versus Aim2–/– stomachs (Figure 2A). The increase in gastric T cells in Aim2–/– was corroborated by immunohistochemical staining of CD3 (Figure 2B) and RT-qPCR of gastric CD3 expression (Figure 2C). Also, consistent with the FACS analysis in Figure 2A, gastric mRNA expression of T cell markers was increased for CD8 but not CD4 mRNA by RT-qPCR (Figure 2D). In contrast, immunohistochemical staining of B220, and gastric mRNA expression of the B cell marker CD19, did not show a significant difference between chronically infected WT versus Aim2–/– stomachs (Supplemental Figure 2, A and B). These findings indicate that Aim2 deficiency increases gastric CD8+ T cell frequency in the inflamed stomach, without affecting the frequency of other immune populations.
Figure 2. Aim2 deficiency increases gastric CD8+ T cell frequency in the chronically inflamed stomach.
(A) FACS analyses of gastric CD11b+Ly6G– myeloid cells (upper), CD11b+Ly6G+ MDSCs (upper), CD4+ versus CD8+ gastric T cells (middle), and B220+IgM+ gastric B cells (lower) in 6-month H. felis–infected WT versus Aim2–/– stomachs relative to uninfected WT controls. Values indicated are mean frequencies ± SEM from 3 mice per group. (B) Representative CD3 immunohistochemistry (brown) of Aim2–/– versus WT 6-month H. felis–infected gastric mucosa. Scale bar: 100 μm. (C) RT-qPCR analyses of gastric CD3 mRNA expression in 6-month H. felis–infected Aim2–/– versus WT stomach relative to uninfected controls. (D) RT-qPCR analyses of gastric CD8 and CD4 mRNA expression in 6-month H. felis–infected Aim2–/– versus WT stomach relative to uninfected controls. Each data point represents 1 mouse. Error bars represent mean ± SEM. N.S., not significant; *P < 0.05; **P < 0.01. Data were compared using one-way ANOVA with Dunnet’s (parametric) multiple comparison tests.
Increased gastric CD8+ T cells of Aim2–/– mice lose their homing receptor expression.
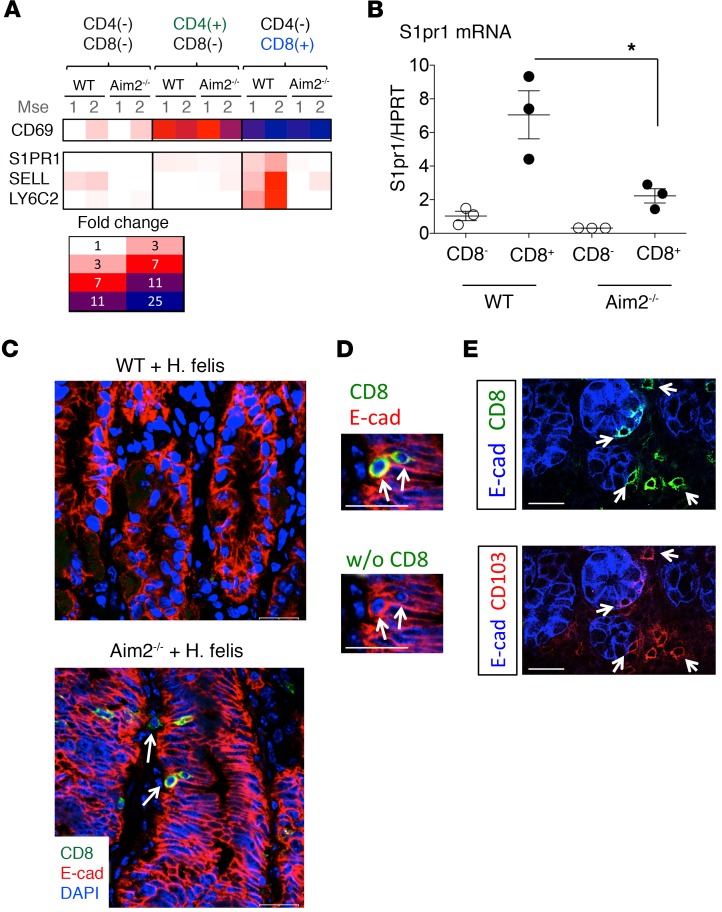
Because gastric CD8+ T cell frequency was affected by Aim2 deletion, we utilized these cells to gain insight about the mechanism of Aim2. We first optimized a FACS protocol in which distinct T cell populations could be sorted from the inflamed stomach for microarray analysis (Supplemental Figure 3). We performed this by gating individual cells from the inflamed stomach for CD3+ cells, then subgating for CD4+ versus CD8+ cells. We then performed microarray analysis for the sorted CD4–CD8–, CD4+CD8–, and CD4+CD8+ cell populations (Supplemental Figure 3A). We observed a majority of T cell–specific genes that were commonly expressed in both CD4+ and CD8+ T cells (Supplemental Figure 3A). However, a number of genes were differentially expressed by either CD8+ or CD4+ T cells (annotated as “Distinct Genes” in Supplemental Figure 3A). The list of distinct genes is illustrated in Supplemental Figure 3B. Gastric CD4 gene expression was enriched in the isolated CD4+ T cell population (Supplemental Figure 3B), whereas CD8A and CD8B1 gene expression was enriched in the gastric CD8+ T cell population (Supplemental Figure 3B). The highly expressed genes in WT gastric CD8+ T cells included killer cell lectin-like receptors and perforin 1, which was indicative of cytotoxic activity (Supplemental Figure 3B), and homing receptor genes sphingosine-1-phosphate receptor 1 (S1PR1), CD62L/L-selectin (SELL), and LY6C2 (Supplemental Figure 3B). When comparing the expression profiles of these CD8+ T cells between Aim2–/– and WT stomachs from mice chronically infected with H. felis, we observed a difference only in the expression of homing receptors (Figure 3A; refer to the comparison of S1PR1, SELL, and LY6C2 expression between WT versus Aim2–/– CD4–CD8+ cells) but not in other CD8+ T cell–specific genes (data not shown). Aim2–/– gastric CD8+ T cells lost S1PR1, SELL, and LY6C2 expression relative to WT (Figure 3A), which was corroborated for S1PR1 by RT-qPCR (Figure 3B). These findings indicate that Aim2 was necessary for homing receptor expression by gastric CD8+ T cells.
Figure 3. Aim2 deficiency leads to downregulation of gastric CD8+ T cell homing receptors characteristic of tissue-resident memory T cells.
(A) Microarray heatmap of homing receptor S1PR1, SELL (CD62L), LY6C2 (LY6C), and CD69 expression in isolated gastric CD8+ versus CD4+ T cells. Mse, mouse. (B) RT-qPCR of S1PR1 homing receptor mRNA expression in isolated gastric CD8+ T cells (versus CD8– control populations) from 6-month H. felis–infected WT versus Aim2–/– stomachs. Each data point represents 1 mouse. Error bars represent the mean ± SEM. *P < 0.05. (C) Immunofluorescence analysis of CD8+ cells (green), epithelial cells (E-cadherin, red), and nuclei (DAPI, blue) in 6-month H. felis–infected Aim2–/– versus WT stomachs. Arrows indicate intraepithelial and stromal localizations of gastric CD8+ cells in the Aim2–/– gastric mucosa. Scale bar: 20 μm. (D) Higher magnification inset from C: coexpression of CD8 (green) and E-cadherin (red) in the chronically infected Aim2–/– gastric mucosa. (E) Coexpression of CD8 (green, upper) and CD103 (red, lower) in the chronically infected Aim2–/– gastric mucosa, as denoted by white arrows. Epithelial cell E-cadherin is labeled in blue. Data were compared using one-way ANOVA with Dunnet’s (parametric) multiple comparison tests.
Gastric CD8+ T cells of Aim2–/– mice exhibit a tissue-resident memory phenotype.
Recent studies have implicated homing receptors S1PR1, CD62L, and LY6C in T cell memory differentiation. Memory T cells exhibit several phenotypes, including central memory T cells (Tcm cells), effector memory T cells (Tem cells), or tissue-resident memory T cells (Trm cells) (29–31). Tcm cells patrol lymph nodes and the white pulp of the spleen and express the abovementioned homing receptors. Tem cells circulate between the blood and nonlymphoid tissue but do not express homing receptors. Trm cells are resident in the tissue, do not recirculate into the blood or secondary lymphoid organs, and lose their homing receptor expression. Thus, homing receptor expression can be used to distinguish between Tcm versus Tem and Trm cells (29–31). However, distinguishing Tem from Trm cells requires additional analysis (29). In a recent review, Mueller and Mackay (29) stated that a defining feature for Trm cells depends on the localization of these cells within the intraepithelial compartment, which distinguishes them from Tem cells. We therefore evaluated the localization of the Aim2–/– CD8+ T cells in the gastric mucosa. We detected Aim2–/– CD8+ T cells occupied a predominantly intraepithelial localization (Figure 3C). Based on a number of reports, the defining features for CD8+ Trm cells include the following: (a) their loss of expression of S1PR1 (32–35), CD62L (35–37), and LY6C (37, 38); (b) their high expression of CD69 (33, 34, 36–39); and (c) their expression of E-cadherin (38) and CD103 (33, 36–40). We observed that, in addition to their loss of S1PR1, SELL (CD62L), and LY6C2 (Figure 3, A and B) and their intraepithelial localization (Figure 3C), Aim2–/–CD8+ cells also expressed high levels of CD69 (Figure 3A), E-cadherin (Figure 3D), and CD103 (Figure 3E). Therefore, based on this evidence we conclude that Aim2–/– deficiency increases CD8+ Trm cell frequency within the chronically inflamed gastric mucosa.
Aim2 deficiency does not intrinsically regulate homing receptor expression within CD8+ T cells.
Because the loss of homing receptor expression has been shown to correlate with, and be necessary for, Trm cell generation (29–32), we hypothesized that Aim2 might be regulating Trm cell generation by regulating these receptors. To determine whether T cell–intrinsic Aim2 regulated the expression of homing receptors, we developed an in vitro assay to assess CD62L downregulation in Aim2–/– versus WT CD8+ T cells. CD8+ T cells were isolated from the spleen by negative selection using magnetic beads and compared between naive, suboptimal, and optimal activation status using anti-CD3 and anti-CD8 (Supplemental Figure 4). We observed that T cell activation reduced CD62L expression in CD8+ T cells (Supplemental Figure 4). While only 11.9% of naive CD8+ T cells were CD62L–, this number increased to 29.3% upon suboptimal activation (with 0.75 μg/mL CD3 antibody) and to 68.3% upon optimal activation (with 10 μg/mL CD3 antibody) (Supplemental Figure 4; naive versus suboptimal activation: P < 0.001; suboptimal versus optimal activation: P < 0.001). However, Aim2 deficiency did not significantly affect the downregulation of CD62L upon activation of these cells relative to WT (Supplemental Figure 4; compare upper versus lower panels), which indicated that CD8+ T cell–intrinsic Aim2 was dispensable for the regulation of homing receptor expression.
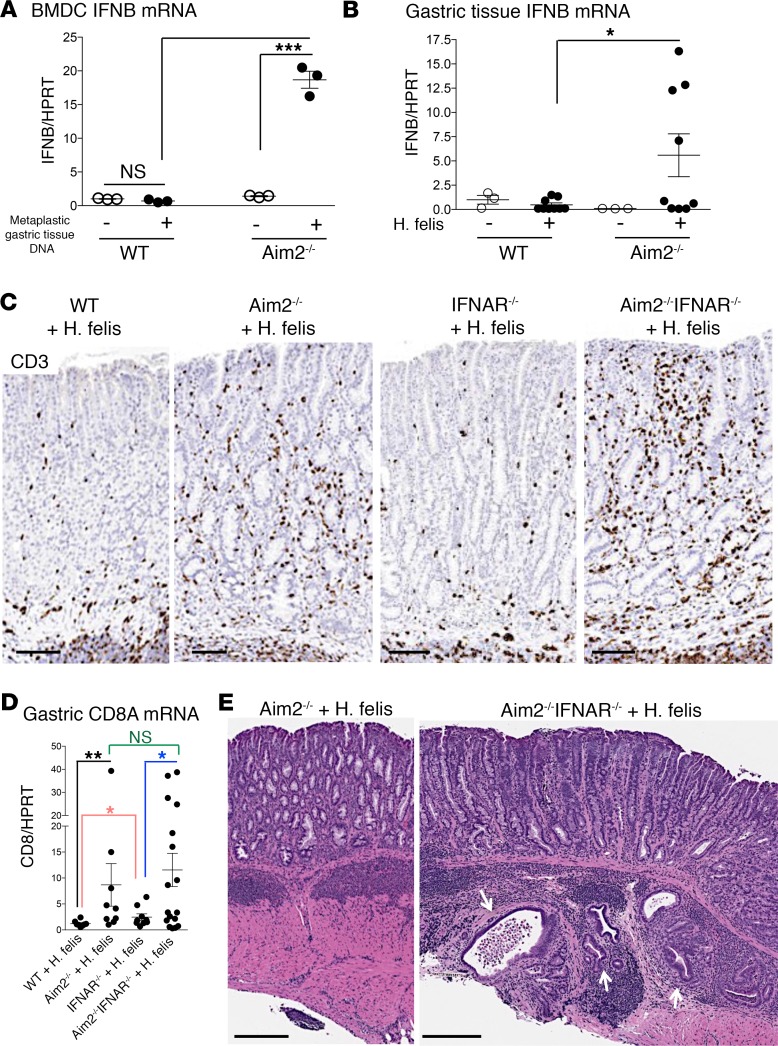
Aim2 regulation of gastric CD8+ T cells is independent of the IFN-βpathway. We investigated the possibility that Aim2 might regulate homing receptor expression in CD8+ T cells via a paracrine mechanism initiated by dendritic cells. Previous reports have shown that Aim2 suppresses IFN-β production by myeloid cells in response to intracellular DNA or extracellular bacterial stimulation (41–43). Moreover, reports have also shown that IFN-β suppresses S1PR1 on T cells via CD69 (44). We therefore hypothesized that Aim2 suppresses IFN-β production by myeloid cells, which downregulates homing receptors CD62L and S1PR1 on CD8+ T cells in a paracrine manner. To test this hypothesis, we first assessed the ability of Aim2 to suppress IFN-β production in dendritic cells stimulated by intracellular DNA (Figure 4A). We transfected BMDCs with 1 μg of metaplastic gastric tissue DNA and measured Ifnb1 (IFN-β gene) expression (Figure 4A). Transfection with metaplastic stomach DNA stimulated IFN-β gene only in Aim2–/– BMDCs, not in WT BMDCs (Figure 4A). This indicated that the loss of Aim2 relieved its inhibition of IFN-β expression in dendritic cells. This mechanism has recently been reported to be mediated by Aim2 antagonism of the stimulator of interferon genes/cyclic GMP-AMP synthase (STING/cGas) pathway (43). Since Aim2–/– dendritic cells produced higher IFN-β, we hypothesized that exogenous IFN-β suppresses homing receptor expression on CD8+ T cells. The induction of Ifnb1 mRNA was also corroborated in the infected Aim2–/– stomach in vivo (Figure 4B). Hence, this data corroborated the notion that Aim2 suppresses IFN-β induction in myeloid cells, as previously reported (41–43). We therefore sought to determine whether the effect of Aim2 deficiency on gastric CD8+ T cells was mediated by IFN-β.
Figure 4. Deficiency in type I IFN signaling does not reverse the increase in gastric CD8+ T cells observed in H.
felis–infected Aim2–/– mice, but rather exacerbates pathology. (A) RT-qPCR of Ifnb1 (IFN-β gene) mRNA expression in Aim2–/– versus WT bone marrow–derived macrophages (BMDCs) transfected with metaplastic gastric DNA from 6-month H. felis–infected stomach, relative to untransfected controls. Each data point represents 1 experiment. (B) RT-qPCR of IFN-β gene mRNA expression in 6-month H. felis–infected Aim2–/– versus WT stomachs, relative to uninfected controls. Each data point represents 1 mouse. (C) Representative CD3 immunohistochemistry (brown) of WT, Aim2–/–, IFNAR1–/–, and Aim2–/– IFNAR1–/– 6-month H. felis–infected gastric mucosa. Scale bar: 100 μm. (D) RT-qPCR analyses of gastric CD3 mRNA expression in 6-month H. felis–infected WT, Aim2–/–, IFNAR1–/–, and Aim2–/– IFNAR1–/– stomachs relative to uninfected controls. (E) Representative H&E image of 6-month H. felis–infected Aim2–/– versus Aim2–/– IFNAR1–/– gastric mucosa. Scale bars: 300 μm (left), 250 μm (right). The white arrows denote dysplastic gastric glands that have invaded the gastric submucosa. Error bars represent the mean ± SEM. N.S., not significant; *P < 0.05; **P < 0.01; ***P < 0.001. Data were compared using one-way ANOVA with Dunnet’s (parametric) multiple comparison tests (A and B). Data were compared using one-way ANOVA with Dunnet’s (nonparametric) multiple comparison tests (C).
To test the contribution of the IFN-β pathway to the gastric Aim2–/– phenotype, we crossed Aim2–/– mice to type I IFN receptor–deficient mice (B6(Cg)-Ifnar1tm1.2Ees/J; IFNAR1–/–) to generate Aim2–/– IFNAR1–/– double-knockout mice. However, contrary to expectations, Aim2–/– IFNAR1–/– double-knockout mice did not abrogate the increase in gastric CD8+ T cell frequency (Figure 4, C and D) or gastric pathology (Figure 4E). In fact, Aim2–/– IFNAR1–/– exacerbated gastric pathology following 6-month H. felis infection (Supplemental Figure 5A) and exhibited herniation of dysplastic lesions into the gastric submucosa (Supplemental Figure 5B). This demonstrated that the Aim2–/– gastric phenotype is not mediated by IFN-β signaling. Next, we sought to determine the effects of dendritic cell–secreted factors, other than IFN-β, that Aim2 deficiency induced, to determine whether those factors were mediating the Aim2-deficient phenotype.
Dendritic cell–secreted factors induced by Aim2 deficiency do not suppress homing receptor expression on CD8+ T cells.
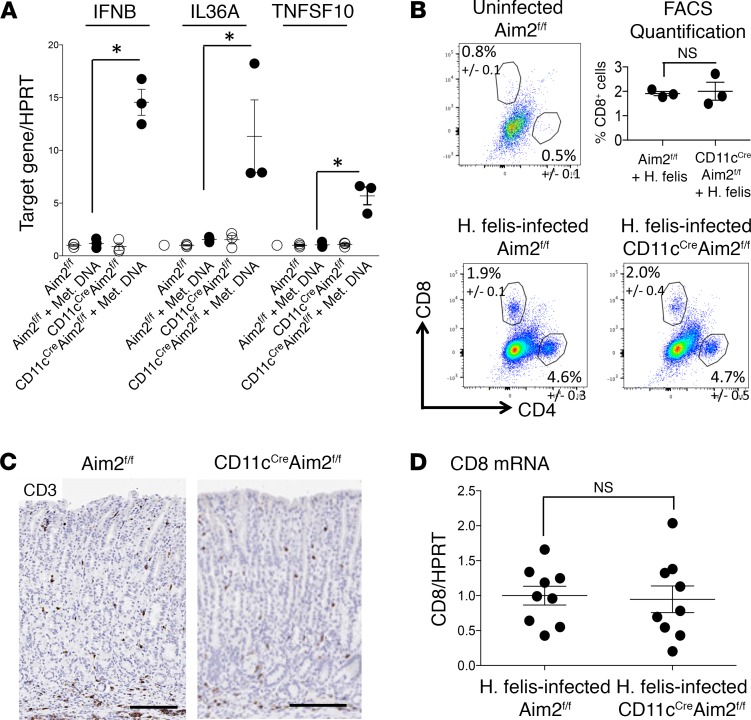
To examine alternative potential mechanisms of Aim2 in dendritic cells that could be contributing to the increase in gastric CD8+ T cells, we performed microarray analysis of WT versus Aim2–/– BMDCs transfected with gastric metaplastic DNA (Supplemental Figure 6). This expression screen revealed a transcriptional “switch” in dendritic cells following stimulation (Supplemental Figure 6). We identified genes for several dendritic cell–secreted factors that were regulated by Aim2, including Ptgs2, Cxcl11, Il1f6, IL6, Tnfsf10, IL27, and TL1A (red font in Supplemental Figure 6). Among these factors, and in addition to IFNb1, only IL36A and TNFSF10 were induced in both the infected Aim2–/– stomach (Supplemental Figure 7A) and Aim2–/– BMDCs (Supplemental Figure 7B). The induction of the remainder of these factors was not recapitulated in the inflamed gastric microenvironment (data not shown). Hence, we sought to determine the effect of IL36A and TNFSF10 on CD8+ T cells’ homing receptor expression. However, treatment of isolated splenic CD8+ T cells with recombinant IL-36α or recombinant TNFSF10 (also known as TNF-related apoptosis-inducing ligand [TRAIL]) did not suppress CD62L homing receptor expression (Supplemental Figure 6, C and D). This indicated that dendritic cells are unlikely serving as the cells in which Aim2 deficiency affect CD8+ T cell function. To test this concept, we generated CD11cCre Aim2fl/fl mice and tested the dendritic cell–intrinsic Aim2 as outlined in the following paragraph.
Dendritic cell–intrinsic Aim2 deficiency does not recapitulate a global Aim2–/– gastric phenotype.
We first validated successful Cre-mediated deletion of Aim2 in dendritic cells of CD11cCre Aim2fl/fl mice by using BMDCs transfected with metaplastic gastric DNA and showing significant increases in IFNB1, IL36A, and TNFSF10 (Figure 5A). However, 6-month H. felis infection of CD11cCre Aim2fl/fl mice did not increase gastric CD8+ T cell frequency relative to Aim2fl/fl controls (Figure 5, B–D). This demonstrated that the gastric CD8+ T cell phenotype we observed in the global Aim2-deficient mice was independent of dendritic cell–intrinsic Aim2.
Figure 5. Dendritic cell–specific deletion of Aim2 does not recapitulate the gastric CD8+ T cell phenotype elicited by global Aim2 deficiency.
(A) RT-qPCR of IFNB1, IL36A, and TNFSF10 in CD11cCre Aim2fl/fl versus Aim2fl/fl BMDCs transfected with gastric metaplastic DNA versus untransfected control. Each data point represents 1 experiment. Error bars represent the mean ± SEM. *P < 0.05. (B) Representative FACS analyses and quantification of gastric CD4+ and CD8+ T cells from H. felis–infected CD11cCre Aim2fl/fl versus Aim2fl/fl relative to uninfected Aim2fl/fl. (C) Representative CD3 immunohistochemistry (brown) of CD11cCre Aim2fl/fl versus Aim2fl/fl 6-month H. felis–infected gastric mucosa. Scale bar: 150 μm. (D) RT-qPCR analyses of gastric CD3 mRNA expression in 6-month H. felis–infected CD11cCre Aim2fl/fl versus Aim2fl/fl stomachs relative to uninfected controls. Each data point represents 1 mouse. N.S., not significant. Data were compared using one-way ANOVA with Dunnet’s (parametric) multiple comparison tests.
Aim2 is highly expressed by gastric B cells.
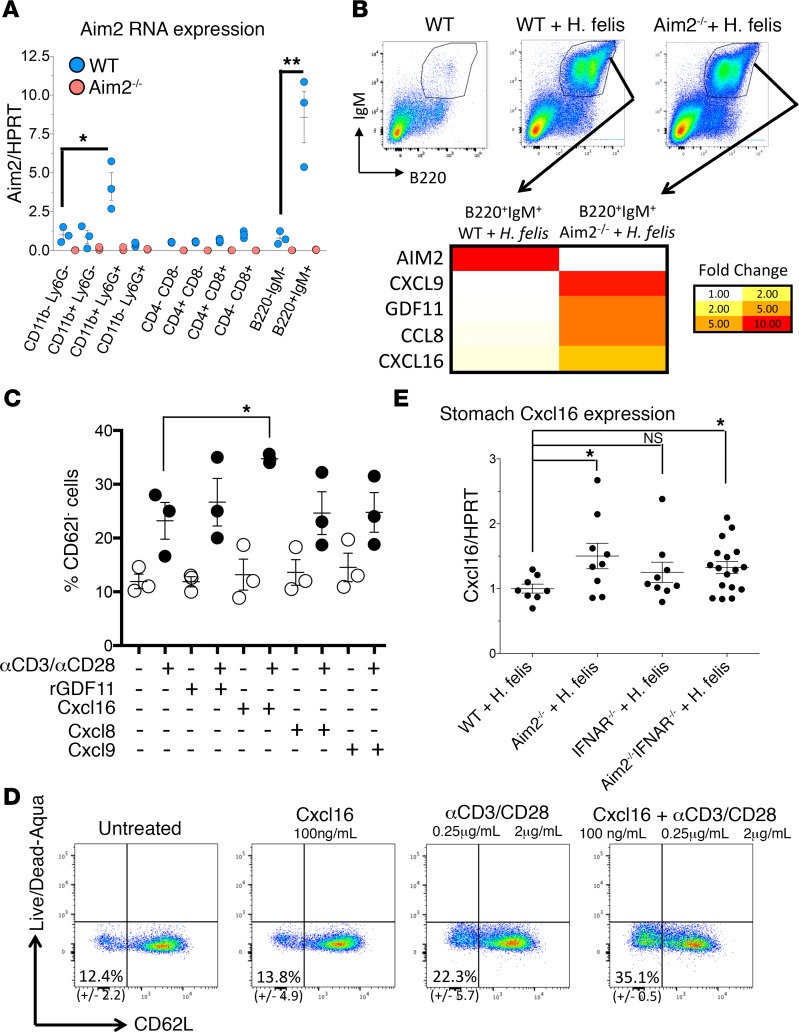
Because Aim2 deficiency in dendritic cells did not recapitulate the global Aim2 knockout, we measured Aim2 gene expression in FACS-sorted gastric immune cells (Figure 6A). While Aim2 gene expression was high in CD11b+ myeloid cells, the expression detected was surprisingly higher in B220+IgM+ B cells (Figure 6A). To confirm the high gene expression of Aim2 by B cells, we utilized JH–/– mice that lack B cells to compare gastric Aim2 gene expression relative to WT following 6-month H. felis infection. As expected, gastric B cells were absent in the JH–/– stomachs following 6-month H. felis infection (Supplemental Figure 8A). The absence of B cells correlated with a dramatic decrease in Aim2 gene expression as detected by microarray (Supplemental Figure 8B), therefore corroborating that gastric B cells were a major source of Aim2.
Figure 6. Aim2 is highest expressed in B cells, and Aim2 deficiency induces the expression of several chemokines, among which CXCL16 suppresses CD62L homing receptor expression on CD8+ T cells.
(A) RT-qPCR of Aim2 mRNA in FACS-isolated gastric immune subpopulations from 6-month H. felis–infected WT (blue) versus Aim2–/– (pink) stomachs. n = 3. (B) Microarray heatmap of FACS-isolated B cells from 6-month H. felis–infected WT versus Aim2–/– gastric mucosa. (C) Plot of FACS-quantified percentages of homing CD62L– splenic CD8+ T cells following treatment with recombinant GDF11, CXCL16, CCL8, or CXCL9 with or without prior anti-CD3/anti-CD28 activation. Each data point represents 1 experiment. (D) Representative FACS plot showing CD62L+ versus CD62L– splenic CD8+ T cells in recombinant CXCL16-treated versus untreated, with or without prior activation with anti-CD3/anti-CD28. (E) RT-qPCR analysis of CXCL16 mRNA expression in the stomach from 6-month H. felis–infected WT, Aim2–/–, and IFNAR1–/– versus Aim2–/– IFNAR1–/– mice. Each data point represents 1 mouse. Error bars represent the mean ± SEM. N.S., not significant; *P < 0.05; **P < 0.01. Data were compared using one-way ANOVA with Dunnet’s (parametric) multiple comparison tests.
Aim2 suppresses CXCL16 production by gastric B cells of the chronically inflamed stomach.
Because we determined that B cells were the major source of Aim2, we sought to determine the effect of Aim2 deficiency on these cells. To gain insight about the function of Aim2 in B cells, we FACS-isolated gastric B220+IgM+ cells and performed microarray analysis (Figure 6B). Several secreted factors — which were differentially expressed when Aim2 was absent — were identified, including growth differentiation factor 11 (GDF11, also known as Bmp11) and chemokines CXCL9, CCL6, and CXCL16 (Figure 6B). However, among these factors, only recombinant CXCL16 significantly increased the frequency of CD62L–CD8+ T cells (i.e., those lacking homing receptor) in splenic CD8+ T cells (Figure 6, C and D). Moreover, CXCL16 gene expression was increased in the gastric tissue of 6-month H. felis–infected mice (Figure 6E), irrespective of IFNAR1 deficiency, which supported its increase in B cells that we observed. In addition, no increase was observed in H. felis–infected CD11cCre Aim2fl/fl gastric tissue, demonstrating that the increase in gastric tissue expression of CXCL16 gene was not mediated by dendritic cell deficiency of Aim2 (Supplemental Figure 9). Collectively, these data indicated that B cell–intrinsic Aim2 could contribute to our observed CD8+ T cell phenotype by suppressing CXCL16 production in gastric B cells of the chronically inflamed stomach. These data correlate with the previously established role of CXCL16 and its receptor, CXCR6, in regulating CD8+ Trm cells (45), in addition to CXCR6 being one of the defining markers of CD8+ Trm cells (46).
Gastric CD8+ T cells produce high levels of IFN-γ. To elucidate the mechanism by which gastric CD8+ T cells stimulate SPEM, we investigated the possibility that these cells produce high levels of IFN-γ. We have previously shown that gastric IFN-γ overexpression is sufficient to induce SPEM (18). We found that gastric CD8+ T cells produced higher levels of IFN-γ than other immune cells, including CD4+ T cells, myeloid cells, and B cells (Figure 7A). Moreover, IFN-γ production was significantly increased in Aim2–/– CD8+ T cells relative to WT (Figure 7A). In light of the higher frequency of gastric CD8+ T cells in the infected Aim2–/– stomachs (Figure 2A), and given that these cells produced higher levels of IFN-γ than WT (Figure 7A), we found that total stomach IFN-γ mRNA was significantly increased in the infected Aim2–/– stomachs relative to WT (Figure 7B). Therefore, we propose that one of the contributing mechanisms by which Aim2–/– exacerbates SPEM is by increasing the frequency of IFN-γ–producing gastric CD8+ T cells.
Figure 7. Gastric CD8+ T cells are major producers of IFN-γ and contribute to higher gastric IFN-γ levels in Aim2–/– than in WT.
(A) RT-qPCR of IFN-γ mRNA in isolated gastric immune cell populations. Populations are isolated from infected stomachs of n = 3 mice per group. WT is shown in blue; Aim2–/– is shown in pink. (B) RT-qPCR of IFN-γ mRNA from total stomach tissue of infected WT versus Aim2–/– relative to uninfected. Each data point represents 1 mouse. Error bars represent the mean ± SEM. *P < 0.05; **P < 0.01. Data were compared using one-way ANOVA with Dunnet’s (parametric) multiple comparison tests (A). Data were compared using one-way ANOVA with Dunnet’s (nonparametric) multiple comparison tests (B).
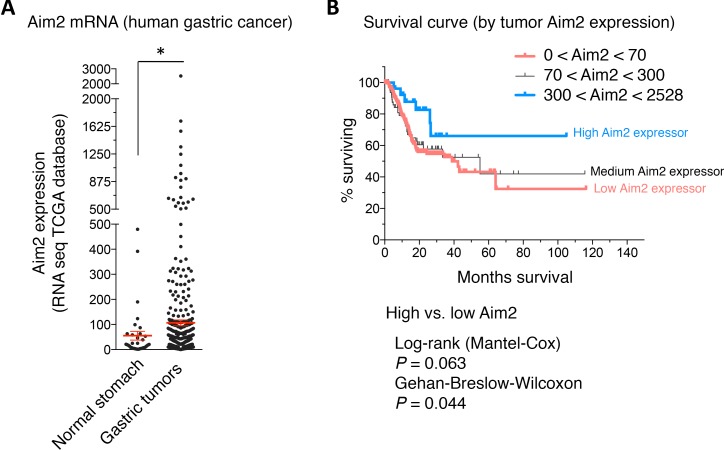
Aim2 is induced in human gastric cancer tissue and correlates with survival.
To correlate our findings to human gastric cancer, we quantified the expression data of Aim2 in gastric cancer versus normal stomach using The Cancer Genome Atlas database (TCGA Research Network: http://cancergenome.nih.gov/) (47). We observed a significant induction of Aim2 mRNA in tissue samples of patients with gastric cancer relative to normal stomach tissue (Figure 8A). Moreover, we observed that high expression of Aim2 in gastric cancer tissue correlated positively with patient survival (Figure 8B). We conclude that Aim2 is induced in human gastric cancer, and its mechanism in regulating Trm cell generation has implications for gastric cancer prognosis.
Figure 8. Aim2 is induced in human gastric cancer and correlates with patient survival.
(A) RNA-Seq expression of Aim2 mRNA from the TCGA database in normal gastric tissue versus gastric tumor tissue. Each data point represents 1 patient. Error bars represent the mean ± SEM. *P < 0.05. (B) Survival curve of gastric cancer patients with high Aim2-expressing versus medium Aim2-expressing versus low Aim2-expressing gastric tumors. Data were compared using Mann-Whitney U Test for nonparametric data.
Discussion
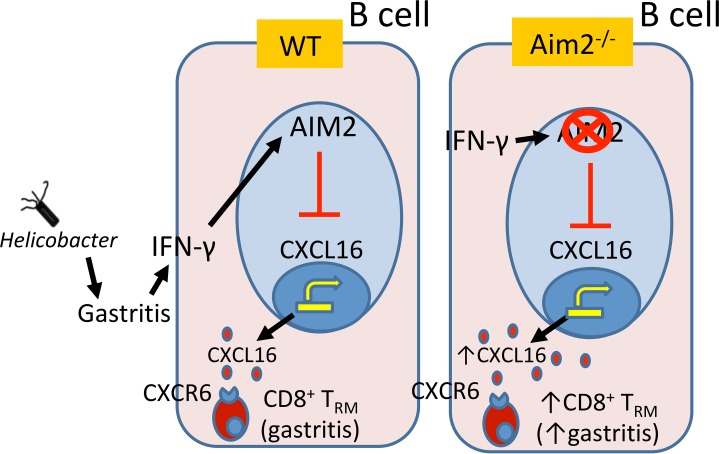
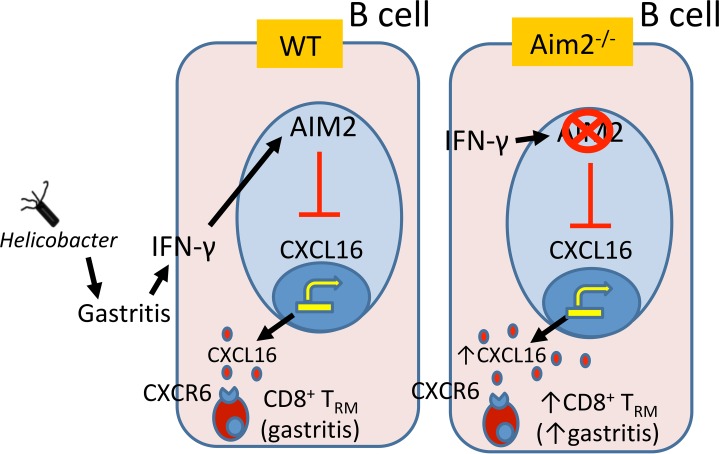
This study elucidates a potentially novel function in which Aim2 acts as an immune checkpoint, which suppresses CD8+ Trm cells in the inflamed gastric mucosa by preventing homing receptor expression (CD62L and S1PR1) on these cells (Figure 9). B cell–intrinsic Aim2 can potentially contribute to this outcome by suppressing CXCL16 production in B cells (hypothetical modeling in Figure 9). The major conclusion is that Aim2 ameliorates Helicobacter-induced SPEM by preventing CD8+ Trm cell accumulation, which is likely mediated by B cell CXCL16 production. The conclusion is supported by the following experimental evidence: (a) Aim2 deficiency relieved the inhibition of CXCL16 production by gastric B cells; (b) exogenous CXCL16 elicited a downregulation of CD8+ T cell homing receptor expression; (c) global Aim2 deficiency, in vivo, led to gastric CD8+ T cell homing receptor downregulation in the inflamed gastric mucosa; (d) IFN-β receptor deficiency or dendritic cell–intrinsic Aim2 deficiency did not recapitulate the gastric CD8+ T cell phenotype of global Aim2-deficient mice; (e) Aim2 deficiency led to the differentiation of gastric CD8+ T cells into Trm cells; (f) the loss of Aim2 led to the accumulation of these gastric mucosal CD8+ Trm cells; (g) gastric CD8+ Trm cells produced high levels of IFN-γ, which is known to induce SPEM (18), and (h) Aim2 was induced in gastric cancer tissue and correlated with patient survival, which indicated that this pathway is relevant to human disease as a protective factor against SPEM. Collectively, this experimental evidence strongly supports the conclusion that Aim2 serves as an immunoregulatory checkpoint in the inflamed gastric microenvironment and functions in an IFN-β– and dendritic cell–independent manner. The mechanism is likely mediated by inhibiting B cell CXCL16 production and superseding this molecule’s ability to promote CD8+ Trm cells.
Figure 9. Hypothetical modeling of the role of Aim2 in gastric CD8+ Trm cell generation.
Aim2 suppresses CXCL16 production in B cells. In chronically inflamed Aim2–/– stomachs, CXCL16 levels are high and can bind CXCR6 receptors on gastric CD8+ T cells, which can induce their CD8+ Trm cell phenotype, leading to their accumulation the gastric mucosa. The increase in gastric CD8+ Trm cells leads to a dramatic increase in IFN-γ and an exacerbation of gastric preneoplastic lesions.
The essential role of inflammation in the development of Helicobacter-induced SPEM has been previously described (11, 12, 18, 23, 48–52), although its mechanisms have not been fully explored. This began with the discovery of T cell deficiency to be sufficient for preventing metaplasia in the stomach (48, 49). It was followed by studies implicating IL-1β mutations in gastric cancer patients (50) and showing that IL-1β overexpression induces gastric dysplasia in mouse models (22). Further studies emphasized the role of myeloid cells (e.g., macrophages and MDSCs) and T cell cytokines (e.g., IFN-γ) in stimulating SPEM (11, 12, 18, 23, 51, 52). However, the mechanisms by which these immune cells stimulated SPEM remained unclear. In this study, we uncovered one inflammatory mechanism that played a major role in the development of SPEM. Because our proposed pathway (a) consists of an inflammasome molecule (Aim2) playing a “noncanonical” role and (b) involves CD8+ T cells, we will describe the cited literature separately for each component in the following paragraph.
First, in terms of T cells, even though these cells have been shown to be necessary for SPEM (48, 49), the mechanisms by which they are regulated and how they elicit their responses in SPEM remain unclear. It has been shown previously that Rag1–/–, TCRβδ–/–, and T-bet–/– mice fail to develop Helicobacter-induced gastric pathology. However, these studies utilized total T cell knockouts and did not distinguish CD4+ versus CD8+ T cells or between distinct T cell memory subtypes (48, 49). Therefore, our proposed mechanisms in this paper are complementary to the cited literature, even though our study additionally distinguishes different T cell subtypes (CD8+ vs. CD4+) and distinct memory phenotypes (Tcm, Tem, or Trm).
Second, in terms of Aim2 function, our data support a noncanonical function for Aim2 and provide evidence for its possible role in B cells to regulate CXCL16 production. However, traditionally, Aim2 has been described to function (a) as part of the inflammasome (13–15) or (b) in regulating IFN-β production (41–43) via the STING/cGAS pathway (43). We will discuss each of these activities in the following 2 paragraphs.
In terms of the inflammasome function, a previous study has documented, using the caspase-1–deficient (Casp1–/–) mouse model, that Casp1 deficiency exacerbated H. felis–induced SPEM (53). These observations in Casp1–/– mice did not correlate with reduced IL-1β signaling in that IL-1 receptor (IL-1R) deficiency ameliorated SPEM. However, the authors postulated that the Casp1–/– mechanism was mediated by reduced IL-18 bioactivity since IL-18 deficiency also exacerbated SPEM. They did not report a change in gastric CD8+ T cell abundance (53). This is complementary to our findings, in which Aim2 did not affect gastric inflammasome activity, and dendritic cell–specific Aim2 deficiency did not recapitulate the global Aim2 phenotype. Our proposition that Aim2 elicits its phenotype independent of the inflammasome is in agreement with the latter-described study that the inflammasome is not documented to regulate gastric CD8+ T cell frequency.
In regard to Aim2’s reported regulation of IFN-β, our finding that dendritic cell–specific Aim2 deletion, which increased IFN-β production dramatically, did not affect gastric CD8+ T cell frequency is surprising. This is because the role of IFN-β has been previously demonstrated in regulating CD8+ T cells. This mechanism has recently been reported by Woo, Corrales, Gajewski, and colleagues (43, 54–57), in which they described the role of IFN-β in stimulating CD8+ T cell priming in the tumor microenvironment. These studies showed that mice deficient in IFN-β, due to STING deletion, were defective in CD8+ T cell priming against immunogenic tumors (57). Moreover, they showed that Aim2, in turn, antagonized the STING/cGAS pathway that induced IFN-β production (43). Even though, in our study, the increase in IFN-β gene expression was detected in both gastric tissue in vivo and dendritic cells in vitro, the Aim2–/– IFNAR1–/– double-knockout model did not reverse the CD8+ T cell phenotype from the 6-month H. felis–infected Aim2–/– mouse. This demonstrated that our proposed mechanism elicited by Aim2 deficiency in the inflamed stomach was not mediated by IFN-β signaling. Overall, Aim2 does not appear to operate according to its previously described functions (i.e., inflammasome or IFN-β) in the chronically inflamed gastric mucosa. We provide evidence that Aim2 might operate by suppressing CXCL16 expression by B cells. These data are consistent with the previously established role of CXCL16 and its receptor, CXCR6, in regulating CD8+ Trm cells (45), in addition to CXCR6 being one of the defining markers of CD8+ Trm cells (46).
The reason CD8+ T cells are important in the study of cancer is these cells exhibit an antitumor activity by killing tumor cells (58). However, this may not account for their actual role in inflammation-induced tumor microenvironments (59). In our H. felis–infected Aim2–/– model, the increase in IFN-γ–producing CD8+ T cells similarly exacerbated —rather than ameliorated — SPEM. We propose that this was due to a dramatic increase in gastric IFN-γ levels. We have previously demonstrated that chronic IFN-γ overexpression in the gastric mucosa sufficiently induces SPEM (18). Interestingly, this damage response due to increased cytokine production is analogous to a type IV autoimmune/hypersensitivity reaction (60–62) in that it induces host tissue destruction. In light of this argument, gastric autoimmunity has been well documented in Helicobacter pylori–infected patients (63–73). Additionally, CD8+ T cells can attack host cells directly (74), which is a hallmark of a type II autoimmune/hypersensitivity reaction, via antibody-dependent cell-mediated cytotoxicity. Therefore, while gastric CD8+ T cells might exhibit an antitumor cytotoxic effect, they might also exacerbate preneoplastic SPEM development by inducing host tissue destruction of gastric parietal cells.
Despite the above findings and reported literature, the mechanisms of adaptive immunity during chronic gastric inflammation, SPEM, gastric dysplasia, and neoplasia remain poorly understood. Recent reports have described gastric follicular structures that develop within the chronically inflamed stomach to behave as tertiary lymphoid organs (TLOs) (75–77). These reports argued that gastric follicular structures operate in a manner analogous to lymph nodes in that they exhibit functions comprising antigen presentation and B cell–T cell interactions, which occur within the inflamed stomach mucosa in situ (75–77). In support of this hypothesis, we have previously observed gastric follicles to contain distinct B cell and T cell zones in the gastric mucosa (unpublished observation). Hence, this idea proposes that, since antigen presentation occurs within the inflamed gastric mucosa, crosspriming of CD8+ T cells might also occur within the stomach. Such processes would therefore not require egress of antigen-presenting cells toward tissue-draining lymph nodes. However, this field remains poorly understood in gastric carcinogenesis and requires further investigation regarding (a) where antigen presentation occurs (i.e., TLOs vs. lymph node), (b) what the nature of the antigen is, which promotes CD8+ T cell activation (i.e., Helicobacter antigen vs. damaged host cells), and (c) how CD8+ T cells promote SPEM (i.e., via autoimmune mechanisms or indirectly via IFN-γ). These investigations are important because they will provide insight into the immune cell mechanisms that counter or promote gastric carcinogenesis.
We conclude that Aim2 is an immunoregulatory molecule that suppresses SPEM by restricting CD8+ Trm cell accumulation in the chronically inflamed stomach, independent of the inflammasome or IFN-β pathway. Aim2 function is likely mediated by inhibiting CXCL16 production from B cells. This study uncovers potentially novel functional insight and new avenues to modulate the protumorigenic immune response in gastric preneoplastic lesions and cancer. The study also provides a potentially novel pathway to modulate the frequency of infiltrating CD8+ T cells (within the mucosa) of chronically inflamed solid organs, such as the stomach in this study.
Methods
Study approval.
All studies were approved by the University of Michigan Institutional Animal Care and Use Committee (PRO00005890). The human data were obtained by analyzing deidentified databases previously generated by the TCGA study (47), which did not require additional human sample collection (47). Hence, IRB approval was described in the previous study for which the samples were originally collected (47).
Detailed experimental procedures are described in the Supplemental Methods.
Author contributions
MEZ and JYK designed the research studies, acquired data, wrote the manuscript, and obtained funding. SB, HG, NK, and MC assisted in designing the research studies. MEZ, MZ, SB, HH, BH, KL, GH, and GDL conducted the experiments and acquired the data. LJS and AAD provided the gastric IFN-γ–overexpressing mouse model. MC provided the JH–/– mouse model. KE performed the pathological scoring analyses. MEZ, SB, HG, NK, KE, MC, and JYK discussed the experiments and data analysis.
Supplementary Material
Acknowledgments
We would like to acknowledge the American Gastroenterological Association/Gastric Cancer Foundation for providing grant N017489 (to MEZ) and the Department of Defense Peer Reviewed Cancer Research Program for providing grant CA160431 (to MEZ), which supported this study. We would also like to acknowledge the NIH for Public Health Service grants NIDDK R01 DK087708-01 (to JYK) and 5P30DK034933 (C. Owyang, Department of Internal Medicine-Gastroenterology, University of Michigan, Ann Arbor, MI, USA), which also supported this study.
Version 1. 02/13/2020
In-Press Preview
Version 2. 03/12/2020
Electronic publication
Footnotes
Conflict of interest: The authors have declared that no conflict of interest exists.
Copyright: © 2020, American Society for Clinical Investigation.
Reference information: JCI Insight. 2020;5(5):e94035.https://doi.org/10.1172/jci.insight.94035.
Contributor Information
Mohamad El-Zaatari, Email: mohamade@med.umich.edu.
Shrinivas Bishu, Email: bishus@umich.edu.
Min Zhang, Email: minzhang@umich.edu.
Helmut Grasberger, Email: helmut@umich.edu.
Guoqing Hou, Email: hougqing@umich.edu.
Li-Jyun Syu, Email: lijyunsy@med.umich.edu.
Nobuhiko Kamada, Email: nkamada@umich.edu.
Marilia Cascalho, Email: marilia@umich.edu.
John Y. Kao, Email: jykao@umich.edu.
References
- 1.Farraye FA, Odze RD, Eaden J, Itzkowitz SH. AGA technical review on the diagnosis and management of colorectal neoplasia in inflammatory bowel disease. Gastroenterology. 2010;138(2):746–774. doi: 10.1053/j.gastro.2009.12.035. [DOI] [PubMed] [Google Scholar]
- 2.Farraye FA, et al. AGA medical position statement on the diagnosis and management of colorectal neoplasia in inflammatory bowel disease. Gastroenterology. 2010;138(2):738–745. doi: 10.1053/j.gastro.2009.12.037. [DOI] [PubMed] [Google Scholar]
- 3.Correa P, Haenszel W, Cuello C, Tannenbaum S, Archer M. A model for gastric cancer epidemiology. Lancet. 1975;2(7924):58–60. doi: 10.1016/s0140-6736(75)90498-5. [DOI] [PubMed] [Google Scholar]
- 4.Kim N, et al. Helicobacter pylori infection and development of gastric cancer in Korea: long-term follow-up. J Clin Gastroenterol. 2008;42(5):448–454. doi: 10.1097/MCG.0b013e318046eac3. [DOI] [PubMed] [Google Scholar]
- 5.Lee K, Hwang H, Nam KT. Immune response and the tumor microenvironment: how they communicate to regulate gastric cancer. Gut Liver. 2014;8(2):131–139. doi: 10.5009/gnl.2014.8.2.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wong BC, et al. Helicobacter pylori eradication to prevent gastric cancer in a high-risk region of China: a randomized controlled trial. JAMA. 2004;291(2):187–194. doi: 10.1001/jama.291.2.187. [DOI] [PubMed] [Google Scholar]
- 7.Schmidt PH, et al. Identification of a metaplastic cell lineage associated with human gastric adenocarcinoma. Lab Invest. 1999;79(6):639–646. [PubMed] [Google Scholar]
- 8.Nam KT, O’Neal RL, Coffey RJ, Finke PE, Barker N, Goldenring JR. Spasmolytic polypeptide-expressing metaplasia (SPEM) in the gastric oxyntic mucosa does not arise from Lgr5-expressing cells. Gut. 2012;61(12):1678–1685. doi: 10.1136/gutjnl-2011-301193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nam KT, Varro A, Coffey RJ, Goldenring JR. Potentiation of oxyntic atrophy-induced gastric metaplasia in amphiregulin-deficient mice. Gastroenterology. 2007;132(5):1804–1819. doi: 10.1053/j.gastro.2007.03.040. [DOI] [PubMed] [Google Scholar]
- 10.Nomura S, Yamaguchi H, Ogawa M, Wang TC, Lee JR, Goldenring JR. Alterations in gastric mucosal lineages induced by acute oxyntic atrophy in wild-type and gastrin-deficient mice. Am J Physiol Gastrointest Liver Physiol. 2005;288(2):G362–G375. doi: 10.1152/ajpgi.00160.2004. [DOI] [PubMed] [Google Scholar]
- 11.Burclaff J, Osaki LH, Liu D, Goldenring JR, Mills JC. Targeted apoptosis of parietal cells is insufficient to induce metaplasia in stomach. Gastroenterology. 2017;152(4):762–766.e7. doi: 10.1053/j.gastro.2016.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Petersen CP, Weis VG, Nam KT, Sousa JF, Fingleton B, Goldenring JR. Macrophages promote progression of spasmolytic polypeptide-expressing metaplasia after acute loss of parietal cells. Gastroenterology. 2014;146(7):1727–38.e8. doi: 10.1053/j.gastro.2014.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hornung V, et al. AIM2 recognizes cytosolic dsDNA and forms a caspase-1-activating inflammasome with ASC. Nature. 2009;458(7237):514–518. doi: 10.1038/nature07725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fernandes-Alnemri T, Yu JW, Datta P, Wu J, Alnemri ES. AIM2 activates the inflammasome and cell death in response to cytoplasmic DNA. Nature. 2009;458(7237):509–513. doi: 10.1038/nature07710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bürckstümmer T, et al. An orthogonal proteomic-genomic screen identifies AIM2 as a cytoplasmic DNA sensor for the inflammasome. Nat Immunol. 2009;10(3):266–272. doi: 10.1038/ni.1702. [DOI] [PubMed] [Google Scholar]
- 16.Rathinam VA, et al. The AIM2 inflammasome is essential for host defense against cytosolic bacteria and DNA viruses. Nat Immunol. 2010;11(5):395–402. doi: 10.1038/ni.1864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tsuchiya K, et al. Involvement of absent in melanoma 2 in inflammasome activation in macrophages infected with Listeria monocytogenes. J Immunol. 2010;185(2):1186–1195. doi: 10.4049/jimmunol.1001058. [DOI] [PubMed] [Google Scholar]
- 18.Syu LJ, et al. Transgenic expression of interferon-γ in mouse stomach leads to inflammation, metaplasia, and dysplasia. Am J Pathol. 2012;181(6):2114–2125. doi: 10.1016/j.ajpath.2012.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Panchanathan R, et al. Aim2 deficiency in mice suppresses the expression of the inhibitory Fcgamma receptor (FcgammaRIIB) through the induction of the IFN-inducible p202, a lupus susceptibility protein. J Immunol. 2011;186(12):6762–6770. doi: 10.4049/jimmunol.1003638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Takai T, Ono M, Hikida M, Ohmori H, Ravetch JV. Augmented humoral and anaphylactic responses in Fc gamma RII-deficient mice. Nature. 1996;379(6563):346–349. doi: 10.1038/379346a0. [DOI] [PubMed] [Google Scholar]
- 21.Dhodapkar KM, et al. Selective blockade of inhibitory Fcgamma receptor enables human dendritic cell maturation with IL-12p70 production and immunity to antibody-coated tumor cells. Proc Natl Acad Sci U S A. 2005;102(8):2910–2915. doi: 10.1073/pnas.0500014102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tu S, et al. Overexpression of interleukin-1beta induces gastric inflammation and cancer and mobilizes myeloid-derived suppressor cells in mice. Cancer Cell. 2008;14(5):408–419. doi: 10.1016/j.ccr.2008.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ding L, et al. Schlafen 4-expressing myeloid-derived suppressor cells are induced during murine gastric metaplasia. J Clin Invest. 2016;126(8):2867–2880. doi: 10.1172/JCI82529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhuang Y, et al. A pro-inflammatory role for Th22 cells in Helicobacter pylori-associated gastritis. Gut. 2015;64(9):1368–1378. doi: 10.1136/gutjnl-2014-307020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sayi A, et al. The CD4+ T cell-mediated IFN-gamma response to Helicobacter infection is essential for clearance and determines gastric cancer risk. J Immunol. 2009;182(11):7085–7101. doi: 10.4049/jimmunol.0803293. [DOI] [PubMed] [Google Scholar]
- 26.Tan MP, et al. CD8+ T cells are associated with severe gastritis in Helicobacter pylori-infected mice in the absence of CD4+ T cells. Infect Immun. 2008;76(3):1289–1297. doi: 10.1128/IAI.00779-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fukui T, et al. Cross-primed CD8+ cytotoxic T cells induce severe Helicobacter-associated gastritis in the absence of CD4+ T cells. Helicobacter. 2007;12(5):486–497. doi: 10.1111/j.1523-5378.2007.00536.x. [DOI] [PubMed] [Google Scholar]
- 28.Fox JG, et al. Local and systemic immune responses in murine Helicobacter felis active chronic gastritis. Infect Immun. 1993;61(6):2309–2315. doi: 10.1128/IAI.61.6.2309-2315.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mueller SN, Mackay LK. Tissue-resident memory T cells: local specialists in immune defence. Nat Rev Immunol. 2016;16(2):79–89. doi: 10.1038/nri.2015.3. [DOI] [PubMed] [Google Scholar]
- 30.Park CO, Kupper TS. The emerging role of resident memory T cells in protective immunity and inflammatory disease. Nat Med. 2015;21(7):688–697. doi: 10.1038/nm.3883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schenkel JM, Masopust D. Tissue-resident memory T cells. Immunity. 2014;41(6):886–897. doi: 10.1016/j.immuni.2014.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Skon CN, Lee JY, Anderson KG, Masopust D, Hogquist KA, Jameson SC. Transcriptional downregulation of S1pr1 is required for the establishment of resident memory CD8+ T cells. Nat Immunol. 2013;14(12):1285–1293. doi: 10.1038/ni.2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mackay LK, et al. Hobit and Blimp1 instruct a universal transcriptional program of tissue residency in lymphocytes. Science. 2016;352(6284):459–463. doi: 10.1126/science.aad2035. [DOI] [PubMed] [Google Scholar]
- 34.Mackay LK, et al. Cutting edge: CD69 interference with sphingosine-1-phosphate receptor function regulates peripheral T cell retention. J Immunol. 2015;194(5):2059–2063. doi: 10.4049/jimmunol.1402256. [DOI] [PubMed] [Google Scholar]
- 35.Wakim LM, et al. The molecular signature of tissue resident memory CD8 T cells isolated from the brain. J Immunol. 2012;189(7):3462–3471. doi: 10.4049/jimmunol.1201305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gebhardt T, Wakim LM, Eidsmo L, Reading PC, Heath WR, Carbone FR. Memory T cells in nonlymphoid tissue that provide enhanced local immunity during infection with herpes simplex virus. Nat Immunol. 2009;10(5):524–530. doi: 10.1038/ni.1718. [DOI] [PubMed] [Google Scholar]
- 37.Masopust D, Vezys V, Wherry EJ, Barber DL, Ahmed R. Cutting edge: gut microenvironment promotes differentiation of a unique memory CD8 T cell population. J Immunol. 2006;176(4):2079–2083. doi: 10.4049/jimmunol.176.4.2079. [DOI] [PubMed] [Google Scholar]
- 38.Hofmann M, Pircher H. E-cadherin promotes accumulation of a unique memory CD8 T-cell population in murine salivary glands. Proc Natl Acad Sci U S A. 2011;108(40):16741–16746. doi: 10.1073/pnas.1107200108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lee YT, et al. Environmental and antigen receptor-derived signals support sustained surveillance of the lungs by pathogen-specific cytotoxic T lymphocytes. J Virol. 2011;85(9):4085–4094. doi: 10.1128/JVI.02493-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wakim LM, Woodward-Davis A, Bevan MJ. Memory T cells persisting within the brain after local infection show functional adaptations to their tissue of residence. Proc Natl Acad Sci U S A. 2010;107(42):17872–17879. doi: 10.1073/pnas.1010201107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jones JW, et al. Absent in melanoma 2 is required for innate immune recognition of Francisella tularensis. Proc Natl Acad Sci U S A. 2010;107(21):9771–9776. doi: 10.1073/pnas.1003738107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fernandes-Alnemri T, et al. The AIM2 inflammasome is critical for innate immunity to Francisella tularensis. Nat Immunol. 2010;11(5):385–393. doi: 10.1038/ni.1859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Corrales L, Woo SR, Williams JB, McWhirter SM, Dubensky TW, Gajewski TF. Antagonism of the STING pathway via activation of the AIM2 inflammasome by intracellular DNA. J Immunol. 2016;196(7):3191–3198. doi: 10.4049/jimmunol.1502538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shiow LR, et al. CD69 acts downstream of interferon-alpha/beta to inhibit S1P1 and lymphocyte egress from lymphoid organs. Nature. 2006;440(7083):540–544. doi: 10.1038/nature04606. [DOI] [PubMed] [Google Scholar]
- 45.Wein AN, et al. CXCR6 regulates localization of tissue-resident memory CD8 T cells to the airways. J Exp Med. 2019;216(12):2748–2762. doi: 10.1084/jem.20181308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kumar BV, et al. Human tissue-resident memory T cells are defined by core transcriptional and functional signatures in lymphoid and mucosal sites. Cell Rep. 2017;20(12):2921–2934. doi: 10.1016/j.celrep.2017.08.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cancer Genome Atlas Research Network Comprehensive molecular characterization of gastric adenocarcinoma. Nature. 2014;513(7517):202–209. doi: 10.1038/nature13480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Roth KA, Kapadia SB, Martin SM, Lorenz RG. Cellular immune responses are essential for the development of Helicobacter felis-associated gastric pathology. J Immunol. 1999;163(3):1490–1497. [PubMed] [Google Scholar]
- 49.Stoicov C, et al. T-bet knockout prevents Helicobacter felis-induced gastric cancer. J Immunol. 2009;183(1):642–649. doi: 10.4049/jimmunol.0900511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.El-Omar EM, et al. Interleukin-1 polymorphisms associated with increased risk of gastric cancer. Nature. 2000;404(6776):398–402. doi: 10.1038/35006081. [DOI] [PubMed] [Google Scholar]
- 51.Schumacher MA, et al. Gastric Sonic Hedgehog acts as a macrophage chemoattractant during the immune response to Helicobacter pylori. Gastroenterology. 2012;142(5):1150–1159.e6. doi: 10.1053/j.gastro.2012.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.El-Zaatari M, et al. Gli1 deletion prevents Helicobacter-induced gastric metaplasia and expansion of myeloid cell subsets. PLoS One. 2013;8(3):e58935. doi: 10.1371/journal.pone.0058935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hitzler I, et al. Caspase-1 has both proinflammatory and regulatory properties in Helicobacter infections, which are differentially mediated by its substrates IL-1β and IL-18. J Immunol. 2012;188(8):3594–3602. doi: 10.4049/jimmunol.1103212. [DOI] [PubMed] [Google Scholar]
- 54.Corrales L, Gajewski TF. Molecular pathways: targeting the stimulator of interferon genes (STING) in the immunotherapy of cancer. Clin Cancer Res. 2015;21(21):4774–4779. doi: 10.1158/1078-0432.CCR-15-1362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Corrales L, McWhirter SM, Dubensky TW, Gajewski TF. The host STING pathway at the interface of cancer and immunity. J Clin Invest. 2016;126(7):2404–2411. doi: 10.1172/JCI86892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Deng L, et al. STING-dependent cytosolic DNA sensing promotes radiation-induced type I interferon-dependent antitumor immunity in immunogenic tumors. Immunity. 2014;41(5):843–852. doi: 10.1016/j.immuni.2014.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Woo SR, et al. STING-dependent cytosolic DNA sensing mediates innate immune recognition of immunogenic tumors. Immunity. 2014;41(5):830–842. doi: 10.1016/j.immuni.2014.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tscharke DC, Croft NP, Doherty PC, La Gruta NL. Sizing up the key determinants of the CD8(+) T cell response. Nat Rev Immunol. 2015;15(11):705–716. doi: 10.1038/nri3905. [DOI] [PubMed] [Google Scholar]
- 59.Corrales L, Matson V, Flood B, Spranger S, Gajewski TF. Innate immune signaling and regulation in cancer immunotherapy. Cell Res. 2017;27(1):96–108. doi: 10.1038/cr.2016.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Uzzaman A, Cho SH. Chapter 28: Classification of hypersensitivity reactions. Allergy Asthma Proc. 2012;33(suppl 1):96–99. doi: 10.2500/aap.2012.33.3561. [DOI] [PubMed] [Google Scholar]
- 61.Rajan TV. The Gell-Coombs classification of hypersensitivity reactions: a re-interpretation. Trends Immunol. 2003;24(7):376–379. doi: 10.1016/S1471-4906(03)00142-X. [DOI] [PubMed] [Google Scholar]
- 62. Gell PGH, Coombs RRA. The Classification of Allergic Reactions Underlying Disease. Oxford, UK: Blackwell Sciences; 1963. [Google Scholar]
- 63.Ito M, et al. Serological comparison of serum pepsinogen and anti-parietal cell antibody levels between Japanese and German patients. Eur J Gastroenterol Hepatol. 2002;14(2):123–127. doi: 10.1097/00042737-200202000-00005. [DOI] [PubMed] [Google Scholar]
- 64.Ito M, et al. Role of anti-parietal cell antibody in Helicobacter pylori-associated atrophic gastritis: evaluation in a country of high prevalence of atrophic gastritis. Scand J Gastroenterol. 2002;37(3):287–293. doi: 10.1080/003655202317284183. [DOI] [PubMed] [Google Scholar]
- 65.Sugiu K, et al. Evaluation of an ELISA for detection of anti-parietal cell antibody. Hepatogastroenterology. 2006;53(67):11–14. [PubMed] [Google Scholar]
- 66.Sugiu K, et al. Anti-parietal cell antibody and serum pepsinogen assessment in screening for gastric carcinoma. Dig Liver Dis. 2006;38(5):303–307. doi: 10.1016/j.dld.2005.10.021. [DOI] [PubMed] [Google Scholar]
- 67.Faller G, Steininger H, Eck M, Hensen J, Hann EG, Kirchner T. Antigastric autoantibodies in Helicobacter pylori gastritis: prevalence, in-situ binding sites and clues for clinical relevance. Virchows Arch. 1996;427(5):483–486. doi: 10.1007/BF00199508. [DOI] [PubMed] [Google Scholar]
- 68.Appelmelk BJ, et al. Potential role of molecular mimicry between Helicobacter pylori lipopolysaccharide and host Lewis blood group antigens in autoimmunity. Infect Immun. 1996;64(6):2031–2040. doi: 10.1128/IAI.64.6.2031-2040.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Negrini R, et al. Antigenic mimicry between Helicobacter pylori and gastric mucosa in the pathogenesis of body atrophic gastritis. Gastroenterology. 1996;111(3):655–665. doi: 10.1053/gast.1996.v111.pm8780570. [DOI] [PubMed] [Google Scholar]
- 70.Barrio R, Roldán MB, Alonso M, Cantón R, Camarero C. Helicobacter pylori infection with parietal cell antibodies in children and adolescents with insulin dependent diabetes mellitus. J Pediatr Endocrinol Metab. 1997;10(5):511–516. doi: 10.1515/jpem.1997.10.5.511. [DOI] [PubMed] [Google Scholar]
- 71.Faller G, et al. Antigastric autoantibodies in Helicobacter pylori infection: implications of histological and clinical parameters of gastritis. Gut. 1997;41(5):619–623. doi: 10.1136/gut.41.5.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Faller G, Steininger H, Appelmelk B, Kirchner T. Evidence of novel pathogenic pathways for the formation of antigastric autoantibodies in Helicobacter pylori gastritis. J Clin Pathol. 1998;51(3):244–245. doi: 10.1136/jcp.51.3.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Claeys D, Faller G, Appelmelk BJ, Negrini R, Kirchner T. The gastric H+,K+-ATPase is a major autoantigen in chronic Helicobacter pylori gastritis with body mucosa atrophy. Gastroenterology. 1998;115(2):340–347. doi: 10.1016/S0016-5085(98)70200-8. [DOI] [PubMed] [Google Scholar]
- 74.Walter U, Santamaria P. CD8+ T cells in autoimmunity. Curr Opin Immunol. 2005;17(6):624–631. doi: 10.1016/j.coi.2005.09.014. [DOI] [PubMed] [Google Scholar]
- 75.Buckley CD, Barone F, Nayar S, Bénézech C, Caamaño J. Stromal cells in chronic inflammation and tertiary lymphoid organ formation. Annu Rev Immunol. 2015;33:715–745. doi: 10.1146/annurev-immunol-032713-120252. [DOI] [PubMed] [Google Scholar]
- 76.Pitzalis C, Jones GW, Bombardieri M, Jones SA. Ectopic lymphoid-like structures in infection, cancer and autoimmunity. Nat Rev Immunol. 2014;14(7):447–462. doi: 10.1038/nri3700. [DOI] [PubMed] [Google Scholar]
- 77.Neyt K, Perros F, GeurtsvanKessel CH, Hammad H, Lambrecht BN. Tertiary lymphoid organs in infection and autoimmunity. Trends Immunol. 2012;33(6):297–305. doi: 10.1016/j.it.2012.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.