Abstract
Objective:
Protective effects of ischemic postconditioning (PostC) decrease/disappear with age and chronic heart diseases. Similarly, low serum melatonin levels have been reported in the same risk groups. The aims of this study were to investigate the effects of melatonin on the protection of PostC in ischemia–reperfusion (I/R)-induced infarct size and roles of uncoupling protein (UCP) 3, irisin, and nuclear factor kappa B (NFkB) levels.
Methods:
Rats were pinealectomized (Px) or sham operated (non-Px) 2 months before the I/R studies. The left main coronary artery was occluded for 30 min followed by 120 min reperfusion. PostC was induced with three cycles of R/I (10 s each) after ischemia.
Results:
The infarct size was found to be significantly higher in Px rats (54.68±1.5%) than in the control group (35.1±2.5%). PostC and melatonin administrations to non-Px rats significantly reduced the infarct size. On the other hand, PostC did not create a significant effect in Px rats, but protection was provided when PostC was co-administrated with melatonin. While significant decreases were detected in the UCP3 levels, irisin and NFkB levels increased with I/R and Px. Treatment with PostC and melatonin in non-Px groups and their co-administration in Px groups were found to return all the genes close to normal levels.
Conclusion:
The physiological and pharmacological concentrations of melatonin may play a role in the protection of PostC. In cases when physiological melatonin is reduced, such as aging and heart diseases, this protection may decrease, and this effect may be restored by melatonin replacement. PostC and melatonin may regulate energy metabolism and inflammatory mediators and protect mitochondria by affecting the UCP3, irisin, and NFkB levels.
Keywords: reperfusion injury, postconditioning, melatonin, uncoupling protein 3, irisin, nuclear factor kappa B
Introduction
Ischemia–reperfusion (I/R) injury is the essential segment of myocardial infarction (MI), cerebral ischemia, stroke, hemorrhagic shock, and surgical interventions such as organ transplantation, coronary angioplasty, and the pathophysiology resulting from thrombolytic treatment (1). Although the blood flow is restored with rapid interventions and thrombolytic therapies, these treatment protocols are often inefficient (2). Ischemic preconditioning (PreC) and postconditioning (PostC) are considered to be the strongest mechanisms that restrict the size of MI and reduce I/R injury (3). The clinical administration of cardioprotection stimulated by PreC is limited by the unpredictable development of acute coronary syndromes. Therefore, PostC has drawn more attention in surgery (4).
The first use of PostC by applying three cycles of 30 s I/R following coronary artery occlusion in dogs lead to reduction in infarct size (5). It was reported that the protective effects of PostC decreased or disappeared with age, hypercholesterolemia, diabetes, obesity, and cardiac pathologies such as hypertension and left ventricular hypertrophy, which are associated with acute MI (6,7).
Pharmacological PostC trials are available in addition to the mechanically applied ischemic PostC studies. Butorphanol-induced pharmacologic PostC showed protective effects by reducing the I/R-related infarct size and MDA and MPO levels (8).
Melatonin, an important endogenous antioxidant, acts as a potent free-radical scavenger (9). It has a strong therapeutic potential and various physiological functions in cardiovascular diseases in animals and humans (10). Protective effects of PostC decrease/disappear with age and chronic heart disease (11). Similarly, low serum melatonin levels were reported in the same risk groups (12).
The mechanisms of melatonin action on PostC and effects of uncoupling protein 3 (UCP3), irisin, and nuclear factor kappa B (NFkB) levels on the protection of melatonin and PostC in myocardial I/R injury are unknown. In this study, it was aimed:
To show the level of impact of potential PostC protective roles on the infarct size when physiological melatonin is decreased through pinealectomy (Px) and melatonin administration,
To determine whether the activities of mitochondrial membrane protein UCP3 and levels of irisin, which plays a regulatory role in energy metabolism, and levels of NFkB, which plays a central role in the inflammatory cellular stress and is involved in the regulation of mitochondrial dynamics, play a role in PostC; and also to show how these are affected by physiological and pharmacological melatonin concentrations,
To determine whether melatonin could mimic the effects of PostC by comparing the effects of melatonin administrations on the infarct size and molecular mechanism.
Methods
Experimental groups
Eight-week-old male Sprague–Dawley rats weighing 200 g were placed in a quiet and temperature- (21±2°C) and humidity- (60±5%) controlled room in which a 12–12 h light–dark cycle was maintained. Rats were divided into eight groups. Those were the control group, I/R group, I/R-PostC group, I/R-Mel group in non-Px groups and Px group, Px-I/R group, Px-PostC group, and Px-Mel-PostC group (Fig. 1). Melatonin was dissolved in ethanol and further diluted in saline (0.09% NaCl wt/vol). Melatonin (Sigma Chemical Company, St. Louis, MO, USA) was dissolved in pure ethanol at a dose of 10 mg/kg with a final concentration at 1%, and it was administered by intraperitoneally injection for 10 days before I/R experiments. Myocardial tissue UCP3, irisin, and NFkB levels were measured in 7 animals for each group. Infarct size was also analyzed in the same number of rats for each group. All the experiments in this study were performed in accordance with the guidelines for animal research of the National Institutes of Health and were approved by the Local Committee on Animal Research (2014/18-172).
Figure 1.
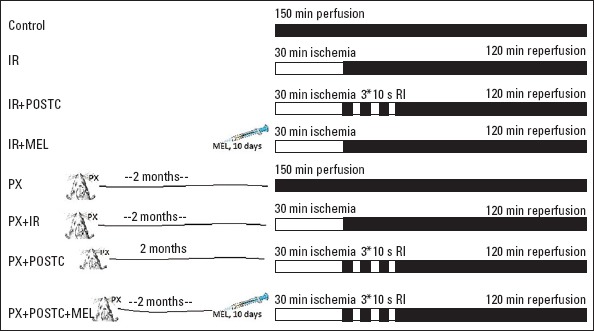
Experimental groups
The number to be included in the sample size was determined by power analysis that suggested at least 7 individuals with an alpha error of 0.05 and a beta error of 0.20 (power=0.80) should be included.
Surgical applications
Pinealectomy
Px was performed as described by Hoffman and Reiter (13). Rats were anesthetized with ketamine hydrochloride (75 mg/kg) and xylazine (8 mg/kg) before the operation. The entire procedure was completed within 15 min. Px was confirmed by the histological evaluation of the gland for each animal.
I/R procedure
Two months after Px, rats were anesthetized with urethane (1.2–1.4 g/kg) administered intraperitoneally. The trachea was cannulated for artificial respiration. Systemic blood pressure (BP) and electrocardiogram (ECG) were monitored from the carotid artery by a Biopac MP36. The chest was opened by a left thoracotomy, followed by sectioning the fourth and fifth ribs, about 2 mm to the left of the sternum. Positive-pressure artificial respiration was started immediately with room air, using a volume of 1.5 mL/100 g body weight at a rate 60 beats/min to maintain normal pCO2, pO2, and pH parameters. After the pericardium was incised, the heart was exteriorized by gentle pressure on the right side of the rib cage. A 6/0 silk suture attached to a 10 mm micro-point reverse-cutting needle was quickly placed under the left main coronary artery. The heart was then carefully replaced in the chest, and the animal was allowed to recover for 20 min. Any animal in which this procedure produced arrhythmias or a sustained decrease in the mean arterial BP to less than 70 mm Hg was discarded. A small plastic snare was threaded through the ligature and placed in contact with the heart. The artery could then be occluded by applying tension to the ligature, and reperfusion was achieved by releasing the tension. The artery was occluded for 30 min and then reperfused for 120 min before the experiment was terminated. These durations of I/R were already successfully used in the same experimental model (14). The ECG changes and heart rate were measured before and during the occlusion and reperfusion. In the rats assigned to PostC, after 30 min of ischemia, the artery kept open for 10 s until reoccluded and then reperfused for 10 s. I/R was repeated in three cycles with 10 s intervals (15). Total experimental time was 150 min in all groups and at the end of the experiment, animals were sacrificed, and the heart tissue was taken for biochemical analysis and risk zone measurement. At the end of each in vivo study, the heart was mounted on a Langendorff apparatus where it was flushed with saline at room temperature for 60 s. The coronary branch was then reoccluded, and 2% Evans blue suspension (Alfa Aesar, Ward Hill) were infused into the perfusate to mark the risk zone (the nonpaint tissue). The heart was then frozen, and a total of 4 transverse slices, 2 mm in size, from each heart, were cut starting from the apex. For the evaluation of tissue death, the slices were incubated in 1% triphenyl tetrazolium chloride (TTC) in a pH 7.4 buffer at 37°C for 20 min. TTC stains from living tissue are of a deep-red color, while necrotic tissue is TTC-negative and appears tan (Fig. 2). The infarct and risk zone, considered to be the area lacking fluorescence under ultraviolet light, were traced. The volume of the infarct and the risk zone was determined by the ImageJ program. The infarct size expressed as the percentage of the risk zone was measured as previously described (14).
Figure 2.
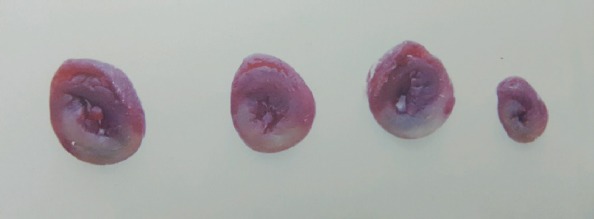
Living tissues were of a deep-red color because of the 1% triphenyl tetrazolium chloride (TTC) stains, while the necrotic tissue was TTC-negative and appeared tan
Quantitative real-time polymerase chain reaction analysis (qRT-PCR)
Total RNA was extracted from the heart using a commercially available Trizol Reagent (Life Technologies, catalog no. 15596) according to the manufacturer’s instructions. Briefly, 50 mg of heart tissue was removed from the freezer and immediately immersed in 1 mL of Trizol Reagent. The heart was homogenized using an automated homogenizer (Next Advance, Averill Park, USA). To carry out the PCR array, total RNA from heart samples in each experimental group was pooled (3 µg total). cDNA from pools was synthesized using High-Capacity RNA-to-cDNA kit (Invitrogen, Carlsbad, USA).
Total RNA was converted to cDNA using High-Capacity cDNA Reverse Transcription Synthesis Kits (Applied Biosystem, USA) using 1 µg of total RNA. The cDNA synthesis was performed in a gradient thermal cycler (Biometra, Germany 07-850) with a profile of 25°C for 10 min, 37°C for 120 min, and 85°C for 5 min, 4°C for 60 min. All samples were run together, and several negative controls that did not contain either RNA (no template controls) or the reverse transcriptase enzyme (RT negative) were run simultaneously, to control for RNA and genomic DNA contamination, respectively. A Real-Time PCR analysis was performed with the ABI Prism 7500 Fast Real-Time PCR Instrument (Applied Biosystems, Foster City, CA) using UCP 3, irisin, NFkB and GAPDH genes Tag Man Assay and Tag Man Master Mix (Applied Biosystems, Foster City, CA 94404). All results were standardized to the levels of GAPDH. The assay was performed by three independent experiments with triplicate. To compare the transcript levels between different groups, the 2-ΔΔCt method was used (16).
Western blot analysis
Frozen heart tissues from the left ventricle were weighed and added to the RIPA lysis buffer (1;9,w;v) (Santa Cruz Biotechnology Inc., USA) with a cocktail protease inhibitor. Then, this mixture was homogenized with an automatic tissue homogenizer (Next Advanced Inc., Averill Park, NY, USA). Homogenized samples were centrifuged at 10.000xg for 10 min to obtain the supernatant and then recentrifuged to form a clear lysate, and all procedures were carried out at +4°C according to the manufacturer’s instruction. Subsequently, sample pools were formed for each group from these obtained clear lysates. The amount of protein in each sample was determined with a fluorometer (Qubit fluorometer, Invitrogen, USA) using a Quant-iT protein assay kit (Invitrogen, USA). Thirty micrograms of the total protein from each sample pool were loaded on gels (NuPAGE 4%–12% Bis-Tris Protein Gel; Invitrogen, USA) for electrophoresis, transferred to a PVDF membrane, and probed with rabbit polyclonal antibody against NFκB-p105 (cat no: ARG20501, Arigo Biolaboratories, Taiwan, China, at 1:125–1:1000 dilution), rabbit monoclonal antibody against irisin 20-30 kD (FNDC5, cat no: ab174833, Abcam, USA, at 1:1000–1:5000 dilution), and probed with rabbit polyclonal antibody against UCP3-30-40 kD (cat no: 10750-1-AP, Proteintech, USA, at 1:1000–1:5000 dilution). Probed with mouse monoclonal antibody against β-Actin was used as the reference protein (cat no: MA1115, Boster Biological Technology, USA, at 1:200–1:400 dilution). The blot was incubated with horseradish peroxidase-conjugated secondary antibody, and the protein bands were visualized by chromogenic substrates using the WesternBreeze Chromogenic Immunodetection Kit (Invitrogen, USA). Protein expression levels were assessed using the Image J software, which is used to compare the density of bands on western blots.
Statistical analysis
Data are expressed as arithmetic means±SEM; a p-value <0.05 was considered to be statistically significant. Normality of the distribution within the groups was evaluated using the Shapiro–Wilk test. Raw data for each experiment satisfied the assumptions of normality and homogeneity of variance, and parametric analyses (one-way independent measure ANOVAs and t-tests) were performed, along with Tukey’s HSD post-hoc test, where appropriate. Conversely, nonparametric alternatives (Kruskal–Wallis, with subsequent Mann–Whitney U or Dunnett’s T3 where indicated) were used when assumptions were violated. The t-test was used to evaluate the differences between the normalized relative quantities of genes.
Results
There was no significant difference in BP values (mm Hg) between the groups at the beginning and throughout the experiment (Table 1).
Table 1.
Blood pressure measurements (mm Hg)
| End of stabilization | End of ischemia | Reperfusion | |||
|---|---|---|---|---|---|
| 30 min | 60 min | 120 min | |||
| I/R | 77±2.1 | 61±4.0 | 73±2.8 | 70±3.4 | 65±4.0 |
| I/R+PostC | 79±3.8 | 68±2.6 | 76±3.9 | 69±2.5 | 61±3.8 |
| I/R+Mel | 76±4.1 | 64±3.4 | 75±3.1 | 72±4.2 | 64±2.9 |
| Px+I/R | 84±3.3 | 66±3.5 | 82±3.4 | 74±3.7 | 65±2.2 |
| Px+I/R+PostC | 81±4.6 | 60±2.8 | 81±1.2 | 71±2.1 | 64±3.6 |
| Px+I/R+PostC+Mel | 82±2.6 | 65±2.7 | 79±2.2 | 72±1.9 | 66±3.1 |
I/R - ischemia/reperfusion, PostC - postconditioning, mel - melatonin 10 mg/kg, Px: pinealectomy, n=7
PostC and melatonin-reduced I/R injury in cardiac tissue
In non-Px rats, I/R-induced myocardial infarct size was significantly decreased with PostC and melatonin (from 35.1±2.5% to 28.43±1.1% and 26.03±1.0%, respectively) (p<0.05). The reduction in the two treatment groups was not significant when compared to each other. On the other hand, the infarct size was significantly increased in Px rats (54.68±1.5%) in comparison to non-Px rats. In Px rats, PostC decreased the infarct size, but this decrease was not significant (48.9±1.2%) (p>0.05). A significant preservation was observed with pre-PostC melatonin administration (33.46±1.5%) (p<0.05) (Fig. 3).
Figure 3.
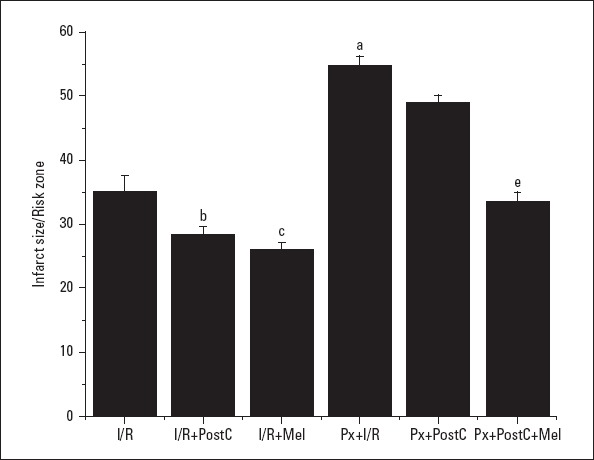
Effects of PostC, melatonin and Px administrations on infarct size. (a) Significant difference when compared to ischemia/reperfusion, (b) Significant difference due to postconditioning, (c) Significant difference due to application of melatonin, (d) Significant difference due to postconditioning in pinealectomized rats, (e) Significant difference due to postconditioning and melatonin co-administration in pinealectomized rats (p<0.05)
I/R - ischemia/reperfusion, PostC - postconditioning, mel - melatonin (10 mg/kg), Px - pinealectomy, n=7
PostC and melatonin regulated energy metabolism and protect mitochondria by affecting UCP3 and irisin levels
We hypothesized that UCP3 is a protective molecule and that it mediates the protective effect of PostC and melatonin. We observed that UCP3 mRNA and protein expressions decreased with I/R (0.08±0.01, 0.48±0.01 times) and Px (0.35±0.17, 0.7±0.02 times, respectively) administrations when compared to the control (1±0.17, 1±0.06, respectively) and increased with PostC (0.28±0.02, 0.74±0.01 times, respectively) and melatonin (0.46±0.15, 0.97±0.07 times, respectively) administrations compared to the I/R group (Fig. 4, 5). In Px groups, the UCP3 mRNA expression decreased with I/R (0.04±0.01, 0.71±0.05 times) significantly when compared with Px groups (0.35±0.17, 0.69±0.02 times) (p<0.05), but there was no significant change in protein expression. PostC (0.06±0.01, 0.61±0.01 times) did not create significant effect in UCP3 levels compared to Px+I/R groups (0.04±0.01, 0.7±0.06 times). Melatonin and PostC co-administration (0.07±0.02, 0.75±0.07 times) increased mRNA and protein expression compared to Px+I/R. Although the increases did not reach significance, there was more rise compared to the Px+PostC group.
Figure 4.
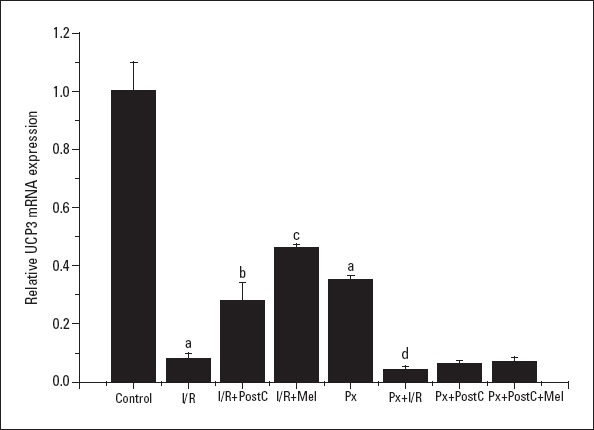
Effects of PostC, melatonin and Px administrations on UCP3 mRNA expression. (a) Significant difference when compared to control, (b) Significant difference due to postconditioning when compared to ischemia/reperfusion, (c) Significant difference due to application of melatonin when compared to ischemia/reperfusion, (d) Significant difference due to I/R in pinealectomized rats (p<0.05)
I/R - ischemia/reperfusion, PostC - postconditioning, mel - melatonin (10 mg/kg), Px - pinealectomy, n=7
Figure 5.
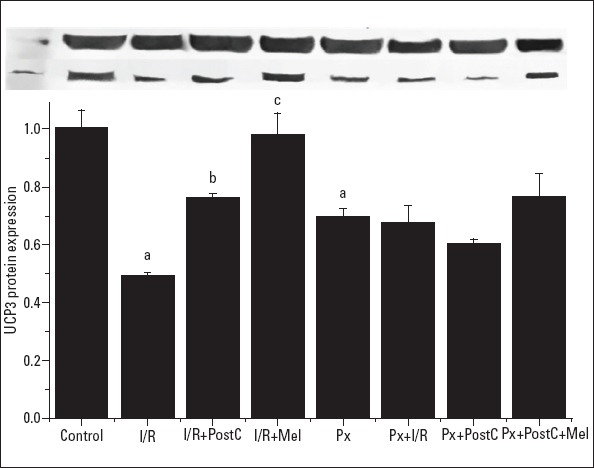
Effects of PostC, melatonin and Px administrations on UCP3 protein expression. (a) Significant difference when compared to control, (b) Significant difference due to postconditioning when compared to ischemia/reperfusion, (c) Significant difference due to application of melatonin when compared to ischemia/reperfusion, (p<0.05)
I/R - ischemia/reperfusion, PostC - postconditioning, mel - melatonin (10 mg/kg), Px - pinealectomy, n=7
Irisin mRNA and protein expressions increased with I/R (I/R: 1.2±0.09, 1.37±0.05; PX+I/R: 1.9±0.19, 1.45±0.09 times, respectively) and Px (1.92±0.22, 1.36±0.02 times) administration, but the increase in the Px group was significant when compared to control, only. However, irisin protein expressions increased by 37% in the I/R group compared to control group (Fig. 6, 7). The levels decreased with PostC (0.19±0.02, 0.75±0.02; Px+PostC 0.18±0.07, 0.72±0.01 times) and melatonin (0.14±0.05, 0.61±0.01 times) when compared to I/R. The PostC treatment reduced the level of irisin significantly in Px groups. However, we saw further reduction when it was co-administered with melatonin in irisin mRNA protein expression (0.12±0.04, 0.74±0.06 times). These results confirmed that irisin has direct effects on the cardiac tissue and plays important roles in protection of PostC and melatonin treatments.
Figure 6.
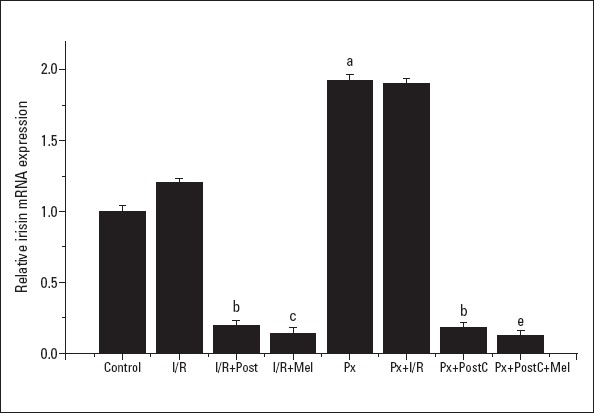
Effects of PostC, melatonin and Px administrations on irisin mRNA expression. (a) Significant difference when compared to control, (b) Significant difference due to postconditioning when compared to ischemia/reperfusion, (c) Significant difference due to application of melatonin when compared to ischemia/reperfusion, (e) Significant difference due to postconditioning and melatonin co-administration when compared to ischemia/reperfusion in pinealectomized rats (p<0.05)
I/R - ischemia/reperfusion, PostC - postconditioning, mel - melatonin (10 mg/kg), Px - pinealectomy, n=7
Figure 7.
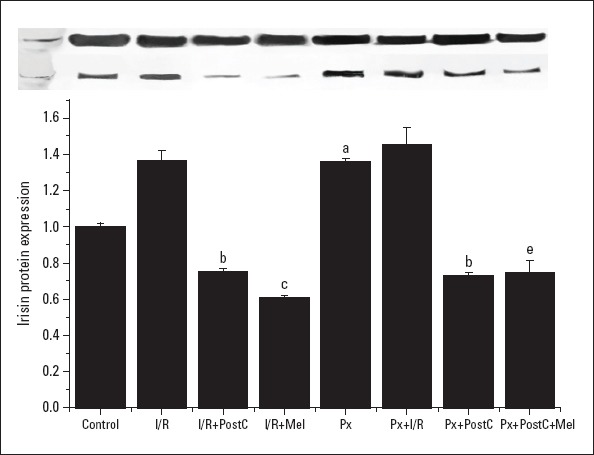
Effects of PostC, melatonin and Px administrations on irisin protein expression. (a) Significant difference when compared to control, (b) Significant difference due to postconditioning when compared to ischemia/reperfusion, (c) Significant difference due to application of melatonin when compared to ischemia/reperfusion, (e) Significant difference due to postconditioning and melatonin co-administration when compared to ischemia/reperfusion in pinealectomized rats (p<0.05)
I/R - ischemia/reperfusion, PostC - postconditioning, mel - melatonin (10 mg/kg), Px - pinealectomy, n=7
PostC and melatonin reduce inflammation by affecting the NFkB pathway
The NFkB level increased with I/R in both Px (compared to Px) and non-Px groups (when compared to control), and decreased with PostC and melatonin (when compared to I/R or Px+I/R) (Fig. 8, 9). In the Px group, there was a greater decrease in the co-administration of PostC and melatonin when compared to Px+PostC group (the mRNA expressions–CONTROL: 1±0.26, I/R: 2.19±0.36, I/R+POSTC: 0.45±0.18, I/R+MEL: 0.44±0.15, PX: 1.11±0.20, PX+I/R: 2.24±0.21, PX+POSTC: 1.11±0.43, PX+ POSTC+MEL: 0.51±0.04 times and the protein expressions: CONTROL: 1±0.03, I/R: 1.88±0.01, I/R+POSTC: 1.44±0.01, I/R+MEL: 1.32±0.19, PX:1.26±0.01, PX+I/R: 2.2±0.05, PX+POSTC:1.67±0.01, PX+ POSTC+MEL: 1.59±0.03 times).
Figure 8.
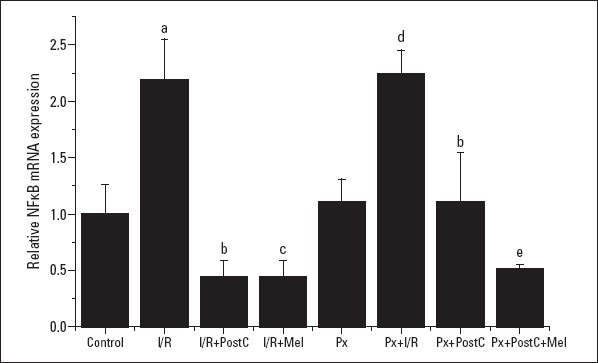
Effects of PostC, melatonin and Px administrations on NFkB mRNA expression. (a) Significant difference when compared to control, (b) Significant difference due to postconditioning when compared to ischemia/reperfusion, (c) Significant difference due to application of melatonin when compared to ischemia/reperfusion, (d) Significant difference due to I/R in Px rats, (e) Significant difference due to postconditioning and melatonin co-administration when compared to ischemia/reperfusion in pinealectomized rats
I/R - ischemia/reperfusion, PostC - postconditioning, mel - melatonin (10 mg/kg), Px - pinealectomy, n=7
Figure 9.
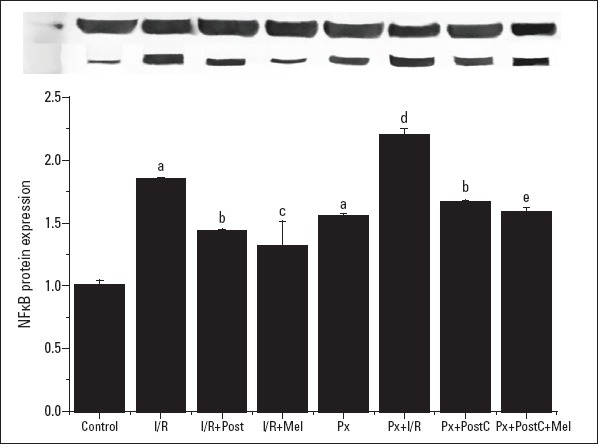
Effects of PostC, melatonin and Px administrations on NFkB protein expression. (a) Significant difference when compared to control, (b) Significant difference due to postconditioning when compared to ischemia/reperfusion, (c) Significant difference due to application of melatonin when compared to ischemia/reperfusion, (d) Significant difference due to I/R in Px rats, (e) Significant difference due to postconditioning and melatonin co-administration when compared to ischemia/reperfusion in pinealectomized rats (p<0.05)
I/R - ischemia/reperfusion, PostC - postconditioning, mel - melatonin (10 mg/kg), Px - pinealectomy, n=7
Discussion
In our study, the infarct size due to I/R decreased with PostC and melatonin significantly in non-Px rats. The infarct size that increased in Px rats significantly when compared to the controls tended to decrease with PostC; however, the difference was not significant. On the other hand, a significant protective effect was observed when melatonin was administered before PostC in Px rats.
The protective effects of melatonin in I/R were demonstrated in many studies (17-19). It was also shown in the studies that melatonin reduces the I/R-related cardiac arrhythmias, the infarct size, and oxidative changes and that it increases survival (18). The infarct size increased significantly with Px in the present study. Our previous study has shown that the infarct size was large in Px hearts when compared to the control group, and preservation of endogenous melatonin levels was found to have cardioprotective effects on the I/R injury (17). In doxorubicin-induced cardiotoxicity, melatonin treatment in both Px and non-Px rats significantly reduced MDA levels (19). In our study, we performed pharmacological melatonin replacement in a 10 mg/kg dose in rats that were deprived of physiological melatonin by Px. Liu et al. (20) compared single doses of 2.5, 5, and 10 mg/kg (intraperionally) melatonin and showed that 10 mg/kg melatonin could effectively protect myocardium without affecting other physiological functions in rats. Simko et al. (21) have reported that pretreatment with 10 mg/kg melatonin for 7 days provided cardioprotective effect in the isoproterenol-induced cardiac injury model. Şehirli et al. (22) with a similar I/R protocol have demonstrated that 10 mg/kg (intraperionally) melatonin for 4 weeks ameliorates ischemic heart failure in rats. In addition, in our previous study, it was shown that the infarct size increased in the Px group and decreased with melatonin supplementation in the Px+mel group (14).
We observed that PostC significantly reduced the I/R-related infarct size in non-Px rats. PostC reduced the I/R injury by reducing the infarct size and improving the cardiac function in the rat model (23). According to a clinical study, PostC has been applied in cardiopulmonary bypass surgery in children who had been operated for congenital malformations, and the troponin I and creatine kinase MB, and the requirement for post-operative inotropic drugs significantly decreased (24).
We showed that the PostC protection disappeared with Px, and significant protection was observed again when melatonin and PostC were administered together. Similarly, ischemic PostC and sevoflurane PostC reduced the infarction by 50% in non-diabetic rats, but this effect completely disappeared in diabetic rats (25). In another study, sevoflurane PostC significantly reduced the infarct size in young rats; however, the protection was not found to be significant in older rats (26). Our results were consistent with our hypothesis. If melatonin replacement treatment is performed when physiological melatonin is decreased, the protective effect of PostC can be restored.
Excessive amounts of reactive oxygen species (ROS) caused by oxygenation of tissues together with reperfusion are the most important cause of I/R injury. One of the most important sources of cellular ROS is mitochondria and excessive ROS production is a major cause of mitochondrial dysfunction paradoxically. Mitochondria plays a critical role in oxidative stress, ion hemostasis, membrane potential modulation, and cell life (27). UCP3 is a member of mitochondrial anion carrier proteins family. This gene facilitates the transfer of anions from inner to outer and protons from the outer to the inner in the mitochondrial membrane. It also reduces the mitochondrial membrane potential in mammalian cells. It is thought that the protein product of this gene protects mitochondria against lipid-induced oxidative stress (28). In the present study, the UCP3 level significantly decreased with I/R. Similarly, UCP3 protein expressions significantly decreased with doxorubicin-induced heart failure (29). We found that the UCP3 level significantly increased with melatonin and PostC administration in non-Px groups. In the Px groups, PostC and PostC+melatonin did not lead to a significant increase in the UCP3 level. To the best of our knowledge, there is no study investigating the effect of melatonin and PostC on the UCP3 levels. However, UCP3 plays a role in the cardioprotective effect of PreC (30). It was reported that the UCP3 knockout mice had two times larger infarctions, and the protective effect of PreC disappeared in these rats (31).
Irisin, a myokine related to exercise and energy metabolism, plays a role in the regulation of mitochondrial thermogenesis and ischemic reactions in cardiomyocytes. The elevation of irisin in the tissue is associated with many pathologies such as MI, chronic kidney disease, and obesity (32). In our study, the irisin levels increased with I/R in the non-Px group; however, this increase was not significant. It has been determined that the irisin level increased in doxorubicin-related MI (33). Aronis et al. (34) showed that elevated irisin was associated with adverse cardiovascular events in coronary artery diseases developing after coronary intervention. We observed that the irisin increased to a higher level with Px when compared to the I/R injury. Similarly, Emanuele et al. (35) reported that the serum irisin was higher in the healthy elderly when compared to younger healthy and patients with acute MI. In the only study that investigated the effect of melatonin on the irisin level, the circulating irisin levels were not influenced by melatonin in both diabetic fat and lean rats (36). In our study, PostC and pharmacological melatonin administration significantly reduced the irisin level, both in non-Px and Px rats. PostC following Px showed a protective effect on the irisin level, and this protection was more pronounced with the co-administration of PostC and melatonin. Irisin can lead to these effects that affect UCPs levels (37), superoxide dismutase2 activity, and apoptosis in cardiomyocytes (38), modulate neurons and control vagal tone (39), or suppress the opening of mPTP (40). In addition, irisin downregulates the expression receptor activators of NFkB (41).
NFkB regulates the expression of proinflammatory cytokines and mitochondrial respiration (42) and is activated in response to oxidative stress (43). However, the decrease in the ROS level helps to decrease the NFkB level indirectly and reduces inflammations, and mitochondrial integrity is maintained possibly (44). In addition, it has been shown that the mTOR activity is limited by NFkB inhibition (45) and that autophagy-related genes induced by inhibition of NFkB interacting with mTOR may play a vital role in reducing myocardial I/R damage (46). Pharmacological supression of the NFkB activity improves defects in mitochondrial respiratory function, reduces the loss of UCP3, and decreases the increase in ROS (47). In addition, the NFkB pathway plays a role in the regulation of mitochondrial dynamics and the OPA1 expression (48) and is influenced by mitochondrial markers ANT and VDAC, contributing to enhanced oxidative stress caused by cardiac dysfunction in type II diabetes (49). NFkB increased after hemorrhagic shock (50), and the NFkB knockout mice reduced I/R injury sensitivity (51). Similarly, while significant increases were detected in the NFkB mRNA level with I/R and Px, its level decreased with PostC and melatonin in non-Px groups. Tilstra et al. (52) showed that the NFkB transcriptional activity is increased in various tissues with aging and degenerative diseases associated with aging, and inhibition of NFkB delays the occurrence of age-related symptoms and pathologies. It was shown that NFkBp50 and NFkBp52 were increased in the heart of old mice (53). According to a study, melatonin reduces the upregulation of various proinflammatory cytokines, such as interleukins and TNFα, by preventing translocation of NFkB to the nucleus (54). The NFkB levels also decreased with PostC and melatonin in the present study. This may be related to a decrease in the antioxidant and antiproliferative effect capacity because of a decreasing melatonin level with aging. In addition, the administration of a selective NFkB inhibitor attenuated cardioprotective effects of remote PreC and erythropoietin PreC (55).
Volatile anesthetics showed a conditioning effect on the myocardium in cardiac surgery. In a meta-analysis, the use of volatile anesthetics resulted in a significant decrease in post-operative MI, and the in-hospital mortality incidence, and troponin levels, post-operative inotropic need, duration of intensive care unit stay, and duration of mechanic ventilation (3). In the non-Px group, both PostC and melatonin were observed to reduce the infarct size significantly and affect the biochemical values similarly. Although melatonin showed a better protection and no significant difference was observed between the two groups, melatonin may be an agent mimicking the effect of conditioning, such as of volatile anesthetics.
In the present study, non-Px group treatments aimed to protect the mitochondria and energy metabolism by increasing UCP3 and decreasing irisin mRNA and protein expressions. However, the treatments also reduced the level of ROS to protect the tissue from the destructive effects of oxidative stress. The decrease in the ROS level helped to reduce the NFkB level indirectly and reduced inflammations and mitochondrial integrity was possibly maintained. Non-Px groups represent healthy adults, and this grouping enabled us to compare the effects of melatonin and PostC. Thus, we wanted to examine whether melatonin could be a pharmacological conditioning agent.
The Px group symbolizes physiological conditions in which melatonin decreases, such as age, and the Px I/R group represents the pathological conditions associated with melatonin reduction. By comparing the infarct size and biochemical parameters in Px groups, we can see that the protective effect of PostC disappeared when physiological melatonin decreased, and this effect was observed to reverse with melatonin replacement.
Conclusion
UCP3, irisin, and NFkB expression levels may play an important role in the cardioprotective effect of PostC and melatonin.
The protective effect of PostC disappeared when physiological melatonin decreased, and this effect was seen to reverse with melatonin replacement. It is suggested that cardioprotection may be provided through preserving the melatonin endogenous level by modifying the lifestyle, and melatonin replacement may be recommended in the presence of predisposing conditions for cardiac diseases in which the melatonin level decreases.
It is suggested that melatonin may be an agent that leads to pharmacological conditioning as melatonin and PostC are protective, showing similar rates and similar parameters.
Acknowledgment:
This study is supported by TUBITAK (115S323) and FUBAP (TF.14.70).
Footnotes
Conflict of interest: None declared.
Peer-review: Externally peer-reviewed.
Authorship contributions: Concept – G.A., E.Ş.; Design – G.A., E.Ş.; Supervision – G.A., E.Ş.; Funding – G.A., E.Ş.; Materials – G.A., E.Ş.; Data collection and/or processing – G.A., E.Ş.; Analysis and/or interpretation – G.A., H.F.G., A.T., E.Ş.; Literature search – G.A., H.F.G., A.T., E.Ş.; Writing – G.A., E.Ş.; Critical review – G.A., H.F.G., A.T., E.Ş.
References
- 1.Verma S, Fedak PWM, Weisel RD, Butany J, Rao V, Maitland A, et al. Fundamentals of reperfusion injury for the clinical cardiologist. Circulation. 2002;105:2332–6. doi: 10.1161/01.cir.0000016602.96363.36. [DOI] [PubMed] [Google Scholar]
- 2.Lopez AD, Murray CC. The global burden of disease 1990-2020. Nat Med. 1998;4:1241–3. doi: 10.1038/3218. [DOI] [PubMed] [Google Scholar]
- 3.Bousselmi R, Lebbi MA, Ferjani M. Myocardial ischemic conditioning:Physiological aspects and clinical applications in cardiac surgery. J Saudi Heart Assoc. 2014;26:93–100. doi: 10.1016/j.jsha.2013.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Marzilli M, Morrone D, Guarini G. Postconditioning. Heart Metab. 2012;54:20–4. [Google Scholar]
- 5.Zhao ZQ, Corvera JS, Halkos ME, Kerendi F, Wang NP, Guyton RA, et al. Inhibition of myocardial injury by ischemic postconditioning during reperfusion:comparison with ischemic preconditioning. Am J Physiol Heart Circ Physiol. 2003;285:H579–88. doi: 10.1152/ajpheart.01064.2002. [DOI] [PubMed] [Google Scholar]
- 6.Laskey WK, Selzer F, Jacobs AK, Cohen HA, Holmes DR, Jr, Wilensky RL, et al. Importance of the postdischarge interval in assessing major adverse clinical event rates following percutaneous coronary intervention. Am J Cardiol. 2005;95:1135–9. doi: 10.1016/j.amjcard.2005.01.038. [DOI] [PubMed] [Google Scholar]
- 7.Heusch G. Reduction of infarct size by ischaemic post-conditioning in humans:fact or fiction?Eur Heart J. 2012;33:13–5. doi: 10.1093/eurheartj/ehr341. [DOI] [PubMed] [Google Scholar]
- 8.Wu Y, Wan J, Zhen W-Z, Chen LF, Zhan J, Ke JJ, et al. The effect of butorphanol postconditioning on myocardial ischaemia reperfusion injury in rats. Interact Cardiovasc Thorac Surg. 2014;18:308–12. doi: 10.1093/icvts/ivt516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Reiter RJ, Tan DX, Galano A. Melatonin reduces lipid peroxidation and membrane viscosity. Front Physiol. 2014;5:377. doi: 10.3389/fphys.2014.00377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jiki Z, Lecour S, Nduhirabandi F. Cardiovascular Benefits of Dietary Melatonin:A Myth or a Reality?Front Physiol. 2018;9:528. doi: 10.3389/fphys.2018.00528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shahzad T, Kasseckert SA, Iraqi W, Johnson V, Schulz R, Schlüter KD, et al. Mechanisms involved in postconditioning protection of cardiomyocytes against acute reperfusion injury. J Mol Cell Cardiol. 2013;58:209–16. doi: 10.1016/j.yjmcc.2013.01.003. [DOI] [PubMed] [Google Scholar]
- 12.Reiter RJ, Tan DX, Kim SJ, Qi W. Melatonin as a pharmacological agent against oxidative damage to lipids and DNA. Proc West Pharmacol Soc. 1998;41:229–36. [PubMed] [Google Scholar]
- 13.Hoffman RA, Reiter RJ. Rapid pinealectomy in hamsters and other small rodents. Anat Rec. 1965;153:19–21. doi: 10.1002/ar.1091530103. [DOI] [PubMed] [Google Scholar]
- 14.Sahna E, Acet A, Ozer MK, Olmez E. Myocardial ischemia-reperfusion in rats:reduction of infarct size by either supplemental physiological or pharmacological doses of melatonin. J Pineal Res. 2002;33:234–8. doi: 10.1034/j.1600-079x.2002.02924.x. [DOI] [PubMed] [Google Scholar]
- 15.Fang J, Chen L, Wu L, Li W. Intra-cardiac remote ischemic post-conditioning attenuates ischemia-reperfusion injury in rats. Scand Cardiovasc J. 2009;43:386–94. doi: 10.1080/14017430902866681. [DOI] [PubMed] [Google Scholar]
- 16.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–8. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 17.Sahna E, Olmez E, Acet A. Effects of physiological and pharmacological concentrations of melatonin on ischemia-reperfusion arrhythmias in rats:can the incidence of sudden cardiac death be reduced? J Pineal Res. 2002;32:194–8. doi: 10.1034/j.1600-079x.2002.1o853.x. [DOI] [PubMed] [Google Scholar]
- 18.Sahna E, Parlakpinar H, Turkoz Y, Acet A. Protective effects of melatonin on myocardial ischemia/reperfusion induced infarct size and oxidative changes. Physiol Res. 2005;54:491–5. [PubMed] [Google Scholar]
- 19.Sahna E, Parlakpinar H, Ozer MK, Ozturk F, Ozugurlu F, Acet A. Melatonin protects against myocardial doxorubicin toxicity in rats:role of physiological concentrations. J Pineal Res. 2003;35:257–61. doi: 10.1034/j.1600-079x.2003.00084.x. [DOI] [PubMed] [Google Scholar]
- 20.Liu LF, Qin Q, Qian ZH, Shi M, Deng QC, Zhu WP, et al. Protective effects of melatonin on ischemia-reperfusion induced myocardial damage and hemodynamic recovery in rats. Eur Rev Med Pharmacol Sci. 2014;18:3681–6. [PubMed] [Google Scholar]
- 21.Simko F, Bednarova KR, Krajcirovicova K, Hrenak J, Celec P, Kamodyova N, et al. Melatonin reduces cardiac remodeling and improves survival in rats with isoproterenol-induced heart failure. J Pineal Res. 2014;57:177–84. doi: 10.1111/jpi.12154. [DOI] [PubMed] [Google Scholar]
- 22.Şehirli AÖ, Koyun D, Tetik Ş, Özsavcı D, Yiğiner Ö, Çetinel Ş. Melatonin protects against ischemic heart failure in rats. J Pineal Res. 2013;55:138–48. doi: 10.1111/jpi.12054. [DOI] [PubMed] [Google Scholar]
- 23.Zhang L, Ma J, Liu H. Protective effect of ischemic postconditioning against ischemia reperfusion-induced myocardium oxidative injury in IR rats. Molecules. 2012;17:3805–17. doi: 10.3390/molecules17043805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Luo W, Li B, Lin G, Huang R. Postconditioning in cardiac surgery for tetralogy of Fallot. J Thorac Cardiovasc Surg. 2007;133:1373–4. doi: 10.1016/j.jtcvs.2007.01.028. [DOI] [PubMed] [Google Scholar]
- 25.Drenger B, Ostrovsky IA, Barak M, Nechemia-Arbely Y, Ziv E, Axelrod JH. Diabetes blockade of sevoflurane postconditioning is not restored by insulin in the rat heart:phosphorylated signal transducer and activator of transcription 3- and phosphatidylinositol 3-kinase-mediated inhibition. Anesthesiology. 2011;114:1364–72. doi: 10.1097/ALN.0b013e31820efafd. [DOI] [PubMed] [Google Scholar]
- 26.Li H, Zhou C, Chen D, Fang N, Yao Y, Li L. Failure to protect against myocardial ischemia-reperfusion injury with sevoflurane postconditioning in old ratsin vivo. Acta Anaesthesiol Scand. 2013;57:1024–31. doi: 10.1111/aas.12156. [DOI] [PubMed] [Google Scholar]
- 27.Sifuentes-Franco S, Pacheco-Moisés FP, Rodríguez-Carrizalez AD, Miranda-Díaz AG. The Role of Oxidative Stress, Mitochondrial Function, and Autophagy in Diabetic Polyneuropathy. J Diabetes Res. 2017;2017:1673081. doi: 10.1155/2017/1673081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bézaire V, Seifert EL, Harper ME. Uncoupling protein-3:clues in an ongoing mitochondrial mystery. FASEB J. 2007;21:312–24. doi: 10.1096/fj.06-6966rev. [DOI] [PubMed] [Google Scholar]
- 29.Bugger H, Guzman C, Zechner C, Palmeri M, Russell KS, Russell RR., 3rd Uncoupling protein downregulation in doxorubicin-induced heart failure improves mitochondrial coupling but increases reactive oxygen species generation. Cancer Chemother Pharmacol. 2011;67:1381–8. doi: 10.1007/s00280-010-1441-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ozcan C, Palmeri M, Horvath TL, Russell KS, Russell RR., 3rd Role of uncoupling protein 3 in ischemia-reperfusion injury, arrhythmias, and preconditioning. Am J Physiol Heart Circ Physiol. 2013;304:H1192–200. doi: 10.1152/ajpheart.00592.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen Y, Liu J, Zheng Y, Wang J, Wang Z, Gu S, et al. Uncoupling protein 3 mediates H2O2preconditioning-afforded cardioprotection through the inhibition of MPTP opening. Cardiovasc Res. 2015;105:192–202. doi: 10.1093/cvr/cvu256. [DOI] [PubMed] [Google Scholar]
- 32.Ho MY, Wen MS, Yeh JK, Hsieh IC, Chen CC, Hsieh MJ, et al. Excessive irisin increases oxidative stress and apoptosis in murine heart. Biochem Biophys Res Commun. 2018;503:2493–8. doi: 10.1016/j.bbrc.2018.07.005. [DOI] [PubMed] [Google Scholar]
- 33.Aydin S, Eren MN, Kuloglu T, Aydin S, Yilmaz M, Gul E, et al. Alteration of serum and cardiac tissue adropin, copeptin, irisin and TRPM2 expressions in DOX treated male rats. Biotech Histochem. 2015;90:197–205. doi: 10.3109/10520295.2014.977949. [DOI] [PubMed] [Google Scholar]
- 34.Aronis KN, Moreno M, Polyzos SA, Moreno-Navarrete JM, Ricart W, Delgado E, et al. Circulating irisin levels and coronary heart disease:association with future acute coronary syndrome and major adverse cardiovascular events. Int J Obes (Lond) 2015;39:156–61. doi: 10.1038/ijo.2014.101. [DOI] [PubMed] [Google Scholar]
- 35.Emanuele E, Minoretti P, Pareja-Galeano H, Sanchis-Gomar F, Garatachea N, Lucia A. Serum irisin levels, precocious myocardial infarction, and healthy exceptional longevity. Am J Med. 2014;127:888–90. doi: 10.1016/j.amjmed.2014.04.025. [DOI] [PubMed] [Google Scholar]
- 36.Jiménez-Aranda A, Fernández-Vázquez G, Campos D, Tassi M, Velasco-Perez L, Tan DX, et al. Melatonin induces browning of inguinal white adipose tissue in Zucker diabetic fatty rats. J Pineal Res. 2013;55:416–23. doi: 10.1111/jpi.12089. [DOI] [PubMed] [Google Scholar]
- 37.Erden Y, Tekin S, Sandal S, Onalan EE, Tektemur A, Kirbag S. Effects of central irisin administration on the uncoupling proteins in rat brain. Neurosci Lett. 2016;618:6–13. doi: 10.1016/j.neulet.2016.02.046. [DOI] [PubMed] [Google Scholar]
- 38.Wang Z, Chen K, Han Y, Zhu H, Zhou X, Tan T, et al. Irisin Protects Heart Against Ischemia-Reperfusion Injury Through a SOD2-Dependent Mitochondria Mechanism. J Cardiovasc Pharmacol. 2018;72:259–69. doi: 10.1097/FJC.0000000000000608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Brailoiu E, Deliu E, Sporici RA, Brailoiu GC. Irisin evokes bradycardia by activating cardiac-projecting neurons of nucleus ambiguus. Physiol Rep. 2015;3:pii:e12419. doi: 10.14814/phy2.12419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang H, Zhao YT, Zhang S, Dubielecka PM, Du J, Yano N, et al. Irisin plays a pivotal role to protect the heart against ischemia and reperfusion injury. J Cell Physiol. 2017;232:3775–85. doi: 10.1002/jcp.25857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ma Y, Qiao X, Zeng R, Cheng R, Zhang J, Luo Y, et al. Irisin promotes proliferation but inhibits differentiation in osteoclast precursor cells. FASEB J. 2018;32:5813–23. doi: 10.1096/fj.201700983RR. [DOI] [PubMed] [Google Scholar]
- 42.Mauro C, Leow SC, Anso E, Rocha S, Thotakura AK, Tornatore L, et al. NF-κB controls energy homeostasis and metabolic adaptation by upregulating mitochondrial respiration. Nat Cell Biol. 2011;13:1272–9. doi: 10.1038/ncb2324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Burma O, Onat E, Uysal A, Ilhan N, Erol D, Ozcan M, et al. Effects of rosuvastatin on ADMA, rhokinase, NADPH oxidase, caveolin-1, hsp 90 and NFkB levels in a rat model of myocardial ischaemia-reperfusion. Cardiovasc J Afr. 2014;25:212–6. doi: 10.5830/CVJA-2014-038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Morgan MJ, Liu ZG. Crosstalk of reactive oxygen species and NF-κB signaling. Cell Res. 2011;21:103–15. doi: 10.1038/cr.2010.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Okamoto T, Ozawa Y, Kamoshita M, Osada H, Toda E, Kurihara T, et al. The Neuroprotective Effect of Rapamycin as a Modulator of the mTOR-NF-κB Axis during Retinal Inflammation. PLoS One. 2016;11:e0146517. doi: 10.1371/journal.pone.0146517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Huang WQ, Wen JL, Lin RQ, Wei P, Huang F. Effects of mTOR/NF-κB signaling pathway and high thoracic epidural anesthesia on myocardial ischemia-reperfusion injury via autophagy in rats. J Cell Physiol. 2018;233:6669–78. doi: 10.1002/jcp.26320. [DOI] [PubMed] [Google Scholar]
- 47.Nisr RB, Shah DS, Ganley IG, Hundal HS. Proinflammatory NFkB signalling promotes mitochondrial dysfunction in skeletal muscle in response to cellular fuel overloading. Cell Mol Life Sci. 2019;76:4887–904. doi: 10.1007/s00018-019-03148-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Laforge M, Rodrigues V, Silvestre R, Gautier C, Weil R, Corti O, et al. NF-κB pathway controls mitochondrial dynamics. Cell Death Differ. 2016;23:89–98. doi: 10.1038/cdd.2015.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mariappan N, Elks CM, Sriramula S, Guggilam A, Liu Z, Borkhsenious O, et al. NF-kappaB-induced oxidative stress contributes to mitochondrial and cardiac dysfunction in type II diabetes. Cardiovasc Res. 2010;85:473–83. doi: 10.1093/cvr/cvp305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lin KH, Liu CL, Kuo WW, Paul CR, Chen WK, Wen SY, et al. Early Fluid Resuscitation by Lactated Ringer's Solution Alleviate the Cardiac Apoptosis in Rats with Trauma-Hemorrhagic Shock. PloS One. 2016;11:e0165406. doi: 10.1371/journal.pone.0165406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhang XQ, Tang R, Li L, Szucsik A, Javan H, Saegusa N, et al. Cardiomyocyte-specific p65 NF-κB deletion protects the injured heart by preservation of calcium handling. Am J Physiol Heart Circ Physiol. 2013;305:H1089–97. doi: 10.1152/ajpheart.00067.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tilstra JS, Clauson CL, Niedernhofer LJ, Robbins PD. NF-κB in Aging and Disease. Aging Dis. 2011;2:449–65. [PMC free article] [PubMed] [Google Scholar]
- 53.Forman K, Vara E, García C, Kireev R, Cuesta S, Escames G, et al. Effect of a combined treatment with growth hormone and melatonin in the cardiological aging on male SAMP8 mice. J Gerontol A Biol Sci Med Sci. 2011;66:823–34. doi: 10.1093/gerona/glr083. [DOI] [PubMed] [Google Scholar]
- 54.Cuesta S, Kireev R, Forman K, García C, Escames G, Ariznavarreta C, et al. Melatonin improves inflammation processes in liver of senescence-accelerated prone male mice (SAMP8) Exp Gerontol. 2010;45:950–6. doi: 10.1016/j.exger.2010.08.016. [DOI] [PubMed] [Google Scholar]
- 55.Diwan V, Kant R, Jaggi AS, Singh N, Singh D. Signal mechanism activated by erythropoietin preconditioning and remote renal preconditioning-induced cardioprotection. Mol Cell Biochem. 2008;315:195–201. doi: 10.1007/s11010-008-9808-3. [DOI] [PubMed] [Google Scholar]


